Virus inactivation under the photodynamic effect of phthalocyanine zinc(II) complexes
-
Mimi Remichkova
Abstract
Various metal phthalocyanines have been studied for their capacity for photodynamic effects on viruses. Two newly synthesized water-soluble phthalocyanine Zn(II) complexes with different charges, cationic methylpyridyloxy-substituted Zn(II)- phthalocyanine (ZnPcMe) and anionic sulfophenoxy-substituted Zn(II)-phthalocyanine (ZnPcS), were used for photoinactivation of two DNA-containing enveloped viruses (herpes simplex virus type 1 and vaccinia virus), two RNA-containing enveloped viruses (bovine viral diarrhea virus and Newcastle disease virus) and two nude viruses (the enterovirus Coxsackie B1, a RNA-containing virus, and human adenovirus 5, a DNA virus). These two differently charged phthalocyanine complexes showed an identical marked virucidal effect against herpes simplex virus type 1, which was one and the same at an irradiation lasting 5 or 20 min (Δlog=3.0 and 4.0, respectively). Towards vaccinia virus this effect was lower, Δlog=1.8 under the effect of ZnPcMe and 2.0 for ZnPcS. Bovine viral diarrhea virus manifested a moderate sensitivity to ZnPcMe (Δlog=1.8) and a pronounced one to ZnPcS at 5- and 20-min irradiation (Δlog=5.8 and 5.3, respectively). The complexes were unable to inactivate Newcastle disease virus, Coxsackievirus B1 and human adenovirus type 5.
1 Introduction
The photodynamic process utilizes visible light which is absorbed by a photosensitizer, which converts it into an excited state. The excited state of photosensitizers undergoes intersystem crossing to the long-live triplet state which reacts with the molecular oxygen inducing singlet oxygen, superoxide and free radicals. They can oxidize proteins, nucleic acids and lipids, thus leading to damages of bioorganic molecules [1], [2]. The photosensitizing activity of different dyes has been extensively studied for their capability to inactivate bacteria, fungi and viruses [3], [4], [5], [6], [7]. Many compounds with photoactive properties have been synthesized since the first positive results about the bactericidal effect of acridine hydrochloride, published a century ago. Phenothiazine dyes such as methylene blue and toluidine blue, several cyanine dyes, porphyrins, phthalocyanines and their metal complexes are known as photosensitive compounds. At present, it is known that the photodynamic effect depends on the charge of the photosensitizer, its lipophylicity, the molecular extinction coefficient, the redox potential of the triplet excited state, the spin state and the metal bond in metal chelate photosensitizers [8], [9].
Various phthalocyanines, porphyrin-like synthetic pigments, were studied for their capacity for photodynamic inactivation (PDI) of viruses (vesicular stomatitis virus, VSV), either intracellular or free virions [3]. Cationic phthalocyanines with aluminum as a central metal demonstrated a marked capacity to inactivate Sindbis virus, VSV, and human immunodeficiency virus type 1 (HIV-1) in red blood concentrates [10]. An analogous photodynamic effect towards bovine viral diarrhea virus (BVDV), VSV, HIV-1 and the pseudorabies virus in in vitro red cells was established by the cationic porphyrin Tri-P(4), structurally closed to the cationic phthalocyanines [11].
The aim of this paper is to study the effect of two differently charged phthalocyanine Zn(II) complexes on viruses belonging to different taxonomic families.
2 Materials and methods
2.1 Photosensitizers
Phthalocyanine zinc(II) complexes: 2,9,16,23-tetrakis(3-methylpyrydyloxy) phtalocyanine-Zn(II) (ZnPcMe) was synthesized by [12], and 2,9,16,23-tetrakis(4-sulfophenoxy)phthalocyanine-Zn(II) (ZnPcS) ZnPcS as described in previous papers [12], [13].
