Abstract
Background
To investigate the interaction of pendimethalin (PM), a commonly used herbicide, with various mammalian serum albumins.
Methods
The interactions of PM with serum albumins of bovine (BSA), sheep (SSA), porcine (PSA) and rabbit (RbSA) were studied using fluorescence quenching titration and site marker displacement experiments.
Results
A comparison of the PM-induced quenching of the fluorescence of these albumins with that published for human serum albumin (HSA) showed similarity between BSA and HSA. The PM binding affinity of these albumins was found to follow the order: SSA>BSA>RbSA>PSA. Warfarin (WFN) displacement results also suggested similar displacing action of PM on WFN-BSA complex, when compared to the published results on WFN-HSA complex.
Conclusion
The results suggested close similarity between BSA and HSA in terms of PM binding characteristics and hence bovine can be selected as a suitable animal model for further toxicological studies of PM.
Öz
Amaç
Yaygın olarak kullanılan bir herbisit olan pendimethalin (PM) ile çeşitli memeli serum albüminlerinin etkileşimini araştırmak.
Gereç ve Yöntem
PM’nin sığır (BSA), koyun (SSA), domuz (PSA) ve tavşan (RbSA) serum albüminleri ile etkileşimleri floresan söndürme titrasyonu ve yer işareti deplasman deneyleri kullanılarak incelenmiştir.
Bulgular
Bu albüminlerin PM kaynaklı floresan söndürmesi ile insan serum albümin (HSA) için yayınlanmış değerlerin karşılaştırması, BSA ve HSA arasında benzerlik olduğunu gösterdi. Bu albüminlere PM bağlanma afinitesinin şu sırayı takip ettiği bulunmuştur: SSA>BSA>RbSA>PSA. Varfarin (WFN) deplasman sonuçları, WFN-HSA kompleksi üzerine yayınlanmış sonuçlarla karşılaştırıldığında, WFN-BSA kompleksi üzerinde PM’nin benzer yer değiştirme aktivitesini ortaya koymuştur.
Sonuç
Sonuçlar, PM bağlanma özellikleri açısından BSA ve HSA arasında yakın benzerlik olduğunu ve dolayısıyla sığırın, PM’nin ileri toksikolojik çalışmaları için uygun bir hayvan modeli olarak seçilebileceğini göstermiştir.
Introduction
Pendimethalin (3,4-dimethyl-2,6-dinitro-N-pentan-3-ylaniline, PM) belongs to a class of dinitroanilinic herbicides and is being used to protect food crops (Figure 1). Its use in agriculture is widespread and is approved in most countries. However, PM is also known for its adverse effects towards various living organisms, which include soil fauna and fishes [1], [2], [3], [4]. The degradation of PM in soil has been described as a slow process with a half-life period of 78–111 days [5]. The common use of PM in growing crops and its persistency in the soil contribute to soil and water contamination, through which it gets access to human and animal systems [6], [7]. Environmental Protection Agency of the United States of America (US-EPA) has classified PM as a Group C possible human carcinogen [8]. Liver enlargement and hepatic lesions have been noticed in dogs upon oral administration [9]. Toxicity studies of PM have shown increased level of reactive oxygen species in Chinese hamster lung fibroblast, V79 cells [10]. PM has also been found genotoxic to V79 cells as well as human peripheral lymphocytes [10]. Increased levels of antioxidant enzymes in rats exposed to PM have also indicated cellular toxicity of PM [11].
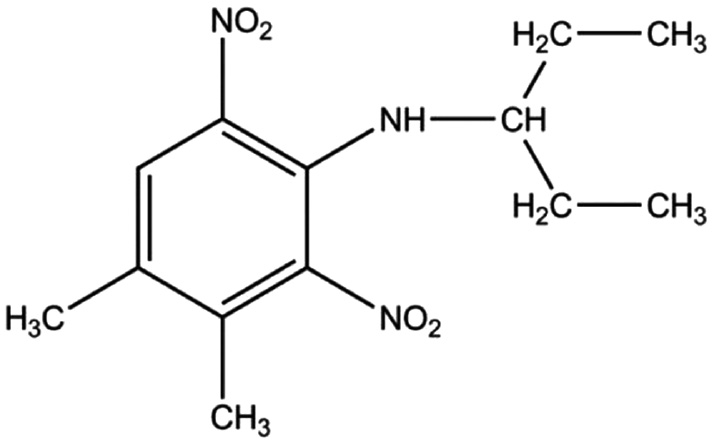
Chemical structure of PM.
