Computed tomography donor liver volumetry before liver transplantation in infants ≤10 kg: does the estimated graft diameter affect the outcome?
-
Nagoud Schukfeh
, Maren Schulze
Abstract
Aim of the study
Living donor liver transplantation (LDLT) is regularly performed in small-sized infants. Computed tomography (CT)-based donor liver volumetry is used to estimate the graft size. The aim of our study was to assess the results of CT liver volumetry and their impact on the clinical outcome after LDLT in extremely small-sized infants.
Patients and methods
In this study, we included all patients with a body weight of ≤10 kg who underwent living related liver transplantation at our centre between January 2004 and December 2014. In all cases of LDLT, a preoperative CT scan of the donor liver was performed, and the total liver and graft volumes were calculated. The graft shape was estimated by measuring the ventro-dorsal (thickness), cranio-caudal, and transversal (width) diameter of segment II/III. We assessed the impact of CT donor liver volumetry and other risk factors on the outcome, defined as patient and graft survival.
Results
In the study period, a total of 48 living related liver transplantations were performed at our centre in infants ≤10 kg [20 male (42%), 28 female (58%)]. The mean weight was 7.3 kg (range 4.4–10 kg). Among the recipients, 33 (69%) received primary abdominal closure and 15 (31%) had temporary abdominal closure. The patient and graft survival rates were 85% and 81%, respectively. In CT volumetry, the mean estimated graft volume was 255 mL (range 140–485 mL) and the actual measured mean graft weight was 307 g (range 127–463 g). The mean ventro-dorsal diameter of segment II/III was 6.9 cm (range 4.3–11.2 cm), the mean cranio-caudal diameter was 9 cm (range 5–14 cm), and the mean width was 10.5 cm (range 6–14.7 cm). The mean graft-body weight ratio (GBWR) was 4.38% (range 1.41–8.04%). A high graft weight, a GBWR >4%, and a large ventro-dorsal diameter of segment II/III were risk factors for poorer patient survival.
Conclusion
Preoperative assessment of the graft size is a crucial investigation before LDLT. For extremely small-sized recipients, not only the graft weight but also the graft shape seems to affect the outcome.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, computed tomography; GBWR, graft-body weight ratio; INR, international normalized ratio; LDLT, living donor liver transplantation; LLS, left lateral segment; LT, liver transplantation; MELD, model for end-stage liver disease; γGT, gamma-glutamyl-transpeptidase.
Introduction
Liver transplantation (LT) has become an established treatment for children with end-stage liver disease. Among the group of very small liver recipients, the most frequent disease leading to LT is biliary atresia, followed by several other chronic liver diseases [1], [2]. This special group of patients often requires transplantation before reaching a body weight of 10 kg. Therefore, performing LT in these patients remains a surgical challenge due to vascular complications, hypercoagulation, and, most important, size mismatching. Precise surgical planning including preoperative hepatic volumetry is required to avoid large-for-size grafts resulting in increased intra-abdominal pressure.
In addition, the organ supply from a deceased donor for infant recipients is much smaller than the actual demand, which makes it difficult to find adequate organs matching the small size of these patients and tightens the major problem of massive organ shortage in LT [1]. Besides new approaches like using ABO-incompatible or split grafts [3], [4], the most important possibility to overcome the organ shortage for infants and to avoid mortality while on the waiting list remains living donor LT (LDLT). In order to receive grafts that better match the size of small recipients, Kitajima et al. [5] recently reported on LDLT using reduced-thickness left lateral segment (LLS) grafts.
In order to assess the expected graft size, computed tomography (CT)-based volumetry of the donor liver has become an established examination prior to LDLT. Thus far, there are no reports investigating the clinical impact of the results of CT liver volumetry before LDLT in small-sized infants. As it is important to know aspects of volumetry that actually influence the course and the outcome of LDLT, in the present study, we investigated which parameters of pretransplant liver volumetry affect the outcome in extremely small recipients. The primary endpoint of our study was the outcome after LDLT; the secondary endpoints were identifying risk factors that influence the outcome after LDLT.
Materials and methods
The study design was reviewed and approved by the Local Research Ethical Committee (no. 17-7412-BO). In this study, we included all paediatric patients with a body weight ≤10 kg undergoing LDLT between January 2004 and December 2014 at one university transplant centre. The medical records of all recipients and donors were analysed retrospectively. Two patients were excluded from the study: one patient died during the operation, whereas another patient needed to be left anhepatic after the first LT and received a second LT on the same day.
