Effect of oral hydration therapy on amniotic fluid index and maternal-neonatal outcomes in pregnant women with oligohydramnios: a systematic review and meta-analysis
-
Anita Yadav
, Priyanka Yadav
, Kalyani P. Deshmukh
, Anusha Kamath
, Chanchal Goyal
, Avinash Prakash
und Aravind P. Gandhi
Abstract
Objectives
Maternal oral hydration therapy is a non-invasive approach to improving AFI, but its effectiveness remains uncertain. This systematic review and meta-analysis were therefore undertaken to assess the effectiveness of maternal oral hydration therapy in increasing AFI in pregnancies complicated by oligohydramnios and to evaluate its impact on maternal and neonatal outcomes.
Methods
A systematic search of Cochrane Library, Embase, PubMed, Scopus, and Web of Science was conducted. Eligible studies included randomized controlled trials (RCTs), quasi-experimental studies, and analytical observational studies. All studies published till 22 November 2024 in the above databases were included. No restrictions were placed on geographic location or study setting. Two reviewers independently undertook the screening. Relevant studies were identified, screened, and duplicates removed using NESTED Knowledge. Risk of bias was assessed using NOS, JBI, and ROBINS-I tools. Statistical analyses, including meta-analysis using a random-effects model, were conducted in R Studio and Comprehensive Meta-Analysis (CMA) software. Heterogeneity was assessed using the I2 statistic.
Results
Out of the 12 included studies four qualified for meta-analysis. Pooled results showed a significant increase in AFI at 2 hours (mean difference: 0.996; 95 % CI: 0.781–1.210), 1 day (0.853; 95 % CI: 0.532–1.174), 2 days (1.649; 95 % CI: 0.943–2.356), and 1 week (2.232; 95 % CI: 0.943–3.520). However, high heterogeneity was observed due to variations in fluid type, volume, and frequency.
Conclusions
It can be concluded that oral hydration therapy significantly increases AFI and is a simple intervention for managing oligohydramnios, especially in resource-limited settings.
Introduction
Amniotic fluid is vital for foetal growth, providing cushioning, enabling movement, and supporting lung and musculoskeletal development. It is assessed via ultrasonography using the amniotic fluid index (AFI), with oligohydramnios defined as an AFI below 8 cm or a single deepest vertical pocket (SDVP) under 2 cm [1], 2]. Affecting approximately 0.5–5 % of gestations, this condition is linked to increased maternal and neonatal risks, including foetal growth restriction, umbilical cord compression, meconium aspiration syndrome, preterm birth, and higher rates of operative delivery, such as caesarean section [3]. Beyond perinatal risks, reduced amniotic fluid may limit foetal movement, leading to musculoskeletal abnormalities, and increase umbilical cord compression, causing hypoxia and foetal distress. Early detection and management are essential to improving maternal and neonatal outcomes [4].
Maternal oral hydration therapy has emerged as a promising, non-invasive intervention for oligohydramnios. Research has shown that both oral and intravenous hydration therapy can enhance amniotic fluid levels, increase uteroplacental perfusion, and support foetal well-being [5], 6]. Oral hydration therapy stands out as an accessible, low-cost strategy, especially beneficial in resource-constrained settings where advanced medical interventions such as amnioinfusion may not be feasible [7]. Its physiological benefits are attributed to increased maternal plasma volume, improved uteroplacental blood flow, and augmented foetal urine production, the latter being a key determinant of amniotic fluid volume [8].
Clinical trials have highlighted the rapid and sustained effects of oral hydration on amniotic fluid levels, with improvements often observed within hours and lasting for several days [9], 10]. These studies have also reported favorable perinatal outcomes, such as reduced incidences of fetal distress, improved Apgar scores, and decreased neonatal intensive care unit (NICU) admissions [11]. Additionally, the simplicity and safety profile of oral hydration therapy make it an attractive option for outpatient management, potentially reducing the need for costly hospitalizations and invasive procedures.
Despite these promising findings, several limitations in the current literature warrant attention. Inconsistencies in the reported efficacy and variability in hydration protocols remain significant challenges. While some research suggests that isotonic or hypotonic fluids may offer superior benefits compared to plain water, no consensus exists on the optimal type, volume, or frequency of fluid intake [2], 12]. Another notable limitation is the predominant focus on third-trimester oligohydramnios. There is a lack of data on the use of hydration therapy in earlier stages of pregnancy, where intervention may yield different outcomes, or in pregnancies complicated by additional maternal conditions such as preeclampsia, gestational diabetes, or chronic hypertension. This systematic review and meta-analysis (SRMA) seeks to bridge these gaps by compiling the available evidence on oral hydration therapy in increasing amniotic fluid levels and enhancing maternal–neonatal outcomes in pregnancies affected by oligohydramnios.
Methods
This SRMA adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines [13] (Supplementary Table S1). We carried out a SRMA of randomized controlled trials, quasi-experimental studies and analytical observational studies. In addition, the study protocol was prospectively registered in the International Prospective Register of Systematic Reviews (Registration No. CRD42024617122).
Eligibility criteria
The research question addressed in the SRMA – “What is the effect of oral water intake on the amniotic fluid index (AFI) and feto-maternal outcomes in pregnant women with oligohydramnios?” – was used to identify the eligible population, intervention incorporated, comparator group, and outcomes documented (Supplementary Table S2). We included any study irrespective of its design (except for reviews) that reported the Amniotic Fluid Index in pregnant women with oligohydramnios. We placed no limitations on the geographic location of studies or the study setting (facility based or community based). Detailed eligibility criteria are enumerated in Supplementary Table S2.
Study population
Studies performed among pregnant women of any age diagnosed with oligohydramnios (AFI<8 cm) without pre-existing comorbidities were included.
Intervention/exposure: Oral water intake (over and above the routine intake).
Comparator/control: Studies with a comparator group of pregnant women with oligohydramnios who continued to receive routine care and were not advised additional hydration as in the exposure group, were included in the SRMA. Studies with single-arm that assessed baseline and endline outcomes among pregnant women with oligohydramnios (before and after exposure) were also included.
