Abstract
Functional magnetic resonance imaging (fMRI) stands as a pivotal tool in advancing our comprehension of Schizophrenia, offering insights into functional segregations and integrations. Previous investigations employing either task-based or resting-state fMRI primarily focused on large main regions of interest (ROI), revealing the thalamus and superior temporal gyrus (STG) as prominently affected areas. Recent studies, however, unveiled the cytoarchitectural intricacies within these regions, prompting a more nuanced exploration. In this study, resting-state fMRI was conducted on 72 schizophrenic patients and 74 healthy controls to discern whether distinct thalamic nuclei and STG sub-regions exhibit varied functional integrational connectivity to main networks and to identify the most affected sub-regions in Schizophrenia. Employing seed-based analysis, six sub-ROIs – four in the thalamus and two in the STG – were selected. Our findings unveiled heightened positive functional connectivity in Schizophrenic patients, particularly toward the anterior STG (aSTG) and posterior STG (pSTG). Notably, positive connectivity emerged between the medial division of mediodorsal thalamic nuclei (MDm) and the visual network, while increased functional connectivity linked the ventral lateral nucleus of the thalamus with aSTG. This accentuated functional connectivity potentially influences these sub-regions, contributing to dysfunctions and manifesting symptoms such as language and learning difficulties alongside hallucinations. This study underscores the importance of delineating sub-regional dynamics to enhance our understanding of the nuanced neural alterations in Schizophrenia, paving the way for more targeted interventions and therapeutic approaches.
1 Introduction
Schizophrenia is a complex neuropsychiatric disorder characterized by a constellation of symptoms, including delusions, hallucinations, impaired emotional reactivity, social withdrawal, and cognitive dysfunction. These symptoms significantly impair psychosocial functioning and pose a considerable burden on individuals, their families, and society at large. While the pathogenesis of schizophrenia remains incompletely understood, emerging evidence suggests it is a neurodevelopmental disorder influenced by genetic, developmental, and environmental factors. These factors contribute to structural and functional alterations in the brain’s neural circuitry, particularly affecting thalamic connectivity, which is crucial to the disorder’s symptomatology [1,2].
Dysfunction in thalamic connectivity is increasingly recognized as central to the neural circuit abnormalities observed in schizophrenia [3]. The thalamus, a crucial subcortical structure, plays a vital role in sensory processing, motor functions, and cognitive and emotional processing. It comprises over 60 interconnected nuclei that form extensive circuits with both cortical and subcortical regions [4,5]. Notably, alterations in thalamic volume and connectivity, particularly with the prefrontal cortex, somatosensory regions, cerebellum, and temporal cortex, have been documented in schizophrenia [6,7,8,9,10,11,12,13,14,15,16,17,18]. However, these studies often consider the thalamus as a whole, overlooking the distinct roles and connectivity patterns of its individual nuclei [19]. Similarly, the superior temporal gyrus (STG) is implicated in schizophrenia, especially concerning auditory hallucinations and cognitive deficits [20,21,22,23,24,25,26,27,28]. Yet, research has not adequately differentiated the functions and connectivity of the STG’s cytoarchitectonic subregions.
Our study aims to address these gaps by focusing on the functional connectivity of specific, underexplored thalamic nuclei and STG subregions in individuals with schizophrenia. This approach is grounded in the premise that the unique anatomical and functional characteristics of these subregions may underlie specific aspects of the disorder’s symptomatology. Our selection of the mediodorsal thalamic nuclei (MDm), ventral anterior (VA) nucleus, anterior pulvinar nucleus (PuA), ventral lateral (VL) nucleus of the thalamus, and the anterior and posterior subregions of the STG (aSTG and pSTG) is based on their potential relevance to the diverse symptoms of schizophrenia, as highlighted in prior research and our preliminary findings. For instance, the MDm’s role in the thalamo-prefrontal cortical circuitry is crucial for cognitive control and emotional processing, which are core aspects disrupted in schizophrenia. The MDm’s connectivity with the prefrontal cortex underpins its involvement in executive function and working memory, facets profoundly affected in schizophrenia, as underscored by recent studies [29,30]. The VA and VL nuclei, integral to motor control and connected with the premotor and motor cortices, are relevant to schizophrenia beyond mere anatomical associations, given the disorder’s link with motor dysfunctions. Our examination of VA and VL aims to shed light on their potential contributions to schizophrenia’s motor symptoms, informed by research emphasizing the thalamic regions’ significance in motor functions [31]. The PuA, linked to somatosensory regions, plays a role in sensory integration, which is often compromised in schizophrenia. Including PuA in our study allows us to investigate its involvement in the disorder’s altered sensory processing, as discussed in research on thalamic contributions to sensory and cognitive functions [32]. The aSTG and pSTG are crucial for language and social cognition, domains where deficits are commonly seen in schizophrenia. Exploring these STG subregions can offer insights into the neural underpinnings of schizophrenia-related communication and social interaction challenges, with support from studies highlighting the cortical regions’ importance in cognitive and social functions [33].
The rationale for focusing on these specific thalamic nuclei and STG subregions, despite the broader involvement of various brain regions in schizophrenia, is derived from their documented deviations in schizophrenia patients and their lack of representation in existing studies [34]. Our decision to explore the designated thalamic and STG subfields is based on their pivotal roles in cognitive control, motor function, emotional regulation, and sensory processing – all commonly disrupted in schizophrenia. Thus, our study’s objectives are refined to investigate the resting-state functional connectivity of these selected thalamic nuclei and STG subregions with the entire brain, aiming to elucidate their differential roles and connectivity patterns in schizophrenia. In doing so, we aspire to contribute novel insights into the neural foundations of schizophrenia and identify potential therapeutic targets, leveraging the comprehensive dataset from The Center for Biomedical Research Excellence (COBRE).
2 Materials and methods
2.1 Subject recruitment and scanning
The dataset of this study consists of 72 patients with schizophrenia (60 male, right-handed, age ranged 18–65 years) and 74 healthy controls (51 male, right-handed, age ranged 18–65 years). Patients with a history of neurological disorder, history of mental retardation, history of severe head trauma with more than 5 min loss of consciousness, and a history of substance abuse within the last 12 months were excluded. The data were downloaded from The COBRE, which is a non-restricted public dataset and is available freely. This dataset is a part of the International Neuroimaging Data-Sharing Initiative under the 1000 Functional Connectomes Project. Full Information on this dataset is available at http://cobre.mrn.org/as well as in the following studies [35–38]. COBRE is a publically available dataset distributed with “Creative Commons License: Attribution – Non-Commercial,” and written informed consent was obtained from all subjects in accordance with the institutional review board (IRB) protocols of the University.
All the participants were scanned with a Siemens TIM 3.0 Tesla scanner. A multi-echo MPRAGE (MEMPR) sequence was used with the following parameters: TR/TE/TI = 2,530/[1.64, 3.5, 5.36, 7.22, 9.08]/900 ms, flip angle = 7°, FOV = 256 mm × 256 mm, slab thickness = 176 mm, matrix = 256 × 256 × 176, voxel size = 1 mm × 1 mm × 1 mm, number of echoes = 5, pixel bandwidth = 650 Hz, and total scan time = 6 min. Using five echoes, the TR, TI, and time to encode partitions for the MEMPR are similar to that of a conventional MPRAGE, resulting in similar GM/WM/CSF contrast. Resting-state functional magnetic resonance imaging (rs-fMRI) data were collected with single-shot full k-space echo-planar imaging (EPI) with ramp sampling correction using the intercomissural line (AC-PC) as a reference (TR: 2 s, TE: 29 ms, matrix size: 64 × 64, 32 slices, and voxel size: 3 × 3 × 4 mm3). During the acquisition process, all subjects were instructed to keep their eyes open and stare at the fixation cross.
2.2 rs-fMRI preprocessing
The functional data preprocessing was conducted using the SPM12 and CONN toolbox implemented on MATLAB [39]. Initially, the first few volumes of each functional MRI dataset were discarded to allow for signal stabilization and reduce the impact of initial transient effects. Skull stripping was performed on both functional and structural images to remove non-brain tissues. Motion correction was applied to account for head movements during scanning, and the functional images were realigned and unwrapped.
The T1-weighted structural images were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) regions, providing valuable information for further analyses. The functional images were then normalized to the standard Montreal Neurological Institute (MNI) space using the structural data. Temporal band-pass filtering was applied to retain signals within the desired frequency range while minimizing noise.
Additionally, the global signal was regressed out to further reduce confounding effects. Outlier correction was performed using the Artifact Detection Tools implemented in CONN to detect and correct data outliers. Finally, the images were smoothed using an 8-mm full-width half-maximum (FWHM) isotropic Gaussian kernel. Temporal processing with data denoising was also carried out to neutralize the effects of artifacts and confounding parameters from the BOLD signal in regions of no interest.
