Electrochemical sensing of hydrogen peroxide on a carbon paste electrode modified by a silver complex based on the 1,3-bis(1H-benzimidazole-2-yl)propane ligand
Abstract
A new carbon paste electrode (Ag-CPE) modified with a nitrogen heterocyclic silver(I) complex, [Ag2(BBP)2](pic)2·CH3OH (BBP = 1,3-bis(1H-benzimidazol-2-yl)propane, pic = picrate), was developed as a highly sensitive and simple electrochemical sensor for the determination of hydrogen peroxide. The Ag(I) complex was prepared by an interface reaction and characterized by elemental analysis, IR and UV/Vis spectra and single crystal X-ray diffraction. The Ag(I) complex shows a dinuclear cluster structure, which is formed by two BBP ligands bridging two Ag (I) centers and an Ag–Ag interaction (d Ag–Ag = 3.0875 Å). Cyclic voltammetry and chronoamperometry studies showed that the electrochemical sensing performance of Ag-CPE for H2O2 was improved in 0.2 m phosphate buffer solution (PBS, pH = 6). The electrochemical H2O2 sensor Ag-CPE exhibits a wide linear detection range from 0.5 to 4.0 mm and a lower detection limit of 0.39 μm with a relatively high sensitivity of 6.77 μA mm −1. Moreover, the sensor also shows good anti-interference properties and stability. The results prove that the Ag(I) complex based on the bis(benzimidazole) ligand may be an efficient component of electrode materials.
1 Introduction
In the past few years, hydrogen peroxide has been widely used in the fields of chemistry, biology, clinical control, and environmental protection, and biological studies have shown that H2O2 is closely integrated in the metabolism of humans, which has led widespread interest in the detection of H2O2 [1], [2], [3], [4], [5], [6]. The development of H2O2 sensors with low cost, high sensitivity and good biocompatibility has important practical significance [7]. Currently, the detection of hydrogen peroxide (H2O2) can be achieved by many different analytical methods, including chemoluminescence [8], spectrophotometry [9] and fluorescence analysis [10], but these technologies are generally limited in their application because they are time-consuming, have low stability, require high cost and may not be environmentally friendly [11]. Electrochemical methods for detecting H2O2 sensors have attracted much attention due to their high sensitivity, low cost, fast signal response, and easy operation [12]. Many research groups are committed to the development and manufacture of electrochemical technology for the detection of H2O2 [13].
Metal complexes have become the focus of chemistry and materials researchers owing to their characteristics of structural tunability and wide application [14], [15], [16], [17], [18], [19], [20]. Compared with other metals ions, Ag(I) requires less energy for its valence state to change, which favors its role as an electron transport center for redox reactions [21, 22]. Thus Ag(I) complexes have great potential in the field of electrode materials. Carbon paste electrodes (CPE) have been widely used in electrochemical research not only because of their simple and low-cost preparation methods, but also of the renewal of the electrode surface, the low residual current and the wide potential range [23], [24], [25]. However, the sensitivity of carbon paste electrodes is reduced [26]. Therefore, modifiers are often added to improve the electrochemical performance of CPEs [27]. Based on the above facts, there is a great potential for use of Ag(I) complexes as modifiers for carbon paste electrode to achieve effective detection of H2O2.
In previous work, we studied the basic electrochemical properties of a series of linear bis(benzimidazole) ligands and their Ag(I) complexes [28, 29]. In order to further study the electrochemical recognition of hydrogen peroxide by Ag(I) complexes, a new dinuclear Ag(I) complex based on a flexible bisbenzimidazole derivative was synthesized and characterized. The electrical sensing performance of H2O2 was studied. The results of this study confirm that the new Ag-CPE is a good basis for a simple and inexpensive electrochemical method for the determination of H2O2.
2 Experimental
2.1 Materials and instruments
All chemicals and solvents were reagent grade and were used without further purification. C, H, and N contents were determined using a Carlo Erba 1106 elemental analyzer. IR spectra were recorded from 4000 to 400 cm−1 with a Nicolet FT-VERTEX 70 spectrophotometer using KBr pellets. Electronic spectra were recorded on a Lab-Tech UV Bluestar instrument. 1H NMR spectra were obtained with a Mercury plus 400 MHz NMR spectrometer with TMS as internal standard. All electrochemical experiments were performed on a LK2005A electrochemical workstation using a conventional three-electrode system.