The toxicological effects of PM on the human system can be predicted from the animal model study, which may provide useful information on the compound’s toxicity [12], [13]. This makes the selection of an animal model an important subject. In principle, this selection is based on the similarity of the toxicokinetics/toxicodynamics of PM, including its transport in the blood circulation, between animal and human systems [14]. Most of the exogenous compounds including toxins are being transported in the circulation through serum albumin, which carries them to different target organs for further metabolism [15]. Therefore, it is imperative to check the similarity in the transport of any ligand in the blood circulation of human and animal systems [16]. Recently, we have published data on the PM binding characteristics of human serum albumin (HSA) [17]. However, results on PM binding to the mammalian albumins are lacking. In view of this, present study was undertaken and binding analysis of PM with serum albumins of different mammalian species was made. Here, we present our results on the binding characteristics of PM towards serum albumins of bovine (BSA), porcine (PSA), sheep (SSA) and rabbit (RbSA), using fluorescence spectroscopy as well as warfarin displacement experiments.
Materials and methods
Materials
Different serum albumins, viz. BSA (Lot # 011M7406V), SSA (Lot # 117K7540), PSA (Lot # 084K7636) and RbSA (Lot # 105K7565) were obtained from Sigma-Aldrich Co., (St. Louis, MO, USA). PM and warfarin (WFN) were also supplied by Sigma-Aldrich Co., (St. Louis, MO, USA). Other chemicals used were of analytical grade quality.
Sample preparation
Serum albumin stock solutions were prepared by dissolving ~22 mg of lyophilized powder in 10 mL of 60 mM sodium phosphate buffer, pH 7.4. The protein concentrations of different stock solutions were determined spectrophotometrically on a Shimadzu UV-2450 double beam spectrophotometer (Shimadzu Corp., Kyoto, Japan), using molar absorption coefficients at 280 nm, i.e. 43 827 M−1⋅cm−1 for BSA, 43 385 M−1⋅cm−1 for both PSA and RbSA and 42 925 M−1⋅cm−1 for SSA [18].
PM and WFN stock solutions were prepared by dissolving a known amount of these compounds in a fixed volume of absolute ethanol. The stock solutions were diluted before use to the desired concentrations, using 60 mM sodium phosphate buffer, pH 7.4.
PM-albumin titration
Binding of PM to different serum albumins was studied in the same way as described earlier [17], by titrating a fixed amount of serum albumin (3 μM) with increasing concentrations (0.9–4.5 μM with 0.9 μM intervals) of PM. The total volume of the mixture in each tube was made to 3.0 mL with 60 mM sodium phosphate buffer, pH 7.4. The fluorescence spectra were recorded after 1 h incubation at room temperature, in the wavelength range, 310–400 nm, after exciting the samples at 295 nm. Jasco FP-6500 spectrofluorometer (Jasco Corp., Tokyo, Japan) was used for fluorescence measurements using quartz cuvette of 1 cm path length.
Analysis of the titration data
The fluorescence data, obtained above were subjected to the inner filter effect correction, as suggested by Lakowicz [19]:
where Fcor and Fobs refer to the values for the corrected and the observed fluorescence intensity, respectively. Aex and Aem are the absorbance values at the excitation (295 nm) and the emission wavelengths (310–400 nm), respectively.
Values of the Stern-Volmer constant, Ksv were obtained by treating the fluorescence quenching data according to the following equation [19].
where F0 and F are the fluorescence intensity values of the serum albumin, obtained in the absence and the presence of the quencher (PM), respectively. [Q] refers to the quencher concentration, kq, the bimolecular quenching rate constant and τ0 is the fluorescence lifetime of serum albumins, taken as 10−8 s [19].
The double logarithmic plot, based on the following equation [20] was used to determine the values of the association constant, Ka.
where [LT] and [PT] are the total ligand and protein concentrations, respectively.
The Gibbs free energy change (∆G) of the binding reaction was obtained from the following equation, using the values of the gas constant, R and absolute temperature, T as 8.3145 J⋅mol−1⋅K−1 and 298 K, respectively.
