Synthesis of dibenzothiazepine analogues by one-pot S-arylation and intramolecular cyclization of diaryl sulfides and evaluation of antibacterial properties
-
Yasutaka Shimotori
, Masayuki Hoshi
Abstract
Dibenzothiazepine analogues containing lactam, amidine and imine moieties were prepared from 2-aminophenyl disulfides via one-pot S-arylation. The S-arylation involved cleavage of an S-S bond of disulfides and SNAr reaction in aqueous ammonia solution of L-cysteine to afford diaryl sulfides. Dibenzothiazepine analogues having lactam and amidine moieties were obtained by cyclization of the corresponding diaryl sulfides under acidic conditions. One-pot S-arylation of 2-bromo-5-nitrobenzaldehyde gave dibenzothiazepine analogues with an imine moiety in one step through intramolecular cyclization. Compounds with antibacterial activities against Staphylococcus aureus and Escherichia coli were obtained.
Introduction
Heterocyclic compounds exhibit various biological activities including antibacterial [1], [2], [3], [4], [5], antitumor [6], [7], [8], [9], anti-inflammatory [10], [11], [12], [13], [14] and antiviral properties [15], [16], [17], [18], [19]. Important bioactive compounds are sulfur-containing heterocycles. Penicillin and cephalosporin C [20] used as antibacterial agents, and ritonavir [21], [22] used as an anti-HIV therapeutic agent are well-known sulfur-containing cyclic compounds. In addition, benzothiazepines exhibit antimicrobial [23], [24], [25], antiviral [26], cytostatic [27], anticonvulsant [28], [29] and antipsychotic activities [30]. Previously, we have reported synthesis of sulfides from aryl disulfides [31], [32] by cleavage of an S-S bond of aryl disulfides using ammonium thioglycolate, followed by S-arylation and alkylation in one-pot treatment. In this study, the S-S bond cleavage of 2-aminophenyl disulfides was carried out using L-cysteine instead of thioglycolic acid. Diaryl sulfides were synthesized by a one-pot SNAr reaction using nitroarenes. Various dibenzothiazepine analogues were synthesized by cyclization of the corresponding diaryl sulfides. Furthermore, antibacterial activities of the synthesized compounds against Staphylococcus aureus and Escherichia coli were investigated.
Results and discussion
Diltiazem and its derivatives, benzothiazepines having a lactam moiety, are used as antiarrhythmic drugs [33]. Benzothiazepines with an amidine moiety exhibit antipsychotic activity [34]. Based on these facts, we considered synthesizing various dibenzothiazepine analogues (Scheme 1). Dibenzothiazepines with lactam could be obtained by intramolecular cyclization of the corresponding diaryl sulfides substituted with a methyl ester. Similarly, those with imine could be obtained from the corresponding diaryl sulfides substituted with a formyl group. Furthermore, those with amidine could be obtained by the Pinner reaction at amino and cyano groups. Diaryl sulfides are readily available by one-pot S-arylation of disulfides with disubstituted nitroarenes [31], [32].
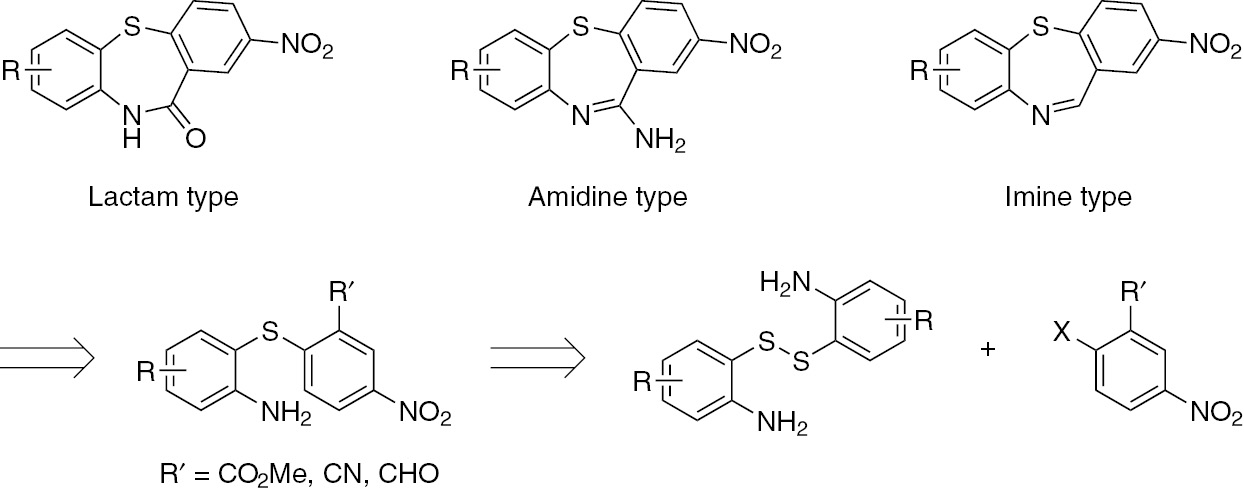
Retrosynthetic analysis of three types of dibenzothiazepine analogues.
The effect of a leaving group on the SNAr reaction in 2-aminophenyl disulfide and various nitroarenes was investigated. The nitro group, which is a strong electron withdrawing group for anion stabilization, is required to decrease the electron density at the benzene ring [35], [36], [37], [38], [39]. Various leaving groups of nitroarene were investigated. The S-S bond of bis(2-aminophenyl) disulfide was cleaved in aqueous ammonia solution of L-cysteine. Subsequently, one-pot S-arylation was performed between thiolate anion and various nitroarenes (Scheme 2). In the case of using an arylating agent with a bromo group at ortho- or para-position of the nitro group, S-arylation progressed with 48% and 69% yields, respectively. By contrast, S-arylation was not observed using 1-bromo-3-nitrobenzene. When the thiolate nucleophile attacks the ipso position containing a bromo substituent, σ-complex is formed as an intermediate. The bromo group at the ortho- or para-position stabilizes the σ-complex by a resonance effect, and no resonance effect is possible at the meta-position. The same feature may be operative in the case of dinitrobenzenes.
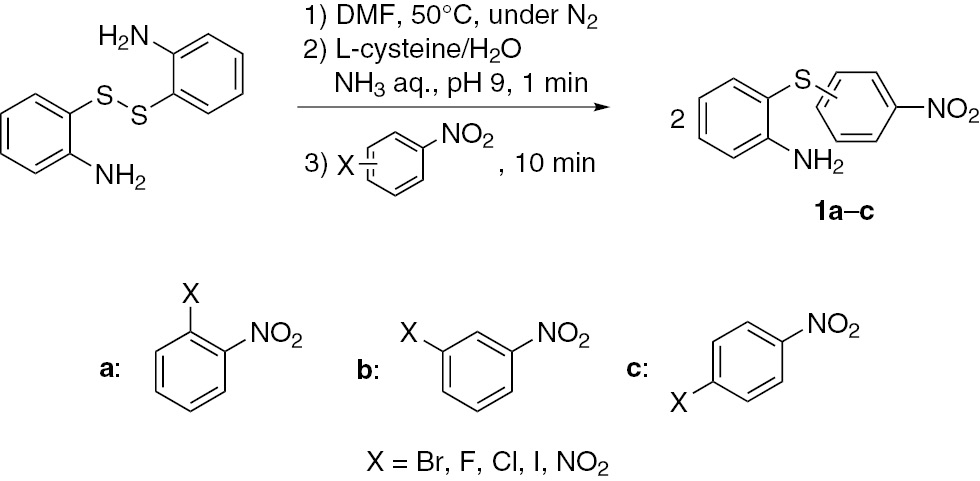
SNAr reaction of 2-aminophenyl disulfide and nitroarenes.
The use of 1,2-dinitrobenzene and 1,4-dinitrobenzene gave the corresponding diaryl sulfides with 99% yields, and no S-arylation was observed using 1,3-dinitrobenzene. Among halogeno groups at the para-position, the presence of the fluoro group gave rise to the highest 99% yield, and the use of iodophenyl gave rise to a low yield of 36%. This analysis largely agrees with the order of degree of electronegativity. As the electronegativity increases, the positive charge at the ipso-position increases, which enhances electrophilicity. On the other hand, the yields are higher when using an arylating agent with a sterically smaller leaving group. Apparently, the addition of thiolate anion at the ipso-position is a rate-controlling step.
Methyl [2-(2-aminophenyl)sulfanyl]-5-nitrobenzoates 2 are precursors to dibenzothiazepine analogues having a lactam moiety. These compounds were prepared by one-pot S-arylation of 2-aminophenyl disulfides with methyl 2-bromo-5-nitrobenzoate (Scheme 3). All diaryl sulfides 2 were obtained in a quantitative yield. With 1-bromo-4-nitrobenzene used as an arylating agent, the conversion was not 100% after 10 min and the yield of 2-aminophenyl 4-nitrophenyl sulfide (1c) was 69%. By contrast, when using methyl 2-bromo-5-nitrobenzoate, all diaryl sulfides 2 were quantitatively obtained after 10 min regardless of the substituents. In nitroarenes with a methyl ester group at the ortho-position to the leaving group, the ester group acts as an electron withdrawing group, which decreases the electron density at the carbon atom at the ipso-position of the halogen. This feature facilitates the nucleophilic attack of thiolate anion, increasing the yields of diaryl sulfides 2.
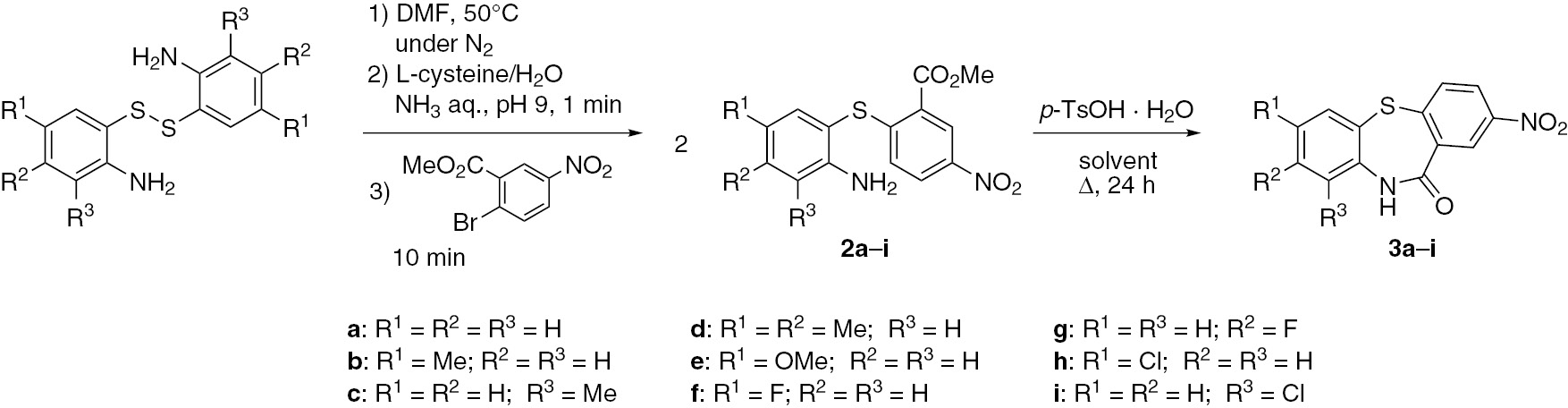
Synthesis of lactam-containing dibenzothiazepines 3.
Lactam dibenzothiazepine analogues 3 were obtained by intramolecular cyclization of the corresponding diaryl sulfides 2 (Scheme 3). The cyclization was carried out in the presence of p-TsOH·H2O as an acid catalyst under reflux for 24 h. With 0.2 and 0.4 equivalents of p-TsOH·H2O, the cyclization of methyl [2-(2-aminophenyl)sulfanyl]-5-nitrobenzoate (2a) in o-xylene yielded 2-nitrodibenzo[b,f][1,4]thiazepin-11(10H)-one (3a) in the respective yields of 40% and 95%. When the reaction was conducted under reflux with 0.4 equivalent of p-TsOH·H2O in toluene instead of o-xylene, the yield of 3a dropped to 15%. It can be suggested that the reaction requires 0.4 equivalent of acid and should be conducted at least at 140°C. Other lactam dibenzothiazepine analogues 3b–i were synthesized under similar conditions. Excepting 3d and 3f, the remaining dibenzothiazepines 3 were obtained with a yield of more than 80%.
The amidine dibenzothiazepine analogues 5 were synthesized as shown in Scheme 4 starting with diaryl sulfides 4 substituted with a nitrile group. 2-Bromo-5-nitrobenzonitrile as an arylating agent was added after the cleavage of the S-S bond of 2-aminophenyl disulfides, and the mixture was stirred for 10 min (Scheme 4). 2-Aminophenyl-2-cyano-4-nitrophenyl sulfide (4a) was obtained in a 62% yield. When the amount of the arylating agent was increased to 3.0 equivalents and the reaction was conducted for 10 min under otherwise similar conditions, the yield of 4a increased to 84%. After the reaction time was extended to 30 min, the yield of 4a was further increased to 95%. S-arylation of the remaining 2-aminophenyl disulfides was carried out under similar conditions to furnish diaryl sulfides 4b–i with yields of about 80%.
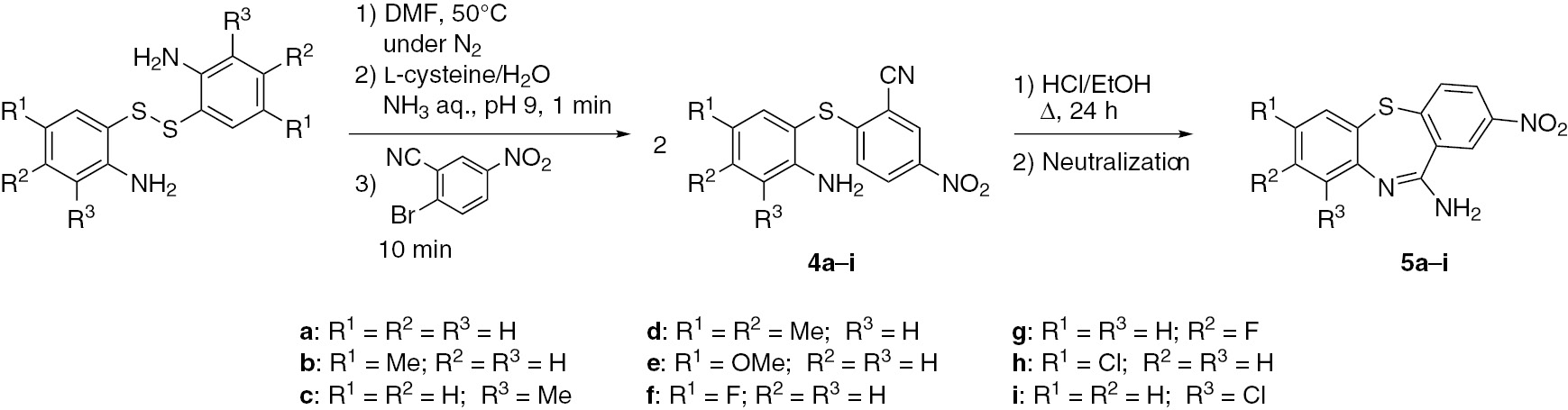
Synthesis of dibenzothiazepine analogues with amidine moiety (5).
