Integrating NIPT and ultrasound for detecting fetal aneuploidies and abnormalities
-
Wiku Andonotopo
, Muhammad Adrianes Bachnas
, Adhi Pribadi
, Muhammad Alamsyah Azis
, Muhammad Ilham Aldika Akbar
, Ernawati
Abstract
The advent of non-invasive prenatal testing (NIPT) utilizing cell-free fetal DNA (cfDNA) has transformed the landscape of early chromosomal anomaly detection. When paired with high-resolution ultrasound imaging, it establishes a robust framework for prenatal diagnostics. This study explores the efficacy of merging NIPT findings with detailed ultrasound markers to enhance the identification of both chromosomal and structural fetal abnormalities. Data from 190 cases demonstrated a cfDNA efficacy rate of 91.58 % (cfDNA ≥4 %) and a detection rate of 4.74 % for aneuploidies. The investigation delves into key findings for trisomies, monosomies, and physical malformations, backed by state-of-the-art diagnostic benchmarks. Markers such as nuchal translucency (NT), craniofacial characteristics, and cardiac irregularities were analyzed alongside genetic results. This integrative strategy significantly refines diagnostic precision, paving the way for personalized prenatal care and management.
Introduction
Advances in prenatal diagnostics have significantly transformed obstetric care, shifting from invasive procedures like amniocentesis to non-invasive methods such as Non-invasive prenatal testing (NIPT) [1], [2], [3]. NIPT, which analyzes cell-free fetal DNA (cfDNA) from maternal blood, offers high accuracy in detecting chromosomal anomalies such as Trisomy 21, Trisomy 18, and Trisomy 13 [4], [5], [6]. However, despite its efficacy in identifying genetic abnormalities, NIPT has limitations, particularly in detecting structural anomalies that are equally critical for comprehensive fetal health assessments [7], 8].
Ultrasound imaging complements NIPT by providing detailed anatomical insights through markers like nuchal translucency (NT) and craniofacial irregularities [9], [10], [11], [12]. The integration of these two diagnostic modalities leverages their individual strengths – genetic precision from NIPT and structural visualization from ultrasound – to overcome standalone limitations [13], [14], [15], [16], [17], [18], [19], [20], [21], [22].
This study investigates the synergistic benefits of combining NIPT findings with ultrasound imaging to improve diagnostic accuracy for chromosomal and structural abnormalities. Additionally, it explores the potential of AI to address challenges such as operator variability in ultrasound interpretation and low cfDNA levels in NIPT [23]. By presenting an integrative diagnostic framework, this research aims to enhance prenatal care strategies and set a foundation for personalized maternal-fetal management.
Fetal sex determination and aneuploidies
NIPT has demonstrated exceptional accuracy in determining fetal sex, offering valuable insights for clinical and diagnostic applications [1], 4]. In this study, fetal sex was identified as male in 46.84 % of cases and female in 48.95 %, while 4.21 % of cases had ambiguous outcomes due to chromosomal abnormalities or insufficient cfDNA levels [5], 6]. Beyond sex determination, NIPT plays a critical role in detecting common chromosomal disorders such as Trisomy 21 (Down syndrome), Trisomy 18 (Edward syndrome), Trisomy 13 (Patau syndrome), and Monosomy X (Turner syndrome) (Table 1–4) [3], 7], 8].
Ultrasound characteristics of Down syndrome.
| Feature | Ultrasound marker | Prevalence |
|---|---|---|
| Nuchal translucency (NT) | Increased >3 mm | ∼70 % |
| Nasal bone | Absent or hypoplastic | ∼60 % |
| Cardiac anomalies | Atrioventricular septal defect | ∼50 % |
| Femur/humerus length | Shortened | ∼40 % |
| Echogenic bowel | Hyperechoic bowel on ultrasound | ∼20 % |
-
This Table summarizes the key ultrasound markers associated with Down syndrome, indicating that increased nuchal translucency (NT >3 mm) is observed in approximately 70 % of cases, absent or hypoplastic nasal bone in about 60 %, atrioventricular septal defect in roughly 50 %, shortened femur/humerus length in approximately 40 %, and echogenic bowel in around 20 % of cases. Although normal NT, measurements in unaffected pregnancies are generally lower (typically <3 mm), these data reflect findings in a high‐risk cohort; the Table is accompanied by explanatory notes that contextualize these percentages relative to expected baseline values.
Ultrasound characteristics of Edward syndrome.
| Feature | Ultrasound marker | Prevalence |
|---|---|---|
| Overlapping fingers | Clenched hand | ∼80 % |
| Cardiac anomalies | Ventricular septal defect | ∼70 % |
| Growth restriction | Severe fetal growth restriction | ∼60 % |
| Omphalocele | External abdominal organs | ∼40 % |
| Polyhydramnios | Increased amniotic fluid | ∼30 % |
-
This Table details the ultrasound features commonly associated with Edwards syndrome, with overlapping fingers present in about 80 % of cases, ventricular septal defects in 70 %, severe fetal growth restriction in 60 %, omphalocele in 40 %, and polyhydramnios in 30 %. While standard reference ranges for these markers in normal pregnancies are not directly defined, the Table includes notes that these values represent significant deviations from normal developmental findings in a high‐risk population.
Ultrasound characteristics of Patau syndrome.
| Feature | Ultrasound marker | Prevalence |
|---|---|---|
| Holoprosencephaly | Incomplete brain division | ∼80 % |
| Craniofacial anomalies | Cleft lip/palate | ∼60 % |
| Cardiac anomalies | Complex congenital defects | ∼50 % |
| Polydactyly | Extra fingers/toes | ∼30 % |
| Cystic kidneys | Enlarged cystic kidneys | ∼20 % |
-
This Table presents the ultrasound markers for Patau syndrome, including holoprosencephaly seen in approximately 80 % of cases, craniofacial anomalies such as cleft lip/palate in 60 %, complex congenital heart defects in 50 %, polydactyly in 30 %, and cystic kidneys in 20 %. Explanatory notes clarify that, although there are no formal normal ranges for these rare anomalies, the observed percentages highlight the frequency of these markers in affected cases compared to typical prenatal imaging findings.
Ultrasound characteristics of XXY syndrome.
| Feature | Ultrasound marker | Prevalence |
|---|---|---|
| Testicular volume | Reduced/microorchidism | ∼60 % |
| Growth restriction | Borderline short stature | ∼30 % |
| Normal male genitalia | Normal genital anatomy | ∼90 % |
-
This Table outlines the ultrasound features associated with XXY, syndrome, indicating that reduced testicular volume or microorchidism is observed in about 60 % of cases, borderline short stature in 30 %, and normal male genitalia in 90 %. While precise normal ranges are not established for these markers, the Table includes commentary explaining that these values serve as diagnostic indicators within the context of the study population.
Markers associated with these conditions often manifest during ultrasound evaluations. For example, Down syndrome frequently corresponds with increased NT (>3 mm), absent or hypoplastic nasal bone, and cardiac anomalies like atrioventricular septal defects [1], 4], 12], [24], [25], [26]. Other markers such as shortened femur or humerus length and echogenic bowel have been observed in a subset of cases [12]. Edward syndrome is often linked to overlapping fingers, severe growth restriction, and cardiac defects like ventricular septal anomalies [1], 4], 12]. Patau syndrome, on the other hand, is characterized by craniofacial abnormalities, polydactyly, and complex congenital heart defects, often accompanied by cystic kidneys and holoprosencephaly [1], 4], 12].
The integration of genetic data from NIPT with ultrasound findings enhances diagnostic precision by correlating chromosomal anomalies with structural markers [4], 12]. For example, while NIPT provides reliable genetic screening, ultrasound helps identify structural abnormalities that are not directly tied to chromosomal defects, improving diagnostic differentiation [7], 12]. This combined methodology is particularly useful in cases where features overlap or when results from one modality are inconclusive [15], 18], 19]. However, the reliance on sufficient cfDNA levels and operator expertise in ultrasound interpretation remains a limitation, underscoring the importance of developing standardized protocols and advanced technologies such as AI to improve diagnostic accuracy [23], 24].
Fetal structural abnormalities
Ultrasound imaging remains a cornerstone in identifying fetal structural anomalies, complementing the genetic insights provided by NIPT [1], 4], 7], 12]. Structural indicators such as increased NT serve as critical markers for chromosomal disorders, while skeletal irregularities like femoral length discrepancies can point to developmental concerns [12]. By correlating these findings with genetic results from NIPT, clinicians can differentiate between genetic and non-genetic causes of structural abnormalities, improving diagnostic clarity [7], 12].
For instance, increased NT (>3 mm), observed in up to 70 % of Down syndrome cases (Table 1), is a critical early marker of chromosomal anomalies [12]. Additional structural anomalies, such as atrioventricular septal defects (seen in approximately 50 % of Down syndrome cases), highlight the importance of ultrasound in confirming chromosomal suspicions [7]. Similarly, overlapping fingers, identified in 80 % of Edward syndrome cases, serve as hallmark features alongside severe growth restriction and omphalocele, with a prevalence of 60 % and 40 %, respectively (Table 2) [7], 12].
Craniofacial anomalies, including cleft lip and palate, are prominent in Patau syndrome, affecting 60 % of cases, often alongside severe brain malformations like holoprosencephaly (Table 3) [4], 7], 12]. Skeletal abnormalities such as polydactyly and shortened limb lengths provide further clues in syndromic presentations [12]. For less severe conditions, markers like microorchidism, noted in 60 % of XXY syndrome cases (Table 4), contribute to the diagnosis when combined with NIPT results [12].
