Abstract
Heavy metals are toxic, non-biodegradable pollutants that pose serious risks to human health and the environment, even at trace concentrations. The contamination of drinking water and groundwater by heavy metals requires urgent attention. Nanotechnology has advanced significantly over the past decade, offering innovative solutions for water purification, particularly through the adsorption of heavy metal ions using nanomaterials. This study focuses on the synthesis of magnetic nanoparticles, their adsorption capacity, and the desorption process. Additionally, the effects of key experimental parameters – such as contact time, ion concentration, pH, temperature, ionic strength, and adsorbent dose – on the removal efficiency of metal ions are examined. The findings underscore the potential of magnetic nanoparticles for effective heavy metal remediation in water.
1 Introduction
One of the most significant natural resources on the planet is water. 1 Because heavy metal ions can physiologically accumulate in the environment and enter food chains, the pollution of water resources by heavy metals from industrial wastes has a substantial impact on life. 2 Furthermore, because microorganisms cannot decompose into the environment, heavy metals might potentially have negative impacts on other ecological receptors and the environment. Heavy metals are defined as elements that have atomic weights that range from 63.5 to 200.6 and densities that are greater than 5 g/cm3.3 3 Furthermore, the existence of highly toxic water contaminants like Arsenic (V), Cadmium (II), Mercury (II), Nickel (II), Lead (II), Chromium (VI), Manganese (II), etc., which are in the form of heavy metal ions, can have detrimental effects on living things. 4 When the amounts of certain heavy metal ions exceed the threshold levels, they have the potential to become carcinogenic and even deadly.5 5 Furthermore, due to the widespread distribution of these pollutants, removing heavy metal ions from water is challenging. 6 The adsorption technique is thought to be safe, effective, clean, and technically feasible. 7 Adsorbent materials are easy to create and use, have a high porosity, a wide surface area, and a strong resistance to harmful chemicals. 8 This particular research review focuses on water contamination by heavy metals and methods of heavy metal removal, 9 since water quality is improving with rising technological advancements1 10 and water purification is crucial to minimize hazardous effects and disruption of ecological equilibrium.
To increase the effectiveness of heavy metal removal from water systems, nanotechnology has recently been combined with a number of cutting-edge methods. 11 , 12 , 13 Because of their distinct sizes and physical characteristics, nanoscale materials have various advantages when used, making nanoscience one of the most significant areas of research and development in modern science. 14 Organic contaminants, colloids, and tiny particles are difficult to separate using traditional techniques; however, magnetic nanoparticles enable this. 15 Due to their ease of separation after use, magnetic absorbent materials have become increasingly in demand in recent times for the removal of pollutants. 16 , 17 Thus, the purpose of this work is to review and explain the use of magnetic nanoparticles in adsorption to remove heavy metals from water and wastewater. The operating parameters that control this process are also covered, including how the adsorption process is affected by the treatment solution’s pH, contact time, temperature, initial heavy metal concentration, and adsorbent dosage.
2 Nanoparticles
2.1 Definition of a nanoparticle
Nanoparticles are defined as synthetic particles with one or more dimensions of less than 100 nm by the American Society for Testing and Materials (ASTM, 2006) and the British Standards Institute (BSI, 2005) in recent work. 18 “Unique properties which differentiate the nanoparticles from the original materials, typically developed at a critical scale of 100 nm” is a comment that goes along with this definition. As a result, the newly indicated features completely rely on the fact that at the nanoparticle scale. The macroscopic solid’s qualities differ from its physicochemical characteristics. It is caused by both the quantum size effects and their high (surface/volume) ratio. 19
2.2 Synthesis and characterization of magnetic nanoparticles
The steps of the adsorption process are synthesis, magnetic nanoparticle characterization, and, in the end, adsorption evaluation using certain techniques, which are detailed below. Since characterization is used to visualize and analyze the outcomes of the first stage, synthesis, the two processes are connected. Chemical techniques such as coprecipitation, 20 fractional emulsions, 21 The synthesis of sol–gel, 22 , 23 acoustic and chemical reactions, 24 processes involving hydrothermal energy, 25 the process of hydrolysis, 26 , 27 thermolysis of beginning substances, 28 An infusion of flow, 29 , 30 the synthesis of electrospray, 31 the solvothermal technique, 32 and ablation using a laser3 33 have all been used over the years to perform bottom-up or top-down approaches for the synthesis of nanoparticles. Moreover, a number of variables, including temperature and pH, influence the stability and size of nanoparticles. The synthesis techniques mentioned above could be used to create nanoparticles of various sizes and forms 34
2.3 Carbon-based nanomaterials
Because of their unique chemical and physical characteristics, carbonaceous functional materials are effective types of nano adsorbents. Due to their enormous potential, numerous attempts have been made in the past to produce different carbon-based nanostructures, such as graphene, carbon-based nanocomposites, and carbon nanotubes (CNTs) for the purpose of eliminating different types of pollutants or treating wastewater contamination; they are attracting the attention of current researchers. CNTs are the most promising of these because of their electrical conductivity, huge surface area, small size, and hollow cylindrical form. SWCNTs, which stand for single-walled carbon nanotubes, and MWCNTs, which stand for multi-walled carbon nanotubes, are the two principal varieties of carbon nanotubes (CNTs). Several pieces of study have addressed the utilization of carbon nanotubes (CNTs) for the purpose of removing heavy metal ions from waste water. For example, Rahbari and Goharrizi reported that lead (II) was able to adsorb from water onto carbon nanotubes CNTs with an adsorption capacity of 70.1 mg/g during the experiment. 35 The application of CNTs is for removing ions by adsorptive with removal capabilities for Copper (II) > lead (II) > Cobalt (II) > Zinc (II) > Manganese (II) was also proven by Stafiej and Pyrzynska. 36 A different study found that MnO2-coated oxidized multiwalled carbon nanotubes (MnO2/oMWCNTs) had a capacity of adsorption 41.6 mg/g and were able to successfully remove cadmium (II) ions from aqueous solution. 37 Because they have better qualities than their equivalents or traditional carbonaceous materials, carbon-layered silicate nanocomposites have recently attracted a lot of scientific interest. An ecologically friendly montmorillonite/carbon (MMT/C) adsorbent with a maximum adsorption capacity of 247.85 mg/g was created for the removal of lead (II) in research by Zhu et al.3 38 Another carbon-based nanomaterial that has drawn a lot of interest is graphene, which is used in environmental cleanup. Graphene oxide has a high hydrophilic character because of the existence of oxygen-containing functional groups on its surface, which causes it to disperse finely in water. Given its unique functional groups and large surface area, graphene oxide is a viable option for wastewater purification. These nanoparticles work well against a variety of contaminants found in wastewater. The process of removing heavy metal ions involves complexing metal ions with graphene’s oxide binding site by adsorption. The graphene’s delocalized π-electron system is impacted by organic contaminants such as dyes. 39 GO nanosheets were produced by Zhao et al. to remove Cadmium (II) and Cobalt (II) adsorptively. The results showed that the adsorption capabilities for Cadmium (II) and Cobalt (II) were 106.3 and 68.2 mg/g, respectively. Zeng et al. developed a 3D MnO2 graphene oxide hydrogel in a different scientific investigation. 40
2.4 Advantages of nanoparticles
There is a high surface area to volume ratio when nanoparticle size decreases. It is largely responsible for the characteristics involving interactions at the interface between the thing under consideration and its surroundings. Due to this characteristic, the material is more chemically reactive, which makes it suitable for applications involving heterogeneous catalysis. 41 Beyond their tiny size, nanomaterials frequently exhibit certain characteristics, including surface and quantum effects. Their peculiar adsorption capacity, which is advantageous for removing heavy metal ions, is a result of these characteristics. An enormous amount of research has been done thus far on nanomaterials to explore potential uses in the treatment of heavy metal-contaminated water. As a viable substitute for adsorbing heavy metals from wastewater, they have shown a lot of promise. 42
The remarkable rate of adsorption that nano adsorbents may hold in a short period has piqued curiosity. Moreover, organic and inorganic contaminants based on nano adsorbents can be removed from water by using them as a separation medium. 43 A survey of the literature reveals that numerous attempts have already been made to clean wastewater, using nanoparticles as an adsorbent to produce effective outcomes. For the nano-size adsorbents to be commercialized for decontaminating water, a few obstacles must be completely overcome. These obstacles include selectivity, stability, longevity of the material, and excellent adsorption measurements. Toxic ions and chemicals from wastewater must be controlled. Hence, an active strategy for treating wastewater and creating novel nano adsorbents is desperately needed. 44 Because they may easily be separated from wastewater by utilizing both the magnetite core and the organic or inorganic shells and because of their superior absorption and heavy metal-absorbing powers. The ability to remove heavy metals from modified magnetite nanoparticles with envelopes as their fundamental structure has shown significant promise. 43
3 Different nanomaterial kinds for the removal of heavy metals
Inorganic and carbon-based nanoparticles are the two categories into which nanomaterials are divided. 45 In the realm of environmental cleanup, they have found widespread application. The most widely used and researched nanomaterials among them are carbon nanotubes (CNTs), titanium dioxide nanoparticles (TiO2 NPs), and nano zero-valent iron (NZVI). 46 , 47 The uses and best practices for the removal of heavy metals from water by using nanomaterials are collected in Table 1.
