Abstract
Water treatment plays a crucial role in meeting the growing demand for water and preventing future shortages. The unique and adaptable arbitrary, physical, and chemical properties of carbon nanotubes (CNTs) make them an attractive candidate for use in water treatment. CNTs are employed in environmental applications because of their exceptional adsorbent, mechanical, and chemical characteristics. Functional groups chemically or physically modify pure CNTs, improving their desalination and extraction capabilities. The advantages of CNT-based composites, such as antifouling performance, excellent selectivity, and higher water permeability, reassure us of their effectiveness in water treatment. This review comprehensively discusses the structural features and synthetic methods of CNTs. The functionalization and the pros and cons of functionalized CNT materials are also discussed. Pharmaceutical compounds are often manufactured using batch processes, resulting in the production of various products in wastewater. The occurrence of pharmaceutical compounds in drinking water arises from two distinct origins: the pharmaceutical industry’s manufacturing procedures and the widespread use of pharmaceutical compounds, which leads to their presence in urban and agricultural wastewater. This review discusses role of CNT-based nanomaterials in effectively removing pharmaceutical waste from wastewater through adsorption and photocatalytic processes. Lastly, the future approach is discussed to develop CNT-based nanomaterials better.
1 Introduction
The growing human population, chemical dependence, and the fact that the majority of micropollutants (MPs) are not biodegradable. There are a lot of human activities that release synthetic MPs into water bodies every day. 1 , 2 Some examples of these activities are shipping, motorboats, port operations, oil spills, ballast water discharge, and the release of industrial, municipal, and untreated wastewater. 3 Most of these MPs linger for a long time, which means they may damage ecosystems. These micropollutants (MPs) are extremely dangerous to aquatic life because of their persistent nature, which can also have negative impacts on human health due to bioaccumulation in the food chain. 4 , 5 Furthermore, especially in densely populated areas, the ongoing migration of MPs into water bodies makes it harder to guarantee clean and secure water supplies. 6 The development and implementation of cutting-edge water treatment techniques, that can efficiently remove MPs from wastewater before it reaches the environment, are necessary to address this issue. Nanomaterials have now been used for the cleansing purposes of wastewater. 7 , 8 , 9 , 10 , 11
The extraordinary mechanical, electrical, thermal, and chemical capabilities of carbon nanotubes (CNTs) have been known since their discovery in 1991. Numerous fields have explored the potential uses of carbon nanotubes (CNTs), such as nanocomposites, microelectronics, energy storage, medicinal devices, and the removal and treatment of contaminants. 12 Adsorption and photocatalytic treatment of pharmaceuticals compounds (PHCs), and endocrine disrupting compounds (EDCs) have been the subject of relatively few investigations, despite the fact that numerous studies have shown that CNTs efficiently adsorb a variety of contaminants. 13 Water and wastewater experts are starting to become worried about the presence of pharmaceuticals and EDCs in water supplies. 14 An instance of this is the detection of pharmaceuticals in freshwater resources at ng L−1 levels, which are the parts per trillion thresholds. Complete removal of pharmaceuticals and EDCs from wastewater are still beyond the capabilities of currently available designed treatment systems, which typically include preliminary and primary treatment, secondary biological treatment, disinfection, and other similar procedures. Due to the persistence of these compounds in the treated effluent, wastewater treatment facilities (WWTPs) continue to release micro-pollutants into the environment. 15
Due to nanoparticles’ many advantageous characteristics, such as their high specific surface area, enormous pore volume, and high porosity, the adsorptive process may reap significant benefits. 16 Among the many desirable qualities of nano-adsorbents are their high reactivity, catalytic potential, abundance of active sites, and potential for surface functionalization, which allows for exceptional selectivity. 17 So far, the majority of the adsorption research has been on metal-based nano-adsorbents such as metallic organic frameworks, polymeric nano-adsorbents, and carbon nanotubes (CNTs). 18
The many functionalization choices, mechanical and chemical stability, and geometrical properties of carbon nanotubes (CNTs) make them a potential material for the removal of organic components. 19 The amount of graphene sheets within a carbon nanotube (CNT) determines its primary kind; single-wall, double-wall, and multi-wall CNTs are the three most common varieties. 20 Such nanostructures may have sizes ranging from micrometres to nanometers. An abundance of literature on the topic of carbon nanotubes (CNTs) as PHC adsorbents attests to their efficacy in the removal of a variety of medications, including carbamazepine, sodium diclofenac, tetracycline, ciprofloxacin, ofloxacin, norfloxacin, amoxicillin, sulfamethazine, and pefloxacin. 21
Van der Waals interactions allow for a strong adsorbent-drug interaction, which is directly proportional to CNT efficiency. 22 However, the same feature may concurrently make the nano-adsorbent useless. It is common practice to functionalize CNTs to enhance certain physical-chemical characteristics of materials. 23 Surface functionalization of nanomaterials is a typical technique that involves oxidation procedures with agents containing the target chemical group. 24 , 25 When looking at alternate green functionalization routes, the approach becomes more enticing due to its environmentally friendly appeal and the potential drop in manufacturing costs. Synthesis via these pathways generally makes use of agro-industrial byproducts. 26
Two recent studies 27 , 28 have addressed the removal of pharmaceuticals from carbon materials, but previous research did not focus on CNTs specifically but rather provided an overview of diverse materials. While Nasrollahzadeh et al. 29 and Raza et al. 30 reviewed sustainable carbonaceous materials for water treatment, they neglected to focus on CNTs or the adsorption process and photocatalytic process, so it is evident that a study assessing the advancements in scientific research regarding conventional and green CNTs as adsorbents is required. The authors state that most literature studies on drug removal methods using green-functionalized carbon nanotubes are lacking. Since different types of functionalization and synthesis directly affect CNT performance in the adsorption process of PHCs, synthesizing this research is important to fill this knowledge gap.
The goal of this study is to examine and argue the pros and cons of different types of CNT functionalization for PHC removal by comparing papers that detail the adsorption and photocatalytic treatment of these materials. 31 , 32 An exhaustive literature search of recently published works in this area was the first step. This work summarized the many forms of CNT functionalization that are now available and critically evaluated the effectiveness of various CNTs for adsorption of medicinal compounds; including CNTs produced/functionalized by both adsorption and photocatalytic procedures. Finally, we considered the unanswered questions and potential future research topics in this area. The authors hope that by publishing this review, they will help bring more attention to the removal of PHCs in general and to the area of carbon nanotube adsorption in particular, which is an emerging field.
2 Structural features
The dimensionality of carbon nanomaterials affects several aspects such as durability, linking type, electrical conductivity, surface area, and other structural characterizations. Graphene-based nanomaterials, nanodiamonds, g-C3N4 and carbon nanotubes are often used as experimental models to assess the efficacy of wastewater treatment. 33
2.1 Zero-dimensional carbon nanomaterial
A nitrogen dioxide molecule has a tetrahedral arrangement of carbon atoms that are sp 3-hybridized. The three distinct layers that comprise a nanodiamond are as follows: a surface layer that contains different functional groups; a carbon encasement that is not homogenous nor uniform and has a thickness ranging from 0.1 to 1.0 nm; and finally, a carbon diamond core that comprises about 70–90 % of the NDs. 34 Presently, diffraction and high-resolution transmission electron microscopy methods are being used to observe the relevant structural features, such as sp 3 and sp 2 nano-twinned stacking domains, concentric carbon cages, and carbon nanoscale patterns, 35 Rapidly emerging nanodiamond have become a prominent nanomaterial in wastewater treatment methods due to their remarkable properties, including high thermal conductivity, surface modification capabilities, resistance to environmental degradation, and strong mechanical characteristics. 36 The ability to produce nanodevices at a low cost gives them a significant advantage over other sophisticated nanomaterials. 35
Quantitative correlation suggests that the nucleophilic carbonyl groups on the surface of NDs act as catalysts for the generation of oxygen. Thus, the unique sp 3/sp 2 nandiamond might be a great solution for treating wastewater. 37
2.2 Carbon nanomaterial with one dimension
The cylindrical, tube-like forms of carbon hybrids called carbon nanotubes are easily identifiable. The amount of graphene layers a carbon nanotube has determines its classification. The several types of carbon nanotubes with different numbers of layers of graphene: double-walled, numerous-walled, single-walled, and triple-walled (Figure 1). 38 According to researchers carbon nanotubes have a variety of electrical, electronic, and mechanical properties because of their physically modifiable sp 2 carbon structure. 39 , 40 , 41

Various types of carbon nanotubes. 42
2.3 Two-dimensional CNTs
Graphene’s carbon atoms are neatly organized in a sp 2 hybridized-hexagonal honeycomb crystal structure. Because of its unusual configuration, graphene has exceptional mechanical stability, excellent electron transport, and enhanced thermal conductivity. 43 Among the many graphene derivatives put to use in green technologies, graphene oxide and reduced graphene oxide stand out as the most common and well known. Gelatin nanoparticles have the power to adsorb numerous kinds of contaminants, including both organic and inorganic chemicals, via means of hydrogen bonding, hydrophobic interactions, and electrostatic attraction. Graphene-based adsorbents used for the removal of organic contaminants represent the first use of confined fluids in the nanoconfinement hypothesis. Recent studies shown that, after the synthesis of a nanographene, triangulene dimer displayed intrinsic magnetic characteristics. 44 , 45
3 Carbon nanotube synthesis methods
In recent years, carbon nanotubes have been created using a variety of techniques, including chemical vapour deposition, laser ablation, electric arc discharge. It is possible to alter the form and content of CNTs by adjusting the process parameters of production processes. Due to its simplicity, adaptability, cost-effectiveness, and ability to produce exceptionally pure carbon nanotubes with adjustable characteristics, chemical vapour deposition is a widely used and highly favoured technique. 46 , 47
Nanotube production first made use of arc discharge. The arc discharge method, which involves passing a direct current between two electrodes, is used to create an arc. Prior to being filled with inert gas, the electrodes are inserted into a vacuum chamber. The chamber’s plasma helps the deposition process forward by continually providing gas, allowing the electrodes to accumulate carbon quickly. Both the arc current and the pressure of the inert gas must be carefully regulated for this technique to work. Turning on the power maintains a steady pressure for the source filling the reaction chamber with inert gas. Then the positive and negative electrodes approach each other, eventually collide, and an arc is formed. When electrodes arc, it produces plasma, which is very hot. A further process involves the accumulation of negatively charged carbon nanotubes on the cathodes. An approach to fabricating SWCNTs and MWCNTs. 48
Carbon nanotubes may also be manufactured using laser ablation, an alternate method. When electric arc technology has its limits, the laser ablation approach is used to overcome them. This method may be used to synthesize both single-walled and multi-walled carbon nanotubes. Using this method consistently increases the amount of nanotubes produced. The carbon target is vaporized using a pulsed laser during the production process. Pulsed laser ablation on a graphite target in the presence of inert gas and catalyst. The end products are CNTs or carbon nanotubes. Laser ablation synthesis is sensitive to several environmental factors, including laser power, temperature, inert gas selection, pressure, catalyst, fluid dynamics, and wavelength around the carbon target. 49
A very effective process for creating carbon nanotubes is chemical vapor deposition. The creation of a disorganized and misaligned graphene sheath is the cause of the dispersion. A low degree of crystallinity in carbon nanotubes is produced using the CVD process. Alternatively, chemical vapor deposition using plasma generated by an inductively-coupled magnetic field is used. In this technique, the plasma from the ICP apparatus is present during the creation of the nanotubes. Extremely stable below 500 degrees Celsius, this approach manages to generate carbon nanotube crystallization. Low temperatures are necessary for plasma-assisted chemical vapor deposition, for the manufacture of carbon nanotubes. This synthesis, which is carried out at low temperatures, has significant benefits for the microstructures. Additionally, the number of nanotubes polluted is lowered when using plasma-enhanced chemical vapor deposition for nanotube manufacture as opposed to chemical vapor deposition synthesis. 50 Significant progress has been made in the field of nanotechnology with the creation of carbon nanotubes and carbon nanofibers. As a consequence of their adaptability in manufacture, nanotubes have found several applications. According to researchers, there are five main ways to synthesize carbon nanotubes. 51 , 52 , 53
3.1 Laser ablation
Nanotubes may also be synthesized via a process called laser ablation. Nanotubes are synthesized by laser treatment, as the name suggests. Laser ablation technique is the most sophisticated form to eliminate the faults of the electric arc approach. 54 Both single-walled and multi-walled carbon nanotube manufacture may benefit from this. This method is often used for the mass manufacturing of nanotubes. In order to ablate the carbon target during the synthesis process, a pulsed laser is required. 55 In this setup, a catalyst and an inert gas are used to perform pulsed laser ablation on a graphite target. As a byproduct, carbon nanotubes are created (Figure 2).

Laser ablation method.
3.2 Arc discharge method
The technique of arc discharge is the most ancient and commonly used approach for the synthesis of carbon nanotubes. It also produces fullerene molecules, which are carbon soot. The creation of plasma from the electrical breakdown of a gas is the basis for this method. To create carbon nanotubes with minimum structural flaws, this approach utilizes high temperatures (over 1700 °C) to evaporate carbon atoms in plasma. One electrode is the anode and the other is the cathode, and they are both located inside the chamber. Graphite powder and a catalyst are mixed together in the anode. Single-walled carbon nanotubes are favored by the catalyst over multi-walled carbon nanotubes in terms of growth. 56 A pure rod of graphite is used to make the cathode. Initially, a gaseous atmosphere (often argon and hydrogen) is used to keep the electrodes at a safe distance from one another. Next, a potential drop of 25 V is applied, resulting in a 60–100 A or 50–150 A electric arc between the electrodes. 46 , 57 The temperature in the gap between the electrodes is sustained above 1,700–4,000 °C, and the flow of inert gas is maintained at a pressure of 50–600 Torr. By increasing the temperature of the electrodes, a plasma is generated. As a result of the temperature differential, carbon evaporates off the positive anode and solidifies as filamentous carbon on the cathode. The time of the answer might be anything from 30 s to 10 min. The machine is allowed to cool during the last stage of the reaction. Then the carbon nanotubes and soot that have formed on the chamber walls are collected, filtered, and their structure is analyzed by means of an electron microscope. Flow rate, inert gas pressure, and metal concentration are the most important process factors for producing a high yield of carbon nanotubes. Small tubes, 0.6–1.4 nm for single-walled nanotubes and 10 nm for multi-walled nanotubes, are often manufactured. 46 High temperatures are used throughout the production process, low pressure is applied, and expensive noble gases are required. 58 Researchers used the arc discharge method to create carbon nanotubes. They used a NaCl solution containing Fe and Ni catalyst particles to react with graphite electrodes. As a consequence, they generated CNTs with lengths ranging from 100–300 nm and widths between 25 and 30 nm. 59 Researchers used a low-frequency bipolar pulsed arc discharge approach to synthesize single-walled carbon nanotubes. They used an innovative bipolar pulsed current circuit that reliably delivered a steady current and pulse. Researchers found that although the quantity of soot created rose with increasing frequency, the quality of the resultant SWNTs did not vary significantly (Figure 3a). 60

Overview of experimental setup and treatment effects on material dispersion and hydration in advanced composites. (a) Diagram of the experimental arrangement for the bipolar pulsed arc discharge reactor 60 (b) influence of ozone treatment on the dispersion of carbon nanotubes and the hydration process in UHPC/CNT composites. 62
3.3 Vapor-assisted ozone treatment
It has been shown that a vapor-assisted ozone treatment may be used to add functionality to carbon nanotubes. This method may introduce several oxygen groups, the most common of which is the carboxyl group, at temperatures very similar to those of room temperature. 61 In an ozone treatment method, solvent vapors including H2O2, C2H5OH, and H2O were used to alter the carbon nanotubes. By lowering the functionalization temperature to roughly 40 °C and progressively raising the partial vapor pressure, the amount of oxygenated groups on the CNTs was greatly enhanced. The ozone treatment, done under the vapor, requires the use of compressed air as the carrier gas. This strategy for functionalization is kind to the environment. 61 In addition, the suspension of carbon nanotubes was formed by utilizing an ozone treatment to build composites of ultra-high performance concrete and carbon nanotubes (Figure 3b). 62 Carbon nanotubes may be more easily dispersed in water after being treated with ozone, which forms oxygen-containing carboxylic groups on the CNTs’ surfaces. Therefore, it is possible that early-stage nucleation may be significantly influenced by improving the interfacial contact between carbon nanotubes and ultra-high-performance concrete. Ozone treatment facilitated the dispersion of carbon nanotubes by creating many nucleation sites and elevating steric repulsion. This resulted in better hydration in the beginning and higher compressive strength in the end. 62
3.4 Microwave
Carbon nanotubes may now be synthesized and used in a wide variety of nano applications with the use of microwave radiation, a novel and inexpensive method. It has been proposed that a common microwave oven may be used as a plasma reactor to produce carbon nanotubes in a manner that is simple, fast, energy-efficient, and solvent-free. Because of the microwave’s novel high-density carbon brushes, heating method may be produced in a matter of seconds, without the need of a furnace or dangerous gaseous carbon. By carefully choosing the substrate and catalyst, carbon nanotubes may be synthesized at room temperature from organic polymers with a low melting point. 63
3.5 Ultrasonic irradiation
Direct modification of single-walled carbon nanotubes using polymers grafted with poly(ethylene glycol) in aqueous solution has been documented in a research. The Diels–Alder click reaction responsible for this change proceeds at room temperature with the aid of ultrasonic irradiation (Figure 4). 64

Synthesis mechanism of PEG-PSMF copolymers and PEG-PSMF/SWCNTs.
