Proceedings of cell-free noncoding RNA biomarker studies in liquid biopsy
-
Yumin Zhu
, Zhi John Lu
and Ning Gu
Abstract
Liquid biopsy has great application value in the field of precision medicine because of its non-invasiveness, sensitivity and dynamics. Cell-free RNA molecules are one of the emerging biomarkers that can be used for liquid biopsy, and cell-free non-coding RNAs have become main RNA molecular markers because of their high abundance and stability, as well as their regulatory roles in basic development. It provides clues for the diagnosis, prognosis and monitoring of a variety of complex diseases, including rheumatic and immune diseases. This article describes the characterization of cell-free non-coding RNAs and bioinformatics strategies, and summarizes cell-free non-coding RNA biomarkers associated with rheumatic and immune diseases. Prospects and reflections are made on the further research and clinical application of cell-free RNA markers.
Introduction
Cell-free RNA (cfRNA) was first discovered in plasma and serum in 1972. In 2006 and 2007, two groundbreaking works demonstrated that RNAs are present in microvesicles and exosomes, respectively, and that these RNAs can be secreted outside cells to act as signaling molecules to influence the behavior of recipient cells. This process can occur between adjacent cells and can be regulated over long distances.[1,2] These two works opened the prelude for cfRNA research and brought new perspectives for exploring signal transduction between cells. Subsequently, cfRNAs were found to be present in almost all biological body fluids, including blood, saliva, urine, breast milk, cerebrospinal fluid, amniotic fluid, ascites, bile, and pleural effusion.[3] The development of new technologies has created opportunities for the identification of cfRNA biomarkers. For example, a multinational research team led by Stanford University in the U. S. spent ten years developing RARE-seq, an optimized cfRNA detection method. This technology overcomes the critical limitation of conventional approaches in capturing trace cfRNA signals, enabling highly sensitive and accurate detection of low-concentration cfRNA in bodily fluids. It paves a new way for non-invasive molecular diagnostics.[4] The results of high-throughput sequencing also showed that in addition to mRNA, cfRNA also contains a variety of non-coding RNA (ncRNA) types, such as microR-NA (miRNA), piwi-interacting RNAs (piRNAs), tRNA, long non-coding RNA (lncRNA), nucleolar small RNA, etc.[5] The broad-spectrum existence and diversity of cfRNAs suggest that cfRNAs, acting as a genetic, epigenetic, and translational regulator, may have great significance and impact on human health by participating in important biological processes, regulating normal growth and development, as well as the occurrence of cancer and disease.[6]
Common biomarkers in liquid biopsies include proteins, DNA, RNA, and metabolites. DNA and protein biomarkers play important roles in liquid biopsies. Meanwhile, RNA, which has a special and important status in the central dogma of molecular biology, has been found to act as a possible strongly advantageous biomarker in the occurrence and development of disease by increasing numbers of studies. As a biomarker, cfRNA has several benefits including high sensitivity, high tissue specificity, and low cost of testing, and has drawbacks including instability and the limited detection technology. From the theoretical perspective, RNA differs from DNA for having multiple copies in a single cell and multiple transcriptional regulation forms, and thus can reflect the dynamics of cell states and regulatory processes inside cells. Therefore, large-scale body fluid cfRNA expression profiling can provide information on both genomic differences and transcriptomic dynamic changes, which can be used as direct and accurate biomarkers for non-invasive detection of human health and disease states.[7,8] In addition, the tissue specificity of cfRNA helps overcome the tissue-origin-untraceable defect in circulating tumor DNA (ctDNA) detection, which showed great scientific research value and application prospects. Related studies have shown that cell-free RNAs (cfRNAs) in the blood are more sensitive than cfDNAs in disease detection, and researchers can further identify the tissue source of cfRNAs and evaluate the clinical status of patients through bioinformatics algorithms.[9,10] From the technical perspective, the detection of protein biomarker requires specific antibodies for each marker, and the detection of ctDNA mutations requires ultra-high sequencing depth, both of which are relatively high cost. In contrast, cfRNA sequences can be captured and tracked at high sensitivity and specificity by simple and economical polymerase chain reaction (PCR) techniques.
Cell-free ncRNA is the most common type of cfRNA, including miRNA, piRNA, snRNA, lncRNA, circular RNA (circRNA), etc. Due to the protection of cell membrane-like structures and RNA binding proteins, and their own specific structures, cell-free ncRNAs can resist the degradation of RNases in a variety of body fluids, and thus exist stably.[11] This article reviews the characteristics of cell-free ncRNAs, bioinformatics analysis tools, as well as research advances in rheumatic and immune diseases. Finally, the challenges and future research directions of cell-free ncRNAs are discussed.
A Brief Overview of the Different Types of Cell-free ncRNAs
The evolution of high-throughput sequencing and other technologies has resulted in the identification of a wide range of different classes and sizes of ncRNA, while the biological importance of these ncRNAs also has received much attention. NcRNAs are classified into two broad categories based on length, including small ncRNAs ranging from few to 200 nucleotides (nt) and lncRNAs longer than 200 nt.[12] MiRNAs of approximately 22 nt in size are generally located in intergenic or intronic regions, which are the most abundant class of small ncRNAs known. The human genome encodes over 1, 000 miRNAs, and more than 30% of genes encoding mammalian proteins contain conserved miRNA target sites. Consequently, miRNAs impact a variety of physiological processes in mammals, including epithelial regeneration, cardiac function, ovulation, reproductive health, and cancer progression.[12] These miRNAs function via diverse mechanisms that regulate gene expression in the cytoplasm and nucleus and mediate gene silencing at the post-transcriptional level.[13] Moreover, miRNAs can also function as inter-cellular communication molecules by virtue of being secreted in extracellular vesicles or acting as hormones.[14]
piRNAs are a novel group of small ncRNA molecules with size of 24–31 nt that frequently bind to members of the piwi family of proteins to fulfil regulatory roles.[15] Contrary to ubiquitous miRNAs, piRNAs are predominantly expressed in the animal gonad.[16] The representative function of piRNAs is to silence transposons at transcriptional and post-transcriptional levels in animal germ cells.[17] The role of the PIWI-piRNA machinery in regulating protein-coding genes in germ cells has apparent gradually surfaced, and the biological merits of the PIWI-piRNA complex have been characterized in germ cells.[17] In fact, there is also a significant amount of piRNA expressed albeit at low levels in somatic tissues, the functional role of which remains to be elucidated.[18]
snRNAs are located in the nucleus with the length of ~60–200 nt in length, and constitute conserved non-coding RNAs in eukaryotes.[19] The biosynthesis of most snRNAs involves 3’-terminal nucleolytic cleavage of the nascent transcript by DNA-dependent RNA polymerase II.[20] SnRNAs work with several proteins to form spliceosomes (snRNPs) that are involved in alternative splicing.[21] In eukaryotes, snRNAs play a role in fundamental cellular events such as the regulation of gene expression and ribosomal RNA processing.[22]
LncRNAs are a highly diverse group of ncRNAs that account for the largest proportion of the non-coding transcriptome. In general, lncRNAs can be transcribed from virtually every locus in the human genome, and the majority of lncRNAs can be transcribed from intergenic, exonic, or distal proteins coded for in the genome by RNA polymerase II.[12] The diversity of lncRNAs can be reflected in their functions, including transcriptional and translational regulation of neighboring and distal genes. LncRNAs can bind directly to DNA or interact with other RNAs. In addition, lncRNAs can also be used as scaffolds or guides through interactions with proteins, which can facilitate protein co-localization or promote protein-protein interactions.[14] In the cytoplasm, lncRNAs also perform essential functions including the regulation of translation, metabolism and signal transduction.[23]
CircRNAs formed by post-splicing of precursor mRNAs (premRNAs) range from 100 nt to over 4 kb in length and are a special type of endogenous ncRNA that can be derived from exons, introns, exon-intron junctions, or intergenic regions of the genome.[24,25] In addition to the effect of back-splicing itself on typical splicing, circRNAs have been reported to modulate gene expression in the nucleus, act as decoys for miR-NAs and proteins, and serve as scaffolds for circRNA-protein complexes. CircRNAs in the nucleus are associated with the regulation of transcription, selective splicing, and chromatin cyclization.[26] While entering the cytoplasm, some circular RNAs can act as competing endogenous RNA (ceRNAs), which are defined as miRNA sponges that bind miRNAs, thus blocking them from binding and repressing target mRNAs.[27] Considering the stable and unique structure of circRNAs, further studies are warranted to enrich their biological functions.
