Abstract
The electrical conductivities of granites with different chemical compositions [XA = (Na2O + K2O + CaO)/SiO2 = 0.10, 0.13, 0.14, and 0.16 in weight percent] were measured at 623-1173 K and 0.5 GPa in a multi-anvil high-pressure apparatus using a Solartron-1260 Impedance/Gain Phase analyzer within a frequency range of 10-1-106 Hz. The conductivity of the granite sample with XA = 0.13 was also measured at 0.5-1.5 GPa. The results indicate that pressure has a very weak influence on the electrical conductivity in the stability field of granite, whereas increases in temperature and the value of XA produce dramatic increases in the electrical conductivity. For the granite samples with XA = 0.16 and 0.13, the activation enthalpies are 1.0 eV above 773 K and 0.5 eV below 773 K, suggesting that impurity conduction is the dominant conduction mechanism in the lower-temperature region. For the granites with XA = 0.14 and 0.10, the activation enthalpy is 1.0 eV over the whole temperature range, suggesting that only one conduction mechanism dominates the conductivity. Based on the value of activation enthalpy (~1.0 eV) and the dependence of electrical conductivity and activation enthalpy on XA at high temperatures, we propose that intrinsic conduction is the dominant conduction mechanism in all samples, and that K+, Na+, and Ca2+ in feldspar are the probable charge carriers controlling the conductivity. All conductivity data at high temperatures can be fitted to the general formula
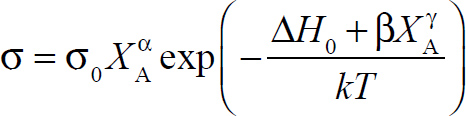
where σ0 is the pre-exponential factor; α, β, and γ are constants; ΔH0 is the activation enthalpy at very small values of XA; k is the Boltzmann constant; and T is the temperature. The present results suggest that the granite with various chemical compositions is unable to account for the high conductivity anomalies under stable mid- to lower-crust and southern Tibet.
© 2014 by Walter de Gruyter Berlin/Boston
Artikel in diesem Heft
- Highlights and Breakthroughs
- An alternative to alteration and melting processes in the Earth: Reaction between hydrogen (H2) and oxide components in the Earth in space and time
- The crystallographic and petrogenetic significance of pegmatite phosphates
- Small grains and big implications: Accessory Ti- and Zr-minerals as petrogenetic indicators in HP and UHP marbles
- What Lurks in the Martian Rocks and Soil? Investigations of Sulfates, Phosphates, and Perchlorates
- Natural Fe-bearing oxides and sulfates from the Rio Tinto Mars analog site: Critical assessment of VNIR reflectance spectroscopy, laser Raman spectroscopy, and XRD as mineral identification tools
- Dissolution rates of amorphous Al- and Fe-phosphates and their relevance to phosphate mobility on Mars
- Stability and spectroscopy of Mg sulfate minerals: Role of hydration on sulfur isotope partitioning
- Synthesis and characterization of the Mars-relevant phosphate minerals Fe- and Mg-whitlockite and merrillite and a possible mechanism that maintains charge balance during whitlockite to merrillite transformation
- Minerals in the Human Body
- Determination of the concentration of asbestos minerals in highly contaminated mine tailings: An example from abandoned mine waste of Crètaz and Èmarese (Valle d’Aosta, Italy)
- Spinels Renaissance: The past, present, and future of those ubiquitous minerals and materials
- Pressure-volume equation of state for chromite and magnesiochromite: A single-crystal X-ray diffraction investigation
- The systematics of the spinel-type minerals: An overview
- Chemistry and Mineralogy of Earth’s Mantle
- Formation of SiH4 and H2O by the dissolution of quartz in H2 fluid under high pressure and temperature
- Identifying the spin transition in Fe2+-rich MgSiO3 perovskite from X-ray diffraction and vibrational spectroscopy
- Mantle-derived guyanaite in a Cr-omphacitite xenolith from Moses Rock diatreme, Utah
- Pluton assembly and the genesis of granitic magmas: Insights from the GIC pluton in cross section, Sierra Nevada Batholith, California
- First-principles molecular dynamics simulations of MgSiO3 glass: Structure, density, and elasticity at high pressure
- A new UHP metamorphic complex in the ~1.8 Ga Nagssugtoqidian Orogen of West Greenland
- Visible and short-wave infrared reflectance spectroscopy of REE fluorocarbonates
- Volatile abundances of coexisting merrillite and apatite in the martian meteorite Shergotty: Implications for merrillite in hydrous magmas
- Sb5+ and Sb3+ substitution in segnitite: A new sink for As and Sb in the environment and implications for acid mine drainage
- First-principles elasticity of monocarboaluminate hydrates
- Substitution of Ti3+ and Ti4+ in hibonite (CaAl12O19)
- Exploring the effect of lithium on pegmatitic textures: An experimental study
- XANES measurements of Cr valence in olivine and their applications to planetary basalts
- Z-contrast imaging and ab initio study on “d” superstructure in sedimentary dolomite
- Influence of temperature, pressure, and chemical composition on the electrical conductivity of granite
