Exploring inorganic phosphors: basics, types, fabrications and their luminescence properties for LED/WLED/displays
-
Junaid Ur Rahman
, Shahab Khan
, Vicky Jain
Abstract
The utilization of phosphors in lighting and display applications has garnered significant attention due to their unique luminescent properties and versatile crystal structures. This review article comprehensively examines recent advances in the synthesis, characterization, and applications of nitride and sulfide phosphors. This article addresses various phosphor crystal structures, including perovskite, garnet, nitride sulfide, fabrications strategies, and their impact on the optical and electronic properties. Furthermore, the review highlights the role of doping and activator ions in tailoring the emission characteristics of nitride and sulfide phosphors, enabling precise control over color rendering and efficiency. Additionally, the article also discusses emerging trends in phosphor technology, such as the development of novel synthesis methods and the integration of phosphors into next-generation lighting and display devices. The basic properties of phosphor materials like CRI, CIE chromaticity coordinates, quantum efficiencies are well discussed. Overall, this article provides valuable insights into the current state of research and future directions in the field of phosphors offering potential avenues for further advancements in lighting and display technologies.
1 Introduction
In recent years, the advancement of lighting technology has undergone a transformative journey, propelled by the discovery and utilization of inorganic phosphors. These remarkable compounds, with their inherent luminescent properties, have emerged as pivotal components in the development of Light light-emitting diodes (LEDs) and White Light light-emitting diodes (WLEDs), revolutionizing the landscape of illumination across various domains. 1 This review explores into the intricate realm of inorganic phosphors, unraveling their fundamental characteristics, luminescence mechanisms, and diverse applications within the LED and WLED domains. With a keen focus on the synthesis methodologies, structural configurations, and doping strategies, this exploration aims to provide a comprehensive understanding of the underlying principles governing the luminescent behavior of inorganic phosphors. Moreover, this review endeavors to elucidate the pivotal role played by inorganic phosphors in enhancing the efficiency, color rendering, and spectral properties of LED and WLED devices. Through a meticulous examination of their applications in various fields including solid-state lighting, displays, optoelectronics, and beyond, this article seeks to underscore the multifaceted utility and promising prospects of inorganic phosphors in modern illumination technologies. By synthesizing the latest advancements and key insights from the realm of inorganic phosphors, this review aspires to furnish researchers, engineers, and enthusiasts alike with a comprehensive resource that illuminates the fascinating interplay between materials science, luminescence phenomena, and the burgeoning landscape of LED and WLED applications. 2
The quest in recent decades for more energy-efficient, environmentally sustainable, and aesthetically pleasing lighting solutions has led to significant advancements in the field of illumination technology. Among the myriad innovations driving this evolution, the utilization of inorganic phosphors stands out as a pivotal milestone. These compounds, with their remarkable luminescent properties, have revolutionized the landscape of lighting, particularly in the development of Light Emitting Diodes (LEDs) and White Light Emitting Diodes (WLEDs). 3 This review embarks on an illuminating journey through the realm of inorganic phosphors, delving into their fundamental characteristics, luminescence mechanisms, synthesis methodologies, structural configurations, doping strategies, and diverse applications within the LED and WLED domains. The genesis of inorganic phosphors can be traced back to the early 20th century, with pioneering research laying the groundwork for their synthesis and characterization. Initially employed in cathode-ray tube displays and fluorescent lamps, the potential of inorganic phosphors remained largely untapped until the advent of solid-state lighting technologies. The emergence of LEDs as a viable alternative to traditional incandescent and fluorescent lighting systems catalyzed a resurgence of interest in inorganic phosphors, owing to their ability to efficiently convert blue or ultraviolet light into various colors across the visible spectrum. 2
At the heart of inorganic phosphors’ luminescent prowess lies a complex interplay of electronic transitions, energy transfer mechanisms, and crystal field effects. Understanding the intricacies of these phenomena is essential for tailoring the optical properties of phosphor materials to meet the diverse requirements of modern lighting applications. From the dopant ions responsible for imparting specific emission wavelengths to the host lattices that dictate the efficiency and stability of luminescence, every aspect of phosphor design is meticulously engineered to achieve optimal performance. Synthesizing inorganic phosphors encompasses a diverse array of techniques, ranging from traditional solid-state reactions and sol–gel methods to more advanced approaches such as hydrothermal synthesis and microwave-assisted processing. Each method offers unique advantages in terms of scalability, controllability, and purity, thereby catering to the varied needs of phosphor fabrication across industrial and research settings. Moreover, the choice of precursor materials, reaction conditions, and post-synthesis treatments profoundly influence the crystallinity, morphology, and luminescent properties of the resulting phosphor particles. 4
Structurally, inorganic phosphors exhibit a rich diversity of crystal symmetries, including but not limited to perovskites, garnets, oxides, sulfides, and nitrides. The intricate arrangement of atoms within these crystalline frameworks governs the electronic band structure, defect distribution, and phonon dynamics, all of which exert a profound influence on the luminescence behavior of phosphor materials. Tailoring the composition, stoichiometry, and crystallographic orientation of these host lattices offers a versatile toolkit for modulating the emission spectra, quantum efficiency, and thermal stability of inorganic phosphors. Doping represents a key strategy for enhancing the luminescent properties of inorganic phosphors, wherein controlled incorporation of dopant ions imparts unique optical characteristics to the host material. Transition metal ions, rare earth elements, and lanthanide series elements are commonly employed as dopants, owing to their ability to undergo radiative transitions across a broad range of energy levels. By judiciously selecting dopant species, concentration levels, and local coordination environments, researchers can engineer phosphor materials with tailored emission colors, lifetimes, and quantum yields, thereby expanding the design space for next-generation LED and WLED devices.
The applications of inorganic phosphors span a myriad of domains, encompassing solid-state lighting, displays, optoelectronics, sensors, biomedical imaging, and beyond. In the realm of solid-state lighting, phosphor-converted LEDs have emerged as the cornerstone of energy-efficient illumination, offering superior color rendering, spectral tunability, and longevity compared to conventional light sources. Moreover, the advent of WLEDs, which combine blue LEDs with yellow phosphors or multi-component phosphor blends, has further broadened the scope of phosphor-based lighting solutions, enabling the replication of natural daylight and customizable color temperatures for diverse indoor and outdoor environments. Beyond lighting, inorganic phosphors find applications in display technologies, where they serve as the emissive components in liquid crystal displays (LCDs), organic light-emitting diodes (OLEDs), and quantum dot displays. The ability of phosphor materials to emit light with high color purity, stability, and efficiency makes them indispensable for achieving vibrant, lifelike imagery in televisions, smartphones, tablets, and other electronic devices. Additionally, phosphor-based sensors play a vital role in environmental monitoring, industrial process control, and biomedical diagnostics, leveraging the sensitivity of luminescence signals to detect and quantify analytes with high accuracy and precision. 5
Looking ahead, the future of inorganic phosphors holds immense promise, fueled by ongoing research efforts aimed at enhancing their performance, versatility, and sustainability. Novel synthesis techniques, such as sonochemical synthesis, aerosol-assisted methods, and template-directed assembly, are opening new avenues for fabricating phosphor materials with tailored nanostructures, hierarchical morphologies, and enhanced optical properties. Furthermore, the integration of inorganic phosphors with emerging technologies such as quantum dots, perovskite nanocrystals, 6 and two-dimensional materials promises to unlock unprecedented opportunities for advancing the frontier of solid-state lighting, display technologies, and beyond. 7 In conclusion, the remarkable journey of inorganic phosphors from fundamental research to practical applications underscores their indispensable role in shaping the future of illumination. As we continue to unravel the mysteries of luminescence phenomena and push the boundaries of materials science, the transformative potential of phosphor-based technologies in enhancing energy efficiency, visual comfort, and environmental sustainability remains as bright as the light they emit. Through collaborative interdisciplinary efforts spanning chemistry, physics, engineering, and beyond, we can harness the full spectrum of inorganic phosphors’ capabilities to illuminate the world with brilliance and beauty.
1.1 Inorganic phosphors
Inorganic phosphors have emerged as pivotal materials in modern lighting and display technologies due to their unique luminescent properties. These materials, typically composed of metal cations and anions, exhibit luminescence when excited by external stimuli, such as light or electricity. In Table 1 some examples of Me3Ln(PO4)3 types phosphors are listed with their properties. The luminescent behavior of inorganic phosphors arises from electronic transitions within the crystal lattice, which result in the emission of photons at specific wavelengths. This chapter provides an in-depth exploration of the synthesis, structure, and luminescence properties of inorganic phosphor materials, highlighting their importance in various applications, including light-emitting diodes (LEDs), displays, and sensors.
Examples of some common Me3Ln(PO4)3 type Inorganic phosphors, their constituents and properties.
| General | Me | Ln | Doped/codoped activator ions | Resultant | Properties | Ref |
|---|---|---|---|---|---|---|
| Me 3 Ln(PO 4 ) 3 | Ba | Y | Sm+3 | Ba3Y(PO4)3:Sm3+ | Photoluminescence | 8 |
| Sr | Y | Dy+3 | Sr3Y(PO4)3:Dy3+ | Photoluminescence | 9 | |
| Sr | Gd | Tb+3 | Sr3Bi(PO4)3:Tb3+ | Photoluminescence | 10 | |
| Sr | Gd | Eu2+/Mn2+ | Sr3Gd(PO4)3:Eu2+/Mn2+ | Energy transfer between the codoped activator ions | 11 | |
| Sr | Lu | Eu2+/Mn2+ | Sr3Lu(PO4)3:Eu2+/Mn2+ | 12 | ||
| Sr | La | Sm3+/Eu3+ | Sr3La(PO4)3:Sm3+/Eu3+ | 13 | ||
| Ba | La | Eu3+/Tb3+ | Ba3La(PO4)3:Eu3+/Tb3+ | 14 | ||
| Ba | Lu | Ce3+/Tb3+ | Ba3Lu(PO4)3:Ce3+/Tb3+ | 15 | ||
| Ba | La | Eu3+ | Ba3La(PO4)3 | High thermal stabilities | 16 | |
| Sr | La | Eu3+ | Sr3La(PO4)3 | 17 | ||
| Ba | Lu | Eu3+ | Ba3Lu(PO4)3 | 18 | ||
| Ba | Y | Sm+3 | Ba3Y(PO4)3 | 8 | ||
| Sr | Gd | Dy+3 | Sr3Gd(PO4)3 | 19 | ||
| Sr | La | Eu2+/Tb3+ | Sr3La(PO4)3 | 20 | ||
| Sr | Bi | Eu2+/Tb3+ | Sr3Bi(PO4)3 | 21 | ||
| Sr | La | Eu2+/Tb3+ | Sr3La(PO4)3 | 22 , 23 |
2 Synthesis of inorganic phosphors
The synthesis of inorganic phosphor materials encompasses a wide range of techniques, each offering unique advantages in terms of control over particle size, morphology, and composition. Traditional solid-state reactions involve the direct thermal decomposition of precursor compounds to form crystalline phosphor particles. Sol–gel methods, on the other hand, rely on the hydrolysis and condensation of metal alkoxides to produce phosphor materials with tailored chemical compositions and surface properties. Hydrothermal synthesis, involving the reaction of aqueous precursors under elevated temperature and pressure conditions, enables the fabrication of phosphor nanoparticles with enhanced crystallinity and uniformity. Other techniques, such as coprecipitation, microwave-assisted processing, and flame spray pyrolysis, offer additional avenues for synthesizing inorganic phosphors with diverse structures and properties.
The synthesis of inorganic phosphors encompasses a diverse array of techniques, each offering unique advantages in terms of control over particle size, morphology, crystallinity, and luminescence properties. The choice of synthesis method depends on various factors, including the desired composition, crystal structure, and intended application of the phosphor material. In this section, we will explore some of the commonly employed synthesis techniques for inorganic phosphors. 24 , 25
2.1 Solid-state reaction
Solid-state reaction is one of the most traditional and widely used methods for synthesizing inorganic phosphors. In this method, precursor compounds containing the constituent elements of the phosphor are mixed in stoichiometric ratios and heated at high temperatures (typically above 1,000 °C) in a furnace (Figure 1). The thermal decomposition and chemical reactions between the precursors result in the formation of crystalline phosphor particles. Solid-state reaction offers simplicity, scalability, and versatility, making it suitable for producing a wide range of phosphor materials, including oxides, sulfides, and nitrides. However, it may require long reaction times and high temperatures, leading to the formation of agglomerated particles and phase impurities. 26 Solid-state reaction is a traditional method for synthesizing inorganic phosphors, offering several advantages, along with some disadvantages and challenges.
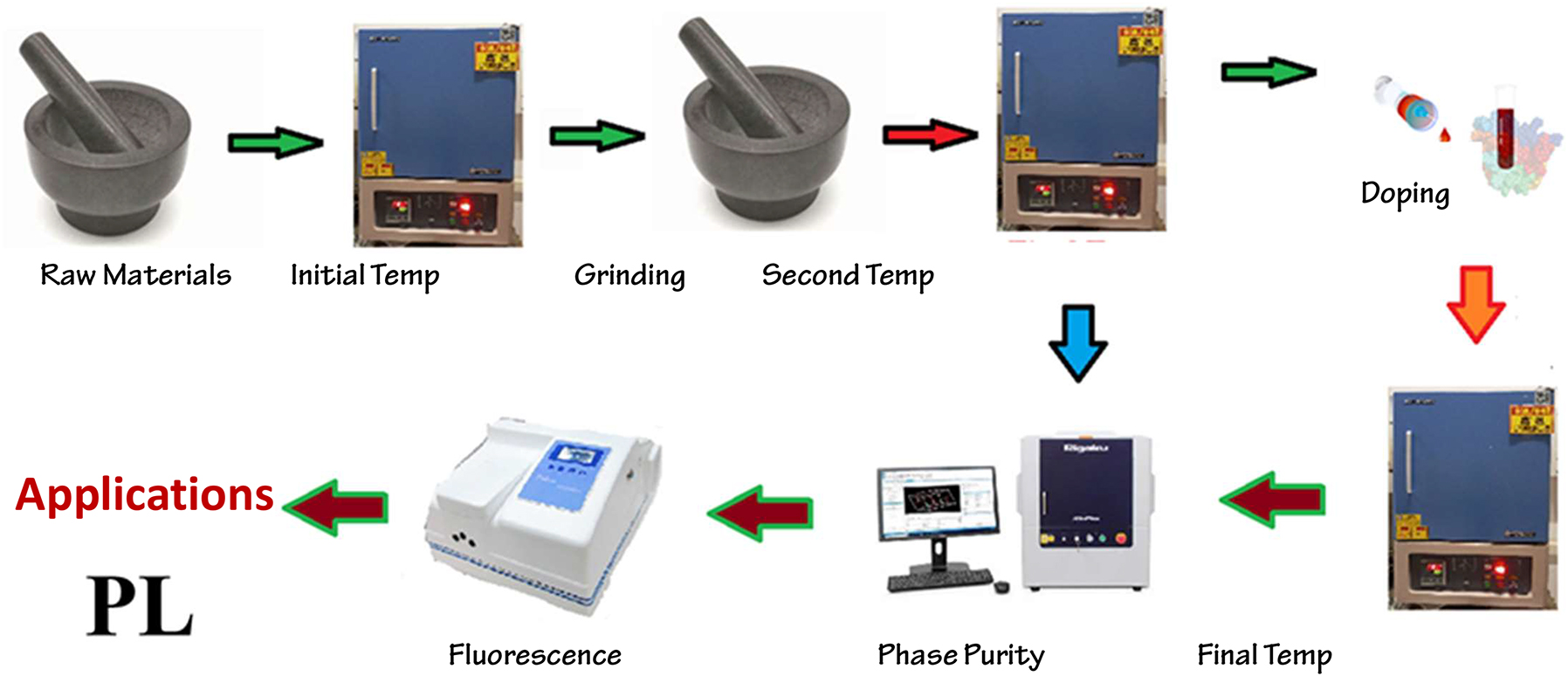
Synthetic route of inorganic phosphor materials via solid state reactions.
