Abstract
Objective
Nanoparticles (NPs) in haze are potentially hazardous to health, which is more severe in the winter. Brown adipose tissue (BAT) plays important roles in obesity, insulin resistance, and diabetes. Though the toxicology of NPs has been intensively studied, few studies have been reported on the antagonistic effects between Silicon dioxide(SiO2) NPs and cold exposure in brown adipocytes.
Materials and methods
We evaluated changes by quantitative real-time reverse-transcriptase polymerase chain reaction (qRT-PCR) on metabolism genes, plasticity genes and the inflammatory responses genes in brown adipocytes in vitro.
Results
The expression of adipogenic genes PRDM16, Dio2, PGC-1α and UCP1 was upregulated upon cold exposure (P < 0.05), but downregulated by SiO2 NPs (P < 0.05). The results demonstrated that there was antagonistic effect between SiO2 NPs and cold exposure on the plasticity genes and metabolism genes in brown adipocytes, where the main effects of SiO2 NPs or cold exposure on the plasticity genes and metabolism genes were significant (P < 0.05). Moreover, the levels of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α were upregulated by SiO2 NPs or cold exposure (P < 0.05). The factorial analysis indicated that there was also antagonistic effect between SiO2 NPs and cold exposure on the toxic effects in brown adipocytes, in which the main effects of cold exposure and/or SiO2 NPs on the toxic effects were significant (P < 0.05).
Conclusion
SiO2 NPs inhibit the effect of cold exposure on metabolic genes and inflammatory responses genes in brown adipocytes.
1 Introduction
There is a widespread concern on the problem of haze in recent years, owing to its major impact on public health. Ultrafine particulate matter (PM0.1), which is equivalent to nanoparticles (NPs), has been found more harmful to human health[1]. NPs have been widely used in industry, biomedicine, and commercial applications, which raises a concern about their safety and potential threats to the environment and human health[2-3]. On an equal-mass basis, NPs induce stronger toxic responses than do larger-sized particles of the same chemical composition[4]. Inhaled NPs can induce systemic and local tissue toxicological reactions, such as oxidative stress and inflammation responses, enhance blood coagulation, impair cellular defense and immune function, by different pathways including translocation into the blood circulation thereby to other organs as well[5-7]. In a word, NPs exposure can cause unexpected remote adverse effects by direct or indirect mechanisms[8].
Silicon NPs require a detailed understanding on toxicological effects at the nanoscale level because of their widely use in industry and daily life[9]. Particulate matter, which is a serious air pollution problem especially in winter, is present in the air year-round[10]. The composition of particulate matter, where silicon accounts for a significant proportion, is complicated and associated with the risk of PM2.5–caused mortality[11]. Silicon dioxide (SiO2), one of the main components of haze, is an important member of the silicon family. SiO2 NPs (silica) can be transported into the bloodstream after the translocation across biological barriers and become deposited in target organs where they can exert potential toxic effects[12]. Changes of ambient temperature are the most common survival challenge that all endothermic organisms are facing[13]. Though the toxic effects of SiO2 NPs have been intensively studied, investigation on the combined toxic effects of SiO2 NPs and cold exposure has been sparse. In our previous work, rats co-treated with SiO2 NPs and cold exposure produced strong systemic inflammatory responses[14].
Adipose tissues, including white adipose tissue (WAT) and brown adipose tissue (BAT), play important roles in obesity, insulin resistance, and diabetes. The overexpansion of WAT causes obesity, and BAT uncouples oxidation of fatty acids from ATP production. The plasticity of WAT has been demonstrated in cold exposure[15]. There has been a global resurgence of research interest in BAT since its presence and functionality in humans was confirmed. It is known that the hazard of NPs to human health is more serious in wintertime because of increasing air pollution. However, the combined effects of NPs and cold exposure at the cellular level remained unclear. Our previous studies suggest that SiO2 NPs in the air inhibit the effect of cold-induced WAT/BAT changes in plasticity and metabolism[16-17]. The present study aimed to evaluate the combined effects of SiO2 NPs and cold exposure at the cellular level in brown adipocytes.
The genes, such as UCP1, PRDM16, PGC1α and Dio2, are metabolic markers of BAT. UCP1 is a mitochondrial protein enriched in brown adipocytes. The extraordinary thermogenic potential of brown adipocytes is conferred by UCP1. Once activated, UCP1 uncouples the mitochondrial oxidation of fatty acids from adenosine triphosphate synthesis, thereby initiating heat production[18-19]. PRDM16, a major transcription factor in BAT adipogenesis, plays a key role in triggering brown adipocyte differentiation, mitochondrial biogenesis and expression of UCP1[20-21]. Similar to PRDM16, PGC-1α is a peroxisome proliferator-activated receptor γ (PPARγ) transcriptional co-activator that confers the brown adipocyte with its energy-burning phenotype. It is directly involved in the stimulation of UCP1 expression through its ability to co-activate PPARγ[22-23]. Dio2 is expressed by brown adipocytes in order to make triiodothyronine available to sustain the elevated metabolism of BAT[24-25]. Expression analysis of inflammatory markers in the adipose tissue has implicated macrophages as the primary source of proinflammatory mediators such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β in adipose tissue[26-27]. Chronic inflammation, specifically mediated by macrophage activation, plays a crucial role in the development of obesity-related insulin resistance[28]. Proinflammatory cytokines induce insulin resistance. TNF-α has been shown to alter adipogenesis[29]. Adiponectin (ADPN), the most abundant peptide secreted by adipocytes, enhances insulin sensitivity. Leptin, another important adipocyte-derived peptide hormone plays an important role in the regulation of food intake and thermogenesis[30]. In the present work, the expression of these genes was detected. The results showed that in brown adipocytes, SiO2 NPs have antagonistic effect with cold exposure on the metabolism genes and plasticity genes, in which the main effects of SiO2 NPs or cold exposure on the metabolism genes and plasticity genes were significant (P < 0.05). Moreover, there was also antagonistic effect between SiO2 NPs and cold exposure on the toxic effects in brown adipocytes, where the main effects of SiO2 NPs and/or cold exposure on the toxic effects were significant (P < 0.05).
