Antituberculosis, antimicrobial, antioxidant, cytotoxicity and anti-inflammatory activity of Schiff base derived from 2,3-diaminophenazine moiety and its metal(II) complexes: structural elucidation, computational aspects, and biological evaluation
-
Saleh M. Bufarwa
, Reem M. El-Sefait
, Dalal K. Thbayh
, Mustapha Belaidi
, Rehab K. Al-Shemary
, Rema. M. Abdusamea
, Marei M. El-Ajaily
, Béla Fiser
, Hanan A. Bader
, Abdulsalam A. Saleh
und Mohamad M. Bufarwa
Abstract
Enticed by the present scenario of infectious diseases, four new Co(II), Ni(II), Cu(II), and Cd(II) complexes of Schiff base ligand were synthesized from 6,6′-((1E-1′E)(phenazine-2,3-dielbis(azanylidene)-bis-(methanylidene)-bis-(3-(diethylamino)phenol)) (H 2 L) to ascertain as effective drug for antituberculosis, anti-inflammatory, antioxidant, cytotoxic and antimicrobial activities. The organic ligand and its metal(II) complexes were characterized by numerous physical and spectroscopic methods, which showed that the complexes have a general formula, [ML], (where M = Co(II) (C1), Ni(II) (C2), Cu(II) (C3) and Cd(II) (C4)), for metal complexes have been proposed and have a square planar geometry, are amorphous in nature, and are thermally stable. Data highlight obtained from activity testing against tuberculosis, inflammation, and oxidants that all compounds are significantly active against these symptoms. Also, was to evaluate the effectiveness of various compounds against bacterial and fungal strains. Specifically, four bacterial strains (Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa) and two fungal strains (Aspergillus flavus and Candida albicans) were tested and compared to the results of some standard drugs. The results revealed that compound C4 was more effective against bacterial strains than the comparison standard drugs. In addition, C3 was found to be the most effective of the comparison antibiotics against fungi, while the other compounds showed moderate antifungal activity. Moreover, to support the vitro results, certain computational studies as molecular docking studies, DFT, MESP, and AMEDT were also conducted to confirm the effectiveness of an organic ligand and its complexes against tuberculosis. These studies revealed that C4 is the most effective against tuberculosis and has desirable effects such as absorption, no degradation and no hepatotoxicity, etc.
1 Introduction
Although the use of antibiotics has improved public health around the world, 1 inappropriate antibiotic usage has resulted in the emergence of bacteria that are increasingly resistant to most antibiotics, 2 which means the fight against bacterial infections continues. 3 From this vantage point, the fact that bacterial resistance is a concern everywhere on the globe has made it a major study focus. 4 For instance, it has been discovered that the methicillin-resistant Staphylococcus aureus and the vancomycin-resistant Enterococcus are resistant to both drugs. 5 The emergence of antibiotic-resistant bacteria and the limited efficacy of current antibiotics have made alternative strategies for combating bacterial infections essential to maintaining public health. 6 A recent report by the World Health Organization (WHO) highlights tuberculosis infection (TB) as one of the leading causes of death worldwide. 7 This infection is caused by a group of bacteria known as Mycobacterium tuberculosis. 8 Shockingly, it has been found that around one person out of three is exposed to latent tuberculosis, which is capable of becoming active tuberculosis. 9 Tuberculosis is a disease that can spread among both humans and animals, including bovine tuberculosis, which is common in developing countries. 10 It is a chronic and infectious disease that affects livestock, wild animals, and humans alike, making it a significant public health concern. 11 Since their first synthesis by Hugo Schiff in 1864, Schiff bases have played a significant role in the development of chemistry. 12 , 13 When one equivalent of a diamine derivative is reacted with two equivalents of a salicylaldehyde derivative, a specific type of Schiff base called Salen is formed. 13 The stability of salen complexes containing transition elements has made them a subject of numerous chemistry, physics, and medicine studies. Salen compounds act as tetradentate chelating ligands that form solid metal complexes. 14 The metal ion coordinates through four coordination sites, comprising three chelating rings with two oxygen and two nitrogen atoms. 15 Moreover, many studies have identified that Schiff base complexes of transition metals exhibit biological effects such as antibacterial, 16 antifungal, 17 anti-inflammatory, 18 antioxidant, 19 and anti-tumor activities. 20 Cytotoxicity data revealed that these exhibit significant non-toxic behavior, leading to the conclusion that they can be safely used as anti-inflammatory and anti-cancer agents. 20 Phenazines are a significant group of natural substances that are found in nature. They are crucial for medicinal purposes because of their excellent healing properties. 21 Over the last century, scientists have discovered more than 6,000 phenazine-based compounds, and this number is constantly increasing due to their clinical effectiveness. 22 One specific phenazine-based compound of great interest to chemists and biochemists is 2,3-diaminophenazine, which is an interesting aromatic compound possessing high electron density. 23 Some phenazine derivatives have been reported as antituberculosis agents, such as turbomycin and clofazimine, which have high activity against M. tuberculosis, in addition to their analogs, such as clofazimine with a C2-pyridyl substituent. 22 , 24 So, during the search for an effective anti-infective agent, Schiff base compounds containing diaminophenazine moiety were synthesized due to their biological significance. Schiff base was synthesized by condensing two equivalents of 4-diethylaminosalicylaldehyde with 2,3-diaminophenazine to form 6,6′-((1E-1′E)(phenazine-2,3-dielbis(azanylidene)bis-(methanylidene)bis(3-(diethylamino)phenol) in methanol, which was further treated with Co(II), Ni(II), Cu(II) and Cd(II) nitrate to form the four complexes. The organic ligand and its complexes underwent characterization using various physical and spectroscopic techniques, including molar conductivity, melting point, magnetic susceptibility measurement, thermogravimetric analysis (TGA), elemental analysis (CHN), atomic absorption spectroscopy (AAS), nuclear magnetic resonance (1H and 13C NMR), Fourier transform infrared (FT-IR), UV–Visible absorption (UV–Vis), electronic spin resonance (ESR) and mass spectra. Furthermore, the synthesized compounds were assessed for their antituberculosis, antibacterial, antifungal, antioxidant, anti-inflammatory, and cytotoxic activity and compared to standard drugs. To validate and support the present research, various computational studies were performed. These studies included an examination of the partial docking of compounds as well as the effects of absorption by absorption, distribution, metabolism, extraction, and toxicity (ADMET). Also, highlighted calculations of energy levels (HOMO and LUMO) and electronic distribution, as well as lengths of bonds and angles through discrete Fourier transform (DFT) studies.
2 Experimental
2.1 Chemicals
To conduct the current research, high-purity analytical reagents were used in the experiments including the following chemicals: 4-diethylaminosalicylaldehyde (98 %, Aldrich), 2,3-diaminophenazine (90 %, Aldrich), cobalt nitrate hexahydrate (98 %, Fluka), nickel nitrate hexahydrate (98.5 %, Fluka), copper nitrate trihydrate (99 %, Aldrich), cadmium nitrate tetrahydrate (98 %, Aldrich), dimethyl sulfoxide (99 %, Ficher), N,N-dimethyl formamide (99.9 %, Merck), methanol anhydrous (99.8 %, Aldrich), and ethanol (99.8 %, Fluka).
2.2 Apparatus
Molar conductivity measurements were obtained for solid compounds in dimethylformaldehyde (DMF) solution at room temperature by Jenway 4510 conductivity meter. Magnetic susceptibility calculations were performed using the Faraday method (Faraday equilibrium) at room temperature. The Perkin-Elmer 2400 CHN was utilized for elemental analysis. Also, the metallic content was determined using a Thermo Scientific iCE 3300 spectrometer atomic absorption spectrometer with an auto-sampler model. The FT-IR spectra were recorded using a Thermo Scientific 6700 and a KBr disc. The Beckman Coulter DU 800 spectrometer was used to obtain electronic spectra and dimethylsulfoxide (DMSO) as solvent. The mass spectra of compounds were performed by a direct input unit (DI-50) with the Shimadzu QP-5050 GC-MS. The 1H and 13C NMR spectra were recorded using a Bruker high-performance Avance III NMR spectrometer at 400 MHz, with DMSO‑d6 as the solvent. The ESR spectra of the powder complexes were recorded at room temperature using a Jeol JES-FE 2XG spectrometer. Most of the analyses were carried out by the microanalysis team located at Cairo University in Giza, Egypt. To determine the antimicrobial and antioxidant activity, biological tests were conducted at the Department of Microbiology at Omar Al-Mukhtar University. Cytotoxicity and anti-tuberculosis activity were evaluated at the Faculty of Veterinary Medicine in collaboration with the Animal Health Center in the Laboratory of Preventive Medicine and Public Health.