2.3 ROI selections
This study was conducted with the aim of investigating the connectivity between selected seed regions and broader brain functional networks, utilizing rs-fMRI as the method of analysis. A total of six seeds, situated bilaterally across the hemispheres, were chosen for this analysis. These seeds comprise the medial division of the medidorsal thalamic nuclei (MDm), the VA nucleus of the thalamus, the PuA of the thalamus, the VL nucleus of the thalamus, the aSTG, and the pSTG. To outline the scope of our investigation, Table 1 details the targeted networks and ROI. These selections were rigorously defined, employing a combination of tools such as cytoarchitectonic probability anatomy maps and the Automated Anatomical Labelling Atlas 3 [40–44], ensuring a precise and scientifically grounded selection process. The rationale behind the seed selection was their clear identification within the atlas.
Demographics of all subjects
| Group | Gender | Count | Mean age (years) | Std age (years) | Min age (years) | Max age (years) | Median age (years) | Right-handed count | Left-handed count | Both-handed count |
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy | Male | 51 | 36.4 | 11.8 | 18 | 65 | 35 | 49 | 1 | 1 |
| Female | 23 | 34.5 | 11.1 | 18 | 58 | 33 | 22 | 0 | 1 | |
| Patients | Male | 58 | 37.5 | 14 | 18 | 64 | 36 | 48 | 8 | 2 |
| Female | 14 | 40.9 | 13.5 | 20 | 65 | 40.5 | 12 | 2 | 0 |
In addition to the seeds, our study also focused on a series of targeted brain regions, encompassing a wide array of functional networks. These were meticulously identified and are documented in Table 2, based on criteria set forth in the CONN toolbox. Among these networks are the default mode, attention, sensorimotor, visual, salience, dorsal attention, frontoparietal, cerebellar, and language networks. The delineation of these networks was obtained using an independent component analysis (ICA) conducted on data from 497 participants in the Human Connectome Project.
Targeted selected networks with the regions that constitute them; the coordinates of these regions are also shown
| Network | Region (sub-region) | MNI coordinates (x, y, z) | Key findings |
|---|---|---|---|
| Default mode | Medial prefrontal cortex (MPFC) | (1, 55, −3) | Increased connectivity with MDm and VA nuclei in schizophrenia patients; disrupted connectivity patterns with sensorimotor networks |
| Lateral parietal (LP) left | (−39, −77, 33) | Higher connectivity with visual networks in schizophrenia patients | |
| LP right | (47, −67, 29), | Similar pattern as left LP with stronger connectivity in schizophrenia | |
| Posterior cingulate cortex (PCC) | (1, −61, 38) | Altered connectivity patterns with both MDm and VA nuclei in schizophrenia patients | |
| Sensorimotor | Lateral left | (−55, −12, 29) | Elevated connectivity with anterior and posterior thalamic nuclei in schizophrenia patients |
| Lateral right | (56, −10, 29) | Similar to left lateral sensorimotor with stronger connectivity in schizophrenia patients | |
| Superior | (0, −31, 67) | Increased connectivity with lateral sensorimotor and visual networks in schizophrenia patients | |
| Visual | Medial | (2, −79, 12) | Altered connectivity in schizophrenia patients compared to controls |
| Occipital | (0, −93, −4) | No significant differences in connectivity between schizophrenia patients and healthy controls | |
| Lateral left | (−37, −79, 10) | Altered connectivity patterns with other visual regions in schizophrenia patients | |
| Lateral right | (38, −72, 13) | Similar pattern as left lateral visual with connectivity alterations | |
| Salience | Anterior cingulate cortex (ACC) | (0, 22, 35) | Reduced connectivity with MDm and VA nuclei in schizophrenia patients |
| Insula left | (−44, 13, 1) | Disrupted connectivity with default mode and visual networks in schizophrenia patients | |
| Insula right | (47, 14, 0) | Similar pattern to left insula with altered connectivity in schizophrenia patients | |
| Rostral lateral prefrontal cortex (RPFC) left | (−32, 45, 27) | Reduced connectivity with thalamic nuclei, particularly in VL and MDm, in schizophrenia patients | |
| RPFC right | (32, 46, 27) | Similar pattern as left RPFC with connectivity deficits in schizophrenia patients | |
| Supramarginal gyrus (SMG) left | (−60, −39, 31) | Connectivity disruptions affecting both sensorimotor and visual networks in schizophrenia patients | |
| SMG right | (62, −35, 32) | Similar disruptions as observed in the left hemisphere | |
| Dorsal attention | Frontal eye fields (FEF) left | (−27, −9, 64) | Reduced connectivity with visual and sensorimotor networks in schizophrenia patients |
| FEF right | (30, −6, 64) | Similar to left FEF, showing reduced connectivity in schizophrenia patients | |
| Intraparietal sulcus (IPS) left | (−39, −43, 52) | Altered connectivity affecting visual-spatial processing in schizophrenia patients | |
| IPS right | (39, −42, 54) | Similar pattern as left IPS with connectivity changes in schizophrenia patients | |
| Fronto-Parietal | Lateral prefrontal cortex (LPFC) left | (−43, 33, 28) | Disrupted connectivity with thalamic nuclei, affecting cognitive functions in schizophrenia patients |
| Posterior parietal cortex (PPC) left | (−46, −58, 49) | Elevated connectivity with visual networks in schizophrenia patients | |
| LPFC right | (41, 38, 30) | Similar pattern as left LPFC with connectivity alterations in schizophrenia patients | |
| PPC right | (52, −52, 45) | Similar disruptions observed in the left hemisphere | |
| Language | Inferior frontal gyrus (IFG) left | (−51, 26, 2) | Increased connectivity with thalamic and cerebellar networks in schizophrenia patients |
| IFG right | (54, 28, 1) | Similar pattern to left IFG with stronger connectivity in schizophrenia patients | |
| pSTG left | (−57, −47, 15) | Elevated connectivity with thalamic nuclei, especially MDm and VL, in schizophrenia patients | |
| pSTG right | (59, −42, 13) | Similar pattern to left pSTG with no significant differences in healthy controls | |
| Cerebellar | Anterior | (0, −63, −30) | Increased connectivity with thalamic and sensorimotor networks in schizophrenia patients |
| Posterior | (0, −79, −32) | Similar pattern as anterior cerebellar network with no significant changes in healthy controls |
2.4 Statistical analysis
Statistical analyses were performed on two distinct levels. Initially, at the individual subject level, the analysis involved the use of weighted bivariate correlation models applying general linear methods, focusing on connectivity matrices that describe interactions between predefined ROIs. These matrices were generated based on the Fisher-transformed bivariate correlation coefficients for ROI pairs’ time-series data. Subsequently, at a broader scale, the analysis was used to assess and contrast the functional connectivity metrics at a collective level. This step aimed to discern and differentiate the rs-fMRI networks associated with various sub-regions across subjects. Results were standardized employing a correction for the false discovery rate (FDR) set at p < 0.05, adhering to multivariate statistics through a parametric analysis approach (MVPA omnibus test) [39,45]. In this context, inferences at the cluster level were made by leveraging multivariate parametric statistical inferences on functional network connectivity, taking into account clusters or networks comprising interconnected ROIs. The process entailed a comprehensive evaluation of all inter-ROI connections, examining both internal and external network connectivity [46]. By applying a multivariate parametric general linear model to all connections, this approach facilitated a detailed analysis. The output is a map depicting F-statistical tests for each network pair. The FDR threshold for clusters signifies the ratio of expected false discoveries among network pairs showing equivalent or more significant effects within the entire array of functional connectivity network pairs [47]. Preferring FDR over the family-wise error rate is advantageous for its enhanced sensitivity in peak detection while minimizing the likelihood of false positives [48]. For further information, refer to the mentioned articles or visit the CONN toolbox website: https://web.conn-toolbox.org.
-
Ethical approval: The research related to human use has complied with all the relevant national regulations and institutional policies in accordance with the tenets of the Helsinki Declaration and has been approved by the authors’ IRB or equivalent committee. This study was approved by the Faculty of Applied Medical Sciences Committee (code 01-2022 – Date of Approval 07-01-2022).
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
3 Results
Functional connectivity between and among the ROIs of the healthy and schizophrenic subjects is shown in Figures 1–6, respectively. The figures show the results as a ring of functional connectivity and matrix connectivity. A summary of direct comparisons between connectivity in patients with schizophrenia and healthy controls is shown in Figures 7–9. A summary of the connected networks for each ROI is given next. A summary of key findings is also shown in Table 3.

The functional connectivity between chosen seeds and target areas demonstrated at the collective level in healthy individuals. Red lines signify positive connections, whereas blue lines represent negative ones. The intensity of the line colors reflects the statistical significance, with the T-bar displayed in the upper right corner.