2 Materials and methods
2.1 Sample preparation
SiO2 NPs (shape, crystal structure; size, 20.2 ± 6.4 nm; composition, SiO2 > 99.0%), purchased from Shanghai Run He Nano Co. Ltd. (Shanghai, China), were prepared in 2 mg/mL phosphate buffered saline (PBS), followed by ultrasonication at < 50°C for 8 h. Using transmission electron microscopy (JEM-100CX II, JEOL Co., Tokyo, Japan), the shape and size of the SiO2 NPs were measured (Fig. 1). After sonication, aliquots of the stock suspensions were immediately serially diluted in cell culture medium to obtain the desired test concentrations. The zeta (ζ) potential of SiO2 NPs (-11.76 ± 0.58 mV) was analyzed by dynamic light scattering (Malvern Zeta sizer Nano ZS, Worcestershire, UK).
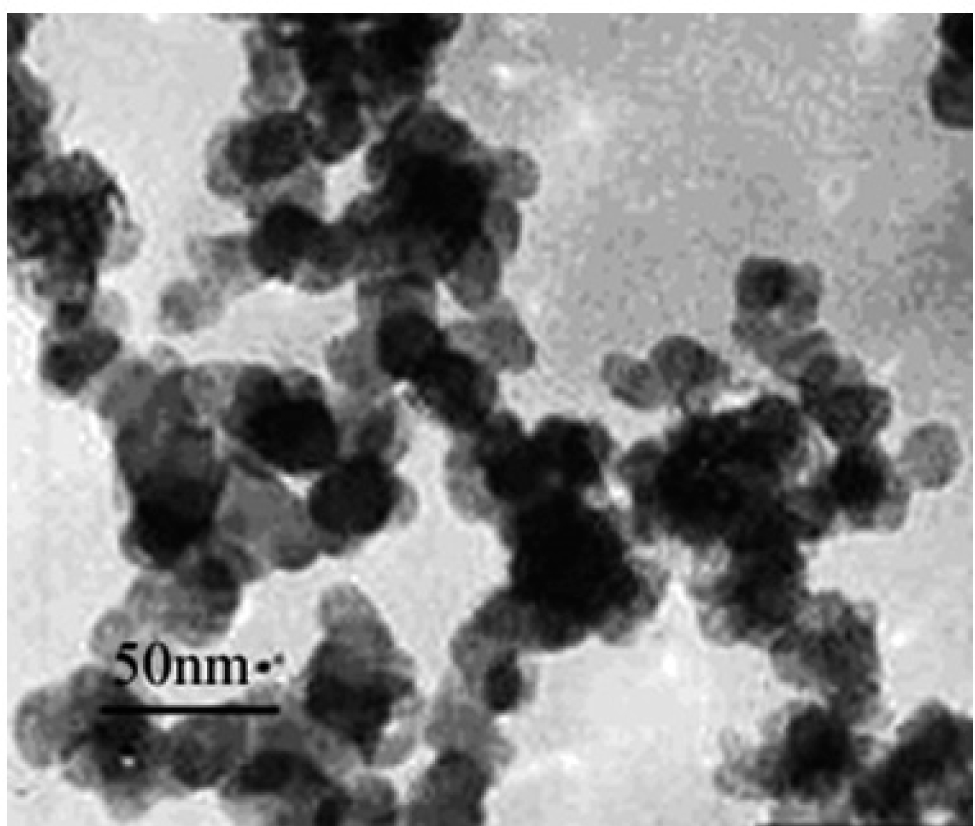
Image of SiO2 NPs by transmission electron microscopy
2.2 Cell isolation and culture
Brown adipocytes were isolated from the BAT near scapular area of 1-2-day-old Sprague-Dawley (SD) newborn rats. The parental SD female and male rats were housed in stress free, clean and animal friendly conditions in a standard environment (relative humidity 60% ± 10%, room temperature 21 ± 2°C) with a 12 h dark/ light cycle and 10-15 air changes per hour. Food and water were freely accessible. All newborn rats lived with their parental female rats until they were used for experiment. The tissue was cut into 1 mm × 1 mm pieces and digested using 2 mg/mL collagenase and 20 g/L bovine serum albumin (BSA) in Dulbecco’s modified eagle medium (DMEM) in a shaking water bath at 37°C for 1 h. After digestion, the digested tissue was filtered through a 100-μm nylon screen, and cells were centrifuged at 300 × g for 5 min. The pellet consisting of precursor cells was washed once in isolation buffer and centrifuged again. Cells were resuspended in 2 mL of culture medium (DMEM containing 25 mmol/L glucose, 20 mmol/L HEPES, 20% FBS, 100 units/mL penicillin/streptomycin), seeded on 35 mm plates, and grown in a humidified atmosphere of 5% CO2 and 95% air with the medium changed every day. Preadipocytes were grown to confluence for differentiation in culture medium supplemented with 20 nmol/L insulin and 1 nmol/L T3 (differentiation medium). Confluent cells were incubated in differentiation medium further supplemented with 0.125 mmol/L indomethacin, 0.5 μmol/L dexamethasone, and 0.5 mmol/L isobutylmethylxanthine (induction medium) for 24 h. Subsequently, the cells were maintained in differentiation medium for 4-5 days until a fully differentiated phenotype with massive accumulation of multilocular fat droplets had emerged.All experiments involving the care and use of animals were performed in accordance with the guidelines and regulations concerning the ethics of science research in the Institute of Environmental and Operational Medicine and approved by the Ethics Review Board of Institute of Environmental and Operational Medicine (AMMS-04-2020-017). The animals, which were provided by the Laboratory Animal Center of Institute of Environmental and Operational Medicine, were treated humanely and with regard for alleviation of suffering.
2.3 Oil red O staining
After washed twice with PBS, the culture dishes were fixed with 10% buffered formalin at room temperature for at least 1 h. Cells were then stained at room temperature for 2 h with the filtered oil red O solution (0.5 g of oil red O in 100 mL of isopropyl alcohol), washed twice with water, and visualized.