Intramolecular cyclization of diaryl sulfides 4 between the amino and nitrile groups was carried out by the Pinner reaction (Scheme 4). In a model reaction, 2-aminophenyl 2-cyano-4-nitrophenyl sulfide (4a) was added to hydrogen chloride in ethanol and the mixture was heated under reflux for 24 h. Various concentrations of hydrogen chloride were investigated. In the presence of 5, 10, 20 and 30 wt% of hydrogen chloride in ethanol, 2-nitrodibenzo[b,f][1,4]thiazepin-11-amine (5a) was obtained in the respective yields of 28, 26, 38 and 61%. As can be seen, the yields increased with increasing concentration of HCl. Accordingly, synthesis of other products 5b–i from 2-aminophenyl-2-cyano-4-nitrophenyl sulfides 4 was conducted using 30 wt% hydrogen chloride ethanolic solution. Dibenzothiazepines 5b–i were obtained regardless of the nature of substituents R1, R2 and R3. When diaryl sulfides 4f with R1=F and 4h with R1=Cl were used, the yields of 5f and 5h were improved by at least 10% as compared with the yield of 5a. On the other hand, the yields of dibenzothiazepines 5b–e having methyl or methoxy groups decreased by about 20–30% as compared with that for 5a. An electron donating group such as methyl or methoxy increases basicity of the amino group, which makes it easier to form hydrochloride. This feature reduces the reactivity of the amino group and decreases the yields of 5.
Preparation of [2-(2-aminophenyl)sulfanyl]-5-nitrobenzaldehydes 6 and dibenzothiazepine analogues with imine moiety 7 involved the use of 2-bromo-5-nitrobenzaldehyde as an arylating agent (Scheme 5). After stirring at 50°C for 10 min, no diaryl sulfide 6a could be isolated and the cyclized form, 2-nitrodibenzo[b,f][1,4]thiazepine (7a), was obtained in a yield of 48%. The reaction temperature was studied to improve the yield. The yield was increased to 67% for the reaction conducted at 70°C for 10 min. However, extending the time to 30 min did not increase the yield.
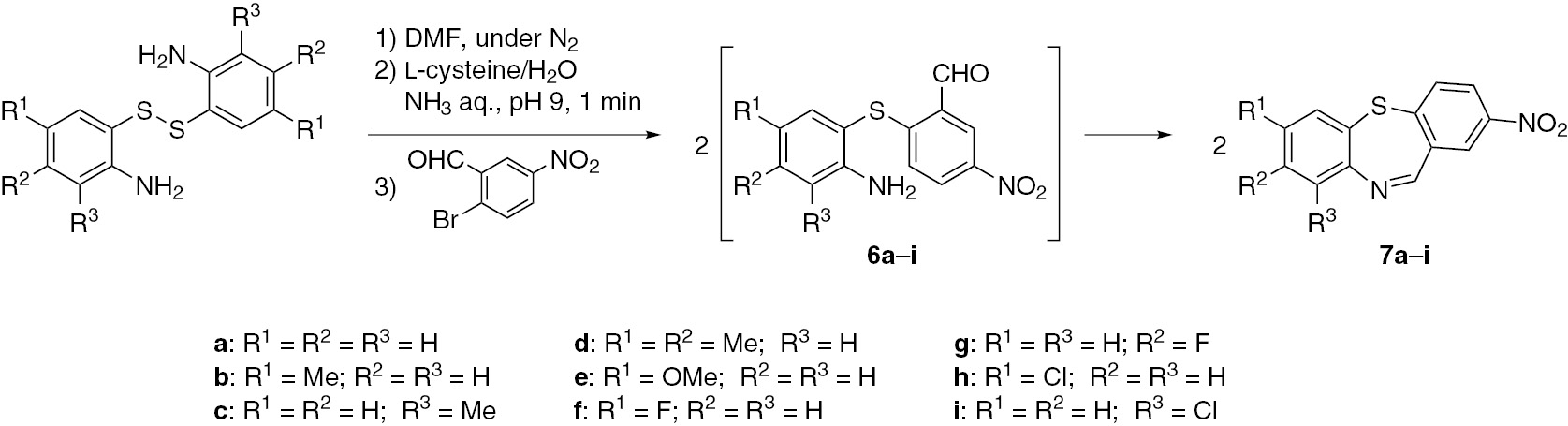
Synthesis of dibenzothiazepine analogues with imine moiety 7.
When the reaction was carried out at 90°C for 10 min, the yield was 62% and many by-products were observed by thin layer chromatography (TLC) analysis. The reaction time of 10 min at 70°C was taken as the optimum conditions. Synthesis of other dibenzothiazepine analogues with imine moiety 7b–i is also shown in Scheme 5. In all cases, the S-arylation, regardless of the substituent on the benzene ring of thiolate anion, was spontaneously followed by intramolecular cyclization, and dibenzothiazepines 7 were obtained in about 50–60% yields.
The antibacterial activities of the products against S. aureus (Gram positive) and E. coli (Gram negative) bacteria were investigated (Table 1). A total of 45 diaryl sulfides 2 and 4 and dibenzothiazepines 3, 5 and 7 were tested. All diaryl sulfides 2 and dibenzothiazepines 3 and 7 show no activity against both S. aureus and E. coli regardless of the substituents R1–R3. All diaryl sulfides 4 show no antibacterial activity against E. coli. However, compound 4a reduced S. aureus to about 0.2% compared with the negative control. Moreover, 4g and 4h show even stronger activities reducing S. aureus to about 0.03 and 0.1%, respectively. Because not all diaryl sulfides 4 exhibit antibacterial activities, the apparent correlation between the presence of a cyano group and antibacterial activity cannot be confirmed. As 2g and 2h are not active, the fluorine atom (R2) and the chlorine atom (R1) are not necessarily involved in the activity.
Antibacterial activities of compounds 4 and 5 against S. aureus and E. coli.
| Sample | R1 | R2 | R3 | S. aureus (CFU/mL) | E. coli (CFU/mL) | Sample | R1 | R2 | R3 | S. aureus (CFU/mL) | E. coli (CFU/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4a | H | H | H | 2.0×105 | 1.2×109 | 5a | H | H | H | 9.8×107 | 1.5×109 |
| 4b | Me | H | H | 3.2×106 | 1.1×109 | 5b | Me | H | H | 2.8×105 | 4.2×107 |
| 4c | H | H | Me | 6.4×107 | 1.1×109 | 5c | H | H | Me | 8.8×107 | 1.8×109 |
| 4d | Me | Me | H | 2.0×107 | 1.4×109 | 5d | Me | Me | H | 3.6×107 | 8.5×107 |
| 4e | OMe | H | H | 4.6×107 | 1.4×109 | 5e | OMe | H | H | 3.4×105 | 6.7×107 |
| 4f | F | H | H | 7.4×106 | 1.3×109 | 5f | F | H | H | 1.2×108 | 1.6×109 |
| 4g | H | F | H | 2.6×104 | 1.7×109 | 5g | H | F | H | 5.4×107 | 2.4×109 |
| 4h | Cl | H | H | 9.5×104 | 8.0×108 | 5h | Cl | H | H | 5.6×107 | 1.2×109 |
| 4i | H | H | Cl | 1.1×108 | 1.9×109 | 5i | H | H | Cl | 8.6×107 | 1.5×109 |
Negative control with S. aureus is 8.5×107 (CFU/mL) and with E. coli is 1.5×109 (CFU/mL).
Dibenzothiazepines with amidine moiety 5b and 5e show activities against both S. aureus and E. coli decreasing the bacteria to about 0.4 and 3–4%, respectively, compared to the negative control. Dibenzothiazepine 5d shows antibacterial activity only against E. coli. Furthermore, three dibenzothiazepines with lactam moiety, 3b, 3d and 3e, and three dibenzothiazepines with amidine moiety, 7b, 7d and 7e, are not active.
Conclusions
The S-S bonds of various aryl disulfides are efficiently cleaved in the presence of L-cysteine. When methyl 2-bromo-5-nitrobenzoate is used as an arylating agent, diaryl sulfides 2 are quantitatively obtained in a one-pot reaction. Similarly, the use of 2-bromo-5-nitrobenzonitrile furnishes diaryl sulfides 4 in high yield. Dibenzothiazepines having lactam and amidine moieties, 3 and 5, were synthesized from the corresponding diaryl sulfides, 2 and 4, under acidic conditions. On the other hand, dibenzothiazepines with imine moiety 7 were obtained from the aryl disulfides in a one-pot procedure that involves spontaneous intramolecular cyclization of the intermediate products 6. All diaryl sulfides 2 and dibenzothiazepines 3 and 7 show no antibacterial activity against both S. aureus and E. coli. However, diaryl sulfides 4a, 4g and 4h are highly active against S. aureus. Two dibenzothiazepines with amidine moiety, 5b and 5e, show antibacterial activities against both S. aureus and E. coli.
Experimental
Unless stated otherwise, proton nuclear magnetic resonance (1H NMR) spectra (600 MHz) and carbon-13 nuclear magnetic resonance (13C NMR) spectra (150 MHz) were recorded in dimethyl sulfoxide-d6 (DMSO-d6) on a JNM-ECA-600 spectrometer (JEOL, Tokyo, Japan). Structural determination of all compounds was performed using correlation spectroscopy (COSY), heteronuclear multiple quantum coherence (HMQC) and heteronuclear multiple bond correlation (HMBC) NMR techniques. Infrared spectra were obtained in KBr pellets on an Fourier-transform infrared spectroscopy (FTIR) 460plus spectrometer (JASCO Corp., Tokyo, Japan). Electrospray ionization (ESI) high resolution mass spectra were recorded on an AccuTOF GCv 4G instrument (JEOL). Melting points were recorded on a MP-500D micro-melting-point apparatus from Yanaco Technical Science Co., Ltd. (Kyoto, Japan) and are uncorrected.
General procedure for the preparation of 2-aminophenyl 2-nitrophenyl sulfides 1a–c
A solution of bis(2-aminophenyl) disulfide (0.10 g, 0.40 mmol) in 2.5 mL dimethyl formamide (DMF) was treated at 50°C under nitrogen with L-cysteine (0.15 g, 1.21 mmol), and the pH of the mixture was adjusted to 9 using ammonia water. After stirring for 1 min, 1,2-dinitrobenzene or 1,4-dinitrobenzene (0.15 g, 0.89 mmol) was added, and the mixture was continuously stirred until bis(2-aminophenyl) disulfide was completely consumed, as monitored by TLC. Then, the mixture was diluted with water and extracted with Et2O or AcOEt. The organic layer was washed with brine, dried with MgSO4, filtered and concentrated under reduced pressure. The crude product 1a,c was purified by column chromatography on silica gel eluting with hexane-ethyl acetate, 9:1.
2-Aminophenyl 2-nitrophenyl sulfide (1a)
Yield 99%; yellow solid; mp 80–81°C; Rf 0.50 (hexane-AcOEt, 3:1); IR: 3468 (N-H), 3373 (N-H), 3099 (Ar, C-H), 1612 (Ar, C=C), 1511 (ArNO2, (N=O)2), 1331 (ArNO2, (N=O)2), 1042 (Ar-S-Ar), 854 (ArNO2, C-N), 724 cm−1 (Ar, C-H); 1H NMR (CDCl3): δ 4.28 (s, 2H, NH2), 6.80 (t, J=7.5 Hz, 1H, H-5′), 6.83–6.86 (m, 2H, H-3′, H-6), 7.21 (t, J=7.7 Hz, 1H, H-4), 7.29–7.36 (m, 2H, H-4′, H-5), 7.41 (d, J=8.0 Hz, 1H, H-6′), 8.23 (d, J=8.0 Hz, 1H, H-3); 13C NMR (CDCl3): δ 112.0 (C-1′), 115.5 (C-3′), 119.1 (C-5′), 125.0 (C-4), 126.0 (C-3), 127.2 (C-6), 132.3 (C-4′), 133.7 (C-5), 137.2 (C-1), 137.7 (C-6′), 145.1 (C-2), 149.3 (C-2′).
2-Aminophenyl 4-nitrophenyl sulfide (1c)
Yield 99%; yellow solid; mp 63–64°C; Rf 0.49 (hexane-AcOEt, 3:1); IR: 3457 (N-H), 3357 (N-H), 1616 (Ar, C=C), 1497 (Ar, C=C), 1477 (Ar, C=C), 1336 (ArNO2, (N=O)2), 853 (ArNO2, C-N), 837 (Ar, C-H), 746 cm−1 (Ar, C-H); 1H NMR: δ 5.47 (ss, 2H, NH2), 6.65 (td, J=3.3, 7.5 Hz, 1H, H-5′), 6.88 (dd, J=3.2, 8.3 Hz, 1H, H-3′), 7.16–7.19 (m, 2H, H-2, H-6), 7.24–7.28 (m, 1H, H-4′), 7.34 (dd, J=3.1, 7.5 Hz, 1H, H-6′), 8.10–8.12 (m, 2H, H-3, H-5); 13C NMR: δ 108.9 (C-1′), 115.2 (C-3′), 116.8 (C-5′), 123.9 (C-3, C-5), 125.5 (C-2, C-6), 131.9 (C-4′), 137.1 (C-6′), 144.6 (C-4), 147.3 (C-1), 150.6 (C-2′).
Methyl [2-(2-aminophenyl)sulfanyl]-5-nitrobenzoate (2a)
Yield 99%; yellow solid; mp 105–106°C; Rf 0.10 (hexane-AcOEt, 9:1); IR: 3470 (N-H), 3373 (N-H), 1711 (-C(=O)-O-), 1611 (Ar, C=C), 1573 (ArNO2, (N=O)2), 1513 (Ar, C=C), 1477 (Ar, C=C), 1335 (ArNO2, (N=O)2), 1260 (-C(=O)-O-), 1132 (-C-C(=O)-O-), 1048 (Ar-S-Ar), 902 (ArNO2, C-N), 837 (Ar, C-H), 738 cm−1 (Ar, C-H); 1H NMR (CDCl3): δ 4.03 (s, 3H, -C(=O)OCH3), 4.28 (s, 2H, NH2), 6.83–6.86 (m, 2H, H-3′, H-5′), 6.89 (d, J=8.6 Hz, 1H, H-3), 7.34 (td, J=1.7, 7.7 Hz, 1H, H-4′), 7.42 (d, J=7.4 Hz, 1H, H-6′), 8.06 (dq, J=1.2, 8.9 Hz, 1H, H-4), 8.88 (m, 1H, H-6); 13C NMR (CDCl3): δ 52.8 (-C(=O)OCH3), 111.8 (C-1′), 115.7 (C-5′), 119.4 (C-3′), 126.4–126.6 (C-3, C-4, C-6), 126.7 (C-1), 132.5 (C-4′), 137.5 (C-6′), 144.4 (C-5), 149.3 (C-2′), 151.1 (C-2), 165.2 (-C(=O)OCH3). HRMS (FD). Calcd for C14H12N2O4S, [M]+: m/z 304.0518. Found: m/z 304.0519.
