Abstract
Objective
The fruit stalk of Schisandra chinensis (Turcz.) Baill. (S. chinensis) has been found to contain bioactive components similar to the fruit of S. chinensis. Here, we report a recent discovery about new nortriterpenoids with a novel skeleton and anti-gastric cancer activity, which were isolated from the fruit stalk of S. chinensis.
Methods
The chemical components of ethyl acetate extract from 70% ethanol extract from S. chinensis fruit stalk were separated, purified, and identified by liquid chromatography methods (silica gel, ODS, HPLC) and extensive spectroscopic analyses (NMR, IR, UV, MS, CD).
Results
Two new nortriterpenoids, schilancitrilactone M and 25-hydroxyl schindilactone D (1 and 2), along with ten known nortriterpenoids (3–12) were isolated from the fruit stalk of S. chinensis. The isolated compounds were tested for their cytotoxic activities against MGC-803 cells, and the results showed that compounds 6–8 possessed significant activities with IC50 of 9.01, 11.77, and 2.74 μmol/L, respectively.
Conclusion
Twelve nortriterpenoids including two new compounds were isolated from the fruit stalk of S. chinensis for the first time. Among them, compounds 6–8 showed significant anti-gastric cancer activities. We postulated that the fruit stalk of S. chinensis could be used as an anti-gastric cancer drug.
1 Introduction
The fruit of Schisandra chinensis (Turcz.) Baill. (S. chinensis), as a genuine traditional chinese drugs in Northeast China, is mainly distributed in cold regions such as Heilongjiang, Jilin, and Liaoning. The 2020 edition of Chinese Pharmacopoeia recorded that it has the effect of astringing and controlling, supplementing qi while nourishing fluid, tonifying kidney, and calming heart. Its root, stem, leaf, fruit stalk and other non-medicinal parts were often removed as impurities. However, it was found that these non-medicinal parts also contained bioactive components similar to the fruit of S. chinensis[1–2]. In particular, a series of nortriterpenoids with novel and complex skeletons as well as broad biological activities (anti-tumor, anti-HIV and anti-HBV activities) were found from the stem and leaf of Schisandraceae plants which has attracted extensive attention from scholars[3]. Through the literature retrieving, more than 200 nortriterpenoids have been identified in Schisandraceae, including over 20 different skeleton types. Meanwhile, the chemical synthesis of nortriterpenoids has also been exploited[4]. To date, there have not been any reports on nortriterpenoids and their biological activities in the fruit stalk of S. chinensis. Thus, we carried out the present study on the constituents of nortriterpenoids in the fruit stalk of S. chinensis. Two new nortriterpenoids, schilancitrilactone M and 25-hydroxyl schindilactone D (1 and 2), together with ten known nortriterpenoids (3–12) (Fig. 1) were isolated and characterized. The cytotoxicity of these compounds against human gastric cancer cell line MGC-803 cells was tested, and the results showed that compounds 6–8 possessed significant anti-gastric cancer activities with IC50 values of 9.01, 11.77, and 2.74 μmol/L, respectively.