The premade CNT-based nano systems showed improved drug loading capacity and efficiently delivered doxorubicin to targeted tumor cells. Under acidic circumstances (pH = 5.5), as opposed to physiological conditions (pH = 7.4), these nanosystems delivered the drug at an optimal pace. Normal cell lines showed no signs of toxicity when exposed to the functionalized single-walled carbon nanotubes delivered by this approach. The drug showed significant anti-tumor effects on HeLa cancer cells, however, and this opens up fascinating possibilities for targeted chemotherapy in cancer/tumor treatment. 64 Ultrasonic irradiation has been used to chemically treat carboxylated–COOH– multiwalled carbon nanotubes with thiamine to create MWCNTs-poly(vinyl chloride)/thiamine nanocomposites with improved mechanical qualities and remarkable heat stability. 65 Polymers may be grafted onto multiwalled nanotubes via the Diels–Alder process in water with the help of heating, stirring, and ultrasound. Consequently, a large increase in reaction rate was attained, reaching roughly 12 times greater than the rate obtained with traditional heating and stirring. 66 The effectiveness of the grafting procedure was not sacrificed to accomplish this improvement. As a consequence, this methodology offers an easy and ecologically benign way for direct functionalization that may be utilized on a wider scale. Sonicating CH3I, CH2Cl2, and CHCl3 in the presence of silicon nanowires under ambient circumstances led to the formation of MWCNTs. It was found that ultrasonic irradiation facilitated the dissociation of solvent molecules and their subsequent contact with the silicon nanowires’ hydrogen-terminated surfaces. 67 Furthermore, ultra-pure SWCNTs were synthesized via a sonochemical method. The solution mixture was subjected to 20 min of pulsed high-intensity ultrasound. Silica particles acted as nucleation sites, while ultrasonic energy helped break down ferrocene to produce Fe nanoparticles, which stimulated the development of nanotubes. 68 In spite of promising early findings, carbon nanotubes made with the use of ultrasound cannot compete with carbon nanotubes made using more traditional techniques. Therefore, ultrasound-assisted CNT manufacturing is unlikely to find widespread use in industry without significant improvements and optimization. Surface modifications and dispersion/solubilization of carbon nanotubes using ultrasound-assisted methods have nonetheless seen widespread use. 69
3.6 Plasma-enhanced chemical vapor deposition
By producing a glow discharge within the chamber, nanotubes may be produced using plasma enhanced chemical vapor deposition. 70 The temperature range for thermal chemical vapor deposition is 700–1,000 °C. 71 Despite its many benefits and adaptability, this strategy has two major flaws. To begin, it’s possible that the tubes aren’t oriented properly or aren’t perfectly straight. Furthermore, the substrate material degrades due to the increased temperature.
The synthesis of aligned nanotube at low temperatures utilizing PECVD techniques is the current subject of several studies. 72 Carbon nanotubes may be manufactured in an ordered array by plasma-enhanced chemical vapor deposition. According to a 2,000 study, plasma processing is the most promising and beneficial approach for manufacturing nanotubes at low temperatures. The PECVD method is attractive because it has the potential to yield nanotubes and nanofibers that may be used as electron emission sources. Displays, parallel electron beam lithography, microwave amplifiers are just a few applications for these electron emitters. 73 The PECVD method relies heavily on plasma. It is vital to grasp the notion of plasma and the technique of creating plasma via glow discharge inductively coupled plasma.
According to many studies, plasma is the fourth state of matter. 74 , 75 According to studies, 76 , 77 Langmuir initially used the term “plasma” in 1928. Electric gas discharge is the most frequent technique for producing plasma in the laboratory. 50 The discharge occurs inside an electric field produced by a combination of techniques. In the simplest case, two electrodes are housed within a glass tube. A power source is connected to the electrodes. Glow discharge plasma occurs when gases are present within a vessel at a specific low pressure. Non-equilibrium discharges are characterized by a large temperature difference between electrons, ions, and neutrals in the plasma. Inductively Coupled Plasma is utilized to create the plasma for our experiments. The electric field necessary for the discharge is produced by winding a coil around the mostly borosilicate glass vessel wall. Because of this, placing electrodes within the chamber is unnecessary. When an electromagnetic field is introduced to the coil, an electric field is created in the reaction chamber, which maintains ionization and leads to gas breakdown. The chemical energy required by the enclosed beings comes from the dispersed gaseous state. If the process conditions are optimized, most of the substance will be deposited on the walls. These days, IC-PECVD is often used in conjunction with other techniques to create carbon nanotubes. Aligned carbon nanotubes are the primary focus of this study, which focuses on their production using the IC-PECVD method.
The underlying idea is based on the method of ionizing a mixture of gaseous vapor using a plasma reactor in order to synthesis carbon nanotubes that are aligned in a specific way. 78 A copper wire of 8 mm in diameter was wound into five turns to form the inductor. An inductor and radio frequency generator operating at 13.56 MHz were coupled via a matching box to minimize power loss due to reflections off the load. For this purpose, we use a plasma chamber that is 85 mm tall and 44 mm wide.
4 Properties of carbon nanotubes
Carbon nanotubes are far stronger than steel or Kevlar because of their superior sp 2 bond, which is even stronger than diamond’s sp 3 link. Carbon nanotubes are ideal for improving the functionality of several polymers and ceramics used in the manufacturing of everyday items due to their high strength and low flexibility. Following is a summary of the most salient features of carbon nanotubes.
4.1 Chemical characteristics
Graphene makes up the structure of carbon nanotubes, and chemical amplification happens all through the manufacturing process. CNT’s reaction is proportional to the pi-orbital discrepancy brought on by the increased curvature. 79 Carbon nanotubes’ sidewalls and end caps diverged to this degree. As the diameter becomes smaller, the reactivity gets higher. The reaction is done on both the sidewall and end cap of the carbon nanotube. Carbon nanotubes are a good example since their solubility and reactivity in different solvents may be controlled. Nanotubes are impure, making research into their chemical modification difficult.
4.2 Mechanical properties
Carbon nanotubes are now widely acknowledged as nature’s most durable materials. This chemical component is the most stable and adaptable since it has a C–C covalent bond and a perfect hexagonal network structure. This property aids in the calculation of Young’s modulus. When two carbon atoms come together, they create a covalent sp 2 bond that is very stable. The predicted range for the young modulus of SWNT is between 1 Tpa and 1.8 Tpa. The fabrication of scanning microscopy probe tips, for example, makes use of moduli with such huge values. Multi-walled carbon nanotubes have qualities that are affected by the number of sidewalls, whereas single-walled nanotubes have attributes that are governed by their diameter and chirality.
Experiments that can endure stress in the tubes are likely dispersed within an epoxy matrix with a value of 1.3, as suggested by the existence of an exterior graphite shell. The cohesiveness of SWNT inner tubes is much lower than that of individual linkages. 80 Carbon nanotubes have remarkable promise in aerospace because of their excellent mix of mechanical qualities and lightweight nature. Carbon nanotubes are employed in the notion of the “space elevator,” a cable imagined by Arthur C. Clarke that links Earth to space. CNTs take on the structure of the metallic or semiconducting substance from which they are made. When compared to silicon, copper’s conductivity is far higher. Multiple gadgets, such as scanning-probe microscopes, sensors, flat-panel displays, make use of carbon nanotubes. 81 Because of its unique properties, carbon nanotubes may be used in a variety of contexts. 82
4.3 Optical properties
Counterpropagating nonlinear transmission is a fundamental component in the science of optics. This is tied to their linear. 83 According to theoretical studies, the optical impact of the chiral nanotube decreases with increasing nanotube size. Only about a third of nanotubes, those that act like metals, can be explained by the tight-binding paradigm. Semiconducting nanotubes make up the remaining two-thirds, with varying characteristics depending on their indices (n, m). 84 From 1 nm to 2 nm, the band gap decreases as the diameter of the cylinder increases. This causes an optical absorption at 1,550 nm, which is in line with the visible spectrum. The utilization of optical properties is common in fiber optic technology. There is a possibility that optical devices will be developed as a consequence of optical work. 85
4.4 Thermal properties
Nanotubes exhibit a phenomenon known as “ballistic conduction” and have exceptionally high heat conductivity. Due to the strength of the in-plane C–C bonds in graphene, superconductivity in CNT occurs at temperatures below around −253 °C (−423.4 °F). Carbon-carbon bonding provides the material resilience and axial stress resistance. The end product is a remarkable adaptability to off-axis forces. It has a greater thermal conductivity than copper at normal temperature, up to 6000 W/m/K. 86 At low temperatures, when the 1D quantum of the phonon band structure is also measured, it has been shown that single-walled nanotubes, 3D graphite, and 2D graphene all have different specific heats. 87 The thermal conductivity of nanotubes is exceptionally high, even in large-scale samples. When it comes to conducting heat, pyrolytic graphite really shines. In contrast to minerals like mica or large-sized gem graphite, which do not display the same degree of thermal conductivity as strongly oriented pyrolytic graphite, diamond has the highest documented thermal conductivity among materials. Metal’s thermal conductivity is exceptional, with 385 W m1 K1. Carbon nanotubes have a high heat tolerance, functioning at temperatures up to 2,800 °C in a vacuum and roughly 750 °C in air. Unlike regular graphite fibers, carbon nanotubes have a rather uniform thermal property in all directions. However, it is also highly anisotropic, meaning its thermal characteristics vary depending on the direction one looks at it. 88 Nanotubes’ thermal conductivity varies with both temperature and environmental factors.
4.5 Electronic characteristics of CNT
Symmetry plays a vital role in defining the electrical band structure via a number of different symmetrical features.
That n and m are equivalent may be shown from the equation n = m. The statement that n is a metal is also included. If n is an even number and m is an odd number, then the difference between the two is 3j, where j is an integer greater than zero. The tube’s diameter, together with the values of n and m, establish the bandgap. 89 The elasticity of carbon nanotubes is measured in terms of Young’s modulus. The modulus of carbon nanotubes is around 1800 GPa, whereas that of steel is approximately 210 GPa. CNT’s tensile strength is 20 times that of steel, making it a very powerful material. The maximum stress before a material begins to deform permanently may be measured by a metric called the yield stress. Carbon nanotubes are stronger in tension than steel and can withstand more force before breaking. This material has a higher electrical current flow density than metals like copper, with 4,109 A/cm2. 90 Dispersion and flaws in the inner walls of carbon nanotubes brought on by lattice vibrations affect the CNTs’ electrical characteristics. Similar to how bulk materials behave, this causes changes in their resistance, capacitance, and inductance. Anisotropic composite materials are desirable for potential uses. 91
5 Methods for modifying carbon nanotubes for use in wastewater purification
Carbon nanotubes possess distinctive features in terms of their form, physicochemical composition, and adsorption properties. There are some difficulties associated with pure carbon nanotubes, including the tendency for carbon nanotubes to bundle or agglomerate. These phenomena have negative impacts on both the efficiency of carbon nanotubes and the manufacturing process of membranes. 92 , 93 , 94 The primary factors contributing to the inadequate dispersion of carbon nanotube in various solvents are the presence of van der Waals forces and strong intermolecular interactions, resulting in the formation of CNT clusters or tightly bound bundles. 95 According to study, the process of functionalization enhances the chemical reactivity and adsorption capabilities of carbon nanotubes by augmenting their dispersion in an aqueous medium. According to the findings, several physical and chemical techniques may be used to achieve functionalization of carbon nanotubes. 96 , 97
In order to facilitate the binding of bioactive compounds to carbon nanotubes, modifications may be noticed at the terminations or lateral surfaces of carbon nanotubes. The use of carbon nanotubes in this procedure is intended to improve the pollutant’s dispersion or elimination. This is done in an effort to boost the performance of carbon nanotubes in a number of water treatment applications and to make membrane fabrication easier. Covalent functionalization of carbon nanotubes with phenol groups by the 1, 3-dipolar cycloaddition technique dramatically improved undernanotubes dispersion in polar liquids. This enhancement in dispersion permitted the successful integration of CNTs into the polymer matrix. This research looked at how well functionalized multi-walled carbon nanotubes may selectively adsorb Zn2+ and Pb2+ ions from wastewater. Researchers report that the increased surface area and ligand interaction sites of multi-walled carbon nanotubes may be responsible for their improved adsorption capability. 96
Hydrochloric acid, hydrogen peroxide, potassium permanganate, nitric acid, sodium hypochlorite, and sulfuric acid, are only some of the oxidizing chemicals that have been utilized in the oxidative treatment of carbon nanotubes to create an oxidative characteristic on pure carbon nanotubes. When it comes to removing harmful pollutants from water, oxidized polymers excel at both dispersing them and soaking them up. Carbon nanotubes may be made more malleable by modifying their surface with metal oxides like Fe2O3, MnO2, and Al2O3. This modification strategy has been found to boost the ability of CNTs to efficiently remove aquatic contaminants. 98