2.2 Synthesis of BBP
The BBP ligand was synthesized according to the literature method [30], [31], [32], [33]. Yield: 1.08 g, 78%. M.p. 270 °C. – IR (KBr pellet, cm−1): ν = 3431(w), 1439(s), 1268(m), 747(s). – UV/Vis (DMF): λ = 277, 283 nm – 1H NMR (400 MHz, DMSO-d 6): δ = 7.12 (m, 4H, H-ph), 7.48 (q, 4H, H-ph), 2.93 (d, 4H, CH2), 2.31 (q, 2H, CH2). – Elemental analysis for C17H16N4 (276.14): calculated (%) C 73.89, H 5.84, N 20.27; found C 73.80, H 5.83, N 20.26.
2.3 Synthesis of [Ag2(BBP)2](pic)2·CH3OH
A solution of BBP (0.0138 g, 0.05 mmol) in CH3OH (3 mL) was carefully layered onto a solution of silver(I) picrate (0.0168 g, 0.05 mmol) in CH2Cl2-CH3OH (6 mL, v/v = 1 : 1) in a glass tube. The solution was left to stand for two weeks at room temperature in the dark, and block colorless crystals were obtained. Yield: 49.5 mg, 79%. – IR (KBr pellet, cm−1): ν = 3436(w), 1444(s), 1358(s), 1163(w), 788(s), 738(m), 702(m). – UV/Vis (DMF): λ = 275, 281, 380 nm – Elemental analysis for C47H40Ag2N14O15 (1254.09): calculated (%) C 44.92, H 3.21, N 15.60; found C 45.01, H 3.23, N 15.58.
2.4 X-ray structure determination
A suitable single crystal of the Ag(I) complex was mounted on a glass fiber, and the intensity data was collected on a Bruker APEX II area detector with graphite-monochromatized MoKα radiation (λ = 0.71073 Å) at T = 153 K. Data reduction and cell refinement were performed using the Smart and Saint programs. The absorption corrections were carried out by an empirical method [34], [35], [36], [37]. The crystal structure of the Ag(I) complex has been solved by Direct Methods and refined by full-matrix least-squares against F 2 of the data using the Olex 2 program [38, 39]. All hydrogen atoms were located geometrically and were subsequently refined in a riding-model approximation with C–H distances from 0.97 to 0.99 Å. The crystal data and experimental parameters relevant to the structure determination are listed in Table 1. Selected bond lengths and angles are presented in Table 2.
Crystallographic data and data collection parameters for the Ag(I) complex.
| Complex | [Ag2(BBP)2](pic)2·CH3OH |
|---|---|
| Molecular formula | C47H40Ag2N14O15 |
| Molecular weight M r | 1256.67 |
| Crystal system | Triclinic |
| Space group |
P
|
| a, Å | 7.712(3) |
| b, Å | 13.745(5) |
| c, Å | 24.236(8) |
| α, deg | 81.200(6) |
| β, deg | 89.768(6) |
| γ, deg | 74.784(6) |
| V, Å3 | 2448.1(15) |
| Z | 2 |
| ρ cald, g cm−3 | 1.71 |
| Absorption coefficient, mm−1 | 0.9 |
| F(000), e | 1268.0 |
| Crystal size, mm3 | 0.41 × 0.36 × 0.31 |
| 2θ range for data collection, deg | 3.11–50.098 |
| Index ranges hkl | −9/+9, −16/+16, −23/+28 |
| Reflections collected | 12,587 |
| Independent reflections | 8579 |
| R int | 0.0251 |
| Refinement method | Full-matrix least-squares on F 2 |
| Data/parameters | 8579/695 |
| Goodness-of-fit on F 2 | 1.017 |
| Final R 1/wR 2 [I > 2 σ(I)] | 0.0431/0.1007 |
| Final R 1/wR 2 (all data) | 0.0677/0.1143 |
| Largest diff. peak/hole, e Å−3 | 0.82/−0.51 |
Selected bond lengths (Å) and angles (deg) of the Ag(I) complex.
