Abstract
Objectives
The objective was to demonstrate superiority of a fully vs. semi-automated approach (5D CNS+™) and to verify operators could handle and benefit from a fully automated rendering volumetric datasets to generate a complete fetal neurosonogram.
Methods
A total of 136 stored three-dimensional (3D) volumes of the brain of unselected, structurally normal fetuses were examined. Two operators applied both software versions for detailed assessment of the fetal central nervous system (CNS). The procession time was measured for each operator and for both program versions. The number of correctly calibrated planes were evaluated and necessity for manual adjustment of the planes was registered.
Results
The intraclass correlation coefficient was 0.507 (0.307–0.648) for semi-automated and 0.782 (0.693–0.846) for fully automated 5D CNS+™. The acquisition time of application for semi-automated 5D CNS+™ was 27.70 s ± 6.28 s for operator 1 and 33.20 s ± 9.67 s for operator 2, for fully automated 5D CNS+™ 10.89 s ± 0.85 s for operator 1 and 10.79 s ± 0.60 s for operator 2 (p<0.0001). The statistical analysis for manually corrected planes by both operators between both software algorithms showed a Bland-Altman-Bias of 1.44/9 planes for operator 1 and 1.45/9 planes for operator 2.
Conclusions
The fully automated 5D CNS+™ algorithm applied on 3D volume datasets provides examiners regardless their expertise not only enormous time efficiency, but also diagnostic confidence in evaluating details of the fetal CNS. This tremendously simplifies application in clinical routine.
Introduction
Decidedly, artificial intelligence (AI)-based applications will significantly transform future fetal imaging and shape the way of working in prenatal clinical routine [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. It is well-known that AI – as an adjunctive tool – can aid in optimizing prenatal diagnostics’ efficiency and productivity by shortening the examination time, reducing the physician’s daily workload with iterative, resource-intensive processes while improving diagnostic objectivity, reproducibility and accuracy [2], 4], 13], 14]. On the one hand, operators can particularly benefit from the supportive use of AI in the comprehensive assessment of highly complex three-dimensional (3D) anatomical structures such as the fetal heart or the fetal brain with concrete landmarks suitable for image recognition, which are frequently affected by structural defects during their organogenesis; on the other hand, it is exactly this complexity, such as motion or rapid continuous structural progression, causing challenges that need to be taken into account [5], [15], [16], [17], [18].
The overall prevalence of congenital brain anomalies – the second most common group of anomalies in fetuses after cardiac malformations – has recently been estimated to be 9.8 to 14.9 per 10,000 live births [19], [20], [21], [22]. However, their detection rate still remains rather unsatisfactory [23].
According to the currently updated international guidelines of the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG), a fetal neurosonogram comprises nine differently orientated diagnostic standard planes (SP) [24], 25]. For the all-embracing assessment of fetal structural neurodevelopment, the acquisition of SPs as well as standard biometric measurements are crucial [26].
Applications for AI-assisted tools in fetal neurosonography are abundant. Current research approaches to the application of AI-assisted methods in the context of fetal neurosonography beyond the first trimester are heterogeneous and, with some exceptions, useful applications in clinical practice are still rare [15].
A workflow-based volumetric software approach for fetal neurosonography already firmly established in clinical practice is 5D CNS+™ (Samsung Healthcare, Gangwon-do, South Korea). The software reliably enables the simultaneous reconstruction of all nine diagnostic fetal brain planes required for a comprehensive neurosonogram [27], 28].
The objective of this study was to scrutinize the benefit of the fully automated AI-enhanced 5D CNS+™ tool for comprehensive fetal neurosonography for both a beginner and an expert with particular focus on its clinical applicability. We further intended to discuss the additional value of future requirements for the characteristics of information content obtained from an optimal standardized 3D volume of the fetal CNS far beyond existing tools.
Materials and methods
Subjects
In this prospective study, a total of 136 stored 3D volumes of the brain of an unselected mixed risk collective of structurally normal singleton second- and third-trimester fetuses without congenital brain anomalies were examined. All volumes used were acquired from women undergoing targeted ultrasound survey in the tertiary prenatal ultrasound unit at of a university hospital between 2023 and 2024 and were acquired in the transthalamic plane required for biometric assessment of biparietal diameter (BPD) and head circumference (HC), resulting in a triplanar orthogonal reconstruction of fetal central nervous structures. All the examinations were performed transabdominally using a Samsung Hera W10 ultrasound system equipped with a transabdominal probe with 1–8 MHz (S-Vue™-Transducer CV1-8A), operating with the AI-enhanced post-processing software 5D CNS+™ (Samsung Healthcare, Gangwon-do, Republic of Korea). Each of the stored volumes were analyzed offline by two operators with different levels of expertise: The minimum standard for a sonographer in gynecology and obstetrics, DEGUM level 1 (German Society for Ultrasound in Medicine) (J.L.S., operator 2), certifies familiarity with the basics of ultrasound diagnostics, and DEGUM level 3 (J.W., operator 1), characterizes a proven expert far beyond basic knowledge. Both, semi- and fully automated 5D CNS+™, were applied separately and independently for comprehensive assessment of the fetal central nervous system (CNS). Beforehand, the expert selected each volume to be analyzed and confirmed its applicability for further analysis by considering certain quality requirements (e.g. absence of artifacts like fetal movements, a clearly identifiable cerebellum, minimal or absent shadowing with sufficient image sharpness and no blurring). However, both performed the analysis blinded to each other, although each operator was able to decide individually the order of the software version selected. It was not intended and practically impossible that both operators were blinded to the software types in use. Informed consent was obtained from all participants. A statement about institutional review board (IRB) approval or exemption is not available and was not required considering a retrospective analysis of anonymized data.
Acquisition of 3D volumes and application of 5D CNS+™ software versions
As already described elsewhere, as for optimal use of the semi-automated 5D CNS+™ software tool after 3D volume acquisition, a horizontal alignment of the falx in the axial acquisition plane (a plane) and, if necessary, depth adjustment of the cutting section is required manually [27], 28]. Subsequently, two seeds between the anteriormost third of the thalami (1st seed) and the cavum septi pellucidi (2nd seed) were placed and all nine diagnostic planes – axial, coronal and sagittal – were calculated and displayed automatically. In contrast, the innovative algorithm of fully automated 5D CNS+™ tool requires no further manual intervention after volume acquisition, as it automatically identifies the SPs of the fetal CNS and measures its key diagnostic features by automatic segmentation and plane-navigation. Both software versions enable biometric measurements automatically in all reconstructed planes with manual adjustment, if needed. The processing time from the beginning of the volume reconstruction to the initial display of the nine-image template was measured for each operator and for both program versions. Furthermore, the number of correctly calibrated planes was analyzed, the need for manual plane adjustment was noted and the time taken until the final result with adequately reconstructed planes has been achieved (Figure 1, Supplementary Material, Video-Clip S1).
