Abstract
A facile access to 3-alkyl-5-N-allylamino-2-N′-benzoylimino-1,3,4-thiadiazoles via Dimroth rearrangement is reported. Alkylation of 5-allylamino-4-benzoyl-1,2,4-triazole-3-thione with alkyl halides in basic alcohol solution is regioselective. A mechanism for the formation of a 1,3,4-thiadiazole ring by 1,2,4-triazole recyclization is proposed. The obtained compounds were characterized by NMR, elemental analysis and X-ray diffraction analysis.
Introduction
1,3,4-Thiadiazoles possess a wide range of biological activities including antimicrobial [1–3], antifungal [4], antituberculosis [5], anti-inflammatory [6], anticancer [7], antioxidant [8] and antidepressant activity [9]. Some drugs (Figure 1) containing an amino-substituted 1,3,4-thiadiazole ring, are used in medicinal practice [2, 3].
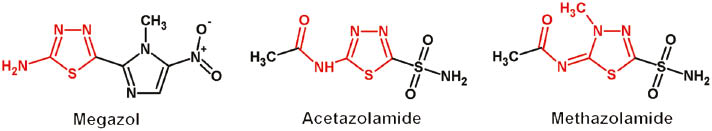
Drugs containing amino-substituted 1,3,4-thiadiazole ring.
1,3,4-Thiadiazoles are available by (i) cyclization of acylhydrazines [9], thiocarbazides [10], thiosemicarbazides [11], dithiocarbazates [12], thioacylhydrazines [13] and bithioureas [14], (ii) by rearrangement of other heterocycles, like 1,3,4-oxadiazole [15], 1,3,4-thiadiazine [16] and imidazoloiminophosphorane [17] and (iii) by tandem 1,3-dipolar cycloaddition reaction [18]. Herein, we summarize a fortuitous synthesis of substituted 5-amino-2-imino-1,3,4-thiadiazoles from 1,2,4-triazole derivatives in the hope that this procedure will enrich the list of simple and versatile methods for thiadiazole ring formation and can help chemists in preparation of methazolamide-like pharmaceuticals.
Previously, we have shown [19, 20] that 5-allylamino-1,2,4-triazole-3-thiones are useful intermediate products in ring anellation reactions; we have also shown that the presence of an aroyl substituent in the 4-position of 1,2,4-triazole-3-thiones allows for use of these compounds as ligands for complexation of heavy metals cations [21]. Our group has continued to investigate the chemical properties of the 5-amino-4-aroyl-1,2,4-triazole-3-thione system, particularly its behavior in basic medium toward treatment with alkyl halides. Herein, we wish to report a facile synthetic method for construction of substituted 5-amino-2-imino-1,3,4-thiadiazoles by alkylation of 5-allylamino-4-benzoyl-1,2,4-triazole-3-thione with primary alkyl halides in basic alcohol solution. We believe that this reaction proceeds by Dimroth rearrangement.
Results and discussion
The starting allylaminotriazole 2 was obtained by cyclization of bis-thiourea 1, obtained in turn by the reaction of benzoyl isothiocyanate with allyl thiosemicarbazide according to the literature [19]. The reaction of triazole 2 with a primary alkyl halide was carried out in methanol in the presence of potassium hydroxide. Analysis of the NMR spectra of the obtained products indicated the formation of N-alkylated 1,3,4-thiadiazole 3 in each case instead of S-alkylation.
