Synthesis, characterization and in vitro antimicrobial assessment of some novel 4H-1, 4-benzothiazines and their sulfone derivatives
-
Naveen Gautam
, Ajay Kumar Bishnoi
Abstract
In recent years, synthesis and biological evaluation of novel 4H-1,4-benzothiazines and their sulfone derivatives have gained momentum due to their medicinal and industrial importance. Our studies focused on the design and synthesis of new antimicrobial agents, and for this purpose a series of novel 4H-1,4-benzothiazines and their sulfone derivatives were synthesized and their in vitro antimicrobial assessment was carried out against a representative panel of Gram-positive and Gram-negative bacteria strains and selected fungi species. The reported 4H-1,4-benzothiazines were prepared by condensation followed by oxidative cyclization of substituted 2-aminobenzenethiols with compounds containing active methylene groups. It is believed that the reaction proceeds via intermediary of the enaminoketone system. The sulfone derivatives were synthesized by oxidation of 4H-1,4-benzothiazines using 30% hydrogen peroxide in glacial acetic acid. Structure determination was done by spectral and elemental investigations.
Introduction
Multiple drug resistance against antimicrobial agents is one of the most serious problems of today’s medicinal scenario. Examples of drug-resistant pathogens are methicillin/oxacillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and the penicillin-resistant Streptococcus pneumonia. Unfortunately, microbes develop resistance against newer agents after a short period of their clinical use. Thus, the pipeline of new antimicrobial agents is drying up. Therefore, there is a need to develop new and different antimicrobial agents which can be used as drugs to treat chronic conditions. It is well established that the benzothiazine system has potential to be developed into novel antibacterial and anticancer agents [1–5]. Knowing the importance of the benzothiazine template, we have synthesized several substituted 4H-1,4-benzothiazines [6, 7] and their sulfone derivatives [8–10] that exhibit antibacterial and antifungal activity [11–15]. Similar compounds herein reported were easily prepared by condensation and oxidative cyclization of 2-aminobenzenethiols with β-diketones/β-ketoesters in dimethyl sulfoxide (DMSO). These intermediate products were further converted into sulfone derivatives by oxidation under reflux conditions using 30% hydrogen peroxide in glacial acetic acid (Scheme 1). To reflect the potency of the synthesized compounds as antimicrobial agents, activity index (AI) and minimum inhibitory concentration (MIC) against different Gram-positive, Gram-negative and fungi strains belonging to the Microbial Type Culture Collection (MTCC) is reported using the agar well diffusion method and the broth microdilution method, respectively.
Results and discussion
Chemistry
The starting substituted 2-aminobenzenethiols 1a–d (Scheme 1) were synthesized by alkaline hydrolysis of corresponding 2-aminobenzothiazoles, which in turn were prepared by the cyclization of substituted phenylthiourea. The phenylthiourea derivatives were obtained by the reaction of ammonium thiocyanate with substituted anilines. A mixture of 2-aminobenzenethiols 1a–d and β-diketones/β-ketoesters 2a–d was heated under reflux conditions in DMSO, which led to condensation and oxidative cyclization. Oxidation of 2-aminobenzenethiols produced bis-(2-aminophenyl)disulfide 3, which in turn underwent cyclization to form 4H-1,4-benzothiazines 4a–e by scission of a sulfur-sulfur bond. This cleavage is due to high reactivity of the α-position of the enaminoketone system towards nucleophillic attack. Compounds 4a–e were converted into their corresponding sulfones 5a–e by treatment with 30% hydrogen peroxide in glacial acetic acid.

Synthesis of 4H-1,4-benzothiazines 4a–e and their sulfone derivatives 5a–e.
Spectral data and elemental analysis support the structures of synthesized compounds. In the IR spectra of compounds 4a–e, a single peak is seen in the region of 3340–3310 cm-1 due to NH stretching vibration. These compounds also exhibit a band at 1655–1605 cm-1 due to C=O stretching vibrations. A slight shift towards higher frequencies is observed for sulfone derivatives 5a–e due to an increased electron-accepting ability of sulfones compared with the parent system. Compounds 5a–e exhibit intense peaks in the regions of 1385–1245 cm-1, 1190–1145 cm-1 and 585–545 cm-1, which can be ascribed to vibrations of the sulfonyl group. The signals for C-S stretching vibrations in the region of 1065–1035 cm-1 are also observed in the IR spectra of compounds 5a–e. The given structures of products 4a–e and 5a–e are also fully consistent with their 1H NMR spectra. A multiplet in the region of δ 8.02–6.13 is due to the presence of aromatic protons in all compounds 4a–e and 5a–e. A singlet in the region δ 9.03–8.12 can be ascribed to the N-H function. The molecular ion peaks in the mass spectra of 4H-1,4-benzothiazines and their sulfone derivatives are fully consistent with their molecular weights. The base peak is for the ion R4CO+, as predicted for the generation of the most stable cation.
