Environmentally benign synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates using CeO2 nanoparticles as a reusable and robust catalyst
Abstract
In this work, we report the synthesis and characterization of CeO2 nanoparticles as an efficient catalyst for the preparation of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates via one-pot four-component condensation reaction of dimethyl acetylenedicarboxylate, hydrazine hydrate, malononitrile, and aldehydes in aqueous medium. The use of a non-hazardous organic solvent, easy recovery of the catalyst, compatibility with various functional groups, and high yield of the products make the protocol attractive, greener, and economic.
1 Introduction
Pyranopyrazoles are important classes of organic compounds due to their wide range of biological and pharmacological activities. The synthesis of pyranopyrazoles is an interesting challenge because compounds with these scaffolds were reported to possess a multiplicity of pharmacological properties such as anticancer activity against several cell lines [1], analgesic [2], Chk1 inhibitors [3], and molluscicidal activities [4]. Pyrano[2,3-c]pyrazole is isomeric with pyrano[4,3-c]pyrazole, pyrano[3,2-c]pyrazole, and pyrano[3,4-c]pyrazole. Practically, the construction of pyrano[2,3-c]pyrazole structures has been established through different modes of reaction and cyclization: two-component, three-component, and four-component reactions. Based on the two-component synthesis, Sharanina et al. in 1982 developed the three-component synthesis of pyrano[2,3-c]pyrazoles from the reaction of substituted pyrazol-5-ones, aldehydes, and malononitrile in the presence of morpholine as a catalyst [5]. Synthesis of bioactive compounds should be facile, flexible, and green in organic synthesis. Therefore, looking for efficient and concise methods for the synthesis of pyranopyrazoles is considered very important. Multicomponent reactions supply a practical method for the synthesis of various compounds with pharmaceutical and biological activities [6]. The combination of multicomponent reactions with a heterogeneous catalyst could improve their effectiveness from operating cost and environmental points of view.
The chemical synthesis productivity can be increased by nano-sized catalysts due to small size and high surface to volume ratios [7]. Among various nanoparticles (NPs), cerium NPs have received considerable attention because of their unique properties and potential applications in diverse fields [8]. CeO2 has received much attention because of its many attractive characteristics, such as unique UV absorption ability [9], ferromagnetic properties [10], and major part of catalyst formulation for the dehydrogenation of ethylbenzene to styrene [11].
There are many methods for the preparation of CeO2 NPs such as sol–gel method [12], thermal hydrolysis [13], homogeneous precipitation [14], and so on. In the present research, CeO2 NPs were fabricated by a simple co-precipitation technique. Compared with other techniques, the co-precipitation method is a simple and attractive procedure for the preparation of CeO2 NPs. Recently, γ-alumina [15], piperidine [16], imidazole [17], glycine [18], l-proline or KF-alumina [19], and triethylamine (under solvent-free conditions) [20] were reported for the synthesis of pyrano[2,3-c]pyrazole derivatives.
Herein, we report the use of CeO2 NPs as an efficient catalyst for the preparation of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates by the four-component reaction of dimethyl acetylenedicarboxylate, hydrazine hydrate, malononitrile, and aromatic aldehydes in water at room temperature (Scheme 1).
![Scheme 1: Synthesis of pyrano[2,3-c]pyrazoles using CeO2 NPs.](/document/doi/10.1515/znb-2016-0119/asset/graphic/j_znb-2016-0119_scheme_001.jpg)
Synthesis of pyrano[2,3-c]pyrazoles using CeO2 NPs.
2 Results and discussion
The catalyst was prepared by the co-precipitation method using aqueous ammonia solution as the precipitating agent. Ce(NO3)3·6H2O was used as a starting material for the synthesis of CeO2 NPs. This method is easy and inexpensive. The XRD pattern for CeO2 NPs is shown in Fig. 1.