Advantages:
Solid-state reaction is a relatively simple and straightforward method that involves mixing precursor compounds and heating them to high temperatures in a furnace. It does not require complex equipment or specialized techniques, making it accessible to researchers and manufacturers.
Solid-state reaction can be used to synthesize a wide range of inorganic phosphors, including oxides, sulfides, and nitrides. It offers versatility in terms of composition, allowing for the incorporation of various dopant ions and modifiers to tailor the properties of the phosphor material.
Solid-state reaction can be easily scaled up for large-scale production, making it suitable for industrial manufacturing processes. The simplicity of the method and the availability of raw materials contribute to its scalability and cost-effectiveness.
Disadvantages:
Solid-state reaction typically requires heating precursor compounds to high temperatures (above 1,000 °C) to initiate the chemical reactions and facilitate the formation of crystalline phosphor particles. These high temperatures can lead to energy consumption, material degradation, and phase impurities.
Solid-state reaction often involves long reaction times to ensure complete conversion of precursor compounds into the desired phosphor phase. Prolonged heating at high temperatures may increase processing time and production costs, particularly for large-scale synthesis.
Solid-state reaction may lack precise control over particle size, morphology, and composition, leading to the formation of agglomerated particles and phase impurities. Achieving uniform and monodisperse phosphor particles may require additional processing steps or optimization of reaction parameters.
Challenges:
One of the major challenges in solid-state reaction is achieving phase-pure phosphor materials without the formation of unwanted phases or impurities. Controlling reaction kinetics, stoichiometry, and processing conditions is crucial for minimizing phase segregation and ensuring the desired crystal structure.
Another challenge is achieving uniform distribution of dopant ions and modifiers within the phosphor lattice. Inhomogeneous distribution can lead to variations in luminescence properties, affecting the performance and reliability of phosphor-based devices.
Improving the energy efficiency of solid-state reaction processes is a key challenge, particularly in light of increasing environmental concerns and energy conservation efforts. Developing alternative synthesis methods or optimizing reaction conditions to reduce energy consumption and minimize waste generation is essential for sustainable phosphor manufacturing.
2.2 Sol–gel method
The sol–gel method involves the hydrolysis and condensation of metal alkoxides or metal salts in a liquid solvent to form a colloidal suspension, or sol. By controlling the reaction conditions, such as pH, temperature, and solvent composition, the sol can be processed further to form a gel or dried to yield a powder. 27 Subsequent calcination of the gel or powder at elevated temperatures results in the formation of crystalline phosphor particles. The sol–gel method offers precise control over the chemical composition, particle size, and morphology of the phosphor material, enabling the synthesis of homogeneous and nanostructured phosphors with enhanced luminescence properties. Moreover, it allows for the incorporation of dopant ions and modifiers to tailor the optical characteristics of the phosphor (Figure 2). The sol–gel method is a versatile technique for synthesizing inorganic phosphors, offering several advantages along with some disadvantages and challenges: 28
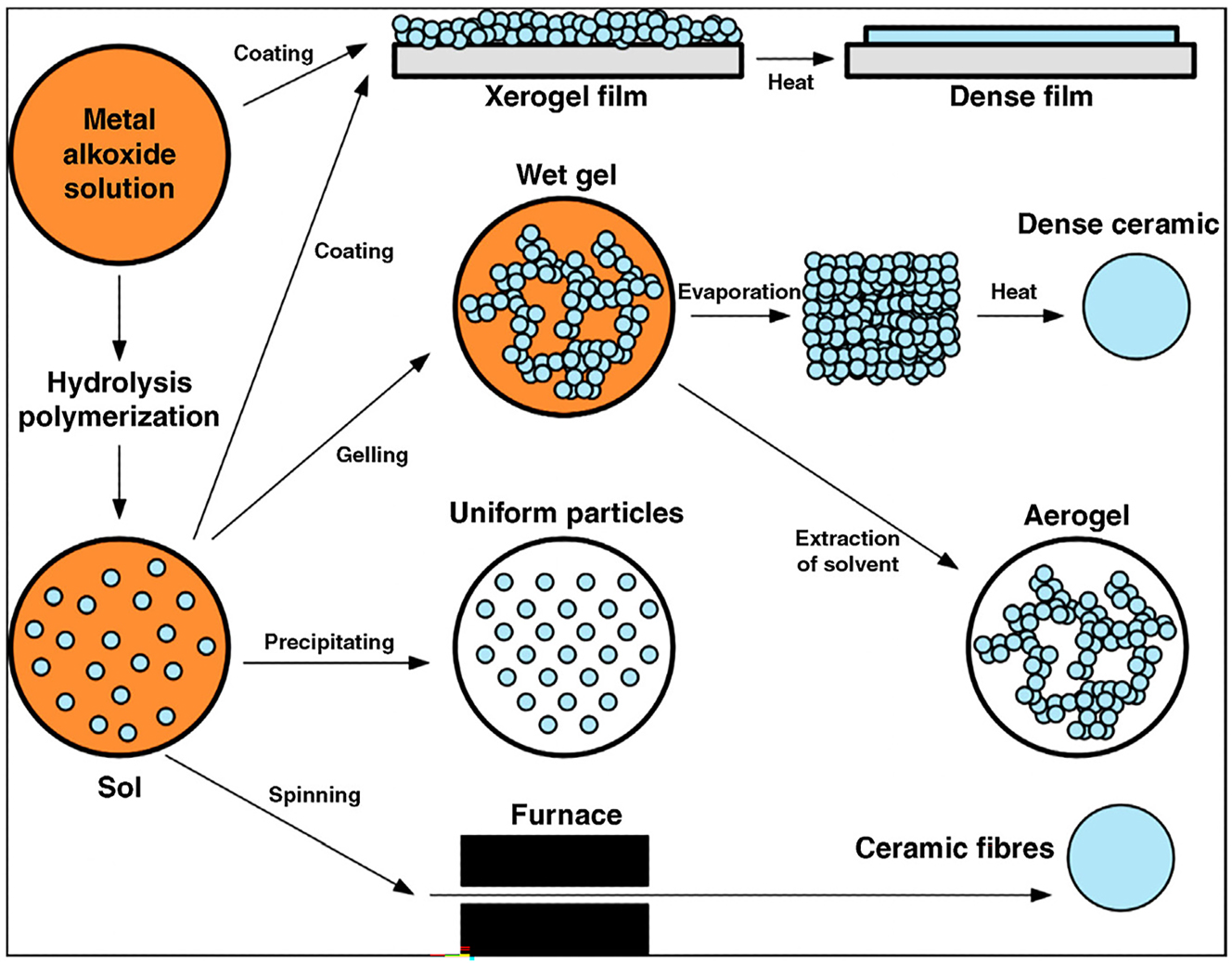
Schematic representation of the different stages and routes of the sol–gel technology. 29
Advantages:
The sol–gel method enables precise control over the composition, particle size, morphology, and crystallinity of the phosphor material. By adjusting parameters such as pH, temperature, and precursor concentrations, researchers can tailor the properties of the phosphor to meet specific requirements.
Sol–gel synthesis results in homogeneous distribution of dopant ions and modifiers within the phosphor lattice, leading to uniform luminescence properties. This ensures consistent performance and reliability of phosphor-based devices, such as LEDs and displays.
The sol–gel method allows for the synthesis of nanostructured phosphors with controlled particle size and surface properties. Nanoscale phosphor particles exhibit unique optical properties, such as enhanced luminescence efficiency and tunable emission spectra, making them suitable for advanced applications in lighting, sensing, and imaging.
Sol–gel synthesis can be used to fabricate a wide range of inorganic phosphors, including oxides, sulfides, and nitrides. It offers versatility in terms of precursor selection and processing conditions, allowing for the incorporation of various dopant ions and modifiers to tailor the optical characteristics of the phosphor material.
Disadvantages:
The sol–gel method involves multiple steps, including hydrolysis, condensation, drying, and calcination, which can make the process complex and time-consuming. Achieving optimal synthesis conditions and controlling reaction kinetics require careful attention to detail and expertise in materials chemistry.
The sol–gel method may involve the use of expensive precursors and reagents, increasing the cost of phosphor synthesis compared to other methods. Additionally, the need for specialized equipment, such as vacuum systems and controlled atmosphere furnaces, can further contribute to the overall cost of production.
Achieving reproducible results with the sol–gel method can be challenging due to variations in reaction kinetics, precursor reactivity, and environmental conditions. Small changes in synthesis parameters can have significant effects on the properties of the resulting phosphor material, requiring meticulous optimization and quality control.
Challenges:
Scaling up the sol–gel synthesis process for large-scale production remains a challenge due to the complexity of the method and the need for precise control over reaction conditions. Developing cost-effective and scalable synthesis strategies while maintaining product quality and consistency is essential for commercialization of sol–gel-derived phosphor materials.
Ensuring the long-term stability and reliability of sol–gel-derived phosphors under harsh operating conditions, such as high temperatures and humidity, poses a challenge. Improving the chemical and thermal stability of phosphor materials through advanced synthesis techniques and surface modification strategies is critical for expanding their practical applications.
Addressing the environmental impact of sol–gel synthesis, including the generation of hazardous waste and the consumption of energy and resources, is a key challenge. Developing greener synthesis protocols and alternative precursors with lower environmental footprint is essential for sustainable phosphor manufacturing.
2.3 Hydrothermal synthesis
Hydrothermal synthesis involves the reaction of precursor compounds in an aqueous solution under elevated temperature and pressure conditions inside a sealed autoclave. The hydrothermal environment promotes the rapid nucleation and growth of crystalline phases, resulting in the formation of highly crystalline and uniformly sized phosphor particles. 30 The controlled release of reactants and the confinement of reaction intermediates within the autoclave facilitate the formation of phase-pure and stoichiometric phosphors with enhanced crystallinity and luminescence properties. Hydrothermal synthesis is particularly well-suited for producing complex and metastable phosphor materials, such as perovskites, garnets, and nanocrystalline structures, with precise control over particle size, shape, and surface properties. Hydrothermal synthesis is a powerful method for synthesizing inorganic phosphors, offering numerous advantages along with some disadvantages and challenges. 31 A.B. Chavan et al. 32 design SrMnO4:Dy3+ phosphor with blue emission via hydrothermal process, where NaOH was used as PH regulator. 32 While X. Yu et al. 33 reported white light emitting phosphor of Dy3+ with Ca3(PO4)2 by this process. It was also concluded that we can synthesize phosphor even at low temperature by hydrothermal process. 33
Advantages:
Hydrothermal synthesis enables controlled nucleation and growth of crystalline phases under high-temperature and high-pressure conditions. This results in the formation of highly crystalline and uniformly sized phosphor particles with precise control over particle size, morphology, and crystal structure.
The hydrothermal environment restricts the access of impurities and contaminants, leading to the formation of phase-pure phosphor materials. This ensures high purity and crystallinity of the synthesized phosphors, minimizing the presence of defects and non-radiative recombination centers that can degrade luminescence properties.
Hydrothermal synthesis allows for the incorporation of dopant ions and modifiers to tailor the optical properties of the phosphor material. By controlling reaction parameters such as temperature, pressure, pH, and precursor concentrations, researchers can fine-tune the emission spectra, quantum efficiency, and thermal stability of the synthesized phosphors to meet specific application requirements. 32
Hydrothermal synthesis can be used to fabricate a wide range of inorganic phosphors, including oxides, sulfides, and nitrides. It offers versatility in terms of precursor selection and reaction conditions, enabling the synthesis of complex and metastable phosphor materials with tailored properties for various applications.
Disadvantages:
Hydrothermal synthesis requires specialized equipment, such as autoclaves capable of withstanding high pressures and temperatures, which can be expensive to purchase and maintain. Additionally, the need for corrosion-resistant materials and safety precautions adds to the overall cost of operation.
Hydrothermal synthesis involves heating precursor solutions to high temperatures under pressure, consuming significant amounts of energy. The high energy requirements make hydrothermal synthesis relatively expensive and may limit its scalability for large-scale production.
Hydrothermal synthesis involves multiple steps, including precursor preparation, reaction setup, and post-synthesis treatments, which can make the process complex and time-consuming. Achieving optimal synthesis conditions and controlling reaction kinetics require expertise in materials chemistry and experimental techniques.
Challenges:
Scaling up hydrothermal synthesis for commercial production remains a challenge due to the complexity of the method and the need for precise control over reaction parameters. Developing cost-effective and scalable synthesis strategies while maintaining product quality and consistency is essential for industrial applications.
Achieving reproducible results with hydrothermal synthesis can be challenging due to variations in reaction conditions, precursor reactivity, and crystal growth kinetics. Optimizing synthesis parameters and understanding the underlying mechanisms governing crystal growth are critical for achieving consistent and reliable synthesis of phosphor materials.
Addressing the environmental impact of hydrothermal synthesis, including energy consumption, waste generation, and the use of hazardous reagents, is a key concern. Developing greener synthesis protocols and alternative reaction pathways with reduced environmental footprint is essential for sustainable phosphor manufacturing.
