Abstract
To survey the probability of the re-utilization of nickel containing slag corroded magnesia and alumina-graphite refractories, different amounts of nickel oxide (NiO) were used as one of raw materials to synthesize nickel containing magnesium aluminum oxynitride (MgAlON) composites, and the sintering and oxidation behavior have been explored in this work. The results reveal that with the increasing NiO additive, submicron metallic Ni grains are segregated from the (Ni,Mg)AlON spinel, and these grains are beneficial to improve thermal shock resistance of MgAlON. Though the oxidation reaction of (Ni,Mg)AlON starts at lower temperature than MgAlON, the metallic Ni grains in the plate can improve the oxidation resistance. Hence, the metallic nickel containing (Ni,Mg)AlON composite has not only superior thermal shock resistance but also excellent oxidation resistance, and it is of great potential to utilize in plant. So, the fabrication of metallic nickel containing (Ni,Mg)AlON composite should be a feasible way to reuse the nickel slag corroded magnesia and alumina graphite refractories.
Introduction
Refractories are used by a variety of companies, including metal, ceramic, cement and glass producers to withstand severe service conditions, such as high temperatures, corrosive liquids and gases, abrasion, mechanical, thermal induced stresses, and so on [1]. When refractory materials have reached their service lives, the spent refractories are typically disposed of in a landfill wasting valuable natural resources. Hence, development of novel methods to reuse these materials is of great social and economy potential [1, 2, 3].
Till now, only some of un-corroded spent refractories have been reused as low rank raw materials for refractories, and there are almost no methods to reuse corroded refractories, even there are some useful elements, such as nickel, magnesium, aluminum, and so on. These elements are components of the recently developed high performance refractory, magnesium aluminum oxynitride (MgAlON), and the MgAlON has attracted lots of researchers’ interests for its high mechanical strength at high temperatures, good stability under room temperature, high resistance to slag and liquid metal [4, 5, 6, 7, 8, 9, 10]. Though MgAlON can be easily synthesized from corroded spent refractories with magnesia, alumina, and graphite through carbothermal reduction and nitridation method, its poor oxidation resistance and thermal shock resistance at high temperature are key reasons to restrict the utilization of MgAlON in plant.
Recently, Ye et al. [11] has explored the impurities on the preparation and oxidation behavior of MgAlON, and they propose that impurities have some effect on the synthesis and oxidation behavior of MgAlON. Since the element of nickel exists in the slag for stainless steel manufacture, the nickel element might have some beneficial effect on improving the oxidation resistance of MgAlON. Hence, different amounts of nickel oxide (NiO) were used as one of raw materials, and properties of the synthesized composites have been surveyed in the present work.
Experimental procedure
Fine alumina (Al2O3>99.9 %,<0.5 μm), aluminum nitride (AlN>99.0 %,<1.0 μm), magnesia (MgO>99.0 %,<0.5 μm), and nickel oxide (NiO>99.0 %,<5.0 μm) with high purity was selected as the raw materials in present work. To reveal the effect of NiO on the preparation and oxidation of MgAlON, extra 2 % and 5 % NiO were added, and according to Table 1, all the raw materials were meticulously weighted, and ball-milled. Then, green compacts were formed by uniaxial compressed at 50 MPa, and subsequently the samples were hot-pressed in vacuum atmosphere at 1700°C for 20 min under the pressure of 100 MPa.
Composition of sample (mass%).
| Sample No. | NiO | MgO | Al2O3 | AlN |
|---|---|---|---|---|
| S0 | 0 | 10.00 | 82.50 | 7.50 |
| S1 | 1.96 | 9.80 | 80.89 | 7.35 |
| S2 | 4.76 | 9.52 | 78.58 | 7.14 |
The oxidation experiment of powders and plates was conducted on thermo-gravimeter (TG) and MoSi2 electric furnace under dry air. The non-isothermal tests of powders were warmed up at 5°C min−1, and as to the isothermal oxidation of plates, they were placed into the furnace later after that had arrived the setting temperature for 30 min.
As to the water quench test, the plates were firstly dried at 110°C for 50 min, then they were put into a MoSi2 electric furnace at 1400°C and held for 20 min, and then quenched into water at 20°C. Those for next quench test were dried before returning to the furnace at 1400°C. This procedure was repeated until obvious destruction crack, and the number of quenches was taken as the measure of thermal shock resistance. To obtain an average quench number, more than 10 samples were tested.
Moreover, microstructure and element distribution were characterized by field emission scanning electron microscope (FE-SEM) equipped with energy-dispersive spectroscope (EDS). Phase composition was determined by X-ray diffraction (XRD). The contents of Mg and Al were measured by X-ray fluorescence spectroscope (XRFS), and O, N by oxygen and nitrogen analyzer.
Results
Figure 1 shows the microstructure and EDS result of synthesized sample determined by FE-SEM. It can be seen that the sample without NiO additive was nearly full dense. As to the sample with NiO additive, white grains less than 1 μm were uniformly distributed among gray MgAlON matrix. The EDS analysis shown in Figure 1(d) revealed that the white grains were nearly pure metallic Ni. Moreover, lots of submicron pores laid around Ni grains. The detected chemical composition of the synthesized samples was listed in Table 2. Compared to the chemical composition of raw materials, it was known that the amount of N and Ni in NiO added composites was ambiguously less than that in their original materials.