| Bond lengths | Bond angles | ||
|---|---|---|---|
| Ag(2)–Ag(1) | 3.0875(10) | N(4)–Ag(2)–Ag(1) | 99.98(10) |
| Ag(2)–N(4) | 2.094(3) | N(1)–Ag(1)–Ag(2) | 82.01(9) |
| Ag(1)–N(2) | 2.126(3) | N(2)–Ag(1)–Ag(2) | 91.14(10) |
| Ag(2)–N(3) | 2.106(3) | N(3)–Ag(2)–Ag(1) | 76.40(10) |
| Ag(1)–N(1) | 2.122(3) | N(4)–Ag(2)–N(3) | 175.85(13) |
| N(1)–Ag(1)–N(2) | 170.94(13) | ||
CCDC 2088230 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
2.5 Preparation of the CPE modified by the Ag(I) complex
Ag-CPE was prepared by manually grinding 10 mg of the Ag(I) complex and 50 mg of carbon powder with a mortar and pestle to make them uniformly mixed. Afterwards, 20 μL of liquid paraffin was added dropwise and stirred sufficiently to form a carbon paste. The resulting paste was filled into the inner cavity of the tetrafluoroethylene electrode (3 mm diameter). A copper rod of appropriate size was inserted into one end of the tetrafluoroethylene electrode tube to make it conductive, and the carbon paste surface was polished with butter paper. For the preparation of an unmodified electrode (CPE) the amount of Ag(I) complex was replaced with the same amount of graphite.
2.6 Electrochemical responses toward H2O2
Electrochemical measurement was carried out using a conventional three-electrode system, with Ag-CPE as the working electrode, Ag/AgCl as the reference electrode, and a platinum plate as the auxiliary electrode. The supporting electrolyte was 0.2 m PBS (pH = 6). The voltage was selected for electrochemical detection of H2O2, and different concentrations of H2O2 are added to the stirred 0.2 m PBS (pH = 6). The corresponding current response signal was recorded by chronoamperometry.
3 Results and discussion
3.1 General characterization and crystal structure
The Ag(I) complex was obtained by the interface reaction between the flexible ligand BBP and silver picrate. The results of elemental analysis of the Ag(I) complex agree with the theoretical composition. The compound is soluble in polar aprotic solvents such as DMF and DMSO, partly soluble in ethanol, methanol and ethyl acetate, but insoluble in Et2O and petroleum ether. The IR and UV spectra show that the characteristic absorption peaks are significantly shifted compared with that of the BBP ligand, which confirms that the nitrogen atom from BBP ligand is coordinated to the central metal silver atom [40], [41], [42], [43], [44], [45], [46], [47], [48].
The X-ray diffraction studies have shown that the Ag(I) complex crystallizes in the triclinic space group P
2·CH3OH with ellipsoids shown at the 30% probability level (hydrogen atoms and methanol are omitted for clarity). (b) The simplified frame-shaped structure and the Ag1–Ag2 distance. Coordination model of the (c) Ag1 and the (d) Ag2 atoms.](/document/doi/10.1515/znb-2021-0089/asset/graphic/j_znb-2021-0089_fig_001.jpg)
(a) The crystal structure of [Ag2(BBP)2](pic)2·CH3OH with ellipsoids shown at the 30% probability level (hydrogen atoms and methanol are omitted for clarity). (b) The simplified frame-shaped structure and the Ag1–Ag2 distance. Coordination model of the (c) Ag1 and the (d) Ag2 atoms.
3.2 Cyclic voltammetry studies
Cyclic voltammetry (CV) responses of different electrodes have been investigated in 0.2 m PBS (pH = 6) at a scan rate of 0.025→0.5 Vs−1. As shown in Figure 2a, Ag-CPE has an anode peak at 0.39 V and a cathode peak at −0.05 V, which can be attributed to the redox couple of Ag+/Ag2+. Obviously, the two well-defined redox peak currents increase proportionally with the gradual increase in scan rate. The linear relationship between the peak current and the square root of the scan rate indicates that the electrochemical reaction on the Ag-CPE is mainly a process controlled by diffusion (Figure 2b) [50].

(a) Cyclic voltammograms of an Ag-CPE in 0.2 m PBS (pH = 6) at different scan rates. (b) Peak current versus square root of scan rate (0.025→0.5 Vs−1).
3.3 Electrochemical detection of H2O2
Optimization of the experimental parameters. Since the current response of the modified electrode is closely related to the potential, the induced voltage for H2O2 was screened by chronoamperometry. In the voltage range of −0.1 to −0.5 V, the influence of the applied potential on the ampere response of the Ag-CPE when 1 mm H2O2 was continuously added to 0.2 m PBS (pH = 6) was studied (Figure 3). With the increase of the applied potential, the current response continues to improve. When the potential reaches −0.2 V, the electrochemical signal shows negligible noise and is accompanied by an obvious signal generation. Therefore, it is necessary to select −0.2 V as the applied potential for detecting H2O2.