Warfarin displacement studies
Equimolar concentrations (3 μM each) of WFN, Sudlow’s site I marker and albumin were incubated for 1 h at room temperature (25°C). Increasing concentrations (0–4.5 μM with 0.9 μM intervals) of PM were then added to WFN-albumin mixture and the fluorescence spectra were recorded in the wavelength range, 360–480 nm after 1 h incubation at room temperature, using an excitation wavelength of 335 nm.
Results and discussion
Fluorescence quenching titration
The fluorescence quenching titration results of different serum albumins, i.e. BSA, SSA, PSA and RbSA, obtained in the presence of increasing PM concentrations are shown in Figure 2A–D. Quantitative differences in the values of the fluorescence intensity, obtained with these albumins can be ascribed to the number of tryptophan (Trp) residues in these proteins, that contribute towards the fluorescence intensity upon excitation at 295 nm [19]. BSA, SSA and PSA with two Trp residues produced higher fluorescence intensity (Figure 2A–C, Table 1) compared to RbSA (Figure 2D, Table 1), which possesses single Trp residue [21]. On the other hand, the emission maxima of these albumins were found to fall in the range, 341–345 nm, characteristic of Trp residue(s) (Table 1) [22].
![Figure 2: Fluorescence spectra of different serum albumins excited at 295 nm in the absence and presence of various PM concentrations. [Albumin]=3 μM, [PM]=0–4.5 μM with 0.9 μM intervals (1–6), T=25°C. (A) BSA, (B) SSA, (C) PSA and (D) RbSA.](/document/doi/10.1515/tjb-2018-0031/asset/graphic/j_tjb-2018-0031_fig_002.jpg)
Fluorescence spectra of different serum albumins excited at 295 nm in the absence and presence of various PM concentrations. [Albumin]=3 μM, [PM]=0–4.5 μM with 0.9 μM intervals (1–6), T=25°C. (A) BSA, (B) SSA, (C) PSA and (D) RbSA.
Fluorescence characteristics of different mammalian serum albumins in the absence and presence of PM.
| Albumin | Emission maxima (nm) | Fluorescence intensity (a.u.) | % Quenching ([PM]/[albumin]=1.5:1) |
|---|---|---|---|
| SSA | 341 | 378 | 50.9±2.3 |
| BSA | 345 | 424 | 36.3±2.6 |
| RbSA | 343 | 125 | 26.0±1.6 |
| PSA | 343 | 384 | 19.4±1.6 |
PM, pendimethalin; SSA, sheep serum albumin; BSA, bovine serum albumin; RbSA, rabbit serum albumin; PSA, porcine serum albumin.
As evident from Figure 2A–D, addition of PM to these albumins produced quenching in the fluorescence intensity in a concentration dependent manner. Such quenching was suggestive of the binding of PM to these albumins, as similar quenching was observed with the binding of many ligands including PM to serum albumins [17], [23], [24], [25], [26], [27]. Values of the fluorescence intensity at the emission maxima, obtained at different PM concentrations were transformed into the relative fluorescence intensity, as described earlier [28]. Figure 3 shows change in the relative fluorescence intensity at the emission maxima with increasing PM concentrations, obtained with different serum albumins. All these albumins showed gradual decrease in the fluorescence intensity with increasing PM concentrations. However, a comparison of the quenching pattern, obtained with these albumins suggests lesser extent of the fluorescence quenching with PSA and RbSA compared to that observed with SSA and BSA at the highest PM/albumin molar ratio (1.5:1). Quantitatively, about 51% and 36% quenching was observed with SSA and BSA, respectively, compared to RbSA and PSA, showing 26% and 19% quenching, respectively (Figure 3, Table 1). Interestingly, magnitude of the fluorescence quenching characteristics of BSA (36%) was found similar to that observed with HSA (39%) [17].

Comparison of the fluorescence quenching behavior of different serum albumins at their emission maxima with increasing PM concentrations.
