Influence of structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity
-
Katarzyna Wińska
, Małgorzata Grabarczyk
Abstract
The aim of this article is influence of the structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity. This work was based on the general remark that even the smallest change in the structure of a compound can affect its biological properties. The results of the biotransformation of four bicyclic unsaturated lactones with one or two methyl groups in the cyclohexene ring was tested using fifteen fungal strains (Fusarium species, Penicillium species, Absidia species, Cunninghamella japonica, and Pleurotus ostreatus) and five yeast strains (Yarrowia lipolytica, Rhodorula marina, Rhodorula rubra, Candida viswanathii, and Saccharomyces cerevisiae). During these transformations, new epoxylactone and hydroxylactone were obtained. The relationship between the substrate structure and the ability of the microorganisms to transform them were analysed. Only compounds with C–O bond of lactone ring in the equatorial position were transformed by fungus. All presented here lactones were examined also for their antimicrobial activity. It turned out that these compounds exhibited growth inhibition of bacteria and fungi, mainly Bacillus subtilis, Candida albicans, Aspergillus niger, and Penicillium expansum.
1 Introduction
Among naturally occurring terpenoid lactones, it is possible to find those in which an exo or endocyclic double bond is present in the molecule [1], [2], [3], [4]. Sometimes, these compounds have different interesting biological properties such as antimicrobial [5], antiproliferative [6], cytotoxic [7], [8], or antifeedant effects [9]. Lactones with a double bond are sometimes characterized by an interesting odor such as coconut [10], [11], butter cake, milky almond [12], green tea [13], or celery leaves or stem [14].
Biotransformed unsaturated terpenoid lactones [4], [15], [16], [17] as well as mono-[18] or sesquiterpenoid [19], [20] compounds can undergo several different reactions: hydrogenation of the double bond [15], [16], epoxidation [15], [21], or hydroxylation [15], [17], [18], [19], [20]. The hydroxy group can be positioned at different places in the molecule. The first possibility is to obtain the diol by using one of two possible methods: opening the epoxide ring [15] or directly by dihydroxylation of the double bond [15], [18], [19], [20]. The second possibility is to introduce the hydroxy group in an allylic position [15], [17], [18], [20], [21].
As we know from the literature mentioned above, biotransformation of unsaturated terpenoid compounds may yield different, interesting derivatives such as epoxylactones or hydroxylactones. For this reason, we have chosen to carry out biotransformation of some unsaturated bicyclic lactones. Our goal was to determine the influence of the number (one or two) and position of methyl groups present in the molecule on the type of products formed during biotransformation. We hypothesized that the unsaturated lactones we chose as well as the products of their biotransformation would be characterized by interesting antimicrobial activities. Our previous experience had shown that the hydroxylactones obtained by biotransformation were able to inhibit the growth of pathogenic bacteria and fungi [22], [23], [24].
2 Materials and methods
2.1 Chemical analysis
The progress of chemical reactions and biotransformations as well as the purity of isolated products were monitored by TLC-technique on silica gel-coated aluminum plates (DC-AlufolienKieselgel 60 F254, Merck, Darmstadt, Germany). Chromatograms were developed with a mixture of hexane, acetone, and diethyl ether in various ratios (for unsaturated lactones hexane : diethyl ether 4:1, for products biotransformation hexane : acetone 3:1). Compounds were detected by spraying the plates with 1% Ce(SO4)2, 2% H3[P(Mo3O10)4] in 10% H2SO4 or 20% ethanolic H2SO4, containing 0.1% of anisaldehyde, followed by heating to 120 °C. Preparative column chromatography was performed on silica gel (Kieselgel 60, 230–400 mesh ASTM, Merck, Darmstadt, Germany) with a mixture of hexane and acetone (for unsaturated lactones hexane : acetone 4:1, for products biotransformation hexane : acetone 3:1) as eluents. GC analysis was carried out on an Agilent Technologies 6890N instrument(Varian, Agilent Technologies, Santa Clara, CA, USA) using a DB-17 column (cross-linked methyl silicone gum, 30 m×0.32 mm×0.25 μm) or on a Varian CP3380 instrument (Varian, Agilent Technologies, Santa Clara, CA, USA) using Thermo TR-5 (30 m×0.32 mm×1.0 μm) capillary columns. The enantiomeric compositions of the products obtained during biotransformation were determined by GC analysis using the chiral column CP-cyclodextrin-B-325 (30 m×0.25 mm×0.25 μm) under the following conditions: injector 200 °C, detector (FID) 250 °C, column temperature 140 °C, hold 45 min; ramp 140–200 °C at rate 20 °C/min and hold 1 min at 200 °C. The molar masses of the obtained compounds were confirmed by high-resolution mass spectrometry analysis using a Waters LCT Premier XE instrument (ESI ionization) (Waters Division, Milford, Massachusetts).
1H NMR and 13C NMR spectra were recorded in a CDCl3 solution on a BrukerAvance DRX 300 MHz spectrometer (Bruker, Billerica, MA, USA) or on a BrukerAvance™ 600 MHz spectrometer (Bruker, Billerica, MA, USA). Chemical shifts are reported in reference to the residual solvent signal (δH=7.26). IR spectra were recorded on a Thermo-Nicolet IR300 FT-IR spectrometer (Waltham, MA, USA). Optical rotations were determined on a P-2000 polarimeter (Jasco Easton, PA, USA) in chloroform solutions, with concentrations denoted in g/100 mL. The melting points were determined on a Boetius apparatus. The refractive index was measured on Carl Zeiss Abbe and Pulfrichrefractometer (Jena, Germany).
2.2 Synthesis of substrates
Two new racemic unsaturated lactones 2a and 2b were synthesized from the δ-iodo-γ-lactones 1a [25] and 1b [26] according to the procedure described earlier [27]. Two unsaturated lactones 2c and 2d were obtained in an analogous manner [27] (Scheme 1).