A connectivity matrix in healthy subjects, featuring the chosen seeds (the first 12 rows) and the targeted neural networks, including statistical metrics of connection strength on the left. Right: Presents another matrix, where the connectivity levels are adjusted by a statistical normalization threshold to clarify distinctions between strong, weak, and negative connections for illustrative purposes.

A matrix of the healthy subjects, where the connectivity levels are adjusted by a statistical normalization threshold to clarify distinctions between strong, weak, and negative connections for illustrative purposes.

The functional connectivity between selected seeds and target regions at a group level in subjects with schizophrenia. Positive connections are marked with red lines, and negative connections are marked with blue lines. The line colors vary according to the statistical significance, and the T-bar is located in the top right-hand corner.

The matrix of the functional connectivity in the schizophrenic subjects of the selected seeds (top 12 rows) and the targeted brain networks is shown along with the statistical indications of the strength of the connection (left).
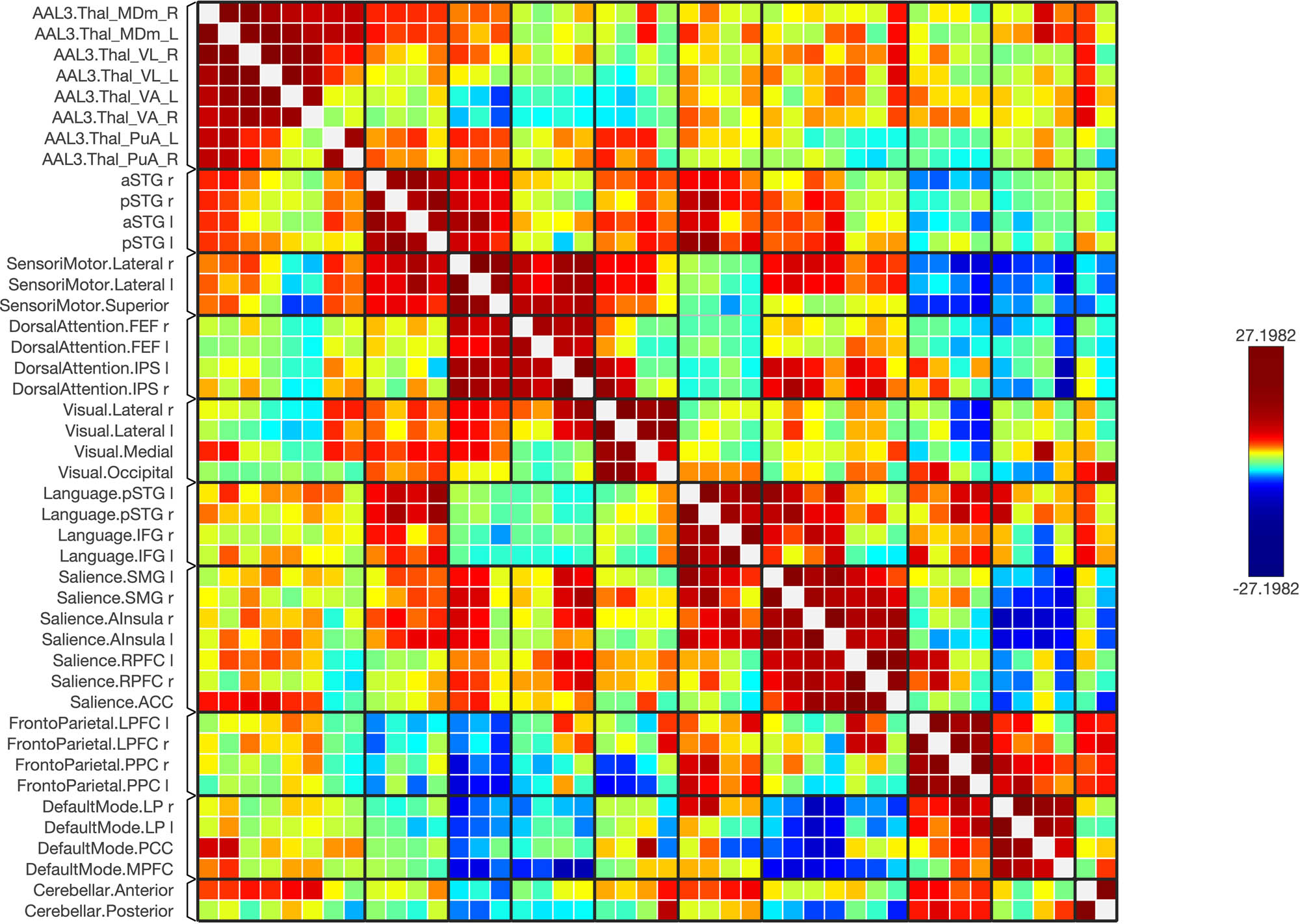
A matrix of the schizophrenic subjects; the matrix of the connectivity was set with a statistical equalizer threshold to easily distinguish strong, weak, and negative connections for illustration.

The functional connectivity of the selected seeds and target regions is shown at the group level in the direct comparison between schizophrenic > control subjects. The lines of the connections in red indicate +ve means SCz > HC, and blue −ve means HC > SCz. The colors of the lines are proportional to statistical strength, and the T-bar is shown in the top right-hand corner.

The matrix of the functional connectivity in the schizophrenic > control subjects of the selected seeds (top 12 rows) and the targeted brain networks is shown along with the statistical indications of the strength of the connection (left). Right: the matrix of the connectivity was set with a statistical equalizer threshold to easily distinguish strong, weak, and negative connections for illustration.

The matrix of the functional connectivity in the schizophrenic > control subjects of the selected seeds (top 12 rows) and the targeted brain networks is shown. Also, the matrix of the connectivity was set with a statistical equalizer threshold to easily distinguish strong, weak, and negative connections for illustration.
Targeted selected networks with the regions that constitute them and key findings
| Network | Regions | Key findings |
|---|---|---|
| Default mode | MPFC | Increased connectivity with MDm and VA nuclei in schizophrenia patients; disrupted connectivity patterns with sensorimotor networks |
| LP (L) | Higher connectivity with visual networks in schizophrenia patients | |
| LP (R) | Similar pattern as the left LP with stronger connectivity in schizophrenia | |
| PCC | Altered connectivity patterns with both MDm and VA nuclei in schizophrenia patients | |
| Sensorimotor | Medial | Increased connectivity with lateral sensorimotor and visual networks in schizophrenia patients |
| Occipital | No significant differences in connectivity between schizophrenia patients and healthy controls | |
| Lateral (L) | Elevated connectivity with anterior and posterior thalamic nuclei in schizophrenia patients | |
| Lateral (R) | Similar to the left lateral sensorimotor with stronger connectivity in schizophrenia patients | |
| Salience | ACC | Reduced connectivity with MDm and VA nuclei in schizophrenia patients |
| Insula (L) | Disrupted connectivity with default mode and visual networks in schizophrenia patients | |
| Insula (R) | Similar pattern to the left insula with altered connectivity in schizophrenia patients | |
| RPFC (L) | Reduced connectivity with thalamic nuclei, particularly in the VL and MDm, in schizophrenia patients | |
| RPFC (R) | Similar pattern as the left RPFC with connectivity deficits in schizophrenia patients | |
| SMG (L) | Connectivity disruptions affecting both sensorimotor and visual networks in schizophrenia patients | |
| SMG (R) | Similar disruptions as observed in the left hemisphere | |
| Dorsal Attention | FEF (L) | Reduced connectivity with visual and sensorimotor networks in schizophrenia patients |
| FEF (R) | Similar to the left FEF, showing reduced connectivity in schizophrenia patients | |
| IPS (L) | Altered connectivity affecting visual-spatial processing in schizophrenia patients | |
| IPS (R) | Similar pattern as the left IPS with connectivity changes in schizophrenia patients | |
| Fronto-parietal | LPFC (L) | Disrupted connectivity with thalamic nuclei, affecting cognitive functions in schizophrenia patients |
| PPC (L) | Elevated connectivity with visual networks in schizophrenia patients | |
| LPFC (R) | Similar pattern as the left LPFC with connectivity alterations in schizophrenia patients | |
| PPC (R) | Similar disruptions observed in the left hemisphere | |
| Language | IFG (L) | Increased connectivity with thalamic and cerebellar networks in schizophrenia patients |
| IFG (R) | Similar pattern to the left IFG with stronger connectivity in schizophrenia patients | |
| pSTG (L) | Elevated connectivity with thalamic nuclei, especially MDm and VL, in schizophrenia patients | |
| pSTG (R) | Similar pattern to the left pSTG with no significant differences in healthy controls | |
| Cerebellar | Anterior | Increased connectivity with thalamic and sensorimotor networks in schizophrenia patients |
| Posterior | Similar pattern as the anterior cerebellar network with no significant changes in healthy controls |
3.1 Connectivity of the medial division of mediodorsal thalamic nuclei (MDm)
Using the right MDm as a seed, functional connectivity to the following regions was higher in schizophrenia patients compared to healthy controls: bilateral aSTG, bilateral pSTG, the right lateral visual networks (LVN), the right medial visual network (MVN), the right dorsal attention network, bilateral sensorimotor and default mode networks (DMN). In contrast, the following regions had lower connectivity with the right MDm in schizophrenia patients than healthy controls: the left MDm, bilateral VL thalamic nuclei, and bilateral VA nuclei. When looking at the left MDm as a seed, functional connectivity was higher to the following regions in schizophrenia patients compared to healthy subjects: bilateral aSTG, bilateral pSTG, the left LVN, the left MVN, and DMN. In contrast, connectivity was lower in schizophrenia patients in comparison to healthy controls in the following regions: the right MDm, bilateral VL nuclei, bilateral VA nuclei, and the visual occipital network.
