Abstract
Context
The mechanisms by which osteopathic cranial manipulative medicine (OCMM) promotes health and healing have yet to be fully elucidated. One commonly utilized OCMM technique, compression of the fourth ventricle (CV4), has been theorized to balance autonomic nervous system (ANS) activity. There is growing evidence that the ANS also plays a significant mechanistic role in acupuncture. Potential connections between OCMM and acupuncture meridian theory largely remain unknown.
Objectives
By measuring specific electrical parameters at acupuncture points that have been shown to correlate with ANS activity, the objectives of this study were to: 1) determine if CV4 has any influence on the bioelectric properties of the acupuncture meridian system; and 2) determine if CV4 affects the ANS.
Methods
A total of 77 males and females ages 18–78 years, all volunteers recruited by local flyers and personal or phone contact, were randomized into CV4 (n=40) and Sham (n=37) groups. All CV4 participants were treated by the same physician utilizing standard CV4 protocol. The Sham treatment, performed by a different physician, consisted of the supine participant’s occiput resting passively on the physician’s finger pads for a similar duration as those in the CV4 group. Among several devices developed to assess ANS activity at acupuncture points, evidence suggests that the Apparatus for Meridian Identification (AMI) is the most accurate and valid. Utilizing the AMI, bioelectric skin parameters were measured immediately before and after CV4 or Sham treatments. Student’s or Welch’s t tests and Wilcoxon tests were utilized for analysis of normally and non-normally distributed data, respectively.
Results
Statistical significance was determined with a p value less than 0.05. Sham treatments showed insignificant (p=0.754) before vs. after differences in ANS activity measured at acupuncture points, whereas CV4 treatment significantly (p=0.00015) affected ANS activity.
Conclusions
This research suggests that CV4 has demonstrable biophysical effects on the acupuncture meridian system occurring via the ANS, and that the underlying mechanisms of OCMM and acupuncture may be related. Further studies are needed to clarify this.
Osteopathic cranial manipulative medicine (OCMM) is employed therapeutically to correct numerous dysfunctions of the cranial, facial, and mandibular regions as well as the eye, ear, nose, and throat (EENT) region, improve cranial nerve function, restore autonomic balance, reduce pain and stress, and improve sleep [1, 2]. Its clinical acceptance and use, however, have been hampered by an incomplete understanding of the underlying mechanisms and effects. Studies to elucidate the nature of these factors have been relatively small and heterogeneous, often leading to inconclusive results [3], [4], [5].
OCMM studies can be particularly difficult to design and interpret because of manipulation’s effects on multiple structures and systems. For example, OCMM’s benefits are theorized to act, in part, through alteration of the primary respiratory mechanism (PRM). The PRM is defined as a rhythmic motion that is palpable at multiple interactive and interdependent areas including the cranium, sacrum, meninges, other parts of the central nervous system (CNS), and throughout all parts of the body. The cranial rhythmic impulse (CRI), which is palpated at the head, is one manifestation of the PRM [1, 6]. In addition to involving multiple anatomical structures, multiple physiologic oscillators have been implicated as sources for these motions, including cerebrospinal fluid fluctuation [7], cranial arterial blood flow [8], [9], [10], and even bioenergetic influences [11, 12].
The autonomic nervous system (ANS) is a potential underlying mechanism for the PRM. In fact, the most well-studied OCMM technique, compression of the fourth ventricle (CV4), has been shown to affect several outcome measures of ANS activity. These include changes in the measures of heart rate variability (HRV) [13, 14] and skin conductance in a study of 32 healthy adults (vs. placebo) who received CV4 and rib raising [14], increased salivary amylase concentration immediately after treatment in a study of 90 people who were randomized to sham or CV4 [15], reduced blood pressure and increased parasympathetic activity in hypertensive men 15 min after CV4 in a study of 30 men divided into normotensive or hypertensive groups [16], decreased sleep latency changes in muscle sympathetic nerve activity in a CV4 vs. sham vs. control study of 20 healthy adult volunteers [17], and quantitative electroencephalogram (qEEG) changes in a study of the alpha band after CV4 in 10 healthy adults [18]. CV4 studies also indicate that ANS activity tends to move from a predominantly sympathetic to a more parasympathetic neurophysiological state after CV4 treatment [6, 16, 18].
CV4 involves applying external manual pressure on the tissues in the posteroinferior squamous portions of the occiput just superficial to the fourth ventricle during the extension phase of the cranial rhythmic motion cycle. Pressure is maintained through a “still point” during which time cranial movement pauses briefly and then restarts [1]. CV4 has been hypothesized to reduce the fourth ventricle’s volume, leading to cerebral spinal fluid (CSF) dispersion to other parts of the CNS and mobilization of the tissues surrounding the fourth ventricle. Tissue and fluid mobilization at the level of the floor of the fourth ventricle can theoretically affect the ANS directly because it contains many autonomic nuclei and nerve tracts [1, 6].
Over the past several decades, an increasing number of osteopathic physicians in the United States have utilized osteopathic manipulative medicine (OMM) and acupuncture successfully for the treatment of a variety of medical conditions [19]. In our practices, we have found synergistic effects of combining the two modalities, especially for pain syndromes [20]. In addition to pain relief, we have noted similar subjective relaxation responses reported by patients receiving CV4 or acupuncture treatments.
Interestingly, there is increasing evidence that the ANS also plays a significant mechanistic role in acupuncture. Robinson [21] provided a comprehensive discussion of the neuroanatomy and neurophysiology of acupuncture points (acupoints) and the acupuncture meridian system. The physiological and healing effects of acupuncture can partially be explained by alterations in ANS activity stimulated by needle insertion into various acupoints [21], [22], [23], [24], [25]. Activation of sensory-afferent A-delta and C-fibers can elicit somatosomatic and somatovisceral reflexes, as well as directly affect specific brain centers [21, 26], [27], [28]. Somatovisceral reflexes at dorsal Shu and ventral Mu acupoints, a majority of which correspond to Chapman Reflex points [29], have direct effects on organs [26, 30].
In the acupuncture model, 14 paired primary meridians or channels, corresponding to specific organs, connect traditional acupoints [31] on the body. Located superficially in the dermis, these meridian channels are thought to transmit “vital energy” or “qi,” which appears to possess bioelectromagnetic properties, to and from the organs [31], [32], [33]. True acupoints exhibit increased electrical conductivity and lower resistance compared to nonacupuncture points [34, 35]. They are found at specific anatomical locations and are relatively consistent among individuals with varying anatomies. Ting (Jing-Well) acupoints, located at the proximal corners of fingernails and toenails, represent the beginning or end of the paired acupuncture meridians [21].