Characteristics of Cell-free ncRNA
Location: RNA is specifically localized in different regions of the cell, starting in the nucleus and then exported to the cytoplasm. Since the extracellular space and body fluids contain a large number of RNases, it was generally considered to be RNAs free. In recent years, an increasing number of studies have found that cell-free ncRNA exists in various encapsulated forms in body fluids, such as extracellular vesicles (EV), including exosomes[28] and microvesicles,[29] as well as lipoprotein particles[30] and argonaute 2 (AGO2) protein complexes[31] (Figure 1). EV are heterogeneous structures surrounded by the cell membrane and can be secreted by many types of cells carrying abundant ncRNAs, mainly divided into exosomes and microvesicles, which are shed directly from the endosomal system or originate from the cell membrane, respectively.[32] EVs protect ncRNAs from degradation and are an important carrier and source of cell-free ncRNAs. At the same time, ncRNAs can also be released from cells without EVs and are detected both in the extracellular space and in body fluids as complexes with the protein AGO2[31,33] or high-density lipoproteins (HDLs).[30,34] In addition, platelets formed by the shedding of cytoplasm from megakaryocytes also contain a large number of ncRNAs, which can be used to distinguish early and late stage cancer patients from healthy individuals.[35]
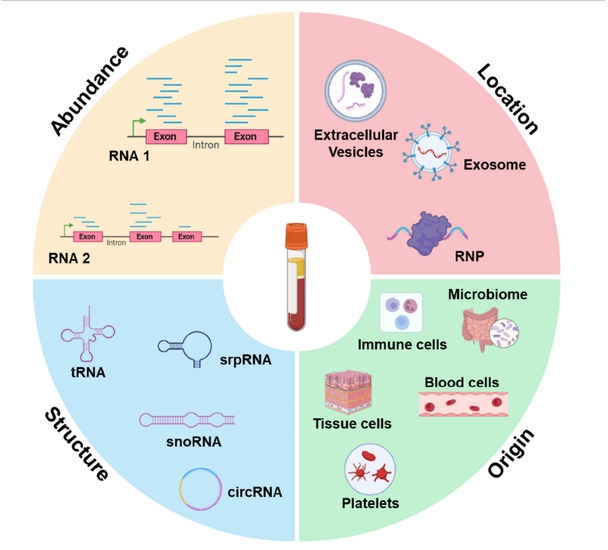
Characteristics of cell-free ncRNAs.
Structure: The cell-free ncRNAs might be protected from degradation for their specific structures. For example, Zhu et al. found that the 5’ region enriched with secondary structure in SNORD3B-1 can stably exist in plasma, and its abundance can be used as a biomarker for early diagnosis of liver cancer.[36] Tan et al identified that the S domain of srpRNA RN7SL1 was rich in exosomes and showed reliable performance in hepatocellular carcinoma (HCC) diagnosis and prognosis.[37] Moreover, circRNAs are emerging as biomarkers in liquid biopsy for their covalently closed cyclic structure might be responsible for their stability in plasma unlike linear RNAs.[38] Abundant full length tRNAs and tRNA fragments (tRFs) have recently garnered attention as a promising source of biomarkers and a novel mediator in cell-to-cell communication in eukaryotes, tRNAs/ex-tRFs may be protected from degradation by RNases folding into highly stable intermolecular tetramers stabilized by G-quadruplex structures.[39,40]
Origin: Previous studies have shown that cell-free ncRNA is mainly derived from broken dead cells or live cell signaling mediated by exosomes, such as blood cells, immune cells, microbes, etc.[41, 42, 43] Cell-free ncRNA released into the circulation by human tissue cells, cancer cells, which offers an opportunity to detect disease in body fluids such as plasma, since overexpression of pathological tissue specific transcripts may lead to amplification of pathological tissue derived RNA signals in blood. With the development of RNA sequencing technology and bioinformatics methods, cell-free ncRNA shows unique potential in detecting disease and predicting pathological tissue of origin.[44]
Abundance: Abundance is the most easily detectable and most reflective feature of cell-free ncRNA regulation. Numerous studies have found that abnormal expression of cell-free ncRNA in body fluids can distinguish cancer patients from normal people, which is expected to be used for diagnosis, prediction, and monitoring of diseases. For example, Zhou et al. found a set of miRNAs for the diagnosis of hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC).[45] miR-1, miR-133, and miR-208 were significantly up-regulated in plasma after myocardial infarction, while miR-126 was down-regulated in plasma of patients with atherosclerosis.[46] miR-29 and miR-124 were significantly up-regulated in the plasma of patients with Alzheimer’s disease, while miR-9 and miR-132 were down-regulated in the plasma of patients with Parkinson’s disease.[47] Notably, repeat-derived cfRNA, including simple repeat RNAs and transposable elements RNAs are often present at low levels or undetectable in healthy individuals, but it’s highly enriched in the plasma of tumor patients.[48] In the following sections, aberrant expression of cell-free ncRNAs will be described in more detail in a variety of diseases.