Thus, the 1H NMR spectra of alkylated products 3 do not contain the peak at 12–13 ppm which is a signal of NH-proton of triazole ring seen in the spectrum of a triazole 2, and the corresponding proton signals of an alkyl group are observed in the aliphatic area. A signal for protons of the exocyclic N-CH2 group in thiadiazole 3b–f appears at about 4.27–5.46 ppm. This is in contrast to a higher-field resonance of S-CH2 group protons (at 3.76–3.98 ppm) observed for S-alkylated 3-mercapto-1,2,4,-triazoles [22]. The 13C NMR spectra of compounds 3 reveal low-field signals of heterocyclic nodal carbons at 172 ppm (thiadiazole C-2) and 161 ppm (thiadiazole C-5) in addition to a signal at 159 ppm for a carbonyl function. If these products of alkylation were the isomeric triazoles A (Scheme 1), the nodal C-3 and C-5 triazole signals would have appeared at a higher field (smaller chemical shift) [23–25]. The structure of thiadiazole 3c was also unambiguously confirmed by X-ray crystallographic analysis of its dibromo derivative 4. Compound 4 was used because a crystal of 3 suitable for X-ray analysis could not be prepared (Scheme 2). Thus, the bromination of thiadiazole 3c leads to a simple addition product 4 without halocyclization in contrast to related reports [19, 20, 26–33] but in agreement with a similar transformation [34]. X-ray crystallographic analysis of dibromide 4 (Figure 2 and Tables 1S, 2S) provides confirmation of the structure of the alkylation products (Scheme 1). A mechanism for the formation of the thiadiazole ring is suggested in Scheme 3. It is proposed that recyclization is carried out through the Dimroth rearrangement with cleavage of a triazole ring under the action of hydroxide anion. The next stage is the formation of the 1,3,4-thiadiazole ring and then alkylation of its anion form with formation of products 3. This unexpected rearrangement under basic conditions can be explained by the high stability of 5-amino-substituted 4-carbonyl-1,3,4-thiadiazoles [16].
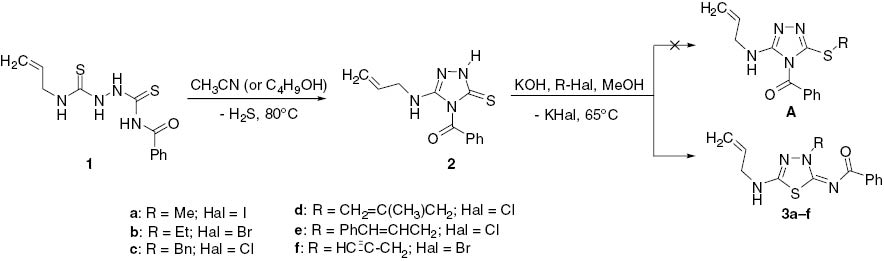
Synthetic approach to 1,3,4-thiadiazoles 3.
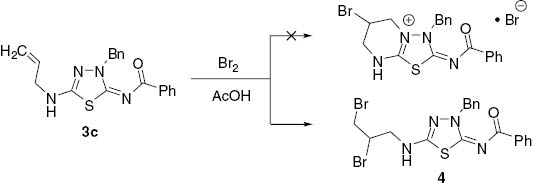
Synthesis of thiadiazole 4.
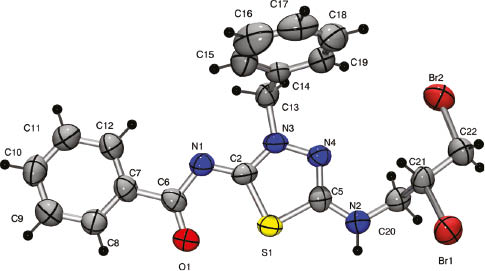
Structure of compound 4 as obtained by X-ray diffraction analysis.
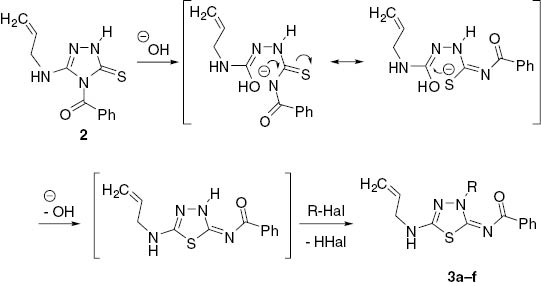
Mechanistic proposal for the formation of the 1,3,4-thiadiazole ring.