Functionalized carbon nanotubes have their ability to remove contaminants from water tested. 99 For phenol removal in electrocatalytic water treatment, study investigated the use of metal oxide-modified carbon nanotube filters. Modified carbon nanotube filters were almost twice as effective as unmodified CNT filters. 100 Researchers performed a research that modified carbon nanotubes were utilized to recover hydrocarbons from water (Figure 5a). 101 Recent research by Abdullah et al. indicates that functionalized carbon nanotubes are useful for cleaning up oil spills in water. Study described the use of functionalized carbon nanotubes in desalination. 102 Researchers conducted research in which they desalinated water using Rim-modified carbon nanotubes. Researchers report that Zwitterion customized/polyamide nanocomposite membranes have recently been utilized for water desalination using a membrane format. 103 In order to purify wastewater from the petroleum industry of phenol and benzene pollutants, a composite polymer membrane containing carbon nanotubes was synthesized and evaluated in this research. Based on the findings obtained, the integration of f-CNT into the composite membrane acted as a strengthening agent, leading in the production of a membrane material that demonstrated good separation qualities. 104 Hydroxamic acid analogs were produced with MWCNTs in a separate investigation (Figure 5b). The Pb(II) particles were successfully chelated thanks to the amazing coordination sites present in the analogues, such as hydroxyl, carboxyl groups, and amine. 105
6 Functionalization
Due to their chemical characteristics, CNTs are notoriously difficult to disperse and dissolve in aqueous solution, water, and the vast majority of organic solvents and aqueous solvents. Significant research into enhancing the chemical properties of carbon nanotubes has been performed to address these concerns. 101 Covalent and noncovalent interactions, such as van der Waals forces, may alter the surface of CNTs. The technique used is determined by the means by which the CNTs are linked to the active molecules. 101
6.1 Covalent functionalization
Researchers used multiwalled carbon nanotubes with functional groups added to create a Fe/Mn bimetallic heterogenized catalyst by covalently connecting Mn and Fe porphyrins to the nanotubes’ surfaces. In terms of catalytic efficiency the bimetallic nanohybrid dominates its monometallic analogue. 106 A sp 2 carbon framework is used to link functional groups including carboxyl, amino, and hydroxyl. Covalent functionalization typically involves linking these entities to the surface of carbon nanotubes. 107 This improves their solubility and dispersion in a wide variety of solvents such as dimethylformamide chloroform, 1,3-dimethyl-2-imidazoleidinone, water, dimethyl sulfoxide, and ethyl alcohol. 108 By establishing covalent connections between zinc porphyrin and single-walled carbon nanotubes researchers have successfully transformed carbon nanotubes with porphyrin dendrons using click chemistry. 109 To the contrary, researchers looked into cycloaddition, radical and oxidation processes to learn more about covalent surface modification of carbon nanotubes. The goal was to evaluate how well these strategies worked for chemically altering the CNTs’ surface. 110 Phase engineering by covalent functionalization was used to produce monolayered transition metal dichalcogenides. 111 Researchers also applied this procedure and underlined that modified carbon nanotubes displayed changed dispersibility and enhanced hydrophilicity following functionalization. Because of this modification, the suspension is much more stable than that of pure CNTs, making them ideal for use in electrochemical sensing (Figure 6a). 112 Thus, researchers adopted them for usage in hydrogen storage. Creating surface defects that allow a higher surface area for hydrogen adsorption is a key function played by chemical modifications in enhancing the density of hydrogen storage (Figure 6b). 113
6.2 Non-covalent functionalization
Instead of using covalent functionalization, carbon nanotubes rely on non-covalent functionalizations to coat molecules of surfactant or polymers. The chemical structure of the – network of CNTs is not altered, save for the length restriction brought on by the Sonication utilized in the functionalization technique, 114 and the physical characteristics of nanotubes are well retained by the non-covalent approach. It is mostly composed of biomacromolecules, polymer, surfactants coatings. Hydrophobic parts of the associated micelles and polymer should be encapsulated inside the carbon nanotubes to form supramolecular complexes. 115 Because of the non-covalent dispersion, soluble nanotubes maintain its aromatic structure and electrical properties. Polyaniline, polythiophene, polypyrrole, and their derivatives are advantageous materials for surface modification of CNTs because of the existence of diverse oxidation structures. 116
6.2.1 Surfactants
Cationic, nonionic, and anionic, surfactants have all been used in an attempt to disperse nanotubes. CNT suspensions with concentrations of 0.1 and 0.5 mg/mL were made using the surfactants sodium dodecyl sulfate and Triton X-100, respectively. This moratorium lasted for no more than seven days. Sodium dodecylbenzene sulfonate was utilized as an alternative because it showed increased stability for a month, resulting in a suspension concentration of 10 mg/mL, which allowed for better results. Combining the extended lipid chains of sodium dodecylbenzenesulfonate with the k-k combination of aromatic moieties found in carbon nanotubes increases the complex’s efficacy. When single carbon nanotubes are present, atomic force microscopy and transmission electron microscopy have shown that the surfactant remains linked to the cylinders. A long amphiphilic lipid chain generates a micelle-like structure, with molecules packed into a chamber that is either perpendicular to or angled with respect to the cylinders. Another way to promote the adsorption/dispersion of carbon nanotubes includes synthesizing a succinimidyl ester from 1-pyrenebutanoic acid. Protein building blocks, such those found in streptavidin and ferritin react quickly and strongly with this molecule. For biological uses, the relatively low solubility of carbon nanotubes (between 0.1 and 0.7 Mg/mL) is ideal. It’s possible that surfactants may help dissolve CNT, but they’re also poisonous and have been shown to damage cell membranes. Since surfactants might have unintended consequences for living organisms, their usage in biomedical settings is limited. 117
6.2.2 Polymers
Medication delivery systems often use polymers as molecular carriers. Solubilization does not result in enhanced scattering capabilities from CNTs. They are not as effective as surfactants, but they do provide an alternative. The dispersion causes the polymer to wrap around the cylinders. The scattering effectiveness is due to the hydrophilic component of nonionic polymers, notably copolymers of poly(oxyethylene). The positively charged tetra alkyl ammonium groups on the surface of the nanotubes were exposed while the hydrophobic polymer backbone enveloped them, making them soluble in water. 117 The effect of these fluorescent polymers on mammalian cells has also been studied. Poly (vinyl pyrrolidone) was chemically bonded to a variety of fluorescent pigments. Carbon nanotubes and fluorescent polymers were joined in a solution containing 1 % SDS to form supramolecular structures. The potential for these structures to serve as cutting-edge molecular sensors is encouraging. 118
6.2.3 Biopolymers
K-k interactions, like those often seen in double-stranded DNA, the self-assembly mechanism may be associated with the widespread distribution of nanotubes Nucleic acids are great building blocks for supramolecular complexes due to the K-stacking interactions between aromatic bases and the surface of carbon nanotubes. In the milligram per milliliter level, DNA-nanotubes These complexes are allowing ion-exchange chromatography, and very stable to be used for purification. The unique class of biopolymers known as amphiphilic peptides has been shown to efficiently disseminate CNTs. Amino acids including tryptophan, phenylalanine, tyrosine, and histidine have a significant impact on a peptide’s ability to dissolve in water. Very specific peptides are arranged in such a way that they overlap onto the nanotubes, which is an attractive method for ensuring solubility and may serve as a useful tool for evaluating separation, leading to enhanced performance and application. More recently, cyclic peptides have been demonstrated to have similar properties. 119 , 120
6.3 Medicinal chemistry of CNTs
Size, aggregation, surface functionalization, chemical composition, shape, and solubility are only few of the physicochemical features of carbon nanotubes that affect nanoparticle biodistribution and pharmacokinetics. 121 Intraperitoneal injection of iodine-labeled, oxidatively functionalized, single-walled carbon nanotubes was employed. 122 The intraperitoneal technique was compared to others, including the subcutaneous and oral (by stomach incubation) approaches. This technique allowed for the distribution of carbon nanotubes throughout the body, rather than only in specific locations. Favored tissues and organs include the kidney, stomach, and bone. 123 During the safety examination, it was determined that 94 % of the nanotubes were eliminated in the urine, while the remaining 6 % were excreted in the stool. Our research has shown that the tissues are resilient and unaffected by the experimental perturbations. Additionally, the structure was functionalized with single-walled carbon nanotubes and multi-walled carbon nanotubes utilizing a 1, 3-dipolar cycloaddition procedure, another surface chemistry approach. After that, this technique was compared to only using single-walled nanotubes. Diethylenetriaminepena acetate and radiolabeled indium are used to functionalize carbon nanotubes. In this study, we use DTPA to measure the effect of biodistribution and blood flow on the surface area of degree. Both forms of functionalized DTPA-SWNT are found in the kidneys, muscles, skin, bones, and blood, as shown by the biodistribution study. There was a considerable improvement in tissue cleanliness and blood flow after using any kind of nanotube. 124 Functionalization using diethylenetriaminepentaacetic acid was performed on both single- and multi-walled carbon nanotubes. It was shown that following intravenous administration, DTPA was removed in the urine via the renal system. The DTPA-CNT was observed without any defects in voided urine using TEM.
7 CNT-based materials for adsorption process pharmaceuticals
The extensive misuse of antibiotics has resulted in significant environmental issues. Several biopolymer gels, which are often used as adsorbents, have subpar adsorption capabilities and insufficient mechanical characteristics. 125 Jie Ma et al. explored the synergistic effects of incorporating carbon nanotubes (CNTs) and graphene oxide (GO) into sodium alginate (SA) to enhance the adsorption efficiency and overall characteristics of conventional adsorbents. Carbon nanotubes/l-cysteine@graphene oxide/sodium alginate (CNTs/l-cys@GO/SA) triple-network composite hydrogels were synthesized using hydrogen peroxide and l-cysteine (l-cys) (Figure 7a). These triple-network composite hydrogels have the ability to enhance their adsorption capacity by using their three-dimensional structure, in contrast to both standard hydrogels and the double-network hydrogels now under development. The presence of an independent triple-network structure enhances the spatial dimensions, resulting in a greater number of pores and sites for adsorption of pollutants, hence enabling the effective removal of ciprofloxacin. The CNTs/l-cys@GO/SA hydrogels had an adsorption capacity of 181 and 200 mg/g at 25 and 15 °C respectively in a weakly acidic environment. Indeed, the hydrogels composed of carbon nanotubes (CNTs), l-cysteine (l-cys), and graphene oxide (GO) with sulfonic acid (SA) have superior characteristics at low temperature conditions. The adsorption capacities of CNTs/SA, CNTs/GO/SA, and CNTs/l-cys@GO/SA hydrogels were 107, 156.6, and 160.2 mg g−1 at an initial concentration of 200 mg l−1, respectively, which is close to the primary adsorption capacities of 109, 158.8, and 181 mg g−1, as shown in Figure 7b. The experimental setup used a ciprofloxacin solution with a concentration of 10 mg L−1 to examine the hydrogels’ capacity to remove the antibiotic from wastewater at a low concentration. The adsorption capacity for 12 h of three different hydrogels was compared using a four-fold repetition. The regeneration performance was outstanding on the first three occasions, as demonstrated in Figure 7c. Furthermore, the triple-network hydrogels had shown enhanced thermal stability, mechanical characteristics, and swelling ability. The independent multilayer network preserves the exceptional characteristics of the original materials while expanding the internal volume of hydrogels. The potential of these multinetwork hydrogels for wastewater treatment was significant in terms of pollutant removal. Furthermore, the CNTs/l-cys@GO/SA hydrogels had a greater ability to adsorb ciprofloxacin when exposed to weakly acidic conditions, low temperatures, and low concentrations of inorganic salts. 126
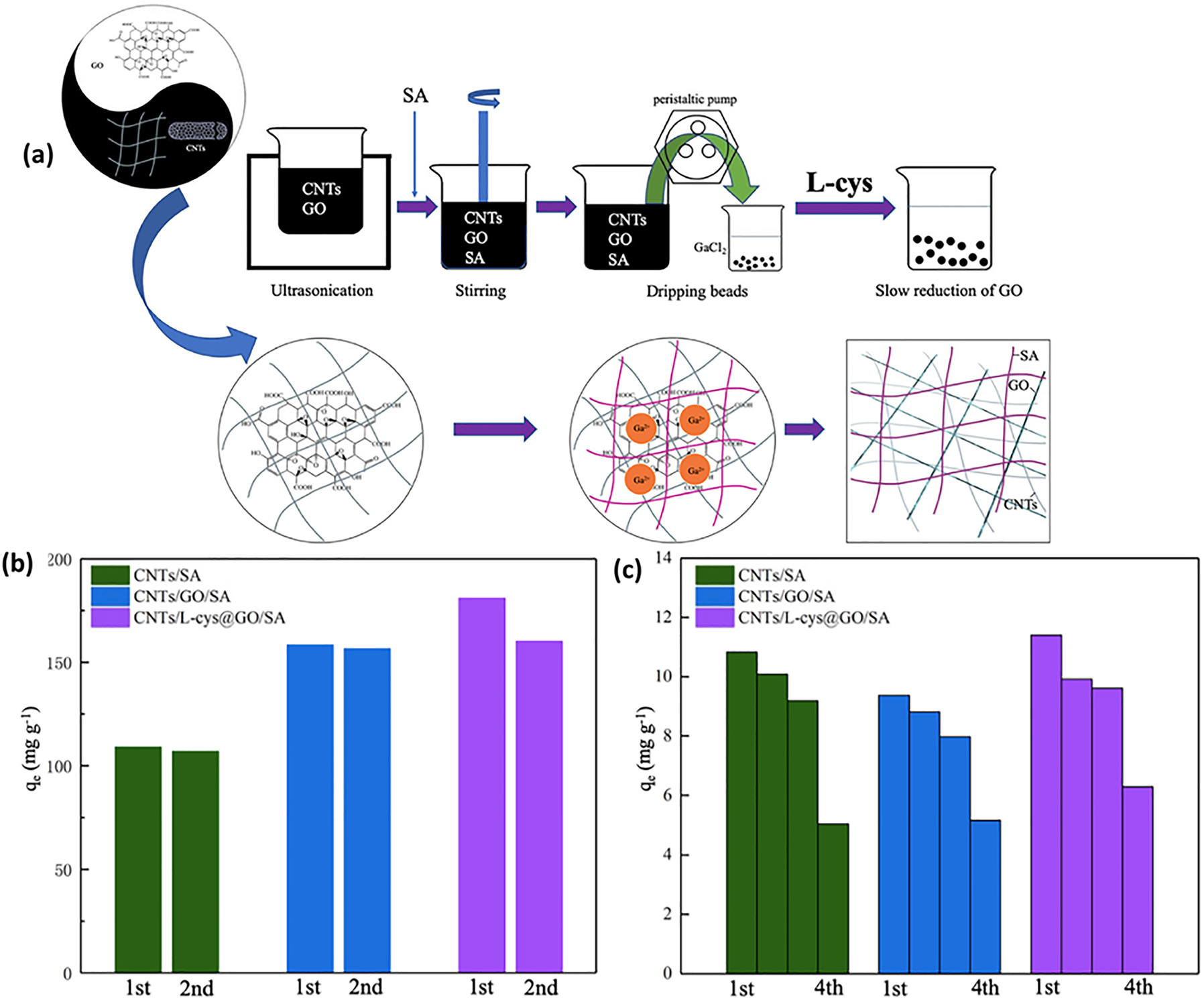
Schematic of hydrogel formation process and regeneration performance of different hydrogels after multiple cycles with varying ciprofloxacin concentrations. (a) Illustrative schematic showing the formation process of the CNTs/l-cys@GO/SA triple-network hydrogel. Regeneration performance of three different hydrogels after multiple cycles using ciprofloxacin solution of (b) 200 mg L−1 and (c) 10 mg L−1. 126
Ildiko Lung et al. manifested the effectiveness of a nanocomposite of carboxy-carbon nanotubes (CNT-COOH), manganese(IV) oxide (MnO2), and iron(II,III) oxide (Fe3O4) in adsorbing cymoxanil. He studied the effect of pH (2–10), temperature (20–60 °C), starting cymoxanil concentration (7–20 mg L−1), adsorbent dosage (0.5–5 g L−1), and contact time (2–30 min) on the adsorption process to find the best conditions. They analyzed the experimental data using the isotherm and kinetic models. There was a maximum adsorption capacity of 10.672 mg g−1, indicating that the Langmuir isotherm model offered the greatest fit. Not only that, but the pseudo-second order kinetic model describes the cymoxanil adsorption process. The result suggested that a nanocomposite of carbon nanotubes, metal oxides, and ferric oxide could be useful in the removal of cymoxanil from water. 127
The adsorption of pharmaceuticals from wastewater by carbon nanotubes (CNT) is an issue of substantial experimental attention. These nanomaterials reveal intriguing designs for interactions with this class of pollutant of growing concern (CECs). Cristiane Ferraz de Azevedo et al. described the interaction between single (SWCNT) and multi-walled carbon nanotubes (MWCNT) and the diclofenac potassium (DP) anti-inflammatory by adsorption and spectroscopic tests. Raman and Infrared spectroscopy techniques were applied to elucidate the interaction between both CNT and DP molecules. Scanning electron microscope was used to see the morphology of prepared materials (Figure 8a and b) additionally, batch adsorption studies were carried out to examine CNT for removing DP from aqueous solutions. The carbon-based nanostructures give rapid kinetics of adsorption of DP. Also, the maximum adsorption capacity (Qmax) for adsorption of the DP medicine at 40 °C, yielding high values such as 77.54 and 150.37 mg g−1 for MWCNT and SWCNT, respectively. The interaction between diclofenac anti-inflammatory and CNT was spontaneous and endothermic, being regulated by physisorption. In addition, utilizing the thermodynamic and spectroscopic measurements, a mechanism based especially on hydrogen-bond and π-π interactions was hypothesized to explain the adsorption of DP onto both CNT. 128

SEM images of (A) SWCNT and (B) MWCNT.
