A 39-year-old man was admitted with persistent abdominal and back pain, accompanied by nausea and vomiting for 19 days. Five years prior, he had been diagnosed with popliteal vein thrombosis and was subsequently confirmed to have antiphospholipid syndrome (APS). He had been on long-term oral warfarin therapy, which he had discontinued without medical guidance. At the onset of current symptoms, laboratory investigations at a local hospital revealed hyponatremia (serum sodium 123 mmol/L), markedly prolonged activated partial thromboplastin time (APTT, 102.3 s), and elevated inflammatory markers (C-reactive protein [CRP], 47.55 mg/L), and mild enlargement of the left adrenal gland on contrast-enhanced abdominal computed tomography. Low molecular weight heparin (LMWH) was interrupted due to evidence of adrenal hemorrhage (AH) and hematuria. Upon admission to our hospital, repeat enhanced CT revealed new-onset right-sided AH in addition to the left, appearing as a slightly hyperdense, non-enhancing area (Figure 1A, 1B, asterisk). Another wedge-shaped hypo-enhanced lesion was seen at the right kidney margin, suggesting ischemia (Figure 1C, yellow arrow). On delayed imaging, a patchy low-density shadow in the left adrenal vein (Figure 1D, red arrow) raised suspicion of a thrombosis. The diagnosis of APS was confirmed by persistently high titers of anticardiolipin antibody-IgG (ACL-IgG, 135.1 IU/mL), anti-β2 glycoprotein I antibody-IgG (aβ2GPI-IgG, 825.9 IU/mL), and lupus anticoagulant (LA, 2.16). Serum cortisol was decreased and adrenocorticotropin (ACTH) was increased. Microvascular involvement was also detected in myocardium (elevated enzymes and ischemic changes on perfusion magnetic resonance imaging [MRI]), skin (painful purplish-red rash on toes), and pancreas (elevated pancreatic enzymes), leading to the diagnosis of catastrophic APS (CAPS). Macrovascular involvement was thoroughly excluded. Despite the presence of bilateral AH, therapeutic LMWH was resumed, and methylprednisolone pulse therapy (1000 mg/d for 3 days) was applied, followed by oral prednisone (1.0 mg/kg/d, gradually tapered). Symptoms improved and laboratory parameters returned to normal. LMWH was transitioned to warfarin (international normalized ratio [INR] maintained 2–3), and cyclophosphamide (CTX, 100 mg/d orally) and hydroxychloroquine (HCQ, 0.2 g twice a day) were added. The patient’s condition remained stable during 4-month follow-up.
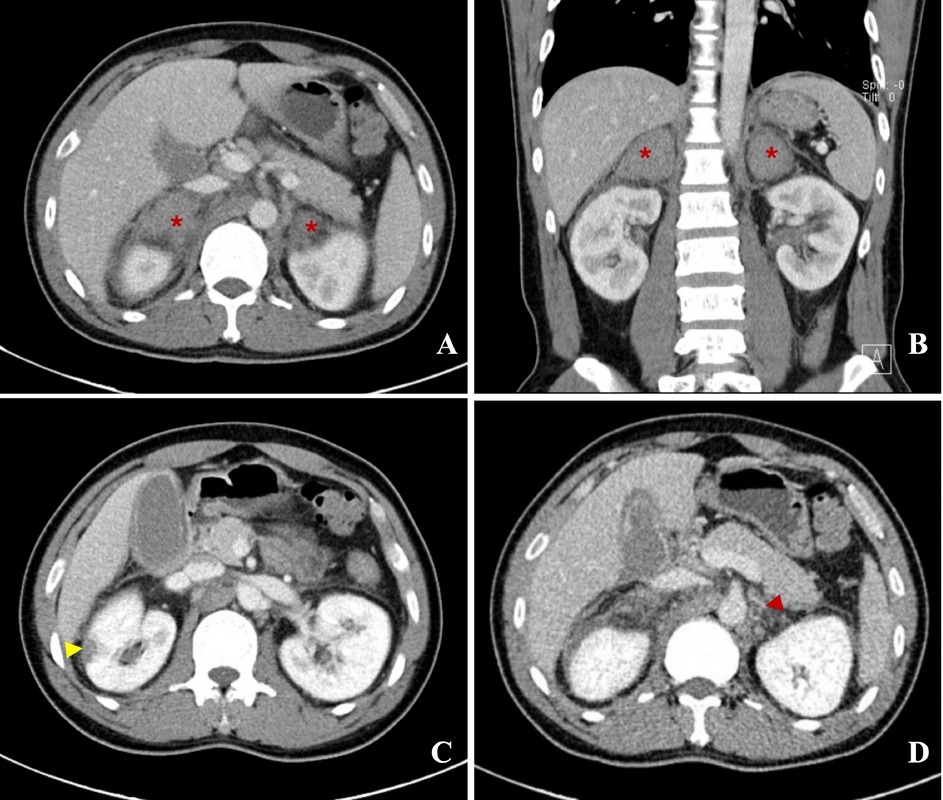
A, B: Transverse and coronal section of enhanced abdominal tomography (CT), showed clearly bilateral adrenal hemorrhage (AH), manifesting as slightly high-density shadow with no enhancement, marked with asterisk. C: A wedge-shaped hypo-enhanced lesion indicating ischemic change was seen at marginal of right kidney, marked with yellow arrow. D: A patchy low-density shadow was found in left adrenal vein, marked by red arrow, which might be a thrombosis.