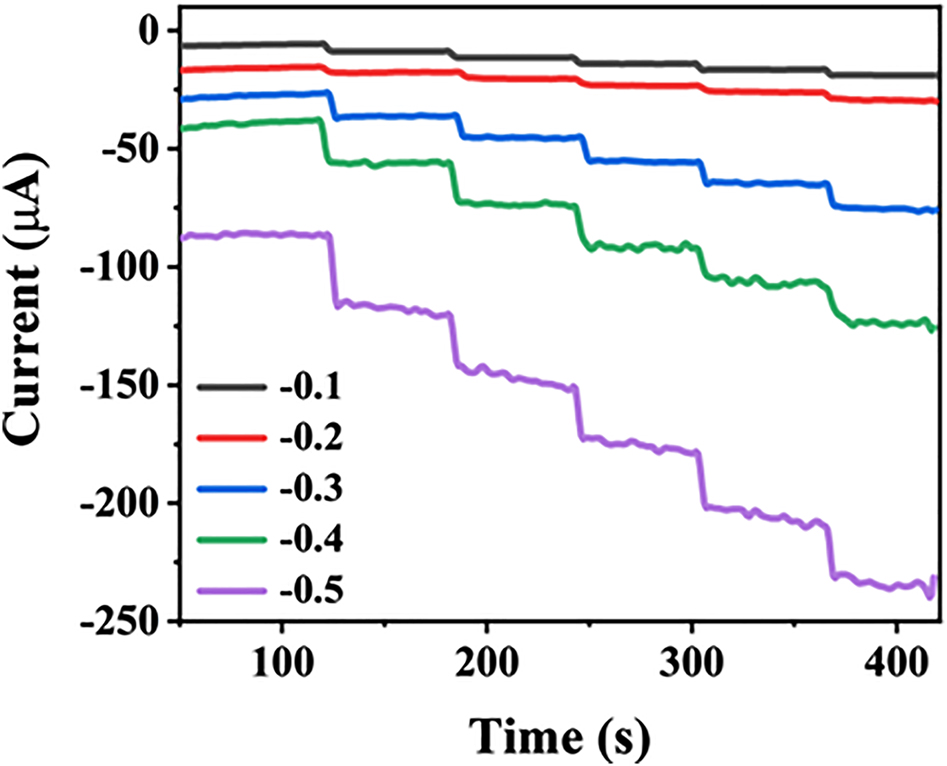
The current-time response of the Ag-CPE with continuously adding 1 mm H2O2 into 0.2 m PBS (pH = 6) at −0.1 ∼ −0.5 V.
3.3.1 Chronoamperometric titration
For the continuous addition of different concentrations of H2O2 to a stirred 0.2 m PBS solution (pH = 6) under an applied potential of −0.2 V, the electrochemical response of the Ag-CPE to the measured amount of H2O2 is depicted in Figure 4a [51], [52], [53]. When adding 5 μm H2O2, the curve shows a significant decline, which indicates the current response at low H2O2 concentration. The current response achieves more than 98% of the steady-state current within 4 s, indicating a speedy amperometric response to H2O2. Simultaneously, the response current gradually increases with the increase of the H2O2 concentration. According to the current value analysis, the linear relationship between H2O2 concentration and response current can be fitted as shown in Figure 4b [54], [55], [56], [57]. The sensor displays a linear response to H2O2 in the concentration range between 0.5 μm and 4.0 mm with a correlation coefficient of 0.999. The detection limit is estimated as 0.39 μm and a higher sensitivity of 6.77 μA mm −1 is found (S/N = 3). The concentration and response current show a good linear relationship, which indicates that the Ag(I) complex synthesized using BBP as a ligand can be used as a modifier for the construction of H2O2 electrochemical sensors [58]. The Michaelis-Menten constant (K M) is calculated to be 0.289 mm (Figure 5) from the slope and the intercept of the plot of the reciprocals of the steady-state current versus H2O2 concentration based on the Lineweaver-Burk equation [59]. It is well known that the smaller K M indicates higher catalytic activity of the electrode, and the low K M value in this work indicates that the Ag-CPE exhibits a high catalytic affinity [60].

(a) Current response of the Ag-CPE for the addition of H2O2 (concentration range from 0.5 to 4 mm) into 0.2 m PBS (pH = 6) at −0.2 V. (b) Linear fitting curve obtained from 0.5 to 4 mm.