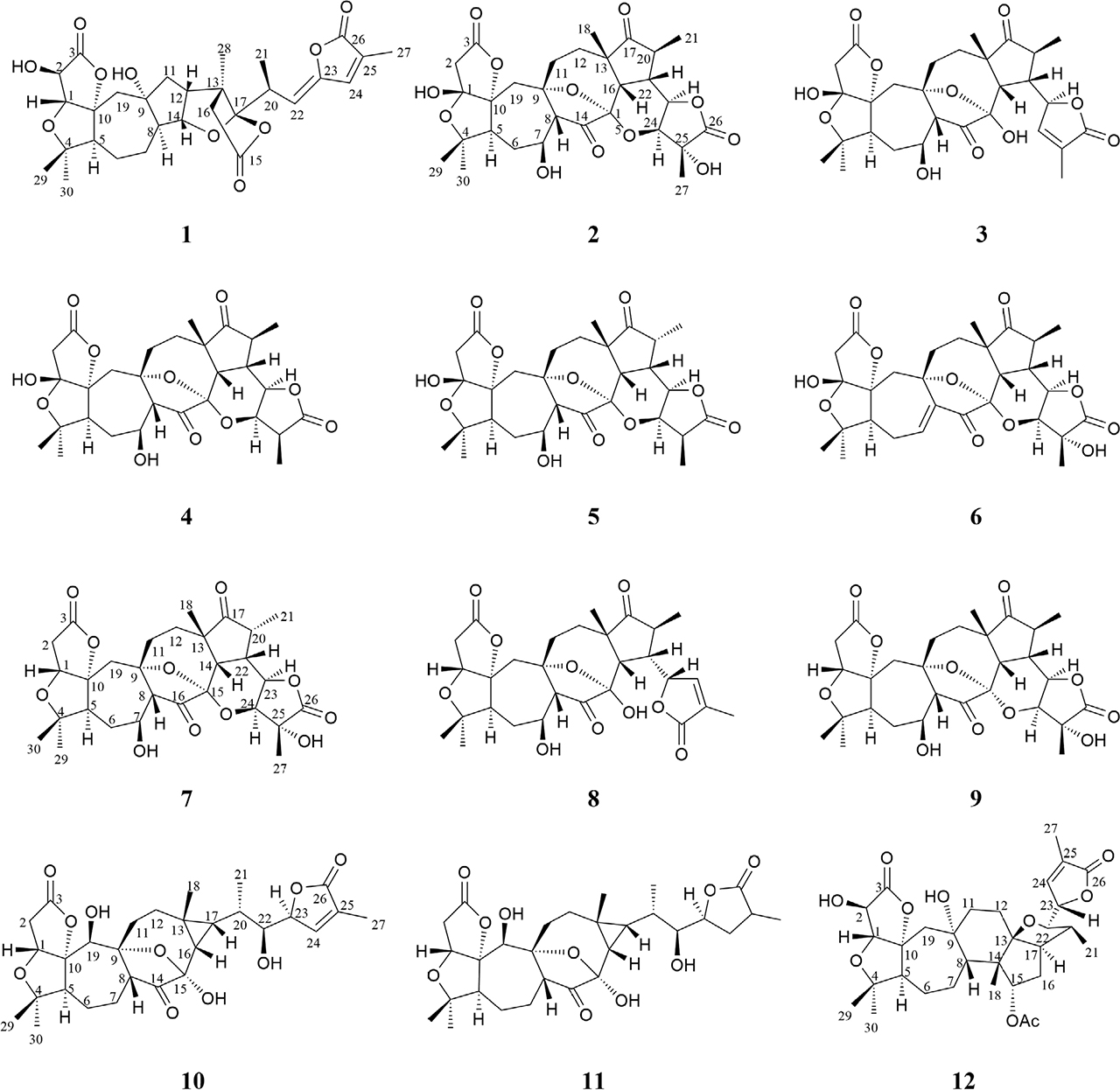
Structures of compounds 1–12 from Schisandra chinensis
2 Materials and Methods
2.1 General experimental procedures
Optical rotations were measured by a JASCO P-2000 digital polarimeter. NMR spectra using C5D5N as solvent and tetramethylsilane (TMS) as an internal standard were obtained on a DPX 400 instrument. HRESIMS were acquired on an AB SCIEX TripleTOF* 5600+ mass spectrometer. IR data were obtained on a Thermo Scientific Nicolet | IS10. An Alliance 2998 PDA Detector was used to measure UV data. A Bio-Logic MOS-450 instrument was employed to record CD spectra. Semi-preparative HPLC was performed on a Waters 600 unit equipped with a Waters 2414 RI detector, utilizing a SunFireTM C18 column (19×150 mm, 10 μmol/L). Epoch 2 microplate reader (BioTek Instruments, Inc, USA) was used to measure the absorbance for investigating the effects of nortriterpenoids on the rate of proliferation of MGC-803 cells. Silica gel (80–100 mesh; 200–300 mesh, Qingdao Haiyang Chemical Corporation, China), MCI gel (37–75 μmol/L, Mitsubishi Chemical Corporation, Japan), and ODS gel (50 μmol/L, YMC, Japan) were used for column chromatography. Silica gel F254 plates (Merck, Germany) were prepared for TLC.