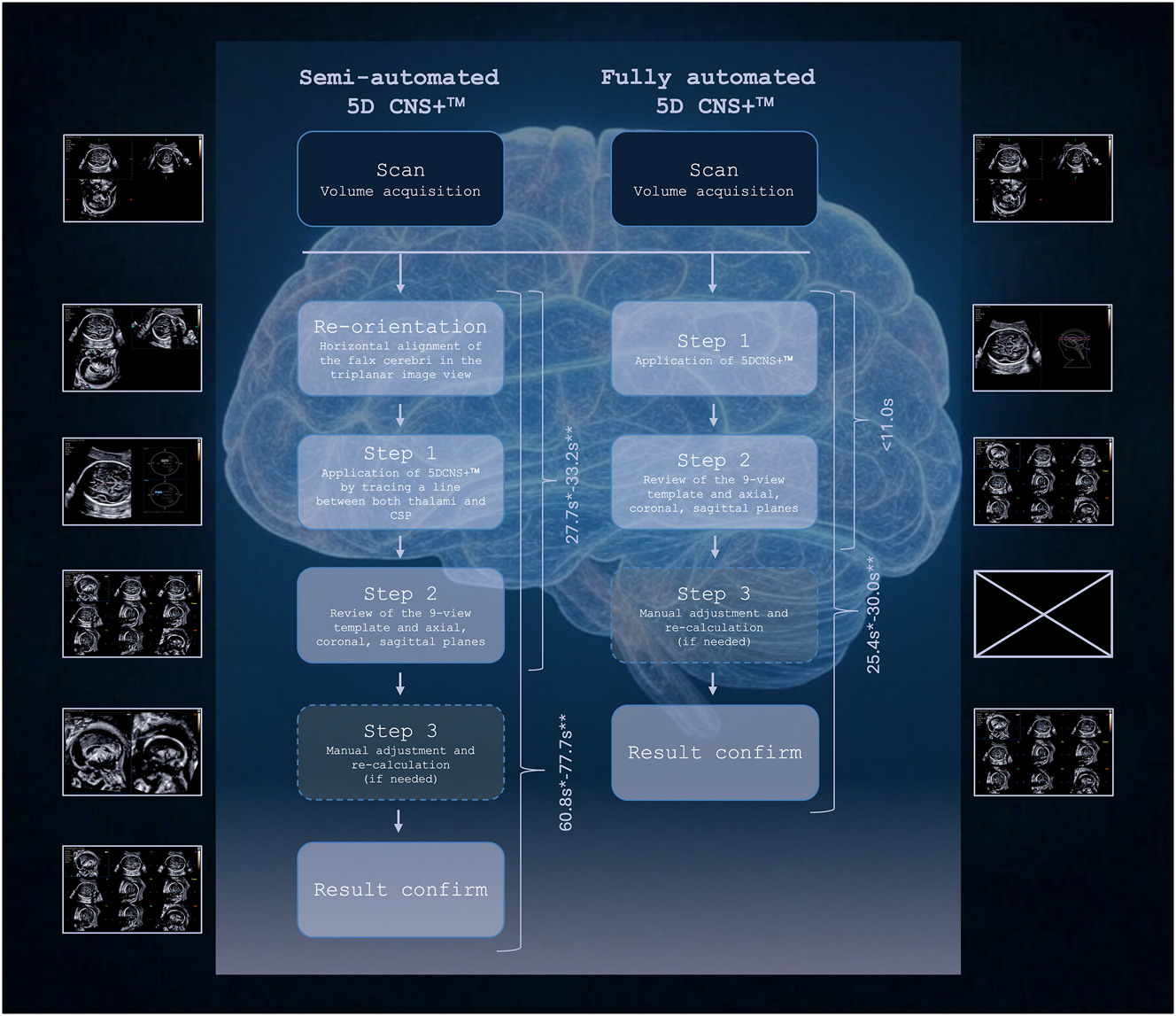
Flowchart of both software versions illustrating the workflow with reduced steps when applying fully automated 5D CNS+™ on stored 3D volumes compared to semi-automated 5D CNS+™. Most remarkably, volume reconstructions were accomplished in less than 11 s compared to semi-automated 5D CNS+™ irrespective of the operator’s expertise and with a significantly reduced need of manual plane adjustments (*: operator 1; **: operator 2). CSP, cavum septi pellucidi. Generated in parts by Grok 3.
Statistics
Descriptive statistics and Wilcoxon matched-pairs signed rank test were applied. A statistical level of p<0.05 was assumed to be significant. The median of the differences, including the 95 % confidence intervals (CI), was calculated as a measure of a scale-related effect size. The intraclass correlation coefficient (ICC) was calculated for interoperator reproducibility. ICC values were calculated using a two-way mixed effects model for absolute agreement. The data were compared between semi- and fully automated 5D CNS+™ application for both operators using a Bland-Altman plot (average between the planes to be corrected manually after processing with semi- and fully automatic 5D CNS+™ of both operators against the difference between these two) to test the agreement between both software algorithms. GraphPad Prism 10 for Mac (version 10.4.1, GraphPad Software Inc., La Jolla, CA, USA), SPSS Statistics (version 29.0.2.0, IBM Corporation, Armonk, NY, USA) and Microsoft 365 Excel for Mac (version 16.93.1, Microsoft Corp., Redmond, WA, USA) were used.
Results
The mean maternal age was 32 years (ranging from 23 to 43 years), and the mean maternal pre-pregnancy body mass index was 24.52 kg/m2 (16.90–44.05 kg/m2). The gestational age (GA) ranged from 17.4 to 33.7 weeks (average 23.1 weeks) (Table 1).
Clinical characteristics of the study population (n=136).
| Characteristics | Mean (range) |
|---|---|
| Maternal age, years | 32.60 (23–43) |
| Nulliparous, % | 44.86 |
| Primiparity, % | 40.44 |
| BMI prior to pregnancy, kg/m2 | 24.52 (16.90–44.05) |
| Gestational age at targeted ultrasound, weeks of gestation | 23.10 (17 + 3–33 + 5) |
| Fetal cephalic presentation, % | 59.56 |
The ICC between both operators of correctly depicted planes after application of both software algorithms without requirement for manual correction was 0.507 (0.307–0.648) for semi-automated 5D CNS+™ and 0.782 (0.693–0.846) for fully automated 5D CNS+™.
The acquisition time of application of both software algorithms until all nine planes are obtained without manual correction for semi-automated 5D CNS+™ was 27.70 s ± 6.28 s for operator 1 and 33.20 s ± 9.67 s for operator 2, for fully automated 5D CNS+™ 10.89 s ± 0.85 s for operator 1 and 10.79 s ± 0.60 s for operator 2 (p<0.0001) (Figure 2A). The median of the differences for operator 1 was −15.88 s (95 % CI from −17.68 s to −14.92 s), for operator 2 –20.84 s (95 % CI from −22.45 s to −18.35 s).