2.3 Synthesis of ligand
The Schiff base ligand was prepared by 4-diethylaminosalicyaldehyde (1.93 g, 10 mmol) was added to 25 mL of hot methanol, followed by the addition of 2,3-diaminophenazine (1.05 g, 5 mmol) dissolved in 25 mL of methanol (Figure 1). 13 , 15 The mixture was heated and refluxed at 90 °C for 3 h. After condensation, the mixture was left to cool down to room temperature, resulting in the formation of orange precipitated crystals. The crystals were then isolated by filtration.
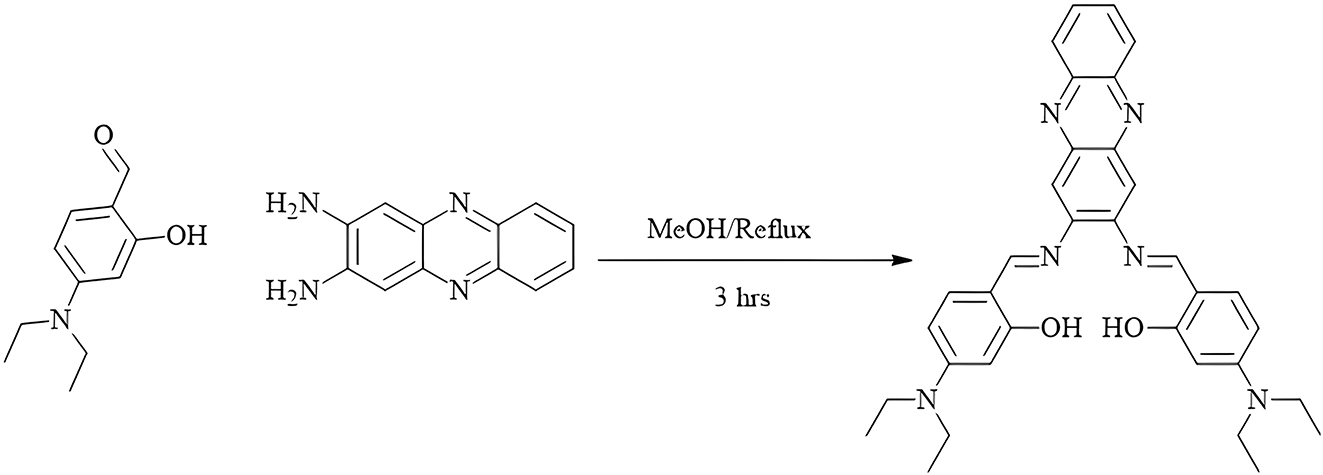
Synthesis of 2,3-diaminophenazine Schiff base (H 2 L).
Characterization of 6,6′-((1E-1′E)-(phenazine-2,3-diylbis(azanylylidene))bis-(metha-nylylidene))bis(3-(diethylamino)phenol) (H 2 L): color: orange; yield: 70 %; m.p.: 132 °C; elemental analysis for organic ligand, m.wt: 560.702 g mol−1 (C34H36N6O2) calculated: C, 72.83 %; H, 6.47 %; N, 14.99 %; found: C, 72.80 %; H, 6.40 %; N, 5.05 %. FT-IR (KBr, cm−1): 3,047 ν(C–H)arm; 3,359 ν(O–H); 1,244 ν(C–N)phe; 1,610 ν(C=N)azo; 1,025 ν(C–O). 1H NMR (300 MHz, DMSO‑d6) δ 13.46 (m, 1H, OH), δ 13.36 (m, 1H, OH), δ 8.62 (m, 2H, CH=N), δ 8.52 (dd, J = 6.2, 3.4 Hz, 2H, Ar–H), δ 8.34 (s, 4H, Ar–H), δ 7.73 (dd, J = 6.2, 3.3 Hz, 2H, Ar–H), δ 7.38 (d, J = 2.1 Hz, 1H, Ar–H), δ 7.30 (d, J = 8.9 Hz, 1H, Ar–H), δ 7.09 (dd, J = 8.7, 2.2 Hz, 2H, Ar–H), δ 6.89 (J = 8.7, 2.5 Hz, 2H, Ar–H), δ 4.79 (q, J = 6.9 Hz, 4H, CH2), δ 2.49 (t, J = 7.1 Hz, 6H, CH3). 13C NMR (125 MHz, CDCl3) δ 187.16, 183.09, 175.56, 169.66, 168.65, 168.01, 157.51, 156.52, 150.17, 149.16, 148.98, 146.03, 144.00, 71.80, 39.52.
2.4 Synthesis of complexes: a general produce
The complexes were prepared by mixing: Co(NO3)2·6H2O (1.456 g, 5 mmol); Ni(NO3)2·6H2O (1.454 g, 5 mmol); Cu(NO3)2·2H2O (1.208 g, 5 mmol) and Cd(NO3)2·4H2O (1.542 g, 5 mmol) with the (2.803 g, 5 mmol) of organic ligand H 2 L (molar ratio 1:1) in solvent mixture DMF/EtOH/H2O (volume ratio 10:3:3 cm3). 15 The mixture was returned at 90 °C for 24 h, the prepared complexes were left to cool at room temperature, then the crystals were filtered. Different analytical methods were used characterize the ligand and its complexes with metal ions. The results obtained for the metal complexes were listed and summarized as follows:
2.4.1 Complex Co(II)L (C1)
Yield 72 %, color: dark wine, m.p.: 192 °C, m.wt: 617.21 g mol−1, Λm: 17.9 Ω−1 cm−1 mol−1; elemental analysis for complex (C34H34CoN6O2) calculated: C, 66.12 %; H, 5.55 %; N, 13.61 % and M, 9.54 %; found: C, 66.05 %; H, 5.52 %, N, 13.59 % and M, 9.57 %. FT-IR (KBr, cm−1): 3,056 ν(C–H)arm; 1,247 ν(C–O); 1,618 ν(C=N); 443 ν(Co–N); 525 ν(Co–O).
2.4.2 Complex Ni(II)L (C2)
Yield 73 %, color: orange, m.p.: 184 °C, m.wt: 617.37 g mol−1, Λm:17.4 Ω−1 cm−1 mol−1; elemental analysis for complex (C34H34NiN6O2) calculated: C, 66.12 %; H, 5.55 %; N, 13.61 % and M, 9.51 %; found: C, 66.11 %; H, 5.49 %, N, 13.68 % and M, 9.48 %. FT-IR (KBr, cm−1): 3,047 ν(C–H)arm; 1,258 ν(C–O); 1,618 ν(C=N); 441 ν(Ni–N); 526 ν(Ni–O).
2.4.3 Complex Cu(II)L (C3)
Yield 73 %, color: dark brown, m.p: 188 °C, m.wt: 622.23 g mol−1, Λm: 16.8 Ω−1 cm−1 mol−1; elemental analysis for complex (C34H34CuN6O2) calculated: C, 65.63 %; H, 5.51 %; N, 13.51 % and M, 10.21 %; found: C, 50.95 %; H, 5.54 %, N, 13.49 % and M, 10.18 %. FT-IR (KBr, cm−1): 3,059 ν(C–H)arm; 1,244 ν(C–O); 1,619 ν(C=N); 443 ν(Cu–N); 523 ν(Cu–O).
2.4.4 Complex Cd(II)L (C4)
Yield 78 %, color: dark orange, m.p: 266 °C, m.wt: 671.08 g mol−1, Λm: 18.5 Ω−1 cm−1 mol−1; elemental analysis for complex (C34H34CdN6O2) calculated: C, 60.85 %; H, 5.11 %; N, 12.52 % and M, 16.75 %; found: C, 61.05 %; H, 5.07 %, N, 12.48 % and M, 16.83 %. FT-IR (KBr, cm−1): 3,053 ν(C–H)arm; 1,245 ν(C–O); 1,620 ν(C=N); 428 ν(Cd–N); 574 ν(Cd–O). 1H NMR (300 MHz, DMSO‑d6) δ 8.72 (m, 2H, CH=N), δ 8.45 (dd, J = 6.2, 3.4 Hz, 2H, Ar–H), δ 8.29 (s, 4H, Ar–H), δ 7.70 (dd, J = 6.2, 3.3 Hz, 2H, Ar–H), δ 7.37 (d, J = 2.1 Hz, 1H, Ar–H), δ 7.28 (d, J = 8.9 Hz, 1H, Ar–H), δ 7.01 (dd, J = 8.7, 2.2 Hz, 2H, Ar–H), δ 6.85 (J = 8.7, 2.5 Hz, 2H, Ar–H), δ 4.79 (q, J = 6.9 Hz, 4H, CH2), δ 2.49 (t, J = 7.1 Hz, 6H, CH3). 13C NMR (125 MHz, CDCl3) 177.93, 172.58, 169.34, 168.81, 159.58, 157.21, 150.43, 149.87, 149.05, 146.63, 144.27, 71.96, 42.74.