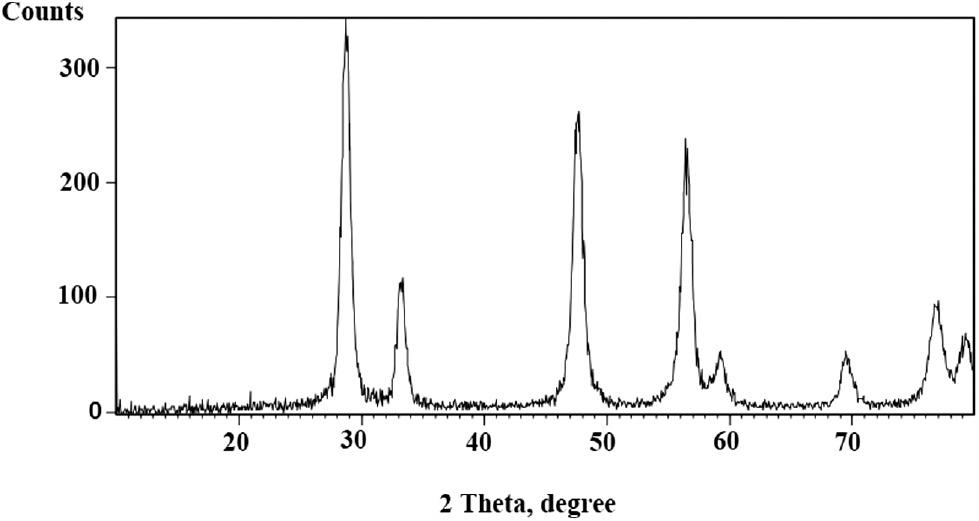
The XRD pattern of CeO2 NPs.
The pattern agrees well with the reported pattern for CeO2 NPs (JCPDS No. 43-1002). The crystalline size was calculated from full-width at half-maximum (FWHM) using Scherrer’s formula and was observed to be 11 nm.
In order to optimize the reaction conditions for the synthesis of pyrano[2,3-c]pyrazoles, the reaction of 4-nitrobenzaldehyde with dimethyl acetylenedicarboxylate, hydrazine hydrate, and malononitrile was used as a model reaction at room temperature. Several reactions were scrutinized using different solvents, such as ethanol, acetonitrile, water, and dichloromethane. In the presence of water the products were obtained in high yield. The model reaction was considered in the presence of different catalysts. The best result was obtained in using CeO2 NPs. To optimize the catalyst quantity, the reaction was carried out with different quantities of the CeO2 NPs and 6 mol% of CeO2 NPs was found to be optimal (Table 1). When the reaction was carried out using CeO2 bulk and CeO2 NPs as the catalyst, the product could be obtained in a moderate to good yield. As expected, the increased surface area due to small particle size increased the reactivity of the catalyst. This factor is responsible for the accessibility of the substrate molecules on the catalyst surface. The catalyst was recovered from the reaction mixture by either filtration or centrifugation and washed successively with ethanol and water. The recycling of the nano-CeO2 catalyst was examined, and it was found that product yields decreased to a small extent on each reuse (run 1, 91%; run 2, 91%; run 3, 90%; run 4, 89%; run 5, 88%, run 6, 87%, run 7, 87%).
Optimization of reaction condition using different catalysts.a
| Entry | Solvent | Catalyst | Mol, % | Time, min | Yield, %b |
|---|---|---|---|---|---|
| 1 | CH2Cl2 | InCl3 | 3 | 90 | 33 |
| 2 | CH3CN | Et3N | 20 | 70 | 37 |
| 3 | EtOH | CuO | 10 | 65 | 41 |
| 4 | EtOH | CaO | 5 | 60 | 44 |
| 5 | H2O | ZrO2 | 10 | 65 | 49 |
| 6 | CH3CN | Ce(NO3)3 | 3 | 55 | 51 |
| 7 | CH3CN | (NH4)2Ce(NO3)6 | 3 | 55 | 53 |
| 8 | H2O | Nd2O3 | 5 | 55 | 48 |
| 9 | H2O | CeO2 bulk | 8 | 40 | 60 |
| 10 | EtOH | CeO2 NPs | 6 | 25 | 76 |
| 11 | H2O | CeO2 NPs | 3 | 25 | 84 |
| 12 | H2O | CeO2NPs | 6 | 25 | 91 |
| 13 | H2O | CeO2 NPs | 9 | 25 | 91 |
aReaction conditions: hydrazine hydrate (1 mmol), malononitrile (1 mmol), dimethyl acetylenedicarboxylate (1 mmol), and 4-nitrobenzaldehyde (1 mmol).
bIsolated yields.
