Impact of introduction of the growth assessment protocol in a South Indian tertiary hospital on SGA detection, stillbirth rate and neonatal outcome
Abstract
Objectives
India has a high rate of stillbirths, and many deaths are due to fetal growth restriction and potentially preventable. Screening and identification of the small for gestational age (SGA) fetus during the antenatal period has been shown to reduce stillbirths. We set out to evaluate the impact of implementing the Growth Assessment Protocol (GAP), a programme designed for screening for SGA.
Methods
Observational study comparing two-time epochs; before (years 2011–2014) and after (years 2015–2018) introduction of GAP. The programme includes identification of risk factors, risk categorization, serial fundal height measurement, customised fetal growth charts and appropriate referral protocols. Fetal growth charts and birth centiles were generated based on the hospital database of normal outcome pregnancies, customised to women’s ethnicity, parity, height, and weight. The protocol was introduced following training of obstetric and midwifery care providers. We evaluated SGA detection rates, stillbirth rates (from 28 weeks) and neonatal morbidity at term.
Results
There were 26,199 and 31,498 births, with 115 and 108 stillbirths in the pre and post-GAP implementation periods, respectively. SGA detection rates increased from 51.1 to 67.1%, representing a 31% improvement (p<0.001). Overall stillbirth rates declined from 4.4 to 3.4 per 1000 births (RR 0.78 CI 95% 0.60–1.02) and at term from 1.5 to 0.6 (RR 0.37 CI 95% 0.20–0.66). Neonatal intensive care admission and neonatal encephalopathy in term neonates also decreased significantly.
Conclusions
Introduction of the GAP programme in an Indian tertiary maternity service was associated with improved antenatal detection of SGA and reduced stillbirth rates and neonatal morbidity.
Introduction
Stillbirth is a tragic outcome of pregnancy, and India has one of the highest rates and largest number of stillbirths [1]. The global variation of stillbirth rates from 4 to 50 per thousand highlights that there is much scope for improvement [2, 3].
Stillbirths can be prevented through diagnosis of fetal growth restriction and timely intervention [4]. Small for gestational age (SGA) babies are at increased risk [5], and antenatal detection has been shown to reduce stillbirth risk [6]. Yet routine antenatal care has been shown to identify only a minority of babies that were SGA at birth.
Improvement in SGA detection rate and reduction in stillbirths requires a vigorous approach with screening, surveillance and confirmatory tests. In routine antenatal care, conventional symphysio-fundal height (SFH) assessment is done with the use of hands, without a measuring tape and no chart for plotting. Serial measurements of SFH with a measuring tape and plotting on a chart enables a clinician to suspect slow fetal growth and request an ultrasound for fetal growth assessment [7, 8].
The diagnosis of SGA was made with the use of population based charts not based on an Indian population. In addition to ethnicity, maternal characteristics such as height, early pregnancy weight and parity affect fetal growth and birthweight standards and hence the definition of SGA [9]. Small for gestational age (SGA) by customised standard is more strongly associated with growth restriction, placental pathology and adverse outcome [10, 11] and a reduced false positive rate [12] when compared to uncustomised, population based standards.
A comprehensive program (Growth Assessment Protocol – GAP) including customised charts has been shown to decrease stillbirth rates in the UK [4, 13, 14]. We wanted to evaluate the impact of such a program on SGA detection rate and stillbirth rate in our centre.
Materials and methods
Setting
The study was conducted at a privately run tertiary referral hospital with approximately 10,000 deliveries annually. The participating women came from a varied social background and included both private and public patients. Expectant mothers were usually referred from community clinics in early pregnancy, with investigations, antenatal and intrapartum care provided mostly by medical staff in the centre. Routine visits in uncomplicated pregnancies included 4 weekly visits in the first and second trimester, 2–3 weekly from 28 weeks and weekly from 36 weeks onwards. During each visit the care for uncomplicated pregnancies was provided by a team of midwife and obstetrician.
Study design and population
The evaluation included a prospective study cohort following implementation of GAP (2015–2019), and assessment against a pre-GAP cohort (2011–14). All singleton pregnancies booked before 24 weeks were included and all multifetal pregnancies, those with fetal anomalies, and referrals for emergency care were excluded.
Practice pre-GAP implementation
Usual practice included routine dating of pregnancy, early pregnancy risk assessment and regular antenatal check-ups, including in low risk women by regular assessment of fundal height at 2–3 week intervals by manual palpation without tape, and no formal training. The examination was recorded as an estimation of whether the size was appropriate for gestational age. In addition, all women had a single routine scan to estimate fetal weight (EFW) at 34–36 weeks gestation. In women considered high risk, ultrasound biometry was performed at usually 3–4 weekly intervals during the third trimester, with EFWs plotted on standard Hadlock growth charts [15].
Implementation
The Growth Assessment Protocol (GAP) [16] was introduced in January 2015 starting with training, with embedment into routine practice during 2015. GAP training was provided through online self-paced training modules with certification based on an inbuilt evaluation protocol. Subsequently GAP was introduced in a practice session to the entire team of obstetric care providers (60 doctors and 30 midwives) with follow up sessions to respond to any queries. Education included general knowledge of evidence-based risk assessment for SGA and stillbirth and protocols for SGA detection and management according to RCOG [5] and GAP guidelines [17]. Training included standardised measurement of SFH [8], generation and use of customised charts, plotting exercises, and referral pathways for ultrasound scan assessment.