Binding characteristics of PM-albumin interaction
Dynamic or static quenching mechanisms seem to be responsible for the ligand-induced quenching of the protein fluorescence. Molecular collisions between the fluorophore and the quencher may lead to dynamic quenching, while static quenching is resulted from the complex formation between the fluorophore and the quencher [19]. In order to characterize PM-induced quenching, fluorescence quenching data were treated according to Eq. 2 and the KSV values were obtained (Table 2) from the slope of the linear Stern-Volmer plots (Figure 4A). In line to the quenching pattern (Table 1), SSA and BSA showed higher KSV values compared to those obtained with PSA and RbSA (Table 2). The values of the bimolecular quenching rate constant, kq, as obtained from the KSV values had fallen in the order of 1013 M−1⋅s−1 for SSA and BSA, and 1012 M−1⋅s−1 for RbSA and PSA. Values of kq higher than 1010 M−1⋅s−1 (diffusion-controlled limit) simply reflected complex formation between the ligand and the protein [19]. Thus, it appears that PM-induced fluorescence quenching might have resulted from static quenching mechanism, which supported the complex formation between PM and serum albumins.
Binding parameters for the interaction between PM and different serum albumins.
| Albumin | KSV (M–1) | Ka (M–1) | ∆G (kJ mol–1) |
|---|---|---|---|
| SSA | (2.44±0.04)×105 | (3.27±0.24)×105 | −31.5 |
| BSA | (1.29±0.14)×105 | (1.71±0.15)×105 | −29.9 |
| RbSA | (8.10±0.14)×104 | (9.74±0.31)×104 | −28.5 |
| PSA | (5.57±0.30)×104 | (8.88±0.08)×104 | −28.2 |
KSV, Stern-Volmer constant; Ka, association constant; ∆G, Gibbs free energy change; SSA, sheep serum albumin; BSA, bovine serum albumin; RbSA, rabbit serum albumin; PSA, porcine serum albumin.

(A) Stern-Volmer plots and (B) double logarithmic plots for the fluorescence quenching data of different serum albumins by PM. The symbols used for various serum albumins are the same as used in Figure 3.
Binding affinity of PM towards different serum albumins was determined by analyzing the fluorescence quenching data according to Eq. 3. The values of the association constant, Ka for different PM-albumin systems were obtained from the resulting double logarithmic plots (Figure 4B) and are given in Table 2. Based on the PM binding affinity, these albumins can be arranged in the order: SSA>BSA>RbSA>PSA. Although the sequence and protein characteristics of these albumins show high degree of similarity [29], binding affinity of ligands varies considerably among the species according to the degree of phylogenetic relationship [30]. Such species differences in ligand-albumin interaction have been reported earlier [26], [31], [32], [33], [34]. However, the binding affinity of BSA (Table 2) was revealed to be similar to the binding affinity of HSA [17]. The Gibbs free energy change (∆G) of the binding reaction was calculated using Eq. 4 and the negative values of ∆G for all PM-albumin systems (Table 2) suggested that the binding reactions were energetically feasible [35].
WFN displacement results
Warfarin is a well-defined marker that binds to HSA at site I, located in subdomain IIA [36]. Therefore, it is commonly used in characterizing the binding site of ligands on serum albumins [30]. As PM was found to bind to site I of HSA, based on site marker displacement and molecular modeling study [17], a comparison of the WFN displacing action of PM on the complexes of WFN with other serum albumins was made. Spectrum 1 in Figure 5 shows the fluorescence spectrum of WFN-SSA complex, when excited at 335 nm. The spectrum was characterized by the presence of an emission maxima at 383 nm. Addition of increasing concentrations of PM displaced WFN from its binding site, as reflected from the progressive decrease in the fluorescence intensity with increasing PM concentrations (spectra 2–6 in Figure 5). In this range of wavelength, the fluorescence signals produced by SSA, PM and PM-SSA mixture (spectra ‘b’–‘d’ in Figure 5) were insignificant. Even free WFN (spectrum ‘a’ in Figure 5) produced relatively weaker signal at 383 nm compared to WFN-SSA complex. WFN displacement results, obtained with other serum albumins were qualitatively similar to those obtained with SSA, but showed significant differences in the extent of fluorescence quenching. This can be clearly seen from the decrease in the relative fluorescence intensity of different WFN-albumin (1:1) complexes at 383 nm with increasing PM concentrations, as shown in Figure 6. Values of the percentage fluorescence quenching of different WFN-albumin complexes induced by 4.5 μM PM are listed in Table 3. Complexes of WFN with BSA and RbSA showed close similarity to HSA in terms of percentage quenching, as 27% and 31% quenching were observed with BSA and RbSA, respectively, against 34% quenching observed with HSA [17]. On the other hand, SSA showed relatively higher degree (48%) of quenching, whereas a lesser degree (23%) of quenching was displayed by PSA.