Bold text signifies best yield in 25 min for the reaction.
With these promising results in hand, we turned to explore the scope of the reaction using diverse aromatic aldehydes as substrates under the optimized reaction conditions (Table 2). As is shown in Table 2, aromatic aldehydes bearing electron-donating or -withdrawing substituents gave the desired methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates in good to excellent yields and short reaction times. Several groups such as F, OMe, Me, and NO2 are compatible with the reaction conditions.
Synthesis of pyrano[2,3-c]pyrazole-3-carboxylates with CeO2 NPs.a
| Entry | Ar | Product | Time, min | Yield, %b | M.p., °C | M.p., °C [Ref.] |
|---|---|---|---|---|---|---|
| 1 | 4-NO2-C6H4 | 5a | 25 | 91 | 242–243 | 244–245 [21] |
| 2 | 2-F-C6H4 | 5b | 28 | 88 | 248–250 | – |
| 3 | 4-OMe-C6H4 | 5c | 34 | 84 | 238–240 | – |
| 4 | 4-Me-C6H4 | 5d | 32 | 88 | 242–244 | – |
| 5 | 3-NO2-C6H4 | 5e | 29 | 89 | 234–235 | 236–237 [21] |
| 6 | C6H5 | 5f | 31 | 85 | 231–232 | 231–232 [21] |
| 7 | 2,3-(OMe)2-C6H3 | 5g | 39 | 82 | 239–241 | – |
| 8 | 2-OMe-C6H4 | 5h | 36 | 84 | 210–212 | 212–214 [21] |
| 9 | 4-Br-C6H4 | 5i | 32 | 85 | 245–247 | 247–248 [21] |
aAll the reactions were carried out in water.
bIsolated yields.
2.1 The proposed reaction mechanism
A plausible mechanism for the preparation of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates using CeO2 NPs is shown in Scheme 2. First, we assumed that the reaction occurs via a Knoevenagel condensation between malononitrile and aldehyde to form the intermediate I and concomitant formation of pyrazole derivative II by the reaction of hydrazine hydrate and dimethyl acetylenedicarboxylate. Finally, Michael addition of I, II, followed by cyclization and tautomerization to give the pyranopyrazoles. CeO2 NPs coordinate with the active groups (particular C=O, C≡N, and active H), thus increasing the reactivity of the groups. Theoretically, nanoscale heterogeneous catalysts should present higher surface areas which are mainly responsible for their catalytic activity. These surface atoms behave as the centers where the chemical reactions could be catalytically activated.
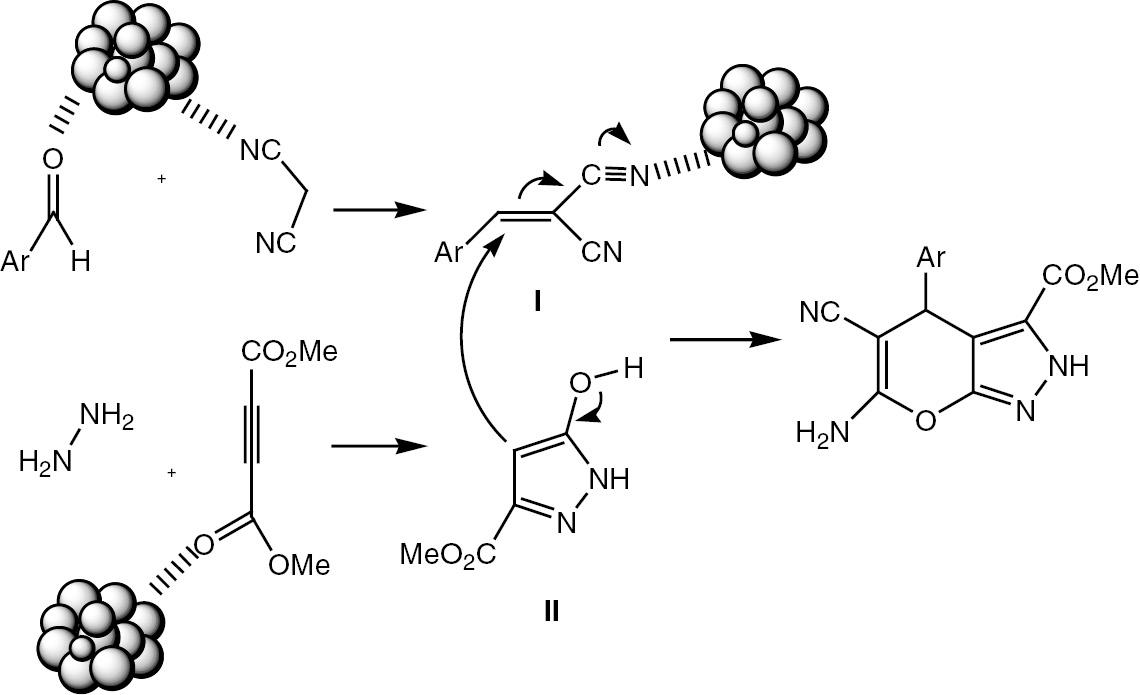
Schematic mechanism for the catalytic activity of CeO2 NPs in the synthesis of title compounds 5a–i.
3 Conclusions
