Abstract
Claisen-Schmidt condensation of ferrocenecarboxaldehyde (2b) with 2-acetylfuran (4) yielded (E)-3-ferrocenyl-1-(2-furyl)prop-2-en-1-one (E-5) together with 1,5-di(2-furyl)-3-ferrocenylpentane-1,5-dione (6). Reactions of the ferrocenyl chalcones 3a,b and 5 with hydrazine hydrate, phenyl hydrazine, ethyl acetoacetate, ethyl cyanoacetate and malononitrile, were also studied. Possible reaction mechanisms were discussed and structures of the new products were unambiguously characterized by common analytical and spectroscopic methods.
Introduction
The bioorganometallic chemistry of ferrocene has aroused great interest and its study has been encouraged by potential biological applications [1]. The ferrocenyl moiety has been incorporated into the structure of a number of biologically active molecules such as antibiotic [2], anticancer [3] and antimalarial drugs [4]; resulting in an increase of activity. These compounds can be used for synthesizing an array of pharmacologically active derivatives [5], particularly against human immuno-deficiency virus (HIV) [6] and microbes [7]. In addition, ferrocene derivatives possess numerous applications in chemical sensing, asymmetric catalysis, material science and industrial chemistry [8]. From another side, 1,3-diaryl-2-propen-1-ones (chalcones) have been reported to possess a broad range of biological activities such as antimalarial, antimicrobial, antitumor, antioxidant, antihyperglycemic and anti-HIV properties [9–11]. Replacement of one of the two aryl groups by a ferrocenyl moiety has been reported [11–13]. The resulting ferrocenyl chalcones are dramatically more effective than the original organic molecules [11, 14] which, in particular, is due to their excellent stability in aqueous aerobic media and favorable electrochemical properties [11, 15]. In the present communication, we have studied the reactivity of ferrocenyl chalcones 3a,b and 5 towards hydrazines and active methylene compounds. The presence of a furyl or a benzofuranyl moiety in the reacting chalcones (3b or 5) or in the reaction products would boost the biological activity [16–19].
Results and discussion
The general plan employed to prepare the ferrocenyl chalcones 3a,b (Scheme 1) and 5 (Scheme 2) called for the Claisen-Schmidt condensation [20] between the appropriate aldehyde and an acetyl substrate. The resulting chalcones 3a[21], 3b and 5 [22, 23] were purified by crystallization.
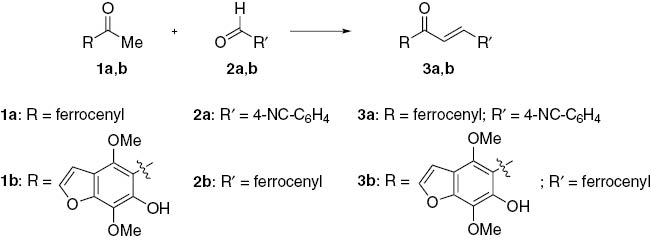

Claisen-Schmidt condensation of ferrocenecarboxaldehyde (2b) with 2-acetylfuran (4) has been reported to proceed in aqueous NaOH [22], or under solvent-free conditions [23] yielding chalcone 5 as the sole reaction product. In the present investigation, condensation of 2b with 4 in ethanol in the presence of 10% NaOH gave a mixture of two products which were separated by column chromatography (Scheme 2).
The first eluted product (40%) was (E)-3-ferrocenyl-1-(2-furyl)prop-2-en-1-one, (E-5). A characteristic feature of the 1H NMR spectrum of 5 is the pair of doublets at 7.16 ppm and 7.60 ppm (J = 15.3 Hz) consistent with the presence of the chalcone moiety with the E-configuration [24]. No trace for the Z-isomer was detected by NMR. The second eluted product (30%) was 1,5-di(2-furyl)-3-ferrocenylpentane-1,5-dione (6). The structure of 6, in addition to spectral analysis, was established by X-ray crystallographic analysis (Figure 1, Tables 1–3). Apparently, the initially formed chalcone 5 undergoes the addition reaction with another molecule of 4 to afford compound 6.
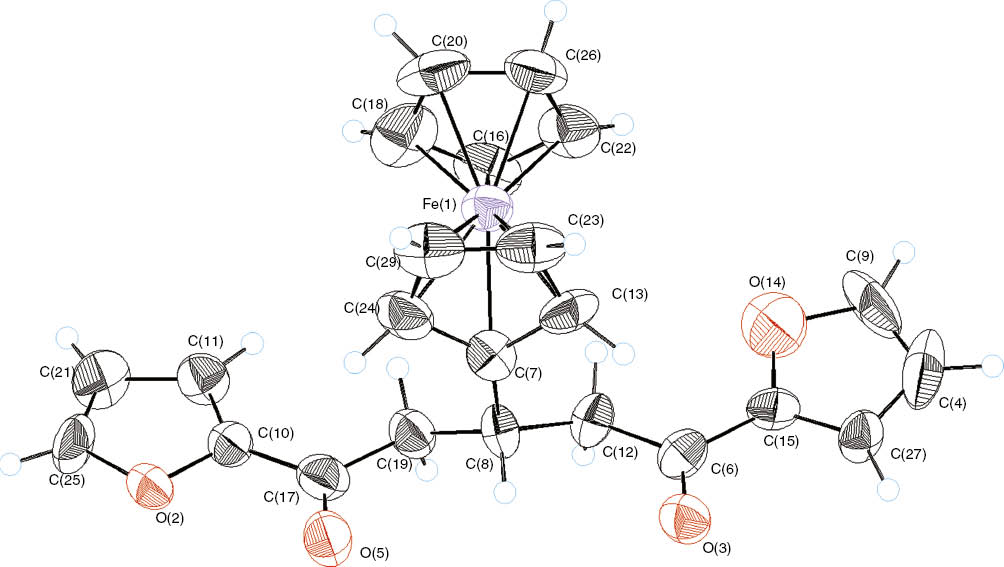
An ORTEP overview of compound 6.
