PRP improves the outcomes of autologous skin graft transplantation on the esophagus by promoting angiogenesis and inhibiting fibrosis and inflammation
-
Ning Xu
Abstract
Background and Objectives
Autologous skin graft (ASG) transplantation is a challenging approach but a promising option for patients to prevent postoperative esophageal stricture. Nonetheless, the current strategies require improvement. We aimed to investigate the effectiveness of the injection of platelet-rich plasma (PRP) before skin graft transplantation for extensive esophageal defects after endoscopic resection.
Methods
Standardized complete circular endoscopic resection (5 cm in length) was performed in 27 pigs allocated into 3 groups. The artificial ulcers were treated with a fully covered esophageal stent (control group), ASG (ASG group), and submucosal injection of PRP with ASG (PRP-ASG group). Macroscopic evaluation and histological analysis of the remolded esophagus were performed 7, 14, and 28 days after surgery.
Results
The macroscopic evaluation indicated that submucosal injection of PRP before transplantation effectively promoted the survival rate of skin grafts and decreased the rate of mucosal contraction compared with those treated with ASG or stent alone. Histological analysis of submucosal tissue showed that this modified strategy significantly promoted wound healing of reconstructed tissues by enhancing angiogenesis, facilitating collagen deposition, and decreasing inflammation and fibrogenesis.
Conclusions
These findings suggested that PRP might be used as a biological supplement to increase the esophageal skin graft survival rate and improve submucosal tissue remolding in a clinically relevant porcine model. With extremely low mucosal contraction, this novel combination strategy showed the potential to effectively prevent stenosis in extensive esophageal ulcers.
Introduction
Esophageal cancer is a common malignant tumor and can be detected and treated at an early stage using advanced endoscopy with auxiliary tools.[1] Endoscopic submucosal tunnel dissection (ESTD), an innovative, effective treatment for large superficial esophageal neoplasms (SENs), has been developed into an optimal choice for treatment.[2] However, for extensive en bloc resection of lesions, a major complication is artificial ulcer constriction that occurs owing to extensive mucosal defects. Almost all patients who underwent endoscopic resection for wholly circumferential SENs finally developed postoperative stenosis, leading to dysphagia and considerably influencing the quality of life in patients.[3]
Oral or local administration of glucocorticoids is listed as the common treatment method in the clinic to overcome this problem, but to prolong the effect, repeated treatment is needed.[4] Other approaches, such as transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets or acellular dermal matrix to overcome the loss of epithelium, have shown good outcomes in animal models but only for small or semi circumferential ulcers.[5,6] Currently, no standard method has been established for esophageal stricture prevention after endoscopic resection of large circumferential SENs.
The presence of epithelium is essential for wound healing, and loss of esophageal epithelium may have a considerable effect on tissue inflammation infiltration and fibrogenesis. Therefore, we developed autologous skin graft (ASG) surgery to prevent esophageal stricture after complete circular ESTD,[7] and the effectiveness of this method in preventing esophageal stenosis reached 63.2%, with an average ulceration length of 9.8 cm in 19 patients.[8] However, the low survival rate of the skin graft was the main barrier to its stricture prevention effect on the remaining 36.8% of patients.
Platelets contain high quantities of growth factors, including transforming growth factor β-1 (TGFβ-1) and vascular endothelial growth factor (VEGF), which are able to stimulate angiogenesis and matrix remodeling.[9] Autologous PRP has been used to improve the survival rate of skin flaps and enhance bone regeneration in the fields of plastic surgery and orthopedics.[10] In this study, by applying PRP to promote the survival of esophageal skin grafts and using histopathological analysis, we demonstrated a successful modified strategy to prevent esophageal strictures after endoscopic resections of extensive mucosa in a clinically relevant porcine model.
Materials and methods
Animals
Twenty-seven adult male Bama miniature pigs weighing 25–30 kg were randomly divided into three groups. Nine of them were arranged to place fully covered esophageal stents (FCES) alone (control group). Autologous skin-grafting surgery was performed in the remaining 18 pigs. Animals with/without the application of PRP were subdivided into the PRP-ASG group and ASG group, respectively. All animals were fasted two days prior to the procedure and given water ad libitum. Three pigs in each group were euthanized on Days 7, 14, and 28. All experimental procedures were approved by the Institutional Animal Ethics Committee of the First Medical Center of PLA General Hospital.
Preoperative preparation
Light anesthesia was induced using intramuscular injections of xylazine hydrochloride (25 mg/kg) and midazolam (0.25 mg/kg). General anesthesia, introduced with 3–4% sevoflurane and maintained with 0.5–2.0% sevoflurane, was achieved by endotracheal intubation. The animals were placed in the left lateral position, and the right side of the pig dorsum was shaved and then disinfected with 70% ethanol and iodine tincture.
Artificial esophageal defect
A schematic overview of the modified autologous skin-grafting surgery on the esophagus is shown in Scheme 1.
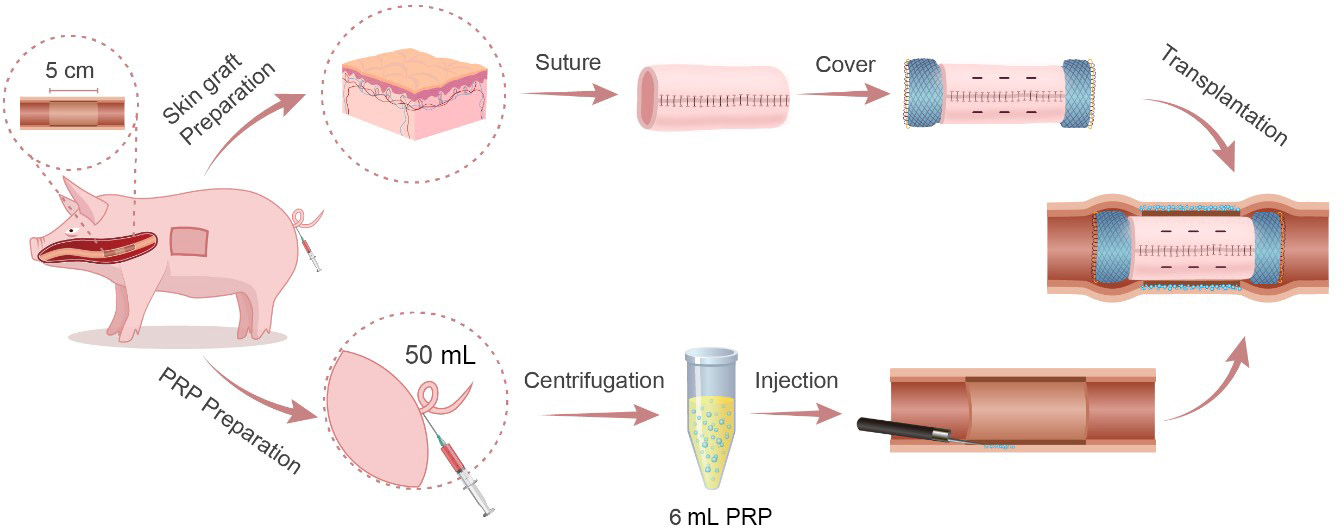
Schematic diagram of the surgical procedure.
