Abstract
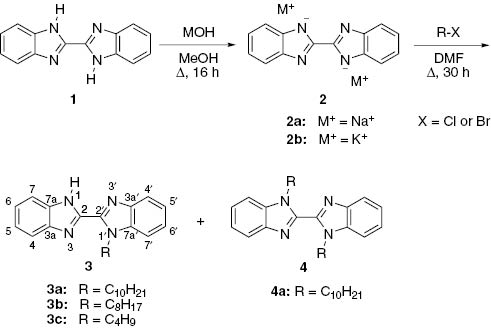
New synthesis of 1-alkyl-1H,1′H-2,2′- bibenzo[d]imidazoles 3 by alkylation of a disodium derivative of 1H,1′H-2,2′-bibenzo[d]imidazole 2a with alkyl halides is proposed. It is noteworthy that 1,1′-dialkylated byproducts 4 are formed in yields of <5%. The structure of all obtained compounds has been confirmed by 1H NMR, 13C NMR, and HRMS or elemental analysis.
Introduction
New, selective extractants have been designed for recovery of metals from primary and secondary raw materials in hydrometallurgical processes. Owing to the presence of four nitrogen atoms in 1H,1′H-2,2′-bibenzo[d]imidazole (1), this compound can form various types of complexes with d-electron metal ions [1–4]. The limited solubility of 1H,1′H-2,2′-bibenzo[d]imidazole (1) in commercial hydrocarbon solvents makes it impractical to use the compound as an extractant. The introduction of hydrophobic substituents into the molecule of compound 1 increases solubility and enables the use of such derivatives in the extraction stage in technological processes. Our previous studies have shown that the complexing and extracting abilities of hydrophobic derivatives of 1H,1′H-2,2′-bibenzo[d]imidazole (1) and other heterocyclic compounds (e.g. hydrophobic amides and esters of pyridinecarboxylic acids) depend on the structure, position, and number of substituents in aromatic rings. To accurately investigate the usefulness of monoalkyl- (3) and dialkyl-1H,1′H-2,2′-bibenzo[d]imidazoles (4) in the extraction processes, it is necessary to obtain pure compounds with well-defined structures. There are some reports in the literature describing only disubstituted derivatives of 1 as the extractants of metal ions [5]. ACORGA ZNX 50, a reagent with a bis(alkoxycarbonyl) substitution of 1H,1′H-2,2′-bibenzo[d]imidazole (1) was proposed previously as a selective extractant for zinc(II) over iron(III) from chloride solutions [6, 7].
The objective of our work was the synthesis of 1-alkyl-1H,1′H-2,2′-bibenzo[d]imidazoles 3. Alkylation of disodium 2,2′-bibenzo[d]imidazole-1,1′-diide (2a) turned out to be a good method to obtain the desired compounds 3. To the best of our knowledge, this is the first work that shows how to obtain hydrophobic mono-substituted compounds 3. The literature describes alkylation of 1H,1′H-2,2′-bibenzo[d]imidazole (1) conducted in the presence of a base. Alkyl halides with highly hydrophobic (octadecyl) and less hydrophobic (methyl, ethyl) groups have been used to obtain 1,1′-disubstituted derivatives of 1 [8–11]. Furthermore, the reaction of compound 1 with an alkyl dihalide has been used for the synthesis of 1,1′-bridged derivatives 1 [12, 13]. The disubstituted derivatives 4 have also been obtained from 1-alkylbenzimidazoles as substrates [14–17].
There are two reports describing the preparation of 1-methyl-1H,1′H-2,2′-bibenzo[d]imidazole. Fieselmann and co-workers have described the synthesis of this compound by alkylation of 1 with methyl iodide in the presence of sodium methoxide. The alkylation reaction was conducted in a glass pressure vessel heated at 95°C at the pressure of 4 atm giving a mixture of both 1-methyl-1H,1′H-2,2′-bibenzo[d]imidazole and 1,1′-dimethyl-1H,1′H-2,2′-bibenzo[d]imidazole [18]. The mono-methylated derivative has also been obtained by Nguyen and co-workers by an iron sulfide-catalyzed condensation between 2-aminonitrobenzenes and 2-methylbenzimidazoles [19]. Neither of the two methods has been used to prepare more hydrophobic 1-alkyl-1H,1′H-2,2′-bibenzo[d]imidazoles 3.