2.5 Antimicrobial activity
In order to evaluate the antimicrobial activity of the ligand and its complexes, the agar-well diffusion method was used to test compounds and compare the efficacy with tetracycline, ciprofloxacin, and amphotericin B as standard drugs. 12 , 13 For the objective of comparison, the standard drugs and complexes were subject to identical concentrations and conditions. The antimicrobial activity of the complexes was subsequently assessed in vitro against two kinds of fungal: A. flavus and C. albicans strains as well as four bacterial strains: B. subtilis, S. aureus (+Gram), E. coli, and P. aeruginosa (−Gram).
2.6 Antioxidant activity
The assay measured the radical scavenging effect of stabilized 2,2-diphenyl-1-picrylhydrazyl (DPPH). 13 The 1 μg of ligand and its complexes were dissolved in 1 mL of DMSO, and their antioxidant activities were compared to ascorbic acid. 250 μL of each solution was added to 1 mL of DMSO/DPPH solution, which was prepared by dissolving 6 mg DPPH in 50 mL DMSO. The total volume was then adjusted to 3 mL by adding DMSO. The mixtures were incubated for 30 min at room temperature. The absorbance was measured at 517 nm wavelength, and the blank sample containing 3 mL of DMSO and DPPH was measured as well. The measurements were done in triplicate, and the value of radical scavenging activity was calculated using the following equation:
Where: Acontrol: DPPH absorbance of radical + DMSO after 30 min.
Asample: DPPH absorbance + sample after 30 min.
Where: AE: antiradical efficiency.
IC50: concentration that had 50 % inhibition.
2.7 Cytotoxicity assay
The cytotoxicity assay was conducted using brine shrimp larvae, according to the protocol developed by Mayer et al. 25 This method has been found to correlate well with cytotoxic activity, while it is also cost-effective. Shrimp eggs were incubated in a rectangular dish filled with seawater with the help of the Department of Preventive Medicine and Public Health Laboratory at Omar Al-Mukhtar University. To create an uneven section in the plate, a perforated tool was used. About 50 mg of eggs were sprayed into a large dark chamber, while the smaller chamber was exposed to normal light. After two days, the larvae were collected in a pipette with a lighted side. To perform the test, stock solutions of each test compound were prepared by dissolving 10 mg of the compound in 10 mL of DMSO. Different concentrations of compounds were added to separate vials and the final volume was adjusted to 10 mL with seawater after two days. Once the shrimp larvae were ready, we added 10 larvae to each vial and incubated them for 24 h. The vials were then observed using a magnifying glass and the number of surviving individuals in each vial was counted. The test was performed in duplicate and Finney computer software was used to analyses the data and determine the LD50. The results were compared with a control group positive for the anticancer drug bleomycin.
2.8 Anti-inflammatory activity
The anti-inflammatory activity of the organic ligand and its complexes was evaluated using a BSA (bovine serum albumin, 98 %, Aldrich),) assay. 26 The test was conducted in triplicate with aspirin and sodium diclofenac as standard drugs. Compounds were prepared in DMSO with dilute phosphate buffer (0.2 M, pH 7.4) at concentrations of 15, 25, 50, 100, and 200 µg L−1. 1 mL of BSA (1 mM) in phosphate buffer was dissolved in 4 mL containing various concentrations of the compounds. The BSA was denatured in a water bath at 70 °C for 15 min after incubating the solutions at 37 °C for 20 min. After allowing the solutions to cool at ambient temperature, the test solutions exhibited turbidity. Finally, the absorbance was measured at 660 nm using a UV–Vis spectrometer, and the % denaturation was determined as a control in the absence of any drug and the inhibition of BSA was assessed using the following formula:
Where: X: absorbance of the control.
Y: absorbance of the compounds.
2.9 Anti-tritubercular activity
All compounds were evaluated in vitro for anti-TB using the proportion method. 27 To perform this method, a Lowenstein–Jensen (LJ) medium was prepared by mixing fresh chicken eggs with mineral salt and adding 2 % malachite green. A stock solution of the test compound was created in DMF and then filtered through a 1 µg porous membrane. It was then diluted to 1, 2 and 3 μg/mL. Each solution was transferred to a sterile universal container and put in isolation at 85 °C for 45 min. Control experiments were also conducted without the presence of the test compound. Mel (LJ) was inoculated with 10−4 CFU mL−1 of standard M.TB H 37RV and the clinical isolate. They were successively incubated at 37 °C for 28 days. The tested compounds were also incubated for 14 days and compared with pyrazinamide (PZA) as a standard.
2.10 Computational studies
The computational studies mentioned below were performed on the organic ligand and its metal complexes against active anti-tuberculosis compounds to support in vitro results.
2.10.1 Generation and optimization of compounds
The molecular editor Gauss View 6.0. was used to create and optimize the three-dimensional structure of the organic ligand and its metal complexes. To obtain the optimization of exchange and correlation functions, B3LYP was applied with basic set 6-31G (d,p)/LANL2DZ.
2.10.2 Obtaining the three-dimensional structure of the target
The target structure for pfndh was obtained from the Protein Data Bank (PDB) (www.rcsb.org) to serve as the intended target.
2.10.3 Molecular docking
The significance of molecular docking lies in its ability to predict the conformation of compounds within the active sites or the target molecular binding site, as well as to predict the binding aspects of a specific interaction. Using the AutoDock tool, 28 , 29 molecular docking was carried out on an organic ligand and its complexes with a specific target of the M. tuberculosis structure (PDB ID:1DQZ) obtained from the Protein Data Bank.
2.10.4 DFT and MESP analysis
Molecular properties and analysis of chemical reactions to predict the ability of compounds to interact with each other rely largely on theoretical methods. These methods are frequently used to determine the molecular structure of manufactured compounds because they are efficient and accurate. Therefore, some theoretical studies were carried out to obtain additional information about the computer calculations of the prepared compounds. Related fields were also studied based on density functional theory (DFT). 30 Studying the relationship between the geometry and electronic properties of chemical compounds that contain the highest occupied molecular orbitals (HOMO) and lowest occupied molecular orbitals (LUMO) orbitals is crucial, along with some quantity parameters. In addition, the bond lengths and angles of the prepared compounds were calculated computationally.
2.10.5 ADMET analysis
The AdmetSAR web server was used to anticipate the absorption, metabolism, and carcinogenicity of the ligand and its metal complexes. To do this, the structures of the compounds were uploaded onto the server and the drug-like compounds were assessed based on their ADMET characteristics. 31
3 Results and dissection
3.1 Chemistry
Metal ion complexes of Co(II), Ni(II), Cu(II) and Cd(II) were prepared in the solid state. They are soluble in some organic solvents, such as DMF and DMSO, and not soluble in water. The low molar conductivity of the complexes was measured in 10−3 M in DMF solution at ambient temperature, indicating that they are non-electrolyte in nature. 32 The thermal stability of the complexes was determined up to 253 °C using TGA. The square planar geometry of the N and O atoms of the Schiff base ligand in the complexes was determined using various spectral analysis methods.
3.2 Spectral and physical methods
The current study, investigated the nature of chemical bonding between atoms, using thermal decomposition and various spectral and physical techniques for analysis, as shown below.
3.3 Elemental analysis
The elemental components and the purity of the prepared complexes were determined by (C %, H %, N % and M %) elemental analysis and are mentioned in (Table 1).