Outcomes: Primary outcome includes Amniotic Fluid Index, feto-maternal outcomes, mode of delivery and pregnancy-related complications. Appropriate effect measures, including odds ratio, risk ratio, hazard ratio, mean difference, proportions, prevalence, and incidence rate, were abstracted from the literature.
Study selection process
The study selection process was conducted in three stages:
Initial screening: Two independent reviewers (AY and PY) conducted a preliminary screening of titles, abstracts, and keywords based on the literature search. Studies meeting the essential eligibility requirements were chosen for comprehensive text review.
Detailed assessment: The full texts of the shortlisted studies were thoroughly evaluated by AY and PY to determine their compliance with the predefined eligibility criteria.
Final selection: Any disagreements during the selection process were resolved through discussion, with the involvement of a senior author (AG) to ensure consensus. In cases where essential study details were missing, the corresponding authors were contacted. If no response was received, the study was excluded from the review.
Search strategy
A thorough search strategy was implemented among the major electronic databases: PubMed, Scopus, Web of Science, and Cochrane. All studies published until 22 November 2024 in the above databases were included. Boolean operators and combinations of key terms were utilized and the detailed search strategy has been enumerated in Supplementary Table S3. Only English-language articles involving human participants were included. The search was supplemented with manual screening of reference lists of included studies to ensure completeness.
Data extraction process
Based on the keywords, articles were listed from the databases. The duplicates were reviewed and removed using NESTED Knowledge [14]. Any discrepancies in the eligibility of the studies were fixed in discussion with a third reviewer (AG). The reasons for excluded articles were documented.
Data were retrieved manually using a pre-designed, structured data abstraction form in MS Excel, which included essential details such as the author, publication year, and methodological aspects of each study. The extracted information covered study design, setting, sample size, type of exposure, outcome assessment methods, and quality-related parameters. Key study characteristics recorded included demographics, intervention specifics (fluid volume, duration, and administration setting), and maternal, fetal, and pregnancy-related outcomes. Data entry was conducted by AY and AK, with PY and AG performing a secondary review to ensure accuracy and consistency.
Risk of bias assessment
Study quality was evaluated using design-specific tools such as the “Newcastle-Ottawa Scale (NOS)” [15] for analytical observational studies (case control studies), “Joanna Briggs Institute (JBI)” [16] critical appraisal tools for Quasi Experimental studies and ROBINS-I for Non-Randomized Controlled Trials [17].
Statistical analysis
A “random-effects model (REM)” was used to calculate pooled estimates for outcomes with maximum likelihood estimators. Inter-study heterogeneity was quantified using the I2 statistic. Significant heterogeneity prompted the calculation of prediction intervals [18]. Publication bias assessment by Doi plots, and the LFK (Luis Furuya-Kanamori) index were planned but were not applicable as fewer studies qualified for the outcomes in the meta-analyses. Statistical evaluations were carried out using R Studio, following established coding procedures [19], 20], and comprehensive meta-analysis (CMA) software (for pre-post studies) with a significant threshold of p<0.05. For pre-post studies, a correlation of 0.5 was assumed between the pre and post AFI values for the studies. Sensitivity analysis was also conducted for correlation of 0.1 and 0.9 between pre and post AFI values.
Certainty in the evidence
The certainty of pooled estimates for each outcome was assessed and summarized using GRADEpro software according to the “Grading of Recommendations, Assessment, Development, and Evaluations (GRADE)” methodology [21].
Registration and ethical statement
The protocol was registered in the PROSPERO registry (CRD42024608801). Ethical approval is not required, as the SRMA is based on data from previously published literature.
Results
Study selection
A total of 231 studies were identified across five databases. After removing 124 duplicates, 107 studies remained for screening. Of these, 84 were excluded due to incorrect population (n=36), intervention (n=39), outcomes (n=2), study design (n=6), and lack of abstracts (n=1). Following full-text screening, 12 studies met the inclusion criteria for systematic review, with four included in the meta-analysis (Figure 1).
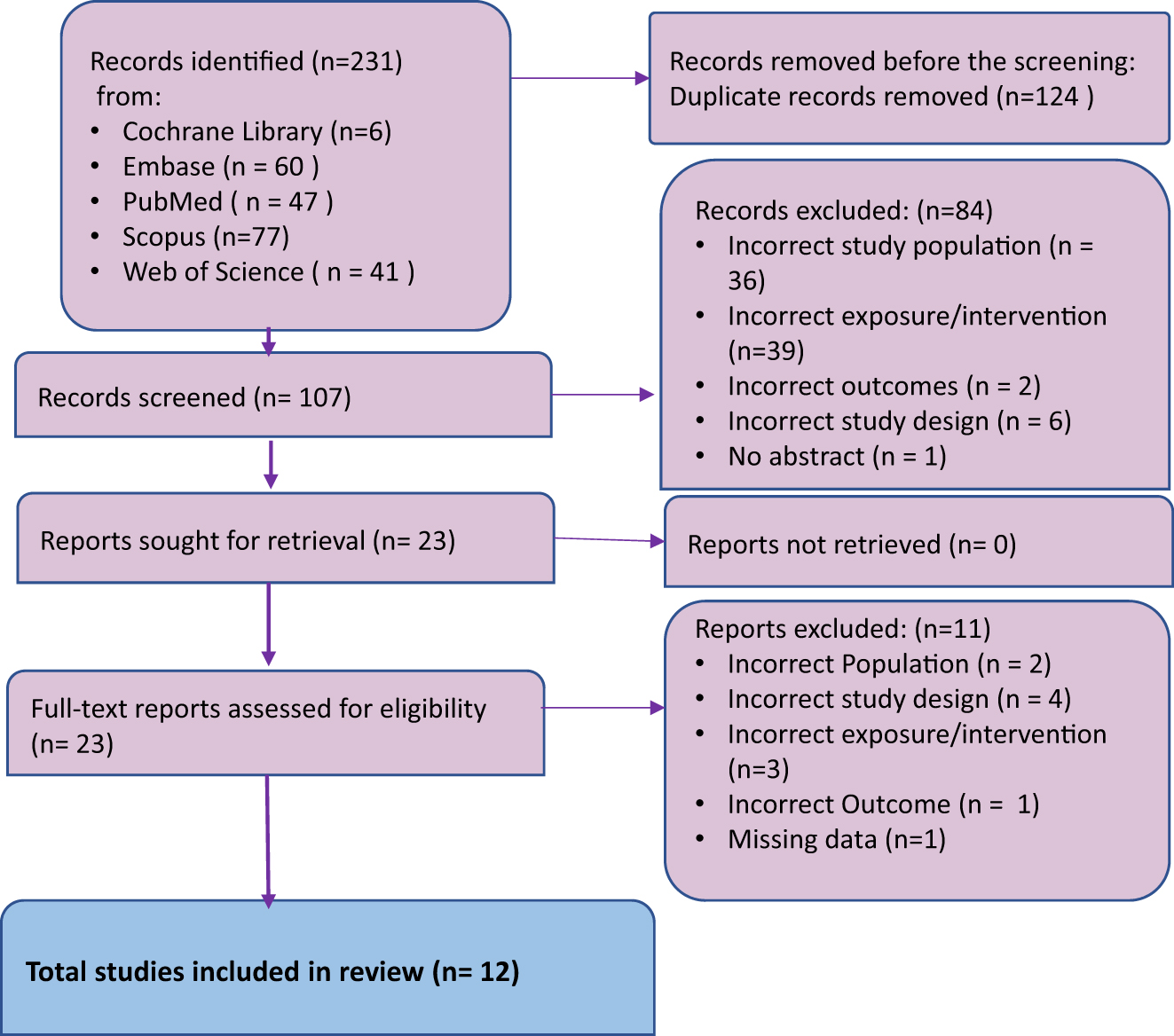
PRISMA flowchart for study selection process.