Results and discussion
1-Alkyl-1H,1′H-2,2′-bibenzo[d]imidazoles 3 were obtained from 1 in a two-step synthesis through the intermediary of product 2 (Scheme 1) that was generated by the reaction of 1 with sodium or potassium hydroxide. The analysis of the literature suggests that methanol is the most suitable solvent for the deprotonation reaction, and the next choices are dichloromethane, acetonitrile, or dimethylformamide (DMF) [20]. First reactions were attempted in methanol under reflux conditions. It was observed that an increase in the reaction time above 16 h (Table 1, entries 2, 3 and 8, 9) and an increase in the molar ratio of hydroxide to the substrate 1 from 2 to 4 (Table 1, entries 1–3 and 7–9, 4 and 10, 5 and 11) had no significant impact on the yield of salt 2. Slightly lower yields of 2 are observed in cases where potassium hydroxide is used instead of sodium hydroxide (Table 1, entries 4 and 5, 10 and 11). The yields in methanol are increased with increasing the temperature (Table 1, entries 2 and 4, 8 and 10). On the other hand, the superior solubility of compound 1 in DMF results in a reduced yield because isolation of salt 2a from the remaining starting material is difficult (Table 1, entry 6). Spectral analyses confirmed that crude salts 2a,b could be used for the subsequent alkylation reaction without further purification. The 1H NMR spectra of sodium salt 2a and potassium salt 2b indicate the absence of a singlet at 13.5 ppm assigned to the N-H protons in substrate 1.
Synthesis of salt 2 from compound 1.
| Number | (1):MOH (mol/mol) | M | Solvent | T (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 1:2 | Na | MeOH | 65 | 12 | 39 |
| 2 | 1:2 | Na | MeOH | 65 | 16 | 47 |
| 3 | 1:2 | Na | MeOH | 65 | 26 | 44 |
| 4 | 1:2 | Na | MeOH | 75 | 16 | 60 |
| 5 | 1:2 | K | MeOH | 75 | 16 | 51 |
| 6 | 1:2 | Na | DMF | 125 | 30 | 15 |
| 7 | 1:4 | Na | MeOH | 65 | 12 | 42 |
| 8 | 1:4 | Na | MeOH | 65 | 16 | 45 |
| 9 | 1:4 | Na | MeOH | 65 | 20 | 46 |
| 10 | 1:4 | Na | MeOH | 75 | 16 | 58 |
| 11 | 1:4 | K | MeOH | 75 | 16 | 52 |
Alkylation of salt 2 was carried out at the reflux temperature of the reaction mixture using DMF or methanol as a solvent. When DMF was used in the alkylation reaction, 1-alkyl derivative of 1 was obtained as the main product (Table 2, entry 2) and no formation of 1,1′-dialkyl derivative of 1 was observed. Molecular modeling was used to analyze the reaction outcome. The Becke-style three-parameter density functional theory (DFT) and the Lee-Yang-Parr correlation functional B3LYP/6-31G(d,p) basis set were used to optimize molecules and compare their stability. The planar conformer s-trans of 1H,1′H-2,2′-bibenzo[d]imidazole was found to be definitely more stable (at 11.6 kcal/mol) than the nonplanar s-cis conformer because of the steric hindrance of (N)H atoms, causing the system to lose its planarity and resonance. The calculations showed also that 1-methyl-substituted s-trans-1H,1′H-2,2′-bibenzo[d]imidazole is a planar molecule. However, benzimidazole moieties of s-trans conformer of 1,1′-dimethyl-1H,1′H-2,2′-bibenzo[d]imidazole are not positioned in the same plane (Figure 1). One can expect that, for the extensive substituents (isobutyl, octyl, and decyl), the stability of such structures without coupling will be limited. The effective resonance-stabilization of planar s-trans-1-alkyl-1H,1′H-2,2′-bibenzo[d]imidazole is the cause of its great stability that protects it against further substitution reaction.