This has led to the development of multiple devices that have been validated to measure ANS response at acupoints after acupuncture treatments. These devices include the Dermatron, Neurometer, Prognos, and the Apparatus for Meridian Identification (AMI) [32, 36, 37]. Among these devices, the AMI may be the most reliable and valid, correlating the most with the galvanic skin response, a known standard for ANS response in the skin [36, 38, 39]. The AMI appears to be able to identify changes in individual meridian activity by measuring bioelectric skin responses at Ting points [32, 40], [41], [42], [43]. Utilizing the AMI to measure bioelectric parameters before and after CV4 treatments would provide a way to further assess the effects of cranial manipulation both on the ANS and the acupuncture meridian system.
The goal of this study was to assess the effect of CV4 on the ANS bioelectric skin response at Ting acupoints as measured by the AMI. We postulated that this response could be significant, establishing potential correlations between the mechanisms underlying acupuncture and CV4, and better understanding the potential clinical applications of CV4.
Methods
Participants
From November 2017 to June 2018, a total of 80 males and females from the Erie, Pennsylvania area, aged 18–78 years, were recruited by local flyers placed around the College of Osteopathic Medicine campus and health system and by word-of-mouth marketing initiated by the investigators. Potential participants were given a phone number to contact, and upon calling, were made aware of the exclusion criteria and randomly assigned, by block randomization utilizing Microsoft Excel, to an appointment day and time. Upon arrival to the study site, potential participants were screened for exclusion criteria, provided informed consent forms, and block-randomized, utilizing Microsoft Excel, to sham and CV4 groups. The study was approved by the Institutional Review Board at Lake Erie College of Osteopathic Medicine under the protocol #24–177 and Clinical Trial Registry Number NCT05190731. Funding for the study was generously provided by John M. Ferretti, DO. No compensation or reimbursements were provided for participation.
The exclusion criteria included individuals younger than 18 years old, individuals experiencing known or identifiable acute illness of any kind, individuals with a medical history of stroke or transient ischemic attack in the past 6 months, intracranial aneurysm, intracranial hemorrhage, increased intracranial pressure, or seizure disorder. Participants of childbearing potential were asked about their pregnancy status, and if uncertain, underwent a urine pregnancy test at the study site, due to hypothetical CV4 contraindications in pregnant individuals (Figure 1).
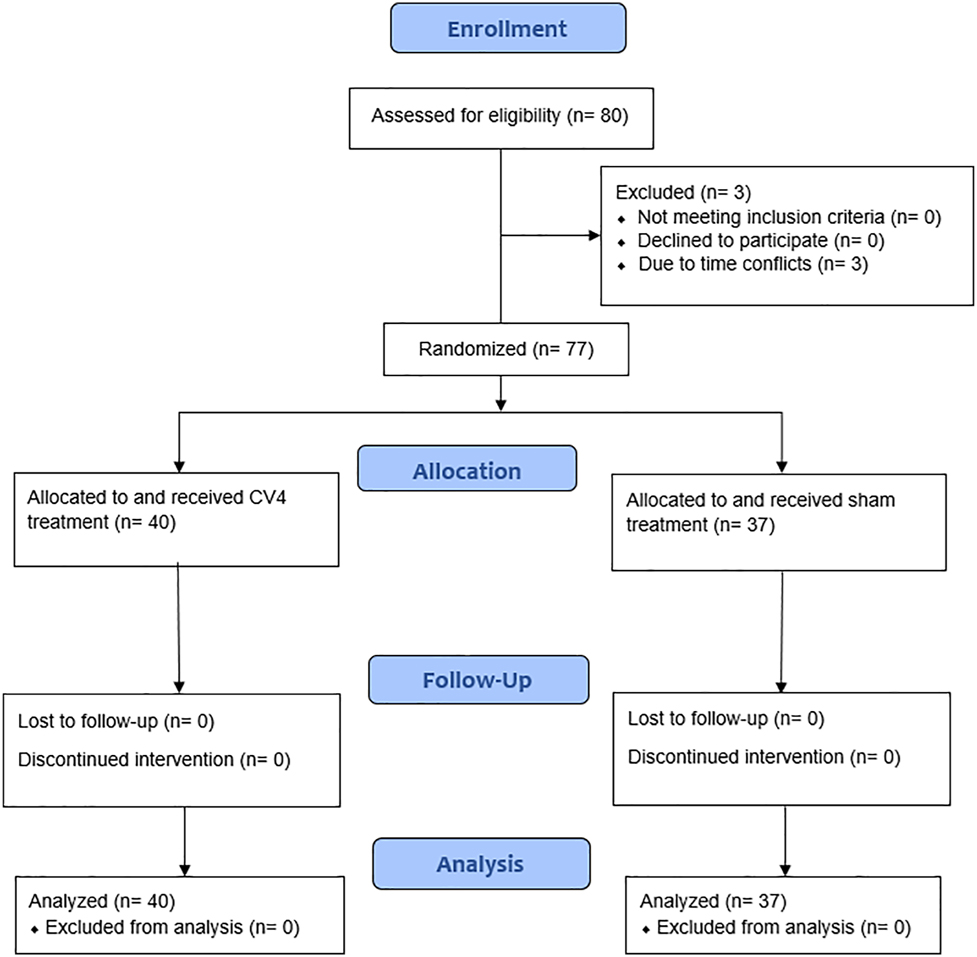
Participant flow diagram. Available patients were randomized into sham and CV4 treatment groups, in which data before and after treatment was assessed during a single session. Formatted utilizing the CONSORT 2010 flow diagram.
Research design and treatment protocol
Eligible participants were randomized into CV4 and sham treatment groups utilizing a block randomization generator. Each subject lay supine on two similar treatment tables while receiving either sham or CV4 treatments. AMI measurements were taken immediately before and within 15 min after CV4 and sham treatments. The AMI is displayed in Figure 2. AMI measurements involved the placement of 7 × 7 mm metal active electrode patches with conductive gel at all Ting acupoints on the fingers and toes of each participant (Figure 3), and a ground electrode placed on the dorsal aspect of the forearm about 3 inches above the wrist.