Conclusions
3-Alkyl-5-N-allylamino-2-N′-benzoylimino-1,3,4-thiadiazoles are easily accessible by treatment of 5-alkylamino-4-benzoyl-1,2,4-triazol-3-thiones with alkyl halides in hydroxide alcohol solution.
Experimental
1H NMR (500 MHz) and 13C NMR (100 MHz) spectra were recorded on Brucker Avance 500 and Varian VXR 400 instruments, respectively, in DMSO-d6. The melting points were determined on a Stuart SMP30 instrument. Elemental analyses were performed on Elementar Vario MICRO cube analyzer. All reagents were obtained from commercial suppliers and used without any further purification.
X-ray diffraction analysis
The structure of 4 was obtained by X-ray diffraction studies performed at room temperature (293(2) K) on an automatic diffractometer Oxford Diffraction Xcalibur, interpreted by a direct method and refined by the full-matrix least-squares technique in the anisotropic approximation for non-hydrogen atoms using the SHELX-97 program package [35]. The program WinGX [36] was used to analyze the structure and to produce the illustrations. All C–H hydrogen atoms were placed in calculated positions and refined as a riding model. For all distances, bonds and valence angles the expected values were found in the molecule of dibromide 4.
X-ray crystal data [35]: M 510.25, colorless crystal, crystal size ca. 0.55×0.03×0.02 mm. Monoclinic, space group P21/c, a = 5.6367(5) A, b = 19.4588(15) A, c = 18.6097(19) A, β = 95.511(8)°, V = 2031.7(3) A3, Z = 4, Dcalc. = 1.668 g/cm3, μ = 4.109 mm-1, F(000) = 1016. The intensity data were collected within the range of 3.04 ≤ θ ≤ 28.36°, using Mo-Kα radiation (λ = 0.71078 Å). The intensities of 14314 reflections were collected (4324 unique reflections). The multi-scan absorption correction (the minimum and maximum transmission 0.211 and 0.922) was applied. In the refinement, 4324 reflections (1791 reflections with I ≥ 2σ(I)) were used. Convergence was obtained at RF = 0.1597 and wR2 = 0.0952 for all reflections and RF = 0.0475 and wR2 = 0.0677, GOF = 0.984.
Synthesis of 5-allylamino-3-R-1,3,4-thiadiazol-2(3H)-ylidenebenzamide 3a–f
5-Allylamino-4-benzoyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (2, 5.0 mmol) was stirred in methanol (20 mL) containing potassium hydroxide (6.0 mmol) untill a clear solution was formed. An alkylating agent (6.0 mmol) was then added and the mixture was heated under reflux for 2 h. After cooling the precipitated product 3 was filtered, washed with cold water-methanol solution (1:1), dried and crystallized from water-ethanol (1:2) mixture.
5-Allylamino-3-methyl-1,3,4-thiadiazol-2(3H)-ylidenebenzamide (3a)
This compound was obtained from 2 and methyl iodide; yield 60%; light-yellow crystals; mp 169.8–170.5°C; 1H NMR: δ 8.20 (d, 2H), 7.80 (t, 1H), 7,50 (m, 3H), 5.92 (m, 1H), 5.28 (d, 1H), 5.17 (d, 1H), 3.87 (m, 2H), 3.81 (s, 3H); 13C NMR: δ 172.3, 161.0, 158.5, 136.8, 134.7, 132.0, 129.3, 128.6, 116.7, 45.9, 45.9, 37.6. Anal. Calcd for C13H14N4OS: C, 56.91; H, 5.14; N, 20.42; S, 11.69. Found: C, 56.62; H, 5.30; N, 20.26; S, 11.45.