Synthesis of compounds 3a–c from salt 2 and alkyl halide R-X.
| Number | R | X | (2) | (2):R-X (mol/mol) | Solvent | T (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | decyl | Cl | Na | 1:2 | MeOH | 65 | 30 | 0 |
| 2 | decyl | Cl | Na | 1:2 | DMF | 150 | 30 | 10 |
| 3 | decyl | Cl | Na | 1:3 | DMF | 150 | 30 | 19 |
| 4 | decyl | Cl | Na | 1:4 | DMF | 150 | 30 | 61 |
| 5 | decyl | Br | Na | 1:4 | DMF | 150 | 30 | 74 |
| 6 | decyl | Br | K | 1:4 | DMF | 150 | 30 | 71 |
| 7 | decyl | Cl | Na | 1:4 | DMF | 150 | 40 | 55 |
| 8 | octyl | Cl | Na | 1:2 | DMF | 150 | 30 | 37 |
| 9 | octyl | Cl | Na | 1:4 | DMF | 150 | 30 | 76 |
| 10 | octyl | Br | K | 1:4 | DMF | 150 | 30 | 72 |
| 11 | isobutyl | Br | Na | 1:4 | DMF | 150 | 30 | 24 |
![Figure 1 B3LYP/6-31G(d,p) optimized structures of the s-trans conformers for 1-methyl-1H,1′H-2,2′-bibenzo[d]imidazole (A) and 1,1′-dimethyl-1H,1′H-2,2′-bibenzo[d]imidazole (B).](/document/doi/10.1515/hc-2015-0236/asset/graphic/j_hc-2015-0236_fig_001.jpg)
B3LYP/6-31G(d,p) optimized structures of the s-trans conformers for 1-methyl-1H,1′H-2,2′-bibenzo[d]imidazole (A) and 1,1′-dimethyl-1H,1′H-2,2′-bibenzo[d]imidazole (B).
Increasing the molar ratio of the alkyl halide to sodium salt 2a from 2:1 to 4:1 significantly increases the yield of the formation of a monoalkyl derivative 3 (Table 2, entries 2–4, 8 and 9). On the other hand, increasing the reaction time enhances the formation of a dialkyl derivative 4, which makes the purification of the main products 3 difficult. It is important that byproduct 4 is formed in a yield of <5%.
Byproduct 4a, 1,1′-didecyl-1H,1′H-2,2′-bibenzo[d]imidazole, was isolated from the reaction mixture and its structure was confirmed by 1H NMR, 13C NMR, and HRMS.
Conclusion
New 1-alkyl-1H,1′H-2,2′-bibenzo[d]imidazoles 3a–c were synthesized by alkylation of substrate 1. The alkylation does not produce a significant amount of disubstituted byproduct 4.
Experimental
Melting points were determined using a Boetius hot stage apparatus. The low- and high-resolution mass spectra were recorded on a Intectra Mass AMD 402 spectrometer with electron-impact ionization and 70 eV energy. The 1H NMR and the 13C NMR spectra were measured on a Varian Gemini 400 spectrometer (400 and 100 MHz, respectively) in CDCl3, using tetramethylsilane (TMS) as an internal standard. Elemental analysis was performed on a Vario EL III (Elementar) instrument. All reagents were of analytical grade. Reaction progress and purity of the compounds were monitored by thin layer chromatography (TLC) using aluminum plates coated with silica gel (E. Merck). Silica gel 60 (E. Merck 70–230 mesh) was used for flash chromatography. Synthesis of 1H,1′H-2,2′-bibenzo[d]imidazole (1) has been described in our previous work [21].
General procedure for the synthesis of 2,2′- bibenzo[d]imidazole-1,1′-diide salts 2a,b
A mixture of 1H,1′H-2,2′-bibenzo[d]imidazole (1) (30 g, 0.128 mol), sodium hydroxide or potassium hydroxide (0.256 mol), and methanol (400 mL) was heated at 75°C for 16 h. After cooling, the remaining starting material was filtered off and washed with methanol. The solution was concentrated under reduced pressure and the brown-red solid residue was washed with methanol (3×50 mL) and dried at 110–120°C to give a brown-red powder of product 2a,b.