![Figure 2:
Apparatus for meridian identification (AMI) equipment setup. AMI was invented, developed, and researched by Hiroshi Motoyama, PhD [32, 33]. The 7 × 7 mm metal active electrode patches with conductive gel are utilized at the Ting points on the fingers and toes, while the ground electrodes are placed on the forearm. (Photo courtesy of California Institute for Human Science, Encinitas, CA).](/document/doi/10.1515/jom-2022-0111/asset/graphic/j_jom-2022-0111_fig_002.jpg)
Apparatus for meridian identification (AMI) equipment setup. AMI was invented, developed, and researched by Hiroshi Motoyama, PhD [32, 33]. The 7 × 7 mm metal active electrode patches with conductive gel are utilized at the Ting points on the fingers and toes, while the ground electrodes are placed on the forearm. (Photo courtesy of California Institute for Human Science, Encinitas, CA).
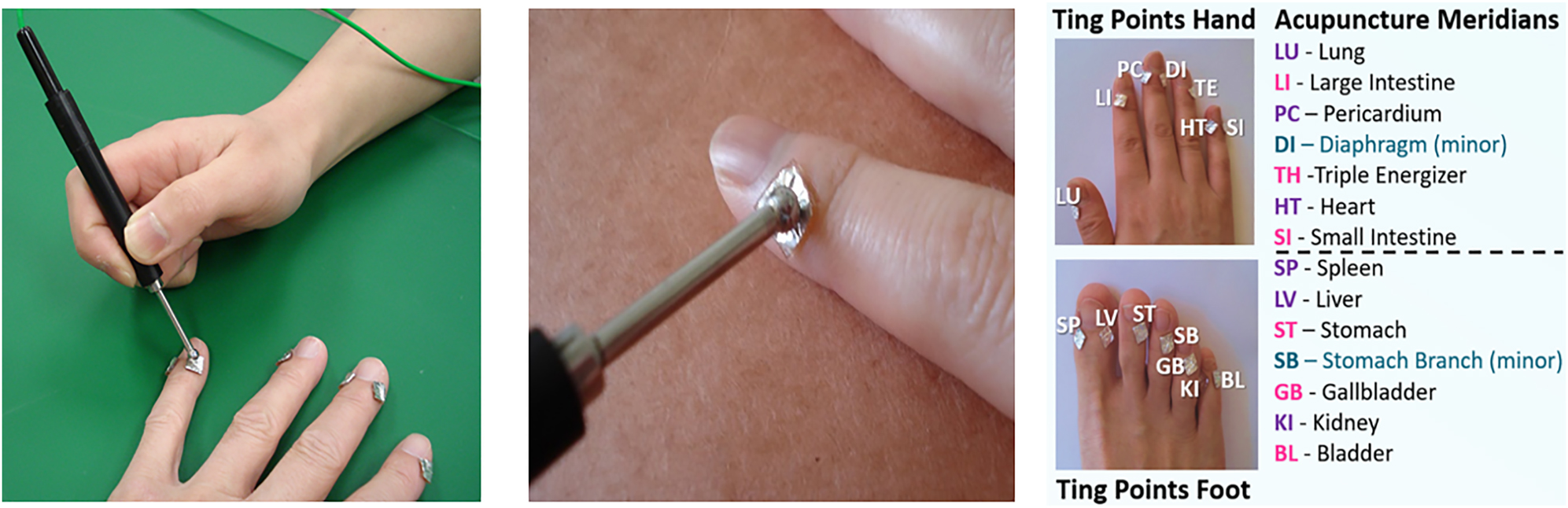
The two left images show the placement of the 7 × 7 mm metal active electrode patches at the Ting points on the hand. The probe electrode is placed on the metal patches and distributes a 3 V DC pulse for 512 µsec. Each Ting acupoint measured in this study is indicated in the image on the right. (Photos courtesy of California Institute for Human Science, Encinitas, CA).
Subject orientation to the AMI measurement process and AMI measurements were performed by biophysicist Gaétan Chevalier, PhD, who had extensive experience with the AMI in research settings.
The AMI applied a 3 V DC pulse for 512 µsec and was held in place at the Ting point for approximately 1 s until the 512-µsec pulse was completed (Figure 3). The AMI measured the current generated between this point and a ground electrode on the forearm. The current response peaks to a maximum value within a few µsec after the initial 3 V pulse was applied and, after approximately 40 µsec, decreases to a steady state value for the rest of the 512 µsec. This low steady state current is known as the after polarization (AP) current (Figure 4), which has been found to reliably correlate with ANS activity [32, 33].
![Figure 4:
Apparatus for meridian identification (AMI) measurements. This graph, representing current vs. time, shows the peak tissue current response to polarization by 3 V DC square pulse (called before polarization [BP] current), the area under the curve representing the total electrical charge of ions mobilized throughout polarization or the integral electrical charge (called IQ), and the steady state current at the end of polarization, termed the after polarization (AP) current [32, 33]. The AP current has been correlated with autonomic nervous system (ANS) activity.](/document/doi/10.1515/jom-2022-0111/asset/graphic/j_jom-2022-0111_fig_004.jpg)
Apparatus for meridian identification (AMI) measurements. This graph, representing current vs. time, shows the peak tissue current response to polarization by 3 V DC square pulse (called before polarization [BP] current), the area under the curve representing the total electrical charge of ions mobilized throughout polarization or the integral electrical charge (called IQ), and the steady state current at the end of polarization, termed the after polarization (AP) current [32, 33]. The AP current has been correlated with autonomic nervous system (ANS) activity.
CV4 and sham treatments were performed by osteopathic physicians board-certified in Special Proficiency in Osteopathic Manipulative Medicine (C-SPOMM) and Neuromusculoskeletal Medicine (NMM). All CV4 treatments were performed by the same physician according to the standardized protocol [1]. The physician’s hands were placed underneath the occiput with the thenar eminences in contact on the occipital squama medial to the lambdoidal and occipitomastoid sutures. Inherent PRM motion was identified and thenar eminences followed the occipital motion into the extension phase until a still point was attained. This position was held until the still point released (usually approximately 3 min) and normal cranial motion ensued (Video 1). The physician left the room, and within 10–15 min, the subject was remeasured with the AMI and the data recorded.
Video CV4 Treatment JOM-2022-0111.
Similarly, all sham treatments were performed by the same physician, who was different from the physician who performed the CV4 treatments. The patient’s occiput rested passively on the physician’s finger pads for 3 min, after which the same AMI measurement protocol was followed as in the CV4 group (Video 2).
Video Sham Treatment JOM-2022-0111.
Statistical analysis
Data from the AMI were organized into the meridians that corresponded to the Ting points from which AMI measurements were collected. Measurements were reported as averages of the left and right sides (for each participant).