In conclusion, we have developed a straightforward and efficient approach to methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates by a simple one-pot four-component condensation reaction of dimethyl acetylenedicarboxylate, hydrazine hydrate, malononitrile, and aldehydes using CeO2 NPs in aqueous medium. These compounds will provide promising candidates for biological applications and drug discovery. The attractive features of this protocol are the simple procedure, use of recyclable nanocatalyst, easy workup, and the use of water as a solvent that is considered to be relatively environmentally benign.
4 Experimental
4.1 Chemicals and instruments
All organic materials were purchased commercially from Sigma-Aldrich and Merck and were used without further purification. Ce(NO3)3·6H2O (99.999%) was obtained from Sigma-Aldrich.
All melting points are uncorrected and were determined in capillary tubes on a Boetius melting point microscope. FT-IR spectra were recorded with KBr pellets using a Magna-IR 550 Nicolet spectrometer. NMR spectra were recorded on a Bruker 400 MHz spectrometer with tetramethylsilane as an internal standard. Powder XRD was carried out on a Philips X’pert diffractometer with monochromatized CuKα radiation (λ=1.5406 Å). The microscopic morphology of products was visualized by SEM (MIRA 3 TESCAN).
4.2 Preparation of CeO2 nanoparticles
CeO2 NPs were prepared according to the procedure reported in the literature [22] with some modification by a co-precipitation technique with post-annealing in air. Briefly, 3 g of highly pure Ce(NO3)3·6H2O was dissolved in the mixture of 50 mL deionized water and 20 mL alcohol. Then, the appropriate amount of aqueous ammonia solution (28 wt%) was added to the above solution till the pH value reached 8. Thereafter, the mixture was stirred for 4 h at room temperature and then dried at 80°C for 6 h. The solid was treated at 700°C for 2 h to obtain the CeO2 NPs. The pattern agrees well with the reported pattern for CeO2 NPs (JCPDS No. 43-1002). The crystalline size was calculated from FWHM using Scherrer’s formula and resulted in an average diameter of 11 nm.
4.3 General procedure for the preparation of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylate derivatives (5a–i)
A mixture of malononitrile (1 mmol), and aldehydes (1 mmol), hydrazine hydrate (1 mmol), dimethyl acetylenedicarboxylate (1 mmol), and 6 mol% CeO2 NPs in H2O (4 mL) was stirred at room temperature for the specific time. The reaction was monitored by thin-layer chromatography. After cooling, the precipitated solid was filtered and washed with H2O. The product was dissolved in hot methanol and the catalyst was filtered. After cooling, the crude products were precipitated. The precipitate was washed with methanol to afford the pure product and dried well in vacuo. The structures of the products were fully established on the basis of their 1H NMR, 13C NMR, and FT-IR spectra.
