Abstract
Objectives
Studies have shown that fibrinolysis activity is insufficient in COVID-19 patients. Plasminogen activator inhibitor-1 (PAI-1) is an important antifibrinolytic molecule that plays a key role in the fibrinolytic system. In our study; we aimed to evaluate serum PAI-1 and other biochemical parameters of COVID-19 patients in terms of disease course and mortality.
Methods
A total of 40 COVID-19 patients were hospitalized in the service and intensive care unit (ICU) of our hospital from October to December 2020 and 20 healthy volunteers were included in our study. The patients were grouped as those who transferred to the ICU from the service and transferred to service from the ICU. The first and second values of the same patients in both the service and the ICU were analyzed by SPSS.
Results
The PAI-1 levels of the patients in the ICU were significantly higher than the levels of the same patients in the service and the healthy control group (p<0.001). IL-6, ferritin, and D-dimer levels in the ICU of the same patients were significantly higher than the levels of service and healthy control group (p<0.001). A positive correlation was found between initial serum PAI-1 and D-dimer levels in patients hospitalized in the service (p=0.039) and initial serum ferritin and IL-6 levels in the ICU (p=0.031).
Conclusions
In our study, we found that PAI-1 levels increased significantly with the increase in mortality in COVID-19 patients.
Introduction
In December 2019, a coronavirus affecting the respiratory tract of patients with pneumonia was detected in Wuhan, China. Due to its highly contagious and deadly nature, this common novel coronavirus disease has become a worldwide pandemic with the name COVID-19 (Coronavirus disease 2019). Approximately 536 million cases detected in the world so far, and 6.3 million deaths from COVID-19 are stated [1].
Acute phase reactants due to inflammation increase in patients diagnosed with COVID-19. Cytokine storm, called macrophage activation syndrome (MAS), is characterized by hyperferritinemia and high proinflammatory cytokine levels [2]. It is also known that this situation leads to the disruption of the anticoagulant pathways [3]. In order to restore the disrupted gas exchange by these microthrombi to normal, the fibrinolytic system is activated and fibrin degradation products such as D-dimer increase [4]. In addition, high levels of plasminogen activator inhibitor (PAI-1) are produced by endothelial cells and activated platelets [5, 6].
As the severity of the disease increases, the pulmonary coagulation system is rapidly activated and the fibrinolytic system becomes unable to compensate for this situation. Especially in COVID-19 patients in the ICU (intensive care unit), insufficient fibrinolysis activity was observed compared to the healthy population [7]. As a result of all these, increased oxygen requirement, renal failure, ischemia, thrombosis, acute respiratory distress syndrome (ARDS), disseminated intravascular coagulopathy [8], multiple organ failure, and even serious complications up to death can be seen [4]. It is valuable to evaluate the impaired fibrinolysis system of COVID-19 patients and to examine the effect of this condition on the clinical course and mortality of the disease. More research is needed to elucidate the mechanisms that cause this course in patients with a worsening clinical condition.
PAI-1 is one of the most important and fastest inhibitors of the fibrinolytic system in plasma. The main function of PAI-1 is to inhibit plasminogen activators by cleaving a specific arginine-valine peptide bond at the protease site [9].
At this point investigating the correlation between PAI-1 levels which play an acute phase reactant role in inflammation in the control step of the fibrinolytic system, and prognosis and mortality in COVID-19 patients can be valuable in follow-up and treatment and can be a guide in preventing sudden complications. Studies examining PAI-1 levels in COVID-19 patients are quite limited.
In our study, we aimed to determine the initial PAI-1 levels of COVID-19 patients who were hospitalized in the service and then transferred to the ICU and the hospitalized in ICU and then transferred to the service and evaluate the relationship between the PAI-1 levels and ferritin, D-dimer, IL-6 levels and the clinical course of the disease.
Materials and methods
Design and study groups
This prospective study was approved by the Institutional Ethics Committee (April 22, 2020, No: 2,726) and was conducted at the University of Health Sciences, Sisli Etfal Training and Research Hospital. Consent was obtained from all participants or their relatives in the study. The study included COVID-19 patients who had SARS-CoV-2 RNA detected in their nasopharyngeal or oropharyngeal swab specimens by reverse transcription polymerase chain reaction (RT-PCR) (Biospeedy® SARS-CoV-2 Double Gene RT-qPCR Kit, Bioeksen) on BioradCFX96 Touch Real Time (ABD, CA, USA) device. These patients were hospitalized in Sisli Hamidiye Etfal Training and Research Hospital COVID-19 service and ICU between October and December 2020. Being under 18 years of age, malignancy, chronic kidney disease, cardiovascular system disease, coagulopathy, organ transplantation, use of immunomodulators, and pregnancy were determined as exclusion criteria. After completing the patient group, serum samples were collected from healthy volunteers of similar gender and age at the rate of 1/2 of the total number of patients for the control group. As a result, 40 patients and 20 controls were included in the study by performing a power analysis. A total of two blood samples were taken from each patient on the first day of hospitalization to both the service and the ICU. The patients were grouped as those who were hospitalized at the ICU and then transferred to the service and who were hospitalized at the service and then transferred to the ICU.
The following criteria [10] were evaluated and decided for hospitalization at ICU in the COVID-19 cases in our hospital.