Physical features of ligand (HL) along with its complexes.
| Symbol | Molecular formula | M wt | Color | Yield % | Λm | M.p. °C | Cal (found) % | |||
|---|---|---|---|---|---|---|---|---|---|---|
| C | C | H | N | M | ||||||
| HL | C34H36N6O2 | 560.69 | Orange | 70 | – | 132 | 72.83 | 6.47 | 14.99 | – |
| (72.80) | (6.40) | (15.05) | – | |||||||
| C1 | C34H34N6NiO2 | 617.37 | Orange | 73 | 17.9 | 184 | 66.15 | 5.55 | 13.61 | 9.51 |
| (66.04) | (5.49) | (13.57) | (9.48) | |||||||
| C2 | C34H34CoN6O2 | 617.21 | Dark wine | 72 | 17.4 | 192 | 66.12 | 5.55 | 13.61 | 9.54 |
| (66.05) | (5.52) | (13.59) | (9.57) | |||||||
| C3 | C34H34CuN6O2 | 622.22 | Dark brown | 74 | 16.8 | 188 | 65.63 | 5.51 | 13.51 | 10.21 |
| (65.60) | (5.48) | (13.46) | (10.25) | |||||||
| C4 | C34H34CdN6O2 | 671.08 | Dark orange | 73 | 18.5 | 266 | 60.85 | 5.11 | 12.52 | 16.75 |
| (60.93) | (4.98) | (12.60) | (16.80) | |||||||
3.4 Molar conductivity
The molar conductivity of the prepared complexes was measured using a 10−3 M dimethylformamide (DMF) solution. The obtained results showed that the C1, C2, C3, and C4 complexes have low molar conductivity values (17.9, 17.4, 16.8, and 18.5 Ω−1 cm2 mol−1). These values confirmed that the complexes are non-electrolytic in nature. 32
3.5 Mass spectra
The peaks of the complexes’ mass spectra were recorded by correlating the peaks of the molecular ions (m/z) with the molecular mass of the complexes. The bonds of the complexes show molecular ionic peaks at m/z 617.2, 616.2, 621.2, and 672.1 for C1, C2, C3, and C4, respectively, which is consistent with the proposed molecular mass values for complexes (Figures S1–S4).
3.6 FT-IR spectra
Table 2 presents the FT-IR data for the ligand and their corresponding metal complexes. In the FT-IR spectra, the free Schiff base ligand H 2 L displays a sharp band at 1,630 cm−1, which is attributed to the ν(C=N) azomethine group. 14 Additionally, the broadband at 3,359 cm−1 indicates the presence of the ν(OH) phenolic group in the ligand. 23 , 33 Furthermore, the range of 1,237 cm−1 shows the occurrence of ν(C–O) phenolic. The broadband is observed in regions 1,547 and 1,258 cm−1 due to the (C=C) and (C–N), respectively. 34 Upon chelating, the bands are shifted higher or lower indicating the participation of the phenolic oxygen atom in coordination with the metal ion. Based on the non-electrolytic nature of metal complexes, it can be concluded that metal ions coordinate with phenolic oxygen due to their bivalency satisfaction. 35 The absence of broadband attributed to ν(OH) phenolic in the FT-IR spectra of metal complexes indicates the coordination of the central metal bonds of C1, C2, C3, and C4 by the phenolic oxygen atom. 36 This shift is attributed to the decrease in the property of the double bond between ν(C=N) azomethine, which occurred due to the entry of nitrogen into coordination with the metal ion. 37 The ν(M−O) and ν(M–N) complexes have frequency patterns of 525, 526, 523, and 541 cm−1; and 443, 441, 443 and 487 cm−1, respectively. 38 , 39
FT-IR spectra characterization of organic ligand and its complexes.
| Compound | ν(OH)phenolic | ν(C=C)aromatic | ν(C=N)azomethine | ν(C–O)phenolic | ν(MN) | ν(MO) |
|---|---|---|---|---|---|---|
| H2L | 3,359 | 1,574 | 1,630 | 1,237 | – | – |
| C1 | – | 1,557 | 1,594 | 1,188 | 443 | 525 |
| C2 | – | 1,561 | 1,581 | 1,207 | 441 | 526 |
| C3 | – | 1,556 | 1,589 | 1,194 | 443 | 523 |
| C4 | – | 1,559 | 1,615 | 1,175 | 487 | 541 |
3.7 Electronic absorption spectra and magnetic moments
The stereochemistry of the organic ligand H 2 L and its complexes is well examined by UV–Vis spectra, which were evaluated in DMSO at ambient temperature (Figure S6). The absorption band recorded at (273 nm, 36,630 cm−1) was assigned to π→π* transitions that correspond to the phenolic ring of the ligand. 40 The absorption band at (406 nm, 24,630 cm−1) is attributed to n→π transitions of azomethine. 41 compared with the organic ligand, the Co(II) and Ni(II) complexes showed three absorption bands, while the Cu(II) complex contained two absorption bands, while the Cd(II) complex showed one absorption band due to the d10 electronic configuration and charge transfer from the ligand to the Cd(II) ion and are mentioned in (Table 3). 42 Magnetic moment values for C1, C2, and C3 complexes were found in the range of 2.1, 1.6, and 1.5 BM, respectively, and the C4 complex did not show a value for the magnetic moment due to the electrical configuration of d10. 43
Electronic spectra data of ligand and its complexes.
| Compound | λ (nm) and υ (cm−1) | Band assignments | µeff (BM) | Suggested structure |
|---|---|---|---|---|
| H 2 L | 273 nm, 36,630 cm−1 | π→π* benzene ring | – | – |
| 406 nm, 24,630 cm−1 | n→π* azomethine | |||
| C1 | 257 nm, 38,910 cm−1 | HL chromophore (π→π*) | 2.1 | Square planar |
| 387 nm, 25,839 cm−1 | (C.T) 4T1g(F)→4T1g(P) | |||
| 944 nm, 10,593 cm−1 | 4T1g(F)→4A2g(P) (d–d) | |||
| 1,213 nm, 8,244 cm−1 | 4T1g(F)→4T2g(P) (d–d) | |||
| C2 | 253 nm, 39,525 cm−1 | HL chromophore (π→π*) | 1.6 | Square planar |
| 385 nm, 25,974 cm−1 | (C.T) 1A1g→1A2g | |||
| 438 nm, 22,831 cm−1 | 2A1g→1Eg | |||
| 618 nm, 16,181 cm−1 | 1A1g→1A2g | |||
| C3 | 255 nm, 39,215 cm−1 | HL chromophore (π→π*) | 1.5 | Square planar |
| 376 nm, 26,595 cm−1 | (C.T) 2B1g→2Eg | |||
| 580 nm, 17,241 cm−1 | 2B1g→2A1g | |||
| C4 | 284 nm, 35,211 cm−1 | HL chromophore (π→π*) | Dia. | Square planar |
| 380 nm, 26,315 cm−1 | (C.T) (L→M) |
3.8 1H NMR and 13C NMR spectra
1H and 13C NMR analysis provides the positions of proton and carbon signals for the Schiff base ligand H 2 L and its C4 (Table 4, Figures S7 and S8). Two phenol δ OH signals were observed at 13.43 and 13.46 ppm, while the δ CH=N signal of the azomethine proton appeared at 8.62 ppm in the 1H NMR spectra of the ligand. 23 Fourteen aromatic δ C–H protons were recorded in the range of 4.79 ppm, and there were four aliphatic δ CH2 protons and six δ CH3 protons in the ligand. 44 In the C4, the phenol proton at δ OH 13.43 and 13.46 ppm in the ligand disappears due to its entry into the complex. 13 Moreover, the signal shift of the azomethine group of the ligand indicates the formation of the complex via the nitrogen atom of azomethine. In the 13C NMR spectra, a signal appears at 187.16 ppm attributed to two azomethine carbons in the ligand. Signals also appear at 175.56–144.00 ppm are attributed to the carbons in the aromatic ring of quinoxaline, C27, C28, C29, C30, C31, C32, C33, C35, C37, C38, C39, C40 and C41. The signal for the aliphatic CH3 group appears at 39.52 ppm, C11, C12, C25 and C26, while the aliphatic CH2 signal appears at 71.80 ppm, C10, C11, C23 and C24. 42
1H NMR and 13C NMR data in ppm of organic ligand and its Cd complexe.