Customised charts
GROW (gestation related optimal weight) chart software [18] was made available by the Perinatal Institute, UK (www.perinatal.org.uk) as part of the GAP program [16]. It included normal pregnancy coefficients derived from our own local, routinely collected dataset of 7372 pregnancies, using previously described methods [19]. The GROW application produced a printed chart (Figure 1), customised for maternal characteristics including her height, weight in early pregnancy, parity and ethnic origin. The software also provided mandatory postnatal audit functions to record whether SGA was suspected and detected antenatally, before calculating the birthweight centile following delivery.
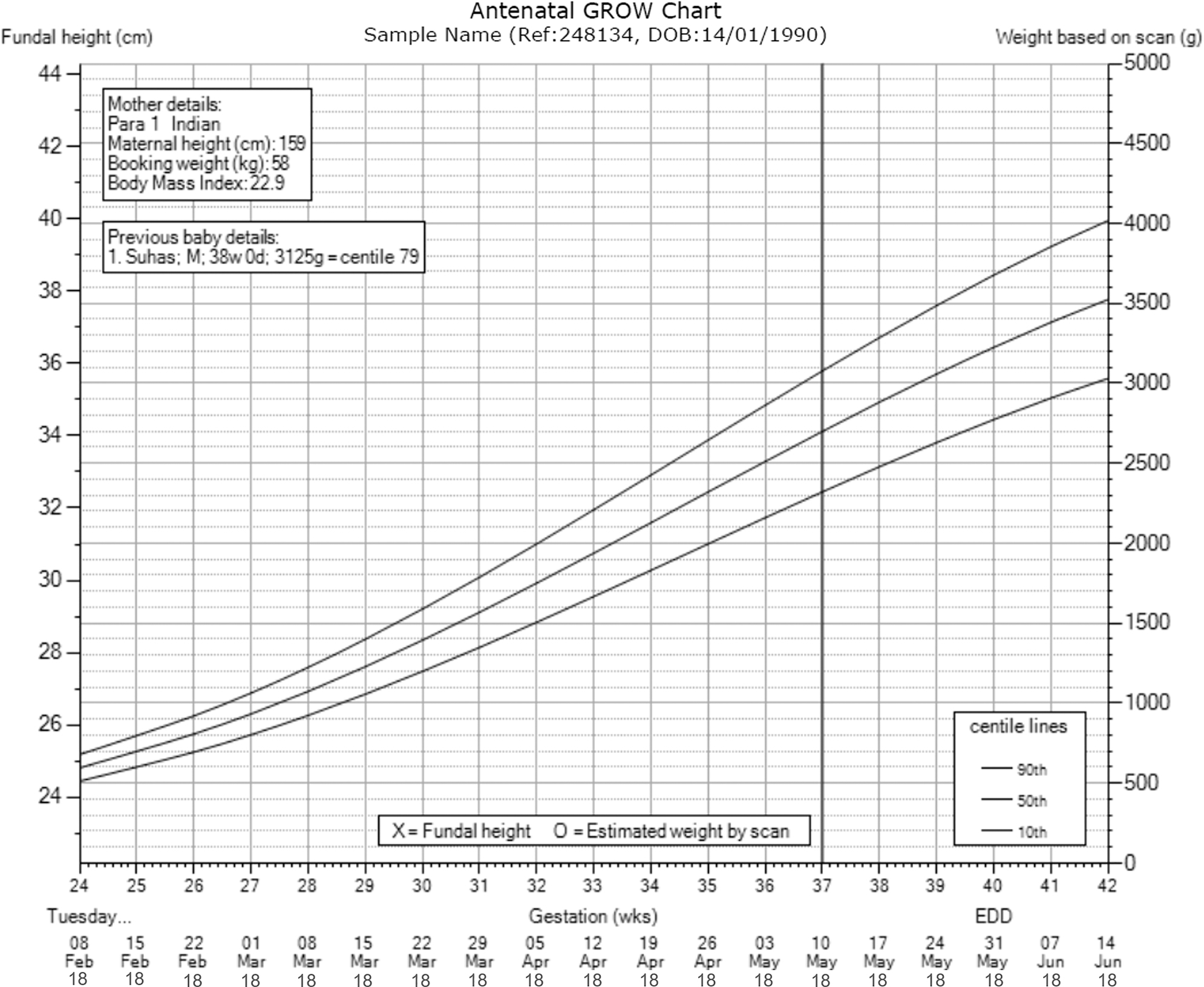
Customised GROW chart.
Post-GAP practice
Following early pregnancy risk assessment, GROW charts were generated before 24 weeks of gestation for all singleton pregnancies, adjusted for ethnicity, maternal height, early pregnancy weight, and parity. All mothers in the study were of Indian ethnic origin. For the low risk population, defined according to SGA risk assessment criteria, serial measurement and plotting of SFH was undertaken at 2 weekly visits from 24–26 weeks of pregnancy [8]. Referral for ultrasound assessment of EFW was done if there was static or slow growth based on SFH measurements, with subsequent investigations and management according to RCOG guidelines [5]. The routine third trimester scan was continued for all pregnancies as per departmental policy. Women considered high risk for SGA had serial ultrasound EFW measurements plotted on the GROW charts every three to four weeks [17]. The audit function of the GROW software was utilised in regular meetings to assess cases where SGA was missed antenatally, to identify reasons and challenges and ways to overcome them.