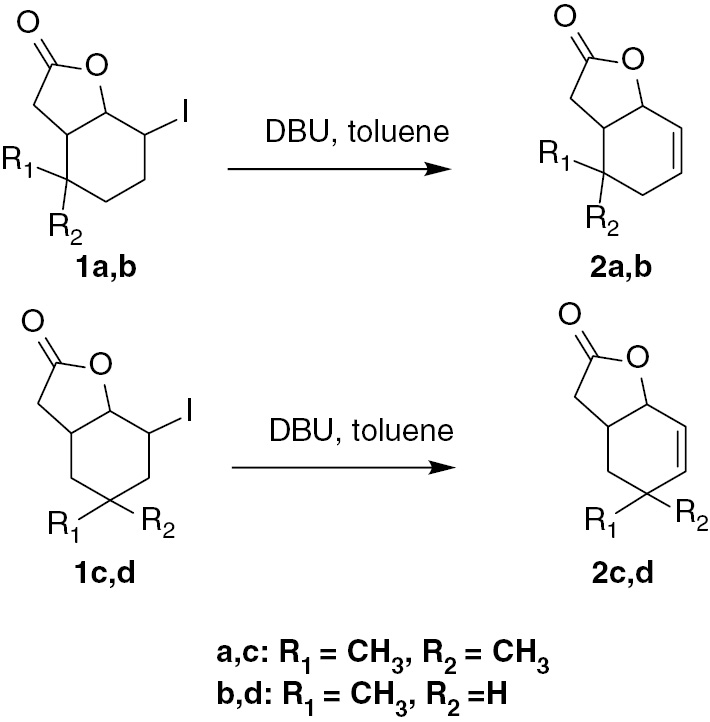
Synthesis of unsaturated lactones 2a–d.
In this article, we present the synthesis and spectral data of new compounds 2a and 2b and the spectral data of compounds 2c and 2d (according to literature) [27]. This data will be useful in studying the relationship between the structure of the substrate and its interaction with microorganisms.
2.2.1 5,5-Dimethyl-9-oxabicyclo[4.3.0]non-2-en-8-one 2a
The mixture of 2.5 g (8.5 mmol) of iodolactone 1a and 2 mL 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) dissolved in 40 mL of toluene was subjected to processing according to a known procedure [27]. The product of this reaction was a colorless liquid (1.3 g, 92% yield).The spectral data of product 2a are as follows: nD: 1.4850. IR (KBr, cm−1) ν 2959, 1779, 1168, 1003. 1H NMR (300 MHz, CDCl3) δ 0.96 (s, 3H, CH3-9), 1.00 (s, 3H, CH3-10), 1.83 (ddd, J 18.4, 4.8, 0.7 Hz, 1H, one of CH2-4), 2.03 (dd, J 18.4, 2.6 Hz, 1H, one of CH2-4), 2.30 (m, 1H, one of CH2-7), 2.36 (m, 1H, one of CH2-7), 2.43 (m, 1H, H-6), 4.96 (m, 1H, H-1), 5.71 (dd, J 10.2, 1.2 Hz, 1H, H-2), 5.88 (ddd, J 10.2, 4.8, 2.6 Hz, 1H, H-3); 13C NMR (75 MHz, CDCl3) δ 26.9 (C-10), 28.1 (C-9), 30.3 (C-7), 31.5 (C-5), 32.0 (C-5), 34.5 (C-4), 44.6 (C-6), 77.0 (C-1), 123.2 (C-2), 130.2 (C-3), 176.9 (C-8). ESIHRMS m/z 167.1068 [M+H]+ (calculated for C10H14O2 [M+H]+, 167.1067).
2.2.2 5-Methyl-9-oxabicyclo[4.3.0]non-2-en-8-one 2b
Iodolactone 1b (2.3 g, 8.2 mmol) and 1 mL 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) were mixed, and the reaction was carried out in an analogous manner as described above. A colorless liquid was obtained (1.1 g, 78% yield). The spectral data of product 2b are given below: nD: 1.4935. IR (KBr, cm−1) ν 2958, 1779, 1171, 948. 1H NMR (300 MHz, CDCl3) δ 1.00 (d, J 6.6 Hz, 3H, CH3-9), 1.53 (m, 1H, H-5), 1.74 (m, 1H, one of CH2-4), 2.16 (m, 1H, one of CH2-4), 2.23 (m, 1H, H-6), 2.51 (dd, J 17.6, 2.3 Hz, 1H, one of CH2-7), 2.73 (dd, J 17.6, 8.2 Hz, 1H, one of CH2-7), 4.82 (m, 1H, H-1), 5.90 (m, 1H, H-2), 6.11 (m, 1H, H-3). 13C NMR (75 MHz, CDCl3) δ 18.9 (C-9), 28.3 (C-5), 31.7 (C-4), 34.1 (C-7), 40.8 (C-6), 44.6 (C-6), 76.2 (C-1), 122.7 (C-2), 133.7 (C-3), 176.5 (C-8). ESIHRMS m/z 153.0916 [M+H]+ (calculated for C9H12O2 [M+H]+, 153.0910).
The spectral data of 4.4-dimethyl-9-oxabicyclo[4.3.0]non-2-en-8-one 2c are as follows: IR (KBr, cm−1) ν 2976, 1772, 1180, 972, 944. 1H NMR (300 MHz, CDCl3) δ 1.00 (s, 3H, CH3-9), 1.05 (s, 3H, CH3-10), 1.28 (dd, J 13.4, 13.2 Hz, 1H, one of CH2-5), 1.50 (ddd, J 13.4, 4.7, 1.0 Hz, 1H, one of CH2-5), 2.25 (d, J 17.3 Hz, 1H, one of CH2-7), 2.66 (dddd, J 13.2, 8.0, 5.2, 4.7, 1H, H-6), 2.86 (dd, J 17.3, 8.0 Hz, 1H, one of CH2-7), 4.70 (dd, J 5.2, 4.3 Hz, 1H, H-1), 5.80 (dd, J 10.0, 4.3 Hz, 1H, H-2), 5.92 (d, 1H, J 10.0, H-3).
The spectral data of 4-methyl-9-oxabicyclo[4.3.0]non-2-en-8-one2d are given below: IR (KBr, cm−1) ν 2957, 1789, 1173, 967, 948. 1H NMR (300 MHz, CDCl3) δ 1.00 (m, 1H, one of CH2-5), 1.06 (d, J 7.2 Hz, 3H, CH3-9), 1.73 (ddd, J 12.6, 4.3, 4.3 Hz, 1H, one of CH2-5), 2.19 (m, 1H, H-4), 2.28 (d, J 17.4 Hz, 1H, one of CH2-7), 2.51 (m, 1H, H-6), 2.86 (dd, J 17.4, 8.1 Hz, 1H, one of CH2-7), 4.73 (m, 1H, H-1), 5.91 (dm, J 10.0 Hz, 1H, H-3), 5.98 (d, J 10.0 Hz, 1H, H-2). 13C NMR (600 MHz, CDCl3) δ 20.8 (C-9), 29.7 (C-4), 33.3 (C-5), 33.8 (C-6), 37.0 (C-7), 75.1 (C-1), 121.7 (C-2), 141.4 (C-3), 176.4 (C-8).