The primary endpoint was the outcome, characterised by patient and graft survival. We focussed on those patients who received LDLT and evaluated the postoperative clinical course by identifying risk factors leading to a poorer outcome.
CT liver volumetry
In cases, the preoperative CT scans of the donor were evaluated and the diameter of segment II/III was assessed in all three dimensions: ventro-dorsal (thickness), cranio-caudal (length), and transversal (width) (Figure 1). The total liver volume was calculated after boundaries of the hepatic lobe were drawn manually on consecutive 5-mm-thick axial portal-venous phase CT images (no gap). Volumes were calculated by multiplying the slice thickness with the sum of all traced areas of the respective hepatic lobe. Manual measurement was favoured over automatic measurements, as automatic programs identify the left hepatic vein as the boundary whereas transplant surgeons choose their resection plane slightly moving into segment IV, so that automatic measurements would deliver too small values for the estimated graft volume.
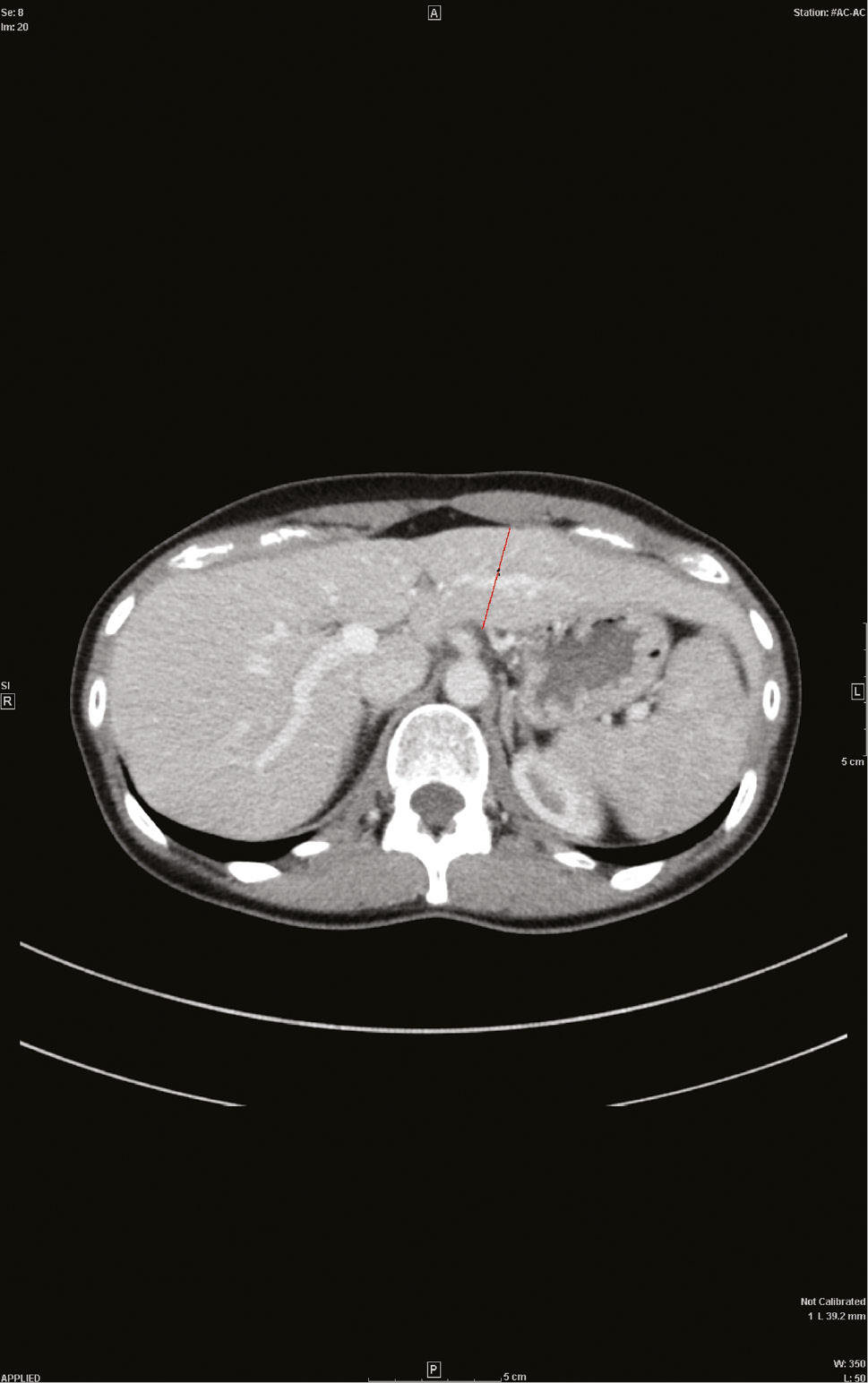
CT of the liver.