Antimicrobial assessment
The in vitro antimicrobial assessment of synthesized compounds was carried out by using the agar well diffusion method for determination of AI and the broth microdilution method for determination of MIC against two strains of bacteria, Micromonospora sp. (Gram-positive) and Zymomonas mobilis (Gram-negative), and two strains of fungi, Aspergillus solani and Fusarium culmorum. Ampicillin sulfate and fluconazole were used as a standard antibacterial and antifungal drug, respectively. Compounds 4c and 5c show good antibacterial activity against the Gram-positive strain Micromonospora sp., whereas compounds 4d, 4e, 5d and 5e show excellent activity against the Gram-negative strain of subject bacteria Z. mobilis. Compound 5a exhibits good antifungal activity against A. solani and compound 5c is an excellent agent against F. culmorum. The remaining compounds show moderate antimicrobial activities. It should be noted that sulfone derivatives of 4H-1,4-benzothiazines exhibit better antimicrobial activities than their parent compounds. This finding can be explained by the presence of the electron-withdrawing group (-SO2) in sulfones, which enhances biological activity. This effect of an electron-withdrawing group has been noted in the literature [16–18].
Conclusions
The synthesis of novel heterocyclic compounds which can be used as potent antibacterial and antifungal agents is described. Compounds 4d and 5d show antibacterial activity against Gram-negative Z. mobilis that is comparable to activity of ampicillin sulfate. Compounds 4e and 5e are much better than ampicillin sulfate in antibacterial activity against Gram-negative Z. mobilis. Compound 5c shows antifungal activity against F. culmorum that is better than that of fluconazole.
Experimental section
Melting points are uncorrected. IR spectra were recorded in KBr on a Shimadzu 8400 S FT-IR spectrophotometer. 1H NMR spectra were recorded on a JEOL AL 300 spectrometer (300 MHz) in DMSO-d6. Fast atom bombardment (FAB) mass spectra were recorded on a JEOL SX 102/DA 600 instrument using Xenon/Argon. The purity of compounds were checked by TLC using silica gel “G” as adsorbent in various solvent systems with visualization by UV light or in an iodine chamber.
General method for synthesis of substituted 4H-1,4-benzothiazines 4a–e
β-Diketone/β-ketoester 2a–d (0.01 mol) was treated with a solution of 2-aminobenzenethiol 1a–d (0.01 mol) in 5 mL of DMSO and the resulting mixture was heated under reflux (190°C) for 20 min, then cooled and concentrated on a rotary evaporator. The residue was washed with petroleum ether and crystallized from methanol.
Ethyl-5,7-dimethyl-3-propyl-4H-1,4-benzothiazine-2-carboxylate (4a)
Yield 57%; mp 125–127°C (dec); IR: 3315, 1620, 1425, 1330, 1040 cm-1; 1H NMR: δ 8.12 (s, 1H), 6.55–6.37 (m, 2H), 2.02 (t, 2H), 1.21 (m, 2H), 1.10 (t, 3H), 4.16 (q, 2H), 1.27 (t, 3H), 1.94 (s, 3H), 2.10 (s, 3H); MS: m/z 291 (M+), 218 (53), 176 (76), 73 (100). Anal. Calcd for C16H21NO2S: C, 65.97; H, 7.21; N, 4.81. Found: C, 66.22; H, 7.26; N, 4.79.
2-(4′-Bromobenzoyl)-7-(4′-chlorophenoxy)-3-methyl-4H-1,4-benzothiazine (4b)
Yield 52%; mp 214–216°C (dec); IR: 3310, 1605, 1270, 1070, 1465, 1350, 1015 cm-1; 1H NMR: δ 8.52 (s, 1H), 7.72–6.28 (m, 11H), 1.83 (s, 3H); MS: m/z 472.5 (M+), 288.5 (54), 246.5 (82), 184 (100). Anal. Calcd for C22H15BrClNO2S: C, 56.05; H, 3.18; N, 2.97. Found: C, 56.80; H, 3.21; N, 2.83.