Crystal structure and data refinement parameters of compound 6.
| Empirical formula | C23H20FeO4 |
| Formula weight | 416.256 |
| Crystal system/space group | Monoclinic/P21/n |
| a/Å | 10.6264 (5) |
| b/Å | 7.7745 (3) |
| c/Å | 23.0193 (13) |
| α/° | 90.00 |
| β/° | 95.886 (2) |
| γ/° | 90.00 |
| V/Å3 | 1891.7 (2) |
| Z | 4 |
| Dcalc (g/cm3) | 1.462 |
| μ (mm-1) | 0.82 |
| Color/shape | Yellow/Prismatic |
| θ range for collection/° | 0.00–31.42 |
| Reflections collected | 5423 |
| Independent reflections | 5090 |
| Data/restraints/parameters | 5090/0/253 |
| Goodness of fit on F2 | 0.580 |
| Final R indices [I > 2σ (I)] | 0.0589 |
| R indices (all data) | 0.3391 |
| Largest difference peak/Hole | 0.328/-0.451 |
Selected bond lengths (Å) of compound 6.
| O2–C25 | 1.363 (7) | C11–C21 | 1.424 (9) |
| O2–C10 | 1.377 (7) | C13–C23 | 1.415 (9) |
| O3–C6 | 1.225 (7) | O14–C15 | 1.383 (8) |
| C4–C9 | 1.286 (11) | C15–C27 | 1.308 (8) |
| C4–C27 | 1.411 (10) | C16–C22 | 1.423 (9) |
| O5–C17 | 1.215 (7) | C16–C18 | 1.421 (10) |
| C6–C15 | 1.464 (9) | C17–C19 | 1.507 (8) |
| C6–C12 | 1.496 (8) | C18–C20 | 1.408 (11) |
| C7–C13 | 1.425 (9) | C20–C26 | 1.418 (10) |
| C7–C24 | 1.434 (9) | C21–C25 | 1.332 (9) |
| C7–C8 | 1.508 (9) | C22–C26 | 1.404 (9) |
| C8–C12 | 1.527 (8) | C23–C29 | 1.419 (10) |
| C8–C19 | 1.536 (8) | C24–C29 | 1.412 (10) |
| C9–O14 | 1.401 (9) | Fe1–C(Cp) avg | 2.031 |
| C10–C11 | 1.342 (8) | Fe1–C(Cp′) avg | 2.030 |
| C10–C17 | 1.459 (9) |
Selected bond angles (°) of compound 6.
| C25–O2–C10 | 105.3 (5) | C27–C15–O14 | 111.0 (6) |
| C9–C4–C27 | 108.3 (7) | C27–C15–C6 | 128.2 (6) |
| O3–C6–C15 | 118.2 (6) | O14–C15–C6 | 120.8 (7) |
| O3–C6–C12 | 124.0 (6) | C22–C16–C18 | 107.8 (7) |
| C15–C6–C12 | 117.8 (6) | O5–C17–C10 | 121.0 (6) |
| C13–C7–C24 | 106.3 (6) | O5–C17–C19 | 121.8 (7) |
| C13–C7–C8 | 125.9 (6) | C10–C17–C19 | 117.1 (6) |
| C24–C7–C8 | 127.8 (7) | C20–C18–C16 | 108.1 (8) |
| C7–C8–C12 | 112.7 (6) | C17–C19–C8 | 114.8 (5) |
| C7–C8–C19 | 112.2 (6) | C18–C20–C26 | 107.7 (8) |
| C12–C8–C19 | 110.3 (5) | C25–C21–C11 | 104.8 (6) |
| C4–C9–O14 | 110.4 (8) | C26–C22–C16 | 107.6 (7) |
| C11–C10–O2 | 109.4 (6) | C13–C23–C29 | 107.8 (8) |
| C11–C10–C17 | 134.3 (7) | C29–C24–C7 | 108.9 (7) |
| O2–C10–C17 | 116.3 (6) | C21–C25–O2 | 112.4 (6) |
| C10–C11–C21 | 108.0 (6) | C22–C26–C20 | 108.8 (8) |
| C6–C12–C8 | 115.4 (5) | C15–C27–C4 | 106.6 (6) |
| C23–C13–C7 | 109.1 (7) | C24–C29–C23 | 107.9 (7) |
| C15–O14–C9 | 103.6 (6) |
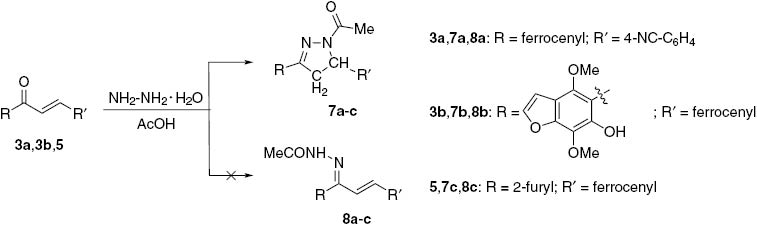
Reaction of ferrocenyl chalcones 3a, 3b and 5 with hydrazine
Chalcone 3a was allowed to react with hydrazine hydrate in boiling acetic acid to give a crystalline substance formulated as 4-(1-acetyl-4,5-dihydro-3-ferrocenyl-1H-pyrazol-5-yl)- benzonitrile (7a). In a similar way, the reaction of chalcone 3b furnished pyrazoline derivative 7b.
The reaction of chalcone 5 under similar conditions (Scheme 3) furnished a crystalline substance formulated as 1-(3-(2-furyl)-4,5-dihydro-5-ferrocenylpyrazol-1-yl)ethanone (7c). The expected hydrazones 8a–c were not found (Scheme 3). Interestingly, products seven are structural analogs of a known antitubercular drug [25].
Reaction of ferrocenyl chalcones 3a and 3b with phenylhydrazine
Heating chalcone 3a with phenylhydrazine in ethanol gave a mixture of two products which were separated by column chromatography. The first product (25%) is 4-(3-ferrocenyl-1-phenyl-1H-pyrazol-5-yl)benzonitrile (9a) and the second product (45%) is 4-(4,5-dihydro-3-ferrocenyl-1-phenyl-1H-pyrazol-5-yl)benzonitrile (10a) (Scheme 4).

Heterocyclization reaction of chalcones 3a,b with phenyl hydrazine.
Similarly, reaction of the ferrocenyl chalcone 3b with phenylhydrazine in ethanol at room temperature gave two products 9b and 10b which were separated by column chromatography. Again, the expected hydrazones 11a,b were not found in the crude mixtures. It appears that compounds 10a,b undergo dehydrogenation [26] to give 9a,b.
Reaction of ferrocenyl chalcones 3a and 3b with ethyl acetoacetate
Chalcone 3a was allowed to react with ethyl acetoacetate in boiling ethanol in the presence of a few drops of piperidine to give a crystalline product for which structure 12 was assigned (Scheme 5). Two products were obtained upon refluxing chalcone 3b with ethyl acetoacetate.

The first compound (20%) was formulated as 8-acetyl-7-ferrocenyl-4,11-dimethoxy-7,8-dihydro-5H-benzofuro[6,5-b]oxocine-5,9(6H)-dione (13). The second compound (35%) was ethyl 2-acetyl-3-ferrocenyl-5-(6-hydroxy-4,7-dimethoxybenzofuran-5-yl)-5-oxo-pentanoate (14). Apparently, the initially formed 1:1 adduct 14 undergoes intramolecular cyclization via loss of ethanol molecule to give compound 13.