Axial portal-venous phase CT image with the ventro-dorsal diameter of a female donor liver.
Correlations between one-dimensional measurements and volumes were analysed.
Surgical technique
The surgical technique for the donor and recipient operation followed previously described principles [1], [6], [7], [8]. All LTs were carried out via an open transverse laparotomy. The graft was anastomosed to the recipients’ inferior vena cava by using the piggy-back technique. Anastomoses of the portal vein and hepatic artery were performed by using the end-to-end technique. The bile flow was maintained by performing a bileo-enteric anastomosis. Immediately after vascular anastomosis, intraoperative duplex ultrasound was performed, and portal venous, hepatic artery, and venous outflow were measured.
Primary abdominal closure was performed when the muscular abdominal wall could be well adapted. If, however, the portal venous or arterial flow was impaired upon approximation and the portal venous flow was <10 mL/min, the abdomen was left open and a silicon foil was inserted, as described before [9]. Therefore, duplex ultrasound was repeatedly performed before and after abdominal wall closure. The decision for temporary abdominal closure was also made according to the surgeons’ evaluation in case of limited intra-abdominal space when the muscular abdominal wall could not be adapted after the surgery. Heparin (50 IE/kg) was administered postoperatively for a minimum of 7 days in uncomplicated cases and then replaced by acetylsalicylic acid (50 mg 3×/week).
Statistical analysis
Statistical analysis was performed using Fisher’s exact test and unpaired Student’s t-test with significance assumed at p<0.05. The correlation between two variables was determined by calculating the Pearson product-moment correlation coefficient. Laboratory data of donors and recipients as well as demographic data are given as mean and range.
Results
LDLT: recipient and donor characteristics
In the study period, 48 infants with a body weight of ≤10 kg [20 male (42%), 28 female (58%)] underwent LDLT in a single university transplant centre. The mean body weight of the 48 paediatric recipients was 7.3 kg (range 4.4–10 kg). The underlying diseases for the LDLT were extrahepatic biliary atresia in 38 cases; hepatoblastoma in three cases; progressive familial intrahepatic cholestasis type 2 in two cases; primary hyperoxaluria in two cases; and Alagille syndrome, toxic liver failure, and cholestatic liver disease of unknown origin in one case each. These patients spent a mean time of 40 days (range 1–345 days) on the waiting list for LT. The mean model for end-stage liver disease score was 15 (range 6–35), and the mean paediatric end-stage liver disease score was 28 (range 22–40). In three cases (6.3%), an ABO-incompatible LDLT was performed.
One donor for LDLT was the grandmother, whereas all other donors were parents of the recipients [fathers 16 (33%), mothers 31 (65%)], with one mother being an unrelated adoptive mother. The mean age of the donors was 31.8 years (range 22.1–48.3 years), and their mean body mass index was 24.4 (range 18–33.4). The mean graft-body weight ratio (GBWR) was 4.38% (range 1.41–8.04%). The mean donor weight-graft weight ratio was 9.9 (range 5.2–15.3).
LDLT: CT volumetry
In all cases, a CT-based volumetry of the donor liver was performed prior to LDLT. The mean estimated total liver volume of the donor was 1579 cm3 (range 865–3060 cm3). As we regularly use the LLS for LDLT, in all cases, liver segment II/III of the donor was measured to estimate the graft volume and diameters. The mean ventro-dorsal diameter of segment II/III was 6.9 cm (range 4.3–11.2 cm); the mean cranio-caudal diameter was 9 cm (range 5–14 cm). The mean transversal diameter, describing the width of the graft, was 10.5 cm (range 6–14.7 cm). The grafts from male donors had a significantly larger thickness (ventro-dorsal diameter, p<0.005) than those from female donors.