2.3 Microorganisms
The fungal and yeast strains used in all biotransformation came from the collection of the Institute of Biology and Botany, Medical University, Wrocław (Fusarium culmorum AM10, Fusarium culmorum AM196, Fusarium avenaceum AM11,Fusarium oxysporum AM13, Fusarium semitectum AM20, Fusarium solani AM203, Penicillium vermiculatum AM30, Penicillium chermesinum AM113, Penicillium frequentas AM351, Absidia coerulea AM93, Absidia glauca AM177, Absidia cylindrospora AM336, Absidia glauca AM524, Cunninghamella japonica AM472, Pleurotus ostreatus AM482, Yarrowia lipolytica AM71, Rhodorula marina AM77, Rhodorula rubra AM82, Candida viswanathii AM120, Saccharomyces cerevisiae AM464). All of these strains were cultivated on Sabouraud’s agar containing 0.5% of aminobac, 0.5% of peptone, 4% of glucose, and 1.5% of agar dissolved in distilled water at 28 °C and stored in refrigerator at 4 °C.
2.4 Screening procedure
Biotransformation was performed in two 300 mL Erlenmeyer flasks containing 100 mL of medium consisting of 3% glucose and 1% peptone. When the culture was grown (3 days), 10 mg of the substrate dissolved in 1 mL of acetone was added to each flask. Incubation of shaken cultures with substrate was continued for 14 days. After 5, 9, and 14 days of incubation, the medium contained unreacted substrate, product and mycelium were extracted with dichloromethane (15 mL) and analyzed by GC (DB-17 column).
2.5 Preparative biotransformation
This step was similar to this described above, with the difference that we used 10 Erlenmeyer flasks (300 mL) containing 3-day cultures of fungal strains to which was added 100 mg of substrate dissolved in 10 mL acetone. After 14 days, the postreaction mixture was extracted with dichloromethane (3×40 mL). The combined organic fractions were dried over anhydrous magnesium sulfate and the solvent was evaporated in vacuo. Pure product was separated from the unreacted substrate and the metabolites of the fungi by using column chromatography (silica gel, hexane : acetone 3:1).
Epoxylactone(2,3-epoxy-5,5-dimethyl-9-oxabicyclo[4.3.0]nonan-8-one) (3a) was obtained as a white solid. The spectral data are given below:
mp: 57‒58 °C. IR (KBr, cm−1) ν 2918, 1771, 1159, 1018. 1H NMR (600 MHz, CDCl3) δ 0.91 (s, 3H, CH3-9), 1.03 (s, 3H, CH3-10), 1.78 (dd, J 15.8, 2.0 Hz, 1H, one of CH2-4), 1.85 (dd, J 15.8, 3.2 Hz, 1H, one of CH2-4), 2.33-2.47 (m, 3H, H-6 and CH2-7), 3.26 (d, J 3.5 Hz, 1H, H-2), 3.33 (m, 1H, H-3), 4.83(d, J 6.3 Hz, 1H, H-1). 13C NMR (151 MHz, CDCl3) δ 27.8 (C-9), 28.6 (C-10), 29.5 (C-5), 30.0 (C-7), 33.7 (C-4), 42.9 (C-6), 50.4 (C-2), 52.9 (C-3), 74.9 (C-1), 176.0 (C-8). ESIHRMS m/z 183.1021 [M+H]+ (calculated for C10H14O3 [M+H]+, 183.1016).
Hydroxylactone(4-hydroxy-5,5-dimethyl-9-oxabicyclo[4.3.0]nonan-8-one) 4a was obtained as a white solid. The spectral data are as follows:
IR (KBr, cm−1) ν3453, 2965, 1772, 1477, 1171, 1001. 1H NMR (600 MHz, CDCl3) δ 0.97 (s, 3H, CH3-9 A), 0.99 (s, 3H, CH3-10 A), 1.02 (s, 3H, CH3-9 B), 1.09 (s, 3H, CH3-10 B), 1.27 (m, 1H, OH A), 2.19 (m, 1H, OH B), 2.36 (dd, J 17.4, 10.9 Hz, 1H, one of CH2-7 B), 2.42 (m, H-6 A), 2.48 (dd, J 17.4, 8.7 Hz, 1H, one of CH2-7 B), 2.60 (dd, J 17.8, 8.9 Hz, 1H, one of CH2-7 A), 2.69 (m, H-6 B), 2.89 (dd, J 17.8, 6.2 Hz, 1H, one of CH2-7 A), 3.90 (s, 1H, H-4 A), 4.09 (s, 1H, H-4 B), 4.87 (m, 1H, H-1 A), 5.00 (m, 1H, H-1 B), 5.83 (m, 1H, H-2 B), 5.92 (m, 2H, H-3 A and H-3 B), 6.00 (m, 1H, H-2 A). 13C NMR (151 MHz, CDCl3) δ 18.7 (C-9 A), 20.8 (C-9 B), 24.7 (C-10 B), 25.7 (C-10 A), 29.5 (C-7 B), 31.4 (C-7 A), 35.3 (C-5 A), 37.0 (C-5 B), 42.5 (C-6 A), 44.7 (C-6 B), 70.7 (C-4 B), 72.7 (C-4 A), 75.7 (C-1 A), 76.5 (C-1 B), 123.6 (C-2 B), 124.8 (C-3 A), 133.8 (C-3 B), 133.98 (C-2 A), 176.3 (C-8 B), 176.8 (C-8 A). ESIHRMS m/z 183.1021 [M+H]+ (calculated for C10H14O3 [M+H]+, 183.1016).
2.6 Bioassay
The tests have been made by using following bacteria strains: Escherichia coli ATCC 10536, Staphylococcus aureus DSM 799, Bacillus subtilis PCM 2850, yeast strains: Candida albicans CBS 767 and filamentous fungi strains: Aspergillus niger KKBMZ X1, Fusarium linii KKBMZ 3A, Penicilliumexpansum CBS 664. Alternariaalternata CBS 1526. The test was performed in the automated Bioscreen C system (Automated Growth Curve Analysis System, Lab Systems, Finland) with procedure analogous to described before [24].
2.7 Statistical analysis
Results of microbiological experiments are shown as mean±S.E. Statistical significance was determined using one-way ANOVA with Dunnett’s multiple comparison test.