Dyspnea and respiratory distress, respiratory rate>30/min, PaO2/FiO2<300,
Increase in oxygen demand during follow-up,
SpO2<90% or PaO2<70 mmHg despite 5 L/min oxygen therapy,
Hypotension,
Tachycardia>100/min,
Acute kidney injury, acute liver function tests,
Patients with immunosuppression and development of acute organ dysfunction such as confusion,
Acute bleeding diathesis, troponin elevation, and arrhythmia,
Lactate>2 mmol,
Presence of prolonged capillary refill time and skin disorders such as cutis marmaratus.
Biochemical analyses
Measurement of IL-6 and ferritin
Venous blood samples were taken from all patients participating in the study after 12 h of overnight fasting.
Samples taken into gel vacuum tubes (Sarstedt, Nümbrecht, Germany) for ferritin and IL-6 parameters were kept at room temperature for 30 min, centrifuged at 3220×g for 10 min and their sera were separated. They were stored in Eppendorf tubes at −80 °C until the day of analysis. Before analysis, they were kept at −20 °C for 12 h and at +4 °C for 12 h, respectively. Sera were thawed at room temperature and homogenized by vortexing and IL-6 and ferritin were performed on the Roche Cobas 8,000 (Basel, Switzerland) device on the day of analysis of all serum samples.
Measurement of D-dimer
Venous blood samples taken into gel vacuum tubes containing 3.2% sodium citrate (Sarstedt, Nümbrecht, Germany) were centrifuged at 3220×g for 10 min and the plasma was separated and D-dimer analysis was performed on the Beckman Coulter AU480 (Pasadena, CA, USA) device on the day of analysis of all serum samples with D-Dimer Latex Assay Kit (Improgen, Istanbul, Turkey).
Measurement of PAI-1
Samples taken into gel vacuum tubes (Sarstedt, Nümbrecht, Germany) for PAI-1 analysis were kept at room temperature for 30 min and their sera were separated by centrifugation at 1000×g for 20 min at +4 °C. They were stored in Eppendorf tubes at −80 °C until the day of analysis. Before analysis, it was kept at −20 °C for 12 h and at +4 °C for 12 h, respectively. Sera were thawed at room temperature and homogenized by vortexing and ELISA was performed on sera on the day of analysis. Bioassay Technology Laboratory brand Human PAI-1 ELISA Kit (Shanghai, China) (Cat. No: E1159Hu) was used for PAI-1 analysis. It was an advantage for us that the kit stability period was convenient and the assay procedure steps were quick and easy. The standard curve range of the kit is 0.05–20 ng/mL, the sensitivity is 0.019 ng/mL. The intra-assay coefficient of variation is 3.7% and the inter-assay coefficient of variation is <10%. Patient samples were diluted 1/50 with Phosphate-Buffer Saline, which is the diluent recommended to us. BIO-TEK ELx50 (Vermont, USA) was used for washes and BIO-TEK ELx500 (Vermont, USA) for readings.
Statistical analysis
SPSS (Statistical Package for the Social Sciences) 21.0 for Windows program was used for statistical analysis. Parametric tests were used for normally distributed data and nonparametric tests were used for data that did not show normal distribution. Kruskal Wallis test was used for the comparison of the independent three groups and the Mann-Whitney U test was used for the comparison of the independent two groups. Wilcoxon test was used for dependent two groups. Diagnostic values of valuable parameters for clinical course and mortality of COVID-19 patients were evaluated by area under the receiver operating characteristic (ROC) curve. Quantitative variables are given as mean, standard deviation, minimum and maximum. The statistical alpha significance level was considered as less than 0.05.
Results
Forty COVID-19 patients and twenty healthy control were included in our study. Twenty-four patients were hospitalized in the service and then these patients were transferred to the ICU. 16 patients were hospitalized in the ICU and then these patients were transferred to the service. According to current data, all 24 patients who passed to the ICU died and all 16 patients who entered the service recovered and were discharged.
Age and gender distributions of the patient group according to the initial hospitalization unit, age and gender distribution of the control group are presented in Table 1.
Age and gender distribution of the patient and control groups.
| Hospitalization unit | Gender | n | Mean of age | Min, years | Max, years |
|---|---|---|---|---|---|
| ± SD, years | |||||
| Service | Female | 11 | 73.9 ± 12.4 | 50 | 88 |
| Male | 13 | 66.2 ± 10.0 | 53 | 83 | |
| Total | 24 | 69.7 ± 11.6 | 50 | 88 | |
| ICU | Female | 5 | 60.4 ± 15.0 | 39 | 80 |
| Male | 11 | 61.3 ± 16.2 | 25 | 80 | |
| Total | 16 | 61.0 ± 15.3 | 25 | 80 | |
| Control group | Female | 6 | 71.5 ± 13.4 | 34 | 78 |
| Male | 14 | 65.9 ± 8.9 | 47 | 77 | |
| Total | 20 | 63.7 ± 10.7 | 34 | 77 |
-
ICU, intensive care unit; SD, standard deviation.
There was no significant difference between the hospitalization unit and age gender (p=0.8) and between the survival status of patients and age gender (p=0.7).
When the PAI-1 and other parameter levels of all patients at the time of admission to the service and intensive care unit were compared with the control group, the PAI-1, IL-6, ferritin, and D-dimer levels of the patients in the ICU were significantly higher than the levels in the service and the healthy control group (p=0.001) (Table 2).