Evaluation
We evaluated the effect of implementation by comparing SGA detection rates and stillbirth rates during the respective periods in the pre and post-GAP cohorts. SGA (<10th customised centile) detection rate was calculated as a proportion of births with an SGA birthweight that had at any stage in the antenatal period one or more ultrasound estimated fetal weights that were SGA. Stillbirth rates were determined according to the WHO definition [20, 21] based on gestational age (delivery of a fetus from 28.0 weeks of pregnancy with no sign of life), without the need for the alternative 1000 g weight limit criterion [2] as our pregnancies had a routine first or second trimester dating scan.
Statistical analysis
Incidence of stillbirth was compared between two groups by calculating relative risk with 95% confidence interval (CI). Univariate and multivariable logistic regression was performed to determine the predictors of stillbirth. Crude and Adjusted relative risk along with 95% CI were presented. Adjustment for the multivariable logistic regression was informed by a directed acyclic graph (Figure S1) which identified maternal age and body mass index (BMI) as confounders. Other variables within the dataset such as birthweight and gestational age were not adjusted for, to avoid the birthweight paradox or collider bias from unobserved confounding [22, 23]. p-value <0.05 was considered statistically significant for all tests. RStudio Version 1.2.1093 was used for statistical analysis [24].
Results
The total number of births from 24 weeks gestation during pre and post-GAP were 26,295 with 115 stillbirths and 31,624 with 108 stillbirths, respectively. Table 1 lists the characteristics of the two cohorts and shows them to be clinically similar. Average gestational age at birth was 38 weeks in both periods and average birth weight was 2.98 and 2.93 kg respectively.
Maternal and fetal characteristics of women in the pre- and post-GAP epochs including all births from 24 weeks.
| Pre-GAP (2011–2014) (n=26295) | Post-GAP (2015–2018) (n=31624) | ||
|---|---|---|---|
| Maternal age | ≤35 years | 25,377 (96.5%) | 30,144 (95.3%) |
| >35 years | 918 (3.5%) | 1,480 (4.7%) | |
| Maternal height, cm | Median (IQR) | 157 (155, 162) | 157 (155, 162) |
| Maternal weight, kg | Median (IQR) | 64.1 (56.6, 72.8) | 64.6 (57.1, 73.3) |
| Maternal BMI | <18.5 | 781 (3.0%) | 798 (2.5%) |
| 18.5–24.9 | 9,232 (35.1%) | 10,799 (34.2%) | |
| 25.0–29.9 | 9,998 (38.0%) | 12,375 (39.1%) | |
| ≥30.0 | 6,284 (23.9%) | 7,652 (24.2%) | |
| Parity | 0 | 15,193 (57.8%) | 17,592 (55.6%) |
| 1 | 7,778 (29.6%) | 9,948 (31.5%) | |
| 2 | 2,263 (8.6%) | 2,843 (9.0%) | |
| 3+ | 1,061 (4.0%) | 1,241 (3.9%) | |
| Gestational age at birth, days | Median (IQR) | 269 (262, 276) | 269 (262, 276) |
| Preterm delivery (<37 weeks) | 12.8% | 12.3% | |
| Average birth weight, kg | (±SD) | 2.98 ± 0.53 | 2.93 ± 0.53 |
-
GAP, growth assessment protocol; BMI, body mass index; IQR, interquartile range; SD, standard deviation.
SGA detection
The overall incidence of SGA by customised centiles in our population was 10.3%. Figure 2 shows (1) the SGA detection rate by quarter for the pre-GAP period (51.1%); (2) the one year period (2015) of implementation, during which charts started to be generated in early pregnancy and the new method and protocol was embedded into practice (49.8%); and (3) post implementation of GAP (67.1%). This represented a 31% increase in detection rates between pre- and post-GAP implementation (p<0.001).