Uses of nanomaterials for environmental heavy metal removal.
| Types of nanomaterials | Environment | Heavy metals target | Highlights of performance | Ref. |
|---|---|---|---|---|
| NZVI_HCS | Water | Lead (II), copper (II), Zinc (II) | For lead (II), copper (II), zinc II, the highest adsorption capabilities were 195.1, 161.9 and 109.7 mg g−1 respectively | 48 |
| NZVI | Water | Lead (II) | At pH 6, NZVI’s highest adsorption capacity was 807.23 mg g−1. | 49 |
| MWCNTs_COOH | Water | Mercury (II), arsenic (III) | The highest removal efficiencies for arsenic (III) and mercury (II) were 72.4 % and 80.5 %, respectively, at pH 7.6–7.9 with an adsorbent dosage of 20 mg L−1 | 50 |
| Mesoporous carbonated titanium dioxide NPs | Water | Strontium (II) | The highest adsorption capabilities of strontium (II) 204.4 mg g−1 at the natural pH by 4C-titanium dioxide | 51 |
4 Mechanisms of heavy metal removal by nanoparticles
There is ongoing discussion over the interaction processes that enable the removal of heavy metal ions from aqueous solutions, as they are currently poorly understood. 52 Sorption, sorption–reduction, or photocatalytic degradation are the most common remediation techniques used today to remove these contaminants. 53 To understand these pollutants’ possible impact on the environment and establish alternative removal routes, it becomes essential to identify the physicochemical characteristics of these pollutants and combine contemporary characterization (such as spectroscopy) with theoretical predictions at the molecular level become crucial. Because of the chemical interactions between functional groups – particularly those with various oxygen-containing groups, hydroxyl groups, or carbonyl groups – and metal ions, sorption is one of the most straightforward processes for removing heavy metal ions from solutions. 54 It is important to note that sorption is defined by the International Union of Pure and Applied Chemistry (IUPAC) as the process of sorping (adsorbed or absorbed) a material (sorbate) on or in another substance (sorbent). 55 Strong surface complexes that form through hydrophobic contacts, π–π-donor–acceptor interactions, hydrogen bond interactions, and electrostatic attraction promote this process. 56 , 57 , 58 , 59 Comparably, sorption-reduction is a technique that turns high-valent metal ions into low-valent metal ions by immobilizing and reducing them. Reduction of the high valent metal ions produces more dense particles or clusters that precipitate more easily. This sorption-reduction process is commonly seen in the reduction of Cr6+ to Cr3+ and Se4+ to Se2+. Low valent metal ions are, therefore much more biocompatible in their native environment. High-valent metal ions are generally thought to be significantly more mobile than low-valent metal ions, which regulates this process. Lastly, although the method of photocatalytic degradation is widely used to eliminate a variety of organic pollutants from solutions, including POPs (persistent organic pollutants), it has also been widely used to eliminate low concentrations of metalloid ions and heavy metals. 60 , 61 This method is predicated on photocatalytic reactions, which are heavily influenced by the catalyst’s shape, mechanisms of mass transfer, visible light absorption, and the distribution of active sites on the surface. 57 , 58 , 59 , 62 Depending on the kind of light source and metal ions, several mechanisms may be involved in the presence of heavy metal ions. 4
5 Factors affecting adsorption
Many factors, such as adsorbent dosage, pH, temperature, contact time, and initial ion concentration, have been studied in scientific investigations to determine how heavy metal adsorption occurs on the surface of nanoparticles.
5.1 Effect of pH
pH is important for the adsorption capacity in particular and for the entire adsorption process. Consequently, research on its impact on the elimination of metal ions is required. It has been demonstrated in a research paper that the adsorptive removal of Mercury II ions by CANPs rises with a pH increase from 2.0 to 5.0. The electrostatic repulsions between the metal cations and the protonated functional moieties on the surface of CANPs prevent their adsorption at low pH levels. Furthermore, the metal ions are in competition for adsorption with a considerable amount of H+ and H3O+ ions in water. 63 Numerous additional studies have also shown that adsorption of heavy metal ions is preferred at intermediate pH levels as opposed to lower ones. For instance, lead (II) and Copper (II) adsorption on the surface of chitosan/TiO2 nanofibers peaked at pH 6.0 and decreased from pH 2.0 to pH 4.0. 64 In a related investigation, it was shown that the percentage of Cadmium (II) adsorption increased noticeably from pH 4.0 to pH 6.0 before stabilizing at pH 9.0 (97 %). Following that, when the pH was raised to 11.0 once more, the percentage of adsorption dropped to 80 %. 65 The findings gathered from these studies are presented in Figure 1a.
