Synthesis and crystal structure of a 3D copper(II)–silver(I) coordination polymer assembled through hydrogen bonding, π–π stacking and metal–π interactions, {[Cu(phen)2(CN)][Ag(CN)2] · 3H2O}n (phen = 1,10-phenanthroline)
-
Sidra Nawaz
and Javed Hussain Shah
Abstract
A copper(II) polymeric complex {[Cu(phen)2(CN)][Ag(CN)2] · 3H2O}n, 1 (phen = 1,10-phenanthroline), has been prepared and structurally characterized. The compound crystallizes in the monoclinic space group P21/c with [Cu(phen)2(CN)]+ and [Ag(CN)2]− units and three water molecules. The cationic and anionic units are linked to each other through M-π and π–π interactions. The array is extended further by hydrogen bonding and π–π interactions to form a 3D network.
1 Introduction
Transition metal cyanides such as [Fe(CN)6]3− [1–6], [Ni(CN)4]2− [7–11], [Au(CN)2]− [12–17], and [Ag(CN)2]− [18–29] are widely used as building blocks for the preparation of supramolecular coordination systems. The rigidity of such frameworks may allow shape and size selective inclusion of organic solvents, water molecules, and aromatic compounds for potential applications in the area of catalysis [1, 12, 29, 30]. Among the cyanidometalates, [Ag(CN)2]− is particularly interesting because the resulting coordination polymers are usually supported by Ag–Ag (argentophilic) interactions [18, 27–29]. The [Ag(CN)2]− anion is labile and may be transformed into other secondary products such as [Ag2(CN)3]− according to the following equilibria [26]:
As a result, a variety of products consisting of [Ag(CN)2]− [18–25], [Ag2(CN)3]− [26], and [Ag3(CN)5]2− anions [27] have been detected in the reactions of [Ag(CN)2]−. In addition, polymers incorporating pentameric units, [{Ag(CN)2}−]5, have also been observed [28]. The Ag–CN system is also of considerable interest because cyanide solutions form the basis for an important method of extracting silver metal from silver ore [31].
We have been investigating the structural properties of M(II)–Ag(I) coordination polymers that contain the [Ag(CN)2]− anion as a bridging unit [18–20]. In the present study, we attempted to prepare a coordination polymer consisting of [Cu(phen)2]2+ and [Ag(CN)2]− ions, but the product isolated was found to be {[Cu(phen)2(CN)][Ag(CN)2] · 3H2O}n, in which the [Ag(CN)2]− anions are bound to the copper complex cations through metal–π and nitrilo–π interactions. The crystal structure is characterized further by significant π–π stacking interactions between aromatic rings and hydrogen bonding to form a 3D network.
2 Experimental section
2.1 Materials and methods
CuCl2 · 2H2O was obtained from BDH Chemical Co. (England). AgNO3 was purchased from Panreac (Spain). Ethylenediamine (en) is a product of Merck Chemical Co. (Germany). K[Ag(CN)2] was prepared by reacting AgNO3 with KCN in a molar ratio of 1:2. The IR spectra were recorded with a Perkin Elmer FTIR spectrophotometer using KBr pellets in the 4000–500 cm−1 range.
2.2 Synthesis
To a solution of (0.170 g, 1 mmol) CuCl2 · 2H2O in 10 mL water was added 0.400 g (2 mmol) of 1,10-phenanthroline (phen) in 15 mL methanol. The solution turned greenish blue. After stirring for 15 min, 2 mmol of K[Ag(CN)2 (0.400 g) in 15 mL distilled water was added and the reaction mixture was stirred for further 30 min at room temperature. After filtration, the filtrate was kept in air for overnight. The blue crystals obtained in the filtrate were washed with methanol and finally dried in air. Yield 20 %, m.p. = 158–160 °C. – IR: ν = 1626, 1583 (C=N), 1519, 1428 (C=C), 2140, 2095 ν(C≡N), 3321 ν(O–H) cm−1.