Means and standard deviations were calculated across each meridian before and after the sham and CV4 treatments. Skewness, Kurtosis, and Omnibus normality tests were utilized for all data sets. Student’s and Welch’s t tests were applied to normally distributed data with equal and unequal variances, respectively, and Wilcoxon t tests were applied to the nonparametric data sets. Significance was set to p<0.05. Tests for outliers were not performed because outliers were not apparent.
Results
Among the 80 participants recruited for the study, three participants did not complete the study due to time conflicts. No participants met the exclusion criteria (Figure 1). Among the final sample (n=77), the average participant age was 47.6 years (range, 18–78 years) with 64% (49) of the participants female (range, 18–78 years); the rest were male (range, 22–75 years). In the CV4 treatment group (n=40), the average participant age was 46.1 years (range, 18–78 years) with 72.5% (29) of participants female (range, 18–78 years); the rest were male (range, 23–68 years). In the sham group (n=37), the average participant age was 49.1 years (range, 21–77 years) with 54% (20) of the participants female (range, 21–77 years); the rest were male (range, 22–75 years).
After CV4 treatment, AMI AP measurements, reported as an average of left and right measurements, were decreased at 13 out of 14 Ting acupoints, and this difference was significant (p<0.05) at 12 of the 13 acupoints that yielded lower AP measurements. At one Ting acupoint (Spleen meridian), the AP measurement was increased after CV4 treatment, and this change was not significantly different. After sham treatment, AP measurements were decreased at five Ting acupoints, increased at two acupoints, and unchanged at seven acupoints; none of these differences were significant (p≥0.05) (Figure 5 and Table 1).
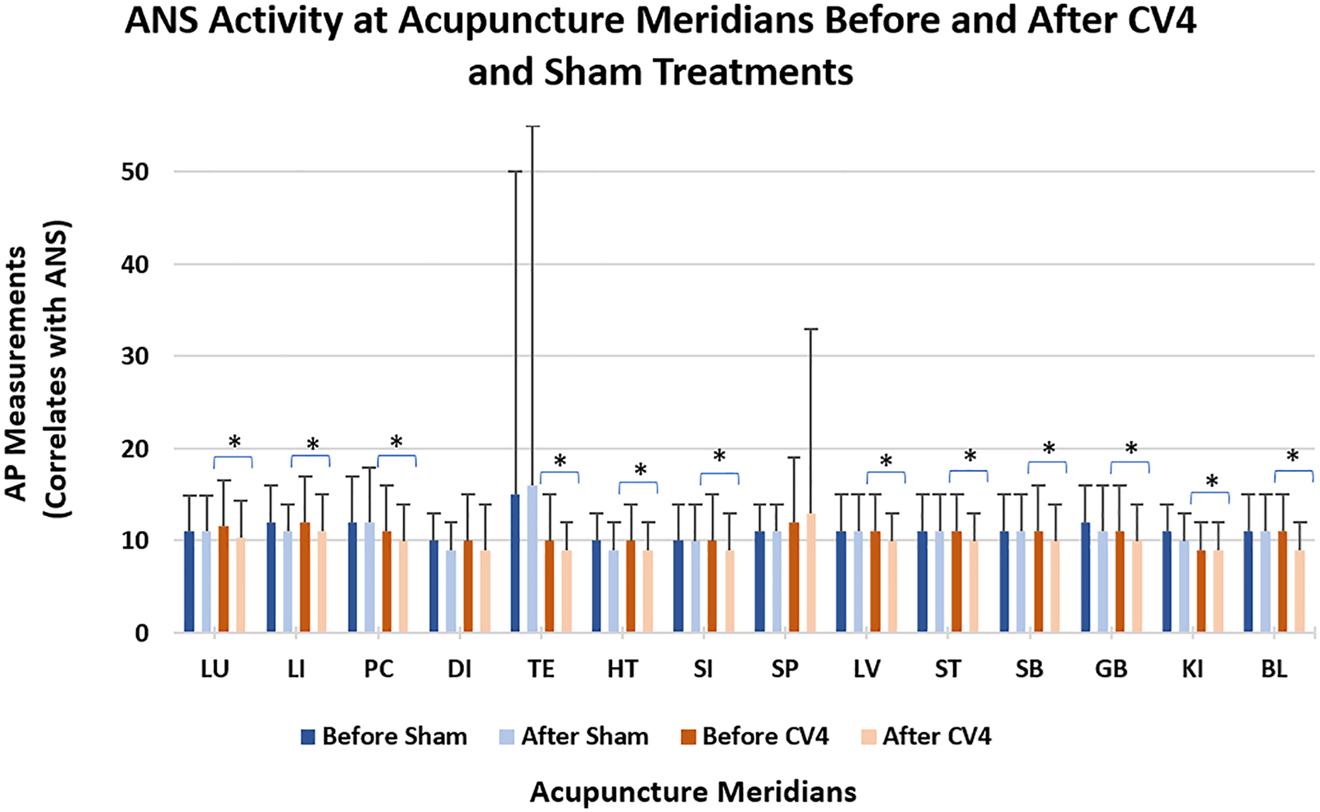
After AP) currents, as measured by the apparatus for meridian identification (AMI), for each Ting acupoint (corresponding to a meridian) before and after CV4 and sham treatments. The before-CV4 AP current was compared to after-CV4 AP current, and the before-sham AP current polarization (was compared to the after-sham AP current, with significance denoted by an asterisk. Statistical significance is determined with a p value of less than 0.05. The lack of an asterisk denotes no significant difference.