ESTD was performed using an endoscope with a single-accessory channel (GIF-Q260J, Olympus, Tokyo, Japan). A transparent cap (D201-11804; Olympus) is routinely placed on the tip of the endoscope. A mixture of 0.9% saline and 0.1% methylene blue was used for submucosal injection. Various electrosurgical knives, including a triangle-tip knife (KD-640 L; Olympus), a dual knife (KD-650Q; Olympus) and an insulated-tip (IT) knife (KD-611 L; Olympus), were applied to complete the procedure. Bleeding was controlled by the application of hemostatic forceps (FD-410LR; Olympus). All pigs underwent esophageal ESTD 30–35 cm from the incisor. Circular dots were made at 30 cm and 35 cm to reveal the oral and anal margins with electrosurgical knives, respectively. Then, a dual knife was used to separate the mucosa transversely after the submucosal fluid cushion reached a feasible level on the anal end and the oral end in turn. A dual knife was used to form a submucosal tunnel with the assistance of repeated submucosal injections from the oral side to the anal side. Next, lateral mucosal resection close to the markings was completed by an IT knife from the anal to the oral side until a length of 5 cm circumferential esophageal mucosa was removed (Figure 1A).
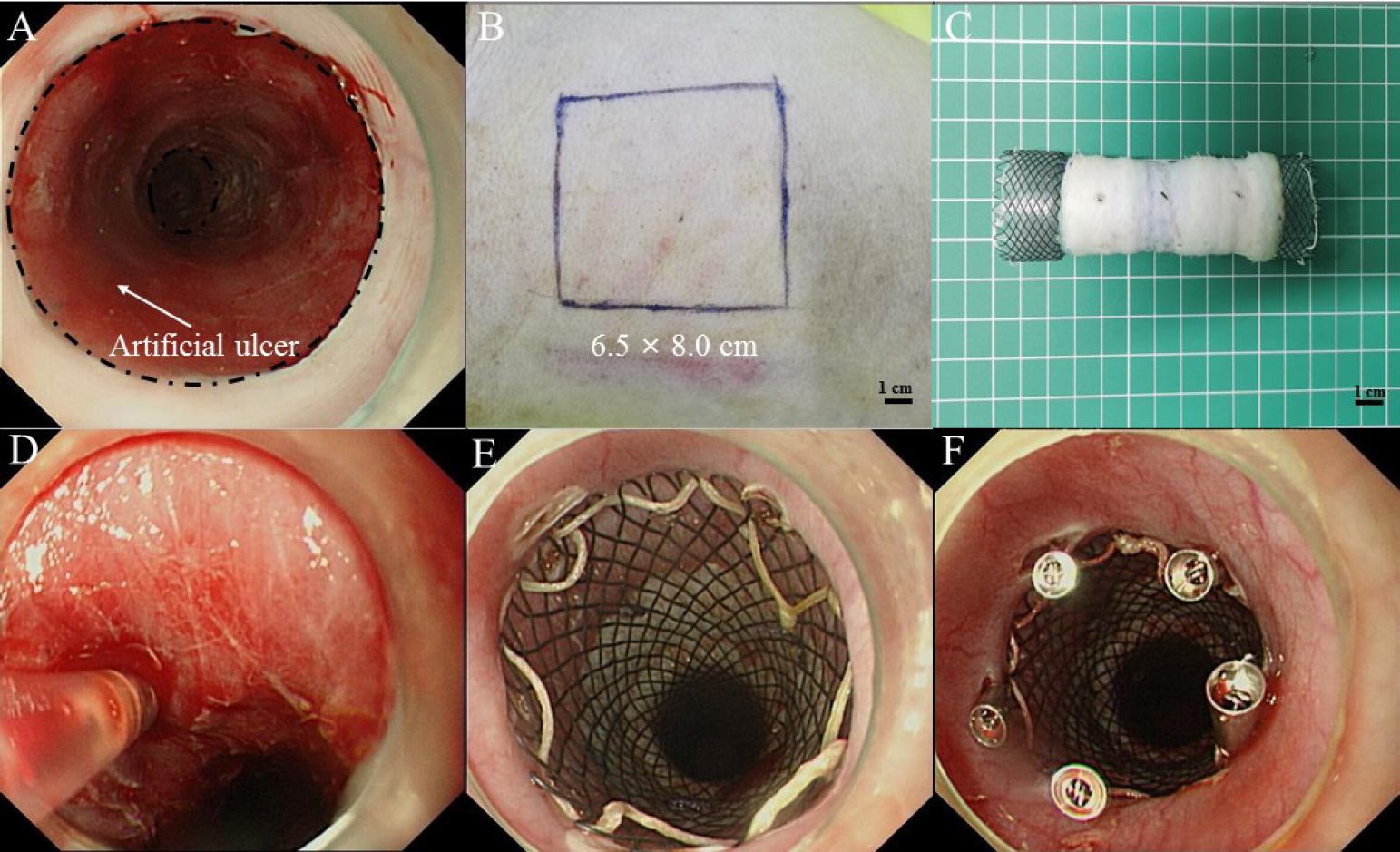
Artificial circumferential mucosal defect followed by endoscopic injection of PRP and transplantation of ASG. (A) Artificial circumferential mucosal defect on esophagus (5 cm in length). (B) Split-thickness skin graft was harvested from the right back of the pig. (C) The graft was sewn into an “oversleevelike” skin and was covered the outside of esophageal stent. (D) Submucosal injection of PRP. (E) The stent was placed at the location of the esophageal defect. (F) Using endoscopic clips to fix the stent. PRP: plasma-rich platelet; ASG: autologous skin graft.
Skin graft
Using a Humby knife, a 6.5 × 8.0 cm split-thickness skin graft (Figure 1B) was harvested from the right side of the pig dorsum by a plastic surgeon and then sewn on a FCES (EVO-FC-20-25-12-E, Cook Medical, Bloomington, USA) into an “oversleeve-like” skin with absorbable suture (VICRYL Plus; 4–0; 1.5 Ph. Eur) (Figure 1C). A No. 11 scalpel was used to make well-distributed holes in the skin graft at 2 cm intervals.
Preparation and endoscopic injection of PRP
Fifty milliliters of blood was collected from the tail vein of pigs in the PRP-ASG group and then transferred to a test tube containing 0.5 mL heparin (No. 1 Biochemical & Pharmaceutical Co., Ltd., Shanghai, China). Two steps were used to obtain the PRP: centrifugation at 2000 × g for 10 min to obtain a supernatant and recentrifugation at 2350 g for 10 min to remove the upper two-thirds of the plasma and retain the lower one-third as PRP (approximately 6 mL).[11] Prior to the transplantation of the autologous skin graft, 0.5 mL PRP was slowly injected into the uncovered submucosal layer or muscular layer at 12 regular intervals via the submucosal injection needle (NM-200 L; Olympus) (Figure 1D).
Stent placement
An FCES (with/without skin graft) was deployed in vivo to the location of the esophageal defect (Figure 1E). The specific processes were as follows. Grasping forceps were passed through the biopsy channel of the endoscope to tightly grasp the distal steel lasso loop of the FCES. Then, under direct visualization of the endoscope, the FCES was delivered to the esophageal ulcer. Finally, the middle section of the FCES was fit into the esophageal defect by releasing the steel lasso loop. Furthermore, to prevent stent migration, five or more endoscopic clips (Rocc-D-26-195; Micro-Tech, Nanjing, China) were applied to fix the stent to the esophageal wall (Figure 1F).
Postoperative care and follow-up
All animals were administered intramuscular injections of 80 mg/day gentamicin (Baiyunshan Tianxin Pharmaceutical, Ltd., Guangzhou, China) for 3 days postoperatively. They were all fasted for 1 day and fed a liquid diet for 5 days until they were able to consume a semisolid diet. Prior to euthanasia, all of them underwent a repeat endoscopy each week to define the position of the stent. If stent migration occurred, endoscopic forceps were used to place the stent in the original position, and then, the stent was fixed with clips. The total number of clips used before killing was recorded.