2.3 X-ray structure determination
X-ray diffraction data of 1 were collected with an Oxford Gemini S diffractometer [MoKα (λ = 0.71073 Å)] at 110 K. The structure was solved by direct methods with Shelxs-2013 [32] and refined by full-matrix least-squares procedures on F2 using the program Shelxl-2013 [32]. All non-hydrogen atoms were refined anisotropically. All C-bonded hydrogen atoms were geometrically placed and refined isotropically in riding modes using default Shelxl-2013 parameters. The positions of O-bonded hydrogen atoms were taken from difference Fourier maps and refined isotropically. Crystal and structural refinement data of 1 are summarized in Table 1.
Crystal data and numbers pertinent to data collection and structural refinement data of 1.
| Formula | C27H22AgCuN7O3 |
| Formula weight | 663.92 |
| Crystal size, mm3 | 0.3 × 0.2 × 0.1 |
| Temperature, K | 110 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a, Å | 14.4057(4) |
| b, Å | 13.2496(3) |
| c, Å | 14.8489(4) |
| β, deg | 112.814(3) |
| V, Å3 | 2612.48(13) |
| Z | 4 |
| ρcalcd, gcm−3 | 1.688 |
| μ (MoKα), mm−1 | 1.608 |
| F(000), e | 1332 |
| 2θ range, deg | 2.977–24.995 |
| h, k, l limits | −11:17, −15:13, −17:17 |
| Max./min. transmission | 1.0/0.91785 |
| Reflections: collected/unique/Rint | 12156/4584/0.0262 |
| Data/restraints/parameters | 4584/9/376 |
| R1/wR2 [I > 2σ(I)] | 0.0273/0.0638 |
| R1/wR2 (all data) | 0.0348/0.0667 |
| Largest diff. peak/hole, e Å−3 | 0.561/−0.401 |
CCDC 1031221 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre viawww.ccdc.cam.ac.uk/data_request/cif.
3 Results and discussion
The title complex was prepared by the reaction of CuCl2 · 2H2O, phen, and K[Ag(CN)2] mixed in a molar ratio of 1:2:2. In the IR spectrum of 1, two ν(CN) bands are observed, one at 2140 cm−1 and the other one at 2095 cm−1, while for K[Ag(CN)2] only one ν(C≡N) appears at 2140 cm−1. The presence of two bands indicates that the complex contains two kinds of cyanide environments. The IR spectrum of phen has the characteristic bands for ν(C=N) at 1618 and 1589 cm−1 and ν(C=C) at 1505 and 1423 cm−1 [18]. For 1 the ν(C=N) vibrations of phen were observed at 1626 and 1583 cm−1, while the ν(C=C) bands appeared at 1519 and 1428 cm−1. The presence of these bands indicates the coordination of phen to the metal. A broad peak at 3321 cm−1 due to the O–H stretch suggests the presence of hydrogen bonds in the complex.
The molecular structure of the asymmetric unit of 1 along with the crystallographic numbering scheme is illustrated in Fig. 1. Selected bond lengths and bond angles are listed in Table 2. The crystal structure of the complex consists of [Cu(phen)2(CN)]+ cations, [Ag(CN)2]− anions, and three water molecules. The Cu(II) ions are coordinated to four nitrogen atoms of phen ligands and one cyanide carbon atom assuming a distorted square pyramidal geometry. The phen ligands are in the square basal plane, and the CN group is present at the apical position of the square pyramid. The [Ag(CN)2]− anions are connected to the complex cation through metal–π and nitrilo–π interactions as illustrated in Fig. 2. The cis N–Cu–N angles vary from 80.10(8)° to 93.01(8)°, while the trans N–Cu–N angles are 100.70(7)° and 169.55(8)°. The cis N–Cu–C angles are greater than the cis N–Cu–N angles due to the strain of the phen ring. The Cu–C≡N unit is nearly linear with a bond angle of 178.7(2)°. The coordination environment of non-coordinated [Ag(CN)2]− ions is known to be linear. The coordination environment of the [Ag(CN)2]− units is also close to linear with Ag–C≡N angles at 177.0(2)° and 179.3(3)°. The Cu–N bond lengths are with 2.016(3)–2.137(2) Å comparable to those in known structures of copper(II) complexes of phen [33–37].