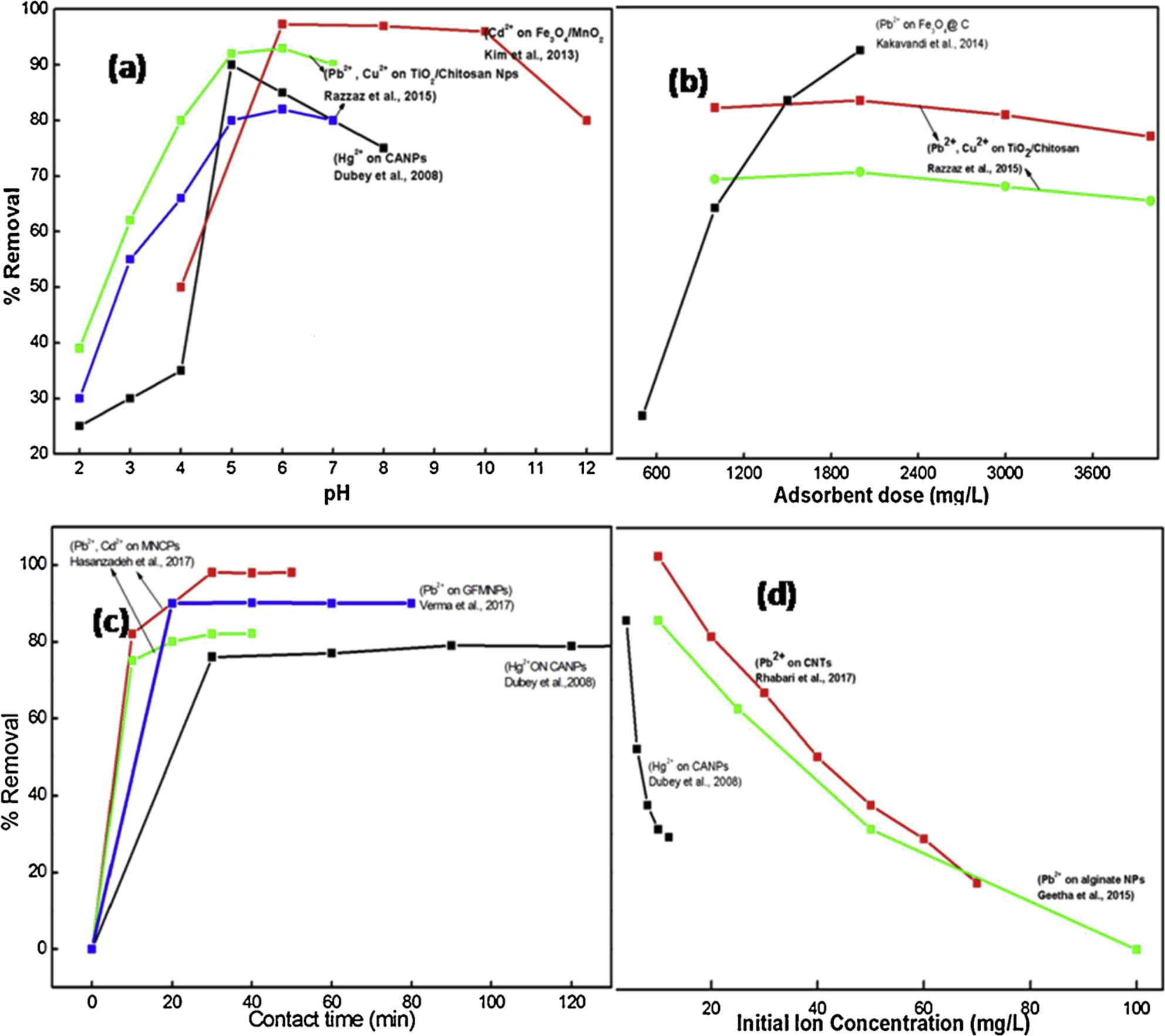
Numerous studies demonstrate how various types of nanosomic materials affect the % removal of heavy metal ions by varying adsorbent doses, pH values, contact times, and initial ion concentrations.
We can infer from the previous description that a moderate pH is ideal for removing heavy metal ions because it causes the sorbent surface to become deprotonated, which increases the number of negatively charged sites. Increased electrostatic attractions between the surface of the adsorbent and metal cations lead to an increase in the adsorption capacity of the adsorbent. As the pH level decreases, however, the number of positively charged adsorption sites increases. This results in an increase in the repulsive interactions that occur between the positively charged metal ions and the surface of the adsorbent, which in turn results in a decrease in the amount of metal ions that are absorbed.6 66 Higher pH levels cause the metal ions to form hydroxylated complexes, which damage the nano adsorbent’s surface and obstruct some of its active sites.
5.2 Effect of contact time
An important factor in the efficient treatment of wastewater is the duration of contact between nanoparticles and the metal ion solution. Removal efficiency has been found to improve with the contact time, which may be explained by an increase in the amount of time that metal ions spend interacting with the active sites on the nano adsorbent. Additionally, removal efficiency grows gradually after the initial stage, which is quick. This is mostly because there are initially more available empty spots on the adsorbent surface, but over time, those spots become less in number. This type of adsorption behavior was demonstrated in a study wherein extending the contact duration from 0 to 90 min improved the removal efficacy for Hg (II) on the surface of CANPs. When adsorption equilibrium was reached in 90 min, the rate of rise in Hg (II) removal efficiency slowed down after increasing quickly in the first 30 min. 63 In a related investigation, it was found that during the first 20 min of contact time, lead (II) and Cadmium (II) adsorbed 91 and 100 % of the total amount of adsorption (48.53 and 53.33 mg/g), respectively, on the surface of MNCPs. After that, the process became independent of contact time because of the equilibrium between the rates of adsorption and desorption. 67 A comparison of several of these research may be found in Figure 1c.
5.3 Effect of temperature
Another crucial element in the adsorption process is solution temperature. Depending on whether the process involves the absorption or development of heat, a subsequent change in temperature can have two distinct effects on the adsorption process. When the temperature rises in an exothermic process, the adsorption capacity falls, while in an endothermic process, it rises. It was discovered through scientific research that the adsorption of Hg (II) on CANPs is exothermic. When the temperature was raised from 10 to 30 °C, the removal effectiveness rose. However, after that, it dropped, reaching a maximum adsorption of about 79.40 % at 30 °C. The increased mobility of metal ions with temperature rise may be the cause of further decline since metal ions may desorb or de-chelate from the adsorbent surface. As a result, when the temperature rose, fewer metal ions were adsorbed on CANPs. Furthermore, in the case of an exothermic adsorption process, the adsorbent’s electrostatic interactions with the Mercury (II) ions become weaker at higher temperatures. 63 In a distinct investigation, it was discovered that Pb (II) adsorption on the Fe3O4 nano adsorbent surface was endothermic at various temperatures. When the temperature was raised, the Pb (II) adsorption effectiveness increased. This was likely caused by an increase in ionic mobility, which led to more ions interacting with the active sites. 68
5.4 Effect of initial ion concentration
Up to a point, increasing the concentration of metal ions enhances the rate of adsorption; however, further increases result in a drop in the removal efficiency. This could be because the solution should contain an ideal concentration of metal ions for a given amount of adsorbent. Low concentrations result in fewer metal ions available for adsorption, which lowers removal capacity; high concentrations, on the other hand, increase the number of ions available for adsorption, increasing the adsorption rate. However, after a certain starting concentration, more ions are available for the same number of adsorption sites, which lowers removal efficiency. For instance, raising the initial Hg (II) concentration on the surface of CANPs from 4 to 12 mg/l increases the adsorption rate, which then falls. 63 Similar research found that when starting metal ion concentrations were raised from 90 to 500 mg/l, the sorption capacity of PVA/ZnO nanofibrous adsorbent for Uranium (VI), Copper (II), and Nickel (II) ions increased. 69 Figure 1d presents a few of these findings.