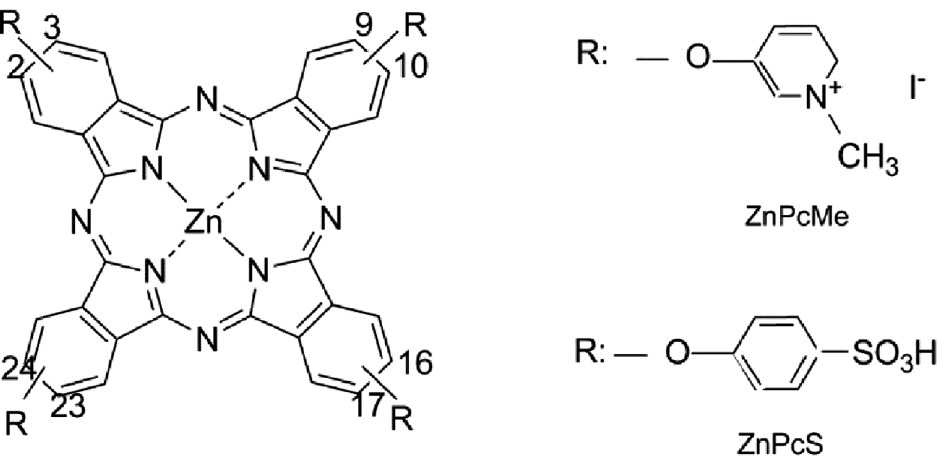
Stock solutions of the phthalocyanines (1 mM) were prepared in DMSO and stored frozen in the dark at –20 °C.
2.2 Viruses, embryonated eggs and cell cultures
Herpes simplex virus (HSV-1, DA strain) was cultivated in Madin-Darbey bovine kidney (MDBK) cells. BVDV (TVM strain) was propagated in the calf trachea cell line. Newcastle disease virus (NDV, Russeff strain) was grown in primary culture of chick embryo fibroblasts (CEF). Vaccinia virus (VV, Bratislava strain) was cultivated on the chorioallantoic membrane of 11-day-old chick embryos (200 cell culture infective doses 50%, CCID50/0.2 mL). The embryonated eggs were incubated at 37 °C for 5 days. After several passages in the chick embryos, the VV strain underwent two passages in Vero cells. Coxsackievirus B1 and human adenovirus type 5 were cultivated in HEp-2 cells. The virus assay was done in cell monolayers in 96-well microplates according to the end-point dilution method [14] and the infectious virus titer was evaluated in CCID50 on the basis of cytopathic effect (CPE) determined microscopically.
2.3 Media
Dulbecco’s modification of Eagle’s medium (DMEM) (Gibco BRL, Paisley, Scotland, UK) supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES [4-(2-hydroxyethyl)-1-piperazine-ethansulphonic acid] and antibiotics (100 IU/mL penicillin, 100 μg/mL streptomycin) was used as a growth medium for the cultivation of all cell lines in a CO2 incubator (5% CO2, HeraCell 150, Heraeus, Hanau, Germany) at 37 °C. Maintenance media for cultivation of the viruses and the virus assay contained DMEM supplemented with 2% FBS.
2.4 Photoinactivation procedure
Aliquots of 0.1 mL stock virus were mixed with 0.1 mL solution containing 0.58 μM ZnPcMe or 0.64 μM ZnPcS. They were irradiated for 20 min with light from a diode laser at 635 nm (Lumileads, USA) at a fluence rate of 100 mW/cm2 and a light dose of 50 J/cm2. The fluence rates were controlled with photometer equipment (Spectra Physics, USA). Light doses of 18 and 72 J/cm2 were reached through 5- and 20-min irradiation, respectively, at a fluence rate of 60 mW/cm2 to obtain a PDI effect on viruses. Three types of controls were used: (i) untreated by phthalocyanines not irradiated virus, (ii) untreated irradiated virus and (iii) phthalocyanines treated but not irradiated virus. Following the exposure period the residual infectivity in the samples and in the controls samples was determined by microscopic evaluation of the virus-induced CPE and a modification of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT assay) method [15]. The virucidal activity was expressed as the difference (Δlog) between the infectious virus titer of the test sample and the control (untreated by phthalocyanines). No influence of the photoinactivation procedure on the cell culture media was recorded.
2.5 Statistical analysis
Experiments were performed in triplicate. Data were expressed as mean values±standard deviation (SD). Student’s t-test was used to compare photodynamic irradiation values obtained with the dark and/or light control without a photosensitizer.