| Compound | 1H NMR (DMSO‑d6 – 300 MHZ) | 13C NMR (CDCl3) |
|---|---|---|
| H 2 L | δ 13.43–13.46 (m, s, 2H for OH phenolic ring), δ 8.62 (d, J, 2H for CH=N azomethine), δ 6.89–8.53 (s, m, 14H, for C–H aromatic ring), 4.79 (m, 6H for C–H aliphatic) | δ 187.16, 183.09, 175.56, 169.66, 168.65, 168.01, 157.51, 156.52, 150.17, 149.16, 148.98, 146.03, 144.00, 71.80, 39.52 |
| C4 | δ 8.97 (d, J, 2H for CH=N azomethine), δ 7.47–8.15 (s, m, 14H, for C–H aromatic ring), 4.76 (m, 6H for C–H aliphatic) | δ 180.57, 170.38, 167.75, 162.83, 154.53, 154.47, 149.76, 149.16, 148.67, 145.94, 143.85, 70.92, 39.47 |
3.9 ESR spectra
ESR spectra of a metal complex provide information about the metal and its environment within the complex. 45 This includes details about the geometric structure and location arrangement of the Schiff base and the metal. Figure 2 illustrates the ESR spectra of the copper complex at room temperature. The data suggests that the delocalized electron is in the dx2–y2 orbital and its ground state is 2B1g with square planar geometry. The metal–ligand bonds have a covalent nature with a g‖ value of less than 2.0023. Hathaway’s statement suggests that if G < 4, there are some exchange interactions, while if G > 4, there is little interaction between the copper ions. The properties of the square planar geometry can be determined by the symmetry axis parameter G, which has a value of 1.609, indicating significant exchange interactions between the copper centers. 46 Furthermore, the correlation parameters α2(in-plane σ bonding), β2(in-plane π bonding), and γ2(out-plane π bonding), were predicted based on the values of the basic parameters derived (g‖, g┴ and A‖) as shown in Table 5. The resulting values indicate that K2‖ > K2┴, suggesting the presence of a large external bond in the copper complex, confirming its strong covalent nature.
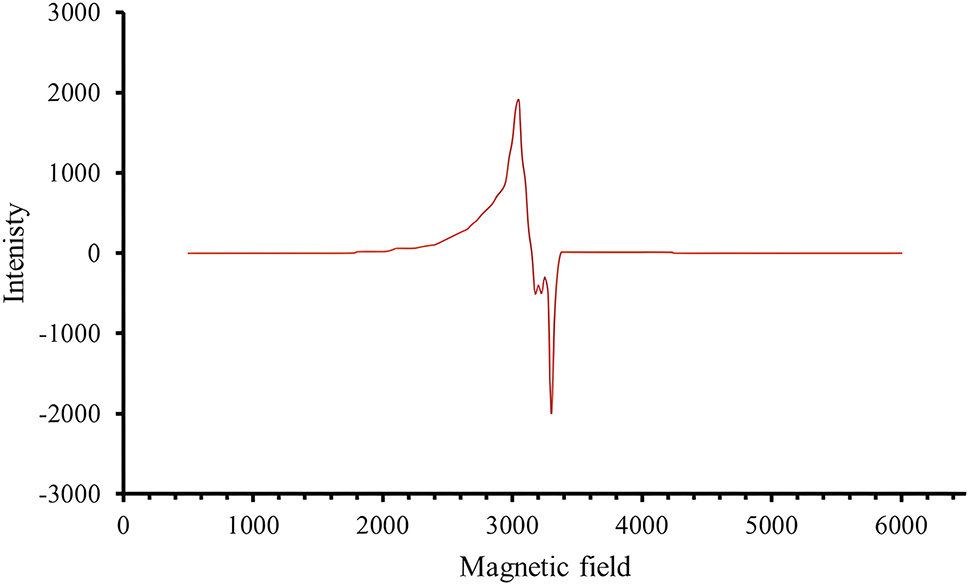
The ESR spectra of H 2 L and C1, C2, C3 and C4 at room temperature.
Spin Hamiltonian parameters of the copper complex at room temperature.
| Complex | g ‖ | g ┴ | A ‖ | α 2 | β 2 | γ 2 | K 2‖ | K 2┴ | µ eff |
|---|---|---|---|---|---|---|---|---|---|
| C3 | 2.0757 | 2.0304 | 0.0187 | 0.650 | 0.196 | 0.302 | 0.127 | 0.046 | 1.5 |
3.10 Thermal analysis
In the current work, we conducted thermal analysis to investigate the thermal stability of Schiff base complexes, synthesized during the research. The aim was to determine the presence or absence of solvent molecules, and whether they were located inside or outside the inner coordination sphere of the complex. 47 , 48 The ligand and its complexes underwent thermogravimetric analysis from 0 to 600 °C with a heating rate of 10 °C min−1. All thermal decomposition data for the ligand and its complexes shown in (Figure 3) are listed in (Table 6). The thermal curve pattern for the decomposition of C1, C2, and C3 complexes converges in three steps, with a slight difference in C4, which has four steps. The complexes begin to decompose in the first step at a temperature range at 218–243 °C with the loss of the two azomethine groups and part of the organic ligand. In the second step, the remaining organic ligand decomposes at 235–520 °C, leaving the metal oxide as a final product behind.
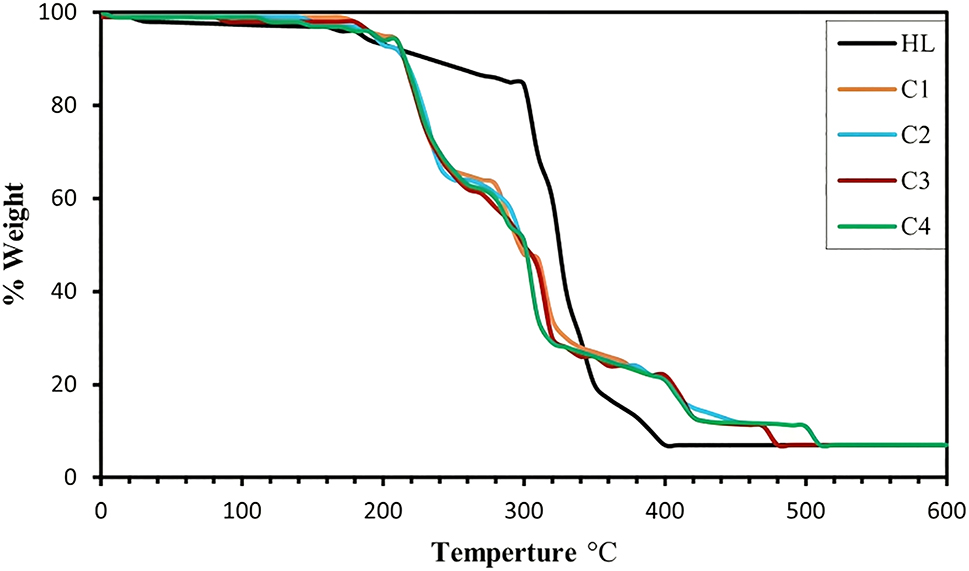
TGA curve of H 2 L and C1, C2, C3 and C4.
Thermal analysis of the decomposition of metal complexes.
| Symbol | Molecular formula (molecular weight) | TG range °C | % weight lost | Assignments | |
|---|---|---|---|---|---|
| Calc. | (Found) | ||||
| H 2 L | C34H36N6O2 (560.69) | 170–400 | 12.84 87.03 |
(12.90) (86.91) |
|
| C1 | C34H34N6CoO2 (617.21) Residue |
218–235 235–320 320–410 410–480 >480 |
8.75 28.84 23.82 26.41 |
(8.69) (28.79) (23.80) (26.40) |
|
| C2 | C34H34N6NiO2 (617.37) Residue |
220–240 240–325 325–420 420–487 >487 |
8.75 28.83 23.81 26.40 |
(8.71) (28.85) (23.80) (26.39) |
|
| C3 | C34H34N6CuO2 (622.22) Residue |
220–239 239–296 296–386 386–494 >494 |
8.68 28.61 23.63 26.20 |
(8.70) (28.60) (23.64) (26.19) |
|
| C4 | C34H34N6CdO2 (671.08) Residue |
219–243 243–320 320–410 410–480 480–520 >520 |
8.05 26.52 21.90 10.89 13.56 |
(8.07) (26.51) (21.87) (10.90) (13.58) |
|
3.11 Geometrical structures
The geometries of ligand and the metal complexes were calculated after optimizing the angle and bond lengths using the Gaussian 09 program package. 49 Density functional theory (DFT) method has been used, the B3LYP functional along with the 6-31G(d) basis set for ligands and LANL2DZ for metals in the solvent phase. 50 Structure data for the selected bond lengths are listed in (Table 7 and 8). The optimized conformations of all complexes are listed in (Figure 4). Structure data for the selected bond lengths are listed in (Table S2). The bond lengths of all complexes of the optimized conformations in (Figure 11) are listed in (Tables S3–S4). The theoretical data showed several points:
The lengths of bonds belonging to the coordinated atoms suffer elongation from the free ligand, due to the entry of MO and MN into coordination.