![Figure 5: Fluorescence spectra of WFN-SSA complex with increasing PM concentrations. [WFN]=[SSA]=3 μM, [PM]=0–4.5 μM with 0.9 μM intervals (1–6), λex=335 nm, T=25°C. The spectra labeled as ‘a’, ‘b’, ‘c’ and ‘d’ refer to the fluorescence spectra of WFN, SSA, PM and PM-SSA mixture, respectively.](/document/doi/10.1515/tjb-2018-0031/asset/graphic/j_tjb-2018-0031_fig_005.jpg)
Fluorescence spectra of WFN-SSA complex with increasing PM concentrations. [WFN]=[SSA]=3 μM, [PM]=0–4.5 μM with 0.9 μM intervals (1–6), λex=335 nm, T=25°C. The spectra labeled as ‘a’, ‘b’, ‘c’ and ‘d’ refer to the fluorescence spectra of WFN, SSA, PM and PM-SSA mixture, respectively.

Fluorescence quenching of different WFN-albumin complexes at 383 nm with increasing PM concentrations. The symbols used for various serum albumins are the same as used in Figure 3.
Fluorescence quenching of different WFN-albumin (1:1) complexes in the presence of 4.5 μM PM.
| Albumin | % Quenching |
|---|---|
| SSA | 47.8±1.4 |
| BSA | 27.4±0.2 |
| RbSA | 31.0±1.1 |
| PSA | 23.3±1.3 |
SSA, sheep serum albumin; BSA, bovine serum albumin; RbSA, rabbit serum albumin; PSA, porcine serum albumin.
Conclusion
Among the serum albumins of different mammalian species used in this study, BSA was found to be closely similar to HSA, as evident from the PM-induced fluorescence quenching pattern, binding affinity as well as WFN displacement results. Thus, bovine can be considered as a suitable animal model for further exploration on toxicological effects of PM.
Acknowledgements
This study was financially supported by the Ministry of Higher Education, Funder Id: 10.13039/501100003093, Government of Malaysia and the University of Malaya, in the form of High Impact Research Grant UM.C/625/1/HIR/MOHE/SC/02. We extend our appreciation to the Dean, Faculty of Science and the Head, Institute of Biological Sciences for providing all necessary facilities.
Conflict of interest: The authors declare no conflict of interest in the preparation of this article.
References
1. Belden JB, Phillips TA, Clark BW, Coats JR. Toxicity of pendimethalin to nontarget soil organisms. Bull Environ Contam Toxicol 2005;74:769–76.10.1007/s00128-005-0648-5Search in Google Scholar PubMed
2. Abd-Algadir MI, Idris OF, Elkhier MK. Effect of pendimethalin herbicide on fish (Tilapia nilotica) skeletal muscles, gills and its influence on human. World J Life Sci Med Res 2011;1:5–10.Search in Google Scholar
3. El-Sayed YS, Samak DH, Abou-Ghanema IY, Soliman MK. Physiological and oxidative stress biomarkers in the freshwater monosex Nile tilapia, Oreochromis niloticus L., exposed to pendimethalin-based herbicide. Environ Toxicol 2015;30: 430–8.10.1002/tox.21919Search in Google Scholar PubMed
4. Tabassum H, Ashafaq M, Khan J, Shah MZ, Raisuddin S, Parvez S. Short term exposure of pendimethalin induces biochemical and histological perturbations in liver, kidney and gill of freshwater fish. Ecol Indic 2016;63:29–36.10.1016/j.ecolind.2015.11.044Search in Google Scholar
5. Zimdahl RL, Catizone P, Butcher AC. Degradation of pendimethalin in soil. Weed Sci 1984;32:408–12.10.1017/S004317450005921XSearch in Google Scholar
6. Edwards CA, Adams RS. Persistent pesticides in the environment. Crit Rev Environ Sci Technol 1970;1:7–67.10.1080/10643387009381563Search in Google Scholar
7. Belden JB, Phillips TA, Henderson KL, Clark BW, Lydy MJ, Coats JR. Persistence, mobility, and bioavailability of pendimethalin and trifluralin in soil. In: Coats JR, Yamamoto H, editors. Environmental fate and effects of pesticides. Washington DC: American Chemical Society, 2003:167–77.10.1021/bk-2003-0853.ch010Search in Google Scholar