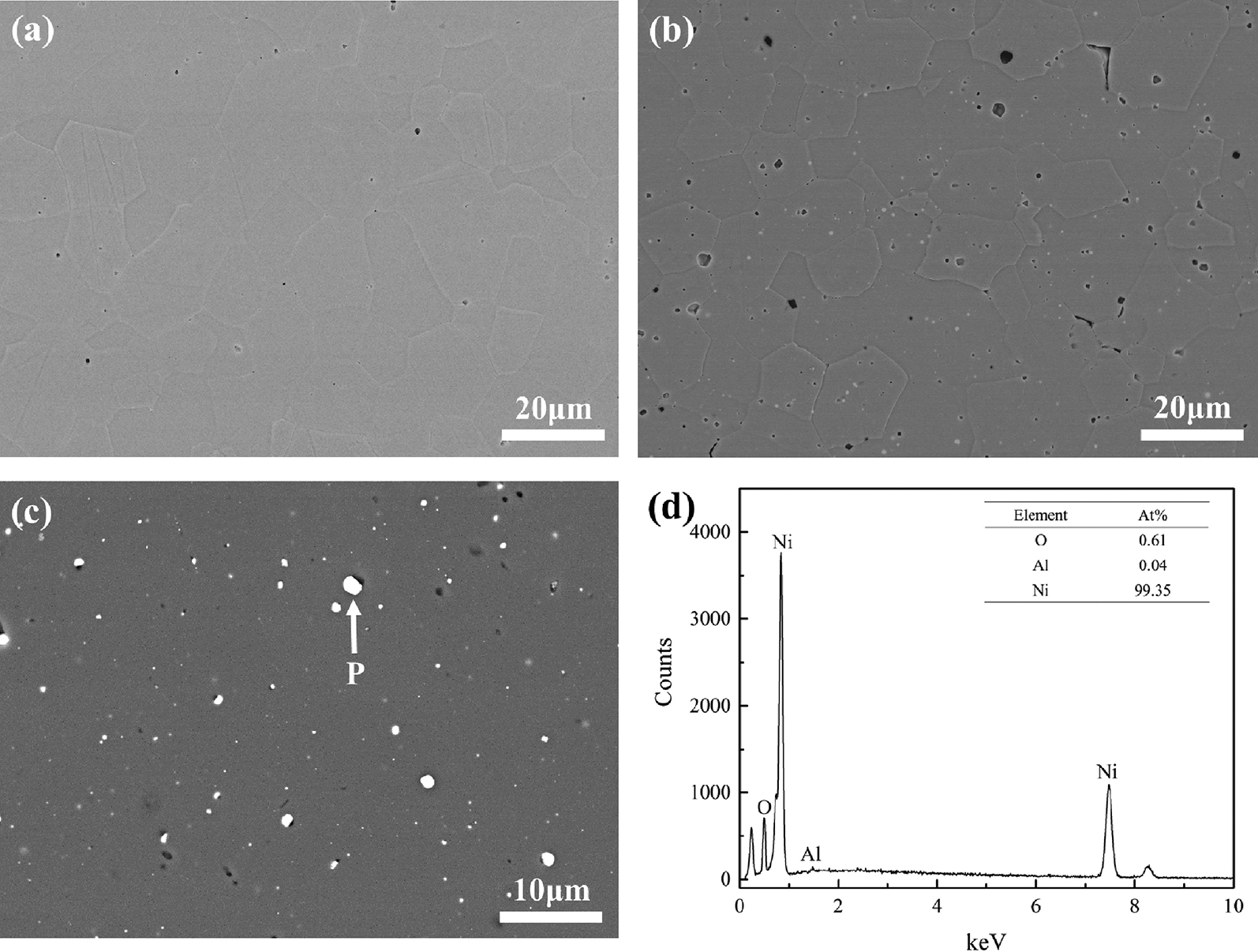
Microstructure of synthesized sample with (a) None, (b) 2 %NiO, and (c) 5 %NiO NiO additive, (d) EDS result of P point in (c).
Chemical composition of sample before and after sintering process (mass%).