5.5 Effect of adsorbent dose
It is essential to the adsorption process. It’s some of the effective adsorption sites that determine the capability of adsorption for removing heavy metal ions, and this number grows when the dose of the adsorbent is increased. However, a higher dose of the adsorbent causes NPs to aggregate, reduces surface area, and reduces sorption sites, all of which lower the adsorption capacity. Numerous studies have been published in the literature that show how the dose of the adsorbent affects the adsorption of heavy metal ions. For adsorbing ions of lead (II) and copper (II), for instance, 2000 mg/l was the ideal concentration of nanofibrous chitosan covered with Titanium dioxide. It has been demonstrated that higher concentrations of nanoparticles (NPs) can cause nanofiber surfaces to become partially inactive due to the accumulation of NPs. Consequently, the adsorbent’s adsorption capability was greatly diminished. 64 When the dosage of Fe3O4@C nanosorbent was increased from 0.5 to 2 g/l, there was a noticeable improvement in adsorption efficiency from 41.7 to 92 %; however, there was a corresponding decrease in adsorption capacity from 41.7 to 22.9 mg/g. The enhanced removal efficiency can be attributed to Pb (II) ions having easier access to the active sites on the Fe3O4@C surface. Conversely, a higher adsorbent concentration may cause interparticle aggregation, which lowers the active surface area of Fe3O4@C and leads to a decreased adsorption capacity with an increase in adsorbent dose. 70 A graphic representation of these findings is shown in Figure 1b.
5.6 Effect of ion strength
The aqueous solution’s ionic strength, which indicates the impact of extra ions on the adsorption of adsorbate molecules on the surface of the adsorbent, is crucial to the adsorption process. Typically, varying quantities of ions such as Na+, Cl−, and so on are added to an adsorbate solution to study it. The rivalry between the target and additional ions for active sites on the adsorbent surface, as well as the screening of the coulombic potential between the adsorbent molecule and the adsorbing ions, determine how much of an impact the strength of the ions has. Both the affinity of the adsorbent and the concentration of additional ions have an impact on the adsorption effectiveness. The ionic strength of the solution does not affect the adsorption efficiency of the adsorbent if it has a greater affinity for the target metal ions than for the added ions. Ionic strength has very little impact on the adsorption of heavy metal ions, according to several observations in the literature. Hasanzadeh et al. demonstrated that the presence of sodium chloride at concentrations of 3 mmol per liter affected the adsorption of two heavy metal ions, namely lead (II) and Cadmium (II), on the surface of MNCPs.6 67 The fact that sodium chloride was found to have negligible influence on the adsorption of heavy metal ions indicate that the adsorbent is more suited to adsorb lead (II) and Cadmium (II) ions than Sodium (I) ions. In a study by Xu et al., 71 the addition of sodium chloride at a concentration of 0.025 mM was shown to slightly increase the adsorption capacity of lead (II) on Fe3O4–SiO2-GSH MNPs; however, additional increases in sodium chloride concentration up to 0.2 mM resulted in a drop in adsorption capacity from 98.87 to 85.72 mg/g (Table 2). This observation makes sense since, at first, sodium chloride encourages the dissociation of functional groups on the adsorbent’s surface, increasing the adsorption capacity. The reason for the later decline was the competition between ions for binding sites. Thus, it can be said that depending on the sodium chloride concentration and the adsorbent surface’s affinity for adsorbate molecules, the impact of ionic strength can range from little to significant.
Adsorption capacity and system conditions.
| Magnetic nanoparticle | Heavy metal ion | Adsorption capacity (mg/g) | Time (min) | pH | Temperature (°C) | Ref. |
|---|---|---|---|---|---|---|
| MnFe2O4 | Zinc (II) | 454.4 | 120 | 6 | 25 | 72 |
| CoFe2O4 | Zinc (II) | 384.6 | 120 | 6 | 25 | 72 |
| SiO2/CuFe2O4/PANI | Copper (II) | 285.71 | 300 | 5.3 | 30 | 73 |
| Fe3O4@Z-NCNT/PC | Lead (II) | 789.87 | 20 | 5.5 | 74 | |
| Fe3O4/NaP/NH2 | Lead (II) | 181.81 | 480 | 5–6 | 60 | 75 |
| Fe3O4/NaP/NH2 | Cadmium (II) | 50.25 | 240 | 5–6 | 70 | 75 |
| APTES-Fe3O4 (3 wt%) | Arsenic (V) | 14.6 | 210 | 2 | 25 | 76 |
| CoFe2O4@SiO2-EDTA | Mercury (II) | 103.3 | 360 | 7 | 25 | 77 |
| MnFe2O4–BC | Cadmium (II) | 181.49 | 7 | 25 | 78 | |
| CoFe2O4@SiO2 | Mercury (II) | 149.3 | 7 | 25 | 79 | |
| rGO-PDTC/Fe3O4 | Copper (II) | 113.64 | 5 | 25 | 80 | |
| Graphene oxide-Fe3O4 | Lead (II) | 373.14 | 10 | 6 | 81 |
6 Conclusions
Nanomaterials, due to their remarkable properties, have proven highly effective in removing heavy metals from water. This review has focused on the synthesis, characterization, and applications of magnetic nanoparticles in heavy metal removal. The impact of several key factors, including pH, adsorbent dose, contact time, temperature, initial ion concentration, and ionic strength, on the adsorption process has been discussed. While these findings demonstrate the potential of magnetic nanoparticles in water purification, the next challenge lies in scaling up these methods for industrial use. Practical considerations such as the cost of large-scale synthesis, the reusability and recovery of nanoparticles, and the long-term environmental impacts of widespread nanoparticle use must be addressed. Additionally, optimizing the efficiency of magnetic nanoparticles for use in diverse water treatment systems will be crucial. Despite these challenges, magnetic nanoparticles hold great promise as a scalable, efficient solution for heavy metal remediation, offering significant benefits for environmental protection and public health.
