Comprehensive succinylome analyses reveal that hyperthermia upregulates lysine succinylation of annexin A2 by downregulating sirtuin7 in human keratinocytes
-
Xueli Niu
and Bing Song
Abstract
Background and Objectives
Local hyperthermia at 44°C can clear multiple human papillomavirus (HPV)-infected skin lesions (warts) by targeting a single lesion, which is considered as a success of inducing antiviral immunity in the human body. However, approximately 30% of the patients had a lower response to this intervention. To identify novel molecular targets for anti-HPV immunity induction to improve local hyperthermia efficacy, we conducted a lysine succinylome assay in HaCaT cells (subjected to 44°C and 37°C water baths for 30 min).
Methods
The succinylome analysis was conducted on HaCaT subjected to 44°C and 37°C water bath for 30 min using antibody affinity enrichment together with liquid chromatography-tandem mass spectrometry (LC-MS/MS). The results were validated by western blot (WB), immunoprecipitation (IP), and co-immunoprecipitation (Co-IP). Then, bioinformatic analysis including Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment, motif characterization, secondary structure, and protein-protein interaction (PPI) was performed.
Results
A total of 119 proteins with 197 succinylated sites were upregulated in 44°C-treated HaCaT cells. GO annotation demonstrated that differential proteins were involved in the immune system process and viral transcription. Succinylation was significantly upregulated in annexin A2. We found that hyperthermia upregulated the succinylated level of global proteins in HaCaT cells by downregulating the desuccinylase sirtuin7 (SIRT7), which can interact with annexin A2.
Conclusions
Taken together, these data indicated that succinylation of annexin A2 may serve as a new drug target, which could be intervened in combination with local hyperthermia for better treatment of cutaneous warts.
Introduction
Cutaneous wart, regarded as a benign tumor, is a proliferative disease caused by human papillomavirus (HPV) infection.[1] The global prevalence of HPV infection was 11.7%, while no completely effective treatment was determined.[2,3] In the clinic, local hyperthermia at 44°C for 30 min could successfully clear cutaneous or anogenital warts as well as cervical HR-HPV infection. This treatment is characterized for the phenomenon that once the targeted lesion responded, other untargeted warts could fall off simultaneously.[4,5,6] It can be predicted that hyperthermia plays a role in regulating antimicrobial immunity against HPV infection. However, approximately 30% of patients showed no response to this treatment. Potential mechanisms are required to be elucidated, which could provide a clue to find new drug targets and improve the treatment efficacy.
Lysine succinylation is a newly identified posttranslational modification (PTM) that describes the process of the transfer of succinyl group (-CO-CH2-CH2-CO2H) from succinyl-CoA to the ε-amino group of lysine residues on the target protein in an enzymatic or nonenzymatic manner.[7] Compared with several well-known PTMs, such as acetylation (42 Da) and methylation (14 Da), succinylation induces greater charge shifts (+1 to –1) and mass changes (100 Da) to the target proteins, indicating its greater potentials in cellular regulation.[8,9] Recently, lysine succinylation has been reported in various biological processes, including reactive oxygen species (ROS) removal,[10] inflammation,[11] metabolism,[11,12] autophagy,[13] and immune response.[14] A previous study showed that succinylation of mitochondrial antiviral signaling protein (MAVS) at lysine 7 could improve its activity in antiviral innate immunity by regulating retinoic acid-inducible (RIG-I )-like receptor (RLR)-mediated type I interferon (IFN) production.[14] In addition, succinylation of pyruvate kinase isozyme typeM2 (PKM2) at K311 could improve its transition from the cytoplasm to the nucleus, subsequently regulating the inflammatory response.[11] However, whether lysine succinylation affects antimicrobial immunity to HPV infection is still unknown.
Succinylation, as a posttranslational modification, is typically regulated by catalyzing enzymes. Mammalian sirtuins (SIRT1 to SIRT7) are a family of seven proteins with a conserved nicotinamide adenine dinucleotide (NAD+)-dependent catalytic domain, which function as class III histone deacetylases (HDACs) in Homo sapiens.[15] Sirtuins could remove the succinyl group from the lysine residue, which play roles as desuccinylases. Among these sirtuins, SIRT5 deficiency was confirmed to increase the level of succinylation on carbamoyl phosphate synthase 1 (CPS1) at K1291, suggesting that lysine succinylation (Ksucc) can be regulated by SIRT5 in vivo. In vitro studies have shown that SIRT5 desuccinylase activity is much higher than its deacetylase activity.[16] SIRT7 is the only member that is primarily located in the nucleolus with weak deacetylase activity.[17] Recently, SIRT7 was found to act as a desuccinylase for H3K122 to regulate the chromatin remodeling process.[18] As for SIRT3, the major mitochondrial protein deacetylase, recent evidence in cells has demonstrated that it may also be able to perform a desuccinylation reaction on histones. [19] Carnitine palmitoyltransferase 1A (CPT1A) and lysine acetyltransferase 2A (KAT2A) possess succinyltransferase activities.[20] CPT1A is a mitochondrial enzyme responsible for the formation of acyl carnitines.[21] CPT1A has lysine succinyl-transferase (LSTase) activity both in vivo and in vitro.[22] KAT2A, a member of the GCN5-related N-acetyltransferase (GNAT) superfamily, binds to succinyl-CoA and acts as a succinyltransferase for histone H3 on lysine 79.[23]
In the present study, we carried out the first proteomic analysis of lysine succinylation in hyperthermia-induced HaCaT cells (subjected to 44°C and 37°C water baths for 30 min as control). A series of bioinformatic analyses were conducted to investigate the potential role of lysine succinylation in hyperthermia-mediated antimicrobial activities. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses demonstrated that 19 and 6 proteins related to immune processes and viral regulation were succinylated in response to heat treatment, respectively. Several lysine residues in annexin A2 were upregulated, while the protein level of desuccinylase SIRT7 was downregulated after hyperthermia. SIRT7 could directly interact with annexin A2, and the findings may provide novel insight into targeted drug design at the level of lysine succinylation that may potentially increase the cure rates or shorten the period of treatment of HPV infectious diseases. This study may improve our understandings of the mechanism underlying the breaking of microbial resistance as well as the successively elicited antimicrobial immunity induced by local hyperthermia in the skin.
Materials and methods
Cell culture and hyperthermia treatment
HaCaT cells were purchased from American Type Culture Collection (Maryland, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Biological Industries, Beit-Haemek, Israel), penicillin (100 U/mL), and streptomycin (100 μg/mL) in a 37°C, 5% CO2 constant temperature incubator.
For hyperthermia treatment, cell culture plates were sealed with parafilm and placed carefully in a circulating water bath for 30 min at 37°C for controls or 44°C for hyperthermia treatment. After these treatments, the cells were returned to a 37°C incubator and cultured for 0–24 h.
Sample preparation
Cells in the control group (37°C) and the heat-treated group (44°C) were harvested and washed with icecold PBS twice. Samples were sonicated on ice using a highintensity ultrasonic processor (Scientz, Ningbo, China) in lysis buffer. The remaining debris was discarded by centrifugation at 12,000 × g at 4°C for 10 min. Finally, the protein concentration was determined with a BCA kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Finally, trypsin (Promega, York, USA) was added at a 1:50 trypsin-to-protein mass ratio for the first digestion overnight and a 1:100 trypsin-to-protein mass ratio for a second 4 h digestion.
High performance liquid chromatography fractionation and affinity enrichment
The peptides were fractionated into 60 fractions by high pH reverse-phase high performance liquid chromatography (HPLC) using a Ther mo Betasil C18 column (5 μm particles, 10 mm ID, 250 mm length). Then, the peptides were combined into 6 fractions and dried by vacuum centrifugation.
The peptides dissolved in NETN buffer (100 mmol/L NaCl, 1 mmol/L ethylene diamine tetraacetic acid (EDTA), 50 mmol/L Tris-HCl, and 0.5% NP-40, pH 8.0) were incubated with prewashed pan-antisuccinyl antibody beads (PTM Biolabs, Hangzhou, China) at 4°C overnight. Then, the beads were washed with NETN buffer and H2O. The bound peptides were eluted from the beads with 0.1% trifluoroacetic acid (TFA) and vacuum-dried.
Liquid chromatography-tandem mass spectrometry analysis
For liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, the resulting peptides were desalted with C18 ZipTips (Millipore, Mass, USA). The peptides were subjected to NSI source followed by tandem mass spectrometry (MS/MS) using Q ExactiveTM Plus (Thermo Fisher Scientific, Mass, USA) coupled online to the UPLC.