The mean estimated graft volume was 255 mL (range 140–485 mL). There was no difference between male and female donors concerning the estimated graft weight. The actual measured mean graft weight at transplantation was 307 g (range 127–463 g). There was no significant difference between the estimated graft volume and the actual measured graft weight. However, the ratio describing the accuracy of the estimated graft volume by CT volumetry (mean estimated graft volume/actual graft weight) was 85% (range 48–168%), indicating that CT volumetry estimated a graft volume that was lower than the actual measured graft weight.
By analysing correlations between one-dimensional measurement, we found the following formulas for estimating the graft volume:
Male donors: graft volume (mL)=83.1+2.2 ∗ ventro-dorsal diameter (deviation±23% (10/90-quantile: −22.8%/18.1%)
Female donors: graft volume (mL)=116.76+0.23 ∗ cranio-caudal diameter ∗ ventro-dorsal diameter [deviation±50/61% (10/90-quantile: −39%/26%].
A formula for the accurate estimation of the graft volume including all three dimensions of segment II/III (ventro-dorsal, cranio-caudal, and transversal) could not be identified.
LDLT: outcome
The mean operation duration for LDLT was 426 min (range 281–1273 min). The mean cold ischaemic time was 92 min (range 6–298 min), whereas the mean warm ischemic time was 45 min (range 15–123 min). After LDLT, 33 recipients (69%) received primary abdominal closure and 15 (31%) received temporary abdominal closure. The serum activities of liver enzymes, bilirubin, and coagulation parameters before and 6 months after transplantation are shown in Table 1. Surgical complications included pleural effusion (n=21), hepatic artery thrombosis (n=12), portal vein thrombosis (n=9), biliary leakage (n=7), chylascites (n=7), biliary stenosis (n=6), intra-abdominal bleeding (n=5), and gastrointestinal bleeding (n=4). After LDLT, the patient and graft survival rates were 85% and 81%, respectively.
Serum levels of different liver markers preoperatively and 6 months after LT.
| Mean (range) | ||
|---|---|---|
| Preoperatively | 6 Months post-LT | |
| Bilirubin (mg/dL) | ||
| Whole cohort | 12.4 (0.1–44) | 2.1 (0.1–34.3) |
| LDLT | 14 (0.2–44) | 1.4 (0.1–21.1) |
| Prothrombin time (quick%) | ||
| Whole cohort | 61 (10–120) | 83 (26–114) |
| LDLT | 64 (16–120) | 85 (28–114) |
| PTT (s) | ||
| Whole cohort | 55 (21–170) | 38 (21–160) |
| LDLT | 56 (23–160) | 40 (21–160) |
| INR | ||
| Whole cohort | 1.57 (0.86–4.78) | 1.07 (0.95–1.33) |
| LDLT | 1.45 (0.86–3.81) | 1.06 (0.95–1.33) |
| γGT (U/L) | ||
| Whole cohort | 171 (10–1829) | 113 (4–2207) |
| LDLT | 162 (10–1829) | 102 (7–2207) |
| ALT (U/L) | ||
| Whole cohort | 290 (15–4946) | 185 (9–2894) |
| LDLT | 166 (23–1874) | 143 (13–2109) |
| AST (U/L) | ||
| Whole cohort | 475 (26–8197) | 529 (20–14253) |
| LDLT | 230 (26–811) | 239 (20–5132) |
LT, Liver transplantation; LDLT, living donor liver transplantation; PTT, partial thromboplastin time; INR, international normalised ratio; γGT, gamma-glutamyl-transpeptidase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Overall, there were five cases of graft loss secondary to vascular complications. The graft loss was caused by hepatic arterial thrombosis in four cases and by hepatic vein thrombosis in one case. In two of these five cases, there was a large-for-size situation with a GBWR >4%. The other three cases had a GBWR <4%, and none had a GBWR >6%.
LDLT: risk factors
In statistical analysis, we identified several clinical and biochemical parameters as risk factors for a poorer patient or graft survival in the group who received LDLT (Table 2). The characteristics of the graft affecting patient survival were a high graft weight, a GBWR of >4%, and a large ventro-dorsal diameter of segment II/III (graft thickness), measured using CT volumetry (Figure 2). A GBWR of >4% affected both patient survival and graft survival. There was no correlation between any other parameter concerning the donor liver measured in CT volumetry and the outcome. However, the occurrence of vascular thrombosis, including hepatic artery and portal vein thrombosis, was identified in multivariable analysis as an independent risk factor for both poorer patient survival and graft survival. There was no correlation between the occurrence of thrombosis and any of the estimated graft diameters.