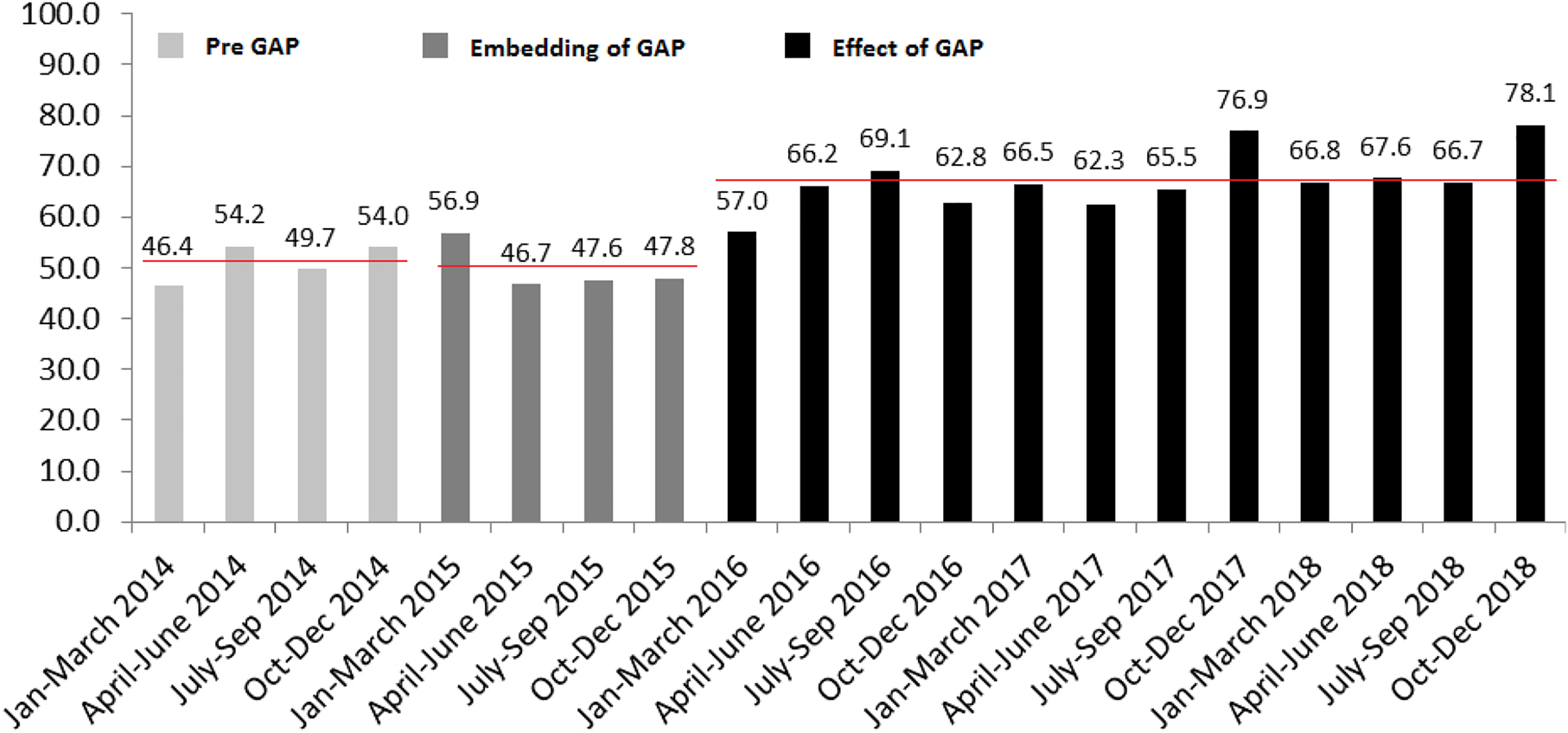
Quarterly antenatal SGA detection rates 2014–2018, for the three study periods.
Averages displayed as horizontal lines for each period: Pre-GAP: 51.1%; Embedding: 49.8%; Post implementation: 67.1%.
Missed cases
Case reviews were undertaken on a monthly basis and provided important feedback by identifying root causes for SGA missed antenatally. The most common reason was failure to identify risk factors and put such pregnancies on an appropriate surveillance pathway with serial ultrasound for fetal growth. Introduction of a screening risk assessment in the electronic medical records helped in risk categorization and prompting for ultrasound assessment. The other common reasons were failure to plot SFH, failure to refer for ultrasound assessment of fetal growth, errors in interpretation and inaccuracies in ultrasound EFW.
Stillbirth rates
Table 2 shows the stillbirth rates which decreased overall from 4.4/1000 in the pre-GAP period to 3.4/1000 post-GAP implementation, reaching borderline significance for the whole cohort (RR 0.78, CI 0.60–1.02; 25% decrease). Stratification by gestational age groups showed that stillbirth rates at term reduced significantly following GAP implementation (1.5/1000 to 0.6/1000, RR 0.37, CI 0.20–0.66, 60% decrease) while they remained similar during early and late preterm periods.
Gestational age specific (from 28 weeks) stillbirth rates during pre-GAP (n=26,199) and post-GAP (n=31,498) period.
| Gestational age (Weeks) | Pre-GAP | Post-GAP | RR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|
| No. of stillbirths/total births | Rate per 1000 births | No. of stillbirths/total births | Rate per 1000 births | |||
| 28 to 31 | 36/272 | 132.4 | 50/363 | 137.7 | 1.04 (0.68–1.60) | 0.855 |
| 32 to 36 | 43/2,652 | 16.2 | 42/2,970 | 14.1 | 0.87 (0.57–1.33) | 0.528 |
| ≥37 | 36/23,275 | 1.5 | 16/28,165 | 0.6 | 0.37 (0.20–0.66) | 0.001 |
| Overall | 115/26,199 | 4.4 | 108/31,498 | 3.4 | 0.78 (0.60–1.02) | 0.065 |
-
GAP, growth assessment protocol; CI, confidence interval; RR, relative risk. Significant p-values in bold.
The logistic regression model found that the Intervention (GAP) significantly decreased the risk of stillbirth (RR=0.75, 95% CI 0.60–0.94). The multivariable model, after adjusting for maternal BMI and age, found identical results to the univariate analysis in that babies in the post-GAP period had 25% less risk of stillbirths as compared to babies in the pre-GAP period.