Acknowledgments
The authors would like to express their gratitude to the Head of the Chemistry Department at Koya University in Iraq.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Koncagül, E.; Tran, M.; Connor, R.; Uhlenbrook, S. The United Nations World Water Development Report 2018: Nature-Based Solutions for Water; Facts and Figures; United Nations Educational, Scientific and Cultural Organization: Paris, 2018.Suche in Google Scholar
2. Chervona, Y.; Arita, A.; Costa, M. Carcinogenic Metals and the Epigenome: Understanding the Effect of Nickel, Arsenic, and Chromium. Metallomics 2012, 4 (7), 619–627; https://doi.org/10.1039/c2mt20033c.Suche in Google Scholar PubMed PubMed Central
3. Afroze, S.; Sen, T. K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water, Air, Soil Pollut. 2018, 229, 1–50; https://doi.org/10.1007/s11270-018-3869-z.Suche in Google Scholar
4. Le, A. T.; Pung, S. Y.; Sreekantan, S.; Matsuda, A.; Huynh, D. P. Mechanisms of Removal of Heavy Metal Ions by ZnO Particles. Heliyon 2019, 5 (4); https://doi.org/10.1016/j.heliyon.2019.e01440.Suche in Google Scholar PubMed PubMed Central
5. Yari, S.; Abbasizadeh, S.; Mousavi, S. E.; Moghaddam, M. S.; Moghaddam, A. Z. Adsorption of Pb (II) and Cu (II) Ions from Aqueous Solution by an Electrospun CeO2 Nanofiber Adsorbent Functionalized with Mercapto Groups. Process Saf. Environ. Protect. 2015, 94, 159–171; https://doi.org/10.1016/j.psep.2015.01.011.Suche in Google Scholar
6. Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable Technologies for Water Purification from Heavy Metals: Review and Analysis. Chem. Soc. Rev. 2019, 48 (2), 463–487; https://doi.org/10.1039/c8cs00493e.Suche in Google Scholar PubMed
7. Elgarahy, A.; Elwakeel, K.; Mohammad, S.; Elshoubaky, G. A Critical Review of Biosorption of Dyes, Heavy Metals and Metalloids from Wastewater as an Efficient and Green Process. Clean. Eng. Tech. 2021, 4, 100209; https://doi.org/10.1016/j.clet.2021.100209.Suche in Google Scholar
8. Ethaib, S.; Erabee, I. K.; Abdulsahib, A. A. Removal of Methylene Blue Dye from Synthetic Wastewater Using Kenaf Core and Activated Carbon. Int. J. Eng. Technol. 2018, 7 (4.19), 909–913; https://doi.org/10.14419/ijet.v7i4.19.28069.Suche in Google Scholar
9. Kim, K. H.; Keller, A. A.; Yang, J. K. Removal of Heavy Metals from Aqueous Solution Using a Novel Composite of Recycled Materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 425, 6–14; https://doi.org/10.1016/j.colsurfa.2013.02.044.Suche in Google Scholar
10. Hao, X.; Chen, G.; Yuan, Z. Water in China. Water Res. 2020, 169, 115256; https://doi.org/10.1016/j.watres.2019.115256.Suche in Google Scholar PubMed
11. Liosis, C.; Papadopoulou, A.; Karvelas, E.; Karakasidis, T. E.; Sarris, I. E. Heavy Metal Adsorption Using Magnetic Nanoparticles for Water Purification: A Critical Review. Materials 2021, 14 (24), 7500; https://doi.org/10.3390/ma14247500.Suche in Google Scholar PubMed PubMed Central
12. Karvelas, E.; Liosis, C.; Karakasidis, T.; Sarris, I. Mixing of Particles in Micromixers under Different Angles and Velocities of the Incoming Water. In Proceedings; MDPI, 2, 577, 2018.10.3390/proceedings2110577Suche in Google Scholar
13. Karvelas, E.; Liosis, C.; Benos, L.; Karakasidis, T.; Sarris, I. Micromixing Efficiency of Particles in Heavy Metal Removal Processes under Various Inlet Conditions. Water 2019, 11 (6), 1135; https://doi.org/10.3390/w11061135.Suche in Google Scholar
14. Akchiche, Z.; Abba, A.; Saggai, S. Magnetic Nanoparticles for the Removal of Heavy Metals from Industrial Wastewater. Algerian J. Chem. Eng. 2021, 1 (1), 8–15.Suche in Google Scholar
15. Predescu, A.; Matei, E.; Predescu, A.; Berbecaru, A. Removal Efficiency on Magnetite (Fe3O4) of Some Multicomponent Systems Present in Synthetic Aqueous Solutions. In Proceedings of the 11th WSEAS International Conference on Electronics, Hardware, Wireless and Optical Communications, and Proceedings of the 11th WSEAS International Conference on Signal Processing, Robotics and Automation, and Proceedings of the 4th WSEAS International Conference on Nanotechnology, 63–67, 2012.Suche in Google Scholar
16. Can, M. M.; Ozcan, S.; Ceylan, A.; Firat, T. Effect of Milling Time on the Synthesis of Magnetite Nanoparticles by Wet Milling. Mater. Sci. Eng., B 2010, 172 (1), 72–75; https://doi.org/10.1016/j.mseb.2010.04.019.Suche in Google Scholar
17. Gazeau, F.; Wilhelm, C. Nanoparticules et stimuli magnétiques pour l’imagerie médicale et la thérapie, nanomagnetism for medical imaging and therapy; Université de Paris Diderot: Paris, 2012.Suche in Google Scholar
18. Goutayer, M. Nano-émulsions pour la vectorisation d’agents thérapeutiques ou diagnostiques: étude de la biodistribution par imagerie de fluorescence in vivo; Université Pierre et Marie Curie-Paris VI: Paris, 2008.Suche in Google Scholar
19. d’Orlyé, F. Caractérisation physicochimique par électrophorèse capillaire de nanoparticules magnétiques, anioniques et cationiques: distribution de taille, densité de charge et coefficient de diffusion collectif; Chimie ParisTech: Paris, 2008.Suche in Google Scholar
20. Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A. R.; Ali, J. S.; Hussain, A. Synthesis, Characterization, Applications, and Challenges of Iron Oxide Nanoparticles. Nanotechnol. Sci. Appl. 2016, 49–67; https://doi.org/10.2147/nsa.s99986.Suche in Google Scholar PubMed PubMed Central
21. Najjar, R., Ed. Microemulsions – An Introduction to Properties and Applications; IntechOpen: Rijeka, 2012.10.5772/36057Suche in Google Scholar
22. Wang, X. L.; Wei, L.; Tao, G. H.; Huang, M. Q. Synthesis and Characterization of Magnetic and Luminescent Fe3O4/CdTe Nanocomposites Using Aspartic Acid as Linker. Chin. Chem. Lett. 2011, 22 (2), 233–236; https://doi.org/10.1016/j.cclet.2010.09.016.Suche in Google Scholar
23. Takai, Z. I.; Mustafa, M. K.; Asman, S.; Sekak, K. A. Preparation and Characterization of Magnetite (Fe3O4) Nanoparticles by Sol-Gel Method. Int. J. Nanoelectron. Mater 2019, 12, 37–46.Suche in Google Scholar