2.4 Coprecipitation
Coprecipitation involves the simultaneous precipitation of cations and anions from aqueous solutions by the addition of a precipitating agent, such as a base or a salt. By adjusting the pH, temperature, and concentration of the reactants, the formation of insoluble precipitates containing the desired phosphor constituents can be induced. The resulting precipitates are typically washed, dried, and calcined to form crystalline phosphor particles. 34 Coprecipitation offers advantages such as simplicity, scalability, and uniformity of particle size and composition. Moreover, it enables the synthesis of composite and doped phosphor materials by incorporating dopant ions or secondary phases during the precipitation process. Coprecipitation is a widely used method for synthesizing inorganic phosphors, offering several advantages along with some disadvantages and challenges. 35
Advantages:
Coprecipitation is a relatively simple and cost-effective method that involves mixing aqueous solutions of precursor salts followed by the addition of a precipitating agent. The method does not require complex equipment or specialized techniques, making it accessible to researchers and manufacturers.
Coprecipitation typically yields a high quantity of product compared to other synthesis methods. The precipitation reaction generates a large number of phosphor particles simultaneously, leading to high production efficiency and throughput.
Coprecipitation results in homogeneous distribution of dopant ions and modifiers within the phosphor lattice, leading to uniform luminescence properties. This ensures consistent performance and reliability of phosphor-based devices, such as LEDs and displays.
Coprecipitation can be used to fabricate a wide range of inorganic phosphors, including oxides, sulfides, and nitrides. It offers versatility in terms of precursor selection and reaction conditions, enabling the synthesis of complex and multicomponent phosphor materials with tailored properties for various applications. 36
Disadvantages:
Coprecipitation may lack precise control over particle size, morphology, and composition, leading to the formation of agglomerated particles and phase impurities. Achieving uniform and monodisperse phosphor particles may require additional processing steps or optimization of reaction parameters.
Coprecipitation may result in the incorporation of impurities or contaminants from the precursor salts or precipitating agent, affecting the chemical purity and optical properties of the synthesized phosphor material. Purification steps, such as washing and annealing, may be required to remove residual impurities and improve material quality.
Coprecipitation is typically carried out under ambient conditions or mild heating, limiting the range of achievable particle sizes and crystal structures. Complex phosphor compositions or metastable phases may be difficult to synthesize using coprecipitation alone, requiring alternative synthesis methods or post-treatment procedures.
Challenges:
Achieving phase-pure phosphor materials with desired crystal structures and properties remains a challenge in coprecipitation. Controlling reaction kinetics, stoichiometry, and processing conditions is crucial for minimizing phase segregation and ensuring the formation of the desired crystalline phase.
Coprecipitated phosphor particles may exhibit surface defects or functional groups that can affect their optical and chemical properties. Developing surface modification strategies, such as coating or doping, is important for enhancing the stability, luminescence efficiency, and functionality of coprecipitated phosphor materials.
Scaling up coprecipitation for large-scale production poses challenges related to reproducibility, process control, and cost-effectiveness. Developing scalable synthesis protocols and optimizing reaction conditions while maintaining product quality and consistency is essential for industrial applications.
2.5 Microwave-assisted processing
Microwave-assisted processing involves the application of microwave irradiation to accelerate chemical reactions and promote the synthesis of inorganic phosphor materials. 37 Microwave heating offers several advantages over conventional thermal methods, including rapid heating rates, uniform heating profiles, and energy efficiency. By coupling microwave irradiation with conventional synthesis techniques, such as solid-state reaction, sol–gel method, or hydrothermal synthesis, the synthesis time can be significantly reduced, while improving the crystallinity, purity, and yield of the phosphor material. Microwave-assisted processing is particularly beneficial for synthesizing nanoscale and metastable phosphors, as it enables precise control over reaction kinetics and thermodynamics. 38 , 39 Microwave-assisted processing is an innovative method for synthesizing inorganic phosphors, offering several advantages along with some disadvantages and challenges.
Advantages:
Microwave-assisted processing allows for rapid and uniform heating of reaction mixtures, resulting in accelerated synthesis rates and reduced processing times compared to conventional heating methods. The selective absorption of microwave energy by polar molecules, such as water and certain precursor compounds, facilitates fast and efficient energy transfer, enabling high-temperature reactions to occur within minutes or even seconds. 40
Microwave-assisted processing typically requires lower energy consumption compared to conventional heating methods, as microwave energy is directly absorbed by the reaction mixture, minimizing heat loss to the surroundings. This leads to improved energy efficiency and reduced operating costs, making microwave-assisted synthesis environmentally friendly and economically viable.
Microwave-assisted processing offers enhanced control over reaction parameters, such as temperature, pressure, and heating rate, leading to improved reproducibility and product quality. Precise modulation of microwave power and irradiation time allows researchers to optimize reaction conditions and tailor the properties of the synthesized phosphor material to meet specific application requirements.
Microwave-assisted processing can be used to synthesize a wide range of inorganic phosphors, including oxides, sulfides, and nitrides. It offers versatility in terms of precursor selection and reaction conditions, enabling the synthesis of complex and multicomponent phosphor materials with tailored properties for various applications.
Disadvantages:
Microwave-assisted processing requires specialized equipment, such as microwave reactors or synthesizers, which can be expensive to purchase and maintain. Additionally, the need for corrosion-resistant materials and safety precautions adds to the overall cost of operation.
Scaling up microwave-assisted synthesis for large-scale production may pose challenges related to reactor design, heat transfer, and process control. Achieving uniform heating and efficient energy transfer in large reaction volumes while maintaining product quality and consistency is essential for industrial applications.
Microwave heating may result in non-uniform temperature distribution within the reaction mixture, leading to localized hot spots and thermal gradients. This can affect reaction kinetics, phase evolution, and product homogeneity, requiring careful optimization of microwave power and irradiation conditions to ensure uniform heating and reaction progress.
Challenges:
Understanding the underlying reaction mechanisms and kinetics of microwave-assisted synthesis is crucial for optimizing reaction conditions and controlling product formation. Investigating the effects of microwave irradiation on precursor decomposition, nucleation, growth, and phase transformation processes is essential for predicting and controlling the properties of the synthesized phosphor material.
Microwave-assisted processing may result in side reactions, decomposition, or phase impurities due to the rapid and intense heating conditions. 41 Developing strategies to enhance reaction selectivity and minimize unwanted by-products or defects is important for achieving high purity and crystallinity of the synthesized phosphor material.
Optimizing microwave-assisted synthesis protocols for specific phosphor compositions, crystal structures, and properties requires systematic experimentation and parameter optimization. Factors such as precursor concentration, solvent choice, reaction temperature, and irradiation time need to be carefully optimized to achieve desired product performance and functionality.
2.6 Flame spray pyrolysis
Flame spray pyrolysis involves the atomization of precursor solutions into fine droplets, which are then ignited and combusted in a high-temperature flame reactor. 42 The rapid heating and cooling rates within the flame reactor promote the formation of nanoscale phosphor particles through homogeneous nucleation and growth. 43 By adjusting the composition of the precursor solution, the flame parameters, and the residence time in the reactor, the size, morphology, and crystallinity of the phosphor particles can be controlled. Flame spray pyrolysis offers advantages such as continuous operation, scalability, and versatility, making it suitable for producing high-purity and well-defined phosphor materials for various applications. Flame spray pyrolysis (FSP) is an advanced technique for synthesizing inorganic phosphors, offering several advantages along with some disadvantages and challenges. 44 , 45
Advantages:
Flame spray pyrolysis enables high production rates, making it suitable for large-scale synthesis of inorganic phosphors. The continuous flow of precursor solutions through a high-temperature flame reactor allows for rapid nucleation and growth of nanoparticles, leading to high throughput and productivity.
FSP produces inorganic phosphor nanoparticles with narrow size distribution and controlled morphology. The high-temperature flame environment promotes rapid and homogeneous nucleation, resulting in uniform particle size and shape. This uniformity enhances the optical properties and performance of the synthesized phosphors in various applications.
FSP can be easily scaled up for industrial production of inorganic phosphors due to its simple and continuous operation. The modular design of flame spray pyrolysis reactors allows for easy adjustment of process parameters and reactor configurations to accommodate different production scales and throughput requirements.
FSP offers versatility in terms of precursor selection and reaction conditions, enabling the synthesis of a wide range of inorganic phosphors, including oxides, sulfides, and nitrides. By adjusting precursor compositions, concentrations, and feed rates, researchers can tailor the properties of the synthesized phosphor material to meet specific application requirements.
Disadvantages:
Flame spray pyrolysis requires specialized equipment, such as a spray nozzle, burner system, and temperature control unit, which can be complex and expensive to set up and maintain. Additionally, the need for precise control over process parameters, such as fuel flow rate, oxygen concentration, and residence time, adds to the complexity of operation.
FSP consumes significant amounts of energy to generate and maintain the high-temperature flame required for nanoparticle synthesis. The high energy requirements may increase operating costs and environmental impact, particularly for large-scale production.
Achieving precise control over particle size, morphology, and composition in FSP can be challenging due to the complex interplay of process parameters and reaction kinetics. Variations in flame temperature, residence time, and precursor droplet size may affect the nucleation and growth of nanoparticles, leading to variations in product quality and performance.
Challenges:
Flame spray pyrolysis may result in the formation of particle agglomerates due to collisions and coalescence of nanoparticles in the high-temperature flame environment. Controlling particle dispersion and preventing agglomeration is essential for maintaining uniformity and stability of the synthesized phosphor material.
Understanding the surface chemistry and reactivity of flame-synthesized phosphor nanoparticles is important for controlling their optical and chemical properties. Surface modification techniques, such as coating or doping, may be required to enhance the stability, luminescence efficiency, and functionality of FSP-derived phosphors for specific applications.
Addressing the environmental impact of flame spray pyrolysis, including energy consumption, emissions, and waste generation, is a key concern. Developing greener synthesis protocols and alternative fuel sources with reduced environmental footprint is essential for sustainable phosphor manufacturing.
3 Structure of inorganic phosphors
The structural diversity of inorganic phosphor materials plays a critical role in determining their luminescence properties and performance in various applications. Common crystal structures include perovskites, garnets, oxides, sulfides, and nitrides, each characterized by specific lattice parameters, symmetry elements, and coordination environments. Perovskite phosphors, with their ABX3 composition, exhibit versatile luminescence behavior and are widely employed in solid-state lighting applications. Garnet phosphors, typified by the A3B5O12 structure, offer excellent thermal stability and are commonly used in high-power LEDs and laser diodes. 46 Oxide phosphors, such as aluminate and silicate compounds, demonstrate superior chemical durability and color purity, making them suitable for display and signage applications. Sulfide and nitride phosphors, though less common, exhibit unique luminescence properties and have garnered interest for emerging optoelectronic devices. The structure of inorganic phosphors plays a crucial role in determining their luminescent properties, stability, and performance in various applications. In this section, we will explore the common crystal structures of inorganic phosphors and their implications for luminescence.
3.1 Perovskite structure
The perovskite structure, named after the mineral calcium titanate (CaTiO3), is one of the most widely studied and versatile crystal structures in inorganic phosphors. It has the general formula ABX3, where A and B are cations occupying octahedral and larger coordination sites, respectively, and X is an anion. The octahedral coordination of the A-site cation creates a network of corner-sharing polyhedra, while the B-site cation occupies the center of the octahedra. The perovskite structure can accommodate a wide range of cations and anions, allowing for extensive compositional variations and dopant incorporation. 47 Perovskite phosphors exhibit diverse luminescence properties, including emission colors ranging from ultraviolet to near-infrared, depending on the composition and dopant ions. For example, europium-doped strontium aluminate (SrAl2O4:Eu2+) phosphors emit long-lasting green luminescence due to the 4f–5d transition of the Eu2+ ions within the perovskite lattice (Figure 3). Similarly, lanthanide-doped perovskite oxides, such as LaAlO3:Ln3+ (Ln = Eu, Tb, Ce), are widely used for their efficient red, green, and blue emissions, respectively, making them suitable for applications in solid-state lighting and displays. 48 Perovskite structure phosphor materials have gained significant attention in recent years due to their unique properties and potential applications in various fields, including lighting, displays, sensing, and photovoltaics. The perovskite structure is characterized by a specific crystal structure known as the perovskite structure, which consists of a three-dimensional network of corner-sharing octahedra. In this structure, a cation occupies the center of the octahedron, while an anion occupies the vertices. The general chemical formula for perovskite structure compounds is ABX3, where A is a large cation (usually an alkaline earth metal or a rare earth metal), B is a smaller transition metal cation, and X is an anion (usually oxygen). However, in perovskite structure phosphor materials, X can also include other anions such as halides (e.g., chloride, bromide, iodide) or other elements (e.g., sulfur, nitrogen). 49
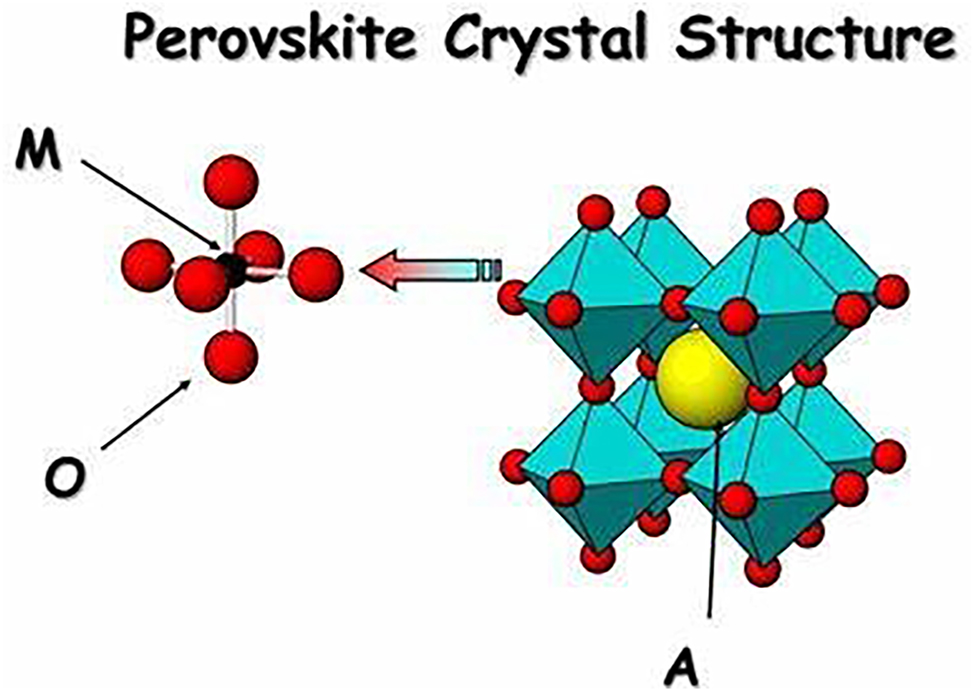
Perovskite crystal structure, which can be followed by the constituents of phosphor materials for more stability.
