Abstract
Single crystals of the phosphides Sm6Rh30P19 and Lu6Rh30P19 were synthesized from the elements with the bismuth flux technique. Polycrystalline Eu6Rh30Sb19 was obtained from the elements by arc-melting and subsequent annealing. The structures of the phosphides were refined from single-crystal X-ray diffractometer data: P63/m, a = 1,555.07(9), c = 383.38(2) pm, wR2 = 0.0500, 1044 F 2 values, 58 variables for Sm6Rh30P19 and a = 1,551.99(15), c = 376.32(3) pm, wR2 = 0.0725, 1022 F 2 values, 58 variables for Lu6Rh30P19. Sm6Rh30P19 and Lu6Rh30P19 crystallize with the Sm6Rh30Si19-type structure, which is closely related to the Yb6Co30P19 type. The rhodium and phosphorus atoms build up three-dimensional [Rh30P19] networks (225–255 pm Rh–P and 276–288 pm Rh–Rh in Sm6Rh30P19) which leave hexagonal prismatic cavities for the rare earth atoms. The phosporus atoms on the c axis have distorted octahedral rhodium coordination and show disorder on the c axis (refinement with a split position). Temperature dependent magnetic susceptibility data of Eu6Rh30Sb19 show Curie-Weiss behavior with an experimental magnetic moment of 7.78(1) µB Eu atom−1 and θ P = −9.6(1) K, compatible with divalent europium. The europium atoms in Eu6Rh30Sb19 are ordered antiferromagnetically at T N = 3.9(4) K which is corroborated by specific heat data. The divalent ground state of europium is manifested in the 151Eu Mössbauer spectrum with an isomer shift value of −10.44(1) mm s−1 at 78 K. The 121Sb Mössbauer spectrum with an isomer shift of −7.12(2) mm s−1 (envelope signal of the four crystallographically independent antimony sites) underpins the antimonide character.
1 Introduction
The rare earth (RE) transition (T) metal phosphorus systems with the 3d transition metals have intensively been studied and a manifold of ternary phosphides RE x T y P z has been structurally characterized. The crystal chemical data are summarized in review articles 1 , 2 and in the Pearson data base. 3 This family of phosphides comprises phosphorus-rich phases with P2 dumb-bells, compounds with extended [T y P z ] δ− polyanionic networks with substantial covalent T−P bonding and many metal-rich phases with isolated (no P–P bonding) phosphorus atoms in trigonal prismatic metal coordination. Several isothermal sections have been studied, e.g., La–Ni–P and Ce–Ni–P, 4 Y–Cu–P, 5 Ce–Cu–P 6 or Nd-Cu-P. 7
The RE-T-P systems with the 4d and especially the 5d transition metals have only scarcely been studied. This might be due to the lower reactivity of the heavier transition metals and also to the high price of the noble metals. So far only 25 RE x Rh y P z phases have been reported, while the Ce–Ni–P system alone contains 15 Ce x Ni y P z phases. 4 The reported RE x Rh y P z phases are RERhP (RE = Sc, La, Ce), 8 , 9 , 10 , 11 RERh2P2 (RE = La, Ce, Pr, Nd, Eu), 12 , 13 RE 2Rh12P7 (RE = Y, La, Nd, Gd, Tb, Dy, Ho, Er, Yb), 14 , 15 , 16 RE 6Rh32P17 (RE = La, Ce), 17 RERh6P4 (RE = Sc, Yb, Lu), 18 RE 6Rh30P19 (RE = Eu, Yb), 19 LaRh4P12 20 and La4Rh8P9. 21
Some of the RE x Rh y P z phosphides have been studied with respect to their magnetic and electrical transport properties. Equiatomic CeRhP 8 is a valence fluctuating compound with a Kondo temperature of around 400–500 K. The skutterudite phase LaRh4P12 was synthesized under high-pressure high-temperature conditions. 20 LaRh4P12 shows a comparatively high transition to superconductivity at around 17 K. The transition temperatures of ScRhP (T C = 2 K) 10 and LaRhP (T C = 2.5 K) 9 are much lower. Surprisingly, the phosphide in this series, EuRh2P2, shows the unusual coexistence of a non-integral europium valence of 2.2 along with antiferromagnetic ordering below 50 K. 13 , 22 , 23 , 24 , 25
Well-shaped single crystals of such phosphides can be grown under lead 26 or bismuth flux 27 conditions. Lead was used for the growth of Eu6Rh30P19 and Yb6Rh30P19 crystals, 19 while bismuth was found suitable for the RERh6P4 (RE = Sc, Yb, Lu) 18 crystals and La4Rh8P9. 21
In continuation of the phase analytical studies of the RE-T-P systems, our bismuth flux experiments resulted in the new rhodium-rich phosphides Sm6Rh30P19 and Lu6Rh30P19 which adopt the Sm6Rh30Si19 type 28 structure. The crystal chemical details of both phosphides are reported herein. The isotypic antimonide Eu6Rh30Sb19 was reported by Jeitschko et al. 29 We have resynthesized a polycrystalline sample of this phase and investigated its magnetic properties and Mössbauer spectroscopic behavior.
2 Experimental
2.1 Synthesis
Starting materials for the synthesis of the phosphide and antimonide samples were samarium and lutetium pieces (Smart Elements), europium pieces (Alfa Aesar), rhodium powder (Agosi), red phosphorus (Hoechst, Knapsack, ultrapure), antimony lumps (Johnson Matthey) and bismuth granules (Chempur, 1–10 mm), all with stated purities better than 99.9 %.
Single crystals of Sm6Rh30P19 and Lu6Rh30P19 were grown from bismuth fluxes. Pieces of the elements were weighed in the atomic ratios of 1:2:2:30 and sealed in evacuated silica ampoules. The latter were placed in a muffle furnace and first heated to 770 K within 20 h to allow for a pre-reaction of the phosphorus. After keeping the temperature for 24 h, the samples were heated to 1,370 K at a rate of 20 K h−1 and annealed for another 100 h, followed by cooling to room temperature at a rate of 2 K h−1. The bismuth flux was dissolved in an equimolar mixture of H2O2 (ACROS 35 %) and glacial acetic acid (VWR International, >99.8 %). The resulting products were rinsed with demineralized water. The Sm6Rh30P19 and Lu6Rh30P19 crystals have columnar shape. By-products were single crystals of IrP2, Ir2P and Sm4Rh13P9. 3
A polycrystalline sample of Eu6Rh30Sb19 was prepared from the elements by arc-melting. Europium pieces, rhodium powder (cold-pressed to a pellet) and antimony pieces were weighed in the ideal 6:30:19 atomic ratio and placed in the water-cooled copper crucible of an arc-melting furnace. 30 The elements were reacted in the arc under an argon pressure of ca. 700 mbar. The argon was purified using titanium sponge (900 K), silica gel, and molecular sieves. The resulting button was turned over and remelted three times to ensure homogeneity. The total weight loss after the arc-melting procedure was smaller than 0.5 %. To enhance crystallinity, the Eu6Rh30Sb19 button was finally sealed in an evacuated silica ampoule and annealed in a high-frequency furnace 31 for 30 min slightly below the (not determined) melting temperature. The Eu6Rh30Sb19 sample is silvery with metallic luster and stable in air over months.
2.2 X-ray diffraction
The polycrystalline Eu6Rh30Sb19 sample was characterized by powder X-ray diffraction: Guinier technique, Enraf-Nonius FR552 camera, CuKα 1 radiation, image plate system (Fujifilm, BAS-1800) and α-quartz (a = 491.30 and c = 540.46 pm) as an internal standard. The refined hexagonal lattice parameters (a = 1,694.4(6), c = 408.5(1) pm, V = 1.0157 nm3) are in good agreement with the data published by Jeitschko et al. (a = 1,693.2(2), c = 408.11(4) pm, V = 1.0133 nm3). 29 The correct indexing of the pattern was ensured with the help of an intensity calculation (Lazy-Pulverix routine 32 ).
Small columnar single crystals of Sm6Rh30P19 and Lu6Rh30P19 were selected under an optical microscope and fixed to thin glass fibers using beeswax. Their quality was tested by Laue photographs on a Buerger camera (white molybdenum radiation, image plate technique, Fujifilm, BAS-1800). Two complete data sets were collected at room-temperature on a STOE IPDS-II diffractometer (graphite-monochromatized MoKα radiation; oscillation mode). Numerical absorption corrections were applied to the data sets. Details about the data collections and the structure refinements are listed in Table 1.
