Abstract
The mixed cation triel borate Ga4In4B15O33(OH)3 was synthesized in a Walker-type multianvil apparatus at high-pressure/high-temperature conditions of 12.5 GPa and 1300°C. Although the product could not be reproduced in further experiments, its crystal structure could be reliably determined via single-crystal X-ray diffraction data. Ga4In4B15O33(OH)3 crystallizes in the tetragonal space group I41/a (origin choice 2) with the lattice parameters a = 11.382(2), c = 15.244(2) Å, and V = 1974.9(4) Å3. The structure of the quaternary triel borate consists of a complex network of BO4 tetrahedra, edge-sharing InO6 octahedra in dinuclear units, and very dense edge-sharing GaO6 octahedra in tetranuclear units.
1 Introduction
In the last decade, extensive research has been carried out in the field of group 13 element borates which yielded numerous new structures featuring interesting properties. For instance, triel borates have been reported to show luminescence when being doped with a suitable activator ion [1], [2], [3], [4], and acentric compounds have been described as possible NLO materials [5], [6], [7]. Especially gallium and indium borates shifted into the research focus due to their photocatalytic properties. During the last 5 years, the results of seven photocatalytic studies of indium or gallium borates have been published [1], [8], [9], [10], [11], [12], [13]. Up to date, 14 borates are known in the systems Ga-B-O(-H) and In-B-O(-H), from which 12 have been published since 2010. This emerging research field has benefited from the advancement of high-pressure techniques. More than half of all existing indium or gallium borates in these (partially hydroxylated) ternary systems were synthesized with a multianvil apparatus and formed under conditions of at least 10 GPa.
Likewise, the new compound Ga4In4B15O33(OH)3 has now been synthesized at high-pressure conditions via a multianvil press. As usual in such dense structures, this mixed cation triel borate solely consists of boron atoms that are coordinated by four oxygen atoms. Ga3+ and In3+ form diverse edge-sharing octahedral entities. Unlike the solid solution In1−xGaxBO3 (0≤x≤0.5) [14], in which the indium position is optionally replaced by gallium, our title compound is the first triel borate containing indium and gallium in crystallographically different positions.
In the following, the synthesis and crystal structure of Ga4In4B15O33(OH)3 are described in detail.
2 Experimental section
2.1 Synthesis
In2O3 (99.9%, ChemPUR, Karlsruhe, Germany), Ga2O3 (99.998%, Strem Chemicals, Kehl, Germany), and H3BO3 (99.5%, Carl Roth, Karlsruhe, Germany) were weighed in a molar ratio of 1:3:8 and ground in an agate mortar. Then, the mixture was encapsulated in gold foil (99.99%, 0.025 mm, Sigma-Aldrich, USA) and transferred into a boron nitride crucible (α-BN, Henze Boron Nitride Products AG, Kempten, Germany). The high-pressure/high-temperature experiment was performed with a multianvil device based on a Walker-type module (Voggenreiter, Mainleus, Germany) that consisted of a 14/8 assembly surrounded by eight tungsten carbide cubes (ha-7% Co, Hawedia, Marklkofen, Germany). The detailed procedure is described in the literature [15], [16], [17]. Ga4In4B15O33(OH)3 formed under conditions of 12.5 GPa and 1300°C. The maximum pressure was built up within 342 min and held for 42 min during the heating period. Within 7 min, the maximum temperature of 1300°C was reached, kept for 5 min and afterwards the temperature was decreased to 900°C within 30 min. The following careful pressure reduction to ambient conditions took 1013 min.