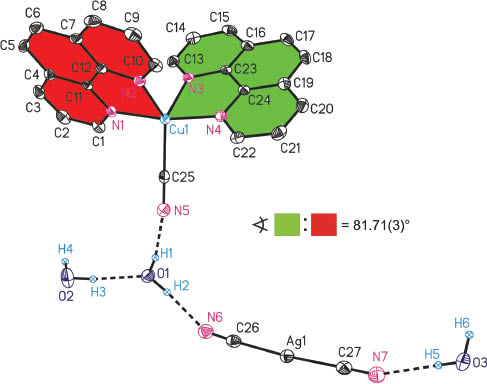
Ortep diagram (50 % probability ellipsoids) of the molecular structure of 1. All carbon-bonded hydrogen atoms are omitted for clarity. Dotted lines indicate hydrogen bonds. The sign 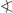 refers to the calculated interplanar angle between mean planes built up by atoms of differently colored areas.
refers to the calculated interplanar angle between mean planes built up by atoms of differently colored areas.
Selected bond lengths (Å) and bond angles (deg) for compound 1.
| Bond distances | Bond angles | ||
|---|---|---|---|
| Cu(1)–C(25) | 1.966(3) | N(1)–Cu(1)–N(2) | 80.10(8) |
| Cu(1)–N(1) | 2.016(2) | N(1)–Cu(1)–N(3) | 92.76(8) |
| Cu(1)–N(2) | 2.137(2) | N(1)–Cu(1)–N(4) | 169.55(8) |
| Cu(1)–N(3) | 2.097(2) | N(2)–Cu(1)–N(3) | 100.70(7) |
| Cu(1)–N(4) | 2.018(2) | N(2)–Cu(1)–N(4) | 93.01(8) |
| Ag(1)–C(26) | 2.061(3) | N(3)–Cu(1)–N(4) | 80.73(8) |
| Ag(1)–C(27) | 2.062(3) | C(25)–Cu(1)–N(1) | 95.94(9) |
| N(1)–C(1) | 1.328(3) | C(25)–Cu(1)–N(2) | 125.97(9) |
| N(1)–C(11) | 1.359(3) | C(25)–Cu(1)–N(3) | 133.32(9) |
| N(3)–C(13) | 1.320(3) | C(25)–Cu(1)–N(4) | 94.47(9) |
| N(3)–C(23) | 1.362(3) | Cu(1)–C(25)–N(5) | 178.7(2) |
| N(5)–C(25) | 1.140(3) | C(26)–Ag(1)–C(27) | 176.05(11) |
| N(6)–C(26) | 1.137(4) | N(6)–C(26)–Ag(1) | 177.0(2) |
| N(7)–C(27) | 1.137(4) | N(7)–C(27)–Ag(1) | 179.3(3) |
![Fig. 2: Illustration of interactions of the [Ag(CN)2]− fragment of 1 with [Cu(phen)2(CN)]+ ions by means of metal–π and nitrilo–π interactions. All carbon-bonded hydrogen atoms are omitted for clarity. Bond distances between interacting atoms: Ag1A–C10B = 3.28 Å, Ag1A–C19 = 3.27 Å, C24–C27A = 3.31 Å, C23–C27A = 3.30 Å, C27A–C24B = 3.51 Å, C19B–C27A = 3.50 Å, N3–N7A = 3.27 Å, C23–N7A = 3.28 Å, N7A–C19B = 3.32 Å. Labels A and B refer to fragments of asymmetric units with the following symmetry codes: A = x, 1.5 − y, z + 1.5; B = 1 − x, y+ ½, 2.5 − z.](/document/doi/10.1515/znb-2014-0264/asset/graphic/j_znb-2014-0264_fig_002.jpg)
Illustration of interactions of the [Ag(CN)2]− fragment of 1 with [Cu(phen)2(CN)]+ ions by means of metal–π and nitrilo–π interactions. All carbon-bonded hydrogen atoms are omitted for clarity. Bond distances between interacting atoms: Ag1A–C10B = 3.28 Å, Ag1A–C19 = 3.27 Å, C24–C27A = 3.31 Å, C23–C27A = 3.30 Å, C27A–C24B = 3.51 Å, C19B–C27A = 3.50 Å, N3–N7A = 3.27 Å, C23–N7A = 3.28 Å, N7A–C19B = 3.32 Å. Labels A and B refer to fragments of asymmetric units with the following symmetry codes: A = x, 1.5 − y, z + 1.5; B = 1 − x, y+ ½, 2.5 − z.