3.2 Connectivity of the VA thalamic nucleus
When looking at the right VA nucleus as a seed, functional connectivity was higher in schizophrenia patients compared to healthy subjects in the following regions: the left pSTG, bilateral LVNs, and DMNs. On the other hand, connectivity was lower in schizophrenia patients compared to healthy controls in the following regions: bilateral MDm, bilateral VL nucleus, and the left VA nucleus. Using the left VA as a seed, functional connectivity was higher in schizophrenia patients compared to healthy subjects in the following regions: bilateral pSTG, bilateral aSTG, and DMNs. Connectivity was, in contrast, lower in schizophrenia in comparison to healthy subjects to bilateral MDm, bilateral VL nuclei, and the right VA nucleus.
3.3 Connectivity of the PuA
When looking at the left PuA as a seed, functional connectivity was mostly higher in schizophrenia patients compared to healthy subjects. This included the bilateral lateral sensorimotor networks. There is no significantly higher connectivity in healthy subjects compared to schizophrenia patients. The right PuA demonstrates no significant higher connectivity in healthy subjects compared to schizophrenia patients and schizophrenia patients compared to healthy subjects.
3.4 Connectivity of the VL nucleus
When looking at the right VL as a seed, functional connectivity to the following regions was higher in schizophrenia patients than healthy subjects: bilateral pSTG, bilateral aSTG, sensorimotor superior and lateral networks, the right MVN, and right LVN. Lower connectivity was seen in the following regions in schizophrenia patients compared to healthy subjects: the right VL nucleus, bilateral VA nuclei, and bilateral MDm nuclei. Using the left VL as a seed, functional connectivity was higher in the following regions in schizophrenia patients compared to healthy subjects: bilateral pSTG, bilateral aSTG, sensorimotor superior and lateral networks, the MVN, and the LVN. Lower connectivity in schizophrenia patients in comparison to healthy subjects was found in the following regions: the right VL nucleus, bilateral VA nuclei, and bilateral MDm nuclei.
3.5 Connectivity of the aSTG
When looking at the right aSTG as a seed, functional connectivity to the following regions was higher in schizophrenia patients compared to healthy subjects: bilateral MDm nuclei, bilateral VL nuclei, the left VA nucleus, and anterior cerebellar networks. Using the left aSTG as a seed, functional connectivity to the following regions was lower in schizophrenia patients compared to healthy subjects: bilateral MDm nuclei, bilateral VL nuclei, the left VA nucleus, and anterior cerebellar networks.
3.6 Connectivity of the pSTG
When looking at the right pSTG as a seed, functional connectivity to the following regions was higher in schizophrenia patients compared to healthy subjects: bilateral MDm nuclei, bilateral VL nuclei, bilateral VA nuclei, and the anterior cerebellar network. There were no regions with significantly lower connectivity to the right pSTG in schizophrenia patients in comparison to healthy subjects. Using the left pSTG as a seed, functional connectivity to the following regions was higher in schizophrenia patients compared to healthy subjects: bilateral MDm nuclei, bilateral VL nuclei, bilateral VA nuclei, and the anterior cerebellar network. There were no regions with significantly lower connectivity with the pSTG in schizophrenia patients in comparison to healthy subjects.
4 Discussion
This investigation into schizophrenia’s neural underpinnings through fMRI data highlights diminished functional connectivity within select thalamic sub-regions (MDm, VA, PuA, and VL) compared to healthy controls. This reduction in connectivity, previously underexplored, suggests disruptions in thalamocortical loops and informational integration, potentially impacting thalamocortical and cortico-striato-thalamo-cortical circuits’ role in differentiating internal from external perceptions, a key mechanism in reality construction and symptom manifestation in schizophrenia [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66].
Our findings align with theories proposing that altered thalamic connectivity affects brain oscillatory activities, impacting the brain’s ability to modulate these activities based on sensory input. The presence of low-frequency oscillations during wakefulness, typically restricted to slow-wave sleep, supports this, suggesting a link between these neural activities and the under-constrained perception characteristic of schizophrenia’s hallucinatory experiences [67,68,69,70,71,72,73].
Consistent with prior research, our study observed increased connectivity between the thalamus and networks such as the DMN, sensorimotor, visual, and cerebellar networks. This enhanced thalamic engagement with sensorimotor regions, previously associated with schizophrenia’s positive symptoms, underscores the potential role of neural integration disruptions in symptomatology [10,12,19,74,75,76,77,78,79,80]. The DMN’s heightened connectivity, implicated in self-referential thoughts and daydreaming, could relate to schizophrenia’s reality distortion symptoms [81,82,83].
Particularly noteworthy is the increased connectivity between the VL and STG, suggesting potential auditory processing disruptions linked to cognitive difficulties in schizophrenia. This aligns with temporal lobe abnormalities associated with the disorder’s positive symptoms [84].
The study further explores the role of specific thalamic nuclei in schizophrenia’s diverse symptoms, emphasizing the importance of thalamic projections in behaviors ranging from arousal to cognitive functions [85]. The mediodorsal thalamic nuclei (MDm) connections with limbic and prefrontal areas and its role in cognitive control highlight the complex interplay between thalamic dysfunction and schizophrenia’s cognitive and emotional symptoms [19,30,31,77,86,87,88,89,90,91,92,93,94,95,96,97].
Similarly, the STG’s involvement in auditory processing and language, coupled with its hyperconnectivity with the MDm, underscores the potential neural basis of auditory hallucinations and related symptoms in schizophrenia [22,23,65,66]. The STG’s role in social cognition further implicates dysconnectivity in the broader spectrum of schizophrenia’s symptoms, including thought disorder and social dysfunction [98,99].
Our study’s interpretations of rs-fMRI FC patterns, suggesting direct associations between thalamic connectivity and specific brain functions or symptoms in schizophrenia, must be approached with caution. The complexity of brain connectivity, involving potentially multisynaptic pathways rather than direct monosynaptic connections, challenges straightforward correlations between observed FC alterations and clinical manifestations of schizophrenia. For instance, the inferred impact of thalamic sub-regions like the VL nucleus on auditory processing areas such as the STG does not account for the lack of direct anatomical connections, underscoring the speculative nature of such conclusions.
Furthermore, the suggestion that decreased intra-thalamic FC reflects disruptions in thalamocortical loops and information integration may overestimate our findings’ specificity. Given the absence of monosynaptic connections between thalamic nuclei, the observed FC likely represents complex, multisynaptic networks, complicating the direct attribution of these connectivity patterns to specific dysfunctions in schizophrenia.
This study’s exploration of schizophrenia’s neural mechanisms through rs-fMRI highlights critical limitations due to the spatial resolution and data smoothing techniques employed. With a resolution of 3 mm × 3 mm × 4 mm, capturing the detailed connectivity of thalamic nuclei, known for their small size, proves challenging. Spatial smoothing, intended to enhance signal-to-noise ratio (SNR) and adjust for anatomical variations, might further obscure the delicate signals from these crucial areas. Although aimed at improving data analysis, such smoothing risks diluting the specificity of connectivity signals, particularly for small brain structures. Future research should pivot toward utilizing higher-resolution rs-fMRI data and refining imaging techniques to improve spatial specificity without sacrificing SNR or extending scan times. Adjusting the approach to spatial smoothing or avoiding it when analyzing small brain regions could yield a more accurate depiction of functional connectivity patterns. Enhancing ROI definition methods will also be vital for improving study outcomes.
This study, investigating the neural mechanisms of schizophrenia through rs-fMRI, acknowledges the methodological limitations imposed by the spatial resolution and data smoothing techniques utilized. Employing a resolution of 3 mm × 3 mm × 4 mm and 8 mm FWHM smoothing aimed to enhance the SNR for analyzing large-scale brain networks but presented challenges in capturing the intricate connectivity of smaller brain structures like thalamic nuclei. The AAL3 atlas demands high-resolution data to fully leverage its detailed parcellation, especially in areas like the thalamus, where precision is crucial for accurate analysis.