5-Allylamino-3-ethyl-1,3,4-thiadiazol-2(3H)-ylidenebenzamide (3b)
This compound was obtained from 2 and ethyl bromide; yield 55%; colorless crystals; mp 129.8–131.1°C; 1H NMR: δ 8.18 (d, 2H), 7.76 (t, 1H), 7.50 (m, 3H), 5.92 (m, 1H), 5.26 (d, 1H), 5.15 (d, 1H), 4.27 (q, 2H), 3.86 (m, 2H), 1.35 (t, 3H); 13C NMR: δ 172.3, 160.5, 158.6, 136.9, 134.7, 132.0, 129.2, 128.6, 116.8, 45.9, 45.5, 13.7. Anal. Calcd for C14H16N4OS: C, 58.31; H, 5.59; N, 19.43; S, 11.12. Found: C, 58.10; H, 5.72; N, 19.18; S, 10.97.
5-Allylamino-3-benzyl-1,3,4-thiadiazol-2(3H)-ylidenebenzamide (3c)
This compound was obtained from 2 and benzyl chloride; yield 66%; colorless crystals; mp 243.5–244.0°C; 1H NMR: δ 8.21 (d, 2H), 7.88 (t, 1H), 7.44 (m, 3H), 5.91 (m, 1H), 5.46 (s, 2H), 5.26 (d, 1H), 5.15 (d, 1H), 3.84 (m, 2H). Anal. Calcd for C19H18N4OS: C, 65.12; H, 5.18; N, 15.99; S, 9.15. Found: C, 65.01; H, 5.32; N, 15.68; S, 9.37.
5-Allylamino-3-methallyl-1,3,4-thiadiazol-2(3H)-ylidenebenzamide (3d)
This compound was obtained from 2 and methallyl chloride; yield 71%; white powder; mp 123.8–124.7°C; 1H NMR: δ 8.17 (d, 2H), 7.79 (t, 1H), 7.49 (m, 3H), 5.92 (m, 1H), 5.26 (d, 1H), 5.15 (d, 1H), 4.93 (s, 1H), 4.82 (s, 2H), 4.80 (s, 1H), 3.84 (m, 2H), 1.71 (s, 3H); 13C NMR: δ 172.4, 161.6, 158.4, 140.1, 136.8, 134.6, 132.2, 129.3, 128.7, 116.7, 113.5, 55.4, 45.9, 20.5. Anal. Calcd for C16H18N4OS: C, 61.12; H, 5.77; N, 17.82; S, 10.20. Found: C, 60.71; H, 6.03; N, 17.16; S, 9.99.
5-Allylamino-3-cinnamyl-1,3,4-thiadiazol-2(3H)-ylidenebenzamide (3e)
This compound was obtained from 2 and cinnamyl chloride; yield 67%; yellow powder; mp 156.4–158.5°C; 1H NMR: δ 8.21 (d, 2H), 7.81 (t, 1H), 7.23–7.55 (m, 8H), 6.70 (d, 1H), 6.45 (m, 1H), 5.91 (m, 1H), 5.28 (d, 1H), 5.15 (d, 1H), 5.04 (d, 2H), 3.86 (m, 2H); 13C NMR: δ 172.4, 161.2, 158.6, 136.8, 136.5, 134.6, 133.7, 132.2, 129.4, 129.2, 128.7, 128.4, 127.0, 123.6, 116.8, 52.2, 46.0. Anal. Calcd for C21H20N4OS: C, 67.00; H, 5.35; N, 14.88; S, 8.52. Found: C, 66.70; H, 5.84; N, 14.46; S, 8.22.
5-Allylamino-3-propargyl-1,3,4-thiadiazol-2(3H)-ylidenebenzamide (3f)
This compound was obtained from 2 and propargyl bromide; yield 48%; colorless crystals; mp 177.3–177.6°C; 1H NMR: δ 8.20 (d, 2H), 7.86 (t, 1H), 7.52 (m, 3H), 5.92 (m, 1H), 5.28 (d, 1H), 5.16 (d, 1H), 5.09 (s, 2H), 3.87 (m, 2H), 3.41 (s, 1H); 13C NMR: δ 172.6, 161.5, 158.4, 136.3, 134.6, 132.5, 129.4, 128.7, 116.9, 78.1, 76.2, 45.9. Anal. Calcd for C15H14N4OS: C, 60.38; H, 4.73; N, 18.78; S, 10.75. Found: C, 60.04; H, 4.98; N, 18.46; S, 10.33.