3 Results
The phthalocyanines ZnPcMe and ZnPcS at the concentrations applied did not show any cytotoxic effect on all cell cultures used in the experiments: MDBK, CT, Vero, HEp-2 and CEF.
3.1 Effect of phthalocyanine Zn(II) complexes on herpes simplex virus type 1
As seen in Table 1, HSV-1 (DA strain) manifested a high sensitivity to photoinactivation in the presence of the two photosensitizers; the infectious virus titer was decreased by 3.00 logs after a 5-min irradiation and by 4.00 logs after a 20-min irradiation. In dark the compounds were inactive during these time intervals.
Effects of ZnPcMe and ZnPcS on HSV-1.
| Experimental group | Virus titer, CCID50/0.1 mLa | Δlogb | Virus titer CCID50/0.1 mLa | Δlogb | ||
|---|---|---|---|---|---|---|
| Without ZnPcMe | With ZnPcMe | Without ZnPcS | With ZnPcS | |||
| No irradiation | 6.5±0.92 | 6.5±0.64 | 0 | 5.5±0.35 | 5.5±0.7 | 0 |
| 5-min irradiation | 6.5±0.4 | 3.5±0.58* | 3.0 | 5.5±0.8 | 2.5±0.64* | 3.0 |
| No irradiation | 6.5±0.86 | 6.5±0.9 | 0 | 5.5±0.67 | 5.5±0.2 | 0 |
| 20-min irradiation | 6.5±0.35 | 2.5±0.72** | 4.0 | 5.5±0.55 | 1.5±0.7** | 4.0 |
aReduction of virus titers after treatment with a photosensitizer. Data are expressed as mean values±SD; *p<0.05; **p<0.01; ***p<0.001 as compared with the dark/or light control without a photosensitizer.
bDifference between the infectious virus titers of the virus sample with a photosensitizer and the respective control without a photosensitizer.
3.2 Effect of phthalocyanine Zn(II) complexes on vaccinia virus
The activity of phthalocyanines on VV is presented in Table 2. A moderate effect, a decrease of the infectious virus content of 2 logs by ZnPcS and 1.8 logs by ZnPcMe following irradiation in the presence of the compounds, was registered. In dark an inactivation effect of 2.4 logs was registered by ZnPcMe after 20 min and of 2.2 logs by ZnPcS after 5 min.
Effects of ZnPcMe and ZnPcS on VV.
| Experimental group | Virus titer CCID50/0.1 mLa | Δlogb | Virus titer CCID50/0.1 mLa | Δlogb | ||
|---|---|---|---|---|---|---|
| Without ZnPcMe | With ZnPcMe | Without ZnPcS | With ZnPcS | |||
| Dark control | 5.5±0.86 | 5.5±0.52 | 0 | 5.7±0.78 | 3.5±0.95* | 2.2 |
| Light control | 5.5±0.91 | 3.3±0.96* | 2.2 | 5.7±0.85 | 3.3±0.92* | 2.4 |
| 5-min irradiation | 5.3±0.68 | 3.5±0.77 | 1.8 | 5.5±0.82 | 3.5±0.36* | 2.0 |
| No irradiation | 4.7±0.44 | 2.3±0.65* | 2.4 | 4.7±0.55 | 2.5±0.65* | 2.2 |
aReduction of virus titers after treatment with a photosensitizer. Data are expressed as mean values±SD; *p<0.05, **p<0.01, ***p<0.001 as compared with the dark/or light control without a photosensitizer.
bDifference between the infectious virus titers of the virus sample with a photosensitizer and the respective control without a photosensitizer.
3.3 Effect of phthalocyanine Zn(II) complexes on bovine viral diarrhea virus
As seen in Table 3, the cationic phthalocyanine ZnPcMe inactivated BVDV only following the irradiation. This effect was a moderate one, not exceeding 2 logs. The other photosensitizer, the anionic ZnPcS, was far more effective: the infectious virus titer was decrease by 5–6 logs. ZnPcS was also capable of inactivating the virus in dark, but this effect did not exceed 2 logs.