The reaction product appeared grey-white and its powder pattern did not match that of any known phase. A suitable single-crystal was isolated from the sample. The solution of the structure of Ga4In4B15O33(OH)3 explains some of the reflections in the powder pattern of the reaction product (see Fig. 1). However, the majority of the reflections is still unknown up to now. All subsequent attempts to purposefully and stoichiometrically synthesize Ga4In4B15O33(OH)3 failed up to now.
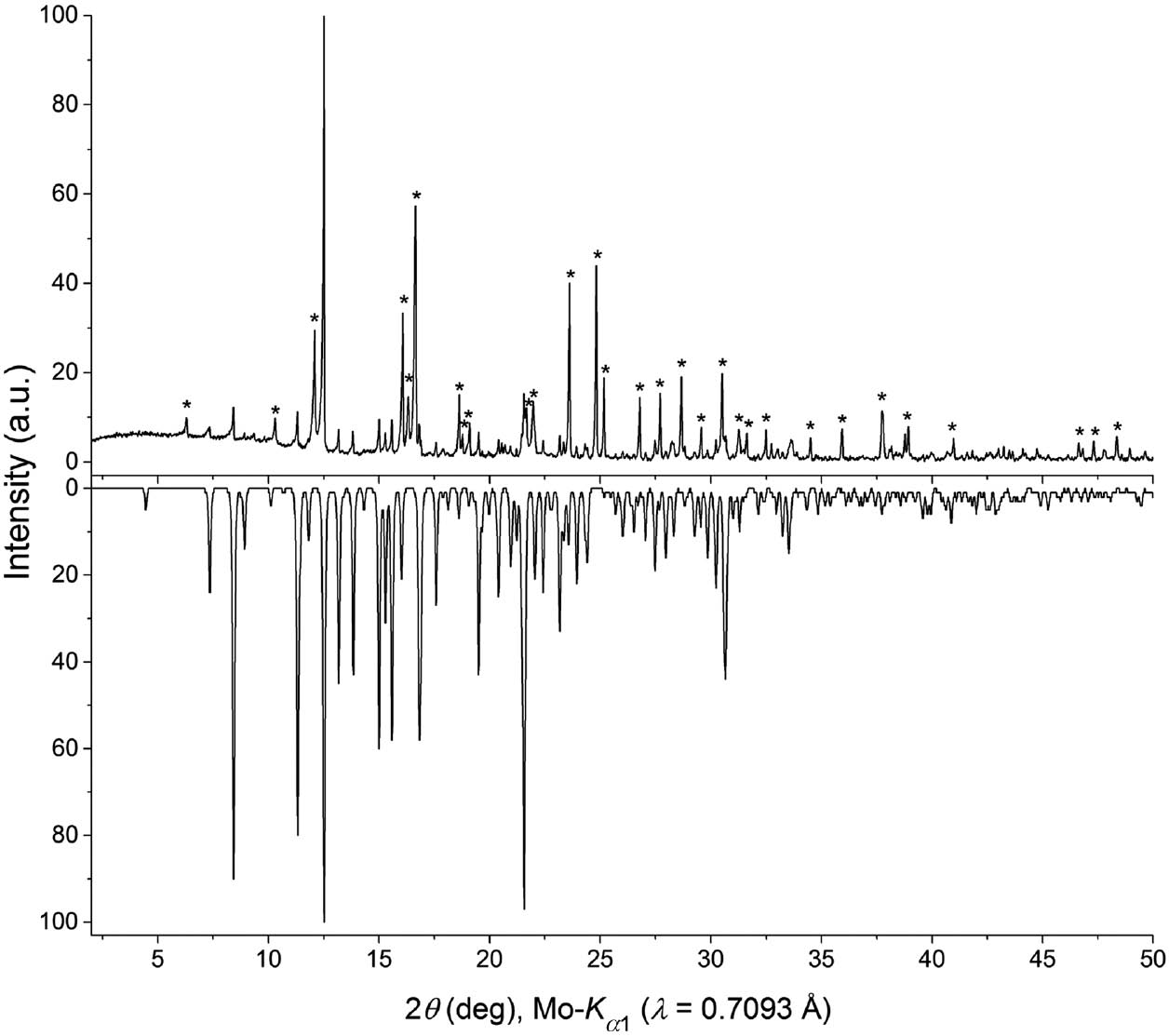
Experimental powder pattern of the reaction product (top) compared to a simulation of the theoretical powder pattern of Ga4In4B15O33(OH)3 based on single-crystal data (bottom). Reflections marked with an asterisk do not result from the title compound.
2.2 X-ray structure determination
At first, the reaction product was analyzed with a Stoe Stadi P powder diffractometer equipped with a Mythen 1 K detector (Dectris, Switzerland). The measurement was carried out with Ge(111)-monochromatized MoKα1 radiation (λ=0.7093 Å) in transmission geometry across a 2θ range of 2.0–60.5°. Figure 1 shows the experimental powder pattern. Most of the reflections could not be associated with any known phases. All reflections that do not match with the simulation of Ga4In4B15O33(OH)3 derived from single-crystal data are marked with asterisks.
A colorless, prismatic single-crystal of Ga4In4B15O33(OH)3 was isolated from the reaction product and measured on a Bruker D8 Quest diffractometer equipped with a Photon 100 CMOS detector. The data was collected at room temperature and a multiscan absorption correction was performed with Sadabs 2014/5 [18]. For the structure solution and parameter refinement, the Shelxs/l-2013 [19], [20] software implemented in the program Wingx-2013.3 [21] was used. The single-crystal was treated as a non-merohedral twin with a domain fraction of 0.165–0.835 and was refined with the twin matrix [010 100 001̅]. Aside from the proton, all atoms in Ga4In4B15O33(OH)3 could be refined anisotropically with site occupancy factors of 1. The proton position was fixed with the DFIX command in a distance of 0.83 Å to O8, since this is the only oxygen atom with otherwise only two coordination partners. Due to charge neutrality reasons, the proton H8 was refined with a site occupancy factor of 0.75. In the end, the refinement of Ga4In4B15O33(OH)3 led to satisfying results with total R (all data) values of R1=0.0192 and wR2=0.0422 as well as a Goodness-of-fit of 1.078.
CCDC 1895780 (Ga4In4B15O33(OH)3) contains the supplementary crystallographic data for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre viawww.ccdc.cam.ac.uk/data_request/cif.
3 Results and discussion
3.1 Crystal structure
Ga4In4B15O33(OH)3 crystallizes in the tetragonal space group I41/a (no. 88, origin choice 2) with four formula units (Z=4) and the lattice parameters a=11.382(2), c=15.244(2) Å, and V=1974.9(4) Å3. Details of the crystal structure refinement can be found in Table 1. Figure 2 shows the asymmetric unit of Ga4In4B15O33(OH)3. The structure consists of five different boron atom positions (corner-sharing BO4 tetrahedra) and one octahedrally coordinated indium and gallium atom position. The proton, which forms a hydroxyl group with the donor atom O8, is the only atom site that shows a diminished occupancy factor of 0.75 for charge neutrality reasons. A complete list of occupancy factors, Wyckoff positions, fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters can be found in Table 2. All anisotropic displacement parameters are listed in Table 3. The structure of Ga4In4B15O33(OH)3 will now be explained in detail starting with the In-O and Ga-O networks.
Crystal data and structure refinement of tetragonal Ga4In4B15O33(OH)3.