Methyl [2-(2-amino-5-methylphenyl)sulfanyl]-5-nitrobenzoate (2b)
Yield 99%; orange solid; mp 117–118°C; Rf 0.48 (hexane-AcOEt, 3:1); IR: 3457 (N-H), 3366 (N-H), 3027 (Ar, C-H), 2954 (CH3), 2925 (CH3), 1719 (-C(=O)-O-), 1615 (Ar, C=C), 1498 (Ar, C=C), 1453 (Ar, C=C), 1339 (ArNO2, (N=O)2), 1304 (ArNH2, C-N), 1254 (-C(=O)-O-), 1131 (-C-C(=O)-O-), 1047 (Ar-S-Ar), 918 (ArNO2, C-N), 741 cm−1 (Ar, C-H); 1H NMR (CDCl3): δ 2.27 (s, 3H, ArCH3), 4.03 (d, J=1.4 Hz, 3H, -C(=O)OCH3), 4.13 (s, 2H, NH2), 6.78 (d, J=7.4 Hz, 1H, H-3′), 6.91 (dd, J=1.1, 8.9 Hz, 1H, H-3), 7.15 (d, J=8.3 Hz, 1H, H-4′), 7.23 (s, 1H, H-6′), 8.06 (d, J=8.9 Hz, 1H, H-4), 8.88 (s, 1H, H-6); 13C NMR (CDCl3): δ 20.2 (ArCH3), 52.8 (-C(=O)OCH3), 111.8 (C-1′), 115.9 (C-3′), 126.3 (C-1), 126.4 (C-4), 126.6 (C-6), 126.8 (C-3), 128.9 (C-5′), 133.3 (C-4′), 137.4 (C-6′), 144.4 (C-2), 146.8 (C-2′), 151.3 (C-5), 165.2 (-C(=O)OCH3). HRMS (FD). Calcd for C15H14N2O4S, [M]+: m/z 318.0674. Found: m/z 318.0677.
Methyl [2-(2-amino-3-methylphenyl)sulfanyl]-5-nitrobenzoate (2c)
Yield 99%; yellow solid; mp 96–97°C; Rf 0.48 (hexane-AcOEt, 3:1); IR: 3488 (N-H), 3393 (N-H), 2950 (CH3), 2862 (CH3), 1727 (-C(=O)-O-), 1612 (Ar, C=C), 1518 (ArNO2, (N=O)2), 1338 (ArNO2, (N=O)2), 1243 (-C(=O)-O-), 969 (Ar-S-Ar), 901 (ArNO2, C-N), 735 (Ar, C-H), 675 cm−1 (Ar, C=C); 1H NMR (CDCl3): δ 2.24 (s, 3H, ArCH3), 4.03 (s, 3H, -C(=O)OCH3), 4.27 (s, 2H, NH2), 6.77 (t, J=7.6 Hz, 1H, H-5′), 6.86 (d, J=8.9 Hz, 1H, H-3), 7.23 (d, J=7.4 Hz, 1H, H-4′), 7.31 (d, J=7.7 Hz, 1H, H-6′), 8.05 (dd, J=2.6, 9.2 Hz, 1H, H-4), 8.87 (d, J=2.6 Hz, 1H, H-6); 13C NMR (CDCl3): δ 18.0 (ArCH3), 52.8 (-C(=O)OCH3), 111.5 (C-1′), 118.8 (C-5′), 123.1 (C-3′), 126.4 (C-1, C-4), 126.6 (C-6), 126.7 (C-3), 133.4 (C-4′), 135.2 (C-6′), 144.3 (C-2), 147.5 (C-2′), 151.3 (C-5), 165.2 (-C(=O)OCH3). HRMS (FD). Calcd for C15H14N2O4S, [M]+: 318.0674. Found: m/z 318.0660.
Methyl [2-(2-amino-4,5-dimethylphenyl)sulfanyl]-5-nitrobenzoate (2d)
Yield 99%; orange solid; mp 166–167°C; Rf 0.49 (hexane-AcOEt, 3:1); IR: 3466 (N-H), 3371 (N-H), 2957 (CH3), 1721 (-C(=O)-O-), 1616 (Ar, C=C), 1516 (ArNO2, (N=O)2), 1456 (Ar, C=C), 1337 (ArNO2, (N=O)2), 1249 (-C(=O)-O-), 1193 (-C-C(=O)-O-), 890 (ArNO2, C-N), 738 (Ar, C-H), 672 cm−1 (Ar, C=C); 1H NMR (CDCl3): δ 2.17 (s, 3H, ArCH3 at C-5′), 2.25 (s, 3H, ArCH3 at C-4′), 4.02 (s, 3H, -C(=O)OCH3), 4.07 (s, 2H, NH2), 6.68 (s, 1H, H-3′), 6.92 (d, J=9.2 Hz, 1H, H-3), 7.15 (s, 1H, H-6′), 8.05 (dd, J=2.6, 8.9 Hz, 1H, H-4), 8.86 (d, J=2.6 Hz, 1H, H-6); 13C NMR (CDCl3): δ 18.5 (ArCH3 at C-5′), 19.9 (ArCH3 at C-4′), 52.7 (-C(=O)OCH3), 108.8 (C-1′), 117.2 (C-3′), 126.3 (C-1, C-4), 126.6 (C-6), 126.8 (C-3), 127.9 (C-4′), 137.7 (C-6′), 141.7 (C-5′), 144.2 (C-2), 147.1 (C-2′), 151.8 (C-5), 165.2 (-C(=O)OCH3). HRMS (FD). Calcd for C16H16N2O4S, [M]+: m/z 332.0831. Found: m/z 332.0841.
Methyl [2-(2-amino-5-methoxyphenyl)sulfanyl]-5-nitrobenzoate (2e)
Yield 99%; yellow solid; mp 115–116°C; Rf 0.49 (hexane-AcOEt, 3:1); IR: 3454 (N-H), 3366 (N-H), 3006 (Ar, C-H), 2957 (CH3), 1709 (-C(=O)-O-), 1494 (Ar, C=C), 1435 (Ar, C=C), 1345 (ArNO2, (N=O)2), 1266 (C-O-C), 1047 (Ar-S-Ar), 900 (ArNO2, C-N), 838 (Ar, C-H), 691 cm−1 (Ar, C=C); 1H NMR (CDCl3): δ 3.75 (s, 3H, ArOCH3), 3.99 (s, 2H, NH2), 4.02 (s, 3H, -C(=O)OCH3), 6.83 (d, J=8.6 Hz, 1H, H-3′), 6.91 (d, J=9.2 Hz, 1H, H-3), 6.97 (m, 2H, H-4′, H-6′), 8.07 (dd, J=2.6, 9.2 Hz, 1H, H-4), 8.88 (d, J=2.3 Hz, 1H, H-6); 13C NMR (CDCl3): δ 52.8 (-C(=O)OCH3), 55.8 (ArOCH3), 112.5 (C-1′), 117.1 (C-3′), 119.8 (C-4′), 120.7 (C-6′), 126.3 (C-1), 126.5 (C-4), 126.6 (C-6), 126.8 (C-3), 143.3 (C-5′), 144.4 (C-2), 150.9 (C-5), 152.8 (C-2′), 165.2 (-C(=O)OCH3). HRMS (FD). Calcd for C15H14N2O5S, [M]+: m/z 334.0623. Found: m/z 334.0622.
Methyl [2-(2-amino-5-fluorophenyl)sulfanyl]-5-nitrobanzoate (2f)
Yield 99%; yellow solid; mp 139–140°C; Rf 0.25 (hexane-AcOEt, 3:1); IR: 3498 (N-H), 3397 (N-H), 3064 (Ar, C-H), 2951 (CH3), 2842 (CH3), 1709 (-C(=O)-O-), 1623 (Ar, C=C), 1521 (ArNO2, (N=O)2), 1489 (Ar, C=C), 1455 (Ar, C=C), 1346 (ArNO2, (N=O)2), 1298 (ArNH2, C-N), 1248 (-C(=O)-O-), 1203 (Ar, C-F), 1134 (-C-C(=O)-O-), 1049 (Ar-S-Ar), 933 (ArNO2, C-N), 783 (Ar, C-H), 680 cm−1 (Ar, C=C); 1H NMR (CDCl3): δ 4.03 (s, 3H, -C(=O)OCH3), 4.16 (s, 2H, NH2), 6.82 (q, J=4.6 Hz, 1H, H-3′), 6.90 (d, J=8.9 Hz, 1H, H-3), 7.09 (td, J=2.8, 8.4 Hz, 1H, H-4′), 7.18 (dd, J=2.9, 8.0 Hz, 1H, H-6′), 8.09 (dd, J=2.6, 8.9 Hz, 1H, H-4), 8.89 (d, J=2.6 Hz, 1H, H-6); 13C NMR (CDCl3): δ 52.8 (-C(=O)OCH3), 112.5 (C-1′), 116.5 (C-3′), 119.7 (C-4′), 122.9 (C-6′), 126.5, 126.6, 126.7 (C-1, C-3, C-4, C-6), 144.6 (C-2), 145.8 (C-2′), 150.0 (C-5), 154.6 (C-5), 156.5 (C-5′), 165.1 (-C(=O)OCH3). HRMS (FD). Calcd for C14H11N2O4SF, [M]+: m/z 322.0424. Found: m/z 322.0428.
Methyl [2-(2-amino-4-fluorophenyl)sulfanyl]-5-nitrobenzoate (2g)
Yield 99%; yellow solid; mp 155–156°C; Rf 0.36 (hexane-AcOEt, 3:1); IR: 3466 (N-H), 3385 (N-H), 3088 (Ar, C-H), 2959 (CH3), 2847 (CH3), 1717 (-C(=O)-O-), 1622 (Ar, C=C), 1575 (ArNO2, (N=O)2), 1486 (Ar, C=C), 1461 (Ar, C=C), 1338 (ArNO2, (N=O)2), 1169 (-C-C(=O)-O-), 906 (ArNO2, C-N), 742 (Ar, C-H), 677 cm−1 (Ar, C=C); 1H NMR (CDCl3): δ 4.03 (s, 3H, -C(=O)OCH3), 4.41 (s, 2H, NH2), 6.55 (m, 2H, H-3′, H-5′), 6.88 (d, J=8.9 Hz, 1H, H-3), 7.39 (t, J=7.4 Hz, 1H, H-6′), 8.08 (dd, J=2.6, 9.2 Hz, 1H, H-4), 8.88 (d, J=2.6 Hz, 1H, H-6); 13C NMR (CDCl3): δ 52.8 (-C(=O)OCH3), 102.3 (C-3′), 106.9 (C-5′), 107.3 (C-1′), 126.5 (C-1, C-3, C-4), 126.7 (C-6), 139.4 (C-6′), 144.5 (C-2), 150.9 (C-2′), 151.0 (C-5), 165.1 (-C(=O)OCH3), 166.7 (C-4′). HRMS (FD). Calcd for C14H11N2O4SF, [M]+: m/z 322.0424. Found: m/z 322.0430.
Methyl [2-(2-amino-5-chlorophenyl)sulfanyl]-5-nitrobenzoate (2h)
Yield 99%; yellow solid; mp 141–142°C; Rf 0.48 (hexane-AcOEt, 3:1); IR: 3491 (N-H), 3394 (N-H), 1790 (-C(=O)-O-), 1616 (Ar, C=C), 1573 (ArNO2, (N=O)2), 1479 (Ar, C=C), 1435 (Ar, C=C), 1346 (ArNO2, (N=O)2), 1267 (-C(=O)-O-), 1150 (-C-C(=O)-O-), 901 (ArNO2, C-N), 784 cm−1 (Ar, C-H); 1H NMR (CDCl3): δ 4.03 (s, 3H, -C(=O)OCH3), 4.30 (s, 2H, NH2), 6.80 (d, J=8.6 Hz, 1H, H-3′), 6.90 (d, J=8.9 Hz, 1H, H-3), 7.29 (dd, J=2.4, 8.7 Hz, 1H, H-4′), 7.42 (d, J=2.6 Hz, 1H, H-6′), 8.09 (dd, J=2.6, 8.9 Hz, 1H, H-4), 8.88 (d, J=2.3 Hz, 1H, H-6); 13C NMR (CDCl3): δ 52.9 (-C(=O)OCH3), 113.2 (C-1′), 116.7 (C-3′), 123.2 (C-5′), 126.5 (C-1), 126.6 (C-3, C-4), 126.7 (C-6), 132.5 (C-4′), 136.5 (C-6′), 144.6 (C-2), 147.9 (C-2′), 149.9 (C-5), 165.1 (-C(=O)OCH3). HRMS (FD). Calcd for C14H11N2O4SCl, [M]+: m/z 338.0128. Found: m/z 338.0130.
Methyl [2-(2-amino-3-chlorophenyl)sulfanyl]-5-nitrobenzoate (2i)
Yield 99%; yellow solid; mp 122–123°C; Rf 0.58 (hexane-AcOEt, 3:1); IR: 3439 (N-H), 3384 (N-H), 2959 (CH3), 2850 (CH3), 1721 (-C(=O)-O-), 1573 (ArNO2, (N=O)2), 1516 (Ar, C=C), 1455 (Ar, C=C), 1341 (ArNO2, (N=O)2), 1264 (-C(=O)-O-), 1150 (-C-C(=O)-O-), 1084 (Ar, C-Cl), 1047 (Ar-S-Ar), 902 (ArNO2, C-N), 784 (Ar, C-H), 681 cm−1 (Ar, C=C); 1H NMR (CDCl3): δ 4.04 (s, 3H, -C(=O)OCH3), 4.71 (s, 2H, NH2), 6.77 (t, J=7.9 Hz, 1H, H-5′), 6.86 (d, J=9.2 Hz, 1H, H-3), 7.37 (d, J=7.4 Hz, 1H, H-4′), 7.45 (d, J=8.0 Hz, 1H, H-6′), 8.09 (dd, J=2.4, 9.0 Hz, 1H, H-4), 8.89 (d, J=2.6 Hz, 1H, H-6); 13C NMR (CDCl3): δ 52.8 (-C(=O)OCH3), 113.2 (C-1′), 118.8 (C-5′), 119.8 (C-3′), 126.5 (C-1), 126.6 (C-3, C-4), 126.7 (C-6), 132.4 (C-6′), 136.1 (C-4′), 144.6 (C-2), 145.9 (C-2′), 150.1 (C-5), 165.1 (-C(=O)OCH3). HRMS (FD). Calcd for C14H11N2O4SCl, [M]+: m/z 338.0128. Found: m/z 338.0122.