Bioinformatics Tools and Databases for Cell-free ncRNAs
The growing evidence about the presence of cell-free ncRNAs and their role in cell-cell communication and liquid biopsies has outlined the need for suitable processes and tools to collect and analyze these data. Therefore, the pipeline of cell-free ncRNA study includes body fluid samples collection, RNA library preparation, Illumina sequencing and bioinformatics analysis (Figure 2). Through this series of processes, a variety of cell-free ncRNA characteristics can be obtained to provide promising biomarkers for liquid biopsy.
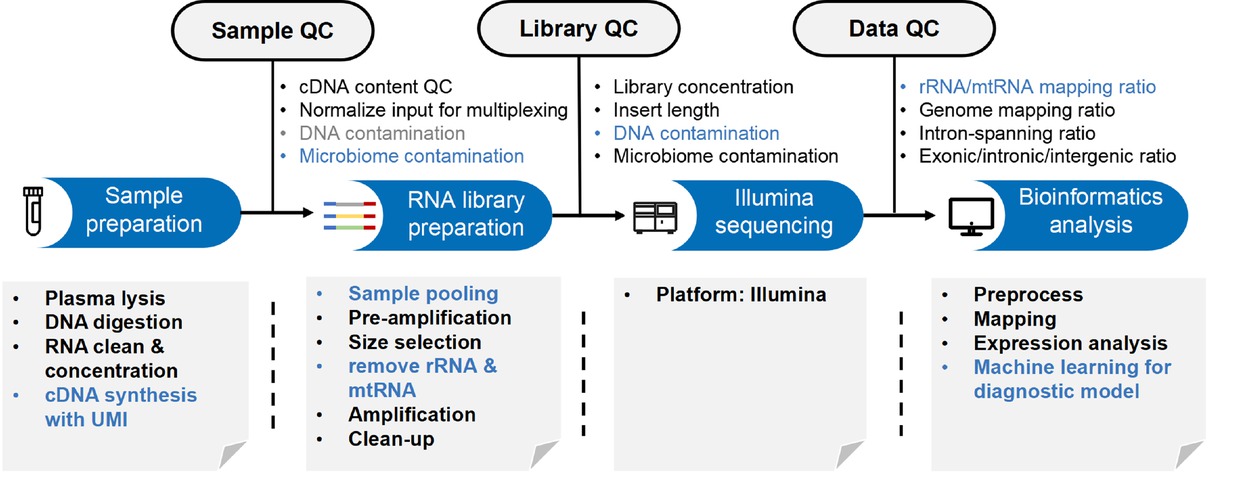
Pipeline of cell-free ncRNA study.
As for bioinformatics tools and databases, exceRpt, a comprehensive analytic platform for cell-free RNA profiling, which has been widely used to process cfRNA-seq data from public datasets.[49] The EVmiRNA database depicts the comprehensive expression profiles of miRNAs in EVs from 462 smRNA sequencing datasets from 17 tissues/diseases, and the biological functions, target genes, pathway regulation and small molecular drug regulations of these miRNAs were analyzed and annotated.[50] liqDB is browsable and interactive database for small RNA-seq profiles in bio-fluids, which provides a user-friendly web interface and useful tool for exploring expression profiles, differential expression analysis, cluster analysis and relevant visualizations of miRNAs from 1607 manually annotated samples.[51] BBCancer is a web-accessible and comprehensive open resource for providing the expression landscape of six type of RNAs, including messenger RNAs (mRNAs), lncRNAs, miRNAs, circRNAs, tRNA derived fragments (tRFRNAs) and piRNAs in 7184 samples including 5040 blood samples such as EVs and circulating tumor cells (CTCs) from normal persons or cancer patients of 15 cancer types.[52] miREV is an online database and tool to uncover potential reference RNAs and biomarkers in small-RNA sequencing data sets from extracellular vesicles enriched samples.[53] exoRBase is a repository of extracellular vesicles long RNAs (exLRs) derived from RNA-seq data analyses in different human fluids. Among them, exoRBase features the integration and visualization of RNA expression profiles, as well as the functional pathway-level changes and the heterogeneity of circulating-EVs origins, which will facilitate the identification of novel exLR signatures from human body fluids and will help discover new circulating biomarkers for the improvement of tumor diagnosis and therapy. EVAtlas database collected small RNA-seq datasets from 2, 030 human EVs, covering 24 conditions and more than 40 diseases. All data were compared by a unified Dynamic reading allocation algorithm (RDAA), and the expression profiles of seven ncRNA types (miRNA, snoRNA, piRNA, snRNA, rRNA, tRNA, and YRNA) were quantified by considering mismatches and multiple mapped read segments.[54, 55, 56] cfOmics is a comprehensive database focusing on extracellular multi-Omics data of multiple diseases, compiling a comprehensive collection of molecular data (including cell-free ncRNAs) from various body fluids, and providing integration, browing, analysis and visualization of multi-omics data[57] (Table 1).
List of bioinformatics analysis tools and databases for cell-free ncRNAs
| Bioinformatics Resources | Description | Web Link | Reference |
|---|---|---|---|
| exceRpt | A Comprehensive Analytic Platform for cell-free RNA Profiling | github.gersteinlab.org/exceRpt/genboree.org | [49] |
| EVmiRNA | a database of miRNA profiling in extracellular vesicles | http://bioinfo.life.hust.edu.cn/EVmiRNA | [50] |
| LiqDB | a small-RNAseq knowledge discovery database for liquid biopsy studies | http://bioinfo5.ugr.es/Liqdb | [51] |
| BBCancer | an expression atlas of blood-based biomarkers in the early diagnosis of cancers | http://bbcancer.renlab.org/ | [52] |
| miREV | An Online Database and Tool to Uncover Potential Reference RNAs and Biomarkers in Small-RNA Sequencing Data Sets from Extracellular Vesicles Enriched Samples | https://www.physio.wzw.tum.de/mirev/ | [53] |
| exoRBase | a database of circRNA, lncRNA and mRNA in human blood exosomes | http://www.exoRBase.org | [75] |
| EVAtlas | a vesicles comprehensive database for ncRNA expression in human extracellular | http://bioinfo.life.hust.edu.cn/EVAtlas | [55] |
| NPInter | a ncRNAs database and that other collects biomolecules information on the interactions between | http://bigdata.ibp.ac.cn/npinter5/ | [54] |
| ncRNADrug | a database of ncRNAs related to drug resistance and drug targeting | http://www.jianglab.cn/ncRNADrug | [56] |
| cfOmics | cfOmics: a cell-free multi-Omics database for diseases | https://cfomics.ncRNAlab.org/ | [57] |
These tools and websites also have certain limitations. For instance, exceRpt is an analysis platform specifically developed for small RNA sequencing, but there is a lack of tools suitable for analyzing cfRNA in long RNA sequencing data.[49] Regarding EVmiRNA, this database primarily focuses on cancers such as prostate cancer and breast cancer, but does not include non-cancer-related data.[50] For LiqDB, although it demonstrates satisfactory integration performance for samples processed with different library preparation protocols, its effectiveness in large-scale sample cohorts may require further validation.[51] Regarding BBCancer, while this database provides valuable insights into the abundance and differential expression profiles of diverse ncRNAs in cancer contexts, it currently lacks dedicated modules for functional enrichment analysis to elucidate the biological significance of these RNAs.[52] For miREV, it similarly lacks modules for functional enrichment analysis of these miRNAs, limiting its utility for in-depth biological interpretation.[53] An additional limitation shared by these tools/databases is the difficulty in maintaining consistent or timely updates, necessitating the continual development of novel tools or databases to meet evolving research demands.