| Element | S0 | S1 | S2 | |||||
|---|---|---|---|---|---|---|---|---|
| Original | Sintered | Original | Sintered | Original | Sintered | |||
| Mg | 6.00 | 6.01 | 5.97 | 5.99 | 5.94 | 5.96 | ||
| Al | 48.62 | 48.70 | 48.41 | 48.53 | 48.10 | 48.30 | ||
| O | 42.82 | 42.90 | 43.07 | 43.42 | 43.43 | 43.86 | ||
| N | 2.56 | 2.39 | 2.55 | 2.21 | 2.53 | 2.09 | ||
| Ni | – | – | 1.56 | 1.39 | 3.89 | 3.54 | ||
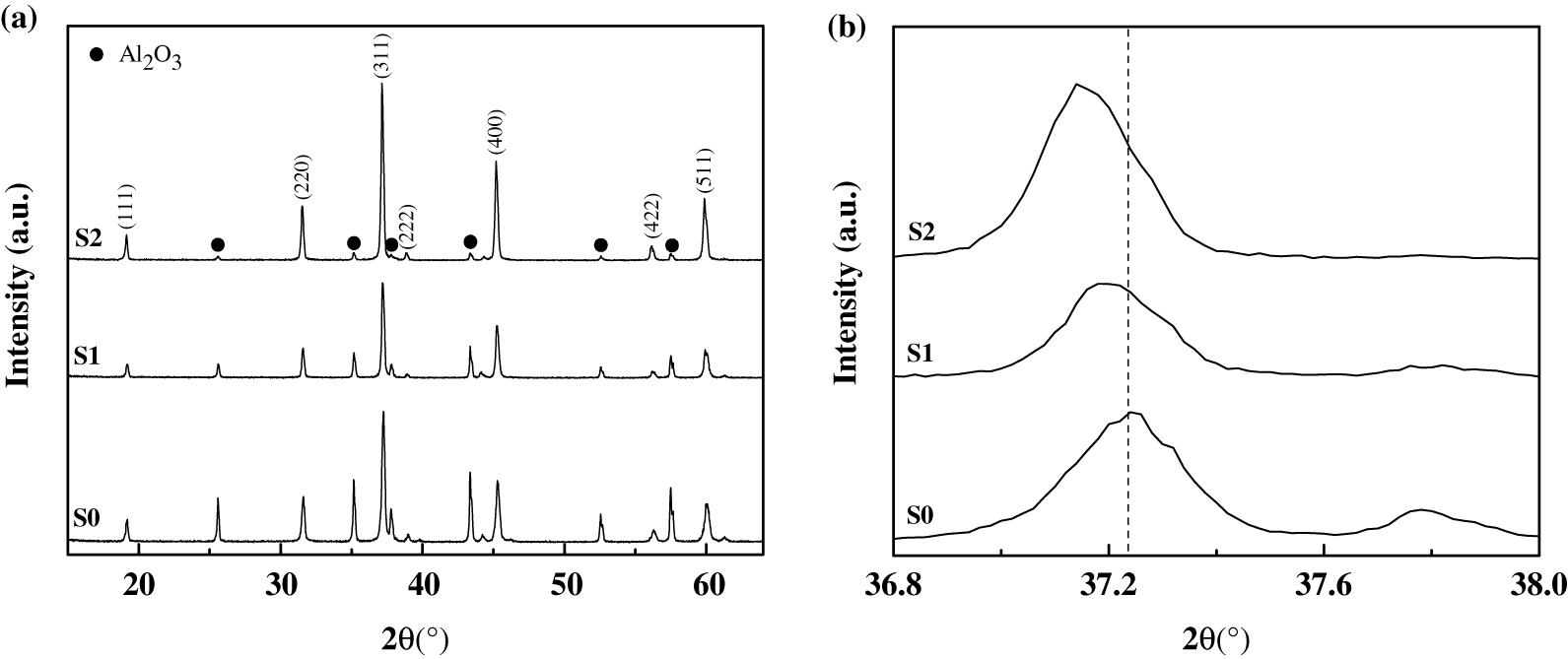
The further XRD results plotted in Figure 2(a) revealed that MgAlON monophase was obtained in the sample without NiO additive (S0), and metallic Ni phase was formed besides MgAlON phase in the sample with 2 % NiO additive (S1). Notably, in the sample with 5 % NiO additive (S2), alumina phase was detected together with Ni and MgAlON phases. As shown in Figure 2(b), with the calibration of high purity silicon, it was found that the diffraction angle of the synthesized MgAlON phase shift to right, and the lattice constant of MgAlON decreased with the increased amount of NiO additive.

(a) XRD results of the synthesized sample and (b) its extended peak.
Figure 3 plots the average water quenching numbers of the synthesized samples. With the increasing of NiO additive, the thermal shock resistance was obviously enhanced. Hence, the formation of metallic Ni grains in MgAlON matrix is favorable to improve the thermal shock resistance of the composite and overcome the inherent shortcomings of ceramics.