2.2 Plant material
The fruit stalk of S. chinensis was collected from Raohe County, Heilongjiang Province, China in August 2015 and authenticated by Prof. Ruifeng Fan, Heilongjiang University of Chinese Medicine, where a voucher specimen with the herbarium (No.20150898) was deposited.
2.3 Extraction and isolation
The dried fruit stalk of S. chinensis (6.3 kg) was extracted three times (two h per time) with 70% ethanol under conditions of reflux. After removing solvents under reduced pressure, the crude extract (2 680.0 g) was suspended in H2O and successively divided by PE, EtOAc and n-BuOH. The resulting EtOAc (290.0 g) was column chromatographed on silica gel column and eluted by CH2CI2-MeOH (1:0 to 0:1, V/V) to afford ten fractions (I-X). Next, fraction VII was decolorized by MCI column and applied to ODS column to obtain five fractions (VII-1-VII-5). Then, fraction VII-2 was column chromatographed over silica gel with CH2CI2-MeOH, to yield compounds 1 (8.0 mg) and 5 (6.0 mg). Fraction VII-3 was column chromatographed on silica gel column and further subjected to semi-HPLC, to afford compounds 2 (10.0 mg), 4 (10.0 mg), 6 (10.0 mg), and 7 (5.0 mg). Fraction VII-4 was separated by ODS column and then purified by semi-HPLC, to yield compounds 3 (7.5 mg), 8 (9.0 mg) and 9 (6.0 mg). Fraction VII-5 was separated by column chromatographed on silica gel column with CH2CI2-MeOH mixtures of increasing polarity and purified by semi-HPLC, to acquire compounds 10 (14.0 mg), 11 (10.0 mg), and 12 (10.0 mg).
Schilancitrilactone M (1): White amorphous powder; +79 (c = 0.10, MeOH); IR (KBr) vmax: 3447, 2973, 2923, 1769, 1675, 1619, 1458, 1374, 1074, 990 cm−1 (Fig. S9); UV (MeOH) λmax: 196, 273 nm (Fig. S10); 1H-NMR and 13C-NMR (Table 1); HR-ESI-MS m/z 545.2380 [M+H]+ (Calcd. for C29H37O10, 545.2387) (Fig. S8).
1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectroscopic data for 1 (δ in ppm, J in Hz) in C5D5N
| No | δC | δH (J in Hz) |
|---|---|---|
| 1 | 87.1 | 4.42 (1H, s) |
| 2 | 73.0 | 4.72 (1H, s) |
| 3 | 176.6 | |
| 4 | 85.3 | |
| 5 | 60.5 | 2.44 (1H, dd, J=4.6, 12.2) |
| 6 | 22.1 | 1.36–1.42 (2H, m) |
| 7 | 24.9 | 1.95 (2H, m) |
| 8 | 54.5 | 2.65(1H, m) |
| 9 | 82.8 | |
| 10 | 99.3 | |
| 11 | 43.8 | 2.13 (1H, o) |
| 1.75 (1H, dd, J=8.4, 12.6) | ||
| 12 | 50.9 | 2.70 (1H, o) |
| 13 | 50.7 | |
| 14 | 85.5 | 4.75 (1H, dd, J=6.0, 8.0) |
| 15 | 173.5 | |
| 16 | 46.3 | 2.85 (2H, o) |
| 17 | 121.9 | |
| 19 | 42.5 | 2.82 (1H, ABd, J=15.6) |
| 2.37 (1H, ABd, J=15.6) | ||
