Abstract
Polycrystalline materials with the compositions CaLa2Zn1−x Ca x Ti2O9 (x = 0.00, 0.15, 0.30, 0.45, 0.90 and 1.00) were synthesized via the conventional solid-state reaction method under ambient conditions. Their crystal structures were determined at room temperature from powder X-ray diffraction (XRPD) using the Rietveld method. For compositions with x = 0.00, 0.15, 0.30, and 0.45, the structures crystallize in the orthorhombic space group Pbnm, with the octahedra tilting in-phase along the [001] direction, and out-of-phase along the [100] and [010] directions of the pseudocubic cell; corresponding to the tilt system a − a − c +. Compositions with x = 0.90 and 1.00 adopt the monoclinic space group P21/n, with the octahedra tilting in-phase along the c axis and out-of-phase along the a and b axes, consistent with the tilt system a − b − c +. In both structural models, the frameworks consist of corner-sharing BO6 octahedra in three dimensions. The structural distortion of the monoclinic phases indicates partial 1:1 B-site ordering between Ti4+ (occupying the 2c site) and a random mixture of 2/3 (Zn2+ 1−x Ca2+ x ) and 1/3 Ti4+ (occupying the 2d site). Along the c axis, the B–O1–B bond angles in the orthorhombic phases (B = Zn(Ca2)/Ti) were calculated to be 156.3° (x = 0.00), 157.3° (x = 0.15), 154.8° (x = 0.30), and 157.2° (x = 0.45). In contrast, the Ti1–O3–B′ bond angles in the monoclinic phases (B′ = Zn(Ca2)/Ti2) were found to be 158.5° (x = 0.90) and 158.3° (x = 1.00). These materials could have promising potential for applications in electrical and dielectric devices.
1 Introduction
Materials based on rare earth complex oxides, which contain transition metals besides titanium, have been extensively studied for their exceptional properties. It has been demonstrated that several complex perovskites with the general formula (A, A′)(B, B′)O3 exhibit high dielectric permittivity, thermally stable electrical properties (including low dielectric loss and stable conductivity at elevated temperatures), negative temperature coefficient (NTC) behavior, and high microwave quality factors. These attributes make them promising candidates for a variety of electrical and dielectric applications. 1 , 2 , 3 , 4 , 5 , 6
When combined in a solid solution with materials that have positive temperature coefficients of the resonant frequency (τ f), the value of τ f can be shifted toward zero. 3 , 7 La2O3–TiO2-based perovskite ceramics have gained particular attention due to their promising potential for microwave resonator applications. 8 , 9 For example, the microwave dielectric properties of La(Zn1/2Ti1/2)O3 were reported. 1 It shows a permittivity of 34, a quality factor of 6,000 at 10 GHz, and a temperature coefficient of the resonant frequency of −55 ppm K−1. The synthesis of these materials, particularly the control of their composition, phase stability, and microstructure, is crucial in optimizing their performance.
Recent studies have investigated the correlation between the structural characteristics of perovskite compounds and their properties. Certain factors, such as the structural phase transition, the concentration of oxygen vacancies, and the size and distribution of cations at the A and B sites, have a significant influence on perovskite properties. 10 , 11 , 12 For example, La(Mg0.5Ti0.5)O3 has been identified as a promising candidate for microwave dielectric applications, due to its distorted monoclinic perovskite structure, caused by octahedral tilting (a − a − c + tilt system), La displacement, and Mg/Ti ordering, which results in favorable properties such as a high quality factor (Q), low dielectric loss, and good temperature stability at microwave frequencies. 8 , 13 Additionally, Ba3CaTa1.5Ti0.5O9, a perovskite-related material, was reported to have enhanced electrical conduction properties, 14 showing the importance of structural characteristics in determining material performance. Understanding these correlations is essential for optimizing the synthesis of perovskite-based materials for specific applications, including photocatalysis, microwave technologies, and catalysis in general.
In this study, we investigated the synthesis and structural characterization of several perovskite compounds incorporating zinc and titanium cations: CaLa2Zn1−x Ca x Ti2O9 (x = 0.00, 0.15, 0.30, 0.45, 0.90 and 1.00), aiming to further enhance their material properties for advanced applications.
2 Experimental section
2.1 Synthesis
Powders of the CaLa2Zn1−x Ca x Ti2O9 perovskite materials (with x = 0.00, 0.15, 0.30, 0.45, 0.90 and 1.00) were prepared using conventional solid-state reaction techniques in an ambient environment. Ca(OH)2 (99.995 %), La2O3 (99.999 %), ZnO (99.9 %), and TiO2 (99.8 %) were used as starting reagents (all received from Sigma-Aldrich) to synthesize the title compositions. The amount of each reagent was weighed according to the stoichiometric coefficient to obtain the appropriate metal ratios in the final products, then mixed and ground in an agate mortar to form a homogeneous powder. The mixtures were placed in alumina crucibles, and then increasingly heated in an ambient environment to different temperature levels; all samples were finally calcined at 1,200 °C for 18 h. During the heat treatment process, the samples were cooled to room temperature, reground and sintered several times to improve their homogeneity. The chemical reaction is as follows:
2.2 Powder X-ray diffraction
The diffraction patterns of the CaLa2Zn1−x Ca x Ti2O9 samples (with x = 0.00, 0.15, 0.30, 0.45, 0.90 and 1.00) were collected at room temperature on a D2 PHASER diffractometer, with Bragg-Brentano geometry, using a copper anti-cathode tube as radiation source (Kα 1, Kα 2) of wavelengths λ(Kα 1) = 1.54056 Å and λ(Kα2) = 1.54439 Å with 30 kV and 10 mA, Soller slits of 0.02 rad on incident and diffracted beams; divergence slit of 0.5°; anti-scattering slit of 1°; receiving slit of 0.1 mm; with a sample spinner, and a Lynxeye-type detector with a maximum overall count rate >1,000,000,000 cps. All the patterns were scanned in steps of 0.020283° (2θ), between 15 and 105° (2θ) with a fixed-time counting of 2 s step−1. The WinPlotr software 15 integrated in the FullProf program 16 was used to analyze the crystal structure of the compounds by means of the Rietveld method. 17 The peak shape was described by a pseudo-Voigt function, and the background level was modeled using a 6 coefficients polynomial function. Instrumental and structural parameters such as scale factor, unit cell parameters, pseudo-Voigt-corrected for asymmetry parameters, FWHM parameters (U, V and W), preferred orientation, atomic coordinates and isotropic displacement parameters were included in the refinement of the crystal structures of the as-synthesized samples.