7-n-Butyl-2-(4′-bromobenzoyl)-3-methyl-4H-1,4-benzothiazine (4c)
Yield 40%; mp 119–121°C (dec); IR: 3315, 1610, 1275, 1080, 1465, 1355, 1035 cm-1; 1H NMR: δ 8.53 (s, 1H), 7.36–6.29 (m, 7H), 2.65 (t, 2H), 1.83 (m, 2H), 1.39 (m, 2H), 1.04 (t, 3H), 1.87 (s, 3H); MS: m/z 402 (M+), 218 (44), 176 (73), 184 (100). Anal. Calcd for C20H20BrNOS: C, 59.85; H, 4.98; N, 3.49; Found: C, 59.78; H, 4.92; N, 3.42.
3-Ethyl-7-fluoro-2-propionyl-4H-1,4-benzothiazine (4d)
Yield 56%; mp 133–135°C (dec); IR: 3340, 1655, 1240, 1455, 1350, 1025 cm-1; 1H NMR: δ 8.63 (s, 1H), 7.23–6.46 (m, 3H), 1.99 (q, 2H), 1.16(t, 3H), 3.14 (q, 2H), 1.56 (t, 3H); MS: m/z 251 (M+), 194 (44), 162 (24), 161 (75), 57 (100). Anal. Calcd for C13H14FNOS: C, 62.15; H, 5.57; N, 5.57. Found: C, 63.32; H, 5.48; N, 5.54.
Isopropyl-7-fluoro-3-methyl-4H-1,4-benzothiazine-2-carboxylate (4e)
Yield 60%; mp 154–156°C (dec); IR: 3335, 1625, 1245, 1455, 1355, 1045 cm-1; 1H NMR: δ 8.69 (s, 1H), 7.47–6.83 (m, 3H), 2.27 (s, 3H), 4.23–4.12 (m, 1H), 1.92 (d, 6H); MS: m/z 267 (M+), 180 (36), 138 (78), 87 (100). Anal. Calcd for C13H14FNO2S: C, 58.42; H, 5.24; N, 5.24. Found: C, 58.65; H, 5.30; N, 5.19.
General method for synthesis of 4H-1,4-benzothiazine 1,1-dioxides (sulfones) 5a–e
A solution of 0.01 mol of 4H-1,4-benzothiazine in 20 mL glacial acetic acid was added to 30% hydrogen peroxide (5 mL) and then the mixture was heated under reflux for 15 min keeping the temperature between 50°C and 55°C. Another lot of 30% hydrogen peroxide (5 mL) was added after 15 min without heating. Then the mixture was heated under reflux (120°C) for an additional 4–5 h, concentrated under reduced pressure and the residue was treated with crushed ice. The resultant solid product 5 was filtered and crystallized from ethanol.
Ethyl-5,7-dimethyl-3-propyl-4H-1,4-benzothiazine-2-carboxylate 1,1-dioxides (5a)
Yield 56%; mp 136–138°C (dec); IR: 3320, 1645, 1185, 1150, 1345, 1290, 1275, 580, 555, 1055 cm-1; 1H NMR: δ 8.42 (s, 1H), 6.95–6.63 (m, 2H), 2.22 (t, 2H), 1.14 (m, 2H), 1.22 (t, 3H), 4.35 (q, 2H), 1.77 (t, 3H), 2.14 (s, 3H), 2.33 (s, 3H); MS: m/z 323 (M+), 250 (51), 208 (77), 73 (100). Anal. Calcd for C16H21NO4S: C, 59.44; H, 6.50; N, 4.33. Found: C, 59.31; H, 6.45; N, 4.30.
2-(4′-Bromobenzoyl)-7-(4′-chlorophenoxy)-3-methyl-4H-1,4-benzothiazine 1,1-dioxides (5b)
Yield 45%; mp 253–255°C (dec); IR: 3355, 1625, 1190, 1160, 1360, 1285, 1265, 565, 545, 1035 cm-1; 1H NMR: δ 8.82 (s, 1H), 8.02–7.28 (m, 11H), 2.13 (s, 3H). MS: m/z 504.5 (M+), 320.5 (52), 278.5 (79), 184 (100). Anal. Calcd for C22H15BrClNO4S: C, 52.48; H, 2.98; N, 2.78. Found: C, 52.66; H, 2.96; N, 2.83.