While the smoothing and resolution settings were beneficial for broader network analyses, they may not provide the necessary spatial precision for detailed identification and analysis of smaller structures, potentially obscuring the nuanced signals essential for understanding schizophrenia’s neural basis. Future research should consider higher-resolution rs-fMRI data and refined imaging techniques to improve spatial specificity without compromising SNR or extending scan times. Adjusting spatial smoothing strategies or avoiding them when analyzing small brain regions could enhance the accuracy of functional connectivity patterns. Enhancing ROI definition methods will also be critical for advancing study outcomes, allowing for a more nuanced exploration of the brain’s connectivity and function. Despite these methodological challenges, it is important to note that the findings of this study still contribute valuable insights into the neural underpinnings of schizophrenia, adding to the broader understanding of this complex condition. The study’s results, while limited by the aforementioned constraints, provide important findings that enrich our knowledge and set the groundwork for future research to build upon, ensuring that neuroimaging research continues to evolve in its capacity to dissect the intricate details of brain function and connectivity.
Moreover, the study’s reliance on an open-access dataset introduces significant limitations in the availability of clinical and demographic details, particularly concerning the selection and characterization of the schizophrenic cohort. The lack of detailed participant information, such as the length of time since diagnosis, specific treatments received, and the presence of other health conditions, restricts our ability to comprehensively link connectivity patterns with schizophrenia symptoms or patient histories. Additionally, the absence of precise definitions for the ‘healthy’ cohort further complicates the interpretation of our findings. These limitations highlight the importance of collecting detailed clinical data, including factors like age, gender, medication history, and other health conditions, which are known to influence brain connectivity. Addressing these methodological and data-related challenges in future studies, including refining the definitions of “schizophrenic” and “healthy” cohorts, will be crucial for advancing our understanding of schizophrenia. Emphasizing the collection of detailed clinical data and employing advanced neuroimaging techniques could foster more nuanced insights into the disorder, potentially informing therapeutic strategies.
While our study focused on the functional connectivity differences between schizophrenia patients and healthy controls, we recognize the potential influence of gender and age differences, which were part of the recruitment regimen. However, our current analysis did not reveal significant findings related to these variables. The broad age range and the inclusion of both male and female participants could introduce variability in connectivity patterns that we were unable to fully explore within the scope of this study. Future research should aim to investigate these factors more thoroughly, as they may offer valuable insights into the heterogeneity of schizophrenia and contribute to a more personalized understanding of the disorder. Including a more detailed analysis of gender-specific and age-related connectivity differences could enhance our understanding of how these demographic variables interact with the neurobiological mechanisms underlying schizophrenia.
While our current study included participants with varying handedness, our variability test suggested no significant impact of handedness on the functional connectivity outcomes, likely due to the small number of left-handed and ambidextrous individuals in our sample. However, handedness is known to influence brain connectivity patterns, and its potential effects should not be overlooked. Future research should consider conducting analyses on larger, more balanced groups of right-handed, left-handed, and ambidextrous participants to better understand how handedness may affect neurobiological mechanisms in schizophrenia. Additionally, excluding non-right-handed participants or separately analyzing them could provide clearer insights and strengthen the validity of the results.
5 Conclusion
In summary, this research delves into the less explored sub-regions of the thalamus, including the medial division of the mediodorsal thalamic nuclei (MDm), the VA nucleus, the VL nucleus, and the left PuA. We discovered that patients with schizophrenia exhibit increased connectivity within these thalamic subdivisions. Furthermore, both the aSTG and pSTG showed enhanced connectivity with these thalamic sub-regions, marking the highest deviation observed in our study. Such abnormal connectivity patterns, particularly in the thalamus and STG, may underpin the hallucinations and cognitive difficulties frequently encountered in schizophrenia.
Acknowledgement
This research work was funded by institutional Fund Projects under grant no (IFPHI-095-142-2020). Therefore, authors gratefully acknowledge technical and financial support from the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
-
Funding information: This research work was funded by institutional Fund Projects under grant no IFPHI-095-142-2020. The authors gratefully acknowledge technical and financial support from the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
-
Author contributions: AA: conceptualization, methodology, data collection, formal analysis, writing – original draft and edits, and supervision. JA: data analysis, interpretation of results, and study contextualization, writing – review and editing. HT: data analysis, study connection, and validation, writing – review and editing.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Restrictions apply to the availability of these data. Data were obtained from the International Neuroimaging Data-Sharing Initiative under the 1000 Functional Connectomes Project. Full Information on this dataset is available at http://cobre.mrn.org/ as well as in the following studies [35–38]. COBRE is a publically available dataset distributed with “Creative Commons License: Attribution – Non-Commercial and is available from http://cobre.mrn.org/ with the permission of COBRE.
References
[1] Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, et al. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull. 2003;29(4):653–69.10.1093/oxfordjournals.schbul.a007037Search in Google Scholar PubMed
[2] Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–32.10.1146/annurev.neuro.25.112701.142754Search in Google Scholar PubMed
[3] Sigurdsson T. Neural circuit dysfunction in schizophrenia: insights from animal models. Neuroscience. 2016;321:42–65.10.1016/j.neuroscience.2015.06.059Search in Google Scholar PubMed
[4] Wang HL, Rau CL, Li YM, Chen YP, Yu R. Disrupted thalamic resting-state functional networks in schizophrenia. Front Behav Neurosci. 2015;9:45.10.3389/fnbeh.2015.00045Search in Google Scholar PubMed PubMed Central
[5] Fama R, Sullivan EV. Thalamic structures and associated cognitive functions: Relations with age and aging. Neurosci Biobehav Rev. 2015;54:29–37.10.1016/j.neubiorev.2015.03.008Search in Google Scholar PubMed PubMed Central
[6] Gröhn C, Norgren E, Eriksson L. A systematic review of the neural correlates of multisensory integration in schizophrenia. Schizophr Res: Cognit. 2022;27:100219.10.1016/j.scog.2021.100219Search in Google Scholar PubMed PubMed Central
[7] van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547–53.10.1038/mp.2015.63Search in Google Scholar PubMed PubMed Central
[8] Faria AV, Zhao Y, Ye C, Hsu J, Yang K, Cifuentes E, et al. Multimodal MRI assessment for first episode psychosis: A major change in the thalamus and an efficient stratification of a subgroup. Hum Brain Mapp. 2021;42(4):1034–53.10.1002/hbm.25276Search in Google Scholar PubMed PubMed Central
[9] Okada N, Fukunaga M, Yamashita F, Koshiyama D, Yamamori H, Ohi K, et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry. 2016;21(10):1460–6.10.1038/mp.2015.209Search in Google Scholar PubMed PubMed Central
[10] Zhang M, Palaniyappan L, Deng M, Zhang W, Pan Y, Fan Z, et al. Abnormal thalamocortical circuit in adolescents with early-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2021;60(4):479–89.10.1016/j.jaac.2020.07.903Search in Google Scholar PubMed
[11] Ramsay IS. An activation likelihood estimate meta-analysis of thalamocortical dysconnectivity in psychosis. Biol Psychiatry: Cognit Neurosci Neuroimaging. 2019;4(10):859–69.10.1016/j.bpsc.2019.04.007Search in Google Scholar PubMed