Crystallographic data and structure refinement of Sm6Rh30P19 and Lu6Rh30P19, space group P63/m, Z = 1.
| Formula | Sm6Rh30P19 | Lu6Rh30P19 |
| Molar mass, g mol−1 | 4,577.8 | 4,741.0 |
| Lattice parameters (single-crystal data) | ||
| a, pm | 1,555.07(9) | 1,551.99(15) |
| c, pm | 383.38(2) | 376.32(3) |
| Cell volume V, nm3 | 0.8029 | 0.7850 |
| Calc. density, g cm−3 | 9.47 | 10.03 |
| Crystal size, µm3 | 30 × 30 × 40 | 10 × 30 × 50 |
| Diffractometer | IPDS-II | IPDS-II |
| Radiation/wavelength, pm | MoKα/71.073 | MoKα/71.073 |
| Transmission max/min | 0.604/0.313 | 0.665/0.370 |
| Absorption coeff., mm−1 | 26.6 | 34.9 |
| Detector dist., mm | 80 | 80 |
| Irradiation time, min | 6 | 10 |
| ω range/increment, deg | 0–180/1.0 | 0–180/1.0 |
| Integr. param. A/B/EMS | 12.5/2.0/0.014 | 12.0/2.6/0.012 |
| F(000), e | 2,007 | 2,061 |
| θ range, deg | 2.62–31.87 | 2.62–31.93 |
| hkl range | ±23, ±23, ±5 | ±23, ±23, ±5 |
| No. refl. | 17,285 | 24,356 |
| Indep. refl./R int | 1,044/0.0343 | 1,022/0.1165 |
| Refl. with I ≥ 3 σ(I)/R σ | 925/0.0083 | 727/0.0416 |
| Data/parameters | 1,044/58 | 1,022/58 |
| Goodness-of-fit on F 2 | 1.68 | 1.36 |
| R1/wR2 (I ≥ 3 σ(I)) | 0.0228/0.0484 | 0.0438/0.0617 |
| R1/wR2 (all data) | 0.0297/0.0500 | 0.0869/0.0725 |
| Extinction coeff. | 1,260(40) | 65(9) |
| Largest diff. peak/hole, e Å−3 | 5.97/−2.59 | 9.51/−9.44 |
2.3 EDX analysis
The Sm6Rh30P19 and Lu6Rh30P19 single crystals studied on the image plate diffractometer were analyzed by EDX. The Zeiss EVO® MA10 scanning electron microscope (operated in variable pressure mode; 60 Pa N2 pressure) was equipped with a LaB6 cathode and an Oxford Instruments INCA® x-act detector. SmF3, LuF3, Rh and GaP were used as standards. The EDX analyses of 9 ± 2 at% Sm: 55 ± 2 at% Rh: 36 ± 2 at% P and 10 ± 2 at% Lu: 54 ± 2 at% Rh: 36 ± 2 at% P were in good agreement with the ideal composition (10.9: 54.5: 34.5). No impurity elements (especially with respect to a potential incorporation of bismuth from the flux) were detected.
2.4 Magnetic measurements
Magnetic data of the Eu6Rh30Sb19 sample was measured with a Vibrating Sample Magnetometer unit (VSM) of a Quantum Design Physical Property Measurement System (PPMS). An Eu6Rh30Sb19 piece was attached to the sample holder rod using Kapton foil and investigated in the temperature range of 2.5–305 K with applied external magnetic fields of up to 80 kOe (1 kOe = 7.96 × 104 A m−1). The data fitting and plotting was performed with OriginPro2017 33 and the graphical editing with the program CorelDraw2024. 34 The heat capacity was measured using a piece of 14.134 mg in the temperature range of 2–300 K without an applied field. The sample was fixed on a pre-calibrated heat capacity puck using N-Apiezon grease.
2.5 Mössbauer spectroscopy
The 121Sb and 151Eu Mössbauer spectroscopic studies on the Eu6Rh30Sb19 sample were performed with Ba121mSnO3 and 151Sm:EuF3 sources (21.53 keV transition, 55 MBq activity, 1 % of the total activity). The sample was ground to a fine powder and mixed with α-quartz to ensure an even distribution of the sample within the PMMA sample holder (∅ 2 cm). The optimal sample thickness (ca. 30 mg) was calculated according to Long et al. 35 The measurement for 121Sb was performed in transmission geometry at 78 K using a liquid-nitrogen bath cryostat. 151Eu spectra were recorded at T = 4.2, 78 and 298 K in a Continuous Flow Cryostat System (Janis Research Co LLC.). Both sources were kept at room temperature. Fitting and plotting of the spectra were done with the WinNormos for Igor6 program package 36 and graphical editing with the program CorelDRAW2024. 34
3 Structure refinements
The data sets of Sm6Rh30P19 and Lu6Rh30P19 showed hexagonal lattices with low Laue symmetry and the systematic extinctions 000l with l = 2n, leading to the possible space groups P63 and P63/m, of which the centrosymmetric one was chosen. The starting atomic parameters were deduced with the charge-flipping algorithm 37 implemented in Superflip, 38 and both structures were refined on F 2 with the Jana2020 software package, 39 , 40 with anisotropic displacement parameters for all sites. Initially, the P4 atoms were placed on the 2b sites; however, they showed strongly enhanced U 33 values and lower occupancy. In parallel to the work of Wurth et al. 19 on the related phosphides Ca6Rh30P19, Eu6Rh30P19 and Yb6Rh30P19, we introduced a split position 4e with 25 % occupancy, which was refined with an isotropic displacement parameter (the crystal chemical consequences are discussed below). Separate refinement of the occupancy parameters of the remaining sites gave no hints for deviations from the ideal compositions. The final difference Fourier synthesis was contourless. Further details on the refinements, the positional, the displacement parameters and the interatomic distances (exemplarily listed for Sm6Rh30P19) are presented in Tables 1–3.
Atomic coordinates and displacement parameters (pm2) for Sm6Rh30P19 and Lu6Rh30P19. The anisotropic displacement factor exponent takes the form: –2π 2[(ha*)2 U 11 + … + 2hka*b*U 12]. U eq is defined as one third of the trace of the orthogonalized U ij tensor. Standard deviations are given in parentheses. U 23 = U 13 = 0.
| Atom | Wyckoff | x | y | z | U 11 | U 22 | U 33 | U 12 | U eq/U iso |
|---|---|---|---|---|---|---|---|---|---|
| Sm 6 Rh 30 P 19 | |||||||||
| Sm1 | 6h | 0.29704(2) | 0.39377(2) | 1/4 | 52(1) | 61(1) | 63(1) | 28(1) | 59(1) |
| Rh1 | 6h | 0.22342(4) | 0.57221(4) | 1/4 | 51(2) | 58(2) | 199(3) | 13(2) | 109(2) |
| Rh2 | 6h | 0.53503(3) | 0.07359(3) | 1/4 | 48(2) | 53(2) | 83(2) | 21(2) | 64(2) |
| Rh3 | 6h | 0.04602(3) | 0.27598(3) | 1/4 | 64(2) | 46(2) | 71(2) | 28(2) | 60(2) |
| Rh4 | 6h | 0.41600(3) | 0.26087(3) | 1/4 | 48(2) | 55(2) | 80(2) | 23(2) | 62(2) |
| Rh5 | 6h | 0.12595(4) | 0.14844(4) | 1/4 | 90(2) | 117(2) | 77(2) | 77(2) | 84(2) |
| P1 | 6h | 0.54900(11) | 0.23142(11) | 1/4 | 57(6) | 54(6) | 92(7) | 23(5) | 70(5) |
| P2 | 6h | 0.25785(11) | 0.11784(11) | 1/4 | 43(6) | 55(6) | 69(7) | 25(5) | 56(5) |
| P3 | 6h | 0.06117(10) | 0.43226(11) | 1/4 | 50(6) | 49(6) | 74(6) | 26(5) | 57(5) |
| 0.25P4 | 4e | 0 | 0 | 0.080(2) | 106(14) | ||||
| Lu 6 Rh 30 P 19 | |||||||||
| Lu1 | 6h | 0.39333(6) | 0.29748(6) | 1/4 | 73(3) | 65(3) | 62(3) | 34(3) | 67(3) |
| Rh1 | 6h | 0.57173(10) | 0.22349(10) | 1/4 | 63(6) | 54(6) | 146(7) | 6(5) | 98(5) |
| Rh2 | 6h | 0.07413(10) | 0.53403(10) | 1/4 | 66(6) | 51(6) | 91(7) | 25(5) | 71(5) |
| Rh3 | 6h | 0.27683(10) | 0.04425(11) | 1/4 | 47(5) | 68(6) | 72(5) | 27(5) | 63(5) |
| Rh4 | 6h | 0.25757(11) | 0.41532(9) | 1/4 | 58(5) | 56(5) | 73(6) | 26(5) | 64(5) |
| Rh5 | 6h | 0.14899(11) | 0.12637(10) | 1/4 | 100(6) | 88(6) | 62(6) | 64(5) | 76(5) |
| P1 | 6h | 0.2293(3) | 0.5469(3) | 1/4 | 51(17) | 76(18) | 78(19) | 35(15) | 67(15) |
| P2 | 6h | 0.1169(3) | 0.2593(3) | 1/4 | 60(17) | 73(18) | 50(20) | 33(15) | 63(15) |
| P3 | 6h | 0.4307(4) | 0.0590(3) | 1/4 | 60(18) | 45(18) | 93(18) | 11(16) | 73(15) |
| 0.25P4 | 4e | 0 | 0 | 0.089(6) | 110(40) | ||||
Interatomic distances (pm) in the structure of Sm6Rh30P19. All distances of the first coordination spheres of the atoms are listed. Standard deviations are ≤0.2 pm.