Effects of ZnPcMe and ZnPcS on BVDV.
| Experimental group | Virus titer CCID50/0.1 mLa | Δlogb | Virus titer CCID50/0.1 mLa | Δlogb | ||
|---|---|---|---|---|---|---|
| Without ZnPcMe | With ZnPcMe | Without ZnPcS | With ZnPcS | |||
| No irradiation | 6.5±0.65 | 6.5±0.72 | 0 | 6.3±0.56 | 4.5±0.84 | 1.8 |
| 5-min irradiation | 5.3±0.50 | 3.5±0.86 | 1.8 | 6.5±0.98 | 1.7±0.76** | 5.8 |
| No irradiation | 6.5±0.44 | 6.5±0.94 | 0 | 6.7±0.93 | 4.5±0.77* | 2.2 |
| 20-min irradiation | 5.3±0.25 | 3.5±0.74 | 1.8 | 5.3±0.65 | 0*** | 5.3 |
aReduction of virus titers after treatment with a photosensitizer. Data are expressed as mean values±SD; *p<0.05, **p<0.01, ***p<0.001 as compared with the dark/or light control without a photosensitizer.
bDifference between the infectious virus titers of the virus sample with a photosensitizer and the respective control without a photosensitizer.
3.4 Effect of phthalocyanine Zn(II) complexes on Newcastle disease virus
Results of the experiments with NDV are presented in Table 4. As seen, this virus is completely insensitive to photoinactivation effects of the phthalocyanine Zn(II) complexes, both in dark and following the irradiation.
Effects of ZnPcMe and ZnPcS on NDV.
| Experimental group | Virus titer CCID50/0.1 mLa | Δlogb | Virus titer CCID50/0.1 mLa | Δlogb | ||
|---|---|---|---|---|---|---|
| Without ZnPcMe | With ZnPcMe | Without ZnPcS | With ZnPcS | |||
| No irradiation | 5.75±0.45 | 6.0±0.87 | 0 | 6.75±0.68 | 6.5±0.91 | 0.25 |
| 5-min irradiation | 5.75±0.83 | 5.5±0.66 | 0.25 | 6.75±0.88 | 5.5±0.89 | 1.25 |
| No irradiation | 5.75±0.96 | 6.0±0.40 | 0 | 7.5±0.55 | 7.25±0.64 | 0.25 |
| 20-min irradiation | 5.5±0.56 | 5.5±0.38 | 0 | 6.75±0.71 | 5.5±0.22 | 1.25 |
aReduction of virus titers after treatment with a photosensitizer. Data are expressed as mean values±SD; *p<0.05, **p<0.01, ***p<0.001 as compared with the dark/or light control without a photosensitizer.
bDifference between the infectious virus titers of the virus sample with a photosensitizer and the respective control without a photosensitizer.
3.5 Effect of the phthalocyanine Zn(II) complex ZnPcMe on Coxsackievirus B1
Coxsackievirus B1 was insusceptible to the photoinactivation effects of the phthalocyanine Zn(II) complex ZnPcMe, both in dark and following the irradiation (Table 5).
Effects of ZnPcMe on Coxsackievirus B1.
| Experimental group | Virus titer, CCID50/0.1 mLa | Δlogb | |
|---|---|---|---|
| Without ZnPcMe | With ZnPcMe | ||
| No irradiation | 7.0±0.17 | 6.50±0.33 | 0.5 |
| 5-min irradiation | 6.78±0.25 | 6.67±0.44 | 0.11 |
| No irradiation | 7.0±0.17 | 7.0±0.17 | 0 |
| 20-min irradiation | 6.83±0.34 | 6.5±0.33 | 0.33 |
aReduction of virus titers after treatment with a photosensitizer. Data are expressed as mean values±SD; *p<0.05; **p<0.01; ***p<0.001 as compared with the dark/or light control without a photosensitizer.
bDifference between the infectious virus titers of the virus sample with a photosensitizer and the respective control without a photosensitizer.
3.6 Effect of the phthalocyanine Zn(II) complex ZnPcMe on human adenovirus type 5
The phthalocyanine Zn(II) complex ZnPcMe did not show a marked inactivating effect against human adenovirus type 5 (Table 6).