Risk factors for poorer patient and graft survival (univariate analysis).
| Risk factor | Patient survival (p-value) | Graft survival (p-value) |
|---|---|---|
| Vascular thrombosis | Yes (0.007)a | Yes (0.017)a |
| MELD | Yes (0.05)a | – |
| Graft weight | Yes (0.006)a | – |
| GBWR >4% | Yes (0.03) | Yes (0.04)a |
| Recipient age at LT | Yes (0.08) | – |
| Donor age | – | Yes (0.04) |
| AP diameter of segment II/III | Yes (0.045) | – |
| Temporary abdominal closure | Yes (0.04) | – |
| Days at waiting list | Yes (0.05) | Yes (0.06) |
MELD, Model for end-stage liver disease; GBWR, graft-body weight ratio; LT, liver transplantation; AP, anteroposterior. aIndependent risk factor (multivariable analysis).
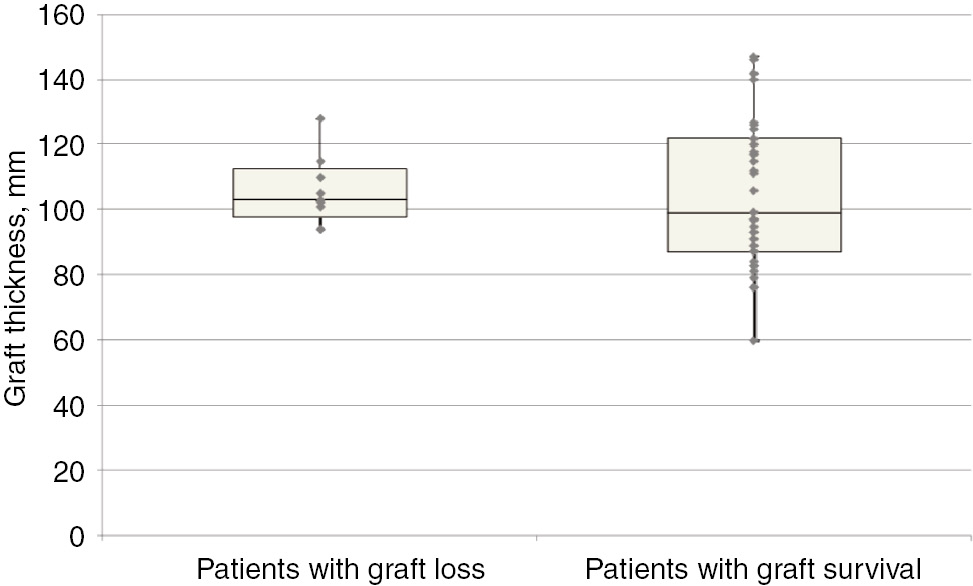
Boxplot showing the correlation between graft thickness and graft survival.
Left box: Patients with graft loss (n=9). Graft loss median graft thickness: 103 mm. Graft loss 25 quartile: 98 mm. Graft loss 75 quartile: 113 mm. Right box: Patients with graft survival (n=39). Graft survival median graft thickness: 99 mm. Graft survival 25 quartile: 87 mm. Graft survival 75 quartile: 122 mm.
Discussion
Preoperative imaging of the liver donor with estimation of the graft size has become a crucial investigation prior to LT. According to Lim et al. [10], imaging-based volumetry of the liver is vital in the preoperative planning for LT, as the liver volume is one key factor in the selection of the appropriate individual for LDLT. Especially in LDLT, precise assessment of the donor liver volume is crucial in determining whether the donor is suitable for LDLT, to ensure safety for both donor and recipient [10]. In addition, preoperative imaging is required to ensure that there is no underlying focal or diffuse liver disease that may make transplantation unsuitable, such as steatosis, cirrhosis, and focal benign or malignant neoplasms.
Currently, the preferred means of imaging-based liver and graft size estimation is CT volumetry, which generally provides good correlation of the estimated volume with the graft weight obtained [10], [11]. Yoon et al. [12] used the actual graft weight as a standard of reference in living liver donors for determination of the accuracy, reproducibility, and improvement in the clinical workflow of a semi-automatic CT virtual surgical planning program in estimating graft volume. The authors reviewed the liver CTs of 105 liver donor candidates, and found a strong correlation between the predicted volumes of the hepatic graft and the actual graft weight. Furthermore, the mean processing time of hepatic volumetry, segmentation, and surgical planning using software was significantly shorter than using manual volumetry.