Disodium 2,2′-bibenzo[d]imidazole-1,1′-diide (2a)
Yield 60%; mp >350°C; 1H NMR (DMSO-d6): δ 7.28 (m, 4H, ArH), 7.64 (m, 4H, ArH); 13C NMR (DMSO-d6): δ 115.5, 122.8, 139.2, 144.0. Anal. Calcd for C14H8N4Na2: C, 60.42; H, 2.90; N, 20.14. Found: C, 60.71; H, 2.86; N, 19.98.
Dipotassium 2,2′-bibenzo[d]imidazole-1,1′-diide (2b)
Yield 51%; mp >350°C; 1H NMR (DMSO-d6): δ 7.25 (m, 4H, ArH), 7.63 (m, 4H, ArH); 13C NMR (DMSO-d6): δ 115.5, 122.9, 139.2, 143.9. Anal. Calcd for C14H8N4K2: C, 54.17; H, 2.60; N, 18.05. Found: C, 53.92; H, 2.58; N, 18.21.
General procedure for the synthesis of 1-alkyl-1H,1′H-2,2′-bibenzo[d]imidazoles 3
A mixture of disodium 2,2′-bibenzo[d]imidazole-1,1′-diide (2a) (10.57 g, 0.038 mol) and DMF (150 mL) was heated under reflux until a clear solution was formed. After cooling to 80oC, the suspension was treated with decyl bromide (33.62 g, 0.152 mol) and the mixture was heated under reflux for 30 h and then concentrated. The residue was extracted with toluene. The organic phase was washed with water and dried over anhydrous magnesium sulfate. The crude product was purified by column chromatography on silica gel eluting with toluene. Pure product 3a–c was obtained without any need for further crystallization.
1-Decyl-1H,1′H-2,2′-bibenzo[d]imidazole (3a)
Yield 74%; mp 114–116°C; 1H NMR (CDCl3): δ 0.87 (t, J = 6.8 Hz, 3H, CH3), 1.24 (s, 10H, 5×CH2), 1.41 (m, 2H, CH2), 1.49 (m, 2H, CH2), 2.03 (m, 2H, CH2), 5.14 (t, J = 7.6 Hz, 2H, NCH2), 7.27 (m, 4H, C5-H, C5′-H, C6-H and C6′-H), 7.39 (t, J = 7.6 Hz, 1H, C7-H), 7.55 (d, J = 8.0 Hz, 1H, C7′-H), 7.70 (d, J = 8.4 Hz, 1H, C4-H), 7.89 (d, J = 7.6 Hz, 1H, C4′-H), 13.57 (s, 1H, NH); 13C NMR (CDCl3): δ 14.1, 22.7, 26.8, 29.3, 29.3, 29.5, 29.5, 29.9, 31.9, 45.4, 110.7, 111.9, 119.5, 120.2, 122.4, 123.2, 123.9, 124.1, 134.1, 136.1, 141.9, 143.5, 143.6, 144.3; MS: m/z 374 (47%, M+), 234 (100%, M+- C10H20). HRMS. Calcd for C24H30N4 (M+): m/z 374.2470. Found: m/z 374.2456.
1-Octyl-1H,1′H-2,2′-bibenzo[d]imidazole (3b)
Yield 76%; mp 85–88°C; 1H NMR (CDCl3): δ 0.86 (t, J = 7.0 Hz, 3H, CH3), 1.26 (s, 6H, 3×CH2), 1.40 (m, 2H, CH2), 1.48 (m, 2H, CH2), 2.03 (m, 2H, CH2), 5.15 (t, J = 7.5 Hz, 2H, NCH2), 7.29 (m, 4H, C5-H, C5′-H, C6-H and C6′-H), 7.40 (t, J = 8.0 Hz, 1H, C7-H), 7.56 (d, J = 8.5 Hz, 1H, C7′-H), 7.70 (d, J = 8.5 Hz, 1H, C4-H), 7.87 (d, J = 8.0 Hz, 1H, C4′-H), 13.85 (s, 1H, NH); 13C NMR (CDCl3): δ 14.1, 22.6, 26.8, 29.2, 29.2, 29.9, 31.8, 45.4, 110.7, 111.9, 119.5, 120.2, 122.5, 123.2, 123.9, 124.1, 134.2, 136.1, 141.9, 143.5, 143.6, 144.3; MS: m/z 346 (47%, M+), 234 (100%, M+- C8H16). HRMS. Calcd for C22H26N4 (M+): m/z 346.2158. Found: m/z 346.2171.