3 Results and discussion
3.1 Crystallite size and dislocation
The crystallite size of all prepared compositions was determined by means of X-ray line broadening using the Scherrer formula. X-ray peak broadening may be due to crystallite size, micro-strain, the instrumental profile, temperature factors, and solid solution inhomogeneity. The Scherrer equation can be described by the following term: 18
where D is the mean size of the crystallite thickness in (nm), K is the Scherrer constant equal to 0.9, λ is the wavelength of the X-rays (CuKα average = 1.542475 Å), θ is the Bragg angle of the most intense X-ray diffraction peak and β is the full-width at half-maximum (FWHM) of the peak (radians) corrected for instrumental broadening.
Thus, the crystallite size was deduced from XRD patterns near the strongest peak in the range 31.35°≤°2θ ≤ 34.26°. Furthermore, the crystallite size can be used to determine the number of vacancies and defects in a crystal, which is known as the dislocation density and can be obtained by:
where δ represents the dislocation density, and D is the size of the average crystallite. The values for the crystallite size (D) and dislocation density (δ) for all compositions are summarized in Table 1.
Rietveld refinement details and crystallite sizes for the CaLa2Zn1−x Ca x Ti2O9 phases.
| x = 0.00 | x = 0.15 | x = 0.30 | x = 0.45 | x = 0.90 | x = 1.00 | |
|---|---|---|---|---|---|---|
| Chemical formula | Ca1/3La2/3Zn1/3Ti2/3O3 | Ca1/3La2/3(Zn0.85Ca0.15)1/3Ti2/3O3 | Ca1/3La2/3(Zn0.7Ca0.3)1/3Ti2/3O3 | Ca1/3La2/3(Zn0.55Ca0.45)1/3Ti2/3O3 | (Ca1/3La2/3)2Ti(Zn0.1Ca0.9)2/3Ti1/3O6 | (Ca1/3La2/3)2TiCa2/3Ti1/3O6 |
| M r | 623.0266 | 619.2270 | 615.4273 | 611.6277 | 600.2287 | 597.6956 |
| Temperature, K | 298 | 298 | 298 | 298 | 298 | 298 |
| d calc, g cm−3 | 5.77 | 5.73 | 5.66 | 5.59 | 5.23 | 5.21 |
| Radiation | CuKα | CuKα | CuKα | CuKα | CuKα | CuKα |
| Wavelengths, Å | ||||||
| λ 1 | 1.54056 | 1.54056 | 1.54056 | 1.54056 | 1.54056 | 1.54056 |
| λ 2 | 1.54439 | 1.54439 | 1.54439 | 1.54439 | 1.54439 | 1.54439 |
| 2θ step scan increment, deg | 0.020283 | 0.020283 | 0.020283 | 0.020283 | 0.020283 | 0.020283 |
| 2θ range, deg | 15–105 | 15–105 | 15–105 | 15–105 | 15–105 | 15–105 |
| Program | FullProf | FullProf | FullProf | FullProf | FullProf | FullProf |
| FWHM parameters | ||||||
| U | 0.057(5) | 0.111(9) | 0.121(10) | 0.202(18) | 0.118(15) | 0.203(24) |
| V | −0.014(5) | −0.037(7) | −0.042(8) | −0.086(14) | −0.037(10) | −0.099(16) |
| W | 0.007(1) | 0.012(1) | 0.012(2) | 0.020(3) | 0.011(1) | 0.025(3) |
| No. of reflections | 330/2 | 334/2 | 334/2 | 342/2 | 656/2 | 664/2 |
| No. of ref. param. | 29 | 29 | 29 | 29 | 43 | 34 |
| Crystal system | Orthorhombic | Orthorhombic | Orthorhombic | Orthorhombic | Monoclinic | Monoclinic |
| Space group | Pbnm | Pbnm | Pbnm | Pbnm | P21/n | P21/n |
| a, Å | 5.5192(2) | 5.51819(2) | 5.5256(3) | 5.5314(3) | 5.5894(2) | 5.5873(4) |
| b, Å | 5.5448(2) | 5.55045(2) | 5.5654(3) | 5.5820(3) | 5.6969(2) | 5.7025(4) |
| c, Å | 7.8132(3) | 7.8137(3) | 7.8313(3) | 7.8454(4) | 7.9737(3) | 7.9761(6) |
| β, deg | 90 | 90 | 90 | 90 | 89.885(4) | 89.901(6) |
| Cell volume, Å3 | 239.11(1) | 239.32(1) | 240.83(2) | 242.23(2) | 253.90(2) | 254.13(3) |
| Z | 4 | 4 | 4 | 4 | 2 | 2 |
| Atom number | 5 | 5 | 5 | 5 | 5 | 4 |
| R B, % | 4.61 | 4.39 | 4.99 | 6.09 | 5.32 | 8.94 |
| R F, % | 5.25 | 4.87 | 5.23 | 8.08 | 4.75 | 6.34 |
| R p, % | 6.69 | 6.56 | 7.11 | 8.51 | 7.27 | 8.63 |
| R wp, % | 9.12 | 8.76 | 9.52 | 11.5 | 9.54 | 11.6 |
| R exp, % | 7.82 | 7.79 | 8.59 | 8.46 | 8.27 | 8.27 |
| χ 2 | 1.36 | 1.26 | 1.23 | 1.83 | 1.33 | 1.98 |
| D Sch, nm | 93 | 79 | 82 | 76 | 79 | 72 |
| δ, m−2 | 1.16 × 1014 | 1.61 × 1014 | 1.48 × 1014 | 1.75 × 1014 | 1.59 × 1014 | 1.94 × 1014 |
| <r B site>, nm | 0.0650 | 0.0663 | 0.0676 | 0.0689 | 0.0728 | 0.0737 |
| Tolerance factor t | 0.950 | 0.944 | 0.938 | 0.933 | 0.915 | 0.911 |
3.2 Crystal structure determination and structure description
Figure 1 exhibits the powder X-ray diffraction patterns of the ceramic materials CaLa2Zn1−x Ca x Ti2O9 (x = 0.00, 0.15, 0.30, 0.45, 0.90 and 1.00) collected at room temperature. The patterns of all compositions are successfully identified by data of the PDF2 database 19 integrated into the HighScore plus software 20 and analyzed using the DICVOL program 21 to adopt phases related to the orthorhombic and monoclinic perovskite structures. From the enlarged peaks in the inset of Figure 1, the XRD patterns of the powdered samples display an obvious shift of the peaks towards lower angles (2θ, Bragg position), indicating that a partial substitution of Zn2+ by Ca2+ has an influence on the crystal structure parameters. The shape of the enlarged peaks revealed a phase transition between x = 0.45 and x = 0.90. Minor amounts of unidentified secondary phases were observed in the XRD patterns of certain samples.