| Sm: | 2 | P1 | 297.2 | Rh4: | 1 | P1 | 233.1 |
| 2 | P2 | 299.1 | 1 | P2 | 235.1 | ||
| 2 | P3 | 299.4 | 2 | P3 | 249.9 | ||
| 2 | Rh2 | 306.9 | 2 | Rh3 | 275.7 | ||
| 2 | Rh4 | 307.0 | 1 | Rh2 | 276.7 | ||
| 2 | Rh3 | 307.2 | 2 | Rh1 | 285.0 | ||
| 1 | Rh2 | 334.3 | 2 | Sm | 307.0 | ||
| 1 | Rh3 | 338.3 | 1 | Sm | 339.4 | ||
| 1 | Rh5 | 338.9 | Rh5: | 2 | P4a | 225.1 | |
| 2 | Rh4 | 339.4 | 1 | P2 | 232.6 | ||
| 1 | Rh1 | 346.7 | 2 | P4a | 249.9 | ||
| 1 | Rh1 | 349.1 | 2 | P2 | 249.9 | ||
| Rh1: | 1 | P3 | 236.9 | 2 | Rh3 | 278.7 | |
| 2 | P1 | 253.3 | 1 | Rh3 | 281.9 | ||
| 2 | P1 | 254.5 | 4 | Rh5 | 288.4 | ||
| 2 | Rh1 | 277.6 | 2 | P4a | 335.3 | ||
| 2 | Rh4 | 285.0 | 1 | Sm | 338.9 | ||
| 2 | Rh2 | 285.6 | P1: | 1 | Rh4 | 233.1 | |
| 1 | Sm | 346.7 | 1 | Rh2 | 235.3 | ||
| 1 | Sm | 349.1 | 2 | Rh1 | 253.3 | ||
| Rh2: | 1 | P1 | 235.3 | 2 | Rh1 | 254.5 | |
| 1 | P3 | 235.6 | 2 | Sm | 297.2 | ||
| 2 | P3 | 247.5 | P2: | 1 | Rh5 | 232.6 | |
| 2 | Rh2 | 275.8 | 1 | Rh4 | 235.1 | ||
| 1 | Rh4 | 276.7 | 1 | Rh3 | 236.1 | ||
| 2 | Rh1 | 285.6 | 2 | Rh5 | 249.9 | ||
| 2 | Sm | 306.9 | 2 | Rh3 | 251.0 | ||
| 1 | Sm | 334.3 | 2 | Sm | 299.1 | ||
| Rh3: | 1 | P3 | 232.2 | P3: | 1 | Rh3 | 232.2 |
| 1 | P2 | 236.1 | 1 | Rh2 | 235.6 | ||
| 2 | P2 | 251.1 | 1 | Rh1 | 236.9 | ||
| 2 | Rh4 | 275.7 | 2 | Rh2 | 247.5 | ||
| 2 | Rh5 | 278.7 | 2 | Rh4 | 249.9 | ||
| 1 | Rh5 | 281.9 | 2 | Sm | 299.4 | ||
| 2 | Sm | 307.2 | P4a: | 1 | P4a | 61.4 | |
| 1 | Sm | 338.3 | 1 | P4a | 130.3 | ||
| 2 | P4a | 191.7 | |||||
| 3 | Rh5 | 225.1 | |||||
| 3 | Rh5 | 249.9 | |||||
| 1 | P4a | 235.1 |
-
aNote that the P4 site has only 25 % occupancy.
CCDC 2443532 (Sm6Rh30P19) and CCDC 2443531 (Lu6Rh30P19) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
4 Crystal chemistry
The rhodium-rich phosphides Sm6Rh30P19 and Lu6Rh30P19 crystallize with a slightly modified variant of the Yb6Co30P19 type structure, 41 similar to Eu6Rh30P19 and Yb6Rh30P19. 19 The crystal chemistry of the prototype has been discussed in detail by Jeitschko and Jakubowski-Ripke. 41 In the following discussion we focus on the differences observed for the respective rhodium compounds. Our discussion exemplarily relies on the Sm6Rh30P19 structure.
A view of the Sm6Rh30P19 structure along the c axis is presented in Figure 1. The rhodium and phosphorus atoms build a three-dimensional covalently bonded [Rh30P19] network. This is in agreement with the course of the interatomic distances. The Rh–P distances range from 225–255 pm, comparable to the sum of the covalent radii 42 of 235 pm for Rh + P. Comparable ranges of Rh–P distances have been observed for the closely related structures of ScRh6P4 (228–258 pm) 18 and La4Rh8P9 (229–254 pm). 21 The bonded [Rh30P19] network is additionally stabilized by Rh–Rh bonding. The five crystallographically independent rhodium sites show Rh–Rh distances in the range from 276 to 288 pm, only slightly longer than in fcc rhodium (12 × 269 pm). 43 This is the typical bonding pattern in the large family of metal-rich phosphides. 44
![Figure 1:
View of the Sm6Rh30P19 structure along the c axis. Samarium, rhodium and phosphorus atoms are drawn as medium grey, blue and magenta circles, respectively. The three-dimensional [Rh30P19] network and the octahedral coordination of the P4 atoms (extending along the c axis) are emphasized.](/document/doi/10.1515/znb-2025-0023/asset/graphic/j_znb-2025-0023_fig_001.jpg)
View of the Sm6Rh30P19 structure along the c axis. Samarium, rhodium and phosphorus atoms are drawn as medium grey, blue and magenta circles, respectively. The three-dimensional [Rh30P19] network and the octahedral coordination of the P4 atoms (extending along the c axis) are emphasized.
The [Rh30P19] network leaves hexagonal prismatic cavities that are filled by the larger samarium atoms. The rectangular faces of these prisms are all capped by additional rhodium atoms, leading to coordination number 18, a so-called hexa-capped hexagonal prism. This type of rare earth coordination is frequently found in metal-rich rare earth transition metal phosphides. An overview is given in the review by Kuz’ma and Chykhrij. 1
Another striking structural motif that is evident from Figure 1 is the infinite row of face-sharing P4@Rh56 octahedra that extends along the c axis. Figure 2 shows a cutout of such a row. This substructure is responsible for the crystal chemial differences with respect to the prototype Yb6Co30P19.