7-n-Butyl-2-(4′-bromobenzoyl)-3-methyl-4H-1,4-benzothiazine 1,1-dioxides (5c)
Yield 47%; mp 109–111°C (dec); IR: 3350, 1640, 1185, 1150, 1355, 1290, 1245, 570, 545, 1065 cm-1; 1H NMR: δ 8.63 (s, 1H), 7.46–6.39 (m, 7H), 2.75 (t, 2H), 1.93 (m, 2H), 1.49 (m, 2H), 1.14 (t, 3H), 1.97 (s, 3H); MS: m/z 434 (M+), 250 (41), 208 (77), 184 (100). Anal. Calcd for C20H20BrNO3S: C, 55.42; H, 4.61; N, 3.23. Found: C, 55.58; H, 4.65; N, 3.27.
3-Ethyl-7-fluoro-2-propionyl-4H-1,4-benzothiazine 1,1-dioxides (5d)
Yield 60%; mp 140–142°C (dec); IR: 3360, 1680, 1190, 1145, 1385, 1295, 1260, 575, 560, 1045 cm-1; 1H NMR: δ 9.03 (s, 1H), 7.33–6.76 (m, 3H), 2.39 (q, 2H), 1.66 (t, 3H), 3.54 (q, 2H), 2.26 (t, 3H); MS: m/z 283 (M+), 226 (41), 162 (22), 161 (76), 57 (100). Anal. Calcd for C13H14FNO3S: C, 55.12; H, 4.94; N, 4.94. Found: C, 55.27; H, 4.89; N, 4.97.
Isopropyl-7-fluoro-3-methyl-4H-1,4-benzothiazine-2-carboxylate 1,1-dioxides (5e)
Yield 34%; mp 158–160°C (dec); IR: 3340, 1640, 1180, 1155, 1355, 1285, 1265, 585, 565, 1065 cm-1; 1H NMR: δ 8.88 (s, 1H), 7.77–6.13 (m, 3H), 2.55 (s, 3H), 4.63 (m, 1H), 2.22 (d, 6H); MS: m/z 299 (M+), 212 (38), 170 (81), 87 (100). Anal. Calcd for C13H14FNO4S: C, 52.17; H, 4.68; N, 4.68. Found: C, 52.37; H, 4.67; N, 4.59.
Antimicrobial assessment
MIC The broth microdilution method was used to determine the MICs (μg/mL) of the compounds in accordance with the NCCLS 1992 manual. The strains used for quality control were Micromonospora sp. MTCC 3296 (Gram-positive) and Z. mobilis MTCC 88 (Gram-negative) bacteria. Aspergillus solani MTCC 2101 and F. culmorum MTCC 349 were the reference strains for testing antifungal activities of the compounds. The standard antibacterial and antifungal drugs used were ampicillin sodium salt and fluconazole, respectively. DMSO was used for preparing solutions of test compounds and standard drugs. For preparing stock solution, each synthesized drug was diluted to obtain concentration of 1000 μg/mL. For primary screening, 500, 250 and 125 μg/mL concentrations of the synthesized drugs were taken. The active drugs found in this primary screening were further tested against all microorganisms in a second set of dilution in which the drugs were again diluted to obtain 100, 50, 25, 20, 15 μg/mL concentrations. The highest dilution showing at least 99% inhibition was recorded as MIC. Thus, the lowest concentration of each compound in the tube showing no growth (i.e., no turbidity) of inoculated bacteria/fungi was taken as MIC of that compound. Luria broth (Himedia) medium was used to carry out antibacterial activities of the bacterial strains. The reference strains of fungi were cultivated, at pH 6.9, in sabouraud dextrose agar (Himedia), with an inoculum of 108 cfu/mL, by the spectrophotometric method. An aliquot (10 μL) of this prepared culture was added to each tube of the serial dilution and then incubated at 37°C for 24 h at 3.2602 × g (150 rpm) on a rotary shaker. After incubation, MIC values were recorded. The MICs of tested compounds in μg/mL against certain strains of bacteria and fungi are shown in Tables 1 and 2.