3 Results and discussion
During our previous studies, we noticed that some of the tested compounds were not converted by microorganisms. That prompted us to compare the properties of selected compounds with their spatial structure. In order for this, four unsaturated structurally related lactones with difference in position and number of methyl groups in cyclohexene ring 2a–d were chosen. These substrates were subjected to a screening biotransformation in order to verify the ability of microorganisms to convert these compounds into other products. Fifteen fungal strains and five yeast strains were used for this step. It turned out that only one of the substrates was transformed. Positive results of these tests are presented in Table 1.
Positive results of screening biotransformation of lactone (2a) after 14 days of incubation (in % according to GC).
| Entry | Microorganism | Screening biotransformation/% | ||
|---|---|---|---|---|
| (2a) | (3a) | (4a) | ||
| 1 | Fusarium culmorumAM10 | 14.8 | 85.2 | – |
| 2 | Fusarium culmorum AM196 | 41.5 | 58.5 | – |
| 3 | Fusarium avenaceumAM11 | 89.0 | 11.0 | – |
| 4 | Fusarium oxysporumAM13 | 86.5 | 13.5 | – |
| 5 | Fusarium solani AM203 | 86.4 | 13.6 | – |
| 6 | Penicillium vermiculatum AM30 | 66.6 | 33.4 | – |
| 7 | Absidia coerulea AM93 | 87.0 | 13.0 | – |
| 8 | Absidia cylindrospora AM336 | 54.0 | – | 29.0+17.0 |
As it is shown in Table 1, of the 20 tested microorganisms, only some were capable of converting one of the chosen substrates. Lactone 2a, with cyclohexene ring containing two methyl groups, was transformed by seven microorganisms into the same product – epoxylactone 3a. The best biotransformation results were obtained for strains of F. culmorum AM10 (entry 1) and AM196 (entry 2). In other cases, the degree of conversion did not exceeded 20% except for P. vermiculatum AM30 (entry 6), which created 33% of product. The other compounds, lactones 2b, 2c, and 2d, were not converted at all. Knowing from past experiences [21] that the A. cylindrospora AM336 strain was able to transform lactones 2c and 2d, the ability of this strain to convert lactones 2a and 2b, which were not tested earlier, was also checked. In the case of lactone 2a instead of epoxylactone 3a, a cis–trans mixture of hydroxylactone 4a was obtained (entry 8). Lactone 2b was also not transformed by this strain.
The obtained results were a complete surprise. Firstly, biotransformation of lactones 2c and 2d has been described in the literature [21]: A. cylindrospora strain was able to catalyze the epoxidation of these substrates. Secondly, Fusariumoxy sporum catalyzed hydroxylation most often, but epoxidation very seldomly [21], [22]. For this reactions, such enzymes as chloroperoxidase, unspecific peroxygenase (UPO, EC 1.11.2.1.), or intracellular P450 monooxygenases [28] may be responsible.
Hitherto chloroperoxidase and peroxygenase have not been detected in Fusarium strains. It seems to be probable that monooxygenases were responsible for the biotransformation described in this article. Some P450 monooxygenases have been identified in Fusarium. CYP55A1 (EC 1.7.1.14, class IX P450 enzymes), which is specific for NADH and participates in denitrification. CYP58 (class II P450 enzymes) catalyses epoxidation in mycotoxin synthesis. In the detoxification/degradation of xenobiotics, the first published and best studied P450 is pisatin demethylase (PDA, CYP57A1). Hydroxylation of fatty acids is catalyzed by P450foxy (CYP505A1, EC 1.14.14.1) – a catalytically self-sufficient flavocytochrome, which belongs to class VIII of the P450 enzymes. It is important to note that P450foxy (CYP505A1) shares several characteristics (e.g., primary structure organization, strong preference for NADPH over NADH as the electron donor, function, catalytic rate) with P450BM3 (CYP102A1) from Bacillus megaterium. The main difference between these two enzymes is intracellular localization. CYP505A1 was recovered from the membrane fraction, whereas CYP102A1 is a soluble protein like other bacterial P450s [29].
Very recently, a diastereoselective epoxidation of cis-1,2,3,6-tetrahydrophthalate was described, which was catalyzed by Bacillus megaterium P450BM3[30]. Based on this information, it seems to be probable that CYP505A1 could catalyze the epoxidation described in our article.
Strains of Absidia are known for their ability to hydroxylate structurally different substrates, such as steroids [31] and lactones [32] among others. Despite this fact, there are no reports in the scientific literature on the specific enzymes responsible for hydroxylation, which were isolated from the genus Absidia. Hydroxylation in the allylic position of the substrate may be catalyzed by an enzyme homologous to monooxygenases P450BM3. Recently, hydroxylation of other compounds in this position catalyzed by CYPBM3 has been described [33].
In the next step, three strains: F. culmorum AM10 (entry 1), F. culmorum AM196 (entry 2), and A. cylindrospora AM336 (entry 8) were used to conduct biotransformation of 2a on the preparative scale. The obtained results are shown in Figure 1.
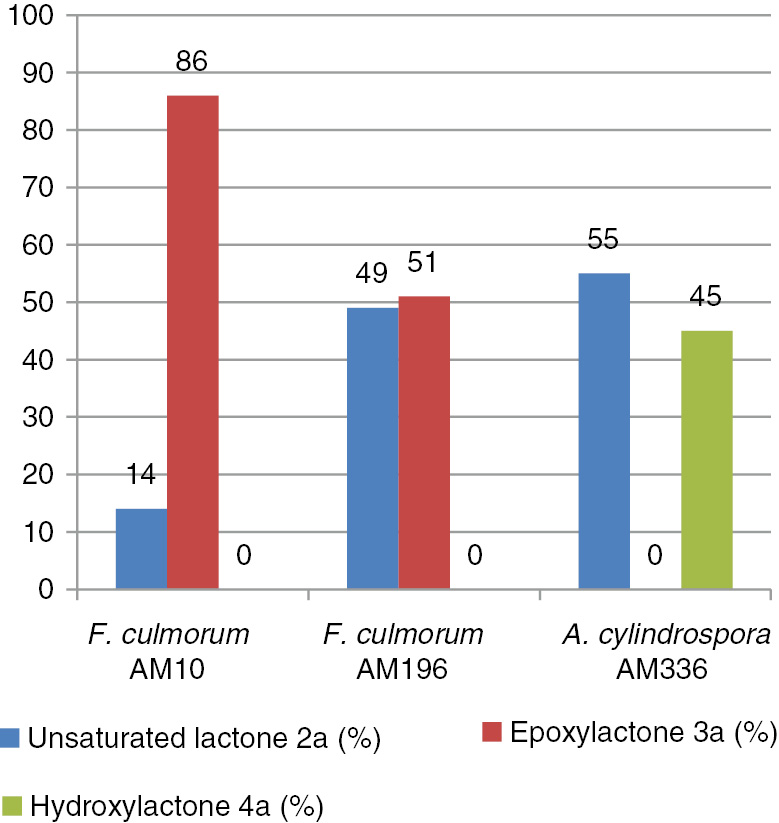
Results of preparative biotransformations (by GC-FID) of lactone (2a).