| Empirical formula | Ga4In4B15O33(OH)3 |
| Molar mass, g mol−1 | 1479.33 |
| Crystal system | tetragonal |
| Space group | I41/a (no. 88, origin choice 2) |
| Single-crystal diffractometer | Bruker D8 Quest Kappa |
| Radiation | MoKα (λ=0.71073 Å) |
| a, Å | 11.382(2) |
| c, Å | 15.244(2) |
| V, Å3 | 1974.9(4) |
| Formula units per cell Z | 4 |
| Calculated density, g cm−3 | 4.98 |
| Crystal size, mm3 | 0.05×0.04×0.02 |
| Temperature, K | 297(2) |
| Detector distance, mm | 50 |
| Exposure time, ° s−1 | 130 |
| Absorption coefficient, mm−1 | 10.2 |
| F(000), e | 2744 |
| θ range, deg | 2.2–36.0 |
| Range in hkl | ±18; ±18; ±25 |
| Reflections total/independent | 28476/2348 |
| Rint | 0.0450 |
| Reflections with I≥2 σ(I) | 2260 |
| Rσ | 0.0202 |
| Data/ref. parameters/restraints | 2348/139/1 |
| Absorption correction | multi-scan |
| Final R1/wR2 [I≥2σ(I)] | 0.0175/0.0417 |
| Final R1/wR2 (all data) | 0.0192/0.0422 |
| Goodness-of-fit on Fi2 | 1.078 |
| Largest diff. peak/hole, e Å−3 | 0.53/–1.78 |
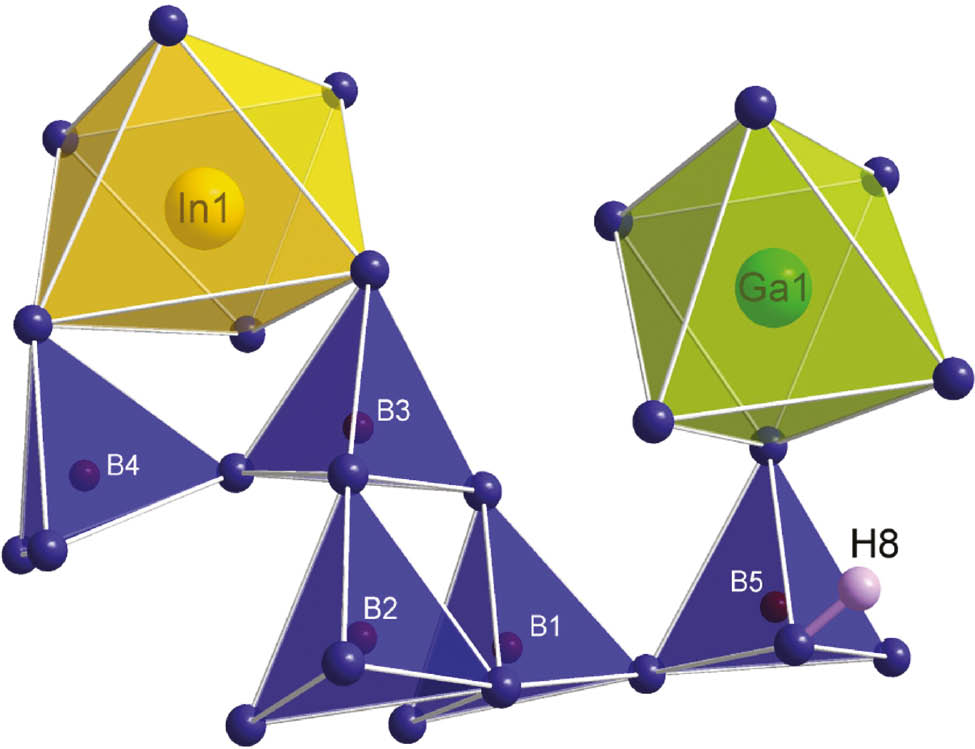
Asymmetric unit of Ga4In4B15O33(OH)3 consisting of five different boron atom positions forming corner-sharing BO4 tetrahedra, an InO6 octahedron, a GaO6 octahedron, and a hydroxyl group (atoms O8 and H8).
Wyckoff positions, occupancy factors, atomic coordinates, isotropic Uiso or equivalent isotropic displacement parameters Ueq (Å2) of Ga4In4B15O33(OH)3.
| Atom | Wyckoff position | Occupancy | x | y | z | Ueq (Uiso for H8) |
|---|---|---|---|---|---|---|
| In1 | 16f | 1 | 0.23415(2) | 0.65795(2) | 0.32996(2) | 0.00510(3) |
| Ga1 | 16f | 1 | 0.41303(2) | 0.64704(2) | 0.70048(2) | 0.00335(4) |
| B1 | 16f | 1 | 0.1577(2) | 0.9068(2) | 0.6132(2) | 0.0037(3) |
| B2 | 8e | 1 | 0 | ¾ | 0.6196(2) | 0.0041(5) |
| B3 | 16f | 1 | 0.1624(2) | 0.7336(2) | 0.5071(2) | 0.0042(3) |
| B4 | 4b | 1 | 0 | ¾ | ⅜ | 0.0069(7) |
| B5 | 16f | 1 | 0.3358(2) | 0.5774(2) | 0.5190(2) | 0.0041(3) |
| O1 | 16f | 1 | 0.0752(2) | 0.8315(2) | 0.67198(9) | 0.0048(2) |
| O2 | 16f | 1 | 0.0798(2) | 0.6775(2) | 0.56575(9) | 0.0052(2) |
| O3 | 16f | 1 | 0.2279(2) | 0.8245(2) | 0.5570(2) | 0.0049(2) |
| O4 | 16f | 1 | 0.1010(2) | 0.7899(2) | 0.43024(9) | 0.0052(2) |
| O5 | 16f | 1 | 0.2484(2) | 0.6495(2) | 0.47177(9) | 0.0043(2) |
| O6 | 16f | 1 | 0.0894(2) | 0.9828(2) | 0.55999(9) | 0.0049(2) |
| O7 | 16f | 1 | 0.2430(2) | 0.9697(2) | 0.66984(9) | 0.0044(2) |
| O8 | 16f | 1 | 0.4032(2) | 0.5060(2) | 0.45766(9) | 0.0059(2) |
| O9 | 16f | 1 | 0.4178(2) | 0.6616(2) | 0.56737(9) | 0.0048(2) |
| H8 | 16f | 0.75 | 0.446(6) | 0.553(6) | 0.432(4) | 0.09(3) |
Ueq is defined as one third of the trace of the orthogonalized Uij tensor (standard deviations in parentheses).