2-Aminophenyl 2-cyano-4-nitrophenyl sulfide (4a)
Yield 95%; yellow solid; mp 131–132°C; Rf 0.06 (hexane-AcOEt, 9:1); IR: 3471 (N-H), 3366 (N-H), 3105 (Ar, C-H), 2231 (C≡N), 1626 (Ar, C=C), 1574 (ArNO2, (N=O)2), 1516 (Ar, C=C), 1447 (Ar, C=C), 1339 (ArNO2, (N=O)2), 1307 (ArNH2, C-N), 1148 (-C-C(=O)-O-), 1011 (Ar-S-Ar), 908 (ArNO2, C-N), 759 cm−1 (Ar, C-H); 1H NMR (CDCl3): δ 4.32 (s, 2H, NH2), 6.84 (t, J=7.6 Hz, 1H, H-5′), 6.88 (m, 2H, H-3′, H-6), 7.37 (t, J=7.7 Hz, 1H, H-4′), 7.43 (d, J=7.7 Hz, 1H, H-6′), 8.13 (d, J=8.9 Hz, 1H, H-5), 8.44 (s, 1H, H-3); 13C NMR (CDCl3): δ 109.1 (C-1′), 110.3 (C-2), 114.9 (-C≡N), 116.2 (C-3′), 119.7 (C-5′), 126.4 (C-6), 127.4 (C-5), 128.7 (C-3), 133.3 (C-4′), 137.7 (C-6′), 144.9 (C-1), 149.4 (C-2′), 152.0 (C-4). HRMS (FD). Calcd for C13H9N3O2S, [M]+: m/z 271.0415. Found: m/z 271.0420.
2-Amino-5-methylphenyl 2-cyano-4-nitrophenyl sulfide (4b)
Yield 96%; orange solid; mp 111–112°C; Rf 0.33 (hexane-AcOEt, 3:1); IR: 3459 (N-H), 3361 (N-H), 2921 (CH3), 2860 (CH3), 2224 (C≡N), 1628 (Ar, C=C), 1568 (ArNO2, (N=O)2), 1500 (Ar, C=C), 1454 (Ar, C=C), 1338 (ArNO2, (N=O)2), 1306 (ArNH2, C-N), 915 cm−1 (ArNO2, C-N); 1H NMR (CDCl3): δ 2.27 (s, 3H, ArCH3), 4.17 (s, 2H, NH2), 6.80 (d, J=8.0 Hz, 1H, H-3′), 6.89 (d, J=8.9 Hz, 1H, H-6), 7.19 (d, J=8.3 Hz, 1H, H-4′), 7.24 (s, 1H, H-6′), 8.14 (dd, J=2.1, 9.0 Hz, 1H, H-5), 8.45 (d, J=2.0 Hz, 1H, H-3); 13C NMR (CDCl3): δ 20.1 (ArCH3), 108.8 (C-1′), 110.0 (C-2), 114.8 (-C≡N), 116.2 (C-3′), 126.3 (C-6), 127.2 (C-5), 128.5 (C-3), 129.1 (C-5′), 134.0 (C-4′), 137.3 (C-6′), 144.6 (C-1), 146.8 (C-2′), 152.0 (C-4). HRMS (FD). Calcd for C14H11N3O2S, [M]+: m/z 285.0572. Found: m/z 285.0571.
2-Amino-3-methylphenyl 2-cyano-4-nitrophenyl sulfide (4c)
Yield 76%; yellow solid; mp 122–123°C; Rf 0.38 (hexane-AcOEt, 3:1); IR: 3462 (N-H), 3363 (N-H), 2915 (CH3), 2857 (CH3), 2236 (C≡N), 1634 (Ar, C=C), 1514 (Ar, C=C), 1467 (Ar, C=C), 1343 (ArNO2, (N=O)2), 1299 (ArNH2, C-N), 1082 (Ar-S-Ar), 768 (Ar, C-H), 694 cm−1 (Ar, C=C); 1H NMR (CDCl3): δ 2.25 (s, 3H, ArCH3), 4.30 (s, 2H, NH2), 6.78 (t, J=7.6 Hz, 1H, H-5′), 6.85 (d, J=8.9 Hz, 1H, H-6), 7.27 (d, J=6.9 Hz, 1H, H-4′), 7.32 (d, J=7.7 Hz, 1H, H-6′), 8.13 (dd, J=2.6, 8.9 Hz, 1H, H-5), 8.45 (d, J=2.3 Hz, 1H, H-3); 13C NMR (CDCl3): δ 18.1 (ArCH3), 108.5 (C-1′), 110.0 (C-2), 114.8 (-C≡N), 119.0 (C-5′), 123.5 (C-3′), 126.2 (C-6), 127.2 (C-5), 128.5 (C-3), 134.0 (C-4′), 135.2 (C-6′), 144.6 (C-1), 147.5 (C-2′), 152.1 (C-4). HRMS (FD). Calcd for C14H11N3O2S, [M]+: m/z 285.0572. Found: m/z 285.0574.
2-Amino-4,5-dimethylphenyl 2-cyano-4-nitrophenyl sulfide (4d)
Yield 84%; orange solid; mp 149–150°C; Rf 0.34 (hexane-AcOEt, 3:1); IR: 3457 (N-H), 3375 (N-H), 3050 (Ar, C-H), 2921 (CH3), 2852 (CH3), 2232 (C≡N), 1621 (Ar, C=C), 1564 (ArNO2, (N=O)2), 1515 (Ar, C=C), 1454 (Ar, C=C), 1337 (ArNO2, (N=O)2), 1309 (ArNH2, C-N), 794 cm−1 (Ar, C-H); 1H NMR (CDCl3): δ 2.18 (s, 3H, ArCH3 at C-5′), 2.26 (s, 3H, ArCH3 at C-4′), 4.09 (s, 2H, NH2), 6.70 (s, 1H, H-3′), 6.89 (d, J=9.2 Hz, 1H, H-6), 7.17 (s, 1H, H-6′), 8.12 (dd, J=2.4, 9.0 Hz, 1H, H-5), 8.44 (d, J=2.6 Hz, 1H, H-3); 13C NMR (CDCl3): δ 18.5 (ArCH3 at C-5′), 20.0 (ArCH3 at C-4′), 105.8 (C-1′), 109.8 (C-2), 114.9 (-C≡N), 117.4 (C-3′), 126.2 (C-6), 127.1 (C-5), 128.2 (C-4′), 128.4 (C-3), 137.6 (C-6′), 142.6 (C-5′), 144.5 (C-4), 147.1 (C-2′), 152.6 (C-1). HRMS (FD). Calcd for C15H13N3O2S, [M]+: m/z 299.0728. Found: m/z 299.0728.
2-Amino-5-methoxyphenyl 2-cyano-4-nitrophenyl sulfide (4e)
Yield 75%; orange solid; mp 149–150°C; Rf 0.28 (hexane-AcOEt, 3:1); IR: 3466 (N-H), 3375 (N-H), 2229 (C≡N), 1570 (ArNO2, (N=O)2), 1498 (Ar, C=C), 1454 (Ar, C=C), 1348 (ArNO2, (N=O)2), 1299 (C-O-C), 1032 (Ar-S-Ar), 914 cm−1 (ArNO2, C-N); 1H NMR (600 MHz, CDCl3): δ 3.77 (s, 3H, ArOCH3), 4.02 (s, 2H, NH2), 6.86 (d, J=8.6 Hz, 1H, H-3′), 6.91 (d, J=9.2 Hz, 1H, H-6), 6.99 (dd, J=2.9, 8.0 Hz, 1H, H-4′), 7.02 (d, J=2.9 Hz, 1H, H-6′), 8.16 (dd, J=2.3, 8.9 Hz, 1H, H-5), 8.47 (d, J=2.6 Hz, 1H, H-3); 13C NMR (CDCl3): δ 55.9 (ArOCH3), 109.4 (C-1′), 110.1 (C-2), 114.8 (-C≡N), 117.5 (C-3′), 120.6 (C-4′, C-6′), 126.3 (C-6), 127.3 (C-5), 128.5 (C-3), 143.3 (C-2′), 144.8 (C-1), 151.6 (C-4), 152.8 (C-5′). HRMS (FD). Calcd for C14H11N3O3S, [M]+: m/z 301.0521. Found: m/z 301.0530.
2-Amino-5-fluorophenyl 2-cyano-4-nitrophenyl sulfide (4f)
Yield 89%; yellow solid; mp 160–161°C; Rf 0.20 (hexane-AcOEt, 3:1); IR: 3452 (N-H), 3357 (N-H), 2237 (C≡N), 1631 (Ar, C=C), 1567 (ArNO2, (N=O)2), 1494 (Ar, C=C), 1454 (Ar, C=C), 1342 (ArNO2, (N=O)2), 1301 (ArNH2, C-N), 913 cm−1 (ArNO2, C-N); 1H NMR (CDCl3): δ 4.19 (s, 2H, NH2), 6.85 (q, J=4.5 Hz, 1H, H-3′), 6.92 (d, J=8.9 Hz, 1H, H-6), 7.13 (t, J=6.9 Hz, 1H, H-4′), 7.20 (d, J=7.7 Hz, 1H, H-6′), 8.17 (d, J=8.9 Hz, 1H, H-5), 8.47 (s, 1H, H-3); 13C NMR (CDCl3): δ 109.5 (C-1′) 110.5 (C-2), 114.6 (-C≡N), 116.9 (C-3′), 120.6 (C-4′), 122.9 (C-6′), 126.4 (C-6), 127.3 (C-5), 128.6 (C-3), 145.1 (C-1), 145.8 (C-4), 150.6 (C-2′), 154.5 (C-5′). HRMS (FD). Calcd for C13H8N3O2SF, [M]+: m/z 289.0321. Found: m/z 289.0316.
2-Amino-4-fluorophenyl 2-cyano-4-nitrophenyl sulfide (4g)
Yield 75%; yellow solid; mp 126–127°C; Rf 0.36 (hexane-AcOEt, 3:1); IR: 3451 (N-H), 3353 (N-H), 1632 (Ar, C=C), 1569 (ArNO2, (N=O)2), 1452 (Ar, C=C), 1342 (ArNO2, (N=O)2), 1145 (Ar, C-F), 914 cm−1 (ArNO2, C-N); 1H NMR (CDCl3): δ 4.43 (s, 2H, NH2), 6.58 (m, 2H, H-3′, H-5′), 6.88 (d, J=8.9 Hz, 1H, H-6), 7.42 (t, J=7.3 Hz, 1H, H-6′), 8.16 (dd, J=2.3, 8.9 Hz, 1H, H-5), 8.46 (d, J=2.6 Hz, 1H, H-3); 13C NMR (CDCl3): δ 102.6 (C-3′), 104.4 (C-1′), 107.2 (C-5′), 110.3 (C-2), 114.7 (-C≡N), 126.1 (C-6), 127.3 (C-5), 128.6 (C-3), 139.6 (C-6′), 145.0 (C-1), 151.0 (C-2′), 151.5 (C-4), 164.8 (C-4′). HRMS (FD). Calcd for C13H8N3O2SF, [M]+: m/z 289.0321. Found: m/z 289.0321.
2-Amino-5-chlorophenyl 2-cyano-4-nitrophenyl sulfide (4h)
Yield 83%; orange-colored solid; mp 146–147°C; Rf 0.25 (hexane-AcOEt, 3:1); IR: 3456 (N-H), 3358 (N-H), 2242 (C≡N), 1629 (Ar, C=C), 1515 (Ar, C=C), 1455 (Ar, C=C), 1340 (ArNO2, (N=O)2), 1299 (ArNH2, C-N), 1152 (Ar, C-Cl), 1053 (Ar-S-Ar), 794 (Ar, C-H), 726 cm-1 (Ar, C=C); 1H NMR (CDCl3): δ 4.33 (s, 2H, NH2), 6.82 (d, J=8.6 Hz, 1H, H-3′), 6.92 (d, J=9.2 Hz, 1H, H-6), 7.32 (dd, J=2.3, 8.6 Hz, 1H, H-4′), 7.45 (d, J=2.3 Hz, 1H, H-6′), 8.18 (dd, J=2.3, 8.9 Hz, 1H, H-5), 8.47 (d, J=2.3 Hz, 1H, H-3); 13C NMR (CDCl3): δ 110.2 (C-1′), 110.5 (C-2), 114.6 (-C≡N), 117.1 (C-3′), 123.5 (C-5′), 126.4 (C-6), 127.4 (C-5), 128.6 (C-3), 133.2 (C-4′), 136.5 (C-6′), 145.1 (C-1), 147.9 (C-2′), 150.5 (C-4). HRMS (FD). Calcd for C13H8N3O2SCl, [M]+: m/z 305.0026. Found: m/z 305.0022.
2-Amino-3-chlorophenyl 2-cyano-4-nitrophenyl sulfide (4i)
Yield 71%; yellow solid; mp 126–127°C; Rf 0.58 (hexane-AcOEt, 3:1); IR: 3442 (N-H), 3346 (N-H), 2237 (C≡N), 1627 (Ar, C=C), 1512 (Ar, C=C), 1456 (Ar, C=C), 1344 (ArNO2, (N=O)2), 1304 (ArNH2, C-N), 1147 (Ar, C-Cl), 1058 (Ar-S-Ar), 914 cm−1 (ArNO2, C-N); 1H NMR (CDCl3): δ 4.75 (s, 2H, NH2), 6.79 (t, J=7.9 Hz, 1H, H-5′), 6.88 (d, J=8.9 Hz, 1H, H-6), 7.40 (d, J=7.7 Hz, 1H, H-3′), 7.49 (d, J=7.7 Hz, 1H, H-4′), 8.18 (dd, J=2.3, 8.9 Hz, 1H, H-5), 8.48 (d, J=2.3 Hz, 1H, H-3); 13C NMR (CDCl3): δ 110.2 (C-1′), 110.4 (C-2), 114.6 (-C≡N), 119.0 (C-5′), 120.2 (C-3′), 126.2 (C-6), 127.4 (C-5), 128.6 (C-3), 133.1 (C-4′), 136.2 (C-6′), 145.0 (C-1), 145.9 (C-2′), 150.7 (C-4). HRMS (FD). Calcd for C13H8N3O2SCl, [M]+: m/z 305.0026. Found: m/z 305.0025.
2-Nitrodibenzo[b,f][1,4]thiazepine (7a)
Yield 67%; yellow solid; mp 148–149°C; Rf 0.50 (hexane-AcOEt, 3:1); IR: 3051 (Ar, C-H), 1599 (Ar, C=C), 1567 (ArNO2, (N=O)2), 1520 (Ar, C=C), 1460 (Ar, C=C), 1345 (ArNO2, (N=O)2), 1058 (Ar-S-Ar), 945 (Ar, C-H), 914 (ArNO2, C-N), 845 (Ar, C-H), 778 cm−1 (Ar, C-H); 1H NMR: δ 7.31 (m, 2H, H-8, H-9), 7.47 (m, 2H, H-6, H-7), 7.73 (d, J=8.6 Hz, 1H, H-4), 8.28 (t, J=5.6 Hz, 1H, H-3), 8.50 (d, J=2.6 Hz, 1H, H-1), 9.04 (s, 1H, H-11); 13C NMR: δ 125.4 (C-1), 126.7 (C-3), 127.1 (C-5a), 127.2 (C-9), 128.4 (C-8), 130.7 (C-7), 133.2 (C-4), 133.5 (C-6), 137.8 (C-11a), 146.7 (C-4a), 148.1 (C-2), 148.5 (C-9a), 161.5 (C-11). HRMS (FD). Calcd for C13H8N2O2S, [M]+: m/z 256.0306. Found: m/z 256.0315.