General evaluation
Weight loss was recorded from the body weights measured before endoscopic resection and those before euthanasia. Comparisons were performed between the three groups. Dysphagia was scored by using a 5-point scale before euthanasia: 4 = complete dysphagia, 3 = able to swallow liquids only, 2 = able to swallow a semisolid diet, 1 = able to swallow some solid food, 0 = normal swallowing (able to eat a normal diet).[12]
Macroscopic assessment
The entire esophagus was removed and dissected longitudinally after the animals were euthanized. To evaluate the survival rate of skin grafts, photographs of the entire esophagus taken in each group were analyzed by ImageJ software (National Institutes of Health, USA). The rate of skin graft survival was calculated by the formula (A0-A1) /A0 × 100%, where A0 and A1 were the total wound area and surviving skin graft area, respectively.[13] The degree of stenosis at the artificial ulcer was expressed as the lateral mucosal contraction rate and was calculated using the following formula {1-[Length of the short at site of maximal contraction/ (Length of short axis at a normal mucosal site on upper side + Length of short axis at a normal mucosal site on lower side] × ½) } × 100%.[14]
Histological analysis of reconstructed tissues
The esophageal specimens were fixed in 10% formalin for at least 48 h and then dissected routinely to prepare paraffin blocks. Three micrometer-thick sections were used to perform hematoxylin and eosin (H & E) staining with conventional methods. In each sample, 12 locations (× 200 magnification) with regular intervals were randomly viewed by two senior histologists who were blinded to the origin of the sections, and then the total number of inflammatory cells was recorded and compared between the three groups.
Evaluation of angiogenesis
Cluster of differentiation 34 (CD34; GeneTex, USA) and VEGF (Abcam, Cambridge, UK) were detected by immunohistochemistry (IHC). The sections were deparaffinized in xylene 3 times and rehydrated in a graded series of ethanol with decreasing concentrations. We used Tris-buffered saline (TBS; pH 7.4) for rinsing. Then, the cells were blocked with 5% serum for 30 min before incubation with porcine primary antibodies against CD34 and VEGF at 4°C overnight. Tissue slices were further incubated in secondary antibody at 25°C for 50 min, visualized with diaminobenzidine and counterstained with hematoxylin. Twelve random fields (× 200 magnification) of each section were captured by optical microscopy. The integrated optical density (IOD) of VEGF was detected using ImageJ software.[15] The microvessel density (MVD) was assessed by two histologists counting the number of positive vessels directly from CD34-stained images.
Evaluation of submucosal tissue fibrogenesis
Expression of alpha smooth muscle actin (α-SMA; Novus Biologicals, USA) was detected by IHC. The images were taken at 200× magnification with 12 regular intervals and then used to measure the IOD. Masson’s trichrome staining was used to measure the thickness of submucosal tissue, detect the percent of collagen content and grade the damage of fibrosis. Submucosal tissue thickness was defined as the distance between the basal cells of the epithelium and the esophageal muscular layer and was calculated from 12 regular intervals in each sample. The collagen volume fraction was identified as the ratio of collagen to the remolded tissue. Images of Masson’s trichrome staining (× 200) were taken by optical microscopy, and the collagen content was semiquantitatively evaluated by analyzing the IOD via ImageJ software.[16] The damage of fibrosis was graded using the following scale: 3 = transmural fibrosis of the muscularis propria, 2 = atrophy or fibrosis present but confined to the outer longitudinal muscle layer, 1 = atrophy or fibrosis present but confined to the inner circular muscle layer, and 0 = no atrophic or fibrotic change in the muscularis propria evident in any of the examined sections.[17] Collagen I (COL-1) and collagen III (COL-3) fibers were shown by picrosirius red connective tissue staining. Under polarized light, COL-1 was identified by its thick fibers and compact and dense fibrils, which were yellow to light red colored. COL-3 formed green fine fibers with thin fibrils.[18] These sections were visualized using a microscope digital camera, and the ratio of COL-1/ COL-3 was detected using Image-Pro Plus software (Media Cybernetics, USA).
Statistical analysis
Results are described as the means ± standard deviations or median. For comparison of quantitative variables between the different groups, ANOVA with repeated measures was performed. Nonnormal quantitative variables were compared using the Kruskal-Wallis test or chi-square tests. A P value < 0.05 was considered significant. All statistical analyses were performed using SPSS for Windows 26.0 (IBMCorp, Armonk, NY).
Results
Macroscopic appearance and clinical evaluation
Circumferential ESTD was performed safely in each group. Endoscopic observation after stent removal (Figure 2A) and macroscopic appearance (Figure 2B) of the reconstructed esophagus on Day 28 showed the presence of the artificial ulcer and confirmed that the majority of the grafted skin survived. The average survival rate of the grafted skins was significantly higher in the PRP-ASG group than in the ASG group on Days 7, 14, and 28 (98.6%, 94.8%, 92.2% vs. 72.1%, 60.6%, 55.1%, P < 0.01) (Figure 2C). The mucosal contraction rates on Days 7, 14, and 28 were 2.37 ± 0.47%, 5.23 ± 0.47% and 7.83 ± 0.51% in the control group, 2.03 ± 0.63%, 3.00 ± 0.36% and 4.43 ± 0.25% in the ASG group, and 1.70 ± 0.27%, 1.87 ± 0.21% and 2.13 ± 1.10% in the PRP-ASG group, respectively. The PRP-ASG group had a significantly lower mucosal contraction rate on Day 28 than the other two groups (P = 0.013), with no difference on Days 7 and 14 (Figure 2D).