2.4 Quantitative real-time reverse-transcriptase polymerase chain reaction (qRT-PCR)
To quantify the relative mRNA levels of the genes of interest in brown adipocyte, qRT-PCR was performed using primers from SYBR GREEN Gene Expression Assays and the 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Relative expression levels were established using the comparative threshold cycle (CT) method outlined in the manufacturer’s instructions. All expression levels were normalized to the expression level of housekeeping gene (β-actin), and reported as a relative fold change over the control. Relative mRNA expression was determined by the ΔΔ-Ct method using the expression level of the gene encoding the TATA-binding protein levels as the endogenous control. The brown adipocytes for qRT-PCR were cultured at 37°C and at 31°C with the concentrations of SiO2 NPs 0 and 100 μg/mL, respectively[31]. The brown adipocytes were divided into four groups, such as the control group(37°C, SiO2 NPs 0 μg/mL), the SiO2 NPs group(37°C, SiO2 NPs 100 μg/ mL), the cold exposure group(31°C, SiO2 NPs 0 μg/mL) and the combined group(31°C, SiO2 NPs 100 μg/mL). The primer pairs were as follows:
UCP1, 5’-TGTGCAATGACCATGTACACCAA-3’ (forward) and 5’-GCACACAAACATGATGACGTTCC-3’ (reverse);
PGC-1α, 5’-CACCGTAAATCTGCGGGATG-3’ (forward) and 5’-TATCCATTCTCAAGAGCAGCGAAAG-3’ (reverse);
Dio2, 5’-CTGTGGTTGGATGTAGTCACACGA-3’ (forward) and 5’-CTTTGCACCAGGACCCAAATG-3’ (reverse);
PRDM16, 5’-CAAAGGGAAGCCAGCAGAG-3’ (forward) and 5’-GAGGCGGGAAGAAGGAATG-3’ (reverse);
TNF-α, 5’-TTCCAATGGGCTTTCGGAAC-3’ (forward) and 5’-AGACATCTTCAGCAGCCTTGTGAG-3’ (reverse);
IL-1β, 5’-CCCTGAACTCAACTGTGAAATAGCA-3’ (forward) and 5’-CCCAAGTCAAGGGCTTGGAA-3’ (reverse);
IL-6, 5’-ATTGTATGAACAGCGATGATGCAC-3’ (forward) and 5’-CCAGGTAGAAACGGAACTCCAGA-3’ (reverse);
ADPN, 5’-CAAGGCCGTTCTCTTCACCT-3’ (forward) and 5’-CCCCATACACTTGGAGCCAG-3’ (reverse);
Leptin, 5’-TGACAAACAGGTTACAGGACCAGA-3’ (forward) and 5’-ACGAAACCCGATCCAGTTCA-3’ (reverse);
β-actin, 5’-CCTAAGGCCAACCGTGAAAA-3’ (forward) and 5’-CAGAGGCATACAGGGACAACAC-3’ (reverse).
2.5 Statistical analyses
All quantitative variables are expressed as the mean ± standard error of mean (SEM). Statistical analyses were conducted using the SPSS software (IBM SPSS Statistics version 21.0, IBM Co., New York, USA). Analysis of variance (ANOVA) was used to establish the statistical significance of differences between the experimental groups and control. One-way ANOVA with Bonferroni’s correction was used for multiple comparisons. UNIANOVA was used to analyze the main effect and the interaction between SiO2 NPs and cold exposure. P values < 0.05 were considered significant and < 0.01 highly significant.
3 Results
3.1 SiO2 NPs antagonize the effect of cold exposure on the metabolism genes and plasticity genes in brown adipocytes
The brown adipocytes, stained with oil red O (Fig. 2), verified that the isolation, differentiation, and culture from BAT were successful. With SiO2 NPs treatment or cold exposure, the expression of adipogenic genes significantly changed in brown adipocytes (Fig. 3). In Fig. 3A, the expression of PRDM16, a marker gene for brown adipocytes, was downregulated significantly (P < 0.05) in the SiO2 NPs group, but upregulated significantly (P < 0.01) in the cold exposure group, compared with the control group. As expected based on the opposing effects between SiO2 NPs and cold exposure, co-treatment with two rendered a loss of the effects seen with SiO2 NPs or cold exposure alone. Similar pattern of changes of the mRNA levels of Dio2, PGC-1α and UCP1 genes was consistently observed. Specifically, Dio2, a gene critical for thermogenesis in response to dropping temperature, sympathetic stimulation, or overfeeding in BAT, was downregulated (P < 0.05) by SiO2 NPs alone but upregulated (P < 0.05) by cold exposure, whereas SiO2 NPs/cold exposure combination produced relatively moderate but statistically significant (P < 0.05) effect with a smaller magnitude of downregulation compared to SiO2 NPs alone and less upregulation compared to cold exposure alone. As illustrated in Fig. 3C and 3D, the expression of PGC-1α and UCP1 was significantly downregulated by SiO2 NPs alone (UCP1: P < 0.01; PGC-1α: P < 0.05) but increased (P < 0.05) by cold exposure alone, and the effects of SiO2 NPs or cold exposure alone were neutralized by their combination (UCP1: P < 0.01, PGC-1α: P < 0.05). The factorial analysis showed that there were antagonistic effects between SiO2 NPs and cold exposure on the plasticity genes and metabolism genes in brown adipocytes, where the main effect of SiO2 NPs or cold exposure on the plasticity genes and metabolism genes were significant (P < 0.05).