Neonatal outcomes
Table 3 lists the five neonatal outcome measures that were compared between pre- and post-GAP periods in term babies (delivery ≥37 weeks). Following implementation of GAP, admissions to neonatal intensive care reduced by 40% (RR=0.60, 95% CI 0.54–0.67), and 45% fewer babies developed neonatal encephalopathy (RR=0.55, 95% CI 0.34–0.90).
Neonatal outcomes in term babies in the pre- and post-GAP period.
| Outcomes | Control period pre-GAP, n (%) n=23,275 | Study period post-GAP, n (%) n=28,165 | RR (95%CI) | p-Value |
|---|---|---|---|---|
| APGAR score <7 at 5 min | 78 (0.34) | 109 (0.39) | 1.15 (0.86–1.54) | 0.331 |
| Admission to NICU | 836 (3.59) | 608 (2.16) | 0.60 (0.54–0.67) | <0.001 |
| Meconium aspiration | 57 (0.24) | 53 (0.19) | 0.77 (0.53–1.12) | 0.166 |
| Neonatal encephalopathy | 39 (0.17) | 26 (0.09) | 0.55 (0.34–0.90) | 0.017 |
| Neonatal death (/1000) | 19 (0.82) | 24 (0.85) | 1.04 (0.57–1.91) | 0.889 |
-
NICU, Neonatal Intensive Care Unit; RR, relative risk; CI, confidence interval. Significant p-values in bold.
Discussion
This is to our knowledge the first study in a low or middle income country (LMIC) that evaluated the effect of a programme aimed at stillbirth prevention through improved antenatal detection of fetal growth restriction. We observed a significant increase in antenatal detection of SGA, and an associated reduction of stillbirths at term. These findings are consistent with reports that improved antenatal detection of SGA, as a marker for fetal growth restriction, is associated with a significant reduction in stillbirths [4]. They furthermore show that the same principle can be implemented and prove effective in our environment.
Application of the customised standard
The GROW standard customised to our population defined 10.3% of births as SGA. In contrast, applying the Hadlock growth standard [15] to the same births designates a more than three times higher number of cases (31.7%) as SGA, which raises questions of the applicability of Hadlock in our population. While we do not have data on induction rates, it is likely that pregnancies designated SGA pre-GAP may have been induced unnecessarily, as has been observed in pregnancies of Indian mothers in a different setting [25]. This may be a reason why the prematurity rate did not increase, being 12.8% before and 12.3% after GAP implementation (Table 1).
SGA detection rate
With these elements and the underlying protocol, training and audit, GAP increased SGA detection rates from 51.1 to 67.1%. Such improvement is consistent with reports from Australia [26, 27], New Zealand [28] and the UK [4]. The SGA detection rates improved with GAP training, constant monitoring and positive feedback, and retraining of staff in SFH measurements, plotting and interpretation of charts. SGA detection rates had not been monitored before GAP and hence there was no focus on improvement, but with implementation they became a new quality indicator of antenatal care. The streamlining of risk categorization may also have been a reason for improvement of SGA detection rates.
The GAP package
In addition to the customised charts being a more appropriate standard by which to assess fetal growth and birthweight, other elements of GAP may have contributed to the improved outcomes, such as training and general awareness of potential avoidability of adverse outcome, more rigorous early pregnancy risk assessment, standardised fundal height measurement in low risk pregnancies with referral pathways, and serial scanning in high risk pregnancy.
Case assessment of missed cases helped to identify and address problems. Importantly, such case reviews can only be undertaken if there is a clear protocol against which to perform the audit.
These various elements of audit and feedback, together with comprehensive training took up to a year to fully embed, as it concerned some fundamental changes in practice. This was reflected in the delayed increase in SGA detection following implementation (Figure 2).
Pregnancy outcome
While the decline in overall stillbirth rates reached only borderline significance (RR 0.78, CI 0.60–1.02) (Table 2), stillbirths at term reduced by almost two-thirds (RR 0.37, CI 0.20–0.66). These results are consistent with the focus of the GAP program to screen for and detect late onset fetal growth restriction, combined with guidelines to consider delivery of the SGA baby at term [5].
The findings of fewer NICU admissions and neonatal encephalopathy may also be linked to better SGA detection rates, as it would have led to increased monitoring before and during labour, or earlier delivery of fetuses identified as at-risk.
Strengths and limitations
A strength of this study is that it represents a real world implementation of GAP, as a longitudinal evaluation in practice. Being a single centre, there were no variations of service protocols during the study period other than that intended by the intervention, i.e., the GAP programme. Being a busy centre allowed sufficient data to be generated to investigate the effect on relatively rare outcomes including stillbirth and neonatal morbidity. A limitation of our data is that information was not routinely collected about referrals for investigations such as ultrasound scans.
An important consideration in longitudinal, observational studies is the potential influence of background changes that may at least in part explain the observed results – here the reduction in term stillbirths. According to UNICEF data [29], there has been a decrease in overall (28+ weeks) stillbirth rates in India during the period of our study, from an average of 18.5/1000 for our pre-GAP years (2011–2014) to 15.7/1000 for 2015–2018. Various factors are considered to be important contributors to the high national stillbirth rate and need to be addressed. The majority of stillbirths occur in pregnancies in rural environments with limited access to facilities, or being un-booked and receiving little or no care antenatally [30, 31] or during labour [29].