7-Methyl-2-nitrodibenzo[b,f][1,4]thiazepine (7b)
Yield 60%; yellow solid; mp 133–134°C; Rf 0.51 (hexane-AcOEt, 3:1); IR: 3045 (Ar, C-H), 2923 (CH3), 2850 (CH3), 1601 (Ar, C=C), 1569 (ArNO2, (N=O)2), 1525 (Ar, C=C), 1468 (Ar, C=C), 1347 (ArNO2, (N=O)2), 941 (Ar, C-H), 915 (ArNO2, C-N), 741 cm−1 (Ar, C-H); 1H NMR: δ 2.26 (s, 3H, ArCH3), 7.18 (d, J=8.0 Hz, 1H, H-9), 7.23 (d, J=8.3 Hz, 1H, H-8), 7.26 (s, 1H, H-6), 7.69 (d, J=8.6 Hz, 1H, H-4), 8.25 (dd, J=2.3, 8.6 Hz, 1H, H-3), 8.45 (d, J=2.6 Hz, 1H, H-1), 8.96 (s, 1H, H-11); 13C NMR: δ 20.5 (ArCH3), 125.3 (C-1), 126.6 (C-3), 127.2 (C-5a), 131.4 (C-9), 133.1 (C-8), 133.6 (C-7), 137.9 (C-4), 138.4 (C-6), 146.2 (C-11a), 146.5 (C-4a), 148.1 (C-2, C-9a), 160.1 (C-11). HRMS (FD). Calcd for C14H10N2O2S, [M]+: m/z 270.0463. Found: m/z 270.0458.
9-Methyl-2-nitrodibenzo[b,f][1,4]thiazepine (7c)
Yield 63%; dark yellow solid; mp 137–138°C; Rf 0.59 (hexane-AcOEt, 3:1); IR: 3043 (Ar, C-H), 2925 (CH3), 2852 (CH3), 1598 (Ar, C=C), 1566 (ArNO2, (N=O)2), 1449 (Ar, C=C), 1373 (Ar, C-N), 1339 (ArNO2, (N=O)2), 1005 (Ar-S-Ar), 943 (Ar, C-H), 916 (ArNO2, C-N), 773 (Ar, C-H), 699 cm−1 (Ar, C=C); 1H NMR: δ 2.27 (s, 3H, ArCH3), 7.13 (t, J=7.6 Hz, 1H, H-6), 7.26 (m, 2H, H-7, H-8), 7.68 (d, J=8.6 Hz, 1H, H-4), 8.22 (dd, J=2.6, 8.6 Hz, 1H, H-3), 8.42 (d, J=2.6 Hz, 1H, H-1), 9.06 (s, 1H, H-11); 13C NMR: δ 19.0 (ArCH3), 124.9 (C-1), 126.4 (C-3), 127.4 (C-5a), 128.1 (C-9), 131.1 (C-8), 132.1 (C-7), 133.1 (C-4), 135.1 (C-6), 137.9 (C-11a), 146.6 (C-4a), 147.0 (C-2), 148.1 (C-9a), 160.5 (C-11). HRMS (FD). Calcd for C14H10N2O2S, [M]+: m/z 270.0463. Found: m/z 270.0456.
7,8-Dimethyl-2-nitrodibenzo[b,f][1,4]thiazepine (7d)
Yield 49%; yellow solid; mp 210–211°C; Rf= 0.59 (hexane-AcOEt, 3:1); IR: 3056 (Ar, C-H), 2919 (CH3), 2857 (CH3), 1597 (Ar, C=C), 1567 (ArNO2, (N=O)2), 1525 (Ar, C=C), 1464 (Ar, C=C), 1344 (ArNO2, (N=O)2), 1023 (Ar-S-Ar), 942 (Ar, C-H), 912 (ArNO2, C-N), 879 (Ar, C-H), 769 cm−1 (Ar, C-H); 1H NMR (600 MHz, DMSO-d6): δ 2.17 (s, 3H, ArCH3), 2.19 (s, 3H, ArCH3), 7.09 (s, 1H, H-9), 7.22 (s, 1H, H-6), 7.69 (d, J=8.6 Hz, 1H, H-4), 8.25 (dd, J=2.6, 8.6 Hz, 1H, H-3), 8.45 (d, J=2.6 Hz, 1H, H-1), 8.96 (s, 1H, H-11); 13C NMR: δ 18.3 (ArCH3), 18.8 (ArCH3), 122.8 (C-1), 124.6 (C-3), 125.9 (C-9), 127.4 (C-4), 132.3 (C-5a), 133.2 (C-6), 136.6 (C-8), 137.3 (C-11a), 138.7 (C-7), 145.7 (C-9a), 146.2, 147.4 (C-2, C-4a), 160.1 (C-11). HRMS (FD). Calcd for C15H12N2O2S, [M]+: m/z 284.0619. Found: m/z 284.0631.
7-Methoxy-2-nitrodibenzo[b,f][1,4]thiazepine (7e)
Yield 68%; yellow solid; mp 164–165°C; Rf 0.40 (hexane-AcOEt, 3:1); IR: 3007 (Ar, C-H), 2977 (CH3), 2837 (CH3), 1590 (Ar, C=C), 1511 (ArNO2, (N=O)2), 1475 (Ar, C=C), 1344 (ArNO2, (N=O)2), 1236 (C-O-C), 1056 (C-O-C), 1039 (Ar-S-Ar), 945 (Ar, C-H), 912 (ArNO2, C-N), 741 (Ar, C-H), 696 cm−1 (Ar, C=C); 1H NMR: δ 3.76 (s, 3H, ArOCH3), 7.02 (m, 2H, H-6, H-8), 7.24 (d, J=8.3 Hz, 1H, H-9), 7.70 (d, J=8.6 Hz, 1H, H-4), 8.27 (dd, J=2.3, 8.6 Hz, 1H, H-3), 8.45 (d, J=2.3 Hz, 1H, H-1), 8.91 (s, 1H, H-11); 13C NMR: δ 55.6 (ArOCH3), 116.2 (C-6), 116.9 (C-8), 124.6 (C-1), 125.9 (C-3), 126.8 (C-5a), 128.2 (C-9), 132.6 (C-4), 137.3 (C-11a), 141.5 (C-9a), 145.1 (C-4a), 147.5 (C-2), 158.9 (C-7, C11). HRMS (FD). Calcd for C14H10N2O3S, [M]+: m/z 286.0412. Found: m/z 286.0418.
7-Fluoro-2-nitrodibenzo[b,f][1,4]thiazepine (7f)
Yield 54%; yellow solid; mp 138–139°C; Rf 0.50 (hexane-AcOEt, 3:1); IR: 3100 (Ar, C-H), 1600 (Ar, C=C), 1569 (ArNO2, (N=O)2), 1520 (Ar, C=C), 1475 (Ar, C=C), 1349 (ArNO2, (N=O)2), 1215 (Ar, C-F), 1066 (Ar-S-Ar), 943 (Ar, C-H), 915 (ArNO2, C-N), 768 (Ar, C-H), 714 cm−1 (Ar, C=C); 1H NMR: δ 7.27–7.38 (m, 3H, H-6, H-8, H-9), 7.72 (d, J=8.6 Hz, 1H, H-4), 8.29 (dd, J=2.6, 8.6 Hz, 1H, H-3), 8.48 (d, J=2.3 Hz, 1H, H-1), 9.01 (s, 1H, H-11); 13C NMR: δ 117.2, 119.1 (C-6, C-8), 124.7 (C-1), 126.2 (C-3), 127.8 (C-5a), 128.3 (C-9), 137.0 (C-11a), 144.7 (C-9a), 145.0 (C-4a), 147.7 (C-2), 159.8 (C-9a), 160.7 (C-11), 161.8 (C-7). HRMS (FD). Calcd for C13H7N2O2SF, [M]+: m/z 274.0212. Found: m/z 274.0211.
8-Fluoro-2-nitrodibenzo[b,f][1,4]thiazepine (7g)
Yield 66%; yellow solid; mp 141–142°C; Rf 0.59 (hexane-AcOEt, 3:1); IR: 3094 (Ar, C-H), 1594 (Ar, C=C), 1574 (ArNO2, (N=O)2), 1525 (Ar, C=C), 1465 (Ar, C=C), 1346 (ArNO2, (N=O)2), 1222 (Ar, C-F), 910 (ArNO2, C-N), 771 cm−1 (Ar, C-H); 1H NMR: δ 7.13 (m, 2H, H-7, H-9), 7.49 (dd, J=6.2, 9.2 Hz, 1H, H-6), 7.71 (dd, J=2.9, 8.6 Hz, 1H, H-4), 8.27 (d, J=8.6 Hz, 1H, H-3), 8.49 (s, 1H, H-1), 9.06 (s, 1H, H-11); 13C NMR: δ 113.0, 114.7 (C-7, C-9), 122.3 (C-5a), 124.7 (C-1), 126.1 (C-3), 132.4 (C-4), 134.4 (C-6), 137.0 (C-11a), 145.9 (C-4a), 147.5 (C-2), 149.3 (C-9a), 162.0 (C-11), 163.7 (C-8). HRMS (FD). Calcd for C13H7N2O2SF, [M]+: m/z 274.0212. Found: m/z 274.0223.
7-Chloro-2-nitrodibenzo[b,f][1,4]thiazepine (7h)
Yield 63%; yellow solid; mp 159–160°C; Rf 0.64 (hexane-AcOEt, 3:1); IR: 3046 (Ar, C-H), 1601 (Ar, C=C), 1521 (Ar, C=C), 1456 (Ar, C=C), 1347 (ArNO2, (N=O)2), 1057 (Ar-S-Ar), 949 (Ar, C-H), 915 (ArNO2, C-N), 740 (Ar, C-H), 709 cm−1 (Ar, C=C); 1H NMR: δ 7.32 (d, J=8.6 Hz, 1H, H-9), 7.50 (dd, J=2.4, 8.4 Hz, 1H, H-8), 7.56 (d, J=2.3 Hz, 1H, H-6), 7.74 (d, J=8.6 Hz, 1H, H-4), 8.31 (dd, J=2.6, 8.6 Hz, 1H, H-3), 8.51 (d, J=2.3 Hz, 1H, H-1), 9.06 (s, 1H, H-11); 13C NMR: δ 124.8 (C-1), 126.2 (C-3), 127.9 (C-9), 128.1 (C-7), 130.0 (C-8), 131.8 (C-5a), 131.9 (C-6), 132.8 (C-4), 137.0 (C-11a), 145.2 (C-4a), 146.7 (C-9a), 147.7 (C-2), 161.5 (C-11). HRMS (FD). Calcd for C13H7N2O2SCl, [M]+: m/z 289.9917. Found: m/z 289.9927.
9-Chloro-2-nitrodibenzo[b,f][1,4]thiazepine (7i)
Yield 47%; yellow solid; mp 167–168°C; Rf 0.59 (hexane-AcOEt, 3:1); IR: 3061 (Ar, C-H), 1602 (Ar, C=C), 1531 (ArNO2, (N=O)2), 1508 (Ar, C=C), 1456 (Ar, C=C), 1348 (ArNO2, (N=O)2), 1135 (Ar, C-Cl), 941 (Ar, C-H), 901 (ArNO2, C-N), 769 (Ar, C-H), 740 (Ar, C-H), 699 cm−1 (Ar, C=C); 1H NMR: δ 7.29 (d, J=8.0 Hz, 1H, H-7), 7.47 (d, J=8.0 Hz, 1H, H-6), 7.57 (d, J=7.7 Hz, 1H, H-8), 7.75 (d, J=8.6 Hz, 1H, H-4), 8.30 (s, 1H, H-3), 8.55 (s, 1H, H-1), 9.19 (s, 1H, H-11). 13C NMR: δ 124.5 (C-1), 126.3 (C-3), 128.3 (C-7), 129.3 (C-9), 130.1 (C-5a), 131.0, 131.7 (C-6, C-8), 132.8 (C-4), 136.9 (C-11a), 144.1 (C-9a), 145.5 (C-4a), 147.7 (C-2), 162.2 (C-11). HRMS (FD). Calcd for C13H7N2O2SCl, [M]+: m/z 289.9917. Found: m/z 289.9923.
Synthesis of 2-nitrodibenzo[b,f][1,4]thiazepin-11(10H)-one derivatives (3)
A representative example is the synthesis of 2-nitrodibenzo[b,f][1,4]thiazepin-11(10H)-one (3a). Methyl [2-(2-aminophenyl)sulfanyl]-5-nitrobenzoate (2a, 0.10 g, 0.33 mmol), 15 mL of o-xylene and 0.13 mmol of p-TsOH·H2O were placed in a 50-mL flask. The mixture was heated under reflux at 125°C overnight, then cooled to room temperature, neutralized using aqueous saturated sodium bicarbonate solution and extracted three times with AcOEt. The organic layer was washed several times with brine and dried over with MgSO4. After filtration and concentration, the residue was purified by column chromatography on silica gel eluting with n-hexane/ethyl acetate (4/1, v/v) to give compound 3a (0.09 g, 95%).
2-Nitrodibenzo[b,f][1,4]thiazepin-11(10H)-one (3a)
Yield 95%; dark orange solid; mp 263–264°C; Rf 0.20 (hexane-AcOEt, 3:1); IR: 3288 (N-H), 3179 (N-H), 3045 (Ar, C-H), 1653 (-NHC(=O)-), 1598 (Ar, C=C), 1568 (ArNO2, (N=O)2), 1521 (Ar, C=C), 1457 (Ar, C=C), 1344 (ArNO2, (N=O)2), 905 (ArNO2, C-N), 843 (Ar, C-H), 741 (Ar, C-H), 705 cm−1 (Ar, C=C); 1H NMR: δ 7.19 (t, J=7.6 Hz, 1H, H-7), 7.29 (d, J=7.4 Hz, 1H, H-9), 7.42 (t, J=7.2 Hz, 1H, H-8), 7.60 (d, J=6.9 Hz, 1H, H-6), 7.81 (d, J=8.6 Hz, 1H, H-4), 8.26 (dd, J=2.6, 8.6 Hz, 1H, H-3), 8.38 (d, J=2.6, Hz, 1H, H-1), 10.97 (s, 1H, H-10); 13C NMR: δ 123.4 (C-9), 125.8 (C-7), 125.9, 126.0 (C-1, C-3), 127.3 (C-5a), 130.3 (C-8), 132.7 (C-4, C-6), 138.6 (C-11a), 139.4 (C-9a), 144.2 (C-4a), 147.4 (C-2), 166.4 (C-11). HRMS (FD). Calcd for C13H8N2O3S, [M]+: m/z 272.0256. Found: m/z 272.0255.