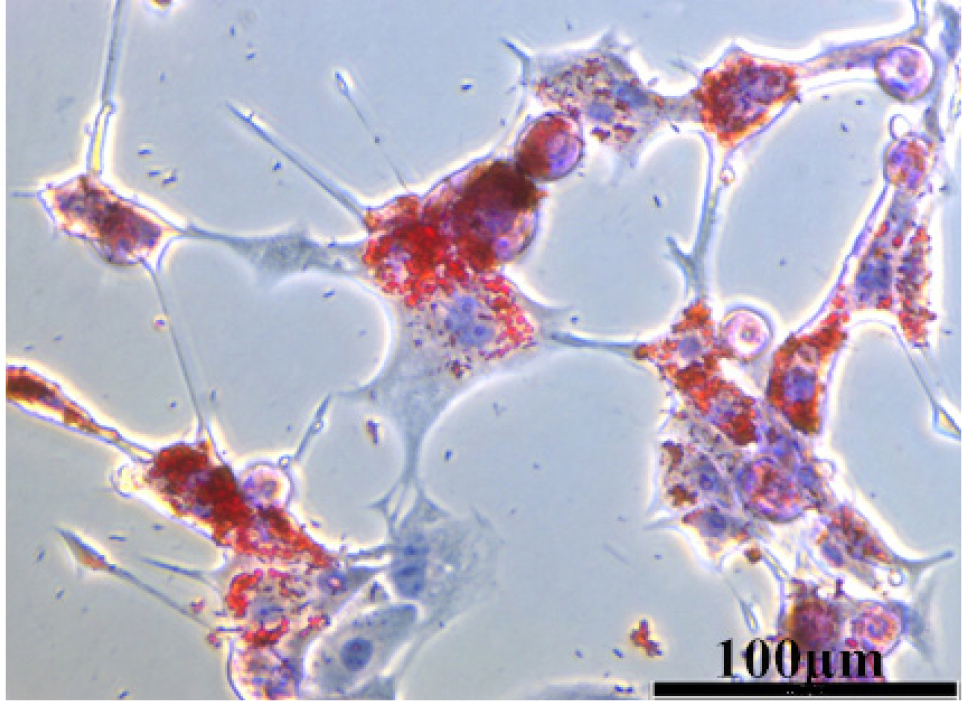
The oil red O staining of brown adipocytes
The brown adipocytes were isolated from the brown adipose tissue, induced in differentiation medium, and stained with oil red O.
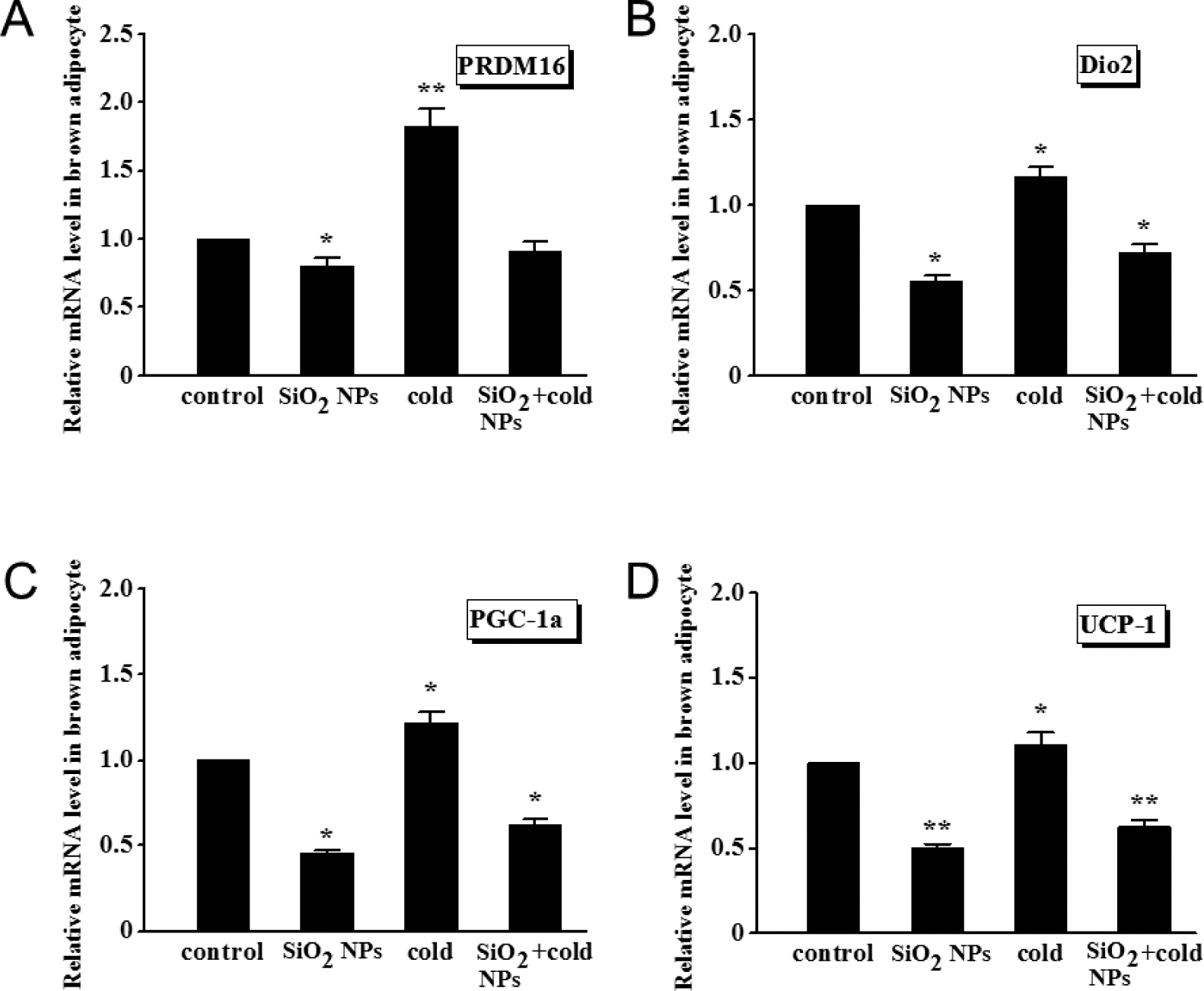
Adipogenic gene expression determined by qRT-PCR in brown adipocytes
(A) PRDM16. (B) Dio2. (C) PGC-1a. (D) UCP1. Values represent mean ± SEM, and significant differences compared with controls are indicated by *P < 0.05, **P < 0.01.