Reaction of ferrocenyl chalcone 3b with ethyl cyanoacetate
The reaction of chalcone 3b with ethyl cyanoacetate in boiling ethanol furnished a mixture of two substances which were separated by column chromatography (Scheme 6) These are structures 15 (36%) and 16 (22%).

Reaction of ferrocenyl chalcone 3a with malononitrile
Compound 3a was allowed to react with malononitrile in boiling ethanol to give crystalline 4-(1,1-dicyano-4-oxo-4-ferrocenylbutan-2-yl)benzonitrile (17). On the basis of the experimental data the dihydropyridinyl structures 18 and/or 19 were safely excluded (Scheme 7).
Conclusions
The present work describes simple methods for the preparation of new ferrocene derivatives by the reaction of ferrocenyl chalcones 3a,b and 5 with hydrazines and active methylene reagents. Of particular interest is the reaction of phenylhydrazine with ferrocenyl chalcones 3a and 3b which yields a mixture of pyrazolyl and dihydropyrazolyl products in each case. Generally, the dihydropyrazolyl form is solely isolated in reactions of other chalcones with the same reagent [27–31].

Experimental
Solvents were purified and dried according to the usual procedures. Chalcone 3a[21] was prepared as previously described. The reactions were monitored (TLC) and purity of the isolated products analyzed by using aluminum sheets coated with silica gel with fluorescent indicator F254 [Fluka]. Column chromatography was performed on silica gel with grain size 0.063–0.200 mm (Merck). Uncorrected melting points were determined on an Electrothermal Digital Melting Point Apparatus. Elemental analytical data were obtained at the analytical laboratory of the National Research Centre. The IR spectra were recorded in KBr disks on a Jasco Fourier Transform Infrared Spectrophotometer model FT/IR-300E. The 1H NMR and 13C NMR spectra were recorded on a JEOL 500 AS or a Varian Mercury VX-300 spectrometer. Electron-impact mass spectra (EI-MS) were determined at 70 eV on a Finnigan MAT SSQ 7000 spectrometer. X-ray diffraction analysis: The intensity data were performed with a κ-CCD Enraf Nonius FR 590 single crystal diffractometer, temperature 298 K, wavelength Mo Kα (0.71073 Å). The structure was solved by direct methods using the SIR92 program [32] and refined using maXus [33]. The molecular graphics were made with ORTEP [34]. Crystallographic data (CIF) for the structure reported in this article have been deposited in the Cambridge Crystallographic Data Centre (CCDC) as supplementary publication No. CCDC 1425801. Copies of the data can be obtained, free of charge, upon application to the CCDC, 12 Union Road, Cambridge CB 12EZ, UK (FAX: + 44(1223)336-033; E-mail: deposit@ccdc.cam.ac.uk).
Preparation of (E)-1-ferrocenyl-3-(4-cyanophenyl)-2-propen-1-one (3a)
A solution of 4-cyanobenzaldehyde (2a, 1 mmol) in N,N-dimethylformamide (5 mL) was added gradually to a solution of acetylferrocene (1a, 1 mmol) in alcoholic KOH (2%, 15 mL) and the mixture was stirred at room temperature for 12 h. The separated solid was filtered, washed with water, dried and crystallized from DMF/H2O to give compound 3a as deep red crystals, mp 219–221°C [Ref. [21] mp 222°C (ethanol)]; yield 43%; IR: 3080, 3055, 4022, 2235, 1645, 1620 cm-1.
Preparation of (E)-3-ferrocenyl-1-(6-hydroxy-4,7-dimethoxybenzofuran-5-yl)prop-2-en-1-one (3b)
A solution of ferrocenealdehyde (2b, 1 mmol) in N,N-dimethylformamide (5 mL) was added gradually to a solution of khellinone (1b, 1 mmol) in alcoholic KOH (2%, 15 mL) and the mixture was stirred at room temperature for 12 h. The separated solid was filtered, washed with water, dried and crystallized from DMF/H2O to give compound 3b as dark brown crystals; mp 195–197°C; IR: 3435, 3086, 3045, 3025, 2985, 2890, 1635, 1615, 1590 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 3.88 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 4.17 (s, 5H, ferrocene), 4,60 (s, 2H, ferrocene), 4.62 (s, 2H, ferrocene), 6.72 (d, J = 14.0 Hz, 1H, O=C–CH=C), 6.96 (d, J = 3.0 Hz, 1H, furyl), 7.13 (d, J = 14.0 Hz, 1H, O=C–C=CH), 7.36 (d, J = 3.0 Hz, 1H, furyl), 9.95 (s, 1H, OH, D2O exchangeable). Anal. Calcd for C23H20FeO5 (432.25): C, 63.91; H, 4.66; Fe, 12.92. Found: C, 64.02; H, 4.64.
Reaction of ferrocenaldehyde with 2-acetylfuran
A solution of NaOH (10%, 10 mL) was added dropwise to a mixture of ferrocenecarboxaldehyde (1 mmol) and 2-acetylfuran (excess) in 20 mL ethanol while cooling and stirring. The mixture was stirred at room temperature for an additional 12 h. The precipitate formed was subjected to column chromatography to give compounds 5 and 6.
(E)-3-Ferrocenyl-1-(2-furyl)prop-2-en-1-one (5)
Eluent: petroleum ether/acetone (95/3, v/v); red crystals; yield 40%; mp 107–110°C [Ref. [22] mp 108–110°C]; MS: m/z 306 (94%).
1,5-Di(2-furyl)-3-ferrocenylpentane-1,5-dione (6)
Eluent: petroleum ether/acetone (95/5, v/v); yellow crystals; yield 30%; mp 134°C; IR: 3124, 2927, 1660, 1564, 1465 cm-1; 1H NMR (300 MHz, CDCl3): δ 3.20 (d, J = 6.1 Hz, 4H, CH(CH2)2), 3.80 (quintet, J = 6.1 Hz, 1H, CH(CH2)2), 4.06 (d, J = 1.5 Hz, 2H, ferrocene), 4.08 (d, J = 1.5 Hz, 2H, ferrocene), 4.12 (s, 5H, ferrocene), 6.52 (dd, J = 3.8, 1.5 Hz, 2H, furyl), 7.22 (d, J = 3.8 Hz, 2H, furyl), 7.56 (d, J = 1.5 Hz, 2H, furyl); 13C NMR (75 MHz, CDCl3): δ 30.7, 40.4, 67.5, 68.8, 68.9, 93.06, 113.1, 119.1, 148.4, 152.6, 187.8; MS: m/z 416 (100%) [M+]. Anal. Calcd for C23H20FeO4 (416.25): C, 66.37; H, 4.84; Fe, 13.42. Found: C, 66.44; H, 4.82.