Effects of ZnPcMe on human adenovirus type 5.
| Experimental group | Virus titer, CCID50/0.1 mLa | Δlogb | |
|---|---|---|---|
| Without ZnPcMe | With ZnPcMe | ||
| No irradiation | 3.58±0.4 | 3.47±0.34 | 0.11 |
| 5-min irradiation | 3.65±0.47 | 2.79±0.26 | 0.86 |
| No irradiation | 3.58±0.4 | 1.81±0.24 | 1.77 |
| 20-min irradiation | 2.85±0.1 | 1.94±0.13 | 0.91 |
aReduction of virus titers after treatment with a photosensitizer. Data are expressed as mean values±SD; *p<0.05; **p<0.01; ***p<0.001 as compared with the dark/or light control without a photosensitizer.
bDifference between the infectious virus titers of the virus sample with a photosensitizer and the respective control without a photosensitizer.
4 Discussion
Our study on the photodynamic activity of a cationic (ZnPcMe) and an anionic (ZnPcS) phthalocyanine Zn(II) complexes manifested a marked effect against viruses belonging to different taxonomic groups (BVDV, HSV-1 and VV). As seen, all mentioned viruses possess a lipid containing envelope. Ben-Hur et al. [3] found that the efficacy of the virus inactivation depends on the substituents on the Pc macrocycle. However, the structure-activity and the charge-activity relationship in the photosensitizing inactivation of the studied viruses have not been clearly established. The non-enveloped (nude) viruses tested, Coxsackievirus B1 and human adenovirus type 5, were insensitive to the photodynamic action of ZnPcMe.
Previous investigations of ours [13] showed that the phthalocyanine Zn(II) complexes ZnPcMe and ZnPcS possess (i) absorption properties with a strong Q-bond in the far-red range (670–690 nm), (ii) a high photosensitizing efficiency due to the generation of singlet oxygen, and (iii) an appropriate values of fluorescence quantum yield. The phototreatment data indicate that both singlet oxygen (type II reaction) and hydroxyl radical (type I reaction) play a role in viral inactivation [16], [17], [18].
Our data showed that lipid-enveloped viruses differ in their sensitivity to phthalocyanine photosensitization. The phthalocyanine Zn(II) complexes tested demonstrated a marked virucidal activity against HSV-1, even following an irradiation of 5 min. Smetana et al. [19] carried out profound investigations manifesting a marked anti-herpesvirus effect of 11 different phthalocyanine derivatives. Their ultrastructural examinations on the mode of the photosensitization capacity of phthalocyanine derivatives against HSV-1, HSV-2 and varicella zoster virus demonstrated a damage of the viral envelope which prevented viral adsorption and/or penetration.
Vaccinia virus was the only virus that was sensitive to the ZnPcMe and ZnPcS complexes in the dark. These results are in line with the data of [20], which show that sulfonated phthalocyanine classes are light-independent inhibitors of VV infection in cell culture.
The difference between the two photosensitizers was demonstrated especially in the treatment of BVDV. The anionic ZnPcS was twice more active than the cationic ZnPcMe. It was suggested by [3] that the efficacy of viral inactivation of this virus by phthalocyanines depends on the extent of sulfonation.
The inability of NDV to be inactivated by photodynamic treatment and the diverse effect of the phthalocyanine complexes against various enveloped viruses are in accordance with the difference in the viral envelope and composition of the studied viruses. The insusceptibility of the uncoated (nude) viruses to the effect of the phthalocyanine complexes excludes the protein capsid and the viral genome as targets of the photodynamic activity. Evidently, lipid envelopes could be considered as the potential targets for photosensitizer binding.
In conclusion, phthalocyanine Zn(II) complexes could be used as virus-inactivating agents in the hospital hygiene strategies for photodynamic disinfection of surfaces, instruments and biological fluids.
Acknowledgments
The authors thank Dr. Ivana Roeva, Dr. Yordanka Neycheva and Ms. Krassimira Beshkova for their excellent assistance. This work is supported by grant B-1534/05 from the Bulgarian National Science Fund.