41
The short c axis of the Sm6Rh30P19 structure does not allow for occupancy of every octahedral void, since this would result in too short P–P distances of 191.7 pm (half the c axis). A way out of this dilemma would be a symmetry reduction to the non-centrosymmetric space group
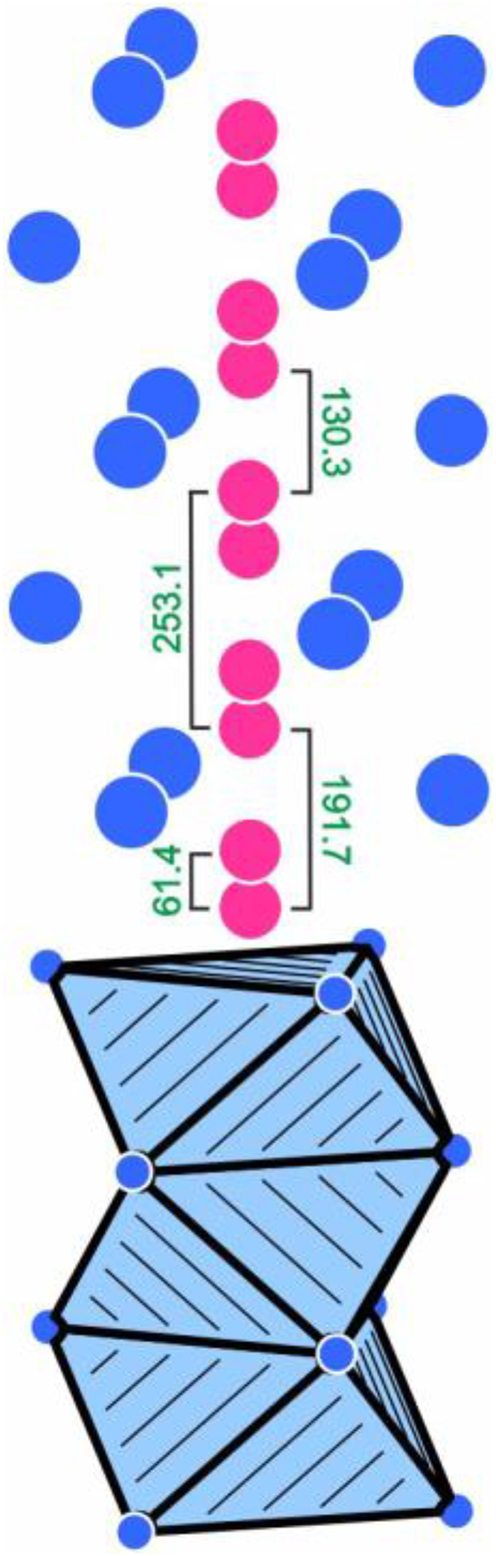
Cutout of the Sm6Rh30P19 structure showing the strands of face sharing P4@Rh56 octahedra that extend along the c axis. Rhodium and phosphorus atoms are drawn as blue and magenta circles, respectively. The different distances (pm) induced by the split refinement are indicated. Note that this 4e site for the P4 atoms is a split position with only 25 % occupancy. In a chemically reasonable ordered model, only P–P distances of ≥253 pm are realized. For details see text.
The phosphorus atoms P1, P2 and P3 have trigonal prismatic metal coordination (Figure 3). For both, P2 and P3, the rectangular faces of the trigonal prisms are capped by additional rhodium atoms, leading to coordination number nine in the form of tricapped trigonal prisms, the typical phosphorus coordination in metal-rich phosphides with isolated (i.e., no P–P bonding) P atoms. 44 Three trigonal prisms filled with the P1 atoms form compact units by sharing common edges. This leads to an emtpy Rh6 prism, shaded in yellow color in Figure 3. These Rh6 prisms are too small to be occupied by an additional atom. The central atom would have much too short distances (172 pm) to the P1 atoms. Geometrically one can describe the Sm6Rh30P19 structure with a shamrock-like condensation pattern of trigonal, P-centered prisms. An overview of these shamrock-like building units is given by Zaremba et al. 50 and a general classification of structures with condensed trigonal prisms as building units was published by Gladyshevskii and Grin. 51
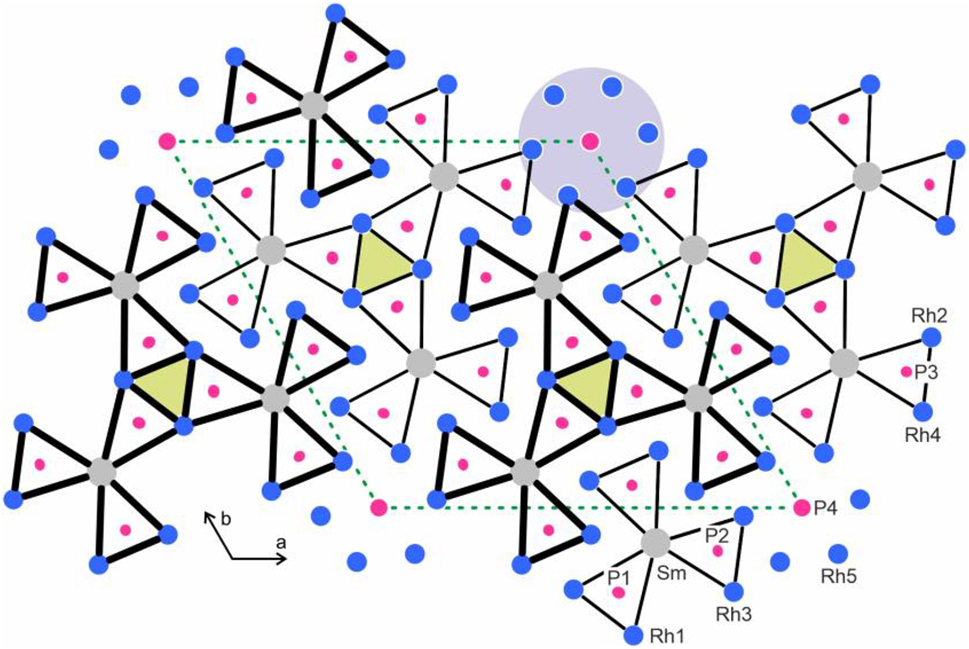
Projection of the Sm6Rh30P19 structure along the short unit cell axes. Samarium, rhodium and phosphorus atoms are drawn as medium grey, blue and magenta circles, respectively. All atoms (except P4) lie on mirror planes at x = 1/4 and 3/4, accentuated by thin and thick lines. The trigonal prisms around the phosphorus atoms are highlighted. Atom designations are given at the lower right-hand part. The blue grey circle emphasizes the row of condensed P4@Rh56 octahedra shown in Figure 2 and the empty Rh6 prisms are shaded in yellow.
Finally, we turn to the isotypic antimonide Eu6Rh30Sb19. 29 The structure of the europium compound was studied on the basis of single-crystal X-ray diffractometer data in space group P63/m and showed the same microdomain problems as Sm6Rh30P19 and Lu6Rh30P19. We have re-synthesized a polycrystalline sample of Eu6Rh30Sb19 for physical property studies which are reported in the following.
5 Magnetic properties of Eu6Rh30Sb19
The course of the cell volumes of the RE 6Rh30Sb19 series 29 showed a strong positive deviation for Eu6Rh30Sb19, indicating at least partially divalent europium. We have therefore performed magnetic susceptibility measurements in order to clarify the magnetic ground state of Eu6Rh30Sb19. The temperature dependence of the magnetic susceptibility of Eu6Rh30Sb19 measured at 10 kOe is presented in Figure 4. The compound shows Curie-Weiss behavior above 25 K. A fit of the data (25–300 K range) with the Curie-Weiss law yielded an experimental magnetic moment of 7.78(1) µB Eu atom−1 and a Weiss constant of −9.6(1) K. The experimentally derived moment is slightly smaller than that of the free ion value of Eu2+ (7.94 µB). 52 Such a slight moment reduction has been observed for a variety of intermetallic europium compounds. 53 , 54 , 55 The negative Weiss constant is indicative of antiferromagnetic interactions in the paramagnetic range.