In contrast, the average stillbirth rate in our institution was already much lower pre-GAP (4.4/1000, Table 2). Ours was an urban population with ready access to care facilities, a low (3–4%) rate of un-booked pregnancies and few (<5%) of stillbirths occurring during labour. The likely improvements responsible for the national decline, such as the push to improve access to facilities, would not have affected our results. The reduction in our stillbirth rates to 3.4/1000 post implementation was due to a 63% reduction in term stillbirths (Table 2), consistent with the increased antenatal detection of at-risk SGA babies.
Conclusions
The GAP program including customised growth charts proved a simple, inexpensive care bundle that has increased identification of SGA and reduced term stillbirth rates and adverse neonatal outcomes. This protocol has scope to improve maternity services and perinatal outcomes in a country like India where resources need to be utilized in a most optimal manner.
Acknowledgments
We would like to thank Prof Jason Gardosi and his team at Perinatal Institute, Birmingham, UK for developing customised fetal growth charts for our population, for sharing the GAP protocol, training and certification, and continued support and guidance. We also thank the entire team at Fernandez Hospital for making this study possible.
-
Research funding: None declared.
-
Author contributions: Author A1, A4 has conceptualized the study and played primary role in compiling, analysis, and interpretation of the data. All the drafts were prepared, reviewed and final draft was approved by all authors. Author A2 has contributed to data collection and in writing up the manuscript. A3 had performed data cleaning, analysis and preparation of the results section and reviewed the manuscript for statistical and methodological aspects. A4 was the primary coordinator for the GAP protocol and responsible clinical lead for the project training and evaluation. A4 also had reviewed the citations, performed quality check of the document. All authors have reviewed the results and contributed to preparation and review of drafts. All the authors have read and approved final version of the manuscript. All the authors take complete responsibility for the content of the manuscript.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: The Fernandez Hospital Ethical Committee (Reg No. ECR/933/Inst/TG/2017) reviewed and approved the protocol (Protocol Ref. No. 02_2021, at Fernandez Hospital, Hyderabad, India).
References
1. Hug, L, You, D, Blencowe, H, Mishra, A, Wang, Z, Fix, MJ, et al. Global, regional, and national estimates and trends in stillbirths from 2000 to 2019: a systematic assessment. Lancet 2021;398:772–85. https://doi.org/10.1016/S0140-6736(21)01112-0.Suche in Google Scholar PubMed PubMed Central
2. Blencowe, H, Cousens, S, Jassir, FB, Say, L, Chou, D, Mathers, C, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Global Health 2016;4:e98–108. https://doi.org/10.1016/S2214-109X(15)00275-2.Suche in Google Scholar PubMed
3. Lawn, JE, Blencowe, H, Waiswa, P, Amouzou, A, Mathers, C, Hogan, D, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 2016;387:587–603. https://doi.org/10.1016/S0140-6736(15)00837-5.Suche in Google Scholar PubMed
4. Hugh, O, Williams, M, Turner, S, Gardosi, J. Reduction of stillbirths in England from 2008 to 2017 according to uptake of the Growth Assessment Protocol: 10-year population-based cohort study. Ultrasound Obstet Gynecol 2021;57:401–8. https://doi.org/10.1002/uog.22187.Suche in Google Scholar PubMed
5. Royal College of Obstetricians and Gynaecologists. The investigation and management of the small for gestational age fetus. Green Top Guidel No 31; 2013. Available from: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg31/.Suche in Google Scholar
6. Gardosi, J, Madurasinghe, V, Williams, M, Malik, A, Francis, A. Maternal and fetal risk factors for stillbirth: population based study. BMJ 2013;346:f108. https://doi.org/10.1136/bmj.f108.Suche in Google Scholar PubMed PubMed Central
7. Kean, L, Liu, D. Antenatal care as a screening tool for the detection of small for gestational age babies in the low risk population. J Obstet Gynaecol 1996;16:77–82. https://doi.org/10.3109/01443619609007744.Suche in Google Scholar
8. Morse, K, Williams, A, Gardosi, J. Fetal growth screening by fundal height measurement. Best Pract Res Clin Obstet Gynaecol 2009;23:809–18. https://doi.org/10.1016/j.bpobgyn.2009.09.004.Suche in Google Scholar PubMed
9. Figueras, F, Gardosi, J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol 2011;204:288–300. https://doi.org/10.1016/j.ajog.2010.08.055.Suche in Google Scholar PubMed
10. Gardosi, J, Francis, A, Turner, S, Williams, M. Customized growth charts: rationale, validation and clinical benefits. Am J Obstet Gynecol 2018;218(2 Suppl):S609–18. https://doi.org/10.1053/j.semperi.2003.12.002.Suche in Google Scholar PubMed