In the solid state, the compound 1 forms a 3D network which comprises both intermolecular hydrogen bonds and different types of π interactions. Thereby, all hydrogen atoms of the three crystallographically independent H2O molecules, cf. Figure 1, are involved in hydrogen bonds to cyano ligands coordinated to the Cu1 or Ag1 atoms. Selected data of the hydrogen bonds are given in Table 3. A view on a selected cut-off of the 3D network of 1 is shown in Fig. 2, displaying the metal–π and nitrilo–π interactions. As this illustration cannot show individual interactions, Figs. 2–4 illustrate the π–π interactions in more detail, whereby the same labeling code has been applied.
Selected bond lengths (Å) and angles (deg) of hydrogen bonds of 1.a
| Donor–H…Acceptor | D…A | ∠ D–H…A |
|---|---|---|
| O1–H1…N5 | 2.799(3) | 167(4) |
| O1–H2…N6 | 2.844(3) | 175(4) |
| O2–H3…O1 | 2.817(3) | 173(3) |
| O2–H4…O1i | 2.843(3) | 177(2) |
| O3–H5…N7 | 2.903(4) | 165(3) |
| O3–H6…O2ii | 2.834(3) | 167(2) |
aSymmetry codes: i = −x, 2 − y, −z; ii = 1 + x, y, z.
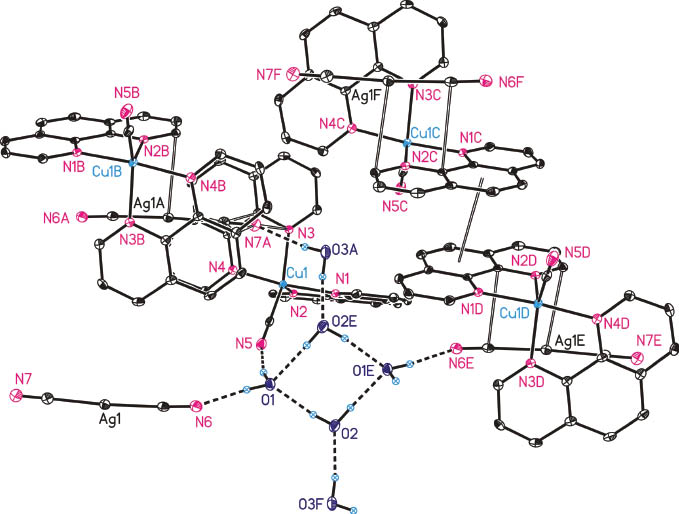
Selected part of the 3D network formed by 1 in the solid state. All carbon bonded hydrogen atoms are omitted for clarity. Dotted lines indicate intermolecular hydrogen bonds and open bonds indicate principal π interactions. Labels A to F refer to selected fragments with the following symmetry operations: A = 1 − x, 1 − y, 3 − z; B = 1 − x, y+ ½, 2.5 − z; C = x, ½ − y, z − ½; D = 2 − x, y+ ½, 2.5 − z; E = 2 − x, 1 − y, 3 − z; F = x, y, z + 1.
![Fig. 4: Illustration of the interactions of [Cu(phen)2(CN)]+ of 1 with each other by means of π–π stacking of two C6H2 aromatic rings in two different perspective views. All carbon-bonded hydrogen atoms are omitted for clarity. Label A refers to a symmetry-generated molecule of [Cu(phen)2(CN)]+, symmetry code: A = 2 − x, 1 − y, 2 − z. The sign refers to the interplanar angle between interacting C6H2 aromatic rings and D refers to the distance of the geometrical centroids of the atoms C4–C7, C11, C12 and C4A–C7A, C11A, C12A, respectively.](/document/doi/10.1515/znb-2014-0264/asset/graphic/j_znb-2014-0264_fig_004.jpg)
Illustration of the interactions of [Cu(phen)2(CN)]+ of 1 with each other by means of π–π stacking of two C6H2 aromatic rings in two different perspective views. All carbon-bonded hydrogen atoms are omitted for clarity. Label A refers to a symmetry-generated molecule of [Cu(phen)2(CN)]+, symmetry code: A = 2 − x, 1 − y, 2 − z. The sign 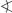 refers to the interplanar angle between interacting C6H2 aromatic rings and D refers to the distance of the geometrical centroids of the atoms C4–C7, C11, C12 and C4A–C7A, C11A, C12A, respectively.
refers to the interplanar angle between interacting C6H2 aromatic rings and D refers to the distance of the geometrical centroids of the atoms C4–C7, C11, C12 and C4A–C7A, C11A, C12A, respectively.