References
1. Horowitz B, Williams B, Rywkin S, Prince AM, Pascual D, Geacintov N, et al. Inactivation of viruses in blood with aluminum phthalocyanine derivatives. Transfussion 1991;31:102–8.10.1046/j.1537-2995.1991.31291142938.xSearch in Google Scholar
2. North J, Neyndorff H, Levy JG. New trends in photobiology: photosensitizers as virucidal agents. J Photochem Photobiol B 1993;17:99–108.10.1016/1011-1344(93)80002-QSearch in Google Scholar
3. Ben-Hur E, Hoeben RC, Van Ormondt H, Dubbelman TM, Van Steveninck J. Photodynamic inactivation of retroviruses by phthalocyanine: the effects of sulphonation, metal ligand and fluoride. J Photochem Photobiol B 1992;13:145–52.10.1016/1011-1344(92)85053-WSearch in Google Scholar
4. Kussovski V, Mantareva V, Angelov I, Orozova P, Wöhrle D, Schnurpfeil G, et al. Photodynamic inactivation of Aeromonas hydrophila by cationic phthalocyanines with different hydrophobicity. FEMS Microbiol Lett 2009;294:133–40.10.1111/j.1574-6968.2009.01555.xSearch in Google Scholar PubMed
5. Ragàs X, Sánchez-García D, Ruiz-González R, Dai T, Agut M, Hamblin MR, et al. Cationic porphycenes as potential photosensitizers for antimicrobial photodynamic therapy. J Med Chem 2010;53:7796–803.10.1021/jm1009555Search in Google Scholar PubMed PubMed Central
6. Boehm AB, Yamahara KM, Love DC, Peterson BM, McNeill K, Nelson KL. Correction to covariation and photoinactivation of traditional and novel indicator organisms and human viruses at a sewage-impacted marine beach. Environ Sci Technol 2011;45:1160.10.1021/es103947kSearch in Google Scholar
7. Mantareva V, Kussovski V, Angelov I, Wöhrle D, Dimitrov R, Popova E, et al. Non-aggregated Ga(III)-phthalocyanines in the photodynamic inactivation of planktonic and biofilm cultures of pathogenic microorganisms. Photochem Photobiol Sci 2011;10:91–102.10.1039/B9PP00154ASearch in Google Scholar
8. Margolis-Nino H, Ben-Hur E, Gottelieb P, Robinson R, Oetjen J, Horowitz B. Inactivation by phthalocyanine photosensitization of multiple forms of human immunodeficiency virus in red cell concentrates. Transfusion 1996;36:743–50.10.1046/j.1537-2995.1996.36896374381.xSearch in Google Scholar PubMed
9. Song R, Witvrouw M, Schols D, Robert A, Balzarini J, De Clerq E, et al. Anti-HIV activities of anionic metalloporphyrins and related compounds. Antivir Chem Chemother 1997;8:85–97.10.1177/095632029700800202Search in Google Scholar
10. Rywkin S, Lenny L, Goldstein J, Geacintov NE, Margolis-Nunno H, Horowitz B. Importance of type I and type II mechanisms in the photodynamic inactivation of viruses in blood with aluminum phthalocyanine derivatives. Photochem Photobiol 1992;56:463–9.10.1111/j.1751-1097.1992.tb02189.xSearch in Google Scholar PubMed
11. Trannoy LL, Terpstra FG, De Korte D, Lagerberg JW, Verhoeven AJ, Brand A, et al. Differential sensitivities of pathogens in red cell concentrates to Tri-P(4)-photoinactivation. Vox Sang 2006;91:111–8.10.1111/j.1423-0410.2006.00791.xSearch in Google Scholar
12. Wöhrle D, Iskander N, Graschew G, Sinn H, Friedrich EA, Maierborst W, et al. Synthesis of positively changed phthalocyanines and their activity in the photodynamic therapy of cancer cells. Photochem Photobiol 1990;51:351–6.10.1111/j.1751-1097.1990.tb01721.xSearch in Google Scholar
13. Mantareva V, Kussovski V, Angelov I, Borisova E, Avramov L, Schnurpfeil G, et al. Photodynamic activity of water-soluble phthalocyanine zinc(II) complexes against pathogenic microorganisms. Bioorg Med Chem 2007;15:4829–35.10.1016/j.bmc.2007.04.069Search in Google Scholar
14. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Higiene 1938;27:493–7.10.1093/oxfordjournals.aje.a118408Search in Google Scholar
15. Sudo K, Konno K, Yokota T, Shigeta S. A sensitive assay system screening antiviral compounds against herpes simplex virus type 1 and type 2. J Virol Methods 1994;49:169–78.10.1016/0166-0934(94)90041-8Search in Google Scholar