Some key characteristics of perovskite structure phosphor materials include:
Perovskite structure phosphors offer tunable optical, electronic, and magnetic properties through compositional variation and dopant incorporation. The flexibility in composition allows researchers to tailor the bandgap, emission wavelength, and luminescence efficiency of perovskite structure phosphors for specific applications.
Perovskite structure phosphors exhibit high luminescence quantum efficiency, making them promising candidates for efficient light emission and energy conversion. The defect-tolerant nature of perovskite materials enables efficient radiative recombination of charge carriers, resulting in bright and stable luminescence.
Perovskite structure phosphors can emit light across a wide range of wavelengths, from ultraviolet to near-infrared, depending on the composition and structure of the material. This broad emission spectrum makes perovskite phosphors suitable for various applications, including white light generation and color-tunable lighting.
Perovskite structure phosphors can be synthesized using solution-based methods, such as solution combustion, sol–gel, and precipitation, enabling facile and cost-effective synthesis of phosphor nanoparticles, thin films, and coatings. Solution processability allows for large-area deposition and compatibility with flexible substrates, offering opportunities for scalable manufacturing and device integration.
Perovskite structure phosphors exhibit excellent thermal and chemical stability under ambient conditions, making them suitable for long-term device operation in harsh environments. The robustness of perovskite materials against degradation ensures the durability and reliability of phosphor-based devices for practical applications.
Perovskite structure phosphor materials have shown great promise in various optoelectronic and photonic devices, including light-emitting diodes (LEDs), displays, lasers, photodetectors, and solar cells. Ongoing research efforts are focused on further improving the performance, stability, and scalability of perovskite phosphors for commercialization and widespread adoption in emerging technologies.
3.2 Garnet structure
The garnet structure, characterized by the general formula A3B5O12, is another common crystal structure found in inorganic phosphors. In garnet phosphors, the A-site cation occupies dodecahedral coordination sites, while the B-site cation occupies octahedral coordination sites within the crystal lattice. The close-packed arrangement of oxygen atoms creates tunnels or channels through which the cations are arranged in a three-dimensional network. 46 Garnet phosphors exhibit excellent thermal stability, chemical durability, and photoluminescence properties, making them suitable for high-power LED applications. For instance, yttrium aluminum garnet (YAG:Ce3+) phosphors, doped with cerium ions as activators, are widely used as yellow phosphors in white LED devices. 50 The 4f–5d transition of the Ce3+ ions within the garnet lattice results in efficient yellow emission, which, when combined with blue LEDs, produces white light with high color rendering index (CRI) and luminous efficacy. The garnet structure refers to a specific crystal structure known as the garnet structure (Figure 4), which is commonly found in a class of materials called garnets. Garnets are a group of silicate minerals that share the same crystal structure characterized by a cubic unit cell. The garnet structure is named after the mineral garnet, which is one of the most well-known members of this mineral group. The general chemical formula for garnet structure compounds is A3B2(CO4)3, where A is typically a divalent cation such as calcium (Ca), magnesium (Mg), or iron (Fe), B is a trivalent cation such as aluminum (Al), iron (Fe), or chromium (Cr), and CO4 represents a group of four oxygen atoms. However, the garnet structure can accommodate a wide range of cations and anions, leading to a diverse family of materials with various compositions and properties. 51
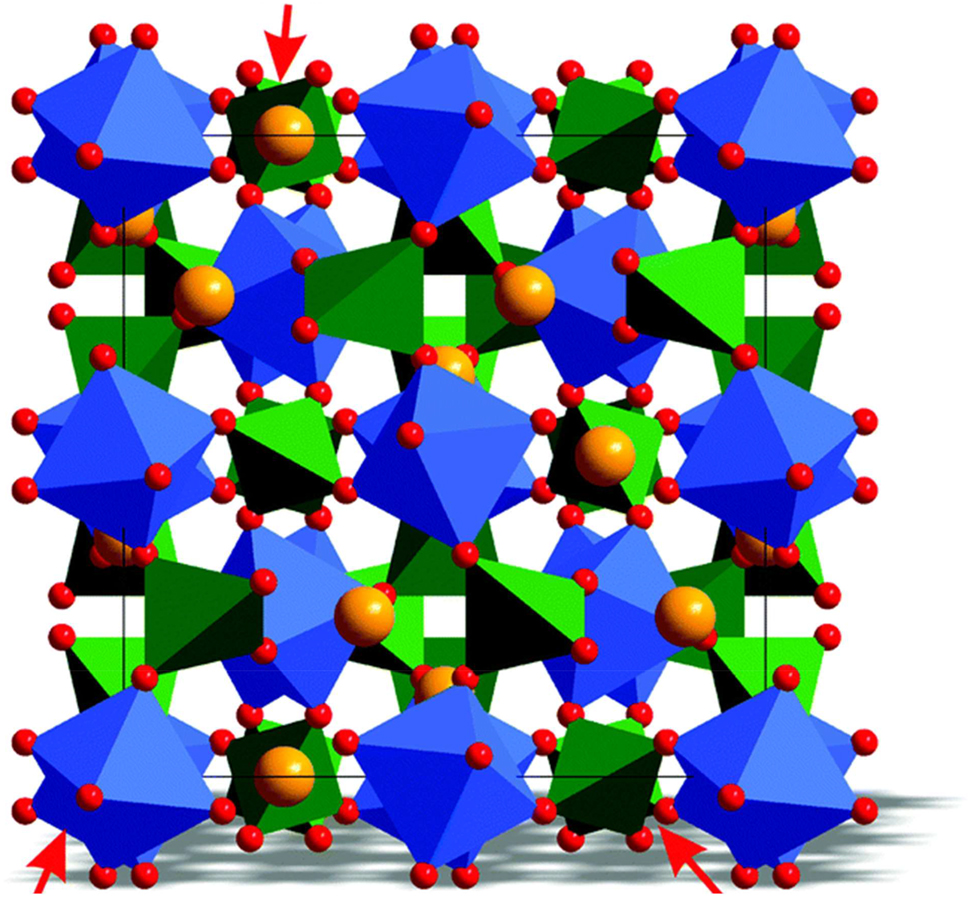
Garnet-type structure which can be followed by phosphors.
Key features of the garnet structure include:
The garnet structure belongs to the cubic crystal system, characterized by a unit cell with cubic symmetry. The lattice parameters of the garnet structure can vary depending on the specific composition of the garnet material, but the overall cubic symmetry remains consistent.
In the garnet structure, the cations (A and B) are arranged in corner-sharing polyhedral units, forming a three-dimensional network. Each cation is surrounded by oxygen atoms, which are shared between adjacent polyhedra, creating a continuous framework throughout the crystal lattice.
The garnet structure allows for the substitution of cations at both the A and B sites, as well as the substitution of anions such as oxygen. This flexibility in composition leads to the formation of solid solutions with a wide range of properties, including variations in color, hardness, and optical behavior.
Garnet structure materials exhibit interesting optical and magnetic properties due to the interaction between the cations and the surrounding oxygen atoms. Some garnet materials are transparent and exhibit strong optical absorption and emission characteristics, making them useful in optical and laser applications. Others display magnetic behavior, with potential applications in magnetic storage and spintronics.
Garnet structure materials have diverse applications in various fields, including optics, electronics, telecommunications, and materials science. For example, yttrium aluminum garnet (YAG) is widely used as a host material for solid-state lasers, phosphors in LED lighting, and scintillator detectors in medical imaging.
Overall, the garnet structure provides a versatile framework for the design and synthesis of functional materials with tailored properties for specific applications. Understanding the structure-property relationships in garnet materials is essential for optimizing their performance and exploring new functionalities in emerging technologies.
3.3 Oxide structures
Oxide phosphors encompass a wide range of crystal structures, including perovskites, garnets, spinels, and scheelites, among others. These structures are characterized by close-packed arrangements of oxygen atoms, with metal cations occupying specific coordination sites within the lattice. 52 The optical properties of oxide phosphors are influenced by factors such as crystal field effects, charge transfer transitions, and dopant concentration. 53 For example, zinc sulfide-based oxide phosphors, such as ZnS:Cu,Ag, emit blue-green luminescence due to the radiative recombination of electron-hole pairs trapped at defect sites within the crystal lattice. 54 These phosphors are commonly used in cathode-ray tube (CRT) displays and fluorescent lamps. Similarly, magnesium aluminate spinel (MgAl2O4) phosphors doped with rare earth ions exhibit intense blue or green luminescence, making them suitable for applications in LED-based displays and signage. 55 Oxide structures refer to a broad class of materials composed primarily of oxygen atoms bonded to one or more metal cations. Oxides are ubiquitous in nature and have a wide range of structural motifs, each with unique properties and applications. Some common oxide structures include:
Ionic oxides consist of positively charged metal cations and negatively charged oxide ions arranged in a regular lattice structure. Examples include sodium oxide (Na2O) and magnesium oxide (MgO). Ionic oxides often exhibit high melting points, electrical insulating properties, and are commonly used as ceramics and refractory materials.
The perovskite oxides has the chemical formula ABX3, where A is a larger cation (often an alkaline earth metal), B is a smaller transition metal cation, and X is an anion (typically oxygen). Perovskite oxides have a cubic crystal structure with the A cations occupying the corners of the unit cell, the B cations in the center, and the X anions at the face centers. Perovskite oxides are known for their diverse properties, including ferroelectricity, ferromagnetism, and superconductivity, and have applications in catalysis, fuel cells, and electronic devices.
Spinel oxides have the general formula AB2O4, where A and B are metal cations. The spinel structure consists of oxygen ions arranged in a face-centered cubic lattice, with the A cations occupying tetrahedral sites and the B cations occupying octahedral sites. Spinel oxides exhibit a range of properties, including semiconducting, magnetic, and optical behavior, and are used in various applications such as pigments, catalysts, and electronic components.
The corundum structure is characterized by a close-packed arrangement of oxygen atoms with metal cations occupying two-thirds of the octahedral sites. This structure is commonly found in aluminum oxide (Al2O3), where the aluminum ions occupy two-thirds of the octahedral sites, resulting in a hexagonal close-packed arrangement. Corundum oxides are hard, transparent, and have high melting points, making them suitable for use as abrasives, refractory materials, and gemstones.
Periclase or rock salt structure is a simple cubic arrangement of oxide ions with metal cations occupying all of the octahedral sites. This structure is observed in oxides such as magnesium oxide (MgO) and calcium oxide (CaO), which exhibit high ionic conductivity and are used in ceramics, refractories, and as catalyst supports.
Some oxides adopt layered structures, where metal-oxygen layers are stacked on top of each other. Examples include layered double hydroxides (LDHs) and transition metal oxides such as molybdenum oxide (MoO3) and vanadium pentoxide (V2O5). Layered oxides exhibit interesting electronic and electrochemical properties and find applications in batteries, catalysis, and sensors.
oxide structures encompass a diverse range of materials with varying compositions, structures, and properties. Understanding the structural characteristics of oxides is essential for tailoring their properties and harnessing their potential in a wide range of technological applications, including energy storage, electronics, catalysis, and environmental remediation.
3.4 Nitride and sulfide structures
Nitride and sulfide phosphors represent another class of inorganic materials with unique crystal structures and luminescence properties. Nitride phosphors, such as silicon nitride (Si3N4), gallium nitride (GaN), and aluminum nitride (AlN), exhibit wide bandgap energies and efficient luminescence in the visible and ultraviolet regions of the spectrum. These materials are of interest for applications in UV-LEDs, solid-state lighting, and optoelectronic devices. 56
Sulfide phosphors, including zinc sulfide (ZnS), cadmium sulfide (CdS), and calcium sulfide (CaS), are known for their broad emission spectra and high quantum efficiency. These phosphors are commonly used as activators in fluorescent lamps, cathode-ray tubes, and phosphor-converted LEDs. For example, cadmium sulfide-based phosphors doped with manganese ions (CdS:Mn) emit red luminescence due to the 4T1-6A1 transition of the Mn2+ ions, making them suitable for red-emitting phosphor applications. 57 The structure of inorganic phosphors plays a critical role in determining their luminescence properties, stability, and performance in various applications. Understanding the crystallographic arrangement of atoms within the phosphor lattice enables researchers to design and engineer materials with tailored optical characteristics and enhanced functionality. From perovskites and garnets to oxides, nitrides, and sulfides, the diverse array of crystal structures offers a versatile toolkit for advancing the frontier of solid-state lighting, displays, and optoelectronic devices. Nitride and sulfide structures are widely utilized in the field of phosphors due to their unique luminescent properties and diverse crystal structures.
Nitride compounds with the wurtzite crystal structure, such as gallium nitride (GaN) and indium gallium nitride (InGaN), are extensively used in phosphor applications, particularly in light-emitting diodes (LEDs). These materials exhibit direct bandgaps and efficient light emission, making them suitable for producing blue, green, and even white light in LEDs. By varying the composition and doping levels, the emission wavelength of wurtzite nitride phosphors can be tuned across the visible spectrum.
Nitride materials with the zinc blende crystal structure, including aluminum nitride (AlN) and indium nitride (InN), are also employed as phosphors in LEDs and other optoelectronic devices. Zinc blende nitride phosphors exhibit strong luminescence properties and are often used to produce blue and ultraviolet (UV) light in LEDs. These materials offer advantages such as high thermal stability and excellent optical properties, contributing to the efficiency and color quality of LED lighting systems.
Sulfide compounds with various crystal structures are utilized as phosphors in different applications. For example, zinc sulfide (ZnS) phosphors are commonly used in cathode ray tubes (CRTs), fluorescent lamps, and X-ray screens due to their strong luminescence properties and wide bandgap. Zinc sulfide phosphors can emit light in the visible spectrum, ranging from blue to red, depending on the dopants and processing conditions.
Layered sulfide structures, such as transition metal dichalcogenides (TMDs) like molybdenum disulfide (MoS2) and tungsten disulfide (WS2), have also emerged as promising phosphor materials. These materials exhibit unique optical properties, including quantum confinement effects and strong photoluminescence, making them suitable for applications in displays, sensors, and photodetectors. 58 Layered sulfide phosphors offer opportunities for tuning the emission wavelength and enhancing the efficiency of light conversion processes.