The present study describes the crystal structure of a unique example of polymeric copper(II) complexes, namely {[Cu(phen)2(CN)][Ag(CN)2] · 3H2O}n (1). The complex forms in the solid state a 3D network through hydrogen bonding and different types of π interactions.
Acknowledgments
Financial support from Pakistan Council for Science and Technology, Islamabad, is gratefully acknowledged.
References
[1] R. Lescouezec, L. M. Toma, J. Vaissermann, M. Verdaguer, F. S. Delgado, C. Ruiz-Perez, F. Lloret, M. Julve, Coord. Chem. Rev.2005, 249, 2691.10.1016/j.ccr.2005.09.017Search in Google Scholar
[2] H. Miyasaka, H. Takahashi, T. Madanbashi, K. Sugiura, R. Clerac, H. Nojiri, Inorg. Chem.2005, 44, 5969.10.1021/ic0505753Search in Google Scholar
[3] M. Nayak, P. Kundu, P. Lemoine, R. Koner, H. H. Wei, S. Mohanta, Polyhedron2006, 25, 2007.10.1016/j.poly.2005.12.024Search in Google Scholar
[4] A. Rodriguez-Dieguez, R. Kivekaes, R. Sillanpaeae, J. Cano, F. Lloret, V. McKee, H. Stoeckli-Evans, E. Colacio, Inorg. Chem.2006, 45, 10537.Search in Google Scholar
[5] H.-X. Zhang, Y.-X. Tong, Z.-N. Chen, K.-B. Yu, B.-S. Kang, J. Organomet. Chem.2000, 598, 63.10.1016/S0022-328X(99)00679-8Search in Google Scholar
[6] E. Colacio, M. Ghazi, H. Stoeckli-Evans, F. Lloret, J. M. Moreno, C. Perez, Inorg. Chem.2001, 40, 4876.10.1021/ic0103446Search in Google Scholar
[7] E. Colacio, F. Lloret, M. Navarrete, A. Romerosa, H. Stoeckli-Evans, J. Suarez-Varela, New J. Chem.2005, 29, 1189.Search in Google Scholar
[8] D. Ghoshal, A. K. Ghosh, T. K. Maji, J. Ribas, G. Mostafa, E. Zangrando, N. R. Chaudhuri, Inorg. Chim. Acta2006, 359, 593.10.1016/j.ica.2005.09.056Search in Google Scholar
[9] T. Akitsu, Y. Einaga, Inorg. Chim. Acta2008, 361, 36.10.1016/j.ica.2007.06.034Search in Google Scholar
[10] J. Paharova, J. Cernak, R. Boca, Z. Zak, Inorg. Chim. Acta2003, 346, 25.10.1016/S0020-1693(02)01388-9Search in Google Scholar
[11] A. Karadag, H. Pasaoglu, G. Kastas, O. Buyukgungor, Acta Crystallogr. 2004, C60, m581.10.1107/S0108270104021493Search in Google Scholar
[12] A. Deak, T. Tunyogi, C. Jobbágy, Z. Károly, P. Baranyai, G. Palinkas, Gold Bull.2012, 45, 35.10.1007/s13404-012-0041-1Search in Google Scholar
[13] D. B. Leznoff, B. Y. Xue, R. J. Batchelor, F. W. B. Einstein, B. O. Patrick, Inorg. Chem. 2001, 40, 6026.10.1021/ic010756eSearch in Google Scholar
[14] D. B. Leznoff, B. Y. Xue, C. L. Stevens, A. Storr, R. C. Thompson, B. O. Patrick, Polyhedron2001, 20, 1247.10.1016/S0277-5387(01)00601-5Search in Google Scholar
[15] D. B. Leznoff, B. Y. Xue, B. O. Patrick, V. Sanchez, R. C. Thompson, Chem. Commun.2001, 259.10.1039/b007342nSearch in Google Scholar
[16] E. Colacio, F. Lloret, R. Kivekas, J. S. Varela, M. R. Sundberg, R. Uggla, Inorg. Chem. 2003, 42, 560.10.1021/ic025949wSearch in Google Scholar
[17] M. J. Katz, V. K. Michaelis, P. M. Aguiar, R. Yson, H. Lu, H. Kaluarachchi, R. J. Batchelor, G. Schreckenbach, S. Kroeker, H. H. Patterson, D. B. Leznoff, Inorg. Chem.2008, 47, 6353.10.1021/ic800425fSearch in Google Scholar