Mean values of the independent groups according to hospitalization unit and values of healthy control, for malea and femaleb.
| Parameters | Reference range | Service median (IQR) n=24 | ICU median (IQR) n=16 | Control median (IQR) n=20 | p-Value |
|---|---|---|---|---|---|
| PAI-1, ng/mL | 87.4 (21.4) | 95.7 (21.5) | 3.3 (2.1) | 0.001 | |
| IL-6, pg/mL | <7 | 40.5 (86.8) | 109.5 (318.2) | 2.6 (4.1) | 0.001 |
| Ferritin, μg/L | 11–307a 24–336b | 812 (997) | 2,615 (3,314) | 64 (77) | 0.001 |
| D-dimer, μg/L | <500 | 946 (1,437) | 1,573 (1,806) | 351 (325) | 0.001 |
-
PAI-1, plasminogen activator inhibitor-1; IL-6, interleukin-6; IQR, interquantile range.
When patients are grouped as those who are transferred to ICU from the service (group 1) and transferred to service from the ICU (group 2); a significant difference was found between service and ICU PAI-1 values of the first group (p=0.001) and the second group (p=0.004).
The mean values and significance levels of PAI-1 and other parameters of the same patients grouped according to hospitalization unit changes are given in Table 3.
Mean values of same patients dependent grouped according to hospitalization unit changes.
| Parameters | Group 1 (patients transferring to the ICU from the service) | ||
|---|---|---|---|
| Service median (IQR) n=24 | ICU median (IQR) n=24 | p-Value | |
| PAI-1, ng/mL | 86.1 (27.1) | 97.7 (30.7) | 0.001 |
| IL-6, pg/mL | 66.1 (114.7) | 350.7 (507.8) | 0.001 |
| Ferritin, μg/L | 1,348 (787) | 3,622 (7,394) | 0.001 |
| D-dimer, μg/L | 884 (1,881) | 1,484 (1,682) | 0.008 |
| Group 2 (patients transferring to the service from the ICU) | |||
|---|---|---|---|
| ICU median (IQR) n=16 | Service median (IQR) n=16 | p-Value | |
| PAI-1, ng/mL | 97.5 (17.5) | 87.1 (12.5) | 0.004 |
| IL-6, pg/mL | 57.2 (70.9) | 6.7 (27.0) | 0.034 |
| Ferritin, μg/L | 935 (2,110) | 585 (434) | 0.002 |
| D-dimer, μg/L | 2,023 (2,143) | 1,222 (1,264) | 0.023 |
-
PAI-1, plasminogen activator inhibitor-1; IL-6, interleukin-6; ICU, intensive care unit; IQR, interquartile range.
When the correlation between values is evaluated; a positive correlation was found between initial serum D-dimer and PAI-1 levels in patients hospitalized in the service (p=0.039) and ferritin and IL-6 levels in patients hospitalized in the ICU (p=0.031).
ROC curve
In order to evaluate the survival status of patients in ICU; serum PAI-1 value was calculated as 91.0 ng/mL cut-off level, sensitivity as 67% and specificity as 65%, serum IL-6 value was calculated as 91.3 pg/mL cut-off level, sensitivity as 79% and specificity as 80%, serum ferritin value was calculated as 2073 μg/L cut-off level, sensitivity as 75% and specificity as 90%, serum D-dimer value was calculated as 1,399 μg/L cut-off level sensitivity as 63% and specificity as 70% (Figure 1).
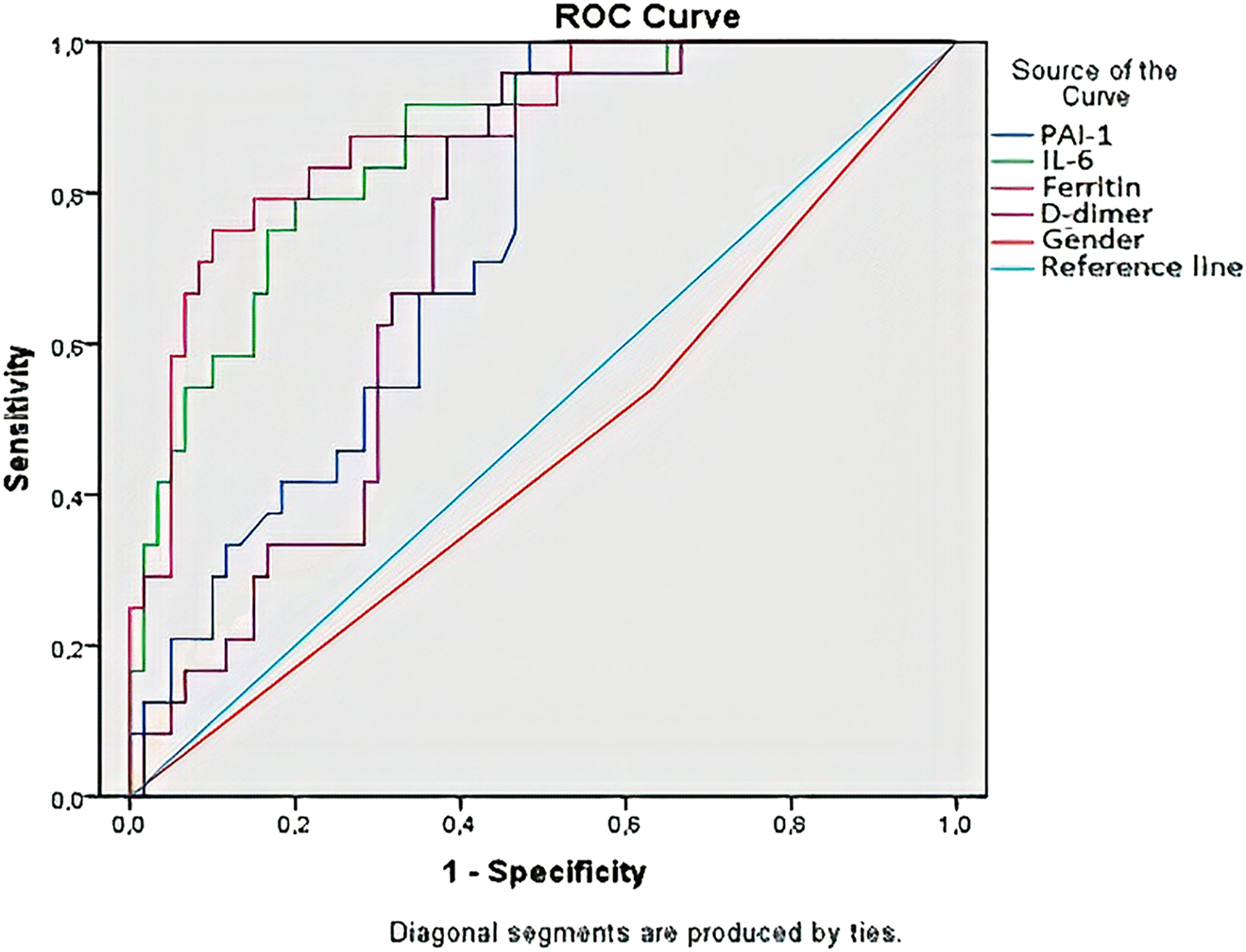
Evaluation of the relationship of the survival status of the patients with gender and measured parameters and the sensitivity and specificity of these values with the ROC curve.
Discussion
Our study is a prospective-controlled study that includes the follow-up of PAI-1 levels of COVID-19 patients and examines the relationship between these levels and some important parameters that correlate with the clinical course and mortality of the patients. In our study, a significant difference was observed between the serum PAI-1 levels of COVID-19 patients at their first hospitalization to the service and ICU. The values of ICU were significantly higher than service and healthy volunteers. When the patients were grouped according to the first hospitalization units, especially increasing PAI-1 levels of patients transferred to the ICU from the service had a very high statistical significance. All of the patients who transferred to the ICU from the service unit died, therefore, we found that the serum PAI-1 levels of those who died tended to increase. These findings forced us to think that the PAI-1 molecule may be a critical marker in patients diagnosed with COVID-19 whose clinical course is worsening.