Doping sulfide and nitride phosphors with various activator ions enables precise control over their luminescent properties. For instance, rare earth ions such as europium (Eu), cerium (Ce), and terbium (Tb) are commonly used as activators in nitride and sulfide phosphors to produce specific emission colors and improve luminescence efficiency. By doping with different activators and adjusting the synthesis conditions, researchers can tailor the emission characteristics of nitride and sulfide phosphors for specific applications, including display technologies and lighting systems.
Nitride and sulfide structures offer a rich variety of phosphor materials with diverse luminescent properties and crystal structures. These materials play a crucial role in modern lighting, display, and optoelectronic technologies, driving advances in energy efficiency, color quality, and functionality. Continued research and development in nitride and sulfide phosphors are expected to lead to further improvements in performance and expand their applications in emerging technologies.
4 Luminescence properties of inorganic phosphors
The luminescence properties of inorganic phosphor materials are governed by a complex interplay of electronic transitions, energy transfer mechanisms, and defect states within the crystal lattice. The emission spectrum of a phosphor material is determined by the energy levels of the dopant ions and the host lattice, as well as the nature of the excitation source. Photoluminescence, wherein photons are emitted following the absorption of light, is the most common luminescence mechanism observed in inorganic phosphors. Other mechanisms, including cathodoluminescence (stimulated by electron beams), electroluminescence (induced by electrical currents), and thermoluminescence (activated by heat), offer additional avenues for harnessing the luminescent properties of phosphor materials in diverse applications. Quantum efficiency, lifetime, and color purity are key parameters that govern the performance of inorganic phosphors in LED and display devices, with ongoing research efforts focused on enhancing these attributes through the design of novel materials and doping strategies. 59 The luminescence properties of inorganic phosphors are governed by a complex interplay of electronic transitions, energy transfer mechanisms, and defect states within the crystal lattice. Understanding these properties is crucial for tailoring phosphor materials to meet the requirements of various applications, including lighting, displays, sensors, and biomedical imaging. In this section, we will explore the key luminescence properties of inorganic phosphors:
4.1 Emission spectrum
The emission spectrum of an inorganic phosphor refers to the range of wavelengths of light emitted when the phosphor is excited by an external energy source, such as ultraviolet (UV) or blue light. The emission spectrum is influenced by the energy levels of the dopant ions and the host lattice, as well as the nature of the excitation source. Different phosphor compositions and crystal structures can result in emission spectra spanning the ultraviolet, visible, and near-infrared regions of the electromagnetic spectrum. For example, europium-doped strontium aluminate (SrAl2O4:Eu2+) phosphors emit green luminescence due to the 4f–5d transition of the Eu2+ ions within the crystal lattice. Similarly, lanthanide-doped perovskite oxides, such as LaAlO3:Ln3+ (Ln = Eu, Tb, Ce), exhibit red, green, and blue emissions, respectively, making them suitable for applications in solid-state lighting and displays. 60
4.2 Quantum efficiency
The quantum efficiency of an inorganic phosphor refers to the ratio of the number of photons emitted to the number of photons absorbed by the phosphor material. It is a measure of the phosphor’s ability to convert absorbed energy into emitted light and is typically expressed as a percentage. High quantum efficiency is desirable for phosphor materials used in lighting and display applications, as it indicates efficient energy conversion and minimal losses due to non-radiative processes. Phosphors with high quantum efficiency exhibit intense and uniform luminescence, leading to bright and vibrant displays with high color purity and contrast. Quantum efficiency can be influenced by factors such as crystal structure, dopant concentration, defect states, and surface morphology. Improving the quantum efficiency of phosphor materials is a key focus of research aimed at enhancing the performance and energy efficiency of solid-state lighting and display devices. 26 The relationship among external quantum efficiency, internal quantum efficiency, injection and extraction efficiency is as below:
External Quantum Efficiency = [Internal Quantum Efficiency] × [Injection Efficiency] × [Extraction Efficiency].
4.3 Lifetime
The lifetime of luminescence refers to the duration over which the phosphor emits light following excitation. It is determined by the radiative and non-radiative decay processes occurring within the phosphor material. Radiative decay involves the emission of photons, while non-radiative decay involves processes such as phonon-assisted relaxation, energy transfer to impurity centers, and surface quenching. Phosphors with long lifetimes are desirable for applications requiring sustained luminescence, such as fluorescent lamps, cathode-ray tubes, and phosphor-converted LEDs. Long lifetimes ensure stable and uniform illumination, minimizing flicker and color shift over time. The lifetime of a phosphor can be influenced by factors such as dopant concentration, crystal structure, defect density, and temperature. Improving the lifetime of phosphor materials is essential for enhancing the reliability and longevity of lighting and display devices. 61
4.4 Color rendering index (CRI) and CIE chromaticity coordinate
The color rendering index (CRI) is a quantitative measure of the ability of a light source to accurately reproduce the colors of objects compared to a reference light source. It is particularly important for applications such as indoor lighting, where accurate color representation is essential for visual comfort, aesthetics, and task performance. Phosphors with high CRI values exhibit broad and uniform emission spectra, enabling them to render colors faithfully and accurately. CRI values are influenced by factors such as the spectral distribution of the emitted light, the color temperature of the light source, and the color sensitivity of the human eye. In general, phosphors with a balanced emission spectrum spanning the visible spectrum (i.e., red, green, and blue regions) are preferred for achieving high CRI values. Improving the CRI of phosphor materials is a key objective in the development of next-generation lighting technologies, such as phosphor-converted LEDs and white light-emitting diodes (WLEDs). 62 For CIE mainly three colors coordinates are used, upto date three model are proposed to representation CIE coordinates which are CIE-19931, CIE-1960, and CIE-1976 as shown in Figure 5. 63
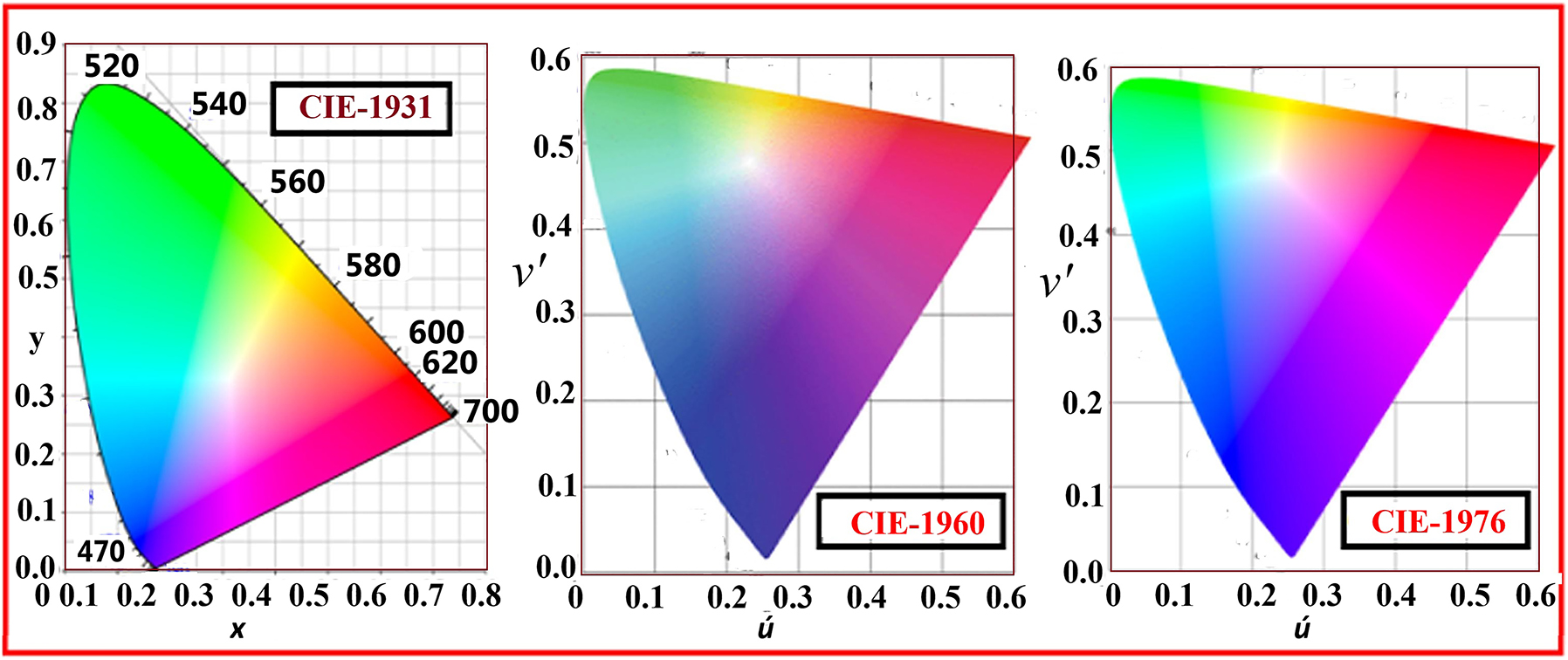
The type color RBG CIE chromaticity diagram CIE-19931, CIE-1960, and CIE-1976. 63
4.5 Thermal stability
The thermal stability of inorganic phosphors refers to their ability to maintain their luminescent properties at elevated temperatures. Phosphors used in lighting and display applications are often subjected to high operating temperatures, which can affect their efficiency, color stability, and longevity. Thermal stability is influenced by factors such as the chemical composition, crystal structure, dopant concentration, and defect density of the phosphor material. Phosphors with high thermal stability exhibit minimal degradation in luminescence properties when exposed to elevated temperatures, ensuring consistent performance over the lifetime of the device. Improving the thermal stability of phosphor materials is essential for enhancing the reliability and efficiency of LED-based lighting and display systems, particularly in high-power and high-temperature environments.
5 Applications of inorganic phosphors
Inorganic phosphor materials find wide-ranging applications in solid-state lighting, displays, sensors, and biomedical imaging. Phosphor-converted LEDs, wherein blue or ultraviolet light is absorbed by a phosphor layer and re-emitted at longer wavelengths, offer energy-efficient alternatives to traditional lighting sources, with applications spanning residential, commercial, and automotive lighting. White light-emitting diodes (WLEDs), employing multi-component phosphor blends or quantum dot converters, enable the replication of natural daylight and customizable color temperatures for various indoor and outdoor environments. In displays, phosphor materials serve as the emissive components in liquid crystal displays (LCDs), organic light-emitting diodes (OLEDs), and quantum dot displays, facilitating the generation of vibrant, high-resolution imagery in televisions, smartphones, tablets, and other electronic devices. Phosphor-based sensors, leveraging the sensitivity of luminescent signals to detect and quantify analytes, find applications in environmental monitoring, industrial process control, and biomedical diagnostics, offering rapid, selective, and cost-effective solutions for a wide range of sensing applications. Inorganic phosphors find wide-ranging applications across various fields due to their unique luminescent properties, stability, and versatility. From lighting and displays to sensors and biomedical imaging, inorganic phosphors play a crucial role in a multitude of technologies.