N-[3-Benzyl-5-(2,3-dibromopropylamino)-3H-[1,3,4]thiadiazol-2-ylidene]benzamide (4)
A solution of bromine (10.0 mmol) in acetic acid was dropwise added to the solution of triazole 3c (10.0 mmol) in acetic acid with constant stirring at room temperature during 30 min. The precipitated product was filtered, washed with acetone and crystallized from a water–ethanol–dioxane (1:2:1) mixture to give compound 4 as fine white needles suitable for X-ray diffraction analysis; yield 74%; mp 285.4–287.2°C; 1H NMR: δ 8.21 (d, 2H), 8.03 (t, 1H), 7.30–7.58 (m, 8H), 5.47 (s, 2H), 4.61 (m, 1H), 3.98 (m, 2H), 3.80 (m, 1H), 3.66 (m, 1H). Anal. Calcd for C19H18Br2N4OS: C, 44.72; H, 3.56; N, 10.98; S, 6.28. Found: C, 44.98; H, 3.82; N, 10.81; S, 6.09.
Acknowledgments:
This research was partly supported by the International Visegrad Fund [contract number: 51501563].
References
[1] Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. 1,3,4-Thiadiazole: synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610.10.1021/cr400131uSearch in Google Scholar PubMed
[2] Jain, A. K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R. K. 1,3,4-Thiadiazole and its derivatives: a review on recent progress in biological activities. Chem. Biol. Drug Des. 2013, 81, 557–576.10.1111/cbdd.12125Search in Google Scholar PubMed
[3] Khalilullah, H.; Khan, M. U.; Mahmood, D.; Akhtar, J.; Osman, G. 1,3,4-Thiadiazole: a biologically active scaffold. Int. J. Pharm. Pharm. Sci. 2014, 6, 8–15.Search in Google Scholar
[4] Upadhyay, P. K.; Mishra, P. Design, synthesis and antifungal evaluation of novel substituted 1,3,4-oxadiazoles, and 1,3,4-thiadiazoles. Int. J. Pharm. Pharm. Sci. 2015, 7, 466–470.Search in Google Scholar
[5] Syed, M. A.; Ramappa, A. K.; Alegaon, Sh. Synthesis and evaluation of antitubercular and antifungal activity of some novel 6-(4-substituted aryl)-2-(3,5-dimethyl-1H-pyrazol-1-yl) imidazo[2,1-b] [1,3,4] thiadiazole derivatives. Asian J. Pharm. Clin. Res. 2013, 6, 47–51.Search in Google Scholar
[6] Al-Mekhlafi, S.; Alkadi, H.; El-Sayed, M.-I. K. Synthesis and anti-inflammatory activity of novel Ketoprofen and Ibuprofen derivatives. J. Chem. Pharm. Res. 2015, 7, 503–510.Search in Google Scholar
[7] Dawood, K. M.; Gomha, S. M. Synthesis and anti-cancer activity of 1,3,4-thiadiazole and 1,3-thiazole derivatives having 1,3,4-oxadiazole moiety. J. Heterocycl. Chem. 2014, 52, 1400–1405.10.1002/jhet.2250Search in Google Scholar
[8] Seelolla, G.; Ponneri, V. Synthesis, antimicrobial, and antioxidant activities of some fused heterocyclic [1,2,4]Triazolo[3,4-b][1,3,4]thiadiazole derivatives. J. Heterocycl. Chem. 2015, published ahead of print; DOI: 10.1002/jhet.2348.10.1002/jhet.2348Search in Google Scholar
[9] Augustine, J. K.; Vairaperumal, V.; Narasimhan, S.; Alagarsamy, P.; Radhakrishnan, A. Propylphosphonic anhydride (T3P): an efficient reagent for the one-pot synthesis of 1,2,4-oxadiazoles, 1,3,4-oxadiazoles, and 1,3,4-thiadiazoles. Tetrahedron2009, 65, 9989–9996.10.1016/j.tet.2009.09.114Search in Google Scholar