Anisotropic displacement parameters Uij (Å2) of Ga4In4B15O33(OH)3 (standard deviations in parentheses).
| Atom | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|
| In1 | 0.00709(6) | 0.00417(6) | 0.00403(5) | –0.00103(4) | –0.00059(4) | 0.00039(4) |
| Ga1 | 0.00390(9) | 0.00340(9) | 0.00274(8) | –0.00011(7) | 0.00015(6) | 0.00007(6) |
| B1 | 0.0030(8) | 0.0048(8) | 0.0032(7) | –0.0006(6) | 0.0002(6) | 0.0004(6) |
| B2 | 0.003(2) | 0.005(2) | 0.004(2) | –0.0005(9) | 0 | 0 |
| B3 | 0.0049(8) | 0.0040(8) | 0.0037(8) | 0.0001(6) | 0.0003(6) | 0.0004(6) |
| B4 | 0.008(2) | 0.008(2) | 0.005(2) | 0 | 0 | 0 |
| B5 | 0.0047(8) | 0.0043(8) | 0.0034(7) | 0.0005(6) | 0.0003(6) | 0.0001(6) |
| O1 | 0.0053(6) | 0.0058(6) | 0.0035(5) | –0.0023(5) | 0.0009(4) | –0.0002(4) |
| O2 | 0.0057(6) | 0.0038(6) | 0.0061(5) | –0.0006(4) | 0.0025(4) | 0.0003(4) |
| O3 | 0.0035(6) | 0.0055(6) | 0.0058(5) | –0.0007(4) | 0.0009(4) | –0.0024(4) |
| O4 | 0.0051(6) | 0.0048(6) | 0.0057(5) | –0.0012(5) | –0.0009(4) | 0.0006(4) |
| O5 | 0.0048(6) | 0.0046(6) | 0.0035(5) | 0.0014(5) | 0.0001(4) | –0.0002(4) |
| O6 | 0.0039(6) | 0.0059(6) | 0.0049(5) | 0.0002(5) | –0.0001(4) | 0.0007(4) |
| O7 | 0.0038(6) | 0.0040(6) | 0.0053(5) | –0.0004(5) | –0.0019(4) | –0.0012(4) |
| O8 | 0.0058(6) | 0.0055(6) | 0.0064(5) | 0.0000(5) | 0.0022(4) | –0.0012(4) |
| O9 | 0.0045(6) | 0.0047(6) | 0.0050(5) | –0.0003(5) | –0.0004(4) | –0.0003(4) |
Both metal atoms are coordinated octahedrally by oxygen atoms. Due to the symmetry of the space group, indium forms edge-sharing octahedra in dinuclear units caused by an inversion axis, and gallium assembles dense units of four edge-sharing octahedra induced by the 4̅ axis. The whole unit cell contains four isolated tetranuclear Ga–O units and eight isolated dinuclear In-O units. Figure 3 shows both octahedral units and their arrangement in the unit cell of Ga4In4B15O33(OH)3. All metal-oxygen distances are in good accordance with values reported in the literature (Ga–O=1.928(2)–2.126(2) Å, Ø=2.00 Å; In–O=2.121(2) –2.219(2) Å, Ø=2.17 Å) [1], [7], [9], [22], while the axial angles with average values of 168.6° for O–Ga–O and 170.3° for O–In–O are a bit smaller than the expected one of 180°. A complete list of distances and angles is given in Tables 4 and 5.
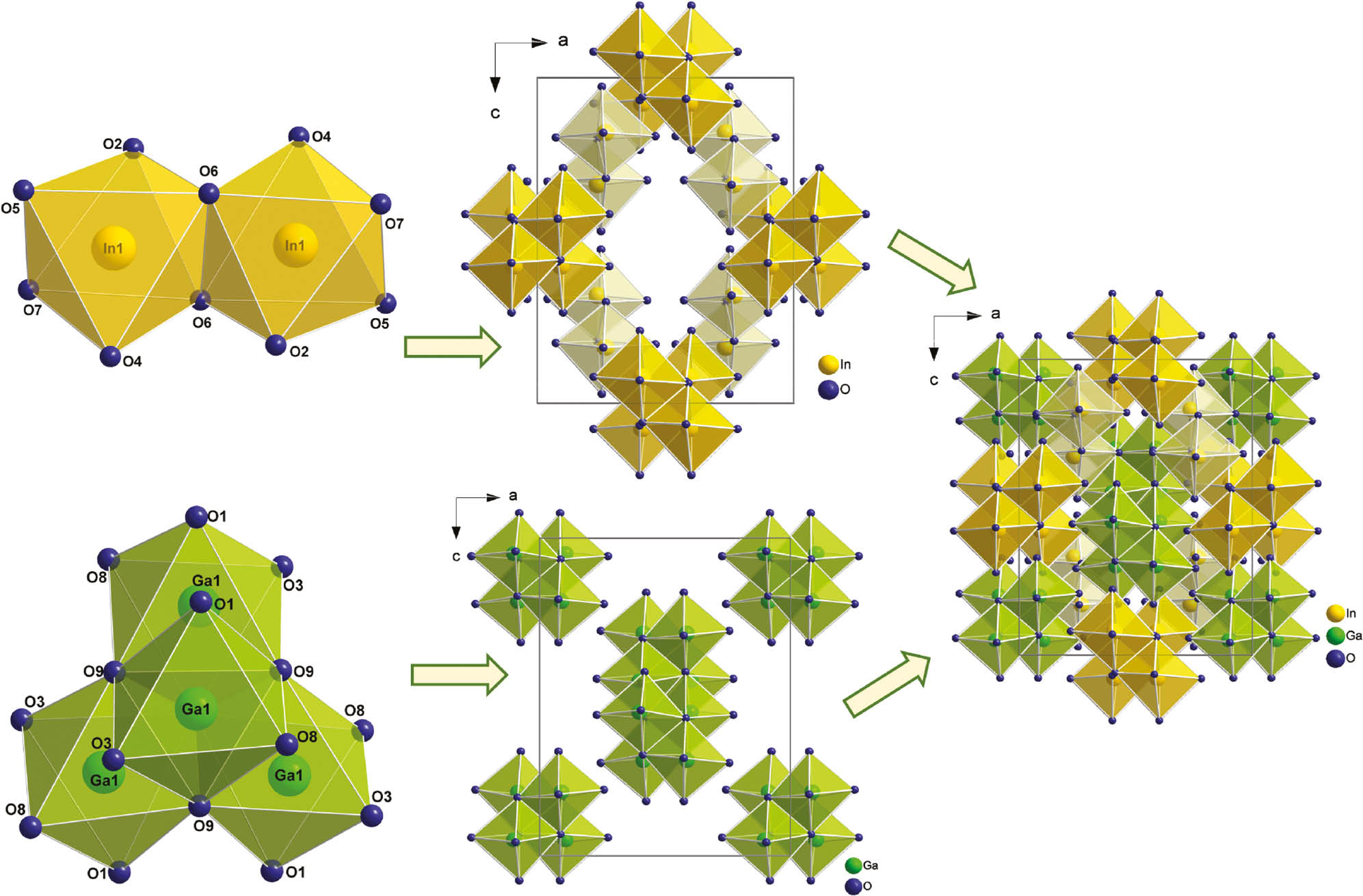
Isolated edge-sharing InO6 octahedra (top left, yellow) and GaO6 octahedra (bottom left, green) and their arrangement in the unit cell of Ga4In4B15O33(OH)3 (middle and right).