| 20 | 36.5 | 3.52 (1H, m) |
| 21 | 16.3 | 1.31 (3H, d, J=6.8) |
| 22 | 114.3 | 5.59 (1H, d, J=9.8) |
| 23 | 148.2 | |
| 24 | 138.5 | 6.98 (1H, s) |
| 25 | 129.6 | |
| 26 | 170.8 | |
| 27 | 10.4 | 1.83 (3H, s) |
| 28 | 19.0 | 1.14 (3H, s) |
| 29 | 22.2 | 1.03 (3H, s) |
| 30 | 28.8 | 1.24 (3H, s) |
25-hydroxyl schindilactone D (2): White amorphous powder; −17 (c = 0.10, MeOH); IR (KBr) vmax: 3458, 3436, 3361, 2965, 2919, 2873, 2851, 1778, 1738, 1665, 1459, 1208, 1111, 1011 cm−1 (Fig. S20); UV (MeOH) λmax: 195, 244 nm (Fig. S21); 1H-NMR and 13C-NMR (Table 2); HR-ESI-MS m/z 594.2576 [M+NH4]+ (Calcd. for C29H40NO12, 594.2551) (Fig. S19).
1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectroscopic data for 2 (δ in ppm, J in Hz) in C5D5N
| No | δC | δH (J in Hz) |
|---|---|---|
| 1 | 108.7 | |
| 2 | 43.5 | 3.04(1H, ABd, J=17.8) |
| 3.13 (1H, ABd, J=17.8) | ||
| 3 | 173.4 | |
| 4 | 84.2 | |
| 5 | 58.9 | 2.64 (1H, m) |
| 6 | 36.4 | 2.26 (2H, m) |
| 7 | 68.4 | 4.60 (1H, m) |
| 8 | 60.2 | 2.93 (1H, d, J=9.8) |
| 9 | 81.7 | |
| 10 | 97.0 | |
| 11 | 42.4 | 1.73 (1H, m) |
| 2.08 (1H, m) | ||
| 12 | 31.2 | 1.59 (1H, m) |
| 1.84 (1H, m) | ||
| 13 | 50.2 | |
| 14 | 209.8 | |
| 15 | 99.1 | |
| 16 | 45.0 | 2.88 (1H, o) |
| 17 | 220.4 | |
| 18 | 26.0 | 0.93 (3H, s) |
| 19 | 40.7 | 2.61 (1H, ABd, 16.3) |
| 2.83 (1H, ABd, 16.3) | ||
| 20 | 44.6 | 2.75 (1H, dq, J=7.0, 12,0) |
| 21 | 14.9 | 1.05 (3H, d, J=7.0) |
| 22 | 40.3 | 2.88 (1H, m) |
| 23 | 75.1 | 5.30 (1H, br. s) |
| 24 | 73.3 | 5.22 (1H, d, J=1.8) |
| 25 | 76.7 | |
| 26 | 177.7 | |
| 27 | 17.6 | 1.71 (3H, s) |
| 29 | 25.1 | 1.39 (3H, s) |
| 30 | 29.5 | 1.26 (3H, s) |
2.4 Cytotoxicity assay
The cellular toxicity of compounds 1–12 on MGC-803 cells was evaluated by CCK-8 assay. Briefly, cells were seeded in 96-well plates and incubated in DMEM medium containing 10% fetal bovine serum and 1% penicillin-streptomycin in a humidified atmosphere of 5% CO2 at 37°C for 24 h. After adherence of the cells to the bottom surface of the plates, 100 μL of various concentrations of compounds 1–12 were added and incubated under the same conditions for another 24 h. Cisplatin was used as a positive drug. Subsequently, CCK-8 solution (10 μL) was added to the culture medium and incubated for 4 h. Finally, cell viability or cytotoxicity was determined based on absorbance values at 490 nm obtained on an Epoch2 microplate reader (Bio-Tek) and concentration-dependent decreases of cell viability were evaluated to determine IC50 values of the test compounds.