Database search
The resulting mass spectrometry data were processed using the MaxQuant search engine v.1.5.2.8 (Max Planck Institute of Biochemistry, Munich, Germany). Tandem mass spectra were searched against the SwissProt Human database concatenated with the reverse decoy database. Carbamidomethyl on Cys was specified as a fixed modification, and succinylation on Lys was specified as a variable modification.
Bioinformatics analysis
GO annotation was obtained from the UniProt-GOA database (www. http://www.ebi.ac.uk/GOA/), InterProScan database (http://www.ebi.ac.uk/interpro/), and DAVID database (https://david.ncifcrf.gov/). The subcellular localization was analyzed by WoLF PSORT software. For the functional descriptions of the protein domain, the InterProScan database was used. Protein pathways were annotated by the KEGG database and KEGG online service tools KEGG mapper. Motif-x was used to predict specific Ksucc motifs. Analysis of secondary structure was conducted by NetSurfP v1.0 (DTU Health Tech, Lyngby, Denmark).
Isolated tissue and hyperthermia treatment
Fresh condyloma acuminata (CA) tissues were obtained from patients undergoing surgical treatment at the First Hospital of China Medical University. The lesion was equally divided into two parts and separately placed in cell culture dishes containing DMEM plus 10% fetal bovine serum. For hyperthermia management, the tissue was treated in the same way as HaCaT cells described above. Subsequently, tissue samples were fixed with paraformaldehyde 4 h after hyperthermia treatment and then used for immunohistochemical assays. This part of the study was approved by the Ethics Committee of the First Hospital of China Medical University (Approval No. 2018-135-2), and informed consent was obtained from the patients.
Western blotting
Cultured cell samples were treated with RIPA lysis buffer (Beyotime, Shanghai, China) containing protease inhibitors and phosphatase inhibitors (Beyotime, Shanghai, China) and centrifuged at 15,000 × g at 4°C for 10 min. The supernatant was carefully aspirated, mixed with SDS–PAGE loading buffer after protein quantification by BCA assay, boiled at 100°C for 5 min, and then subjected to SDS–PAGE detection. After being transferred to a polyvinylidene difluoride membrane (PVDF), nonspecific binding was blocked with QuickBlock blocking solution (Beyotime, Shanghai, China) for 15 min. The blots were probed with the following primary antibodies: fatty acid synthase (FASN) antibody (Cell Signaling Technology, Mass, USA), KATA2 antibody (Cell Signaling Technology, Mass, USA), succinyl lysine antibody (PTM Biolabs, Hangzhou, China), SIRT5 antibody (Cell Signaling Technology, Mass, USA), SIRT3 antibody (Cell Signaling Technology, Mass, USA), SIRT7 antibody (Cell Signaling Technology, Mass, USA) and annexin A2 antibody (Proteintech, Wuhan, China).
Plasmid construction and transfection
SIRT7 overexpression plasmids were constructed by Shanghai Heyuan Biotechnology Co., Ltd. According to the protocols provided by the manufacturer, plasmid DNA was transfected with Lipofectamine 3000 Transfection Reagent (Thermo Fisher Scientific, Mass, USA), and cells were harvested 48 h after transfection.
Co-immunoprecipitation
Cultured cells were lysed by immunoprecipitation (IP) buffer (0.25% sodium deoxycholate, 50 mmol/L Tris, 1 mmol/L EDTA, 1% Triton X-100, 1% NP-40, and 150 mmol/L NaCl) after washing twice with ice-cold PBS. The supernatants of digested cells were transferred into new EP tubes, mixed with primary antibody and magnetic beads (Bimake, Houston, USA), and incubated overnight on a rotator at 4°C. The next day, the magnetic bead particles were washed 5 times with cold IP buffer, and then protein loading buffer was added and boiled at 100°C for 5 min.
Immunohistochemical analysis
Immunohistochemical (IHC) staining was performed to detect the expression of SIRT7 in CA tissues after in vitro hyperthermia. Briefly, CA tissues (hyperthermia-treated group or controls) were fixed with 4% paraformaldehyde and then embedded in paraffin. Tissue sections were prepared for antigen retrieval with 0.01 mol/L sodium citrate antigen retrieval solution in a pressure cooker for 2 min. Endogenous peroxidase activity was inactivated using 3% H2O2. Then, nonspecific interactions were blocked with 10% goat serum. SIRT7 antibody at a dilution of 1:200 was incubated with the slides overnight at 4°C. The following steps were performed according to the protocols of the EliVision kit (Maxim, Fuzhou, China). The slides were examined using light microscopy (Olympus IX51, Tokyo, Japan).
Statistical analysis
Two-tailed Fisher’s exact tests were applied to GO, KEGG, and protein domain enrichment analyses. Categories with a corrected P value < 0.05 were considered significant.
Results
Proteome-wide analysis of succinylation proteins and sites identified in HaCaT cells
Hyperthermia clears multiple remote cutaneous warts by targeting a single lesion through eliciting anti-HPV immune responses.[24] The clinical treatment of children’s perianal warts showed that local hyperthermia removed multiple lesions by treating one place of lesions (Figure 1A). Lysine succinylation is a novel PTM and has already been confirmed to be involved in multiple cellular processes. Here, we conducted a comprehensive succinylome in HaCaT cells (both the control and hyperthermia-treated groups) and identified 387 proteins with 969 succinylated lysine sites altogether (Figure 1B), of which 119 proteins with 197 lysine sites were upregulated in response to hyperthermia treatment (44°C) (Figure 1C).
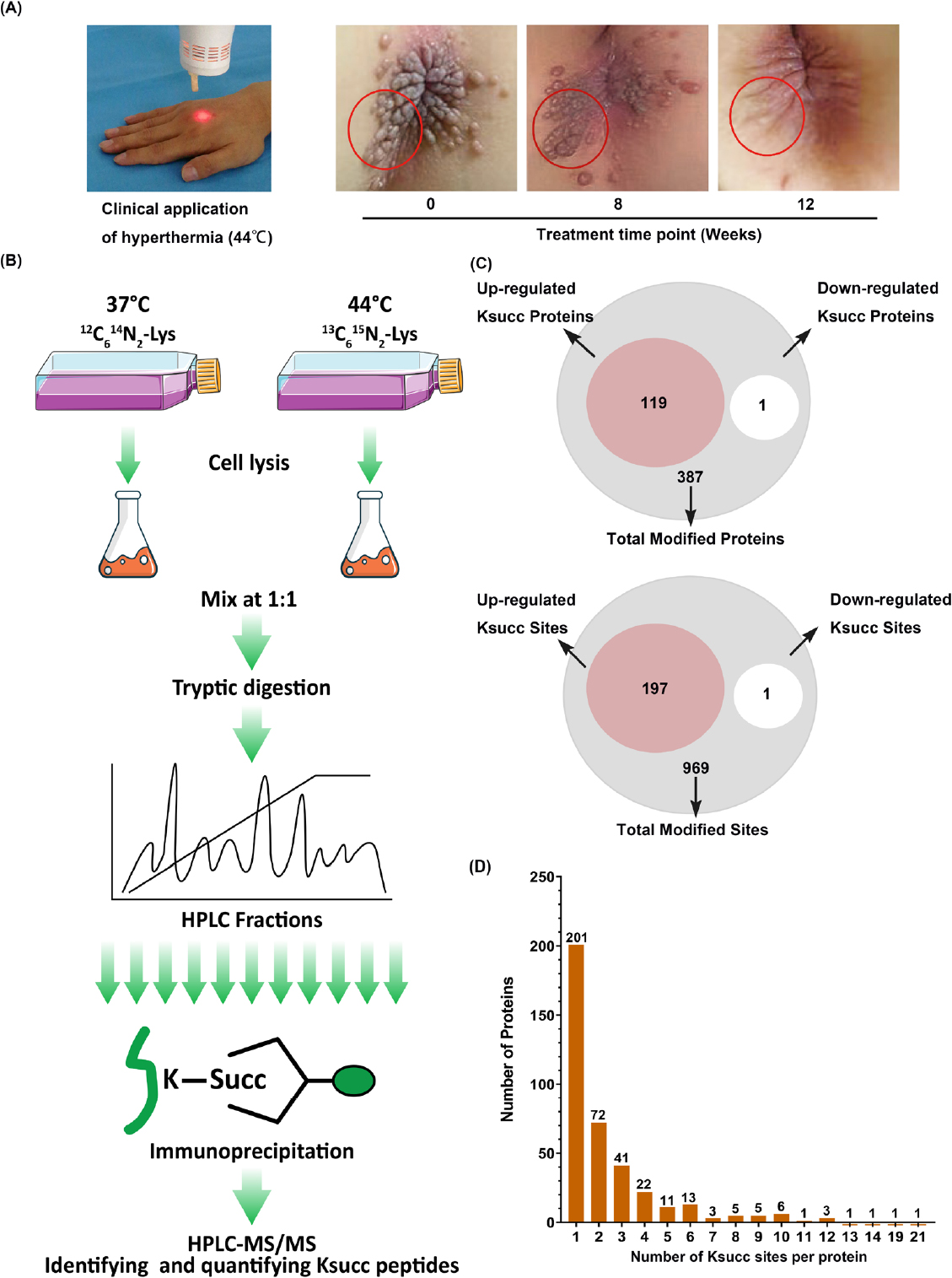
The clinic treatment of cutaneous warts and total profiling of lysine succinylome in HaCaT cells after response to hyperthermia. (A) Local hyperthermia treatment in children’s perianal warts. The red circle is therapeutic target for multiple verrucous lesions. (B) Experimental workflow for the identification of succinylated proteins and sites in HaCaT cells (with or without 44°C treatment). (C) Quantification of succinylated sites of HaCaT cells in response to hyperthermia. (D) Distribution of succinylation proteins based on their number of succinylated sites. Ksucc: lysine succinylation; MS/MS: mass spectrometry.