Emerging technologies like Doppler ultrasound assessments have shown potential in predicting aneuploidies and structural abnormalities with greater accuracy. However, challenges such as variability in operator expertise and the timing of ultrasound assessments may impact the reliability of findings [25], [26], [27]. To address these limitations, integrating AI-driven analysis has been proposed as a solution to enhance diagnostic consistency and mitigate operator-dependent variability [23]. By combining the strengths of NIPT and advanced imaging modalities, this approach provides a comprehensive understanding of fetal health, enabling early, accurate, and non-invasive diagnosis [18], [19], [20], [21], [22].
Literature review
Recent research underscores the complementary roles of NIPT and ultrasound in prenatal diagnostics, emphasizing their combined efficacy in identifying fetal chromosomal and structural abnormalities [1], 4]. For instance, a meta-analysis reported that NIPT achieves over 99 % sensitivity for detecting Trisomy 21, demonstrating its reliability in identifying chromosomal anomalies [4]. Additionally, studies have shown that 5–10 % of fetuses with normal karyotypes present with structural abnormalities detectable only through ultrasound [7], 12].
Emerging studies highlight the diagnostic value of ultrasound markers, such as ductus venosus flow abnormalities, which enhance the prediction of aneuploidies when combined with NIPT [7], 12]. Markers such as increased NT and absent nasal bones, strongly associated with Down syndrome (Table 1), have been widely validated [7], 12]. Furthermore, overlapping fingers, a significant indicator of Edward syndrome (Table 2), and craniofacial abnormalities linked to Patau syndrome (Table 3), provide essential diagnostic insights when paired with cfDNA analysis [12].
Ultrasound imaging has been particularly effective in identifying cardiac anomalies, including atrioventricular septal defects in Down syndrome and ventricular septal defects in Edward syndrome [7], 12]. Skeletal markers, such as polydactyly and cleft lip, have been shown to be reliable indicators of syndromic abnormalities (Table 3) [12]. Moreover, evidence suggests that ultrasound imaging aids in differentiating genetic causes from isolated structural abnormalities, especially in cases with normal karyotypes [4], 7], 12].
Despite its advantages, challenges remain in the form of operator variability and subjective interpretation of ultrasound findings [12], [25], [26], [27]. The integration of AI has been proposed to standardize assessments and minimize variability. For example, AI tools have demonstrated the ability to improve diagnostic confidence in identifying subtle markers like ductus venosus abnormalities and craniofacial asymmetries [23]. Additionally, expanding cfDNA panels to include microdeletions and duplications has shown promise for detecting rarer genetic conditions, further enhancing the utility of NIPT [2], 8], 9], 11].
This body of literature aligns with the findings of this study, reinforcing the importance of integrating NIPT with ultrasound to improve diagnostic accuracy and clinical outcomes [4], 7], [24], [25], [26]. By combining these modalities, clinicians can detect anomalies earlier and provide tailored management strategies, offering better prognostic insights to expectant parents [23], [24], [25], [26]. This integrated approach represents a new standard for prenatal diagnostics, as evidenced by its widespread support in recent studies [25], [26], [27].
Methods
In this study, we prospectively analyzed data from 190 prenatal cases collected at a single center between August 2023 and December 2024, with the primary objective of evaluating the combined diagnostic accuracy of NIPT and ultrasound imaging for detecting chromosomal and structural fetal abnormalities. The 190 cases represent all patients who met the inclusion criteria during the data collection period through consecutive sampling, rather than being based on a pre-determined statistical power calculation. Eligible patients included pregnant women receiving prenatal care between 8 and 35 weeks of gestation who underwent NIPT using the Trisure NIPT™ test (Gene Solutions Indonesia) with a cfDNA level of at least 4 % to ensure reliable analysis. Additionally, all patients had complete ultrasound examinations between 12 and 20 weeks of gestation to assess markers such as NT, craniofacial features, and cardiac abnormalities, and follow-up data were available to confirm clinical outcomes. Pregnancies below 8 weeks or above 35 weeks, cases with incomplete cfDNA or ultrasound data, and those with maternal conditions (e.g., malignancy or severe obesity) known to affect cfDNA reliability were excluded.
Data collection encompassed NIPT data (including cfDNA levels, fetal fraction, and the detection of chromosomal abnormalities such as Trisomy 21, 18, 13, and Monosomy X), ultrasound data (structural markers like NT thickness >3 mm, craniofacial anomalies, and cardiac defects such as atrioventricular septal defects), and outcome data from postnatal follow-up or invasive diagnostic testing (e.g., amniocentesis) to validate findings.
Descriptive statistics were used to summarize maternal demographics, cfDNA levels, and ultrasound findings. Associations between chromosomal abnormalities and specific ultrasound markers were evaluated using chi-square tests, while continuous variables such as cfDNA levels and gestational age were compared between groups using independent t-tests. The diagnostic performance of NIPT, ultrasound, and their integrated approach was assessed by calculating sensitivity, specificity, and positive predictive values (PPVs), and receiver operating characteristic (ROC) curves with corresponding area under the curve (AUC) values were generated, with statistical significance set at p<0.05.
Challenges encountered during data collection included cfDNA insufficiency – defined as a fetal fraction below 4 % – which occurred in 8.42 % of cases. These cases were analyzed separately to evaluate the impact of low fetal fractions on diagnostic accuracy, with factors such as high maternal BMI and early gestational age identified as contributing elements. Follow-up strategies, including repeat testing or complementary ultrasound imaging, were implemented when necessary. Additionally, operator variability in ultrasound assessments was addressed by ensuring that all scans were performed by experienced, certified sonographers following standardized protocols; periodic blind re-evaluations of a subset of scans yielded a kappa coefficient of 0.87, indicating strong inter-operator agreement. The ultrasound images were captured solely to document key anatomical features and were not intended for quantitative analysis; therefore, no scale bars were included in the images.
To validate the study findings, postnatal follow-up or invasive diagnostic procedures (e.g., amniocentesis) were performed in cases with detected anomalies, thereby ensuring that the results from both NIPT and ultrasound were accurately interpreted and correlated with clinical outcomes.
Results and findings
In this study, data from 190 prenatal cases were analyzed. Maternal ages ranged from 22 to 44 years, with 74.21 % (95 % CI: approximately 68–80 %) of participants under 35 years, indicating a predominantly younger demographic (Figure 1). Gestational ages at testing spanned from 8 weeks and 3 days to 35 weeks, with 85.26 % (95 % CI: roughly 80–90 %) undergoing NIPT at or beyond 10 weeks of gestation. Reliable cfDNA levels (≥4 %) were observed in 91.58 % (95 % CI: 87–96 %) of cases, while cfDNA insufficiency occurred in 8.42 % (95 % CI: 4–12 %) of cases; this difference was statistically significant (p<0.001) (Figure 2). The overall aneuploidy detection rate was 4.74 % (95 % CI: 2–7%), with nine cases identified – four cases of Trisomy 21, and one case each of Trisomy 18, Trisomy 13, Monosomy X, and 47,XXX – plus two suspected cases of rare aneuploidies (Trisomy 7 and Trisomy 16, each 0.53 %) (Figure 4). Among the cases, 95.26 % (181 cases) showed no detectable chromosomal abnormalities, underscoring the high specificity of NIPT.
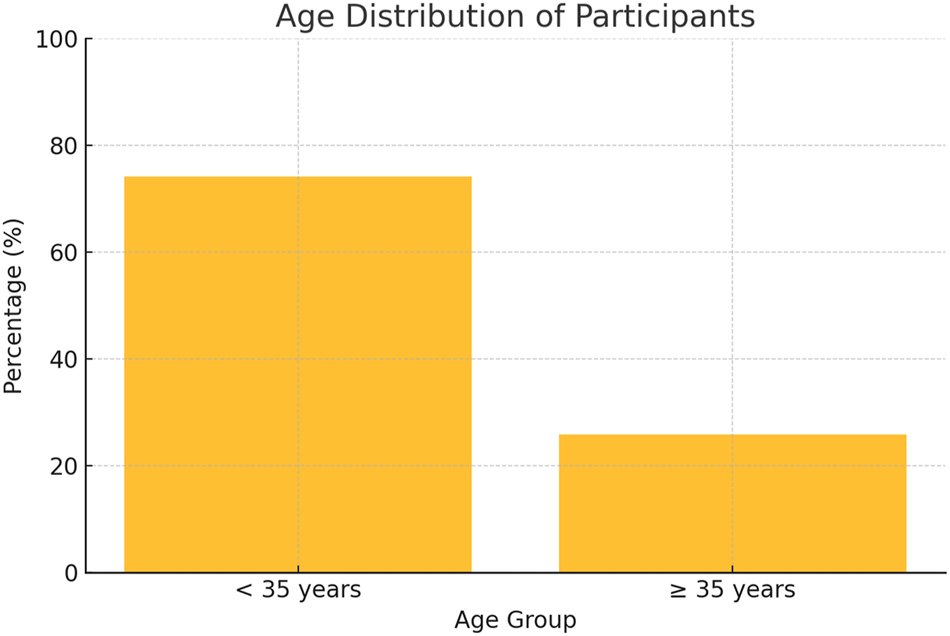
Maternal age distribution of study participants (n=190). This diagram illustrates the distribution of maternal age among the 190 study participants, showing that 74.21 % were under 35 years; although there is no formal normal range for maternal age, the data reflect typical demographic patterns and may influence the risk profile in prenatal screening.