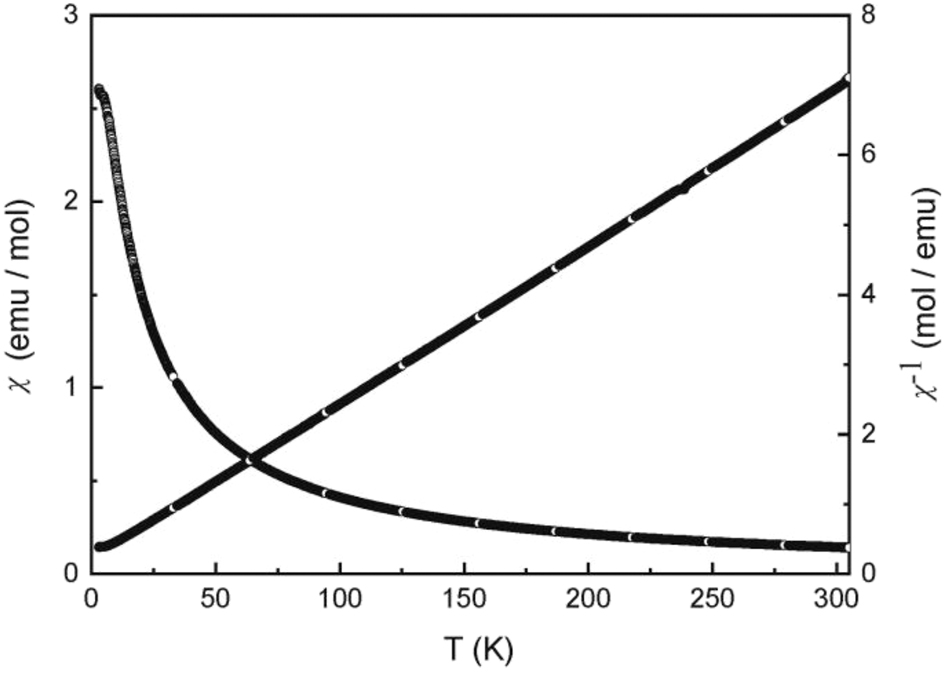
Temperature dependence of the magnetic susceptibility (χ and χ −1 values) of Eu6Rh30Sb19 measured at 10 kOe.
The low-field measurement at 100 Oe (Figure 5) shows an anomaly in the low-temperature regime, but without a clear sign for antiferromagnetism. We have then measured the temperature dependence of the heat capacity of Eu6Rh30Sb19. A clear λ-type anomaly is evident in the low-temperature regime (Figure 6), manifesting the magnetic ordering temperature of 3.9(4) K. The nature of magnetic ordering becomes clear from the magnetization measurements (Figure 7). At 10 and 50 K we observe a linear increase of the magnetization with increasing field as is expected for a paramagnetic material. The 4 K isotherm shows a weak deviation from a linear increase at around 40 kOe, most likely resulting from a spin-flip transition, and this underpins the antiferromagnetic ground state. The magnetization at 4 K and 80 kOe amounts to 5.33(1) µB Eu atom−1, lower than the theoretical value of 7 µB for Eu2+. 52 Thus, much higher fields are necessary to achieve full parallel spin alignment.
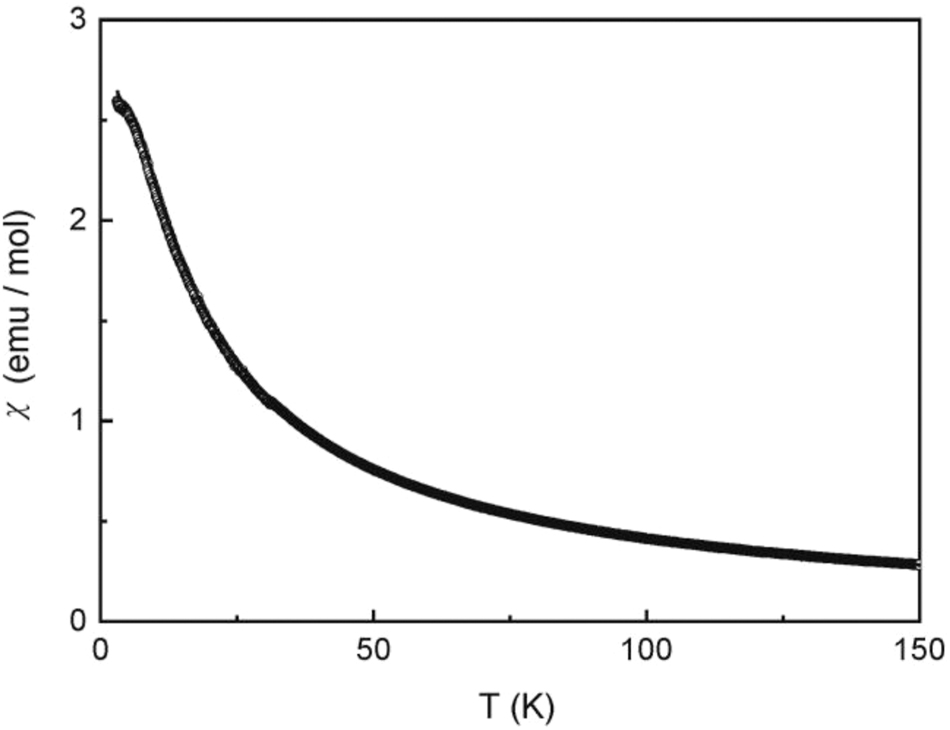
Low-field magnetic susceptibility of Eu6Rh30Sb19 measured at 100 Oe in zero-field cooling and field-cooling mode.
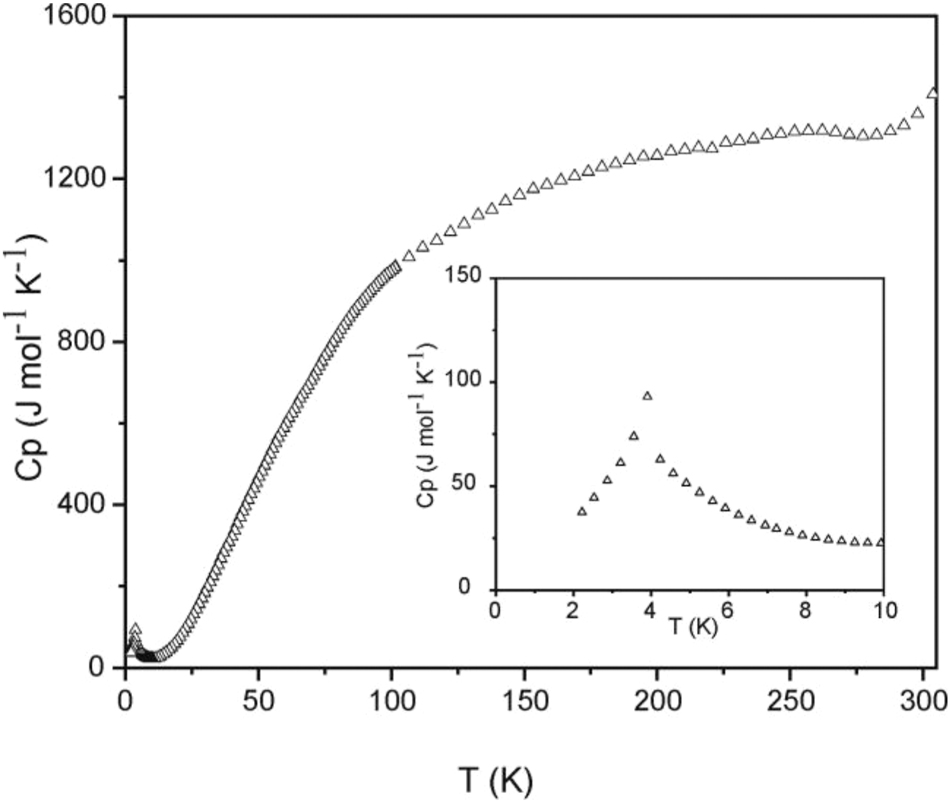
Heat capacity of Eu6Rh30Sb19 measured without external field. The low-temperature behavior is shown in the insert.