24. Wang, Y.; Nkurikiyimfura, I.; Pan, Z. Sonochemical Synthesis of Magnetic Nanoparticles. Chem. Eng. Commun. 2015, 202 (5), 616–621; https://doi.org/10.1080/00986445.2013.858039.Suche in Google Scholar
25. Ge, S.; Shi, X.; Sun, K.; Li, C.; Uher, C.; Baker Jr, J. R.; Banaszak Holl, M. M.; Orr, B. G. Facile Hydrothermal Synthesis of Iron Oxide Nanoparticles with Tunable Magnetic Properties. J. Phys. Chem. C 2009, 113 (31), 13593–13599; https://doi.org/10.1021/jp902953t.Suche in Google Scholar PubMed PubMed Central
26. Oćwieja, M.; Węgrzynowicz, A.; Maciejewska-Prończuk, J.; Michorczyk, P.; Adamczyk, Z.; Roman, M.; Bielańska, E. Preparation of Iron Oxide Nanoparticles Doped by Chromium for Application in Water–Gas Shift Reaction. Colloids Surf. A Physicochem. Eng. Asp. 2017, 523, 71–80; https://doi.org/10.1016/j.colsurfa.2017.04.004.Suche in Google Scholar
27. Compeán-Jasso, M.; Ruiz, F.; Martínez, J.; Herrera-Gómez, A. Magnetic Properties of Magnetite Nanoparticles Synthesized by Forced Hydrolysis. Mater. Lett. 2008, 62 (27), 4248–4250; https://doi.org/10.1016/j.matlet.2008.06.053.Suche in Google Scholar
28. Stefanescu, M.; Stefanescu, O.; Stoia, M.; Lazau, C. Thermal Decomposition of Some Metal-Organic Precursors: Fe2O3 Nanoparticles. J. Therm. Anal. Calorim. 2007, 88 (1), 27–32; https://doi.org/10.1007/s10973-006-8003-6.Suche in Google Scholar
29. Salazar-Alvarez, G.; Muhammed, M.; Zagorodni, A. A. Novel Flow Injection Synthesis of Iron Oxide Nanoparticles with Narrow Size Distribution. Chem. Eng. Sci. 2006, 61 (14), 4625–4633; https://doi.org/10.1016/j.ces.2006.02.032.Suche in Google Scholar
30. El-Amin, M. F.; Saad, A. M.; Salama, A.; Sun, S. Modeling and Analysis of Magnetic Nanoparticles Injection in Water-Oil Two-phase Flow in Porous Media under Magnetic Field Effect. Geofluids 2017, 2017; https://doi.org/10.1155/2017/3602593.Suche in Google Scholar
31. Agostini, P.; Meffre, A.; Lacroix, L. M.; Ugnati, D.; Ondarçuhu, T.; Respaud, M.; Lassagne, B. Electrospray Deposition of Isolated Chemically Synthesized Magnetic Nanoparticles. J. Nanoparticle Res. 2016, 18, 1–10; https://doi.org/10.1007/s11051-015-3312-y.Suche in Google Scholar
32. Atabaev, T. S.; Kim, H. K.; Hwang, Y. H. Fabrication of Bifunctional Core-Shell Fe3O4 Particles Coated with Ultrathin Phosphor Layer. Nanoscale Res. Lett. 2013, 8, 1–6; https://doi.org/10.1186/1556-276x-8-357.Suche in Google Scholar
33. Rivera-Chaverra, M. J.; Restrepo-Parra, E.; Acosta-Medina, C. D.; Mello, A.; Ospina, R. Synthesis of Oxide Iron Nanoparticles Using Laser Ablation for Possible Hyperthermia Applications. Nanomaterials 2020, 10 (11), 2099; https://doi.org/10.3390/nano10112099.Suche in Google Scholar PubMed PubMed Central
34. Park, J. Y.; Lee, Y. J.; Khanna, P. K.; Jun, K. W.; Bae, J. W.; Kim, Y. H. Alumina-Supported Iron Oxide Nanoparticles as Fischer–Tropsch Catalysts: Effect of Particle Size of Iron Oxide. J. Mol. Catal. Chem. 2010, 323 (1–2), 84–90; https://doi.org/10.1016/j.molcata.2010.03.025.Suche in Google Scholar
35. Rahbari, M.; Goharrizi, A. S. Adsorption of Lead (II) from Water by Carbon Nanotubes: Equilibrium, Kinetics, and Thermodynamics. Water Environ. Res. 2009, 81 (6), 598–607; https://doi.org/10.2175/106143008x370511.Suche in Google Scholar PubMed
36. Stafiej, A.; Pyrzynska, K. Adsorption of Heavy Metal Ions with Carbon Nanotubes. Sep. Purif. Technol. 2007, 58 (1), 49–52; https://doi.org/10.1016/j.seppur.2007.07.008.Suche in Google Scholar
37. Liu, D.; Zhu, Y.; Li, Z.; Tian, D.; Chen, L.; Chen, P. Chitin Nanofibrils for Rapid and Efficient Removal of Metal Ions from Water System. Carbohydr. Polym. 2013, 98 (1), 483–489; https://doi.org/10.1016/j.carbpol.2013.06.015.Suche in Google Scholar PubMed
38. Zhu, K.; Jia, H.; Wang, F.; Zhu, Y.; Wang, C.; Ma, C. Efficient Removal of Pb (II) from Aqueous Solution by Modified Montmorillonite/Carbon Composite: Equilibrium, Kinetics, and Thermodynamics. J. Chem. Eng. Data 2017, 62 (1), 333–340; https://doi.org/10.1021/acs.jced.6b00676.Suche in Google Scholar
39. Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-layered Graphene Oxide Nanosheets as Superior Sorbents for Heavy Metal Ion Pollution Management. Environ. Sci. Technol. 2011, 45 (24), 10454–10462; https://doi.org/10.1021/es203439v.Suche in Google Scholar PubMed
40. Zeng, T.; Yu, Y.; Li, Z.; Zuo, J.; Kuai, Z.; Jin, Y.; Wang, Y.; Wu, A.; Peng, C. 3D MnO2 Nanotubes@ Reduced Graphene Oxide Hydrogel as Reusable Adsorbent for the Removal of Heavy Metal Ions. Mater. Chem. Phys. 2019, 231, 105–108; https://doi.org/10.1016/j.matchemphys.2019.04.019.Suche in Google Scholar
41. Andrieux-Ledier, A. Elaboration de nanoparticules d’argent par réduction de sels métallo-organiques: contrôle de taille, stabilité, organisation et propriétés physiques; Université Pierre et Marie Curie-Paris VI, 2012.Suche in Google Scholar
42. Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9 (3), 424; https://doi.org/10.3390/nano9030424.Suche in Google Scholar PubMed PubMed Central
43. Yaqoob, A.; Parveen, T.; Umar, K. Mohamad Ibrahim MN Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12 (495.10), 3390.10.3390/w12020495Suche in Google Scholar
44. Guo, Y.; Zhang, X.; Sun, X.; Kong, D.; Han, M.; Wang, X. Nanoadsorbents Based on NIPAM and Citric Acid: Removal Efficacy of Heavy Metal Ions in Different Media. ACS Omega 2019, 4 (10), 14162–14168; https://doi.org/10.1021/acsomega.9b00573.Suche in Google Scholar PubMed PubMed Central
45. Stone, V.; Nowack, B.; Baun, A.; van den Brink, N.; von der Kammer, F.; Dusinska, M.; Handy, R.; Hankin, S.; Hassellöv, M.; Joner, E.; Fernandes, T. F. Nanomaterials for Environmental Studies: Classification, Reference Material Issues, and Strategies for Physico-Chemical Characterisation. Sci. Total Environ. 2010, 408 (7), 1745–1754; https://doi.org/10.1016/j.scitotenv.2009.10.035.Suche in Google Scholar PubMed
46. Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon Nanotubes as Adsorbents in Environmental Pollution Management: A Review. Chem. Eng. J. 2011, 170 (2-3), 395–410; https://doi.org/10.1016/j.cej.2010.08.045.Suche in Google Scholar
