6a
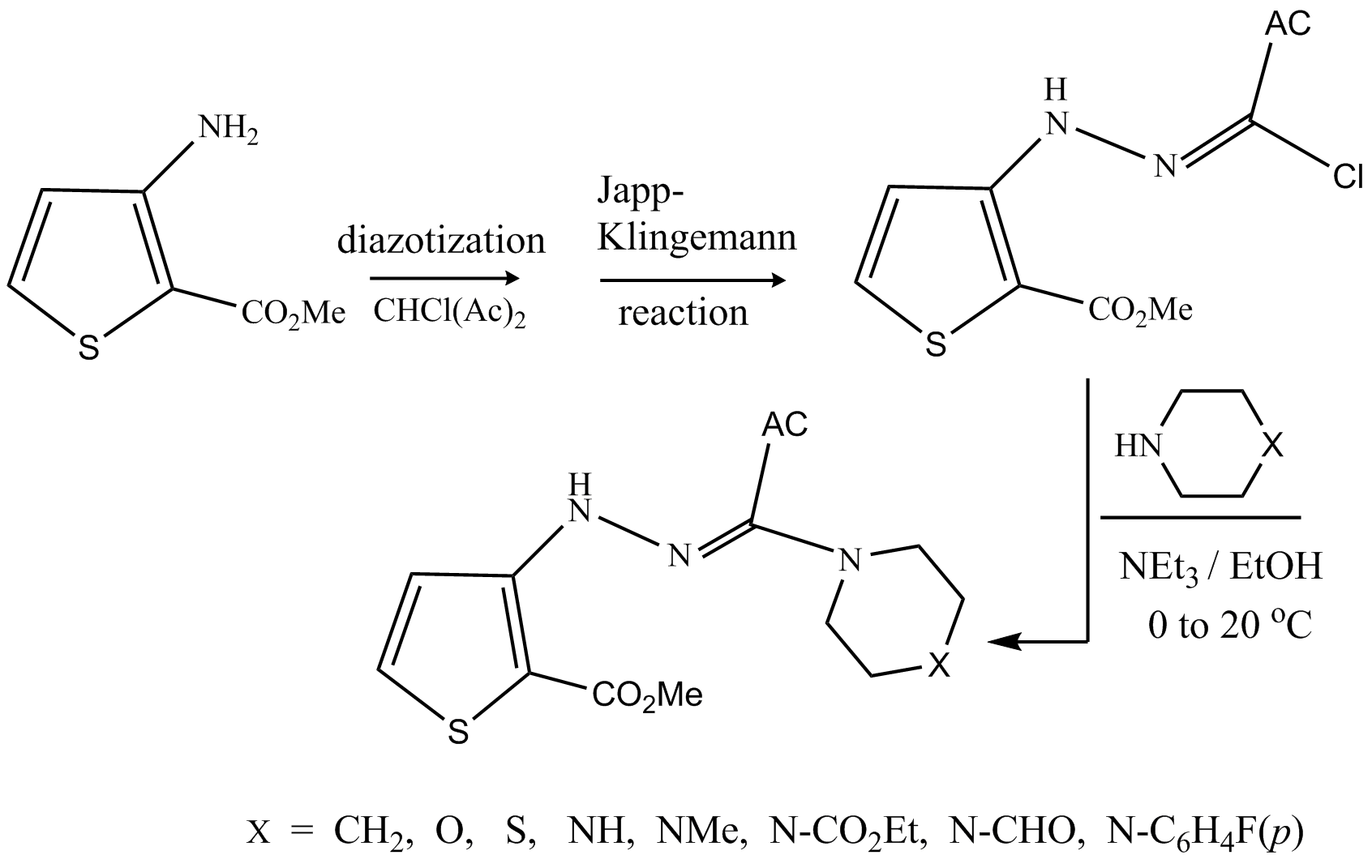
A set of new N2-(thien-3-yl)amidrazones (-h) incorporating N-piperazines and related congeners has been synthesized by reacting the hydrazonoyl chloride 4(derived from 3-aminothiophene- 2-carboxylate) with the appropriate sec-cyclic amine. The antitumor activity of these compounds was evaluated on breast cancer (MCF-7) and leukemic (K562) cell lines by a cell viability assay utilizing the tetrazolium dye (MTT). The amidrazone 6d encompassing the N-piperazine moiety, was the most active against MCF-7 and K562 with IC50 of 7.28 and 9:91 μM, respectively.
Graphical Abstract
Synthesis and Antitumor Activity of Some N2-(Thien-3-yl)amidrazones
© 1946 – 2014: Verlag der Zeitschrift für Naturforschung
Articles in the same Issue
- Synthesis, Structure and Thermal Decomposition of a New Iodine Inclusion Compound in the 2,2-Dimethylpropane-1,3-diamine/HI/I2 System
- Rapid Microwave Synthesis and Structural Phase Diagram of LnxY1−xMnO3
- Gold-Tin Ordering in SrAu2Sn2
- The Stannides EuPd2Sn2, EuPt2Sn2, EuAu2Sn2, and Eu5.4Sn5.6 – Structure and Magnetic Properties
- Line-Shape Analyses of Solid-state 17O NMR Spectra for Hexagonal Ice
- A Novel Alkali-Metal Hydrido-tris(pyrazolyl)borate (Tp*) Complex. Isolation and Crystal Structure of [(Me2CO)3(NaTp*)2]
- Synthesis and Structure of an Aluminum Bis(3-chloropentanedionate) Isopropoxide: [Al(μ-OiPr)(3-Clacac)2]2
- Carbon-modified MgH2: Experimental and ab-initio Investigations
- Synthesis and Antitumor Activity of Some N2-(Thien-3-yl)amidrazones
- Synthesis of New TGX-221 Analogs
- Crystal Structure Explains Crystal Habit for the Antiviral Drug Rimantadine Hydrochloride
- The Reaction of Cyanamidium Salts with Ylidenecyanamide Derivatives
- Iridoid Glycosides from Lagotis alutacea
- Solid-state and Calculated Electronic Structure of 4-Acetylpyrazole
Articles in the same Issue
- Synthesis, Structure and Thermal Decomposition of a New Iodine Inclusion Compound in the 2,2-Dimethylpropane-1,3-diamine/HI/I2 System
- Rapid Microwave Synthesis and Structural Phase Diagram of LnxY1−xMnO3
- Gold-Tin Ordering in SrAu2Sn2
- The Stannides EuPd2Sn2, EuPt2Sn2, EuAu2Sn2, and Eu5.4Sn5.6 – Structure and Magnetic Properties
- Line-Shape Analyses of Solid-state 17O NMR Spectra for Hexagonal Ice
- A Novel Alkali-Metal Hydrido-tris(pyrazolyl)borate (Tp*) Complex. Isolation and Crystal Structure of [(Me2CO)3(NaTp*)2]
- Synthesis and Structure of an Aluminum Bis(3-chloropentanedionate) Isopropoxide: [Al(μ-OiPr)(3-Clacac)2]2
- Carbon-modified MgH2: Experimental and ab-initio Investigations
- Synthesis and Antitumor Activity of Some N2-(Thien-3-yl)amidrazones
- Synthesis of New TGX-221 Analogs
- Crystal Structure Explains Crystal Habit for the Antiviral Drug Rimantadine Hydrochloride
- The Reaction of Cyanamidium Salts with Ylidenecyanamide Derivatives
- Iridoid Glycosides from Lagotis alutacea
- Solid-state and Calculated Electronic Structure of 4-Acetylpyrazole