Researchers assessed the efficacy of green-modified carbon nanotubes (CNTs) as an adsorbent material for the elimination of two antibiotics (ciprofloxacin and ofloxacin) in water-based solutions. Analysis of the material pre and post adsorption revealed that the crystalline structure of the CNTs remained intact throughout the process. Consequently, adsorption is confined to the surface through interaction with the C=C, O–H, C–O, and CO–O groups of the adsorbent. This interaction is further intensified by the presence of iron nanoparticles on the nanotubes’ surface. The MWCNT-E + OFL exhibited superior heat resistance in comparison to the other systems under investigation, suggesting the establishment of stable links between the adsorbent and this medication. The rate of reaction was rapid for both pollutants in all tested systems (either single or competitive), achieving equilibrium within a time frame of 20–30 min. All kinetic models examined exhibited a strong fit to the data for both medicines, suggesting that both chemisorption and physisorption had a role in influencing the process. In the single adsorption of CIP, intraparticle diffusion was identified as the limiting phase, whereas for OFL, external diffusion was the limiting step (Figure 9a). However, in the binary removal of pollutants, both intraparticle diffusion and external diffusion were found to be important. The most effective approach for ANN modelling was utilizing a neural network design consisting of [3 5 5 2] neurons. This architecture was trained using the Log-sigmoid transfer function and the Bayesian regularization training technique. The ANN model had superior performance compared to the PSO model in accurately predicting both single and binary data, exhibiting a decreased Mean Relative Deviation (MRD) from the experimental values (Figure 9b). This suggests that the use of ANN is a promising method for modelling competitive adsorption data. Ultimately, the findings demonstrated that green-modified nanotubes exhibit high efficacy in eliminating emerging pollutants from water-based solutions, even when present in small amounts. 129

Overview of adsorption mechanisms and enhanced artificial neural network (ANN) design for improving CIP/OFL adsorption on nanotubes. (a) Potential mechanisms involved in the adsorption process of CIP and OFL and (b) enhanced design for the artificial neural network (ANN) used in the adsorption of CIP/OFL on nanotubes. 129
The adsorbent MCNTs–UiO–66–NH2, which is environmentally safe, demonstrated excellent water stability within a pH range of 1–10. It possesses a magnetic strength of 23.68 emu g−1 and can effectively adsorb Ibu and Nap in water within a pH range of 1–10 or a salinity range of 0–0.2 mol L−1. The adsorption of Ibu (Nap) achieved a state of equilibrium within a period of 2 h. At a solid-liquid ratio of 1 g L−1, the MCNTs-UiO-66-NH2 material demonstrated an adsorption capacity of 139 mg g−1 for Ibu and 136 mg g−1 for Nap at a temperature of 303 K. The regenerative capacity of exhausted MCNTs–UiO–66–NH2 maintained consistently over 90 % even after undergoing five cycles using NaHCO3 as the eluent. MCNTs–UiO–66–NH2 have shown the ability to completely eliminate Ibu and Nap from simulated wastewater (with initial concentrations of 10 mg L−1) within a pH range of 3–9. The isotherm and kinetic studies indicated that both processes involved monolayer chemisorption. Based on XPS analysis and static adsorption investigation, it was inferred that the two adsorption mechanisms may include complexation, π–π conjugation, electrostatic interaction, and H–bonding. To summaries, MCNTs–UiO–66–NH2 can be used to remove Ibu and Nap from real water sources. 130 The detail comparison of various CNT-based materials for photocatalytic degradation is given in Table 1 and 2.
Carbon nanotube-based adsorbents for pharmaceutical with different parameters.
| Adsorbent | C o (μg/L) | Source of water | Surface area (m2 g) | Sorption capacity (mg/g) | Pharmaceutical | Reference |
|---|---|---|---|---|---|---|
| g-C3N4/H-ZSM-5 | 600 | Synthetic water | 172 | 4 | Fipronil | 131] |
| MWNTs, SWNTs, MWNTs-O | 50–2000 | Synthetic water | 283,1020, 287 | 81, 232, 19 | Ibuprofen | 132] |
| SnO2/Ni@NCNT | 50,000 | – | 155–217 m | >70 % | Cephalexin | 133] |
| MWNTs | 100,000 | Synthetic water | 174 | 600–800 | Asulfapyridine | 134] |
| SWNTs | 2,960 | Synthetic landfill Leachate |
407, 233 | 24.9–120 | Hormones 17a-ethinyl estradiol | [135], [136], [137 |
| BNNS | 20,000 | – | 20,000 | 15–36 | Ofloxacin | 138], [139] |
| MWNTs-H | 20,000–100,000 | Synthetic water | 228 | 85 | Tylosin | 140] |
| MWNTs-COOH | 90,000 | Synthetic water | 200–400 | 13.9c | Carbamazepine | 141] |
| SWNTs | 10–20,000 | Synthetic water i.e., DOM | 380 | 130 | Antiepileptics | 142], [143] |
| Acid-treated SWNTs, SWNTs |
228 | Synthetic water | 407 | 41.4, 52.8 | Bisphenol A | 144] |
| MWCNTs | 50–1,200 | – | – | 2.0–10.0 | Sulfapyridine | 145], [146] |
Multiwalled carbon nanotube-based adsorbents for pharmaceutical with different parameters.
| Adsorbent | pH | Pharmaceutical | Maximum adsorption | Removal efficiency (%) | Isotherm model | References |
|---|---|---|---|---|---|---|
| CNTs/l-cys/SA/GO | 4, 6 | Ciprofloxacin | 200 | – | Freundlich–Langmuir | 126] |
| m-MWCNT-CYS m-MWCNT-HYD m-MWCNT-HA |
4 | Ibuprofen | 1.15 7.56 11.8 |
61.5 98.4 93 |
Langmuir | 147] |
| Fe3O4/MWCNT MWCNT |
– | Sulfamethazine | 136.99 196.85 |
75.81 95.30 |
136.99 196.85 |
148] |
| L-MCNT60-100 nm (1:1) | 5 | Ibuprofen Sodium Diclofenac Ketoprofen |
7.9 9 17.9 |
– | Langmuir | 149] |
| MWCNT–COOH | 1–3 | Ibuprofen | 12.88 | – | – | 150] |
| MWCNT | – | Ciprofloxacin hydrochloride | 1.7446 | 88.96 | Freundlich | 151] |
| MWCNT SWCNT N-CNT MWCNT-COOH |
11 | Dimetridazole Metronidazole |
101 84 |
– | Redlich–Peterson | 152] |
| PMCNT FMCNT |
6 | Aspirin | 41 58 |
– | – | 153] |
| O-MWCNT | 3 | Enrofloxacin | – | – | Freundlich | 154] |
| SWCNT MWCNT H-MWCNT H-MWCN |
2 5 2 5 |
Sulfamethazine | 426.3 81.5 105.2 111.6 |
85–90 | Langmuir | 155] |
| PAni/MCNT | 2 | Meloxicam | 221.2 | 93.5 | Dual site Langmuir–Freundlich | 156] |
| MWCNT | 7 3 3 9 3 |
Seven sulfonamides Carbamazepine Diclofenac Three chloramphenicols, Ibuprofen |
– | – | Freundlich | 157] |
| MWCNT | 7 | Triclosan Acetaminophen Ibuprofen |
3.73 3.83 3.09 |
93.3 77.3 93.2 |
– | 158] |
8 CNT-based materials for adsorption process pharmaceuticals
The structural diversity of pharmaceutical chemicals has led to their detection in a wide range of water sources, including groundwater, surface water, wastewater, and drinking water. Hospital effluent, urban agricultural runoff, treated and untreated industrial wastewater, and other sources often release these chemicals into the environment. 159 Patients’ excretion and the improper disposal of unwanted pharmaceuticals make hospitals a significant contributor to pharmaceutical discharge into the environment. 160 Another important source of environmental contamination is the improper disposal of pharmaceutical waste from industrial processes. Many different medications can end up in water, which is bad for everyone’s health. As an example, paracetamol, an analgesic, increases the risk of kidney cancer, liver damage, and asthma, whereas sulfamethoxazole, an antibiotic, induces genetic mutations and chronic effects even at low doses. 160 In general, medications have negative impacts, such as causing environmental systems to develop tolerance to microbes and disrupting the endocrine system. It is difficult to analyse, identify, and remove pharmaceuticals from aquatic systems because they are often present at extremely low quantities. The biggest problem with researching drug incidence and destiny is that there aren’t enough sensitive and robust tools for quantitative analysis, which makes detection very difficult. 161
Researchers covered the structural, morphological, and photocatalytic properties of stretchable composites made up of carbon nanotubes (CNTs), silicon rubber, and Ti@TiO2:W nanoparticles (TiWNi NPs) with an average size of 37 ± 2 nm. Under the microscope, the TiWNi nanoparticles sparkled like jewels on the surface of the precisely aligned carbon nanotube strands. Anatase and rutile phases, organised in a cubic form, made up the composite composition of the TiWNi NPs. We evaluated TiWNi powders and stretchable composites for their photocatalytic degradation of the anti-inflammatory drug diclofenac (DCF) using UV-visible light. In Figure 10a it can see the effects of 0 % and 100 % strain on the stretchable CNT + TiWNi composite during the photocatalytic process. Figure 10b displays the DCF deterioration curves versus time (3 h). After 180 min, or 3 h, the maximum DCF degradation efficiencies got as follows: 2.2 % for the bare silicon elastomer substrate (SSR), 34.6 % for the CNT composite (stretched at 0 %), 91.9 % for the TiWNi powders, CNT + TiWNi (stretched at 0 %), CNT + TiWNi (stretched at 50 %), and 100 % for the CNT + TiWNi (stretched at 100 %), respectively. Figure 10c displays the time-dependent reduction of the typical DCF absorption band at 276 nm for both the CNT + TiWNi composite (stretched at 100 %) and the TiWNi powders. The stretched CNT + TiWNi composite showed a quicker disappearance of the DCF absorption band, suggesting a greater degradation rate. Figure 10d showed the results of the reuse tests that proved the stability of the TiWNi powders and the CNT + TiWNi composite in degrading DCF. They were able to accomplish this by extending the material to introduce additional flaws, particularly oxygen vacancies. Delaying electron-hole recombination and speeding up DCF breakdown, these flaws acted as electron drains. Raman absorbance measurements validated the existence of such defects. In addition, according to the scavenger’s research, the ·OH and O2− radicals were the reactive oxygen species (ROS) that attacked and destroyed the DCF molecules. Results from the study confirm that stretchable CNT/TiWNi composites can effectively remove pharmaceutical contaminants from water. Another advantage over photocatalytic powders is the possibility of manually separating these composites from the purified water. 162

Images, degradation curves, absorption spectra, and performance after multiple cycles of CNT+TiWNi composites and TiWNi powders in the photocatalytic degradation of diclofenac. (a) Images of CNT + TiWNi composites stretched at (i, ii) 0 % and (iii) 100 %, (b) diclofenac (DCF) degradation curves using TiWNi powders, CNT, and CNT + TiWNi composites (subjected at different strains or having different content of TiWNi powder on their surface), (c) DCF absorption spectra as a function of time during the photocatalytic degradation by using TiWNi powders (inset) and CNT + TiWNi composites, and (d) DCF degradation percentages after multiple cycles of usage for the TiWNi powders and CNT + TiWNi composites. 162
To activate peroxymonosulfate for antipyrine breakdown, a hybrid catalyst system was used, which consisted of MWCNT and Co–TiO2 by simple physical mixing. In 12 min, the antipyrine elimination rate rose from 53.24 % to 100 % in the presence of 2 mM peroxymonosulfate, Co–TiO2, and a half-and-half combination of MWCNTs and Co–TiO2, respectively, with a weight of 0.2 g/L. They went even deeper into the effects of changing several factors, such as the catalyst dose, peroxymonosulfate dose, antipyrine concentration, and starting pH. Quenching experiments and EPR analysis revealed the catalytic mechanism that underpinned the synergistic impact. The reasons given included the acceleration of Co3+/Co2+ due to produced superoxide and the transfer of electrons between Co–TiO2 and MWCNT 163 (Figure 11).

Proposed mechanism of (a) Co–TiO2/PMS/LED process and (b) MWCNT/PMS/LED process. 163
Na Tian et al. reported the effective synthesis and use of a ternary ZnO@spinel cobalt ferrite@carbon nanotube magnetic photocatalyst (ZSCF@CNT) for the degradation of the antibiotic Cefixime (CFX) when exposed to ultraviolet light. Experimenters used XPS, XRD, FESEM-EDX, TEM, BET, VSM, UV–vis DRS, and PL studies to study the morphological, optical, structural, and physicochemical characteristics of ZSCF@CNT. When tested under UVC irradiation and PMS, the ternary ZSCF@CNT photocatalyst showed faster removal of CFX than either the individual components or the binary composite catalysts. They found that the best circumstances were a pH of 7.0, catalyst at 0.3 g/L, and PMS at 3.0 mM, which allowed us to eliminate all of the CFX (10 mg/L) in around 20 min. The radical scavenger experiments revealed that the ZSCF@CNT/PMS/UVC system featured a number of radicals and non-radical species, such as sulphate, hydroxyl, and superoxide radicals, as well as singlet oxygen and electrons. The enhanced catalytic activity and stability of the ZSCF@CNT photocatalyst were due to its increased surface area, less agglomeration formation, and effective separation of photogenerated electron-hole pairs. As a photocatalyst for the activation of PMS for the treatment of genuine aqueous matrices, ZSCF@CNT showed promise in the experiments conducted in real water matrices. 164 The detail comparison of various CNT-based materials for photocatalytic degradation is given in Table 3.
CNT based materials for photocatalysis process pharmaceutical’s.
| Photocatalyst | Pollutants | time | Light source | Maximum decomposition ability | Synthesis method | Reference |
|---|---|---|---|---|---|---|
| WO3/CNT | 60 mg L−1 tetracycline | 60 min | US and visible light irradiations | η = 100 % | Sol–gel/hydrothermal method | 165] |
| FCNT–Bi2O2CO3/g-C3N4 | Tetracycline | 60 min | 300 W Xe lamp (λ ≥ 400 nm) | η = 97 % | One-step hydrothermal method | 166] |
| N-CNT/mpg-C3N4 | 10 mg L−1 MO | 30 min | 300 W Xe lamp (λ > 400 nm) | η = 88 % | One-step hard template method | 167] |
| CNTs/ZnO/MoS2 | 100 ppm aniline | 120 min | 50 W LED lamps | η = 76 % | Hydrothermal method | 168] |
| MWCNTs–CuNiFe2O4 | Ampicillin | 30 min | UV light irradiation | η = 100 % | Coprecipitation method | 169] |
| CNT/CuS–MD | 5 mg L−1 RhB | 120 min | 200 W Xe lamp | η = 92.4 % | In situ coprecipitation method | 170] |
| CNT-based CuFe12O19 | 20 ppm erythrosine | 50 min | UV irradiation | η = 88.24 % | Sol–gel and microwave | 171] |
| Ag3PO4@MWCNTs@PANI | 25 mg L−1 phenol | 20 min | 300 W Xe lamp (λ > 420 nm) | η = 100 % | In situ precipitation method | 172] |
9 Challenges and Prospects
9.1 Challenges
Although carbon nanotubes (CNTs) show great promise in the treatment of water, there are major barriers to their widespread use in industry. These include the high cost of CNT synthesis and the difficulties in scaling up production for larger uses.
Another important issue is the effect that the production and disposal of CNTs would have on the environment. It is unclear how long-term CNT use may affect ecosystems, and releasing CNTs into the environment may be hazardous to human health and aquatic life.
A recurring problem that can lessen CNTs’ efficacy in water treatment procedures is fouling, or the buildup of undesirable elements on their surface. Adsorption capacity and photocatalytic effectiveness are reduced by fouling, which raises maintenance and operating expenses.
9.2 Prospects
Research on more economical and efficient ways to synthesise carbon nanotubes (CNTs) is ongoing and could help address some of the issues related to cost and scalability. The utilization of less expensive carbon sources and innovations like chemical vapor deposition (CVD) should increase the accessibility of CNTs for large-scale applications.
The performance and sustainability of CNT-based materials could be greatly enhanced by the creation of new functionalization methods that are environmentally friendly and efficient.
As our knowledge of CNT-based materials advances, we may see new uses for them that go beyond the elimination of pharmaceuticals. In order to handle other water contaminants like heavy metals or persistent organic pollutants, CNTs may be extremely important, which would increase their usefulness and commercial potential. Furthermore, as the world’s water shortage worsens, there will probably be a greater need for sophisticated water treatment technology, which will create a powerful commercial incentive for the continuous development of CNT-based solutions.
10 Conclusions
Constant and growing emissions of pharmaceuticals compounds (PHCs) are depleting the world’s water supply. For long-term environmental management, it is critical to develop effective, environmentally acceptable methods of removing PHCs from water. Water contamination from organic matter pollutants poses serious threats to the health of aquatic organisms, plants, and humans alike. There are a lot of proposed technologies for decontaminating wastewater, but most of them are either complicated or too expensive to be practical. Carbon nanotubes (CNTs) have found use in environmental management for a variety of pollutants through their adsorption properties. Several chemical and physical characteristics, including CNTs’ nanosize, big surface area, and hollow structure, make them an effective material for PHCs removal. Since they are simple to work with, CNTs are being explored as a possible solution for PHCs removal from wastewater. However, there are a number of problems with using CNTs on a big scale, including impurity, increased production costs, hydrophobicity, and environmental discharge. The sorption conditions, pollutant type, and CNT properties all play a significant role in the PHCs elimination process. How and how well CNTs remove PHCs from water depends on the kind of pollutant and the characteristics of the CNTs themselves. Thus, carbon nanotubes (CNTs) can have their structures and characteristics altered by chemical, physical, biological, or mechanical activation/modification to produce sorbents that are very effective at removing a specific pollutant.