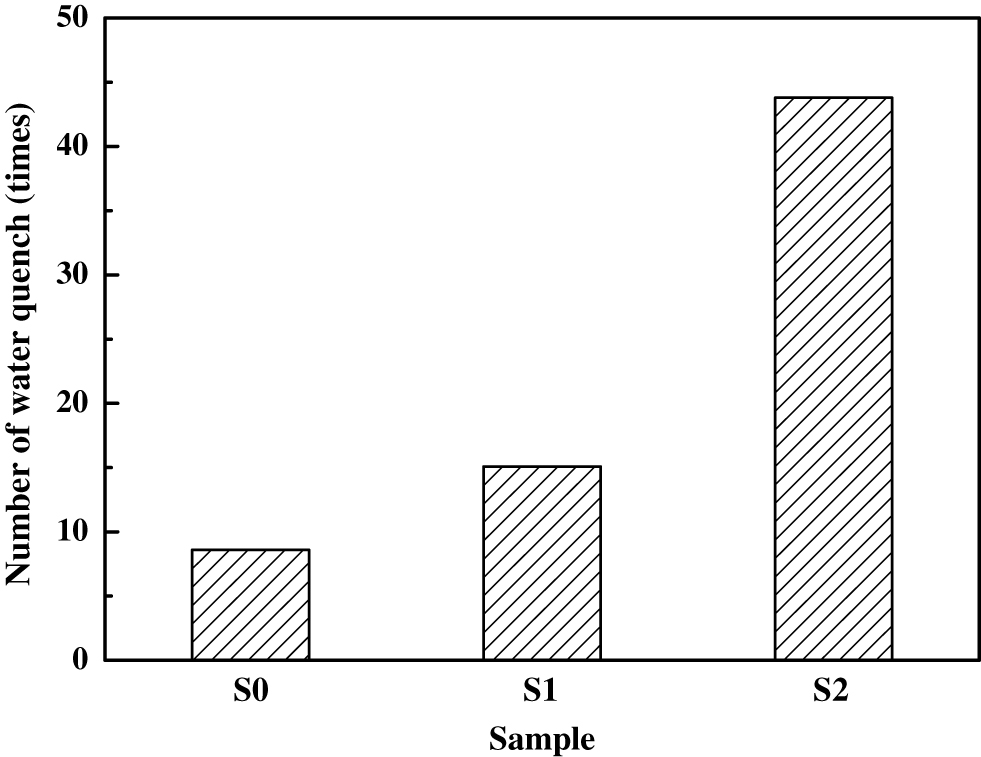
Average water quench numbers of the synthesized sample.
Figure 4 shows the mass gain of powders with the increasing heating temperature and the prolonging soaking time. In the sample without NiO additive, the oxidation reaction occurred above 750°C, and it was accelerated at temperatures higher than 1150°C. With the content of NiO increasing in the raw materials, the beginning temperature of oxidation reaction decreased to 550°C for 2 %NiO, and 350°C for 5 %NiO. On the other hand, the final mass gain of the sample was increased with the increasing amount of NiO additive.

Non-isothermal and isothermal mass gain of powders under air atmosphere.
Figure 5(a) indicates the phase composition of powders oxidized at 1300°C for 2 h. Interestingly, it was found that with the increasing of NiO additive, the amount of alumina in the oxidized powders was decreased, and it was contrast to that in the synthesized composites shown in Figure 2. Simultaneously, another notably phenomena of oxidized powders determined by XRD is shown in Figure 5(b). It was found that oxidized product of spinel phase shift to left, and it was not similar as that of the synthesized powder. Hence, the lattice constant of spinel phase had an increase.
(a) XRD results of the powders oxidized at 1300°C for 1 h under air atmosphere, and (2) its extended peak.
Figure 6 reveals the isothermal oxidation behavior of plates at 1400°C. In the sample without additive and with low content of NiO additive, the mass gain obeyed linear laws. With the increasing NiO additive, the mass gain turned into parabolic rate law. As parabolic law means that the oxidation reaction is limited by gas diffusion and a linear law when controlled by interface reaction [12, 13], the oxidation resistance of the sample with 5 %NiO was obviously improved for the oxidation of plate was limited by gas diffusion, and the detailed mechanism in the following part.
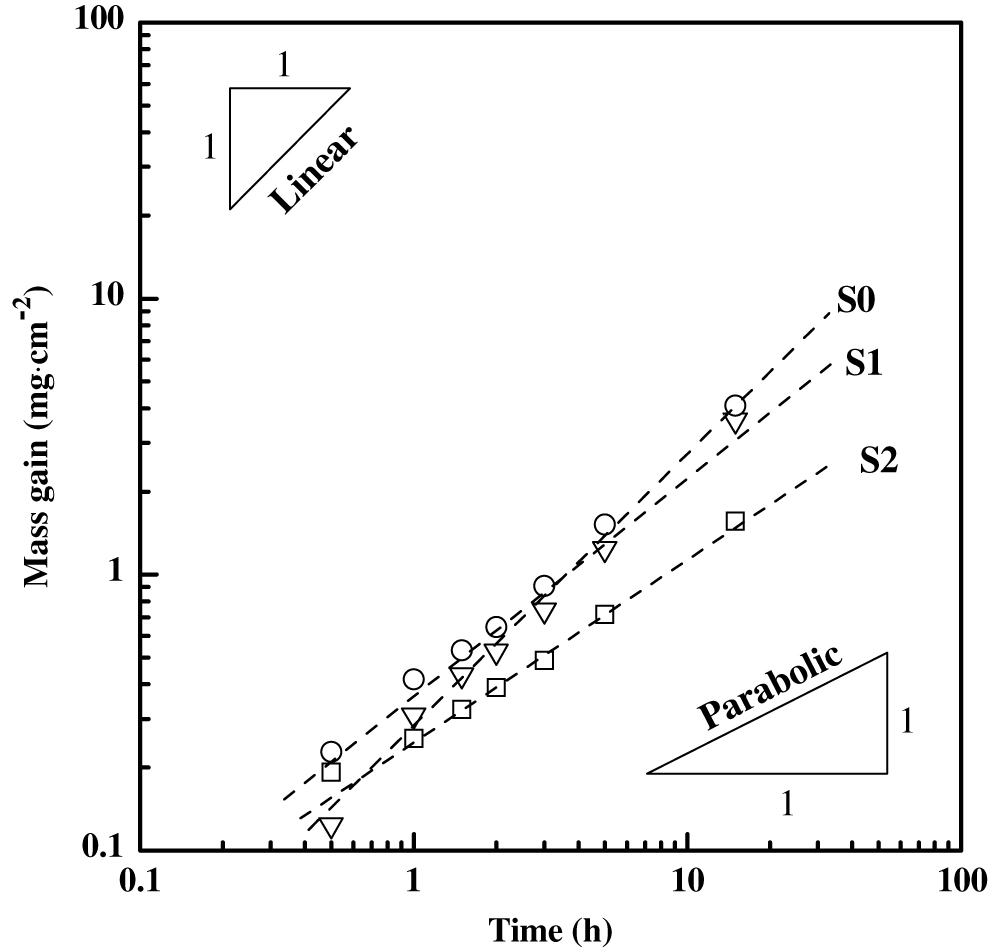
Mass gain of plate under air atmosphere at 1400°C.