During both preparative biotransformations carried on lactone 2a using Fusarium strains as a biocatalyst, the same product – a new epoxylactone 3a, was obtained. F. culmorum AM10 gave this product with a high degree of conversion (86.1%); after purification, 25.8 mg (23.5%) of pure epoxylactone 3a was obtained. F. culmorum AM196 was a less efficient biocatalyst, giving only 51.2% of epoxylactone 3a and consequently 11.0 mg (10.0%) of pure compound 3a. The transformation conducted by A. cylindrospora AM336 strain provided another product–hydroxylactone 4a. This product was created as a cis–trans mixture in a ratio of 67:33; named B and A, respectively. Hydroxylactone 4a was obtained with a yield of 7.6 mg (6.9%) (Scheme 2).
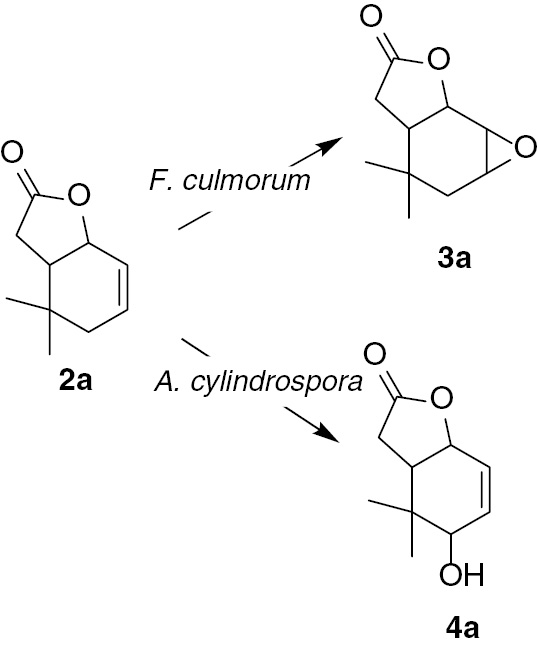
Biotransformation of unsaturated lactone (2a).
Since one of our goals was to obtain product characterized by high optical purity, we examined enantiomeric excess and also optical rotation for both of the epoxylactones obtained from the biotransformation. Results of these measurements are as follows: 3a from F. culmorum AM10 ee3.7%,
Hydroxylactone 4a was obtained in a small amount (almost 8 mg) and also as a mixture of two isomers, so we were not able to separate them during the purification. Therefore, we focused only on determining its structure.
In order to find the correlation between the structure and its ability to biotransformation, we compared spectral data (1H NMR, 13C NMR, COSY, HMQC, and IR) of substrates and products. Analysis of the 1H NMR spectra of these lactones indicated some differences in the structures of these molecules. In all of them, the cyclohexene ring took the form of a deformed chair. In the case of 2a, proton H-1 was in the axial position and proton H-6 was in the equatorial position, which was confirmed by their shapes – wide and narrow multiplets, respectively, whereas in molecules of lactone 2b, the proton H-1 was in the equatorial position (narrow multiplet) and proton H-6 was in the axial position (wide multiplet) (Figure 2).
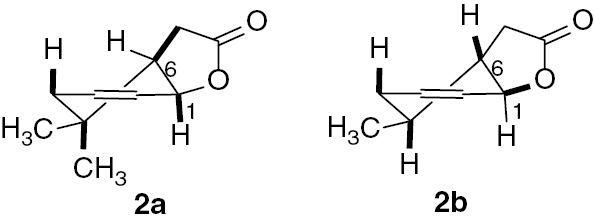
Structures of lactones (2a) and (2b).
Looking at 1H NMR spectra of unsaturated lactones 2c and 2d, we could see that the shapes of their molecules were similar to the shape of lactone 2b. In these cases, the signals coming from proton H-6 were doublet of doublet of doublet of doublet with coupling constants equal to 13.2, 8.0, 5.2, and 4.7 Hz [for 2c] and wide multiplet [for 2d], which indicated their axial position. In the other case, the signals of proton H-1 were doublet of doublet with a small coupling constant (5.2 and 4.3 Hz) and narrow multiplet for 2c and 2d, respectively, suggesting their equatorial position (Figure 3).
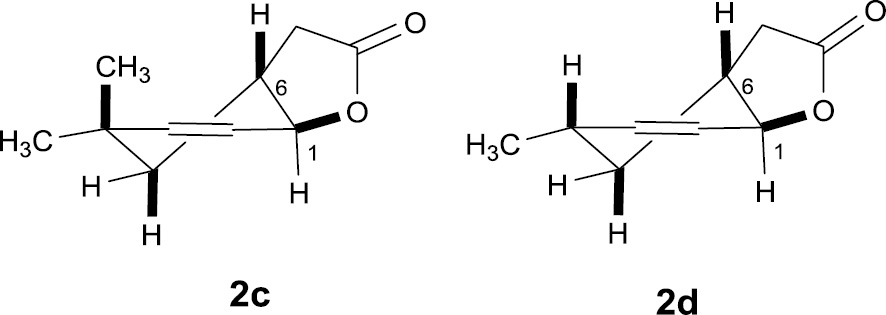
Structures of lactones (2c) and (2d).
Differences and similarities in the structures of our four lactones could bring about the differences observed during the biotransformation. Lactone 2a was transformed into epoxylactone 3a by several strains and into a cis-trans mixture of hydroxylactone 4a by one microorganism. In the case of lactones 2b, 2c, and 2d, we did not observe even a trace of product.
As a result of biotransformation, we obtained epoxylactone 3a in which the γ-lactone ring was retained (absorption band at 1771 cm−1 on IR spectra). Analysis of the NMR spectra indicated that the cyclohexene ring was in the chair conformation. The signal of proton H-1 as a doublet with coupling constant 6.3 Hz and the wide multiplet of proton H-6 suggested their axial and equatorial positions, respectively (similar to the substrate). The signal of proton H-2 as a doublet with a coupling constant of 3.5 Hz indicated its equatorial position. Two doublets from CH2-4 with coupling constants equal to 15.8, 3.2 Hz and 15.8, 2.0 Hz implied an axial orientation of the oxirane ring (Figure 4).