The average and standard deviation (SD) after polarization (AP) values for each meridian before and after sham and CV4 treatments. Also shown are the before-vs.-after p values before-vs.-after from comparing CV4 and sham treatment as described in the Methods section.
| Ting point | Before sham | After sham | Before CV4 | After CV4 | Before vs. after treatment | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-Values | ||||||||||
| Average | SD | Average | SD | Average | SD | Average | SD | Sham | Control | |
| Lung | 11.0 | 4.0 | 11.1 | 3.8 | 11.6 | 5.0 | 10.4 | 3.9 | 0.757352 | 0.006419∗ |
| Large intestine | 11.6 | 3.6 | 11.2 | 3.5 | 11.8 | 4.7 | 11.1 | 4.0 | 0.928286 | 0.027638∗ |
| Pericardium | 11.7 | 5.0 | 12.1 | 6.0 | 11.0 | 5.0 | 10.3 | 3.9 | 0.789266 | 0.008426∗ |
| Diaphragm | 9.7 | 3.4 | 9.3 | 3.3 | 9.7 | 4.6 | 9.4 | 5.3 | 0.057149 | 0.36992 |
| Triple energizer | 15.0 | 34.8 | 15.6 | 39.4 | 9.7 | 4.6 | 8.8 | 3.5 | 0.337644 | 0.001624∗ |
| Heart | 9.7 | 3.3 | 9.4 | 3.1 | 9.9 | 4.0 | 8.9 | 3.4 | 0.49443 | 0.001745∗ |
| Small intestine | 10.0 | 4.1 | 9.7 | 3.9 | 10.2 | 4.8 | 9.2 | 3.9 | 0.411014 | 0.001506∗ |
| Spleen | 11.2 | 3.5 | 11.0 | 3.3 | 12.0 | 7.4 | 13.1 | 19.8 | 0.390915 | 0.242,082 |
| Liver | 11.4 | 4.0 | 11.1 | 3.7 | 11.0 | 3.7 | 10.3 | 3.4 | 0.30184 | 0.003529∗ |
| Stomach | 11.1 | 4.4 | 10.8 | 4.2 | 10.8 | 4.2 | 9.5 | 3.4 | 0.471689 | 0.001211∗ |
| Stomach branch | 10.9 | 4.3 | 10.9 | 4.4 | 10.9 | 5.5 | 9.5 | 4.0 | 0.75934 | 0.002419∗ |
| Gall bladder | 11.6 | 4.1 | 11.5 | 4.7 | 11.1 | 4.7 | 9.9 | 3.7 | 0.348811 | 0.000154∗ |
| Kidney | 10.6 | 3.0 | 10.2 | 3.2 | 9.4 | 2.9 | 8.8 | 3.1 | 0.202632 | 0.014716∗ |
| Urinary bladder | 11.4 | 4.0 | 10.6 | 4.0 | 10.8 | 3.8 | 9.5 | 3.3 | 0.116928 | 0.000059∗ |
Discussion
Results of this study appear to show that CV4 treatment has significant bioelectric effects on the acupuncture meridian system of individuals as measured by the AMI. Specifically, overall reduction of AP currents after CV4 treatment indicates a change of ANS activity beyond that provided by simple relaxation, as modeled by the sham treatment in our study, because sham treatments yielded no significant changes. These findings also correlate with conclusions of others studying the effects of CV4 utilizing other autonomic markers [13], [14], [15], [16], [17], [18]. Furthermore, reductions in AMI AP measurements suggest a shift toward a more parasympathetic state.
Although the ANS has been implicated as a likely major mechanism underlying OCMM, multiple groups have identified other potential mechanisms not measured in this study. The therapeutic effects of CV4 have been shown to activate astrocytes, modulate synaptic transmission, and improve amyloid beta clearance in a model of aged rats who received 7 days of CV4 treatment under isoflurane anesthetic [44]. Work to better identify other possible mechanisms is in its nascency, and even though there have been multiple studies implicating the ANS, it is still unclear how this may occur. In fact, there may be a mechanism underlying the effect of CV4 on the ANS and other systems such as synaptic transmission. For example, changes in gene expression related to cholinergic neurotransmission and multiple neurological disorders have been measured from RNA samples in 12 elderly rats after 7 days of CV4 treatment [45]. However, our focus in measuring ANS activity at Ting points not only supports an underlying ANS mechanism, but also suggests a strong clinical correlate between treatments targeting acupuncture meridians and OCMM.
Gender distribution differed notably between the CV4 and sham treatment groups; 72.5% (29) were female in the CV4 treatment group and 54% (20) were female in the sham group. Gender differences in autonomic activity and reactivity have been noted but not well defined. Also, gender-relevant factors, such as weight, estrogen concentrations, and baseline ANS activity, may have affected our findings, but while some studies have found that women do show more pronounced sympathetic activity, the findings have not been consistent. For example, Cankar and Finderle [46] found more autonomic response in 10 healthy, premenopausal women when compared to 10 healthy men by measuring cutaneous vascular response to hand cooling. However, Convertino [47], when looking at gender differences in ANS activity associated with blood pressure regulation in 7 females and 10 males, found that women have less responsiveness. In future studies, we plan to utilize block randomization to also ensure equal gender distribution across the treatment groups.
It is unclear why a nonsignificant increase in AP current was seen only at one acupoint after CV4 treatment (SP or Spleen). In Traditional Chinese Medicine, the Spleen meridian is associated with the immune system and vascular and lymphatic drainage and flow. Superficially, it courses superiorly from the first toe medial Ting point along the medial lower extremity through major venous and lymphatic drainage pathways to terminate at the sixth intercostal space in the midaxillary line [21]. Its deep projections are thought to travel to the spleen itself and then terminate in the regions of the nose, throat, and tongue [48]. Acupuncture treatment of the spleen points often results in decreased edema and congestion [21].
The glymphatic-lymphatic CSF filtering system in the CNS [49–51] may be responsible in part for the Spleen Ting point data recorded here. Hypothetically, increased CSF pressure secondary to CV4 may increase its drainage into the lymphatics of the head and neck. This could result in pressure changes that activate vessel and lymph node autonomic nerve fiber reflex activity specifically affecting the spleen meridian [51].
As in past studies, our study suggests that there are ANS changes after OCMM on human subjects. Our study is novel in correlating these changes to acupuncture meridians through our use of the AMI. This link could be further established by seeing if acupuncture treatment at Ting or other acupuncture points affects CRI and by utilizing the AMI to correlate these possible CRI changes. As we gain a better understanding of how the underlying mechanisms of OCMM and acupuncture are related, we may gain further understanding of the underlying mechanisms of other osteopathic treatment models—specifically biophysical and bioenergetic [12, 52], [53], [54]. Further work objectively measuring the effects of osteopathic treatments in acupuncture meridians may lead to insights into how to alter OCMM and other osteopathic treatments to achieve many of the therapeutic effects obtained through acupuncture.
Limitations
Limitations include not knowing subjects’ medical histories; thus, the effect of underlying medical conditions on our results is unknown. However, all subjects were from the community, thus there were no participants who were having acute illnesses requiring urgent or emergent care at the time of their participation.