XRD patterns of CaLa2Zn1−x Ca x Ti2O9 perovskite materials collected at room temperature. The inset shows an enlarged view of the peaks around 46° and 48°.
Previous studies have reported that the starting composition CaLa2ZnTi2O9 crystallizes in the orthorhombic space group Pbnm, while the final composition CaLa2CaTi2O9 adopts a monoclinic structure, space group P21/n. 22 , 23 The crystal structures of all the prepared compositions were determined using the Rietveld refinement method. 17
Table 1 provides the crystal structure parameters, data collection and details of the Rietveld refinements, including lattice parameters, cell volume, crystal system, space group and various statistical agreement factors. The values of the reliability factors, R B, R F, R p, R wp and R exp (%), and the goodness-of-fit (χ 2), are small and indicate that we obtained good quality refinements. The quality of the fits is also confirmed by Figures 2 and 3, which provide excellent agreement between the experimental (dots) and theoretical (solid line) patterns. For the composition x = 0.90, the impurity was included in the refinement as a second phase crystallizing in the orthorhombic space group Pbnm (no. 62) with lattice parameters a = 5.5430 Å, b = 5.6056 Å and c = 7.8660 Å. The crystal structure views of the orthorhombic and monoclinic phases, shown in Figures 4 and 5, were generated using VESTA software. 24
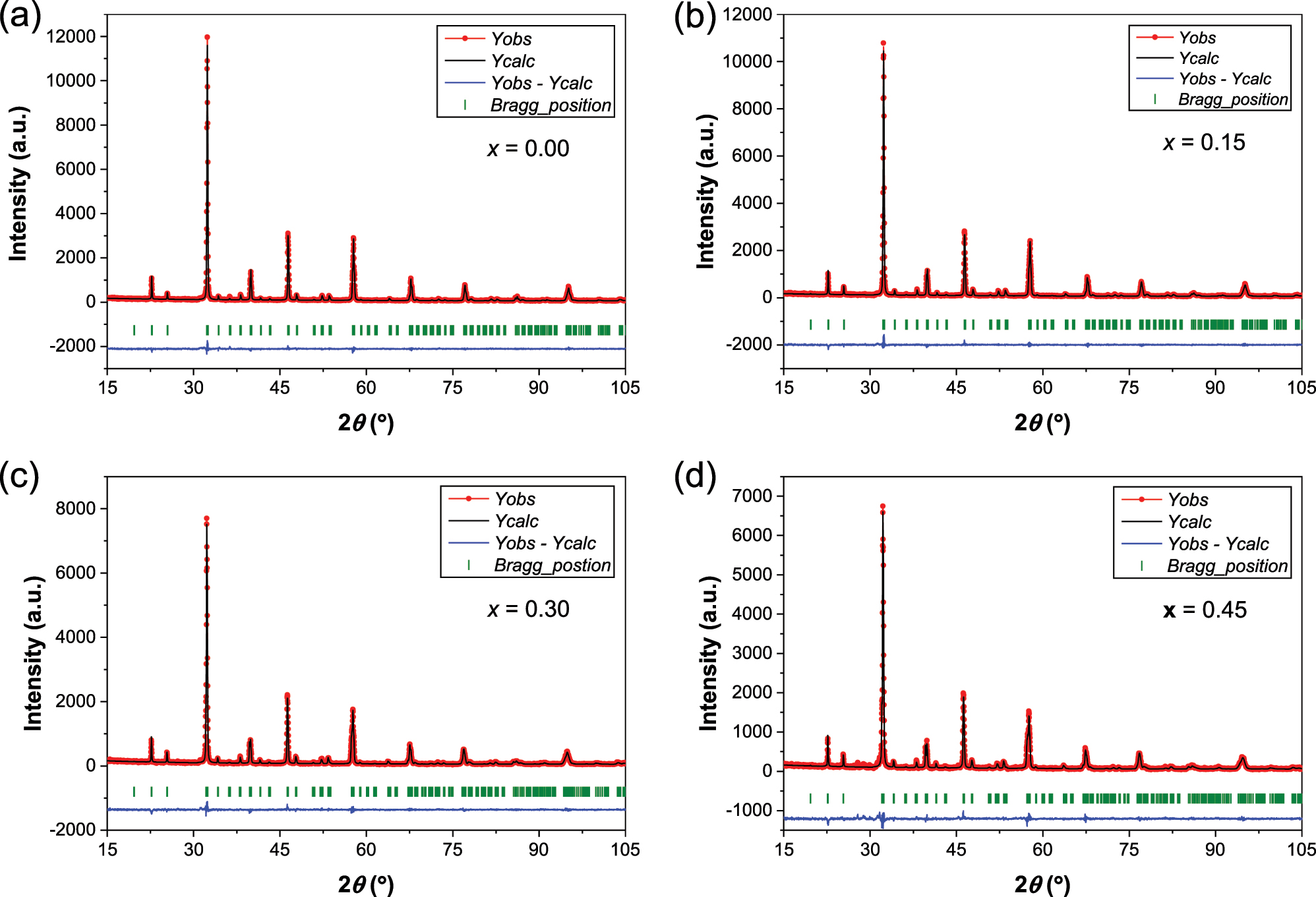
Final Rietveld plots of the orthorhombic CaLa2Zn1−x Ca x Ti2O9 phases. The experimental data are represented by red dots, and the calculated pattern is shown as a black solid line. Green vertical ticks indicate the Bragg positions of the main phase. The blue line at the bottom represents the difference between the observed and calculated patterns.

Final Rietveld plots of the monoclinic CaLa2Zn1−x Ca x Ti2O9 phases. The experimental data are represented by red dots, and the calculated pattern is shown as a black solid line. Green vertical ticks indicate the Bragg positions of the main phase and impurity phases. The blue line at the bottom represents the difference between the observed and calculated patterns.