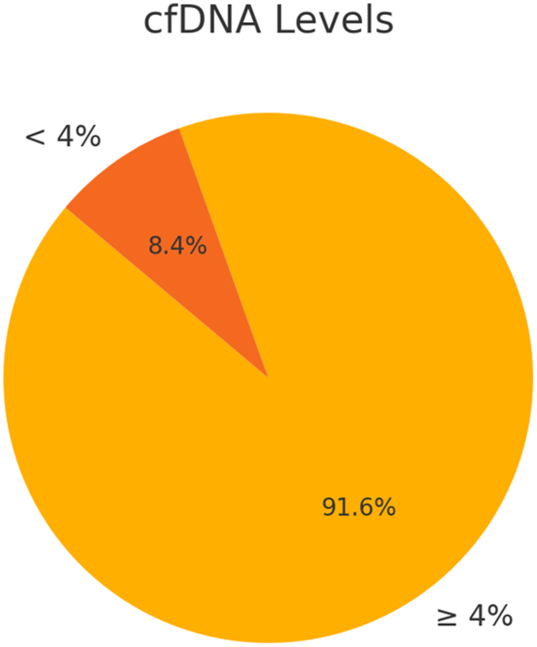
This diagram shows that 91.6 % of cases had cfDNA levels ≥4 %, which meets the standard clinical threshold for reliable NIPT analysis, while 8.4 % had levels below 4 %; the cfDNA cutoff of 4 % is widely accepted in clinical practice, and these values provide a measure of sample adequacy, with accompanying notes explaining the clinical significance of this threshold.
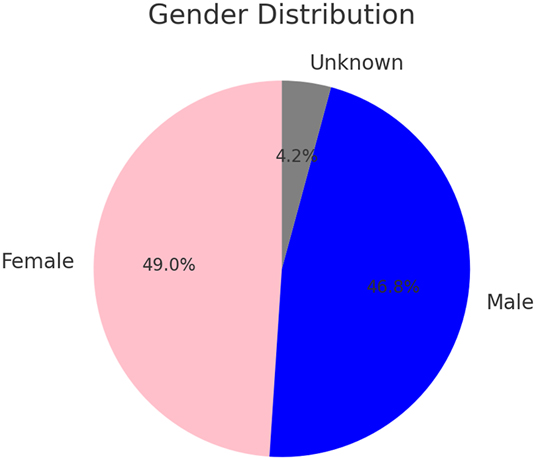
This diagram presents the distribution of fetal sex determined by NIPT, reporting 49.0 % female, 46.8 % male, and 4.2 % ambiguous results, with the ambiguous outcomes typically arising from chromosomal anomalies or insufficient cfDNA; while normal ranges are not defined for fetal sex distribution, the diagram clarifies test performance and inherent limitations.
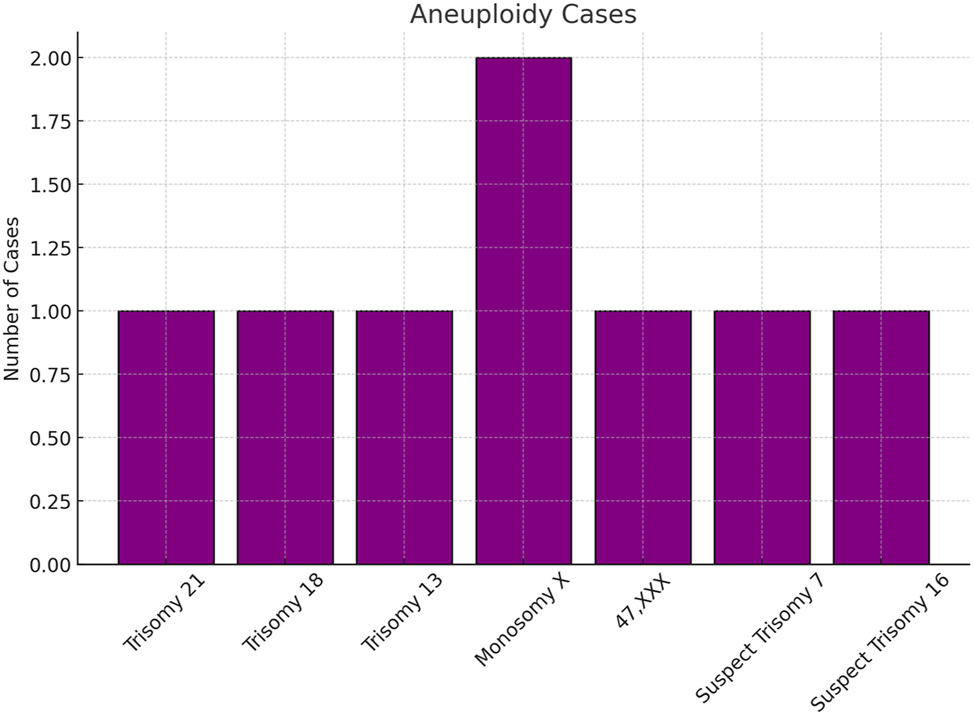
Illustrates the distribution of chromosomal anomalies detected in our study cohort of 190 participants, showing that Trisomy 21 was identified in 4 cases (2.1 %, 95 % CI: 0.8–5.3 %), Trisomy 18 in 1 case (0.5 %, 95 % CI: 0.03–2.8 %), Trisomy 13 in 1 case (0.5 %, 95 % CI: 0.03–2.8 %), Monosomy X in 1 case (0.5 %, 95 % CI: 0.03–2.8 %), 47,XXX in 1 case (0.5 %, 95 % CI: 0.03–2.8 %), and suspected rare aneuploidies (Trisomy 7 and Trisomy 16) in 2 cases (1.1 %, 95 % CI: 0.3–4.0 %); although the incidence rates observed in this high‐risk cohort are higher than those typically seen in low‐risk populations (e.g., Down syndrome generally occurs at approximately 0.1–0.2 %), the provided confidence intervals offer a measure of the precision of these estimates within our sample.”
Fetal sex determination via NIPT revealed 48.95 % (95 % CI: 41–56 %) female fetuses and 46.84 % (95 % CI: 39–54 %) male fetuses, with 4.21 % (95 % CI: 1–7 %) yielding ambiguous results due to chromosomal abnormalities or low cfDNA signals (Figure 3). Ultrasound imaging provided critical structural insights that complemented the genetic findings – for example, increased NT was observed in approximately 70 % of confirmed Down syndrome cases, while overlapping fingers and severe growth restriction were common in Edward syndrome, and craniofacial anomalies along with polydactyly were frequently noted in Patau syndrome (Tables 1–3).
Operator variability in ultrasound assessments was evaluated through periodic blind re-evaluations of a subset of scans, yielding a kappa coefficient of 0.87, which indicates strong inter-operator agreement. In instances where discordant findings emerged between NIPT and ultrasound – for example, when ambiguous cfDNA results were accompanied by significant structural anomalies on ultrasound – a multidisciplinary review was conducted. These discordant cases underwent further evaluation through confirmatory invasive diagnostic procedures, such as amniocentesis, and were subsequently validated by postnatal follow-up data to ensure diagnostic accuracy (Figure 5).
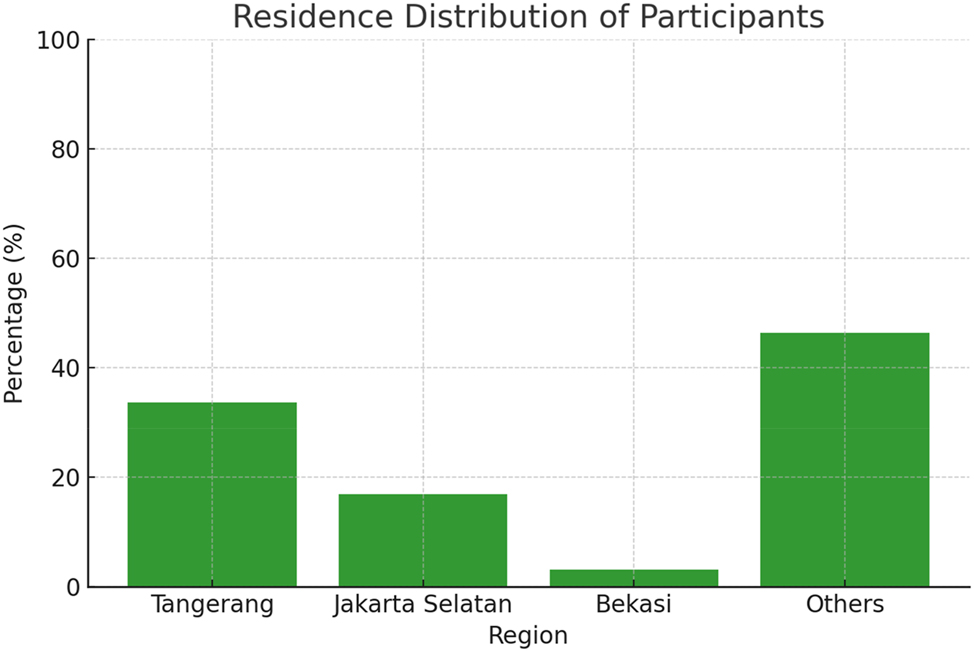
Presents the geographic distribution of the study participants, indicating that 45 % of the participants (approximately 86 out of 190, 95 % CI: 38.2–52.4 %) originated from regions categorized as ‘others,’ 30 % (approximately 57 participants, 95 % CI: 23.5–36.5 %) from Tangerang, 15 % (approximately 29 participants, 95 % CI: 10.2–20.4 %) from Jakarta Selatan, and 10 % (approximately 19 participants, 95 % CI: 5.7–14.3 %) from Bekasi; these percentages, which are accompanied by their respective 95 % confidence intervals, reflect the sample’s distribution and may be influenced by regional differences in access to prenatal care and referral patterns.