Study characteristics
The individual study demographics are given in Table 1. This review included 12 studies: 8 RCTs, 2 NRCTs, 1 case-control, and 1 prospective interventional study from India, Italy, Japan, Iran, Pakistan, Iraq, and Georgia, with sample sizes of 10–200 participants. Mean maternal age ranged from 22.9 to 33.62 years in the intervention group and 23.4–31 years in controls. Primary maternal outcomes included AFI (measured from 1 h to 4 weeks), mode of delivery, hemoglobin concentration, and urine specific gravity. Neonatal outcomes assessed birth weight, APGAR scores, NICU admissions, perinatal death, and ventilatory support.
Baseline characteristics of studies included.
| Author, year | Country | No. of patients | Mean age, years | Outcomes | ||
|---|---|---|---|---|---|---|
| E | C | E | C | |||
| Randomized controlled trials | ||||||
|
|
||||||
| Doi et al. (1998) [9] | Japan | 21 | 21 | 29.3 | 28.6 | Maternal: AFI at 1 h, plasma osmolality, serum sodium level, hematocrit, Hb concentration |
| Lorzadeh et al. (2008) [22] | Iran | 20 | 20 | 25.25 ± 4.27 | 24.9 ± 5.73 | Maternal: AFI at 1 h, change in AFI |
| Singh et al. (2022) [23]) | India | 25 | 25 | 26.44 ± 3.37 | 24.88 ± 2.55 | Maternal: AFI at 2 and 24 h, 7 days, mode of delivery Neonatal: birth weight, APGAR at 1 and 5 min |
| Salvi et al. (2022)a [12] | India | 20 | 22.9 | Maternal: AFI at 4 and 48 h, mode of delivery | ||
| Patil et al. (2018)a [24] | India | 36 | 22.9 ± 4.1 | Maternal: AFI at 2 and 24 h, mode of delivery, liquor colour Neonatal: NICU admissions and APGAR at 1 and 5 min |
||
| Deka et al. (2001)a [1] | India | 25 | Not mentioned | Maternal: AFI at 3 h, urine specific gravity | ||
| Malik et al. (2021)a [25] | Pakistan | 50 | 33.62 ± 5.45 | Maternal: AFI pre and post hydration (time not mentioned) | ||
| Patrelli et al. (2012)a [8] | Italy | 66 | 26.1 ± 6.0 | Maternal: AFI at 7 days, type of delivery Fetal: fetal birthweight, APGAR score, NICU admissions |
||
|
|
||||||
| Non-randomized controlled trials | ||||||
|
|
||||||
| Malhotra et al. (2004)a [7] | India | 15 | 26.6 ± 3.2 | Maternal: AFI at 3, 24, and 48 h | ||
| Flack et al. (1995) [26] | Georgia | 10 | 10 | 31 (25–38) (median) | 27 (23–34) (median) | Maternal: AFI at 2 h, plasma and urine osmolality, Doppler flow velocimetry (maternal and fetal) |
|
|
||||||
| Case-control study | ||||||
|
|
||||||
| Rashid et al. (2021) [10] | Pakistan | 100 | 100 | Not mentioned | Maternal: AFI at 1 h Fetal: birth weight, APGAR at 1 & 5 min, NICU admissions |
|
|
|
||||||
| Prospective interventional study (pre- post) | ||||||
|
|
||||||
| Chaudhary et al. (2022) [2] | India | 75 | 25.96 | Maternal: AFI at 2 h, 2 and 7 days, mode of delivery Neonatal: birthweight, APGAR at 1 and 5 min, NICU admissions, CPAP/ventilatory support |
||
-
E, experimental; C, control; AFI, amniotic fluid index; NICU, neonatal intensive care unit; CPAP, continuous positive airway pressure. aThese studies were RCT/NRCT, but involved groups with different interventions and no control group or a control group with no oligohydramnios so were analysed as pre-post.
The pooled analysis of two RCTs [9], 22] assessing maternal oral hydration’s impact on AFI after 1 h included 41 participants per group (Table 2, Figure 2). The overall mean difference (MD) was 1.10 (95 % CI: −1.05 to 3.25) with high heterogeneity (I2=94.6 %, p<0.0001). The wide prediction interval (−22.59 to 24.79) indicates variability. Sensitivity analysis (Supplementary Figure S1) confirmed substantial heterogeneity, with Lorzadeh et al. [22], significantly influencing the pooled estimate (SMD: 1.10, 95 % CI: −1.05 to 3.25).