3 Results
Compound 1 was isolated as white amorphous powder and an ion peak of [M+H]+ was observed by HR-ESI-MS at m/z 545.2380 (Calcd. for C29H37O10, 545.2387), indicating the molecular formula of C29H36O10.
In the 1H-NMR (400 MHz, C5D5N) spectrum (Fig. S1) of compound 1, a typical AB coupled signal δH 2.82 (1H, ABd, J=15.6 Hz) and 2.37 (1H, ABd, J=15.6 Hz) was observed. 13C-NMR (Fig. S2) and DEPT 135 spectrums (Fig. S3) revealed that compound 1 is composed of 29 carbons, including 5 primary carbons, 5 secondary carbons, 9 tertiary carbons and 10 quaternary carbons (3 ester carbonyl ones). HSQC spectrum (Fig. S4) analysis assigned all protons to their respective carbons unambiguously. The NMR data indicated that 1 is a highly oxygenated nortriterpenoid.
Meanwhile, the 1H-NMR and 13C-NMR spectrums revealed that 1 is highly similar to schilancitrilactone A[5] with main differences in ring A and ring B. The typical ABX coupling system formed by H-1 and H-2 of schilancitrilactone A was replaced by the signals δH (4.42 1H, s) and 4.72 (1H, s) in the low field region, indicating that there was a hydroxy that was further positioned to C-2. The supportive evidence came from the HMBC (Fig. S6) correlations from H-2 to C-1, C-10 and from H-1 to C-2, C-3, C-10, C-19, together with the 1H-1H COSY (Fig. S5) correlations of H-1/H-2 (Fig. 2). In addition, both 1H-NMR signals of H-1 and H-2 were unimodal, indicating that the dihedral angle between H-1 and H-2 was close to 90°, which further indicated that 2-OH should be β-oriented, since H-1 was biogenetically assigned as β[6].
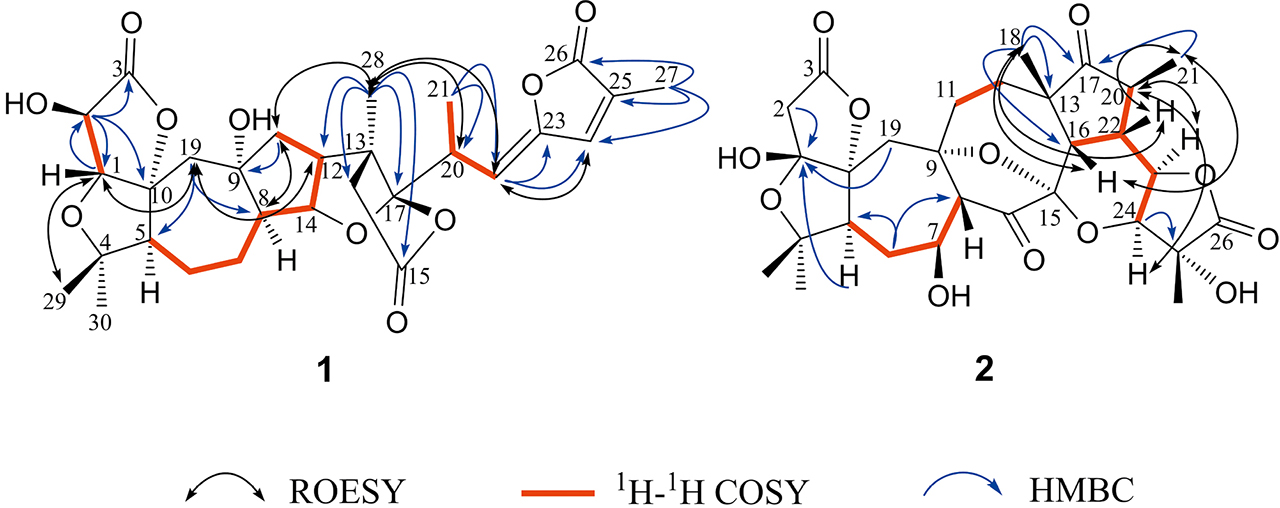
Key HMBC, 1H-1H COSY and ROESY of compounds 1–2
Because of the γ-gauche of hydroxyl group, after the 29-CH3 in schilancitrilactone A was oxidized, 30-CH3 was also moved to a higher field. Therefore, both C-29 and C-30 of compound 1 were not oxidized, and they were general methyls. Meanwhile, the signals H-1/29-CH3 in ROESY (Fig. S7) confirmed that 29-CH3 should be β-oriented. In addition, the absolute configuration at C-20 in compound 1 was determined as S, deduced from the Cotton effects in the CD (Fig. S11) spectrum similar to those of schilancitrilactone L[7], which showed a positive Cotton effect at 268 nm. Furthermore, the ROESY correlation of H-22 with H-24 indicated that the geometry of the C(22) = C(23) of 1 is Z. The ROESY correlations of Me-28 with H-20 and H-11α, H-8 with H-5 and H-11α suggested that Me-28, H-20, H-8 and H-5 were all positioned on the same face of the molecule and therefore assigned as α-oriented, whereas the ROESY