[12] Giraldo-Chica M, Woodward ND. Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophr Res. 2017;180:58–63.10.1016/j.schres.2016.08.005Search in Google Scholar PubMed PubMed Central
[13] Culbreth AJ, Wu Q, Chen S, Adhikari BM, Hong LE, Gold JM, et al. Temporal-thalamic and cingulo-opercular connectivity in people with schizophrenia. NeuroImage: Clin. 2021;29:102531.10.1016/j.nicl.2020.102531Search in Google Scholar PubMed PubMed Central
[14] Ferri J, Ford JM, Roach BJ, Turner JA, van Erp TG, Voyvodic J, et al. Resting-state thalamic dysconnectivity in schizophrenia and relationships with symptoms. Psychol Med. 2018;48(15):2492–9.10.1017/S003329171800003XSearch in Google Scholar PubMed
[15] Fryer SL, Ferri JM, Roach BJ, Loewy RL, Stuart BK, Anticevic A, et al. Thalamic dysconnectivity in the psychosis risk syndrome and early illness schizophrenia. Psychol Med. 2022;52(13):2767–75.10.1017/S0033291720004882Search in Google Scholar PubMed
[16] Dorph-Petersen K-A, Lewis DA. Postmortem structural studies of the thalamus in schizophrenia. Schizophr Res. 2017;180:28–35.10.1016/j.schres.2016.08.007Search in Google Scholar PubMed PubMed Central
[17] Gong J, Luo C, Li X, Jiang S, Khundrakpam BS, Duan M, et al. Evaluation of functional connectivity in subdivisions of the thalamus in schizophrenia. Br J Psychiatry. 2019;214(5):288–96.10.1192/bjp.2018.299Search in Google Scholar PubMed
[18] Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46(1):39–46.10.1016/j.neuroimage.2009.01.045Search in Google Scholar PubMed
[19] Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24(12):3116–30.10.1093/cercor/bht165Search in Google Scholar PubMed PubMed Central
[20] Steullet P. Thalamus-related anomalies as candidate mechanism-based biomarkers for psychosis. Schizophr Res. 2020;226:147–57.10.1016/j.schres.2019.05.027Search in Google Scholar PubMed
[21] Hazlett EA, Buchsbaum MS, Byne W, Wei TC, Spiegel-Cohen J, Geneve C, et al. Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. Am J Psychiatry. 1999;156(8):1190–9.10.1176/ajp.156.8.1190Search in Google Scholar PubMed
[22] Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, et al. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160(1):156–64.10.1176/appi.ajp.160.1.156Search in Google Scholar PubMed PubMed Central
[23] Narayanaswamy JC, Kalmady SV, Venkatasubramanian G, Gangadhar BN. Clinical correlates of superior temporal gyrus volume abnormalities in antipsychotic-naive schizophrenia. J Neuropsychiatry Clin Neurosci. 2015;27(2):e128–33.10.1176/appi.neuropsych.14030049Search in Google Scholar PubMed
[24] Zhou B, Tan C, Tang J, Chen X. Brain functional connectivity of functional magnetic resonance imaging of patients with early-onset schizophrenia. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35(1):17–24.Search in Google Scholar
[25] Ohi K, Matsuda Y, Shimada T, Yasuyama T, Oshima K, Sawai K, et al. Structural alterations of the superior temporal gyrus in schizophrenia: detailed subregional differences. Eur Psychiatry. 2016;35:25–31.10.1016/j.eurpsy.2016.02.002Search in Google Scholar PubMed
[26] Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D'Angelo D, Mauro CJ, et al. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res. 2010;117(1):13–20.10.1016/j.schres.2009.09.030Search in Google Scholar PubMed PubMed Central
[27] Orlov ND, Giampietro V, O'Daly O, Lam SL, Barker GJ, Rubia K, et al. Real-time fMRI neurofeedback to down-regulate superior temporal gyrus activity in patients with schizophrenia and auditory hallucinations: a proof-of-concept study. Transl Psychiatry. 2018;8(1):1–10.10.1038/s41398-017-0067-5Search in Google Scholar PubMed PubMed Central
[28] Pearlson GD. Superior temporal gyrus and planum temporale in schizophrenia: a selective review. Prog Neuro-psychopharmacol Biol Psychiatry. 1997;21(8):1203–29.10.1016/S0278-5846(97)00159-0Search in Google Scholar
[29] Parnaudeau S, Bolkan SS, Kellendonk C. The mediodorsal thalamus: An essential partner of the prefrontal cortex for cognition. Biol Psychiatry. 2018;83(8):648–56.10.1016/j.biopsych.2017.11.008Search in Google Scholar PubMed PubMed Central
[30] DeNicola AL, Park MY, Crowe DA, MacDonald AW, Chafee MV. Differential roles of mediodorsal nucleus of the thalamus and prefrontal cortex in decision-making and state representation in a cognitive control task measuring deficits in Schizophrenia. J Neurosci. 2020;40(8):1650–67.10.1523/JNEUROSCI.1703-19.2020Search in Google Scholar PubMed PubMed Central
[31] Pergola G, Danet L, Pitel AL, Carlesimo GA, Segobin S, Pariente J, et al. The regulatory role of the human mediodorsal thalamus. Trends Cognit Sci. 2018;22(11):1011–25.10.1016/j.tics.2018.08.006Search in Google Scholar PubMed PubMed Central
[32] Copeland CS, Neale SA, Salt TE. Neuronal activity patterns in the mediodorsal thalamus and related cognitive circuits are modulated by metabotropic glutamate receptors. Neuropharmacology. 2015;92:16–24.10.1016/j.neuropharm.2014.12.031Search in Google Scholar PubMed PubMed Central
[33] Ouhaz Z, Fleming H, Mitchell AS. Cognitive functions and neurodevelopmental disorders involving the prefrontal cortex and mediodorsal thalamus. Front Neurosci. 2018;12:33.10.3389/fnins.2018.00033Search in Google Scholar PubMed PubMed Central
[34] Kaur A, Basavanagowda DM, Rathod B, Mishra N, Fuad S, Nosher S, et al. Structural and functional alterations of the temporal lobe in schizophrenia: A literature review. Cureus. 2020;12(10):e11177.10.7759/cureus.11177Search in Google Scholar PubMed PubMed Central
[35] Mayer AR, Ruhl D, Merideth F, Ling J, Hanlon FM, Bustillo J, et al. Functional imaging of the hemodynamic sensory gating response in schizophrenia. Hum Brain Mapp. 2013;34(9):2302–12.10.1002/hbm.22065Search in Google Scholar PubMed PubMed Central
[36] Qureshi MNI, Oh J, Cho D, Jo HJ, Lee B. Multimodal discrimination of schizophrenia using hybrid weighted feature concatenation of brain functional connectivity and anatomical features with an extreme learning machine. Front Neuroinform. 2017;11:59.10.3389/fninf.2017.00059Search in Google Scholar PubMed PubMed Central
[37] Calhoun VD, Sui J, Kiehl K, Turner J, Allen E, Pearlson G. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Front Psychiatry. 2012;2:75.10.3389/fpsyt.2011.00075Search in Google Scholar PubMed PubMed Central
[38] Hanlon FM, Houck JM, Pyeatt CJ, Lundy SL, Euler MJ, Weisend MP, et al. Bilateral hippocampal dysfunction in schizophrenia. Neuroimage. 2011;58(4):1158–68.10.1016/j.neuroimage.2011.06.091Search in Google Scholar PubMed PubMed Central
[39] Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76.10.1146/annurev-clinpsy-032511-143049Search in Google Scholar PubMed
[40] Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–35.10.1016/j.neuroimage.2004.12.034Search in Google Scholar PubMed
[41] Rolls ET, Huang CC, Lin CP, Feng J, Joliot M. Automated anatomical labelling atlas 3. Neuroimage. 2020;206:116189.10.1016/j.neuroimage.2019.116189Search in Google Scholar PubMed
[42] Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex. 2008;18(4):846–67.10.1093/cercor/bhm116Search in Google Scholar PubMed
[43] Scheperjans F, Eickhoff SB, Hömke L, Mohlberg H, Hermann K, Amunts K, et al. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb cortex. 2008;18(9):2141–57.10.1093/cercor/bhm241Search in Google Scholar PubMed PubMed Central
[44] Scheperjans F, Palomero-Gallagher N, Grefkes C, Schleicher A, Zilles K. Transmitter receptors reveal segregation of cortical areas in the human superior parietal cortex: relations to visual and somatosensory regions. Neuroimage. 2005;28(2):362–79.10.1016/j.neuroimage.2005.06.028Search in Google Scholar PubMed
[45] Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–41.10.1089/brain.2012.0073Search in Google Scholar PubMed
[46] Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39(4):1666–81.10.1016/j.neuroimage.2007.11.001Search in Google Scholar PubMed PubMed Central
[47] Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodol). 1995;57(1):289–300.10.1111/j.2517-6161.1995.tb02031.xSearch in Google Scholar
[48] Chumbley J, Worsley K, Flandin G, Friston K. Topological FDR for neuroimaging. Neuroimage. 2010;49(4):3057–64.10.1016/j.neuroimage.2009.10.090Search in Google Scholar PubMed PubMed Central
[49] Shepherd GM, Yamawaki N. Untangling the cortico-thalamo-cortical loop: cellular pieces of a knotty circuit puzzle. Nat Rev Neurosci. 2021;22(7):389–406.10.1038/s41583-021-00459-3Search in Google Scholar PubMed PubMed Central
[50] Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry. 2010;68(1):17–24.10.1016/j.biopsych.2010.04.007Search in Google Scholar PubMed PubMed Central
[51] Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26(4):317–30.10.1016/j.jchemneu.2003.10.003Search in Google Scholar PubMed