Pooled outcomes of studies.
| Outcome | No. of studies | Pooled estimate mean difference 95 % CI | I2 | Prediction interval | p-Value | Studies |
|---|---|---|---|---|---|---|
| Amniotic fluid index | ||||||
|
|
||||||
| AFI at 1 h | 2 | 1.10 [−1.05 to 3.25] | 94.6 | −22.59 to 24.79 | <0.0001 | Doi et al. [9], Lorzadeh et al. [22] |
| AFI at 2 ha | 2 | 0.996 [0.781–1.210] | 0 | – | 0.000 | Chaudhary et al. [2], Patil et al. [24] |
| AFI at 1 daya | 2 | 0.853 [0.532–1.174] | 0 | – | 0.000 | Patil et al. [24], Malhotra et al. [7] |
| AFI at 2 daysa | 3 | 1.649 [0.943–2.356] | 75.384 | −6.553 to 9.852 | 0.000 | Malhotra et al. [7], Chaudhary et al. [2], Salvi et al. [12] |
| AFI at 1 weeka | 3 | 2.232 [0.943–3.520] | 95.843 | −3.973 to 8.437 | 0.001 | Chaudhary et al. [2], Malik et al. [25], Patrelli et al. [8] |
-
AFI, amniotic fluid index; CI, confidence interval; I2, heterogeneity statistic. aPre-post analysis was done for these outcomes in comprehensive meta-analysis software.
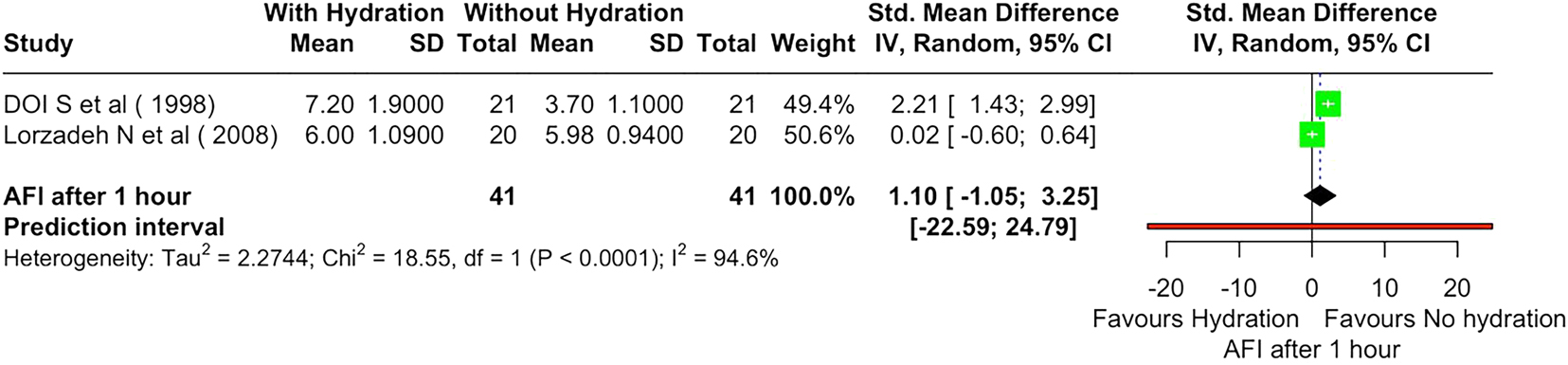
Forest plot showing effect of material hydration on amniotic fluid index (AFI) after 1 h.
AFI at 2 h
The pooled mean difference using both fixed- and random-effects models was 0.996 (95 % CI: 0.781–1.210), with a highly significant p-value (<0.001), indicating a statistically significant increase in AFI following oral hydration after 2 h (Table 2, Supplementary Figure S2), with I2=0 %, p=0.924. The pooled mean difference remained consistent across both sensitivity analyses, with values of 0.996 (95 % CI: 0.711–1.281, p<0.001) at r=0.05 and 0.992 (95 % CI: 0.891–1.094, p<0.001) at r=0.95 (Supplementary Figure S3).
AFI at day 1
The pooled MD was 0.853 (95 % CI: 0.532–1.174, p<0.001), indicating a significant increase in AFI at 1 day following the intervention (Table 2, Supplementary Figure S4) with I2 value of 0 %. On sensitivity analysis for r=0.05, the pooled MD was 0.877 (95 % CI: 0.431–1.323, p<0.001), confirming a statistically significant increase in AFI. For r=0.95, the pooled MD was 0.596 (95 % CI: 0.502–0.690, p<0.001), showing a slightly reduced but still significant impact of the intervention (Supplementary Figure S5)
AFI at day 2
The pooled MD was 1.649 (95 % CI: 0.943–2.356, p<0.001) under REM, showing a significant AFI increase by day 2 (Table 2, Supplementary Figure S6) with moderate heterogeneity (I2=75.38 %, p=0.017). Sensitivity analysis (Supplementary Figure S7) confirmed stability, with no single study overly influencing results. Further analysis under varying correlation assumptions (r=0.05 and r=0.95) (Supplementary Figure S8) maintained statistical significance, reinforcing a consistent intervention effect.
AFI at 1 week
The pooled MD (r=0.5) under a random-effects model was 2.232 (95 % CI: 0.943–3.520, p=0.001), showing a significant AFI increase one-week post-intervention (Table 2, Supplementary Figure S9) with high heterogeneity (I2=95.84 %, p<0.001). Leave-one-out sensitivity analysis confirmed statistical significance (p<0.05) across all exclusions (Supplementary Figure S10). Sensitivity analysis under different correlation assumptions (r=0.05 and r=0.95) also remained significant (Supplementary Figure S11). AFI significantly increased at 2 h, 1 day, 2 days, and 1 week, but not at 1 h (Table 2). High heterogeneity was noted across studies.
Other outcomes
Other pregnancy outcomes in cases of oligohydramnios, including AFI after 3 h of oral hydration therapy, mode of delivery, placental weight, urine specific gravity, maternal plasma osmolality, serum sodium level, hematocrit, hemoglobin concentration, fetal renal artery pulsetality index, umblical artery PH birth weight, APGAR scores at 1 and 5 min, and NICU admissions, did not meet the minimum requirement of at least two studies (with same study design or intervention), for inclusion in the meta-analysis. The following narrative synthesis presents findings from individual studies on these additional outcomes.