8. U.S. Environmental Protection Agency (US-EPA). Reregistration Eligibility Decision (RED) – Pendimethalin List A Case 0187. Washington DC: US EPA-National Service Center for Environmental Publications (NSCEP), 1997:1–227.Search in Google Scholar
9. Integrated Risk Information System Chemical Assessment Summary. Pendimethalin. CASRN 40487-42-1. Available at: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0292_summary.pdf. (Accessed: 14 May 2018).Search in Google Scholar
10. Kilic ZS, Aydin S, Bucurgat UU, Basaran N. In vitro genotoxicity assessment of dinitroaniline herbicides pendimethalin and trifluralin. Food Chem Toxicol 2018;113:90–8.10.1016/j.fct.2018.01.034Search in Google Scholar PubMed
11. Osman KA, Salama AK, Salama MS, Albakary AS. Oxidative stress and pharmacokinetics of pendimethalin in female rats. J Clin Toxicol 2016;6:1000330.10.4172/2161-0495.1000330Search in Google Scholar
12. Cicchetti F, Drouin-Ouellet J, Gross RE. Environmental toxins and Parkinson’s disease: what have we learned from pesticide-induced animal models? Trends Pharmacol Sci 2009;30:475–83.10.1016/j.tips.2009.06.005Search in Google Scholar PubMed
13. Thiel C, Schneckener S, Krauss M, Ghallab A, Hofmann U, Kanacher, T, et al. A systematic evaluation of the use of physiologically based pharmacokinetic modeling for cross-species extrapolation. J Pharm Sci 2015;104:191–206.10.1002/jps.24214Search in Google Scholar PubMed
14. Lin JH. Species similarities and differences in pharmacokinetics. Drug Metab Dispos 1995;23,1008–21.10.1016/S0090-9556(25)06742-XSearch in Google Scholar
15. Peters T, Jr. All about albumin: biochemistry, genetics, and medical applications. San Diego: Academic Press, 1996.Search in Google Scholar
16. Poór M, Li Y, Matisz G, Kiss L, Kunsági-Máté S, Kőszegi T. Quantitation of species differences in albumin–ligand interactions for bovine, human and rat serum albumins using fluorescence spectroscopy: a test case with some Sudlow’s site I ligands. J Lumin 2014;145:767–73.10.1016/j.jlumin.2013.08.059Search in Google Scholar
17. Lee WQ, Affandi IS, Feroz SR, Mohamad SB, Tayyab S. Evaluation of pendimethalin binding to human serum albumin: insights from spectroscopic and molecular modeling approach. J Biochem Mol Toxicol 2016;31:e21839.10.1002/jbt.21839Search in Google Scholar
18. Khan JM, Chaturvedi SK, Khan RH. Elucidating the mode of action of urea on mammalian serum albumins and protective effect of sodium dodecyl sulfate. Biochem Biophys Res Commun 2013;441:681–8.10.1016/j.bbrc.2013.10.055Search in Google Scholar
19. Lakowicz JR. Principles of fluorescence spectroscopy, 3rd ed. New York: Plenum, 2006.10.1007/978-0-387-46312-4Search in Google Scholar
20. Bi S, Ding L, Tian Y, Song D, Zhou X, Liu X, et al. Investigation of the interaction between flavonoids and human serum albumin. J Mol Struct 2004;703:37–45.10.1016/j.molstruc.2004.05.026Search in Google Scholar
21. Pajot P. Fluorescence of proteins in 6-M guanidine hydrochloride. Eur J Biochem 1976;63:263–9.10.1111/j.1432-1033.1976.tb10228.xSearch in Google Scholar
22. Vivian JT, Callis PR. Mechanisms of tryptophan fluorescence shifts in proteins. Biophys J 2001;80:2093–109.10.1016/S0006-3495(01)76183-8Search in Google Scholar
23. Han XL, Mei P, Liu Y, Xiao Q, Jiang FL, Li R. Binding interaction of quinclorac with bovine serum albumin: a biophysical study. Spectrochim Acta Pt A- Molec Biomolec Spectr 2009;74:781–7.10.1016/j.saa.2009.08.018Search in Google Scholar PubMed
24. Samari F, Hemmateenejad B, Shamsipur M, Rashidi M, Samouei H. Affinity of two novel five-coordinated anticancer Pt (II) complexes to human and bovine serum albumins: a spectroscopic approach. Inorg Chem 2012;51:3454–64.10.1021/ic202141gSearch in Google Scholar PubMed