The peptides contained different numbers of succinylated sites varying from 1 to 21. Of all succinylation proteins identified in our data, 51.9% (201/387) had a single site, 18.6% (72/387) contained two succinylated sites, 10.6% (41/387) possessed three modified sites, and 3.6% (14/387) had 10 or more succinylation peptide sites (Figure 1D).
Among the 387 identified proteins, the most extensively succinylated was HSPD1, with 21 modified lysine sites. In addition, endoplasmin (HSP90B1), alpha-enolase (ENO-1), annexin A2, and serine beta-lactamase-like protein (LACTB) contained 14, 12, 10, and 7 succinylated sites, respectively.
Functional annotation and enrichment analysis of identified succinylated proteins
To investigate the potential roles of succinylated proteins in HaCaT cells, we annotated all the identified proteins (387) using gene ontology (GO) functional characterization based on biological process (BP), cellular component (CC), and molecular function (MF) (Figure 2A). In terms of the biological process analysis, the two largest categories were related to cellular process (345 proteins) and metabolic process (284 proteins). Notably, 155 and 46 succinyl proteins were involved in response to stimuli and immune system processes, indicating that succinylated proteins may play a role in the immune response to exogenous stimuli. Within the molecular function category, the two largest groups belonged to binding (230 proteins) and catalytic activity (207 proteins), suggesting that lysine succinylation may alter the structure and functional activity of modified proteins.
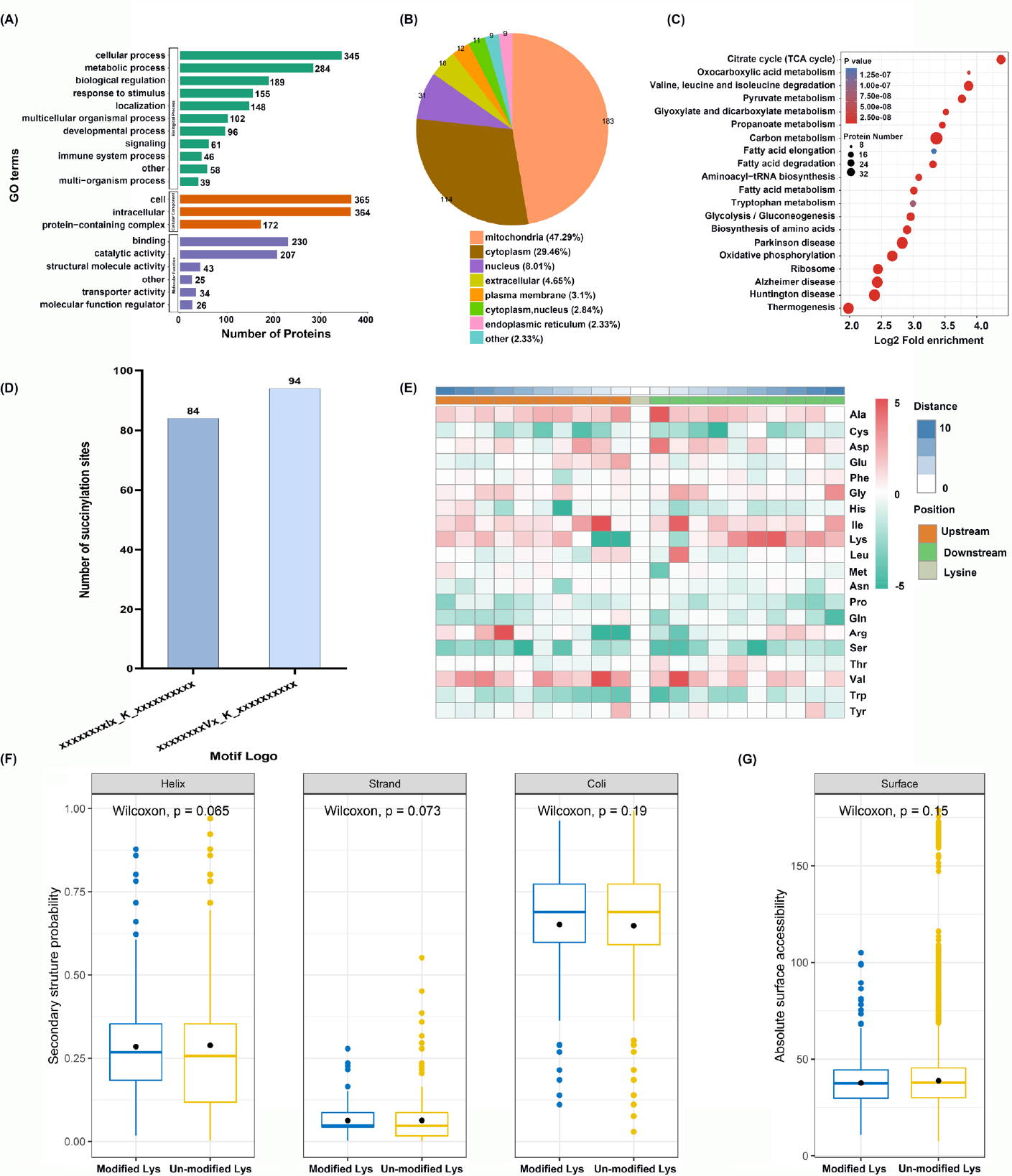
Functional enrichment analysis of upregulated Ksucc proteins and structural analysis of identified succinyl proteins in HaCaT cells in response to hyperthermia. (A) GO characterization of succinylated proteins based on BP, CC, and MF. (B) The subcellular location of succinylated proteins. The enrichment analysis of succinylproteins in terms of (C) KEGG pathway. (D) Number of succinylated peptides in each motif. (E) Heat map of the amino acid surrounding succinyl-lysine. Properties of modified lysine based on (F) secondary structure probability and (G) absolute surface accessibility. Helix: alpha helix; Strand: beta-strand; Coil: coiled coil; BP: biological process; CC: cellular component; MF: molecular function; GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes.
The subcellular localization may provide a clue for predicting protein function.[25] We found that most succinylated proteins were in the mitochondria (47.29%), followed by the cytoplasm (29.46%) and nucleus (8.01%) (Figure 2B). All the above results indicated that succinylated proteins were distributed extensively and affected various cellular processes in HaCaT cells.
The KEGG enrichment analysis showed that succinylated proteins were more likely to be enriched in the thermogenesis, carbon metabolism, oxidative phosphorylation, glycolysis/ gluconeogenesis, and fatty acid metabolism pathways (Figure 2C). It can be predicted that lysine succinylation of proteins may influence the thermal process and energy metabolism in HaCaT cells.
Motif analysis and structural properties of identified succinyl proteins
To identify the specific succinyl-peptide patterns, we characterized the frequencies of position-specific amino acids flanking succinyllysine (10 amino acids upstream and downstream of the centered Ksu site) by the Motif-X program. Two preferred motifs, I* Ksu and V* Ksu, were identified (* indicates a random amino acid residue and Ksu indicates a succinylated lysine site). Nearly 84 and 94 succinylated peptides possessed these two motifs, respectively (Figure 2D). Surrounding succinylated lysine (K) at sites –1 and –2, the basic amino acids arginine (Arg) and another K were less frequent. Four residues, alanine (Ala), aspartic acid (Asp), isoleucine (Ile), and valine (Val), were commonly preferred around the modified lysine (Figure 2E).