3.2 SiO2 NPs antagonize the effect of cold exposure on the inflammatory response genes in brown adipocytes
After SiO2 NPs or cold exposure, the expression of proinflammatory cytokine genes changed significantly in brown adipocytes (Fig. 4). The expression of IL-1β, IL-6 and TNF-α was remarkably (P < 0.05) upregulated by either SiO2 NPs alone or cold exposure alone; yet, co-treatment with SiO2 NPs and cold exposure markedly mitigated the upregulation of these proinflammatory cytokine genes. The factorial analysis indicated that the main effects of SiO2 NPs and/ or cold exposure on the inflammatory responses genes were significant (P < 0.05), Moreover, SiO2 NPs and cold exposure have mutually antagonistic effects on the expression of proinflammatory genes in brown adipocytes.
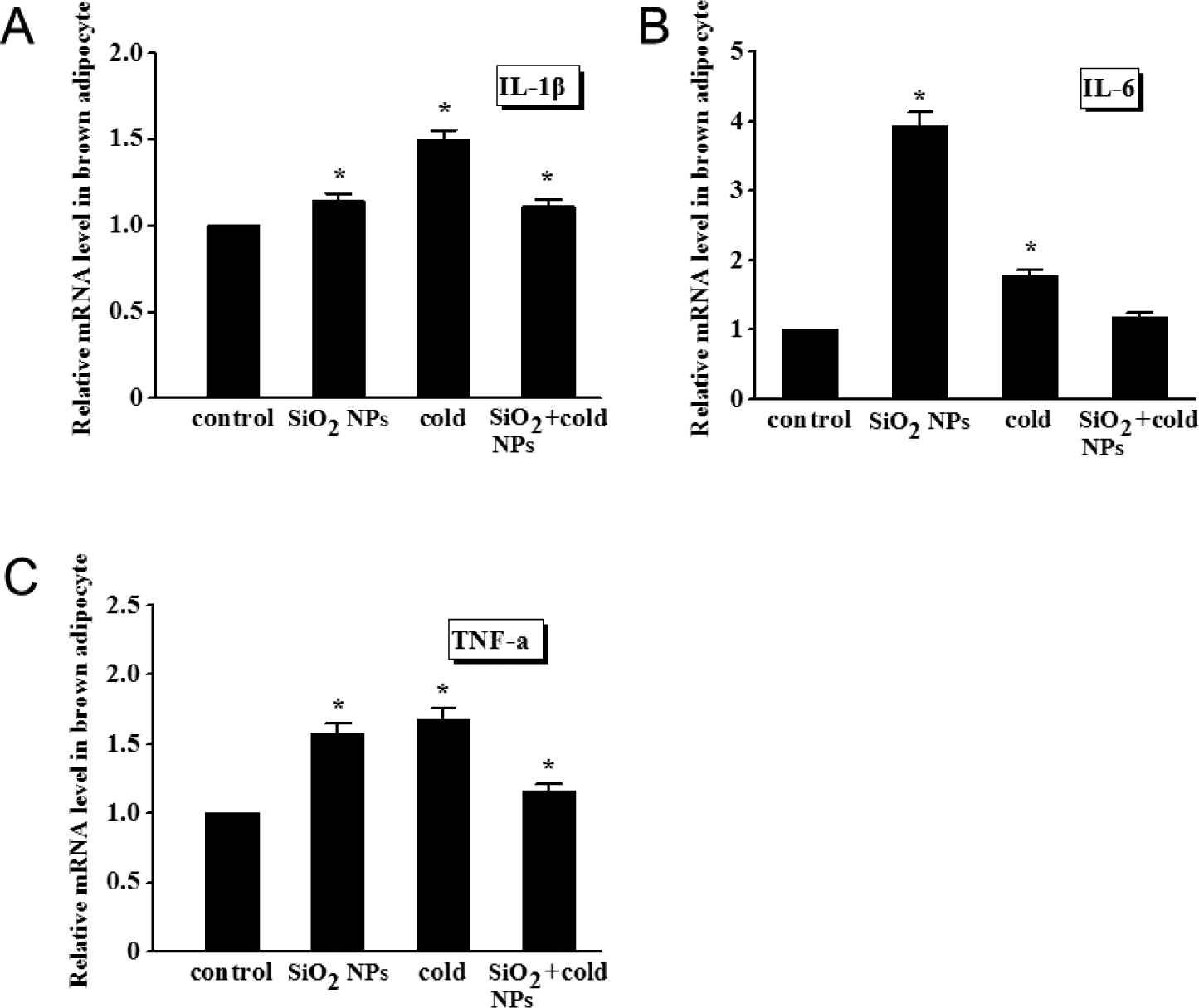
Expression of inflammatory cytokines determined by qRT-PCR in brown adipocytes
(A) IL-1β. (B) IL-6. (C) TNF-α. Values represent mean ± SEM, and significant differences compared with controls are indicated by *P < 0.05, **P < 0.01.
3.3 Antagonistic effects between SiO2 NPs and cold exposure on the expression of secretion genes in brown adipocytes
Fig. 5A demonstrated that the expression of ADPN gene, which correlates with adipocyte differentiation and modulates a number of metabolic processes, was decreased significantly (P < 0.01) by SiO2 NPs alone, but increased significantly (P < 0.01) by cold exposure, compared with non-treated control. Similarly, leptin, which plays a pivotal role in inflammatory responses and in the regulation of energy expenditure and appetite, was considerably decreased (P < 0.01) by SiO2 NPs alone but increased (P < 0.01) by cold exposure (Fig. 5B). As anticipated, SiO2 NPs/cold exposure combination compromised the effects produced by SiO2 NPs alone or cold exposure alone. There were antagonistic effect between SiO2 NPs and cold exposure on leptin and ADPN.