AFI after 3 h
The impact of acute maternal hydration on AFI at 3 h was assessed by Malhotra et al. [7], Deka et al. [1], and Salvi et al. [12]. Malhotra et al. [7] reported an increase from 6.81 to 10.09 cm in oligohydramnios cases. Deka et al. [1] observed a similar rise, confirming hydration’s effectiveness. Salvi et al. [12] highlighted a significant improvement, supporting hydration as a rapid, non-invasive intervention for increasing AFI.
Mode of delivery
Findings from Patrelli et al. [8] indicated that among women receiving hydration therapy, the rate of cesarean section was 30 % in the intervention category, compared to 18 % in the non-intervention category. Similarly, Singh et al. [23] reported that 52 % of women in the control group underwent cesarean section compared to 32 % in the hydrated group, suggesting that hydration therapy may help in reducing cesarean delivery rates.
Birth weight
Hydration therapy did not appear to have a significant impact on birth weight across studies. Patrelli et al. [8] reported a mean birth weight of 3.2 ± 0.55 kg in the hydration group compared to 3.3 ± 0.65 kg in the control group, indicating minimal difference. Singh et al. [23] also observed no significant variation in birth weight between intervention and control groups.
APGAR scores at 1 and 5 min
Singh et al. [23] and Patil et al. [24] reported improved neonatal outcomes with maternal hydration therapy. Singh et al. [23] found that the APGAR score at 1 min was significantly higher in the intervention group (6.52 ± 0.71) compared to controls (5.72 ± 0.84), with a similar trend observed at 5 min (7.96 ± 0.84 vs. 7.28 ± 1.1). Patil et al. [24] also reported favorable APGAR scores in neonates born to mothers receiving hydration therapy, with mean scores of 6.8 ± 0.6 at 1 min and 8.8 ± 0.6 at 5 min. These findings collectively suggest that maternal hydration therapy supports better neonatal adaptation at birth.
NICU admissions
A reduction in NICU admissions was observed across multiple studies following maternal hydration therapy. Singh et al. [23] reported that NICU admission was necessary for just 8 % of newborns in the intervention cohort, whereas 48 % of those in the comparison group required neonatal intensive care. Similarly, Patil et al. [24] noted fewer NICU admissions among neonates whose mothers received hydration therapy. Patrelli et al. [18] and Chaudhary et al. [2] also found a lower incidence of NICU admissions in their intervention groups, reinforcing the potential benefits of maternal hydration in reducing neonatal complications. A summary of the neonatal findings from the included studies is provided in Supplementary Table S8.
Urine specific gravity
The study by Deka et al. [1] measured maternal urine specific gravity following hydration therapy. Findings showed a reduction in urine concentration, indicating improved maternal hydration status, which may correlate with increased amniotic fluid production.
Maternal plasma osmolality and serum sodium levels
Doi et al. [9] examined the impact of maternal fluid intake on amniotic fluid volume and found a notable decrease in maternal plasma osmolality and serum sodium levels after administering hydration treatment. Their study found that maternal hydration using IV hypotonic fluid and oral water significantly increased AFI, correlating with decreases in maternal osmolality. The findings suggest that maternal osmotic changes, rather than volume expansion, primarily drive the increase in AF volume. This mechanism supports the role of hydration therapy in improving fetal urine production and amniotic fluid levels in oligohydramnios cases.
Hematocrit, hemoglobin concentration, fetal renal artery pulsatility index (PI) and umbilical artery pH
Doi et al. [9] investigated maternal hydration’s effects on hematocrit, hemoglobin concentration, fetal renal artery pulsatility index (PI), and umbilical artery pH. The study found a notable reduction in hematocrit and hemoglobin levels post-hydration in the intravenous isotonic fluid group, suggesting maternal plasma volume expansion. However, no significant changes were observed in the fetal renal artery PI or umbilical artery pH across intervention groups. These findings indicate that maternal hydration primarily affects maternal hemodynamics without significantly altering fetal vascular resistance.
Risk of bias assessment
ROB assessment are shown in Supplementary Tables S4–S7. Eight RCT studies were considered to have low risk of bias as per ROB-2 quality assessment tool and two NRCTs had low risk after assessment by ROBINS-I quality assessment tool. One study was graded as good quality based on the Newcastle-Ottawa quality assessment for case-control studies. One study were assessed by the JBI critical appraisal quality assessment tool. All twelve studies had an overall appraisal as good, and hence included in the SRMA.
Certainty of evidence: GRADE profile
The certainty of evidence for the effect of oral hydration therapy on AFI was assessed using the GRADE approach. The certainty for AFI at 1 h was rated as very low (⨁◯◯◯) due to high heterogeneity (I2=94 %) and a wide confidence interval crossing the null effect (SMD 1.1; 95 % CI: −1.05 to 3.25). The presence of serious inconsistency and imprecision further reduces the confidence in the pooled estimate (Supplementary Figure S12).
Discussion
This SRMA highlights the effectiveness of maternal oral hydration therapy in improving AFI in pregnancies complicated by oligohydramnios. The pooled results indicate statistically significant increases in AFI at multiple time points post-intervention, specifically at 2 h, 1 day, 2 days, and 1 week, suggesting that maternal hydration therapy is beneficial in enhancing amniotic fluid dynamics. Our findings align with previous studies that have established hydration therapy as a non-invasive, accessible, and cost-effective intervention for managing oligohydramnios.
The systematic review by Gizzo et al. [6] focused on maternal hydration strategies for improving AFI and concluded that oral hydration was superior to intravenous (IV) hydration, particularly with hypotonic fluids. Their meta-analysis found that AFI improvements were more time-dependent than dose-dependent, meaning that repeated hydration was necessary to maintain AFI increases. Our findings are consistent with this observation, as we also noted short-term increases in AFI, even within 2 h of hydration, reinforcing the idea that maternal hydration is an effective and immediate intervention. However, unlike Gizzo et al. [6], our review included a broader range of studies, demonstrating significant short-term and sustained AFI increases over a one-week period.