47. Fu, F.; Dionysiou, D. D.; Liu, H. The Use of Zero-Valent Iron for Groundwater Remediation and Wastewater Treatment: A Review. J. Hazard Mater. 2014, 267, 194–205; https://doi.org/10.1016/j.jhazmat.2013.12.062.Suche in Google Scholar PubMed
48. Yang, F.; Zhang, S.; Sun, Y.; Cheng, K.; Li, J.; Tsang, D. C. Fabrication and Characterization of Hydrophilic Corn Stalk Biochar-Supported Nanoscale Zero-Valent Iron Composites for Efficient Metal Removal. Bioresour. Technol. 2018, 265, 490–497; https://doi.org/10.1016/j.biortech.2018.06.029.Suche in Google Scholar PubMed
49. Dongsheng, Z.; Wenqiang, G.; Guozhang, C.; Shuai, L.; Weizhou, J.; Youzhi, L. Removal of Heavy Metal Lead (II) Using Nanoscale Zero-Valent Iron with Different Preservation Methods. Adv. Powder Technol. 2019, 30 (3), 581–589; https://doi.org/10.1016/j.apt.2018.12.013.Suche in Google Scholar
50. Alimohammady, M.; Jahangiri, M.; Kiani, F.; Tahermansouri, H. Design and Evaluation of Functionalized Multi-Walled Carbon Nanotubes by 3-Aminopyrazole for the Removal of Hg (II) and as (III) Ions from Aqueous Solution. Res. Chem. Intermed. 2018, 44, 69–92; https://doi.org/10.1007/s11164-017-3091-4.Suche in Google Scholar
51. Mironyuk, I.; Tatarchuk, T.; Naushad, M.; Vasylyeva, H.; Mykytyn, I. Highly Efficient Adsorption of Strontium Ions by Carbonated Mesoporous TiO2. J. Mol. Liq. 2019, 285, 742–753; https://doi.org/10.1016/j.molliq.2019.04.111.Suche in Google Scholar
52. Xiong, C.; Wang, W.; Tan, F.; Luo, F.; Chen, J.; Qiao, X. Investigation on the Efficiency and Mechanism of Cd (II) and Pb (II) Removal from Aqueous Solutions Using MgO Nanoparticles. J. Hazard Mater. 2015, 299, 664–674; https://doi.org/10.1016/j.jhazmat.2015.08.008.Suche in Google Scholar PubMed
53. Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V. H. Heavy Metal Water Pollution: A Fresh Look about Hazards, Novel and Conventional Remediation Methods. Environ. Technol. Innovat. 2021, 22, 101504; https://doi.org/10.1016/j.eti.2021.101504.Suche in Google Scholar
54. Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y. S.; Jiang, Y.; Gao, B. Surface Functional Groups of Carbon-Based Adsorbents and Their Roles in the Removal of Heavy Metals from Aqueous Solutions: A Critical Review. Chem. Eng. J. 2019, 366, 608–621; https://doi.org/10.1016/j.cej.2019.02.119.Suche in Google Scholar PubMed PubMed Central
55. Ettre, L. S. Nomenclature for Chromatography (IUPAC Recommendations 1993). Pure Appl. Chem. 1993, 65 (4), 819–872; https://doi.org/10.1351/pac199365040819.Suche in Google Scholar
56. Hu, J.; Chen, G.; Lo, I. M. Removal and Recovery of Cr (VI) from Wastewater by Maghemite Nanoparticles. Water Res. 2005, 39 (18), 4528–4536; https://doi.org/10.1016/j.watres.2005.05.051.Suche in Google Scholar PubMed
57. Alharbi, N. S.; Hu, B.; Hayat, T.; Rabah, S. O.; Alsaedi, A.; Zhuang, L.; Wang, X. Efficient Elimination of Environmental Pollutants through Sorption-Reduction and Photocatalytic Degradation Using Nanomaterials. Front. Chem. Sci. Eng. 2020, 14, 1124–1135; https://doi.org/10.1007/s11705-020-1923-z.Suche in Google Scholar
58. Hu, B.; Ai, Y.; Jin, J.; Hayat, T.; Alsaedi, A.; Zhuang, L.; Wang, X. Efficient Elimination of Organic and Inorganic Pollutants by Biochar and Biochar-Based Materials. Biochar 2020, 2, 47–64; https://doi.org/10.1007/s42773-020-00044-4.Suche in Google Scholar
59. Liu, C.; Wang, Q.; Jia, F.; Song, S. Adsorption of Heavy Metals on Molybdenum Disulfide in Water: A Critical Review. J. Mol. Liq. 2019, 292, 111390; https://doi.org/10.1016/j.molliq.2019.111390.Suche in Google Scholar
60. Shukor, S. A. A.; Hamzah, R.; Bakar, M. A.; Noriman, N.; Al-Rashdi, A. A.; Razlan, Z.; Shahriman, A.; Zunaidi, I.; Khairunizam, W. Metal Oxide and Activated Carbon as Photocatalyst for Waste Water Treatment. In IOP Conference Series: Materials Science and Engineering; IOP Publishing, Vol. 557, 2019; pp 012066.10.1088/1757-899X/557/1/012066Suche in Google Scholar
61. Cheng, L.; Liu, S.; He, G.; Hu, Y. The Simultaneous Removal of Heavy Metals and Organic Contaminants over a Bi2WO6/mesoporous TiO2 Nanotube Composite Photocatalyst. RSC Adv. 2020, 10 (36), 21228–21237; https://doi.org/10.1039/d0ra03430d.Suche in Google Scholar PubMed PubMed Central
62. Wang, X.; Li, X.; Wang, J.; Zhu, H. Recent Advances in Carbon Nitride-Based Nanomaterials for the Removal of Heavy Metal Ions from Aqueous Solution. J. Inorg. Mater. 2020, 35 (3).Suche in Google Scholar
63. Dubey, R.; Bajpai, J.; Bajpai, A. Chitosan-alginate Nanoparticles (CANPs) as Potential Nanosorbent for Removal of Hg (II) Ions. Environ. Nanotechnol. Monit. Manag. 2016, 6, 32–44; https://doi.org/10.1016/j.enmm.2016.06.008.Suche in Google Scholar
64. Razzaz, A.; Ghorban, S.; Hosayni, L.; Irani, M.; Aliabadi, M. Chitosan Nanofibers Functionalized by TiO2 Nanoparticles for the Removal of Heavy Metal Ions. J. Taiwan Inst. Chem. Eng. 2016, 58, 333–343; https://doi.org/10.1016/j.jtice.2015.06.003.Suche in Google Scholar
65. Kim, E. J.; Lee, C. S.; Chang, Y. Y.; Chang, Y. S. Hierarchically Structured Manganese Oxide-Coated Magnetic Nanocomposites for the Efficient Removal of Heavy Metal Ions from Aqueous Systems. ACS Appl. Mater. Interfaces 2013, 5 (19), 9628–9634; https://doi.org/10.1021/am402615m.Suche in Google Scholar PubMed
66. Huang, S. H.; Chen, D. H. Rapid Removal of Heavy Metal Cations and Anions from Aqueous Solutions by an Amino-Functionalized Magnetic Nano-Adsorbent. J. Hazard Mater. 2009, 163 (1), 174–179; https://doi.org/10.1016/j.jhazmat.2008.06.075.Suche in Google Scholar PubMed
67. Hasanzadeh, R.; Moghadam, P. N.; Bahri-Laleh, N.; Sillanpää, M. Effective Removal of Toxic Metal Ions from Aqueous Solutions: 2-Bifunctional Magnetic Nanocomposite Base on Novel Reactive PGMA-MAn Copolymer@ Fe3O4 Nanoparticles. J. Colloid Interface Sci. 2017, 490, 727–746; https://doi.org/10.1016/j.jcis.2016.11.098.Suche in Google Scholar PubMed