[10] Sayed, A. R. Synthesis of novel thiadiazoles and bis-thiadiazoles from carbonothioic dihydrazide. Tetrahedron Lett. 2010, 51, 4490–4493.10.1016/j.tetlet.2010.06.060Search in Google Scholar
[11] Yang, S.-J.; Lee, S.-H.; Kwak, H.-J.; Gong, Y.-D. Regioselective synthesis of 2-amino-substituted 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives via reagent-based cyclization of thiosemicarbazide intermediate. J. Org. Chem. 2013, 78, 438–444.10.1021/jo302324rSearch in Google Scholar PubMed
[12] Wei, M. X.; Feng, L.; Li, X. Q.; Zhou, X. Z.; Shao, Z. H. Synthesis of new chiral 2,5-disubstituted 1,3,4-thiadiazoles possessing γ-butenolide moiety and preliminary evaluation of in vitro anticancer activity. Eur. J. Med. Chem. 2009, 44, 3340–3344.10.1016/j.ejmech.2009.03.023Search in Google Scholar PubMed
[13] Farrar, J. M.; Patel, M. K.; Kaszynski, P.; Young, V. G. A new thiatriazine isomer: synthesis, tautomerism, and molecular structure of 3,6-diphenyl-4H-1,2,4,5-thiatriazine as a precursor to the 1,2,4,5-thiatriazinyl radical. J. Org. Chem. 2000, 65, 931–940.10.1021/jo991126lSearch in Google Scholar PubMed
[14] Hassan, A. A.; Mourad, A. F. E.; El-Shaieb, K. M.; Abou-Zied, A. H.; Dopp, D. Thermolysis of symmetrical dithiobiurea and thioureidoethylthiourea derivatives. Heteroatom Chem. 2003, 14, 535–541.10.1002/hc.10188Search in Google Scholar
[15] Padmavathi, V.; Reddy, G. D.; Reddy, S. N.; Mahesh, K. Synthesis and biological activity of 2-(bis((1,3,4-oxadiazolyl/1,3,4-thiadiazolyl)methylthio)methylene)malononitriles Eur. J. Med. Chem. 2011, 46, 1367–1373.10.1016/j.ejmech.2011.01.063Search in Google Scholar
[16] Fleischhauer, J.; Beckert, R.; Hornig, D.; Gunther, W.; Gorls, H.; Klimesova, V. Thia- and selena-heterocycles containing cycloamidine substructures: ring contraction reactions of 1,3,4-thia-/selenadiazines. Z. Naturforsch. B. 2008, 63, 415–424.10.1515/znb-2008-0408Search in Google Scholar
[17] Molina P.; Arques A.; Alias Ma. A. Heterocumulene-mediated annelation of a [1,3,4]thiadiazine or [1,3,4]oxadiazine ring into an imidazole ring: preparation and crystal structure of some derivatives of the unknown imidazo[l,5-d][1,3,4]thiadiazine and imidazo[l,5-d[1,3,4]oxadiazine ring systems. Tetrahedron1990, 46, 4353–4370.10.1016/S0040-4020(01)86770-3Search in Google Scholar
[18] Shawali, A. S. 1,3,4-Thiadiazoles of pharmacological interest: recent trends in their synthesis via tandem 1,3-dipolar cycloaddition. J Adv Res2014, 5, 1–17.10.1016/j.jare.2013.01.004Search in Google Scholar PubMed PubMed Central
[19] Fizer, M. M.; Slivka, M. V.; Lendel V. G. New method of synthesis of 3,5,6,7-tetrahydro[1,2,4]triazolo[1,5-a]pyrimidine-2(1H)-thione. Chem. Heterocycl. Comp. 2013, 49, 1243–1245.10.1007/s10593-013-1369-zSearch in Google Scholar
[20] Fizer, M. M.; Slivka, M. V.; Rusanov, E.; Turov, A.; Lendel, V. G. [1,3]Thiazolo[2′,3′:3,4][1,2,4]triazolo[1,5-a]pyrimidines – a new heterocyclic system accessed via bromocyclization. J. Heterocycl. Chem. 2015, 52, 949–952.10.1002/jhet.2073Search in Google Scholar