5.1 Solid-state lighting
One of the most prominent applications of inorganic phosphors is in solid-state lighting (SSL), where they are used to enhance the performance and efficiency of light-emitting diodes (LEDs). Phosphors are employed in phosphor-converted LEDs (PC-LEDs) to convert the blue or ultraviolet light emitted by the LED chip into a broad spectrum of visible light. This enables the generation of white light with adjustable color temperatures and high color rendering index (CRI), making PC-LEDs suitable for a wide range of lighting applications, including residential, commercial, and automotive lighting (Figure 6). 64
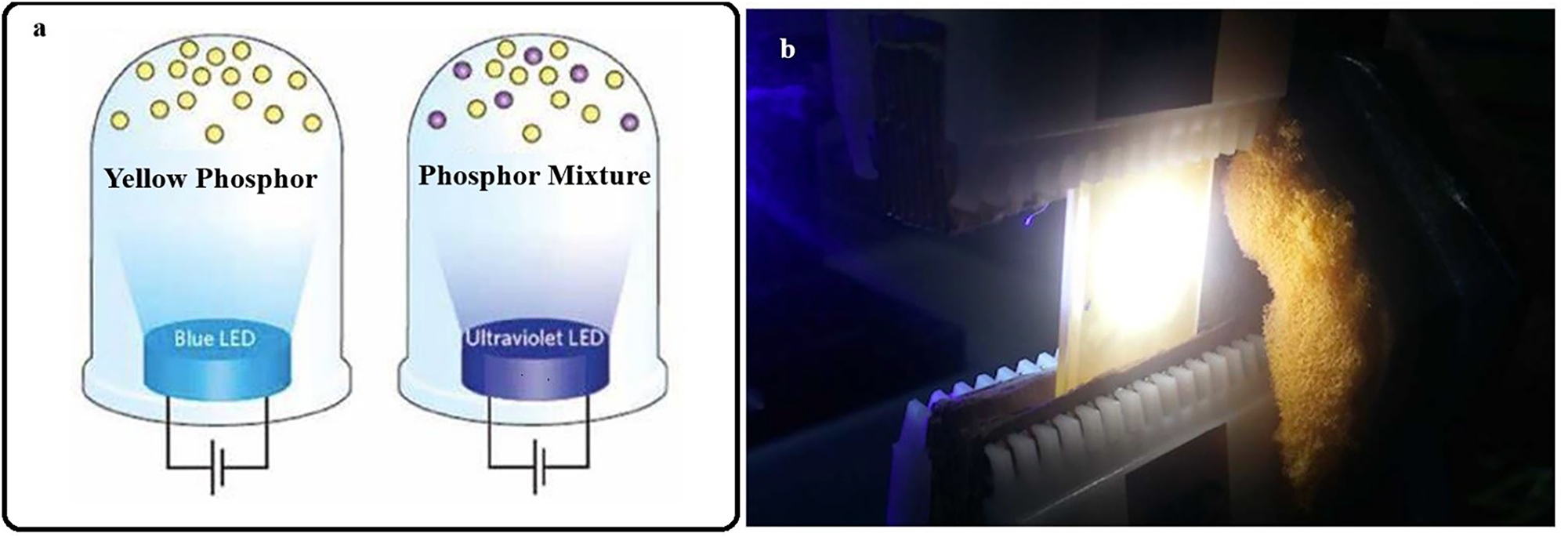
Solid state lighting materials. a) Light emitting of phosphor material under blue LED, and ultraviolet LED. b) Laser-activated remote phosphor system for generation of white light. 66
The basic principle behind PC-LEDs involves the use of a blue LED chip, which emits high-energy blue light. This blue light excites the inorganic phosphors coated on the LED chip, causing them to emit light in the green, yellow, or red regions of the spectrum. By carefully selecting and blending different phosphors, it is possible to produce white light with specific color temperatures, ranging from warm white (2700–3000 K) to cool white (5000–6500 K). The ability to adjust the color temperature and achieve a high CRI makes PC-LEDs highly versatile, as they can mimic natural sunlight and provide pleasant lighting for various environments. One of the key advantages of PC-LEDs is their high efficiency and long lifespan compared to traditional incandescent and fluorescent lighting. 2 The use of inorganic phosphors ensures that the light conversion process is highly efficient, resulting in minimal energy loss as heat. This efficiency translates to lower energy consumption and reduced operational costs, making PC-LEDs an attractive option for residential, commercial, and automotive lighting applications. Additionally, the robust nature of inorganic phosphors contributes to the durability and reliability of PC-LEDs, ensuring consistent performance over time. 2
In residential lighting, PC-LEDs offer the benefits of energy savings and enhanced light quality, creating comfortable and visually appealing living spaces. In commercial settings, such as offices and retail environments, the adjustable color temperatures and high CRI of PC-LEDs contribute to better visual clarity and a more pleasant atmosphere for employees and customers. In automotive lighting, the superior brightness and reliability of PC-LEDs improve visibility and safety on the road. Overall, the application of inorganic phosphors in SSL represents a significant advancement in lighting technology, driving the widespread adoption of energy-efficient and high-performance LED lighting solutions. 65
5.1.1 Laser activated remote phosphor systems
The replacement of LEDS by blue laser blue LDs were emerged in the early 2000s, but latter since 2011 car manufacturer companies has been worked on the combination of blue-emitting laser diode light with a solid-state phosphor for generating white light for car headlights. In 2014 Ledru et al. design laser activated remote phosphor (LARP) as white light source, with 40 lm/W for a CCT of 4000 K (CRI 94) as shown in Figure 6. 66
5.2 Display technologies
Inorganic phosphors play a crucial role in various display technologies, including liquid crystal displays (LCDs), organic light-emitting diodes (OLEDs), and quantum dot displays. Phosphors are used as emissive materials in these displays to generate red, green, and blue (RGB) light, enabling the reproduction of vibrant and high-resolution images with accurate color representation. Phosphor-based displays offer advantages such as high brightness, wide viewing angles, and low power consumption, making them suitable for applications in televisions, smartphones, tablets, and other electronic devices. 2 , 67 The LCDs is consider as mature stage of display technology, LCDs belong to class of panel where liquid crystals that do not emit light directly are used, as light modulating properties. Therefore, it should keep in mind that LCD requires specialized light source. The color gamut of LCD can be design according to color coordinate of RGB emission for WLEDs, while passing through their corresponding filters. Hence a narrow band phosphor material is required for high efficiency of LCDs with larger color gamut (see Figure 7a). Contrast to LCDs, the OLEDs an organic compound is required which can emit light by applying electric current as shown in Figure 7b. While the Figure 7c and d shows the operation of QD-LCD and micro-LED displays by utilization of RBG as narrow band emitters. The overall summary of narrow band emitting phosphor materials are shown in Figure 6e, in term of display technologies. 68
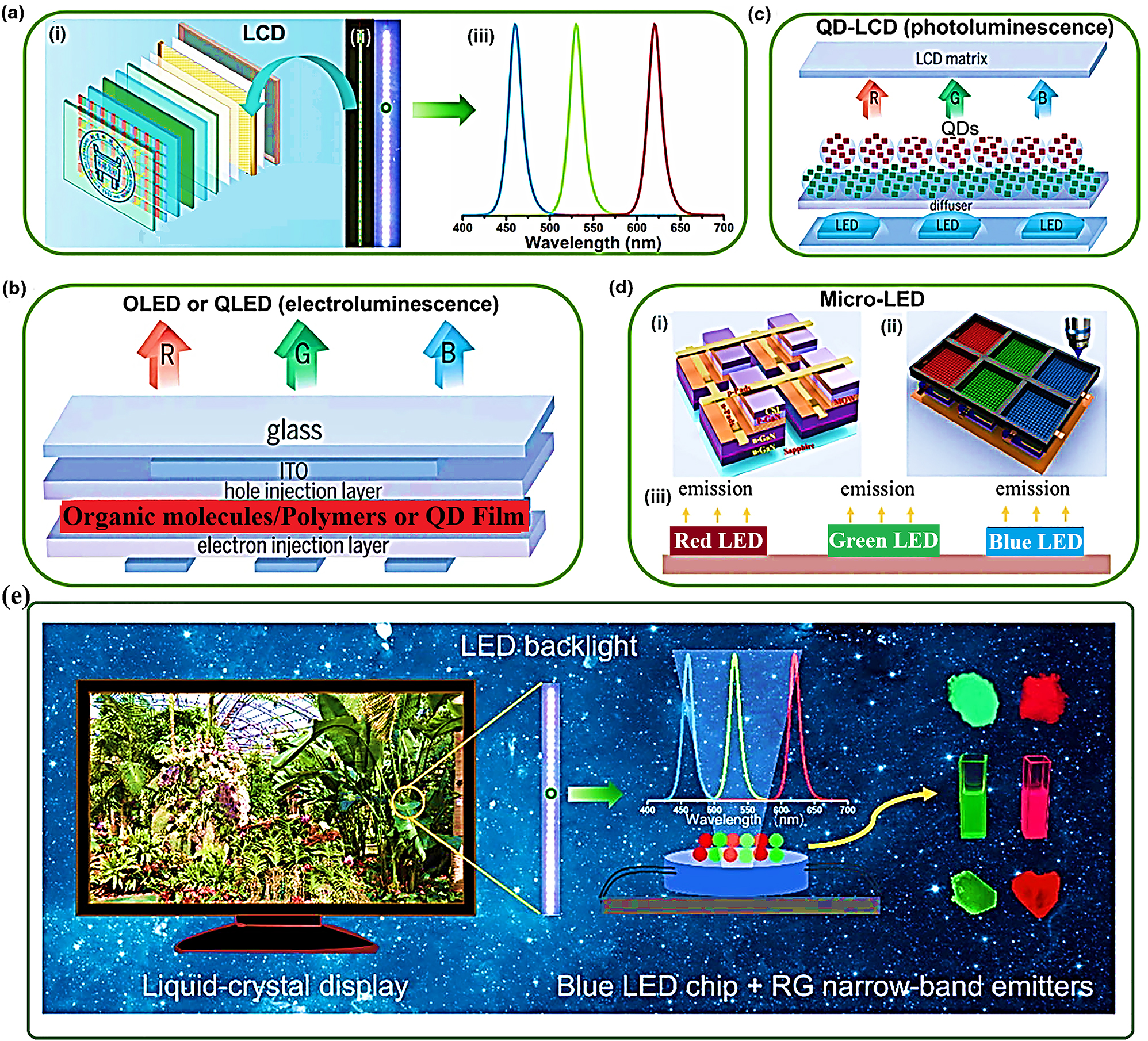
Display technologies and application of solid-state materials. A) i) LCD protype on WLEDS, ii) fabricated and lightened WLEDs, iii) the emission spectra of narrow band emitting phosphor materials used in WLED backlight. B) RBG LED pixel with organic/polymer/QD film as emission layer. C) Operation of QD-LCD display. D) i) Micro-LED structure, ii) RGB pixels for full color pixels by jetting inside, iii) RGB mechanism for micro-LED full color display. E) Conclusion of LCD backlight by the employment of RG narrow-band emitting phosphor materials. 68
5.3 Sensors
Inorganic phosphors are widely used in sensor applications due to their sensitivity to external stimuli and their ability to emit light in response to specific analytes or environmental conditions. Phosphors can be incorporated into sensor devices for detecting gases, heavy metals, toxins, and other pollutants in the environment. Luminescent sensors based on phosphors offer advantages such as high sensitivity, fast response times, and multiplexing capabilities, making them suitable for applications in environmental monitoring, industrial process control, and biomedical diagnostics.
5.4 Biomedical imaging
Inorganic phosphors are also employed in biomedical imaging techniques, such as fluorescence microscopy, bioimaging, and photodynamic therapy. Phosphors can be functionalized with targeting ligands or biomolecules to selectively label cells, tissues, or specific molecular targets for imaging purposes. Luminescent probes based on phosphors offer advantages such as high signal-to-noise ratio, photostability, and tunable emission wavelengths, enabling non-invasive visualization of biological structures and processes with high spatial and temporal resolution. 69 , 70
5.5 Security and anti-counterfeiting
Inorganic phosphors are used in security and anti-counterfeiting applications to authenticate products, documents, and currency. Phosphors with unique luminescent signatures can be incorporated into security features, such as invisible inks, coatings, or tags, which fluoresce under specific excitation conditions. Luminescent security features based on phosphors offer advantages such as high sensitivity, long-term stability, and resistance to tampering, making them suitable for applications in currency authentication, brand protection, and product tracking.
5.6 Radiation detection
Inorganic phosphors are employed in radiation detection devices for monitoring and measuring ionizing radiation, such as X-rays, gamma rays, and alpha particles. Phosphors can be used as scintillators, which emit light when exposed to ionizing radiation, enabling the detection and quantification of radiation doses. Scintillation detectors based on phosphors offer advantages such as high sensitivity, fast response times, and wide dynamic range, making them suitable for applications in medical imaging, homeland security, and nuclear physics research. 71 Inorganic phosphors find diverse applications across various fields, including solid-state lighting, displays, sensors, biomedical imaging, security, and radiation detection. Their unique luminescent properties, stability, and versatility make them indispensable materials in modern technologies, enabling advancements in energy efficiency, visual comfort, environmental monitoring, healthcare, and security. Continued research and innovation in phosphor design, synthesis, and characterization are expected to drive further advancements in the field, leading to the development of more efficient, reliable, and sustainable applications.
6 Future directions and challenges
Despite their widespread use and importance in various applications, inorganic phosphor materials continue to face challenges related to efficiency, stability, and sustainability. Improving the quantum efficiency and color purity of phosphor materials remains a primary focus of research, with efforts aimed at optimizing dopant concentrations, crystal structures, and defect engineering strategies. Enhancing the thermal stability and reliability of phosphor-converted LEDs and WLEDs is another critical area of investigation, particularly for high-power lighting applications. Moreover, the development of environmentally friendly phosphor materials, free from toxic elements such as cadmium and lead, is essential for ensuring the sustainability of phosphor-based technologies. Advances in synthesis techniques, computational modeling, and materials characterization are expected to drive further innovations in the field of inorganic phosphors, unlocking new opportunities for enhancing energy efficiency, visual comfort, and environmental sustainability in lighting and display applications. As inorganic phosphors continue to play a pivotal role in various technologies, including lighting, displays, sensing, and biomedical imaging, the field is poised for significant advancements and innovations in the coming years. However, several challenges and opportunities lie ahead, shaping the future direction of research and development in this field. In this section, we will discuss some of the key future directions and challenges facing the field of inorganic phosphors.
6.1 Efficiency and sustainability
One of the primary objectives in the development of inorganic phosphors is to enhance their efficiency and sustainability. This includes improving the quantum efficiency, lifetime, and thermal stability of phosphor materials to maximize energy conversion and minimize losses due to non-radiative processes. Additionally, there is a growing emphasis on developing environmentally friendly phosphor materials free from toxic elements, such as cadmium and lead, to ensure the sustainability and safety of phosphor-based technologies.
6.2 Tailored properties and functionality
Future research efforts will focus on tailoring the properties and functionality of inorganic phosphors to meet the evolving demands of emerging applications. This includes designing phosphor materials with specific emission spectra, color temperatures, and color rendering properties to address the diverse needs of solid-state lighting and display technologies. Furthermore, there is increasing interest in developing multifunctional phosphor materials capable of integrating additional functionalities, such as sensing, energy harvesting, and photocatalysis, into phosphor-based devices.
6.3 Advanced synthesis and fabrication techniques
Advances in synthesis and fabrication techniques are essential for producing high-quality phosphor materials with tailored nanostructures, hierarchical morphologies, and enhanced optical properties. Future research will focus on exploring advanced synthesis methods, such as sol–gel, hydrothermal synthesis, and aerosol-assisted processing, to achieve precise control over particle size, shape, and composition. Additionally, the integration of additive manufacturing techniques, such as 3D printing and inkjet printing, holds promise for fabricating complex phosphor-based devices with customized designs and functionalities.
6.4 Integration with emerging technologies
Inorganic phosphors are expected to play a crucial role in the integration with emerging technologies, such as quantum dots, perovskite nanocrystals, and two-dimensional materials. Collaborative efforts between researchers in phosphor science and these emerging fields will enable the development of hybrid materials and devices with enhanced performance and functionality. For example, the integration of phosphors with quantum dots can lead to tunable emission colors and improved efficiency in display and lighting applications.
6.5 Application-specific optimization
Future research will focus on application-specific optimization of inorganic phosphors to address the unique requirements of different industries and sectors. This includes tailoring phosphor materials for specific lighting environments, such as residential, commercial, and automotive applications, to optimize color rendering, spectral tunability, and energy efficiency. Additionally, there is increasing interest in developing phosphor-based sensors tailored for environmental monitoring, industrial process control, and biomedical diagnostics, leveraging the sensitivity and selectivity of luminescent signals for real-time detection and quantification of analytes.
6.6 Cost-effectiveness and scalability
Achieving cost-effectiveness and scalability is essential for the widespread adoption of phosphor-based technologies in commercial applications. Future research will focus on developing cost-effective synthesis methods and scalable fabrication processes to reduce production costs and improve the accessibility of phosphor materials. This includes exploring alternative precursors, reaction conditions, and post-synthesis treatments to streamline manufacturing processes and increase production yields. In conclusion, the future of inorganic phosphors holds immense promise, driven by ongoing research efforts aimed at enhancing efficiency, tailoring properties, and integrating with emerging technologies. Addressing challenges related to efficiency, sustainability, synthesis, and scalability will be critical for unlocking the full potential of phosphor-based technologies and realizing their impact across various industries and sectors. Through collaborative interdisciplinary efforts and continued innovation, inorganic phosphors will continue to illuminate the world with brilliance and pave the way for a brighter, more sustainable future.