correlations of H-19β with H-1 and H-12 suggested that H-12 was β-oriented (Fig. 2). The relative configurations of the remaining chiral centers were determined to be the same as those in schilancitrilactone A because of their similar carbon and proton chemical shifts and ROESY correlations. According to the above characteristics, the structure of compound 1 named schilancitrilactone M is shown in Fig. 1.
Compound 2 is white amorphous powder with an ion peak of [M+NH4]+ detected by HR-ESI-MS at m/z 594.2576 (Calcd. for C29H40NO12, 594.2551), indicating that the molecular formula was C29H36O12.
Compared the 1D NMR spectrum (Fig. S12–S13) with the known compound lancifodilactone L[8], compound 2 had the same structure segments of C, D, E, F and G, while a hemiacetal and ester structure at ring A and ring B was oxidized (δC 108.7 C-1, 43.5 C-2). DEPT 135 spectrum (Fig. S14) analysis indicated that C-1 is the oxidation position. The change of the chemical shift of carbon at H-19 was caused by the substitution of 1-OH of compound 2 to produce a γ-gauche, which increased the density of carbon electron cloud at position 19 and moved to the high field (δC 40.7). Moreover, compared with compound 4 named schindilactone D[9], the NMR data of ring A and ring B in compound 2 were consistent with those in compound 4. Furthermore, the planar structure of compound 2 was determined by 2D NMR data (Fig. S15-S17). According to the correlations of 21-CH3 with H-22 and H-16 and 18-CH3 with H-22, H-16 in ROESY (Fig. S18), the same spatial orientation of 18-CH3, 21-CH3, H-22 and H-16 were inferred. The correlation of H-20 with H-23 and H-24 were observed, suggesting that H-23 and H-24 were in a different spatial orientation (Fig. 2). The structure of compound 2 named 25 hydroxyl schindilaractone D is depicted in Fig. 1.
Other ten compounds isolated from the preparations were already known and were identified as schindilactone C (3)[10], schindilactone D (4) [9], schindilactone E (5) [9], schindilactone H (6)[11], lancifodilactone E (7)[12], lancifodilactone I (8)[8], lancifodilactone L (9)[8], preschisanartanin O (10)[13], arisanlactone C (11)[14], wuweizidilactone F (12)[6], based the 1D NMR data from the reported compounds in the literatures.
All compounds (1–12) were tested for their possible anti-cancer activities in MGC-803 gastric cancer cells (Table 3). The results demonstrated that compounds 6–8 exhibited strong cytotoxic effects against MGC-803, as reflected by the reduced cell viability, with IC50 values of 9.01, 11.77 and 2.74 μmol/L, respectively.