Discussion
Figure 7(a) reveals the phase diagram of MgO-Al2O3-AlN-Mg3N2 [14] at 1750°C. The sample without NiO additive (marked as point S0) lies in the completely solid solution area and close to the critical line. Since the sintering temperature in present work is 1700°C and monophase MgAlON has been obtained, the increase of content of Al2O3 might lead to the formation of multiphase composite. Hence, as revealed in Figure 2, alumina is detected in the sample with 5 %NiO additive, even it has not been detected in the sample with 2 %NiO additive.
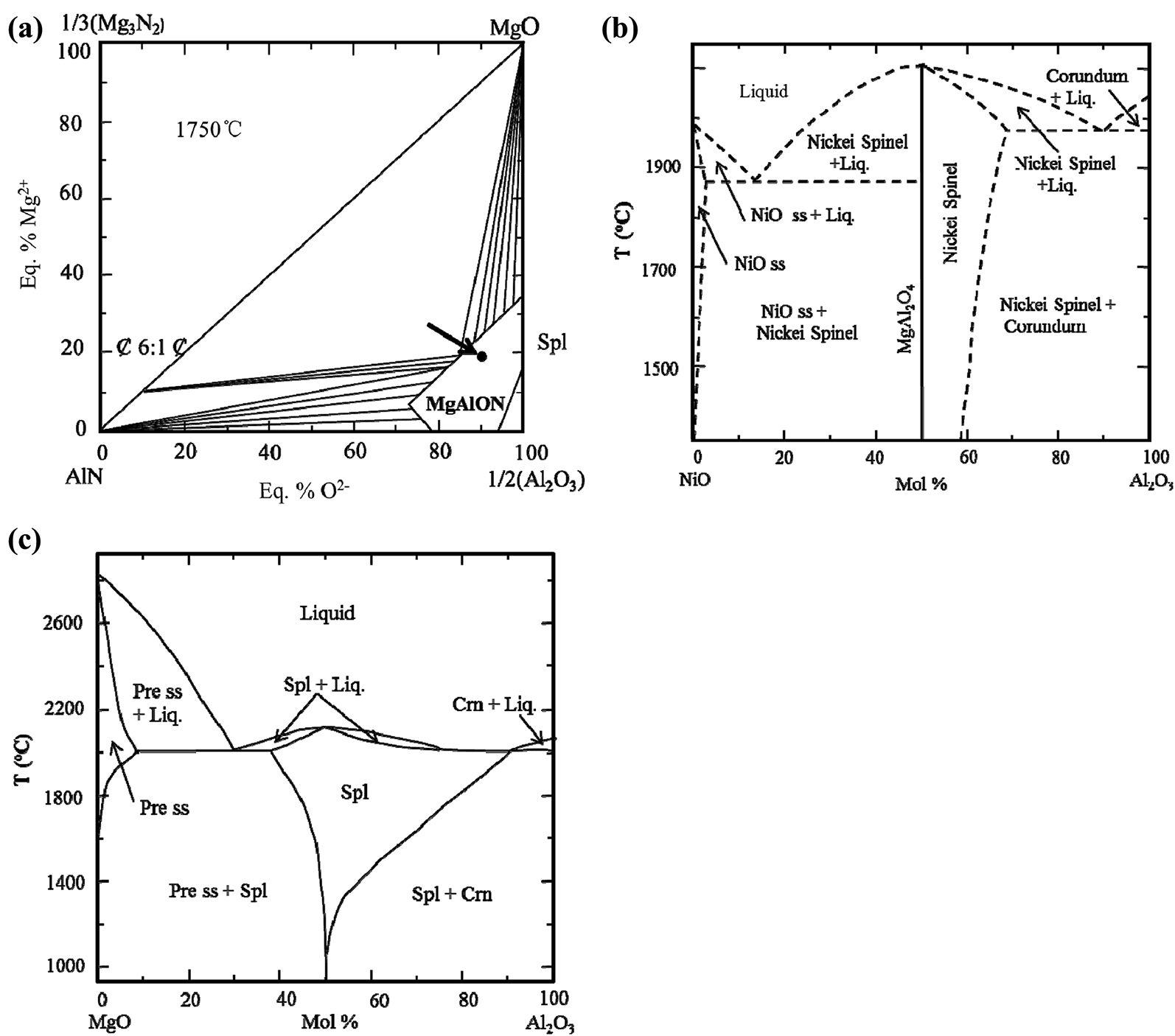
(a) Phase diagram of MgO-Al2O3-AlN-Mg3N2 at 1750°C, (b) Phase diagram of NiO–Al2O3, (c) Phase diagram of MgO–Al2O3.