The MN bond is longer than the MO bond in all complexes.
There was no significant change in the other bond lengths of the atoms.
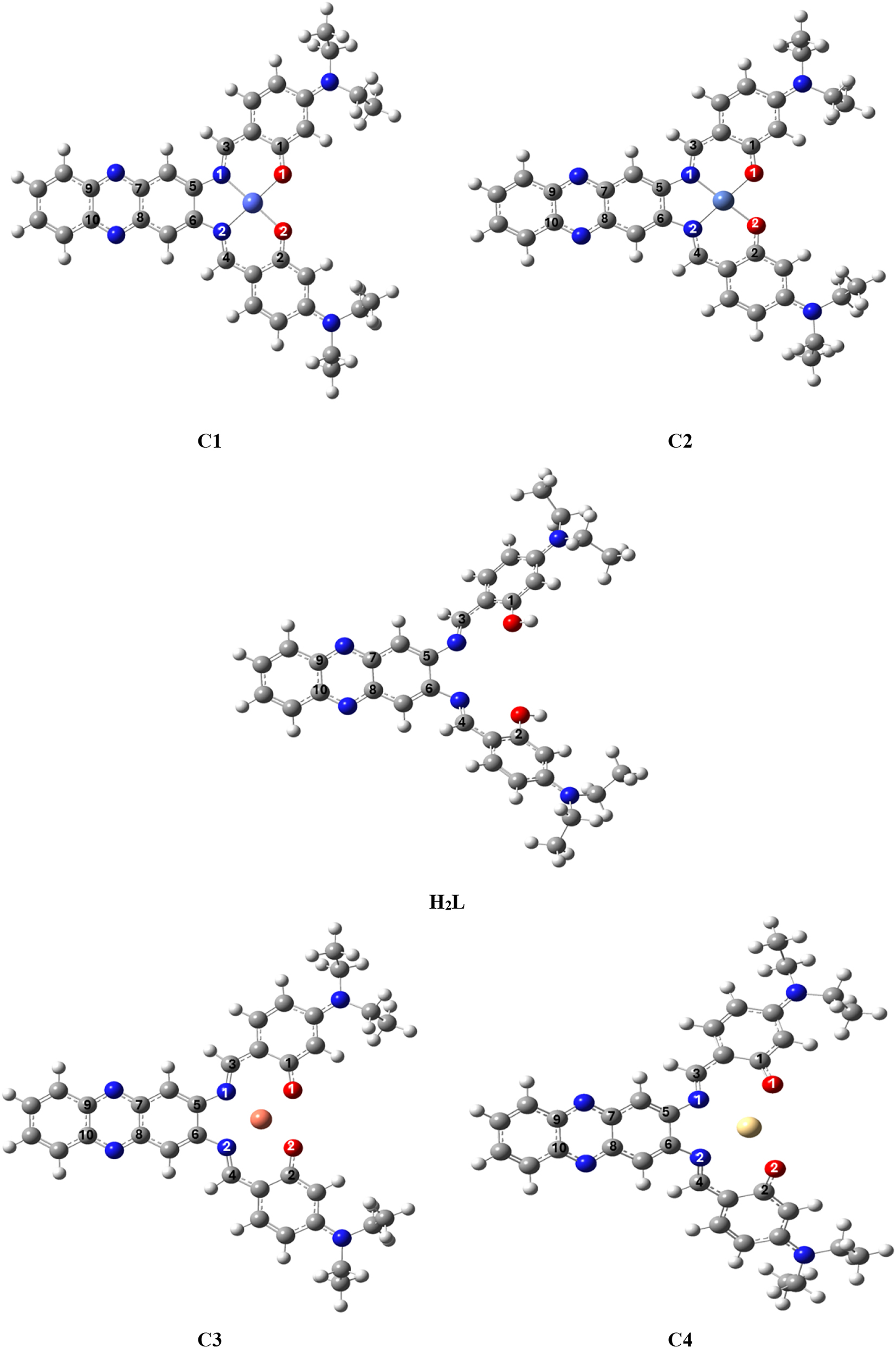
The optimized conformations of H 2 L and C1, C2, C3 and C4.
The two basic orbitals that play a crucial role in chemical stability are the highest molecular orbital, HOMO, and the unoccupied molecular orbital, LUMO. The HOMO refers to the ability to donate an electron, while LUMO refers to the ability to gain an electron as an electron acceptor. The (Table 9 and Figure 5) list the HOMO and LUMO energies obtained from calculations of complexes with C1, C2, and C3. Moreover, additional parameters such as HOMO–LUMO energy gap Eg, absolute electronegative χ, chemical potentials Pi, absolute hardness η, absolute softness σ, global electrophilicity ω, global softness S and additional electronic charge ΔNmax were calculated and are listed in (Table 9). 51 , 52 The energy gap Eg. between HOMO and LUMO is an important metric for predicting the stability of compounds. A lower value of the energy gap Eg. indicates a higher reactivity of the compound. 52 Compared to the other complexes, the C3 complex was found to be the most reactive. The energy gap values of the complexes C1, C2, C3, and C4 are 0.76, 0.73, 0.43, and 2.57 eV, respectively.
The calculated quantum chemical parameters of complexes.
| Complex | HOMO | LUMO | E g | χ | η | σ | Pi | S | ω | ΔN |
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | −5.07 | −2.58 | 2.49 | 3.83 | 1.25 | 0.8 | −3.83 | 0.4 | 5.87 | 3.06 |
| C2 | −5.13 | −2.60 | 2.53 | 3.87 | 1.27 | 0.79 | −3.87 | 0.39 | 5.88 | 3.04 |
| C3 | −5.11 | −2.56 | 2.55 | 3.84 | 1.28 | 0.78 | −3.84 | 0.39 | 5.76 | 3.01 |
| C4 |
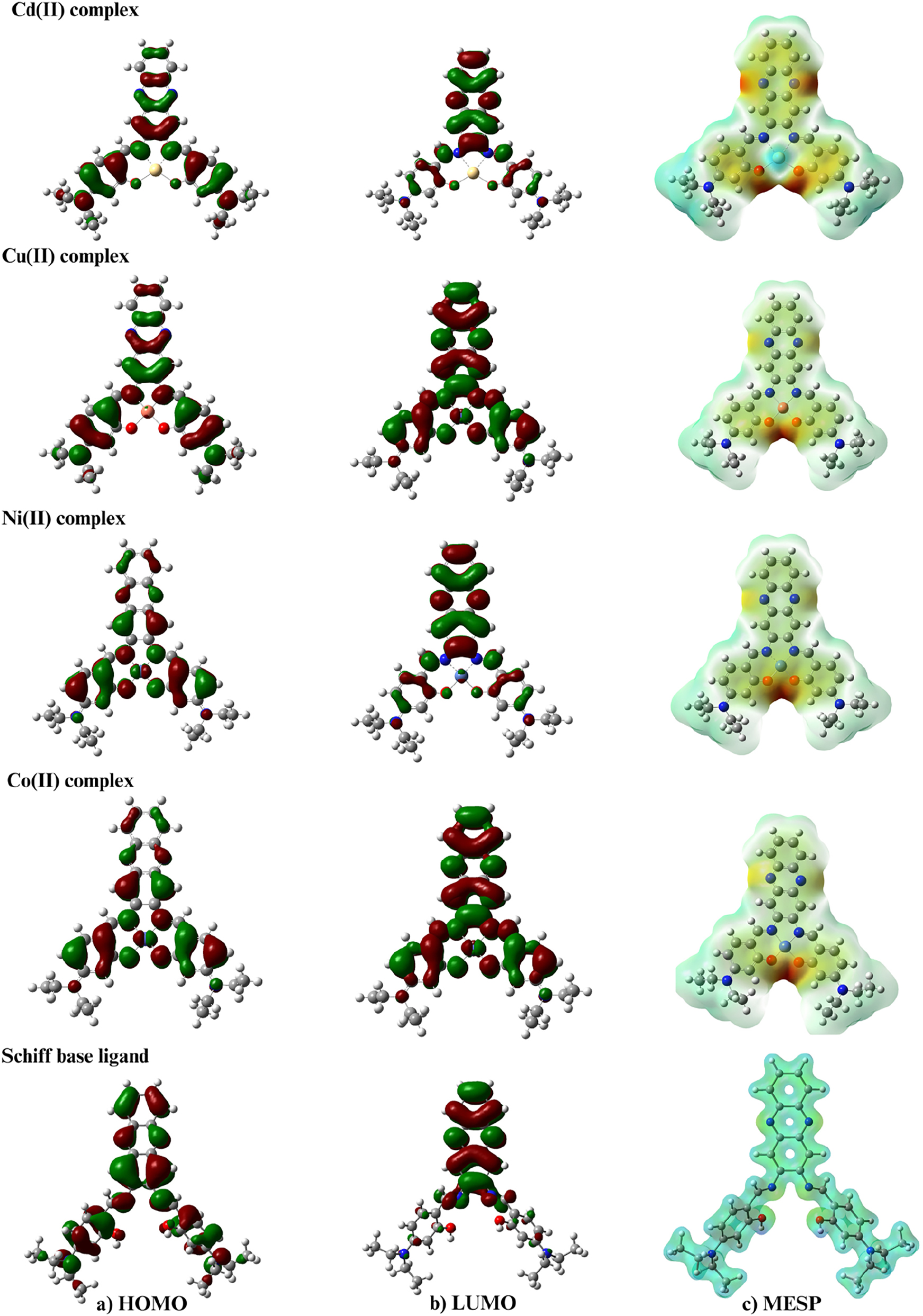
Molecular orbital distribution plots of HOMO, LUMO state, and MESP of the compounds.