We also examined the correlation between serum ferritin, IL-6, and D-dimer levels of patients with PAI-1 levels and prognosis of the COVID-19. Serum ferritin, IL-6, and D-dimer levels of the patients at the time of hospitalization to the ICU were significantly higher than that in the service. Likewise, the healthy control group values were significantly lower than the other groups. We found a correlation between serum IL-6 and ferritin levels of patients who transferred to the ICU from the service and between serum PAI-1 and D-dimer levels of patients transferred to the service from the ICU. Although it was not statistically significant in other parameters (ferritin, IL-6, and D-dimer), we found a positive correlation. The small sample size and the lack of knowledge of the baseline values of the patients may have shown the correlation to be statistically weak. We found that IL-6, ferritin, and D-dimer levels in patients with exitus tended to increase.
Studies on PAI-1, the main molecule in the plasminogen pathway, which is important in fibrinolytic activity in COVID-19 patients are limited and prospective studies are needed. These studies can offer promising options in the follow-up and treatment of COVID-19. In our study, we analyzed the serum PAI-1 levels with serum IL-6, ferritin, and D-dimer levels of COVID-19 patients who were transferred to the ICU from the service and transferred to the service from ICU to investigate the relationship between the plasminogen system whose function is likely to be impaired and the prognosis of the disease.
Fibrinolysis is the result of interactions between a large number of plasminogen activators and inhibitors and these interactions form an enzymatic cascade that causes the degradation of fibrin. The plasminogen activator system plays a key role in a wide variety of physiological and pathological processes such as coagulation, fibrinolysis, and inflammation [11]. PAI-1 is in the control step of the fibrinolytic system.
COVID-19 patients (especially those hospitalized in the ICU) have been found to have insufficient fibrinolysis activity compared to the healthy population [7]. There are several studies in the literature that have examined serum PAI-1 levels in COVID-19 patients. In a study of 78 hospitalized patients diagnosed with COVID-19, they found that serum PAI-1 levels increased, especially in COVID-19 patients with critical comorbidity [12]. Similarly, we found significantly higher PAI-1 levels in the COVID-19 group. Furthermore, we compared both ICU and service values of the same patients. The fact that the serum PAI-1 values of the patients hospitalized in the ICU were higher than the values in the service supports the literature. Also, monitoring the serum PAI-1 levels of the patients at the time of transition to the service and ICU makes our study valuable in terms of evaluating the relationship between these levels and the clinical course of the disease. In a study conducted in Japan and contradicting our results; PAI-1 levels were examined in 24 ARDS patients with COVID-19 pneumonia and 200 ARDS patients with non-COVID-19 pneumonia and PAI-1 levels were within the normal range in COVID-19 patients [13]. Not including the healthy control group and the low number of COVID-19 patients may be the reasons why we found different results from this study.