Although we attempted to minimize the possible effects of the sham treatment, our specific sham model has not been validated. In the future, we plan to attempt to validate it by comparison to other plausible sham models, such as having an individual without NMM training place their hands under the head. Importantly, statistical analyses were performed before-sham values and after-sham values, and then separately between before-CV4 values and after CV-4 values, rather than between the sham and CV4 values. This limited our ability to make direct comparisons between the sham and CV4. However, it did allow for the assessment of the effect of our sham treatment alongside the assessment of the effect of CV4 treatment.
This may be a true effect of CV4 treatment or a function of our small sample size. However, further studies measuring the effect of OCMM on ANS activity measure at Ting acupoints may help clarify this finding.
Conclusions
CV4 cranial manipulation appears to affect the ANS, indicated by changes in the bioelectrical activity at corresponding Ting points as measured by the AMI, whereas the sham treatments did not yield significant differences in bioelectrical activity at the Ting points. This information suggests that mechanisms underlying cranial manipulation techniques may be related to those underlying acupuncture, and that OCMM may be useful in the treatment of many ANS imbalances, improving patient well-being with a holistic approach.
Funding source: Lake Erie College of Osteopathic Medicine, Erie, PA
Award Identifier / Grant number: Grants by John M. Ferretti, DO (LECOM-Health)
Acknowledgments
Measurement of bioelectric activity of acupuncture meridians by the Apparatus for Meridian Identification (AMI) and subsequent data analysis were provided by biophysicist Gaétan Chevalier, PhD, Psy-Tek Labs, Encinitas, CA. The authors also thank him for reviewing our manuscript. The AMI device, photographs of the AMI, and measurement of Ting points were graciously provided by the California Institute for Human Science, Encinitas, CA. The authors would like to thank Diana Speelman, PhD, Director of Research, Lake Erie College of Osteopathic Medicine, Erie, PA, for providing helpful guidance and reviewing our manuscript. The authors would like to thank Kevin Falk, DO, Lake Erie College of Osteopathic Medicine, Erie, PA, for serving as our demonstration model for both still photographs and our videos of CV4 and Sham treatments.
-
Research funding: Funding and support for this research was generously provided through internal institutional grants by John M. Ferretti, DO (LECOM-Health) and the Lake Erie College of Osteopathic Medicine, Erie, PA.
-
Author contributions: J.T.H, A.K, and K.F. provided substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; all authors drafted the article or revised it critically for important intellectual content; all authors gave final approval of the version of the article to be published; and all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
-
Competing interests: None reported.
-
Informed consent: All participants were provided written and electronic informed consent prior to participation.
-
Ethical approval: The study was approved by the Institutional Review Board at Lake Erie College of Osteopathic Medicine (protocol #24–177); ClinicalTrials registry number: NCT05190731.
References
1. King, HH. Osteopathic cranial manipulative medicine. In: Seffinger, MA, editor. Foundations of osteopathic medicine, 4th ed. Philadelphia, PA: Wolters-Kluwer; 2018:885–905 pp.Suche in Google Scholar
2. Feely, RA, editor. Clinical cranial osteopathy: selected readings. Indianapolis, IN: The Cranial Academy; 1988.Suche in Google Scholar
3. Jäkel, A, von Hauenschild, P. Therapeutic effects of cranial osteopathic manipulative medicine: a systematic review. J Am Osteopath Assoc 2011;111:685–93. https://doi.org/10.7556/jaoa.2011.111.12.685.Suche in Google Scholar
4. Zurowska, A, Malak, R, Kolca-Trzesicka, A, Samborski, W, Paprocka-Borowitz, M. Compression of the fourth ventricle using craniosacral osteopathic technique: a systematic review of the clinical evidence. Evidence-Based Complem Alt Med 2017;2017:2974962. https://doi.org/10.1155/2017/2974962.Suche in Google Scholar PubMed PubMed Central
5. Rechberger, V, Biberschick, M, Porthun, J. Effectiveness of an osteopathic treatment on the autonomic nervous system: a systematic review of the literature. Eur J Med Res 2019;24:36. https://doi.org/10.1186/s40001-019-0394-5.Suche in Google Scholar PubMed PubMed Central
6. Magoun, HI. Osteopathy in the cranial field, 3rd ed. Kirksville, MO: The Journal Printing Company; 1976, vol 23–42:110–4 pp.Suche in Google Scholar
7. Sutherland, WG. Chapter 14. Clinical experience in the practice of osteopathy. In: Wales, AE, editor. Teachings in the science of osteopathy. Fort Worth, TX: Sutherland Cranial Teaching Foundation; 1990:166–89 pp.Suche in Google Scholar
8. Glonek, T, Sergueef, N, Nelson, KE. Inherent motion, rhythms and oscillations. In: Seffinger, MA, editor. Foundations of osteopathic medicine, 4th ed. Philadelphia, PA: Wolters-Kluwer 2018:150–66 pp.Suche in Google Scholar
9. Terem, I, Dang, L, Champagne, A, Abderezaei, J, Pionteck, A, Almadan, Z, et al.. 3D amplified MRI (aMRI). Magn Reson Med 2021;86:1674–86. https://doi.org/10.1002/mrm.28797.Suche in Google Scholar PubMed PubMed Central
10. Abderezaei, J, Pionteck, A, Terem, I, Dang, L, Scadeng, M, Morgenstern, P, et al.. Development, calibration, and testing of 3D amplified MRI (aMRI) for the quantification of intrinsic brain motion. Brain Multiphysics 2021;2:1–10. https://doi.org/10.1016/j.brain.2021.100022.Suche in Google Scholar
11. Becker, RE. Diagnostic touch: its principles and applications. Part IV: trauma and stress. In: Feely, RA, editor. Clinical cranial osteopathy: selected readings. Indianapolis: The Cranial Academy; 1988:57–66 pp.Suche in Google Scholar