Reaction of ferrocenyl chalcones 3a, 3b or 5 with hydrazine hydrate
A solution of chalcone 3a, 3b or 5 (1 mmol) and hydrazine hydrate (1.5 mmol) in acetic acid (20 mL) was heated under reflux for 2–5 h. The mixture was cooled to room temperature, poured onto ice-cooled water and left overnight. The resultant precipitate was collected and purified by chromatography on silica gel to give products 7a and 7c for the respective reactions with 3a and 5. Product 7b derived from 3b was crystallized from cyclohexane.
4-(1-Acetyl-4,5-dihydro-3-ferrocenyl-1H-pyrazol-5-yl)benzonitrile (7a)
Eluent: petroleum ether/ethyl acetate (80/20, v/v); orange crystals; yield 69%; mp 88–90°C; IR: 3100, 3051, 2923, 2852, 2221, 1658, 1620, 1495 cm-1; 1H NMR (300 MHz, CDCl3): δ 2.42 (s, 3H, COCH3), 2.94 (dd, Jgem = 17.7, Jvic= 4.8 Hz, 1H, CH2-CH-N pyrazoline, trans–H), 3.71 (dd, Jgem = 17.7, Jvic = 11.7 Hz, 1H, CH2-CH-N pyrazoline, cis–H), 4.12 (s, 5H, ferrocene), 4.43 (s, 2H, ferrocene), 4.58 (s, 1H, ferrocene), 4.65 (d, J = 1.5 Hz, 1H, ferrocene), 5.56 (dd, J = 11.7, 4.8 Hz, 1H, CH2-CH-N pyrazoline), 7.36 and 7.65 (2d, each with J = 8.4 Hz, 4H, aromatic AA′BB′ system); 13C NMR (75 MHz, CDCl3): δ 22.0, 43.6 (CH2), 58.9, 67.6, 68.2, 69.7, 70.7, 70.8, 75.3, 119.18, 126.8, 133.2, 148.3, 156.7, 167.3; MS: m/z 355 [M+ – CH2=C=O]. Anal. Calcd for C22H19FeN3O (397.25): C, 66.52; H, 4.82; Fe, 14.06; N, 10.58. Found: C, 66.63; H, 4.79; N, 10.54.
1-(4,5-Dihydro-3-(6-hydroxy-4,7-dimethoxybenzofuran-5-yl)-5-ferrocenylpyrazol-1-yl)ethanone (7b)
Yellow crystals; mp 166°C; yield 67%; IR: 3420, 3114, 3080, 2983, 2938, 2842, 1647, 1618, 1568 cm-1; 1H NMR (300 MHz, CDCl3): δ 2.29 (s, 3H, COCH3), 4.00 (dd, Jgem= 12.0 Hz, Jvic= 6.0 Hz, 1H, CH2CH-N pyrazoline, trans–H), 4.07 (dd, Jgem = 12.0 Hz, Jvic = 9.0 Hz, 1H, CH2CH-N pyrazoline, cis–H), 4.11–4.20 (m, 14H, 2OCH3, 8H of ferrocene), 4.53 (s, 1H, ferrocene), 5.42 (dd, J = 9.0, 6.0 Hz, 1H, CH2-CH-N pyrazoline), 6.92 (d, J = 3.0 Hz, 1H, furyl), 7.56 (d, J = 3.0 Hz, 1H, furyl), 11.47 (s, 1H, OH, D2O exchangeable); 13C NMR (75 MHz, DMSO-d6): δ 22.1, 43.8, 53.6, 61.0, 65.5, 68.5, 68.6, 70.5, 77.2, 87.1, 104.9, 105.2, 112.2, 129.5, 143.7, 148.3, 148.7, 150.0, 156.5, 167.5; MS: m/z 488 (23%) [M+]. Anal. Calcd for C25H24FeN2O5 (488.31): C, 61.49; H, 4.95; Fe, 11.44; N, 5.74. Found: C, 61.40; H, 4.98; N, 5.70.
1-(3-(2-Furyl)-4,5-dihydro-5-ferrocenylpyrazol-1-yl)ethanone (7c)
Eluent: petroleum ether/ethyl acetate (60/40, v/v); yellow crystals; mp 179°C; yield 71%; IR: 3080, 2925, 2851, 1644, 1482 cm-1 (C=C, C=N); 1H NMR (300 MHz, DMSO-d6): δ 2.14 (s, 3H, COCH3), 3.46 (dd, Jgem = 18.0, Jvic=6.0 Hz, 1H, CH2-CH-N pyrazoline, trans–H), 3.75 (dd, Jgem = 18.0, Jvic=12.0 Hz, 1H, CH2-CH-N pyrazoline, cis–H), 4.01 (s, 1H, ferrocene), 4.12 (s, 2H, ferrocene), 4.19 (s, 5H, ferrocene), 4.38 (s, 1H, ferrocene), 5.34 (dd, J = 12.0, 6.0 Hz, 1H, CH2-CH-N pyrazoline), 6.71 (t, J = 3.0 Hz, 1H, furyl), 7.16 (d, J = 3.0 Hz, 1H, furyl), 7.91 (d, J = 3.0 Hz, 1H, O-CH-C, furyl); 13C NMR (75 MHz, DMSO-d6): δ 22.2, 26.8, 55.0, 66.0, 68.2, 68.3, 68.9, 69.0, 70.4, 87.8, 112.6, 114.4, 145.9, 146.4, 146.8, 167.7; MS: m/z 320 (10%) [M+ – CH2=C=O]. Anal. Calcd for C19H18FeN2O2 (362.20): C, 63.00; H, 5.01; Fe, 15.42; N, 7.73. Found: C, 62.90; H, 5.03; N, 7.69.
Reaction of ferrocenyl chalcone 3a with phenylhydrazine
A solution of chalcone 3a (1 mmol) and phenylhydrazine (1.5 mmol) in absolute ethanol (20 mL) was heated under reflux for 3 h. The volatile materials were removed under reduced pressure and the residue was subjected to chromatography on silica gel using petroleum ether/ethyl acetate as eluent to give products 9a and 10a.
4-(3-Ferrocenyl-1-phenyl-1H-pyrazol-5-yl)benzonitrile (9a)
Eluent: petroleum ether /ethyl acetate (90/10, v/v); yellow crystals; mp146–148°C; yield 25%; IR: 3090, 2220, 1601, 1561, 1494 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 4.13 (s, 5H, ferrocene) 4.29 (d, 2H, J = 3 Hz, ferrocene), 4.34 (d, J = 3 Hz, 2H, ferrocene), 7.28 (s, 1H, pyrazole), 7.30–7.57 (m, 5H, phenyl), 7.93 and 8.12 (2d, each with J = 9.0 Hz, 4H, aromatic AA′BB′ system); 13C NMR (75 MHz, DMSO-d6): δ 67.0, 68.6, 69.2, 69.8, 70.1, 74.3, 78.1, 105.1, 107.5, 110.4, 115.6, 119.4, 125.7, 126.3, 126.8, 129.2, 129.7, 132.9, 133.2, 137.8, 140.3, 144.1, 149.3; MS: m/z 429 (100%) [M+]. Anal. Calcd for C26H19FeN3 (429.29): C, 72.74; H, 4.46; Fe, 13.01; N, 9.79. Found: C, 72.67; H, 4.48; Fe, N, 9.78.