Excessive fibrin-platelet formation in the pulmonary vascular system and lung air spaces of patients with COVID-19 [14, 15] causes excessive production of tissue factors and increased clot formation by alveolar epithelial cells and macrophages. High levels of PAI-1 are produced by endothelial cells, thrombocytes [5, 6], and alveolar macrophages due to the impaired coagulation system [16]. Some studies have shown that PAI-1 levels are higher in the plasma of patients with ARDS than healthy volunteers [17, 18]. In a study on SARS-CoV infection; the fatal and non-fatal SARS-CoV infection was activated in mice and the importance of the impaired activity effect of the urokinase pathway in alveolar damage and acute lung injury was emphasized as a result of proteomic analysis [19]. Wu et al. also found that serum PAI-1 levels increased in patients with a diagnosis of SARS [20].
Plasminogen activators initiate fibrinolysis and are controlled by PAI-1 [9]. In the case study of Wang et al., it was reported that three COVID-19 patients with ARDS and respiratory failure recovered after tissue plasminogen activator (alteplase) treatment, and the PaO2/FiO2 ratio of the patients improved [21]. This study shows the importance of the plasminogen pathway in the mortality of patients diagnosed with COVID-19.
SARS-CoV-2 infection together with inflammation-activated immune cells in the lungs causes impairment of bronchoalveolar hemostasis or excessive clot formation [22]. Moreover, acute phase reactants increase in patients due to inflammation, and tissue damage is increasing due to the resulting cytokine storm [23]. Proinflammatory cytokines play a critical role in abnormal clot formation and platelet hyperactivation, as well as disrupting the function of important physiological anticoagulant pathways [3]. In addition to cytokines, ferritin, which is present in high levels in the circulation, also reflects the acute phase response and plays a critical role in inflammation [24]. Pulmonary microthrombus formations were detected in patients with COVID-19 in postmortem examinations [25]. For the disrupted gas exchange due to these microthrombi to return to normal, the fibrinolytic system is activated and fibrin degradation products such as D-dimer increase [4]. So, it is important to evaluate the coagulation system and immune system together in COVID-19 infection.
In the literature; Ruan et al. showed that those who died due to COVID-19 had serum IL-6 levels nearly twice as high as those who survived [26]. In another study, serum IL-6 levels were associated with increased fibrinogen and D-dimer levels in 16 COVID-19 patients requiring mechanical ventilation and confirmed the link between inflammation and the impaired coagulation system [27]. According to a meta-analysis of 8752 COVID-19 patients, elevated IL-6 levels were associated with the patients’ deteriorating clinical picture. Significant increases in leukocyte count, procalcitonin, D-dimer, lactate dehydrogenase, and ferritin levels were detected before treatment in those with exitus [28]. According to a meta-analysis of 10,614 COVID-19 patients, ferritin levels were significantly higher in severe patients compared to other groups and those who died were significantly higher than those who were not exitus [29]. Yagcı et al. found that serum ferritin values positively correlated with disease severity and mortality in 59 COVID-19 patients [30]. Shah et al. reported in a meta-analysis that 3,682 patients with COVID-19 had significantly higher D-dimer levels and correlated with increased mortality [31]. Our findings are similar to the literature, showing that as the course of the disease worsens, IL-6 and ferritin levels due to increased cytokine storm and inflammation and D-dimer levels due to excessive fibrin degradation increase.
Kang et al. grouped patients with sepsis, ARDS, and burn-induced cytokine release syndrome and examined the levels of IL-6 and other proinflammatory cytokines and PAI-1 in the plasma of these patients; they found that IL-6 levels and PAI-1 levels were positively correlated as in their study. In addition, after tocilisumab treatment of seven COVID-19 patients, it was found that PAI-1 levels decreased with cytokines and the patients’ clinic tended to improve; in addition, the importance of cytokine storm therapy in lowering PAI-1 levels and regression of coagulopathy-endothelial dysfunction findings was emphasized [32]. In our study compared to this study, all patients were diagnosed with COVID-19 and there were no patients receiving tocilisumab treatment. Our patient group included both critically ill and recovering patients. Also, we analyzed the serum PAI-1 levels before the treatment on the first day of hospitalization of the patients in the ICU and service, some of the patients transferred to the service from the ICU recovered and were discharged without receiving tocilisumab treatment. For this reason, we think that it is important to evaluate the follow-up and treatment of patients not only in terms of cytokine storm but also in terms of plasminogen pathway and PAI-1 levels.
The STAT-3 pathway, one of the intracellular signaling pathways, plays a role in T cell differentiation and inflammation [33, 34]. According to the reviews of Matsuyama et al.; the PAI-1 and STAT-3 pathway is likely to interact in COVID-19, promoting coagulopathy and thrombosis. According to this mechanism, through hypoxia inducible factor-1 alpha which increases as a result of hypoxia, PAI-1, which is overproduced in damaged type 2 alveolar cells, induces the secretion of inflammatory cytokines and chemokines by binding to the TLR4 (Toll-Like Receptor 4) on macrophages. This may be the cause of excessive cytokine production in critically ill patients. In addition, it has been hypothesized that the molecule that increases fibrosis by triggering tumor necrosis factor-β1 (TNF-β1) is PAI-1 [35]. In our study, we found that the serum PAI-1 levels of patients with worsening clinical symptoms and increased need for oxygen were significantly increased with parameters of inflammation, cytokine storm, and fibrin degradation product.