Decontaminating wastewater with CNTs tailored to a specific pollutant type is therefore a viable, long-term, sustainable, cost-effective, and environmentally beneficial option. To make CNTs more cheaply and more pure, however, future studies should concentrate on creating novel manufacturing methods and functionalization methodologies. In addition, before using CNTs as a wastewater treatment method, it is necessary to evaluate the likelihood of loaded CNTs removal from water, possible influences on microbial populations, and harmful ecological concerns of CNTs. To ensure that commercial CNT products do not harm the environment, research on their stability and dispersive capabilities in ecosystems is necessary. In addition, there has to be a thorough investigation of the possible effects of long-term exposure of humans and animals to CNT residues in drinking water.
Acknowledgments
Not applicable.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: (for double- anonymized journals: please use initials) All the authors contributed equally to the current research. The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The author states no conflict of interest.
-
Research funding: Not applicable.
-
Data availability: None declared.
References
1. Rehman, M. U.; Nabi, S. U.; Yatoo, A. M.; Ali, S.; Raina, A. A.; Hussain, I.; Rashid, S.; Mushtaq, S.; Masoodi, M. H. Microplastic (MP) Pollution: Environmental Fate, Eco-Toxicity and Sustainable Remediation. Water, Air, Soil Pollut. 2024, 235 (6), 384; https://doi.org/10.1007/s11270-024-07160-y.Search in Google Scholar
2. Qamar, M. A.; Shahid, S.; Javed, M.; Iqbal, S.; Sher, M.; Akbar, M. B. Highly Efficient G-C3N4/Cr-ZnO Nanocomposites with Superior Photocatalytic and Antibacterial Activity. J. Photochem. Photobiol. Chem. 2020, 401, 112776; https://doi.org/10.1016/j.jphotochem.2020.112776.Search in Google Scholar
3. Javed, M.; Iqbal, S.; Qamar, M. A.; Shariq, M.; Ahmed, I. A.; BaQais, A.; Alzahrani, H.; Ali, S. K.; Masmali, N. A.; Althagafi, T. M.; Shakir Khan, M. Fabrication of Effective Co-SnO2/SGCN Photocatalysts for the Removal of Organic Pollutants and Pathogen Inactivation. Crystals 2023, 13 (2), 163; https://doi.org/10.3390/cryst13020163.Search in Google Scholar
4. Zhao, F.; Hao, Y.; Xu, Q.; Li, X.; Cheng, L.; Chen, D.; Shi, X.; Xiao, Y.; Wei, P.; Bi, X. Safety Assessment of Organic Micropollutants in Reclaimed Water: Chemical Analyses, Ecological Risk Assessments, and In Vivo Endocrine-Disrupting Studies. Sci. Total Environ. 2023, 884, 163865; https://doi.org/10.1016/j.scitotenv.2023.163865.Search in Google Scholar PubMed
5. Ansari, K.; Khan, M. A. Introduction to Micropollutants. In Advanced Oxidation Processes for Micropollutant Remediation; CRC Press, 2024; pp 1–11.10.1201/9781003247913-1Search in Google Scholar
6. Sawicka, B. Organic Micropollutants in Wastewaters: Advances in Sustainable Management and Treatment Methods. In Organic Micropollutants in Aquatic and Terrestrial Environments; Springer, 2024; pp 225–247.10.1007/978-3-031-48977-8_11Search in Google Scholar
7. Farhan, A.; Arshad, J.; Rashid, E. U.; Ahmad, H.; Nawaz, S.; Munawar, J.; Zdarta, J.; Jesionowski, T.; Bilal, M. Metal Ferrites-Based Nanocomposites and Nanohybrids for Photocatalytic Water Treatment and Electrocatalytic Water Splitting. Chemosphere 2023, 310, 136835; https://doi.org/10.1016/j.chemosphere.2022.136835.Search in Google Scholar PubMed
8. Al-Gethami, W.; Qamar, M. A.; Shariq, M.; Alaghaz, A. N. M. A.; Farhan, A.; Areshi, A. A.; Alnasir, M. H. Emerging Environmentally Friendly Bio-Based Nanocomposites for the Efficient Removal of Dyes and Micropollutants from Wastewater by Adsorption: A Comprehensive Review. RSC Adv. 2024, 14 (4), 2804–2834; https://doi.org/10.1039/d3ra06501d.Search in Google Scholar PubMed PubMed Central
9. Farhan, A.; Rashid, E. U.; Waqas, M.; Ahmad, H.; Nawaz, S.; Munawar, J.; Rahdar, A.; Varjani, S.; Bilal, M. Graphene-based Nanocomposites and Nanohybrids for the Abatement of Agro-Industrial Pollutants in Aqueous Environments. Environ. Pollut. 2022, 308, 119557; https://doi.org/10.1016/j.envpol.2022.119557.Search in Google Scholar PubMed
10. Farhan, A.; Zahid, M.; Tahir, N.; Mansha, A.; Yaseen, M.; Mustafa, G.; Alamir, M. A.; Alarifi, I. M.; shahid, I. Investigation of Boron-Doped Graphene Oxide Anchored with Copper Sulphide Flowers as Visible Light Active Photocatalyst for Methylene Blue Degradation. Sci. Rep. 2023, 13 (1), 9497; https://doi.org/10.1038/s41598-023-36486-6.Search in Google Scholar PubMed PubMed Central
11. Qamar, M. A.; Shahid, S.; Javed, M.; Sher, M.; Iqbal, S.; Bahadur, A.; Li, D. Fabricated Novel G-C3N4/Mn Doped ZnO Nanocomposite as Highly Active Photocatalyst for the Disinfection of Pathogens and Degradation of the Organic Pollutants from Wastewater under Sunlight Radiations. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125863; https://doi.org/10.1016/j.colsurfa.2020.125863.Search in Google Scholar
12. Asghar, F.; Murtaza, B.; Shakoor, B.; Iqbal, N.; Shafique, M.; Murtaza, R.; Butler, I. S. Properties, Assembly and Characterization of Carbon Nanotubes: Their Application in Water Purification, Environmental Pollution Control and Biomedicines – A Comprehensive Review. Carbon Lett. 2023, 33 (2), 275–306; https://doi.org/10.1007/s42823-022-00432-9.Search in Google Scholar
13. Kurwadkar, S.; Hoang, T. V.; Malwade, K.; Kanel, S. R.; Harper, W. F.Jr.; Struckhoff, G. Application of Carbon Nanotubes for Removal of Emerging Contaminants of Concern in Engineered Water and Wastewater Treatment Systems. Nanotechnol. Environ. Eng. 2019, 4, 1–16; https://doi.org/10.1007/s41204-019-0059-1.Search in Google Scholar
14. Glassmeyer, S. T.; Burns, E. E.; Focazio, M. J.; Furlong, E. T.; Gribble, M. O.; Jahne, M. A.; Keely, S. P.; Kennicutt, A. R.; Kolpin, D. W.; Medlock Kakaley, E. K.; Pfaller, S. L. Water, Water Everywhere, but Every Drop Unique: Challenges in the Science to Understand the Role of Contaminants of Emerging Concern in the Management of Drinking Water Supplies. GeoHealth 2023, 7 (12), e2022GH000716; https://doi.org/10.1029/2022gh000716.Search in Google Scholar PubMed PubMed Central
15. Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A. K.; Bank, M. S.; You, S.; Ok, Y. S. Interactions Between Microplastics, Pharmaceuticals and Personal Care Products: Implications for Vector Transport. Environ. Int. 2021, 149, 106367; https://doi.org/10.1016/j.envint.2020.106367.Search in Google Scholar PubMed
16. Amalina, F.; Krishnan, S.; Zularisam, A.; Nasrullah, M. Biochar and Sustainable Environmental Development Towards Adsorptive Removal of Pollutants: Modern Advancements and Future Insight. Process Saf. Environ. Protect. 2023, 173, 715–728; https://doi.org/10.1016/j.psep.2023.03.069.Search in Google Scholar
17. Srivastava, A.; Dutta, S.; Ahuja, S.; Sharma, R. K. Green Chemistry: Key to Reducing Waste and Improving Water Quality. Handbook Water Purity Qual. 2021, 359–407; https://doi.org/10.1016/b978-0-12-821057-4.00010-0.Search in Google Scholar
18. Singh, D. K.; Mondal, M. K. Nanoadsorbent: An Alternative to Conventional Adsorbent for Water Remediation. In Hazardous Waste Management; Elsevier, 2022; pp 397–420.10.1016/B978-0-12-824344-2.00007-0Search in Google Scholar
19. Gopinath, K. P.; Vo, D. V. N.; Gnana Prakash, D.; Adithya Joseph, A.; Viswanathan, S.; Arun, J. Environmental Applications of Carbon-Based Materials: A Review. Environ. Chem. Lett. 2021, 19, 557–582; https://doi.org/10.1007/s10311-020-01084-9.Search in Google Scholar
20. Madkour, L. H. Carbon Nanocomposites: Emerging Engineering Technologies and Industrial Applications, in Emerging Engineering Technologies and Industrial Applications. IGI Global 2024, 97–146.10.4018/979-8-3693-1335-0.ch005Search in Google Scholar
21. Islam, A.; Teo, S. H.; Taufiq-Yap, Y. H.; Vo, D. V. N.; Ibrahim, M. L.; Hasan, M. M.; Khan, M. A. R.; Nur, A. S.; Awual, M. R. Step towards the Sustainable Toxic Dyes Removal and Recycling from Aqueous Solution-A Comprehensive Review. Resour. Conserv. Recycl. 2021, 175, 105849; https://doi.org/10.1016/j.resconrec.2021.105849.Search in Google Scholar
22. Salahshoori, I.; Namayandeh Jorabchi, M.; Mazaheri, A.; Mirnezami, S. M. S.; Afshar, M.; Golriz, M.; Nobre, M. A. Tackling Antibiotic Contaminations in Wastewater with Novel Modified-MOF Nanostructures: A Study of Molecular Simulations and DFT Calculations. Environ. Res. 2024, 252, 118856; https://doi.org/10.1016/j.envres.2024.118856.Search in Google Scholar PubMed
23. Wang, L.; Chen, G.; Shu, H.; Cui, X.; Luo, Z.; Chang, C.; Zeng, A.; Zhang, J.; Fu, Q. Facile Covalent Preparation of Carbon Nanotubes/amine-Functionalized Fe3O4 Nanocomposites for Selective Extraction of Estradiol in Pharmaceutical Industry Wastewater. J. Chromatogr. A 2021, 1638, 461889; https://doi.org/10.1016/j.chroma.2021.461889.Search in Google Scholar PubMed
24. Charlton-Sevcik, A. K.; Collom, C.; Liu, J. Y.; Hsieh, Y. L.; Stark, N.; Ede, J. D.; Shatkin, J. A.; Sayes, C. M. The Impact of Surface Functionalization of Cellulose Following Simulated Digestion and Gastrointestinal Cell-Based Model Exposure. Int. J. Biol. Macromol. 2024, 271, 132603; https://doi.org/10.1016/j.ijbiomac.2024.132603.Search in Google Scholar PubMed
25. Zha, S.; Liu, H.; Li, H.; Li, H.; Wong, K. L.; All, A. H. Functionalized Nanomaterials Capable of Crossing the Blood–Brain Barrier. ACS Nano 2024, 18 (3), 1820–1845; https://doi.org/10.1021/acsnano.3c10674.Search in Google Scholar PubMed PubMed Central
26. Duarte, E. D.; Oliveira, M. G.; Spaolonzi, M. P.; Costa, H. P.; Silva, T. L.; Silva, M. G. D.; Vieira, M. G. Adsorption of Pharmaceutical Products from Aqueous Solutions on Functionalized Carbon Nanotubes by Conventional and Green Methods: A Critical Review. J. Clean. Prod. 2022, 133743; https://doi.org/10.1016/j.jclepro.2022.133743.Search in Google Scholar
27. Wang, T.; He, J.; Lu, J.; Zhou, Y.; Wang, Z.; Zhou, Y. Adsorptive Removal of PPCPs from Aqueous Solution Using Carbon-Based Composites: A Review. Chin. Chem. Lett. 2022, 33 (8), 3585–3593; https://doi.org/10.1016/j.cclet.2021.09.029.Search in Google Scholar
28. Fallah, Z.; Zare, E. N.; Ghomi, M.; Ahmadijokani, F.; Amini, M.; Tajbakhsh, M.; Arjmand, M.; Sharma, G.; Ali, H.; Ahmad, A.; Makvandi, P.; Lichtfouse, E.; Sillanpää, M.; Varma, R. S. Toxicity and Remediation of Pharmaceuticals and Pesticides Using Metal Oxides and Carbon Nanomaterials. Chemosphere 2021, 275, 130055; https://doi.org/10.1016/j.chemosphere.2021.130055.Search in Google Scholar PubMed PubMed Central
29. Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R. S. Carbon-based Sustainable Nanomaterials for Water Treatment: State-Of-Art and Future Perspectives. Chemosphere 2021, 263, 128005; https://doi.org/10.1016/j.chemosphere.2020.128005.Search in Google Scholar PubMed PubMed Central
30. Raza, A.; Altaf, S.; Ali, S.; Ikram, M.; Li, G. Recent Advances in Carbonaceous Sustainable Nanomaterials for Wastewater Treatments. Sustainable Mater. Technol. 2022, 32, e00406; https://doi.org/10.1016/j.susmat.2022.e00406.Search in Google Scholar
31. Qamar, M. A.; Javed, M.; Shahid, S. Designing and Investigation of Enhanced Photocatalytic and Antibacterial Properties of 3d (Fe, Co, Ni, Mn and Cr) Metal-Doped Zinc Oxide Nanoparticles. Opt. Mater. 2022, 126, 112211; https://doi.org/10.1016/j.optmat.2022.112211.Search in Google Scholar
32. Iqbal, S.; Ahmad, N.; Javed, M.; Qamar, M. A.; Bahadur, A.; Ali, S.; Ahmad, Z.; Irfan, R. M.; Liu, G.; Akbar, M. B.; Qayyum, M. A. Designing Highly Potential Photocatalytic Comprising Silver Deposited ZnO NPs with Sulfurized Graphitic Carbon Nitride (Ag/ZnO/Sg-C3N4) Ternary Composite. J. Environ. Chem. Eng. 2021, 9 (1), 104919; https://doi.org/10.1016/j.jece.2020.104919.Search in Google Scholar
33. Iqbal, S.; Bahadur, A.; Ali, S.; Ahmad, Z.; Javed, M.; Irfan, R. M.; Ahmad, N.; Qamar, M. A.; Liu, G.; Akbar, M. B.; Nawaz, M. Critical Role of the Heterojunction Interface of Silver Decorated ZnO Nanocomposite with Sulfurized Graphitic Carbon Nitride Heterostructure Materials for Photocatalytic Applications. J. Alloys Compd. 2021, 858, 158338; https://doi.org/10.1016/j.jallcom.2020.158338.Search in Google Scholar
34. Wang, R.; Duan, X.; Wang, S.; Ren, N. Q.; Ho, S. H. Production, Properties, and Catalytic Applications of Sludge Derived Biochar for Environmental Remediation. Water Res. 2020, 187, 116390; https://doi.org/10.1016/j.watres.2020.116390.Search in Google Scholar PubMed
35. Németh, P.; McColl, K.; Garvie, L. A. J.; Salzmann, C. G.; Murri, M.; McMillan, P. F. Complex Nanostructures in Diamond. Nat. Mater. 2020, 19 (11), 1126–1131; https://doi.org/10.1038/s41563-020-0759-8.Search in Google Scholar PubMed
36. Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K. H. Nanodiamonds: Emerging Face of Future Nanotechnology. Carbon 2019, 143, 678–699; https://doi.org/10.1016/j.carbon.2018.11.060.Search in Google Scholar
37. Chen, Y.-D.; Liu, F.; Ren, N. Q.; Ho, S. H. Revolutions in Algal Biochar for Different Applications: State-Of-The-Art Techniques and Future Scenarios. Chin. Chem. Lett. 2020, 31 (10), 2591–2602; https://doi.org/10.1016/j.cclet.2020.08.019.Search in Google Scholar