4.4 Physical and spectroscopic data
4.4.1 Methyl 6-amino-5-cyano-2,4-dihydro-4- (4-nitrophenyl)pyrano[2,3-c]pyrazole-3-carboxylate (5a)
Colorless crystals; m.p. 242–243°C. – IR (KBr): ν=3385, 3324, 3265, 2955, 2201, 1488, 1390, 1027 cm−1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm)=3.63 (3H, s, OMe), 4.69 (1H, s, CH), 7.18–7.25 (4H, m, H-Ar), 7.49 (2H, NH2), 13.69 (1H, s, NH). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm)=37.88 (CH), 52.22 (OMe), 58.46 (C), 104.87 (C), 120.84 (CN), 127.69 (2CH), 127.91 (C), 129.27 (2CH), 136.16 (C), 145.47 (C), 155.82 (C), 158.88 (C), 160.65 (C=O). – Analysis for C15H11N5O5: calcd. C 52.79, H 3.25, N 20.52; found C 52.83, H 3.21, N 20.61.
4.4.2 Methyl 6-amino-5-cyano-4-(2-fluorophenyl)-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylate (5b)
Colorless crystals; m.p. 248–250°C. – IR (KBr): ν = 3427, 3299, 3187, 2956, 2193, 1723, 1637, 1601, 1489, 1400, 1044 cm−1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm) = 3.59 (3H, s, OMe), 5.00 (1H, s, CH), 7.08–7.24 (4H, m, CH), 7.23 (2H, NH2), 13.76 (1H, s, NH). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 31.66 (CH), 52.19 (OMe), 56.61 (C), 103.45 (C), 115.65 (CH), 115.87 (CN), 120.57 (CH), 124.82 (CH), 129.19 (CH), 129.27 (C), 130.50 (C), 131.72 (C), 158.79 (C), 159.14 (C), 160.97 (C=O). – Analysis for C15H11FN4O3: calcd. C 57.33, H 3.53, N 17.83; found C 57.27, H 3.46, N 17.76.
4.4.3 Methyl 6-amino-5-cyano-4-(4-methoxylphenyl)-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylate (5c)
Colorless crystals; m.p. 238–240°C. – IR (KBr): ν = 3428, 3323, 3214, 2924, 2194, 1728, 1639, 1602, 1475, 1392, 1110 cm−1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm) = 3.64 (3H, s, OMe), 3.69 (3H, s, OMe), 4.67 (1H, s, CH), 6.81 (2H, NH2), 7.00–7.20 (4H, m, CH), 13.70 (1H, s, NH). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 36.70 (CH), 52.21 (OCH3 ester), 55.41 (OMe), 58.59 (C), 105.05 (C), 114.02 (CN), 120.84 (2CH), 128.87 (2CH), 129.14 (C), 137.53 (C), 155.78 (C), 158.36 (C), 158.90 (C), 160.53 (C=O). – Analysis for C16H14N4O4: calcd. C 58.89, H 4.32, N 17.17; found C 58.80, H 4.28, N, 17.26.
4.4.4 Methyl 6-amino-5-cyano-2,4-dihydropyrano-4- (p-tolyl)pyrano[2,3-c]pyrazole-3-carboxylate (5d)
Colorless crystals, m.p. 242–244°C. – IR (KBr): ν = 3390, 3325, 3215, 2952, 2194, 1724, 1645, 1605, 1480, 1393, 1393, 1049 cm−1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm) = 2.22 (3H, s, Me), 3.63 (3H, s, OMe), 4.67 (1H, s, CH), 6.99–7.15 (4H, m, CH, 2H, NH2), 13.69 (1H, s, NH). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 21.07 (CH3), 37.08 (CH), 52.21 (OCH3), 58.40 (C), 104.87 (C), 120.79 (CN), 127.69 (2CH), 129.27 (2CH), 136.16 (C), 142.47 (C), 155.82 (C), 158.88 (C), 160.60 (C=O). – Analysis for C16H14N4O3: calcd. C 61.93, H 4.55, N 18.06; found C 61.96, H 4.62, N 18.11.