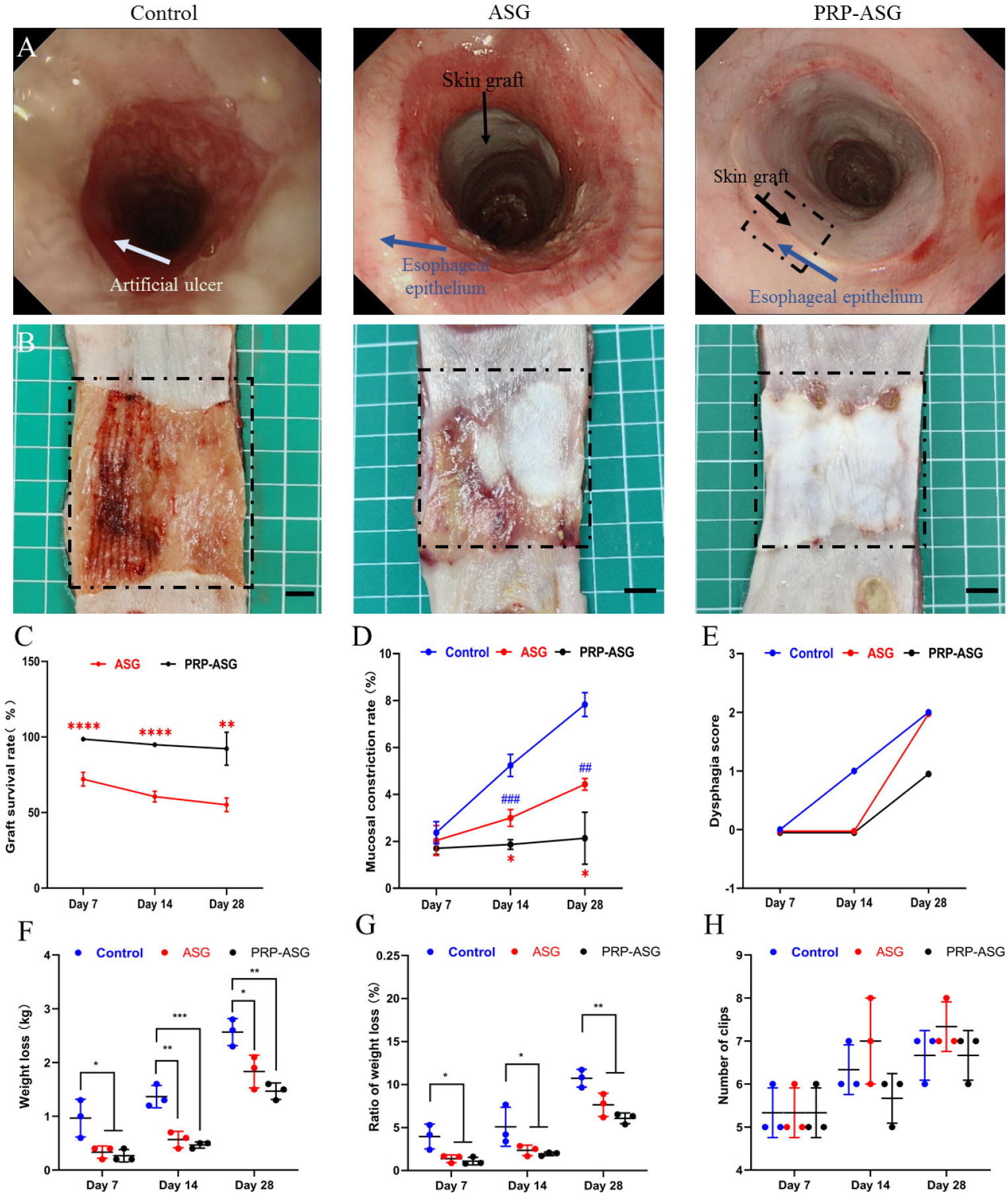
Endoscopic observation and macroscopic appearance of the remolded esophagus on day 28. (A) Typical macroscopic appearance of the esophageal lumen in each group. (B) Endoscopic observation of the esophageal lumen in each group. Statistical analysis of (C) skin graft survival rate, (D) mucosal defect rate, (E) dysphagia score, (F) weight loss, (G) ratio of weight loss, (H) number of endoscopic clips used. ##P < 0.01, ###P < 0.001, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. PRP: plasma-rich platelet; ASG: autologous skin graft.
Although slight swallowing difficulties occurred in each group on Day 28, no pigs developed severe stricture, and the dysphagia score was comparable between the different groups (Figure 2E). Weight loss was significantly higher in the control group than in the ASG group and the PRP-ASG group on Days 7, 14, and 28 (0.97 ± 0.35 kg, 1.37 ± 0.21 kg, 2.57 ± 0.25 kg vs. 0.33 ± 0.12 kg, 0.57 ± 0.15 kg, 1.83 ± 0.31 kg vs. 0.27 ± 0.12 kg, 0.47 ± 0.06 kg, 1.47 ± 0.15 kg, P < 0.05) (Figure 2F). The ratio of weight loss was in agreement with the weight loss (Figure 2G). There was no significant difference in the number of endoscopic clips used among the three groups (Figure 2H).
Histological findings
Histological analysis of recombinant mucosa showed that a stratified and mature epithelium closely resembled the surface of the esophagus, indicating the good growth and histocompatibility of the skin graft (Figure 3A). The inflammatory cell count demonstrated that the PRP-ASG group had a considerably lower accumulation of inflammatory cells than the other two groups on Days 7, 14, and 28 (Figure 3B). As shown in Figure 3C, massive inflammatory cell infiltration was observed in the remaining submucosal layer in the control group. The number of inflammatory cells gradually decreased with time, and leukocyte infiltration was always severe in the control group, moderate in the ASG group, and mild in the PRP-ASG group.
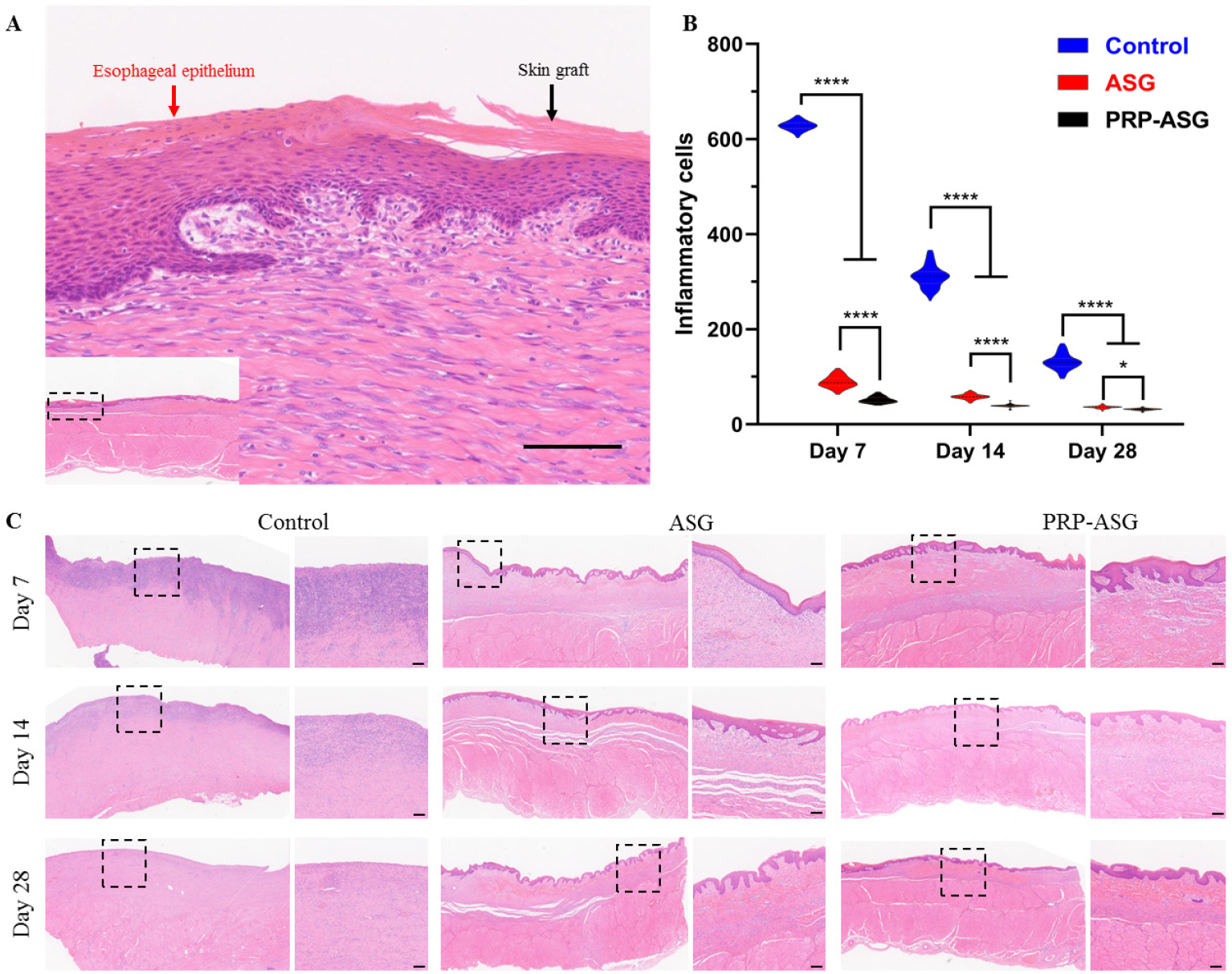
Histopathological finding of the central artificial ulcer sites. (A) Magnified image of the recombinant mucosa. (B) The count of inflammatory cells. *P < 0.05, ****P < 0.0001. (C) Representative images of the central endoscopic resection sites. Scale bar = 100 μm.