4-(4,5-Dihydro-3-ferrocenyl-1-phenyl-1H-pyrazol-5-yl)benzonitrile (10a)
Eluent: petroleum ether/ethyl acetate (60/40, v/v), yellow crystals, mp 160°C; yield 45%; IR: 3084, 3010, 2914, 2223, 1647 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 2.98 (dd, 1H, Jgem = 15.0 Hz, Jvic = 6.0 Hz, CH2-CH-N pyrazoline, trans–H), 3.86 (dd, 1H, Jgem = 15.0 Hz, Jvic = 12.0 Hz, CH2-CH-N pyrazoline, cis–H), 4.12 (s, 5H, ferrocene), 4.41 (d, 2H, J = 3 Hz, ferrocene), 4.62 (d, 1H, J = 3 Hz, ferrocene), 4.69 (t, 1H, J = 3Hz, ferrocene), 5.50 (dd, J = 12.0, 6.0 Hz, 1H, CH2-CH-N pyrazoline), 6.69 (t, 1H, J = 9.0 Hz, aromatic), 6.88 (d, 2H, J = 9.0 Hz, aromatic), 7.14 (t, 2H, J = 9.0 Hz, aromatic), 7.49 (d, 2H, J = 6.0 Hz, aromatic), 7.85 (d, 2H, J = 9.0 Hz, aromatic); 13C NMR (75 MHz, DMSO-d6): δ 44.6, 62.4, 67.0, 67.3, 69.4, 70.0, 77.1, 110.6, 113.0, 118.6, 119.1, 127.4, 128.7, 129.4, 133.4, 144.7, 148.6, 149.3; MS: m/z 431 (100%) [M+]. Anal. Calcd for C26H21FeN3 (431.31): C, 72.40; H, 4.91; Fe, 12.95; N, 9.74. Found: C, 72.49; H, 4.89; Fe, N, 9.70.
Reaction of ferrocenyl chalcone 3b with phenylhydrazine
A mixture of chalcone 3b (1 mmol) and phenyl hydrazine (1.5 mmol) in absolute ethanol (20 mL) was stirred at room temperature for 12 h. The volatile materials were removed under reduced pressure and the residue was subjected to chromatography on silica gel using petroleum ether/acetone as eluent to give the two products 9b and 10b.
5-(5-Ferrocenyl-1-phenyl-1H-pyrazol-3-yl)-4,7-dimethoxybenzofuran-6-ol (9b)
Eluent: petroleum ether/acetone (93/7, v/v); yellow crystals; yield 20%; mp 176°C; IR: 3427, 3117, 2932, 1617, 1494 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 3.90 (s, 1H, ferrocene), 4.09 (s, 3H, OCH3), 4.21(s, 5H, ferrocene), 4.29 (s, 1H, ferrocene), 4.32 (d, 3H, J = 3 Hz, ferrocene), 7.16 (d, 1H, J = 3 Hz, furyl), 7.22–7.57 (m, 5H aromatic and 1H pyrazole), 7.89 (d, 1H, J = 3 Hz, furyl), 11.58 (s, 1H, OH); 13C NMR (75 MHz, DMSO-d6): δ 60.8, 60.8, 68.9, 69.0, 69.3, 70.2, 73.9, 105.7, 106.4, 108.0, 112.6, 126.5, 128.7, 129.3, 129.7, 139.6, 142.7, 144.6, 146.8, 147.4, 148.2, 148.4; MS: m/z 520 (43%) [M+]. Anal. Calcd for C29H24FeN2O4 (520.36): C, 66.94; H, 4.65; Fe, 10.73; N, 5.38. Found: C, 66.88; H, 4.67; N, 5.33.
5-(4,5-Dihydro-5-ferrocenyl-1-phenyl-1H-pyrazol-3-yl)-4,7-dimethoxy)benzofuran-6-ol (10b)
Eluent: petroleum ether/acetone (85/15, v/v); yellow crystals; yield 40%; mp 55°C; IR: 3420, 3086, 2931, 1677, 1597, 1477 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 3.83–5.00 (m, 18H, 2 OCH3, ferrocene, CH2 and CH pyrazoline), 6.56–7.85 (m, 7H, phenyl, furyl), 11.53 (s, 1H, OH); MS: m/z 522 (10%) [M+]. Anal. Calcd for C29H26FeN2O4 (522.37): C, 66.68; H, 5.02; Fe, 10.69; N, 5.36. Found: C, 66.77; H, 5.00; Fe, N, 5.32.
Reaction of ferrocenyl chalcone 3a with ethyl acetoacetate
A mixture of chalcone 3a (1 mmol) and ethyl acetoacetate (1 mmol) in absolute ethanol (20 mL) and a few drops of piperidine was heated under reflux for 8 h. The volatile materials were removed under reduced pressure and the residue was subjected to chromatography on silica gel using n-hexane/ethyl acetate as eluent to give compound 12.
Ethyl 6-(4-cyanophenyl)-4-ferrocenyl-2-oxocyclohex-3-enecarboxylate (12)
Eluent: petroleum ether/ethyl acetate (83/17, v/v); red crystals; mp 200°C; yield 64%; IR: 3095, 2976, 2923, 2869, 2226, 1739, 1641 cm-1; 1H NMR (300 MHz, CDCl3): δ 1.10 (t, 3H, J = 6.0 Hz, CH2-CH3), 1.30 (dt, J = 9.0, 6.0 Hz, 1H, -CH2-CH-CH), 2.79 (dd, 1H, Jgem = 18.0, Jvic = 9.0 Hz, -CH2-, cis–H), 3.01 (dd, J = 18.0, 6.0 Hz, 1H, -CH2-, trans–H), 3.72 (q, J = 6.0 Hz, 2H, OCH2CH3), 3.84 (d, J = 9.0 Hz, 1H, OC–CH(CO)–CH), 4.23 (d, J = 3.0 Hz, 1H, ferrocene), 4.17 (s, 5H, ferrocene), 4.55 (s, 2H, ferrocene), 4.60 (s, 1H, ferrocene), 6.37 (s, 1H, C=CH), 7.45 and 7.75 (2d, each with J = 6.0 Hz, 4H, aromatic AA′BB′ system); 13C NMR (75 MHz, CDCl3): δ 14.04, 35.25, 43.90, 58.94, 61.27, 67.0, 67.80, 69.4, 70.3, 70.7, 72.0, 79.9, 111.5, 118.5, 119.9, 128.3, 132.2, 146.6, 161.4, 169.0, 191.9; MS: m/z 453 (20%) [M+]. Anal. Calcd for C26H23FeNO3 (453.31): C, 68.89; H, 5.11; Fe, 12.32; N, 3.09. Found: C, 68.92; H, 5.08; N, 3.05.