The sintering procedure of magnesia, alumina, and aluminum nitride has been elaborated analyzed by Bandyopadhyay et al., and they propose that an initially formed stoichiometric magnesium aluminate spinel is turned into MgAlON by dissolution of alumina and aluminum nitride, and furthermore, nitrogen diffusion through the MgAlON lattice seems to be rate controlling [15]. As to the NiO-MgO-Al2O3-AlN system, it can be deduced out from Figure 5 that NiO is prone to react with Al2O3 to form cubic solid solution product NiAl2O4 [Figure 7(b)] [16], which is similar as MgAl2O4 and can completely solid solve each other to form (Ni,Mg)Al2O4 spinel [Figure 7(c)] [17].
Subsequently, the solid solution of AlN into spinel to form solid solution compound (Ni,Mg)AlON.
In the course of the solid solution reaction, submicron metallic Ni grains are simultaneously formed. Since the difference of chemical composition between the raw materials and the synthesized products is ambiguous (Table 2), and final mass gains of powders are similar (Figure 4), metallic Ni grains should not be formed through oxidation-reduction reaction. If the reaction occurs, nitrogen or oxygen might be formed and inevitably, these gases are prone to diffuse out the system for low environmental pressure. Hence, the metallic Ni grains might segregate from the (Ni,Mg)AlON compound for some reason. However, it is still unknown and should be further investigate in future.
As to the oxidation behavior of metallic nickel containing (Ni,Mg)AlON composite, metallic Ni is firstly oxidized at temperatures as low as 350°C. From the TG data, it can be concluded that the oxidation temperature of (Ni,Mg)AlON synthesized in present work is about 200°C lower than MgAlON, and the oxidation reaction forms fully solid solution (Ni,Mg)Al2O4 spinel (Figure 4) as follows:
To oxidation behavior of plates, the NiO additive can obviously increase the oxidation resistance. As shown in Figure 8(a), it can be seen from the microstructure in the plate without NiO additive, lots of pores are connected each other, and the oxidized layer has not the effect on the decrease the diffusion rate of oxygen and nitrogen. Though lots of pores also exist in the oxidized layer in the plate with NiO additive [Figures 8(b), (c)], the connection of pores is separated by the formation of (Ni,Mg)Al2O4 spinel, and the increasing of NiO additive is beneficial to decrease the diffusion rate of oxygen and nitrogen. Consequently, the oxidation resistance is increased.
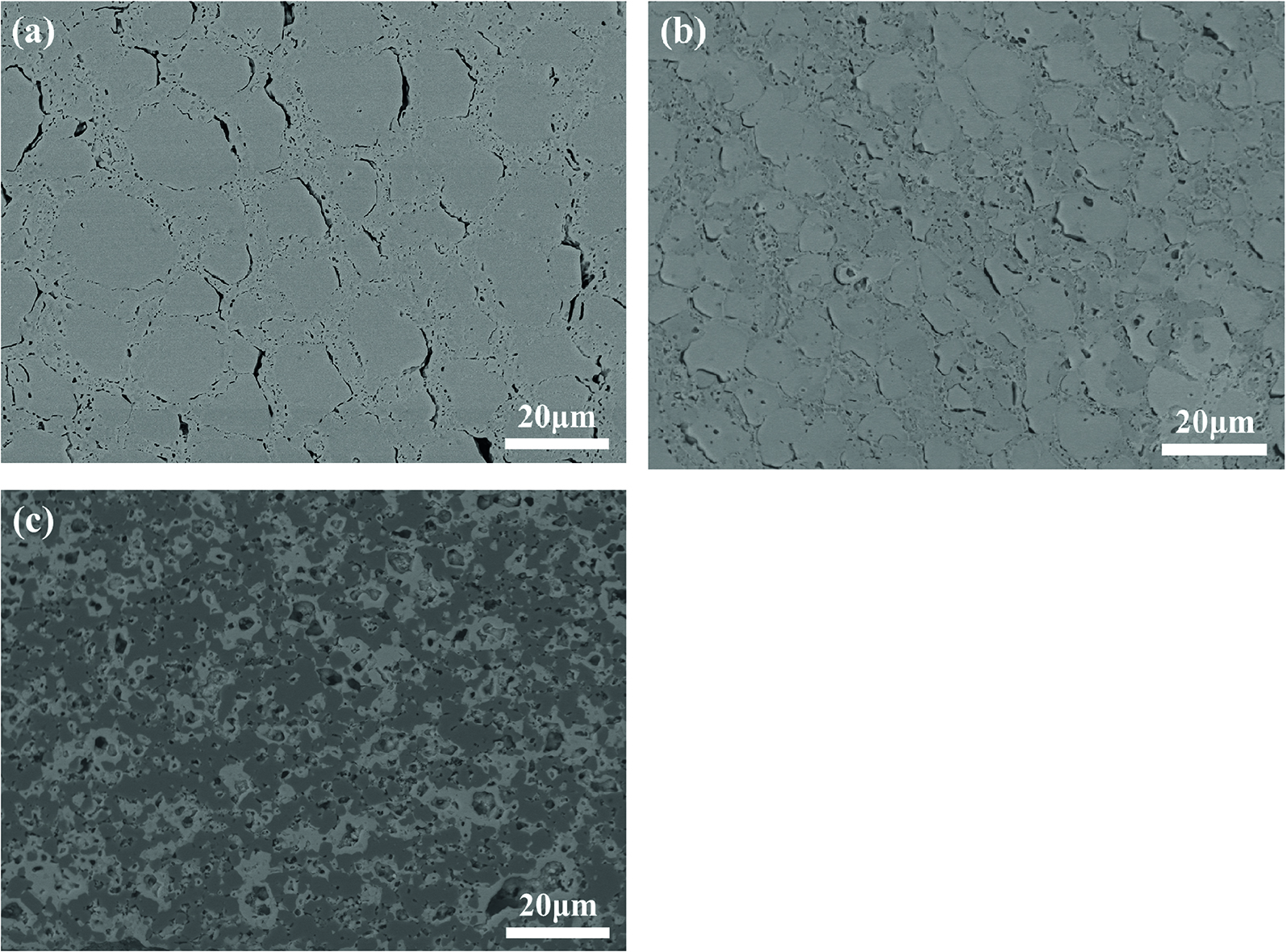
Microstructure of oxidized layer in the sample with (a) None, (b) 2 %NiO, and (c) 5 %NiO NiO additive.
Hence, the metallic nickel containing (Ni,Mg)AlON composite has not only superior thermal shock resistance but also excel oxidation resistance. The fabrication and utilization of metallic nickel containing (Ni,Mg)AlON composite should be a feasible way to reuse the nickel slag corroded magnesia and alumina graphite refractories.