There are some limitations of our study. First of all, the relatively small sample size in our study and the unknown baseline values of the patients can be considered as limitations of our study. In addition, unknown final values of patients with a worsening clinical course before exitus can be considered as a deficiency in terms of follow-up. Secondly, since the patients were included in the study in accordance with the stated exclusion criteria, no remarkable co-morbidity table was given.
Conclusions
In our study, we prospectively investigated PAI-1 levels, which is an important parameter in the fibrinolytic step, together with inflammation (ferritin, IL-6) and fibrin degradation product (D-dimer) parameters in patients diagnosed with COVID-19.
We found that the serum PAI-1 levels of the same patients in the intensive care unit were significantly higher than the PAI-1 levels in the service. All patients who were transferred from the service to the intensive care unit died.
As a result, we found that PAI-1 levels increased significantly with the increase in mortality in COVID-19 patients and decreased in patients whose clinics recovered; also we found that these values correlated with ferritin, IL-6, and D-dimer levels.
We think that our study is one of the few studies in terms of being a prospective study in which PAI-1 was evaluated in the first and later stages of hospitalization of COVID-19 patients. There is no standardization yet in the follow-up and treatment of the clinical symptoms of the disease. More studies are needed to evaluate PAI-1 for follow-up and fibrinolytic therapy options for treatment. Thus, the worsening of the clinical course of the disease can be prevented from the very beginning and mortality and the need for mechanical ventilation can be significantly reduced.
Acknowledgments
We would like to thank the staff working in the Clinical Laboratory, Chest Diseases and Intensive Care Department.
-
Research funding: None declared.
-
Author contributions: Ecem Baltan and Erdinc Serin designed the study. Patient selection and sample supply were provided by Isil Kibar Akilli and Ayse Surhan Cinar. Ecem Baltan and Burak Yasin Avci performed ELISA assay and statistics, interpreted data. Erdinc Serin and Ecem Baltan edited the article, and all authors reviewed and approved the final version of the article.
-
Competing interests: The authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: This prospective study was approved by the Institutional Ethics Committee of Sisli Hamidiye Etfal Training and Research Hospital Health Sciences University (date: April 22, 2020, No: 2,726).
References
1. WHO. COVID-19 weekly epidemiological update, 2022. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20220817_weekly_epi_update_105.pdf?sfvrsn=cfeb4c18_3&download=true.Suche in Google Scholar
2. McGonagle, D, Sharif, K, O’Regan, A, Bridgewood, C. Interleukin-6 use in COVID-19 pneumonia related macrophage activation syndrome. Autoimmun Rev 2020;19:102537. https://doi.org/10.1016/j.autrev.2020.102537.Suche in Google Scholar
3. Dosquet, C, Weill, D, Wautier, JL. Cytokines and thrombosis. J Cardiovasc Pharmacol 1995;25:S13–9. https://doi.org/10.1097/00005344-199500252-00004.Suche in Google Scholar
4. Thachil, J, Agarwal, S. Understanding the COVID-19 coagulopathy spectrum. Anaesthesia 2020;75:1432–6. https://doi.org/10.1111/anae.15141.Suche in Google Scholar
5. Grau, GE, de Moerloose, P, Bulla, O, Lou, J, Lei, Z, Reber, G, et al.. Haemostatic properties of human pulmonary and cerebral microvascular endothelial cells. Thromb Haemostasis 1997;77:585–90. https://doi.org/10.1055/s-0038-1656009.Suche in Google Scholar
6. MacLaren, R, Stringer, KA. Emerging role of anticoagulants and fibrinolytics in the treatment of acute respiratory distress syndrome. Pharmacotherapy 2007;27:860–73. https://doi.org/10.1592/phco.27.6.860.Suche in Google Scholar
7. Panigada, M, Tagliabue, P, Grasselli, G, Novembrino, C, Chantarangkul, V, Pesenti, A, et al.. Hypercoagulability of COVID-19 patients in the intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemostasis 2020;18:1738–42. https://doi.org/10.1111/jth.14850.Suche in Google Scholar
8. Yang, X, Yu, Y, Xu, J, Shu, H, Xia, J, Liu, H, et al.. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. https://doi.org/10.1016/s2213-2600(20)30079-5.Suche in Google Scholar
9. Blasi, F, Carmeliet, P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol 2002;3:932–43. https://doi.org/10.1038/nrm977.Suche in Google Scholar PubMed
10. Bhimraj, A, Morgan, RL, Shumaker, AH, Lavergne, V, Baden, L, Chi-Chung Cheng, V, et al.. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America; 2021. Version 4.3.0. Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/.Suche in Google Scholar
11. Kruithof, EK. Regulation of plasminogen activator inhibitor type 1 gene expression by inflammatory mediators and statins. Thromb Haemostasis 2008;100:969–75. https://doi.org/10.1160/th08-04-0269.Suche in Google Scholar