11. Melamed, N, Hiersch, L, Aviram, A, Keating, S, Kingdom, JC. Customized birth-weight centiles and placenta-related fetal growth restriction. Ultrasound Obstet Gynecol 2021;57:409–16. https://doi.org/10.1002/uog.23516.Suche in Google Scholar PubMed
12. Mongelli, M, Gardosi, J. Reduction of false-positive diagnosis of fetal growth restriction by application of customized fetal growth standards. Obstet Gynecol 1996;88:844–8. https://doi.org/10.1016/0029-7844(96)00285-2.Suche in Google Scholar PubMed
13. Gardosi, J, Giddings, S, Clifford, S, Wood, L, Francis, A. Association between reduced stillbirth rates in England and regional uptake of accreditation training in customised fetal growth assessment. BMJ Open 2013;3:e003942. https://doi.org/10.1136/bmjopen-2013-003942.Suche in Google Scholar PubMed PubMed Central
14. Gardosi, J, Giddings, S, Buller, S, Southam, M, Williams, M. Preventing stillbirths through improved antenatal recognition of pregnancies at risk due to fetal growth restriction. Publ Health 2014;128:698–702.10.1016/j.puhe.2014.06.022Suche in Google Scholar PubMed
15. Hadlock, FP, Harrist, RB, Martinez-Poyer, J. Utero analysis of fetal growth: a sonographic weight standard. Radiology 1991;181:129–33. https://doi.org/10.1148/radiology.181.1.1887021.Suche in Google Scholar PubMed
16. Perinatal, Institute. Growth assessment protocol (GAP) 2013–2022. https://www.perinatal.org.uk/GAP/Programme.Suche in Google Scholar
17. Williams, M, Turner, S, Butler, E, Gardosi, J. Fetal growth surveillance – current guidelines, practices and challenges. Ultrasound 2018;26:69–79. https://doi.org/10.1177/1742271X18760657.Suche in Google Scholar PubMed PubMed Central
18. Gestation, Network. GROW (Gestation Related Optimal Weight) software version 1.1.7, 2015. www.gestation.net.Suche in Google Scholar
19. Gardosi, J, Mongelli, M, Wilcox, M, Chang, A. An adjustable fetal weight standard. Ultrasound Obstet Gynecol 1995;6:168–74. https://doi.org/10.1046/j.1469-0705.1995.06030168.x.Suche in Google Scholar PubMed
20. Lawn, JE, Blencowe, H, Pattinson, R, Cousens, S, Kumar, R, Ibiebele, I, et al. Stillbirths: where? When? Why? How to make the data count? Lancet 2011;377:1448–63. https://doi.org/10.1016/S0140-6736(10)62187-3.Suche in Google Scholar PubMed
21. World Health Organization. Stillbirth; 2022. Available from: https://www.who.int/health-topics/stillbirth.Suche in Google Scholar
22. Hernández-Díaz, S, Schisterman, EF, Hernán, MA. The birth weight “paradox” uncovered? Am J Epidemiol 2006;164:1115–20. https://doi.org/10.1093/aje/kwj275.Suche in Google Scholar PubMed
23. Wang, T, Li, H, Su, P, Yu, Y, Sun, X, Liu, Y, et al. Sensitivity analysis for mistakenly adjusting for mediators in estimating total effect in observational studies. BMJ Open 2017;7:e015640. https://doi.org/10.1136/bmjopen-2016-015640.Suche in Google Scholar PubMed PubMed Central
24. RStudio Team. RStudio: Integrated Development for R. RStudio. Boston, MA: PBC; 2020. Available from: http://www.rstudio.com/.Suche in Google Scholar
25. Giddings, S, Clifford, S, Madurasinghe, V, Gardosi, J. PFM.69 Customised vs. uncustomised ultrasound charts in the assessment of perinatal mortality risk in the South Asian maternity population. Arch Dis Child Fetal Neonatal Ed 2014;99:A104.10.1136/archdischild-2014-306576.298Suche in Google Scholar
26. Roex, A, Nikpoor, P, Eerd, E, Hodyl, N, Dekker, G. Serial plotting on customised fundal height charts results in doubling of the antenatal detection of small for gestational age fetuses in nulliparous women. Aust N Z J Obstet Gynaecol 2012;52:78–82. https://doi.org/10.1111/j.1479-828X.2011.01408.x.Suche in Google Scholar PubMed
27. Jayawardena, L, Sheehan, P. Introduction of a customised growth chart protocol increased detection of small for gestational age pregnancies in a tertiary Melbourne hospital. Aust N Z J Obstet Gynaecol 2019;59:493–500. https://doi.org/10.1111/ajo.12902.Suche in Google Scholar PubMed
28. Cowan, FJ, McKinlay, CJD, Taylor, RS, Wilson, J, McAra‐Couper, J, Garrett, N, et al.. Detection of small for gestational age babies and perinatal outcomes following implementation of the Growth Assessment Protocol at a New Zealand tertiary facility: an observational intervention study. Aust N Z J Obstet Gynaecol 2021;61:339–46. https://doi.org/10.1111/ajo.13283.Suche in Google Scholar PubMed
29. UNICEF DATA. Stillbirths and stillbirth rates; 2020. Available from: https://data.unicef.org/topic/child-survival/stillbirths/.Suche in Google Scholar
30. Sharma, B, Prasad, G, Aggarwal, N, Siwatch, S, Suri, V, Kakkar, N. Aetiology and trends of rates of stillbirth in a tertiary care hospital in the north of India over 10 years: a retrospective study. BJOG An Int J Obstet Gynaecol 2019;126:14–20. https://doi.org/10.1111/1471-0528.15850.Suche in Google Scholar PubMed
31. Mali, RV, Dalal, A, Khursheed, R, Gan, A. Association of stillbirths with maternal and fetal risk factors in a tertiary care hospital in south India. Obstet Gynecol Int 2021;2021:e8033248.10.1155/2021/8033248Suche in Google Scholar PubMed PubMed Central
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/jpm-2022-0111).