![Figure 2:
Semi- vs. fully automated 5D CNS+™: time benefit and manually corrected planes. (A) Acquisition time of application of semi- and fully automated 5D CNS+™ until all nine planes are obtained prior to manually correction for both operators. (B) Planes to be corrected manually after application with semi- and fully automated 5D CNS+™ algorithm for operator 1 and 2 [number of planes, mean with SD].](/document/doi/10.1515/jpm-2025-0188/asset/graphic/j_jpm-2025-0188_fig_002.jpg)
Semi- vs. fully automated 5D CNS+™: time benefit and manually corrected planes. (A) Acquisition time of application of semi- and fully automated 5D CNS+™ until all nine planes are obtained prior to manually correction for both operators. (B) Planes to be corrected manually after application with semi- and fully automated 5D CNS+™ algorithm for operator 1 and 2 [number of planes, mean with SD].
For operator 1 (level 3 expert), planes to be corrected manually after volume processing with both software algorithms were 1.52/9 planes ± 0.94/9 planes (95 % CI from 1.68/9 to 1.36/9) for semi-automated 5D CNS+™ and 0.07/9 planes ± 0.29/9 planes (95 % CI from 0.12/9 to 0.03/9) for fully automated 5D CNS+™, for operator 2 (level 1 examiner) 1.59/9 planes ± 1.01/9 planes (95 % CI from 1.76/9 to 1.42/9) for semi-automated 5D CNS+™ and 0.14/9 planes ± 0.43/9 planes (95 % CI from 0.21/9 to 0.07/9) for fully automated 5D CNS+™ (Figure 2B).
The statistical analysis for manually corrected planes by both operators between software algorithms showed a Bland-Altman-Bias of 1.44/9 planes (standard deviation (SD) of bias: 0.95/9; 95 % CI from 1.65/9 to 1.24/9) and 95 % Limits of Agreement (LoA) from −0.42 (LoA−, 95 % CI from −0.07/9 to −0.77/9) to 3.30 (LoA+, 95 % CI from 3.65 to 2.95) for operator 1 and 1.45/9 planes (SD of bias: 1.06/9; 95 % CI from 1.68/9 to 1.22/9) and 95 % LoA from −0.63 (LoA−, 95 % CI from −0.23/9 to −1.02/9) to 3.53 (LoA+, 95 % CI from 3.92 to 3.13) for operator 2.
Discussion
The fetal brain continues developing via complex sequences of structural changes throughout and beyond the fetal period and CNS anomalies are often identified late in pregnancy [17], 18], 29]. Therefore, a comprehensive assessment of the fetal brain by means of a detailed neurosonogram is of outstanding importance. In this context, ultrasound is the thorough and most powerful diagnostic method. It is undeniable that a manually performed detailed fetal neurosonography is highly operator-dependent, requires specialized skills and prolongs the examination time significantly. AI-driven applications, which prenatal diagnostics will increasingly rely on, are profoundly revolutionizing clinicians’ daily use of ultrasound [30]. Although the development of automated algorithms in fetal ultrasound is still in its early stadium and most algorithms have not yet reached the required level for clinical application, the supportive use of AI in fetal neurosonography could soon be beyond the capabilities of human experts [1], 13].
Our study evaluated the benefits of fully automated AI-enhanced 5D CNS+™ tool compared to the already well-functioning and, in the sonographic workflow, well-established previous semi-automatic software version with at least the same accuracy and reproducibility for standardized, thorough fetal neurosonography for both a beginner and an expert in a busy, tightly scheduled clinical setting.
Especially in the field of a workflow-based volumetric software approach for fetal neurosonography, the basic algorithm of 5D CNS+™ has a leading role in the field in 3D ultrasound volumetric approaches [15]. As a state-of-the-art technique, the algorithm is constantly being advanced and improved. It reliably enables the simultaneous reconstruction of all diagnostic fetal brain planes – axial, coronal and sagittal – required for a comprehensive neurosonogram based on a single 3D volume [27], 28]. Already in 2016, Rizzo et al. confirmed the software 5D CNS+™ to be an accurate and reliable technique for fetal neurosonography. The software improves workflow efficiency for routine targeted ultrasound survey during the second and third trimester and ensures a reduced operator dependency, allowing even less experienced examiners to gain insight into a standardized comprehensive assessment of fetal neuroanatomy according to the ISUOG guidelines [27], 28]. In the recent past, its validity and reliability, its accuracy and efficacy as well as its clinical benefits have been demonstrated several times, even in cases with structural abnormalities compromising the integrity of the fetal CNS [31], [32], [33], [34]. In their article from 2023, Gembicki et al. already declared their interest in the benefit of AI-driven solutions in fetal neurosonography for operators regardless their expertise and suggested the examination of their time spans [33].
Our study showcased that implementation of an optimized fully automated, ready for use algorithm (‘on the fly’) in fetal neurosonography facilitates the calculation of a nine-view template based on a 3D volume in less than 11 s on average following volume acquisition with fewer needed practical steps throughout the examination, with better accuracy and efficiency compared to a semi-automatic approach (Figure 2). In addition to the advantages mentioned above, irrespective of operator’s expertise, less experienced operators benefit in daily clinical routine. On average, operator 1 had to correct 1.44/9 planes less with fully automated 5D CNS+™ than with semi-automated 5D CNS+™, operator 2 had to correct 1.45/9 planes less on average with fully automated 5D CNS+™ compared to semi-automated 5D CNS+™, which in turn further exemplifies the superior accuracy of a fully automated approach. More precisely, we were able to prove that the challenges associated with reconstructing the parasagittal plane (PSP) and the transcerebellar plane (coronal) (TCc) have been resolved in the fully automated version, which has significantly increased its usability. It is to be expected that Samsung’s current ultrasound machine, Hera Z20, will significantly optimize the performance of the software 5D CNS+™ to generate a fetal neurosonogram, with an estimated acquisition time of less than 10 s for all planes.
In the field of fetal neurosonography, other vendors of ultrasound devices also offer built-in volume-based automated software tools with similar features like those of 5D CNS+™: SonoCNS™ (GE Healthcare, Chicago, IL, USA) and Smart Planes CNS™ (Mindray Bio-Medical Electronics Co., Ltd, Shenzhen, China) are semi-automatic post-processing volumetric approaches for diagnostic (axial) plane reconstruction with fetal head biometry and measurement of fetal brain structures. Those tools are limited to the reconstruction of only four diagnostic planes, commonly used for routine scanning of the fetal head and brain (three axial and one midsagittal plane) [16], 33], 35]. However, none of these software solutions offer automatic reconstruction of the nine planes required by international guidelines for a comprehensive fetal neurosonogram and achieve neither the efficiency nor the accuracy with detailed information of the latest version of the 5D CNS+™ algorithm.
Recently, a few research areas in the application of AI-assisted methods in the context of fetal neurosonography have emerged. These comprise primarily the simplified and optimized automatic acquisition of two-dimensional (2D) SPs with their correct orientation and localization within a 3D volume, the automated detection of characteristic brain as well as skull structures as landmarks of orientation with subsequent detection of congenital malformations, the evaluation of the imaging quality and the estimation of the GA by the assessment of neurodevelopmental maturation [1], 2], 13], 36].