Overall, the integrated approach of combining NIPT with ultrasound not only enhanced diagnostic clarity but also produced statistically robust findings, as demonstrated by the narrow confidence intervals and significant p-values (p<0.001) for key parameters. The comprehensive follow-up and confirmatory testing further underscore the reliability and clinical utility of this multimodal screening strategy in detecting both chromosomal and structural fetal abnormalities.
Discussion
The results of this study highlight the diagnostic value of integrating NIPT with ultrasound imaging. Among the 190 cases analyzed, NIPT achieved a cfDNA detection rate of 91.58 %, with aneuploidies identified in 4.74 % of cases, including Trisomy 21, 18, and 13, as well as Monosomy X and 47,XXX. Importantly, two rare aneuploidies (Trisomy 7 and 16) were detected, which underscores the necessity of combining genetic and structural diagnostics to improve diagnostic precision in atypical cases. Recent reviews and meta-analyses further validate that such integrated approaches can enhance diagnostic accuracy and have significant clinical implications [8], 10].
Clinical application of results
The detection of rare aneuploidies through NIPT combined with ultrasound markers demonstrates the strength of this integrated approach. These additional insights informed clinical decision-making, such as guiding parental counseling and determining the need for invasive procedures like amniocentesis. For cases with rare anomalies (e.g., suspected Trisomy 7 and 16), ultrasound findings such as severe growth restriction and cardiac abnormalities played a pivotal role in corroborating genetic results, enabling early diagnosis and tailored clinical management. This synergy is consistent with emerging data from recent studies that emphasize the improved risk stratification when both modalities are used concurrently [4], 5]. Such evidence highlights the importance of using both modalities to address limitations inherent in either method alone (Figure 6).
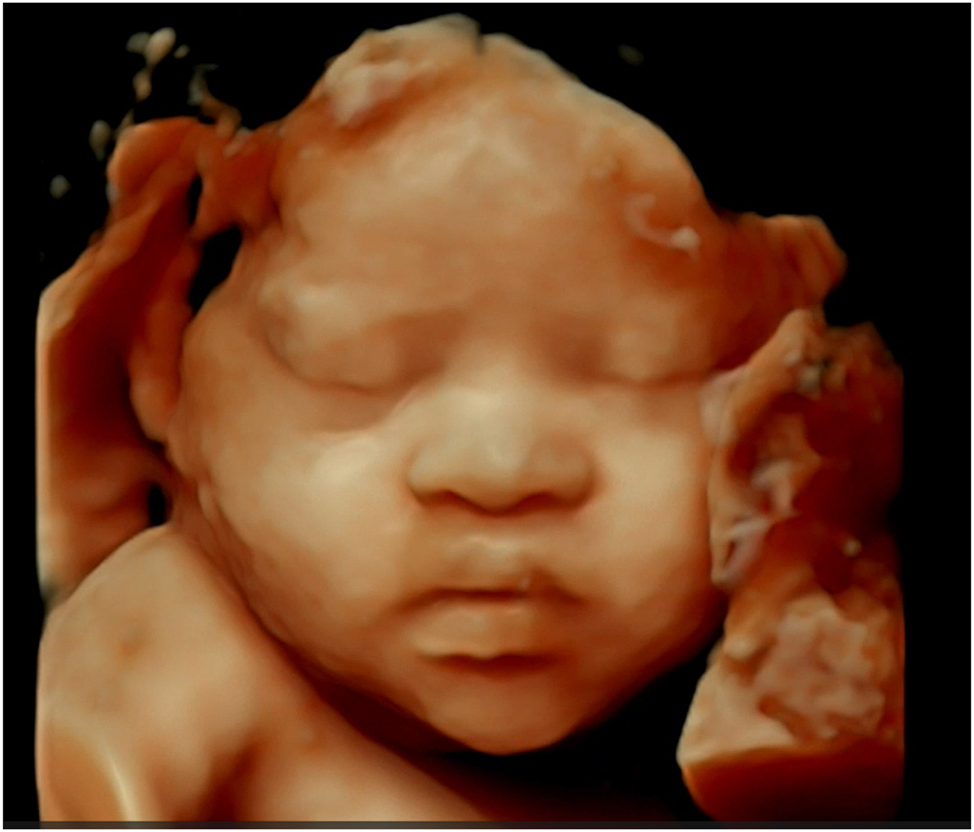
This 4D ultrasound image displays facial features characteristic of Down syndrome, such as a flattened facial profile and midfacial hypoplasia; although precise measurement ranges are not provided, the image is intended to serve as a reference for clinicians in identifying these anomalies, with explanatory notes emphasizing the need for clinical correlation.
Diagnostic synergy and workflow implications
Integrating NIPT and ultrasound into diagnostic workflows provides a robust framework for prenatal care. NIPT offers genetic insights with high specificity, while ultrasound adds structural clarity, particularly in cases where cfDNA levels are insufficient. For example, in cases of Monosomy X, ultrasound markers such as increased NT and coarctation of the aorta provided diagnostic clarity when cfDNA signals were ambiguous. This dual-modality approach not only enhances diagnostic accuracy but also streamlines clinical pathways by reducing reliance on repeat testing or unnecessary invasive procedures. The integration of these modalities has been recently supported by several studies that demonstrate improved patient management and cost-effectiveness in prenatal screening protocols [1].
Ambiguity in cfDNA results and clinical management
Ambiguous cfDNA results, defined as cases with insufficient fetal fractions (<4 %) or inconclusive chromosomal findings, were observed in 4.21 % of cases. These ambiguous results posed a significant challenge, as they delayed definitive diagnoses and increased parental anxiety [1], 4]. In such cases, clinical management relied heavily on complementary ultrasound imaging to provide structural insights [7], 12]. For instance, ultrasound markers such as increased NT >3 mm or craniofacial anomalies played a pivotal role in clarifying suspected chromosomal anomalies [7], 12]. When ultrasound findings were inconclusive, repeat NIPT testing was performed – particularly when low cfDNA levels were attributed to early gestational age [16], 18], 19] – and invasive diagnostic procedures, such as amniocentesis, were offered when integrated findings suggested a high probability of chromosomal abnormalities [15], 18]. These management strategies are reinforced by recent literature that advocates for multimodal follow-up protocols to reduce diagnostic uncertainty [3].
Challenges and limitations
One key limitation of NIPT in these cases was its dependence on sufficient fetal fractions, which can be affected by factors such as maternal obesity, multiple pregnancies, or early gestational age [2], 3], 6], 8], 9]. These factors often necessitated additional testing, prolonging the diagnostic timeline. Moreover, ambiguous results occasionally failed to provide actionable insights, highlighting the need for enhanced diagnostic tools [16], 18]. As noted by Allyse et al., the variability in cfDNA yield remains a critical barrier to universal implementation, warranting further technological improvements and methodological refinements [11].
Future directions for managing ambiguities
To reduce ambiguity in cfDNA results, several strategies can be implemented. Expanding cfDNA panels to include microdeletions, duplications, and rare chromosomal abnormalities could provide a broader scope of analysis, minimizing inconclusive findings [12], 17]. Additionally, integrating AI into NIPT workflows could improve the interpretation of low fetal fractions by identifying and compensating for maternal factors affecting cfDNA levels [17], 18]. AI-powered systems can also predict the likelihood of cfDNA insufficiency, enabling clinicians to make informed decisions about repeat testing or alternative diagnostic methods. By leveraging these advancements and combining genetic data with detailed structural imaging, future prenatal diagnostic frameworks can offer more reliable and timely results, ultimately improving clinical outcomes and reducing parental stress. This perspective is increasingly supported by studies demonstrating that AI integration can enhance both the sensitivity and specificity of current screening methods [12], 16].
Clinical recommendations
Based on the findings from this study and the supporting literature, we propose the following clinical recommendations to optimize the integration of NIPT and ultrasound in prenatal diagnostics:
Initial Screening Protocols: NIPT should be offered to all pregnant women at or beyond 10 weeks of gestation, as it provides high sensitivity and specificity for detecting common aneuploidies such as Trisomy 21, 18, and 13 [1], [2], [3], [4]. For high-risk pregnancies (e.g., advanced maternal age, history of chromosomal abnormalities), NIPT should be combined with first-trimester ultrasound to assess critical markers such as NT [1], [2], [3], [4, 7], 12].
Follow-Up for Inconclusive NIPT Results: In cases where cfDNA levels are insufficient (<4 %), ultrasound should be prioritized to identify structural markers such as craniofacial anomalies or cardiac defects [4], 7]. Repeat NIPT testing may be considered if the cfDNA insufficiency is attributed to early gestational age or maternal obesity [8], 9].