Interatomic distances (Å) in Ga4In4B15O33(OH)3 (standard deviations in parentheses).
| In1–O6 | 2.121(2) | Ga1–O8 | 1.928(2) |
| In1–O6 | 2.134(2) | Ga1–O3 | 1.933(2) |
| In1–O7 | 2.145(2) | Ga1–O1 | 1.964(2) |
| In1–O5 | 2.170(2) | Ga1–O9 | 2.029(2) |
| In1–O2 | 2.216(2) | Ga1–O9 | 2.037(2) |
| In1–O4 | 2.219(2) | Ga1–O9 | 2.126(2) |
| ØIn1–O | 2.17 | ØGa1–O | 2.00 |
| B1–O6 | 1.418(3) | B4–O4 | 1.496(2) 4× |
| B1–O7 | 1.484(3) | ØB4–O | 1.50 |
| B1–O3 | 1.500(3) | ||
| B1–O1 | 1.556(3) | B5–O8 | 1.458(3) |
| ØB1–O | 1.49 | B5–O5 | 1.477(3) |
| B5–O7 | 1.480(3) | ||
| B2–O2 | 1.477(2) 2× | B5–O9 | 1.527(3) |
| B2–O1 | 1.493(2) 2× | ØB5–O | 1.49 |
| ØB2–O | 1.49 | ||
| B3–O2 | 1.446(3) | ||
| B3–O5 | 1.471(3) | ||
| B3–O3 | 1.485(3) | ||
| B3–O4 | 1.507(3) | ||
| ØB3–O | 1.48 |
Interatomic angles (deg) in Ga4In4B15O33(OH)3 (standard deviations in parentheses).
| In1 | Ga1 | ||
| O6–In1–O2 | 75.45(6) | O9–Ga1–O9 | 78.00(6) |
| O6–In1–O6 | 81.01(6) | O9–Ga1–O9 | 80.26(6) |
| O6–In1–O2 | 84.74(6) | O9–Ga1–O9 | 82.83(7) |
| O6–In1–O4 | 85.62(6) | O8–Ga1–O9 | 87.90(6) |
| O7–In1–O5 | 87.17(5) | O1–Ga1–O9 | 90.21(6) |
| O7–In1–O4 | 90.04(6) | O8–Ga1–O1 | 90.44(6) |
| O6–In1–O7 | 90.92(5) | O3–Ga1–O9 | 91.17(6) |
| O7–In1–O2 | 90.94(6) | O3–Ga1–O9 | 92.04(6) |
| O5–In1–O2 | 91.50(5) | O1–Ga1–O9 | 92.91(6) |
| O5–In1–O4 | 98.17(5) | O8–Ga1–O9 | 94.59(6) |
| O6–In1–O5 | 99.97(5) | O8–Ga1–O3 | 96.49(7) |
| O6–In1–O4 | 102.20(6) | O3–Ga1–O1 | 100.19(6) |
| ØO–In1–O90 | 89.8 | ØO–Ga1–O90 | 89.8 |
| O6–In1–O7 | 164.69(6) | O1–Ga1–O9 | 166.98(6) |
| O2–In1–O4 | 170.32(5) | O3–Ga1–O9 | 168.64(6) |
| O6–In1–O5 | 175.76(6) | O8–Ga1–O9 | 170.15(6) |
| ØO–In1–O180 | 170.3 | ØO–Ga1–O180 | 168.6 |
| B1 | B4 | ||
| O7–B1–O3 | 106.5(2) | O4–B4–O4 | 108.47(6) 3× |
| O3–B1–O1 | 107.9(2) | O4–B4–O4 | 108.48(6) |
| O7–B1–O1 | 109.0(2) | O4–B4–O4 | 111.5(2) 2× |
| O6–B1–O1 | 109.6(2) | ØO–B4–O | 109.5 |
| O6–B1–O3 | 110.3(2) | ||
| O6–B1–O7 | 113.4(2) | B5 | |
| ØO–B1–O | 109.4 | O8–B5–O7 | 105.4(2) |
| O5–B5–O9 | 107.4(2) | ||
| B2 | O8–B5–O9 | 109.7(2) | |
| O2–B2–O1 | 106.99(8) 2× | O8–B5–O5 | 110.6(2) |
| O2–B2–O1 | 107.61(8) 2× | O7–B5–O9 | 111.4(2) |
| O2–B2–O2 | 112.4(2) | O5–B5–O7 | 112.4(2) |
| O1–B2–O1 | 115.4(2) | ØO–B5–O | 109.5 |
| ØO–B2–O | 109.5 | ||
| B3 | |||
| O5–B3–O4 | 107.5(2) | ||
| O5–B3–O3 | 107.9(2) | ||
| O2–B3–O3 | 108.5(2) | ||
| O3–B3–O4 | 109.5(2) | ||
| O2–B3–O4 | 111.5(2) | ||
| O2–B3–O5 | 111.8(2) | ||
| ØO–B3–O | 109.5 |
The complex network of exclusively corner-sharing BO4 tetrahedra interconnects the various isolated metal-oxygen units. In doing so, the different BO4 tetrahedra form dreier and vierer rings [23]. B5O4 is the only tetrahedron that does not contribute to a ring system but connects to the proton H8 via O8 instead (see Fig. 4). The complete structure is shown in Fig. 5. The hydrogen bonding situation is illustrated in Fig. 6, angles and distances of the hydrogen bonds are given in Table 6. All B–O bond lengths are within the range of 1.418(3)–1.556(3) Å (average 1.49 Å) and fit to reported distances in tetrahedral borate groups [24]. The average O–B–O angles of 109.4° (for B1) and 109.5° (for B2–5) indicate perfectly shaped polyhedra.