The selected angle length (Å) of complexes.
| Bond | H2L | Bond length (Å) | |||
|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | ||
| C1–O | 1.36783 | 1.30922 | 1.30887 | 1.31157 | 1.30866 |
| C2–O | 1.36783 | 1.30924 | 1.30924 | 1.31157 | 1.30866 |
| C3–N | 1.29520 | 1.32137 | 1.32341 | 1.32486 | 1.31531 |
| C4–N | 1.29520 | 1.32130 | 1.32311 | 1.32488 | 1.31544 |
| C5–N | 1.39340 | 1.40142 | 1.40576 | 1.40673 | 1.39387 |
| C6–N | 1.39343 | 1.40137 | 1.40549 | 1.40672 | 1.39349 |
| C7–N | 1.34072 | 1.34261 | 1.34277 | 1.34281 | 1.34181 |
| C8–N | 1.34071 | 1.34261 | 1.34276 | 1.34281 | 1.34174 |
| C9–N | 1.35023 | 1.34797 | 1.34758 | 1.34712 | 1.34952 |
| C10–N | 1.35023 | 1.34796 | 1.34763 | 1.34712 | 1.34944 |
| O1–M | – | 1.94011 | 1.90472 | 1.86628 | 2.20046 |
| O2–M | – | 1.94144 | 1.90450 | 1.86618 | 2.20065 |
| N1–M | – | 1.99403 | 1.92699 | 1.89204 | 2.37506 |
| N2–M | – | 1.99495 | 1.92721 | 1.89196 | 2.37511 |
Understanding the polarizability, acid-base behavior, structural properties of a molecule, and electrophilic attack sites through electrostatic potentials is crucial and has various applications in quantum chemical calculations. Figure 5 demonstrates the significance of the MESP (Molecular Potential Surface) chart, which was introduced by Gauss View 6.0.1 in the chart, the red, green, and blue colors indicate the negative, neutral, and positive ESP (Electrostatic Potential) regions, respectively. Figure 6 depicts the most positive element of the Cd(II) atom. Generally, the highest negative ESP region is observed in the phenolic oxygen atoms, the two nitrogen atoms of the Schiff base, and the two nitrogen atoms of the phenazine moiety.
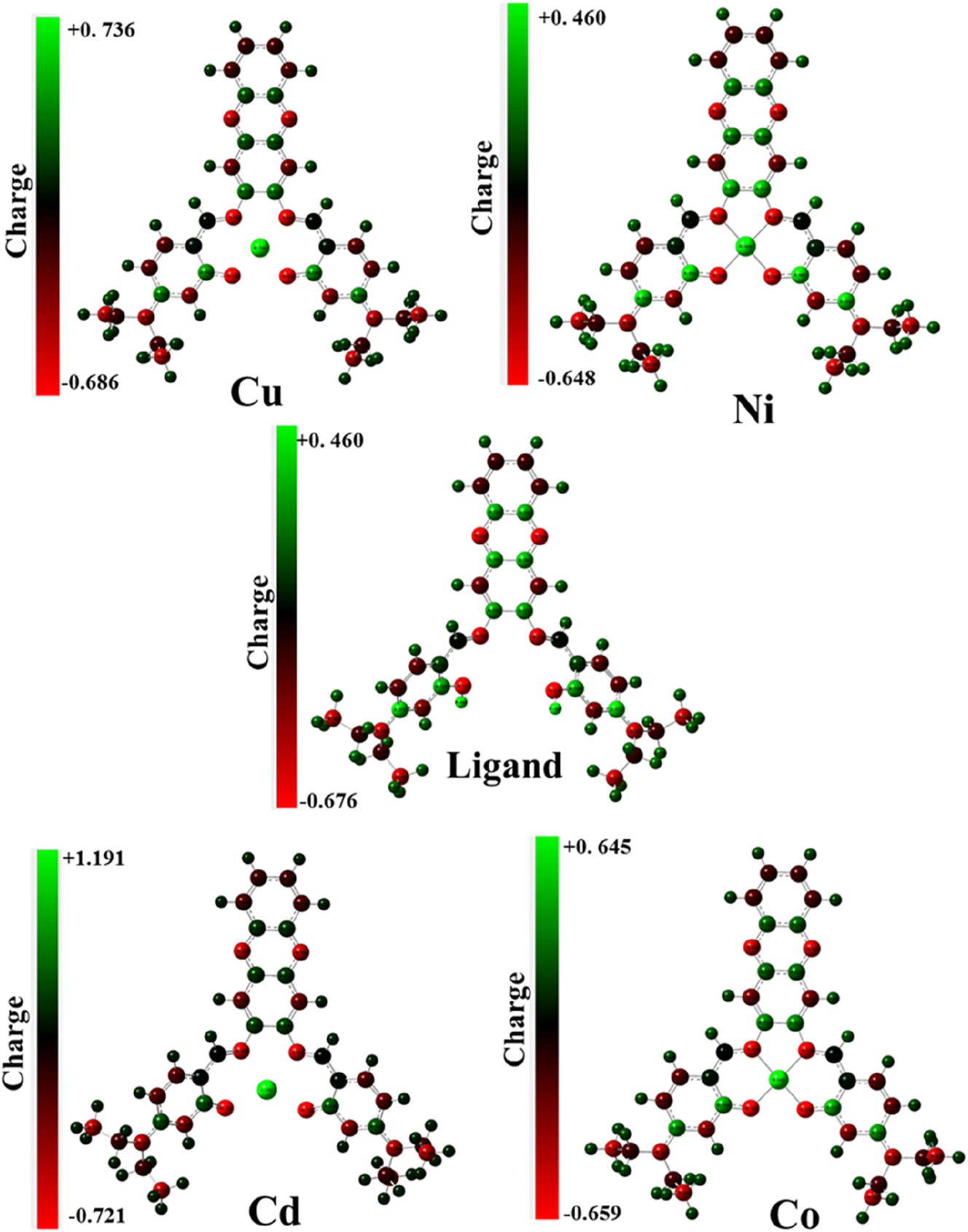
Molecular electrostatic potential map of compounds.
3.12 Biological activity
3.12.1 Antimicrobial activity
The antibacterial and antifungal activities of a ligand and its complexes were tested against six different microorganisms, including B. subtilis, S. aureus, E. coli, P. aeruginosa, A. flavus, and C. albicans. The tests were conducted using a DMSO solvent with a concentration of 1 mg mL−1, and the well-diffusion method was employed to determine the results. The standard drugs Ciprofloxacin, Tetracycline, and Amphotericin B were also screened at the same concentration. The data presented in (Table 10 and Figure 7) demonstrate that the complexes exhibit moderate to good antibacterial activity as compared to standard drugs. The following points provide a summary of the obtained results:
The ligand and its complexes were found to have only slight activity against C. albicans, except the C3 which showed more significant activity.