Views of the orthorhombic (Ca1/3La2/3)4c ((Zn0.7Ca0.3)1/3Ti2/3)4b O3 structure (orthorhombic space group Pbnm; Z = 4). It shows BO6 octahedra sharing the corners in 3D; as well as octahedral tilt effects in accordance with Glazer’s a − a − c + notation. Zn2+/Ca2+(2)/Ti4+ cations are located inside octahedra. Ca2+(1)/La3+ cations are indicated by cyan spheres.
![Figure 5:
Views of the monoclinic [(Ca1/3La2/3)2]4e
[Ti]2c
[Ti1/3(Zn0.1Ca0.9)2/3)]2d
O6 structure (monoclinic space group P21/n; Z = 2). It shows the Ti(1)O6 and B′O6 octahedra sharing the corners in 3D; as well as octahedral tilt effects in accordance with Glazer’s a
−
b
−
c
+ notation. Ti4+(1) cations are located inside the green octahedra. B′ = Zn/Ca2/Ti2 atoms are located inside the blue octahedra. Ca2+(1)/La3+ cations are indicated by yellow spheres.](/document/doi/10.1515/znb-2025-0015/asset/graphic/j_znb-2025-0015_fig_005.jpg)
Views of the monoclinic [(Ca1/3La2/3)2]4e [Ti]2c [Ti1/3(Zn0.1Ca0.9)2/3)]2d O6 structure (monoclinic space group P21/n; Z = 2). It shows the Ti(1)O6 and B′O6 octahedra sharing the corners in 3D; as well as octahedral tilt effects in accordance with Glazer’s a − b − c + notation. Ti4+(1) cations are located inside the green octahedra. B′ = Zn/Ca2/Ti2 atoms are located inside the blue octahedra. Ca2+(1)/La3+ cations are indicated by yellow spheres.
To calculate the Goldschmidt tolerance factor (t) for a perovskite of the form ABO3, the following formula is used:
Here, r A is the effective ionic radii of the A-site cations, r B is the effective ionic radii of the B-site cations and r O is the radius of the oxide ion, typically 0.140 nm. The Shannon effective ionic radii 25 for cations with 12-fold coordination are: Ca2+ (0.134 nm) and La3+ (0.136 nm). For cations with 6-fold coordination the respective radii are: Ti4+ (0.0605 nm), Ca2+ (0.100 nm), and Zn2+ (0.074 nm). The obtained values of the Goldschmidt tolerance factors (Table 1) were calculated based on the effective ionic radii of the A- and B-site cations. These values suggest distorted perovskite structures, consistent with orthorhombic and monoclinic crystal systems. The tolerance factor decreases as the radius of the B-site cation increases.
Orthorhombic phases (x = 0.00, 0.15, 0.30, and 0.45): Rietveld refinement analyses revealed that these compositions crystallize in the orthorhombic space group Pbnm (no. 62) with unit cell parameters related to a
0 (ideal cubic perovskite, a
0 ≈ 3.9 Å) as a ≈ b ≈
Atomic coordinates and isotropic displacement parameters for the orthorhombic phases with space group Pbnm and Z = 4.
| x | y | z | U iso/U eq (Å2) | Occ. (<1) | Wyckoff site | Symmetry | |
|---|---|---|---|---|---|---|---|
| x = 0.00 | |||||||
|
|
|||||||
| Ca, La | 0.9955(8) | 0.0274(2) | 1/4 | 0.0173(5) | 1/3, 2/3 | 4c | .m. |
| Zn, Ti | 1/2 | 0 | 0 | 0.0084(5) | 1/3, 2/3 | 4b | −1 |
| O(1) | 0.074(4) | 0.495(1) | 1/4 | 0.018(2) | 1 | 4c | .m. |
| O(2) | 0.722(2) | 0.295(2) | 0.031(2) | 0.017(2) | 1 | 8d | 1 |
|
|
|||||||
| x = 0.15 | |||||||
|
|
|||||||
| Ca(1), La | 0.9930(6) | 0.0304(2) | 1/4 | 0.0180(5) | 1/3, 2/3 | 4c | .m. |
| Ca(2), Zn, Ti | 1/2 | 0 | 0 | 0.0072(5) | 0.15/3, 0.85/3, 2/3 | 4b | −1 |
| O(1) | 0.070(3) | 0.489(1) | 1/4 | 0.029(2) | 1 | 4c | .m. |
| O(2) | 0.7216(2) | 0.283(2) | 0.036(1) | 0.029(2) | 1 | 8d | 1 |
|
|
|||||||
| x = 0.30 | |||||||
|
|
|||||||
| Ca(1), La | 0.9928(6) | 0.0325(2) | 1/4 | 0.0164(6) | 1/3, 2/3 | 4c | .m. |
| Ca(2), Zn, Ti | 1/2 | 0 | 0 | 0.0006(6) | 0.3/3, 0.7/3, 2/3 | 4b | −1 |
| O(1) | 0.078(3) | 0.487(2) | 1/4 | 0.022(4) | 1 | 4c | .m. |
| O(2) | 0.720(3) | 0.287(2) | 0.033(2) | 0.022(4) | 1 | 8d | 1 |
|
|
|||||||
| x = 0.45 | |||||||
|
|
|||||||
| Ca(1), La | 0.9938(8) | 0.0335(3) | 1/4 | 0.0219(8) | 1/3, 2/3 | 4c | .m. |
| Ca(2), Zn, Ti | 1/2 | 0 | 0 | 0.0013(8) | 0.45/3, 0.55/3, 2/3 | 4b | −1 |
| O(1) | 0.070(4) | 0.4852(2) | 1/4 | 0.044(6) | 1 | 4c | .m. |
| O(2) | 0.714(3) | 0.281(3) | 0.037(2) | 0.044(6) | 1 | 8d | 1 |
The Ca2+(1), La3+ and O2−(1) ions are located on the 4c (x, y, 1/4) sites of C s symmetry. The Ca2+(2), Zn2+ and Ti4+ cations occupy octahedral sites 4b (1/2, 0, 0) of C i symmetry, and the O2−(2) anions are situated at positions 8d (x, y, z) of C 1 symmetry. The Zn2+(Ca2+(2))/Ti4+ cations are surrounded by six neighboring 2 O2−(1) and 4 O2−(2) anions, while the Ca2+(1)/La3+ cations are surrounded by twelve neighboring 4 O2−(1) and 8 O2−(2) anions.