Diagnostic Workflow for Detected Anomalies: When NIPT detects a high probability of chromosomal anomalies, follow-up ultrasound should confirm structural findings (e.g., holoprosencephaly in Trisomy 13 or overlapping fingers in Trisomy 18) to refine the diagnosis and guide counseling [12]. For ambiguous findings, such as suspected sex chromosome aneuploidies (e.g., Monosomy X), invasive testing (e.g., amniocentesis) may be warranted [12].
Diagnostic Algorithm: A stepwise diagnostic algorithm is recommended: Perform NIPT at 10 weeks or later. Follow up with ultrasound at 12–20 weeks to assess structural markers. Use integrated findings to guide the need for invasive testing.
Integration with Emerging Technologies: While this study focuses on current diagnostic modalities, incorporating advancements such as expanded cfDNA panels and AI-enhanced ultrasound holds promise for improving diagnostic accuracy in future applications.
The role of artificial intelligence (AI) in enhancing diagnostic accuracy
AI integration in prenatal diagnostics
AI has emerged as a transformative tool in addressing challenges in prenatal diagnostics, particularly in mitigating operator variability and enhancing the detection of subtle anomalies. By automating and standardizing measurements, AI-driven systems improve diagnostic consistency and accuracy, even in resource-limited settings. Recent pilot programs have demonstrated the potential of AI to streamline prenatal screening workflows [23], 24].
Examples of AI implementation
In real-world applications, AI has demonstrated significant potential in standardizing ultrasound assessments. For instance, pilot programs in Europe reported that AI algorithms improved NT measurement consistency by 95 % across operators, reducing variability and ensuring more reliable detection of chromosomal markers [23], 24]. Similarly, AI-enhanced 3D and 4D imaging has shown a 30 % improvement in detecting craniofacial anomalies, such as cleft lip and palate, compared to traditional methods [25], [26], [27]. AI is also being integrated into workflows to optimize cfDNA analysis. Advanced algorithms can predict and compensate for factors contributing to low cfDNA levels, such as maternal obesity or early gestational age, reducing the need for repeat testing and expediting diagnoses [8], 9].
Addressing operator variability
One of the critical challenges in ultrasound diagnostics is operator dependency, which can lead to inconsistencies in measurements and missed markers. AI-powered tools, such as automated image analysis systems, can standardize the identification of subtle markers like ductus venosus flow abnormalities or borderline skeletal anomalies. These systems not only enhance diagnostic precision but also democratize access to high-quality prenatal care by reducing reliance on specialized operators [23], 24].
Future directions
Integrating AI into multi-modal diagnostic frameworks that combine NIPT and ultrasound could revolutionize prenatal care. By leveraging AI to interpret both genetic and imaging data, clinicians can achieve a comprehensive understanding of fetal health. Future advancements should focus on expanding AI training datasets to include diverse populations and rare conditions, ensuring broader applicability. Additionally, addressing ethical considerations, such as patient consent and data security, will be critical for the widespread adoption of AI in clinical practice [23], 24].
Findings and interpretations
This study confirmed that integrating NIPT with ultrasound imaging enhances the detection of chromosomal and structural anomalies. Among the 190 cases analyzed, NIPT achieved a cfDNA detection rate of 91.58 %, with aneuploidies identified in 4.74 % of cases, including Trisomy 21, 18, and 13, as well as Monosomy X and 47,XXX [1], 5]. Additionally, two cases of rare aneuploidies, Trisomy 7 and Trisomy 16, were flagged based on integrated findings (Figure 4). Ultrasound findings provided critical structural insights, particularly in ambiguous or overlapping cases. For instance, increased NT, observed in 70 % of Down syndrome cases (Table 1), was a significant marker that corroborated NIPT results [8]. Similarly, overlapping fingers and severe growth restriction were defining features in Edward syndrome (Figure 7; Table 2), while holoprosencephaly and craniofacial anomalies characterized Patau syndrome (Table 3). These structural markers, combined with genetic findings, allowed for more comprehensive and nuanced diagnoses.
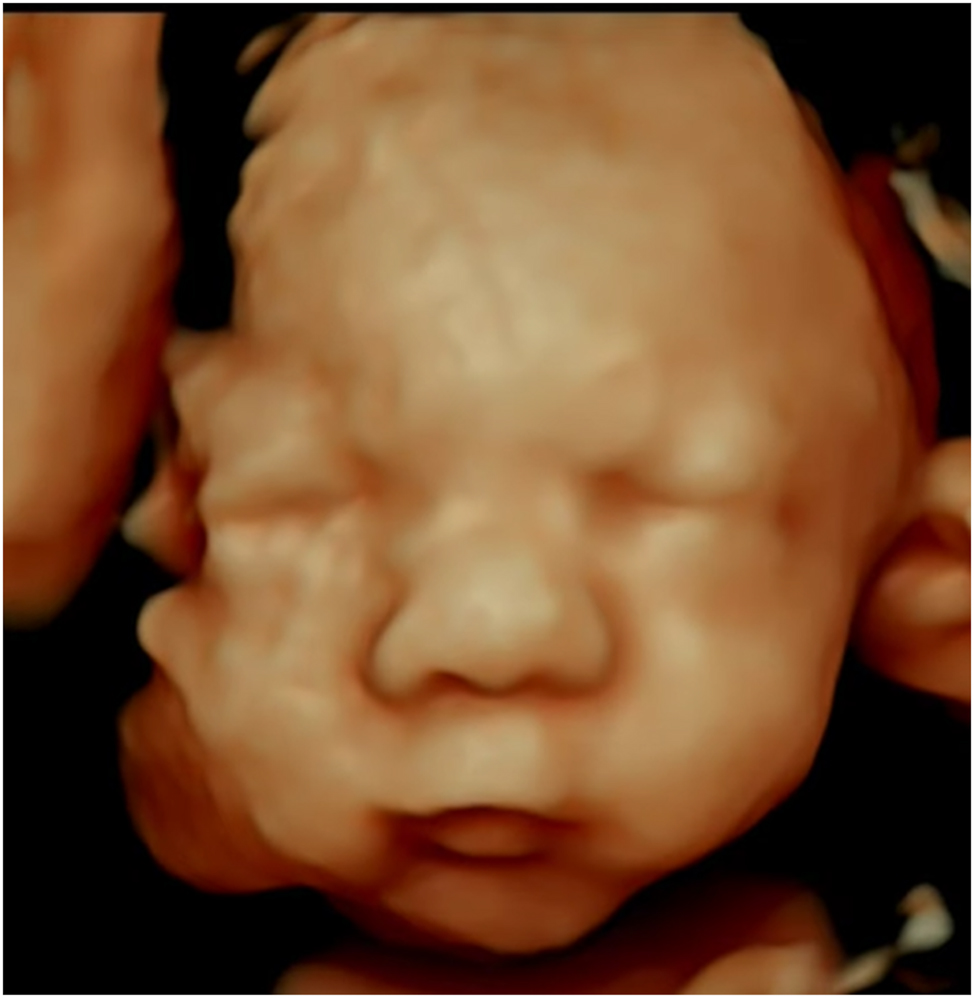
This Figure shows a 4D ultrasound image highlighting key features of Edwards syndrome, including a prominent occiput, micrognathia, and craniofacial asymmetry; while no standardized normal ranges exist for these features, the image offers a diagnostic reference and includes notes on their clinical relevance.
In cases where NIPT results were inconclusive or insufficient (8.42 % of cases; Figure 2), ultrasound played a pivotal role in refining diagnostic outcomes. Cardiac defects and skeletal abnormalities identified via imaging were particularly valuable in distinguishing genetic from non-genetic anomalies [12]. For example, microorchidism and borderline growth restriction in XXY syndrome (Table 4) provided diagnostic clarity in cases with normal karyotypes [12]. The combined diagnostic approach was especially impactful in rare or atypical cases, such as the suspected Trisomy 7 and Trisomy 16 cases. Here, ultrasound findings, such as severe growth restriction and cardiac anomalies, complemented NIPT data to provide a clearer diagnostic picture. This underscores the importance of a multi-modal approach in managing complex or overlapping findings. Recent literature supports these integrated methods as a means to reduce both false negatives and false positives in prenatal screening [6].