4.4.5 Methyl 6-amino-5-cyano-2,4-dihydro-4- (3-nitrophenyl)pyrano[2,3-c]pyrazole-3-carboxylate (5e)
Colorless crystals, m.p. 234–235°C. – IR (KBr): ν = 3399, 3326, 3265, 2955, 2201, 1488 cm−1. – 1H NMR (400 MHz, [D6]DMSO) : δ (ppm) = 3.68 (3H, s, OMe), 4.71 (1H, s, CH), 7.20–7.27 (4H, m, H-Ar), 7.51 (2H, NH2), 13.70 (1H, s, NH). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 37.91 (CH), 52.30 (OMe), 58.47 (C), 104.89 (C), 120.88 (CN), 127.69 (CH), 127.91 (C), 128.8 (CH), 129.31 (CH), 131.12 (CH), 136.26 (C), 145.52 (C), 155.90 (C), 158.91 (C), 160.79 (C=O). – Analysis for C15H11N5O5: calcd. C 52.79, H 3.25, N 20.52; found C 52.70, H 3.18, N 20.61.
4.4.6 Methyl 6-amino-5-cyano-4-phenyl-2,4-dihydropyrano[2,3-c]pyrazole-3- carboxylate (5f)
Colorless crystals; m.p. 231–232°C. – IR (KBr): ν = 3389, 3329, 3268, 2960, 2209, 1729, 1654, 1613 cm−1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm) = 3.59 (3H, s, OMe), 4.690 (1H, s, CH), 7.05–7.28 (5H, m, CH), 7.24 (2H, NH2), 13.74 (1H, s, NH). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 37.45 (CH), 52.16 (OCH3), 58.17 (C), 104.65 (C), 120.73 (CN), 127.10 (CH), 127.79 (CH), 128.68 (CH), 129.18 (C), 145.36 (C), 155.87 (C), 158.84 (C), 160.61 (C=O). – Analysis for C15H12N4O3: calcd. C 60.81, H 4.08, N 18.91; found C 60.73, H 4.16, N 18.96.
4.4.7 Methyl 6-amino-5-cyano-2,4-dihydro-4- (2,3-dimethoxyphenyl)pyrano[2,3-c]pyrazole-3-carboxylate (5g)
Colorless crystals; m.p. 239–241°C. – IR (KBr): ν = 3428, 3290, 3160, 2938, 2188, 1733, 1640, 1605, 1485, 1395, 1050 cm−1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm) = 3.53 (3H, s, OMe), 3.59 (3H, s, OMe), 3.75 (3H, s, OMe), 4.90 (1H, s, CH), 6.58–6.93 (5H, m, H-Ar and NH2), 13.58 (1H, s, NH). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 33.27 (CH), 52.10 (CH3 ester), 55.95 (OMe), 57.36 (OMe), 60.20 (C), 104.73 (C), 111.92 (CN), 121.05 (C), 121.74 (CH), 123.74 (CH), 128.94 (CH), 137.74 (C), 146.88 (C), 152.75 (C), 156.37 (C), 158.95 (C), 161.03 (C=O). – Analysis for C17H16N4O5: calcd. C 57.30, H 4.53, N 15.72; found C 57.42, H 4.42, N 15.79.
4.4.8 Methyl 6-amino-5-cyano-2,4-dihydro-4- (2-methoxyphenyl)pyrano[2,3-c]pyrazole-3-carboxylate (5h)
Colorless crystals; m.p. 210–212°C. – IR (KBr): ν = 3412, 3323, 3269, 2939, 2196, 1719, 1651, 1610, 1488, 1390, 1027 cm−1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm) = 3.57 (3H, s, OMe), 3.67 (3H, s, OMe), 4.99 (1H, s, CH), 6.89–7.17 (4H, m, H-Ar), 7.02 (2H, NH2), 13.76 (1H, s, NH). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 37.31 (CH), 52.25 (CH3 ester), 55.37 (OMe), 57.94 (C), 104.51 (C), 111.99 (CN), 113.90 (CH), 119.93 (CH), 120.75 (CH), 125.98 (C), 129.09 (CH), 129.84.71 (C), 146.96 (C), 158.85 (C), 159.44 (C), 160.70 (C=O). – Analysis for C16H14N4O4: calcd. C 58.89, H 4.32, N 17.17; found: C 58.95, H 4.27, N 17.26.