12. Nougier, C, Benoit, R, Simon, M, Desmurs-Clavel, H, Marcotte, G, Argaud, L, et al.. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J Thromb Haemostasis 2020;18:2215–9. https://doi.org/10.1111/jth.15016.Suche in Google Scholar PubMed PubMed Central
13. Umemura, Y, Yamakawa, K, Kiguchi, T, Nishida, T, Kawada, M, Fujimi, S. Hematological phenotype of COVID-19-induced coagulopathy: far from typical sepsis-induced coagulopathy. J Clin Med 2020;9:75. https://doi.org/10.3390/jcm9092875.Suche in Google Scholar PubMed PubMed Central
14. Tian, S, Hu, W, Niu, L, Liu, H, Xu, H, Xiao, SY. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 2020;15:700–4. https://doi.org/10.1016/j.jtho.2020.02.010.Suche in Google Scholar PubMed PubMed Central
15. Luo, WR, Yu, H, Gou, JZ, Li, XX, Sun, Y, Li, JX, et al.. Histopathologic findings in the explant lungs of a patient with COVID-19 treated with bilateral orthotopic lung transplant. Transplantation 2020;104:e329–31.10.1097/TP.0000000000003412Suche in Google Scholar PubMed
16. Takahashi, K, Uwabe, Y, Sawasaki, Y, Kiguchi, T, Nakamura, H, Kashiwabara, K, et al.. Increased secretion of urokinase-type plasminogen activator by human lung microvascular endothelial cells. Am J Physiol 1998;275:L47–54. https://doi.org/10.1152/ajplung.1998.275.1.l47.Suche in Google Scholar
17. Ware, LB, Matthay, MA, Parsons, PE, Thompson, BT, Januzzi, JL, Eisner, MD. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med 2007;35:1821–8. https://doi.org/10.1097/01.CCM.0000221922.08878.49.Suche in Google Scholar PubMed
18. Groeneveld, AB, Thijs, LG. Increased systemic microvascular permeability in septic shock. Prog Clin Biol Res 1987;236A:487–94.10.1007/978-3-642-83108-9_5Suche in Google Scholar
19. Gralinski, LE, Iii, AB, Jeng, S. Mechanisms of severe acute respiratory syndrome coronavirus. MBio 2013;4:1–12. https://doi.org/10.1128/mBio.00271-13.Suche in Google Scholar PubMed PubMed Central
20. Wu, YP, Wei, R, Liu, ZH, Chen, B, Lisman, T, Ren, DL, et al.. Analysis of thrombotic factors in severe acute respiratory syndrome (SARS) patients. Thromb Haemostasis 2006;96:100–1. https://doi.org/10.1160/TH05-12-0827.Suche in Google Scholar PubMed
21. Wang, J, Hajizadeh, N, Moore, EE, McIntyre, RC, Moore, PK, Veress, LA, et al.. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemostasis 2020;18:1752–5. https://doi.org/10.1111/jth.14828.Suche in Google Scholar PubMed PubMed Central
22. Glas, GJ, Van Der Sluijs, KF, Schultz, MJ, Hofstra, JJ, Van Der Poll, T, Levi, M. Bronchoalveolar hemostasis in lung injury and acute respiratory distress syndrome. J Thromb Haemostasis 2013;11:17–25. https://doi.org/10.1111/jth.12047.Suche in Google Scholar PubMed
23. Ye, Q, Wang, B, Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’’ in COVID-19. J Infect 2020;80:607–13. https://doi.org/10.1016/j.jinf.2020.03.037.Suche in Google Scholar PubMed PubMed Central
24. Recalcati, S, Invernizzi, P, Arosio, P, Cairo, G. New functions for an iron storage protein: the role of ferritin in immunity and autoimmunity. J Autoimmun 2008;30:84–9. https://doi.org/10.1016/j.jaut.2007.11.003.Suche in Google Scholar PubMed
25. Dolhnikoff, M, Duarte-Neto, AN, de Almeida Monteiro, RA, Ferraz da Silva, LF, de Oliveira, EP, Nascimento Saldiva, PH, et al.. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemostasis 2020;18:1517–9. https://doi.org/10.1111/jth.14844.Suche in Google Scholar PubMed PubMed Central
26. Ruan, Q, Yang, K, Wang, W, Jiang, L, Song, J. Clinical predictors of mortality due to COVID -19 based on analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–8. https://doi.org/10.1007/s00134-020-05991-x.Suche in Google Scholar PubMed PubMed Central
27. Ranucci, M, Ballotta, A, Di Dedda, U, Baryshnikova, E, Dei Poli, M, Resta, M, et al.. The procoagulant pattern if patients with COVID -19 acute respiratory distress syndrome. J Thromb Haemostasis 2020;18:1747–51. https://doi.org/10.1111/jth.14854.Suche in Google Scholar PubMed
28. Zhang, P, Shi, L, Xu, J, Wang, Y, Yang, H. Elevated interleukin-6 and adverse outcomes in COVID-19 patients: a meta-analysis based on adjusted effect estimates. Immunogenetics 2020;72:431–7. https://doi.org/10.1007/s00251-020-01179-1.Suche in Google Scholar PubMed PubMed Central
29. Cheng, L, Li, H, Li, L, Liu, C, Yan, S, Chen, H, et al.. Ferritin in the coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Lab Anal 2020;34:1–18. https://doi.org/10.1002/jcla.23618.Suche in Google Scholar PubMed PubMed Central
30. Yağcı, S, Serin, E, Acicbe, Ö, Zeren, Mİ, Odabaşı, MS. The relationship between serum erythropoietin, hepcidin, and haptoglobin levels with disease severity and other biochemical values in patients with COVID-19. Int J Lab Hematol 2021;43:142–51. https://doi.org/10.1111/ijlh.13479.Suche in Google Scholar PubMed PubMed Central
31. Shah, S, Shah, K, Patel, SB, Patel, FS, Osman, M, Velagapudi, P, et al.. Elevated D-dimer levels are associated with increased risk of mortality in coronavirus disease 2019: a systematic review and meta-analysis. Cardiol Rev 2020;28:295–2. https://doi.org/10.1097/crd.0000000000000330.Suche in Google Scholar