An actual clinical impact of preoperative imaging before LDLT was first described by Ringe et al. [13]. In a study on the significance of CT in the donor selection process, the authors found that almost one-third of the donor candidates were rejected because of CT findings. The authors concluded that CT can help reduce the risk for donors and recipients by excluding unsuitable donor livers.
However, these studies did not exclusively address liver donors for infants. One critical challenge in extremely small-sized infants receiving LDLT is the increased risk for developing large-for-size syndrome. In the past, several authors described different approaches, trying to avoid a large-for-size situation in small-sized liver recipients: Ogawa et al. [14] used reduced monosegmental grafts for LDLT in nine extremely small-sized infants, and reported a patient and graft survival at 1 year of 66.7%. They concluded that reduced monosegmental LDLT represents a feasible option for neonates and extremely small infants with liver failure.
Another approach to avoid a large-for-size situation was recently published by Yamada et al. [15]. The group reported on their graft selection strategy being based on preoperative CT volumetry for recipients ≤6 kg. The group’s strategy for graft selection among those patients depended on the GBWR, based on the donor preoperative CT volumetry for the LLS graft. When the predictive GBWR of the LLS graft was ≤5%, they selected the LLS as the liver graft. When the predictive GRWR of the LLS graft was >5%, they selected the S2 monosegment as the liver graft. When the actual GRWR was >4%, they performed partial graft hepatectomy ex vivo to reduce the GRWR to ≤4%. The authors concluded that their graft selection strategy based on the preoperative CT volumetry value is highly useful in patients weighing ≤6 kg. They stated that the use of S2 monosegment grafts is effective and safe in very small infants and particularly in neonates. Recently, Kitajima et al. [5] reported on their experience with reducing the thickness of the LLS and stated that LDLT using reduced LLS is a feasible option with better outcomes when compared with non-reduced grafts.
In contrast to these studies, the study by Schulze et al. [3] showed that using exclusively left lateral grafts from living donors or split grafts for extremely small paediatric liver recipients leads to an excellent outcome without the need for reduction of the grafts or the use of monosegments. The group used temporary abdominal closure in a high proportion of their patients, in 28% of the whole cohort and in 39% of the large-for-size group, and stated that the use of a patch is one possibility to avoid large-for-size syndrome. However, recently, our group found that LDLT is possible without reduction and without the necessity of a patch in some cases of a high GBWR [9]. According to this strategy, our group performed all LDLTs using the LLS without any form of a further graft reduction.
Another key factor in avoiding a large-for-size situation in extremely small-sized liver recipients is CT-based graft size estimation, which is a precise estimation of the graft size. Thus far, there have been no reports on the outcome after LDLT depending on the results of preoperative CT liver volumetry. As this investigation has become a standard procedure, it is important to know which aspects of volumetry actually have clinical relevance, and whether they influence the course and the outcome after LDLT.
In the present study, we provide, for the first time, different diameters of the estimated graft size for recipients ≤10 kg, combining them with clinical data and their impact on the outcome. We found that not only the graft weight but also the estimated thickness of the graft, characterised by the ventro-dorsal diameter of segment II/III, significantly influenced patient survival, whereas the total volume of the donor liver and the volume of the graft had no influence. This goes along with the findings of Schulze et al., who noted that the ventro-dorsal diameter of the graft appeared to be more relevant to potential graft necrosis than the actual graft size [3]. One possible explanation could be the mismatch between the vessels of the graft and the small-sized ones of the neonatal recipient. The difference in the vessel size might rather be a limiting factor in the transplantation procedure than the actual volume of the graft. This clinically relevant aspect in preoperative CT liver volumetry is new and might be beneficial in finding the appropriate graft for extremely small-sized recipients. However, the present study has limitations. We did not differentiate between biliary atresia patients who often show hypoplastic portal veins and cirrhotic livers and therefore might require different surgical strategies as opposed to patients with non-cirrhotic liver diseases. Also, donor-associated factors such as comorbidities or alcohol consumption could have influenced the outcome. The study is also limited by its retrospective nature, and a further prospective study is needed to verify our results. Should the influence of the segment II/III ventro-dorsal diameter on the outcome be confirmed, possibly a cut-off diameter of segment II/III of the donor liver could be recommended before LDLT in extremely small-sized infants to reduce the risk of large-for-size syndrome. However, it is important to note that graft function and survival are not only influenced by graft size but are also affected by other factors. In our present study, the number of days the recipients spent on the waiting list for transplantation, a GBWR >4%, and the occurrence of vascular thrombosis were risk factors for both poorer patient and graft survival, which agrees with the results of previously published studies on parameters affecting the outcome after LT [16], [17], [18].