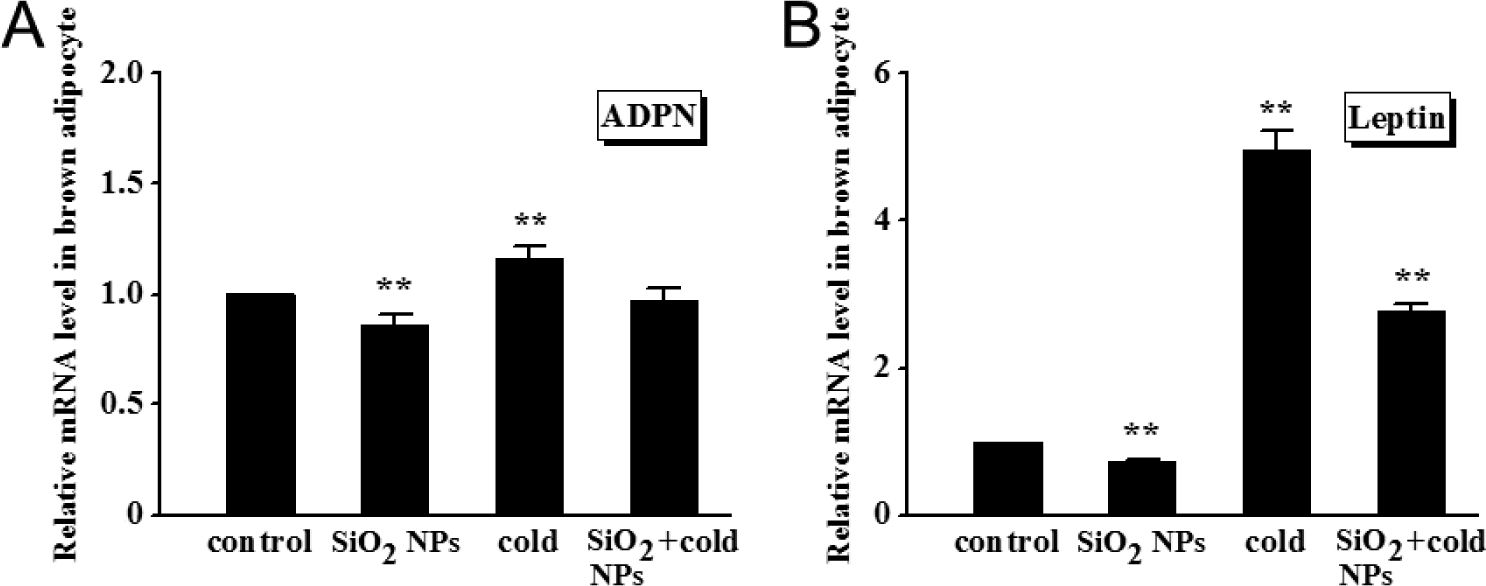
Effect of cold exposure or/and SiO2 NPs on the expression of secretion-related genes in brown adipocytes
(A) ADPN. (B) Leptin. Values represent mean ± SEM, and significant differences compared with controls are indicated by **P < 0.01.
4 Discussion
Hazy weather is common in the northern part of China literally for a year round, especially in wintertime. NPs are the main hazardous pollutants in atmospheric particulate matter and when in combination with other factors such as cold their detrimental effects on human health can be seriously exacerbated. The inhaled NPs can reach many organs through blood circulation, such as the spleen, liver, brain and kidney[7], and their impact on weight control in relation to brown/white adipose tissues has been intensively studied[32-33]. It was suggested that NPs can move across tissue and cellular barriers and interact with DNA[34]. Our previous investigation found that SiO2 NPs inhibit the weight lost and the increase of BAT following cold exposure[17], suggesting that SiO2 NPs impose damaging effects on adipose tissue remodeling. To the best of our knowledge, the present study is the first to assess the effects of SiO2 NPs and cold exposure on gene expression in brown adipocytes. The adipogenic genes including PRDM16, Dio2, PGC-1α and UCP1 were significantly downregulated by SiO2 NPs relative to the control levels, indicating that SiO2 NPs could inhibit the increase of the brown adipocytes. Notably, there seemed to be antagonistic effects between cold exposure and SiO2 NPs on the expression of adipogenic genes in brown adipocytes. The finding is consistent with the results of our previous studies that SiO2 NPs inhibited the cold-induced metabolism activation in white adipocytes and SD rats[16-17].
Cold exposure, which exacerbates asthma and chronic obstructive pulmonary disease (COPD), is a major environmental factor[35]. As an evidence of inflammation of the heart, kidney and blood vessel caused by chronic cold exposure, the mRNA levels of TNF-α and IL-6 increase significantly[36]. The present study showed that the mRNA levels of IL-6, IL-1β and TNF-α were upregulated by cold exposure, suggesting that an inflammatory response may be induced by cold exposure, as well as, in brown adipocytes. These findings were consistent with the results from one of our previous studies showing that cold exposure and SiO2 NPs induced a strong inflammatory response in BAT[14]. Multiple hormones and cytokines can influence instinctual behavior and metabolic pathways[37-38]. A study showed that IL-6 and TNF-α inhibited lipoprotein lipase, and TNF-α induced uncoupling protein expression and stimulated hormone-sensitive lipase[39]. IL-1β and IL-6 can also reduce the activity of adipose tissue lipoprotein lipase[40]. In the present study, the expression of proinflammatory cytokines was increased by cold exposure and SiO2 NPs, which likely influences their secretion and metabolism in brown adipocytes. BAT and WAT have important roles in the development of therapeutics for diabetes and obesity[41]. Meanwhile, the problem of haze in winter is particularly prominent. The present work underlines the potential role of SiO2 NPs exposure in plasticity genes and metabolism genes in brown adipocytes. It suggested that SiO2 NPs inhibit the effect of cold exposure on metabolism genes and plasticity genes.