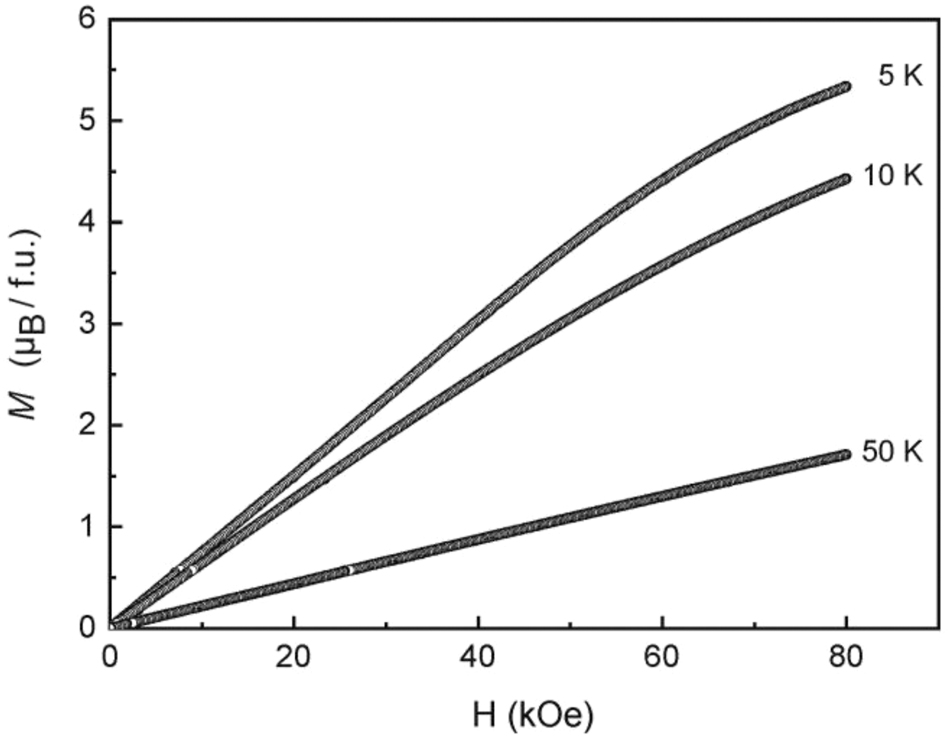
Magnetization isotherms of Eu6Rh30Sb19 measured at T = 5, 10 and 50 K.
6 Mössbauer spectroscopic characterization of Eu6Rh30Sb19
The 151Eu Mössbauer spectra of Eu6Rh30Sb19 are shown in Figure 8 along with transmission integral fits. The underlying fitting parameters are summarized in Table 4. The Mössbauer spectra fully confirm the magnetic data. Eu6Rh30Sb19 has a stable divalent ground state. The isomer shift (78 K data) of −10.44(1) mm s−1 is within the usual range observed for divalent, predominantly covalently bonded intermetallic europium compounds. 56 , 57 The pnictide Zintl phases EuCd2 X 2 (X = P, As, Sb) exbibit a higher degree of ionic bonding and this is expressed in a more negative isomer shift of around −11.7 mm s−1. 58 Evidence for a tiny amount of trivalent europium is observed in the 78 K spectrum. The signal around 0 mm−1 was included as simple Lorentzian in the fit. This small Eu(III) quantity most likely arises from surface oxidation of the sample. The non-cubic site symmetry (m..) of the europium atoms is expressed in a quadrupole splitting parameter of around 2.5 mm s−1. The experimental line widths are in the usual range. The signal observed at 4.2 K (very close to the magnetic ordering temperature) is slightly broadened. The onset of magnetic ordering results in a hyperfine field splitting parameter of 2.7(4) T. Full magnetic hyperfine field splitting will occur at lower temperatures (not accessible with the used experimental setup).
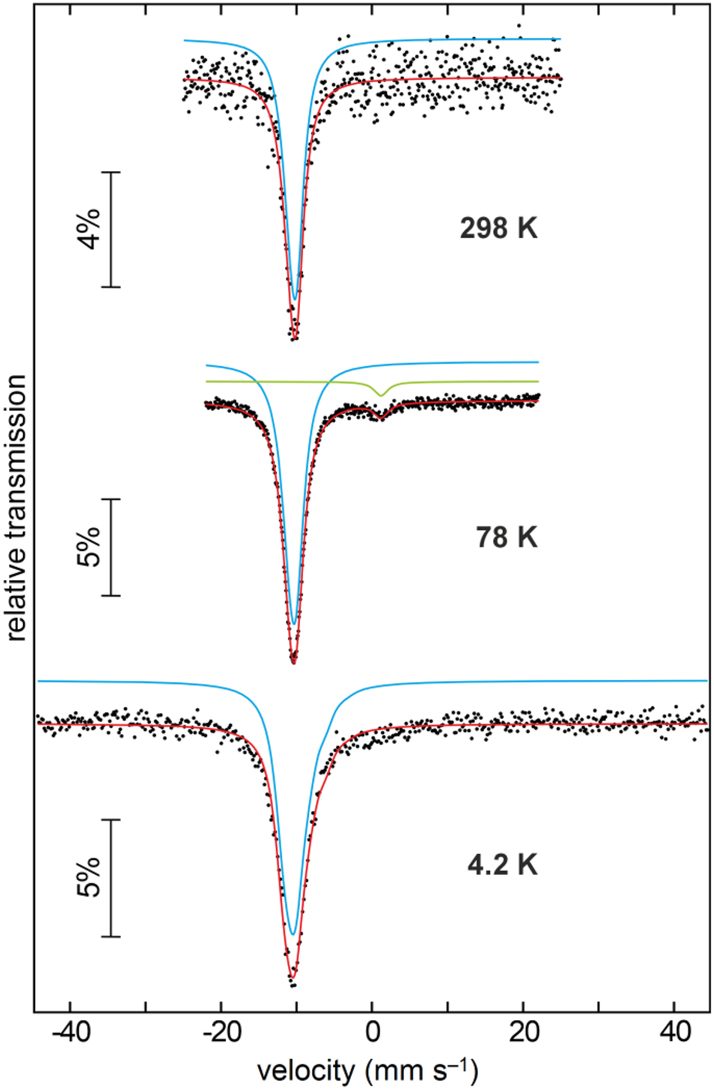
Experimental (dots) and simulated (continuous line) 151Eu Mössbauer spectra of Eu6Rh30Sb19 at T = 4.2, 78 and 298 K.
Fitting parameters of the 121Sb and 151Eu Mössbauer spectroscopic measurements of Eu6Rh30Sb19 at T = 298, 78 and 4.2 K. Numbers in parentheses represent the statistical errors in the last digit, numbers marked with an asterisk were fixed during the fitting procedure. δ isomer shift, Γ experimental line width, ΔE Q electric quadrupole splitting parameter, B hf magnetic hyperfine field.
| Source | T (K) | δ (mm s−1) | Γ (mm s−1) | ΔE Q (mm s−1) | B hf (T) |
|---|---|---|---|---|---|
| 121Sb | 78 | −7.12(2) | 3.72(13) | −0.17(2) | |
| 151Eu | 298 | −10.35(3) | 2.30(16) | 2.5(4) | |
| 78 | −10.44(1) | 2.56(3) | 2.51(9) | ||
| 4.2 | −10.19(2) | 2.6∗ | −5.2(7) | 2.7(4) |
Although the Eu6Rh30Sb19 structure contains four crystallographically independent antimony sites, the 121Sb Mössbauer spectrum of the compound (Figure 9) was well reproduced with a single signal at an isomer shift of −7.12(2) mm s−1. This value clearly manifests the antimonide character. 59 , 60 , 61 , 62 All antimony atoms have a very similar environment and – given the high natural line width of antimony – the observed signal is the envelope of the four sub-signals which cannot be resolved experimentally.
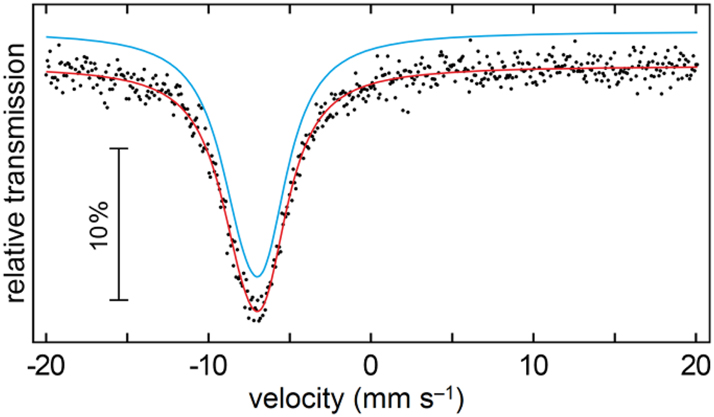
Experimental (dots) and simulated (continuous line) 121Sb Mössbauer spectrum of Eu6Rh30Sb19 at T = 78 K.
Acknowledgements
We thank Dipl.-Ing. U. C. Rodewald for the intensity data collections.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this submitted manuscript and approved the submission.
-
Use of Large Language Models, AI and Machine Learning Tools: Not relevant. Our group is able to think and act independently.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: This research was funded by Universität Münster and Deutsche Forschungsgemeinschaft (INST 211/1034-1).
-
Data availability: Data is available from the corresponding author on well-founded request.