12. Hendryx, JT. Dynamic strain. In: Seffinger, MA, editor. Foundations of osteopathic medicine, 4th ed. Philadelphia, PA: Wolters-Kluwer: 2018:981–90 pp.Suche in Google Scholar
13. Zullow, M, Reisman, S. Measurement of autonomic function during craniosacral manipulation using heart rate variability. In: Proceed IEEE 23rd NE bioeng conf. Durham, NH; 1997:83–4 pp.10.1109/NEBC.1997.594968Suche in Google Scholar
14. Arienti, C, Farinola, F, Ratti, S, Dacco, S, Fasulo, L. Variations of HRV and skin conductance reveal the influence of CV4 and rib raising techniques on autonomic balance: a randomized controlled clinical trial. J Bodyw Mov Ther 2020;24:395–401. https://doi.org/10.1016/j.jbmt.2020.07.00.Suche in Google Scholar
15. Abenavoli, F, Badi, M, Bianchi, G, Biglione, G, Dealessa, C, Grandini, M, et al.. Cranial osteopathic treatment and stress-related effects on autonomic nervous system measured by salivary markers: a pilot study. J Bodyw Mov Ther 2020;24:215–21. https://doi.org/10.1016/j.jbmt.2020.07.017.Suche in Google Scholar PubMed
16. Curi, ACC, Maior Alves, AS, Silva, JG. Cardiac autonomic response after cranial technique of the fourth ventricle (cv4) compression in systemic hypertensive subjects. J Bodyw Mov Ther 2018;22:666–72. https://doi.org/10.1016/j.jbmt.2017.11.013.Suche in Google Scholar PubMed
17. Cutler, MJ, Holland, BS, Stupski, BA, Gamber, RG, Smith, ML. Cranial manipulation can alter sleep latency and sympathetic nerve activity in humans: a pilot study. J Altern Complement Med 2005;11:103–8. https://doi.org/10.1089/acm.2005.11.103.Suche in Google Scholar PubMed
18. Miana, L, do Hugo Vale Bastos, V, Machado, S, Arrias-Carrion, O, Nardi, AE, Almeida, L, et al.. Changes in alpha band activity associated with application of the compression of fourth ventricular (CV-4) osteopathic procedure: a qEEG pilot study. J Bodyw Mov Ther 2013;17:291–6. https://doi.org/10.1016/j.jbmt.2012.10.002.Suche in Google Scholar PubMed
19. Stager, WH. Acupuncture and the osteopathic family physician. Osteopathic Fam Phys 2009;1:84–7. https://doi.org/10.1016/j.osfp.2009.08.005.Suche in Google Scholar
20. Stager, WH. Osteopathic manipulative medicine and acupuncture combined: a retrospective case study to determine if order of treatment makes a difference in outcome for acute mechanical low back pain. AAOJ 2007;17:11–21.Suche in Google Scholar
21. Robinson, NG. Interactive medical acupuncture anatomy, Cann, CC, editor. Jackson, WY: Teton NewMedia; 2016.10.1201/9780429154652Suche in Google Scholar
22. Cabioglu, MT, Surucu, HS. Acupuncture and neurophysiology. Med Acupunct 2009;21:13–20. https://doi.org/10.1089/acu.2009.0638.Suche in Google Scholar
23. Uchida, C, Waki, H, Minakawa, H, Tamei, H, Hisajima, T, Imai, K. Effects of acupuncture sensations on transient heart rate reduction and autonomic nervous system function during acupuncture stimulation. Med Acupunct 2019;31:176–84. https://doi.org/10.1089/acu.2019.1350.Suche in Google Scholar PubMed PubMed Central
24. He, W, Sheng, Z-M, Wang, L, Gaischek, I, Litscher, G. Modulation of autonomic nervous system during and after acupuncture treatment of lumbosacral pain in women: a preliminary clinical observational study. Med Acupunct 2013;25:209–15. https://doi.org/10.1089/acu.2012.0884.Suche in Google Scholar
25. Haker, E, Egekvist, H, Bjerring, P. Effect of sensory stimulation (acupuncture) on sympathetic and parasympathetic activities in healthy subjects. J Auton Nerv Syst 2000;79:52–9. https://doi.org/10.1016/s0165-1838(99)00090-9.Suche in Google Scholar PubMed
26. Helms, JM. Acupuncture energetics. In: A clinical approach for physicians. Berkeley, CA: Medical Acupuncture Publishers; 1995, vol 19–70:91 p.Suche in Google Scholar
27. Stux, G, Pomerantz, B. Acupuncture: textbook and atlas. Berlin: Springer-Verlag; 1987:1–26 pp.10.1007/978-3-642-71742-0Suche in Google Scholar
28. Li, QQ, Shi, GX, Xu, Q, Wang, J, Liu, CZ, Wang, LP. Acupuncture effect and central autonomic regulation. Evid Based Complement Alternat Med 2013;2013:267959. https://doi.org/10.1155/2013/267959.Suche in Google Scholar PubMed PubMed Central
29. Teitelbaum, DE. Osteopathic vertebral manipulation and acupuncture treatment using front Mu and back Shu points. Med Acupunct 2000/2001;12:36–7.Suche in Google Scholar
30. Longhurst, J. Acupuncture’s cardiovascular actions: a mechanistic perspective. Med Acupunct 2013;25:141–8. https://doi.org/10.1089/acu.2013.0960.Suche in Google Scholar PubMed PubMed Central
31. Fang, L, He, T, Xu, Q, Lin, LT, Hui, L, Liu, Y, et al.. What is the acupoint? A preliminary review of acupoints. Pain Med 2015;16:1905–15. https://doi.org/10.1111/pme.12761.Suche in Google Scholar PubMed
32. Motoyama, H. Measurements of ki energy: diagnoses & treatments. Tokyo, Japan: Human Science Press; 1997.Suche in Google Scholar
33. Motoyama, H. Acupuncture meridians. Sci Med 1999;6:48–53.Suche in Google Scholar
34. Matos, LC, Lopes, LT, Freire, VA, Machado, JP, Monteiro, FJ, Greten, HJ. Can the electrical potential of acupoints be used to assess the functional state of meridians and the effects of therapeutics? An exploratory data analysis. J Bodyw Mov Ther 2021;26:309–17. https://doi.org/10.1016/j.jbmt.2020.12.038.Suche in Google Scholar PubMed
35. Ahn, AC, Colbert, AP, Anderson, BJ, Martinsen, ØG, Hammerschlag, R, Cina, S, et al.. Electrical properties of acupuncture points and meridians: a systematic review. Bioelectromagnetics 2008;29:245–56. https://doi.org/10.1002/bem.20403.Suche in Google Scholar PubMed
36. Ahn, AC, Martinsen, ØG. Electrical characterization of acupuncture points: technical issues and challenges. J Altern Complement Med 2007;13:817–24. https://doi.org/10.1089/acm.2007.7193.Suche in Google Scholar PubMed PubMed Central
37. Colbert, AP, Hammerschlag, R, Aickin, M, McNames, J. Reliability of the Prognos electrodermal device for measurements of electrical skin resistance at acupuncture points. J Altern Complement Med 2004;10:610–6. https://doi.org/10.1089/acm.2004.10.610.Suche in Google Scholar PubMed