[21] Fizer, M.; Sukharev, S.; Slivka, M.; Mariychuk, R.; Lendel, V. Preparation of bisthiourea and 5-amino-4-benzoyl-1,2,4-triazol-3-thione complexes of copper (ii), nickel and zinc and their biological evolution. J. Organomet. Chem. 2016, 804, 6–12.10.1016/j.jorganchem.2015.12.024Search in Google Scholar
[22] Slivka, M.; Korol, N.; Rusyn, I.; Lendel, V. Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-S-alkenylthio-4H-1,2,4-triazoles. Heterocycl. Commun. 2015, 21, 397–401.10.1515/hc-2015-0158Search in Google Scholar
[23] Mekheimer R. A., Shaker R. M. Synthesis and reactivity of 3-alkylthio-5-cyanomethyl-4-phenyl-1,2,4-triazoles. J. Chem. Res. (S). 1999, 76–77.10.1039/a806842iSearch in Google Scholar
[24] Elmoghayar, M. R. H.; Ghali, E. A.; Ramiz, M. M. M.; Elnagdi, M. H. Activated nitriles in heterocyclic synthesis, IV. synthesis of 1,3,4-thiadiazole derivatives. Liebigs Ann. Chem. 1985, 10, 1962–1968.10.1002/jlac.198519851005Search in Google Scholar
[25] Katritzky, A. R.; Borowiecka, J.; Fan, W.-Q.; Brannigan, L. H. Some novel S-mono- and S,S′-unsymmetrical di-substituted derivatives of 1,3,4-thiadiazoledithione. J. Heterocycl. Chem. 1991, 28, 1139–1141.10.1002/jhet.5570280451Search in Google Scholar
[26] Khripak, S.; Slivka, M.; Vilkov, R.; Usenko, R.; Lendel, V. Regioselectivity of the monohalogenation of 4-allyl-3-allylamino-1,2,4-triazole-5-thione. Chem. Heterocycl. Comp. 2007, 43, 781–785.10.1007/s10593-007-0126-6Search in Google Scholar
[27] Khripak, S. M.; Plesha, M. V.; Slivka, M. V.; Yakubets, V. I.; Krivovyaz, A. A. Synthesis and reactivity of 1-bromomethyl-5-oxo-4-phenyl-1,2,4,5,6,7,8,9-octahydrobenzo[4,5]thieno[3,2-e][1,3]oxazolo[3,2-a]-pyrimidin-11-ium bromides. Russ. J. Org. Chem. 2004, 40, 1705–1706.10.1002/chin.200521169Search in Google Scholar
[28] Onysko, M. Yu.; Lendel, V. G. Haloheterocyclization of 2-methallyl(propargyl)-thioquinoline-3-carbaldehydes. Chem. Heterocycl. Comp. 2009, 45, 853–855.10.1007/s10593-009-0349-9Search in Google Scholar
[29] Slivka, Mar. V.; Krivovjaz, A. A.; Slivka, M. V.; Lendel, V. G. Stereoselective synthesis of (E)-halogenmethylidene[1,3]thiazolo[3,2-a]thieno[3,2-e]pyrimidinium and Analogous [1,3]oxazolo[3,2-a]thieno[3,2-e]pyrimidinium halogenides from 3-N-Substituted 2-propargylthio(oxy-)thieno-[2,3-d]pyrimidin-4-ones. Heterocycl. Commun. 2013, 19, 189–193.10.1515/hc-2013-0036Search in Google Scholar
[30] Slivka, M. V.; Khripak, S. M.; Britsun, V. N.; Staninets, V. I. Synthesis of 3-substituted 1,2,4-triazolo[3,4-b]thiazolium halides. Russ. J. Org. Chem. 2000, 36, 1033–1038.Search in Google Scholar
[31] Svaljavyn, O. V.; Onysko, M. Yu.; Turov, A. V.; Vlasenko, Yu. G.; Lendel, V. G. Peculiar electrophilic heterocyclization of 5-allyl-6-thioxopyrazolo[3,4-d]pyrimidin-4-one. Chem. Heterocycl. Comp. 2013, 49, 491–495.10.1007/s10593-013-1273-6Search in Google Scholar