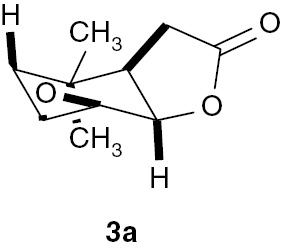
Structure of epoxylactone (3a).
The second product obtained during biotransformation of unsaturated lactone 2a was hydroxylactone 4a. This compound was obtained as a mixture of cis–trans isomers. GC analysis proved that these two isomers were formed in the ratio of 67%:33%, which allowed a precise assignment of the signals in the NMR spectra. Analysis of the NMR spectra indicated that the deformed chair conformation of the cyclohexene ring was retained. The hydroxy group was introduced in an allylic position. The predominant position for the hydroxy group was the cis location (Figure 5).
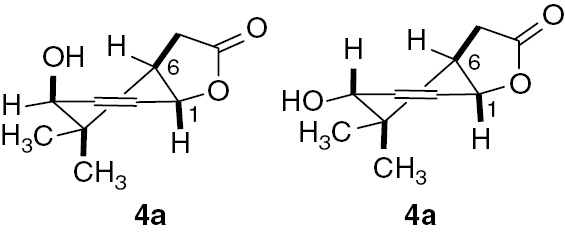
Structure of hydroxylactone (4a).
Analyzing the influence of the substrate’s structure on the degree of biotransformation revealed that the amount of methyl groups in the cyclohexene ring (one or two) does not matter. However, the spatial orientation of the lactone ring relative to the cyclohexene ring was very important. In cases where the C–O bond of the lactone ring was in the equatorial position (compound 2a), two strains of filamentous fungi were capable of epoxidation of the double bond and one strain introduced the hydroxy group in allylic position. In contrast, when the C–O bond of the lactone ring was in the axial position (compounds 2b–d), the tested microorganisms were completely unable to convert the substrates.
Based on the results of biotransformation were examined whether the structure of lactones affects on their toxic properties. Synthetically unsaturated lactones 2a–2d and microbiologically obtained epoxylactone 3a were examined for their antimicrobial activity. This biological test was chosen to determine the ability of these lactones to inhibit the growth of some bacteria, yeast, and fungal strains. Statistical analysis was performed for the results of microbiological tests. Calculations were made for each of the strains separately. Control and further compounds were treated as test variability. The underlying assumption was the null hypothesis – the average values for the control and substances do not differ. The results for a strain of Fusarium were statistically insignificant because it showed no differences between the mean values that were obtained after analysis of variance. For the other strains, the hypothesis was verified by an F-Senedecora test at the significance level of p<0.05. It has been shown that for each strain averages are significantly different. Then Dunnett’s test was checked, in which means are significantly different from the average for the control. Results have been presented in Table 2.
The assessment of the effects of the unsaturated lactones 2a–2d and epoxylactone 3a on the growth of bacteria, yeast and fungi.
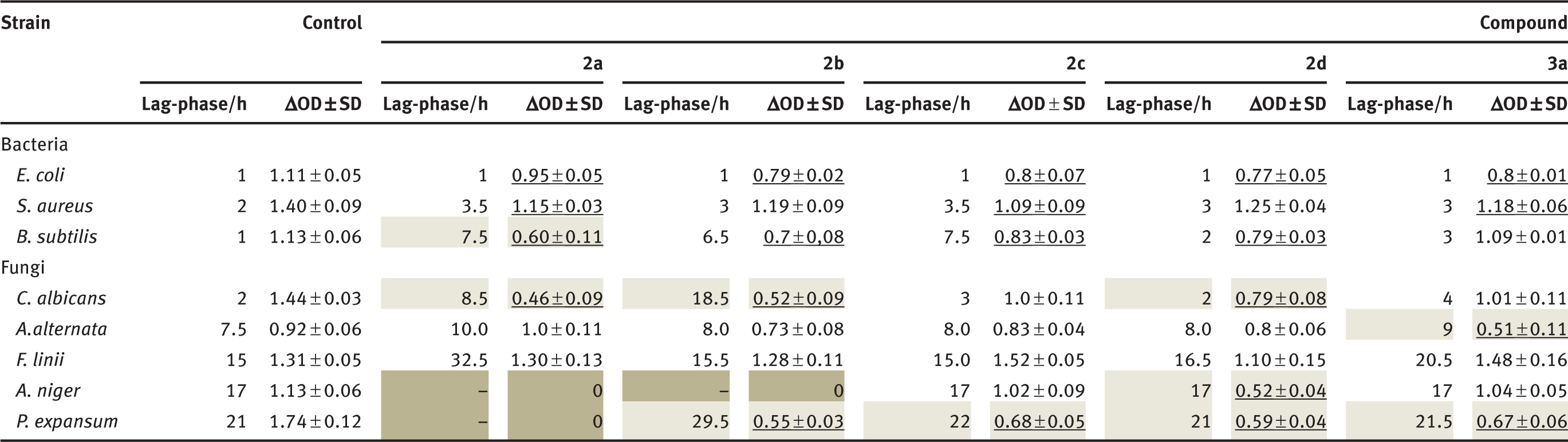
Statistics on a completely randomized design were determined using the one-way analysis of variance (ANOVA) procedure at a level of significance set at p<0.050. A comparison of average growth OD microorganisms relative to the control by Dunnett’s test. Underlined number means the average significantly different from control. Notes: OD (optical density) was measured for λ 550 nm. SD, Standard deviation.
Results presented in Table 2 indicate that the tested compounds affected growth of the selected bacteria strains. The strongest inhibitory effect was observed in the case of lactone 2a against the B. subtilis strain. In this case, the optical density (ΔOD) increase was equal to 0.60, preceded by a 7.5-h duration of the lag-phase, while in the control culture, these values was equal to 1.13 and 1 h, respectively.
The yeast strain C. albicans was relatively sensitive to the effects of the tested compounds. The lowest increase in biomass, compared to a control culture, was ΔOD of 0.46 and 0.52 and were found in the presence of lactones 2a and 2b. It was preceded by a long-lasting lag-phase of 8.5 and 18.5 h, respectively. Despite the short adaptive phase, the growth of the C. albicans strain also was inhibited significantly by lactone 2d.