Furthermore, we analyzed the secondary structure of the identified succinylation proteins to investigate whether lysine succinylation had a tendency for specific structural regions. Approximately 34.8% succinylated sites were detected in the ordered secondary structure. Among them, 28.5% were found in the α-helix, and 6.3% were in the β-sheet. The remaining sites (65.2%) were located at the regions of random coils, indicating that succinylated peptides are more frequently occurred in the unstructured regions of the protein (Figure 2F). In addition, compared with all lysine residues, succinylated sites did not show moderate bias for the protein surface (Figure 2G).
Functional annotation of differentially succinylated proteins in response to hyperthermia treatment
To determine the potential role of lysine succinylation in HaCaT cells in response to hyperthermia treatment, all upregulated succinylated proteins (119 proteins with 197 sites) were submitted to GO analysis (Figure 3A). Based on the classification of MF, the most of succinylation proteins belonged to binding and catalytic activity. Within the CC category, most proteins were related to extracellular exosomes, mitochondria, and cytoplasm, showing that succinylated proteins existed broadly in HaCaT cells. In terms of BP, proteins connected with metabolic process (101 proteins) and response to stimulus (54 proteins) were more likely to be succinylated, which was consistent with the CC analysis that most proteins were in metabolism-related regions. In addition, 19 and 6 proteins were involved in the immune system process and viral transcription, respectively, indicating that the treatment effect of hyperthermia for HPV-infected skin warts could be achieved by regulating lysine succinylation on these proteins.
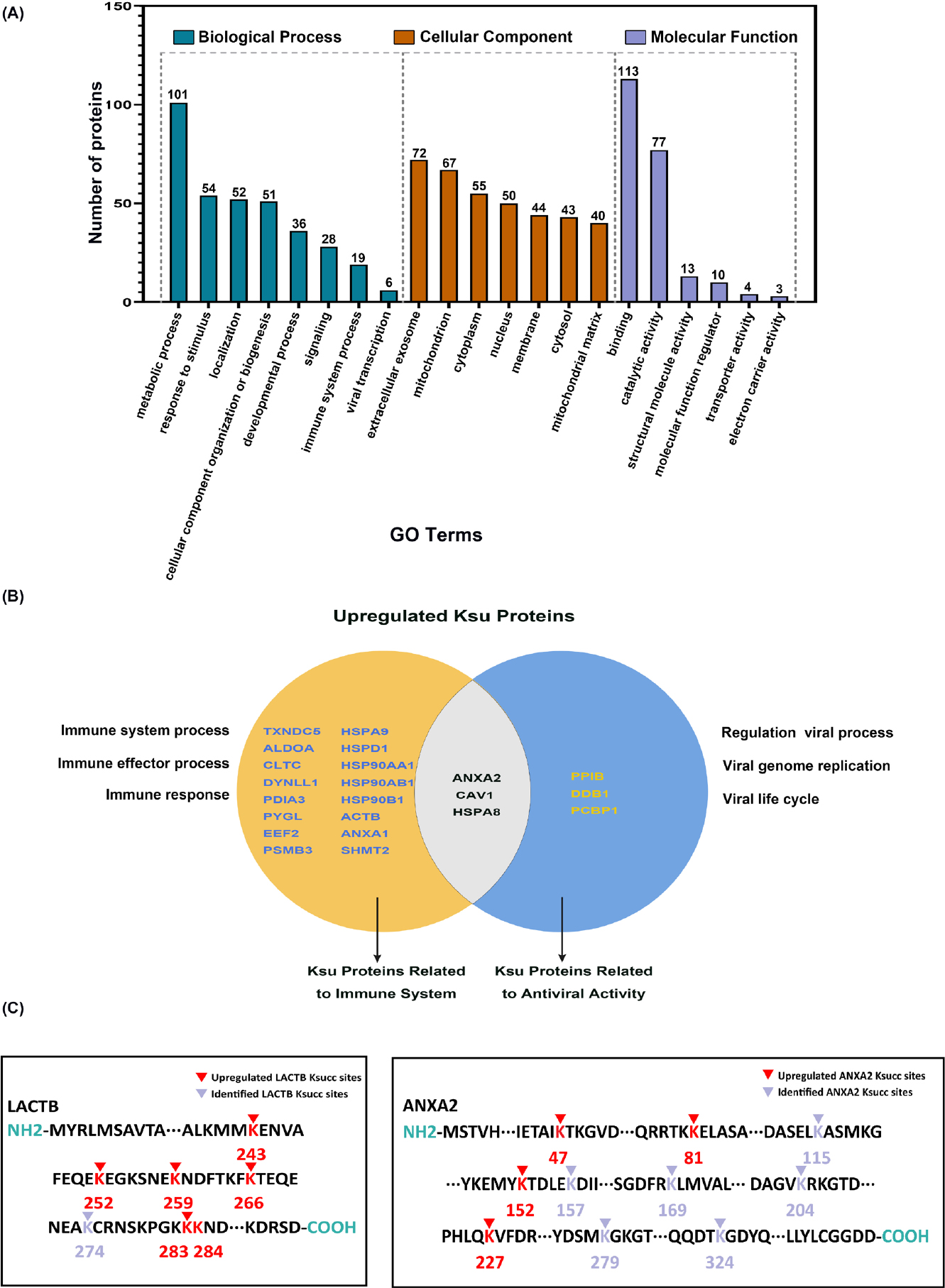
Characterization description of differentially succinylated proteins in response to hyperthermia in HaCaT cells. (A) Functional annotation of differentially succinylated proteins in response to hyperthermia treatment. (B) The integration of upregulated succinylated proteins in immune system process and viral transcription. (C) The Ksucc sites analysis of annexin A2 and LACTB. GO: Gene Ontology; LACTB: serine beta-lactamase-like protein.
Moreover, three succinylated proteins, annexin A2, caveolin 1 (CAV1), and HSPA8, were involved in both the immune system process and viral transcription (Figure 3B). Among them, annexin A2 possessed four upregulated succinylated lysine sites (K47, 81, 152, 227) (Figure 3C, Table 1).
Differentially expressed Ksucc proteins related to both antiviral and immune process
| Protein accession | Protein description | Gene name | Position | heat/control ratio | heat/control P value | Regulation |
|---|---|---|---|---|---|---|
| P07355 | Annexin A2 | ANXA2 | K47 | 1.516 | 0.000415 | Up |
| K152 | 1.533 | 0.000537 | Up | |||
| K227 | 1.545 | 0.00537 | Up | |||
| K81 | 1.521 | 0.0113 | Up | |||
| P11142 | Heat shock cognate 71 kDa protein | HSPA8 | K257 | 1.596 | 0.00327 | Up |
| K108 | 1.633 | 0.00462 | Up | |||
| K246 | 1.580 | 0.0107 | Up | |||
| K597 | 1.540 | 0.0198 | Up | |||
| K319 | 2.005 | 0.0383 | Up | |||
| Q03135 | Caveolin-1 | CAV1 | K26 | 1.698 | 0.0136 | Up |
We sorted the proteins according to the upregulation ratio of succinylation sites in response to hyperthermia treatment. Among them, LACTB, mitochondria ranked first, and multiple sites were upregulated (K243, 259, 266, 283, 284) by more than 3 times (Figure 3C, Table 2).