Cytotoxic activities of compounds 1–12 against MGC-803 cell lines
| Drugs | IC50 (μmol/L) |
|---|---|
| compound 1 | 46.38 ± 3.92 |
| compound 2 | >50 |
| compound 3 | >50 |
| compound 4 | >50 |
| compound 5 | >50 |
| compound 6 | 9.01 ± 3.72 |
| compound 7 | 11.77 ± 2.61 |
| compound 8 | 2.74 ± 0.58 |
| compound 9 | >50 |
| compound 10 | 20.17 ± 3.12 |
| compound 11 | 40.85 ± 3.65 |
| compound 12 | >50 |
| cisplatin | 3.79 ± 0.51 |
IC50 was defined as the concentration that resulted in a 50% decrease in cell number, and the values were within the 0–1 000 μg/mL range. Results are presented as mean ± SD (N = 3 independent replicates).
Cisplatin was used as a positive control.
4 Discussion
In the present study, two new nortriterpenoids, schilancitrilactone M and 25-hydroxyl schindilactone D (1 and 2), together with ten known nortriterpenoids (3–12) were isolated and characterized which assayed for their anti-cancer activities with demonstrating significant effects of compounds 6–8. Notably, compared with 2, 6 was added a double bond at C-7 which would increase the Anti-gastric cancer activity. Compound 9 was essentially similar to compound 7, but it had weaker cytotoxicity in MGC-803 cells than the latter. It is likely because the absolute configuration of 20-bit in compound 9 was the S-configuration. Due to the first position of compound 8 was H instead of OH and the 23-bit of compound 8 was the R-configuration, the Anti-gastric cancer activity of 8 was higher than compound 3. This implied that a schisanartane nortriterpenoid with 1-H, 20S, 23R or a double bond at C-7 is essential for their cytotoxicity against MGC-803.
Supporting information
The HRESIMS, UV, IR, CD, 1D NMR and 2D NMR spectra for compounds 1 and 2 as well as the 1D NMR data for compounds 3–12 are available as supplementary materials.
Acknowledgments
This study was supported by the National Nature Science Foundation (81973440), National Key Research and Development Project (2018YFC1707100; 2018YFC1707103) and Heilongjiang Touyan Innovation Team Program.
Conflict of interests
The authors declare no competing financial interests.
References
[1] Szopa A, Ekiert R, Ekiert H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: a review on the bioactive components, pharmacological properties, analytical and biotechnological studie. Phytochem Rev, 2017; 16: 195–218.10.1007/s11101-016-9470-4Search in Google Scholar PubMed PubMed Central
[2] Jin M H, Liu R J. Analysis of content of the Schisandra chinensis Baill fruit, rattan and fruit handles. J Med Sci, 2005; 1: 28–30.Search in Google Scholar
[3] Jin Y P, Yan S, Liu J X, et al. Reseach progress on nortriterpenoids in plants of Schisandraceae and their pharmacological activities. Zhong Cao Yao, 2014; 45(11): 1643–1650.Search in Google Scholar
[4] Yan B C, Hu K, Sun H D, et al. Recent advances in the synthesis of isodon diterpenoids and schinortriterpenoids. Chinese J Org Chem, 2018; 38: 2259–2280.10.6023/cjoc201806002Search in Google Scholar
[5] Luo X, Shi Y M, Luo R H, et al. Schilancitrilactones A–C: three unique nortriterpenoids from Schisandra lancifolia. Org Lett, 2012; 14(5): 1286–1289.10.1021/ol300099eSearch in Google Scholar PubMed
[6] Huang S X, Yang L B, Xiao W L, et al. Wuweizidilactones A–F: Novel highly oxygenated nortriterpenoids with unusual skeletons isolated from Schisandra chinensis. Chem Eur J, 2007; 13(17): 4816–4822.10.1002/chem.200700346Search in Google Scholar PubMed