Lineweaver-Burk plot of current−1 versus
3.3.1.1 Anti-interference and stability
A chronoamperometry curve was recorded in a 0.2 m PBS (pH = 6) containing 1.0 mm H2O2 (Figure 6a). When 1.0 mm H2O2 was added dropwise again after 900 s, the corresponding response current was 98.2% of the initial response current, proving that the Ag-CPE has excellent stability. In order to test the anti-interference performance of the Ag-CPE, dopamine, uric acid, glucose, ascorbic acid, d-fructose and adenine were sequentially introduced as interference substances. After adding 1.0 mm H2O2, the current has a significant and clear response. When the interfering substance is added 1 min later (Figure 6b), the current has no obvious response. The anti-interference experiment certificated that the Ag-CPE still has good stability in the presence of interference.

For a stirred 0.2 m PBS solution (pH = 6) containing 1 mm H2O2 at −0.2 V, is shown (a) the stability test of the Ag-CPE electrode, and (b) the current response of the Ag-CPE when 1 mm interfering substance was continuously introduced.
4 Conclusions
In summary, a new dinuclear silver(I) complex based on a bisbenzimidazole ligand was synthesized by an interfacial reaction and characterized by elemental analysis, IR and UV/Vis spectroscopy and single crystal X-ray diffraction. The carbon paste electrode modified with the silver(I) complex can be employed as an active material for electrochemical sensing of H2O2. The electrochemical behavior of the Ag-CPE shows excellent sensing performance featuring a wide linear range, high sensitivity, low detection limit, good anti-interference ability, and stability. The electrode may therefore be used as a sensor for detecting H2O2.
Funding source: Lanzhou Jiaotong University http://dx.doi.org/10.13039/501100009014
Award Identifier / Grant number: (Grant No. 152022)
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The present research was supported by Foundation of A Hundred Youth Talents Training Program of Lanzhou Jiaotong University (Grant No. 152022), http://dx.doi.org/10.13039/501100009014.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Miao, Y. E., He, S. X., Zhong, Y. L., Yang, Z., Tjiu, W. W., Liu, T. X. Electrochim. Acta 2013, 99, 117–123; https://doi.org/10.1016/j.electacta.2013.03.063.Suche in Google Scholar
2. Easow, J. S., Selvaraju, T. Electrochim. Acta 2013, 112, 648–654; https://doi.org/10.1016/j.electacta.2013.09.033.Suche in Google Scholar
3. Shu, X. H., Chen, Y., Yuan, H. Y., Gao, S. F., Xiao, D. Anal. Chem. 2007, 79, 3695–3702; https://doi.org/10.1021/ac0624142.Suche in Google Scholar
4. Wang, Q., Zheng, J. B. Microchim. Acta 2010, 169, 361–365; https://doi.org/10.1007/s00604-010-0356-7.Suche in Google Scholar
5. Ramezani, H., Azizi, S. N., Hosseini, S. R. Sensor. Actuator. B Chem. 2017, 248, 571–579; https://doi.org/10.1016/j.snb.2017.04.005.Suche in Google Scholar
6. Khan, M. M., Ansari, S. A., Lee, J., Cho, M. H. Mater. Sci. Eng. C 2013, 33, 4692–4699; https://doi.org/10.1016/j.msec.2013.07.028.Suche in Google Scholar
7. Rui, Q., Lomori, K., Tian, Y., Liu, H., Luo, Y., Sakai, Y. Anal. Chim. Acta 2010, 670, 57–62; https://doi.org/10.1016/j.aca.2010.04.065.Suche in Google Scholar
8. Xie, J. X., Huang, Y. M. Anal. Methods 2011, 3, 1149–1155; https://doi.org/10.1039/c1ay05103b.Suche in Google Scholar