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorials
- Preventing stillbirth: risk factors, case reviews, care pathways
- Managing stillbirth: taking care to investigate the cause and provide care for bereaved families
- Epidemiology and Risk Factors
- Spatial dynamics of fetal mortality and the relationship with social vulnerability
- Stillbirth occurrence during COVID-19 pandemic: a population-based prospective study
- The effect of the Covid pandemic and lockdown on stillbirth rates in a South Indian perinatal centre
- Stillbirths preceded by reduced fetal movements are more frequently associated with placental insufficiency: a retrospective cohort study
- The prevalence of and risk factors for stillbirths in women with severe preeclampsia in a high-burden setting at Mpilo Central Hospital, Bulawayo, Zimbabwe
- Surveillance and Prevention
- Perinatal mortality audits and reporting of perinatal deaths: systematic review of outcomes and barriers
- Stillbirth diagnosis and classification: comparison of ReCoDe and ICD-PM systems
- Facility-based stillbirth surveillance review and response: an initiative towards reducing stillbirths in a tertiary care hospital of India
- Impact of introduction of the growth assessment protocol in a South Indian tertiary hospital on SGA detection, stillbirth rate and neonatal outcome
- Evaluating the Growth Assessment Protocol for stillbirth prevention: progress and challenges
- Prospective risk of stillbirth according to fetal size at term
- Understanding the Pathology of Stillbirth
- Placental findings in singleton stillbirths: a case-control study from a tertiary-care center in India
- Abnormal placental villous maturity and dysregulated glucose metabolism: implications for stillbirth prevention
- Comparison of prenatal central nervous system abnormalities with postmortem findings in fetuses following termination of pregnancy and clinical utility of postmortem examination
- Cardiac ion channels associated with unexplained stillbirth – an immunohistochemical study
- Viral infections in stillbirth: a contribution underestimated in Mexico?
- Audit and Bereavement Care
- Investigation and management of stillbirth: a descriptive review of major guidelines
- Delivery characteristics in pregnancies with stillbirth: a retrospective case-control study from a tertiary teaching hospital
- Perinatal bereavement care during COVID-19 in Australian maternity settings
- Beyond emotional support: predictors of satisfaction and perceived care quality following the death of a baby during pregnancy
- Stillbirth aftercare in a tertiary obstetric center – parents’ experiences
Artikel in diesem Heft
- Frontmatter
- Editorials
- Preventing stillbirth: risk factors, case reviews, care pathways
- Managing stillbirth: taking care to investigate the cause and provide care for bereaved families
- Epidemiology and Risk Factors
- Spatial dynamics of fetal mortality and the relationship with social vulnerability
- Stillbirth occurrence during COVID-19 pandemic: a population-based prospective study
- The effect of the Covid pandemic and lockdown on stillbirth rates in a South Indian perinatal centre
- Stillbirths preceded by reduced fetal movements are more frequently associated with placental insufficiency: a retrospective cohort study
- The prevalence of and risk factors for stillbirths in women with severe preeclampsia in a high-burden setting at Mpilo Central Hospital, Bulawayo, Zimbabwe
- Surveillance and Prevention
- Perinatal mortality audits and reporting of perinatal deaths: systematic review of outcomes and barriers
- Stillbirth diagnosis and classification: comparison of ReCoDe and ICD-PM systems
- Facility-based stillbirth surveillance review and response: an initiative towards reducing stillbirths in a tertiary care hospital of India
- Impact of introduction of the growth assessment protocol in a South Indian tertiary hospital on SGA detection, stillbirth rate and neonatal outcome
- Evaluating the Growth Assessment Protocol for stillbirth prevention: progress and challenges
- Prospective risk of stillbirth according to fetal size at term
- Understanding the Pathology of Stillbirth
- Placental findings in singleton stillbirths: a case-control study from a tertiary-care center in India
- Abnormal placental villous maturity and dysregulated glucose metabolism: implications for stillbirth prevention
- Comparison of prenatal central nervous system abnormalities with postmortem findings in fetuses following termination of pregnancy and clinical utility of postmortem examination
- Cardiac ion channels associated with unexplained stillbirth – an immunohistochemical study
- Viral infections in stillbirth: a contribution underestimated in Mexico?
- Audit and Bereavement Care
- Investigation and management of stillbirth: a descriptive review of major guidelines
- Delivery characteristics in pregnancies with stillbirth: a retrospective case-control study from a tertiary teaching hospital
- Perinatal bereavement care during COVID-19 in Australian maternity settings
- Beyond emotional support: predictors of satisfaction and perceived care quality following the death of a baby during pregnancy
- Stillbirth aftercare in a tertiary obstetric center – parents’ experiences