[18] M. Monim-ul-Mehboob, M. Ramzan, T. Rüffer, H. Lang, S. Nadeem, M. Akhtar, S. Ahmad, Z. Naturforsch.2013, 68b, 161.Search in Google Scholar
[19] S. Ahmad, M. N. Tahir, H. M. Javaid, M. Monim-ul-Mehboob, M. A. Shaheen, R. Mahmood, J. Chem. Crystallogr.2012, 42, 401.10.1007/s10870-011-0261-xSearch in Google Scholar
[20] S. Ahmad, M. M. Mehboob, M. Altaf, H. Stoeckli-Evans, R. Mehmood, J. Chem. Crystallogr.2007, 37, 685.10.1007/s10870-007-9232-7Search in Google Scholar
[21] Y.-P. Ren, L.-S. Long, R.-B. Huang, L.-S. Zheng, Appl. Organomet. Chem.2005, 19, 1071.10.1002/aoc.735Search in Google Scholar
[22] C. Kappenstein, A. Ouali, M. Guerin, J. Cernak, J. Chomic, Inorg. Chim. Acta1988, 147, 189.10.1016/S0020-1693(00)83370-8Search in Google Scholar
[23] J. Cernak, J. Chomic, P. Gravereau, A. Orendacova, M. Orendac, J. Kovac, A. Feher, C. Kappenstein, Inorg. Chim. Acta1998, 281, 134.10.1016/S0020-1693(98)00156-XSearch in Google Scholar
[24] J. Cernak, K. A. Abboud, J. Chomic, M. W. Meisel, M. Orendac, A. Orendacova, A. Feher, Inorg. Chim. Acta2000, 311, 126.Search in Google Scholar
[25] H. X. Zhang, B. S. Kang, L. R. Deng, C. Ren, C. Y. Su, Z. N. Chen, Inorg. Chem. Commun.2001, 4, 41.10.1016/S1387-7003(00)00191-XSearch in Google Scholar
[26] C. J. Shorrock, B. Y. Xue, P. B. Kim, R. J. Batchelor, B. O. Patrick, D. B. Leznoff, Inorg. Chem.2002, 41, 6743.10.1021/ic025850pSearch in Google Scholar
[27] H. X. Zhang, Z. N. Chen, C. Y. Sue, C. Ren, B. S. Kang, J. Chem. Crystallogr.1999, 29, 1239.10.1023/A:1009548705677Search in Google Scholar
[28] H. Zhang, Y. Zhang, C. Wang, L. Cai, Y. Xie, G. Xue, Inorg. Chem. Commun.2006, 9, 555.10.1016/j.inoche.2006.01.026Search in Google Scholar
[29] H. Schmidbaur, A. Schier, Angew. Chem. Int. Ed.2015, 54, 746.10.1002/anie.201405936Search in Google Scholar
[30] T. Iwamoto in Comprehensive Supramolecular Chemistry (Eds.: D. D. MacNicol, F. Toda, R. Bishop), Pergamon Press, Oxford, 1996, chapter 19.Search in Google Scholar
[31] R. J. Lancashire, in Comprehensive Coordination Chemistry, Vol. 5 (Eds.: G. Wilkenson, R. D. Gillard, J. A. McCleverty), Pergamon, Oxford, 1987, p. 775.Search in Google Scholar
[32] G. M. Sheldrick, Acta Crystallogr.2008, A64, 112.10.1107/S0108767307043930Search in Google Scholar
[33] S. Youngme, A. Cheansirisomboon, C. Danvirutai, C. Pakawatchai, N. Chaichit, Inorg. Chem. Commun.2008, 11, 57.10.1016/j.inoche.2007.10.012Search in Google Scholar
[34] R. Carballo, B. Covelo, M. Vazquez-Lopez, A. Castineiras, J. Niclos, Z. Naturforsch.2003, 58b, 151.10.1515/znb-2003-0122Search in Google Scholar
[35] L. Li, D. Liao, Z. Jiang, S. Yan, Polyhedron2000, 19, 2529.Search in Google Scholar
[36] Y. Q. Zheng D. Y. Cheng, J. L. Lin, Z. F. Li, X. W. Wang, Eur. J. Inorg. Chem.2008, 28, 4453.10.1002/ejic.200800309Search in Google Scholar