9. Tang, B., Wang, Y., Liang, H. L., Chen, Z. Z., He, X. W., Shen, H. X. Spectrochim. Acta, Part A 2006, 63, 609–613; https://doi.org/10.1016/j.saa.2005.06.008.Suche in Google Scholar
10. Meng, X. W., Wei, J. F., Ren, X. L., Ren, J., Tang, F. Q. Biosens. Bioelectron. 2013, 47, 402–407; https://doi.org/10.1016/j.bios.2013.03.053.Suche in Google Scholar
11. Li, S., Xiong, J. X., Shen, J. S., Qin, Y., Li, J., Chu, F. Q., Kong, Y., Deng, L. H. J. Appl. Polym. Sci. 2015, 132, 42409; https://doi.org/10.1002/app.42409.Suche in Google Scholar
12. Shi, C. G., Xu, J. J., Chen, H. Y. J. Electroanal. Chem. 2007, 610, 186–192; https://doi.org/10.1016/j.jelechem.2007.07.018.Suche in Google Scholar
13. Sun, X. L., Guo, S. J., Liu, Y., Sun, S. H. Nano Lett. 2012, 12, 4859–4863; https://doi.org/10.1021/nl302358e.Suche in Google Scholar
14. Wu, H. L., Huang, X. C., Yuan, J. K., Kou, F., Jia, F., Liu, B., Wang, K. T. Eur. J. Med. Chem. 2010, 45, 5324–5330; https://doi.org/10.1016/j.ejmech.2010.08.055.Suche in Google Scholar
15. Wu, H. L., Yuan, J. K., Bai, Y., Pan, G. L., Wang, H., Shu, X. B. J. Photochem. Photobiol., B 2012, 107, 65–72; https://doi.org/10.1016/j.jphotobiol.2011.11.010.Suche in Google Scholar
16. Dong, W. K., Zhu, L. C., Ma, J. C., Sun, Y. X., Zhang, Y. Inorg. Chim. Acta 2016, 453, 402–408; https://doi.org/10.1016/j.ica.2016.08.050.Suche in Google Scholar
17. Pu, L. M., Akogun, S. F., Li, X. L., Long, H. T., Dong, W. K., Zhang, Y. Polyhedron 2017, 134, 356–364; https://doi.org/10.1016/j.poly.2017.06.038.Suche in Google Scholar
18. Xia, X. Z., Xia, L. X., Zhang, G., Li, Y. G., Wang, J., Xu, J. H., Wu, H. L. J. Mol. Struct. 2021, 1227, 129726; https://doi.org/10.1016/j.molstruc.2020.129726.Suche in Google Scholar
19. Wang, J. F., Feng, T., Li, Y. J., Sun, Y. X., Dong, W. K., Ding, Y. J. J. Mol. Struct. 2021, 1231, 129950; https://doi.org/10.1016/j.molstruc.2021.129950.Suche in Google Scholar
20. Li, Y. J., Guo, S. Z., Feng, T., Xie, K. F., Dong, W. K. J. Mol. Struct. 2021, 1228, 129796; https://doi.org/10.1016/j.molstruc.2020.129796.Suche in Google Scholar
21. Mao, S. S., Shen, K. S., Shi, X. K., Wu, H. L., Han, X. T., Li, C., Huang, G. Z. Inorg. Chim. Acta 2018, 471, 82–90; https://doi.org/10.1016/j.ica.2017.10.038.Suche in Google Scholar
22. Mao, S. S., Han, X. T., Li, C., Huang, G. Z., Shen, K. S., Shi, X. K., Wu, H. L. J. Coord. Chem. 2018, 71, 3330–3341; https://doi.org/10.1080/00958972.2018.1514116.Suche in Google Scholar
23. Zheng, J. B., Zhou, X. L. Bioelectrochemistry 2007, 70, 408–415; https://doi.org/10.1016/j.bioelechem.2006.05.011.Suche in Google Scholar
24. Huang, T. H., Kuwana, T., Warsinke, A. Biosens. Bioelectron. 2002, 17, 1107–1113; https://doi.org/10.1016/s0956-5663(02)00105-7.Suche in Google Scholar
25. Crespilho, F. N., Rezende, M. O. O. Quim. Nova 2004, 27, 964–969; https://doi.org/10.1590/s0100-40422004000600022.Suche in Google Scholar
26. Thomas, N., Shimna, T., Thomas, J., Thomas, T. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1728–1737; https://doi.org/10.1007/s10904-019-01134-y.Suche in Google Scholar
27. Oren, T., Birel, O., Anik, U. Anal. Lett. 2018, 51, 1680–1693; https://doi.org/10.1080/00032719.2017.1388816.Suche in Google Scholar
28. Qu, Y., Zhao, K., Wang, C., Wu, Y. C., Xia, L. X., Wu, H. L. J. Mol. Struct. 2020, 1203, 127424; https://doi.org/10.1016/j.molstruc.2019.127424.Suche in Google Scholar
29. Wu, H. L., Yang, Z. H., Chen, C. Y., Zhang, J. W., Zhang, H., Peng, H. P., Wang, F. J. Coord. Chem. 2016, 69, 1076–1087; https://doi.org/10.1080/00958972.2016.1145212.Suche in Google Scholar