1-Isobutyl-1H,1′H-2,2′-bibenzo[d]imidazole (3c)
Yield 24%; mp 82–85°C; 1H NMR (CDCl3): δ 1.02 (d, J = 6.4 Hz, 6H, 2×CH3), 2.51 (m, 1H, CH), 4.99 (d, J = 6.0 Hz, 2H, NCH2), 7.25 (m, 4H, C5-H, C5′-H, C6-H and C6′-H), 7.39 (t, J = 6.8 Hz, 1H, C7-H), 7.55 (d, J = 6.8 Hz, 1H, C7′-H), 7.69 (d, J = 6.4 Hz, 1H, C4-H), 7.89 (d, J = 6.4 Hz, 1H, C4′-H), 13.84 (s, 1H, NH); 13C NMR (CDCl3): δ 20.2, 20.2, 29.6, 52.2, 111.1, 111.8, 119.5, 120.3, 122.5, 123.2, 123.8, 124.1, 134.0, 136.6, 141.9, 143.7, 143.7, 144.2; MS: m/z 290 (24%, M)+, 234 (100%, M+- C4H8). HRMS. Calcd for C18H18N4 (M+): m/z 290.1531. Found: m/z 290.1523.
1,1′-Didecyl-1H,1′H-2,2′-bibenzo[d]imidazole (4a)
Yield 4%; mp 60–61°C; 1H NMR (CDCl3): δ 0.87 (t, J = 7.0 Hz, 6H, 2×CH3), 1.19 (s, 16H, 8×CH2), 1.29 (m, 12H, 6×CH2), 1.85 (m, 4H, 2×CH2), 4.89 (t, J = 7.5 Hz, 4H, 2×NCH2), 7.36 (m, 4H, C5-H, C5′-H, C6-H and C6′-H), 7.49 (d, J = 7.5 Hz, 2H, C7-H and C7′-H), 7.86 (d, J = 8.0 Hz, 2H, C4-H and C4′-H); 13C NMR (CDCl3): δ 14.0, 22.6, 26.7, 29.0, 29.2, 29.4, 29.4, 29.9, 31.8, 45.4, 110.7, 120.2, 123.1, 124.2, 128.8, 135.5, 141.9; MS: m/z 514 (100%, M+), 373 (97%, M+- C10H21), 234 (70%, M+- C20H40). HRMS. Calcd for C34H50N4 (M+): m/z 514.4011. Found: m/z 514.4036.
Acknowledgments:
This work was supported by the grants no. 03/32/DS PB/0500, no. 03/32/DS PB/0600, and no. UDA-POIG.02.01.00-30-128/09.