The tested compounds restricted or inhibited strongly the growth of filamentous fungi. Among the cultures tested, the highest sensitivity was shown by the P.expansum strain, whose growth was inhibited by lactone 2a and was significantly reduced in the presence of the other compounds, demonstrated by a decrease in optical density (ΔOD) in the range 0.55–0.68 as compared with the control culture at 1.74.
A strain of A. niger was also highly sensitive; its growth was completely inhibited by lactones 2a and 2b. Although similar in the control culture lag-phase, lactone 2d caused significantly reduced growth of this strain. For comparison, the ΔOD of this strain was 0.52, whereas in the control culture, this parameter was equal to 1.13. In the case of the fungi A. alternata, the strongest inhibitory effect was observed in the presence of epoxylactone3a, in which the ΔOD biomass growth was equal to 0.51 in comparison to the control culture at 0.92.
It is worth noting that lactone 2a, although it caused the longest adaptive phase in culture of this strain, it allowed the highest level of biomass ΔOD at 1.0. The tested compounds generally did not exert inhibitory effects on the growth of the strain F. linii. Only in the presence of lactone 2a, was there a prolongation of the lag-phase to 32 h and a slowdown of growth; yet, the final biomass growth was even higher than for the control culture.
4 Conclusions
Four unsaturated lactones 2a–d were tested on their biotransformation ability by 20 fungal strains. Only one from four substrates, lactone 2a was transformed into epoxylactone 3a and hydroxylactone 4a. Analysis of the NMR spectra of these unsaturated lactones proved that lactone 2a was characterized by a different structure than lactones 2b–2d. The differences in structure may explain why lactone 2a was transformed into two products, and why lactones 2b–2d were not. The biological tests proved that lactone 2a was the best inhibitor for the growth of Aspergillus niger and Penicillium expansum. According to these tests, the presence of one or two methyl groups at carbon C-5 caused the stronger growth inhibition of bacteria and fungi strains. Introduction of the epoxide ring in the place of double bond resulted in reduction of growth inhibition of the tested strains.
References
1. Amoah SK, de Oliveira FL, de Cruz AC, de Souza NM, Campos FR, Barison A, et al. Sesquiterpene lactones from the leaves of Hedyosmum brasiliense (Chloranthaceae). Phytochemistry 2013;87:126–32.10.1016/j.phytochem.2012.11.018Suche in Google Scholar
2. Huang HL, Xu YJ, Liu L, Liu XQ, Shang JN, Han GT, et al. Eremophilane-type sesquiterpene lactones from Ligularia hodgsonii Hook. Phytochemistry 2002;72:514–7.10.1016/j.phytochem.2011.01.008Suche in Google Scholar
3. Xiong Y, Qu W, Sun JB, Wang MH, Liang JY. Eudesmane sesquiterpenoid lactones and abietane diterpenoids from Ajuga forrestii Diels. Phytochemistry Lett 2013;6:457–60.10.1016/j.phytol.2013.05.017Suche in Google Scholar
4. Tori M, Kawahara M, Sono M. Eremophilane-type sesquiterpenes from fresh rhizomes of Petasites japonicus. Phytochemistry 1998;47:401–9.10.1016/S0031-9422(97)00581-5Suche in Google Scholar
5. Fortuna AM, Juárez ZN, Bach H, Nematallah A, Av-Gay Y, Sánchez-Arreola E, et al. Antimicrobial activities of sesquiterpene lactones and inositol derivatives from Hymenoxys robusta. Phytochemistry 2011;72:2413–8.10.1016/j.phytochem.2011.09.001Suche in Google Scholar
6. Wu P, Su MX, Wang Y, Wang GC, Ye WC, Chung HY, et al. Supercritical fluid extraction assisted isolation of sesquiterpene lactones with antiproliferative effects from Centipeda minima. Phytochemistry 2012;76:133–40.10.1016/j.phytochem.2012.01.003Suche in Google Scholar
7. Liu Q, Ahn JH, Kim SB, Lee C, Hwang BY, Lee MK. Sesquiterpene lactones from the roots of Lindera strychnifolia. Phytochemistry 2013;87:112–8.10.1016/j.phytochem.2012.11.004Suche in Google Scholar
8. Popovic V, Heyerick A, Petrovic S, Van Calenbergh S, Karalic I, Niketic M, et al. Sesquiterpene lactones from the extracts of two Balkan endemic Laserpitium species and their cytotoxic activity. Phytochemistry 2013;87:102–11.10.1016/j.phytochem.2012.11.011Suche in Google Scholar
9. Luo SH, Hua J, Niu XM, Liu Y, Li CH, Zhou YY, et al. Defense sesterterpenoid lactones from Leucosceptrum canum. Phytochemistry 2013;86:29–35.10.1016/j.phytochem.2012.11.008Suche in Google Scholar
10. Brenna E, Fuganti C, Serra S. Enantioselective perception of chiral odorants. Tetrahedron: Asym 2003;14:1–42.10.1016/S0957-4166(02)00713-9Suche in Google Scholar
11. Dams I, Białońska A, Ciunik Z, Wawrzeńczyk C. Lactones. 21. Synthesis and odoriferous properties of lactones with the p-menthane system. J Agric Food Chem 2004;52:1630–4.10.1021/jf035272aSuche in Google Scholar
12. Meguro H, Orui H, Nishida Y. Optically active 4-(7-methyl-5,7-butadienyl)-butanolide and its preparation, Jpn Pat No. 59–219273,10.12.1984.Suche in Google Scholar
13. Kawakami M. Topics and progress in tea flavor science. Foods Food Ingredients J Jpn 2002;197:13–26.Suche in Google Scholar
14. Oguro D, Watanabe H. Asymmetric synthesis and sensory evaluation of sedanenolide. Biosci Biotechnol Biochem 2011;75:1502–5.10.1271/bbb.110206Suche in Google Scholar
15. Ma XC, Zheng J, Guo DA. Microbial transformation of dehydrocostuslactone and costunolide by Mucor polymorphosporus and Aspergillus candidus. Enzym Microb Technol 2007;40:1013–9.10.1016/j.enzmictec.2006.07.043Suche in Google Scholar
16. Guan H, You S, Yang L, Wang X, Ni R. Newly detected specific hydrogenation of the conjugated double bond of unsaturated alkaloid lactones by Aspergillus sp. Biotechnol Lett 2005;27:1189–93.10.1007/s10529-005-0015-ySuche in Google Scholar