Another key takeaway from Gizzo et al. [6] was that IV hypotonic solutions produced greater AFI increases compared to isotonic IV fluids. The present meta-analysis also supports that consumption of hypotonic fluids such as plain water, can lead to rapid and significant increase in AFI within hours of intake. This effect is due to reduced maternal plasma osmolality, which suppresses vasopressin secretion and promotes fluid redistribution to the amniotic sac. In contrast, isotonic fluids such as normal saline and Ringer’s lactate primarily expand intravascular volume but may not alter osmolality, which limits their effect on foetal urine output. However, both the present review and Gizzo et al. [6] noted that due to heterogeneous data, it is difficult to determine the tangible medical outcome of oral hydration therapy on perinatal outcomes.
A systematic review by Rosemiarti et al. [27], also emphasized oral hydration as an effective intervention for increasing AFI. Their review demonstrated that additional water intake of 1,500–2,500 mL per day significantly improved AFI levels, findings that closely align with our meta-analysis results. Supporting the theory that maternal hydration therapy is a quick and efficient strategy for managing oligohydramnios, our pooled data showed notable AFI increases at multiple times points, including 2 h, 1 day, 2 days and 1 week.
Rosemiarti et al. [27] also emphasized that oral hydration therapy is a safe, non-invasive alternative to amnioinfusion which is hospital based and associated with side effects such as uterine overdistension, chorioamnionitis and premature rupture of membranes. The present systematic review supports their findings, highlighting maternal hydration as the first line management of oligohydramnios, particularly in outpatient and resource-limited settings. Another important aspect of Rosemiarti et al. [27] was the assessment of the dose-response relationship of hydration volume on AFI change. They observed that a daily minimum intake of 1,500 mL resulted in detectable AFI change, while a higher intake of 2,500 mL produced better improvements. Our findings support this point, as the research we looked at in the present review–such as by Patrelli et al. [8] and Malhotra et al. [7] – also showed how increased fluid intake resulted in sustained improvements in amniotic fluid index (AFI). Nevertheless, inconsistent hydration guidelines pose a hurdle; future research should focus on establishing an approach to determine the effective volume, frequency and type of fluids to optimize clinical outcomes.
A key difference between the present review and that of Rosemiarti et al. [27] is the variation in hydration practices across regions. The present systematic review included studies conducted in India, Japan, Iran, Pakistan, Iraq and Italy which involve varying maternal hydration behaviours, climates and diets. By contrast, Rosemiarti et al. [27] reviewed studies mainly from Western and high-income countries, where the baseline hydration may be different. This is an important consideration: baseline hydration status may affect the response to additional fluid intake in pregnancy. In areas with hotter climates and more dehydration risk, maternal hydration therapy may have more benefit in AFI improvement. Future studies should include geographical and lifestyle factors in the assessment of the effectiveness hydration therapy.
A major controversy in maternal hydration therapy is whether oral or IV hydration is more effective. Gizzo et al. [6] arrived at the conclusion that oral hydration, especially with hypotonic solutions, was the most appropriate approach to enhance AFI. For instance, the present systematic review revealed that oral hydration caused substantial AFI increments within 2 h and that intermittent oral fluid intake was required to maintain such increments. However, the studies included in the present review suggested that IV hydration therapy may be more rapid in effect, especially with the use of hypotonic fluids. Doi et al. [9] compared oral and IV hydration and observed that both methods increased AFI but hypotonic IV hydration was more rapid and pronounced. Likewise, Lorzadeh et al. [22] observed that IV hypotonic fluids enhanced AFI to a significantly greater extent than isotonic solutions. These findings are in concordance with the above-mentioned studies which support the view that although oral hydration is efficient and easily available, IV hydration may be advantageous in situations that demand a quick increase in AFI such as severe oligohydramnios or imminent delivery.
The physiological mechanism of the increase in AFI with maternal hydration is primarily related to plasma osmolality and transplacental fluid movement. Flack et al. [26] showed that maternal hydration results in lowered plasma osmolality which in turn reduces vasopressin secretion. This reduction in vasopressin leads to fetal diuresis, an increase in fetal urine output and, therefore higher AFI. Furthermore, it improves uteroplacental perfusion; as a result, there is better placental exchange of fluids and increase in fetal hydration. This improved perfusion helps in sufficient provision of fluid to the fetus and thus maintains normal AFI levels.
Significant heterogeneity in our pooled estimates, particularly at 1 h (I2=94.6 %) and one week (I2=95.8 %) after hydration, necessitates further investigation. Several potential causes of this heterogeneity were identified. First, a significant factor might be the hydration regimens employed in the different studies. These included differences in the frequency of intake (single-time intake vs. repeated daily intake) and the type of fluids (e.g. plain water, ORS, fruit juices, etc.). The physiological response to additional fluid intake may be influenced by the baseline maternal hydration status and the climate, which differ geographically among the included studies (e.g., Japan, Iran, India and Italy). Subgroup analyses were not possible because of the small number of studies that met the requirements for meta-analysis and the irregular reporting of these variables. To better understand the factors causing heterogeneity, we recognize this limitation and emphasize the necessity of future research to standardize baseline hydration status and hydration protocols.
Even though oral hydration therapy seems like a promising low-cost intervention for treating oligohydramnios, it is important to understand its limitations when applying research to clinical practice. We did not stratify outcomes in the present review based on the severity of oligohydramnios (mild, moderate, or severe) because these classifications were not consistently reported in the included studies. There is a substantial evidence gap because most studies do not specify whether the interventions were carried out in inpatient or outpatient settings. This detail is crucial because the cost and effectiveness of oral hydration can vary significantly depending on the circumstances. To make hydration protocols more applicable in real-world situations, future studies should clearly identify the clinical setting and stratify outcomes according to the severity of the illness.
However, there are several limitations to the present systematic review. The heterogeneity among the incorporated research, in terms of study design and the way the outcomes were measured, was a problem in the interpretation of the results. The heterogeneity in the outcome measures used included different AFI cutoff points for diagnosing oligohydramnios and varying lengths of follow up which may have affected the overall effect sizes. Also, most of the included studies investigated third trimester oligohydramnios, thus, there is a lack of information on the possible advantages of hydration therapy at the initial stages of pregnancy. Further research should be directed towards large randomized controlled trials to fill the gaps and provide recommendations for the use of hydration therapy in oligohydramnios. Hydration protocols should also be standardized, the long-term impacts should also be assessed and its efficacy in high-risk pregnancies should also be evaluated as the next steps.