[37] J. Cernak, F. Gerard, J. Chomic, Acta Crystallogr.1993, C49, 1294.10.1107/S010827019300037XSearch in Google Scholar
©2015 by De Gruyter
Articles in the same Issue
- Frontmatter
- In this Issue
- EPR studies on carboxylic esters, 23 [1]. Preparation of new dialkyl azulenedicarboxylates and EPR-spectroscopic study of their radical anions
- Synthesis and crystal structure of a 3D copper(II)–silver(I) coordination polymer assembled through hydrogen bonding, π–π stacking and metal–π interactions, {[Cu(phen)2(CN)][Ag(CN)2] · 3H2O}n (phen = 1,10-phenanthroline)
- A polyoxometalate-based inorganic–organic hybrid material: synthesis, characterization structure and photocatalytic study
- Hydrogen-bonded assemblies of two organically templated borates: syntheses and crystal structures of [(1,10-phen)(H3BO3)2] and [2-EtpyH][(B5O6(OH)4]
- Catalytic activity of the nanoporous MCM-41 surface for the Paal–Knorr pyrrole cyclocondensation
- A new route for the synthesis of 4-arylacetamido-2-aminothiazoles and their biological evaluation
- Structural and spectroscopic characterization of isotypic sodium, rubidium and cesium acesulfamates
- Nd39Ir10.98In36.02 – A complex intergrowth structure with CsCl- and AlB2-related slabs
- Syntheses, structures and magnetic properties of two mononuclear nickel(II) complexes based on bicarboxylate ligands
- Synthesis and characterization of some new fluoroquinolone-barbiturate hybrid systems
- Synthesis of pyrazoles containing benzofuran and trifluoromethyl moieties as possible anti-inflammatory and analgesic agents
Articles in the same Issue
- Frontmatter
- In this Issue
- EPR studies on carboxylic esters, 23 [1]. Preparation of new dialkyl azulenedicarboxylates and EPR-spectroscopic study of their radical anions
- Synthesis and crystal structure of a 3D copper(II)–silver(I) coordination polymer assembled through hydrogen bonding, π–π stacking and metal–π interactions, {[Cu(phen)2(CN)][Ag(CN)2] · 3H2O}n (phen = 1,10-phenanthroline)
- A polyoxometalate-based inorganic–organic hybrid material: synthesis, characterization structure and photocatalytic study
- Hydrogen-bonded assemblies of two organically templated borates: syntheses and crystal structures of [(1,10-phen)(H3BO3)2] and [2-EtpyH][(B5O6(OH)4]
- Catalytic activity of the nanoporous MCM-41 surface for the Paal–Knorr pyrrole cyclocondensation
- A new route for the synthesis of 4-arylacetamido-2-aminothiazoles and their biological evaluation
- Structural and spectroscopic characterization of isotypic sodium, rubidium and cesium acesulfamates
- Nd39Ir10.98In36.02 – A complex intergrowth structure with CsCl- and AlB2-related slabs
- Syntheses, structures and magnetic properties of two mononuclear nickel(II) complexes based on bicarboxylate ligands
- Synthesis and characterization of some new fluoroquinolone-barbiturate hybrid systems
- Synthesis of pyrazoles containing benzofuran and trifluoromethyl moieties as possible anti-inflammatory and analgesic agents