AH is a rare clinical manifestation of APS (~1%),[1] yet holds diagnostic importance. It has been incorporated as a microvascular manifestation in the 2023 revised APS classification criteria [2] Although APS is a thrombotic disease, adrenal involvement in APS often presents as hemorrhage, attributed to the glands’ unique vascular architecture. Adrenal arteries form a rich supply, while venous drainage is limited, predisposing to thrombosis-induced congestion and hemorrhage.[3] Thrombosis in adrenal veins is difficult to visualize due to their small caliber, making early-stage imaging findings obscure, when combined with nonspecific symptoms, complicating the early detection of AH and adrenal vein thrombosis. However, AH as a manifestation of microvascular APS (MAPS), often implies an underlying CAPS, and is linked to elevated mortality.[4] Therefore, AH signs warrant immediate APS screening. Prompt anticoagulantion is critical, and glucocorticoid pulse therapy and immunosuppressants such as cyclophosphamide might also be necessary if CAPS is suspected.[5,6] This case underscores the clinical significance of APS-related AH and highlights the importance of its early recognition and timely intervention.
Funding statement: This study was supported by the Chinese National Key Technology R& D Program, Ministry of Science and Technology (2021YFC250130), Beijing Municipal Science& Technology Commission (No. Z201100005520022, 23, 25-27), CAMS Innovation Fund for Medical Sciences (CIFMS)(2023-I2M-C& T-B-051).
Acknowledgements
None.
-
Author contributions
L. Liu: Writing-Original draft preparation. C. Huang & Y. Jiang: Writing-Reviewing and Editing. Q. Wang: Supervision-Interpretation of Imaging. All authors have read and agreed to the published version of the manuscript.
-
Ethical approval
Ethical approval was obtained from the institutional ethical committee.
-
Informed consent
Images are de-identified, meanwhile informed consent was obtained from the patient to the publication of the images and clinical information.
-
Conflict of interest
All authors declare no conflict of interest.
-
Use of large language models, AI and machine learning tools
None declared.
-
Data availability statement
No additional data is available.
References
[1] Ruiz-Irastorza G, Crowther M, Branch W, et al. Antiphospholipid syndrome. Lancet. 2010;376:1498–1509.10.1016/S0140-6736(10)60709-XSearch in Google Scholar PubMed
[2] Barbhaiya M, Zuily S, Naden R, et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann Rheum Dis. 2023;82:1258–1270.10.1136/ard-2023-224609Search in Google Scholar PubMed
[3] Jiang W, Chen D, Yang D, et al. Adrenal Hemorrhage in Patients with Systemic Lupus Erythematosus and Antiphospholipid Syndrome: A Case Report and Literature Review. Int J Endocrinol. 2023;2023:6686168.10.1155/2023/6686168Search in Google Scholar PubMed PubMed Central
[4] Meade-Aguilar JA, Figueroa-Parra G, Yang JX, et al. Clinical presentation and outcomes in patients with antiphospholipid syndrome-associated adrenal hemorrhage. A multicenter cohort study and systematic literature review. Clin Immunol. 2024;260:109906.10.1016/j.clim.2024.109906Search in Google Scholar PubMed
[5] Erkan D. Expert Perspective: Management of Microvascular and Catastrophic Antiphospholipid Syndrome. Arthritis Rheumatol. 2021;73:1780–1790.10.1002/art.41891Search in Google Scholar PubMed
[6] Cervera R, Rodríguez-Pintó I, Espinosa G. The diagnosis and clinical management of the catastrophic antiphospholipid syndrome: A comprehensive review. J Autoimmun. 2018;92:1–11.10.1016/j.jaut.2018.05.007Search in Google Scholar PubMed
© 2025 Lingshan Liu, Qin Wang, Ying Jiang, Can Huang, published by De Gruyter on behalf of NCRC-DID
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Review
- Crystal deposition disease of the shoulder at uncommon sites: Diagnostic challenges and nomenclature issues in the absence of synovial fluid analysis
- Original Article
- Features of IgG4-related sclerosing mesenteritis: A Chinese cohort study and literature review
- CD209 gene polymorphism and its clinical correlation with susceptibility to rheumatoid arthritis among Egyptian patients: A case-control study
- Unveiling the dual nature of late-onset systemic lupus erythematosus: A cross-sectional study
- Letter to the Editor
- The mystery of the blue toe
- Spondyloarthritis recognition for primary care: A simplified diagnostic and referral pathway for general physicians
- Development and validation of the manual disease activity score (MDAS) for rheumatoid arthritis
- Images
- Hemorrhage or thrombosis? Adrenal involvements in a patient with antiphospholipid syndrome
Articles in the same Issue
- Review
- Crystal deposition disease of the shoulder at uncommon sites: Diagnostic challenges and nomenclature issues in the absence of synovial fluid analysis
- Original Article
- Features of IgG4-related sclerosing mesenteritis: A Chinese cohort study and literature review
- CD209 gene polymorphism and its clinical correlation with susceptibility to rheumatoid arthritis among Egyptian patients: A case-control study
- Unveiling the dual nature of late-onset systemic lupus erythematosus: A cross-sectional study
- Letter to the Editor
- The mystery of the blue toe
- Spondyloarthritis recognition for primary care: A simplified diagnostic and referral pathway for general physicians
- Development and validation of the manual disease activity score (MDAS) for rheumatoid arthritis
- Images
- Hemorrhage or thrombosis? Adrenal involvements in a patient with antiphospholipid syndrome