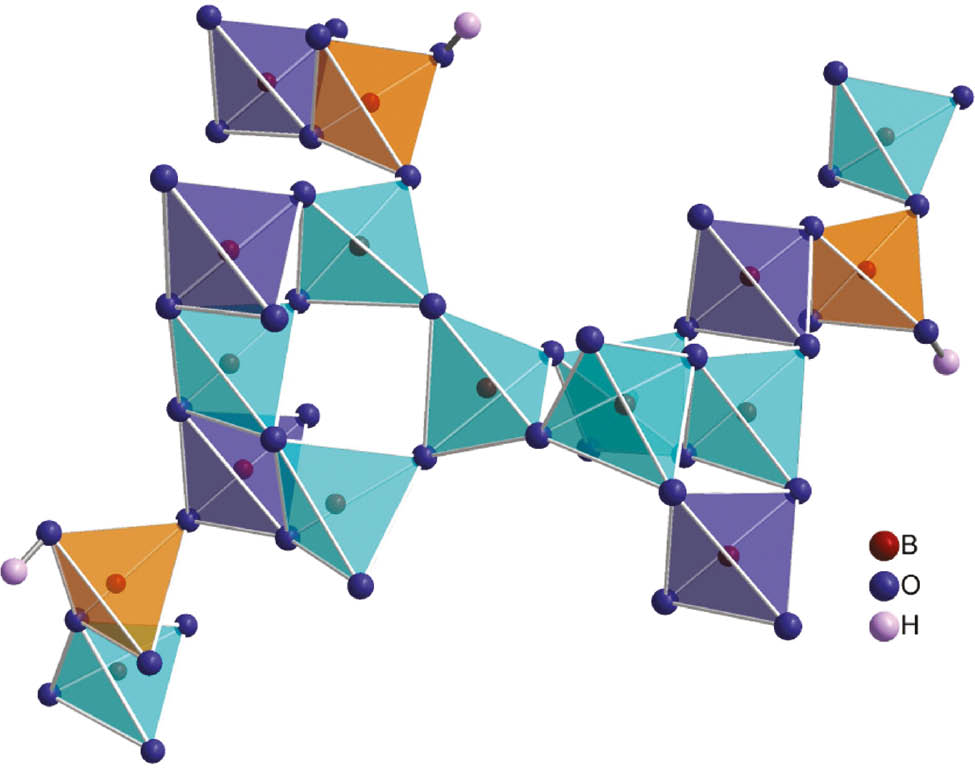
Visualization of the network of corner-sharing B-O tetrahedra in Ga4In4B15O33(OH)3. Tetrahedra centered by the boron atoms B2, B3 and B4 build up vierer rings (turquoise) that are further connected to dreier rings via B1O4 tetrahedra (blue). The ring-systems are interlinked by the B5O4 tetrahedra containing the hydroxyl group (orange).
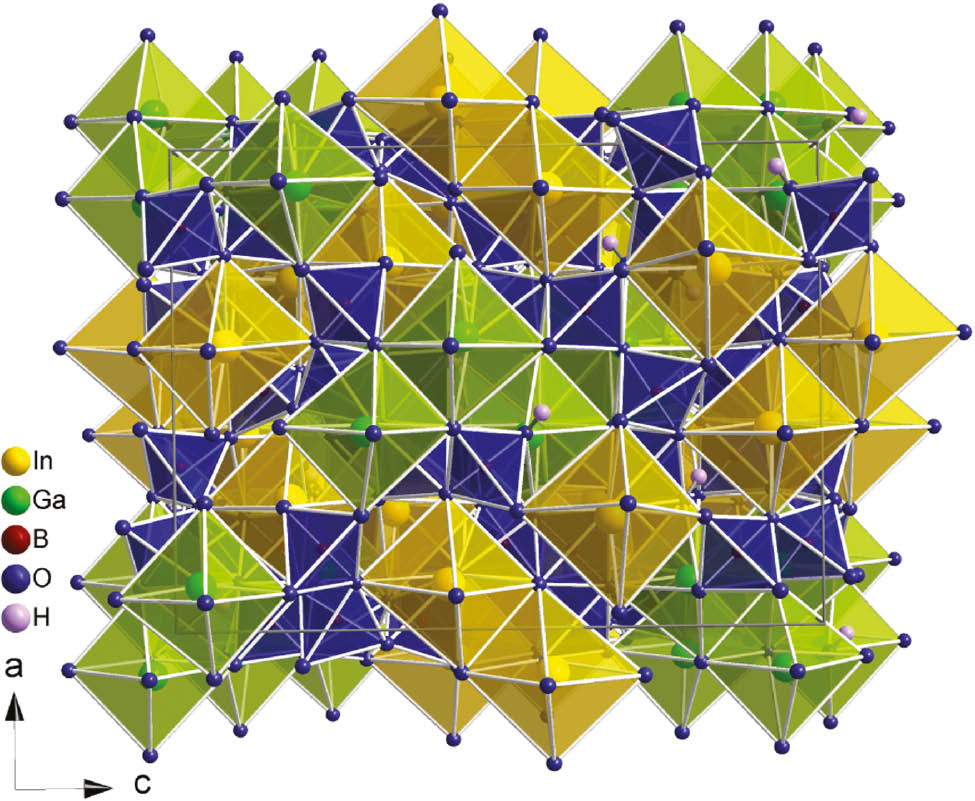
Complete unit cell of Ga4In4B15O33(OH)3.