38. Mehra, N. K.; Mishra, V.; Jain, N. A Review of Ligand Tethered Surface Engineered Carbon Nanotubes. Biomaterials 2014, 35 (4), 1267–1283; https://doi.org/10.1016/j.biomaterials.2013.10.032.Search in Google Scholar PubMed
39. Srivastava, N.; Mishra, Y.; Mishra, V.; Ranjan, A.; Tambuwala, M. M. Carbon Nanotubes in Breast Cancer Treatment: An Insight into Properties, Functionalization, and Toxicity. Anti Cancer Agents Med. Chem. 2023, 23 (14), 1606–1617; https://doi.org/10.2174/1871520623666230510094850.Search in Google Scholar PubMed
40. Obaidullah, I. Carbon Nanotube Membranes for Water Purification: Developments, Challenges, and Prospects for the Future. Sep. Purif. Technol. 2019, 209, 307–337; https://doi.org/10.1016/j.seppur.2018.07.043.Search in Google Scholar
41. Mishra, V.; Kesharwani, P.; Jain, N. K. Biomedical Applications and Toxicological Aspects of Functionalized Carbon Nanotubes. Crit. Rev. Ther. Drug Carrier Syst. 2018, 35 (4); https://doi.org/10.1615/critrevtherdrugcarriersyst.2018014419.Search in Google Scholar
42. Suttee, A.; Mishra, V.; Singh, M.; Nayak, P.; Sriram, P. Vijay, M.; Singh, M.; Nayak, P.; Sriram, P.; Suttee, A. “Carbon nanotubes as emerging nanocarriers in drug delivery: An overview.” Int. J. Pharm. Qual. Assur 11 (2020): 373-378. Int. J. Pharm. Qual. Assur 2020, https://doi.org/10.25258/ijpqa.11.3.11.Search in Google Scholar
43. Pan, X.; Ji, J.; Zhang, N.; Xing, M. Research Progress of Graphene-Based Nanomaterials for the Environmental Remediation. Chin. Chem. Lett. 2020, 31 (6), 1462–1473; https://doi.org/10.1016/j.cclet.2019.10.002.Search in Google Scholar
44. Mishra, S.; Beyer, D.; Eimre, K.; Ortiz, R.; Fernández-Rossier, J.; Berger, R.; Gröning, O.; Pignedoli, C. A.; Fasel, R.; Feng, X.; Ruffieux, P. Collective All-Carbon Magnetism in Triangulene Dimers. Angew. Chem. 2020, 132 (29), 12139–12145; https://doi.org/10.1002/ange.202002687.Search in Google Scholar
45. Su, J.; Telychko, M.; Song, S.; Lu, J. Triangulenes: From Precursor Design to On-Surface Synthesis and Characterization. Angew. Chem. Int. Ed. 2020, 59 (20), 7658–7668; https://doi.org/10.1002/anie.201913783.Search in Google Scholar PubMed
46. Mubarak, N.; Abdullah, E.; Jayakumar, N.; Sahu, J. An Overview on Methods for the Production of Carbon Nanotubes. J. Ind. Eng. Chem. 2014, 20 (4), 1186–1197; https://doi.org/10.1016/j.jiec.2013.09.001.Search in Google Scholar
47. Kesharwani, P.; Mishra, V.; Jain, N. K. Validating the Anticancer Potential of Carbon Nanotube-Based Therapeutics through Cell Line Testing. Drug Discov. Today 2015, 20 (9), 1049–1060; https://doi.org/10.1016/j.drudis.2015.05.004.Search in Google Scholar PubMed
48. Rajaura, R. S.; Singhal, I.; Sharma, K. N.; Srivastava, S. Efficient Chemical Vapour Deposition and Arc Discharge System for Production of Carbon Nano-Tubes on a Gram Scale. Rev. Sci. Instrum. 2019, 90 (12); https://doi.org/10.1063/1.5113850.Search in Google Scholar PubMed
49. Venkataraman, A.; Amadi, E. V.; Chen, Y.; Papadopoulos, C. Carbon Nanotube Assembly and Integration for Applications. Nanoscale Res. Lett. 2019, 14, 1–47; https://doi.org/10.1186/s11671-019-3046-3.Search in Google Scholar PubMed PubMed Central
50. Shoukat, R.; Khan, M. I. Carbon Nanotubes: A Review on Properties, Synthesis Methods and Applications in Micro and Nanotechnology. Microsyst. Technol. 2021, 1–10; https://doi.org/10.1007/s00542-021-05211-6.Search in Google Scholar
51. Huh, Y.; Lee, J. Y.; Cheon, J.; Hong, Y. K.; Koo, J. Y.; Lee, T. J.; Lee, C. J. Controlled Growth of Carbon Nanotubes Over Cobalt Nanoparticles by Thermal Chemical Vapor Deposition. J. Mater. Chem. 2003, 13 (9), 2297–2300; https://doi.org/10.1039/b304582j.Search in Google Scholar
52. Kumar, M.; Ando, Y. Gigas Growth of Carbon Nanotubes. Defence Sci. J. 2008, 58 (4); https://doi.org/10.14429/dsj.58.1670.Search in Google Scholar
53. Kobayashi, Y.; Nakashima, H.; Takagi, D.; Homma, Y. CVD Growth of Single-Walled Carbon Nanotubes Using Size-Controlled Nanoparticle Catalyst. Thin Solid Films 2004, 464, 286–289; https://doi.org/10.1016/j.tsf.2004.06.045.Search in Google Scholar
54. Khan, M. I.; Lin, F. Comparative Analysis and Design of Harmonic Aware Low-Power Latches and Flip-Flops. In 2014 IEEE International Conference on Electron Devices and Solid-State Circuits; IEEE, 2014.10.1109/EDSSC.2014.7061282Search in Google Scholar
55. Singh, S. R.; Jarvis, L. A. Microwave Plasma-Enhanced Chemical Vapour Deposition Growth of Carbon Nanostructures. South Afr. J. Sci. 2010, 106 (5), 1–4; https://doi.org/10.4102/sajs.v106i5/6.183.Search in Google Scholar
56. Janas, D. Perfectly Imperfect: A Review of Chemical Tools for Exciton Engineering in Single-Walled Carbon Nanotubes. Mater. Horiz. 2020, 7 (11), 2860–2881; https://doi.org/10.1039/d0mh00845a.Search in Google Scholar
57. Kukovecz, Á.; Kozma, G.; Kónya, Z. Multi-Walled Carbon Nanotubes. Springer Handbook Nanomater. 2013, 147–188; https://doi.org/10.1007/978-3-642-20595-8_5.Search in Google Scholar
58. Jeon, I.-Y. Functionalization of Carbon Nanotubes. Carbon Nanotubes-Polymer Nanocompos. 2011, 6, 91–110.10.5772/18396Search in Google Scholar
59. Hosseini, A.; Allahyari, M.; Besheli, S. D. Synthesis of Carbon Nanotubes, Nano Fibbers and Nano Union by Electric Arc Discharge Method Using NaCl Accuse as Solution and Fe and Ni Particles and Catalysts. IJEST 2012, 1, 217–229.Search in Google Scholar
60. Maria, K. H.; Mieno, T. Synthesis of Single-Walled Carbon Nanotubes by Low-Frequency Bipolar Pulsed Arc Discharge Method. Vacuum 2015, 113, 11–18; https://doi.org/10.1016/j.vacuum.2014.11.025.Search in Google Scholar
61. Luo, J.; Liu, Y.; Wei, H.; Wang, B.; Wu, K. H.; Zhang, B.; Su, D. S. A Green and Economical Vapor-Assisted Ozone Treatment Process for Surface Functionalization of Carbon Nanotubes. Green Chem. 2017, 19 (4), 1052–1062; https://doi.org/10.1039/c6gc02806c.Search in Google Scholar
62. Jung, M.; Hong, S.-G.; Moon, J. Ozone Treatment on the Dispersion of Carbon Nanotubes in Ultra-high Performance Concrete. Mater. Des. 2020, 193, 108813; https://doi.org/10.1016/j.matdes.2020.108813.Search in Google Scholar
63. Shoukat, R.; Khan, M. I. Carbon Nanotubes/nanofibers (CNTs/CNFs): A Review on State of the Art Synthesis Methods. Microsyst. Technol. 2022, 28 (4), 885–901; https://doi.org/10.1007/s00542-022-05263-2.Search in Google Scholar
64. Cao, X. T.; Patil, M. P.; Phan, Q. T.; Le, C. M.; Ahn, B. H.; Kim, G. D.; Lim, K. T. Green and Direct Functionalization of Poly (Ethylene Glycol) Grafted Polymers onto Single Walled Carbon Nanotubes: Effective Nanocarrier for Doxorubicin Delivery. J. Ind. Eng. Chem. 2020, 83, 173–180; https://doi.org/10.1016/j.jiec.2019.11.025.Search in Google Scholar
65. Mallakpour, S.; Abdolmaleki, A.; Azimi, F. Ultrasonic-assisted Biosurface Modification of Multi-Walled Carbon Nanotubes with Thiamine and its Influence on the Properties of PVC/Tm-MWCNTs Nanocomposite Films. Ultrason. Sonochem. 2017, 39, 589–596; https://doi.org/10.1016/j.ultsonch.2017.05.028.Search in Google Scholar PubMed
66. Le, C. M.; Cao, X. T.; Lim, K. T. Ultrasound-promoted Direct Functionalization of Multi-Walled Carbon Nanotubes in Water via Diels–Alder “Click Chemistry”. Ultrason. Sonochem. 2017, 39, 321–329; https://doi.org/10.1016/j.ultsonch.2017.04.042.Search in Google Scholar PubMed
67. Li, C.; Teo, B. K.; Sun, X. H.; Wong, N. B.; Lee, S. T. Hydrocarbon and Carbon Nanostructures Produced by Sonochemical Reactions of Organic Solvents on Hydrogen-Passivated Silicon Nanowires under Ambient Conditions. Chem. Mater. 2005, 17 (23), 5780–5788; https://doi.org/10.1021/cm050355n.Search in Google Scholar
68. Jeong, S.-H.; Ko, J. H.; Park, J. B.; Park, W. A Sonochemical Route to Single-Walled Carbon Nanotubes Under Ambient Conditions. J. Am. Chem. Soc. 2004, 126 (49), 15982–15983; https://doi.org/10.1021/ja0451867.Search in Google Scholar PubMed
69. Skrabalak, S. E. Ultrasound-Assisted Synthesis of Carbon Materials. Phys. Chem. Chem. Phys. 2009, 11 (25), 4930–4942; https://doi.org/10.1039/b823408f.Search in Google Scholar PubMed
70. Delzeit, L.; McAninch, I.; Cruden, B. A.; Hash, D.; Chen, B.; Han, J.; Meyyappan, M. Growth of Multiwall Carbon Nanotubes in an Inductively Coupled Plasma Reactor. J. Appl. Phys. 2002, 91 (9), 6027–6033; https://doi.org/10.1063/1.1465101.Search in Google Scholar
71. Park, D.; Kim, Y. H.; Lee, J. K. Synthesis of Carbon Nanotubes on Metallic Substrates by a Sequential Combination of PECVD and Thermal CVD. Carbon 2003, 41 (5), 1025–1029; https://doi.org/10.1016/s0008-6223(02)00432-3.Search in Google Scholar
72. Gohier, A.; Minea, T.; Djouadi, A.; Granier, A.; Dubosc, M. Limits of the PECVD Process for Single Wall Carbon Nanotubes Growth. Chem. Phys. Lett. 2006, 421 (1-3), 242–245; https://doi.org/10.1016/j.cplett.2005.12.105.Search in Google Scholar
73. Singh, C.; Shaffer, M. S.; Windle, A. H. Production of Controlled Architectures of Aligned Carbon Nanotubes by an Injection Chemical Vapour Deposition Method. Carbon 2003, 41 (2), 359–368; https://doi.org/10.1016/s0008-6223(02)00314-7.Search in Google Scholar
74. Shoukat, R.; Khan, M. I. Nanotechnology Based Electrical Control and Navigation System for Worm Guidance Using Electric Field Gradient. Microsyst. Technol. 2018, 24, 989–993; https://doi.org/10.1007/s00542-017-3444-3.Search in Google Scholar
75. Khairurrijal, K.; Abdullah, M.; Rosi, M.; Noor, F. A. Structural Characteristics of Carbon Nanotubes Fabricated Using Simple Spray Pyrolysis Method. Indonesian J. Phys. 2008, 19 (3), 91–95; https://doi.org/10.5614/itb.ijp.2008.19.3.5.Search in Google Scholar
76. Yuji, T.; Sung, Y.-M. RF PECVD Characteristics for the Growth of Carbon Nanotubes in a $\hbox {CH} _ {4} $–$\hbox {N} _ {2} $ Mixed Gas. IEEE Trans. Plasma Sci. 2007, 35 (4), 1027–1032; https://doi.org/10.1109/tps.2007.896753.Search in Google Scholar
77. Caughman, J.; Baylor, L. R.; Guillorn, M. A.; Merkulov, V. I.; Lowndes, D. H.; Allard, L. F. Growth of Vertically Aligned Carbon Nanofibers by Low-Pressure Inductively Coupled Plasma-Enhanced Chemical Vapor Deposition. Appl. Phys. Lett. 2003, 83 (6), 1207–1209; https://doi.org/10.1063/1.1597981.Search in Google Scholar
78. Barnard, A. W.; Zhang, M.; Wiederhecker, G. S.; Lipson, M.; McEuen, P. L. Real-time Vibrations of a Carbon Nanotube. Nature 2019, 566 (7742), 89–93; https://doi.org/10.1038/s41586-018-0861-0.Search in Google Scholar PubMed
79. Lordi, V.; Yao, N. Molecular Mechanics of Binding in Carbon-Nanotube–Polymer Composites. J. Mater. Res. 2000, 15 (12), 2770–2779; https://doi.org/10.1557/jmr.2000.0396.Search in Google Scholar
80. Harris, P. J. Carbon Nanotubes and Related Structures: New Materials for the Twenty-First Century; American Association of Physics Teachers, 2004.10.1119/1.1645289Search in Google Scholar
81. Meo, M.; Rossi, M. Prediction of Young’s Modulus of Single Wall Carbon Nanotubes by Molecular-Mechanics Based Finite Element Modelling. Compos. Sci. Technol. 2006, 66 (11–12), 1597–1605; https://doi.org/10.1016/j.compscitech.2005.11.015.Search in Google Scholar
82. Nardelli, M. B.; Fattebert, J. L.; Orlikowski, D.; Roland, C.; Zhao, Q.; Bernholc, J. Mechanical Properties, Defects and Electronic Behavior of Carbon Nanotubes. Carbon 2000, 38 (11–12), 1703–1711; https://doi.org/10.1016/s0008-6223(99)00291-2.Search in Google Scholar
83. Matsuda, K. Fundamental Optical Properties of Carbon Nanotubes and Graphene. In Carbon Nanotubes and Graphene for Photonic Applications; Elsevier, 2013; pp 3–25.10.1533/9780857098627.1.3Search in Google Scholar
84. Yamashita, S.; Inoue, Y.; Maruyama, S.; Murakami, Y.; Yaguchi, H.; Jablonski, M.; Set, S. Y. Saturable Absorbers Incorporating Carbon Nanotubes Directly Synthesized onto Substrates and Fibers and Their Application to Mode-Locked Fiber Lasers. Opt. Lett. 2004, 29 (14), 1581–1583; https://doi.org/10.1364/ol.29.001581.Search in Google Scholar PubMed
85. Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical Properties of Single-Wall Carbon Nanotubes. Synth. Met. 1999, 103 (1–3), 2555–2558; https://doi.org/10.1016/s0379-6779(98)00278-1.Search in Google Scholar
86. Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Dai, H. Thermal Conductance of an Individual Single-Wall Carbon Nanotube above Room Temperature. Nano Lett. 2006, 6 (1), 96–100; https://doi.org/10.1021/nl052145f.Search in Google Scholar PubMed
87. Ruoff, R. S.; Lorents, D. C. Mechanical and Thermal Properties of Carbon Nanotubes. Carbon 1995, 33 (7), 925–930; https://doi.org/10.1016/0008-6223(95)00021-5.Search in Google Scholar
88. Stahl, H.; Appenzeller, J.; Martel, R.; Avouris, P.; Lengeler, B. Intertube Coupling in Ropes of Single-Wall Carbon Nanotubes. Phys. Rev. Lett. 2000, 85 (24), 5186; https://doi.org/10.1103/physrevlett.85.5186.Search in Google Scholar PubMed
89. Chandra, B.; Bhattacharjee, J.; Purewal, M.; Son, Y. W.; Wu, Y.; Huang, M.; Yan, H.; Heinz, T. F.; Kim, P.; Neaton, J. B.; Hone, J. Molecular-Scale Quantum Dots from Carbon Nanotube Heterojunctions. Nano Lett. 2009, 9 (4), 1544–1548; https://doi.org/10.1021/nl803639h.Search in Google Scholar PubMed