4.4.9 Methyl 6-amino-4-(4-bromophenyl)-5-cyano-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylate (5i)
Colorless crystals; m.p. 245–247°C. – IR (KBr): ν = 3405, 3329, 3265, 2966, 2209, 1729, 1654, 1616 cm−1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm) = 3.63 (3H, s, OMe), 4.69 (1H, s, CH), 7.20–7.28 (4H, m, H-Ar), 7.50 (2H, NH2), 13.68 (1H, s, NH). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 37.46 (CH), 52.19 (OCH3), 58.19 (C), 104.66 (C), 118.73 (CN), 127.14 (CH), 127.79 (C), 128.69 (CH), 129.19 (C), 145.37 (C), 155.89 (C), 158.85 (C), 160.61 (C=O). – Analysis for C15H11BrN4O3: calcd. C 48.02, H 2.96, N 14.93; found C 48.11, H 2.90, N 14.89.
Acknowledgment
The authors are grateful to University of Kashan for supporting this work by Grant No.: 463562/VI.
References
[1] N. R. Mohamed, N. Y. Khaireldin, A. F. Fahmy, A. A. El-Sayed, Der Pharma Chem. 2010, 2, 400.Suche in Google Scholar
[2] S. C. Kuo, L. J. Huang, H. Nakamura, J. Med. Chem. 1984, 27, 539.10.1021/jm00370a020Suche in Google Scholar
[3] N. Foloppe, L. M. Fisher, R. Howes, A. Potter, A. G. S. Robertson, A. E. Surgenor, Bioorg. Med. Chem. 2006, 14, 4792.10.1016/j.bmc.2006.03.021Suche in Google Scholar
[4] F. M. Abdelrazek, P. Metz, N. H. Metwally, S. F. El-Mahrouky, Arch. Pharm. 2006, 339, 456.10.1002/ardp.200600057Suche in Google Scholar
[5] L. G. Sharanina, V. K. Promonenkov, V. P. Marshtupa, A. V. Pashchenko, V. V. Puzanova, Yu. A. Sharanin, N. A. Klyuev, L. F. Gusev, A. P. Gnatusina, Chem. Heterocycl. Compd. 1982, 18, 607.10.1007/BF00506154Suche in Google Scholar
[6] J. Safaei-Ghomi, B. Khojastehbakht-Koopaei, H. Shahbazi-Alavi, RSC Adv. 2014, 4, 4610.10.1039/c4ra00853gSuche in Google Scholar
[7] J. Safaei-Ghomi, S. Paymard-Samani, S. Zahedi, H. Shahbazi-Alavi, Z. Naturforsch. 2015, 70b, 819.10.1515/znb-2015-0070Suche in Google Scholar
[8] T. Montini, M. Melchionna, M. Monai, P. Fornasiero, Chem. Rev. 2016, 116, 5987.10.1021/acs.chemrev.5b00603Suche in Google Scholar
[9] S. Tsunekawa, R. Sahara, Y. Kawazoe, A. Kasuya, Mater. Trans. 2000, 41, 1104.10.2320/matertrans1989.41.1104Suche in Google Scholar
[10] Y. Liu, Z. Lockman, A. Aziz, D. J. Macmanus, J. Phys. Condens. Matter. 2008, 20, 165201.10.1088/0953-8984/20/16/165201Suche in Google Scholar
[11] A. Trovarelli, C. D. Leitenburg, M. Boaro, G. Dolcetti, Catal. Today1999, 50, 353.10.1016/S0920-5861(98)00515-XSuche in Google Scholar
[12] C. Laberty-Robert, J. Long, E. M. Lucas, Chem. Mater. 2006, 18, 50.10.1021/cm051385tSuche in Google Scholar
[13] M. Hirano, Y. Fukuda, H. Iwata, Y. Hotta, M. Inagaki, J. Am. Ceram. Soc. 2000, 83, 1287.10.1111/j.1151-2916.2000.tb01371.xSuche in Google Scholar
[14] P. L. Chen, I. W. Chen, J. Am. Ceram. Soc. 1993, 76, 1577.10.1111/j.1151-2916.1993.tb03942.xSuche in Google Scholar
[15] H. Mecadon, M. R. Rohman, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 2011, 52, 2523.10.1016/j.tetlet.2011.03.036Suche in Google Scholar
[16] G. Vasuki, K. Kumaravel, Tetrahedron Lett. 2008, 49, 5636.10.1016/j.tetlet.2008.07.055Suche in Google Scholar