Top 30 upregulated succinylated sites
| Protein accession | Protein description | Gene name | Position | heat/control ratio | heat/control P value | Regulation |
|---|---|---|---|---|---|---|
| P83111 | Serine beta-lactamase-like protein, mitochondrial | LACTB | K266 | 7.357 | 0.000347 | Up |
| K243 | 4.750 | 0.0003 | Up | |||
| K252 | 4.626 | 0.00783 | Up | |||
| K259 | 4.384 | 0.0236 | Up | |||
| K283 | 3.267 | 0.0291 | Up | |||
| K284 | 3.204 | 0.000126 | Up | |||
| P41250 | Glycine-tRNA ligase | GARS | K219 | 2.414 | 0.000158 | Up |
| K204 | 2.404 | 0.0403 | Up | |||
| P07954 | Fumarate hydratase, mitochondrial | FH | K66 | 2.247 | 0.000822 | Up |
| K61 | 2.204 | 0.0000278 | Up | |||
| P34897 | Serine hydroxymethyltransferase, mitochondrial | SHMT2 | K302 | 2.101 | 0.00186 | Up |
| P60709 | Actin, cytoplasmic 1 | ACTB | K326 | 2.020 | 0.00147 | Up |
| P11142 | Heat shock cognate 71 kDa protein | HSPA8 | K319 | 2.005 | 0.0383 | Up |
| P23284 | Peptidyl-prolyl cis-trans isomerase B | PPIB | K67 | 1.968 | 0.0218 | Up |
| P41250 | Glycine-tRNA ligase | GARS | K224 | 1.956 | 0.00424 | Up |
| K646 | 1.911 | 0.00557 | Up | |||
| P34897 | Serine hydroxymethyltransferase, mitochondrial | SHMT2 | K181 | 1.856 | 0.00617 | Up |
| P23284 | Peptidyl-prolyl cis-trans isomerase B | PPIB | K41 | 1.843 | 0.00162 | Up |
| P60709 | Actin, cytoplasmic 1 | ACTB | K50 | 1.816 | 0.0373 | Up |
| Q9Y6N5 | Sulfide:quinone oxidoreductase, mitochondrial | SQRDL | K365 | 1.796 | 0.000387 | Up |
| P23284 | Peptidyl-prolyl cis-trans isomerase B | PPIB | K209 | 1.729 | 0.000408 | Up |
| P55084 | Trifunctional enzyme subunit beta, mitochondrial | HADHB | K254 | 1.728 | 0.00428 | Up |
| P23284 | Peptidyl-prolyl cis-trans isomerase B | PPIB | K129 | 1.727 | 0.00197 | Up |
| P07954 | Fumarate hydratase, mitochondrial | FH | K94 | 1.717 | 0.00153 | Up |
| Q9Y6N5 | Sulfide:quinone oxidoreductase, mitochondrial | SQRDL | K125 | 1.696 | 0.0022 | Up |
| P07954 | Fumarate hydratase, mitochondrial | FH | K122 | 1.685 | 0.0034 | Up |
| P60709 | Actin, cytoplasmic 1 | ACTB | K113 | 1.685 | 0.00944 | Up |
| P34897 | Serine hydroxymethyltransferase, mitochondrial | SHMT2 | K469 | 1.674 | 0.00315 | Up |
| P30101 | Protein disulfide-isomerase A3 | PDIA3 | K494 | 1.655 | 0.0044 | Up |
| P55084 | Trifunctional enzyme subunit beta, mitochondrial | HADHB | K272 | 1.643 | 0.0499 | Up |
Succinylation of global proteins was affected by hyperthermia and showed an obvious increase in annexin A2 and LACTB proteins after heat treatment
To verify the results of proteome analysis, we examined the succinylation of global proteins at different time points in HaCaT cells after hyperthermia treatment. The western blot results showed that the succinylation level of global proteins increased at 30 min after hyperthermia and gradually recovered to untreated levels at 6 h (Figure 4A).
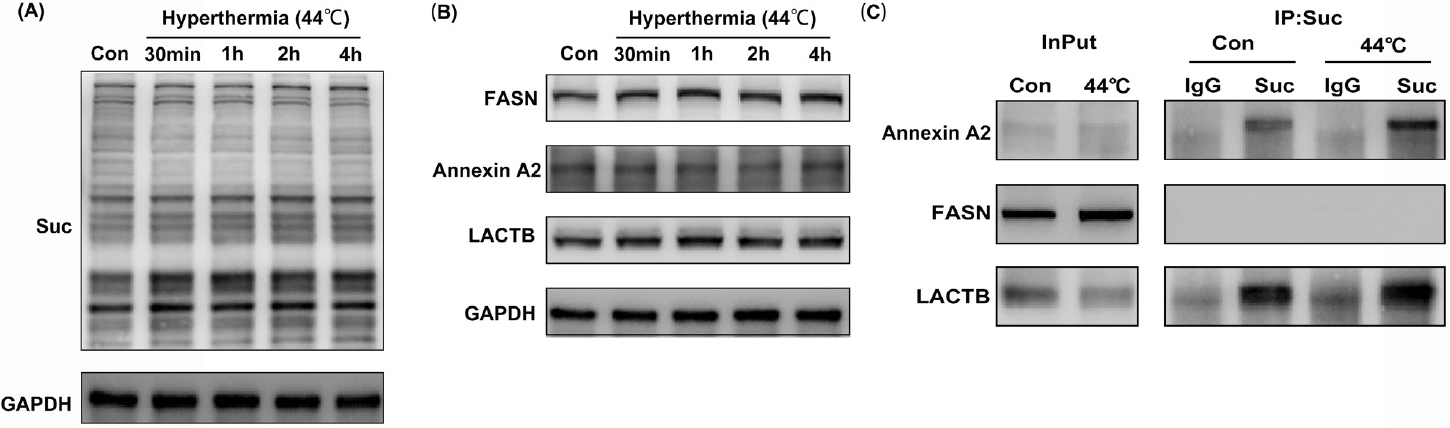
Hyperthermia increase the succinylation levels of total proteins in HaCaT cells. (A) Western blot analysis of the succinylation of total proteins in HaCaT cells in different time points after hyperthermia. (B) Western blot analysis of the expression of LACTB, FASN, and annexin A2 in HaCaT cells in response hyperthermia. (C) Co-immunoprecipitation analysis of the succinylation of LACTB, FASN, and annexin A2 before and after hyperthermia. Suc indicates the lysine succinylation. FASN: fatty acid synthase; LACTB: serine beta-lactamase-like protein; IgG: immunoglobulin G; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
According to proteome analysis, LACTB, annexin A2, and FASN were the most evident molecules in the upregulation ratio of succinylation. Therefore, their expression was also verified by WB. The detection of protein levels showed that LACTB and FASN were not significantly changed in HaCaT cells after hyperthermia, but annexin A2 was decreased (Figure 4B). The succinylation of LACTB, FASN, and annexin A2 was examined by coimmunoprecipitation prior and post to hyperthermia (after 4 h). The data showed that the succinylation of LACTB and annexin A2 was significantly increased after hyperthermia compared to that of the untreated group, which was consistent with the results of our succinylome analysis (Figure 4C). However, the succinylation of FASN was not detected, and it is possible that FASN did not undergo succinylation in HaCaT cells.
Hyperthermia affects succinylation by influencing the expression of SIRT7
First, we examined the expression levels of succinylated enzymes after 44°C hyperthermia treatment. The results indicated that KATA2 and SIRT7 were apparently decreased in expression compared with nonheated cells (Figure 5A). Specifically, KATA2 and SIRT7 were highly decreased at 1 h after heat treatment, gradually began to recover at 4 h, and finally returned to the base level at 12 h (Supplementary Figure 1).