90. Wang, X.-T. The Band Inversion and Topological Insulating State of Heusler Alloys: X 2 RuPb (X= Lu, Y); Acta Physica Sinica, Chinese Physical Society and Institute of Physics, Chinese Academy of Sciences, Vol. 02, 2014; p 023101, https://doi.org/10.7498/aps.63.023101.Search in Google Scholar
91. Peng, H.; Alemany, L. B.; Margrave, J. L.; Khabashesku, V. N. Sidewall Carboxylic Acid Functionalization of Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2003, 125 (49), 15174–15182; https://doi.org/10.1021/ja037746s.Search in Google Scholar PubMed
92. Ihsanullah; Al-Khaldi, F. A.; Abu-Sharkh, B.; Abulkibash, A. M.; Qureshi, M. I.; Laoui, T.; Atieh, M. A. Effect of Acid Modification on Adsorption of Hexavalent Chromium (Cr (VI)) from Aqueous Solution by Activated Carbon and Carbon Nanotubes. Desalination Water Treat. 2016, 57 (16), 7232–7244; https://doi.org/10.1080/19443994.2015.1021847.Search in Google Scholar
93. Lee, J.; Jeong, S.; Liu, Z. Progress and Challenges of Carbon Nanotube Membrane in Water Treatment. Crit. Rev. Environ. Sci. Technol. 2016, 46 (11–12), 999–1046; https://doi.org/10.1080/10643389.2016.1191894.Search in Google Scholar
94. Trulli, M. G.; Sardella, E.; Palumbo, F.; Palazzo, G.; Giannossa, L. C.; Mangone, A.; Comparelli, R.; Musso, S.; Favia, P. Towards Highly Stable Aqueous Dispersions of Multi-Walled Carbon Nanotubes: The Effect of Oxygen Plasma Functionalization. J. Colloid Interface Sci. 2017, 491, 255–264; https://doi.org/10.1016/j.jcis.2016.12.039.Search in Google Scholar PubMed
95. Bounos, G.; Andrikopoulos, K.; Moschopoulou, H.; Lainioti, G.; Roilo, D.; Checchetto, R.; Ioannides, T.; Kallitsis, J.; Voyiatzis, G. Enhancing Water Vapor Permeability in Mixed Matrix Polypropylene Membranes through Carbon Nanotubes Dispersion. J. Membr. Sci. 2017, 524, 576–584; https://doi.org/10.1016/j.memsci.2016.11.076.Search in Google Scholar
96. Oyetade, O. A.; Skelton, A. A.; Nyamori, V. O.; Jonnalagadda, S. B.; Martincigh, B. S. Experimental and DFT Studies on the Selective Adsorption of Pb2+ and Zn2+ from Aqueous Solution by Nitrogen-Functionalized Multiwalled Carbon Nanotubes. Sep. Purif. Technol. 2017, 188, 174–187; https://doi.org/10.1016/j.seppur.2017.07.022.Search in Google Scholar
97. Zhang, Y.; Wu, B.; Xu, H.; Liu, H.; Wang, M.; He, Y.; Pan, B. Nanomaterials-enabled Water and Wastewater Treatment. NanoImpact 2016, 3, 22–39; https://doi.org/10.1016/j.impact.2016.09.004.Search in Google Scholar
98. Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92 (3), 407–418; https://doi.org/10.1016/j.jenvman.2010.11.011.Search in Google Scholar PubMed
99. Lee, K. M.; Wong, C. P. P.; Lai, C. W. Functionalized Carbon Nanotubes for Adsorptive Removal of Water Pollutants. Mater. Sci. Eng., B 2018, 236, 61–69; https://doi.org/10.1016/j.mseb.2018.12.004.Search in Google Scholar
100. Yang, S. Y.; Vecitis, C. D.; Park, H. Electrocatalytic Water Treatment Using Carbon Nanotube Filters Modified with Metal Oxides. Environ. Sci. Pollut. Control Ser. 2019, 26, 1036–1043; https://doi.org/10.1007/s11356-017-8495-6.Search in Google Scholar PubMed
101. Al-Jammal, N.; Abdullah, T. A.; Juzsakova, T.; Zsirka, B.; Cretescu, I.; Vágvölgyi, V.; Sebestyén, V.; Le Phuoc, C.; Rasheed, R. T.; Domokos, E. Functionalized Carbon Nanotubes for Hydrocarbon Removal from Water. J. Environ. Chem. Eng. 2020, 8 (2), 103570; https://doi.org/10.1016/j.jece.2019.103570.Search in Google Scholar
102. Chuah, L.; Aziz, A. R. A.; Yusup, S.; Bokhari, A.; Klemeš, J. J.; Abdullah, M. Z. Performance and Emission of Diesel Engine Fuelled by Waste Cooking Oil Methyl Ester Derived from Palm Olein Using Hydrodynamic Cavitation. Clean Technol. Environ. Policy 2015, 17 (8); https://doi.org/10.1007/s10098-015-0957-2.Search in Google Scholar
103. Hong, Y.; Zhang, J.; Zhu, C.; Zeng, X. C.; Francisco, J. S. Water Desalination Through Rim Functionalized Carbon Nanotubes. J. Mater. Chem. A 2019, 7 (8), 3583–3591; https://doi.org/10.1039/c8ta10941a.Search in Google Scholar
104. Rameetse, M. S.; Aberefa, O.; Daramola, M. O. Effect of Loading and Functionalization of Carbon Nanotube on the Performance of Blended Polysulfone/Polyethersulfone Membrane During Treatment of Wastewater Containing Phenol and Benzene. Membranes 2020, 10 (3), 54; https://doi.org/10.3390/membranes10030054.Search in Google Scholar PubMed PubMed Central
105. Al-Faiyz, Y. S.; Gouda, M. Multi-Walled Carbon Nanotubes Functionalized with Hydroxamic Acid Derivatives for the Removal of Lead from Wastewater: Kinetics, Isotherm, and Thermodynamic Studies. Polymers 2022, 14 (18), 3870; https://doi.org/10.3390/polym14183870.Search in Google Scholar PubMed PubMed Central
106. Nejabat, F.; Rayati, S. Surface Modification of Multi-Walled Carbon Nanotubes to Produce a New Bimetallic Fe/Mn Catalyst for the Aerobic Oxidation of Hydrocarbons. J. Ind. Eng. Chem. 2019, 69, 324–330; https://doi.org/10.1016/j.jiec.2018.09.044.Search in Google Scholar
107. Ngo, C. L.; Le, Q. T.; Ngo, T. T.; Nguyen, D. N.; Vu, M. T. Surface Modification and Functionalization of Carbon Nanotube with Some Organic Compounds. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4 (3), 035017; https://doi.org/10.1088/2043-6262/4/3/035017.Search in Google Scholar
108. Guo, T.; Wu, J.; Gao, H.; Chen, Y. Covalent Functionalization of Multi-Walled Carbon Nanotubes with Spiropyran for High Solubility Both in Water and in Non-Aqueous Solvents. Inorg. Chem. Commun. 2017, 83, 31–35; https://doi.org/10.1016/j.inoche.2017.06.002.Search in Google Scholar
109. Palacin, T.; Khanh, H. L.; Jousselme, B.; Jegou, P.; Filoramo, A.; Ehli, C.; Guldi, D. M.; Campidelli, S. Efficient Functionalization of Carbon Nanotubes with Porphyrin Dendrons via Click Chemistry. J. Am. Chem. Soc. 2009, 131 (42), 15394–15402; https://doi.org/10.1021/ja906020e.Search in Google Scholar PubMed
110. Pavia Sanders, A.; O’Bryan, G. Covalent Surface Modifications of Carbon Nanotubes; Sandia National Lab.(SNL-NM): Albuquerque, NM (United States), 2017.10.2172/1373648Search in Google Scholar
111. Voiry, D.; Goswami, A.; Kappera, R.; Silva, C. D. C. C. E.; Kaplan, D.; Fujita, T.; Chen, M.; Asefa, T.; Chhowalla, M. Covalent Functionalization of Monolayered Transition Metal Dichalcogenides by Phase Engineering. Nat. Chem. 2015, 7 (1), 45–49; https://doi.org/10.1038/nchem.2108.Search in Google Scholar PubMed
112. Abo-Hamad, A.; Hayyan, M.; AlSaadi, M. A.; Mirghani, M. E.; Hashim, M. A. Functionalization of Carbon Nanotubes Using Eutectic Mixtures: A Promising Route for Enhanced Aqueous Dispersibility and Electrochemical Activity. Chem. Eng. J. 2017, 311, 326–339; https://doi.org/10.1016/j.cej.2016.11.108.Search in Google Scholar
113. Rajaura, R. S.; Srivastava, S.; Sharma, P. K.; Mathur, S.; Shrivastava, R.; Sharma, S.; Vijay, Y. Structural and Surface Modification of Carbon Nanotubes for Enhanced Hydrogen Storage Density. Nano-Struct. Nano-Objects 2018, 14, 57–65; https://doi.org/10.1016/j.nanoso.2018.01.005.Search in Google Scholar
114. Zhang, W.; Zhang, Z.; Zhang, Y. The Application of Carbon Nanotubes in Target Drug Delivery Systems for Cancer Therapies. Nanoscale Res. Lett. 2011, 6, 1–22; https://doi.org/10.1186/1556-276x-6-555.Search in Google Scholar
115. Sarojini, S.; Rajasekar, S.; Kumaravelou, K. Carbon Nanotubes, A New Weapon in Health Care Treatment. Int. J. Pharm. Biol. Sci. 2010, 1 (4), 644–649.Search in Google Scholar
116. Reddy, A. L. M.; Rajalakshmi, N.; Ramaprabhu, S. Cobalt-Polypyrrole-Multiwalled Carbon Nanotube Catalysts for Hydrogen and Alcohol Fuel Cells. Carbon 2008, 46 (1), 2–11; https://doi.org/10.1016/j.carbon.2007.10.021.Search in Google Scholar
117. Foldvari, M.; Bagonluri, M. Carbon Nanotubes as Functional Excipients for Nanomedicines: II. Drug Delivery and Biocompatibility Issues. Nanomed. Nanotechnol. Biol. Med. 2008, 4 (3), 183–200; https://doi.org/10.1016/j.nano.2008.04.003.Search in Google Scholar PubMed
118. Pathak, R.; Punetha, V. D.; Bhatt, S.; Punetha, M. Carbon Nanotube-Based Biocompatible Polymer Nanocomposites as an Emerging Tool for Biomedical Applications. Eur. Polym. J. 2023, 112257; https://doi.org/10.1016/j.eurpolymj.2023.112257.Search in Google Scholar
119. Alosime, E. M. A Review on Surface Functionalization of Carbon Nanotubes: Methods and Applications. Discover Nano 2023, 18 (1), 12; https://doi.org/10.1186/s11671-023-03789-6.Search in Google Scholar PubMed PubMed Central
120. Munio, A.; Ambolode, I.; Lc, I. Exploring the Functionality of Cellulose Biopolymer as Carbon Nanotube Composite and Heavy Metals Adsorbent Material: Insights from First-Principles Calculations. Bioint. Res. Appl. Chem. 2023, 13.10.33263/BRIAC136.501Search in Google Scholar
121. Kostarelos, K. Rational Design and Engineering of Delivery Systems for Therapeutics: Biomedical Exercises in Colloid and Surface Science. Adv. Colloid Interface Sci. 2003, 106 (1-3), 147–168; https://doi.org/10.1016/s0001-8686(03)00109-x.Search in Google Scholar PubMed
122. Wang, H.; Wang, J.; Deng, X.; Sun, H.; Shi, Z.; Gu, Z.; Liu, Y.; Zhaoc, Y. Biodistribution of Carbon Single-Wall Carbon Nanotubes in Mice. J. Nanosci. Nanotechnol. 2004, 4 (8), 1019–1024; https://doi.org/10.1166/jnn.2004.146.Search in Google Scholar PubMed
123. Nel, A.; Xia, T.; Mädler, L. Toxic Potential of Materials at the Nanolevel. Science 2006, 311 (5761), 622–627; https://doi.org/10.1126/science.1114397.Search in Google Scholar PubMed
124. Singh, R.; Pantarotto, D.; Lacerda, L.; Pastorin, G.; Klumpp, C.; Prato, M.; Bianco, A.; Kostarelos, K. Tissue Biodistribution and Blood Clearance Rates of Intravenously Administered Carbon Nanotube Radiotracers. Proc. Natl. Acad. Sci. USA 2006, 103 (9), 3357–3362; https://doi.org/10.1073/pnas.0509009103.Search in Google Scholar PubMed PubMed Central
125. Mishra, S.; Sundaram, B. Efficacy and Challenges of Carbon Nanotube in Wastewater and Water Treatment. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100764; https://doi.org/10.1016/j.enmm.2022.100764.Search in Google Scholar
126. Ma, J.; Jiang, Z.; Cao, J.; Yu, F. Enhanced Adsorption for the Removal of Antibiotics by Carbon Nanotubes/Graphene Oxide/Sodium Alginate Triple-Network Nanocomposite Hydrogels in Aqueous Solutions. Chemosphere 2020, 242, 125188; https://doi.org/10.1016/j.chemosphere.2019.125188.Search in Google Scholar PubMed
127. Lung, I.; Soran, M. L.; Stegarescu, A.; Opriş, O. Application of CNT-COOH/MnO2/Fe3O4 Nanocomposite for the Removal of Cymoxanil from Aqueous Solution: Isotherm and Kinetic Studies. Anal. Lett. 2023, 56 (2), 216–230; https://doi.org/10.1080/00032719.2022.2043888.Search in Google Scholar
128. de Azevedo, C. F.; Machado, F. M.; de Souza, N. F.; Silveira, L. L.; Lima, E. C.; Andreazza, R.; Bergamnn, C. P. Comprehensive Adsorption and Spectroscopic Studies on the Interaction of Carbon Nanotubes with Diclofenac Anti-inflammatory. Chem. Eng. J. 2023, 454, 140102; https://doi.org/10.1016/j.cej.2022.140102.Search in Google Scholar
129. Oliveira, M. G.; Spaolonzi, M. P.; Duarte, E. D.; Costa, H. P.; da Silva, M. G.; Vieira, M. G. Adsorption Kinetics of Ciprofloxacin and Ofloxacin by Green-Modified Carbon Nanotubes. Environ. Res. 2023, 233, 116503; https://doi.org/10.1016/j.envres.2023.116503.Search in Google Scholar PubMed
130. Zhang, X.; Gao, C.; Wang, R.; Han, R. Remediation of Ibuprofen and Naproxen in Water by a Green Composite Material of Magnetic Carbon Nanotube–Metal–Organic Framework. J. Environ. Chem. Eng. 2023, 11 (5), 111090; https://doi.org/10.1016/j.jece.2023.111090.Search in Google Scholar
131. Barman, S.; Basu, S. Complete Removal of Endocrine Disrupting Compound and Toxic Dye by Visible Light Active Porous g-C3N4/H-ZSM-5 Nanocomposite. Chemosphere 2020, 241, 124981; https://doi.org/10.1016/j.chemosphere.2019.124981.Search in Google Scholar PubMed
132. Cho, H.-H.; Huang, H.; Schwab, K. Effects of Solution Chemistry on the Adsorption of Ibuprofen and Triclosan onto Carbon Nanotubes. Langmuir 2011, 27 (21), 12960–12967; https://doi.org/10.1021/la202459g.Search in Google Scholar PubMed
133. Duan, P.; Chen, D.; Hu, X. Tin Dioxide Decorated on Ni-Encapsulated Nitrogen-Doped Carbon Nanotubes for Anodic Electrolysis and Persulfate Activation to Degrade Cephalexin: Mineralization and Degradation Pathway. Chemosphere 2021, 269, 128740; https://doi.org/10.1016/j.chemosphere.2020.128740.Search in Google Scholar PubMed
134. Xia, M.; Li, A.; Zhu, Z.; Zhou, Q.; Yang, W. Factors Influencing Antibiotics Adsorption onto Engineered Adsorbents. J. Environ. Sci. 2013, 25 (7), 1291–1299; https://doi.org/10.1016/s1001-0742(12)60215-0.Search in Google Scholar PubMed
135. Joseph, L.; Zaib, Q.; Khan, I. A.; Berge, N. D.; Park, Y. G.; Saleh, N. B.; Yoon, Y. Removal of Bisphenol A and 17α-Ethinyl Estradiol from Landfill Leachate Using Single-Walled Carbon Nanotubes. Water Res. 2011, 45 (13), 4056–4068; https://doi.org/10.1016/j.watres.2011.05.015.Search in Google Scholar PubMed
136. Laddha, U. Carbon Nanotubes: A Novel Approach in Management of Fatal Disease Cancer. In Carbon Nanotubes for Biomedical Applications and Healthcare; Apple Academic Press, 2024; pp 115–130.10.1201/9781003396390-9Search in Google Scholar