Reaction of ferrocenyl chalcone 3b with ethyl acetoacetate
A mixture of chalcone 3b (1 mmol) and ethyl acetoacetate (1 mmol) in absolute ethanol (20 mL) was heated under reflux for 4 h. The volatile materials were removed under reduced pressure and the residue was subjected to chromatography on silica gel to resolve compounds 13 and 14.
8-Acetyl-7-ferrocenyl-4,11-dimethoxy-7,8-dihydro-5H-benzofuro[6,5-b]oxocine-5,9(6H)-dione (13)
Eluent: petroleum ether/acetone (95/5, v/v); yellow crystals; yield 20%; mp 183°C; IR: 3109, 2992, 2937, 1674,1618, 1543 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 1.2 (m, 1H, CH-CH-CH2), 2.43 (s, 3H, CH3CO), 3.02, 3.11 (2d, each with J = 14 Hz, 2H,CH2), 3.89 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 4.22 (s, 5H, ferrocene), 4.35 (s, 2H, ferrocene), 4.38 (s, 2H, ferrocene), 5.34 (d, J = 9.0 Hz, 1H, O=C-CH-C=O), 7.13 (d, J = 3.0 Hz, 1H, CH=CH-O, furyl), 7.91 (d, J = 3.0 Hz, 1H, CH=CH-O, furyl); MS: m/z 516 (2%) [M+]. Anal. Calcd for C27H24FeO7 (516.32): C, 62.81; H, 4.69; Fe, 10.82. Found: C, 62.91; H, 4.66.
Ethyl 2-acetyl-3-ferrocenyl-5-(6-hydroxy-4,7-dimethoxybenzofuran-5-yl)-5-oxo-pentanoate (14)
Eluent: petroleum ether/acetone (80/20, v/v); yellow crystals; yield 35%; mp 68–70°C; IR: 3427, 3100, 2930, 1734, 1663, 1601; 1H NMR (300 MHz, CDCl3): δ 0.88 (q, J = 6.0 Hz, 1H, CH2-CH-CH), 1.27 (t, J = 9.0 Hz, 3H, CH3CH2O), 2.24 (s, 3H, COCH3), 2.90, 3.40 (2dd, each with J = 18.0, 12.0 Hz, 2H, CH2), 3.95 (q, J = 9.0 Hz, 2H, CH3CH2O), 4.35–4.00 (m, 15H, 2OCH3, ferrocene), 5.88 (d, 1H, J = 6.0 Hz, O=C-CH-C=O), 7.53 (d, J = 3.0 Hz, 1H, CH=CH-O, furyl), 6.20 (s, 1H, OH, D2O-exchangeable), 6.88 (d, J = 3.0 Hz, 1H, CH=CH-O, furyl); 13C NMR (75 MHz, CDCl3): δ 14.0, 35.8, 37.9, 61.0, 61.2, 62.0, 65.7, 67.6, 68.5, 77.2, 89.7, 104.9, 113.7, 115.4, 128.0, 129.3, 142.1, 143.4, 144.8, 170.2, 194.1; MS: m/z 560 (5%) [M+ – 2H]. Anal. Calcd for C29H30FeO8 (562.39): C, 61.93; H, 5.38; Fe, 9.93. Found: C, 61.84; H, 5.41.
Reaction of ferrocenyl chalcone 3b with ethyl cyanoacetate
A mixture of 3b (1 mmol) and ethyl cyanoacetate (1 mmol) in absolute ethanol (20 mL) in the presence of a few drops of piperidine was heated under reflux for 6 h. The volatile materials were removed under reduced pressure and the residue was subjected to chromatography on silica gel to give two compounds 15 and 16.
Ethyl 2-cyano-3-ferrocenyl-5-(6-hydroxy-4,7-dimethoxybenzofuran-5-yl)-5-oxo-pentanoate (15)
Eluent: petroleum ether/ethyl acetate (70/30, v/v); yellow crystals; yield 36%; mp 125°C; IR: 3360, 3144, 3093, 2962, 2934, 2835, 2246, 1738, 1628, 1560, 1500 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 1.14–1.16 (m, 4H, CH3CH2-O, CH2-CH-CH), 3.40-3.55 (m, 2H, O=C-CH2-CH), 3.84–4.29 (m, 18H, 2OCH3, CH3CH2-O, NC-CH-C=O, ferrocene), 7.18 (d, J = 3.5 Hz, 1H, CH=CH-O, furyl), 7.90 (d, J = 3.5 Hz, 1H, CH=CH-O, furyl), 10.40 (s, 1H, OH, D2O-exchangeable); MS: m/z 545 (5%) [M+]. Anal. Calcd for C28H27FeNO7 (545.36): C, 61.67; H, 4.99; Fe, 10.24; N, 2.57. Found: C, 61.57; H, 5.03; N, 2.52.
6,9-Dihydro-4,11-dimethoxy-7-ferrocenyl-5,9-dioxo-5H-benzofuro[6,5-b]oxocine-8-carbonitrile (16)
Eluent: petroleum ether/ethyl acetate (5/95, v/v); yellow crystals; yield 22%; mp 200°C; IR: 3157, 3134, 3012, 2945, 2848, 2223, 1725, 1602, 1555 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 2.96 (s, 2H, CH2), 4.11–4.16 (m, 15H, 2OCH3 and ferrocene), 7.02 (d, J = 3.5 Hz, 1H, CH=CH-O, furyl), 7.66 (d, J = 3.5 Hz, 1H, CH=CH-O, furyl). Anal. Calcd for C26H19FeNO6 (497.28): C, 62.80; H, 3.85; Fe, 11.23; N, 2.82. Found: 62.76; H, 3.88; N, 2.80.
Reaction of ferrocenyl chalcone 3a with malononitrile
A mixture of 3a (1 mmol) and malononitrile (1.5 mmol) in absolute ethanol (20 mL) was heated under reflux for 6 h. The volatile materials were removed under reduced pressure and the residue was subjected to chromatography on silica gel to give compound 17.