Compendium of Cell-free ncRNA Biomarkers in Rheumatic and Immune Diseases
Rheumatic and immune diseases mainly include Rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS) and systemic sclerosis (SSc), among others. As for laboratory tests or clinical practice, each rheumatic and immune disease has its own diagnosis method, but there is no generally accepted diagnostic standard. For example, Laboratory tests, such as Complement (C3, C4) and antibodies (ANA, anti-dsDNA, antiphospholipid, etc.) have although been demonstrated to have potential as SLE diagnostic biomarkers, but none of them can accurately diagnose SLE. In clinical practice, the diagnosis of SLE is mainly according to the revised American College of Rheumatology (ACR) classification criteria, which is made based on clinical manifestations and laboratory tests.[58] Aberrant expression of various cell-free ncRNA has been found to distinguish people with rheumatic and immune diseases from healthy individuals (Table 2).
RA
RA is an autoimmune disease characterized by synovial inflammation. Recent studies have shown that the levels of miRNA-21 and miRNA-146a in the peripheral blood serum of RA patients are significantly elevated. miRNA-146a exhibits a significant positive correlation with the DAS-28 score and clinical manifestations in RA patients, including morning stiffness, joint tenderness, and swelling.[59] Another study demonstrated that miR-146b-3p is upregulated in the serum and synovial tissues of RA patients. Silencing miR-146b-3p attenuated TNF-α-induced proliferation and migration of MH7A cells, suggesting that miR-146b-3p not only serves as a diagnostic biomarker for RA but also plays a crucial pro-inflammatory and pro-proliferative role.[60] A study comprising 76 RA patients, 30 SLE patients, 32 SS patients, and 36 healthy controls demonstrated that plasma miR-22–3p and let-7a-5p could effectively discriminate RA patients from healthy individuals, with AUC values reaching 0.812 and 0.832, respectively.[61] Moreover, miR-22–3p and let-7a-5p exhibited strong discriminatory capacity to differentiate RA from other rheumatic diseases, including SLE and SS, with AUC values consistently exceeding 0.75.[61] In addition, the over-expression of let-7a-5p, let-7b-5p, let-7 d-5p, let-7f-5p, let-7 g-5p, let-7i-5p, miR-128–3p, miR-25–3p can be used as biomarkers for diagnose of RA.[62]
A compendium of cell-free ncRNAs with potential diagnostic or prognostic role for patients with immune mediated diseases*
| RNA Species | Disease species | Name | Source | Usage | Up/Down△ | Reference |
|---|---|---|---|---|---|---|
| miRNA | Rheumatoid arthritis | miR-126-3p, let-7 d-5p, miR-221-3p | Serum | Diagnosis | Up | [76] |
| miRNA | Rheumatoid arthritis | miR-125a, miR-125b | Plasma | Prognosis | Up | [77] |
| miRNA | Rheumatoid arthritis | miR-125a-5p, miR-24 | Plasma | Diagnosis | Up | [78] |
| miRNA | Rheumatoid arthritis | miR-146a-5p | Plasma | Diagnosis | Up | [79] |
| miRNA | Rheumatoid arthritis | let-7a-5p, let-7b-5p, let-7 d-5p, let-7f-5p, let-7 g-5p, let-7i-5p, miR-128-3p, miR-25-3p | Exosomes | Diagnosis | Up | [77] |
| miRNA | Rheumatoid arthritis | miR-22-3p and let-7a-5p | plasma | Diagnosis | Up | [61] |
| miRNA | Systemic lupus erythematosus | miR-551b, miR-448 | Serum | Diagnosis | Up | [66] |
| miRNA | Systemic lupus erythematosus | miR-124 | Serum | Diagnosis | Down | [80] |
| miRNA | Systemic lupus erythematosus | miR-125b, miR-101, miR-375 | Plasma | Prognosis | Down | [81] |
| miRNA | Systemic lupus erythematosus | miRNA-21 | Serum | Diagnosis | Up | [67] |
| lncRNA | Systemic lupus erythematosus | GAS5 | plasma | Diagnosis | Down | [63] |
| lncRNA | Sjögren’s syndrome | lnc-DC | plasma | Diagnosis | Up | [69] |
| lncRNA | Sjögren’s syndrome | miR-17-5p and let-7i-5p | Salivary | Diagnosis | Down | [70] |
| miRNA | Systemic sclerosis | miR-214 | plasma | Diagnosis | Down | [72] |
| miRNA | Systemic sclerosis | miR-20a-5p, miR-21-5p | plasma | Diagnosis | Down, up | [73] |
* This table lists the representative cell-free ncRNA biomarkers for pregnancy-related diseases (AUC > 0.8). △Up: Higher abundance in pregnancy-related diseases patient than healthy control; Down: lower abundance in pregnancy-related diseases patient than healthy control.