32. Kang, S, Tanaka, T, Inoue, H, Ono, C, Hashimoto, S, Kioi, Y, et al.. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci U S A 2020;117:22351–6. https://doi.org/10.1073/pnas.2010229117.Suche in Google Scholar PubMed PubMed Central
33. Nagata, K, Shomoda, K. Myeloproliferative diseases caused by JAK2 mutation. Rinsho Byori 2009;57:357–64.Suche in Google Scholar
34. Frank, DA. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett 2007;251:199–10. https://doi.org/10.1016/j.canlet.2006.10.017.Suche in Google Scholar PubMed
35. Matsuyama, T, Kubli, SP, Yoshinaga, SK, Pfeffer, K, Mak, TW. An aberrant STAT pathway is central to COVID-19. Cell Death Differ 2020;27:3209–25. https://doi.org/10.1038/s41418-020-00633-7.Suche in Google Scholar PubMed PubMed Central
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Editorial
- COP27 climate change conference: urgent action needed for Africa and the world
- Review Article
- Clinical laboratory use of liquid chromatography mass spectrometry
- Research Articles
- Verification of enzymatic ethanol analysis method and method comparison with headspace gas chromatography
- Investigation of GHRL (rs4684677), FTO (rs8044769) and PGC1Α (rs8192678) polymorphisms in type 2 diabetic Turkish population
- Roles of OLR1 and IL17A variants on clinical phenotypes of Turkish patients undergoing coronary artery bypass surgery
- Cytokine gene polymorphism frequencies in Turkish population living in Marmara region
- Evaluation of BRCA1/2 gene mutations in patients with high-risk breast and/or ovarian cancer in Turkey
- Impacts of long noncoding RNA MALAT1 on LPS-induced periodontitis via modulating miR-155/SIRT1 axis
- Proteomics characterization of the adenovirus VA1 non-coding RNA on the landscape of cellular proteome
- Modulatory effect of pomegranate extract on TRPA1, TRPM2 and caspase-3 expressions in colorectal cancer induction of mice
- EpCAM is critical for tumor proliferation and oxaliplatin chemoresistance in EpCAMhigh/CD44+ colorectal cancer stem cells
- Prognostic factors in thrombotic thrombocytopenic purpura
- Possible effects of clinoptilolite on small intestinal ischemia-reperfusion injury caused by experimental mesenteric artery occlusion
- The relationship between pregnancies complicated with fetal growth restriction and umbilical cord blood endocan concentrations
- Sildenafil for the treatment of necrotizing enterocolitis: an experimental study
- The importance of LDH/Albumin, LDH/Lymphocyte, and LDH/Platelet ratios in the evaluation of COVID-19 B.1.1.7 variant
- The relationship between serum vitamin D and antibody response following two doses of inactivated COVID-19 vaccine
- The relationship between plasminogen activator inhibitor-1 levels and the course of disease in COVID-19 patients
- Could tear be an alternative specimen for SARS-CoV-2 detection?
- Case Report
- A case of falsely elevated D-dimer result
Artikel in diesem Heft
- Frontmatter
- Editorial
- COP27 climate change conference: urgent action needed for Africa and the world
- Review Article
- Clinical laboratory use of liquid chromatography mass spectrometry
- Research Articles
- Verification of enzymatic ethanol analysis method and method comparison with headspace gas chromatography
- Investigation of GHRL (rs4684677), FTO (rs8044769) and PGC1Α (rs8192678) polymorphisms in type 2 diabetic Turkish population
- Roles of OLR1 and IL17A variants on clinical phenotypes of Turkish patients undergoing coronary artery bypass surgery
- Cytokine gene polymorphism frequencies in Turkish population living in Marmara region
- Evaluation of BRCA1/2 gene mutations in patients with high-risk breast and/or ovarian cancer in Turkey
- Impacts of long noncoding RNA MALAT1 on LPS-induced periodontitis via modulating miR-155/SIRT1 axis
- Proteomics characterization of the adenovirus VA1 non-coding RNA on the landscape of cellular proteome
- Modulatory effect of pomegranate extract on TRPA1, TRPM2 and caspase-3 expressions in colorectal cancer induction of mice
- EpCAM is critical for tumor proliferation and oxaliplatin chemoresistance in EpCAMhigh/CD44+ colorectal cancer stem cells
- Prognostic factors in thrombotic thrombocytopenic purpura
- Possible effects of clinoptilolite on small intestinal ischemia-reperfusion injury caused by experimental mesenteric artery occlusion
- The relationship between pregnancies complicated with fetal growth restriction and umbilical cord blood endocan concentrations
- Sildenafil for the treatment of necrotizing enterocolitis: an experimental study
- The importance of LDH/Albumin, LDH/Lymphocyte, and LDH/Platelet ratios in the evaluation of COVID-19 B.1.1.7 variant
- The relationship between serum vitamin D and antibody response following two doses of inactivated COVID-19 vaccine
- The relationship between plasminogen activator inhibitor-1 levels and the course of disease in COVID-19 patients
- Could tear be an alternative specimen for SARS-CoV-2 detection?
- Case Report
- A case of falsely elevated D-dimer result