In summary, wide spread attention has been attracted on the potential hazard of SiO2 NPs from haze in winter. The work about the combined effect of cold exposure and SiO2 NPs at the cellular level on brown adipocytes has been scarce. Here we reported the antagonistic effect between cold exposure and SiO2 NPs on the expression of metabolism- and plasticity-related genes in brown adipocytes. Moreover, SiO2 NPs and cold exposure both induce an inflammatory response in brown adipocytes. These results will contribute to the further understanding the effects of a combination of haze and winter conditions at the cellular level.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21707165) and the grants of Institute of Environmental and Operational Medicine (BWS17J025, BWS16J0101, WH2017006 and AWS16J022).
Author Contribution
Zhang Y Q and Yang D F designed the study and drafted the manuscript. Zhang L and Li X performed data analysis and data interpretation. Wu S, Zhang G Y and Wei X D performed all the experiments. All participated in the writing and approved the final version of the manuscript.
Ethnical Approval
All experiments involving the care and use of animals were performed in accordance with the guidelines and regulations concerning the ethics of science research in the Institute of Environmental and Operational Medicine and approved by the Ethics Review Board of Institute of Environmental and Operational Medicine (AMMS-04-2020-017).
Conflicts of interest
Yang D F is an Editorial Board Member of the journal. The article was subjected to the journal’s standard procedures, with peer review handled independently of this member and his research groups. There are no conflicts of interest to declare.
References
[1] Lu S L, Feng M, Yao Z K. Physicochemical characterization and cytotoxicity of ambient coarse, fine, and ultrafine particulate matters in Shanghai atmosphere. Atmos Environ, 2011; 45(3): 736-744.10.1016/j.atmosenv.2010.09.020Suche in Google Scholar
[2] Malfatti M A, Palko H A, Kuhn E A, et al. Determining the pharmacokinetics and long-term biodistribution of SiO2 nanoparticles in vivo using accelerator mass spectrometry. Nano Lett, 2012; 12(11): 5532-5538.10.1021/nl302412fSuche in Google Scholar PubMed PubMed Central
[3] Li J Q, Li L, Chen H Q, et al. Application of vitamin E to antagonize SWCNTs-induced exacerbation of allergic asthma. Sci Rep, 2014; 4: 4275.10.1038/srep04275Suche in Google Scholar PubMed PubMed Central
[4] Kang G S, Gillespie P A, Gunnison A, et al. Long-term inhalation exposure to nickel nanoparticles exacerbated atherosclerosis in a susceptible mouse model. Environ Health Perspect, 2011; 119(2): 176-181.10.1289/ehp.1002508Suche in Google Scholar PubMed PubMed Central
[5] Burch W M. Passage of inhaled particles into the blood circulation in humans. Circulation, 2002; 106(20): 141-141.10.1161/01.CIR.0000037134.24080.42Suche in Google Scholar
[6] Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect, 2005; 113(7): 823-839.10.1289/ehp.7339Suche in Google Scholar PubMed PubMed Central
[7] Oberdorster G, Sharp Z, Atudorei V, et al. Extra pulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A, 2002; 65(20): 1531-1543.10.1080/00984100290071658Suche in Google Scholar PubMed
[8] Simeonova P P, Erdely A. Engineered nanoparticle respiratory exposure and potential risks for cardiovascular toxicity: predictive tests and biomarkers. Inhal Toxicol, 2009; 21 Suppl 1: 68-73.10.1080/08958370902942566Suche in Google Scholar PubMed
[9] Wen D. Nanofuel as a potential secondary energy carrier. Energy Environ Sci, 2010; 3: 591-600.10.1039/b906384fSuche in Google Scholar
[10] Zou Y, Wang Y, Zhang Y, et al. Arctic sea ice, Eurasia snow, and extreme winter haze in China. Sci Adv, 2017; 3(3): e1602751.10.1126/sciadv.1602751Suche in Google Scholar PubMed PubMed Central
[11] Dai L, Zanobetti A, Koutrakis P, et al. Associations of fine particulate matter species with mortality in the United States: a multicity time-series analysis. Environ Health Perspect, 2014; 122(8): 837-842.10.1289/ehp.1307568Suche in Google Scholar PubMed PubMed Central
[12] Fruijtier-Poelloth C. The toxicological mode of action and the safety of synthetic amorphous silica-a nanostructured material. Toxicology, 2012; 294(2-3): 61-79.10.1016/j.tox.2012.02.001Suche in Google Scholar PubMed
[13] Jasnic N, Djordjevic J, Djurasevic S, et al. Specific regulation of ACTH secretion under the influence of low and high ambient temperature-the role of catecholamines and vasopressin. J Thermal Biology, 2012; 37: 469-474.10.1016/j.jtherbio.2012.04.003Suche in Google Scholar
[14] Zhang Y, Lin Y, Li X, et al. Silica dioxide nanoparticles combined with cold exposure induce stronger systemic inflammatory response. Environ Sci Pollut Res, 2017; 24(1): 291-298.10.1007/s11356-016-7649-2Suche in Google Scholar PubMed
[15] Smorlesi A, Frontini A, Giordano A, et al. The adipose organ: white-brown adipocyte plasticity and metabolic inflammation. Obes Rev, 2012; 13(Suppl 2): 83-96.10.1111/j.1467-789X.2012.01039.xSuche in Google Scholar PubMed
[16] Zhang Y, Li X, Lin Y, et al. The combined effects of silicon dioxide nanoparticles and cold air exposure on the metabolism and inflammatory responses in white adipocytes. Toxicol Res, 2017; 6(5): 705-710.10.1039/C7TX00145BSuche in Google Scholar PubMed PubMed Central
[17] Lin Y, Li X, Zhang L, et al. Inhaled SiO2 nanoparticles blunt cold exposure induced WAT browning and metabolism activation in white and brown adipose tissue. Toxicol Res, 2016; 5(4): 1106-1114.10.1039/C6TX00015KSuche in Google Scholar
[18] Ricquier D. Respiration uncoupling and metabolism in the control of energy expenditure. Proc Nutr Soc, 2005; 64(1): 47-52.10.1079/PNS2004408Suche in Google Scholar PubMed