References
1. Kuz’ma, Y.; Chykhrij, S. Phosphides. In Handbook on the Physics and Chemistry of Rare Earths; GschneidnerJr.K. A.; Eyring, L., Eds.; Elsevier Science: Amsterdam, Vol. 23, 1996, chapter 156; pp. 285–433.10.1016/S0168-1273(96)23007-7Search in Google Scholar
2. Chykhrij, S. I. Pol. J. Chem. 1999, 73, 1595–1611.Search in Google Scholar
3. Villars, P.; Cenzual, K. Eds. In Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds (release 2023/24); ASM International®; Materials Park: Ohio (USA), 2023.Search in Google Scholar
4. Babizhetskii, V. S.; Kuz’ma, Y. B. Zh. Neorg. Khim. 1994, 39, 322–327.Search in Google Scholar
5. Demchyna, R. O.; Chykhrij, S. I.; Kuz’ma, Yu. B. J. Alloys Compd. 2002, 345, 170–174.10.1016/S0925-8388(02)00432-2Search in Google Scholar
6. Chykhrij, S. I.; Loukashouk, G. V.; Oryshchyn, S. V.; Kuz’ma, Y. B. J. Alloys Compd. 1997, 248, 224–232; https://doi.org/10.1016/s0925-8388(96)02584-4.Search in Google Scholar
7. Demchyna, R. O.; Oryshchyn, S. V.; Kuz’ma, Y. B. J. Alloys Compd. 2001, 322, 176–183.10.1016/S0925-8388(01)01017-9Search in Google Scholar
8. Sasakawa, T.; Suemitsu, T.; Kitagawa, J.; Sologub, O.; Salamakha, P.; Takabatake, T. J. Phys.: Condens. Matter 2002, 14, L267–L272; https://doi.org/10.1088/0953-8984/14/12/106.Search in Google Scholar
9. Qi, Y.; Guo, J.; Lei, H.; Xiao, Z.; Kamiya, T.; Hosono, H. Phys. Rev. B 2014, 89, 024517; https://doi.org/10.1103/physrevb.89.024517.Search in Google Scholar
10. Inohara, T.; Okamoto, Y.; Yamakawa, Y.; Takenaka, K. J. Phys. Soc. Jpn. 2016, 85, 094706; https://doi.org/10.7566/jpsj.85.094706.Search in Google Scholar
11. Nasir, M. T.; Hadi, M. A.; Rayhan, M. A.; Ali, M. A.; Hossain, M. M.; Roknuzzaman, M.; Naqib, S. H.; Islam, A. K. M. A.; Uddin, M. M.; Ostrikov, K. Phys. Status Solidi B 2017, 254, 1700336; https://doi.org/10.1002/pssb.201700336.Search in Google Scholar
12. Madar, R.; Chaudouet, P.; Senateur, J. P.; Zemni, S.; Tranqui, D. J. Less-Common Met. 1987, 133, 303–311; https://doi.org/10.1016/0022-5088(87)90241-4.Search in Google Scholar
13. Wurth, A.; Johrendt, D.; Mewis, A.; Huhnt, C.; Michels, G.; Roepke, M.; Schlabitz, W. Z. Anorg. Allg. Chem. 1997, 623, 1418–1424; https://doi.org/10.1002/zaac.19976230917.Search in Google Scholar
14. Pivan, J.-Y.; Guérin, R.; Sergent, M. C. R. Acad. Sc. Paris, Sér. II 1984, 299, 533–538.Search in Google Scholar
15. Pivan, J. Y.; Guérin, R.; Sergent, M. J. Less-Common Met. 1985, 107, 249–258.10.1016/0022-5088(85)90084-0Search in Google Scholar
16. Dhahri, E. J. Phys.: Condens. Matter 1996, 8, 4351–4360; https://doi.org/10.1088/0953-8984/8/24/004.Search in Google Scholar
17. Pivan, J.-Y.; Guérin, R.; Pena, O.; Padiou, J.; Sergent, M. Mater. Res. Bull. 1988, 23, 513–520.10.1016/0025-5408(88)90159-6Search in Google Scholar
18. Pfannenschmidt, U.; Rodewald, U. C.; Pöttgen, R. Monatsh. Chem. 2011, 142, 219–224.10.1007/s00706-011-0450-5Search in Google Scholar
19. Wurth, A.; Löhken, A.; Mewis, A. Z. Anorg. Allg. Chem. 2002, 628, 661–666.10.1002/1521-3749(200203)628:3<661::AID-ZAAC661>3.0.CO;2-0Search in Google Scholar
20. Shirotani, I.; Sato, S.; Sekine, C.; Takeda, K.; Inagawa, I.; Yagi, T. J. Phys.: Condens. Matter 2005, 17, 7353–7357; https://doi.org/10.1088/0953-8984/17/46/019.Search in Google Scholar
21. Pfannenschmidt, U.; Johrendt, D.; Behrends, F.; Eckert, H.; Eul, M.; Pöttgen, R. Inorg. Chem. 2011, 50, 3044–3051; https://doi.org/10.1021/ic102570x.Search in Google Scholar
22. Barla, A.; Chefki, M.; Huhnt, C.; Braden, M.; Leupold, O.; Rüffer, R.; Sanchez, J. P.; Wurth, A.; Mewis, A.; Abd-Elmeguid, M. M. Phys. Rev. B 2004, 69, 100102; https://doi.org/10.1103/physrevb.69.100102.Search in Google Scholar
23. Huhnt, C.; Michels, G.; Roepke, M.; Schlabitz, W.; Wurth, A.; Johrendt, D.; Mewis, A. Physica B 1997, 240, 26–37; https://doi.org/10.1016/s0921-4526(97)00431-6.Search in Google Scholar
24. Michels, G.; Roepke, M.; Niemöller, T.; Chefki, M.; Abd-Elmeguid, M. M.; Micklitz, H.; Holland-Moritz, E.; Schlabitz, W.; Huhnt, C.; Büchner, B.; Wurth, A.; Mewis, A.; Kataev, V. J. Phys.: Condens. Matter 1996, 8, 4055–4062.10.1088/0953-8984/8/22/009Search in Google Scholar
25. Schmitz, R.; Müller-Hartmann, E. J. Phys.: Condens. Matter 2008, 20, 285229.10.1088/0953-8984/20/28/285229Search in Google Scholar
26. Kanatzidis, M. G.; Pöttgen, R.; Jeitschko, W. Angew. Chem. Int. Ed. 2005, 44, 6996–7023; https://doi.org/10.1002/anie.200462170.Search in Google Scholar PubMed
27. Pöttgen, R. Z. Kristallogr. 2025, 240, 127–139.10.1515/zkri-2025-0006Search in Google Scholar
28. Stepień-Damm, J.; Prots’, Y.; Salamakha, P. S.; Bodak, O. I.; Morozkin, Yu.; Seropegin, Y. D. J. Alloys Compd. 1998, 268, 177–179.10.1016/S0925-8388(97)00615-4Search in Google Scholar
29. Jeitschko, W.; Altmeyer, R. O.; Rodewald, U. C. Z. Anorg. Allg. Chem. 2009, 635, 2440–2446; https://doi.org/10.1002/zaac.200900230.Search in Google Scholar
30. Pöttgen, R.; Gulden, T.; Simon, A. GIT Labor-Fachzeitschrift 1999, 43, 133–136.Search in Google Scholar
31. Niepmann, D.; Prots’, Y. M.; Pöttgen, R.; Jeitschko, W. J. Solid State Chem. 2000, 154, 329–337.10.1006/jssc.2000.8789Search in Google Scholar
32. Yvon, K.; Jeitschko, W.; Parthé, E. J. Appl. Crystallogr. 1977, 10, 73–74.10.1107/S0021889877012898Search in Google Scholar
33. OriginPro 2024 (Version 10.1.0.170); OriginLab Corporation: Northhampton, Massachusetts (USA), 2024.Search in Google Scholar
34. CorelDRAW Graphics Suite 2017 (version 19.0.0.328); Corel Corporation: Ottawa, Ontario (Canada), 2017.Search in Google Scholar
35. Long, G. J.; Cranshaw, T. E.; Longworth, G. Moessbauer Eff. Ref. Data J. 1983, 6, 42–49.Search in Google Scholar
36. Brand, R. A. WinNormos for Igor6 (version for Igor 6.2 or above: 22/02/2017); Universität Duisburg: Duisburg (Germany), 2017.Search in Google Scholar
37. Palatinus, L. Acta Crystallogr. 2013, B69, 1–16.10.1107/S0108768112051361Search in Google Scholar PubMed
38. Palatinus, L.; Chapuis, G. J. Appl. Crystallogr. 2007, 40, 786–790; https://doi.org/10.1107/s0021889807029238.Search in Google Scholar