Angiogenesis evaluation
As shown in Figure 4, the number of CD34-positive microvessels and the proportion of VEGF-positive cells in the submucosal tissue on Days 7 and 14 were high in the PRP-ASG group, whereas the number of CD34-positive microvessels and the proportion of VEGF-positive cells in the ASG group and control group were relatively small. On Day 28, the MVD and expression level were similar to those of the ASG group but lower than those of the control group. Overall, the PRP-ASG group had stronger angiogenesis in the reconstructed tissue within 14 days.
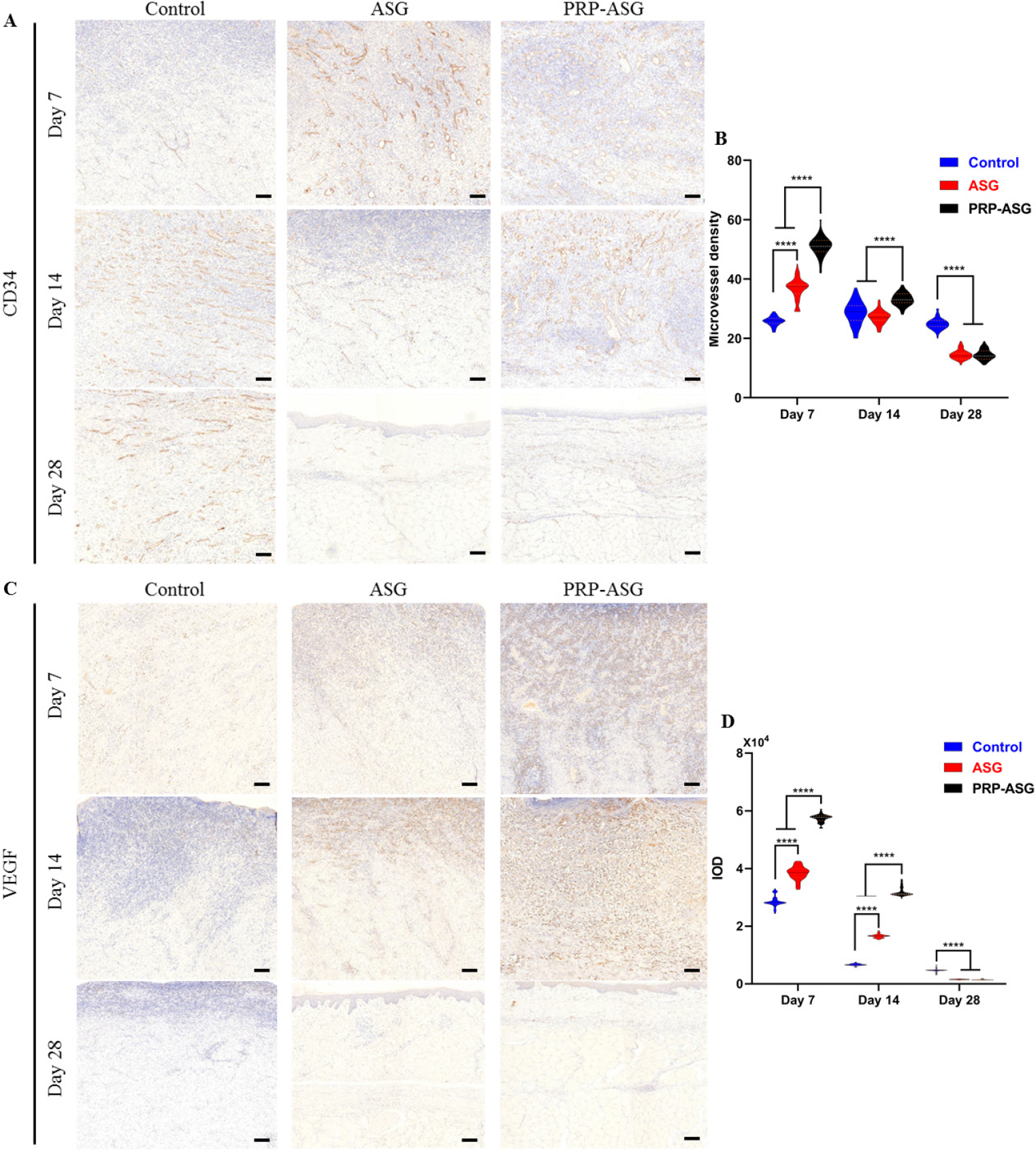
Angiogenesis-related immunohistochemistry staining and histological evaluation on day 7, 14, and 28. (A) CD34-stained sections of the three groups and (B) quantification of microvessel density. (C) VEGF-stained sections of the three groups and (D) quantification of IOD. ****P < 0.0001. Scale bar = 100 μm.
Fibrogenesis assessment
On Masson’s trichrome staining, the wound tissue maturity evaluated by the degree of collagen volume fraction demonstrated that submucosal tissue repair in the PRP-ASG group had a significant advantage compared with the other two groups within 28 days (Figure 5A and B). Picrosirius red staining demonstrated that the ratio of COL-1/COL-3 on Day 28 in the PRP-ASG group was significantly lower than that in the other two groups, and the ratio was in agreement with the ratio of the normal tissue (Figure 5C). The results revealed the presence of large amounts of COL-3 deposition and small amounts of COL-1 deposition in the PRP-ASG group (Figure 5D).
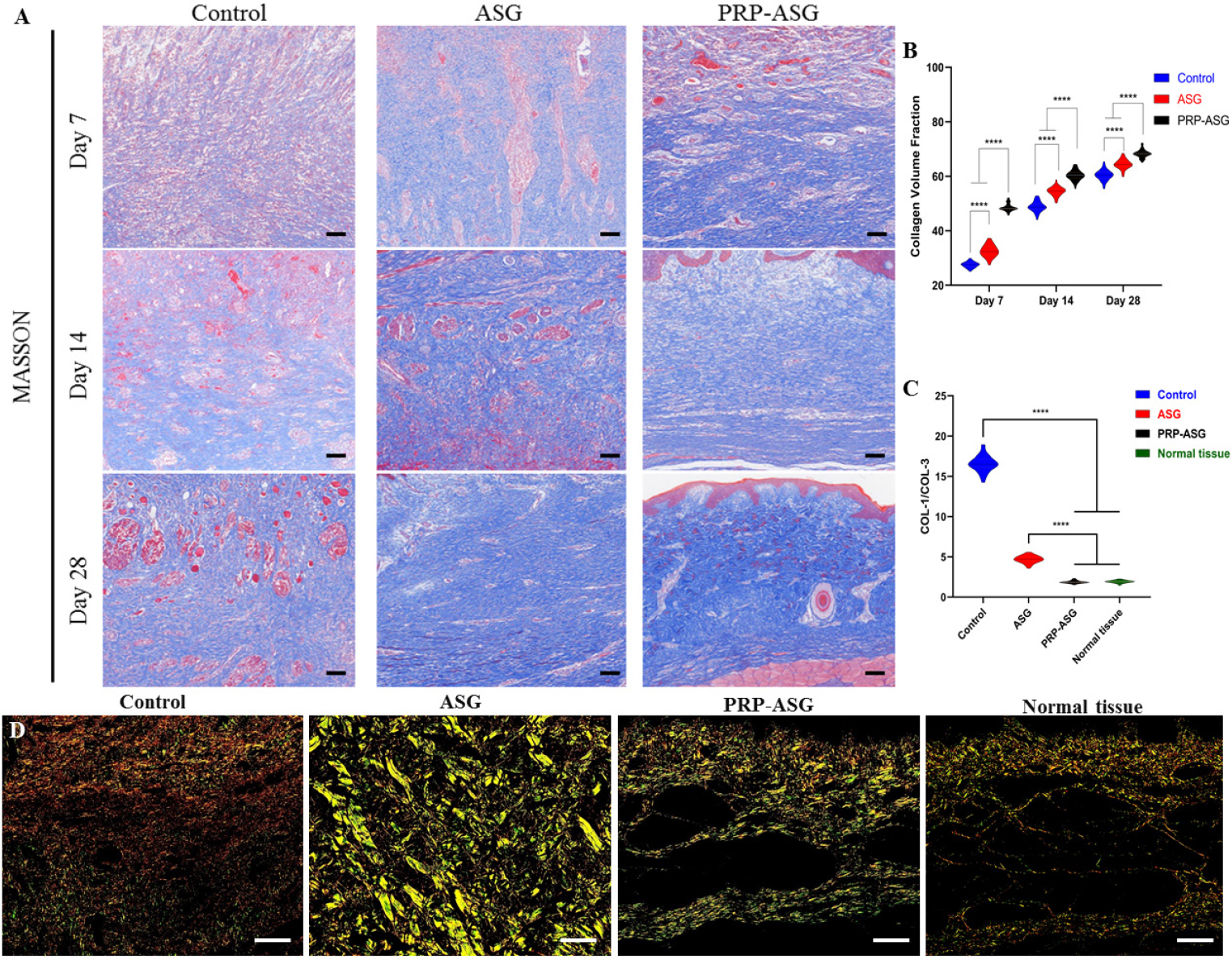
Measurement of collagen volume fraction and collagen birefringence under polarized light microscopy. (A) Representative images of Masson’s trichrome staining in each group and (B) quantification of collagen volume fraction. (C) Analysis of ratio of COL-1 to COL-3 and (D) different types of COL indicated by Picro-sirius red staining. Scale bar = 100 μm. ****P < 0.0001.