References
[1] Galán-Mascarós, J. R.; Dunbar, K. R. A self-assembled 2D molecule-based magnet: the honeycomb layered material {Co3Cl4(H2O)2[Co(Hbbiz)3]2}. Angew. Chem. Int. Ed. 2003, 42, 2289–2293.10.1002/anie.200250152Suche in Google Scholar
[2] Lin, S.; Chen, L.-J.; Xu, H.-H.; Su, J.-B.; Huang, H. Two 2D metal-organic frameworks based on 2,2′-bibenzimidazole ligand with (6,3) net topology. Inorg. Chem. Commun. 2010, 13, 1347–1349.10.1016/j.inoche.2010.07.033Suche in Google Scholar
[3] Zhong, Y.-R.; Cao, M.-L.; Mo, H.-J.; Ye, B.-H. Syntheses and crystal structures of metal complexes with 2,2′-biimidazole-like ligand and chloride: Investigation of X-H···Cl (X = N, O, and C) hydrogen bonding and Cl-π (imidazolyl) interactions. Cryst. Growth Des. 2008, 8, 2282–2290.10.1021/cg700980vSuche in Google Scholar
[4] Mo, H.-J.; Zhong, Y.-R.; Cao, M.-L.; Ou, Y.-C.; Ye, B.-H. Hydrothermal syntheses and structural diversity of cobalt complexes with 2,2′-bibenzimidazole ligand by temperature tuning strategy. Cryst. Growth Des. 2009, 9, 488–496.10.1021/cg800747tSuche in Google Scholar
[5] Devonald, D. P.; Nelson, A. J.; Quan, P. M.; Stewart, D. Process for the extraction of metal values and novel metal extractants. EP Patent 0196153, 1989.Suche in Google Scholar
[6] Cote, G.; Jakubiak, A. Modelling of extraction equilibrium for zinc(II) extraction by a bibenzimidazole type reagent (ACORGA ZNX 50) from chloride solutions. Hydrometallurgy. 1996, 43, 277–286.10.1016/0304-386X(95)00114-VSuche in Google Scholar
[7] Dalton, R. F.; Burgess, A.; Quan, P. M. ACORGA ZNX50 – a new selective reagent for the solvent extraction of zinc from chloride leach solutions. Hydrometallurgy. 1992, 30, 385–400.10.1016/0304-386X(92)90095-HSuche in Google Scholar
[8] Huang, W.-K.; Cheng, C.-W.; Chang, S.-M.; Lee, Y.-P.; Diau, E. W.-G. Synthesis and electron-transfer properties of benzimidazole-functionalized ruthenium complexes for highly efficient dye-sensitized solar cells. Chem. Commun. 2010, 46, 8992–8994.10.1039/c0cc03517cSuche in Google Scholar PubMed
[9] Ninán, O.; Chareyron, R.; Figuereido, O.; Santiago, J. Derivados de bencimidazol cristales líquidos. Rev. Soc. Quím. Perú. 2006, 72, 178–186.Suche in Google Scholar
[10] Wei, T.; Liu, J.; Yao, H.; Lin, Q.; Xie, Y.; Shi, B.; Zhang, P.; You, X.; Zhang, Y. Selective chemosensor of Fe3+ based on fluorescence quenching by 2,2′-bisbenzimidazole derivative in aqueous media. Chin. J. Chem. 2013, 31, 515–519.10.1002/cjoc.201300019Suche in Google Scholar
[11] Guo, H.-L.; Liu, J.-C.; Xiao, C.-H.; Pang, T.; Cao, P. Dimethyl 2,2′-[2,2′-bi(1H-1,3-benzimidazole)-1,1′-diyl]diacetate. Acta Crystallogr. Sect. E. 2012, 68, 2515.10.1107/S1600536812032266Suche in Google Scholar PubMed PubMed Central
[12] Frantz, D. K.; Sullivan, A. A.; Yasui, Y.; Linden, A.; Baldridge K. K.; Siegel, J. S. Synthesis, X-ray crystal structures, and computational studies of 1,1′-bridged 4,4′-diaryl-2,2′-bibenzimidazoles: building blocks for supramolecular structures. Org. Biomol. Chem. 2009, 7, 2347–2352.10.1039/b820502gSuche in Google Scholar PubMed
[13] Shi, Z.; Thummel, R. P. N,N′-Bridged derivatives of 2,2′-bibenzimidazole. J. Org. Chem. 1995, 60, 5935–5945.10.1021/jo00123a034Suche in Google Scholar
[14] Li, Y.; Jin, J.; Qian, W.; Bao, W. An efficient and convenient Cu(OAc)2/air mediated oxidative coupling of azoles via C-H activation. Org. Biomol. Chem. 2010, 8, 326–330.10.1039/B919396KSuche in Google Scholar
[15] Komarov, I. V.; Strizhak, A. V.; Kornilov, M. Yu. Direct phosphorylation of N-protected imidazoles and benzimidazoles – a route to 1H-imidazol(benzimidazol)-2-yl phosphonic and phosphinic acids and their derivatives. Synthetic Commun. 1998, 28, 2355–2370.10.1080/00397919808004288Suche in Google Scholar