17. Grudniewska A,Wawrzeńczyk C. Lactones 41. Synthesis and microbial hydroxylation of unsaturated terpenoid lactones with p-menthane ring systems. Molecules 2013;18:2778–87.10.3390/molecules18032778Suche in Google Scholar
18. Bhatti HN, Khan SS, Khan A, Rani M, Ahmad VU, Choudhary MI. Biotransformation of monoterpenoids and their antimicrobial activities. Phytomedicine 2014;21:1597–626.10.1016/j.phymed.2014.05.011Suche in Google Scholar
19. Miyazawa M, Honjo Y, Kameoka H. Biotransformation of the sesquiterpenoid (+)-γ-Gurjunene using a plant pathogenic fungus, Glomerella cingulata, as a biocatalyst. Phytochemistry 1998;49:1283–5.10.1016/S0031-9422(98)00170-8Suche in Google Scholar
20. Nunes FM, dos Santos GF, Saraiva NN, Trap MA, de Mattos MC, da CConceicao M, et al. New fungi for whole-cell biotransformation of carvone enantiomers. Novel p-menthane-2,8,9-triols production. Appl Catal A 2013;468:88–94.10.1016/j.apcata.2013.08.034Suche in Google Scholar
21. Gładkowski W, Grabarczyk M, Wińska K, Ratuś B, Białońska A,Ciunik Z, et al. Lactones 26 [1]: Stereoselective microbial epoxidation of unsaturated bicyclic γ-lactones with the alkylsubstituted cyclohexane system. J MolCatal B: Enzymatic 2007;49:79–87.10.1016/j.molcatb.2007.08.006Suche in Google Scholar
22. Grabarczyk M, Wińska K, Mączka W, Żarowska B, Maciejewska G, Dancewicz K, et. al. Synthesis, biotransformation and biological activity of halolactones obtained from β-ionone. Tetrahedron 2016;72:637–44.10.1016/j.tet.2015.12.005Suche in Google Scholar
23. Grabarczyk M, Wińska K, Mączka W, Żarowska B, Białońska A, Anioł M. Hydroxy lactones with the gem-dimethylcyclohexane system – Synthesis and antimicrobial activity. Arabian J. Chem doi:10.1016/j.arabjc.2015.02.018.Suche in Google Scholar
24. Grabarczyk M, Mączka W, Wińska K, Żarowska B, Anioł M. Antimicrobial activity of hydroxylactone obtained by biotransformation of bromo- and iodolactone with gem-dimethylcyclohexane ring. J Braz Chem Soc 2013;24:1913–9.10.5935/0103-5053.20130238Suche in Google Scholar
25. Gładkowski W, Mazur M, Białońska A, Wawrzeńczyk C. Lactones 35 . Metabolism of iodolactones with cyclohexane ring in Absidia cylindrospora culture. Enzym Microb Technol 2011;48:326–33.10.1016/j.enzmictec.2010.12.007Suche in Google Scholar
26. Grabarczyk M, Wińska K, Mączka W, Żołnierczyk AK, Żarowska B, Anioł M. Lactones with methylcyclohexane systems obtained by chemical and microbiological methods and their antimicrobial activity. Molecules 2015;20:3335–53.10.3390/molecules20023335Suche in Google Scholar
27. Grabarczyk M, Szumny A, Gładkowski W, Białońska A, Ciunik Z, Wawrzeńczyk C. Lactones 18. Synthesis of bicyclic lactones with methyl-, di- and trimethyl substituted cyclohexane system. Polish J Chem 2005;79:1763–71.10.1002/chin.200611120Suche in Google Scholar
28. Peter S, Kinne M, Ullrich R, Kayser G, Hofrichter M. Epoxidation of linear, branched and cyclic alkenes catalyzed by unspecific peroxygenase. Enzym Microb Technol 2013;52:370–6.10.1016/j.enzmictec.2013.02.013Suche in Google Scholar
29. Cresnar B, Petric S. Cytochrome P450 enzymes in the fungal kingdom. Biochim Biophys Acta 2011;1814:29–35.10.1016/j.bbapap.2010.06.020Suche in Google Scholar
30. Ilie A, Lonsdale R, Agudo R, Reetz MT. A diastereoselective P450-catalyzed epoxidation reaction: anti versus syn reactivity. Tetrahedron Lett 2015;56:3435–7.10.1016/j.tetlet.2015.03.076Suche in Google Scholar
31. Brzezowska E, Dmochowska-Gladysz J, Kolek T. Biotransformation XXXIX. Metabolism of testosterone, androstenedione, progesterone and testosterone derivatives in Absidia coerulea culture. J Steroid Biochem Mol Biol 1996;57:357–62.10.1016/0960-0760(95)00279-0Suche in Google Scholar
32. Gładkowski W, Grabarczyk M, Wińska K, Białońska A, Ciunik Z, Wawrzeńczyk C. Lactones 27 [1]: Transformations of γ-lactones fused to a dimethylcyclohexane ring in Absidia cylindrospora cultures. J Mol Catal B: Enzymatic 2006;39:31–9.10.1016/j.molcatb.2006.01.023Suche in Google Scholar
33. Neufeld K, Henssen B, Pietruszka J. Enantioselective allylic hydroxylation of ω-alkenoic acids and esters by P450 BM3 monooxygenase. Angew Chem Int Ed 2014;53:13253–7.10.1002/anie.201403537Suche in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Aspernolides L and M, new butyrolactones from the endophytic fungus Aspergillus versicolor
- Synthesis, in-vitro cytotoxicity of 1H-benzo[f]chromene derivatives and structure–activity relationships of the 1-aryl group and 9-position
- Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites
- A comparative proteomic analysis for adventitious root formation in lotus root (Nelumbo nucifera Gaertn)
- Curviflorside and curviflorin, new naphthalene glycoside and flavanol from Plicosepalus curviflorus
- Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential
- Influence of structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity
- Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: in vitro evaluations
- Bioenergetic strategy of microalgae for the biodegradation of tyrosol and hydroxytyrosol
Artikel in diesem Heft
- Frontmatter
- Aspernolides L and M, new butyrolactones from the endophytic fungus Aspergillus versicolor
- Synthesis, in-vitro cytotoxicity of 1H-benzo[f]chromene derivatives and structure–activity relationships of the 1-aryl group and 9-position
- Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites
- A comparative proteomic analysis for adventitious root formation in lotus root (Nelumbo nucifera Gaertn)
- Curviflorside and curviflorin, new naphthalene glycoside and flavanol from Plicosepalus curviflorus
- Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential
- Influence of structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity
- Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: in vitro evaluations
- Bioenergetic strategy of microalgae for the biodegradation of tyrosol and hydroxytyrosol