[7] Shi Y M, Hu K, Pescitelli G, et al. Schinortriterpenoids with identical configuration but distinct ECD spectra generated by nondegenerate exciton coupling. Org Lett, 2018; 20(6): 1500–1504.10.1021/acs.orglett.8b00149Search in Google Scholar PubMed
[8] Xiao W L, Huang S X, Zhang L, et al. Nortriterpenoids from Schisandra lancifolia. J Nat Prod, 2006; 69(2): 650–653.10.1021/np060047jSearch in Google Scholar PubMed
[9] Huang S X, Han Q B, Lei C, et al. Isolation and characterization of miscellaneous terpenoids of Schisandra chinensis. Cheminform, 2008; 64(64): 4260–4267.10.1016/j.tet.2008.02.085Search in Google Scholar
[10] Huang S X, Li R T, Liu J P, et al. Isolation and characterization of biogenetically related highly oxygenated nortriterpenoids from Schisandra chinensis. Cheminform, 2007; 9(11): 2079–2082.10.1021/ol070510zSearch in Google Scholar
[11] Xue Y B, Zhang Y L, Yang J H, et al. Nortriterpenoids and lignans from the fruit of Schisandra chinensis. Chem Pharm Bull, 2010; 58(12): 1606–1611.10.1248/cpb.58.1606Search in Google Scholar PubMed
[12] Li R T, Xiang W, Li S H, et al. Lancifodilactones B-E, new nortriterpenes from Schisandra lancifolia. J Nat Prod, 2004; 67(1): 94–97.10.1021/np030339+Search in Google Scholar PubMed
[13] Shi Y M, Wang X B, Li X N, et al. Lancolides, antiplatelet aggregation nortriterpenoids with tricyclo [6.3. 0.02, 11] undecane-bridged system from Schisandra lancifolia. Org Lett, 2013; 15(19): 5068–5071.10.1021/ol402414zSearch in Google Scholar PubMed
[14] Cheng Y B, Liao T C, Lo Y W, et al. Nortriterpene lactones from the fruits of Schisandra arisanensis. J Nat Prod, 2010; 73(7): 1228–1233.10.1021/np100048hSearch in Google Scholar PubMed
© 2022 Yan Liu et al., published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Review
- Dissecting pathophysiology of a human dominantly inherited disease, familial amyloidotic polyneuropathy, by using genetically engineered mice
- Effect of cold on knee osteoarthritis: Recent research status
- Original Article
- Effects of intermittent cold-exposure on culprit plaque morphology in ST-segment elevation myocardial infarction patients: a retrospective study based on optical coherence tomography
- A randomized retrospective clinical study on the choice between endodontic surgery and immediate implantation
- Challenges and improvement in management of neonates born to mothers with COVID-19 in China
- Nortriterpenoids from the fruit stalk of Schisandra chinensis (Turcz.) Baill.
- Altered expression profile of long non-coding RNAs during heart aging in mice
- Cryptotanshinone increases the sensitivity of liver cancer to sorafenib by inhibiting the STAT3/Snail/epithelial mesenchymal transition pathway
Articles in the same Issue
- Review
- Dissecting pathophysiology of a human dominantly inherited disease, familial amyloidotic polyneuropathy, by using genetically engineered mice
- Effect of cold on knee osteoarthritis: Recent research status
- Original Article
- Effects of intermittent cold-exposure on culprit plaque morphology in ST-segment elevation myocardial infarction patients: a retrospective study based on optical coherence tomography
- A randomized retrospective clinical study on the choice between endodontic surgery and immediate implantation
- Challenges and improvement in management of neonates born to mothers with COVID-19 in China
- Nortriterpenoids from the fruit stalk of Schisandra chinensis (Turcz.) Baill.
- Altered expression profile of long non-coding RNAs during heart aging in mice
- Cryptotanshinone increases the sensitivity of liver cancer to sorafenib by inhibiting the STAT3/Snail/epithelial mesenchymal transition pathway