[32] Usenko, R. M.; Slivka, M. V.; Lendel, V. G. Electrophilic heterocyclization of 4,5-disubstituted 3-allylthio-4H-1,2,4-triazoles by the action of halogens. Chem. Heterocycl. Comp. 2011, 47, 1029–1036.10.1007/s10593-011-0870-5Search in Google Scholar
[33] Vas’kevich, R. I.; Khripak, S. M.; Staninets, V. I.; Zborovskii, Yu. L.; Chernega, A. N. Synthesis of fused thiazolothienopyrimidines. Russ. J. Org. Chem. 2000, 36, 1091–1096.Search in Google Scholar
[34] Ukrainets, I. V.; Petrushova, L. A.; Bereznyakova, N. L. Effect of bromination on the pharmacological properties of methyl-1-allyl-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate. Pharm. Chem. J. 2015, 49, 25–28.10.1007/s11094-015-1319-4Search in Google Scholar
[35] Sheldrick, G. M. A short history of SHELX. Acta Cryst. A. 2008, 64, 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
[36] Farrugia, L. J. WinGX suite for small molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838.10.1107/S0021889899006020Search in Google Scholar
Supplemental Material:
The online version of this article (DOI: 10.1515/hc-2015-0279) offers supplementary material, available to authorized users.
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communication
- A facile one-pot synthesis of aryl-substituted fused pyrimidinones
- Research Articles
- Three-component synthesis of new o-hydroxyphenyl-substituted pyrazolo[3,4-b]pyridines promoted by FeCl3
- Reactions of ferrocenyl chalcones with hydrazines and active methylene compounds
- Synthesis of 3-alkyl-5-allylamino-2-benzoylimino-1,3,4-thiadiazoles via Dimroth rearrangement
- Chiral oxazoline ligands with two different six-membered azaheteroaromatic rings – synthesis and application in the Cu-catalyzed nitroaldol reaction
- Stereoselective Michael addition of O-nucleophiles to carbohydrate-based nitro-olefin
- The synthesis of hydrophobic 1-alkyl-1H,1′H-2,2′-bibenzo[d]imidazoles
- Novel synthesis and reactions of pyrazolyl-substituted tetrahydrothieno[2,3-c]isoquinoline derivatives
- Acid-base properties and keto-enol equilibrium of a 5-substituted derivative of 1,3-diethyl-2-thiobarbituric acid
Articles in the same Issue
- Frontmatter
- Preliminary Communication
- A facile one-pot synthesis of aryl-substituted fused pyrimidinones
- Research Articles
- Three-component synthesis of new o-hydroxyphenyl-substituted pyrazolo[3,4-b]pyridines promoted by FeCl3
- Reactions of ferrocenyl chalcones with hydrazines and active methylene compounds
- Synthesis of 3-alkyl-5-allylamino-2-benzoylimino-1,3,4-thiadiazoles via Dimroth rearrangement
- Chiral oxazoline ligands with two different six-membered azaheteroaromatic rings – synthesis and application in the Cu-catalyzed nitroaldol reaction
- Stereoselective Michael addition of O-nucleophiles to carbohydrate-based nitro-olefin
- The synthesis of hydrophobic 1-alkyl-1H,1′H-2,2′-bibenzo[d]imidazoles
- Novel synthesis and reactions of pyrazolyl-substituted tetrahydrothieno[2,3-c]isoquinoline derivatives
- Acid-base properties and keto-enol equilibrium of a 5-substituted derivative of 1,3-diethyl-2-thiobarbituric acid