[19] Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev, 2004; 84(1): 277-359.10.1152/physrev.00015.2003Suche in Google Scholar PubMed
[20] Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab, 2007; 6(1): 38-54.10.1016/j.cmet.2007.06.001Suche in Google Scholar PubMed PubMed Central
[21] Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/ skeletal muscle switch. Nature, 2008; 454(7207): 961-967.10.1038/nature07182Suche in Google Scholar PubMed PubMed Central
[22] Puigserver P, Wu Z, Park C W, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell, 1998; 92(6): 829-839.10.1016/S0092-8674(00)81410-5Suche in Google Scholar
[23] Wu Z, Boss O. Targeting PGC-1 alpha to control energy homeostasis. Expert Opin Ther Targets, 2007; 11(10): 1329-1338.10.1517/14728222.11.10.1329Suche in Google Scholar PubMed
[24] Obregon M J. Thyroid hormone and adipocyte differentiation. Thyroid, 2008; 18(2): 185-195.10.1089/thy.2007.0254Suche in Google Scholar PubMed
[25] Bianco A C, Kim B W. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest, 2006; 116(10): 2571-2579.10.1172/JCI29812Suche in Google Scholar PubMed PubMed Central
[26] Xu H, Barnes G T, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest, 2003; 112(12): 1821-1830.10.1172/JCI200319451Suche in Google Scholar
[27] Lumeng C N, DelProposto J B, Westcott D J, et al. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes, 2008; 57(12): 3239-3246.10.2337/db08-0872Suche in Google Scholar PubMed PubMed Central
[28] Olefsky J M, Glass C K. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol, 2010; 72: 219-246.10.1146/annurev-physiol-021909-135846Suche in Google Scholar PubMed
[29] Xu H, Sethi J K, Hotamisligil G S. Transmembrane tumor necrosis factor TNF-alpha inhibits adipocyte differentiation by selectively activating TNF receptor 1. J Biol Chem, 1999; 274(37): 26287-26295.10.1074/jbc.274.37.26287Suche in Google Scholar PubMed
[30] Galic S, Oakhill J S, Steinberg G R. Adipose tissue as an endocrine organ. Mol Cell Endocrinol, 2010; 316(2): 129-139.10.1016/j.mce.2009.08.018Suche in Google Scholar PubMed
[31] Ye L, Wu J, Cohen P, et al. Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A, 2013; 110(30): 12480-12485.10.1073/pnas.1310261110Suche in Google Scholar PubMed PubMed Central
[32] Enerback S. Human brown adipose tissue. Cell Metab, 2010; 11(4): 248-252.10.1016/j.cmet.2010.03.008Suche in Google Scholar PubMed
[33] Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab, 2010; 11(4): 268-272.10.1016/j.cmet.2010.03.007Suche in Google Scholar PubMed
[34] Bourdon J A, Halappanavar S, Saber A T, et al. Hepatic and pulmonary toxicogenomic profiles in mice intratracheally instilled with carbon black nanoparticles reveal pulmonary inflammation, acute phase response, and alterations in lipid homeostasis. Toxicol Sci, 2012; 127(2): 474-484.10.1093/toxsci/kfs119Suche in Google Scholar PubMed PubMed Central
[35] Li M, Li Q, Yang G, et al. Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)-mediated mechanism. J Allergy Clin Immunol, 2011; 128(3): 626-634.10.1016/j.jaci.2011.04.032Suche in Google Scholar PubMed
[36] Crosswhite P, Sun Z J. Ribonucleic acid interference knockdown of interleukin 6 attenuates cold-induced hypertension. Hypertension, 2010; 55(6): 1484-1491.10.1161/HYPERTENSIONAHA.109.146902Suche in Google Scholar PubMed PubMed Central
[37] Friedman J M. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr, 2009; 89(3): 973S-979S.10.3945/ajcn.2008.26788BSuche in Google Scholar PubMed PubMed Central
[38] Rosen E D, Spiegelman B M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature, 2006; 444(7121): 847-853.10.1038/nature05483Suche in Google Scholar PubMed PubMed Central
[39] Coppack S W. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc, 2001; 60(3): 349-356.10.1079/PNS2001110Suche in Google Scholar PubMed
[40] Hardardottir I, Gruenfeld C, Feingold K R. Effects of endotoxin and cytokines on lipid metabolism. Curr Opin Lipidol, 1994; 5(3): 207-215.10.1097/00041433-199405030-00008Suche in Google Scholar PubMed
[41] Granneman J G, Burnazi M, Zhu Z, et al. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab, 2003; 285(6): E1230-E1236.10.1152/ajpendo.00197.2003Suche in Google Scholar PubMed
© 2023 Yongqiang Zhang et al., published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Artikel in diesem Heft
- The effects of cold region meteorology and specific environment on the number of hospital admissions for chronic kidney disease: An investigate with a distributed lag nonlinear model
- Effects of cold climate on growth and development
- Multiple inducible thermogenic mechanisms in the development of cold acclimatization
- Present situation of rational drug use in plateau area
- Silicon dioxide nanoparticles inhibit the effects of cold exposure on metabolism and inflammatory responses in brown adipocytes
- Hydroxychloroquine induces long QT syndrome by blocking hERG channel
- Cold exposure alters proteomic profiles of the hypothalamus and pituitary in female rats
- Advances in the application of cryotherapy to the treatment of breast cancer
Artikel in diesem Heft
- The effects of cold region meteorology and specific environment on the number of hospital admissions for chronic kidney disease: An investigate with a distributed lag nonlinear model
- Effects of cold climate on growth and development
- Multiple inducible thermogenic mechanisms in the development of cold acclimatization
- Present situation of rational drug use in plateau area
- Silicon dioxide nanoparticles inhibit the effects of cold exposure on metabolism and inflammatory responses in brown adipocytes
- Hydroxychloroquine induces long QT syndrome by blocking hERG channel
- Cold exposure alters proteomic profiles of the hypothalamus and pituitary in female rats
- Advances in the application of cryotherapy to the treatment of breast cancer