SLE
SLE is a clinically heterogeneous disease caused by dysregulation of the immune system and loss of self-tolerance. Emerging evidence highlights the diagnostic potential of lncRNAs in SLE. A study revealed that the expression of GAS5 and lnc7074 was significantly down-regulated in SLE patients compared to healthy subjects, whereas lnc0640 and lnc5150 exhibited elevated levels. These differentially expressed lncRNAs demonstrate high diagnostic accuracy for SLE and exhibit specificity in distinguishing SLE from RA.[63] Another study detected 2, 353 dysregulated lncRNAs in the plasma of SLE patients, among which YPEL4 was associated with the FcγR pathway.[64] YPEL4 may contribute to SLE pathogenesis by stimulating immune cells to release inflammatory mediators.[65] In serum, miR-551b, miR-448 and miRNA-21 were up-regulated in patients with SLE, while miR-124 was down-regulated.[66,67]
SS
Primary SS (pSS) is an autoimmune disease characterized by dry eyes and dry mouth as its predominant clinical manifestations.[68] A study involving 109 healthy controls (HC), 50 SLE patients, 50 RA patients, and 127 pSS patients demonstrated that lnc-DC levels were significantly higher in pSS patients compared to HC, SLE, and RA patients. The AUC value of lnc-DC for distinguishing pSS from healthy individuals reached 0.80.[69] The combination of lnc-DC with anti-SSA and anti-SSB antibodies could further improve the diagnostic efficacy for pSS, achieving an AUC value of 0.84.[69] Salivary miR-17–5p and let-7i-5p also demonstrated high diagnostic performance for pSS, with AUC values reaching 0.87 and 0.91134, respectively. Furthermore, these miRNAs showed significant correlations with salivary flow rate and histopathological features in patients.[70]
SSc
SSc is an autoimmune disease characterized by vascular pathology, chronic inflammation, and fibrosis, primarily affecting connective tissues throughout the body.[71] A study involving 40 female SSc patients and 14 healthy female controls revealed that plasma miR-214 expression was significantly downregulated in SSc patients compared with controls. Notably, miR-214 demonstrated excellent discriminative capacity for SSc identification, with an AUC of 0.80.[72] Furthermore, the combination of plasma miR-20a-5p and miR-21–5p demonstrated significant diagnostic potential for SSc-associated pulmonary arterial hypertension (SSc-PAH), achieving an AUC value of 0.83.[73]
Conclusions and Perspectives
ncRNA includes small RNA and long ncRNA, which are called “dark matter” or “junk RNA” in the genome because they cannot encode proteins. Recent studies have shown that ncRNAs play important roles in many life processes. Cell-free ncRNAs in serum, plasma, saliva, urine, and other body fluids have been implicated in various pathological conditions. Cell-free ncRNAs circulate in body fluids with a highly stable extracellular form due to their secondary structures or their associations with biological macromolecules such as proteins. Given that body fluids are more readily accessible than tissue samples, cell-free ncRNAs are frequently employed as biomarkers in liquid biopsy applications. Nevertheless, there are still many drawbacks and challenges in moving this field forward and implementing clinical applications: Firstly, cfRNA itself is highly fragmented, heterogeneous, and has a low signal-to-noise ratio (the proportion of lesion-derived cfRNA is low), presenting significant challenges for its sensitive detection and clinical application. Secondly, RNA is prone to degradation, posing significant challenges for sample preservation in clinical application. Thirdly, the lack of standardized analytical strategies for cell-free non-coding RNA (cf-ncRNA) hinders comparability across different studies. Lastly, the clinical application of cfRNA requires validation through large-scale, multi-center studies with diverse sample cohorts. There are currently a multitude of methods to isolate RNA from biological fluids, and various protocols and kits affect downstream sequencing and PCR results, and the potential bias of these methods needs to be considered carefully. As well, we are required to overcome technical challenges to consider how to isolate pure cell-free ncRNAs. Future research ought to elucidate the sources, mechanisms of export and uptake of ncRNAs in each of the biofluids evaluated. We can further investigate the biological functions and clinical significance of cell-free ncRNAs by knocking down or overexpressing these RNAs in their cells or animal tissues of origin. The storage of cell-free ncRNA and its verification in multi-center populations with large sample sizes are also challenges in the process of its clinical application. It is also important to continue to develop computational techniques and tools to fully exploit and widely utilize cfRNA data. Based on cell type-associated signature computational methods, cfRNA can trace its cellular origins and characterize cellular pathological changes during disease development.[74] This positions cfRNA as a non-invasive biomarker for monitoring disease progression and in vivo drug responses. For exosome-derived cfRNA, further investigation can reveal whether its active secretion mechanism correlates with rheumatic and immune diseases onset/progression.
Funding statement: This work is supported by National Key Research and Development Program of China (2024YFC2510300, 2024YFC3405900), the National Natural Science Foundation of China (82103870, 82371855, 82341101, 32170671), the Tsinghua University Initiative Scientific Research Program of Precision Medicine (2022ZLA003). This study was also supported by the BioComputing Platform of the Tsinghua University Branch of China National Center for Protein Sciences, the National Natural Science Innovative Research Group Project (61821002), the Frontier Fundamental Research Program of Jiangsu Province for Leading Technology (BK20222002).
Acknowledgements
None.
-
Author contributions
Yumin Zhu: Conceptualization, Writing—Original draft preparation, Writing—Reviewing and Editing. Fengping Wu, Kangping Liu, Shaozhen Xing, Chun Ning, Meng Ning, Heyue Jin, Yun Shao, Zhenye Zhu, Hongke Wang, Binbin Shi, Yajin Mo: Writing—Reviewing and Editing. Xinping Tian, Mengtao Li: Supervision. Jiuliang Zhao, Zhi John Lu, Ning Gu: Conceptualization, Supervision, Project administration.
-
Ethical approval
Not applicable.
-
Informed consent
Not applicable.
-
Conflict of interest
Xinping Tian is the Executive Editor-in-Chief of the journal; Mengtao Li is an Associate Editor-in-Chief; Jiuliang Zhao is an Editorial Board Member. The article was subjected to the standard procedures of peer review process independent of these editors and their research group. The other authors have declared that no conflict of interest exists. Part of the figures were created with biorender.com.
-
Use of large language models, AI and machine learning tools
None declared.
-
Data availability statement
No additional data is available.