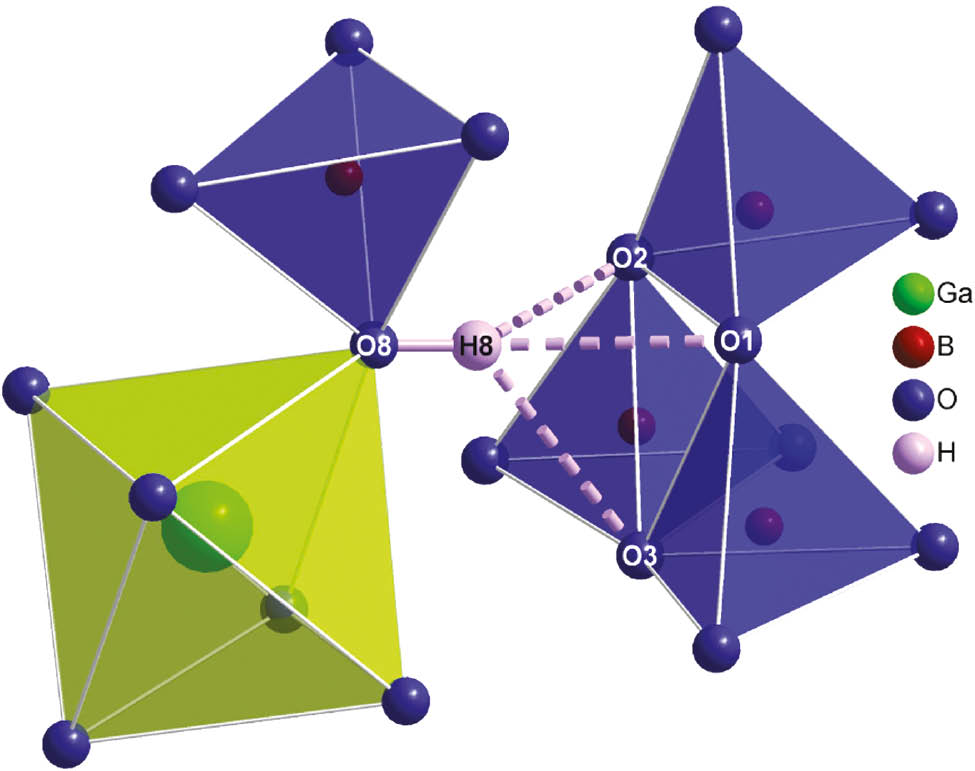
Hydrogen bonds in Ga4In4B15O33(OH)3.
Hydrogen bonds (Å, deg) in Ga4In4B15O33(OH)3 (standard deviations in parentheses).
| D–H | H…A | d(D…A) | D–H–A | |
|---|---|---|---|---|
| O8–H8…O1 | 0.83(2) | 2.08(4) | 2.847(2) | 154(7) |
| O8–H8…O2 | 0.83(2) | 2.22(6) | 2.872(2) | 136(7) |
| O8–H8…O3 | 0.83(2) | 2.42(7) | 3.018(2) | 130(7) |
To evaluate the charge distribution, we performed bond-length/bond-strength calculations (see Table 7) [25], [26]. Solely O9 shows an unusually high charge which can be attributed to the number of bonding partners. While all other oxygen atoms in Ga4In4B15O33(OH)3 coordinate three other atoms, O9 forms quite long but nonetheless definite bonds to three Ga atoms and one B atom.
Charge distribution in Ga4In4B15O33(OH)3, calculated with the bond-length/bond-strength (ΣV) concept.
| In1 | Ga1 | B1 | B2 | B3 | B4 | B5 | O1 | |
| ΣV | 2.94 | 2.92 | 2.93 | 2.94 | 3.01 | 2.85 | 2.94 | –1.86 |
| O2 | O3 | O4 | O5 | O6 | O7 | O8 | O9 | |
| ΣV | –2.00 | –2.02 | –1.83 | –2.00 | –1.97 | –2.00 | –2.16 | –2.61 |
4 Conclusions
A high-pressure/high-temperature experiment in a multianvil press led to the formation of the first mixed cation triel borate in which indium and gallium occupy crystallographically different positions. Although the bulk reaction product was not phase pure, the structure of Ga4In4B15O33(OH)3 could be determined reliably via a single-crystal structure determination. This new hydroxylated high-pressure borate is built up of a complex, dense BO4 tetrahedra network. Indium and gallium form di- or tetranuclear units of edge-sharing octahedra, respectively. This finding complements a series of current publications in the aspiring research field of triel borates.
Acknowledgements
We thank Dr. Klaus Wurst for the recording of the single-crystal data. Although they did not lead to new findings, we warmly thank Daniel Wimmer, BSc, MSc for additional high-pressure experiments on this topic.