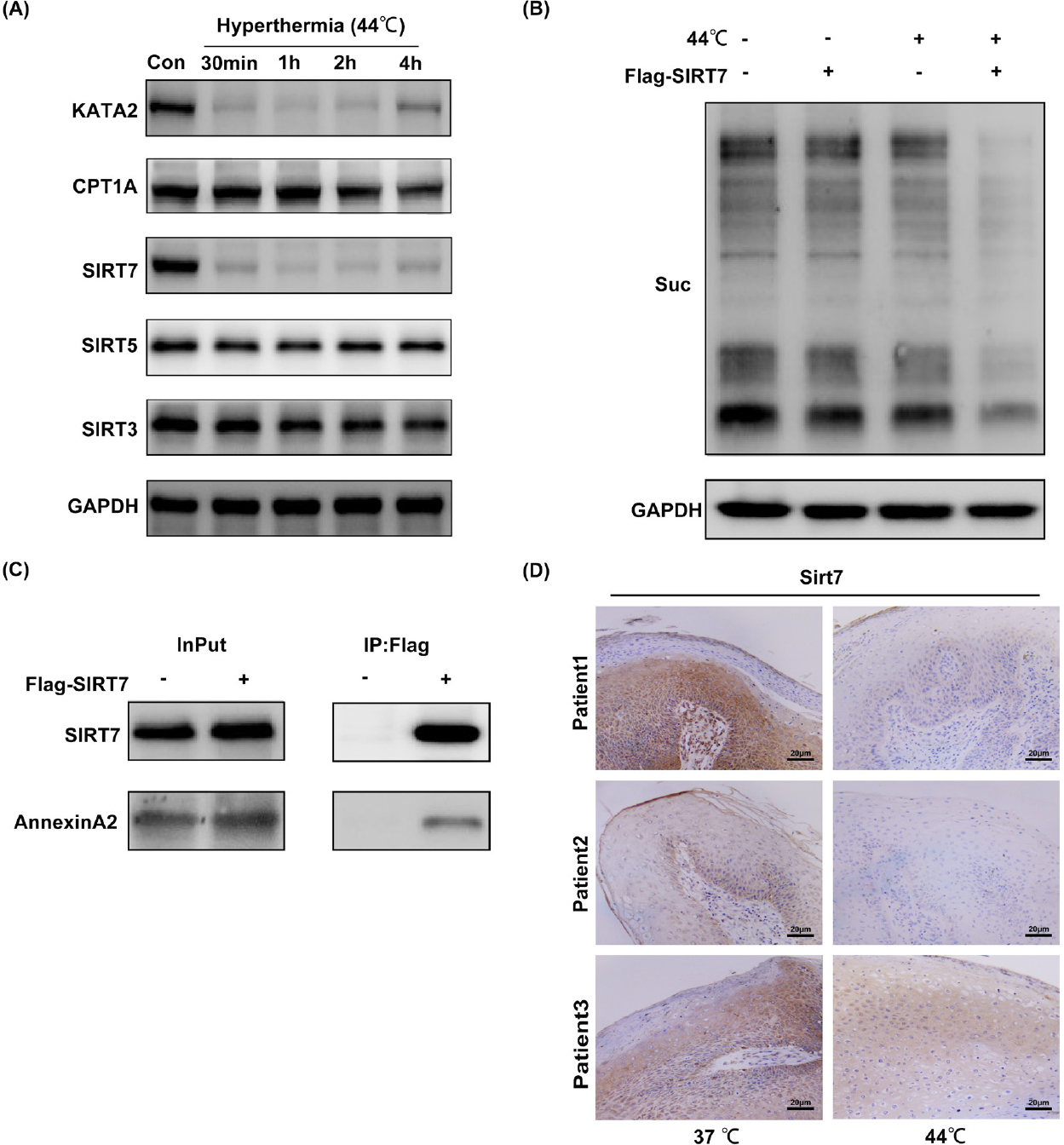
Hyperthermia influencing the succinylation of global proteins by downregulating SIRT7 in HaCat cells. (A) Western blot analysis of the expression of succinylated enzymes after hyperthermia. (B) Western blot analysis of the succinylation of global proteins in overexpression SIRT7. (C) Co-immunoprecipitation analysis of the interaction of SIRT7 with LACTB, FASN, and annexin A2. (D) The immunohistochemical staining of SIRT7 in condyloma acuminatum tissues. Suc indicates the lysine succinylation. - means empty vector plasmid. + means SIRT7 plasmid. Original magnification: 200×. FASN: fatty acid synthase; SIRT7: sirtuin7; LACTB: serine beta-lactamase-like protein; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
To explore the possible reasons for hyperthermia influences on the succinylation of HaCaT cells, SIRT7 overexpression plasmids were constructed. We observed that SIRT7 affected the succinylation of global proteins in HaCaT cells, and succinylation was lowered in the SIRT7 overexpression group than that in the control group (transfected vector plasmid), and this difference was more significant in cells treated with hyperthermia (Figure 5B).
HPV was shown to interact with annexin A2 at both the extracellular plasma membrane and endocytic vesicles, indicating the role of annexin A2 as a receptor for HPV infection, especially in virus attachment, internalization, and trafficking.[26,27,28] To investigate whether SIRT7 might be the regulatory enzyme of annexin A2, co-immunoprecipitation of SIRT7 with annexin A2 was performed, and the results showed that SIRT7 interacted with annexin A2 (Figure 5C).
Hyperthermia downregulates the expression of SIRT7 in CA tissues
Immunohistochemical staining of CA tissue samples showed that SIRT7 was reduced in heat-treated tissue samples as compared with untreated samples (Figure 5D). This result was consistent with the ex vivo experiments in HaCaT cells. In summary, our results implied that hyperthermia reduces the expression of SIRT7 in CA tissues, which in turn affects the succinylation levels of HPV infection-related molecules.
Discussions
In this work, we explored the impact of hyperthermia therapy on the succinylation level and its possible role on antiviral immunity. The succinylome assay identified 119 proteins with 197 lysine sites that were upregulated in response to hyperther mia treatment. Proteomics analysis showed that differential succinylation proteins were related to antimicrobial resistance and changes in bioresponsiveness, indicating that lysine succinylation could participate in antiviral defense activities.
PTMs are important components regulating microbial resistance and immunity.[29,30] New emerging evidences support that PTMs serve as interfaces between viruses, restriction factors, and cellular defense mechanisms, providing potential drug targets for future immunoregulatory therapy.[31] Many restriction factors of viruses, such as IFITM3/2, APOBEC, and SAMHD1, can be subjected to phosphorylation.[32,33,34] Moreover, ubiquitination constitutes a main regulatory mechanism allowing viruses to evade the action of restriction factors.[35,36,37] Although it is well documented that ubiquitination and phosphorylation are increasingly associated with antiviral responses, the role of succinylation and other modifications in antiviral activity are still poorly understood.[38]
SIRT7 exhibits desuccinylase activity in histones and maintains chromatin compaction and genome stability.[18] The expression of SIRT7 was significantly decreased after hyperthermia. SIRT7 reduced the succinylation of global proteins. It was implied that hyperthermia regulated the succinylation of overall proteins through SIRT7 in HaCaT cells. Meanwhile, decreased expression of SIRT7 was obser ved in condyloma acuminata tissues after hyperthermia. The pathological characteristics of warts were hyperplasia of the dermis and epidermis,[39] which could be regarded as benign tumors. A study showed that SIRT7 was significantly increased in highly proliferating tissues and a variety of human cancer cells.[40] Hyperthermia was likely to inhibit HPV-induced hyperplasia of epidermal cells by decreasing SIRT7.
Although no relationship has been discovered between HPV and SIRT7 according to current research, the latest studies have shown that SIRT1, the same family protein of SIRT7, is closely related to the life cycle and replication of HPV. TopBP1 is the pivotal binding partner of E2, and it is essential for E1-E2 replication and the viral life cycle. SIRT1 deacetylates the TopBP1 protein, which can lead to the functional switch from DNA replication to protein repair and cell cycle arrest.[41] In addition, SIRT1 directly regulates E1-E2-mediated DNA replication of HPV16 through PTMs. SIRT1, as a component of the E1-E2 DNA replication complex, is carried into the viral genome through the E1-E2 protein during replication and plays a stabilizing role in the E1-E2 complex.[42] Like SIRT1, SIRT7 could also have an impact on antimicrobial immunity and other bioresponsive factors by influencing succinylation, possibly. Hence, hyperthermia might improve antimicrobial immunity by regulating SIRT7.
Annexin A2 is a multicompartmental protein whose functions are generally influenced by subcellular localization. It often exists as a monomer and participates in various cellular processes, including endocytosis,[43] exocytosis,[26] cytoskeletal rearrangement,[44] and membrane trafficking through lipid microdomain formation.[45] On the plasma membrane, it can form a heterotetrametric complex with S100A10.[46] Several studies have reported that heat stress could stimulate cell surface translocation by phosphorylating annexin A2 at the tyrosine 23 site through Src-like tyrosine kinases.[47,48] Moreover, HPV has been reported to result in Tyr23 phosphorylation and extracellular translocation of annexin A2 to the outer plasma membrane by inducing EGFR-dependent Src kinase activation.[26] We found that the succinylation of annexin A2 was significantly increased after hyperthermia. It was suggested that succinylation could influence the interaction of annexin A2 with HPV as phosphorylation. Phosphorylation and extracellular translocation of annexin A2 allow HPV16 to interact with annexin A2 and S100A10 as heterotetramers, causing HPV16 to enter host cells.[26] Moreover, annexin A2, A2t (composed of two annexin A2 monomers), and S100A10 control the progression of HPV infection from early endosomes to late multivesicular endosomes, inhibits capsid decomposition, and formed complexes with CD63.[27] Hence, succinylation may play regulatory roles in annexin A2-assisted virus entry into host cells. Therefore, the Ksucc sites of annexin A2 may be a potential target for drug design against HPV infection.
However, there are currently no suitable in-vivo models with intact immune functions for HPV infection; moreover, cervical cancer cell lines that contain HPV genome copies are also inadequate for their different epidermal origins, so we found it difficult to tell a appropriate story of succinylation regarding hyperthermia therapy with the use of current available models. In this study, alterations in succinylation were detected by co-immunoprecipitation in LACTB, annexin A2, and FASN due to the lack of site-specific succinylated antibodies for these molecules. In the next step, we will design the site-specific antibodies of succinylation for more comprehensive and in-depth research.