[16] Monguchi, D.; Yamamura, A.; Fujiwara, T.; Somete, T.; Mori, A. Oxidative dimerization of azoles via copper(II)/silver(I)-catalyzed CH homocoupling. Tetrahedron Lett. 2010, 51, 850–852.10.1016/j.tetlet.2009.12.016Suche in Google Scholar
[17] Park, S. B.; Alper, H. Highly efficient, recyclable Pd(II) catalysts with bisimidazole ligands for the Heck reaction in ionic liquids. Org. Lett. 2003, 5, 3209–3212.10.1021/ol030071dSuche in Google Scholar PubMed
[18] Fieselmann, B. F.; Hendrickson, D. N.; Stucky, G. D. Synthesis, electron paramagnetic resonance, and magnetic studies of binuclear bis(η5-cyclopentadienyl) titanium(III) compounds with bridging pyrazolate, biimidazolate, and bibenzimidazolate anions. Inorg. Chem. 1978, 17, 2078–2084.10.1002/chin.197844262Suche in Google Scholar
[19] Nguyen, T. B.; Ermolenko, L.; Al-Mourabit, A. Iron sulfide catalyzed redox/condensation cascade reaction between 2-amino/hydroxy nitrobenzenes and activated methyl groups: A straightforward atom economical approach to 2-hetarylbenzimidazoles and -benzoxazoles. J. Am. Chem. Soc. 2013, 135, 118–121.10.1021/ja311780aSuche in Google Scholar PubMed
[20] Begtrup, M.; Larsen, P. Alkylation, acylation, and silyation of azoles. Acta Chem. Scand. 1990, 44, 1050–1057.10.3891/acta.chem.scand.44-1050Suche in Google Scholar
[21] Mądrzak-Litwa, I.; Borowiak-Resterna, A. The use of diethylene glycol in the synthesis of 2,2′-bibenzimidazole from o-phenylenediamine and oxalic acid. Heterocycl. Commun. 2014, 20, 177–180.10.1515/hc-2013-0241Suche in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Preliminary Communication
- A facile one-pot synthesis of aryl-substituted fused pyrimidinones
- Research Articles
- Three-component synthesis of new o-hydroxyphenyl-substituted pyrazolo[3,4-b]pyridines promoted by FeCl3
- Reactions of ferrocenyl chalcones with hydrazines and active methylene compounds
- Synthesis of 3-alkyl-5-allylamino-2-benzoylimino-1,3,4-thiadiazoles via Dimroth rearrangement
- Chiral oxazoline ligands with two different six-membered azaheteroaromatic rings – synthesis and application in the Cu-catalyzed nitroaldol reaction
- Stereoselective Michael addition of O-nucleophiles to carbohydrate-based nitro-olefin
- The synthesis of hydrophobic 1-alkyl-1H,1′H-2,2′-bibenzo[d]imidazoles
- Novel synthesis and reactions of pyrazolyl-substituted tetrahydrothieno[2,3-c]isoquinoline derivatives
- Acid-base properties and keto-enol equilibrium of a 5-substituted derivative of 1,3-diethyl-2-thiobarbituric acid
Artikel in diesem Heft
- Frontmatter
- Preliminary Communication
- A facile one-pot synthesis of aryl-substituted fused pyrimidinones
- Research Articles
- Three-component synthesis of new o-hydroxyphenyl-substituted pyrazolo[3,4-b]pyridines promoted by FeCl3
- Reactions of ferrocenyl chalcones with hydrazines and active methylene compounds
- Synthesis of 3-alkyl-5-allylamino-2-benzoylimino-1,3,4-thiadiazoles via Dimroth rearrangement
- Chiral oxazoline ligands with two different six-membered azaheteroaromatic rings – synthesis and application in the Cu-catalyzed nitroaldol reaction
- Stereoselective Michael addition of O-nucleophiles to carbohydrate-based nitro-olefin
- The synthesis of hydrophobic 1-alkyl-1H,1′H-2,2′-bibenzo[d]imidazoles
- Novel synthesis and reactions of pyrazolyl-substituted tetrahydrothieno[2,3-c]isoquinoline derivatives
- Acid-base properties and keto-enol equilibrium of a 5-substituted derivative of 1,3-diethyl-2-thiobarbituric acid