References
[1] D. Vitzthum, K. Wurst, J. Pann, P. Brüggeller, M. Seibald, H. Huppertz, Angew. Chem. Int. Ed.2018, 57, 11451.10.1002/anie.201804083Search in Google Scholar PubMed PubMed Central
[2] C. Yin, R. Wang, P. Jiang, R. Cong, T. Yang, J. Solid State Chem.2019, 269, 30.10.1016/j.jssc.2018.09.010Search in Google Scholar
[3] E. Y. Borovikova, K. N. Boldyrev, S. M. Aksenov, E. A. Dobretsova, V. S. Kurazhkovskaya, N. I. Leonyuk, A. E. Savon, D. V. Deyneko, D. A. Ksenofontov, Opt. Mater.2015, 49, 304.10.1016/j.optmat.2015.09.021Search in Google Scholar
[4] P.-L. Xu, C.-C. Jin, T.-T. Deng, E.-R. Wang, J.-W. Cheng, J. Cluster Sci.2017, 28, 1431.10.1007/s10876-016-1148-ySearch in Google Scholar
[5] T. T. Tran, N. Z. Koocher, J. M. Rondinelli, P. S. Halasyamani, Angew. Chem.2017, 129, 3015.10.1002/ange.201612236Search in Google Scholar
[6] D. An, M. Zhang, D. Li, S. Pan, H. Chen, Z. Yang, Y. Zhu, Y. Sun, H. Zhang, Y. Li, J. Mater. Res.2015, 30, 2319.10.1557/jmr.2015.204Search in Google Scholar
[7] D. Vitzthum, L. Bayarjargal, B. Winkler, H. Huppertz, Inorg. Chem.2018, 57, 5554.10.1021/acs.inorgchem.8b00518Search in Google Scholar PubMed
[8] D. Vitzthum, K. Wurst, J. Prock, P. Brüggeller, H. Huppertz, Inorg. Chem.2016, 55, 11473.10.1021/acs.inorgchem.6b02029Search in Google Scholar PubMed
[9] D. Vitzthum, M. Schauperl, C. M. Strabler, P. Brüggeller, K. R. Liedl, U. J. Griesser, H. Huppertz, Inorg. Chem.2016, 55, 676.10.1021/acs.inorgchem.5b02027Search in Google Scholar PubMed
[10] K. Song, M. Yue, W. Gao, R. Cong, T. Yang, J. Alloys Compd.2016, 684, 346.10.1016/j.jallcom.2016.05.194Search in Google Scholar
[11] B. Ma, R. Cong, W. Gao, T. Yang, Catal. Commun.2015, 71, 17.10.1016/j.catcom.2015.08.009Search in Google Scholar
[12] G. Wang, Y. Jing, J. Ju, D. Yang, J. Yang, W. Gao, R. Cong, T. Yang, Inorg. Chem.2015, 54, 2945.10.1021/ic5031087Search in Google Scholar PubMed
[13] W. Gao, Y. Jing, J. Yang, Z. Zhou, D. Yang, J. Sun, J. Lin, R. Cong, T. Yang, Inorg. Chem.2014, 53, 2364.10.1021/ic403175wSearch in Google Scholar PubMed
[14] Y. Yang, K. Song, M. Yue, L. Li, R. Cong, W. Gao, T. Yang, Eur. J. Inorg. Chem.2017, 63.10.1002/ejic.201601071Search in Google Scholar
[15] H. Huppertz, Z. Kristallogr.2004, 219, 330.10.1524/zkri.219.6.330.34633Search in Google Scholar
[16] D. Walker, M. A. Carpenter, C. M. Hitch, Am. Mineral.1990, 75, 1020.Search in Google Scholar
[17] D. Walker, Am. Mineral.1991, 76, 1092.10.1007/978-1-4615-3968-1_10Search in Google Scholar
[18] Sadabs 2014/5, Bruker AXS Inc., Madison, Wisconsin (USA) 2001.Search in Google Scholar
[19] G. M. Sheldrick, Acta Crystallogr.2008, A64, 112.10.1107/S0108767307043930Search in Google Scholar PubMed
[20] G. M. Sheldrick, Acta Crystallogr.2015, C71, 3.Search in Google Scholar
[21] L. J. Farrugia, J. Appl. Crystallogr.2012, 45, 849.10.1107/S0021889812029111Search in Google Scholar
[22] D. Vitzthum, S. A. Hering, L. Perfler, H. Huppertz, Z. Naturforsch.2015, 70b, 207.10.1515/znb-2015-0015Search in Google Scholar
[23] F. Liebau, Structural Chemistry of Silicates, Springer-Verlag, Berlin, 1985.10.1007/978-3-642-50076-3Search in Google Scholar
[24] E. Zobetz, Z. Kristallogr.1990, 191, 45.10.1524/zkri.1990.191.1-2.45Search in Google Scholar
[25] I. D. Brown, D. Altermatt, Acta Crystallogr.1985, B41, 244.10.1107/S0108768185002063Search in Google Scholar
[26] N. E. Brese, M. O’Keeffe, Acta Crystallogr.1991, B47, 192.10.1107/S0108768190011041Search in Google Scholar
© 2019 Hubert Huppertz et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- Coloring in the ZrBeSi-type structure
- Research Articles
- Different substituent effects on the supramolecular arrays in some (E)-halo- and nitro-benzaldehyde oximes: confirmation of attractive π(C=N)···π(phenyl) interactions
- Synthesis, crystal structure, and magnetic properties of a multicage compound: [(Me)2EtNH][Mn(N3)3] with a perovskite-related structure
- A new one-dimensional Cd(II) coordination polymer with a two-dimensional supramolecular architecture: synthesis, structural characterization and fluorescence properties
- Cycloaddition reactions of acetylenic iminium salts and diazoacetates leading to pyrazole iminium salts
- High-pressure synthesis and crystal structure of the mixed-cation borate Ga4In4B15O33(OH)3
- Evaluation of benzthiazolidine-based formamidinium salts for the synthesis of penem-type β-lactams by uncatalysed carbonylation of acyclic diaminocarbenes
- Temperature- and solvate-dependent disorder in the crystal structure of [PNP]+[HSO4]−
- Serendipitous formation and characterization of K2[Pd(NO3)4]·2HNO3
- Phosphanchalkogenide und ihre Metallkomplexe. V. Derivate von [2.2]Paracyclophanylphosphanena
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- Coloring in the ZrBeSi-type structure
- Research Articles
- Different substituent effects on the supramolecular arrays in some (E)-halo- and nitro-benzaldehyde oximes: confirmation of attractive π(C=N)···π(phenyl) interactions
- Synthesis, crystal structure, and magnetic properties of a multicage compound: [(Me)2EtNH][Mn(N3)3] with a perovskite-related structure
- A new one-dimensional Cd(II) coordination polymer with a two-dimensional supramolecular architecture: synthesis, structural characterization and fluorescence properties
- Cycloaddition reactions of acetylenic iminium salts and diazoacetates leading to pyrazole iminium salts
- High-pressure synthesis and crystal structure of the mixed-cation borate Ga4In4B15O33(OH)3
- Evaluation of benzthiazolidine-based formamidinium salts for the synthesis of penem-type β-lactams by uncatalysed carbonylation of acyclic diaminocarbenes
- Temperature- and solvate-dependent disorder in the crystal structure of [PNP]+[HSO4]−
- Serendipitous formation and characterization of K2[Pd(NO3)4]·2HNO3
- Phosphanchalkogenide und ihre Metallkomplexe. V. Derivate von [2.2]Paracyclophanylphosphanena