16. Gábor F, Szolnoki J, Tóth K, Fekete A, Maillard P, Csík G. Photoinduced inactivation of T7 phage sensitized by symmetrically and asymmetrically substituted tetraphenyl porphyrin: comparison of efficiency and mechanism of action. Photochem Photobiol 2001;73:304–11.10.1562/0031-8655(2001)073<0304:PIOTPS>2.0.CO;2Search in Google Scholar
17. Egyeki M, Turóczy G, Majer Z, Tóth K, Fekete A, Maillard P, Csík G. Photosensitized inactivation of T7 phage as surrogate of non-enveloped DNA viruses: efficiency and mechanism of action. Biochim Biophys Acta 2003;1624:115–24.10.1016/j.bbagen.2003.10.003Search in Google Scholar
18. Costa L, Alves E, Carvahlo CM, Tomé JP, Faustino MA, Neves MG, et al. Sewage bacteriophage photoinactivation by cationic porphyrins: a study of charge effect. Photochem Photobiol Sci 2008;7:415–22.10.1039/b712749aSearch in Google Scholar
19. Smetana Z, Mendelson E, Manor J, Van Lier JE, Ben-Hur E, Salzberg S, et al. Photodynamic inactivation of herpes viruses with phthalocyanine derivatives. J Photochem Photobiol B: Biology 1994;22:37–43.10.1016/1011-1344(93)06949-4Search in Google Scholar
20. Chen-Collins A, Dixon D, Vzorov A, Marzilli L, Compans R. Prevention of poxvirus infection by tetrapyrroles. BMC Inf Dis 2003;3:1–10.10.1186/1471-2334-3-9Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Article
- Environmental alterations in biofuel generating molecules in Zilla spinosa
- Short Communication
- Antinociceptive effect of Aristolochia trilobata stem essential oil and 6-methyl-5-hepten-2yl acetate, its main compound, in rodents
- Research Articles
- Bioenergetics of lactate vs. acetate outside TCA enhanced the hydrogen evolution levels in two newly isolated strains of the photosynthetic bacterium Rhodopseudomonas
- Leaves of Cordia boissieri A. DC. as a potential source of bioactive secondary metabolites for protection against metabolic syndrome-induced in rats
- Rapid Communication
- The evaluation of the synergistic effect of 3-(2,4-dihydroxyphenyl)propionic acid and l-ascorbic acid on tyrosinase inhibition
- Research Articles
- Virus inactivation under the photodynamic effect of phthalocyanine zinc(II) complexes
- Cytochalasin P1, a new cytochalasin from the marine-derived fungus Xylaria sp. SOF11
- Synthesis and biological evaluation of newer 1,3,4-oxadiazoles incorporated with benzothiazepine and benzodiazepine moieties
- Caspase-1 from the silkworm, Bombyx mori, is involved in Bombyx mori nucleopolyhedrovirus infection
Articles in the same Issue
- Frontmatter
- Research Article
- Environmental alterations in biofuel generating molecules in Zilla spinosa
- Short Communication
- Antinociceptive effect of Aristolochia trilobata stem essential oil and 6-methyl-5-hepten-2yl acetate, its main compound, in rodents
- Research Articles
- Bioenergetics of lactate vs. acetate outside TCA enhanced the hydrogen evolution levels in two newly isolated strains of the photosynthetic bacterium Rhodopseudomonas
- Leaves of Cordia boissieri A. DC. as a potential source of bioactive secondary metabolites for protection against metabolic syndrome-induced in rats
- Rapid Communication
- The evaluation of the synergistic effect of 3-(2,4-dihydroxyphenyl)propionic acid and l-ascorbic acid on tyrosinase inhibition
- Research Articles
- Virus inactivation under the photodynamic effect of phthalocyanine zinc(II) complexes
- Cytochalasin P1, a new cytochalasin from the marine-derived fungus Xylaria sp. SOF11
- Synthesis and biological evaluation of newer 1,3,4-oxadiazoles incorporated with benzothiazepine and benzodiazepine moieties
- Caspase-1 from the silkworm, Bombyx mori, is involved in Bombyx mori nucleopolyhedrovirus infection