7-Methyl-2-nitrodibenzo[b,f][1,4]thiazepin-11(10H)-one (3b)
Yield 87%; pale orange solid; mp 233–234°C; Rf 0.43 (hexane-AcOEt, 3:1); IR: 3293 (N-H), 3167 (N-H), 3029 (Ar, C-H), 2927 (CH3), 1657 (-NHC(=O)-), 1602 (Ar, C=C), 1527 (ArNO2, (N=O)2), 1498 (Ar, C=C), 1435 (Ar, C=C), 1345 (ArNO2, (N=O)2), 1054 (Ar-S-Ar), 933 (Ar, C-H), 896 (ArNO2, C-N), 741 (Ar, C-H), 708 cm−1 (Ar, C=C); 1H NMR: δ 2.25 (s, 3H, ArCH3), 7.17 (d, J=8.3 Hz, 1H, H-9), 7.22 (d, J=8.0 Hz, 1H, H-8), 7.41 (s, 1H, H-6), 7.79 (d, J=8.6 Hz, 1H, H-4), 8.25 (dd, J=2.6, 8.6 Hz, 1H, H-3), 8.37 (d, J=2.6 Hz, 1H, H-1), 10.86 (s, 1H, H-10); 13C NMR: δ 19.8 (ArCH3), 123.3 (C-9), 125.9 (C-1, C-3), 127.2 (C-5a), 130.8 (C-8), 132.7 (C-4), 132.8 (C-6), 135.5 (C-7), 136.8 (C-9a), 138.7 (C-11a), 144.3 (C-4a), 147.4 (C-2), 166.4 (C-10). HRMS (FD). Calcd for C14H10N2O3S, [M]+: m/z 286.0412. Found: m/z 286.0411.
9-Methyl-2-nitrodibenzo[b,f][1,4]thiazepin-11(10H)-one (3c)
Yield 89%; pale brown solid; mp 212–213°C; Rf 0.48 (hexane-AcOEt, 3:1); IR: 3296 (N-H), 3189 (N-H), 3056 (Ar, C-H), 2958 (CH3), 2921 (CH3), 2857 (CH3), 1662 (-NHC(=O)-), 1606 (Ar, C=C), 1539 (ArNO2, (N=O)2), 1460 (Ar, C=C), 1349 (ArNO2, (N=O)2), 1048 (Ar-S-Ar), 967 (Ar, C-H), 911 (ArNO2, C-N), 778 (Ar, C-H), 698 cm−1 (Ar, C=C); 1H NMR: δ 2.36 (s, 3H, ArCH3), 7.10 (t, J=7.6 Hz, 1H, H-7), 7.27 (d, J=7.4 Hz, 1H, H-8), 7.45 (d, J=7.7 Hz, 1H, H-6), 7.78 (d, J=8.3 Hz, 1H, H-4), 8.22 (d, J=8.6 Hz, 1H, H-3), 8.36 (s, 1H, H-1), 10.39 (s, 1H, H-10); 13C NMR: δ 18.3 (ArCH3), 125.6 (C-1, C-3), 126.0 (C-7), 130.2 (C-5a), 130.6 (C-6), 131.9 (C-8), 132.6 (C-4), 133.5 (C-9), 137.5 (C-9a), 138.8 (C-11a), 144.8 (C-4a), 147.4 (C-2), 166.6 (C-11). HRMS (FD). Calcd for C14H10N2O3S, [M]+: m/z 286.0412. Found: m/z 286.0415.
7,8-Dimethyl-2-nitrodibenzo[b,f][1,4]thiazepin-11(10H)-one (3d)
Yield 58%; dark orange solid; mp 254–255°C; Rf 0.55 (hexane-AcOEt, 3:1); IR: 3288 (N-H), 3174 (N-H), 3050 (Ar, C-H), 2957 (CH3), 2921 (CH3), 2851 (CH3), 1662 (-NHC(=O)-), 1603 (Ar, C=C), 1525 (ArNO2, (N=O)2), 1499 (Ar, C=C), 1432 (Ar, C=C), 1340 (ArNO2, (N=O)2), 902 (ArNO2, C-N), 833 (Ar, C-H), 778 (Ar, C-H), 675 cm−1 (Ar, C=C); 1H NMR: δ 2.15 (s, 3H, ArCH3), 2.16 (s, 3H, ArCH3), 7.03 (s, 1H, H-6), 7.34 (s, 1H, H-9), 7.76 (d, J=8.6 Hz, 1H, H-4), 8.23 (dd, J=2.6, 8.6 Hz, 1H, H-3), 8.34 (d, J=2.6 Hz, 1H, H-1), 10.79 (s, 1H, H-10); 13C NMR: δ 18.3 (ArCH3), 18.8 (ArCH3), 124.2 (C-5a, C-9), 125.7 (C-1, C-3), 131.2 (C-11a), 132.5 (C-4), 133.0 (C-6), 134.2 (C-7), 136.8 (C-8), 138.8 (C-9a), 144.6 (C-4a), 147.3 (C-2), 166.4 (C-11). HRMS (FD). Calcd for C15H12N2O3S, [M]+: m/z 300.0569; found: m/z 300.0581.
7-Methoxy-2-nitrodibenzo[b,f][1,4]thiazepin-11(10H)-one (3e)
Yield 86%; dark yellow solid; mp 222–223°C; Rf 0.21 (hexane-AcOEt, 3:1); IR: 3286 (N-H), 3173 (N-H), 2954 (CH3), 2854 (CH3), 1668 (-NHC(=O)-), 1601 (Ar, C=C), 1569 (ArNO2, (N=O)2), 1525 (Ar, C=C), 1432 (Ar, C=C), 1345 (ArNO2, (N=O)2), 1277 (C-O-C), 1037 (Ar-S-Ar), 893 (ArNO2, C-N), 839 (Ar, C-H), 702 cm−1 (Ar, C=C); 1H NMR: δ 3.75 (s, 3H, ArOCH3), 6.99 (dd, J=2.9, 8.9 Hz, 1H, H-8), 7.14 (d, J=2.9 Hz, 1H, H-6), 7.20 (d, J=8.9 Hz, 1H, H-9), 7.80 (d, J=8.6 Hz, 1H, H-4), 8.25 (dd, J=2.6, 8.6 Hz, 1H, H-3), 8.37 (d, J=2.6 Hz, 1H, H-1), 10.77 (s, 1H, H-10); 13C NMR: δ 55.5 (ArOCH3), 116.4 (C-8), 116.9 (C-6), 124.6 (C-9), 125.8 (C-1, C-3), 128.8 (C-5a), 132.3 (C-9a), 132.8 (C-4), 138.7 (C-11a), 144.1 (C-4a), 147.5 (C-2), 156.7 (C-7), 166.3 (C-11). HRMS (FD). Calcd for C14H10N2O4S, [M]+: m/z 302.0361. Found: m/z 302.0351.
7-Fluoro-2-nitrodibenzo[b,f][1,4]thiazepin-11(10H)-one (3f)
Yield 54%; pale brown solid; mp 209–210°C; Rf 0.24 (hexane-AcOEt, 3:1); IR: 3293 (N-H), 3178 (N-H), 3053 (Ar, C-H), 1668 (-NHC(=O)-), 1604 (Ar, C=C), 1532 (ArNO2, (N=O)2), 1498 (Ar, C=C), 1457 (Ar, C=C), 1347 (ArNO2, (N=O)2), 1264 (Ar, C-F), 932 (Ar, C-H), 902 (ArNO2, C-N), 739 (Ar, C-H), 702 cm−1 (Ar, C=C); 1H NMR: δ 7.04–7.12 (m, 2H, H-8, H-9), 7.63–7.65 (m, 1H, H-6), 7.81 (d, J=8.6 Hz, 1H, H-4), 8.28 (dd, J=2.6, 8.6 Hz, 1H, H-3), 8.38 (d, J=2.6 Hz, 1H, H-1), 11.10 (s, 1H, H-10); 13C NMR: δ 110.3 (C-8), 112.8 (C-6), 122.9 (C-9), 125.9 (C-1), 126.1 (C-4), 131.3 (C-5a), 132.7 (C-3), 134.4 (C-9a), 138.4 (C-11a), 143.7 (C-4a), 147.4 (C-2), 161.5 (C-7), 166.2 (C-11). HRMS (FD). Calcd for C13H7N2O3SF, [M]+: m/z 290.0161. Found: m/z 290.0166.
8-Fluoro-2-nitrodibenzo[b,f][1,4]thiazepin-11(10H)-one (3g)
Yield 99%; brown solid; mp 274–275°C; Rf 0.36 (hexane-AcOEt, 3:1); IR: 3309 (N-H), 3192 (N-H), 3097 (Ar, C-H), 1662 (-NHC(=O)-), 1597 (Ar, C=C), 1568 (ArNO2, (N=O)2), 1522 (Ar, C=C), 1475 (Ar, C=C), 1339 (ArNO2, (N=O)2), 1256 (Ar, C-F), 1054 (Ar-S-Ar), 910 (Ar, C-H), 897 (ArNO2, C-N), 771 (Ar, C-H), 701 cm−1 (Ar, C=C); 1H NMR: δ 7.04–7.12 (m, 2H, H-7, H-9), 7.64 (dd, J=6.2, 8.7 Hz, 1H, H-6), 7.81 (d, J=8.6 Hz, 1H, H-4), 8.28 (dd, J=2.6, 8.6 Hz, 1H, H-3), 8.38 (d, J=2.6 Hz, 1H, H-1), 11.05 (s, 1H, H-10); 13C NMR: δ 110.2 (C-9), 112.7 (C-7), 122.9 (C-5a), 126.0 (C-1), 126.2 (C-3), 132.7 (C-4), 134.5 (C-6), 138.5 (C-11a), 141.3 (C-9a), 143.8 (C-4a), 147.5 (C-2), 162.6 (C-8), 166.3 (C-11). HRMS (FD). Calcd for C13H7N2O3SF, [M]+: m/z 290.0161. Found: m/z 290.0168.
7-Chloro-2-nitrodibenzo[b,f][1,4]thiazepin-11(10H)-one (3h)
Yield 64%; pale brown solid; mp 262–263°C; Rf 0.25 (hexane-AcOEt, 3:1); IR: 3283 (N-H), 3171 (N-H), 3061 (Ar, C-H), 1653 (-NHC(=O)-), 1606 (Ar, C=C), 1570 (ArNO2, (N=O)2), 1530 (Ar, C=C), 1457 (Ar, C=C), 1347 (ArNO2, (N=O)2), 1148 (Ar, C-Cl), 1051 (Ar-S-Ar), 926 (Ar, C-H), 903 (ArNO2, C-N), 796 (Ar, C-H), 742 (Ar, C-H), 703 cm−1 (Ar, C=C); 1H NMR: δ 7.29 (d, J=8.6 Hz, 1H, H-9), 7.48 (dd, J=2.0, 8.6 Hz, 1H, H-8), 7.67 (d, J=2.0 Hz, 1H, H-6), 7.81 (d, J=8.6 Hz, 1H, H-4), 8.28 (dd, J=2.4, 8.4 Hz, 1H, H-3), 8.38 (d, J=2.3 Hz, 1H, H-1), 11.03 (s, 1H, H-10); 13C NMR: δ 124.8 (C-9), 125.9 (C-1), 126.1 (C-3), 129.0 (C-5a), 129.3 (C-7), 130.2 (C-8), 131.9 (C-6), 133.0 (C-4), 138.5 (C-9a, C-11a), 143.2 (C-4a), 147.6 (C-2), 166.2 (C-11). HRMS (FD). Calcd for C13H7N2O3SCl, [M]+: m/z 305.9866. Found: m/z 305.9868.
9-Chloro-2-nitrodibenzo[b,f][1,4]thiazepin-11(10H)-one (3i)
Yield 92%; pale orange solid; mp 243–245°C; Rf 0.38 (hexane-AcOEt, 3:1); IR: 3306 (N-H), 3183 (N-H), 3045 (Ar, C-H), 1653 (-NHC(=O)-), 1602 (Ar, C=C), 1528 (ArNO2, (N=O)2), 1496 (Ar, C=C), 1449 (Ar, C=C), 1341 (ArNO2, (N=O)2), 1080 (Ar, C-Cl), 1050 (Ar-S-Ar), 896 (ArNO2, C-N), 770 (Ar, C-H), 695 cm−1 (Ar, C=C); 1H NMR: δ 7.22 (t, J=7.9 Hz, 1H, H-7), 7.57 (dd, J=7.9 Hz, 1H, H-6), 7.61 (dd, J=1.0, 7.9 Hz, 1H, H-8), 7.81 (d, J=8.6 Hz, 1H, H-4), 8.24 (dd, J=2.6, 8.6 Hz, 1H, H-3), 8.36 (d, J=2.3 Hz, 1H, H-1), 10.65 (s, 1H, H-10); 13C NMR: δ 125.7 (C-1), 126.0 (C-3), 127.3 (C-7), 128.3 (C-5a), 131.2 (C-6), 131.9 (C-8), 132.2 (C-9), 132.9 (C-4), 136.1 (C-9a), 138.1 (C-11a), 143.9 (C-4a), 147.7 (C-2), 166.1 (C-11). HRMS (FD). Calcd for C13H7N2O3SCl, [M]+: m/z 305.9866. Found: m/z 305.9855.
Synthesis of 2-nitrodibenzo[b,f][1,4]thiazepin-11-amine derivatives 5
A representative example is the synthesis of 2-nitrodibenzo[b,f][1,4]thiazepin-11-amine (5a).
A solution of 2-aminophenyl 2-cyano-4-nitrophenyl sulfide (4a, 0.10 g, 0.37 mmol) in 15 mL of 30 wt% HCl-EtOH was heated under reflux at 90°C for 24 h, then cooled to room temperature and concentrated on a rotary evaporator. The residue of 5⋅HCl was dissolved in water and the solution was neutralized using saturated aqueous sodium bicarbonate solution and extracted three times with ethyl acetate. The extract was washed several times with brine and dried over with MgSO4. After filtration and concentration, the crude product was purified by column chromatography on silica gel eluting with n-hexane/ethyl acetate (3:1, v/v) to give analytically pure compound 5a (0.06 g).
2-Nitrodibenzo[b,f][1,4]thiazepin-11-amine (5a)
Yield 61%; yellow solid; mp 214–215°C; Rf 0.13 (hexane-AcOEt, 3:1); IR: 3099 (Ar, C-H), 1600 (Ar, C=C), 1579 (ArNO2, (N=O)2), 1520 (Ar, C=C), 1459 (Ar, C=C), 1338 (ArNO2, (N=O)2), 1028 (Ar-S-Ar), 920 (ArNO2, C-N), 764 (Ar, C-H), 699 cm−1 (Ar, C=C); 1H NMR: δ 6.91 (t, J=6.9 Hz, 1H, H-7), 6.99 (d, J=8.0 Hz, 1H, H-9), 7.11 (s, 2H, NH2), 7.22 (t, J=7.0 Hz, 1H, H-8), 7.36 (d, J=6.6 Hz, 1H, H-6), 7.76 (d, J=8.6 Hz, 1H, H-4), 8.25 (m, 2H, H-1, H-3); 13C NMR: δ 122.6 (C-7), 123.7 (C-1), 125.0 (C-9, C-5a), 125.5 (C-3), 129.7 (C-8), 132.3 (C-6), 132.3 (C-4), 136.4 (C-11a), 146.2 (C-4a), 147.2 (C-2), 149.1 (C-9a), 157.7 (C-11). HRMS (FD). Calcd for C13H9N3O2S, [M]+: m/z 271.0415. Found: m/z 271.0428.