Conclusions
Metallic nickel grains are segregated during the solid solution of nitrogen into magnesium aluminum oxynitride (MgAlON).
The formation of metallic nickel grains is favor to improve the thermal shock resistance of MgAlON.
Though the starting oxidation reaction temperature is decreased in metallic nickel containing composite, the higher amount of metallic Ni grains lead to superior oxidation resistance because the oxidized product (Ni,Mg)Al2O4 spinel decrease gas diffusion rates of nitrogen and oxygen in the matrix.
Funding statement: Natural Science Foundation of China, (Grant / Award Number: ‘51364003’).
Acknowledgments
The authors would like to acknowledge the financial support received from Natural Science Foundation of China (No. 51364003) and Guangxi postdoctoral special funds.
References
[1] H. Fang, J.D. Smith and K.D. Peaslee, Resour. Conserv. Recy., 25 (1999) 111–124.10.1016/S0921-3449(98)00059-7Suche in Google Scholar
[2] A.P. Luz, D.O. Vivaldini, F. López, P.O.R.C. Brant and V.C. Pandolfelli, Ceram. Int., 39 (2013) 8079–8085.10.1016/j.ceramint.2013.03.080Suche in Google Scholar
[3] A.G.M. Othman and W.M.N. Nour, Ceram. Int., 31 (2005) 1053–1059.10.1016/j.ceramint.2004.11.004Suche in Google Scholar
[4] Z.T. Zhang, T. Matsushita, W.C. Li and S. Seetharaman, Metall. Mater. Trans. B, 37 (2006) 421–429.10.1007/s11663-006-0027-6Suche in Google Scholar
[5] A.M. Lejus, Rev. Int. Hautes Temp. Refract., 1 (1964) 53–95.10.1111/j.1651-2227.1964.tb07212.xSuche in Google Scholar
[6] X. Wang, F. Wang and W. Li, J. Inorg. Mater., 18 (2003) 83–90.10.1016/S0921-5093(02)00521-XSuche in Google Scholar
[7] A. Granon, P. Goeuriot, F. Thevenot, J. Guyader, P. L’Haridon and Y. Laurent, J. Eur. Ceram. Soc., 13 (1994) 365–370.10.1016/0955-2219(94)90012-4Suche in Google Scholar
[8] Z.T. Zhang, W.C. Li and S. Seetharaman, Metall. Mater. Trans. B, 37 (2006) 615–621.10.1007/s11663-006-0045-4Suche in Google Scholar
[9] Z.T. Zhang, T. Matsushita, W.C. Li and S. Seetharaman, Metall. Mater. Trans. B, 38 (2007) 231–241.10.1007/s11663-007-9038-1Suche in Google Scholar
[10] Z. Zhang, L. Teng and W. Li, J. Eur. Ceram. Soc., 27 (2007) 319–326.10.1016/j.jeurceramsoc.2006.04.184Suche in Google Scholar
[11] G. Ye, J. Shang, D. Zhang, M. Liang and Y. Chen, J. Am. Ceram. Soc., 93 (2010) 322–325.10.1111/j.1551-2916.2009.03408.xSuche in Google Scholar
[12] T. Narushima, T. Goto, Y. Yokoyama, J. Hagiwara, Y. Iguchi and T. Hirai, J. Am. Ceram. Soc., 77 (1994) 2369–2375.10.1111/j.1151-2916.1994.tb04607.xSuche in Google Scholar
[13] K.L. Luthra, J. Am. Ceram. Soc., 74 (1991) 1095–1103.10.1111/j.1151-2916.1991.tb04348.xSuche in Google Scholar
[14] J. Weiss, P. Greil and L.J. Gauckler, J. Am. Ceram. Soc., 65 (1982) C68–C69.10.1111/j.1151-2916.1982.tb10435.xSuche in Google Scholar