25. Kamtekar N, Pandey A, Agrawal N, Pissurlenkar RR, Borana M, Ahmad B. Interaction of multimicrobial synthetic inhibitor 1,2-bis (2-benzimidazolyl)-1,2-ethanediol with serum albumin: spectroscopic and computational studies. PLoS One 2013;8:e53499.10.1371/journal.pone.0053499Search in Google Scholar PubMed PubMed Central
26. Feroz SR, Rumana A, Malek SN, Tayyab S. A comparative analysis on the binding characteristics of various mammalian albumins towards a multitherapeutic agent, pinostrobin. Exp Anim 2015;64:101–8.10.1538/expanim.14-0053Search in Google Scholar
27. Poureshghi F, Ghandforoushan P, Safarnejad A, Soltani S. Interaction of an antiepileptic drug, lamotrigine with human serum albumin (HSA): Application of spectroscopic techniques and molecular modeling methods. J Photochem Photobiol B 2017;166:187–92.10.1016/j.jphotobiol.2016.09.046Search in Google Scholar
28. Khan MM, Muzammil S, Tayyab S. Role of salt bridge(s) in the binding and photoconversion of bilirubin bound to high affinity site on human serum albumin. Biochim Biophys Acta-Protein Struct Mol Enzymol 2000;1479:103–13.10.1016/S0167-4838(00)00050-9Search in Google Scholar
29. Michaud FT, Garnier A, Lemieux L, Duchesne C. Multivariate analysis of single quadrupole LC-MS spectra for routine characterization and quantification of intact proteins. Proteomics 2009;9:512–20.10.1002/pmic.200800300Search in Google Scholar
30. Day YS, Myszka DG. Characterizing a drug’s primary binding site on albumin. J Pharm Sci 2003;92:333–43.10.1002/jps.10293Search in Google Scholar
31. Kosa T, Maruyama T, Otagiri M. Species differences of serum albumins: I. Drug binding sites. Pharmaceut Res 1997;14:1607–12.10.1023/A:1012138604016Search in Google Scholar
32. Colclough N, Ruston L, Wood JM, MacFaul PA. Species differences in drug plasma protein binding. Med Chem Comm 2014;5:963–7.10.1039/C4MD00148FSearch in Google Scholar
33. Rafols C, Amezqueta S, Fuguet E, Bosch E. Molecular interactions between warfarin and human (HSA) or bovine (BSA) serum albumin evaluated by isothermal titration calorimetry (ITC), fluorescence spectrometry (FS) and frontal analysis capillary electrophoresis (FA/CE). J Pharm Biomed Anal 2018;150:452–9.10.1016/j.jpba.2017.12.008Search in Google Scholar
34. Tayyab S, Khan NJ, Khan MA, Kumar Y. Behavior of various mammalian albumins towards bilirubin binding and photochemical properties of different bilirubin–albumin complexes. Int J Biol Macromol 2003;31:187–93.10.1016/S0141-8130(02)00081-8Search in Google Scholar
35. Ross PD, Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 1981;20:3096–102.10.1021/bi00514a017Search in Google Scholar PubMed
36. Curry S. Beyond expansion: structural studies on the transport roles of human serum albumin. Vox Sang 2002;83:315–9.10.1111/j.1423-0410.2002.tb05326.xSearch in Google Scholar PubMed
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Short Communication
- Acetone-water mixture is a competent solvent to extract phenolics and antioxidants from four organs of Eucalyptus camaldulensis
- Research Articles
- Proteases from Calotropis gigantea stem, leaf and calli as milk coagulant source
- A new method to quantify atmospheric Poaceae pollen DNA based on the trnT-F cpDNA region
- Expression of a functional recombinant vascular endothelial growth factor 165 (VEGF165) in Arabidopsis thaliana
- Computational exploration of antiviral activity of phytochemicals against NS2B/NS3 proteases from dengue virus
- Investigation of antioxidant, cytotoxic, tyrosinase inhibitory activities, and phenolic profiles of green, white, and black teas
- DFR and PAL gene transcription and their correlation with anthocyanin accumulation in Rhodomyrtus tomentosa (Aiton.) Hassk.