Antimicrobial assessment of 4H-1,4-benzothiazines 4a–e.
| Compounds | Micromonospora sp. MTCC 3296 | Zymomonas mobilis MTCC 88 | Aspergillus solani MTCC 2101 | Fusarium culmorum MTCC 349 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IZa (cm) | AI | MIC (μg/mL) | IZ (cm) | AI | MIC (μg/mL) | IZ (cm) | AI | MIC (μg/mL) | IZ (cm) | AI | MIC (μg/mL) | |
| 4a | 1.0 | 0.357 | 36.0 | 1.5 | 0.714 | 28.5 | 2.0 | 0.666 | 21.5 | 1.5 | 0.555 | 27.0 |
| 4b | 1.6 | 0.571 | 33.5 | 1.6 | 0.761 | 27.0 | 1.0 | 0.333 | 28.5 | 1.5 | 0.555 | 27.0 |
| 4c | 1.9 | 0.678 | 32.0 | 1.8 | 0.857 | 26.0 | 1.1 | 0.366 | 26.0 | 2.0 | 0.740 | 22.5 |
| 4d | 1.1 | 0.392 | 36.0 | 2.1 | 1.000 | 22.5 | 1.5 | 0.500 | 24.0 | 1.9 | 0.703 | 24.0 |
| 4e | 1.2 | 0.428 | 35.5 | 2.2 | 1.047 | 20.5 | 1.6 | 0.200 | 23.5 | 2.3 | 0.851 | 22.0 |
| Ampicillin sulfate | 2.8 | – | 28.0 | 2.1 | – | 22.0 | – | – | – | – | – | – |
| Fluconazole | – | – | – | – | – | – | 3.0 | – | 18.0 | 2.7 | – | 20.0 |
aIZ, inhibition zone; AI, activity index; MIC, minimum inhibitory concentration.
Antimicrobial assessment of sulfone derivatives 5a–e.
| Compounds | Micromonospora sp. MTCC 3296 | Zymomonas mobilis MTCC 88 | Aspergillus solani MTCC 2101 | Fusarium culmorum MTCC 349 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IZa (cm) | AI | MIC (μg/mL) | IZ (cm) | AI | MIC (μg/mL) | IZ (cm) | AI | MIC (μg/mL) | IZ (cm) | AI | MIC (μg/mL) | |
| 5a | 1.0 | 0.357 | 36.0 | 1.6 | 0.761 | 27.0 | 2.7 | 0.900 | 21.0 | 1.8 | 0.666 | 24.5 |
| 5b | 1.9 | 0.678 | 32.0 | 1.7 | 0.809 | 26.5 | 2.0 | 0.667 | 27.5 | 1.6 | 0.592 | 26.5 |
| 5c | 2.4 | 0.857 | 30.0 | 1.9 | 0.904 | 24.0 | 2.1 | 0.700 | 26.0 | 2.6 | 0.962 | 20.5 |
| 5d | 1.3 | 0.464 | 35.0 | 2.1 | 1.000 | 22.0 | 2.2 | 0.733 | 20.5 | 2.0 | 0.740 | 23.5 |
| 5e | 1.7 | 0.607 | 33.0 | 2.3 | 1.095 | 20.0 | 2.2 | 0.733 | 20.5 | 2.1 | 0.777 | 21.5 |
| Ampicillin sulfate | 2.8 | – | 28.0 | 2.1 | – | 22.0 | – | – | – | – | – | – |
| Fluconazole | – | – | – | – | – | – | 3.0 | – | 18.0 | 2.7 | – | 20.0 |
aIZ, inhibition zone; AI, activity index; MIC, minimum inhibitory concentration.
AI The AI of compounds was determined by the agar well diffusion method. In this method, nutrient agar and potato dextrose agar plates were swabbed with an 8-h-old broth culture of respective bacteria (Micromonospora sp. MTCC 3296 and Z. mobilis MTCC 88) and fungi (A. solani MTCC 2101 and F. culmorum MTCC 349). Wells were made on agar surface (6 mm in diameter and 2 cm apart) and punctured in the culture medium using sterile cork borers. The plates were then turned upside down. Stock solution of each compound was prepared at a concentration of 1 mg/mL in DMSO. The wells were filled with 100 μL of the compounds and one well was filled with 100 μL of DMSO which served as a control. The compounds were incubated at 37°C for 24 h in the case of bacterial activity and for 48 h at 25°C in the case of fungal activity. The plates were observed for the zone clearance around the wells. The zone of inhibition was calculated by measuring the diameter of the inhibition zone around the well (in cm) including the well diameter. The zone of inhibition exhibited by compounds was then compared with the zone exhibited by the reference compounds (ampicillin sodium salt in the case of bacteria and fluconazole in the case of fungi) to find relative activity. The activity indices of tested compounds against certain bacteria and fungi were calculated as shown below and the results are tabulated in Tables 1 and 2.