[15] S. Bandyopadhyay, G. Rixecker, F. Aldinger and H.S. Maiti, J. Am. Ceram. Soc., 87 (2004) 480–482.10.1111/j.1551-2916.2004.00480.xSuche in Google Scholar
[16] H. Wartenberg and H.J. Reusch, Z. Anorg. Allg. Chem., 207 (1932) 1–20.10.1002/zaac.19322070102Suche in Google Scholar
[17] T.I. Barry, A.T. Dinsdale, J.A. Gisby, B. Hallstedt, M. Hillert, S. Jonsson, B. Sundman and J.R. Taylor, J. Phase Equilib., 13 (1992) 459–475.10.1007/BF02665760Suche in Google Scholar
© 2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Effects of Rare Earth on Mechanical Properties of LZ50 Axle Steels and Its Formation Mechanism
- Formation of Non-metallic Inclusions of Si-killed Stainless Steel during GOR Refining Process
- Application of Direct Resistance Heating in Hot Forging and Analysis of Processing Parameters based on Thermo-electro-mechanical Coupling FEM
- Effect of Long-Term Ageing at 600 °C on Microstructure of ZG1Cr10MoWVNbN Martensitic Heat-Resistant Steel
- Plasma-Assisted Nitriding in Low-Frequency Inductively Coupled Plasma Enhanced with Ferromagnetic Cores
- Modeling of the Hot Flow Behaviors for Ti-6Al-4V-0.1Ru Alloy by GA-BPNN Model and Its Application
- Preparation and Oxidation Behavior of Metallic Nickel Containing MgAlON Composite
- Numerical Investigation on the Strain Evolution of Ti-6Al-4V Alloy during Multi-directional Forging at Elevated Temperatures
- Study of the High-temperature Synthesis of MgAl2O4 Spinel Refractory Raw Materials from Chromium Slag
- Hardness Evolution and High Temperature Mechanical Properties of Laser Welded DP980 Steel Joints
- Microstructure and Properties of Si3N4 Ceramics and 304 Stainless Steel Brazed Joint with Cu/Ag-Cu/Ti Laminated Filler Metal
Artikel in diesem Heft
- Frontmatter
- Effects of Rare Earth on Mechanical Properties of LZ50 Axle Steels and Its Formation Mechanism
- Formation of Non-metallic Inclusions of Si-killed Stainless Steel during GOR Refining Process
- Application of Direct Resistance Heating in Hot Forging and Analysis of Processing Parameters based on Thermo-electro-mechanical Coupling FEM
- Effect of Long-Term Ageing at 600 °C on Microstructure of ZG1Cr10MoWVNbN Martensitic Heat-Resistant Steel
- Plasma-Assisted Nitriding in Low-Frequency Inductively Coupled Plasma Enhanced with Ferromagnetic Cores
- Modeling of the Hot Flow Behaviors for Ti-6Al-4V-0.1Ru Alloy by GA-BPNN Model and Its Application
- Preparation and Oxidation Behavior of Metallic Nickel Containing MgAlON Composite
- Numerical Investigation on the Strain Evolution of Ti-6Al-4V Alloy during Multi-directional Forging at Elevated Temperatures
- Study of the High-temperature Synthesis of MgAl2O4 Spinel Refractory Raw Materials from Chromium Slag
- Hardness Evolution and High Temperature Mechanical Properties of Laser Welded DP980 Steel Joints
- Microstructure and Properties of Si3N4 Ceramics and 304 Stainless Steel Brazed Joint with Cu/Ag-Cu/Ti Laminated Filler Metal