References
[1] Xi X, Li T, Huang Y, et al. RNA Biomarkers: Frontier of Precision Medicine for Cancer. Noncoding RNA. 2017;3:9.10.3390/ncrna3010009Search in Google Scholar PubMed PubMed Central
[2] Li F, Yoshizawa JM, Kim KM, et al. Discovery and Validation of Salivary Extracellular RNA Biomarkers for Noninvasive Detection of Gastric Cancer. Clin Chem. 2018;64:1513–1521.10.1373/clinchem.2018.290569Search in Google Scholar PubMed PubMed Central
[3] Srinivasan S, Yeri A, Cheah PS, et al. Small RNA Sequencing across Diverse Biofluids Identifies Optimal Methods for exRNA Isolation. Cell. 2019;177:446–462.10.1016/j.cell.2019.03.024Search in Google Scholar PubMed PubMed Central
[4] Nesselbush MC, Luca BA, Jeon YJ, et al. An ultrasensitive method for detection of cell-free RNA. Nature. 2025;641:759–768.10.1038/s41586-025-08834-1Search in Google Scholar PubMed
[5] Wei Z, Batagov AO, Schinelli S, et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat Commun. 2017;8:1145.10.1038/s41467-017-01196-xSearch in Google Scholar PubMed PubMed Central
[6] Nemeth K, Bayraktar R, Ferracin M, et al. Non-coding RNAs in disease: from mechanisms to therapeutics. Nat Rev Genet. 2024;25:211–232.Search in Google Scholar
[7] Ngo TTM, Moufarrej MN, Rasmussen MH, et al. Noninvasive blood tests for fetal development predict gestational age and preterm delivery. Science. 2018;360:1133–1136.10.1126/science.aar3819Search in Google Scholar PubMed PubMed Central
[8] Li P, Li SY, Liu M, et al. Value of the expression of miR-208, miR-494, miR-499 and miR-1303 in early diagnosis of acute myocardial infarction. Life Sci. 2019;232:116547.10.1016/j.lfs.2019.116547Search in Google Scholar PubMed
[9] Chen S, Jin Y, Wang S, et al. Cancer type classification using plasma cell-free RNAs derived from human and microbes. Elife. 2022;11:e75181.Search in Google Scholar
[10] Tao Y, Xing S, Zuo S, et al. Cell-free multi-omics analysis reveals potential biomarkers in gastrointestinal cancer patients’ blood. Cell Rep Med. 2023;4:101281.10.1016/j.xcrm.2023.101281Search in Google Scholar PubMed PubMed Central
[11] Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472–484.10.1038/nrclinonc.2013.110Search in Google Scholar PubMed
[12] Beermann J, Piccoli MT, Viereck J, et al. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev. 2016;96:1297–1325.10.1152/physrev.00041.2015Search in Google Scholar PubMed
[13] Lagos-Quintana M, Rauhut R, Lendeckel W, et al. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858.10.1126/science.1064921Search in Google Scholar PubMed
[14] Nemeth K, Bayraktar R, Ferracin M, et al. Non-coding RNAs in disease: from mechanisms to therapeutics. Nat Rev Genet. 2024;25:211–232.10.1038/s41576-023-00662-1Search in Google Scholar PubMed
[15] Liu Y, Dou M, Song X, et al. The emerging role of the piRNA/piwi complex in cancer. Mol Cancer. 2019;18:123.10.1186/s12943-019-1052-9Search in Google Scholar PubMed PubMed Central
[16] Huang X, Fejes Tóth K, Aravin AA. piRNA Biogenesis in Drosophila melanogaster. Trends Genet. 2017;33:882–894.10.1016/j.tig.2017.09.002Search in Google Scholar PubMed PubMed Central
[17] Wang X, Ramat A, Simonelig M, et al. Emerging roles and functional mechanisms of PIWI-interacting RNAs. Nat Rev Mol Cell Biol. 2023;24:123–141.10.1038/s41580-022-00528-0Search in Google Scholar PubMed
[18] Slack FJ, Chinnaiyan AM. The Role of Non-coding RNAs in Oncology. Cell. 2019;179:1033–1055.10.1016/j.cell.2019.10.017Search in Google Scholar PubMed PubMed Central
[19] Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718.10.1016/j.cell.2009.02.009Search in Google Scholar PubMed
[20] Liu Y, Li S, Chen Y, et al. snRNA 3’ End Processing by a CPSF73-Containing Complex Essential for Development in Arabidopsis. PLoS Biol. 2016;14:e1002571.10.1371/journal.pbio.1002571Search in Google Scholar PubMed PubMed Central
[21] Guiro J, Murphy S. Regulation of expression of human RNA polymerase II-transcribed snRNA genes. Open Biol. 2017;7:170073.10.1098/rsob.170073Search in Google Scholar PubMed PubMed Central
[22] Sun J, Li X, Hou X, et al. Structural basis of human SNAPc recognizing proximal sequence element of snRNA promoter. Nat Commun. 2022;13:6871.10.1038/s41467-022-34639-1Search in Google Scholar PubMed PubMed Central
[23] Mattick JS, Amaral PP, Carninci P, et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023;24:430–447.10.1038/s41580-022-00566-8Search in Google Scholar PubMed PubMed Central
[24] Chen B, Huang S. Circular RNA: An emerging non-coding RNA as a regulator and biomarker in cancer. Cancer Lett. 2018;418:41–50.10.1016/j.canlet.2018.01.011Search in Google Scholar PubMed
[25] Zhang P, Wu W, Chen Q, et al. Non-Coding RNAs and their Integrated Networks. J Integr Bioinform. 2019;16:20190027.10.1515/jib-2019-0027Search in Google Scholar PubMed PubMed Central
[26] Li Z, Huang C, Bao C, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–264.10.1038/nsmb.2959Search in Google Scholar PubMed
[27] Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21:475–490.10.1038/s41580-020-0243-ySearch in Google Scholar PubMed
[28] Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659.10.1038/ncb1596Search in Google Scholar PubMed
[29] Ratajczak MZ, Ratajczak J. Innate Immunity Communicates Using the Language of Extracellular Microvesicles. Stem Cell Rev Rep. 2021;17:502–510.10.1007/s12015-021-10138-6Search in Google Scholar PubMed PubMed Central
[30] Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433.10.1038/ncb2210Search in Google Scholar PubMed PubMed Central
[31] Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008.10.1073/pnas.1019055108Search in Google Scholar PubMed PubMed Central
[32] Ohayon L, Zhang X, Dutta P. The role of extracellular vesicles in regulating local and systemic inflammation in cardiovascular disease. Pharmacol Res. 2021;170:105692.10.1016/j.phrs.2021.105692Search in Google Scholar PubMed PubMed Central
[33] Turchinovich A, Weiz L, Langheinz A, et al. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233.10.1093/nar/gkr254Search in Google Scholar PubMed PubMed Central
[34] Allen RM, Zhao S, Ramirez Solano MA, et al. Bioinformatic analysis of endogenous and exogenous small RNAs on lipoproteins. J Extracell Vesicles. 2018;7:1506198.10.1080/20013078.2018.1506198Search in Google Scholar PubMed PubMed Central
[35] In ‘t Veld SGJG, Arkani M, Post E, et al. Detection and localization of early- and late-stage cancers using platelet RNA. Cancer Cell. 2022;40:999–1009.10.1016/j.ccell.2022.08.006Search in Google Scholar PubMed