As shown in Figure 6A, the number and proportion of α-SMA-positive cells were low in the PRP-ASG group, moderate in the ASG group, and high in the control group. Statistical analysis of the IOD of α-SMA indicated that the cell ratio of α-SMA-positive cells to total spindle mesenchymal cells in the PRP-ASG group was significantly lower than that in the ASG group and control group (Figure 6B). At the muscle layer, fibrotic tissue invaded through the inner muscularis in the control group, whereas fibrosis was mild in the two transplanted groups. The damage score for the muscularis propria on Day 28 in the control group was significantly higher than that in the ASG group or PRP-ASG group (3 vs. 0, P < 0.05) (Figure 6C). Perpendicularly, the thickness of the submucosal tissue on Day 28 in the PRP-ASG group was significantly thinner than that in the ASG group and control group (1.24 ± 0.12 μm vs. 1.78 ± 0.11 μm vs. 1.96 ± 0.09 μm, P < 0.0001) (Figure 6D and E).
Discussion
The complication of postoperative stricture has a substantial influence on a patient’s life after endoscopic resection, and efforts have been made to prevent esophageal strictures in recent decades. The repair of postoperative esophageal ulcers involved fibrosis of the submucosa and atrophy of the muscularis, leading to esophageal ulcer shrinkage.[19,20] Therefore, strategies to prevent esophageal stricture require intervention aimed at protecting against mucosal deficiency, reducing inflammation, and preventing excessive fibrosis. The use of esophageal stents, balloon dilation, or steroid injection can partially obtain good results, but repeated interventions for a prolonged period are required to maintain the effect, which will affect the quality of postoperative life and cause an ongoing financial burden.[21] Autologous buccal keratinocytes and adipose tissue-derived stromal cells were injected into the residual submucosal layer in animal models and successfully decreased wound constriction.[22,23] Coverage of the ulcer surface was the main intention of these approaches, but it was difficult to complete full coverage. Other methods, such as acellular dermal matrix or thermoresponsive hydrogel, have been successfully applied in animal models but have not yet been in clinical use.[24,25]
PRP was confirmed to be effective in the healing of dermal collagen grafts on tendon-to-bone and lipofilling treatment for scleroderma patients.[26,27] Moreover, the application of PRP has been demonstrated to be useful in tissue reconstruction.[28] In 2018, we first reported that the endoscopic transplantation of autologous skin grafts was able to prevent esophageal stenosis.[29] Autologous graft skins do survive on the esophageal ulcer postoperatively, and we obtained a good result in clinical research. However, the positive effect of stricture prevention presupposes that the skin graft is alive. In the current study, we improved our strategy by adding autologous PRP and obtained a better outcome in terms of the survival rate of skin grafts. Furthermore, we confirmed that our modified transplantation of skin grafts demonstrated significant efficacy in suppressing inflammation and fibrosis and improving angiogenesis in a porcine model. These findings could be explained by the high capacity of growth factors in PRP, which favored the growth of vascularization and skin grafts. Simultaneously, inhibition of fibrotic formation and inflammatory infiltration toward the muscular layer in esophageal ulcers prevents injury to the muscularis propria, which is significant for tissue repair and reconstruction.
The fibrotic constriction of the postoperative esophageal defect site results in esophageal stenosis. The dysphagia score and mucosal contraction rate are the main indices concerning the prevention effect of strictures,[30] and a high dysphagia score indicates the presence of esophageal strictures. In this study, we showed that the degree of dysphagia was low in each group. The mucosal contraction rate, a more objective and practical index for evaluating esophageal stricture, reflects the actual degree of stenosis in the ulcer. Previous studies using fabricated autologous epidermal cell sheets or allogeneic epithelial cell sheets to prevent esophageal strictures showed contraction rates of 56% and 53.8%, respectively, which were higher than our modified skin graft transplantation.[31,32] On Day 28, the mucosal contraction rate that occurred in the PRP-ASG group was 2.13%, which was negligibly low. Furthermore, due to rare inflammatory cell infiltration and stagnant interstitial fibrosis on Day 28, the esophageal lumen will not contract centripetally even if the stent is removed. Thus, an esophagus with almost no stenosis was reconstructed by our modified strategy.
In a previous study, lesion size and circumferential range were both identified as independent risk factors for esophageal strictures.[33] Stenosis rates after circumferential and noncircumferential resection were reportedly 100% and 56% to 76%, respectively.[34,35] However, the majority of studies have targeted a mucosal defect either semi circumferential in range or short in length,[36] and the effectiveness of their methods might be reduced in patients with extensive lesions. For the stricture prevention strategy of our modified skin graft transplantation, the extensive ulcer did not limit its use. Skin graft and PRP were both harvested from the animals themselves, which were the same as autologous epidermal cell sheets by tissue engineering. They all avoid the rejection associated with allogeneic transplantation. However, for clinical application, regenerative medicine has several limitations, including long required culture time, heavy costs, and strict management by a well-manufacturing practice facility.[37] Within the routine period of the endoscopic procedure, PRP and ASG can be prepared by a technical operator and plastic surgeon, respectively. In addition, the required equipment is commonly used at low cost. Further application of PRP in patients with SENs will soon be carried out. Furthermore, as it does in the field of burns, allogeneic transplantation of porcine skin post-ESTD shows promising prospects due to its great similarity to human skin in dermal architecture and the absence of a panniculus carnosus. With the problem of rejection solved, its application to prevent esophageal strictures will be available.
In conclusion, we showed the effectiveness of a modified skin graft transplantation for preventing esophageal strictures after complete circumferential resection in a porcine model. By adding the endoscopic injection of PRP to the transplantation of ASG surgery, the skin graft survival rate increased, and fibrogenesis of the submucosal remolded tissue decreased. Ensuring patency of the esophageal lumen with minimal mucosal constriction, this novel strategy is promising for clinical applications in preventing postoperative esophageal strictures. However, further research is still necessary to clarify the effectiveness in patients.