Conclusions
This systematic review and meta-analysis suggests that oral hydration is a non-invasive and easily implementable strategy particularly in low-resource settings where amnioinfusion may not be feasible. In pregnancies complicated by oligohydramnios, oral hydration therapy seems to be a straightforward, non-invasive method that can successfully raise amniotic fluid levels. Although some studies documented positive trends for newborns, like higher Apgar scores and fewer NICU admissions, these results were not consistently measured and should be interpreted cautiously. Future studies should be directed towards establishing the best fluid type, quantity and duration of the therapy to improve the current clinical guidance and perinatal outcomes.
Acknowledgments
The authors would like to thank the contribution of the Department of Health Research supported SARANSH (Systematic Reviews And Networking Support in Health) workshop organised by the department of community medicine, All India Institute of Medical Sciences, Nagpur, India and the Technical Resource Centre (Centre for Evidence-Based Guidelines), Department of Community Medicine, AIIMS Nagpur for developing their capacity to undertake the systematic review.
-
Research ethics: Not applicable as it is a systematic review and meta-analysis.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: All data used are provided in the manuscript and the supplementary files.
References
1. Deka, D, Malhotra, B. Role of maternal oral hydration in increasing amniotic fluid volume in pregnant women with oligohydramnios. Int J Gynaecol Obstet 2001;73:155–6. https://doi.org/10.1016/s0020-7292(00)00354-4.Suche in Google Scholar PubMed
2. Chaudhary, A, Varma, U, Goel, S, Tayal, A, Varma, A. Effect of oral and intravenous hydration therapy on amniotic fluid index, maternal and perinatal outcome in borderline oligohydramnios. Int J Reprod Contracept Obstet Gynecol 2022;11:577–82. https://doi.org/10.18203/2320-1770.ijrcog20220191.Suche in Google Scholar
3. Anant, M, Murmu, S, Priya, S. A randomized trial of inpatient and home-based maternal oral hydration therapy in isolated oligohydramnios and its effect on amniotic fluid index and perinatal outcome. Cureus 2023;15:e41326. https://doi.org/10.7759/cureus.41326.Suche in Google Scholar PubMed PubMed Central
4. Mhes, H, Saeed, AM, Alomda, FAE. Effect of maternal intravenous hydration in management of oligohydramnios and the changes in the fetal Doppler. SJMS 2023;2:83–7. https://doi.org/10.55675/sjms.v2i3.52.Suche in Google Scholar
5. Azarkish, F, Janghorban, R, Bozorgzadeh, S, Arzani, A, Balouchi, R, Didehvar, M. The effect of maternal intravenous hydration on amniotic fluid index in oligohydramnios. BMC Res Notes 2022;15:95. https://doi.org/10.1186/s13104-022-05985-6.Suche in Google Scholar PubMed PubMed Central
6. Gizzo, S, Noventa, M, Vitagliano, A, DallAsta, A, Dantona, D, Aldrich, CJ, et al.. An update on maternal hydration strategies for amniotic fluid improvement in isolated oligohydramnios and normohydramnios: evidence from a systematic review of literature and meta-analysis. PLoS One 2015;10:e0144334. https://doi.org/10.1371/journal.pone.0144334.Suche in Google Scholar PubMed PubMed Central
7. Malhotra, B, Deka, D. Duration of the increase in amniotic fluid index (AFI) after acute maternal hydration. Arch Gynecol Obstet 2004;269:173–5. https://doi.org/10.1007/s00404-002-0346-z.Suche in Google Scholar PubMed
8. Patrelli, TS, Gizzo, S, Cosmi, E, Carpano, MG, DiGangi, S, Pedrazzi, G, et al.. Maternal hydration therapy improves the quantity of amniotic fluid and the pregnancy outcome in third-trimester isolated oligohydramnios: a controlled randomized institutional trial. J Ultrasound Med 2012;31:239–44. https://doi.org/10.7863/jum.2012.31.2.239.Suche in Google Scholar PubMed
9. Doi, S, Osada, H, SekiK, SS. Effect of maternal hydration on oligohydramnios: a comparison of three volume expansion methods. Obstet Gynecol 1998;92:525–9. https://doi.org/10.1097/00006250-199810000-00009.Suche in Google Scholar
10. Rashid, S. Assessment of oligohydramnios in pregnant women: outcome of hydration therapy [Internet]. https://www.medforum.pk/article/27-assessment-of-oligohydramnios-in-pregnant-women-outcome-of-hydration-therapy [Accessed 19 Feb 2025].Suche in Google Scholar
11. Shahnazi, M, SayyahMeli, M, Hamoony, F, Sadrimehr, F, GhatreSamani, F, Koshavar, H. The effects of intravenous hydration on amniotic fluid volume and pregnancy outcomes in women with term pregnancy and oligohydramnios: a randomized clinical trial. J Caring Sci 2012;1:123–8. https://doi.org/10.5681/jcs.2012.018.Suche in Google Scholar PubMed PubMed Central
12. Salvi, PP, Gaikwad, V, Sravani, VJ. Study of evaluation of effectiveness of acute maternal hydration therapy with hypotonic fluid (water) in pregnant females with low amniotic fluid. Pravara Med Rev 2022;14:74–9.Suche in Google Scholar
13. Higgins, JPT, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, et al.. editors. Cochrane handbook for systematic reviews of interventions version 6.5 (updated August 2024). London: Cochrane; 2024. Available from: https://www.training.cochrane.org/handbook.Suche in Google Scholar
14. Nested knowledge – powerful evidence synthesis tools for medical researchers [Internet]. https://nested-knowledge.com/ [Accessed 19 Dec 2024].Suche in Google Scholar
15. Wells, GA, Shea, B, O'Connell, D, Peterson, J, Welch, V, Losos, M, et al.. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa: Ottawa Hospital Research Institute Nov 2024. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Accessed 17 Nov 2024].Suche in Google Scholar
16. Martin, J. Critical appraisal checklist for systematic reviews and research syntheses. Adelaide, Australia: Joanna Briggs Institute; 2017.Suche in Google Scholar