[36] Zhu Y, Wang S, Xi X, et al. Integrative analysis of long extracellular RNAs reveals a detection panel of noncoding RNAs for liver cancer. Theranostics. 2021;11:181–193.10.7150/thno.48206Search in Google Scholar PubMed PubMed Central
[37] Tan C, Cao J, Chen L, et al. Noncoding RNAs Serve as Diagnosis and Prognosis Biomarkers for Hepatocellular Carcinoma. Clin Chem. 2019;65:905–915.10.1373/clinchem.2018.301150Search in Google Scholar PubMed
[38] Kristensen LS, Jakobsen T, Hager H, et al. The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol. 2022;19:188–206.10.1038/s41571-021-00585-ySearch in Google Scholar PubMed
[39] Lyons SM, Gudanis D, Coyne SM, et al. Identification of functional tetramolecular RNA G-quadruplexes derived from transfer RNAs. Nat Commun. 2017;8:1127.10.1038/s41467-017-01278-wSearch in Google Scholar PubMed PubMed Central
[40] Torres AG, Martí E. Toward an Understanding of Extracellular tRNA Biology. Front Mol Biosci. 2021;8:662620.10.3389/fmolb.2021.662620Search in Google Scholar PubMed PubMed Central
[41] Li C, Ni YQ, Xu H, et al. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct Target Ther. 2021;6:383.10.1038/s41392-021-00779-xSearch in Google Scholar PubMed PubMed Central
[42] Bauer KM, Round JL, O’Connell RM. No small matter: emerging roles for exosomal miRNAs in the immune system. FEBS J. 2022;289:4021–4037.10.1111/febs.16052Search in Google Scholar PubMed PubMed Central
[43] Chen S, Jin Y, Wang S, et al. Cancer type classification using plasma cell-free RNAs derived from human and microbes. Elife. 2022;11:e75181.10.7554/eLife.75181Search in Google Scholar PubMed PubMed Central
[44] Vorperian SK, Moufarrej MN; Tabula Sapiens Consortium; Quake SR. Cell types of origin of the cell-free transcriptome. Nat Biotechnol. 2022;40:855–861.Search in Google Scholar
[45] Zhou J, Yu L, Gao X, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol. 2011;29:4781–4788.10.1200/JCO.2011.38.2697Search in Google Scholar PubMed
[46] Fichtlscherer S, De Rosa S, Fox H, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684.10.1161/CIRCRESAHA.109.215566Search in Google Scholar PubMed
[47] Burgos KL, Javaherian A, Bomprezzi R, et al. Identification of extracellular miRNA in human cerebrospinal fluid by next-generation sequencing. RNA. 2013;19:712–722.10.1261/rna.036863.112Search in Google Scholar PubMed PubMed Central
[48] Reggiardo RE, Maroli SV, Peddu V, et al. Profiling of repetitive RNA sequences in the blood plasma of patients with cancer. Nat Biomed Eng. 2023;7:1627–1635.10.1038/s41551-023-01081-7Search in Google Scholar PubMed PubMed Central
[49] Rozowsky J, Kitchen RR, Park JJ, et al. exceRpt: A Comprehensive Analytic Platform for Extracellular RNA Profiling. Cell Syst. 2019;8:352–357.10.1016/j.cels.2019.03.004Search in Google Scholar PubMed PubMed Central
[50] Liu T, Zhang Q, Zhang J, et al. EVmiRNA: a database of miRNA profiling in extracellular vesicles. Nucleic Acids Res. 2019;47:D89-D93.10.1093/nar/gky985Search in Google Scholar PubMed PubMed Central
[51] Aparicio-Puerta E, Jáspez D, Lebrón R, et al. liqDB: a small-RNAseq knowledge discovery database for liquid biopsy studies. Nucleic Acids Res. 2019;47:D113-D120.10.1093/nar/gky981Search in Google Scholar PubMed PubMed Central
[52] Zuo Z, Hu H, Xu Q, et al. BBCancer: an expression atlas of blood-based biomarkers in the early diagnosis of cancers. Nucleic Acids Res. 2020;48:D789-D796.10.1093/nar/gkz942Search in Google Scholar PubMed PubMed Central
[53] Hildebrandt A, Kirchner B, Nolte-’t Hoen ENM, et al. miREV: An Online Database and Tool to Uncover Potential Reference RNAs and Biomarkers in Small-RNA Sequencing Data Sets from Extracellular Vesicles Enriched Samples. J Mol Biol. 2021;433:167070.10.1016/j.jmb.2021.167070Search in Google Scholar PubMed
[54] Zheng Y, Luo H, Teng X, et al. NPInter v5.0: ncRNA interaction database in a new era. Nucleic Acids Res. 2023;51:D232-D239.10.1093/nar/gkac1002Search in Google Scholar PubMed PubMed Central
[55] Liu CJ, Xie GY, Miao YR, et al. EVAtlas: a comprehensive database for ncRNA expression in human extracellular vesicles. Nucleic Acids Res. 2022;50:D111-D117.10.1093/nar/gkab668Search in Google Scholar PubMed PubMed Central
[56] Cao X, Zhou X, Hou F, et al. ncRNADrug: a database for validated and predicted ncRNAs associated with drug resistance and targeted by drugs. Nucleic Acids Res. 2024;52:D1393-D1399.10.1093/nar/gkad1042Search in Google Scholar PubMed PubMed Central
[57] Li M, Zhou T, Han M, et al. cfOmics: a cell-free multi-Omics database for diseases. Nucleic Acids Res. 2024;52:D607-D621.10.1093/nar/gkad777Search in Google Scholar PubMed PubMed Central
[58] Zheng X, Zhang Y, Yue P, et al. Diagnostic significance of circulating miRNAs in systemic lupus erythematosus. PLoS One. 2019;14:e0217523.10.1371/journal.pone.0217523Search in Google Scholar PubMed PubMed Central
[59] Moness H, Ibrahim RA, Soliman SA, et al. Association of cell-free DNA, micro-RNA 21, and micro-RNA 146a levels with rheumatoid arthritis activity. Mol Biol Rep. 2025;52:200.10.1007/s11033-025-10266-zSearch in Google Scholar PubMed
[60] Ma L, Liu H, Shao P, et al. Upregulated miR-146b-3p predicted rheumatoid arthritis development and regulated TNF-α-induced excessive proliferation, motility, and inflammation in MH7A cells. BMC Immunol. 2024;25:36.10.1186/s12865-024-00629-9Search in Google Scholar PubMed PubMed Central
[61] Tang J, Lin J, Yu Z, et al. Identification of circulating miR-22–3p and let-7a-5p as novel diagnostic biomarkers for rheumatoid arthritis. Clin Exp Rheumatol. 2022;40:69–77.10.55563/clinexprheumatol/4me6tgSearch in Google Scholar PubMed
[62] Yang X, Wang Z, Zhang M, et al. Differential Expression Profiles of Plasma Exosomal microRNAs in Rheumatoid Arthritis. J Inflamm Res. 2023;16:3687–3698.10.2147/JIR.S413994Search in Google Scholar PubMed PubMed Central
[63] Wu GC, Hu Y, Guan SY, et al. Differential Plasma Expression Profiles of Long Non-Coding RNAs Reveal Potential Biomarkers for Systemic Lupus Erythematosus. Biomolecules. 2019;9:206.10.3390/biom9060206Search in Google Scholar PubMed PubMed Central
[64] Zhang Q, Liang Y, Yuan H, et al. Integrated analysis of lncRNA, miRNA and mRNA expression profiling in patients with systemic lupus erythematosus. Arch Med Sci. 2019;15:872–879.10.5114/aoms.2018.79145Search in Google Scholar PubMed PubMed Central