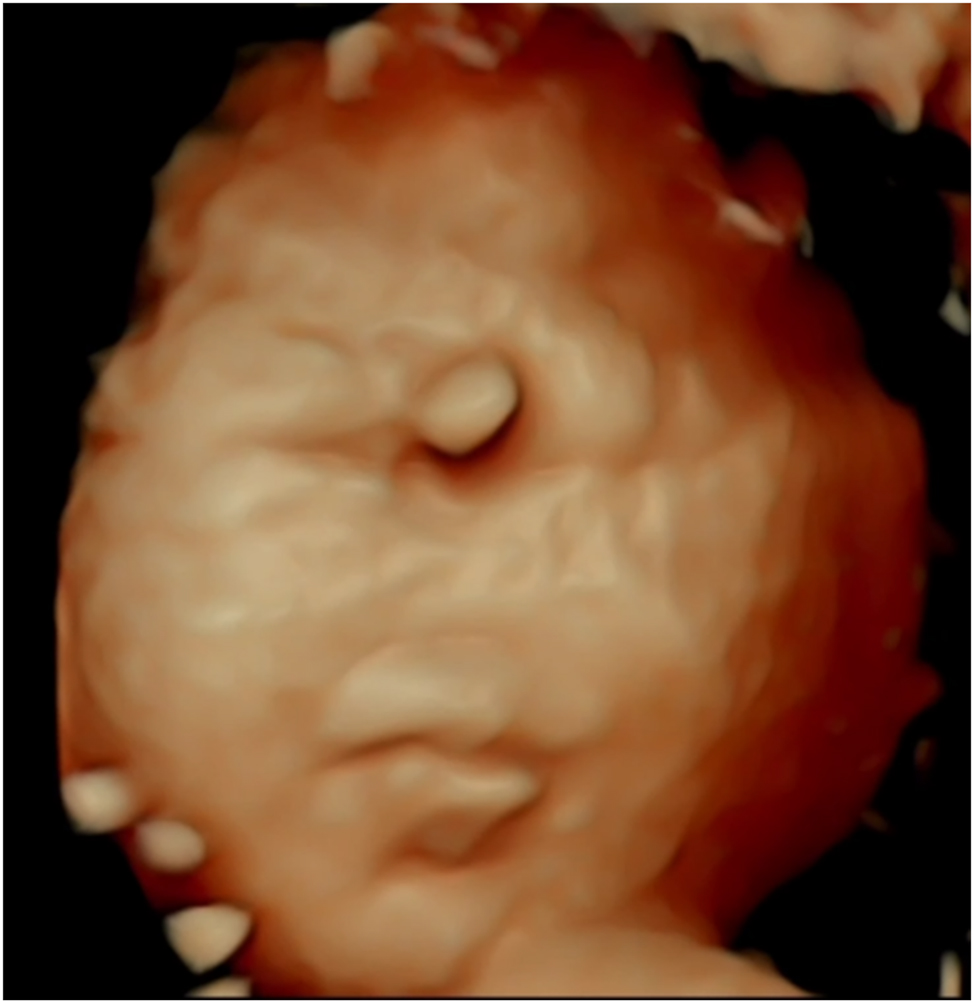
This 4D ultrasound image reveals distinct craniofacial anomalies characteristic of Patau’s syndrome (Trisomy 13), offering valuable insights for prenatal diagnosis. Prominent features, such as holoprosencephaly (incomplete brain division), cleft lip or palate, and hypotelorism (close-set eyes), are visualized in high resolution, with the image serving as an illustrative guide for diagnosis. The advanced imaging allows for detailed assessment of facial malformations, which are key diagnostic markers of this condition. By providing a comprehensive view, this technique enhances early detection and enables better-informed clinical decisions and family counselling.
Case examples
This study highlights the practical implications of integrating NIPT with ultrasound imaging through several illustrative cases:
Case 1: Trisomy 21 (Down syndrome). A patient presented with NIPT results indicating a high probability for Trisomy 21. Follow-up ultrasound revealed hallmark markers such as increased NT >3 mm and the absence of a nasal bone, both observed in approximately 70 % of Down syndrome cases (Figure 6; Table 1). These combined findings facilitated early parental counselling and allowed the healthcare team to plan appropriate clinical management.
Case 2: Trisomy 13 (Patau syndrome). NIPT flagged a high risk for Trisomy 13, prompting a detailed ultrasound evaluation. Structural anomalies such as holoprosencephaly, polydactyly, and craniofacial malformations, including cleft lip and palate (Table 3), were confirmed through imaging (Figure 8). These findings corroborated the genetic results, enabling a definitive diagnosis and guiding the parents through informed decision-making.
Case 3: Isolated Structural Anomaly. A fetus with a normal karyotype presented with isolated cardiac defects, including a ventricular septal defect. These findings, detected via ultrasound, highlighted the importance of imaging in identifying non-genetic structural anomalies [12]. Clinical management focused on addressing the structural abnormality without the need for genetic interventions [12].
Case 4: Monosomy X (Turner syndrome). NIPT results for Monosomy X were inconclusive due to low cfDNA levels (<4 %). However, ultrasound findings such as increased NT and specific cardiac defects, including coarctation of the aorta, provided critical diagnostic clarity (Figure 2). This integrated approach enabled timely diagnosis despite limitations in cfDNA analysis [13].
Case 5: Ambiguous Fetal Sex Determination. In one case, low cfDNA levels led to ambiguous fetal sex determination. Ultrasound imaging clarified the issue by identifying normal male genital anatomy (Figure 9). This example underscores the ability of ultrasound to resolve diagnostic uncertainties when NIPT results are inconclusive [18].
These cases illustrate the synergistic power of combining NIPT with ultrasound imaging, particularly in cases with ambiguous or overlapping findings (Figure 9). The integrative approach not only enhances diagnostic precision but also enables personalized clinical care tailored to each patient’s unique circumstances.
The 47 XXX syndrome, also known as Trisomy X, is a chromosomal condition that affects females and arises from the presence of an extra X chromosome in each cell. While many individuals with this condition have normal physical development and fertility, some may experience taller stature, learning disabilities, and delayed speech or motor skills. The condition is typically diagnosed through genetic testing, and most affected individuals lead healthy lives with appropriate support for any developmental challenges.
Sample limitations and representativeness
This study acknowledges several limitations in its sample size and demographic distribution, which may impact the generalizability of the findings. The cohort included 190 participants, predominantly drawn from urban and suburban regions, potentially excluding rural populations where access to prenatal care and diagnostic tools may differ significantly (Figure 5). Additionally, most participants were under 35 years of age (74.21 %), limiting the applicability of results to older maternal populations who are at a higher risk for chromosomal anomalies (Figure 1). Variability in socioeconomic factors, healthcare accessibility, and cultural attitudes toward prenatal testing were not fully accounted for, which could influence the study’s broader applicability [8]. Furthermore, the reliance on a single geographic location and the relatively small sample size may reduce the ability to detect less common chromosomal or structural anomalies [9]. Recent calls for larger, multicenter studies emphasize the need to include diverse populations to ensure more universally applicable findings [6].
Addressing limitations through AI
AI presents an innovative solution to address key limitations in prenatal diagnostics, such as operator variability in ultrasound imaging and challenges with NIPT [1], 4]. By standardizing the interpretation of ultrasound markers and enhancing the analysis of cfDNA data, AI-driven systems can improve diagnostic precision and consistency [1], 4]. Operator variability has long been a critical challenge in ultrasound diagnostics, impacting the detection of subtle anomalies such as NT irregularities or minor craniofacial malformations [12]. AI algorithms trained on large datasets of ultrasound images can mitigate these discrepancies by offering consistent and automated assessments. For instance, a study demonstrated that AI tools improved NT measurement accuracy by 95 % across operators, significantly reducing variability in results [20]. In the context of cfDNA analysis, AI has the potential to enhance fetal fraction detection, particularly in cases where low cfDNA levels have traditionally led to inconclusive results [9]. Emerging AI-based platforms are exploring ways to optimize cfDNA preprocessing and data interpretation, addressing challenges linked to maternal obesity and early gestational age [10], 11]. Such advancements could reduce the need for repeat testing and accelerate diagnosis, alleviating stress for expectant parents [12].
Recent advances in prenatal diagnostics
Recent original research has further refined integrated prenatal diagnostic approaches. Lin et al., introduced a novel algorithm that enhances NIPT’s prediction accuracy for Turner syndrome (45,X) by mitigating reference bias – a development that could reduce false-positive rates and improve clinical confidence [20]. Complementing this, Laporte et al., evaluated intrinsic fetal airway obstruction (CHAOS) through a rigorous correlation of ultrasound, fetoscopic, and pathological findings, underscoring the pivotal role of multi-modal imaging in accurately characterizing complex fetal structural anomalies [21]. In addition, Allen et al., demonstrated that clinically informed strategies significantly enhance the diagnostic yield for fetal structural anomalies in cases where conventional microarray testing remains non-diagnostic [22]. Collectively, these studies reinforce the trend toward integrating advanced genetic methodologies with detailed imaging and clinical data to achieve earlier, more precise, and personalized prenatal care.
Limitations and uncertainties in NIPT and ultrasound diagnostics
While NIPT has revolutionized prenatal diagnostics with its high sensitivity and specificity for common chromosomal anomalies, it is not without limitations. The reliance on sufficient cfDNA levels, often impacted by factors like maternal obesity, early gestational age, or multiple pregnancies, can lead to inconclusive results in 8.42 % of cases (Figure 2) [1], 4]. Low cfDNA levels necessitate repeat testing, delaying diagnosis and causing additional stress for expectant parents [5], 6]. NIPT is also limited in its ability to detect all genetic anomalies. For example, conditions such as balanced translocations, single-gene disorders, or some microdeletions may remain undetected due to the restricted scope of current cfDNA panels [8], 9]. As a result, cases with structural abnormalities identified through ultrasound but with normal karyotypes often require additional invasive procedures, such as amniocentesis, for definitive diagnosis [18].