39. Petříček, V.; Dušek, M.; Palatinus, L. Z. Kristallogr. 2014, 229, 345–352.10.1515/zkri-2014-1737Search in Google Scholar
40. Petříček, V.; Palatinus, L.; Plášil, J.; Dušek, M. Z. Kristallogr. 2023, 238, 271–282.10.1515/zkri-2023-0005Search in Google Scholar
41. Jeitschko, W.; Jakubowski-Ripke, U. Z. Kristallogr. 1993, 207, 69–79.10.1524/zkri.1993.207.Part-1.69Search in Google Scholar
42. Emsley, J. The Elements; Oxford University Press: Oxford, 1999.Search in Google Scholar
43. Donohue, J. The Structures of the Elements; Wiley: New York, 1974.Search in Google Scholar
44. Pöttgen, R.; Hönle, W.; von Schnering, H. G. Phosphides: Solid State Chemistry. In Encyclopedia of Inorganic Chemistry, 2nd ed.; King, R. B., Ed.; Wiley: New York, Vol. VII, 2005.10.1002/0470862106.ia184Search in Google Scholar
45. Tarasenko, M. S.; Albering, J. H.; Jeitschko, W.; Ibers, J. A. Z. Anorg. Allg. Chem. 2014, 640, 1342–1346; https://doi.org/10.1002/zaac.201300603.Search in Google Scholar
46. Ganglberger, E. Monatsh. Chem. 1968, 99, 557–565; https://doi.org/10.1007/bf00901204.Search in Google Scholar
47. Wurth, A.; Keimes, V.; Johrendt, D.; Mewis, A. Z. Anorg. Allg. Chem. 2001, 627, 2183–2190; https://doi.org/10.1002/1521-3749(200109)627:9<2183::aid-zaac2183>3.0.co;2-j.10.1002/1521-3749(200109)627:9<2183::AID-ZAAC2183>3.0.CO;2-JSearch in Google Scholar
48. Hellmann, A.; Mewis, A. Z. Anorg. Allg. Chem. 2001, 627, 1357–1364; https://doi.org/10.1002/1521-3749(200106)627:6<1357::aid-zaac1357>3.0.co;2-y.10.1002/1521-3749(200106)627:6<1357::AID-ZAAC1357>3.0.CO;2-YSearch in Google Scholar
49. Prots’, Y. M.; Jeitschko, W. Inorg. Chem. 1998, 37, 5431–5438; https://doi.org/10.1021/ic980397w.Search in Google Scholar
50. Zaremba, N.; Krnel, M.; Prots, Yu.; König, M.; Akselrud, L.; Grin, Yu.; Svanidze, E. Inorg. Chem. 2024, 63, 4566–4573; https://doi.org/10.1021/acs.inorgchem.3c03837.Search in Google Scholar
51. Gladyshevskii, E. I.; Grin’, Y. N. Sov. Phys. Crystallogr. 1981, 26, 683–689.Search in Google Scholar
52. Lueken, H. Magnetochemie; Teubner: Stuttgart, 1999.10.1007/978-3-322-80118-0Search in Google Scholar
53. Müllmann, R.; Mosel, B. D.; Eckert, H.; Kotzyba, G.; Pöttgen, R. J. Solid State Chem. 1998, 137, 174–180.10.1006/jssc.1998.7750Search in Google Scholar
54. Pöttgen, R.; Johrendt, D. Chem. Mater. 2000, 12, 875–897; https://doi.org/10.1021/cm991183v.Search in Google Scholar
55. Müllmann, R.; Ernet, U.; Mosel, B. D.; Eckert, H.; Kremer, R. K.; Hoffmann, R.-D.; Pöttgen, R. J. Mater. Chem. 2001, 11, 1133–1140; https://doi.org/10.1039/b100055l.Search in Google Scholar
56. Stein, S.; Heletta, L.; Block, T.; Gerke, B.; Pöttgen, R. Solid State Sci. 2017, 67, 64–71; https://doi.org/10.1016/j.solidstatesciences.2017.03.006.Search in Google Scholar
57. Engel, S.; Gießelmann, E. C. J.; Pöttgen, R.; Janka, O. Rev. Inorg. Chem. 2023, 43, 571–582.10.1515/revic-2023-0003Search in Google Scholar
58. Schellenberg, I.; Pfannenschmidt, U.; Eul, M.; Schwickert, C.; Pöttgen, R. Z. Anorg. Allg. Chem. 2011, 637, 1863–1870; https://doi.org/10.1002/zaac.201100179.Search in Google Scholar
59. Lippens, P. E. Solid State Commun. 2000, 113, 399–403; https://doi.org/10.1016/s0038-1098(99)00501-3.Search in Google Scholar
60. Mishra, R.; Pöttgen, R.; Hoffmann, R.-D.; Fickenscher, Th.; Eschen, M.; Trill, H.; Mosel, B. D. Z. Naturforsch. 2002, 57b, 1215–1223.10.1515/znb-2002-1105Search in Google Scholar
61. Harmening, T.; Eckert, H.; Pöttgen, R. Solid State Sci. 2009, 11, 900–906.10.1016/j.solidstatesciences.2008.12.007Search in Google Scholar
62. Heppke, E. M.; Klenner, S.; Janka, O.; Pöttgen, R.; Bredow, T.; Lerch, M. Inorg. Chem. 2021, 60, 2730–2739; https://doi.org/10.1021/acs.inorgchem.0c03601.Search in Google Scholar PubMed
© 2025 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this issue
- Research Articles
- A MEMS-based gas sensor for H2S detection with enhanced performance using a Ni–In2O3/ZnO nano-composite
- Ultra-sensitive and rapid response H2S microsensor based on Co–ZnO with ppm level detection
- Crystal chemistry and structural phase transition in CaLa2Zn1–x Ca x Ti2O9 (x = 0.00, 0.15, 0.30, 0.45, 0.90 and 1.00)
- RE 10Co3In10 (RE = Y, Gd, Tb, Dy, Ho, Er, Tm) – a new intergrowth structure with CsCl- and AlB2-type slabs
- Crystal structures of phases from the solid solutions DyNiIn1−xSn x
- Tricyanomethanides of the scandium group obtained from aqueous solution: syntheses, crystal structures and Raman spectra of Sc[C(CN)3]3(H2O)3, Y[C(CN)3]3(H2O)2 and La[C(CN)3]3(H2O)4
- Three pnictides with Sm6Rh30Si19-type structure: Sm6Rh30P19, Lu6Rh30P19 and Eu6Rh30Sb19
- The compound (NH4)0.64B4.36O6.72(OH)0.28(NH3)0.36 representing the first ammine/ammonium borate
- Extended investigations on the pressure stability of AlB4O6N:Cr3+
- Synthesis and crystal structures of new phases in the system Hf–Ta–O–N
Articles in the same Issue
- Frontmatter
- In this issue
- Research Articles
- A MEMS-based gas sensor for H2S detection with enhanced performance using a Ni–In2O3/ZnO nano-composite
- Ultra-sensitive and rapid response H2S microsensor based on Co–ZnO with ppm level detection
- Crystal chemistry and structural phase transition in CaLa2Zn1–x Ca x Ti2O9 (x = 0.00, 0.15, 0.30, 0.45, 0.90 and 1.00)
- RE 10Co3In10 (RE = Y, Gd, Tb, Dy, Ho, Er, Tm) – a new intergrowth structure with CsCl- and AlB2-type slabs
- Crystal structures of phases from the solid solutions DyNiIn1−xSn x
- Tricyanomethanides of the scandium group obtained from aqueous solution: syntheses, crystal structures and Raman spectra of Sc[C(CN)3]3(H2O)3, Y[C(CN)3]3(H2O)2 and La[C(CN)3]3(H2O)4
- Three pnictides with Sm6Rh30Si19-type structure: Sm6Rh30P19, Lu6Rh30P19 and Eu6Rh30Sb19
- The compound (NH4)0.64B4.36O6.72(OH)0.28(NH3)0.36 representing the first ammine/ammonium borate
- Extended investigations on the pressure stability of AlB4O6N:Cr3+
- Synthesis and crystal structures of new phases in the system Hf–Ta–O–N