3D ultrasound allows the sonographer to navigate within a volume and to visualize a specific embryonic or fetal anatomical structure in all dimensions, with the ability of post-processing offline analysis [37]. In a systematic review in 2005, Gonçalves et al. addressed the clinical significance of 3D ultrasound for the diagnosis of anomalies of the CNS and questioned if the information contained in the volume dataset by itself would be sufficient to evaluate fetal biometric measurements and diagnose congenital anomalies [38]. The advantages of conducting a comprehensive 3D examination of the fetal brain have been well established since. However, challenges arise from a difficult fetal orientation within the 3D volume and the non-intuitive manual navigation along the x, y and z planes through various structures of interest, and, not least most seriously, from the lack of standardization [31], 39]. Obviously, sonographers require appropriate clinical knowledge and a precise understanding of the fetal neuroanatomy [18]. However, it has been proven that adequate orientation and navigation within a 3D volume is all the more important [2], 36], 40]. Current AI application approaches are focused on the detection of 2D SPs within a 3D volume [1], 2], 17]. Without the ability of spatial thinking in combination with practical skills for handling the ultrasound probe, the application and interpretation of the AI-guided results are not feasible [36], 40].
The common lack of understanding of AI algorithms among clinicians due to the so-called black box character limits the acceptance and application of tools based on these algorithms in daily clinical routine [14], 15], 41], 42]. Responsibility lies with the AI developers, ensuring reliability and generalizability, and with the sonographers implementing those algorithms in the clinical workflow [14], 41]. Marcus et al. emphasize that in ‘explanation-less science’, specifically in AI models, the explainable AI (XAI) would be of central importance and could bring light into the darkness [41], 43]. Sonographers relying on AI algorithms in clinical daily practice should be familiar with various elements of potential bias in the development and implementation of AI algorithms [44], 45]. Nazer et al. outlined potential sources of bias within each step of developing AI algorithms, from framing the problem, data collection, pre-processing, development and validation to their full implementation [44]. AI systems usually rely on the input of giant datasets [2]. A large and diverse high-quality dataset is mandatory, likewise the need for diverse training and test datasets as well as standardization [44], 46]. The performance of AI-driven systems in real-world scenarios is constrained by the quality of the data used to train them [46]. There is also growing awareness of developing standards for transparency of data diversity in health datasets in AI-based applications [47]. However, many algorithms are trained on specific datasets – in part without independent verification of those datasets – resulting in varying performance in different clinical settings or even fundamentally incorrect results due to unreliable models [14], 46]. For example, it is not only in the field of fetal neurosonography – as recently observed – that articles, whose AI models were trained and tested on low-quality data using images that are proven to be incorrect, must be retracted [48]. This fuels the want of confidence.
Finally, there is a need for a careful debate with potential ethical considerations, including informed consent when AI is used in diagnostic processes with subsequent clinical decision-making, and with legal implications like questions of liability, accountability issues, responsibility and data privacy [14], 15], 49]. Those aspects discussed above should be considered when assessing the impact of AI on clinical decision-making.
In terms of anatomy and functionality: It is well known that structural disorders of the CNS might occur at various stages in embryonic or fetal life [17], 18]. However, as long as the midline and cerebellum can be visualized as a kind of basic requirement, the fully automated 5D CNS+™ tool can – independent of fetal position – reconstruct reproducibly a comprehensive neurosonogram in almost all anomalies with preserved gross structure. If these requirements are not fulfilled, the manual (semi-automatic) version can still be used. Manually assisted re-slicing enables an adequate examination of the fetal brain even in abnormal cases, which demonstrates the legitimacy of 5D CNS+™ [32].
In our opinion, the 5D CNS+™ tool can be used for both screening and diagnostics. However, only when examining the corpus callosum, additional manual visualization of the corresponding midsagittal plane may be necessary. In the end, the diagnostic added value can benefit significantly from optimizing the processing capabilities of the raw data of a 3D volume.
Created volumes of the fetal brain contain almost all relevant anatomical information [28]. It would therefore be obvious to extract the maximum clinical output from a volume to evaluate fetal head growth, to predict neurodevelopment, and to diagnose fetal abnormalities in one optimized workflow. Basically, regarding the ongoing development of the 5D CNS+™ software and based on the software Fetal Intelligent Navigation Echocardiography (FINE, 5D Heart™, Samsung Healthcare, Gangwon-do, South Korea), it would be preferable, to be able to extract individual planes and perform specific measurements within them [50].
In future, we expect an idealized AI software tool for the comprehensive analysis of a 3D volume of the fetal brain, both spatially and temporally, to implement the following aspects: the visualization of all standardized sectional planes including the measurement of the associated parameters [24], 25], 27], 28], 31], 33], 35], 51], the angular measurements within the nine templates without additional software [52], [53], [54], [55], the GA estimation based on characteristics of the neuroanatomical appearance with identification of relevant brain regions [56], 57], the visualization of the brain surface with its architecture of the GA-dependent gyri and sulci [52], 53], [58], [59], [60], [61], [62], the calculation of the total brain volume [63], [64], [65], the imaging of the cranial sutures [66] and the implementation of vascular structures [67], 68]. An appropriately optimized algorithm for shadow reduction should be available to assess both hemispheres of the brain equally rather than merely the hemisphere furthest from the bone [69], 70]. After automated segmentation of key core structures [9], 71] an alert is to be expected in the presence of abnormalities following the definition of prominent red flags, which simplifies clinical diagnosis [4] (Figure 3).