[17] A. Siddekha, A. Nizam, M. A. Pasha, Spectrochim. Acta Part A2011, 81, 431.10.1016/j.saa.2011.06.033Suche in Google Scholar PubMed
[18] M. B. Madhusudana Reddy, V. P. Jayashankara, M. A. Pasha, Synth. Commun. 2010, 40, 2930.10.1080/00397910903340686Suche in Google Scholar
[19] H. Mecadon, M. R. Rohman, I. Kharbangar, B. M. Laloo, I. Kharkongor, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 2011, 52, 3228.10.1016/j.tetlet.2011.04.048Suche in Google Scholar
[20] H. M. Al-Matar, K. D. Khalil, A. Y. Adam, M. H. Elnagdi, Molecules2010, 15, 6619.10.3390/molecules15096619Suche in Google Scholar PubMed PubMed Central
[21] A. M. Zonouz, I. Eskandari, H. R. Khavasi, Tetrahedron Lett. 2012, 53, 5519.10.1016/j.tetlet.2012.08.010Suche in Google Scholar
[22] M. Li, R. Zhang, H. Zhang, W. Feng, X. Liu, Micro Nano Lett. 2010, 5, 95.10.1049/mnl.2009.0092Suche in Google Scholar
©2016 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Li2Pt3Se4: a new lithium platinum selenide with jaguéite-type crystal structure by multianvil high-pressure/high-temperature synthesis
- RE4B4O11F2 (RE = Sm, Tb, Ho, Er): four new rare earth fluoride borates isotypic to Gd4B4O11F2
- Regioselective C-3 arylation of coumarins with arylhydrazines via radical oxidation by potassium permanganate
- Two new POM-based compounds containing a linear tri-nuclear copper(II) cluster and an infinite copper(II) chain, respectively
- Environmentally benign synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates using CeO2 nanoparticles as a reusable and robust catalyst
- Synthesis and structural characterization of Ca12Ge17B8O58
- Synthesis of some new hydrazide-hydrazones related to isatin and its Mannich and Schiff bases
- Purpureone, an antileishmanial ergochrome from the endophytic fungus Purpureocillium lilacinum
- Syntheses and crystal structures of two new silver–organic frameworks based on N-pyrazinesulfonyl-glycine: weak Ag···O/N interaction affecting the coordination geometry
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Li2Pt3Se4: a new lithium platinum selenide with jaguéite-type crystal structure by multianvil high-pressure/high-temperature synthesis
- RE4B4O11F2 (RE = Sm, Tb, Ho, Er): four new rare earth fluoride borates isotypic to Gd4B4O11F2
- Regioselective C-3 arylation of coumarins with arylhydrazines via radical oxidation by potassium permanganate
- Two new POM-based compounds containing a linear tri-nuclear copper(II) cluster and an infinite copper(II) chain, respectively
- Environmentally benign synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates using CeO2 nanoparticles as a reusable and robust catalyst
- Synthesis and structural characterization of Ca12Ge17B8O58
- Synthesis of some new hydrazide-hydrazones related to isatin and its Mannich and Schiff bases
- Purpureone, an antileishmanial ergochrome from the endophytic fungus Purpureocillium lilacinum
- Syntheses and crystal structures of two new silver–organic frameworks based on N-pyrazinesulfonyl-glycine: weak Ag···O/N interaction affecting the coordination geometry