7-Methyl-2-nitrodibenzo[b,f][1,4]thiazepin-11-amine (5b)
Yield 32%; yellow solid; mp 204–205°C; Rf 0.11 (hexane-AcOEt, 3:1); IR: 3092 (Ar, C-H), 2919 (CH3), 1599 (Ar, C=C), 1520 (Ar, C=C), 1481 (Ar, C=C), 1339 (ArNO2, (N=O)2), 932 (Ar, C-H), 915 (ArNO2, C-N), 742 (Ar, C-H), 668 cm−1 (Ar, C=C); 1H NMR: δ 2.18 (s, 3H, ArCH3), 6.88 (d, J=8.0 Hz, 1H, H-9), 7.00 (s, 2H, NH2), 7.03 (d, J= 6.9 Hz, 1H, H-8), 7.17 (s, 1H, H-6), 7.73 (d, J=9.2 Hz, 1H, H-4), 8.24 (m, 2H, H-1, H-3); 13C NMR: δ 19.7 (ArCH3), 123.7 (C-1), 124.6 (C-5a), 124.8 (C-9), 125.3 (C-3), 130.4 (C-8), 131.8 (C-7), 132.3 (C-4), 132.4 (C-6), 136.4 (C-11a), 146.1 (C-4a), 146.6 (C-9a), 147.2 (C-2), 157.5 (C-11). HRMS (FD). Calcd for C14H11N3O2S, [M]+: m/z 285.0572. Found: m/z 285.0577.
9-Methyl-2-nitrodibenzo[b,f][1,4]thiazepin-11-amine (5c)
Yield 41%; dark orange solid; mp 234–235°C; Rf 0.21 (hexane-AcOEt, 3:1); IR: 3064 (Ar, C-H), 2856 (CH3), 1600 (Ar, C=C), 1579 (ArNO2, (N=O)2), 1519 (Ar, C=C), 1452 (Ar, C=C), 1337 (ArNO2, (N=O)2), 1051 (Ar-S-Ar), 770 cm−1 (Ar, C-H); 1H NMR: δ 2.20 (s, 3H, ArCH3), 6.81 (t, J=7.6 Hz, 1H, H-7), 7.02 (s, 2H, NH2), 7.09 (d, J=7.2 Hz, 1H, H-8), 7.20 (d, J=7.7 Hz, 1H, H-6), 7.74 (d, J=8.6 Hz, 1H, H-4), 8.24 (m, 2H, H-1, H-3); 13C NMR: δ 18.7 (ArCH3), 122.2 (C-7), 123.5 (C-1), 125.2 (C-3), 125.3 (C-9), 129.9 (C-6), 130.7 (C-8), 132.2 (C-4), 132.8 (C-5a), 136.5 (C-11a), 146.4 (C-4a), 147.2 (C-2), 147.3 (C-9a), 156.9 (C-11). HRMS (FD). Calcd for C14H11N3O2S, [M]+: m/z 285.0572. Found: m/z 285.0579.
7,8-Dimethyl-2-nitrodibenzo[b,f][1,4]thiazepin-11-amine (5d)
Yield 46%; orange solid; mp 229–230°C; Rf 0.10 (hexane-AcOEt, 3:1); IR: 3064 (Ar, C-H), 2959 (CH3), 2924 (CH3), 2857 (CH3), 1593 (Ar, C=C), 1520 (Ar, C=C), 1464 (Ar, C=C), 1372 (Ar, C-N), 1338 (ArNO2, (N=O)2), 1025 (Ar-S-Ar), 896 (ArNO2, C-N), 873 (Ar, C-H), 775 cm−1 (Ar, C-H); 1H NMR: δ 2.08 (s, 6H, ArCH3), 2.10 (s, 6H, ArCH3), 6.79 (s, 1H, H-9), 6.96 (s, 2H, NH2), 7.11 (s, 1H, H-6), 7.71 (s, 1H, H-4), 8.23 (m, 2H, H-1, H-3); 13C NMR: δ 18.1 (ArCH3), 18.9 (ArCH3), 121.7 (C-5a), 123.6 (C-3), 125.2 (C-1), 125.9 (C-9), 130.8 (C-8), 132.1 (C-4), 132.7 (C-6), 136.5 (C-11a), 138.0 (C-7), 146.5 (C-2), 146.8 (C-4a), 147.1 (C-9a), 157.5 (C-11). HRMS (FD). Calcd for C15H13N3O2S, [M]+: m/z 299.0728. Found: m/z 299.0728.
7-Methoxy-2-nitrodibenzo[b,f][1,4]thiazepin-11-amine (5e)
Yield 32%; orange solid; mp 196–197°C; Rf 0.05 (hexane-AcOEt, 3:1); IR: 3095 (Ar, C-H), 2935 (CH3), 2831 (CH3), 1602 (Ar, C=C), 1524 (Ar, C=C), 1480 (Ar, C=C), 1343 (ArNO2, (N=O)2), 1274 (C-O-C), 1032 (Ar-S-Ar), 909 (ArNO2, C-N), 821 (Ar, C-H), 743 (Ar, C-H), 669 cm−1 (Ar, C=C); 1H NMR: δ 3.68 (s, 3H, ArOCH3), 6.83 (d, J=8.9 Hz, 1H, H-8), 6.91 (m, 4H, H-6, H-9, NH2), 7.75 (d, J=9.5 Hz, 1H, H-4), 8.26 (m, 2H, H-1, H-3); 13C NMR: δ 53.3 (ArOCH3), 116.4 (C-8), 123.7 (C-1), 125.2 (C-5a, C-9), 125.3 (C-3), 125.7 (C-6), 132.4 (C-4), 136.4 (C-11a), 142.6 (C-9a), 145.7 (C-4a), 147.3 (C-2), 154.9 (C-7), 157.1 (C-11). HRMS (FD). Calcd for C14H11N3O3S, [M]+: m/z 301.0521. Found: m/z 301.0533.
7-Fluoro-2-nitrodibenzo[b,f][1,4]thiazepin-11-amine (5f)
Yield 75%; dark orange solid; mp 224–225°C; Rf 0.28 (hexane-AcOEt, 3:1); IR: 3079 (Ar, C-H), 1603 (Ar, C=C), 1564 (ArNO2, (N=O)2), 1514 (Ar, C=C), 1471 (Ar, C=C), 1345 (ArNO2, (N=O)2), 914 (ArNO2, C-N), 890 (Ar, C-H), 776 (Ar, C-H), 705 cm−1 (Ar, C=C); 1H NMR: δ 6.98 (dd, J=5.4, 8.9 Hz, 1H, H-8), 7.07 (dd, J=2.9, 8.6 Hz, 1H, H-9), 7.11 (s, 2H, NH2), 7.23 (dd, J=3.0, 8.4 Hz, 1H, H-6), 7.77 (d, J=8.3 Hz, 1H, H-4), 8.27 (m, 2H, H-1, H-3); 13C NMR: δ 116.8 (C-8), 118.2 (C-6), 123.8 (C-3), 125.6 (C-1), 125.7 (C-5a), 126.0 (C-9), 132.6 (C-4), 136.2 (C-11a), 145.3 (C-4a), 145.9 (C-7, C-9a), 147.4 (C-2), 157.7 (C-11). HRMS (FD). Calcd for C13H8N3O2SF, [M]+: m/z 289.0321. Found: m/z 289.0321.
8-Fluoro-2-nitrodibenzo[b,f][1,4]thiazepin-11-amine (5g)
Yield 42%; yellow solid; mp 253–254°C; Rf 0.18 (hexane-AcOEt, 3:1); IR: 3131 (Ar, C-H), 1597 (Ar, C=C), 1514 (Ar, C=C), 1462 (Ar, C=C), 1349 (ArNO2, (N=O)2), 1054 (Ar-S-Ar), 911 (ArNO2, C-N), 773 (Ar, C-H), 718 cm−1 (Ar, C=C); 1H NMR (600 MHz, DMSO-d6): δ 6.75 (m, 2H, H-7, H-9), 7.30 (s, 2H, NH2), 7.39 (t, J=7.7 Hz, 1H, H-6), 7.76 (d, J=8.3 Hz, 1H, H-4), 8.27 (m, 2H, H-1, H-3); 13C NMR (150 MHz, DMSO-d6): δ 110.5 (C-9), 112.0 (C-7), 121.9 (C-5a), 124.6 (C-1), 126.6 (C-3), 133.2 (C-4), 134.5 (C-8), 137.2 (C-11a), 146.8 (C-2), 148.2 (C-4a), 152.0 (C-9a), 159.3 (C-11), 163.0 (C-8). HRMS (FD). Calcd for C13H8N3O2SF, [M]+: m/z 289.0321. Found: m/z 289.0329.
7-Chloro-2-nitrodibenzo[b,f][1,4]thiazepin-11-amine (5h)
Yield 75%; orange-colored solid; mp 221–222°C; Rf 0.15 (hexane-AcOEt, 3:1); IR: 3094 (Ar, C-H), 1601 (Ar, C=C), 1520 (Ar, C=C), 1460 (Ar, C=C), 1344 (ArNO2, (N=O)2), 1051 (Ar-S-Ar), 907 (ArNO2, C-N), 741 (Ar, C-H), 701 cm−1 (Ar, C=C); 1H NMR: δ 6.97 (d, J=8.6 Hz, 1H, H-9), 7.24 (m, 3H, H-8, NH2), 7.41 (d, J=2.6 Hz, 1H, H-6), 7.77 (d, J=8.3 Hz, 1H, H-4), 8.26–8.29 (m, 2H, H-1, H-3); 13C NMR: δ 123.7 (C-3), 125.7 (C-1), 125.9 (C-7), 126.3 (C-9), 129.6 (C-5a), 131.2 (C-8), 132.6 (C-4, C-6), 136.1 (C-11a), 145.2 (C-2), 147.4 (C-4a), 148.1 (C-9a), 158.1 (C-11). HRMS (FD). Calcd for C13H8N3O2SCl, [M]+: m/z 305.0026. Found: m/z 305.0025.
9-Chloro-2-nitrodibenzo[b,f][1,4]thiazepin-11-amine (5i)
Yield 56%; yellow solid; mp 280–281°C; Rf 0.30 (hexane-AcOEt, 3:1); IR: 3120 (Ar, C-H), 1606 (Ar, C=C), 1516 (Ar, C=C), 1471 (Ar, C=C), 1350 (ArNO2, (N=O)2), 1158 (Ar, C-Cl), 905 (ArNO2, C-N), 773 (Ar, C-H), 742 (Ar, C-H), 688 cm−1 (Ar, C=C); 1H NMR: δ 6.89 (t, J=7.9 Hz, 1H, H-7), 7.36 (m, 2H, H-6, H-8), 7.40 (s, 2H, NH2), 7.77 (t, J=4.7 Hz, 1H, H-4), 8.28 (m, 2H, H-1, H-3); 13C NMR: δ 122.7 (C-7), 123.5 (C-1), 125.7 (C-3), 127.5 (C-9), 128.7 (C-9a), 130.4 (C-8), 131.1 (C-6), 132.5 (C-4), 136.1 (C-11a), 145.3 (C-2, C-5a), 147.5 (C-4a), 158.3 (C-11). HRMS (FD). Calcd for C13H8N3O2SCl, [M]+: m/z 305.0026. Found: m/z 305.0029.
Antibacterial activity test
The antibacterial assay was performed according to the protocol of the Clinical and Laboratory Standards Institute (Wayne, PA, USA). In the protocol, E. coli ATCC25922 and S. aureus ATCC29213 are defined as the quality control strains and were used for our antibacterial assay. In the assay, 10 μL of a bacterial cell suspension containing 1×105 colony forming units (CFU) bacteria was inoculated into 1 mL of Mueller-Hinton broth (Becton Dickinson and Company, Sparks, MD, USA) supplemented with 0.5 mg/mL of the test compound. The culture was incubated for 20 h at 35°C under an aerobic condition, and the viable cells in the culture were counted with the modified Miles and Misra method [40]. Briefly, 10 μL aliquots of serial-diluted bacterial suspensions were inoculated into a trypticase soy agar broth. After 20 h incubation at 35°C under an aerobic condition, the numbers of colonies recovered from the diluted suspensions on the agar broth were counted, and the number of viable bacterial cells in the source culture was determined.