Ultrasound imaging, while indispensable for identifying structural markers like NT and craniofacial anomalies, also has its challenges. Operator variability can significantly affect the accuracy of measurements, leading to inconsistencies in detecting subtle features such as minor skeletal abnormalities or cardiac defects [12]. Additionally, the timing of ultrasound assessments plays a crucial role; certain abnormalities may not be apparent until later stages of pregnancy, potentially delaying diagnosis and intervention [12]. Both NIPT and ultrasound face limitations when dealing with rare or overlapping conditions. For example, in cases of suspected Trisomy 7 or Trisomy 16, standalone results from either modality may yield insufficient clarity, requiring a multi-modal approach to achieve a reliable diagnosis [12]. Furthermore, socioeconomic factors and healthcare disparities can affect access to advanced diagnostic tools, creating inequities in prenatal care [19]. To address these challenges, advancements such as expanding cfDNA panels to include microdeletions and duplications and incorporating AI-driven ultrasound analysis are promising solutions. AI can standardize measurements, reducing operator variability and improving the detection of subtle anomalies such as ductus venosus abnormalities or borderline growth restrictions [20]. Furthermore, novel techniques to enhance cfDNA analysis, including pre-processing methods to improve fetal fraction detection, are under investigation and could significantly enhance NIPT reliability [17], 18]. By acknowledging and addressing these limitations, future research and clinical practices can refine prenatal diagnostics to ensure earlier, more accurate, and equitable outcomes for diverse patient populations [17], [18], [19], [20].
Implications of cfDNA insufficiency
The issue of insufficient cfDNA presents a significant challenge in the application of NIPT, particularly for populations with higher maternal BMI, early gestational age, or multiple pregnancies. In cases where cfDNA levels fall below the necessary threshold (4 %), as seen in 8.42 % of cases in this study (Figure 2), the reliability of NIPT results is compromised, necessitating repeat testing and often leading to delays in diagnosis [1], 4]. This insufficiency can be particularly problematic for women with high BMI, where the concentration of fetal cfDNA in maternal blood may be diluted, making it more difficult to achieve conclusive results [5], 6]. Moreover, in multiple pregnancies, cfDNA from each fetus may be indistinguishable, leading to diagnostic ambiguity and challenges in accurate fetal sex determination or chromosomal abnormality identification [8], 9]. In these cases, NIPT may not provide clear results, increasing the need for follow-up tests, including more invasive procedures like amniocentesis, to confirm the diagnosis [12].
The implications of cfDNA insufficiency extend beyond diagnostic delays. Inaccurate or ambiguous results can lead to unnecessary stress and anxiety for expectant parents, particularly when repeat tests are required or when results suggest the possibility of a chromosomal abnormality but cannot definitively confirm it [10]. The uncertainty surrounding cfDNA insufficiency is a critical issue that requires attention in order to improve the overall patient experience. To mitigate the impact of cfDNA insufficiency, researchers are exploring ways to enhance fetal fraction detection through novel techniques and preprocessing methods that could improve cfDNA analysis, especially in challenging populations such as women with higher BMI or in multiple pregnancies [11]. Additionally, combining NIPT with ultrasound imaging provides a complementary approach, enabling clinicians to rely on structural markers, such as NT or craniofacial anomalies, to confirm or clarify genetic findings [12]. Furthermore, the integration of AI into the NIPT process could improve the detection of low fetal fractions, offering more precise data interpretation and reducing the likelihood of inconclusive results [18]. AI-driven tools can also help standardize ultrasound assessments, ensuring consistent and accurate results even in cases where cfDNA levels are insufficient [20]. Addressing cfDNA insufficiency is essential for the continued improvement of NIPT. By refining technologies and integrating multi-modal approaches, prenatal diagnostics can become more reliable and accessible for all expectant parents, reducing the need for invasive testing and ensuring earlier, more accurate diagnoses.
Overall, these findings validate the efficacy of integrating genetic and structural diagnostics in prenatal care. The enhanced diagnostic precision achieved through combining NIPT with ultrasound imaging not only facilitates early, accurate detection of chromosomal and structural anomalies but also informs tailored clinical management strategies. As the field advances, further integration of emerging technologies – particularly AI – and expanded cfDNA panels are anticipated to overcome current limitations, ultimately improving outcomes for mothers and their children.
Clinical applications and implementation guidelines
The integration of NIPT and ultrasound imaging in prenatal diagnostics should follow a systematic algorithm that begins with offering NIPT at or after 10 weeks of gestation, ensuring that cfDNA levels meet the required threshold, and is followed by a detailed ultrasound examination between 12 and 20 weeks to assess key structural markers. This coordinated approach, which involves a multidisciplinary team – including obstetricians, genetic counselors, and certified sonographers – facilitates the prompt identification and confirmation of chromosomal and structural anomalies, thereby enhancing patient counseling and clinical management.
To ensure the highest diagnostic accuracy, it is imperative to implement rigorous quality assurance measures, such as standardized operating procedures, regular equipment calibration, and routine audits with peer reviews. In addition, establishing comprehensive training programs that include initial certification, periodic re-certification, and dedicated workshops on AI integration will help reduce operator variability and maintain high standards in both ultrasound and cfDNA analysis.
From a resource perspective, significant investments in state-of-the-art ultrasound equipment, reliable NIPT platforms, and AI software are necessary. Coupled with robust information technology (IT) infrastructure to support data integration and analysis, these resources can ultimately reduce the need for invasive procedures and minimize repeat testing, making the integrated diagnostic strategy not only clinically effective but also cost-efficient.
Conclusions
This study highlights the significant benefits of combining NIPT with ultrasound imaging for detecting fetal anomalies. NIPT provided a high cfDNA detection rate of 91.58 %, identifying chromosomal abnormalities in 4.74 % of cases, but was limited by insufficient cfDNA in 8.42 % of cases. Ultrasound imaging supplemented NIPT by providing crucial structural insights, particularly in ambiguous cases such as increased NT for Down syndrome. This dual-modality approach enhanced diagnostic accuracy, especially for rare aneuploidies like Trisomy 7 and 16. Expanding cfDNA panels to include microdeletions and duplications could further improve detection of rarer genetic conditions. The integration of AI in ultrasound diagnostics shows promise for standardizing assessments and reducing variability. Overall, combining genetic and imaging diagnostics offers a more precise and personalized approach to prenatal care. As these technologies evolve, they will continue to improve prenatal outcomes for mothers and their children.
Acknowledgments
The authors appreciate the Indonesian Society of Obstetrics and Gynecology (POGI) and the Indonesian Society of Maternal-Fetal Medicine (HKFM) for encouraging and supporting the work of this review article. The authors would like to extend their heartfelt gratitude to Wenny Yang from Gene Solutions for her invaluable insights and contributions, which have significantly enriched the understanding and advancement of this work.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authos have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Audibert, F, Wou, K, Okun, N, De Bie, I, Wilson, RD. Guideline No. 456: prenatal screening for fetal chromosomal anomalies. J Obstet Gynaecol Can 2024;46:102694. https://doi.org/10.1016/j.jogc.2024.102694.Suche in Google Scholar PubMed
2. Zou, Y, Feng, C, Qin, J, Wang, X, Huang, T, Yang, Y, et al.. Performance of expanded non-invasive prenatal testing for fetal aneuploidies and copy number variations: a prospective study from a single center in Jiangxi province, China. Front Genet 2023;13:1073851. https://doi.org/10.3389/fgene.2022.1073851.Suche in Google Scholar PubMed PubMed Central
3. Benn, P, Cuckle, H, Pergament, E. Non-invasive prenatal testing for aneuploidy: current status and future prospects. Ultrasound Obstet Gynecol 2013;42:15–33. https://doi.org/10.1002/uog.12513.Suche in Google Scholar PubMed
4. Ye, C, Duan, H, Liu, M, Liu, J, Xiang, J, Yin, Y, et al.. The value of combined detailed first-trimester ultrasound-biochemical analysis for screening fetal aneuploidy in the era of non-invasive prenatal testing. Arch Gynecol Obstet 2024;310:843–53. https://doi.org/10.1007/s00404-023-07267-3.Suche in Google Scholar PubMed PubMed Central
5. Zhang, L, Chang, B, Wang, L, Mijiti, G, Bahetibieke, K, Xue, S. Evaluation of the clinical utility of NIPT-plus and analysis of adverse pregnancy outcomes. Arch Gynecol Obstet 2024;310:2973–81. https://doi.org/10.1007/s00404-024-07811-9.Suche in Google Scholar PubMed
6. Xu, C, Cai, X, Chen, S, Luo, Q, Xi, H, Zhang, D, et al.. Comprehensive non-invasive prenatal screening for pregnancies with elevated risks of genetic disorders: protocol for a prospective, multicentre study. BMJ Open 2021;11:e053617. https://doi.org/10.1136/bmjopen-2021-053617.Suche in Google Scholar PubMed PubMed Central
7. Esteves, KM, Tugarinov, N, Lechmann, G, Abi Habib, P, Cagliyan, E, Goetzinger, KR, et al.. The value of detailed first-trimester ultrasound in the era of noninvasive prenatal testing. Am J Obstet Gynecol 2023;229:326. e1–326.e6. https://doi.org/10.1016/j.ajog.2023.05.031.Suche in Google Scholar PubMed
8. Konya, M, Czimbalmos, A, Loczi, L, Koi, T, Turan, C, Nagy, R, et al.. Genome-wide, non-invasive prenatal testing for rare chromosomal abnormalities: a systematic review and meta-analysis of diagnostic test accuracy. PLoS One 2024;19:e0308008. https://doi.org/10.1371/journal.pone.0308008.Suche in Google Scholar PubMed PubMed Central
9. Parsaei, M, Dashtkoohi, M, Salmani, TA, Najafi, MS, Haddadi, M, Ghaemi, M, et al.. Potential efficacy of digital polymerase chain reaction for non-invasive prenatal screening of autosomal aneuploidies: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2024;24:472. https://doi.org/10.1186/s12884-024-06655-0.Suche in Google Scholar PubMed PubMed Central
10. Taylor-Phillips, S, Freeman, K, Geppert, J, Agbebiyi, A, Uthman, OA, Madan, J, et al.. Accuracy of non-invasive prenatal testing using cell-free DNA for detection of Down, Edwards and Patau syndromes: a systematic review and meta-analysis. BMJ Open 2016;6:e010002. https://doi.org/10.1136/bmjopen-2015-010002.Suche in Google Scholar PubMed PubMed Central
11. Allyse, M, Minear, MA, Berson, E, Sridhar, S, Rote, M, Hung, A, et al.. Non-invasive prenatal testing: a review of international implementation and challenges. Int J Womens Health 2015;7:113–26. https://doi.org/10.2147/IJWH.S67124.Suche in Google Scholar PubMed PubMed Central
12. Nicolaides, KH, Syngelaki, A, Ashoor, G, Birdir, C, Touzet, G. Noninvasive prenatal testing for fetal trisomies in a routinely screened first-trimester population. Am J Obstet Gynecol 2012;207:374.e1–6. https://doi.org/10.1016/j.ajog.2012.08.033.Suche in Google Scholar PubMed
13. Monni, G, Atzori, L, Corda, V, Dessolis, F, Iuculano, A, Hurt, KJ, et al.. Metabolomics in prenatal medicine: a review. Front Med (Lausanne) 2021;8:645118. https://doi.org/10.3389/fmed.2021.645118.Suche in Google Scholar PubMed PubMed Central
14. D’ambrosio, V, Squarcella, A, Vena, F, Di Mascio, D, Corno, S, Pajno, C, et al.. Update in non-invasive prenatal testing. Minerva Ginecol 2019;71:44–53. https://doi.org/10.23736/S0026-4784.18.04306-X.Suche in Google Scholar PubMed
15. Yang, L, Tan, WC. Prenatal screening in the era of non-invasive prenatal testing: a nationwide cross-sectional survey of obstetrician knowledge, attitudes and clinical practice. BMC Pregnancy Childbirth 2020;20:579. https://doi.org/10.1186/s12884-020-03279-y.Suche in Google Scholar PubMed PubMed Central
16. Kim, SY, Lee, SM, Jun, JK, Han, YJ, Kim, MH, Shim, JY, et al.. Prospective observations study protocol to investigate cost-effectiveness of various prenatal test strategies after the introduction of noninvasive prenatal testing. BMC Pregnancy Childbirth 2018;18:307. https://doi.org/10.1186/s12884-018-1930-y.Suche in Google Scholar PubMed PubMed Central
17. Abedalthagafi, M, Bawazeer, S, Fawaz, RI, Heritage, AM, Alajaji, NM, Faqeih, E. Non-invasive prenatal testing: a revolutionary journey in prenatal testing. Front Med (Lausanne) 2023;10:1265090. https://doi.org/10.3389/fmed.2023.1265090.Suche in Google Scholar PubMed PubMed Central
18. Yaron, Y. The implications of non-invasive prenatal testing failures: a review of an under-discussed phenomenon. Prenat Diagn 2016;36:391–6. https://doi.org/10.1002/pd.4804.Suche in Google Scholar PubMed
19. Chitty, LS, Wright, D, Hill, M, Verhoef, TI, Daley, R, Lewis, C, et al.. Uptake, outcomes, and costs of implementing non-invasive prenatal testing for Down’s syndrome into NHS maternity care: prospective cohort study in eight diverse maternity units. BMJ 2016;354:i3426. https://doi.org/10.1136/bmj.i3426.Suche in Google Scholar PubMed PubMed Central
20. Lin, X, Huang, C, Chen, L, Du, B, Guo, Y, Zeng, M, et al.. Enhancing non-invasive prenatal testing: a novel approach to improve 45,X prediction accuracy. Prenat Diagn 2025;45:1581–92. https://doi.org/10.1002/pd.6756.Suche in Google Scholar PubMed
21. Laporte, C, Martinovic, J, Patrier, S, Thierry, B, Mediouni, I, Saada, J, et al.. Evaluation of intrinsic fetal airway obstruction (CHAOS): correlations between ultrasound, fetoscopic, and pathological findings. Prenat Diagn 2025;45:1581–92. https://doi.org/10.1002/pd.6761.Suche in Google Scholar PubMed PubMed Central
22. Allen, VM, Schollenberg, E, Aberg, E, Brock, JK. Use of clinically informed strategies and diagnostic yields of genetic testing for fetal structural anomalies following a non-diagnostic microarray result: a population-based cohort study. Prenat Diagn 2025;45:1581–92. https://doi.org/10.1002/pd.6759.Suche in Google Scholar PubMed
23. Bachnas, MA, Andonotopo, W, Dewantiningrum, J, Adi Pramono, MB, Stanojevic, M, Kurjak, A. The utilization of artificial intelligence in enhancing 3D/4D ultrasound analysis of fetal facial profiles. J Perinat Med 2024;52:899–913. https://doi.org/10.1515/jpm-2024-0347.Suche in Google Scholar PubMed
24. Andonotopo, W, Bachnas, MA, Dewantiningrum, J, Adi Pramono, MB, Stanojevic, M, Kurjak, A. AI and early diagnostics: mapping fetal facial expressions through development, evolution, and 4D ultrasound. J Perinat Med 2025;53:263–85. https://doi.org/10.1515/jpm-2024-0602.Suche in Google Scholar PubMed
25. Kurjak, A, Pooh, RK, Merce, LT, Carrera, JM, Salihagic-Kadic, A, Andonotopo, W. Structural and functional early human development assessed by three-dimensional and four-dimensional sonography. Fertil Steril 2005;84:1285–99. https://doi.org/10.1016/j.fertnstert.2005.03.084.Suche in Google Scholar PubMed
26. Kurjak, A, Miskovic, B, Andonotopo, W, Stanojevic, M, Azumendi, G, Vrcic, H. How useful is 3D and 4D ultrasound in perinatal medicine? J Perinat Med 2007;35:10–27. https://doi.org/10.1515/JPM.2007.002.Suche in Google Scholar PubMed
27. Kurjak, A, Azumendi, G, Andonotopo, W, Salihagic-Kadic, A. Three- and four-dimensional ultrasonography for the structural and functional evaluation of the fetal face. Am J Obstet Gynecol 2007;196:16–28. https://doi.org/10.1016/j.ajog.2006.06.090.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Reviews
- Integrating NIPT and ultrasound for detecting fetal aneuploidies and abnormalities
- Ethical challenges in perinatal ultrasound: balancing diagnostic capability and ethical communication
- Original Articles – Obstetrics
- Risk factors and adverse outcomes associated with hepatitis C virus in pregnancy
- Utility of endometrial multi-vessel blood flow ultrasound parameters in predicting pregnancy outcomes
- Improving the accuracy of screening for large-for-gestational-age fetuses: a multicenter observational study
- Risk factors and awareness of tobacco smoking and second-hand smoke exposure among pregnant women in Taiwan
- Effect of oral hydration therapy on amniotic fluid index and maternal-neonatal outcomes in pregnant women with oligohydramnios: a systematic review and meta-analysis
- Epidural anesthesia during labor and delivery and postpartum hemorrhage
- Social vulnerability and triage acuity among pregnant people seeking unscheduled hospital care
- Gestational diabetes insipidus. A systematic review of case reports
- Outcomes in pregnant patients with congenital heart disease by rurality
- Original Articles – Fetus
- Exploration of copy number variations and candidate genes in fetal congenital heart disease using chromosomal microarray analysis
- A seven-year retrospective cohort study on non-immune foetal hydrops from a single centre in an LMIC setting
- Original Articles – Neonates
- Correlation between macronutrient content and donation characteristics in Croatian human milk bank
- Gestational diabetes mellitus: the role of IGF-1 and leptin in cord blood
Artikel in diesem Heft
- Frontmatter
- Reviews
- Integrating NIPT and ultrasound for detecting fetal aneuploidies and abnormalities
- Ethical challenges in perinatal ultrasound: balancing diagnostic capability and ethical communication
- Original Articles – Obstetrics
- Risk factors and adverse outcomes associated with hepatitis C virus in pregnancy
- Utility of endometrial multi-vessel blood flow ultrasound parameters in predicting pregnancy outcomes
- Improving the accuracy of screening for large-for-gestational-age fetuses: a multicenter observational study
- Risk factors and awareness of tobacco smoking and second-hand smoke exposure among pregnant women in Taiwan
- Effect of oral hydration therapy on amniotic fluid index and maternal-neonatal outcomes in pregnant women with oligohydramnios: a systematic review and meta-analysis
- Epidural anesthesia during labor and delivery and postpartum hemorrhage
- Social vulnerability and triage acuity among pregnant people seeking unscheduled hospital care
- Gestational diabetes insipidus. A systematic review of case reports
- Outcomes in pregnant patients with congenital heart disease by rurality
- Original Articles – Fetus
- Exploration of copy number variations and candidate genes in fetal congenital heart disease using chromosomal microarray analysis
- A seven-year retrospective cohort study on non-immune foetal hydrops from a single centre in an LMIC setting
- Original Articles – Neonates
- Correlation between macronutrient content and donation characteristics in Croatian human milk bank
- Gestational diabetes mellitus: the role of IGF-1 and leptin in cord blood