[52] Crapse TB, Sommer MA. Corollary discharge circuits in the primate brain. Curr Opin Neurobiol. 2008;18(6):552–7.10.1016/j.conb.2008.09.017Search in Google Scholar PubMed PubMed Central
[53] Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annu Rev Neurosci. 2008;31:317–38.10.1146/annurev.neuro.31.060407.125627Search in Google Scholar PubMed PubMed Central
[54] Frith CD, Blakemore S-J, Wolpert DM. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res Rev. 2000;31(2–3):357–63.10.1016/S0165-0173(99)00052-1Search in Google Scholar
[55] Frith C. The neural basis of hallucinations and delusions. C R Biol. 2005;328(2):169–75.10.1016/j.crvi.2004.10.012Search in Google Scholar PubMed
[56] van de Ven V, Rotarska Jagiela A, Oertel-Knöchel V, Linden D. Reduced intrinsic visual cortical connectivity is associated with impaired perceptual closure in Schizophrenia. Neuroimage Clin. 2017;15:45–52.10.1016/j.nicl.2017.04.012Search in Google Scholar PubMed PubMed Central
[57] Khurana I, Khurana A, Kowlgi NG. Textbook of medical physiology. 3rd edn. India: Elsevier; 2019.Search in Google Scholar
[58] Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;160(2):179–86.10.1192/bjp.160.2.179Search in Google Scholar PubMed
[59] Csernansky JG, Bardgett ME. Limbic-cortical neuronal damage and the pathophysiology of schizophrenia. Schizophr Bull. 1998;24(2):231–48.10.1093/oxfordjournals.schbul.a033323Search in Google Scholar PubMed
[60] Bogerts B. The temporolimbic system theory of positive schizophrenic symptoms. Schizophr Bull. 1997;23(3):423–35.10.1093/schbul/23.3.423Search in Google Scholar PubMed
[61] Tlamsa AP, Brumberg JC. Organization and morphology of thalamocortical neurons of mouse ventral lateral thalamus. Somatosens Mot Res. 2010;27(1):34–43.10.3109/08990221003646736Search in Google Scholar PubMed PubMed Central
[62] Bernard JA, Mittal VA. Cerebellar-motor dysfunction in schizophrenia and psychosis-risk: the importance of regional cerebellar analysis approaches. Front Psychiatry. 2014;5:160.10.3389/fpsyt.2014.00160Search in Google Scholar PubMed PubMed Central
[63] Bédard MA, Agid Y, Chouinard S, Fahn S, Korczyn AD. Mental and behavioral dysfunction in movement disorders. 1st edn. New York: Springer; 2003.10.1385/1592593267Search in Google Scholar
[64] Graves WW, Grabowski TJ, Mehta S, Gupta P. The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. J Cognit Neurosci. 2008;20(9):1698–710.10.1162/jocn.2008.20113Search in Google Scholar PubMed PubMed Central
[65] Tang J, Liao Y, Zhou B, Tan C, Liu W, Wang D, et al. Decrease in temporal gyrus gray matter volume in first-episode, early onset schizophrenia: an MRI study. PLoS One. 2012;7(7):e40247.10.1371/journal.pone.0040247Search in Google Scholar PubMed PubMed Central
[66] Sun J, Maller JJ, Guo L, Fitzgerald PB. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Res Rev. 2009;61(1):14–32.10.1016/j.brainresrev.2009.03.004Search in Google Scholar PubMed
[67] Behrendt R. Hallucinations: Synchronisation of thalamocortical γ oscillations underconstrained by sensory input. Conscious Cogn. 2003;12(3):413–51.10.1016/S1053-8100(03)00017-5Search in Google Scholar PubMed
[68] Behrendt R-P, Young C. Hallucinations in schizophrenia, sensory impairment, and brain disease: a unifying model. Behav Brain Sci. 2004;27(6):771–87.10.1017/S0140525X04000184Search in Google Scholar
[69] Galderisi S, Mucci A, Volpe U, Boutros N. Evidence-based medicine and electrophysiology in schizophrenia. Clin EEG Neurosci. 2009;40(2):62–77.10.1177/155005940904000206Search in Google Scholar PubMed
[70] Siekmeier PJ, Stufflebeam SM. Patterns of spontaneous magnetoencephalographic activity in schizophrenic patients. J Clin Neurophysiol. 2010;27(3):179–90.10.1097/WNP.0b013e3181e0b20aSearch in Google Scholar PubMed PubMed Central
[71] Lehmann D, Faber PL, Pascual-Marqui RD, Milz P, Herrmann WM, Koukkou M, et al. Functionally aberrant electrophysiological cortical connectivities in first episode medication-naive schizophrenics from three psychiatry centers. Front Hum Neurosci. 2014;8:635.10.3389/fnhum.2014.00635Search in Google Scholar PubMed PubMed Central
[72] Llinás RR, Paré D. Of dreaming and wakefulness. Neuroscience. 1991;44(3):521–35.10.1016/0306-4522(91)90075-YSearch in Google Scholar
[73] Lisman J. Low-frequency brain oscillations in schizophrenia. JAMA Psychiatry. 2016;73(3):298–9.10.1001/jamapsychiatry.2015.2320Search in Google Scholar PubMed
[74] Marenco S, Stein JL, Savostyanova AA, Sambataro F, Tan HY, Goldman AL, et al. Investigation of anatomical thalamo-cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology. 2012;37(2):499–507.10.1038/npp.2011.215Search in Google Scholar PubMed PubMed Central
[75] Jiang Y, Patton MH, Zakharenko SS. A case for thalamic mechanisms of schizophrenia: Perspective from modeling 22q11. 2 deletion syndrome. Front Neural Circuits. 2021;15:769969.10.3389/fncir.2021.769969Search in Google Scholar PubMed PubMed Central
[76] Giraldo-Chica M, Rogers BP, Damon SM, Landman BA, Woodward ND. Prefrontal-thalamic anatomical connectivity and executive cognitive function in schizophrenia. Biol Psychiatry. 2018;83(6):509–17.10.1016/j.biopsych.2017.09.022Search in Google Scholar PubMed PubMed Central
[77] Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169(10):1092–9.10.1176/appi.ajp.2012.12010056Search in Google Scholar PubMed PubMed Central
[78] Tu P-C, Lee YC, Chen YS, Li CT, Su TP. Schizophrenia and the brain’s control network: aberrant within-and between-network connectivity of the frontoparietal network in schizophrenia. Schizophr Res. 2013;147(2–3):339–47.10.1016/j.schres.2013.04.011Search in Google Scholar PubMed
[79] Avram M, Brandl F, Bäuml J, Sorg C. Cortico-thalamic hypo-and hyperconnectivity extend consistently to basal ganglia in schizophrenia. Neuropsychopharmacology. 2018;43(11):2239–48.10.1038/s41386-018-0059-zSearch in Google Scholar PubMed PubMed Central
[80] Penner J, Osuch EA, Schaefer B, Théberge J, Neufeld R, Menon RS, et al. Higher order thalamic nuclei resting network connectivity in early schizophrenia and major depressive disorder. Psychiatry Res: Neuroimaging. 2018;272:7–16.10.1016/j.pscychresns.2017.12.002Search in Google Scholar PubMed
[81] Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38.10.1196/annals.1440.011Search in Google Scholar PubMed
[82] Vatansever D, Menon DK, Stamatakis EA. Default mode contributions to automated information processing. Proc Natl Acad Sci U S A. 2017;114(48):12821–6.10.1073/pnas.1710521114Search in Google Scholar PubMed PubMed Central
[83] Galindo L, Bergé D, Murray GK, Mané A, Bulbena A, Pérez V, et al. Default mode network aberrant connectivity associated with neurological soft signs in schizophrenia patients and unaffected relatives. Front Psychiatry. 2017;8:298.10.3389/fpsyt.2017.00298Search in Google Scholar PubMed PubMed Central
[84] Slotnick SD, Schacter DL. The nature of memory related activity in early visual areas. Neuropsychologia. 2006;44(14):2874–86.10.1016/j.neuropsychologia.2006.06.021Search in Google Scholar PubMed
[85] Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev. 2015;54:89–107.10.1016/j.neubiorev.2015.01.014Search in Google Scholar PubMed PubMed Central
[86] Andrews J, Wang L, Csernansky JG, Gado MH, Barch DM. Abnormalities of thalamic activation and cognition in Schizophrenia. Am J Psychiatry. 2006;163(3):463–9.10.1176/appi.ajp.163.3.463Search in Google Scholar PubMed
[87] Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36(4):713–22.10.1093/schbul/sbn145Search in Google Scholar PubMed PubMed Central
[88] Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015;72(9):882–91.10.1001/jamapsychiatry.2015.0566Search in Google Scholar PubMed PubMed Central
[89] Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9(1):357–81.10.1146/annurev.ne.09.030186.002041Search in Google Scholar PubMed
[90] Horga G, Abi-Dargham A. An integrative framework for perceptual disturbances in psychosis. Nat Rev Neurosci. 2019;20(12):763–78.10.1038/s41583-019-0234-1Search in Google Scholar PubMed
[91] Heinks-Maldonado TH, Mathalon DH, Houde JF, Gray M, Faustman WO, Ford JM. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Arch Gen Psychiatry. 2007;64(3):286–96.10.1001/archpsyc.64.3.286Search in Google Scholar PubMed
[92] Ford JM, Mathalon DH. Electrophysiological evidence of corollary discharge dysfunction in schizophrenia during talking and thinking. J Psychiatr Res. 2004;38(1):37–46.10.1016/S0022-3956(03)00095-5Search in Google Scholar PubMed
[93] Ford JM, Roach BJ, Faustman WO, Mathalon DH. Out-of-synch and out-of-sorts: dysfunction of motor-sensory communication in schizophrenia. Biol Psychiatry. 2008;63(8):736–43.10.1016/j.biopsych.2007.09.013Search in Google Scholar PubMed PubMed Central