4-(1,1-Dicyano-4-oxo-4-ferrocenylbutan-2-yl)benzonitrile (17)
Eluent: petroleum ether/acetone (95/5, v/v); red crystals; mp 175°C; IR: 3100, 2885, 2229, 1656, 1502 cm-1; 1H NMR (300 MHz, CDCl3): δ 3.33 (dd, J = 18.0, 6.0 Hz, 1H, O=C-CH-C), 3.50 (dd, 1H, J = 18.0, 9.0 Hz, O=C-CH-C), 3.97 (q, J = 6.0 Hz, 1H, CH2-CH-CH), 4.82 (d, J = 12.0 Hz, 1H, CH(CN)2), 7.65 and 7.79 (2d, each with J = 9.0 Hz, 4H, aromatic AA′BB′ system); 13C NMR (75 MHz, CDCl3): δ 28.0, 40.4, 41.1, 69.3, 70.1, 73.2, 76.9, 77.2, 98.9, 129.0, 133.0, 141.6, 157.3, 172.7 (C=O); MS: m/z 407 (78%) [M+]. Anal. Calcd for C23H17FeN3O (407.25): C, 67.83; H, 4.21; Fe, 13.71; N, 10.32. Found: C, 67.91; H, 4.18; N, 10.28.
References
[1] Gimeno, M. C.; Goitia, H.; Laguna, A.; Luque, M. E.; Villacampa, M. D.; Sepúlveda, C.; Meireles, M. Conjugates of ferrocene with biological compounds. Coordination to gold complexes and antitumoral properties. J. Inorg. Biochem. 2011, 105, 1373–1382.10.1016/j.jinorgbio.2011.07.015Suche in Google Scholar
[2] Edwards, E. I.; Epton, R.; Marr, G. A new class of semi-synthetic antibiotics: ferrocenyl- penicillins and -cephalosporins. J. Organomet. Chem. 1976, 107, 351–357.10.1016/S0022-328X(00)91527-4Suche in Google Scholar
[3] Jaouen, G.; Top, S.; Vessieres, A.; Leclercq, G.; McGlinchey, M. J. The first organometallic selective estrogen receptor modulators (SERMs) and their relevance to breast cancer. Curr. Med. Chem. 2004, 11, 2505–2517.10.2174/0929867043364487Suche in Google Scholar PubMed
[4] Biot, C.; Taramelli, D.; Forfar–Bares, I.; Maciejewski, L. A.; Boyce, M.; Nowogrocki, G.; Brocard, J. S.; Basilico, N.; Olliaro, P.; Egan, T. J. Insights into the mechanism of action of ferroquine. Relationship between physicochemical properties and antiplasmodial activity. Mol. Pharm. 2005, 2, 185–193.10.1021/mp0500061Suche in Google Scholar PubMed
[5] Abu-Hussen, A. A. A.; Linert, W. Synthesis, spectroscopic and biological activity of new mononuclear transition metal complexes of macrocyclic schiff bases derived from 1,1′- diacetylferrocine. Synth. React. Inorg. Met. Org. Chem2009, 39, 13–23.10.1080/15533170802668157Suche in Google Scholar
[6] Kondapi, A. K.; Satyanarayana, N.; Saikrishna, A. D. A study of the Topoisomerase II activity in HIV-1 replication using the ferrocene derivatives as probes. Arch. Biochem. Biophys. 2006, 450, 123–132.10.1016/j.abb.2006.04.003Suche in Google Scholar PubMed
[7] Zhang, J. Preparation, characterization, crystal structure and bioactivity determination of ferrocenyl-thiazoleacylhydrazones. Appl. Organomet. Chem. 2008, 22, 6–11.10.1002/aoc.1338Suche in Google Scholar
[8] Toda, F.; Tanaka, K.; Hamai, K. Aldol condensations in the absence of solvent: acceleration of the reaction and enhancement of the stereose lectivity. J. Chem. Soc. Perkin Trans. I,1990, 3207–3209.10.1039/p19900003207Suche in Google Scholar
[9] Dahr, D. N. The Chemistry of Chalcones and Related Compounds; Wiley: New York, 1981.Suche in Google Scholar
[10] Quintin, J.; Desrivot, J.; Thoret, S.; Le Menez, P.; Cresteil, T.; Lewin, G. Synthesis and biological evaluation of a series of tangeretin-derived chalcones. Bioorg. Med. Chem. Lett.2009, 19, 167–169.10.1016/j.bmcl.2008.10.126Suche in Google Scholar PubMed
[11] Attar S.; O’Brien, Z.; Alhaddad, H.; Golden, M. L.; Calderón–Urrea, A. Ferrocenylchalcones versus organic chalcones: a comparative study of their nematocidal activity. Bioorg. Med. Chem. 2011, 19, 2055–2073.10.1016/j.bmc.2011.01.048Suche in Google Scholar PubMed
[12] Ji, S. J.; Wang, S. Y.; Shen, Z. L.; Zhou, M. F. Facile sythesis of ferrocenylenones in free solvent at room temperature. Chin. Chem. Lett. 2003, 14, 1246–1248.Suche in Google Scholar
[13] Song, Q. B.; Yang, S. D.; Shen, T. H.; Wang, Y. Q.; Ma, Y. X.; Wu, X. L. Synthesis of 3-biaryl-1-ferrocenyl-2-propene-1-ones. Chin. Chem. Lett.2003, 14, 569–571.10.1002/chin.200445190Suche in Google Scholar
[14] Top, S.; Vessiéres, A.; Cabestaing, C.; Laios, I.; Leclercq, G.; Provot, C.; Jaouen, G. Studies on organometallic selective estrogen receptor modulators. (SERMs) Dual activity in the hydroxy-ferrocifen series, J. Organomet. Chem.2001, 637–639, 500–506.10.1016/S0022-328X(01)00953-6Suche in Google Scholar
[15] Metzler–Nolte, N.; Salmain, M. The Bioorganometallic Chemistry of Ferrocene in Ferrocenes. Ligands, Materials and Biomolecules, Ed. John Willey & Sons: West Sussex, 2008; Chapter 13, pp 499–639.10.1002/9780470985663.ch13Suche in Google Scholar
[16] Galal, S. A.; Abd El-All, A. S.; Abdallah, M. M.; El-Diwani, H. I. Synthesis of potent antitumor and antiviral benzofuran derivatives. Bioorg. Med. Chem. Lett., 2009, 19, 2420–2428.10.1016/j.bmcl.2009.03.069Suche in Google Scholar
[17] Mustafa, A. Furopyrans and Furopyrones. John Wiley and Sons; N. Y., 1967, Chapter III: Furochromones, pp 102–159.10.1002/9780470186879Suche in Google Scholar
[18] Mahran, M. R.; Ghoneim, Kh. M.; Sidky, M. M.; Omar, R. S.; Yakout, E. M. A.; Ibrahim, N. M. Reaction of 4-hydroxybergapten and 4-hydroxyisopimpinellin with aromatic aldehydes and dialdehydes. Mass spectrometry of the new (bis-furo-coumarinyl)methyl derivatives”. Bull. Fac. Pharm. Cairo Univ., 1994, 32, 51–58.Suche in Google Scholar
[19] Yosef, H. A. A.; Morsy, N. M.; Mahran, M. R. H.; Aboul-Enein, H. Y. Preparation and reaction of optically active cyanohydrins using the (R)-hydroxynitrilelyase from Prunus amygdalus. J. Iran. Chem. Soc.2007, 4, 46–58.10.1007/BF03245802Suche in Google Scholar