Conclusion
Preoperative assessment of the graft size is a crucial investigation before LDLT. We identified larger graft thickness, estimated by measuring the ventro-dorsal diameter of segment II/III in CT donor liver volumetry, as a risk factor for a poorer patient survival. This new aspect might be helpful in finding more grafts for extremely small-sized recipients despite high donor volumes of segment II/III when its configuration is slim.
Author Statement
Research funding: Authors state no funding involved. Conflict of interest: Authors state no conflict of interest. Informed consent: Informed consent is not applicable. Ethical approval: The research related to human use complied with all the relevant national regulations and institutional policies, and was performed in accordance to the tenets of the Helsinki Declaration. The study design was reviewed and approved by the Local Research Ethical Committee (no. 17-7412-BO).
Author Contributions
Nagoud Schukfeh: conceptualization; data curation; writing – original draft. Maren Schulze: conceptualization; data curation. Anna-Charlotte Tabea Holland: data curation; investigation; methodology. Jens Dingemann: writing – review & editing. Dieter P. Hoyer: formal analysis; methodology. Andreas Paul: supervision; writing – review & editing. Jens M. Theysohn: conceptualization; data curation; investigation; project administration; software; supervision; writing – review & editing.
Publication Funding
The German Society of Surgery funded the article processing charges of this article.
References
[1] Broering DC, Kim JS, Mueller T, Fischer L, Ganschow R, Bicak T, et al. One hundred thirty-two consecutive pediatric liver transplants without hospital mortality: lessons learned and outlook for the future. Ann Surg 2004;240:1002–12.10.1097/01.sla.0000146148.01586.72Suche in Google Scholar PubMed PubMed Central
[2] Schukfeh N, Doerner JM, Heintschel von Heinegg E, Steinmann J, Metzelder ML, Kathemann S, et al. Spectrum of pathogens in native liver, bile, and blood during pediatric liver transplantation. Pediatr Transplant 2014;18:266–71.10.1111/petr.12237Suche in Google Scholar PubMed
[3] Schulze M, Dresske B, Deinzer J, Braun F, Kohl M, Schulz-Jürgensen S, et al. Implications for the usage of the left lateral liver graft for infants ≤10 kg, irrespective of a large-for-size situation – are monosegmental grafts redundant? Transpl Int 2011;24:797–804.10.1111/j.1432-2277.2011.01277.xSuche in Google Scholar PubMed
[4] Schukfeh N, Lenz V, Metzelder ML, Paul A, Mathe Z, Kathemann S, et al. First case studies of successful ABO-incompatible living-related liver transplantation in infants in Germany. Eur J Pediatr Surg 2015;25:77–81.10.1055/s-0034-1387936Suche in Google Scholar PubMed
[5] Kitajima T, Sakamoto S, Sasaki K, Narumoto S, Kazemi K, Hirata Y, et al. Impact of graft thickness reduction of left lateral segment on outcomes following pediatric living donor liver transplantation. Am J Transplant 2018 [Epub ahead of print].10.1111/ajt.14875Suche in Google Scholar PubMed
[6] Broelsch CE, Whitington PF, Emond JC, Heffron TG, Thistlethwaite JR, Stevens L, et al. Liver transplantation in children from living related donors: surgical techniques and results. Ann Surg 1991;214:428–37.10.1097/00000658-199110000-00007Suche in Google Scholar PubMed PubMed Central
[7] Broering DC, Sterneck M, Rogiers X. Living donor liver transplantation. J Hepatol 2003;38:119–35.10.1016/S0168-8278(03)00009-6Suche in Google Scholar
[8] Hoyer DP, Klein C, Kathemann S, Paul A, Mathé Z. Left-lateral living related liver donation – the Essen experience. Zentralbl Chir 2013;141:570–6.10.1055/s-0032-1328346Suche in Google Scholar PubMed
[9] Schukfeh N, Holland AC, Hoyer DP, Gallinat A, Paul A, Schulze M. Liver transplantation in infants with biliary atresia: comparison of primary versus temporary abdominal closure. Langenbecks Arch Surg 2017;402:135–41.10.1007/s00423-016-1525-xSuche in Google Scholar PubMed