- Comparison of phenolic profiles and antioxidant activity of three Ornithogalum L. species
- Increasing the fermentation efficiency of Lactobacillus paracasei ssp. paracasei MIUG BL6 in a rye flour sourdough
- Determination of chemical composition, antibacterial and antioxidant properties of products obtained from carob and honey locust
- Chitinolytic Bacillus subtilis Ege-B-1.19 as a biocontrol agent against mycotoxigenic and phytopathogenic fungi
- Recycling fish skin for utilization in food industry as an effective emulsifier and foam stabilizing agent
- A novel, thermotolerant, extracellular PHB depolymerase producer Paenibacillus alvei PHB28 for bioremediation of biodegradable plastics
- Post-transcriptional regulation of miRNA-15a and miRNA-15b on VEGFR gene and deer antler cell proliferation
- Comparison of pendimethalin binding properties of serum albumins from various mammalian species
- Crocin (active constituent of saffron) improves CCl4-induced liver damage by modulating oxidative stress in rats
- Time dependent change of ethanol consumption biomarkers, ethyl glucuronide and ethyl sulphate, after single dose ethanol intake
- GC-MS analysis and biological activities of Thymus vulgaris and Mentha arvensis essential oil
- Immobilization and some application of α-amylase purified from Rhizoctonia solani AG-4 strain ZB-34
- Letter to the Editor
- Molecular crosstalk between Hog1 and calcium/CaM signaling
Articles in the same Issue
- Frontmatter
- Short Communication
- Acetone-water mixture is a competent solvent to extract phenolics and antioxidants from four organs of Eucalyptus camaldulensis
- Research Articles
- Proteases from Calotropis gigantea stem, leaf and calli as milk coagulant source
- A new method to quantify atmospheric Poaceae pollen DNA based on the trnT-F cpDNA region
- Expression of a functional recombinant vascular endothelial growth factor 165 (VEGF165) in Arabidopsis thaliana
- Computational exploration of antiviral activity of phytochemicals against NS2B/NS3 proteases from dengue virus
- Investigation of antioxidant, cytotoxic, tyrosinase inhibitory activities, and phenolic profiles of green, white, and black teas
- DFR and PAL gene transcription and their correlation with anthocyanin accumulation in Rhodomyrtus tomentosa (Aiton.) Hassk.
- Comparison of phenolic profiles and antioxidant activity of three Ornithogalum L. species
- Increasing the fermentation efficiency of Lactobacillus paracasei ssp. paracasei MIUG BL6 in a rye flour sourdough
- Determination of chemical composition, antibacterial and antioxidant properties of products obtained from carob and honey locust
- Chitinolytic Bacillus subtilis Ege-B-1.19 as a biocontrol agent against mycotoxigenic and phytopathogenic fungi
- Recycling fish skin for utilization in food industry as an effective emulsifier and foam stabilizing agent
- A novel, thermotolerant, extracellular PHB depolymerase producer Paenibacillus alvei PHB28 for bioremediation of biodegradable plastics
- Post-transcriptional regulation of miRNA-15a and miRNA-15b on VEGFR gene and deer antler cell proliferation
- Comparison of pendimethalin binding properties of serum albumins from various mammalian species
- Crocin (active constituent of saffron) improves CCl4-induced liver damage by modulating oxidative stress in rats
- Time dependent change of ethanol consumption biomarkers, ethyl glucuronide and ethyl sulphate, after single dose ethanol intake
- GC-MS analysis and biological activities of Thymus vulgaris and Mentha arvensis essential oil
- Immobilization and some application of α-amylase purified from Rhizoctonia solani AG-4 strain ZB-34
- Letter to the Editor
- Molecular crosstalk between Hog1 and calcium/CaM signaling