Funding statement: This work was supported by a grant from the National Natural Science Foundation of China (No. 82070682) and Beijing Municipal Science & Technology Commission (No. Z181100001718177).
Acknowledgement
The authors thank Dr. Bozhao Li for editing the draft of this manuscript. The authors also thank Miss. Yunxiao Jia, Mr. Bo Peng, Mr. Changqi Zhao, and Miss. Zhaobei Cai for their technical assistance and Dr. Ke Han for the help of statistical analysis.
-
Author Contributions
Xu N and Li LS performed the literature search, obtained ethics approval, led the surgery, and contributed to the protocol, surgical planning, data collection, and drafting of the manuscript; Zou JL conceived the research question; Yue WY contributed to data collection and reviewed the manuscript; Wang PJ, Chai M and, Li L led the surgery; Zhang LH contributed to data collection, particularly the histological examination and transmission electron microscopy; Li X, Cheng YX, Wang ZX, Wang XT, Wang RZ, and Xiang JY contributed to data collection and assisted in surgery; Linghu EQ was the study supervisor and reviewed the manuscript.
-
Ethical Approval
All experimental protocols were approved by the Animal Care and Use Committee of The First Medical Center of PLA General Hospital (2021-X17-11).
-
Informed Consent
Not applicable.
-
Conflict of Interest
The authors declare that there is no conflict of interest.
-
Data Availability Statement
No additional is available.
References
1 Gan T, Yang JL, Zhu LL, Wang YP, Yang L, Wu JC. Endoscopic submucosal multi-tunnel dissection for circumferential superficial esophageal neoplastic lesions (with videos). Gastrointest Endosc 2016;84:143–146.10.1016/j.gie.2016.01.049Search in Google Scholar PubMed
2 Zhai YQ, Li HK, Linghu EQ. Endoscopic submucosal tunnel dissection for large superficial esophageal squamous cell neoplasms. World J Gastroenterol 2016;22:435–445.10.3748/wjg.v22.i1.435Search in Google Scholar PubMed PubMed Central
3 Siersema PD. How to Approach a Patient With Refractory or Recurrent Benign Esophageal Stricture. Gastroenterology 2019;156:7–10.10.1053/j.gastro.2018.11.040Search in Google Scholar PubMed
4 Adler DG, Siddiqui AA. Endoscopic management of esophageal strictures. Gastrointest Endosc 2017;86:35–43.10.1016/j.gie.2017.03.004Search in Google Scholar PubMed
5 Han Y, Guo J, Sun S, Wu W, Wang S, Ge N, et al. Acellular dermal matrix for esophageal stricture prevention after endoscopic submucosal dissection in a porcine model. Gastrointest Endosc 2017;86:1160–1167.10.1016/j.gie.2017.02.038Search in Google Scholar PubMed
6 Ohki T, Yamato M, Murakami D, Takagi R, Yang J, Namiki H, et al. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 2006;55:1704–1710.10.1136/gut.2005.088518Search in Google Scholar PubMed PubMed Central
7 Chai N, Zou J, Linghu E, Chai M, Li L, Wang X, et al. Autologous Skin-Grafting Surgery to Prevent Esophageal Stenosis After Complete Circular Endoscopic Submucosal Tunnel Dissection for Superficial Esophageal Neoplasms. Am J Gastroenterol 2019;114:822–825.10.14309/ajg.0000000000000169Search in Google Scholar PubMed
8 Zou J, Chai N, Linghu E, Li H, Chai M, Shi Y, et al. Autologous skin-grafting surgery to prevent esophageal stenosis after complete circular endoscopic submucosal tunnel dissection: a case-matched controlled study. Surg Endosc 2021;35:5962–5970.10.1007/s00464-020-08081-7Search in Google Scholar PubMed
9 Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol 2009;27:158–167.10.1016/j.tibtech.2008.11.009Search in Google Scholar PubMed
10 Li W, Enomoto M, Ukegawa M, Hirai T, Sotome S, Wakabayashi Y, et al. Subcutaneous injections of platelet-rich plasma into skin flaps modulate proangiogenic gene expression and improve survival rates. Plast Reconstr Surg 2012;129:858–866.10.1097/PRS.0b013e3182450ac9Search in Google Scholar PubMed
11 Aydin O, Karaca G, Pehlivanli F, Altunkaya C, Uzun H, Özden H, et al. Platelet-Rich Plasma May Offer a New Hope in Suppressed Wound Healing When Compared to Mesenchymal Stem Cells. J Clin Med 2018;7:143.10.3390/jcm7060143Search in Google Scholar PubMed PubMed Central
12 Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction. Analysis of technical and functional efficacy. Arch Intern Med 1985;145:1443–1446.10.1001/archinte.145.8.1443Search in Google Scholar
13 Takabayashi Y, Ishihara M, Kuwabara M, Takikawa M, Nakamura S, Hattori H, et al. Improved Survival of Full-Thickness Skin Graft With Low-Molecular Weight Heparin-Protamine Micro/Nanoparticles Including Platelet-Rich Plasma. Ann Plast Surg 2017;78:562–568.10.1097/SAP.0000000000001051Search in Google Scholar PubMed
14 Tang J, Ye S, Ji X, Liu F, Li Z. Deployment of carboxymethyl cellulose sheets to prevent esophageal stricture after full circumferential endoscopic submucosal dissection: A porcine model. Dig Endosc 2018;30:608–615.10.1111/den.13070Search in Google Scholar PubMed
15 Li D, Sun WQ, Wang T, Gao Y, Wu J, Xie Z, et al. Evaluation of a novel tilapia-skin acellular dermis matrix rationally processed for enhanced wound healing. Mater Sci Eng C Mater Biol Appl 2021;127:112202.10.1016/j.msec.2021.112202Search in Google Scholar PubMed
16 Tejiram S, Zhang J, Travis TE, Carney BC, Alkhalil A, Moffatt LT, et al. Compression therapy affects collagen type balance in hypertrophic scar. J Surg Res 2016;201:299–305.10.1016/j.jss.2015.10.040Search in Google Scholar PubMed PubMed Central
17 Honda M, Hori Y, Nakada A, Uji M, Nishizawa Y, Yamamoto K, et al. Use of adipose tissue-derived stromal cells for prevention of esophageal stricture after circumferential EMR in a canine model. Gastrointest Endosc 2011;73:777–784.10.1016/j.gie.2010.11.008Search in Google Scholar PubMed
18 Cuttle L, Nataatmadja M, Fraser JF, Kempf M, Kimble RM, Hayes MT. Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen 2005;13:198–204.10.1111/j.1067-1927.2005.130211.xSearch in Google Scholar PubMed
19 Honda M, Nakamura T, Hori Y, Shionoya Y, Nakada A, Sato T, et al. Process of healing of mucosal defects in the esophagus after endoscopic mucosal resection: histological evaluation in a dog model. Endoscopy 2010;42:1092–1095.10.1055/s-0030-1255741Search in Google Scholar PubMed
20 Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21.10.1038/nature07039Search in Google Scholar PubMed
21 Tsuji Y, Sakaguchi Y, Fujishiro M, Koike K. Preventive measures against stricture after esophageal endoscopic submucosal dissection: Halfway through the journey to the best method. Dig Endosc 2018;30:600–601.10.1111/den.13191Search in Google Scholar PubMed
22 Maeda M, Kanai N, Kobayashi S, Hosoi T, Takagi R, Ohki T, et al. Endoscopic cell sheet transplantation device developed by using a 3-dimensional printer and its feasibility evaluation in a porcine model. Gastrointest Endosc 2015;82:147–152.10.1016/j.gie.2015.01.062Search in Google Scholar PubMed