The bond lengths (Å) and angles (deg) determined from the refined XRD patterns of all orthorhombic structures are given in Table 4. The average <Zn(Ca(2))/Ti–O> interatomic distances obtained at room temperature are very close to those found in other perovskite compounds containing Ca2+, La3+, Zn2+ and Ti4+ cations such as CaLn 2ZnTi2O9 (Ln = La, Pr, Nd, Eu), while those of <Ca(1)/La–O> are slightly larger than those observed in the same compounds. 23
Figure 4 presents the structural views of the orthorhombic phase (space group Pbnm, Z = 4) of the simple perovskite (Ca1/3La2/3)4c ((Zn0.7Ca0.3)1/3Ti2/3)4b O3; it is made up of octahedra which share corners in three dimensions (3D). In the ab plane, the BO6 octahedra with B = Zn/Ca(2)/Ti are linked by O(2) atoms situated at positions (x, y, z). Along the c axis, the BO6 octahedra are connected by O(1) oxygen atoms located at positions (x, y, 1/4). The bond angles B–O(1)–B were calculated as 156.3(13)º, 157.3(10)º, 154.8(10)º and 157.2(13)º, and those for B–O(2)–B were 158.3(7)º, 158.7(6)º, 158.7(8)º, and 157.3(9)º, corresponding to x = 0.00, 0.15, 0.30, and 0.45 respectively. The average bond lengths of <B–O> were calculated as 1.993, 1.991, 1.999, and 2.003 Å, and those of <Ca(1)/La–O> were found to be 2.782, 2.783, 2.791, and 2.796 Å, corresponding to x = 0.00, 0.15, 0.30 and 0.45 respectively. The volume of the BO6 octahedron was calculated as 10.532, 10.527, 10.636, and 10.709 Å3, for compositions x = 0.00, 0.15, 0.30, and 0.45, respectively.
The octahedral tilt system corresponds to the a − a − c + notation; 28 it indicates that the octahedra tilting out-of-phase along the two [100] p and [010] p directions of the pseudocubic cell with equal amplitudes, and in-phase along the [001] p direction with a different amplitude, as determined for the “orthorhombic” Sr1/3La2/3Ni1/3Fe1/3Nb1/3O3 simple perovskite structure, consistent with the space group Pbnm. 29
Monoclinic phases (x = 0.90 and 1.00): These two compositions were analyzed using the Rietveld refinement method and the results revealed that their crystal structures adopt the space group P21/n (no. 14) with unit cell parameters related to a
0 (ideal cubic perovskite, a
0 ≈ 3.9 Å) as a ≈ b ≈
Atomic positions and isotropic displacement parameters for the monoclinic phases with space group P21/n and Z = 2.
| x | y | z | U iso/U eq (Å2) | Occ. | Wyckoff site | Symmetry | |
|---|---|---|---|---|---|---|---|
| x = 0.90 | |||||||
|
|
|||||||
| Ca(1), La | 0.504(1) | 0.5413(3) | 0.2521(4) | 0.0160(7) | 1/3, 2/3 | 4e | 1 |
| Ti(1) | 0 | 1/2 | 0 | 0.0057(9) | 1 | 2c | −1 |
| Ca(2), Zn, Ti(2) | 1/2 | 0 | 0 | 0.0057(9) | 1.8/3, 0.2/3, 1/3 | 2d | −1 |
| O(1) | 0.187(4) | 0.233(4) | −0.043(4) | 0.056(4) | 2 | 4e | 1 |
| O(2) | 0.283(4) | 0.678(4) | −0.018(4) | 0.056(4) | 2 | 4e | 1 |
| O(3) | 0.437(4) | 0.977(2) | 0.271(3) | 0.056(4) | 2 | 4e | 1 |
|
|
|||||||
| x = 1.00 | |||||||
|
|
|||||||
| Ca(1), La | 0.5067(9) | 0.5412(3) | 0.2540(5) | 0.0038* | 1/3, 2/3 | 4e | 1 |
| Ti(1) | 0 | 1/2 | 0 | 0.0033(9) | 1 | 2c | −1 |
| Ca(2), Ti(2) | 1/2 | 0 | 0 | 0.0033(9) | 2/3, 1/3 | 2d | −1 |
| O(1) | 0.186(4) | 0.260(5) | −0.017(4) | 0.061(4) | 2 | 4e | 1 |
| O(2) | 0.298(5) | 0.698(5) | −0.045(5) | 0.061(4) | 2 | 4e | 1 |
| O(3) | 0.436(4) | 0.977(3) | 0.262(5) | 0.061(4) | 2 | 4e | 1 |
-
*Fixed in 0.0038 Å2 to guarantee the convergence of the refinement.
The isotropic displacement parameters U iso (Å2) for the A-site cations were refined by constraining them to be equivalent, with a similar constraint applied to the B-site cations and oxygen atoms. It is noteworthy that the U iso values for the B-site cations are consistently lower than those for the A-site cations.
The anomalous isotropic displacement factors can be explained by assuming a positional disorder (static or dynamic) of the Ca2+(1)/La3+ cations. Due to the discrepancy between calculated and observed patterns for composition x = 1.0, the U iso value for the Ca2+/La3+ atoms was fixed at 0.0038 Å2.
The selected interatomic distances (Å) and angles (deg.) of the monoclinic structures are shown in Table 4. The average <Ca(1)/La–O>, <Ti(1)–O> and <Zn(Ca(2))/Ti(2)–O> bond lengths obtained at room temperature after a Rietveld refinement are approximately the same as those reported for the perovskite compounds CaLa2Ni2WO9, 31 CaLa2CaTi2O9 22 and La2ZnTiO6. 32 The obtained interatomic distances are close to those calculated using Shannon’s effective ionic radii: for a coordination number of 12 they are Ca2+–O2− (2.74 Å) and La3+–O2− (2.76 Å), for a coordination number of 6 they are Ca2+–O2− (2.40 Å), Zn2+–O2− (2.14 Å) and Ti4+–O2− (2.005 Å). 25 The Ti4+(1) cations at site 2c (0, 1/2, 0), or the Zn2+(Ca2+(2))/Ti4+(2) cations at site 2d (1/2, 0, 0) are surrounded by six oxygen anions 2 O2−(1), 2 O2−(2) and 2 O2−(3), while the Ca2+(1)/La3+ cations at site 4e (x, y, z) are surrounded by twelve anions 4 O2−(1), 4 O2−(2) and 4 O2−(3).