137. Ang, M. C.-Y.; Saju, J. M.; Porter, T. K.; Mohaideen, S.; Sarangapani, S.; Khong, D. T.; Wang, S.; Cui, J.; Loh, S. I.; Singh, G. P.; Chua, N. H.; Strano, M. S.; Sarojam, R. Decoding Early Stress Signaling Waves in Living Plants Using Nanosensor Multiplexing. Nat. Commun. 2024, 15 (1), 2943; https://doi.org/10.1038/s41467-024-47082-1.Search in Google Scholar PubMed PubMed Central
138. Singla, P.; Yadav, S.; Goel, N.; Singhal, S. Morphologically Different Boron Nitride Nanomaterials as Efficient Antibiotic Carriers: Adsorption Isotherm and Kinetics Appraisal. Anal. Chem. Lett. 2018, 8 (2), 163–176; https://doi.org/10.1080/22297928.2017.1423244.Search in Google Scholar
139. Gadore, V.; Mishra, S. R.; Singh, A. K.; Ahmaruzzaman, M. Advances in Boron Nitride-Based Nanomaterials for Environmental Remediation and Water Splitting: A Review. RSC Adv. 2024, 14 (5), 3447–3472; https://doi.org/10.1039/d3ra08323c.Search in Google Scholar PubMed PubMed Central
140. Lu, Y.; Jiang, M.; Wang, C.; Wang, Y.; Yang, W. Effects of Matrix and Functional Groups on Tylosin Adsorption onto Resins and Carbon Nanotubes. Water, Air, Soil Pollut. 2013, 224, 1–12; https://doi.org/10.1007/s11270-013-1536-y.Search in Google Scholar
141. Cai, N.; Larese-Casanova, P. Application of Positively-Charged Ethylenediamine-Functionalized Graphene for the Sorption of Anionic Organic Contaminants from Water. J. Environ. Chem. Eng. 2016, 4 (3), 2941–2951; https://doi.org/10.1016/j.jece.2016.06.004.Search in Google Scholar
142. Lerman, I.; Chen, Y.; Chefetz, B. Adsorption of Contaminants of Emerging Concern by Carbon Nanotubes: Influence of Dissolved Organic Matter. In Functions of Natural Organic Matter in Changing Environment; Springer, 2013.10.1007/978-94-007-5634-2_138Search in Google Scholar
143. Barik, D.: A Tool for Functionalization. In Click Chemistry; 2024, CRC Press; pp 123–150.10.1201/9781003403340-7Search in Google Scholar
144. Zaib, Q.; Khan, I. A.; Saleh, N. B.; Flora, J. R. V.; Park, Y. G.; Yoon, Y. Removal of Bisphenol A and 17β-Estradiol by Single-Walled Carbon Nanotubes in Aqueous Solution: Adsorption and Molecular Modeling. Water, Air, Soil Pollut. 2012, 223, 3281–3293; https://doi.org/10.1007/s11270-012-1109-5.Search in Google Scholar
145. Wei, J.; Sun, W.; Pan, W.; Yu, X.; Sun, G.; Jiang, H. Comparing the Effects of Different Oxygen-Containing Functional Groups on Sulfonamides Adsorption by Carbon Nanotubes: Experiments and Theoretical Calculation. Chem. Eng. J. 2017, 312, 167–179; https://doi.org/10.1016/j.cej.2016.11.133.Search in Google Scholar
146. Oladipo, A. A. Carbon Materials as Adsorbents and Catalysts. In Advanced Materials for Pharmaceutical Wastewater Treatment; CRC Press, 2024; pp 117–146.10.1201/9781003340164-6Search in Google Scholar
147. Lung, I.; Soran, M. L.; Stegarescu, A.; Opris, O.; Gutoiu, S.; Leostean, C.; Lazar, M. D.; Kacso, I.; Silipas, T. D.; Porav, A. S. Evaluation of CNT-COOH/MnO2/Fe3O4 Nanocomposite for Ibuprofen and Paracetamol Removal from Aqueous Solutions. J. Hazard Mater. 2021, 403, 123528; https://doi.org/10.1016/j.jhazmat.2020.123528.Search in Google Scholar PubMed
148. Carrales-Alvarado, D. H.; Leyva-Ramos, R.; Rodríguez-Ramos, I.; Mendoza-Mendoza, E.; Moral-Rodríguez, A. E. Adsorption Capacity of Different Types of Carbon Nanotubes towards Metronidazole and Dimetridazole Antibiotics from Aqueous Solutions: Effect of Morphology and Surface Chemistry. Environ. Sci. Pollut. Control Ser. 2020, 27, 17123–17137; https://doi.org/10.1007/s11356-020-08110-x.Search in Google Scholar PubMed
149. El-Sheikh, A. H.; Qawariq, R. F.; Abdelghani, J. I. Adsorption and Magnetic Solid-phase Extraction of NSAIDs from Pharmaceutical Wastewater Using Magnetic Carbon Nanotubes: Effect of Sorbent Dimensions, Magnetite Loading and Competitive Adsorption Study. Environ. Technol. Innovat. 2019, 16, 100496; https://doi.org/10.1016/j.eti.2019.100496.Search in Google Scholar
150. Oyetade, O. A.; Martincigh, B. S.; Skelton, A. A. Interplay Between Electrostatic and Hydrophobic Interactions in the pH-dependent Adsorption of Ibuprofen onto Acid-Functionalized Multiwalled Carbon Nanotubes. J. Phys. Chem. C 2018, 122 (39), 22556–22568; https://doi.org/10.1021/acs.jpcc.8b06841.Search in Google Scholar
151. Avcı, A.; İnci, İ.; Baylan, N. Adsorption of Ciprofloxacin Hydrochloride on Multiwall Carbon Nanotube. J. Mol. Struct. 2020, 1206, 127711; https://doi.org/10.1016/j.molstruc.2020.127711.Search in Google Scholar
152. Hanbali, G.; Jodeh, S.; Hamed, O.; Bol, R.; Khalaf, B.; Qdemat, A.; Samhan, S. Enhanced Ibuprofen Adsorption and Desorption on Synthesized Functionalized Magnetic Multiwall Carbon Nanotubes from Aqueous Solution. Materials 2020, 13 (15), 3329; https://doi.org/10.3390/ma13153329.Search in Google Scholar PubMed PubMed Central
153. Elamin, M. R.; Abdulkhair, B. Y.; Elzupir, A. O. Insight to Aspirin Sorption Behavior on Carbon Nanotubes from Aqueous Solution: Thermodynamics, Kinetics, Influence of Functionalization and Solution Parameters. Sci. Rep. 2019, 9 (1), 12795; https://doi.org/10.1038/s41598-019-49331-6.Search in Google Scholar PubMed PubMed Central
154. Chen, Z.; Han, R.; Zhong, C. Adsorption of Fluoroquinolone by Carbon Nanotubes: A Combined Experimental and Density Functional Theory Study. Chem. Pap. 2020, 74 (11), 3847–3856; https://doi.org/10.1007/s11696-020-01204-3.Search in Google Scholar
155. Zhuang, S.; Zhu, X.; Wang, J. Adsorptive Removal of Plasticizer (Dimethyl Phthalate) and Antibiotic (Sulfamethazine) from Municipal Wastewater by Magnetic Carbon Nanotubes. J. Mol. Liq. 2020, 319, 114267; https://doi.org/10.1016/j.molliq.2020.114267.Search in Google Scholar
156. Dutra, F. V. A.; Pires, B. C.; Nascimento, T. A.; Borges, K. B. Functional Polyaniline/Multiwalled Carbon Nanotube Composite as an Efficient Adsorbent Material for Removing Pharmaceuticals from Aqueous Media. J. Environ. Manag. 2018, 221, 28–37; https://doi.org/10.1016/j.jenvman.2018.05.051.Search in Google Scholar PubMed
157. Zhao, H.; Liu, X.; Cao, Z.; Zhan, Y.; Shi, X.; Yang, Y.; Zhou, J.; Xu, J. Adsorption Behavior and Mechanism of Chloramphenicols, Sulfonamides, and Non-Antibiotic Pharmaceuticals on Multi-Walled Carbon Nanotubes. J. Hazard Mater. 2016, 310, 235–245; https://doi.org/10.1016/j.jhazmat.2016.02.045.Search in Google Scholar PubMed
158. Wang, Y.; Wei, X.; Zhang, R.; Wu, Y.; Farid, M. U.; Huang, H. Comparison of Chemical, Ultrasonic and Thermal Regeneration of Carbon Nanotubes for Acetaminophen, Ibuprofen, and Triclosan Adsorption. RSC Adv. 2017, 7 (83), 52719–52728; https://doi.org/10.1039/c7ra08812d.Search in Google Scholar
159. Ruziwa, D. T.; Oluwalana, A. E.; Mupa, M.; Meili, L.; Selvasembian, R.; Nindi, M. M.; Sillanpaa, M.; Gwenzi, W.; Chaukura, N. Pharmaceuticals in Wastewater and Their Photocatalytic Degradation Using Nano-Enabled Photocatalysts. J. Water Proc. Eng. 2023, 54, 103880; https://doi.org/10.1016/j.jwpe.2023.103880.Search in Google Scholar
160. Üstün Odabaşi, S.; Boudraà, İ.; Aydin, R.; Büyükgüngör, H. Photocatalytic Removal of Pharmaceuticals by Immobilization of TiO2 on Activated Carbon by LC–MS/MS Monitoring. Water, Air, Soil Pollut. 2022, 233 (4), 111; https://doi.org/10.1007/s11270-022-05579-9.Search in Google Scholar
161. Ashraf, M.; Ahammad, S. Z.; Chakma, S. Advancements in the Dominion of Fate and Transport of Pharmaceuticals and Personal Care Products in the Environment – A Bibliometric Study. Environ. Sci. Pollut. Control Ser. 2023, 30 (23), 64313–64341; https://doi.org/10.1007/s11356-023-26796-7.Search in Google Scholar PubMed PubMed Central
162. Valadez-Renteria, E.; Perez-Gonzalez, R.; Gomez-Solis, C.; Diaz-Torres, L. A.; Encinas, A.; Oliva, J.; Rodriguez-Gonzalez, V. A Novel and Stretchable Carbon-nanotube/Ni@TiO2:W Photocatalytic Composite for the Complete Removal of Diclofenac Drug from the Drinking Water. J. Environ. Sci. 2023, 126, 575–589; https://doi.org/10.1016/j.jes.2022.05.028.Search in Google Scholar PubMed
163. Zhang, Y.; Chu, W. Cooperation of Multi-Walled Carbon Nanotubes and Cobalt Doped TiO2 to Activate Peroxymonosulfate for Antipyrine Photocatalytic Degradation. Sep. Purif. Technol. 2022, 282, 119996; https://doi.org/10.1016/j.seppur.2021.119996.Search in Google Scholar
164. Tian, N.; Giannakis, S.; Akbarzadeh, L.; Hasanvandian, F.; Dehghanifard, E.; Kakavandi, B. Improved Catalytic Performance of ZnO via Coupling with CoFe2O4 and Carbon Nanotubes: A New, Photocatalysis-Mediated Peroxymonosulfate Activation System, Applied towards Cefixime Degradation. J. Environ. Manag. 2023, 329, 117022; https://doi.org/10.1016/j.jenvman.2022.117022.Search in Google Scholar PubMed
165. Isari, A. A.; Mehregan, M.; Mehregan, S.; Hayati, F.; Rezaei Kalantary, R.; Kakavandi, B. Sono-Photocatalytic Degradation of Tetracycline and Pharmaceutical Wastewater Using WO3/CNT Heterojunction Nanocomposite under US and Visible Light Irradiations: A Novel Hybrid System. J. Hazard Mater. 2020, 390, 122050; https://doi.org/10.1016/j.jhazmat.2020.122050.Search in Google Scholar PubMed
166. Fu, Y.; Zhang, Y.; Xie, X.; Wang, H.; Wei, L.; Ma, M.; Yan, Q. Functionalized Carbon Nanotube Bridge Interface Drove Bi2O2CO3/g-C3N4 S-Scheme Heterojunction with Enhanced Visible-Light Photocatalytic Activity. Sep. Purif. Technol. 2021, 274, 119032; https://doi.org/10.1016/j.seppur.2021.119032.Search in Google Scholar
167. Liu, J.; Song, Y.; Xu, H.; Zhu, X.; Lian, J.; Xu, Y.; Zhao, Y.; Huang, L.; Ji, H.; Li, H. Non-metal Photocatalyst Nitrogen-Doped Carbon Nanotubes Modified Mpg-C3N4: Facile Synthesis and the Enhanced Visible-Light Photocatalytic Activity. J. Colloid Interface Sci. 2017, 494, 38–46; https://doi.org/10.1016/j.jcis.2017.01.010.Search in Google Scholar PubMed
168. Ghasemipour, P.; Fattahi, M.; Rasekh, B.; Yazdian, F. Developing the Ternary ZnO Doped MoS2 Nanostructures Grafted on CNT and Reduced Graphene Oxide (RGO) for Photocatalytic Degradation of Aniline. Sci. Rep. 2020, 10 (1), 4414; https://doi.org/10.1038/s41598-020-61367-7.Search in Google Scholar PubMed PubMed Central
169. Al-Musawi, T. J.; Rajiv, P.; Mengelizadeh, N.; Sadat Arghavan, F.; Balarak, D. Photocatalytic Efficiency of CuNiFe2O4 Nanoparticles Loaded on Multi-Walled Carbon Nanotubes as a Novel Photocatalyst for Ampicillin Degradation. J. Mol. Liq. 2021, 337, 116470; https://doi.org/10.1016/j.molliq.2021.116470.Search in Google Scholar
170. Wang, Y.; Jiang, F.; Chen, J.; Sun, X.; Xian, T.; Yang, H. In Situ Construction of CNT/CuS Hybrids and Their Application in Photodegradation for Removing Organic Dyes. Nanomaterials 2020, 10 (1), 178; https://doi.org/10.3390/nano10010178.Search in Google Scholar PubMed PubMed Central
171. Xu, Y.; Liu, J.; Xie, M.; Jing, L.; Xu, H.; She, X.; Li, H.; Xie, J. Construction of Novel CNT/LaVO4 Nanostructures for Efficient Antibiotic Photodegradation. Chem. Eng. J. 2019, 357, 487–497; https://doi.org/10.1016/j.cej.2018.09.098.Search in Google Scholar
172. Lin, Y.; Wu, S.; Yang, C.; Chen, M.; Li, X. Preparation of Size-Controlled Silver Phosphate Catalysts and Their Enhanced Photocatalysis Performance via Synergetic Effect with MWCNTs and PANI. Appl. Catal. B Environ. 2019, 245, 71–86; https://doi.org/10.1016/j.apcatb.2018.12.048.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Advancements in CNT-based materials for optimized pharmaceutical removal via adsorption and photocatalysis
- Review on removal of heavy metals from industrial effluents by adsorption
- Cutting-edge techniques in low-temperature electrochemical water splitting: advancements in hydrogen production
- Biological activities of metal complexes with Schiff base
- Potential of organometallic complexes in medicinal chemistry
- Advancement in schiff base complexes for treatment of colon cancer
- Magnetic nanoparticles for efficient heavy metal removal: synthesis, adsorption capacity, and key experimental parameters
- Coal-based carbon/graphene quantum dots: formation mechanisms and applications
- Advancements in transition metal-catalyzed 1,2,3-triazole synthesis via azide–alkyne cycloaddition
- Gold complexes: a new frontier in the battle against lung cancer
Articles in the same Issue
- Frontmatter
- Advancements in CNT-based materials for optimized pharmaceutical removal via adsorption and photocatalysis
- Review on removal of heavy metals from industrial effluents by adsorption
- Cutting-edge techniques in low-temperature electrochemical water splitting: advancements in hydrogen production
- Biological activities of metal complexes with Schiff base
- Potential of organometallic complexes in medicinal chemistry
- Advancement in schiff base complexes for treatment of colon cancer
- Magnetic nanoparticles for efficient heavy metal removal: synthesis, adsorption capacity, and key experimental parameters
- Coal-based carbon/graphene quantum dots: formation mechanisms and applications
- Advancements in transition metal-catalyzed 1,2,3-triazole synthesis via azide–alkyne cycloaddition
- Gold complexes: a new frontier in the battle against lung cancer


![Figure 6:
Covalently functionalized (a) [Fe/Mn(THPP)@MWCNT],
112
and (b) carbon nanotubes.
113](/document/doi/10.1515/revic-2024-0060/asset/graphic/j_revic-2024-0060_fig_006.jpg)