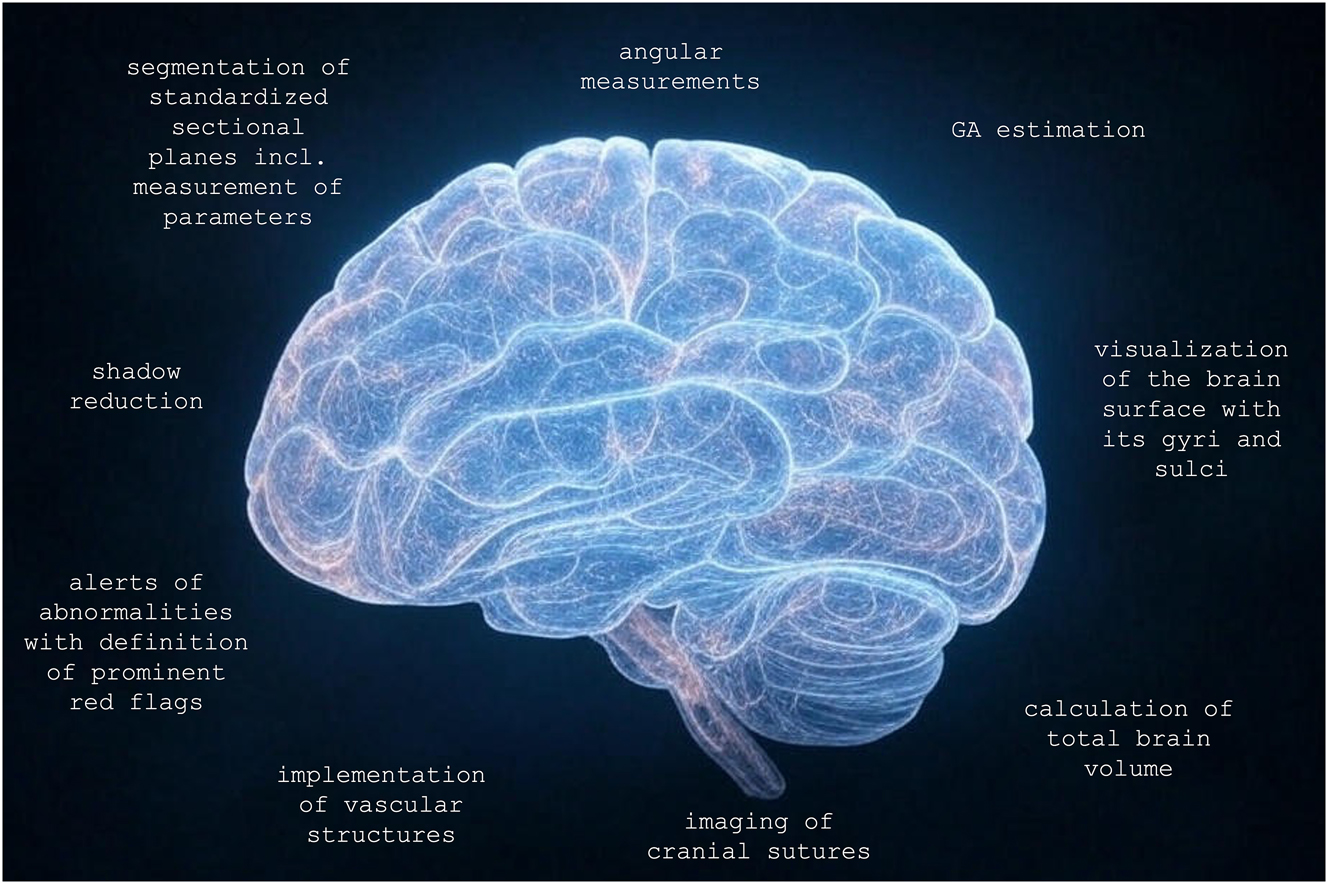
Optimized 3D volume and further post-processing aspects. GA, gestational age. Generated in parts by Grok 3.
In summary, it can be stated that the current commercially available software applications leave their potential untapped in terms of processing the information stored in a 3D volume and fall short of their potential.
Conclusions
Both versions of 5D CNS+™ supply reliable and thorough insights into fetal cerebral anatomy by reconstructing all standard views for fetal neurosonography, as recommended by national and international guidelines. The advance of 5D CNS+™ has led to the development of a fully automated algorithm (‘on the fly’), which ensures standardization, enhances reproducibility and streamlines clinical workflows by significantly reducing both examination time and operator dependency. By intelligent navigation, the algorithm can precisely identify and reconstruct diagnostic planes, improving the accuracy of fetal brain structure quantification. As a workflow-based volumetric software solution, fully automated 5D CNS+™ can be set as a state-of-the-art application in the comprehensive assessment of fetal neurosonography. By defining a benchmark, the software tool should be implemented into the daily workflow of fetal sonographers.
-
Research ethics: Not applicable.
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: Grok 3 was used to enhance Figures 1 and 3.
-
Conflict of interest: The authors M.G. and J.W. have received lecture fees from Samsung HME. J.L.S., A. R. and A.W. state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The data presented in this study are available from the corresponding author upon request. The data are not publicly available due to data privacy restrictions.
References
1. Horgan, R, Nehme, L, Abuhamad, A. Artificial intelligence in obstetric ultrasound: a scoping review. Prenat Diagn 2023;43:1176–219. https://doi.org/10.1002/pd.6411.Suche in Google Scholar PubMed
2. Jost, E, Kosian, P, Jimenez Cruz, J, Albarqouni, S, Gembruch, U, Strizek, B, et al.. Evolving the era of 5D ultrasound? A systematic literature review on the applications for artificial intelligence ultrasound imaging in obstetrics and gynecology. JCM 2023;12:6833. https://doi.org/10.3390/jcm12216833.Suche in Google Scholar PubMed PubMed Central
3. Bastiaansen, WAP, Klein, S, Koning, AHJ, Niessen, WJ, Steegers-Theunissen, RPM, Rousian, M. Computational methods for the analysis of early-pregnancy brain ultrasonography: a systematic review. eBioMedicine 2023;89:104466. https://doi.org/10.1016/j.ebiom.2023.104466.Suche in Google Scholar PubMed PubMed Central
4. Ramirez Zegarra, R, Ghi, T. Use of artificial intelligence and deep learning in fetal ultrasound imaging. Ultrasound Obstet Gynecol 2023;62:185–94. https://doi.org/10.1002/uog.26130.Suche in Google Scholar PubMed
5. Weichert, J, Welp, A, Scharf, JL, Dracopoulos, C, Becker, WH, Gembicki, M. The use of artificial intelligence in automation in the fields of gynaecology and obstetrics – an assessment of the state of play. Geburtshilfe Frauenheilkd 2021;81:1203–16. https://doi.org/10.1055/a-1522-3029.Suche in Google Scholar PubMed PubMed Central
6. Liu, S, Wang, Y, Yang, X, Lei, B, Liu, L, Li, SX, et al.. Deep learning in medical ultrasound analysis: a review. Engineering 2019;5:261–75. https://doi.org/10.1016/j.eng.2018.11.020.Suche in Google Scholar
7. Ahn, KH, Lee, KS. Artificial intelligence in obstetrics. Obstet Gynecol Sci 2022;65:113–24. https://doi.org/10.5468/ogs.21234.Suche in Google Scholar PubMed PubMed Central
8. Komatsu, M, Sakai, A, Dozen, A, Shozu, K, Yasutomi, S, Machino, H, et al.. Towards clinical application of artificial intelligence in ultrasound imaging. Biomedicines 2021;9:720. https://doi.org/10.3390/biomedicines9070720.Suche in Google Scholar PubMed PubMed Central
9. Lin, M, He, X, Guo, H, He, M, Zhang, L, Xian, J, et al.. Use of real‐time artificial intelligence in detection of abnormal image patterns in standard sonographic reference planes in screening for fetal intracranial malformations. Ultrasound Obstet Gynecol 2022;59:304–16. https://doi.org/10.1002/uog.24843.Suche in Google Scholar PubMed
10. Malani, SN, Shrivastava, D, Raka, MS. A comprehensive review of the role of artificial intelligence in obstetrics and gynecology. Cureus [Internet]. 2023 [cited 2023 May 17]; Available from: https://www.cureus.com/articles/122226-a-comprehensive-review-of-the-role-of-artificial-intelligence-in-obstetrics-and-gynecology.10.7759/cureus.34891Suche in Google Scholar PubMed PubMed Central
11. Yousefpour, SR, Karami, F, Karami, E. Enhancing fetal anomaly detection in ultrasonography images: a review of machine learning-based approaches. Biomimetics 2023;8:519. https://doi.org/10.3390/biomimetics8070519.Suche in Google Scholar PubMed PubMed Central
12. Bouachba, A, De Jesus Neves, J, Royer, E, Bartin, R, Salomon, LJ, Grevent, D, et al.. Artificial intelligence, radiomics and fetal ultrasound: review of literature and future perspectives. Ultrasound Obstet Gynecol 2025;uog.29172.10.1002/uog.29172Suche in Google Scholar PubMed
13. Xiao, S, Zhang, J, Zhu, Y, Zhang, Z, Cao, H, Xie, M, et al.. Application and progress of artificial intelligence in fetal ultrasound. JCM 2023;12:3298. https://doi.org/10.3390/jcm12093298.Suche in Google Scholar PubMed PubMed Central
14. Stefanini, B, Giamperoli, A, Terzi, E, Piscaglia, F. Artificial intelligence in ultrasound: pearls and pitfalls in 2024. Ultraschall Med 2024;45:444–8. https://doi.org/10.1055/a-2368-9201.Suche in Google Scholar PubMed