Selected interatomic distances (Å) and angles (deg) for the orthorhombic (x = 0.00, 0.15, 0.30, and 0.45) and monoclinic (x = 0.90 and 1.00) phases.
| x = 0.00 | x = 0.15 | x = 0.30 | x = 0.45 | ||
|---|---|---|---|---|---|
| Ca1/La–O1 | (×1) | 2.986(8) | 3.038(8) | 3.073(9) | 3.089(4) |
| (×1) | 2.626(9) | 2.578(8) | 2.572(9) | 2.556(4) | |
| (×1) | 3.15(2) | 3.12(2) | 3.16(2) | 3.13(2) | |
| (×1) | 2.38(2) | 2.42(2) | 2.39(2) | 2.43(2) | |
| Ca1/La–O2 | (×2) | 2.72(1) | 2.647(9) | 2.67(1) | 2.66(2) |
| (×2) | 2.71(1) | 2.77(1) | 2.74(1) | 2.76(2) | |
| (×2) | 2.46(1) | 2.469(9) | 2.48(1) | 2.47(2) | |
| (×2) | 3.23(1) | 3.238(9) | 3.26(1) | 3.28(2) | |
| <Ca1/La–O> mean | 2.782 | 2.783 | 2.791 | 2.796 | |
| Zn(Ca2)/Ti–O1 | (×2) | 1.996(5) | 1.992(3) | 2.006(4) | 2.001(4) |
| Zn(Ca2)/Ti–O2 | (×2) | 2.06(1) | 2.009(8) | 2.02(1) | 1.99(2) |
| (×2) | 1.93(1) | 1.973(6) | 1.97(2) | 2.02(2) | |
| <Zn(Ca2)/Ti–O> mean | 1.993 | 1.991 | 1.999 | 2.003 | |
| B–O1–B | 156.3(13) | 157.3(10) | 154.8(10) | 157.2(13) | |
| B–O2–B | 158.3(7) | 158.7(6) | 158.7(8) | 157.3(9) | |
| B = Zn(Ca2)/Ti |
| x = 0.90 | x = 1.00 | ||
|---|---|---|---|
| Ca1/La–O1 | (×1) | 3.43(3) | 3.24(3) |
| (×1) | 2.78(3) | 2.67(3) | |
| (×1) | 2.722(7) | 2.79(3) | |
| (×1) | 2.48(3) | 2.70(3) | |
| Ca1/La–O2 | (×1) | 2.60(3) | 2.80(4) |
| (×1) | 3.37(3) | 3.48(3) | |
| (×1) | 2.54(3) | 2.41(4) | |
| (×1) | 2.89(3) | 2.73(3) | |
| Ca1/La–O3 | (×1) | 3.24(1) | 3.24(2) |
| (×1) | 2.51(1) | 2.52(2) | |
| (×1) | 2.50(2) | 2.50(2) | |
| (×1) | 3.15(2) | 3.14(2) | |
| <Ca1/La–O> mean | 2.852 | 2.852 | |
| Ti1–O1 | (×2) | 1.877(3) | 1.72(3) |
| Ti1–O2 | (×2) | 1.88(2) | 2.04(3) |
| Ti1–O3 | (×2) | 1.87(2) | 1.94(4) |
| <Ti1–O> mean | 1.875 | 1.901 | |
| B′–O1 | (×2) | 2.22(2) | 2.30(3) |
| B′–O2 | (×2) | 2.20(2) | 2.09(3) |
| B′–O3 | (×2) | 2.19(2) | 2.12(4) |
| <B ′–O> mean | 2.207 | 2.172 | |
| Ti1–O1–B’ | 153.4(16) | 165.1(16) | |
| Ti1–O2–B′ | 154.8(14) | 149.9(19) | |
| Ti1–O3–B′ | 158.5(12) | 158.3(13) | |
| B′ = Zn(Ca2)/Ti2 |
-
The bold values represent the average interatomic distances obtained after Rietveld refinement.
Figure 5 shows the structural visualization of the monoclinic phase (space group P21/n, Z = 2) of the double perovskite [(Ca1/3La2/3)2]4e [Ti]2c [Ti1/3(Zn0.1Ca0.9)2/3)]2d O6; it consists of alternating octahedra sharing corners in three dimensions (3D) by means of oxygen atoms. Along the c axis, the octahedra Ti(1)O6 and B′O6 with B′ = Zn/Ca(2)/Ti(2) are connected by oxygen atoms O(3) of 4e (x, y, z) positions. Due to the special positions occupied by the Ti(1) and B′ atoms at sites 2c (0, 1/2, 0) and 2d (1/2, 0, 0), respectively, and by the O(3) oxygen atoms at sites 4e (x, y, z), the Ti(1)–O(3)–B′ bond angles were found to be 158.5(12)° and 158.3(13)° for compositions x = 0.9 and 1.0 respectively. In the ab plane, the octahedra are linked by oxygen atoms O(1) and O(2) at sites 4e (x, y, z), where the bond angles Ti(1)–O(1)–B′ and Ti(1)–O(2)–B′ of the composition x = 0.9 are 153.4(16)° and 154.8(14)°, respectively, and those of x = 1.0 are 165.1(16)° and 149.9(19)°, respectively.
The structure contains octahedra tilted in-phase along the [001] p direction of the pseudocubic cell and out-of-phase along the [100] p and [010] p directions with unequal amplitudes, which corresponds to the a − b − c + Glazer’s notation as derived by Fuertes et al. 33 for the 2:1 ordering in La3Co2 MO9 (M = Nb or Ta) triple perovskites, consistent with the monoclinic space group P21/n. Each Ti4+O6 octahedron at sites 2c consists of 6 shorter bond lengths between Ti4+ and O2− with an average of 1.875 Å (x = 0.9) and 1.901 Å (x = 1.0). In contrast, the octahedron B′O6 (B′ = Zn/Ca(2)/Ti(2)) features 6 longer bonds between B′ and O, with an average of 2.207 Å (x = 0.9) and 2.172 Å (x = 1.0). The volumes of the Ti(2c)O6 octahedron are calculated to be 8.774 Å3 (for x = 0.9) and 9.019 Å3 (for x = 1.0), clearly compressed compared with that of the B′(2d)O6 octahedron, which is 14.241 Å3 (x = 0.9) and 13.529 Å3 (x = 1.0).