7 Conclusions
Inorganic phosphor materials represent a versatile and indispensable class of materials with diverse applications in lighting, displays, sensors, and beyond. Synthesis methods, structural design, and doping strategies play crucial roles in tailoring the luminescence properties and performance of phosphor materials for specific applications. Ongoing research efforts aimed at enhancing the efficiency, stability, and sustainability of phosphor-based technologies hold promise for advancing the frontier of illumination and optoelectronics, paving the way for a brighter, more energy-efficient future. Through interdisciplinary collaborations and continued innovation, inorganic phosphor materials will continue to illuminate the world with brilliance and beauty, transforming the way we perceive and interact with light in the modern era. Inorganic phosphors stand as indispensable materials in a wide array of applications, ranging from solid-state lighting and displays to sensing, biomedical imaging, and security. Their luminescent properties, chemical stability, and versatility make them essential components in numerous technologies, enabling energy-efficient illumination, high-resolution imaging, sensitive detection, and secure authentication. As we look to the future, several key trends and challenges shape the trajectory of research and development in the field of inorganic phosphors. Efforts are focused on enhancing efficiency, sustainability, and tailoring properties to meet the evolving demands of emerging applications. Advanced synthesis and fabrication techniques, integration with emerging technologies, and application-specific optimization are driving innovation in phosphor design and manufacturing. Moreover, the pursuit of cost-effectiveness and scalability is crucial for the widespread adoption of phosphor-based technologies in commercial applications. Through interdisciplinary collaboration and continued innovation, inorganic phosphors will continue to illuminate the world with brilliance, transforming the way we perceive and interact with light in the modern era. By addressing challenges and seizing opportunities, we can harness the full potential of phosphor-based technologies to create a brighter, more sustainable future for generations to come.
Funding source: Deanship of Scientific Research at King Khalid University
Award Identifier / Grant number: RGP-2/527/45
-
Research ethics: Not applicable.
-
Author contributions: The initial data were collected, analyzed, and organized by Junaid Ur Rahman. Vicky Jain and Asha Rajiv validated the contents and wrote the CIE details of the phosphors. The Figure, Table, and pattern were improved by Shivakrishna Dasi and Khaled Fahmi Fawy. Pardeep Kumar Jindal and R. Sivarnjani added the advantages and disadvantages of phosphor material along with future perspectives, while the manuscript was evaluated, revised, and finalized by Shahab Khan.
-
Competing interests: Not applicable.
-
Research funding: The authors express their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia, for this work through a research group program under grant number RGP-2/527/45.
-
Data availability: Not applicable.
References
1. Ye, S.; Xiao, F.; Pan, Y.; Ma, Y.; Zhang, Q. Phosphors in Phosphor-Converted White Light-Emitting Diodes: Recent Advances in Materials, Techniques and Properties. Mater. Sci. Eng. R Rep. 2010, 71 (1), 1–34; https://doi.org/10.1016/j.mser.2010.07.001.Suche in Google Scholar
2. Nair, G. B.; Swart, H.; Dhoble, S. A Review on the Advancements in Phosphor-Converted Light Emitting Diodes (Pc-LEDs): Phosphor Synthesis, Device Fabrication and Characterization. Prog. Mater. Sci. 2020, 109, 100622; https://doi.org/10.1016/j.pmatsci.2019.100622.Suche in Google Scholar
3. Li, J.; Yan, J.; Wen, D.; Khan, W. U.; Shi, J.; Wu, M.; Su, Q.; Tanner, P. A. Advanced Red Phosphors for White Light-Emitting Diodes. J. Mater. Chem. C 2016, 4 (37), 8611–8623; https://doi.org/10.1039/c6tc02695h.Suche in Google Scholar
4. Ghosh, S.; Sagayam, K. M.; Haldar, D.; Anton Jone, A. A.; Acharya, B.; Gerogiannis, V. C.; Kanavos, A. A Review on the Types of Nanomaterials and Methodologies Used for the Development of Biosensors. Adv. Nat. Sci. Nanosci. Nanotechnol. 2024, 15 (1), 013001; https://doi.org/10.1088/2043-6262/ad21e8.Suche in Google Scholar
5. Chen, H.-W.; Lee, J.-H.; Lin, B.-Y.; Chen, S.; Wu, S.-T. Liquid Crystal Display and Organic Light-Emitting Diode Display: Present Status and Future Perspectives. Light Sci. Appl. 2018, 7 (3), 17168; https://doi.org/10.1038/lsa.2017.168.Suche in Google Scholar PubMed PubMed Central
6. Khan, S.; Ullah, I.; Ajmal, S.; Saqib, N.; Rahman, F. U.; Ali, S. Advancements in Nanohybrids: From Coordination Materials to Flexible Solar Cells. J. Polym. Sci. Eng. 2024, 7 (1), 4276; https://doi.org/10.24294/jpse.v7i1.4276.Suche in Google Scholar
7. Khan, S.; Ur Rahman, F.; Ullah, I.; Khan, S.; Gul, Z.; Sadiq, F.; Ahmad, T.; Husaain, M. S. S.; Ali, I.; Israr, M. Water Desalination, and Energy Consumption Applications of 2D Nano Materials: Hexagonal Boron Nitride, Graphenes, and Quantum Dots. Rev. Inorg. Chem. 2024, 44, 619–636.10.1515/revic-2024-0013Suche in Google Scholar
8. Xu, Q.; Xu, D.; Sun, J. Preparation and Luminescence Properties of Orange–Red Ba3Y (PO4)3: Sm3+ Phosphors. Opt. Mater. 2015, 42, 210–214; https://doi.org/10.1016/j.optmat.2014.12.035.Suche in Google Scholar
9. Wang, J.; Wang, J.; Duan, P. Luminescent Properties of Dy3+ Doped Sr3Y (PO4)3 for White LEDs. Mater. Lett. 2013, 107, 96–98; https://doi.org/10.1016/j.matlet.2013.06.001.Suche in Google Scholar
10. Wang, Z.; Li, P. Luminescent Property of a Novel Green Emitting Phosphor Sr3Bi (PO4)3: Tb3+. Spectrochim. Acta Mol. Biomol. Spectrosc. 2014, 129, 584–587; https://doi.org/10.1016/j.saa.2014.04.010.Suche in Google Scholar PubMed
11. Guo, N.; Zheng, Y.; Jia, Y.; Qiao, H.; You, H. A Tunable Warm-White-Light Sr3Gd(PO4)3: Eu2+, Mn2+ Phosphor System for LED-Based Solid-State Lighting. New J. Chem. 2012, 36 (1), 168–172; https://doi.org/10.1039/c1nj20532c.Suche in Google Scholar
12. Guo, N.; Zheng, Y.; Jia, Y.; Qiao, H.; You, H. Warm-white-emitting from Eu2+/Mn2+-Codoped Sr3Lu (PO4) 3 Phosphor with Tunable Color Tone and Correlated Color Temperature. J. Phys. Chem. C 2012, 116 (1), 1329–1334; https://doi.org/10.1021/jp209891b.Suche in Google Scholar
13. Wang, Z.; Lou, S.; Li, P. Luminescent Properties and Energy Transfer of Sr3La (PO4) 3: Sm3+, Eu3+ for White LEDs. J. Alloys Compd. 2014, 586, 536–541; https://doi.org/10.1016/j.jallcom.2013.10.023.Suche in Google Scholar
14. Zhang, C.; Liang, H.; Zhang, S.; Liu, C.; Hou, D.; Zhou, L.; Zhang, G.; Shi, J. Efficient Sensitization of Eu3+ Emission by Tb3+ in Ba3La (PO4) 3 under VUV-UV Excitation: Energy Transfer and Tunable Emission. J. Phys. Chem. C 2012, 116 (30), 15932–15937; https://doi.org/10.1021/jp304717z.Suche in Google Scholar
15. Jin, C.; Ma, H.; Liu, Y.; Liu, Q.; Dong, G.; Yu, Q. Tunable Luminescence Properties and Energy Transfer in Ba3Lu (PO4) 3: Ce3+, Tb3+ Phosphors. J. Alloys Compd. 2014, 613, 275–279; https://doi.org/10.1016/j.jallcom.2014.06.043.Suche in Google Scholar
16. Yu, R.; Noh, H. M.; Moon, B. K.; Choi, B. C.; Jeong, J. H.; Jang, K.; Yi, S. S.; Jang, J. K. Synthesis and Luminescence Properties of a Novel Red-Emitting Phosphor Ba3La (PO4) 3: Eu3+ for Solid-State Lighting. J. Alloys Compd. 2013, 576, 236–241; https://doi.org/10.1016/j.jallcom.2013.04.150.Suche in Google Scholar
17. Zhang, X.; Zhou, C.; Song, J.; Zhou, L.; Gong, M. High-brightness and Thermal Stable Sr3La (PO4) 3: Eu3+ Red Phosphor for NUV Light-Emitting Diodes. J. Alloys Compd. 2014, 592, 283–287; https://doi.org/10.1016/j.jallcom.2014.01.018.Suche in Google Scholar
18. Zhang, X.; Zhang, J.; Gong, M. Synthesis and Luminescent Properties of UV-Excited Thermal Stable Red-Emitting Phosphor Ba3Lu (PO4) 3: Eu3+ for NUV LED. Opt. Mater. 2014, 36 (4), 850–853; https://doi.org/10.1016/j.optmat.2013.12.024.Suche in Google Scholar
19. Xu, Q.; Sun, J.; Cui, D.; Di, Q.; Zeng, J. Synthesis and Luminescence Properties of Novel Sr3Gd (PO4) 3: Dy3+ Phosphor. J. Lumin. 2015, 158, 301–305; https://doi.org/10.1016/j.jlumin.2014.10.034.Suche in Google Scholar
20. Bai, Q.; Wang, Z.; Li, P.; Xu, S.; Li, T.; Yang, Z. Tunable Blue-Green Emitting and Energy Transfer of a Eu 2+/Tb 3+ Codoped Sr 3 La (PO 4) 3 Phosphor for Near-UV White LEDs. New J. Chem. 2015, 39 (11), 8933–8939; https://doi.org/10.1039/c5nj01903f.Suche in Google Scholar
21. Liu, Y.; Lan, A.; Jin, Y.; Chen, G.; Zhang, X. Sr3Bi (PO4) 3: Eu2+, Mn2+: Single-phase and Color-Tunable Phosphors for White-Light LEDs. Opt. Mater. 2015, 40, 122–126; https://doi.org/10.1016/j.optmat.2014.12.007.Suche in Google Scholar
22. Zhang, X.; He, P.; Zhou, L.; Pang, Q.; Shi, J.; Gong, M. Sr3La (PO4) 3: Eu2+, Mn2+: a Single-Phased Color-Tunable Phosphor and its Energy Transfer Behavior. J. Lumin. 2015, 157, 352–356; https://doi.org/10.1016/j.jlumin.2014.09.011.Suche in Google Scholar
23. Liu, W.; Wang, X.; Li, J.-G.; Zhu, Q.; Li, X.; Sun, X. Gel-combustion Assisted Synthesis of Eulytite-type Sr3Y (PO4) 3 as a Single Host for Narrow-Band Eu3+ and Broad-Band Eu2+ Emissions. Ceram. Int. 2017, 43 (17), 15107–15114; https://doi.org/10.1016/j.ceramint.2017.08.039.Suche in Google Scholar
24. Bai, G.; Tsang, M. K.; Hao, J. Tuning the Luminescence of Phosphors: beyond Conventional Chemical Method. Adv. Opt. Mater. 2015, 3 (4), 431–462; https://doi.org/10.1002/adom.201400375.Suche in Google Scholar
25. Zhang, C.; Lin, J. Defect-related Luminescent Materials: Synthesis, Emission Properties and Applications. Chem. Soc. Rev. 2012, 41 (23), 7938–7961; https://doi.org/10.1039/c2cs35215j.Suche in Google Scholar PubMed
26. Höppe, H. A. Recent Developments in the Field of Inorganic Phosphors. Angew. Chem. Int. Ed. 2009, 48 (20), 3572–3582; https://doi.org/10.1002/anie.200804005.Suche in Google Scholar PubMed
27. Styskalik, A.; Skoda, D.; Barnes, C. E.; Pinkas, J. The Power of Non-hydrolytic Sol–Gel Chemistry: A Review. Catalysts 2017, 7 (6), 168; https://doi.org/10.3390/catal7060168.Suche in Google Scholar
28. Corriu, R.; Anh, N. T. Molecular Chemistry of Sol–Gel Derived Nanomaterials; John Wiley & Sons: France, 2009.10.1002/9780470742778Suche in Google Scholar
29. Khan, S.; Ullah, I.; Rahman, M. U.; Khan, H.; Shah, A. B.; Althomali, R. H.; Rahman, M. M. Inorganic-polymer Composite Electrolytes: Basics, Fabrications, Challenges and Future Perspectives. Rev. Inorg. Chem. 2024, 44, 347–375.10.1515/revic-2023-0030Suche in Google Scholar
30. Feng, S.; Xu, R. New Materials in Hydrothermal Synthesis. Acc. Chem. Res. 2001, 34 (3), 239–247; https://doi.org/10.1021/ar0000105.Suche in Google Scholar PubMed
31. Lei, F.; Yan, B. Hydrothermal Synthesis and Luminescence of CaMO4: RE3+ (M= W, Mo; RE= Eu, Tb) Submicro-Phosphors. J. Solid State Chem. 2008, 181 (4), 855–862; https://doi.org/10.1016/j.jssc.2008.01.033.Suche in Google Scholar
32. Chavan, A.; Gawande, A.; Gaikwad, V.; Jain, G.; Deore, M. Hydrothermal Synthesis and Luminescence Properties of Dy3+ Doped SrMoO4 Nano-Phosphor. J. Lumin. 2021, 234, 117996; https://doi.org/10.1016/j.jlumin.2021.117996.Suche in Google Scholar
33. Yu, X.; Yuan, D.; Mi, X. Hydrothermal Synthesis and Luminescent Properties of Ca3 (PO4) 2: Dy3+ White-Emitting Phosphors. J. Alloys Compd. 2021, 857, 157585; https://doi.org/10.1016/j.jallcom.2020.157585.Suche in Google Scholar
34. Cushing, B. L.; Kolesnichenko, V. L.; O’connor, C. J. Recent Advances in the Liquid-phase Syntheses of Inorganic Nanoparticles. Chem. Rev. 2004, 104 (9), 3893–3946; https://doi.org/10.1021/cr030027b.Suche in Google Scholar PubMed
35. Yan, B.; Gu, J. Controlled Chemical Co-precipitation and Solid Phase Synthesis, Microstructure and Photoluminescence of La3PO7: Eu3+ Phosphors. J. Non-Cryst. Solids 2009, 355 (14–15), 826–829; https://doi.org/10.1016/j.jnoncrysol.2009.04.024.Suche in Google Scholar