68. Nassar, N. N. Rapid Removal and Recovery of Pb (II) from Wastewater by Magnetic Nanoadsorbents. J. Hazard Mater. 2010, 184 (1–3), 538–546; https://doi.org/10.1016/j.jhazmat.2010.08.069.Suche in Google Scholar PubMed
69. Hallaji, H.; Keshtkar, A. R.; Moosavian, M. A. A Novel Electrospun PVA/ZnO Nanofiber Adsorbent for U (VI), Cu (II) and Ni (II) Removal from Aqueous Solution. J. Taiwan Inst. Chem. Eng. 2015, 46, 109–118; https://doi.org/10.1016/j.jtice.2014.09.007.Suche in Google Scholar
70. Kakavandi, B.; Kalantary, R. R.; Jafari, A. J.; Nasseri, S.; Ameri, A.; Esrafili, A.; Azari, A. Pb (II) Adsorption onto a Magnetic Composite of Activated Carbon and Superparamagnetic Fe3O4 Nanoparticles: Experimental and Modeling Study. Clean: Soil, Air, Water 2015, 43 (8), 1157–1166; https://doi.org/10.1002/clen.201400568.Suche in Google Scholar
71. Xu, P.; Zeng, G. M.; Huang, D. L.; Yan, M.; Chen, M.; Lai, C.; Jiang, H.; Wu, H. P.; Chen, G. M.; Wan, J. Fabrication of Reduced Glutathione Functionalized Iron Oxide Nanoparticles for Magnetic Removal of Pb (II) from Wastewater. J. Taiwan Inst. Chem. Eng. 2017, 71, 165–173; https://doi.org/10.1016/j.jtice.2016.11.031.Suche in Google Scholar
72. Asadi, R.; Abdollahi, H.; Gharabaghi, M.; Boroumand, Z. Effective Removal of Zn (II) Ions from Aqueous Solution by the Magnetic MnFe2O4 and CoFe2O4 Spinel Ferrite Nanoparticles with Focuses on Synthesis, Characterization, Adsorption, and Desorption. Adv. Powder Technol. 2020, 31 (4), 1480–1489; https://doi.org/10.1016/j.apt.2020.01.028.Suche in Google Scholar
73. Taleb, M. A.; Kumar, R.; Al-Rashdi, A. A.; Seliem, M. K.; Barakat, M. Fabrication of SiO2/CuFe2O4/polyaniline Composite: A Highly Efficient Adsorbent for Heavy Metals Removal from Aquatic Environment. Arab. J. Chem. 2020, 13 (10), 7533–7543; https://doi.org/10.1016/j.arabjc.2020.08.028.Suche in Google Scholar
74. Jafari, Z.; Avargani, V. M.; Rahimi, M. R.; Mosleh, S. Magnetic Nanoparticles-Embedded Nitrogen-Doped Carbon Nanotube/porous Carbon Hybrid Derived from a Metal-Organic Framework as a Highly Efficient Adsorbent for Selective Removal of Pb (II) Ions from Aqueous Solution. J. Mol. Liq. 2020, 318, 113987; https://doi.org/10.1016/j.molliq.2020.113987.Suche in Google Scholar
75. Zendehdel, M.; Ramezani, M.; Shoshtari-Yeganeh, B.; Cruciani, G.; Salmani, A. Simultaneous Removal of Pb (II), Cd (II) and Bacteria from Aqueous Solution Using Amino-Functionalized Fe3O4/NaP Zeolite Nanocomposite. Environ. Technol. 2019, 40 (28), 3689–3704; https://doi.org/10.1080/09593330.2018.1485750.Suche in Google Scholar PubMed
76. Rowley, J.; Abu-Zahra, N. H. Synthesis and Characterization of Polyethersulfone Membranes Impregnated with (3-aminopropyltriethoxysilane) APTES-Fe3O4 Nanoparticles for as (V) Removal from Water. J. Environ. Chem. Eng. 2019, 7 (1), 102875; https://doi.org/10.1016/j.jece.2018.102875.Suche in Google Scholar
77. Xia, K.; Guo, Y.; Shao, Q.; Zan, Q.; Bai, R. Removal of Mercury (II) by EDTA-Functionalized Magnetic CoFe2O4@ SiO2 Nanomaterial with Core-Shell Structure. Nanomaterials 2019, 9 (11), 1532; https://doi.org/10.3390/nano9111532.Suche in Google Scholar PubMed PubMed Central
78. Wang, Y. Y.; Ji, H. Y.; Lu, H. H.; Liu, Y. X.; Yang, R. Q.; He, L. L.; Yang, S. M. Simultaneous Removal of Sb (III) and Cd (II) in Water by Adsorption onto a MnFe2O4–biochar Nanocomposite. RSC Adv. 2018, 8 (6), 3264–3273; https://doi.org/10.1039/c7ra13151h.Suche in Google Scholar PubMed PubMed Central
79. Wang, X.; Zhang, Z.; Zhao, Y.; Xia, K.; Guo, Y.; Qu, Z.; Bai, R. A Mild and Facile Synthesis of Amino Functionalized CoFe2O4@ SiO2 for Hg (II) Removal. Nanomaterials 2018, 8 (9), 673; https://doi.org/10.3390/nano8090673.Suche in Google Scholar PubMed PubMed Central
80. Fu, W.; Huang, Z. Magnetic Dithiocarbamate Functionalized Reduced Graphene Oxide for the Removal of Cu (II), Cd (II), Pb (II), and Hg (II) Ions from Aqueous Solution: Synthesis, Adsorption, and Regeneration. Chemosphere 2018, 209, 449–456; https://doi.org/10.1016/j.chemosphere.2018.06.087.Suche in Google Scholar PubMed
81. Guo, T.; Bulin, C.; Li, B.; Zhao, Z.; Yu, H.; Sun, H.; Ge, X.; Xing, R.; Zhang, B. Efficient Removal of Aqueous Pb (II) Using Partially Reduced Graphene Oxide-Fe3O4. Adsorpt. Sci. Technol. 2018, 36 (3–4), 1031–1048; https://doi.org/10.1177/0263617417744402.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Advancements in CNT-based materials for optimized pharmaceutical removal via adsorption and photocatalysis
- Review on removal of heavy metals from industrial effluents by adsorption
- Cutting-edge techniques in low-temperature electrochemical water splitting: advancements in hydrogen production
- Biological activities of metal complexes with Schiff base
- Potential of organometallic complexes in medicinal chemistry
- Advancement in schiff base complexes for treatment of colon cancer
- Magnetic nanoparticles for efficient heavy metal removal: synthesis, adsorption capacity, and key experimental parameters
- Coal-based carbon/graphene quantum dots: formation mechanisms and applications
- Advancements in transition metal-catalyzed 1,2,3-triazole synthesis via azide–alkyne cycloaddition
- Gold complexes: a new frontier in the battle against lung cancer
Artikel in diesem Heft
- Frontmatter
- Advancements in CNT-based materials for optimized pharmaceutical removal via adsorption and photocatalysis
- Review on removal of heavy metals from industrial effluents by adsorption
- Cutting-edge techniques in low-temperature electrochemical water splitting: advancements in hydrogen production
- Biological activities of metal complexes with Schiff base
- Potential of organometallic complexes in medicinal chemistry
- Advancement in schiff base complexes for treatment of colon cancer
- Magnetic nanoparticles for efficient heavy metal removal: synthesis, adsorption capacity, and key experimental parameters
- Coal-based carbon/graphene quantum dots: formation mechanisms and applications
- Advancements in transition metal-catalyzed 1,2,3-triazole synthesis via azide–alkyne cycloaddition
- Gold complexes: a new frontier in the battle against lung cancer