38. Jessel-Kenyon, J, Pfeiffer, L, Brenton, M. A statistical comparison of repeatability in three commonly used bioelectronic devices: kirlian photography, the segmental electrogram, and the AMI of Motoyama. Acupunct Med 1998;16:40–2. https://doi.org/10.1136/aim.16.1.40.Suche in Google Scholar
39. Muehsam, D, Chevalier, G, Barsotti, T, Gurfein, BT. An overview of biofield devices. Global Adv Health Med 2015;4:42–51. https://doi.org/10.7453/gahmj.2015.022.suppl.Suche in Google Scholar PubMed PubMed Central
40. Comunetti, A, Laage, S, Schiessl, N, Kistler, A. Characterization of human skin conductance at acupuncture points. Experientia 1995;51:328–31. https://doi.org/10.1007/bf01928888.Suche in Google Scholar PubMed
41. Reichmanis, M, Marino, AA, Becker, RO. Electrical correlates of acupuncture points. IEEE Trans Biomed Eng 1975;22:533–5. https://doi.org/10.1109/tbme.1975.324477.Suche in Google Scholar PubMed
42. Ahn, AC, Wu, J, Badger, GJ, Hammerschlag, R, Langevin, HM. Electrical impedance along connective tissue planes associated with acupuncture meridians. BMC Compl Alternative Med 2005;5:10. https://doi.org/10.1186/1472-6882-5-10.Suche in Google Scholar PubMed PubMed Central
43. Kramer, S, Winterhalter, K, Schober, G, Becker, U, Wiegele, B, Kutz, DF, et al.. Characteristics of electrical skin resistance at acupuncture points in healthy humans. J Altern Complement Med 2009;15:495–500. https://doi.org/10.1089/acm.2008.0331.Suche in Google Scholar PubMed
44. Tobey, H, Lucas, T, Bledsoe, D, Mykins, M, Campbell, C, Berr, S, et al.. Effect of osteopathic cranial manipulative medicine on an aged rat model of Alzheimer Disease. J Am Osteopath Assoc 2019;119:712–22. https://doi.org/10.7556/jaoa.2019.121.Suche in Google Scholar PubMed PubMed Central
45. Anandakrishnan, R, Tobey, H, Nguyen, S, Sandoval, O, Klein, BG, Costa, BM. Cranial manipulation affects cholinergic expression in aged rats. J Osteopath Med 2022;122:95–103. https://doi.org/10.1515/jom-2021-0183.Suche in Google Scholar PubMed
46. Cankar, K, Finderle, Ž. Gender differences in cutaneous vascular and autonomic nervous response to local cooling. Clin Auton Res 2003;13:214–20. https://doi.org/10.1007/s10286-003-0095-5.Suche in Google Scholar PubMed
47. Convertino, VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 1998;275:R1909–20. https://doi.org/10.1152/ajpregu.1998.275.6.r1909.Suche in Google Scholar
48. Helms, JM. Acupuncture energetics. In: A clinical approach for physicians. Berkeley, CA: Medical Acupuncture Publishers; 1995:198 p.Suche in Google Scholar
49. Hitscherich, K, Smith, K, Cuoco, JA, Ruvolo, KE, Mancini, JD, Leheste, JR, et al.. The glymphatic-lymphatic continuum: opportunities for osteopathic manipulative medicine. JAOA 2016;116:170–7. https://doi.org/10.7556/jaoa.2016.033.Suche in Google Scholar PubMed
50. Plog, BA, Nedergaard, M. The glymphatic system in CNS health and disease: past, present and future. Annu Rev Pathol 2018;13:379–94. https://doi.org/10.1146/annurev-pathol-051217-111018.Suche in Google Scholar PubMed PubMed Central
51. Bachmann, SB, Gsponer, D, Montoya-Zegarra, JA, Schneider, M, Scholkmann, F, Tacconi, C, et al.. A distinct role of the autonomic nervous system in modulating the function of lymphatic vessels under physiological and tumor-draining conditions. Cell Rep 2019;27:3305–14. https://doi.org/10.1016/j.celrep.2019.05.05.Suche in Google Scholar
52. Hendryx, JT. The bioenergetic model in osteopathic diagnosis and treatment: an FAAO thesis, part 1. AAOJ 2014;24:12–20.Suche in Google Scholar
53. Hendryx, JT. The bioenergetic model in osteopathic diagnosis and treatment: an FAAO thesis, part 2. AAOJ 2014;24:10–20.Suche in Google Scholar
54. Hendryx, JT, O’Brien, RL. Dynamic strain-vector release: an energetic approach to OMT. AAOJ 2003;10:19–29.Suche in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Behavioral Health
- Original Article
- Adverse childhood experience categories and subjective cognitive decline in adulthood: an analysis of the Behavioral Risk Factor Surveillance System
- General
- Original Article
- Understanding and preference toward DOs and OMT before and after an osteopathic principles and practice fellow lecture series
- Musculoskeletal Medicine and Pain
- Original Article
- Revisiting chronic low back pain: evidence that it is not non-specific
- Neuromusculoskeletal Medicine (OMT)
- Original Article
- Connecting the dots: alterations in bioelectric activity at acupuncture Ting (Jing-Well) points following CV4 cranial manipulation
- Pediatrics
- Brief Report
- Evaluation of academic detailing to educate clinicians regarding childhood lead poisoning prevention: a pilot study
- Public Health and Primary Care
- Original Article
- Effects of face masks on oxygen saturation at graded exercise intensities
- Clinical Image
- Annular bullous lesions in a child from Uganda: chronic bullous disease of childhood
Artikel in diesem Heft
- Frontmatter
- Behavioral Health
- Original Article
- Adverse childhood experience categories and subjective cognitive decline in adulthood: an analysis of the Behavioral Risk Factor Surveillance System
- General
- Original Article
- Understanding and preference toward DOs and OMT before and after an osteopathic principles and practice fellow lecture series
- Musculoskeletal Medicine and Pain
- Original Article
- Revisiting chronic low back pain: evidence that it is not non-specific
- Neuromusculoskeletal Medicine (OMT)
- Original Article
- Connecting the dots: alterations in bioelectric activity at acupuncture Ting (Jing-Well) points following CV4 cranial manipulation
- Pediatrics
- Brief Report
- Evaluation of academic detailing to educate clinicians regarding childhood lead poisoning prevention: a pilot study
- Public Health and Primary Care
- Original Article
- Effects of face masks on oxygen saturation at graded exercise intensities
- Clinical Image
- Annular bullous lesions in a child from Uganda: chronic bullous disease of childhood