References
[1] Karnik, A. V.; Malviya, N. J.; Kulkarni, A. M.; Jadhav, B. L. Synthesis and in vitro antibacterial activity of novel heterocyclic derivatives of 18-nor-equilenin. Eur. J. Med. Chem.2006, 41, 891–895.10.1016/j.ejmech.2006.01.018Suche in Google Scholar PubMed
[2] McAllister, L. A.; Montgomery, J. I.; Abramite, J. A.; Reilly, U.; Brown, M. F.; Chen, J. M.; Barham, R. A.; Che, Y.; Chung, S. W.; Menard, C. A.; et al. Heterocyclic methylsulfone hydroxamic acid LpxC inhibitors as Gram-negative antibacterial agents. Bioorg. Med. Chem. Lett.2012, 22, 6832–6838.10.1016/j.bmcl.2012.09.058Suche in Google Scholar PubMed
[3] Saikia, P.; Kaishap, P. P.; Goswami, J.; Singh, A. K.; Boruah, H. P. D.; Gogoi, S.; Boruah, R. C. Synthesis of steroidal and nonsteroidal vicinal heterocyclic alcohols, N-(1-cycloalkenyl)heterocycles and their antibacterial studies. Steroids2014, 84, 36–45.10.1016/j.steroids.2014.03.011Suche in Google Scholar PubMed
[4] Suresh, L.; Kumar, P. S. V.; Chandramouli, G. V. P. An efficient one-pot synthesis, characterization and antibacterial activity of novel chromeno-pyrimidine derivatives. J. Mol. Struct.2017, 1134, 51–58.10.1016/j.molstruc.2016.12.030Suche in Google Scholar
[5] Yi, Y.; Xu, X.; Liu, Y.; Xu, S.; Huang, X.; Liang, J.; Shang, R. Synthesis and antibacterial activities of novel pleuromutilin derivatives with a substituted pyrimidine moiety. Eur. J. Med. Chem.2017, 126, 687–695.10.1016/j.ejmech.2016.11.054Suche in Google Scholar PubMed
[6] Li, D. Z.; Li, Y.; Chen, X. G.; Zhu, C. G.; Yang, J.; Liu, H. Y.; Pan, X. D. Synthesis and antitumor activity of heterocyclic acid ester derivatives of 20S-camptothecins. Chinese Chem. Lett.2007, 18, 1335–1338.10.1016/j.cclet.2007.09.014Suche in Google Scholar
[7] Tan, S.; Yin, H.; Chen, Z.; Qian, X.; Xu, Y. Oxo-heterocyclic fused naphthalimides as antitumor agents: synthesis and biological evaluation. Eur. J. Med. Chem.2013, 62, 130–138.10.1016/j.ejmech.2012.12.039Suche in Google Scholar PubMed
[8] Chen, T.-C.; Wu, C.-L.; Lee, C.-C.; Chen, C.-L.; Yu, D.-S.; Huang, H.-S. Structure-based hybridization, synthesis and biological evaluation of novel tetracyclic heterocyclic azathioxanthone analogues as potential antitumor agents. Eur. J. Med. Chem.2015, 103, 615–627.10.1016/j.ejmech.2014.09.050Suche in Google Scholar PubMed
[9] Jukić, M.; Rastija, V.; Opačak-Bernardi, T.; Stolić, I.; Krstulović, L.; Bajić, M.; Glavaš-Obrovac, L. Antitumor activity of 3,4-ethylenedioxythiophene derivatives and quantitative structure-activity relationship analysis. J. Mol. Struct.2017, 1133, 66–73.10.1016/j.molstruc.2016.11.074Suche in Google Scholar
[10] Shaaban, M. R.; Saleh, T. S.; Mayhoub, A. S.; Mansour, A.; Farag, A. M. Synthesis and analgesic/anti-inflammatory evaluation of fused heterocyclic ring systems incorporating phenylsulfonyl moiety. Bioorg. Med. Chem.2008, 16, 6344–6352.10.1016/j.bmc.2008.05.011Suche in Google Scholar PubMed
[11] Reddy, S. A. M.; Mudgal, J.; Bansal, P.; Vasanthraju, S. G.; Srinivasan, K. K.; Rao, C. M.; Kutty, N. G. Antioxidant, anti-inflammatory and anti-hyperglycaemic activities of heterocyclic homoprostanoid derivatives. Bioorg. Med. Chem.2011, 19, 384–392.10.1016/j.bmc.2010.11.016Suche in Google Scholar PubMed
[12] Malvar, D. C.; Ferreira, R. T.; Castro, R. A.; Castro, L. L.; Freitas, A. C. C.; Costa, E. A.; Florentino, I. F.; Mafra, J. C. M.; Souza, G. E. P.; Vanderlinde, F. A. Antinociceptive, anti-inflammatory and antipyretic effects of 1.5-diphenyl-1H-pyrazole-3-carbohydrazide, a new heterocyclic pyrazole derivative. Life Sci.2014, 95, 81–88.10.1016/j.lfs.2013.12.005Suche in Google Scholar PubMed
[13] Abdelazeem, A. H.; El-Saadi, M. T.; El-Din, A. G. S.; Omar, H. A.; El-Moghazy, S. M. Design, synthesis and analgesic/anti-inflammatory evaluation of novel diarylthiazole and diarylimidazole derivatives towards selective COX-1 inhibitors with better gastric profile. Bioorg. Med. Chem.2017, 25, 665–676.10.1016/j.bmc.2016.11.037Suche in Google Scholar PubMed
[14] Chougala, B. M.; Samundeeswari, S.; Holiyachi, M.; Shastri, L. A.; Dodamani, S.; Jalalpure, S.; Dixit, S. R.; Joshi, S. D.; Sunagar, V. A. Synthesis, characterization and molecular docking studies of substituted 4-coumarinylpyrano[2,3-c]pyrazole derivatives as potent antibacterial and anti-inflammatory agents. Eur. J. Med. Chem.2017, 125, 101–116.10.1016/j.ejmech.2016.09.021Suche in Google Scholar PubMed
[15] Zoidis, G.; Fytas, C.; Papanastasiou, I.; Foscolos, G. B.; Fytas, G.; Padalko, E.; Clercq, E. D.; Naesens, L.; Neyts, J.; Kolocouris, N. Heterocyclic rimantadine analogues with antiviral activity. Bioorg. Med. Chem.2006, 14, 3341–3348.10.1016/j.bmc.2005.12.056Suche in Google Scholar PubMed
[16] Hashem, A. I.; Youssef, A. S. A.; Kandeel, K. A.; Abou-Elmagd, W. S. I. Conversion of some 2(3H)-furanones bearing a pyrazolyl group into other heterocyclic systems with a study of their antiviral activity. Eur. J. Med. Chem.2007, 42, 934–939.10.1016/j.ejmech.2006.12.032Suche in Google Scholar PubMed
[17] Francesco, M. E. D.; Avolio, S.; Pompei, M.; Pesci, S.; Monteagudo, E.; Pucci, V.; Giuliano, C.; Fiore, F.; Rowley, M.; Summa, V. Synthesis and antiviral properties of novel 7-heterocyclic substituted 7-deaza-adenine nucleoside inhibitors of Hepatitis C NS5B polymerase. Bioorg. Med. Chem.2012, 20, 4801–4811.10.1016/j.bmc.2012.05.067Suche in Google Scholar PubMed
[18] Barbosa, V. A.; Baréa, P.; Mazia, R. S.; Ueda-Nakamura, T.; Costa, W. F.; Foglio, M. A.; Ruiz, A. L. T. G.; Carvalho, J. E.; Vendramini-Costa, D. B.; Nakamura, C. V.; et al. Synthesis and evaluation of novel hybrids β-carboline-4-thiazolidinones as potential antitumor and antiviral agents. Eur. J. Med. Chem.2016, 124, 1093–1104.10.1016/j.ejmech.2016.10.018Suche in Google Scholar PubMed
[19] Hao, Y.; Zhou, G.; Wu, W.; Zhang, Y.; Tao, L.; Yao, J.; Xu, W. Synthesis and antiviral evaluation of novel N-6 substituted adenosine analogues. Tetrahedron Lett.2017, 58, 190–193.10.1016/j.tetlet.2016.11.059Suche in Google Scholar
[20] Vardakas, K. Z.; Apiranthiti, K. N.; Falagas, M. E. Antistaphylococcal penicillins versus cephalosporins for definitive treatment of methicillin-susceptible Staphylococcus aureus bacteraemia: a systematic review and meta-analysis. Int. J. Antimicrob. Agents2014, 44, 486–492.10.1016/j.ijantimicag.2014.09.002Suche in Google Scholar PubMed
[21] Nowacki, M. R. Lopinavir/ritonavir and rifampin: is coadministration possible? HIV & AIDS Rev.2007, 7, 15–18.10.1016/S1730-1270(10)60046-XSuche in Google Scholar
[22] Mikuła, T.; Dąbrowska, M.; Wiercińska-Drapało, A. Is ritonavir-boosted protease inhibitors (PIs/r) monotherapy noninferior to classic combined antiretroviral therapy (cART)? HIV & AIDS Rev.2010, 9, 61–64.10.1016/S1730-1270(10)60097-5Suche in Google Scholar
[23] Wang, L.; Zhang, P.; Zhang, X.; Zhang, Y.; Li, Y.; Wang, Y. Synthesis and biological evaluation of a novel series of 1,5-benzothiazepine derivatives as potential antimicrobial agents. Eur. J. Med. Chem.2009, 44, 2815–2821.10.1016/j.ejmech.2008.12.021Suche in Google Scholar
[24] Zhang, P.; Wang, L. Z.; Wu, H. S.; Lan, J. M.; Li, Y.; Wang, Y. X. The synthesis and biological evaluation of a series of novel 2-COOC2H5/COONa substituted 1,5-benzothiazepine derivatives as antimicrobial agents. Chinese Chem. Lett.2009, 20, 660–662.10.1016/j.cclet.2009.01.003Suche in Google Scholar
[25] Mor, S.; Pahal, P.; Narasimhan, B. Synthesis, characterization, biological evaluation and QSAR studies of 11-p-substituted phenyl-12-phenyl-11a,12-dihydro-11H-indeno[2,1-c][1,5]benzothiazepines as potential antimicrobial agents. Eur. J. Med. Chem.2012, 57, 196–210.10.1016/j.ejmech.2012.09.003Suche in Google Scholar
[26] Li, T.; Zhang, J.; Pan, J.; Wu, Z.; Hu, D.; Song, B. Design, synthesis, and antiviral activities of 1,5-benzothiazepine derivatives containing pyridine moiety. Eur. J. Med. Chem.2017, 125, 657–662.10.1016/j.ejmech.2016.09.069Suche in Google Scholar
[27] Ambrogi, V.; Furlani, A.; Grandolini, G.; Papaioannou, A.; Perioli, L.; Scarcia, V.; Tuttobello, L. Synthesis, antimicrobial and cytostatic activities of some derivatives of indolo[2,3-b]-1,5-benzothiazepine, a novel heterocyclic ring system. Eur. J. Med. Chem.1993, 28, 659–667.10.1016/0223-5234(93)90024-9Suche in Google Scholar
[28] Sarro, G. D.; Chimirri, A.; Sarro, A. D.; Gitto, R.; Grasso, S.; Zappalà, M. 5H-[1,2,4]Oxadiazolo[5,4-d][1,5]benzothiazepines as anticonvulsant agents in DBA/2 mice. Eur. J. Med. Chem.1995, 30, 925–929.10.1016/0223-5234(96)88311-5Suche in Google Scholar
[29] Garg, N.; Chandra, T.; Archana; Jain, A. B.; Kumar, A. Synthesis and evaluation of some new substituted benzothiazepine and benzoxazepine derivatives as anticonvulsant agents. Eur. J. Med. Chem.2010, 45, 1529–1535.10.1016/j.ejmech.2010.01.001Suche in Google Scholar PubMed
[30] Kaur, H.; Kumar, S.; Chaudhary, A.; Kumar, A. Synthesis and biological evaluation of some new substituted benzoxazepine and benzothiazepine as antipsychotic as well as anticonvulsant agents. Arabian J. Chem.2012, 5, 271–283.10.1016/j.arabjc.2010.09.011Suche in Google Scholar
[31] Enjo, N.; Lu, R.; Miyakoshi, T. Synthesis of asymmetric diaryl sulfides by SNAr reaction of substituted nitrobenzene with aryl disulfides. Current Org. Synthesis2013, 10, 961–968.10.2174/157017941006140206105221Suche in Google Scholar
[32] Lu, R.; Itabashi, S.; Enjo, N.; Miyakoshi, T. Mechanism and method for synthesis of sulfides by thiol-disulfide exchange reaction. Current Org. Synthesis2014, 11, 295–300.10.2174/1570179411666140123234712Suche in Google Scholar
[33] Yadav, A.; Awasthi, A.; Rao, N. K. Mechanistic aspects of benzothiazepines: a class of antiarrhythmic drugs. Eur. J. Med. Chem.2009, 44, 1–6.10.1016/j.ejmech.2008.03.002Suche in Google Scholar PubMed
[34] Pawar, S.; Roy, A.; Wagh, S. Synthesis and pharmacological evaluation of some new dibenzo[b,f][1,4]thiazepines. J. Pharm. Res.2013, 6, 756–760.10.1016/j.jopr.2013.07.011Suche in Google Scholar
[35] Ohta, K.; Goto, T.; Endo, Y. New synthetic method of 1,2-diaryl-1,2-dicarba-closo-dodecaboranes employing aromatic nucleophilic substitution (SNAr) reaction. Tetrahedron Lett.2005, 46, 483–485.10.1016/j.tetlet.2004.11.074Suche in Google Scholar
[36] Danikiewicz, W.; Bieńkowski, T.; Kozłowska, D.; Zimnicka, M. Aromatic nucleophilic substitution (SNAr) reactions of 1,2- and 1,4-halonitrobenzenes and 1,4-dinitrobenzene with carbanions in the gas phase. J. Am. Soc. Mass Spectrom.2007, 18, 1351–1363.10.1016/j.jasms.2007.04.005Suche in Google Scholar PubMed
[37] Yap, J. L.; Hom, K.; Fletcher, S. Ortho-selectivity in the nucleophilic aromatic substitution (SNAr) reactions of 3-substutited, 2,6-dichloropyridines with alkali metal alkoxides. Tetrahedron Lett.2011, 52, 4172–4176.10.1016/j.tetlet.2011.06.007Suche in Google Scholar
[38] Jones-Mensah, E.; Magolan, J. Aryl methyl sulfides via SNAr using DMSO as the source of the thiomethyl moiety. Tetrahedron Lett.2014, 55, 5323–5326.10.1016/j.tetlet.2014.07.058Suche in Google Scholar
[39] Alam, M. P.; Jagodzinska, B.; Campagna, J.; Spilman, P.; John, V. C-O bond formation in a microfluidic reactor: high yield SNAr substitution of heteroaryl chlorides. Tetrahedron Lett.2016, 57, 2059–2062.10.1002/chin.201633066Suche in Google Scholar
[40] Miles, A. A.; Misra, S. S.; Irwin, J. Q. The estimation of the bactericidal power of the blood. J. Hygiene1938, 38, 732–749.10.1017/S002217240001158XSuche in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Synthesis of benzofuro[3,2-b]furo[2,3-d]pyridin-4(5H)-ones, derivatives of a novel heterocyclic system
- Reactions of 3H-furan-2-ones and 2H-chromen-2-ones with pyrazole-3(5)-diazonium salts
- Synthesis of 1,4-oxathian-2-ones by triton B-catalyzed one-pot reaction of epoxides with ethyl mercaptoacetate
- The regioselective catalyst-free synthesis of bis-quinoxalines and bis-pyrido[2,3-b]pyrazines by double condensation of 1,4-phenylene-bis-glyoxal with 1,2-diamines
- [1,3]Thiazolo[3,2-b][1,2,4]triazol-7-ium salts: synthesis, properties and structural studies
- Crystal structure and molecular docking studies of 1,2,4,5-tetraaryl substituted imidazoles
- Design, synthesis and cytotoxicity evaluation of indibulin analogs
- Synthesis of dibenzothiazepine analogues by one-pot S-arylation and intramolecular cyclization of diaryl sulfides and evaluation of antibacterial properties
- Synthesis and preliminary anti-inflammatory evaluation of xanthone derivatives
- Synthesis and antimicrobial evaluation of 3-(4-arylthieno[2,3-d]pyrimidin-2-yl)- 2H-chromen-2-ones
- Corrigendum
- Corrigendum to: Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Synthesis of benzofuro[3,2-b]furo[2,3-d]pyridin-4(5H)-ones, derivatives of a novel heterocyclic system
- Reactions of 3H-furan-2-ones and 2H-chromen-2-ones with pyrazole-3(5)-diazonium salts
- Synthesis of 1,4-oxathian-2-ones by triton B-catalyzed one-pot reaction of epoxides with ethyl mercaptoacetate
- The regioselective catalyst-free synthesis of bis-quinoxalines and bis-pyrido[2,3-b]pyrazines by double condensation of 1,4-phenylene-bis-glyoxal with 1,2-diamines
- [1,3]Thiazolo[3,2-b][1,2,4]triazol-7-ium salts: synthesis, properties and structural studies
- Crystal structure and molecular docking studies of 1,2,4,5-tetraaryl substituted imidazoles
- Design, synthesis and cytotoxicity evaluation of indibulin analogs
- Synthesis of dibenzothiazepine analogues by one-pot S-arylation and intramolecular cyclization of diaryl sulfides and evaluation of antibacterial properties
- Synthesis and preliminary anti-inflammatory evaluation of xanthone derivatives
- Synthesis and antimicrobial evaluation of 3-(4-arylthieno[2,3-d]pyrimidin-2-yl)- 2H-chromen-2-ones
- Corrigendum
- Corrigendum to: Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells