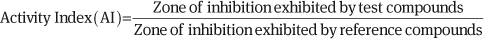
The authors are extremely thankful to the Department of Chemistry, University of Rajasthan, Jaipur for providing necessary facilities. The financial support by UGC and CSIR, New Delhi is duly acknowledged. The authors are also grateful to the Institute of Applied Sciences and Biotechnology (Chemind Biosolutions), Jaipur for providing assistance in carrying out antimicrobial assessment.
References
[1] Gould, J. C. The determination of bacterial sensitivity to antibiotics. Edinb. Med. J. 1952, 59, 178–184.Suche in Google Scholar
[2] Gupta, R. R.; Kumar, R.; Gautam, R. K. Synthesis of new fluorinated 4H-1,4-benzothiazines as possible anticancer agents. J. Fluor. Chem. 1985, 28, 381–385.Suche in Google Scholar
[3] Gupta, R. R.; Kumar, R. Synthesis of 6-(trifluoromethyl)-4H-1,4-benzothiazines as possible anticancer agents. J. Fluor. Chem. 1986, 31, 19–24.Suche in Google Scholar
[4] Gupta, R. R.; Jain, M.; Rathore, R. S.; Gupta, A. Synthetic and spectral investigation of fluorinated phenothiazines and 4H-1,4-benzothiazines as potent anticancer agents. J. Fluor. Chem. 1993, 62, 191–200.Suche in Google Scholar
[5] Niewiadomy, A.; Matysiak, J.; Karpińska, M. M. Synthesis and anticancer activity of new 2-aryl-4H-3,1-benzothiazines. Archiv. Pharm. 2011, 344, 224–230.Suche in Google Scholar
[6] Mukherji, S. K.; Jain, M.; Gupta, A.; Saraswat, V.; Gupta, R. R. Synthesis and spectral studies of 3,4-dihydro-3-oxo-2H-1,4-benzothiazines-2-acetic acid. Ind. J. Chem. 1994, 33B, 990–991.Suche in Google Scholar
[7] Gautam, N.; Gautam, D. C.; Gupta, R. R. Single step synthesis of substituted 4H-1,4-benzothiazines. Heterocycl. Commun. 1997, 3, 401–404.Suche in Google Scholar
[8] Gautam, N.; Gautam, D. C. Synthesis of 7-bromo-3,5-dimethyl-4H-1,4-benzothiazine sulfones. Orient. J. Chem. 2006, 22, 457–460.Suche in Google Scholar
[9] Gautam, V.; Sharma, M.; Panwar, M.; Gautam, N.; Kumar, A.; Sharma, I. K.; Gautam, D. C. Synthetic methodology, spectral elucidation, and antioxidative properties of benzothianes and their sulfones. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 3090–3109.Suche in Google Scholar
[10] Gautam, N.; Ajmera, N.; Gupta, S.; Gautam, D. C. Synthesis, spectral characterization and biological evaluation of 4H-1,4-benzothiazines, their sulfones and ribofuranosides. Eur. J. Chem. 2012, 3, 106–111.Suche in Google Scholar
[11] Gautam, N.; Hans D.; Gautam, D. C. Antifungal activity of some 4H-1,4-benzothiazine compounds. Orient. J. Chem. 2005, 21, 299–302.Suche in Google Scholar
[12] Gupta, S.; Ajmera, N.; Meena, P.; Gautam, N.; Kumar, A.; Gautam, D. C. Synthesis and biological evaluation of new 1,4-thiazine containing heterocyclic compounds. Jourdan J. Chem. 2009, 4, 209–221.Suche in Google Scholar
[13] Patel, N. B.; Patel, H. R. Synthesis and antibacterial and antifungal studies of novel nitrogen containing heterocycles from 5-ethylpyridin-2-ethanol. Indian J. Pharm. Sci. 2010, 72, 613–620.Suche in Google Scholar
[14] Pawar, Y.; Sonawane, A.; Nagle, P.; Mahulikar, P.; More, D. Synthesis of 1,4benzothiazine compound containing isatin moieties as antimicrobial agent. Int. J. Curr. Pharm. Res. 2011, 3, 47–51.Suche in Google Scholar
[15] Sharma, P. K.; Fogla, A.; Rathore, B. S.; Kumar, M. Synthesis and antimicrobial activity of structurally flexible heterocycles with the 1,4-thiazine heterosystem. Res. Chem. Intermed. 2011, 37, 1103–1111.Suche in Google Scholar
[16] Sharma, P.; Rane, N.; Gurram, V. K. Synthesis and QSAR studies of pyrimido (4,5-d) pyrimidine-2,5-dione derivatives as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2004, 14, 4185–4190.Suche in Google Scholar
[17] Guven, O. O.; Erdogan, T.; Goker, H.; Yildiz, S. Synthesis and antimicrobial activity of some novel phenyl and benzimidazole substituted benzyl ethers. Bioorg. Med. Chem. Lett. 2007, 17, 2233–2236.Suche in Google Scholar
[18] Masunari, A.; Tavares, L. C. A new class of nifuroxazide analogues: synthesis of 5-nitrothiophene derivatives with antimicrobial activity against multidrug-resistant Staphylococcus aureus. Bioorg. Med. Chem. 2007, 15, 4229–4236.Suche in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Masthead
- Masthead
- Reviews
- Synthesis and applications of benzothiazole containing cyanine dyes
- Synthesis and chemistry of structurally unique hexasubstituted pyrazolines
- Research Articles
- Synthesis and characterization of heteroarylthio derivatives of 5,17-di-tert-butyl-11,23-diamido-25, 27-diprotected calix[4]arene
- New heterocyclic chalcones. Part 6. Synthesis and cytotoxic activities of 5- or 6-(3-aryl- 2-propenoyl)-2(3H)-benzoxazolones
- Novel benzofuran derivatives: synthesis and antitumor activity
- Synthesis, characterization and in vitro antimicrobial assessment of some novel 4H-1, 4-benzothiazines and their sulfone derivatives
- Efficient synthesis, X-ray diffraction study and antimicrobial activity of some novel thiazolidin-4-ones and perhydro-1,3-thiazin-4-ones
- A simple and efficient synthesis of novel naphthyridine-1-H-pyrazole-4-carboxylic acid esters/carbaldehydes using Vilsmeier-Haack reagent
- Melamine-formaldehyde resin supported H+-catalyzed three-component synthesis of 1,8-dioxo-decahydroacridine derivatives in water and under solvent-free conditions
- A simple and efficient procedure for synthesis of symmetrical bis(4-amino-4H-1,2,4-triazole-5-thiols)
- Poly(ethylene)glycol/AlCl3 as a new and efficient system for multicomponent Biginelli-type synthesis of pyrimidinone derivatives
- Synthesis, crystal structure, and bioactivity of N-dichloroacetyl diazabicyclo compounds
Artikel in diesem Heft
- Masthead
- Masthead
- Reviews
- Synthesis and applications of benzothiazole containing cyanine dyes
- Synthesis and chemistry of structurally unique hexasubstituted pyrazolines
- Research Articles
- Synthesis and characterization of heteroarylthio derivatives of 5,17-di-tert-butyl-11,23-diamido-25, 27-diprotected calix[4]arene
- New heterocyclic chalcones. Part 6. Synthesis and cytotoxic activities of 5- or 6-(3-aryl- 2-propenoyl)-2(3H)-benzoxazolones
- Novel benzofuran derivatives: synthesis and antitumor activity
- Synthesis, characterization and in vitro antimicrobial assessment of some novel 4H-1, 4-benzothiazines and their sulfone derivatives
- Efficient synthesis, X-ray diffraction study and antimicrobial activity of some novel thiazolidin-4-ones and perhydro-1,3-thiazin-4-ones
- A simple and efficient synthesis of novel naphthyridine-1-H-pyrazole-4-carboxylic acid esters/carbaldehydes using Vilsmeier-Haack reagent
- Melamine-formaldehyde resin supported H+-catalyzed three-component synthesis of 1,8-dioxo-decahydroacridine derivatives in water and under solvent-free conditions
- A simple and efficient procedure for synthesis of symmetrical bis(4-amino-4H-1,2,4-triazole-5-thiols)
- Poly(ethylene)glycol/AlCl3 as a new and efficient system for multicomponent Biginelli-type synthesis of pyrimidinone derivatives
- Synthesis, crystal structure, and bioactivity of N-dichloroacetyl diazabicyclo compounds