15. Weichert, J, Scharf, JL. Advancements in artificial intelligence for fetal neurosonography: a comprehensive review. JCM 2024;13:5626. https://doi.org/10.3390/jcm13185626.Suche in Google Scholar PubMed PubMed Central
16. Mlodawski, J, Zmelonek-Znamirowska, A, Mlodawska, M, Detka, K, Białek, K, Swiercz, G. Repeatability and reproducibility of artificial intelligence-acquired fetal brain measurements (SonoCNS) in the second and third trimesters of pregnancy. Sci Rep 2024;14:25076. https://doi.org/10.1038/s41598-024-77313-w.Suche in Google Scholar PubMed PubMed Central
17. Cabezas, M, Diez, Y, Martinez-Diago, C, Maroto, A. A benchmark for 2D foetal brain ultrasound analysis. Sci Data 2024;11:923. https://doi.org/10.1038/s41597-024-03774-3.Suche in Google Scholar PubMed PubMed Central
18. Rouleau, C, Gasner, A, Bigi, N, Couture, A, Perez, MJ, Blanchet, P, et al.. Prevalence and timing of pregnancy termination for brain malformations. Arch Dis Child Fetal Neonatal Ed 2011;96:F360–4. https://doi.org/10.1136/adc.2010.201483.Suche in Google Scholar PubMed
19. Morris, JK, Wellesley, DG, Barisic, I, Addor, MC, Bergman, JEH, Braz, P, et al.. Epidemiology of congenital cerebral anomalies in Europe: a multicentre, population-based EUROCAT study. Arch Dis Child 2019;104:1181–7. https://doi.org/10.1136/archdischild-2018-316733.Suche in Google Scholar PubMed
20. Tagliabue, G, Tessandori, R, Caramaschi, F, Fabiano, S, Maghini, A, Tittarelli, A, et al.. Descriptive epidemiology of selected birth defects, areas of Lombardy, Italy, 1999. Popul Health Metr 2007;5:4. https://doi.org/10.1186/1478-7954-5-4.Suche in Google Scholar PubMed PubMed Central
21. Yasin, F, Mehmood, Q, Shahid, H, Munawar, A, Saadoon, AA. The epidemiology of congenital brain anomalies. In: AlAli, KF, Hashim, HT, editors. Congenital brain malformations: clinical and surgical aspects [Internet]. Cham: Springer Nature Switzerland; 2024 [cited 2024 Jul 14]:7–18 pp.10.1007/978-3-031-58630-9_2Suche in Google Scholar
22. Milani, HJF, Barreto, EQDS, Araujo Júnior, E, Peixoto, AB, Nardozza, LMM, Moron, AF. Ultrasonographic evaluation of the fetal central nervous system: review of guidelines. Radiol Bras 2019;52:176–81. https://doi.org/10.1590/0100-3984.2018.0056.Suche in Google Scholar PubMed PubMed Central
23. Pluym, ID, Afshar, Y, Holliman, K, Kwan, L, Bolagani, A, Mok, T, et al.. Accuracy of automated three‐dimensional ultrasound imaging technique for fetal head biometry. Ultrasound Obstet Gynecol 2021;57:798–803. https://doi.org/10.1002/uog.22171.Suche in Google Scholar PubMed
24. Malinger, G, Paladini, D, Haratz, KK, Monteagudo, A, Pilu, GL, Timor‐Tritsch, IE. ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 1: performance of screening examination and indications for targeted neurosonography. Ultrasound Obstet Gynecol 2020;56:476–84. https://doi.org/10.1002/uog.22145.Suche in Google Scholar PubMed
25. Paladini, D, Malinger, G, Birnbaum, R, Monteagudo, A, Pilu, G, Salomon, LJ, et al.. ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 2: performance of targeted neurosonography. Ultrasound Obstet Gynecol 2021;57:661–71. https://doi.org/10.1002/uog.23616.Suche in Google Scholar PubMed
26. Yeung, PH, Aliasi, M, Papageorghiou, AT, Haak, M, Xie, W, Namburete, AIL. Learning to map 2D ultrasound images into 3D space with minimal human annotation. Med Image Anal 2021;70:101998. https://doi.org/10.1016/j.media.2021.101998.Suche in Google Scholar PubMed
27. Rizzo, G, Aiello, E, Elena Pietrolucci, M, Arduini, D. The feasibility of using 5D CNS software in obtaining standard fetal head measurements from volumes acquired by three-dimensional ultrasonography: comparison with two-dimensional ultrasound. J Matern Fetal Neonatal Med 2016;29:2217–22. https://doi.org/10.3109/14767058.2015.1081891.Suche in Google Scholar PubMed
28. Rizzo, G, Capponi, A, Persico, N, Ghi, T, Nazzaro, G, Boito, S, et al.. 5D CNS+ software for automatically imaging axial, sagittal, and coronal planes of normal and abnormal second‐trimester fetal brains. J Ultrasound Med 2016;35:2263–72. https://doi.org/10.7863/ultra.15.11013.Suche in Google Scholar PubMed
29. Yaron, Y, Ofen Glassner, V, Mory, A, Zunz Henig, N, Kurolap, A, Bar Shira, A, et al.. Exome sequencing as first‐tier test for fetuses with severe central nervous system structural anomalies. Ultrasound Obstet Gynecol 2022;60:59–67. https://doi.org/10.1002/uog.24885.Suche in Google Scholar PubMed PubMed Central
30. Yan, L, Li, Q, Fu, K, Zhou, X, Zhang, K. Progress in the application of artificial intelligence in ultrasound-assisted medical diagnosis. Bioengineering 2025;12:288. https://doi.org/10.3390/bioengineering12030288.Suche in Google Scholar PubMed PubMed Central
31. Welp, A, Gembicki, M, Rody, A, Weichert, J. Validation of a semiautomated volumetric approach for fetal neurosonography using 5DCNS+ in clinical data from > 1100 consecutive pregnancies. Childs Nerv Syst 2020;36:2989–95. https://doi.org/10.1007/s00381-020-04607-5.Suche in Google Scholar PubMed PubMed Central
32. Welp, A, Gembicki, M, Dracopoulos, C, Scharf, JL, Rody, A, Weichert, J. Applicability of a semiautomated volumetric approach (5D CNS+TM) for detailed antenatal reconstruction of abnormal fetal CNS anatomy. BMC Med Imag 2022;22:154. https://doi.org/10.1186/s12880-022-00888-1.Suche in Google Scholar PubMed PubMed Central
33. Gembicki, M, Welp, A, Scharf, JL, Dracopoulos, C, Weichert, J. A clinical approach to semiautomated three-dimensional fetal brain biometry—comparing the strengths and weaknesses of two diagnostic tools: 5DCNS+TM and SonoCNSTM. JCM 2023;12:5334. https://doi.org/10.3390/jcm12165334.Suche in Google Scholar PubMed PubMed Central