30. Li, G. R., Liu, J., Pan, Q., Song, Z. B., Luo, F. L., Wang, S. R., Zhang, X. L., Zhou, X. Chem. Biodivers. 2009, 6, 2200–2208; https://doi.org/10.1002/cbdv.200800281.Suche in Google Scholar
31. Calderazzo, F., Englert, U., Hu, C., Marchetti, F., Pampaloni, G., Passarelli, V., Romano, A., Santi, R. Inorg. Chim. Acta 2003, 344, 197–206; https://doi.org/10.1016/s0020-1693(02)01304-x.Suche in Google Scholar
32. Inamdar, S. M., More, V. K., Mandal, S. K. Tetrahedron Lett. 2013, 54, 579–583; https://doi.org/10.1016/j.tetlet.2012.11.091.Suche in Google Scholar
33. Mukhopadhyay, C., Ghosh, S., Butcher, R. J. ARKIVOC 2010, 9, 75–96; https://doi.org/10.3998/ark.5550190.0011.908.Suche in Google Scholar
34. Sheldrick, G. M. Acta Crystallogr. 2015, C71, 3–8.Suche in Google Scholar
35. Li, J. J., Abramov, Y. A., Doherty, M. F. ACS Cent. Sci. 2017, 3, 726–733; https://doi.org/10.1021/acscentsci.7b00130.Suche in Google Scholar
36. Gruene, T., Hahn, H. W., Luebben, A. V., Meilleur, F., Sheldrick, G. M. J. Appl. Crystallogr. 2014, 47, 462–466; https://doi.org/10.1107/s1600576713027659.Suche in Google Scholar
37. Slater, T., Chen, Y., Auton, G., Zaluzec, N., Haigh, S. Microsc. Microanal. 2016, 22, 440–447; https://doi.org/10.1017/s1431927616000064.Suche in Google Scholar
38. Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. K., Puschmann, H. Acta Crystallogr. 2015, A71, 59–75; https://doi.org/10.1107/s2053273314022207.Suche in Google Scholar
39. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
40. Wu, H. L., Xia, L. X., Qu, Y., Zhao, K., Wang, C., Wu, Y. C. Appl. Organomet. Chem. 2019, 34, 5297; https://doi.org/10.1002/aoc.5297.Suche in Google Scholar
41. Wang, Z. H., Wang, D. F., Zhang, T., Huang, R. B., Zheng, L. S. J. Mol. Struct. 2014, 1064, 27–31; https://doi.org/10.1016/j.molstruc.2014.01.030.Suche in Google Scholar
42. Wu, Y. C., Han, X. T., Qu, Y., Zhao, K., Wang, C., Huang, G. Z., Wu, H. L. J. Mol. Struct. 2019, 1191, 95–100; https://doi.org/10.1016/j.molstruc.2019.04.108.Suche in Google Scholar
43. Xia, L. X., Zhang, G., Xia, X. Z., Li, Y. G., Wang, J., Xu, J. H., Wu, H. L. Z. Naturforsch. 2020, 75b, 353–357; https://doi.org/10.1515/znb-2019-0198.Suche in Google Scholar
44. Xu, Y. L., Mao, S. S., Shen, K. S., Shi, X. K., Wu, H. L., Tang, X. Inorg. Chim. Acta 2018, 471, 17–22; https://doi.org/10.1016/j.ica.2017.10.023.Suche in Google Scholar
45. Pu, L. M., Long, H. T., Zhang, Y., Bai, Y., Dong, W. K. Polyhedron 2017, 128, 57–67; https://doi.org/10.1016/j.poly.2017.02.033.Suche in Google Scholar
46. Zhang, G., Xia, X. Z., Xu, J. H., Wu, H. L., Xia, L. X., Qu, Y., Han, X. T. J. Mol. Struct. 2021, 1226, 129337; https://doi.org/10.1016/j.molstruc.2020.129337.Suche in Google Scholar
47. Li, X. Y., Chen, L., Gao, L., Zhang, Y., Akogun, S. F., Dong, W. K. RSC Adv. 2017, 7, 35905–35916; https://doi.org/10.1039/c7ra06796h.Suche in Google Scholar
48. Dong, W. K., Lan, P. F., Zhou, W. M., Zhang, Y. J. Coord. Chem. 2016, 69, 1272–1283; https://doi.org/10.1080/00958972.2016.1168520.Suche in Google Scholar
49. Schmidbaur, H., Schier, A. Angew. Chem. Int. Ed. 2015, 54, 746–784; https://doi.org/10.1002/anie.201405936.Suche in Google Scholar
50. Dong, S., Zhang, D., Suo, G., Wei, W., Huang, T. Anal. Chim. Acta 2016, 934, 203–211; https://doi.org/10.1016/j.aca.2016.05.040.Suche in Google Scholar