Conclusions
In this study, we observed the effect of hyperthermia on succinylation through succinylation proteomics and preliminarily explored the possible mechanisms of succinylation in virus defense and antimicrobial immunity. Pan-proteome-wide succinylation was upregulated in HaCaT cells in response to hyperthermia. After hyperthermia, the desuccinylase SIRT7 was decreased in HaCaT cells and condyloma acuminatum tissues, which implied that hyperthermia was likely to affect succinylation by downregulating SIRT7. Subsequent functional analysis indicated that differential proteins were involved in viral replication, life cycle regulation, and the immune system, especially annexin A2, a key molecule for HPV endocytosis. In summary, this study was the first to explore the relevance of succinylation with hyperthermia in an antimicrobial immunity context, and the promising results suggested that lysine succinylation sites of annexin A2 possess huge potential available to drug targets for eliminating HPV in hyperthermia treatment of cutaneous warts.
Funding statement: This research was funded by the National Natural Science Foundation of China, grant number 81872538, 82173401 and U1908206.
-
Availability of Data and Materials
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.
-
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of the First Hospital of China Medical University (Approval No. 2018-135-2), and informed consent was obtained from the patients.
-
Conflict of Interest
None declared.
References
1 Ringin SA. The Effectiveness of Cutaneous Wart Resolution with Current Treatment Modalities. J Cutan Aesthet Surg 2020;13:24–30.10.4103/JCAS.JCAS_62_19Search in Google Scholar PubMed PubMed Central
2 Ding W, Ma Y, Ma C, Malone DC, Ma A, Tang W, et al. The Lifetime Cost Estimation of Human Papillomavirus-related Diseases in China: A Modeling Study. J Transl Int Med 2021;9:200–11.10.2478/jtim-2021-0039Search in Google Scholar PubMed PubMed Central
3 Sarier M, Ceyhan AM, Sepin N, Ozel E, Inal MM, Kukul E, et al. HPV infection in urology practice. Int Urol Nephrol 2020;52:1–8.10.1007/s11255-019-02302-2Search in Google Scholar PubMed
4 Gao X, Gao D, Sun X, Huo W, Hong Y, Li X, et al. Non-ablative controlled local hyperthermia for common warts. Chin Med J (Engl) 2009;122:2061–3.Search in Google Scholar
5 Huo W, Gao X-H, Sun X-P, Qi R-Q, Hong Y, Mchepange UO, et al. Local hyperthermia at 44 degrees C for the treatment of plantar warts: a randomized, patient-blinded, placebo-controlled trial. J Infect Dis 2010;201:1169–72.10.1086/651506Search in Google Scholar PubMed
6 Yang Y, Zhang L, Zhang Y, Huo W, Qi R, Guo H, et al. Local Hyperthermia at 44°C Is Effective in Clearing Cervical High-Risk Human Papillomaviruses: A Proof-of-Concept, Randomized Controlled Clinical Trial. Clin Infect Dis 2021;73:1642–9.10.1093/cid/ciab369Search in Google Scholar PubMed
7 Alleyn M, Breitzig M, Lockey R, Kolliputi N. The dawn of succinylation: a posttranslational modification. Am J Physiol Cell Physiol 2018;314:C228–32.10.1152/ajpcell.00148.2017Search in Google Scholar PubMed PubMed Central
8 Zhang Z, Tan M, Xie Z, Dai L, Chen Y. Identification of lysine succinylation as a new post-translational modification 2011;14:58–63.10.1038/nchembio.495Search in Google Scholar PubMed PubMed Central
9 Qian H, Zhang Y, Wu B, Wu S, You S, Zhang N, et al. Structure and function of HECT E3 ubiquitin ligases and their role in oxidative stress. J Transl Int Med 2020;8:71–9.10.2478/jtim-2020-0012Search in Google Scholar PubMed PubMed Central
10 Lin ZF, Xu HB, Wang JY, Lin Q, Ruan Z, Liu FB, et al. SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem Biophys Res Commun 2013;441:191–5.10.1016/j.bbrc.2013.10.033Search in Google Scholar PubMed
11 Wang F, Wang K, Xu W, Zhao S, Ye D, Wang Y, et al. SIRT5 Desuccinylates and Activates Pyruvate Kinase M2 to Block Macrophage IL-1β Production and to Prevent DSS-Induced Colitis in Mice. Cell Rep 2017;19:2331–44.10.1016/j.celrep.2017.05.065Search in Google Scholar PubMed
12 Xiangyun Y, Xiaomin N, Linping G, Yunhua X, Ziming L, Yongfeng Y, et al. Desuccinylation of pyruvate kinase M2 by SIRT5 contributes to antioxidant response and tumor growth. Oncotarget 2017;8:6984–93.10.18632/oncotarget.14346Search in Google Scholar PubMed PubMed Central
13 Polletta L, Vernucci E, Carnevale I, Arcangeli T, Rotili D, Palmerio S, et al. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy 2015;11:253–70.10.1080/15548627.2015.1009778Search in Google Scholar PubMed PubMed Central
14 Liu X, Zhu C, Zha H, Tang J, Rong F, Chen X, et al. SIRT5 impairs aggregation and activation of the signaling adaptor MAVS through catalyzing lysine desuccinylation. EMBO J 2020;39:e103285.10.15252/embj.2019103285Search in Google Scholar PubMed PubMed Central
15 Wu D, Li Y, Zhu KS, Wang H, Zhu W-G. Advances in Cellular Characterization of the Sirtuin Isoform, SIRT7. Front Endocrinol (Lausanne) 2018;9:652.10.3389/fendo.2018.00652Search in Google Scholar PubMed PubMed Central
16 Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 2011;334:806–9.10.1126/science.1207861Search in Google Scholar PubMed PubMed Central
17 Michishita E, Park JY, Burneskis JM, B arrett JC, Horikawa I. Evolutionarily Conserved and Nonconserved Cellular Localizations and Functions of Human SIRT Proteins. Mol Biol Cell. 2005;16:4623–35.10.1091/mbc.e05-01-0033Search in Google Scholar PubMed PubMed Central
18 Li L, Shi L, Yang S, Yan R, Zhang D, Yang J, et al. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat Commun 2016;7:12235.10.1038/ncomms12235Search in Google Scholar PubMed PubMed Central
19 Bao X, Wang Y, Li X, Li X-M, Liu Z, Yang T, et al. Identification of “erasers” for lysine crotonylated histone marks using a chemical proteomics approach. Elife 2014;3:e02999.10.7554/eLife.02999Search in Google Scholar PubMed PubMed Central
20 Sreedhar A, Wiese EK, Hitosugi T. Enzymatic and metabolic regulation of lysine succinylation. Genes Dis 2020;7:166–71.10.1016/j.gendis.2019.09.011Search in Google Scholar PubMed PubMed Central
21 Lee K, Kerner J, Hoppel CL. Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J Biol Chem 2011;286:25655–62.10.1074/jbc.M111.228692Search in Google Scholar PubMed PubMed Central
22 Kurmi K, Hitosugi S, Wiese EK, Boakye-Agyeman F, Gonsalves WI, Lou Z, et al. Carnitine Palmitoyltransferase 1A Has a Lysine Succinyltransferase Activity. Cell Rep 2018;22:1365–73.10.1016/j.celrep.2018.01.030Search in Google Scholar PubMed PubMed Central
23 Wang Y, Guo YR, Liu K, Yin Z, Liu R, Xia Y, et al. KAT2A coupled with the α-KGDH complex acts as a histone H3 succinyltransferase. Nature 2017;552:273–7.10.1038/nature25003Search in Google Scholar PubMed PubMed Central
24 Liu RJ, Niu XL, Yuan JP, Chen HD, Gao XH, Qi RQ. DnaJA4 is involved in responses to hyperthermia by regulating the expression of F-actin in HaCaT cells. Chin Med J (Engl) 2020;134:456–62.10.1097/CM9.0000000000001064Search in Google Scholar PubMed PubMed Central
25 Yuan H, Chen J, Yang Y, Shen C, Xu D, Wang J, et al. Quantitative succinylproteome profiling of Chinese hickory (Carya cathayensis) during the grafting process. BMC Plant Biol 2019;19:467.10.1186/s12870-019-2072-8Search in Google Scholar PubMed PubMed Central
26 Dziduszko A, Ozbun MA. Annexin A2 and S100A10 Regulate Human Papillomavirus Type 16 Entry and Intracellular Trafficking in Human Keratinocytes. J Virol 2013;87:7502–15.10.1128/JVI.00519-13Search in Google Scholar PubMed PubMed Central
27 Taylor JR, Fernandez DJ, Thornton SM, Skeate JG, Lühen KP, Da Silva DM, et al. Heterotetrameric annexin A2/S100A10 (A2t) is essential for oncogenic human papillomavirus trafficking and capsid disassembly, and protects virions from lysosomal degradation. Sci Rep 2018;8:11642.10.1038/s41598-018-30051-2Search in Google Scholar PubMed PubMed Central