[94] Nawani H, Bose A, Agarwal SM, Shivakumar V, Chhabra H, Subramaniam A, et al. Modulation of corollary discharge dysfunction in schizophrenia by tDCS: preliminary evidence. Brain Stimul: Basic Transl Clin Res Neuromodulation. 2014;7(3):486–8.10.1016/j.brs.2014.01.003Search in Google Scholar PubMed
[95] Pack CC. Eye movements as a probe of corollary discharge function in schizophrenia. ACS Chem Neurosci. 2014;5(5):326–8.10.1021/cn5000869Search in Google Scholar PubMed PubMed Central
[96] Richard A, Churan J, Whitford V, O'Driscoll GA, Titone D, Pack CC. Perisaccadic perception of visual space in people with schizophrenia. J Neurosci. 2014;34(14):4760–5.10.1523/JNEUROSCI.4744-13.2014Search in Google Scholar PubMed PubMed Central
[97] Feinberg I. Corollary discharge, hallucinations, and dreaming. Schizophrenia Bull. 2011;37(1):1–3.10.1093/schbul/sbq115Search in Google Scholar PubMed PubMed Central
[98] Mellem MS, Jasmin KM, Peng C, Martin A. Sentence processing in anterior superior temporal cortex shows a social-emotional bias. Neuropsychologia. 2016;89:217–24.10.1016/j.neuropsychologia.2016.06.019Search in Google Scholar PubMed PubMed Central
[99] Rajarethinam, RP, DeQuardo JR, Nalepa R, Tandon R. Superior temporal gyrus in schizophrenia: a volumetric magnetic resonance imaging study. Schizophr Res. 2000;41(2):303–12.10.1016/S0920-9964(99)00083-3Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Brain expression profiles of two SCN1A antisense RNAs in children and adolescents with epilepsy
- Silibinin suppresses glioblastoma cell growth, invasion, stemness, and glutamine metabolism by YY1/SLC1A5 pathway
- Early exercise intervention promotes myelin repair in the brains of ischemic rats by inhibiting the MEK/ERK pathway
- Comparative analysis of CRASH and IMPACT in predicting the outcome of 340 patients with traumatic brain injury
- Association between FOXP3 polymorphisms and expression and neuromyelitis optica spectrum disorder risk in the Northern Chinese Han population
- Trehalose improves the movement ability of Aβarc Drosophila by restoring the damaged mitochondria
- The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling
- Single cocaine exposure attenuates the intrinsic excitability of CRH neurons in the ventral BNST via Sigma-1 receptors
- Effect of dopamine on limbic network connectivity at rest in Parkinson’s disease patients with freezing of gait
- FT4-to-FT3 ratio is a novel prognostic marker in subacute combined spinal cord degeneration patients
- Suanzaoren decoction exerts its antidepressant effect via the CaMK signaling pathway
- Acute ischemic STROKE – from laboratory to the Patient’s BED (STROKELABED): A translational approach to reperfusion injury. Study Protocol
- Thyroid hormone T3 induces Fyn modification and modulates palmitoyltransferase gene expression through αvβ3 integrin receptor in PC12 cells during hypoxia
- Activating α7nAChR suppresses systemic inflammation by mitigating neuroinflammation of the medullary visceral zone in sepsis in a rat model
- Amelioration of behavioral and histological impairments in somatosensory cortex injury rats by limbal mesenchymal stem cell transplantation
- TTBK2 T3290C mutation in spinocerebellar ataxia 11 interferes with ciliogenesis
- In a rodent model of autism, probiotics decrease gut leakiness in relation to gene expression of GABA receptors: Emphasize how crucial the gut–brain axis
- A data science approach to optimize ADHD assessment with the BRIEF-2 questionnaire
- Cystatin C alleviates unconjugated bilirubin-induced neurotoxicity by promoting bilirubin clearance from neurocytes via exosomes, dependent on hepatocyte UGT1A1 activity
- Macrophage accumulation in dorsal root ganglion is associated with neuropathic pain in experimental autoimmune neuritis
- Identifying key biomarkers and therapeutic candidates for post-COVID-19 depression through integrated omics and bioinformatics approaches
- The hidden link: Investigating functional connectivity of rarely explored sub-regions of thalamus and superior temporal gyrus in Schizophrenia
- A pilot evaluation of the diagnostic accuracy of ChatGPT-3.5 for multiple sclerosis from case reports
- Review Articles
- Adaptation of the layer V supraspinal motor corticofugal projections from the primary (M1) and premotor (PM) cortices after CNS motor disorders in non-human primates: A survey
- Comorbidity in spinal cord injury in Iran: A narrative review
- Lipid-based nanoparticles for drug delivery in Parkinson’s disease
- Disgust sensitivity and psychopathic behavior: A narrative review
- Rapid Communications
- Long COVID elevated MMP-9 and release from microglia by SARS-CoV-2 Spike protein
- Internal consistency of the Mental Health Professional Culture Inventory: A pilot study in Romanian population
- Retraction
- Retraction of “Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease”
- Corrigendum
- Corrigendum to “The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling”
- Corrigendum to “Tongxinluo promotes axonal plasticity and functional recovery after stroke”
Articles in the same Issue
- Research Articles
- Brain expression profiles of two SCN1A antisense RNAs in children and adolescents with epilepsy
- Silibinin suppresses glioblastoma cell growth, invasion, stemness, and glutamine metabolism by YY1/SLC1A5 pathway
- Early exercise intervention promotes myelin repair in the brains of ischemic rats by inhibiting the MEK/ERK pathway
- Comparative analysis of CRASH and IMPACT in predicting the outcome of 340 patients with traumatic brain injury
- Association between FOXP3 polymorphisms and expression and neuromyelitis optica spectrum disorder risk in the Northern Chinese Han population
- Trehalose improves the movement ability of Aβarc Drosophila by restoring the damaged mitochondria
- The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling
- Single cocaine exposure attenuates the intrinsic excitability of CRH neurons in the ventral BNST via Sigma-1 receptors
- Effect of dopamine on limbic network connectivity at rest in Parkinson’s disease patients with freezing of gait
- FT4-to-FT3 ratio is a novel prognostic marker in subacute combined spinal cord degeneration patients
- Suanzaoren decoction exerts its antidepressant effect via the CaMK signaling pathway
- Acute ischemic STROKE – from laboratory to the Patient’s BED (STROKELABED): A translational approach to reperfusion injury. Study Protocol
- Thyroid hormone T3 induces Fyn modification and modulates palmitoyltransferase gene expression through αvβ3 integrin receptor in PC12 cells during hypoxia
- Activating α7nAChR suppresses systemic inflammation by mitigating neuroinflammation of the medullary visceral zone in sepsis in a rat model
- Amelioration of behavioral and histological impairments in somatosensory cortex injury rats by limbal mesenchymal stem cell transplantation
- TTBK2 T3290C mutation in spinocerebellar ataxia 11 interferes with ciliogenesis
- In a rodent model of autism, probiotics decrease gut leakiness in relation to gene expression of GABA receptors: Emphasize how crucial the gut–brain axis
- A data science approach to optimize ADHD assessment with the BRIEF-2 questionnaire
- Cystatin C alleviates unconjugated bilirubin-induced neurotoxicity by promoting bilirubin clearance from neurocytes via exosomes, dependent on hepatocyte UGT1A1 activity
- Macrophage accumulation in dorsal root ganglion is associated with neuropathic pain in experimental autoimmune neuritis
- Identifying key biomarkers and therapeutic candidates for post-COVID-19 depression through integrated omics and bioinformatics approaches
- The hidden link: Investigating functional connectivity of rarely explored sub-regions of thalamus and superior temporal gyrus in Schizophrenia
- A pilot evaluation of the diagnostic accuracy of ChatGPT-3.5 for multiple sclerosis from case reports
- Review Articles
- Adaptation of the layer V supraspinal motor corticofugal projections from the primary (M1) and premotor (PM) cortices after CNS motor disorders in non-human primates: A survey
- Comorbidity in spinal cord injury in Iran: A narrative review
- Lipid-based nanoparticles for drug delivery in Parkinson’s disease
- Disgust sensitivity and psychopathic behavior: A narrative review
- Rapid Communications
- Long COVID elevated MMP-9 and release from microglia by SARS-CoV-2 Spike protein
- Internal consistency of the Mental Health Professional Culture Inventory: A pilot study in Romanian population
- Retraction
- Retraction of “Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease”
- Corrigendum
- Corrigendum to “The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling”
- Corrigendum to “Tongxinluo promotes axonal plasticity and functional recovery after stroke”