4 Conclusions
The polycrystalline ceramic phases CaLa2Zn1−x Ca x Ti2O9 (x = 0.00, 0.15, 0.30, 0.45, 0.90 and 1.00) were prepared using the conventional solid-state reaction route. Their crystal structures were determined at room temperature from powder X-ray diffraction (XRPD) recordings using the Rietveld refinement method. The structures of the following compositions (x = 0.00, 0.15, 0.30 and 0.45) crystallize in the Pbnm orthorhombic space group; with the octahedra tilted out-of-phase along the [100] p and [010] p directions of the pseudocubic cell, and in-phase along the [001] p direction. The tilt system is corresponding to the Glazer’s notation a − a − c +. However, the structures of the compositions (x = 0.90 and 1.00) crystallize in the monoclinic space group P21/n with the octahedra tilted out-of-phase along the a, b axes and in-phase along the c axis, which corresponds to the tilt system a − b − c +. For both models, the structure is composed of octahedra with shared corners in three dimensions (3D). Along the c axis the bond angles B–O1–B in the orthorhombic phases with B = Zn(Ca2)/Ti were calculated as 156.3° (x = 0.00), 157.3° (x = 0.15), 154.8° (x = 0.30), and 157.2° (x = 0.45), whereas the monoclinic phases the bond angles Ti1–O3–B′ with B′ = Zn(Ca2)/Ti2 were found to be 158.5° (x = 0.90) and 158.3° (x = 1.00). In the orthorhombic phases, the three elements Zn2+, Ca2+(2) and Ti4+ occupy only one crystallographic B site, and the two elements Ca2+(1) and La3+ have only one crystallographic A site in the perovskite structure (A 1/3 A′2/3)4c (B 1/3 B′2/3)4b O3. The structural distortion of monoclinic phases exhibits partial 1:1 B-perovskite site ordering between Ti4+ in the 2c site and a random mixture (2/3(Zn2+ 1−x Ca2+ x ); 1/3Ti4+) in the 2d site. These materials could have promising potential for applications in electrical and dielectric devices.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The raw data can be obtained on request from the corresponding author. CSD 2452906-2452911 contains the supplementary crystallographic data for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
References
1. Cho, S. Y.; Seo, M. K.; Hong, K. S.; Park, S. J.; Kim, I. T. Mater. Res. Bull. 1997, 32, 725–735; https://doi.org/10.1016/s0025-5408(97)00040-8.Search in Google Scholar
2. Keith, G. M.; Kirk, C. A.; Sarma, K.; Alford, N. M.; Cussen, E. J.; Rosseinsky, M. J.; Sinclair, D. C. Chem. Mater. 2004, 16, 2007–2015; https://doi.org/10.1021/cm035317n.Search in Google Scholar
3. Feteira, A.; Iddles, D.; Price, T.; Muir, D.; Reaney, I. M. J. Am. Ceram. Soc. 2011, 94, 817–821; https://doi.org/10.1111/j.1551-2916.2010.04173.x.Search in Google Scholar
4. El Hachmi, A.; El Ouahbi, S.; Ounza, Y.; Biswas, G.; Lassri, H.; Manoun, B.; Sadoune, Z. Solid State Commun. 2024, 387, 115534; https://doi.org/10.1016/j.ssc.2024.115534.Search in Google Scholar
5. Xie, J.; Qin, Q.; Li, Y.; Zhao, P.; Chang, A.; Zhang, H. Ceram. Int. 2023, 49, 19682–19690; https://doi.org/10.1016/j.ceramint.2023.03.082.Search in Google Scholar
6. Raengthon, N.; Cann, D. P. J. Electroceram. 2012, 28, 165–171; https://doi.org/10.1007/s10832-012-9700-0.Search in Google Scholar
7. Cho, S. Y.; Youn, H. J.; Lee, H. J.; Hong, K. S. J. Am. Ceram. Soc. 2001, 84, 753–758; https://doi.org/10.1111/j.1151-2916.2001.tb00737.x.Search in Google Scholar
8. Lee, D. Y.; Yoon, S. J.; Yeo, J. H.; Nahm, S.; Paik, J. H.; Whang, K. C.; Ahn, B. G. J. Mater. Sci. Lett. 2000, 19, 131–134; https://doi.org/10.1023/a:1006603615193.10.1023/A:1006603615193Search in Google Scholar
9. Houivet, D.; El Fallah, J.; Bernard, J.; Rouland, F.; Haussonne, J. M. J. Eur. Ceram. Soc. 2001, 21, 1715–1718; https://doi.org/10.1016/s0955-2219(01)00100-5.Search in Google Scholar
10. Jia, T.; Zeng, Z.; Zhang, X.; Ohodnicki, P.; Chorpening, B.; Hackett, G.; Lekse, J.; Duan, Y. Phys. Chem. Chem. Phys. 2019, 21, 20454–20462; https://doi.org/10.1039/c9cp03883c.Search in Google Scholar PubMed
11. Vogt, U. F.; Holtappels, P.; Sfeir, J.; Richter, J.; Duval, S.; Wiedenmann, D.; Züttel, A. Fuel Cells 2009, 9, 899–906; https://doi.org/10.1002/fuce.200800116.Search in Google Scholar
12. Hur, S. G.; Kim, T. W.; Hwang, S. J.; Choy, J. H. J. Photochem. Photobiol. A 2006, 183, 176–181; https://doi.org/10.1016/j.jphotochem.2006.03.014.Search in Google Scholar
13. Seabra, M. P.; Ferreira, V. M. Mater. Res. Bull. 2002, 37, 255–262; https://doi.org/10.1016/s0025-5408(01)00748-6.Search in Google Scholar
14. Jaiswal, S. K.; Yoon, K. J.; Son, J. W.; Lee, J. H. J. Alloys Compd. 2019, 775, 736–741; https://doi.org/10.1016/j.jallcom.2018.10.185.Search in Google Scholar
15. Roisnel, T.; Rodríguez-Carvajal, J. Mater. Sci. Forum 2001, 378, 118–123.10.4028/www.scientific.net/MSF.378-381.118Search in Google Scholar
16. Rodríguez-Carvajal, R. FullProf2000, A Program for Rietveld Refinement and Pattern Matching Analysis. In Satellite Meeting on Powder Diffraction of the 15th International Congress of the IUCr, Toulouse (France), 1990; pp. 127.Search in Google Scholar