28 Woodham AW, Da Silva DM, Skeate JG, Raff AB, Ambroso MR, Brand HE, et al. The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection. PLoS One 2012;7:e43519.10.1371/journal.pone.0043519Search in Google Scholar PubMed PubMed Central
29 Tasneem AA, Luck NH. Autoimmune hepatitis: Clinical characteristics and predictors of biochemical response to treatment. J Transl Int Med 2020;8:106–11.10.2478/jtim-2020-0016Search in Google Scholar PubMed PubMed Central
30 Gomes C, Ginzberg D, Wong RJ. Delays and gaps in progressing through the hepatitis C virus cascade of care: An underserved safety-net hospital experience. J Transl Int Med 2020;8:261–7.10.2478/jtim-2020-0039Search in Google Scholar PubMed PubMed Central
31 Chamontin C, Bossis G, Nisole S, Arhel NJ, Maarifi G. Regulation of Viral Restriction by Post-Translational Modifications. Viruses 2021;13:2197.10.3390/v13112197Search in Google Scholar PubMed PubMed Central
32 Cribier A, Descours B, Valadão ALC, Laguette N, Benkirane M. Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep 2013;3:1036–43.10.1016/j.celrep.2013.03.017Search in Google Scholar PubMed
33 Demorest ZL, Li M, Harris RS. Phosphorylation directly regulates the intrinsic DNA cytidine deaminase activity of activation-induced deaminase and APOBEC3G protein. J Biol Chem 2011;286:26568–75.10.1074/jbc.M111.235721Search in Google Scholar PubMed PubMed Central
34 Narayana SK, Helbig KJ, McCartney EM, Eyre NS, Bull RA, Eltahla A, et al. The Interferon-induced Transmembrane Proteins, IFITM1, IFITM2, and IFITM3 Inhibit Hepatitis C Virus Entry. J Biol Chem 2015;290:25946–59.10.1074/jbc.M115.657346Search in Google Scholar PubMed PubMed Central
35 Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med 2003;9:1404–7.10.1038/nm945Search in Google Scholar PubMed
36 Pardieu C, Vigan R, Wilson SJ, Calvi A, Zang T, Bieniasz P, et al. The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathog 2010;6:e1000843.10.1371/journal.ppat.1000843Search in Google Scholar PubMed PubMed Central
37 The retroviral accessory proteins S2, Nef, and glycoMA use similar mechanisms for antagonizing the host restriction factor SERINC5. J Biol Chem 2019;294:7013–24.10.1074/jbc.RA119.007662Search in Google Scholar PubMed PubMed Central
38 Zheng P, Zheng X, Takehiro H, Cheng ZJ, Wang J, Xue M, et al. The prognostic value of Krebs von den Lungen-6 and surfactant protein-A levels in the patients with interstitial lung disease. J Transl Int Med 2021;9:212–22.10.2478/jtim-2021-0040Search in Google Scholar PubMed PubMed Central
39 Hogendoorn GK, Bruggink SC, de Koning MNC, Eekhof J a. H, Hermans KE, Rissmann R, et al. Morphological characteristics and human papillomavirus genotype predict the treatment response in cutaneous warts. Br J Dermatol 2018;178:253–60.10.1111/bjd.15758Search in Google Scholar PubMed
40 Kumari P, Tarighi S, Braun T, Ianni A. SIRT7 Acts as a Guardian of Cellular Integrity by Controlling Nucleolar and Extra-Nucleolar Functions. Genes (Basel) 2021;12:1361.10.3390/genes12091361Search in Google Scholar PubMed PubMed Central
41 Boner W, Taylor ER, Tsirimonaki E, Yamane K, Campo MS, Morgan IM. A Functional interaction between the human papillomavirus 16 transcription/replication factor E2 and the DNA damage response protein TopBP1. J Biol Chem 2002;277:22297–303.10.1074/jbc.M202163200Search in Google Scholar PubMed
42 Das D, Smith N, Wang X, Morgan IM. The Deacetylase SIRT1 Regulates the Replication Properties of Human Papillomavirus 16 E1 and E2. J Virol 2017;91:e00102–17.10.1128/JVI.00102-17Search in Google Scholar PubMed PubMed Central
43 Emans N, Gorvel JP, Walter C, Gerke V, Kellner R, Griffiths G, et al. Annexin II is a major component of fusogenic endosomal vesicles. J Cell Biol 1993;120:1357–69.10.1083/jcb.120.6.1357Search in Google Scholar PubMed PubMed Central
44 Aliyu IA, Ling K-H, Md Hashim N, Chee H-Y. Annexin A2 extracellular translocation and virus interaction: A potential target for antivirus-drug discovery. Rev Med Virol 2019;29:e2038.10.1002/rmv.2038Search in Google Scholar PubMed
45 Babiychuk EB, Draeger A. Annexins in cell membrane dynamics. Ca(2+)-regulated association of lipid microdomains. J Cell Biol 2000;150:1113–24.10.1083/jcb.150.5.1113Search in Google Scholar PubMed PubMed Central
46 Hajjar KA. The Biology of Annexin A2: From Vascular Fibrinolysis to Innate Immunity. Trans Am Clin Climatol Assoc 2015;126:144–55.Search in Google Scholar
47 Christensen MV, Høgdall CK, Jochumsen KM, Høgdall EVS. Annexin A2 and cancer: A systematic review. Int J Oncol 2018;52:5–18.10.3892/ijo.2017.4197Search in Google Scholar PubMed
48 Deora AB, Kreitzer G, Jacovina AT, Hajjar KA. An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. J Biol Chem 2004;279:43411–8.10.1074/jbc.M408078200Search in Google Scholar PubMed
© 2023 Xueli Niu, Yiping Zhao, Tao Zhang, Yuzhe Su, Zhendong Wei, Kangle Fu, Jingyi Li, Mingsui Tang, Wenyu Wan, Xinghua Gao, Hongduo Chen, Ruiqun Qi, Bing Song, published by De Gruyter on behalf of Scholar Media Publishing
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Guideline and Consensus
- Chinese expert consensus on blood lipid management in patients with diabetes (2024 edition)
- Review Article
- Role of viral hepatitis in pregnancy and its triggering mechanism
- Original Article
- Machine learning-based phenogroups and prediction model in patients with functional gastrointestinal disorders to reveal distinct disease subsets associated with gas production
- Recognition of differently expressed genes and DNA methylation markers in patients with Lupus nephritis
- PRP improves the outcomes of autologous skin graft transplantation on the esophagus by promoting angiogenesis and inhibiting fibrosis and inflammation
- Identification of functional heterogeneity of immune cells and tubular-immune cellular interplay action in diabetic kidney disease
- Actin-related protein 2/3 complex subunit 1B promotes ovarian cancer progression by regulating the AKT/PI3K/mTOR signaling pathway
- Comprehensive succinylome analyses reveal that hyperthermia upregulates lysine succinylation of annexin A2 by downregulating sirtuin7 in human keratinocytes
- Inhibition of heterogeneous nuclear ribonucleoproteins A1 and oxidative stress reduces glycolysis via pyruvate kinase M2 in chronic thromboembolic pulmonary hypertension
Articles in the same Issue
- Guideline and Consensus
- Chinese expert consensus on blood lipid management in patients with diabetes (2024 edition)
- Review Article
- Role of viral hepatitis in pregnancy and its triggering mechanism
- Original Article
- Machine learning-based phenogroups and prediction model in patients with functional gastrointestinal disorders to reveal distinct disease subsets associated with gas production
- Recognition of differently expressed genes and DNA methylation markers in patients with Lupus nephritis
- PRP improves the outcomes of autologous skin graft transplantation on the esophagus by promoting angiogenesis and inhibiting fibrosis and inflammation
- Identification of functional heterogeneity of immune cells and tubular-immune cellular interplay action in diabetic kidney disease
- Actin-related protein 2/3 complex subunit 1B promotes ovarian cancer progression by regulating the AKT/PI3K/mTOR signaling pathway
- Comprehensive succinylome analyses reveal that hyperthermia upregulates lysine succinylation of annexin A2 by downregulating sirtuin7 in human keratinocytes
- Inhibition of heterogeneous nuclear ribonucleoproteins A1 and oxidative stress reduces glycolysis via pyruvate kinase M2 in chronic thromboembolic pulmonary hypertension