- Ti- and Zr-minerals in calcite-dolomite marbles from the ultrahigh-pressure Kimi Complex, Rhodope mountains, Greece: Implications for the P-T evolution based on reaction textures, petrogenetic grids, and geothermobarometry
- Ab initio thermodynamic and thermophysical properties of sapphirine end-members in the join Mg4Al8Si2O20-Mg3Al10SiO20
- The relation between Li ↔ Na substitution and hydrogen bonding in five-periodic singlechain silicates nambulite and marsturite: A single-crystal X-ray study
- Natural analogs of belite sulfoaluminate cement clinkers from Negev Desert, Israel
- Australian sedimentary opal-A and its associated minerals: Implications for natural silica sphere formation
- The 2H and 3R polytypes of sabieite, NH4Fe3+(SO4)2, from a natural fire in an oil-bearing shale near Milan, Ohio
- Letter
- Te-rich raspite, Pb(W0.56Te0.44)O4, from Tombstone, Arizona, U.S.A.: The first natural example of Te6+ substitution for W6+
- New Mineral Names
- Book Review
Artikel in diesem Heft
- Highlights and Breakthroughs
- An alternative to alteration and melting processes in the Earth: Reaction between hydrogen (H2) and oxide components in the Earth in space and time
- The crystallographic and petrogenetic significance of pegmatite phosphates
- Small grains and big implications: Accessory Ti- and Zr-minerals as petrogenetic indicators in HP and UHP marbles
- What Lurks in the Martian Rocks and Soil? Investigations of Sulfates, Phosphates, and Perchlorates
- Natural Fe-bearing oxides and sulfates from the Rio Tinto Mars analog site: Critical assessment of VNIR reflectance spectroscopy, laser Raman spectroscopy, and XRD as mineral identification tools
- Dissolution rates of amorphous Al- and Fe-phosphates and their relevance to phosphate mobility on Mars
- Stability and spectroscopy of Mg sulfate minerals: Role of hydration on sulfur isotope partitioning
- Synthesis and characterization of the Mars-relevant phosphate minerals Fe- and Mg-whitlockite and merrillite and a possible mechanism that maintains charge balance during whitlockite to merrillite transformation
- Minerals in the Human Body
- Determination of the concentration of asbestos minerals in highly contaminated mine tailings: An example from abandoned mine waste of Crètaz and Èmarese (Valle d’Aosta, Italy)
- Spinels Renaissance: The past, present, and future of those ubiquitous minerals and materials
- Pressure-volume equation of state for chromite and magnesiochromite: A single-crystal X-ray diffraction investigation
- The systematics of the spinel-type minerals: An overview
- Chemistry and Mineralogy of Earth’s Mantle
- Formation of SiH4 and H2O by the dissolution of quartz in H2 fluid under high pressure and temperature
- Identifying the spin transition in Fe2+-rich MgSiO3 perovskite from X-ray diffraction and vibrational spectroscopy
- Mantle-derived guyanaite in a Cr-omphacitite xenolith from Moses Rock diatreme, Utah
- Pluton assembly and the genesis of granitic magmas: Insights from the GIC pluton in cross section, Sierra Nevada Batholith, California
- First-principles molecular dynamics simulations of MgSiO3 glass: Structure, density, and elasticity at high pressure
- A new UHP metamorphic complex in the ~1.8 Ga Nagssugtoqidian Orogen of West Greenland
- Visible and short-wave infrared reflectance spectroscopy of REE fluorocarbonates
- Volatile abundances of coexisting merrillite and apatite in the martian meteorite Shergotty: Implications for merrillite in hydrous magmas
- Sb5+ and Sb3+ substitution in segnitite: A new sink for As and Sb in the environment and implications for acid mine drainage
- First-principles elasticity of monocarboaluminate hydrates
- Substitution of Ti3+ and Ti4+ in hibonite (CaAl12O19)
- Exploring the effect of lithium on pegmatitic textures: An experimental study
- XANES measurements of Cr valence in olivine and their applications to planetary basalts
- Z-contrast imaging and ab initio study on “d” superstructure in sedimentary dolomite
- Influence of temperature, pressure, and chemical composition on the electrical conductivity of granite
- Ti- and Zr-minerals in calcite-dolomite marbles from the ultrahigh-pressure Kimi Complex, Rhodope mountains, Greece: Implications for the P-T evolution based on reaction textures, petrogenetic grids, and geothermobarometry
- Ab initio thermodynamic and thermophysical properties of sapphirine end-members in the join Mg4Al8Si2O20-Mg3Al10SiO20
- The relation between Li ↔ Na substitution and hydrogen bonding in five-periodic singlechain silicates nambulite and marsturite: A single-crystal X-ray study
- Natural analogs of belite sulfoaluminate cement clinkers from Negev Desert, Israel
- Australian sedimentary opal-A and its associated minerals: Implications for natural silica sphere formation
- The 2H and 3R polytypes of sabieite, NH4Fe3+(SO4)2, from a natural fire in an oil-bearing shale near Milan, Ohio
- Letter
- Te-rich raspite, Pb(W0.56Te0.44)O4, from Tombstone, Arizona, U.S.A.: The first natural example of Te6+ substitution for W6+
- New Mineral Names
- Book Review