17. Rietveld, H. M. J. Appl. Crystallogr. 1969, 2, 65–71; https://doi.org/10.1107/s0021889869006558.Search in Google Scholar
18. Scherrer, P. Nachr. Ges. Wiss. Goettingen, Math.-Phys. Kl. 1918, 1918, 98–100.Search in Google Scholar
19. Gates-Rector, S.; Blanton, T. Powder Diffr. 2019, 34, 352–360; https://doi.org/10.1017/s0885715619000812.Search in Google Scholar
20. Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. Powder Diffr. 2014, 29, S13–S18; https://doi.org/10.1017/s0885715614000840.Search in Google Scholar
21. Louër, D.; Boultif, A. Powder Diffr. 2014, 29, S7–S12; https://doi.org/10.1017/s0885715614000906.Search in Google Scholar
22. Aatiq, A. Solid State Sci. 2003, 5, 745–749; https://doi.org/10.1016/s1293-2558(03)00076-1.Search in Google Scholar
23. Aatiq, A.; Boukhari, A. Mater. Lett. 2004, 58, 2406–2411; https://doi.org/10.1016/j.matlet.2004.02.023.Search in Google Scholar
24. Momma, K.; Izumi, F. J. Appl. Crystallogr. 2011, 44, 1272–1276; https://doi.org/10.1107/s0021889811038970.Search in Google Scholar
25. Shannon, R. D. Acta Crystallogr. 1976, A32, 751–767.10.1107/S0567739476001551Search in Google Scholar
26. He, C.; Li, Y.; Wang, F.; Pang, X.; Hou, Z.; Liu, W.; Liang, D.; Tang, B.; Fang, Z.; Shi, Z.; Chen, J. J. Mater. Sci.: Mater. Electron. 2024, 35, 2081; https://doi.org/10.1007/s10854-024-13789-6.Search in Google Scholar
27. Cockcroft, J. A Hypertext Book of Crystallographic Space Group Diagrams and Tables; Birkbeck College; University of London: London (UK), 1999. http://img.chem.ucl.ac.uk/sgp/large/062ez2.htm (accessed 2025-02-12).Search in Google Scholar
28. Glazer, A. M. Acta Crystallogr. 1972, B28, 3384–3392.10.1107/S0567740872007976Search in Google Scholar
29. El Hachmi, A.; Sadoune, Z. Powder Diffr. 2024, 39, 235–244; https://doi.org/10.1017/s0885715624000289.Search in Google Scholar
30. Hahn, T.; Ed. International Tables for Crystallography, Volume A: Space-Group Symmetry, 5th ed.; International Union of Crystallography: Chester; Springer: Dordrecht, 2005.10.1107/97809553602060000502Search in Google Scholar
31. Chin, C. M.; Sena, R. P.; Hunter, E. C.; Hadermann, J.; Battle, P. D. J. Solid State Chem. 2017, 251, 224–232.10.1016/j.jssc.2017.04.023Search in Google Scholar
32. Aguadero, A.; Alonso, J. A.; Martínez-Lope, M. J.; Fernández-Díaz, M. T. Solid State Sci. 2011, 13, 13–18.10.1016/j.solidstatesciences.2010.10.006Search in Google Scholar
33. Fuertes, V. C.; Blanco, M. C.; Franco, D. G.; De Paoli, J. M.; Sanchez, R. D.; Carbonio, R. E. Mater. Res. Bull. 2011, 46, 62–69; https://doi.org/10.1016/j.materresbull.2010.09.033.Search in Google Scholar
© 2025 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this issue
- Research Articles
- A MEMS-based gas sensor for H2S detection with enhanced performance using a Ni–In2O3/ZnO nano-composite
- Ultra-sensitive and rapid response H2S microsensor based on Co–ZnO with ppm level detection
- Crystal chemistry and structural phase transition in CaLa2Zn1–x Ca x Ti2O9 (x = 0.00, 0.15, 0.30, 0.45, 0.90 and 1.00)
- RE 10Co3In10 (RE = Y, Gd, Tb, Dy, Ho, Er, Tm) – a new intergrowth structure with CsCl- and AlB2-type slabs
- Crystal structures of phases from the solid solutions DyNiIn1−xSn x
- Tricyanomethanides of the scandium group obtained from aqueous solution: syntheses, crystal structures and Raman spectra of Sc[C(CN)3]3(H2O)3, Y[C(CN)3]3(H2O)2 and La[C(CN)3]3(H2O)4
- Three pnictides with Sm6Rh30Si19-type structure: Sm6Rh30P19, Lu6Rh30P19 and Eu6Rh30Sb19
- The compound (NH4)0.64B4.36O6.72(OH)0.28(NH3)0.36 representing the first ammine/ammonium borate
- Extended investigations on the pressure stability of AlB4O6N:Cr3+
- Synthesis and crystal structures of new phases in the system Hf–Ta–O–N
Articles in the same Issue
- Frontmatter
- In this issue
- Research Articles
- A MEMS-based gas sensor for H2S detection with enhanced performance using a Ni–In2O3/ZnO nano-composite
- Ultra-sensitive and rapid response H2S microsensor based on Co–ZnO with ppm level detection
- Crystal chemistry and structural phase transition in CaLa2Zn1–x Ca x Ti2O9 (x = 0.00, 0.15, 0.30, 0.45, 0.90 and 1.00)
- RE 10Co3In10 (RE = Y, Gd, Tb, Dy, Ho, Er, Tm) – a new intergrowth structure with CsCl- and AlB2-type slabs
- Crystal structures of phases from the solid solutions DyNiIn1−xSn x
- Tricyanomethanides of the scandium group obtained from aqueous solution: syntheses, crystal structures and Raman spectra of Sc[C(CN)3]3(H2O)3, Y[C(CN)3]3(H2O)2 and La[C(CN)3]3(H2O)4
- Three pnictides with Sm6Rh30Si19-type structure: Sm6Rh30P19, Lu6Rh30P19 and Eu6Rh30Sb19
- The compound (NH4)0.64B4.36O6.72(OH)0.28(NH3)0.36 representing the first ammine/ammonium borate
- Extended investigations on the pressure stability of AlB4O6N:Cr3+
- Synthesis and crystal structures of new phases in the system Hf–Ta–O–N