17. Sterne, JA, Hernán, MA, Reeves, BC, Savović, J, Berkman, ND, Viswanathan, M, et al.. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919.10.1136/bmj.i4919Suche in Google Scholar PubMed PubMed Central
18. Gandhi, AP, Shamim, MA, Padhi, BK. Steps in undertaking meta-analysis and addressing heterogeneity in meta-analysis. The Evidence 2023;1:78–92.Suche in Google Scholar
19. R: the R project for statistical computing. [Internet]. https://www.r-project.org/ [Accessed 17 Nov 2024].Suche in Google Scholar
20. Shamim, MA, Gandhi, AP, Dwivedi, P, Padhi, BK. How to perform meta-analysis in R: a simple yet comprehensive guide. The Evidence 2023;1:93–113.Suche in Google Scholar
21. GRADEpro, GDT: GRADEpro guideline development tool [Software]. Ontario: McMaster University and Evidence Prime; 2025. Available from: gradepro.org.Suche in Google Scholar
22. Lorzadeh, N, Kazemirad, S, Lorzadeh, M, Najafi, S. Comparison of the effect of oral and intravenous fluid therapy on women with oligohydramnios. Res J Obstet Gynecol 2008;1:25–9. https://doi.org/10.3923/rjog.2008.25.29.Suche in Google Scholar
23. Singh, S, Patil, SK, Vijan, T. Effect of maternal oral hydration in oligohydramnios. Indian J Obstet Gynecol Res 2022;9:221–6. https://doi.org/10.18231/j.ijogr.2022.043.Suche in Google Scholar
24. Patil, N, V, A. A randomized controlled trial to compare the efficacy of three different methods of maternal hydration for oligohydramnios. J Krishna Inst Med Sci Univ 2018;7:47–54.Suche in Google Scholar
25. Malik, M, Irshaad, S, Bokhari, N, Qazi, W, Raza, A, Bashir, K. Effects of oral fluids and intravenous fluids in the improvement of amniotic fluid index during third trimester of pregnancy. PAFMJ 2021;71:179–83. https://doi.org/10.51253/pafmj.v71i1.2790.Suche in Google Scholar
26. Flack, NJ, Sepulveda, W, Bower, S, Fisk, NM. Acute maternal hydration in third-trimester oligohydramnios: effects on amniotic fluid volume, uteroplacental perfusion, and fetal blood flow and urine output. Am J Obstet Gynecol 1995;173:1186–91. https://doi.org/10.1016/0002-9378(95)91350-5.Suche in Google Scholar PubMed
27. Rosemiarti, T, Siregar, P, Hardinsyah, H, Pardede, SO, Santoso, BI, Riza, RA, et al.. An additional adequate water intake increases the amniotic fluid index in pregnant women with oligohydramnios: a systematic review. J Gizi Dan Pangan 2022;17:47–56. https://doi.org/10.25182/jgp.2022.17.1.47-56.Suche in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpm-2025-0176).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Reviews
- Integrating NIPT and ultrasound for detecting fetal aneuploidies and abnormalities
- Ethical challenges in perinatal ultrasound: balancing diagnostic capability and ethical communication
- Original Articles – Obstetrics
- Risk factors and adverse outcomes associated with hepatitis C virus in pregnancy
- Utility of endometrial multi-vessel blood flow ultrasound parameters in predicting pregnancy outcomes
- Improving the accuracy of screening for large-for-gestational-age fetuses: a multicenter observational study
- Risk factors and awareness of tobacco smoking and second-hand smoke exposure among pregnant women in Taiwan
- Effect of oral hydration therapy on amniotic fluid index and maternal-neonatal outcomes in pregnant women with oligohydramnios: a systematic review and meta-analysis
- Epidural anesthesia during labor and delivery and postpartum hemorrhage
- Social vulnerability and triage acuity among pregnant people seeking unscheduled hospital care
- Gestational diabetes insipidus. A systematic review of case reports
- Outcomes in pregnant patients with congenital heart disease by rurality
- Original Articles – Fetus
- Exploration of copy number variations and candidate genes in fetal congenital heart disease using chromosomal microarray analysis
- A seven-year retrospective cohort study on non-immune foetal hydrops from a single centre in an LMIC setting
- Original Articles – Neonates
- Correlation between macronutrient content and donation characteristics in Croatian human milk bank
- Gestational diabetes mellitus: the role of IGF-1 and leptin in cord blood
Artikel in diesem Heft
- Frontmatter
- Reviews
- Integrating NIPT and ultrasound for detecting fetal aneuploidies and abnormalities
- Ethical challenges in perinatal ultrasound: balancing diagnostic capability and ethical communication
- Original Articles – Obstetrics
- Risk factors and adverse outcomes associated with hepatitis C virus in pregnancy
- Utility of endometrial multi-vessel blood flow ultrasound parameters in predicting pregnancy outcomes
- Improving the accuracy of screening for large-for-gestational-age fetuses: a multicenter observational study
- Risk factors and awareness of tobacco smoking and second-hand smoke exposure among pregnant women in Taiwan
- Effect of oral hydration therapy on amniotic fluid index and maternal-neonatal outcomes in pregnant women with oligohydramnios: a systematic review and meta-analysis
- Epidural anesthesia during labor and delivery and postpartum hemorrhage
- Social vulnerability and triage acuity among pregnant people seeking unscheduled hospital care
- Gestational diabetes insipidus. A systematic review of case reports
- Outcomes in pregnant patients with congenital heart disease by rurality
- Original Articles – Fetus
- Exploration of copy number variations and candidate genes in fetal congenital heart disease using chromosomal microarray analysis
- A seven-year retrospective cohort study on non-immune foetal hydrops from a single centre in an LMIC setting
- Original Articles – Neonates
- Correlation between macronutrient content and donation characteristics in Croatian human milk bank
- Gestational diabetes mellitus: the role of IGF-1 and leptin in cord blood