[10] Lim MC, Tan CH, Cai J, Zheng J, Kow AW. CT volumetry of the liver: where does it stand in clinical practice? Clin Radiol 2014;69:887–95.10.1016/j.crad.2013.12.021Suche in Google Scholar PubMed
[11] Radtke A, Sotiropoulos GC, Nadalin S, Molmenti EP, Schroeder T, Lang H, et al. Preoperative volume prediction in adult living donor liver transplantation: how much can we rely on it? Am J Transplant 2007;7:672–9.10.1111/j.1600-6143.2006.01656.xSuche in Google Scholar PubMed
[12] Yoon JH, Lee JM, Jun JH, Suh KS, Coulon P, Han JK, et al. Feasibility of three-dimensional virtual surgical planning in living liver donors. Abdom Imaging 2015;40:510–20.10.1007/s00261-014-0231-9Suche in Google Scholar PubMed
[13] Ringe KI, Ringe BP, von Falck C, Shin HO, Becker T, Pfister ED, et al. Evaluation of living liver donors using contrast enhanced multidetector CT – the radiologists impact on donor selection. BMC Med Imaging 2012;12:21.10.1186/1471-2342-12-21Suche in Google Scholar PubMed PubMed Central
[14] Ogawa K, Kasahara M, Sakamoto S, Ito T, Taira K, Oike F, et al. Living donor liver transplantation with reduced monosegments for neonates and small infants. Transplantation 2007;83:1337–40.10.1097/01.tp.0000263340.82489.18Suche in Google Scholar PubMed
[15] Yamada N, Sanada Y, Hirata Y, Okada N, Wakiya T, Ihara Y, et al. Selection of living donor liver grafts for patients weighing 6kg or less. Liver Transpl 2015;21:233–8.10.1002/lt.24048Suche in Google Scholar PubMed
[16] Agopian VG, Petrowsky H, Kaldas FM, Zarrinpar A, Farmer DG, Yersiz H, et al. The evolution of liver transplantation during 3 decades: analysis of 5347 consecutive liver transplants at a single center. Ann Surg 2013;258:409–21.10.1097/SLA.0b013e3182a15db4Suche in Google Scholar PubMed
[17] Cacciarelli TV, Esquivel CO, Moore DH, Cox KL, Berquist WE, Concepcion W, et al. Factors affecting survival after orthotopic liver transplant in infants. Transplantation 1997;64:242–8.10.1097/00007890-199707270-00011Suche in Google Scholar PubMed
[18] Iglesias J, López JA, Ortega J, Roqueta J, Asensio M, Margarit C. Liver transplantation in infants weighing under 7 kilograms: management and outcome of PICU. Pediatr Transplant 2004;8:228–32.10.1111/j.1399-3046.2004.00128.xSuche in Google Scholar PubMed
Supplemental Material
The article (https://doi.org/10.1515/iss-2017-0047) offers reviewer assessments as supplementary material.
©2018 Schukfeh N., et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Original Articles
- Young surgeons’ challenges at the start of their clinical residency: a semi-qualitative study
- Intermittent Pringle maneuver may be beneficial for radiofrequency ablations in situations with tumor-vessel proximity
- Computed tomography donor liver volumetry before liver transplantation in infants ≤10 kg: does the estimated graft diameter affect the outcome?
- Reversible biliary occlusion in a small animal model: first description of a new technique
- Short- and long-term outcomes for the surgical treatment of acute pulmonary embolism
- Serum markers for early detection of patients with mesenteric ischemia after cardiac surgery
- Case Report
- Acute heart failure due to giant left atrium: remote ECLS implantation for interhospital transfer and bridging to decision
Artikel in diesem Heft
- Original Articles
- Young surgeons’ challenges at the start of their clinical residency: a semi-qualitative study
- Intermittent Pringle maneuver may be beneficial for radiofrequency ablations in situations with tumor-vessel proximity
- Computed tomography donor liver volumetry before liver transplantation in infants ≤10 kg: does the estimated graft diameter affect the outcome?
- Reversible biliary occlusion in a small animal model: first description of a new technique
- Short- and long-term outcomes for the surgical treatment of acute pulmonary embolism
- Serum markers for early detection of patients with mesenteric ischemia after cardiac surgery
- Case Report
- Acute heart failure due to giant left atrium: remote ECLS implantation for interhospital transfer and bridging to decision