[65] Wu H, Chen S, Li A, et al. LncRNA Expression Profiles in Systemic Lupus Erythematosus and Rheumatoid Arthritis: Emerging Biomarkers and Therapeutic Targets. Front Immunol. 2021;12:792884.10.3389/fimmu.2021.792884Search in Google Scholar PubMed PubMed Central
[66] Jin F, Hu H, Xu M, et al. Serum microRNA Profiles Serve as Novel Biomarkers for Autoimmune Diseases. Front Immunol. 2018;9:2381.10.3389/fimmu.2018.02381Search in Google Scholar PubMed PubMed Central
[67] Ibrahim MRK, Waly NG, Moness H, et al. Serum miRNA-21, miRNA-146a and plasma cell free DNA as novel biomarkers for assessing systemic lupus erythematosus activity. Mol Biol Rep. 2023;50:10025–10036.10.1007/s11033-023-08845-zSearch in Google Scholar PubMed PubMed Central
[68] Brito-Zerón P, Baldini C, Bootsma H, et al. Sjögren syndrome. Nat Rev Dis Primers. 2016;2:16047.10.1038/nrdp.2016.47Search in Google Scholar PubMed
[69] Chen Y, Chen Y, Zu B, et al. Identification of Long Noncoding RNAs lnc-DC in Plasma as a New Biomarker for Primary Sjögren’s Syndrome. J Immunol Res. 2020;2020:9236234.10.1155/2020/9236234Search in Google Scholar PubMed PubMed Central
[70] Sembler-Møller ML, Belstrøm D, Locht H, et al. Distinct microRNA expression profiles in saliva and salivary gland tissue differentiate patients with primary Sjögren’s syndrome from non-Sjögren’s sicca patients. J Oral Pathol Med. 2020;49:1044–1052.10.1111/jop.13099Search in Google Scholar PubMed
[71] Pattanaik D, Brown M, Postlethwaite BC, et al. Pathogenesis of Systemic Sclerosis. Front Immunol. 2015;6:272.10.3389/fimmu.2015.00272Search in Google Scholar PubMed PubMed Central
[72] Dichev V, Mehterov NH, Kazakova MH, et al. Serum protein levels of YKL-40 and plasma miR-214 expression in patients with systemic sclerosis. Mod Rheumatol. 2021;31:1010–1018.10.1080/14397595.2020.1859726Search in Google Scholar PubMed
[73] Wuttge DM, Carlsen AL, Teku G, et al. Circulating plasma microRNAs in systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology (Oxford). 2021;61:309–318.10.1093/rheumatology/keab300Search in Google Scholar PubMed PubMed Central
[74] Vorperian SK, Moufarrej MN; Tabula Sapiens Consortium; Quake SR. Cell types of origin of the cell-free transcriptome. Nat Biotechnol. 2022;40:855–861.10.1101/2021.05.05.441859Search in Google Scholar
[75] Li S, Li Y, Chen B, et al. exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018;46:D106-D112.10.1093/nar/gkx891Search in Google Scholar PubMed PubMed Central
[76] Cunningham CC, Wade S, Floudas A, et al. Serum miRNA Signature in Rheumatoid Arthritis and “At-Risk Individuals”. Front Immunol. 2021;12:633201.10.3389/fimmu.2021.633201Search in Google Scholar PubMed PubMed Central
[77] Cheng P, Wang J. The potential of circulating microRNA-125a and microRNA-125b as markers for inflammation and clinical response to infliximab in rheumatoid arthritis patients. J Clin Lab Anal. 2020;34:e23329.10.1002/jcla.23329Search in Google Scholar PubMed PubMed Central
[78] Murata K, Furu M, Yoshitomi H, et al. Comprehensive microRNA analysis identifies miR-24 and miR-125a-5p as plasma biomarkers for rheumatoid arthritis. PLoS One. 2013;8:e69118.10.1371/journal.pone.0069118Search in Google Scholar PubMed PubMed Central
[79] Safari F, Damavand E, Rostamian AR, et al. Plasma Levels of MicroRNA-146a-5p, MicroRNA-24–3p, and MicroRNA125a-5p as Potential Diagnostic Biomarkers for Rheumatoid Arthritis. Iran J Allergy Asthma Immunol. 2021;20:326-337.10.18502/ijaai.v20i3.6334Search in Google Scholar PubMed
[80] Li Y, Fan H, Sun J, et al. Circular RNA expression profile of Alzheimer’s disease and its clinical significance as biomarkers for the disease risk and progression. Int J Biochem Cell Biol. 2020;123:105747.10.1016/j.biocel.2020.105747Search in Google Scholar PubMed
[81] Kay SD, Carlsen AL, Voss A, et al. Associations of circulating cell-free microRNA with vasculopathy and vascular events in systemic lupus erythematosus patients. Scand J Rheumatol. 2019;48:32–41.10.1080/03009742.2018.1450892Search in Google Scholar PubMed
© 2025 Yumin Zhu, Fengping Wu, Kangping Liu, Shaozhen Xing, Chun Ning, Meng Ning, Heyue Jin, Yun Shao, Zhenye Zhu, Hongke Wang, Binbin Shi, Yajin Mo, Xinping Tian, Mengtao Li, Jiuliang Zhao, Zhi John Lu, Ning Gu, published by De Gruyter on behalf of NCRC-DID
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Editorial
- How will we treat systemic lupus erythematosus in the next 5 years?
- Guideline
- 2025 Chinese guidelines for the diagnosis and treatment of systemic lupus erythematosus
- Review
- Proceedings of cell-free noncoding RNA biomarker studies in liquid biopsy
- Original Article
- Investigating the role of tripartite motif containing-21 and interleukin-6 in pro-Inflammatory symptom-associated heterogeneity within primary Sjögren’s syndrome
- Reevaluating risk assessment in connective tissue disease-associated pulmonary arterial hypertension: The prognostic superiority of stroke volume index
- Prevalence and characteristics of concomitant septic and crystal-induced arthritis: A hospital database and literature review
- Association of HLA-B and HLA-DR gene polymorphisms with rheumatoid arthritis: A cross-sectional study in Yunnan Chinese Han population
- Letter to the Editor
- Association of ficolin single nucleotide polymorphism with systemic lupus erythematosus in the Chinese Han Population
- Images
- The storm inside: Abdominal and urinary complications in lupus
Articles in the same Issue
- Editorial
- How will we treat systemic lupus erythematosus in the next 5 years?
- Guideline
- 2025 Chinese guidelines for the diagnosis and treatment of systemic lupus erythematosus
- Review
- Proceedings of cell-free noncoding RNA biomarker studies in liquid biopsy
- Original Article
- Investigating the role of tripartite motif containing-21 and interleukin-6 in pro-Inflammatory symptom-associated heterogeneity within primary Sjögren’s syndrome
- Reevaluating risk assessment in connective tissue disease-associated pulmonary arterial hypertension: The prognostic superiority of stroke volume index
- Prevalence and characteristics of concomitant septic and crystal-induced arthritis: A hospital database and literature review
- Association of HLA-B and HLA-DR gene polymorphisms with rheumatoid arthritis: A cross-sectional study in Yunnan Chinese Han population
- Letter to the Editor
- Association of ficolin single nucleotide polymorphism with systemic lupus erythematosus in the Chinese Han Population
- Images
- The storm inside: Abdominal and urinary complications in lupus