36. Khan, S.; Rahman, M.; Marwani, H. M.; Althomali, R. H.; Rahman, M. M. Bicomponent Polymorphs of Salicylic Acid, Their Antibacterial Potentials, Intermolecular Interactions, DFT and Docking Studies. Z. Phys. Chem. 2023, 238 (01), 1–16; https://doi.org/10.1515/zpch-2023-0378.Suche in Google Scholar
37. Zhu, Y.-J.; Chen, F. Microwave-assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114 (12), 6462–6555; https://doi.org/10.1021/cr400366s.Suche in Google Scholar PubMed
38. Levin, E. E.; Grebenkemper, J. H.; Pollock, T. M.; Seshadri, R. Protocols for High Temperature Assisted-Microwave Preparation of Inorganic Compounds. Chem. Mater. 2019, 31 (18), 7151–7159; https://doi.org/10.1021/acs.chemmater.9b02594.Suche in Google Scholar
39. Miranda de Carvalho, J.; Pedroso, C. C. S.; Saula, M. S. d. N.; Felinto, M. C. F. C.; Brito, H. F. d. Microwave-assisted Preparation of Luminescent Inorganic Materials: A Fast Route to Light Conversion and Storage Phosphors. Molecules 2021, 26 (10), 2882; https://doi.org/10.3390/molecules26102882.Suche in Google Scholar PubMed PubMed Central
40. Khan, S.; Ajmal, S.; Hussain, T.; Rahman, M. U. Clay-Based Materials for Enhanced Water Treatment: Adsorption Mechanisms, Challenges, and Future Directions. J. Umm Al-Qura Univ. Appl. Sci. 2023, 1–16. https://doi.org/10.1007/s43994-023-00083-0.Suche in Google Scholar
41. Khan, S. Phase Engineering and Impact of External Stimuli for Phase Tuning in 2D Materials. Adv. Energy Convers. Mater. 2023, 5 (1), 40–55; https://doi.org/10.37256/aecm.5120243886.Suche in Google Scholar
42. Gröhn, A. J.; Pratsinis, S. E.; Sánchez-Ferrer, A.; Mezzenga, R.; Wegner, K. Scale-up of Nanoparticle Synthesis by Flame Spray Pyrolysis: the High-Temperature Particle Residence Time. Ind. Eng. Chem. Res. 2014, 53 (26), 10734–10742; https://doi.org/10.1021/ie501709s.Suche in Google Scholar
43. Khan, S.; Ullah, I.; Khan, H.; Rahman, F. U.; Rahman, M. U.; Saleem, M. A.; Nazir, S.; Ali, A.; Ullah, A. Green Synthesis of AgNPs from Leaves Extract of Saliva Sclarea, Their Characterization, Antibacterial Activity, and Catalytic Reduction Ability. Z. Phys. Chem. 2024, 238 (5), 931–947; https://doi.org/10.1515/zpch-2023-0363.Suche in Google Scholar
44. Gültekin, S.; Yıldırım, S.; Yılmaz, O.; Keskin, İ. Ç.; Katı, M. İ.; Çelik, E. Structural and Optical Properties of SrAl2O4: Eu2+/Dy3+ Phosphors Synthesized by Flame Spray Pyrolysis Technique. J. Lumin. 2019, 206, 59–69; https://doi.org/10.1016/j.jlumin.2018.10.011.Suche in Google Scholar
45. Yildirim, S.; Asal, E. C. K.; Ertekin, K.; Celik, E. Luminescent Properties of Scintillator Nanophosphors Produced by Flame Spray Pyrolysis. J. Lumin. 2017, 187, 304–312; https://doi.org/10.1016/j.jlumin.2017.03.034.Suche in Google Scholar
46. Maglia, F.; Buscaglia, V.; Gennari, S.; Ghigna, P.; Dapiaggi, M.; Speghini, A.; Bettinelli, M. Incorporation of Trivalent Cations in Synthetic Garnets A3B5O12 (A= Y, Lu− La, B= Al, Fe, Ga). J. Phys. Chem. B 2006, 110 (13), 6561–6568; https://doi.org/10.1021/jp055713o.Suche in Google Scholar PubMed
47. Yashima, M.; Ali, R. Structural Phase Transition and Octahedral Tilting in the Calcium Titanate Perovskite CaTiO3. Solid State Ionics 2009, 180 (2-3), 120–126; https://doi.org/10.1016/j.ssi.2008.11.019.Suche in Google Scholar
48. Bierwagen, J.; Delgado, T.; Jiranek, G.; Yoon, S.; Gartmann, N.; Walfort, B.; Pollnau, M.; Hagemann, H. Probing Traps in the Persistent Phosphor SrAl2O4: Eu2+, Dy3+, B3+-A Wavelength, Temperature and Sample Dependent Thermoluminescence Investigation. J. Lumin. 2020, 222, 117113; https://doi.org/10.1016/j.jlumin.2020.117113.Suche in Google Scholar
49. Moure, C.; Peña, O. Recent Advances in Perovskites: Processing and Properties. Prog. Solid State Chem. 2015, 43 (4), 123–148; https://doi.org/10.1016/j.progsolidstchem.2015.09.001.Suche in Google Scholar
50. Lin, Y.-C. Dynamic Local Structural Symmetries and Luminescenc Properties of the Yellow Emitting Phosphor Ce 3+-Doped Y 3 Al 5 O 12. Chalmers Tek. Hogskola 2016, 2016, 27753643.Suche in Google Scholar
51. Simura, R.; Sawamura, K.; Yamane, H.; Sakakura, T. Occupancies of Y in Garnet-type Ca0. 8Y3. 4Zr0. 8Ga3. 0O12 Crystal. J. Ceram. Soc. Jpn. 2022, 130 (5), 359–362; https://doi.org/10.2109/jcersj2.22003.Suche in Google Scholar
52. Nazir, S.; Zhang, J. M.; Junaid, M.; Saleem, S.; Ali, A.; Ullah, A.; Khan, S. Metal-based Nanoparticles: Basics, Types, Fabrications and Their Electronic Applications. Z. Phys. Chem. 2024, 238, 965–995.10.1515/zpch-2023-0375Suche in Google Scholar
53. Swati, G.; Jaiswal, V. V.; Haranath, D. Rare-earth Doping in Afterglow Oxide Phosphors: Materials, Persistence Mechanisms, and Dark Vision Display Applications. In Spectroscopy of Lanthanide Doped Oxide Materials; Elsevier: India, 2020; pp. 393–425.10.1016/B978-0-08-102935-0.00012-5Suche in Google Scholar
54. Sharma, M.; Sen, S.; Gupta, J.; Ghosh, M.; Pitale, S.; Gupta, V.; Gadkari, S. Tunable Blue-Green Emission from ZnS (Ag) Nanostructures Grown by Hydrothermal Synthesis. J. Mater. Res. 2018, 33 (23), 3963–3970; https://doi.org/10.1557/jmr.2018.358.Suche in Google Scholar
55. Ganesh, I. A Review on Magnesium Aluminate (MgAl2O4) Spinel: Synthesis, Processing and Applications. Int. Mater. Rev. 2013, 58 (2), 63–112.10.1179/1743280412Y.0000000001Suche in Google Scholar
56. Azadmand, M. PA-MBE Growth and Characterization of Nitride Semiconductors, from InGaN Thin-films to GaN and AlN Self-assembled Nanowires; Universita degli Studi di Milano-Bicocca: Milan, 2009.Suche in Google Scholar
57. Reddy, C. P. LUMINESCENCE PROPERTIES OF NaMg4 (PO4) 3: Mn2+ CERAMIC POWDERS. Rasayan J. Chem. 2022, 15 (3). https://doi.org/10.31788/rjc.2022.1536722.Suche in Google Scholar
58. Gul, Z.; Salman, M.; Khan, S.; Shehzad, A.; Ullah, H.; Irshad, M.; Zeeshan, M.; Batool, S.; Ahmed, M.; Altaf, A. A. Single Organic Ligands Act as a Bifunctional Sensor for Subsequent Detection of Metal and Cyanide Ions, a Statistical Approach toward Coordination and Sensitivity. Crit. Rev. Anal. Chem. 2023, 1–17. https://doi.org/10.1080/10408347.2023.2186165.Suche in Google Scholar PubMed
59. Zhou, G.; Ren, Q.; Molokeev, M. S.; Zhou, Y.; Zhang, J.; Zhang, X.-M. Unraveling the Ultrafast Self-Assembly and Photoluminescence in Zero-Dimensional Mn2+-Based Halides with Narrow-Band Green Emissions. ACS Appl. Electron. Mater. 2021, 3 (9), 4144–4150; https://doi.org/10.1021/acsaelm.1c00606.Suche in Google Scholar
60. Chen, R.; Wang, Y.; Hu, Y.; Hu, Z.; Liu, C. Modification on Luminescent Properties of SrAl2O4: Eu2+, Dy3+ Phosphor by Yb3+ Ions Doping. J. Lumin. 2008, 128 (7), 1180–1184; https://doi.org/10.1016/j.jlumin.2007.11.094.Suche in Google Scholar
61 Lin, Y.-C.; Karlsson, M.; Bettinelli, M. Inorganic Phosphor Materials for Lighting. Photoluminescent Mater. Electroluminescent Dev. 2017, 309–355. https://doi.org/10.1007/978-3-319-59304-3_10.Suche in Google Scholar
62. Ahn, Y. N.; Kim, K. D.; Anoop, G.; Kim, G. S.; Yoo, J. S. Design of Highly Efficient Phosphor-Converted White Light-Emitting Diodes with Color Rendering Indices (R 1− R 15)≥ 95 for Artificial Lighting. Sci. Rep. 2019, 9 (1), 16848; https://doi.org/10.1038/s41598-019-53269-0.Suche in Google Scholar PubMed PubMed Central
63. Mehare, M.; Mehare, C. M.; Swart, H.; Dhoble, S. Recent Development in Color Tunable Phosphors: A Review. Prog. Mater. Sci. 2023, 133, 101067; https://doi.org/10.1016/j.pmatsci.2022.101067.Suche in Google Scholar
64. Xia, Z.; Xu, Z.; Chen, M.; Liu, Q. Recent Developments in the New Inorganic Solid-State LED Phosphors. Dalton Trans. 2016, 45 (28), 11214–11232; https://doi.org/10.1039/c6dt01230b.Suche in Google Scholar PubMed
65. Liu, Z.; Lin, C. H.; Hyun, B. R.; Sher, C. W.; Lv, Z.; Luo, B.; Jiang, F.; Wu, T.; Ho, C. H.; Kuo, H. C.; He, J. H. Micro-light-emitting Diodes with Quantum Dots in Display Technology. Light Sci. Appl. 2020, 9 (1), 83; https://doi.org/10.1038/s41377-020-0268-1.Suche in Google Scholar PubMed PubMed Central
66. Ledru, G.; Catalano, C.; Dupuis, P.; Zissis, G. Efficiency and Stability of a Phosphor-Conversion White Light Source Using a Blue Laser Diode. AIP Adv. 2014, 4 (10). https://doi.org/10.1063/1.4899187.Suche in Google Scholar
67. Khan, S.; Iqbal, A. Organic Polymers Revolution: Applications and Formation Strategies, and Future Perspectives. J. Polym. Sci. Eng. 2023, 6 (1), 3125; https://doi.org/10.24294/jpse.v6i1.3125.Suche in Google Scholar
68. Zhao, M.; Zhang, Q.; Xia, Z. Narrow-band Emitters in LED Backlights for Liquid-Crystal Displays. Mater. Today 2020, 40, 246–265; https://doi.org/10.1016/j.mattod.2020.04.032.Suche in Google Scholar
69. Ding, F.; Zhan, Y.; Lu, X.; Sun, Y. Recent Advances in Near-Infrared II Fluorophores for Multifunctional Biomedical Imaging. Chem. Sci. 2018, 9 (19), 4370–4380; https://doi.org/10.1039/c8sc01153b.Suche in Google Scholar PubMed PubMed Central
70. Ullah, A.; Shah Bukhari, K.; Khan, S.; Farooq, F.; Wahab, A.; Hussain, T.; Saleem, S.; Babar, N. Diversification via Coupling Reactions and Biological Activities of Pyrimidine Derivatives. ChemistrySelect 2023, 8 (47), e202303072; https://doi.org/10.1002/slct.202303072.Suche in Google Scholar
71. Nikl, M.; Yoshikawa, A. Recent R&D Trends in Inorganic Single‐crystal Scintillator Materials for Radiation Detection. Adv. Opt. Mater. 2015, 3 (4), 463–481; https://doi.org/10.1002/adom.201400571.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- A review of coordination compounds: structure, stability, and biological significance
- Effluent wastewater technologies for textile industry: a review
- Efficient removal of Cr(VI) ions from industrial wastewater using carbon-based adsorbents functionalized with boronic acid
- Exploring inorganic phosphors: basics, types, fabrications and their luminescence properties for LED/WLED/displays
- Recent advances on hydrogen generation based on inorganic metal oxide nano-catalyst using water electrolysis approach
- Antituberculosis, antimicrobial, antioxidant, cytotoxicity and anti-inflammatory activity of Schiff base derived from 2,3-diaminophenazine moiety and its metal(II) complexes: structural elucidation, computational aspects, and biological evaluation
- Carbon-based nanomaterials: synthesis, types and fuel applications: a mini-review
- Recent advances in carbon quantum dots for antibiotics detection
- Modern innovations in the provision and efficient application of 2D inorganic nanoscale materials
- Bioinorganic metal nanoparticles and their potential applications as antimicrobial, antioxidant and catalytic agents: a review
Artikel in diesem Heft
- Frontmatter
- A review of coordination compounds: structure, stability, and biological significance
- Effluent wastewater technologies for textile industry: a review
- Efficient removal of Cr(VI) ions from industrial wastewater using carbon-based adsorbents functionalized with boronic acid
- Exploring inorganic phosphors: basics, types, fabrications and their luminescence properties for LED/WLED/displays
- Recent advances on hydrogen generation based on inorganic metal oxide nano-catalyst using water electrolysis approach
- Antituberculosis, antimicrobial, antioxidant, cytotoxicity and anti-inflammatory activity of Schiff base derived from 2,3-diaminophenazine moiety and its metal(II) complexes: structural elucidation, computational aspects, and biological evaluation
- Carbon-based nanomaterials: synthesis, types and fuel applications: a mini-review
- Recent advances in carbon quantum dots for antibiotics detection
- Modern innovations in the provision and efficient application of 2D inorganic nanoscale materials
- Bioinorganic metal nanoparticles and their potential applications as antimicrobial, antioxidant and catalytic agents: a review