[20] Vogel, A. I.; Furniss, B. S.; Hannaford, A. J.; Smith, P. W. G.; Tatchell, A. R. Vogel’s Textbook of Practical Organic Chemistry, Fifth Ed., Longman Scientific and Technical. Longman Group, UK Ltd: England, 1989, 1032–1035.Suche in Google Scholar
[21] Wu, X.; Wilairat, P.; Go, M. L. Antimalarial activity of ferrocenylchalcones. Bioorg. Med. Chem. Lett. 2002, 12, 2299–2302.10.1016/S0960-894X(02)00430-4Suche in Google Scholar
[22] Toma, S. Acylation of ferrocene analogues chalcones. Collect. Czech. Chem. Commun.1969, 34, 2235–2248.10.1135/cccc19692235Suche in Google Scholar
[23] Jaime, J. V. B. Solvent-free synthesis of ferrocenylchalcones. Int. J. Chem. Tech. Res.2014, 6, 138–146.Suche in Google Scholar
[24] Nowakowska, Z. Structural Assignment of stilbenethiols and chalconethiols and differentiation of their isomeric derivatives by means of 1H- and 13C NMR spectroscopy. Spectroscopy Lett., 2005, 38, 477–485.10.1081/SL-200062816Suche in Google Scholar
[25] Mahajan, A.; Kremer, L.; Louw, S.; Guéradel, Y.; Chibale, K.; Biot, C. Synthesis and in vitro antitubercular activity of ferrocene-based hydrazones. Bioorg. Med. Chem. Lett.2011, 21, 2866–2868.10.1016/j.bmcl.2011.03.082Suche in Google Scholar PubMed
[26] Makhlouf, A. A.; Kamel, M. M.; Anwar, M. M.; Haiba, M. E.; Mohei El-Deen, E. M. Synthesis of some new 1,8-naphthyridine-3-carboxamides of antimicrobial activity. Egypt. Pharmaceut. J.2005, 4, 371–383.Suche in Google Scholar
[27] El-Khawaga, A. M.; Hassan, K. M.; Khalaf, A. A. Studies on ferrocene derivatives(VII). Synthesis of some new ferrocenyl dibromides, benzofuranes and indoles. Z. Naturforsch.1981, 86b, 119–122.10.1515/znb-1981-0125Suche in Google Scholar
[28] Samshuddin, S.; Narayana, B.; Sarojini, B. K.; Yathirajan, H. S.; Raghavendra, R. Synthesis, characterization and biological evaluation of functionalized derivatives of versatile synthon 4,4′-difluoro chalcone. Der. Pharm. Chemica. 2012, 4, 1445–1457.Suche in Google Scholar
[29] Osman, S. A.; Yosef, H. A. A.; Hafez, T. S.; El-Sawy, A. A.; Mousa, H. A.; Hassan, A. S. Synthesis and antibacterial activity of some novel chalcones, pyrazoline and 3-cyanopyidine derivatives based on Khellinone as well as Ni(II), Co(II) and Zn(II) complexes. Aust. J. Basic Appl. Sci., 2012, 6, 852–863.Suche in Google Scholar
[30] Sharma, S.; Kaur, S.; Bansal, T.; Gaba, J. Review on synthesis of bioactive pyrazoline derivatives. Chem. Sci. Trans.2014, 3, 861–875.Suche in Google Scholar
[31] Suwito, H.; Jumina, M.; Novi Kristanti, A.; Puspaningsih, N. N. T. Chalcones: Synthesis, structure diversity and pharmacological aspects. J. Chem. Pharm. Res.2014, 6, 1076–1088.Suche in Google Scholar
[32] Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M. C.; Polidori, G.; Camall, M. SIRPOW.92 – a program for automatic solution of crystal structures by direct methods optimized for powder data. J. Appl. Cryst. 1994, 27, 435–436.10.1107/S0021889894000221Suche in Google Scholar
[33] Macky, S.; Gilmore, C. J.; Edwards, C.; Stewart, N.; Shankland, K. MaXus Computer Program for the Solution and Refinement of Crystal Structures, Brucker Nonius, The Netherlands; MacScience, Japan and The University of Glasgow, Glasgow, UK, 1999.Suche in Google Scholar
[34] Johnoson, C. K. ORTEP-II, A. Fortran Thermal-Ellipsoid Program, Report ORNL-5138. Oak Ridge National Laboratory, Oak Ridge, Tennessee, USA, 1976.Suche in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Preliminary Communication
- A facile one-pot synthesis of aryl-substituted fused pyrimidinones
- Research Articles
- Three-component synthesis of new o-hydroxyphenyl-substituted pyrazolo[3,4-b]pyridines promoted by FeCl3
- Reactions of ferrocenyl chalcones with hydrazines and active methylene compounds
- Synthesis of 3-alkyl-5-allylamino-2-benzoylimino-1,3,4-thiadiazoles via Dimroth rearrangement
- Chiral oxazoline ligands with two different six-membered azaheteroaromatic rings – synthesis and application in the Cu-catalyzed nitroaldol reaction
- Stereoselective Michael addition of O-nucleophiles to carbohydrate-based nitro-olefin
- The synthesis of hydrophobic 1-alkyl-1H,1′H-2,2′-bibenzo[d]imidazoles
- Novel synthesis and reactions of pyrazolyl-substituted tetrahydrothieno[2,3-c]isoquinoline derivatives
- Acid-base properties and keto-enol equilibrium of a 5-substituted derivative of 1,3-diethyl-2-thiobarbituric acid
Artikel in diesem Heft
- Frontmatter
- Preliminary Communication
- A facile one-pot synthesis of aryl-substituted fused pyrimidinones
- Research Articles
- Three-component synthesis of new o-hydroxyphenyl-substituted pyrazolo[3,4-b]pyridines promoted by FeCl3
- Reactions of ferrocenyl chalcones with hydrazines and active methylene compounds
- Synthesis of 3-alkyl-5-allylamino-2-benzoylimino-1,3,4-thiadiazoles via Dimroth rearrangement
- Chiral oxazoline ligands with two different six-membered azaheteroaromatic rings – synthesis and application in the Cu-catalyzed nitroaldol reaction
- Stereoselective Michael addition of O-nucleophiles to carbohydrate-based nitro-olefin
- The synthesis of hydrophobic 1-alkyl-1H,1′H-2,2′-bibenzo[d]imidazoles
- Novel synthesis and reactions of pyrazolyl-substituted tetrahydrothieno[2,3-c]isoquinoline derivatives
- Acid-base properties and keto-enol equilibrium of a 5-substituted derivative of 1,3-diethyl-2-thiobarbituric acid