34. Caro-Alquiros, LR, Gonzaga, ZG, Quinio, IB. A sonographic evaluation on agreement and time efficiency of fetal central nervous system biometry using semi-automated five-dimensional ultrasound versus standard two-dimensional ultrasound in a Philippine tertiary hospital. Philippine J Obstet Gynecol 2024;48:90–7. https://doi.org/10.4103/pjog.pjog_13_24.Suche in Google Scholar
35. Meng, L, Zhao, D, Yang, Z, Wang, B. Automatic display of fetal brain planes and automatic measurements of fetal brain parameters by transabdominal three‐dimensional ultrasound. J Clin Ultrasound 2020;48:82–8. https://doi.org/10.1002/jcu.22762.Suche in Google Scholar PubMed
36. Cho, HC, Sun, S, Park, SW, Kwon, JY, Seo, JK. Artificial intelligence for fetal ultrasound. In: Seo, JK, editor. Deep learning and medical applications [Internet]. Singapore: Springer Nature Singapore; 2023 [cited 2024 May 2]:215–81 pp. (Mathematics in Industry; vol. 40). Available from: https://link.springer.com/10.1007/978-981-99-1839-3_5.10.1007/978-981-99-1839-3_5Suche in Google Scholar
37. Tonni, G, Grisolia, G. Simulator, machine learning, and artificial intelligence: time has come to assist prenatal ultrasound diagnosis. J Clin Ultrasound 2023;51:1164–5. https://doi.org/10.1002/jcu.23512.Suche in Google Scholar PubMed
38. Gonçalves, LF, Lee, W, Espinoza, J, Romero, R. Three- and 4-dimensional ultrasound in obstetric practice: does it help? J Ultrasound Med 2005;24:1599–624. https://doi.org/10.7863/jum.2005.24.12.1599.Suche in Google Scholar PubMed PubMed Central
39. Kalache, KD, Eder, K, Esser, T, Proquitté, H, Stoltenburg-Didinger, G, Hartung, JP, et al.. Three-dimensional ultrasonographic reslicing of the fetal brain to assist prenatal diagnosis of central nervous system anomalies. J Ultrasound Med 2006;25:509–14. https://doi.org/10.7863/jum.2006.25.4.509.Suche in Google Scholar PubMed
40. Di Vece, C, Dromey, B, Vasconcelos, F, David, AL, Peebles, D, Stoyanov, D. Deep learning-based plane pose regression in obstetric ultrasound. Int J CARS 2022;17:833–9. https://doi.org/10.1007/s11548-022-02609-z.Suche in Google Scholar PubMed PubMed Central
41. Marcus, E, Teuwen, J. Artificial intelligence and explanation: how, why, and when to explain black boxes. Eur J Radiol 2024;173:111393. https://doi.org/10.1016/j.ejrad.2024.111393.Suche in Google Scholar PubMed
42. Riva, A, Guerra, M, Di Gangi, S, Veronese, P, Vida, VL. Artificial intelligence for the prenatal ultrasound diagnosis of congenital heart disease: a narrative review. Clin Exp Obstet Gynecol 2024;51:244. https://doi.org/10.31083/j.ceog5111244.Suche in Google Scholar
43. Borys, K, Schmitt, YA, Nauta, M, Seifert, C, Krämer, N, Friedrich, CM, et al.. Explainable AI in medical imaging: an overview for clinical practitioners – saliency-based XAI approaches. Eur J Radiol 2023;162:110787. https://doi.org/10.1016/j.ejrad.2023.110787.Suche in Google Scholar PubMed
44. Nazer, LH, Zatarah, R, Waldrip, S, Ke, JXC, Moukheiber, M, Khanna, AK, et al.. Bias in artificial intelligence algorithms and recommendations for mitigation. PLOS Digit Health 2023;2:e0000278. https://doi.org/10.1371/journal.pdig.0000278.Suche in Google Scholar PubMed PubMed Central
45. Ambroise, GG, Oster, J, Dap, M, Morel, O, Hossu, G. Artificial intelligence and fetal ultrasound biometry: challenges and perspectives. Diagn Intervent Imag 2023;104:200–1. https://doi.org/10.1016/j.diii.2023.01.008.Suche in Google Scholar PubMed
46. Mohammed, S, Budach, L, Feuerpfeil, M, Ihde, N, Nathansen, A, Noack, N, et al.. The effects of data quality on machine learning performance. [Internet]. arXiv; 2024 [cited 2025 Feb 4]. Available from: http://arxiv.org/abs/2207.14529.Suche in Google Scholar
47. Arora, A, Alderman, JE, Palmer, J, Ganapathi, S, Laws, E, McCradden, MD, et al.. The value of standards for health datasets in artificial intelligence-based applications. Nat Med 2023;29:2929–38. https://doi.org/10.1038/s41591-023-02608-w.Suche in Google Scholar PubMed PubMed Central
48. Sreelakshmy, R, Titus, A, Sasirekha, N, Logashanmugam, E, Begam, RB, Ramkumar, G, et al.. [Retracted] an automated deep learning model for the cerebellum segmentation from fetal brain images. BioMed Res Int 2022;2022:8342767. https://doi.org/10.1155/2022/8342767.Suche in Google Scholar PubMed PubMed Central
49. Mennella, C, Maniscalco, U, De Pietro, G, Esposito, M. Ethical and regulatory challenges of AI technologies in healthcare: a narrative review. Heliyon 2024;10:e26297. https://doi.org/10.1016/j.heliyon.2024.e26297.Suche in Google Scholar PubMed PubMed Central
50. Yeo, L, Romero, R. New and advanced features of fetal intelligent navigation echocardiography (FINE) or 5D heart. J Matern Fetal Neonatal Med 2020:1–19. https://doi.org/10.1080/14767058.2020.1759538.Suche in Google Scholar PubMed PubMed Central
51. Ambroise, GG, Hossu, G, Bertholdt, C, Noble, P, Morel, O, Grangé, G. Artificial intelligence assistance for fetal head biometry: assessment of automated measurement software. Diagn Intervent Imag 2018;99:709–16. https://doi.org/10.1016/j.diii.2018.08.001.Suche in Google Scholar PubMed
52. Poon, LC, Sahota, DS, Chaemsaithong, P, Nakamura, T, Machida, M, Naruse, K, et al.. Transvaginal three‐dimensional ultrasound assessment of Sylvian fissures at 18–30 weeks’ gestation. Ultrasound Obstet Gynecol 2019;54:190–8. https://doi.org/10.1002/uog.20172.Suche in Google Scholar PubMed
53. Pooh, RK, Machida, M, Nakamura, T, Uenishi, K, Chiyo, H, Itoh, K, et al.. Increased Sylvian fissure angle as early sonographic sign of malformation of cortical development. Ultrasound Obstet Gynecol 2019;54:199–206. https://doi.org/10.1002/uog.20171.Suche in Google Scholar PubMed PubMed Central
54. Spinelli, M, Di Meglio, L, Mosimann, B, Di Naro, E, Surbek, D, Raio, L. The vermian-crest angle: a new method to assess fetal vermis position within the posterior fossa using 3-dimensional multiplanar sonography. Fetal Diagn Ther 2019;46:223–30. https://doi.org/10.1159/000494721.Suche in Google Scholar PubMed
55. Volpe, P, Contro, E, De Musso, F, Ghi, T, Farina, A, Tempesta, A, et al.. Brainstem–vermis and brainstem–tentorium angles allow accurate categorization of fetal upward rotation of cerebellar vermis. Ultrasound Obstet Gynecol 2012;39:632–5. https://doi.org/10.1002/uog.11101.Suche in Google Scholar PubMed
56. Namburete, AIL, Stebbing, RV, Kemp, B, Yaqub, M, Papageorghiou, AT, Alison Noble, J. Learning-based prediction of gestational age from ultrasound images of the fetal brain. Med Image Anal 2015;21:72–86. https://doi.org/10.1016/j.media.2014.12.006.Suche in Google Scholar PubMed PubMed Central
57. Burgos-Artizzu, XP, Coronado-Gutiérrez, D, Valenzuela-Alcaraz, B, Vellvé, K, Eixarch, E, Crispi, F, et al.. Analysis of maturation features in fetal brain ultrasound via artificial intelligence for the estimation of gestational age. Am J Obstet Gynecol MFM 2021;3:100462. https://doi.org/10.1016/j.ajogmf.2021.100462.Suche in Google Scholar PubMed
58. Cohen-Sacher, B, Lerman-Sagie, T, Lev, D, Malinger, G. Sonographic developmental milestones of the fetal cerebral cortex: a longitudinal study. Ultrasound Obstet Gynecol 2006;27:494–502. https://doi.org/10.1002/uog.2757.Suche in Google Scholar PubMed
59. Pistorius, LR, Stoutenbeek, P, Groenendaal, F, De Vries, L, Manten, G, Mulder, E, et al.. Grade and symmetry of normal fetal cortical development: a longitudinal two‐ and three‐dimensional ultrasound study. Ultrasound Obstet Gynecol 2010;36:700–8. https://doi.org/10.1002/uog.7705.Suche in Google Scholar PubMed
60. Husen, SC, Koning, IV, Go, ATJI, Van Graafeiland, AW, Willemsen, SP, Groenenberg, IAL, et al.. Three-dimensional ultrasound imaging of fetal brain fissures in the growth restricted fetus. PLoS One 2019;14:e0217538. https://doi.org/10.1371/journal.pone.0217538.Suche in Google Scholar PubMed PubMed Central
61. Putra, M, Hamidi, OP, Driver, C, Peek, EE, Bolt, MA, Gumina, D, et al.. Corpus callosum length and cerebellar vermian height in fetal growth restriction. Fetal Diagn Ther 2024;51:255–66. https://doi.org/10.1159/000538123.Suche in Google Scholar PubMed
62. Xue, J, Xue, J, Ru, Y, Zhang, G, Yin, H, Liu, D. Ultrasound assessment of insular development in adequate-for-gestational-age fetuses and fetuses with early-onset fetal growth restriction using 3D-ICRV technology. Front Med 2024;11:1393115. https://doi.org/10.3389/fmed.2024.1393115.Suche in Google Scholar PubMed PubMed Central
63. Zhou, M, Li, X, Huang, T, Wang, M, Giorgio, A. Analysis of automatic fetal intracranial volume (ICV) measurement based on the optimized ultrasound Smart ICV method at 16–34 weeks of gestation. Quant Imag Med Surg 2024;14:9361–73. https://doi.org/10.21037/qims-24-1379.Suche in Google Scholar PubMed PubMed Central
64. Caspi, Y, De Zwarte, SMC, Iemenschot, IJ, Lumbreras, R, De Heus, R, Bekker, MN, et al.. Automatic measurements of fetal intracranial volume from 3D ultrasound scans. Front Neuroimag 2022;1:996702. https://doi.org/10.3389/fnimg.2022.996702.Suche in Google Scholar PubMed PubMed Central
65. Zeng, S, Zhou, QC, Zhou, JW, Li, M, Long, C, Peng, QH. Volume of intracranial structures on three‐dimensional ultrasound in fetuses with congenital heart disease. Ultrasound Obstet Gynecol 2015;46:174–81. https://doi.org/10.1002/uog.14677.Suche in Google Scholar PubMed
66. Tutschek, B, Blaas, H ‐GK, Abramowicz, J, Baba, K, Deng, J, Lee, W, et al.. Three‐dimensional ultrasound imaging of the fetal skull and face. Ultrasound Obstet Gynecol 2017;50:7–16. https://doi.org/10.1002/uog.17436.Suche in Google Scholar PubMed
67. Danon, E, Weisz, B, Achiron, R, Pretorius, DH, Weissmann-Brenner, A, Gindes, L. Three-dimensional ultrasonographic depiction of fetal brain blood vessels: 3DUS of fetal brain vessels. Prenat Diagn 2016;36:407–17. https://doi.org/10.1002/pd.4791.Suche in Google Scholar PubMed
68. Pooh, RK. Normal anatomy by three-dimensional ultrasound in the second and third trimesters. Semin Fetal Neonatal Med 2012;17:269–77. https://doi.org/10.1016/j.siny.2012.06.003.Suche in Google Scholar PubMed
69. Perez-Gonzalez, J, Arámbula-Cosío, F, Guzmán, M, Camargo, L, Gutierrez, B, Mateus, D, et al.. Spatial compounding of 3-D fetal brain ultrasound using probabilistic maps. Ultrasound Med Biol 2018;44:278–91. https://doi.org/10.1016/j.ultrasmedbio.2017.09.001.Suche in Google Scholar PubMed
70. Perez–Gonzalez, J, Arámbula Cosío, F, Huegel, JC, Medina-Bañuelos, V. Probabilistic learning coherent point drift for 3D ultrasound fetal head registration. Comput Math Methods Med 2020;2020:1–14. https://doi.org/10.1155/2020/4271519.Suche in Google Scholar PubMed PubMed Central
71. Torres, HR, Morais, P, Oliveira, B, Birdir, C, Rüdiger, M, Fonseca, JC, et al.. A review of image processing methods for fetal head and brain analysis in ultrasound images. Comput Methods Progr Biomed 2022;215:106629. https://doi.org/10.1016/j.cmpb.2022.106629.Suche in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpm-2025-0188).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