51. Wang, N., Liang, S., Zhang, L. J., Cao, P. F., Xu, L., Lin, M. Colloids Surf., A 2020, 603, 125199; https://doi.org/10.1016/j.colsurfa.2020.125199.Suche in Google Scholar
52. Safavi, A., Maleki, N., Farjami, E. Electroanalysis 2009, 21, 1533–1538; https://doi.org/10.1002/elan.200804577.Suche in Google Scholar
53. Lu, W. B., Luo, Y. L., Chang, G. H., Sun, X. P. Biosens. Bioelectron. 2011, 26, 4791–4797; https://doi.org/10.1016/j.bios.2011.06.008.Suche in Google Scholar
54. Luo, H. M., Liu, D., Gao, Q., Xu, L. Inorg. Chem. Commun. 2020, 118, 107996; https://doi.org/10.1016/j.inoche.2020.107996.Suche in Google Scholar
55. Kempahanumakkagari, S., Vellingiri, K., Deep, A., Kwon, E. E., Bolan, N., Kim, K. H. Coord. Chem. Rev. 2018, 357, 105–129; https://doi.org/10.1016/j.ccr.2017.11.028.Suche in Google Scholar
56. Ojani, R., Raoof, J. B., Norouzi, B. Electroanalysis 2008, 20, 1996–2002; https://doi.org/10.1002/elan.200804278.Suche in Google Scholar
57. Zhou, L. L., Tang, L. Z., Zhang, Y. X., Zhan, S. Z. Polyhedron 2015, 92, 124–129; https://doi.org/10.1016/j.poly.2015.03.013.Suche in Google Scholar
58. Jia, N. M., Huang, B. Z., Chen, L., Tan, L., Yao, S. Z. Sens. Actuators, B 2014, 195, 165–170; https://doi.org/10.1016/j.snb.2014.01.043.Suche in Google Scholar
59. Tomczak, J. M., Tomczak, E. W. FEBS Lett. 2019, 593, 2742–2750; https://doi.org/10.1002/1873-3468.13531.Suche in Google Scholar
60. Matthews, J. N. S., Allcock, G. C. Stat. Med. 2004, 23, 477–491; https://doi.org/10.1002/sim.1612.Suche in Google Scholar
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- In this issue
- Research Articles
- Antimycobacterial cycloartane derivatives from the roots of Trichilia welwistchii C. DC (Meliaceae)
- Three metal(II) complexes constructed using the 2-(1H-benzo[d]imidazol-2-yl)quinoline ligand
- Low-perchlorate blue-flame pyrotechnic compositions
- A convenient synthesis of trifluoromethyl-substituted quinolino[8,7-h]quinolines and quinolino[7,8-h]quinolines
- Curie temperature adjustment in the solid solution Gd1–xY x PtMg
- Synthesis of oligotetramethylene oxides with terminal amino groups as curing agents for an epoxyurethane oligomer
- Synthesis, characterization and optoelectronic properties of 2D hybrid RPbX4 semiconductors based on an isomer mixture of hexanediamine-based dications
- Electrochemical sensing of hydrogen peroxide on a carbon paste electrode modified by a silver complex based on the 1,3-bis(1H-benzimidazole-2-yl)propane ligand
Artikel in diesem Heft
- Frontmatter
- In this issue
- Research Articles
- Antimycobacterial cycloartane derivatives from the roots of Trichilia welwistchii C. DC (Meliaceae)
- Three metal(II) complexes constructed using the 2-(1H-benzo[d]imidazol-2-yl)quinoline ligand
- Low-perchlorate blue-flame pyrotechnic compositions
- A convenient synthesis of trifluoromethyl-substituted quinolino[8,7-h]quinolines and quinolino[7,8-h]quinolines
- Curie temperature adjustment in the solid solution Gd1–xY x PtMg
- Synthesis of oligotetramethylene oxides with terminal amino groups as curing agents for an epoxyurethane oligomer
- Synthesis, characterization and optoelectronic properties of 2D hybrid RPbX4 semiconductors based on an isomer mixture of hexanediamine-based dications
- Electrochemical sensing of hydrogen peroxide on a carbon paste electrode modified by a silver complex based on the 1,3-bis(1H-benzimidazole-2-yl)propane ligand