23 Hochberger J, Koehler P, Wedi E, Gluer S, Rothstein RI, Niemann H, et al. Transplantation of mucosa from stomach to esophagus to prevent stricture after circumferential endoscopic submucosal dissection of early squamous cell. Gastroenterology 2014;146:906–909.10.1053/j.gastro.2014.01.063Search in Google Scholar PubMed
24 Zhang B, Zhang Y, Wang Y, Yang F, Sheng S, Wang Z, et al. Acellular Dermal Matrix Prevents Esophageal Stricture After Full Circumferential Endoscopic Submucosal Dissection in a Porcine Model. Front Bioeng Biotechnol 2022;10:884502.10.3389/fbioe.2022.884502Search in Google Scholar PubMed PubMed Central
25 Coffin E, Grangier A, Perrod G, Piffoux M, Marangon I, Boucenna I, et al. Extracellular vesicles from adipose stromal cells combined with a thermoresponsive hydrogel prevent esophageal stricture after extensive endoscopic submucosal dissection in a porcine model. Nanoscale 2021;13:14866–14878.10.1039/D1NR01240ASearch in Google Scholar PubMed
26 Virzì F, Bianca P, Giammona A, Apuzzo T, Di Franco S, Mangiapane LR, et al. Combined platelet-rich plasma and lipofilling treatment provides great improvement in facial skin-induced lesion regeneration for scleroderma patients. Stem Cell Res Ther 2017;8:236.10.1186/s13287-017-0690-3Search in Google Scholar PubMed PubMed Central
27 Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, et al. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA 2021;326:2021–2030.10.1001/jama.2021.19415Search in Google Scholar PubMed PubMed Central
28 Nicoli F, Balzani A, Lazzeri D, Gentile P, Chilgar RM, Di Pasquali C, et al. Severe hidradenitis suppurativa treatment using platelet-rich plasma gel and Hyalomatrix. Int Wound J 2015;12:338–343.10.1111/iwj.12117Search in Google Scholar PubMed PubMed Central
29 Chai N, Zhang W, Linghu E, Han Y, Chai M, Li Z, et al. Autologous Skin-Grafting Surgery for the Prevention of Esophageal Stenosis After Complete Circular Endoscopic Submucosal Tunnel Dissection. Am J Gastroenterol 2018;113:938.10.1038/s41395-018-0142-4Search in Google Scholar PubMed
30 Yamamoto Y, Kikuchi D, Nagami Y, Nonaka K, Tsuji Y, Fujimoto A, et al. Management of adverse events related to endoscopic resection of upper gastrointestinal neoplasms: Review of the literature and recommendations from experts. Dig Endosc 2019;31:4–20.10.1111/den.13388Search in Google Scholar PubMed
31 Na HK, Lee JH, Shim IK, Jung HY, Kim DH, Kim CJ. Allogeneic epithelial cell sheet transplantation for preventing esophageal stricture after circumferential ESD in a porcine model: preliminary results. Scand J Gastroenterol 2021;56:598–603.10.1080/00365521.2021.1897669Search in Google Scholar PubMed
32 Kanai N, Yamato M, Ohki T, Yamamoto M, Okano T. Fabricated autologous epidermal cell sheets for the prevention of esophageal stricture after circumferential ESD in a porcine model. Gastrointest Endosc 2012;76:873–881.10.1016/j.gie.2012.06.017Search in Google Scholar PubMed
33 Chen M, Dang Y, Ding C, Yang J, Si X, Zhang G. Lesion size and circumferential range identified as independent risk factors for esophageal stricture after endoscopic submucosal dissection. Surg Endosc 2020;34:4065–4071.10.1007/s00464-020-07368-zSearch in Google Scholar PubMed PubMed Central
34 Sato H, Inoue H, Kobayashi Y, Maselli R, Santi EG, Hayee B, et al. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc 2013;78:250–257.10.1016/j.gie.2013.01.008Search in Google Scholar PubMed
35 Ishihara R. Prevention of esophageal stricture after endoscopic resection. Dig Endosc 2019;31:134–145.10.1111/den.13296Search in Google Scholar PubMed
36 Shibagaki K, Ishimura N, Oshima N, Mishiro T, Fukuba N, Tamagawa Y, et al. Esophageal triamcinolone acetonide-filling method: a novel procedure to prevent stenosis after extensive esophageal endoscopic submucosal dissection (with videos). Gastrointest Endosc 2018;87:380–389.10.1016/j.gie.2017.08.016Search in Google Scholar PubMed
37 Ohki T, Yamato M, Ota M, Takagi R, Murakami D, Kondo M, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012;143:582–588.10.1053/j.gastro.2012.04.050Search in Google Scholar PubMed
© 2024 Ning Xu, Longsong Li, Jiale Zou, Wenyi Yue, Pengju Wang, Mi Chai, Li Li, Lihua Zhang, Xiao Li, Yaxuan Cheng, Zixin Wang, Xueting Wang, Runzi Wang, Jingyuan Xiang, Enqiang Linghu, Ningli Chai, published by De Gruyter on behalf of Scholar Media Publishing
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Guideline and Consensus
- Chinese expert consensus on blood lipid management in patients with diabetes (2024 edition)
- Review Article
- Role of viral hepatitis in pregnancy and its triggering mechanism
- Original Article
- Machine learning-based phenogroups and prediction model in patients with functional gastrointestinal disorders to reveal distinct disease subsets associated with gas production
- Recognition of differently expressed genes and DNA methylation markers in patients with Lupus nephritis
- PRP improves the outcomes of autologous skin graft transplantation on the esophagus by promoting angiogenesis and inhibiting fibrosis and inflammation
- Identification of functional heterogeneity of immune cells and tubular-immune cellular interplay action in diabetic kidney disease
- Actin-related protein 2/3 complex subunit 1B promotes ovarian cancer progression by regulating the AKT/PI3K/mTOR signaling pathway
- Comprehensive succinylome analyses reveal that hyperthermia upregulates lysine succinylation of annexin A2 by downregulating sirtuin7 in human keratinocytes
- Inhibition of heterogeneous nuclear ribonucleoproteins A1 and oxidative stress reduces glycolysis via pyruvate kinase M2 in chronic thromboembolic pulmonary hypertension
Articles in the same Issue
- Guideline and Consensus
- Chinese expert consensus on blood lipid management in patients with diabetes (2024 edition)
- Review Article
- Role of viral hepatitis in pregnancy and its triggering mechanism
- Original Article
- Machine learning-based phenogroups and prediction model in patients with functional gastrointestinal disorders to reveal distinct disease subsets associated with gas production
- Recognition of differently expressed genes and DNA methylation markers in patients with Lupus nephritis
- PRP improves the outcomes of autologous skin graft transplantation on the esophagus by promoting angiogenesis and inhibiting fibrosis and inflammation
- Identification of functional heterogeneity of immune cells and tubular-immune cellular interplay action in diabetic kidney disease
- Actin-related protein 2/3 complex subunit 1B promotes ovarian cancer progression by regulating the AKT/PI3K/mTOR signaling pathway
- Comprehensive succinylome analyses reveal that hyperthermia upregulates lysine succinylation of annexin A2 by downregulating sirtuin7 in human keratinocytes
- Inhibition of heterogeneous nuclear ribonucleoproteins A1 and oxidative stress reduces glycolysis via pyruvate kinase M2 in chronic thromboembolic pulmonary hypertension