The ligand and its complexes exhibited higher activity against bacterial strains and, in some cases, higher than the standard drugs tested. iii. In some cases, the data showed that activity increased after complexation compared to the free ligand. 53 This can be attributed to the chelation theory, which proposes that complexation reduces the polarity of the bond between the central atom and the ligand, making it easier for the complexes to penetrate the lipid layer of the cell membrane. 54
The selected bond length (Å) of complexes.
| H2L | Start atom | End atom | C1 | Start atom | End atom | C2 | Start atom | End atom | C3 | Start atom | End atom | C4 | Start atom | End atom |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CNC 122.38 |
C21 | N1 | N–Co–N 86.25 |
N1 | N2 | N–Ni–N 86.84 |
N1 | N2 | N–Cu–N 87.52 |
N1 | N2 | N–Cd–N 86.99 |
N1 | N2 |
| CNH | C23 | H35 | N–Co–O 94.41 |
N1 | O1 | N–Ni–N 94.40 |
N1 | O1 | N–Cu–O 94.24 |
N1 | O1 | N–Cd–O 93.57 |
N1 | O1 |
| CNH | C23 | H35 | N–Co–O 179.41 |
N1 | O2 | N–Ni–O 178.74 |
N1 | O2 | N–Cu–O 176.26 |
N1 | O2 | N–Cd–O 178.83 |
N1 | O2 |
| CNC | C22 | N2 | N–Co–O 179.34 |
N2 | O2 | N–Ni–O 178.74 |
N2 | O1 | N–Cu–O 176.26 |
N2 | O1 | N–Cd–O 178.83 |
N2 | O1 |
| COH 123.83 |
C1 | H37 | O–Co–O 85.00 |
O1 | O2 | N–Ni–O 94.41 |
N2 | O2 | O–Cu–O 83.81 |
N2 | O2 | O–Cd–O 93.57 |
N2 | O2 |
| COH 123.83 |
C7 | H38 | Co–NC 125.22 |
Co | C21 | Ni–NC 126.19 |
Ni | C21 | Cu–NC 125.57 |
Cu | C21 | Cd–NC 124.86 |
Cd | C21 |
| Co–NC 125.29 |
Co | C23 | Ni–NC 121.71 |
Ni | C23 | Cu–NC 109.59 |
Cu | C23 | Cd–NC 126.86 |
Cd | C23 | |||
| Co–OC 130.59 |
Co | C1 | Ni–OC 133.91 |
Ni | C1 | Cu–OC 132.04 |
Cu | C1 | Cd–OC 131.15 |
Cd | C1 | |||
| Co–OC 130.67 |
Co | C7 | Ni–OC 133.91 |
Ni | C7 | Cu–OC 132.03 |
Cu | C7 | Cd–OC 131.15 |
Cd | C7 |
Antimicrobial activity of the investigated complexes.
| Compound | Antimicrobial activity (inhibition zone diameter in mm) | |||||
|---|---|---|---|---|---|---|
| B. subtilis (+G) | S. aureus (+G) | E. coli (−G) | P. aeruginosa (−G) | A. flavus (fungus) | C. albicans (fungus) | |
| DMSO, control | NA | NA | NA | NA | – | – |
| Ciprofloxacin | 7 | 5 | 7 | 7 | – | – |
| Tetracycline | 27 | 28 | 32 | 23 | – | – |
| Amphotericin B | – | – | – | – | 21 | 20 |
| HL | 25 | 30 | 18 | 14 | 7 | 5 |
| C1 | 36 | 33 | 24 | 22 | 5 | 5 |
| C2 | 28 | 30 | 24 | 26 | 8 | 8 |
| C3 | 28 | 32 | 25 | 24 | 14 | 14 |
| C4 | 38 | 37 | 28 | 16 | 7 | 27 |
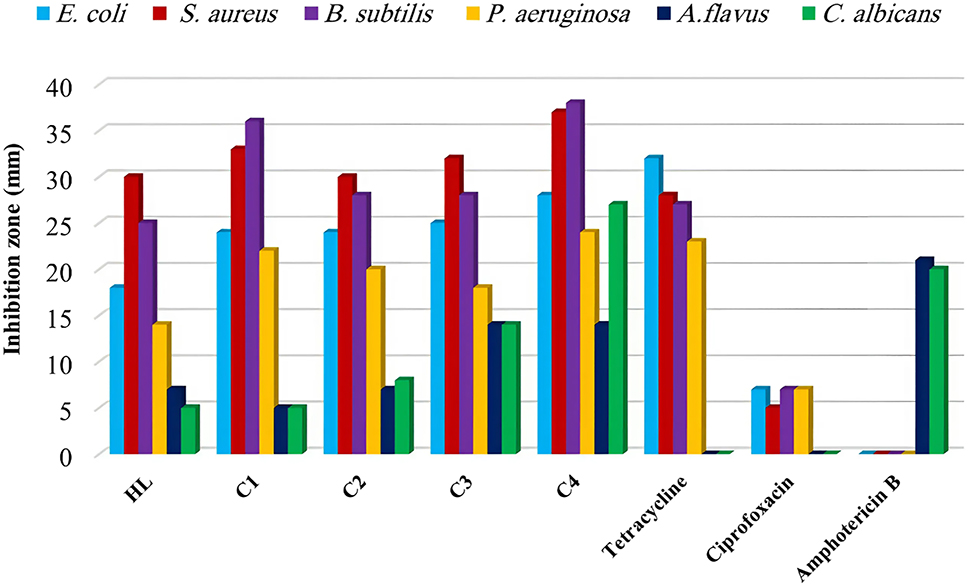
Antimicrobial activity of the compounds and standard drugs.
3.12.2 Antioxidant activity
The antioxidant activity of the ligand and its complexes was evaluated by comparing them with ascorbic acid, which was used as a standard. The DPPH assay results for the ligand and its complexes as shown in (Figure 8) indicated that they had higher antioxidant activity than the standard. The IC50 values revealed that the ligand and its complexes exhibited antioxidant activity in the following order: C3 > C2 > C1 > C4 > H 2 L.
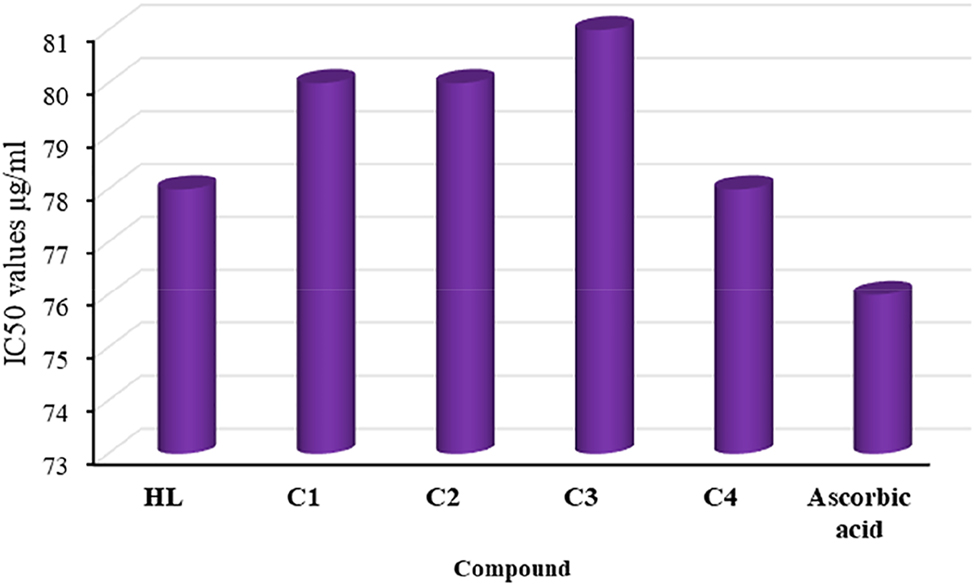
Antioxidant activity of compounds and standard (ascorbic acid).
3.12.3 Cytotoxicity assay
The brine shrimp cytotoxicity assay is a good tool for biomonitoring, used to determine the ability of cells to kill cancer cells. 20 The results of the cytotoxicity test for compound H 2 L and its compounds with metal(II) are summarized in (Table 11). Among all the compounds tested, the C4 and C2 compounds exhibited the highest cytotoxicity, with LD50 values of 1.157 × 10−4 and 1.836 × 10−4, respectively. On the other hand, the H 2 L, C3, and C1 compounds were less effective against A. salina.
Cytotoxicity assay of ligand and their complexes.
| Compound | LD50 (M mL−1) |
|---|---|
| HL | 2.307 × 10−4 |
| C1 | 2.216 × 10−4 |
| C2 | 1.836 × 10−4 |
| C3 | 2.263 × 10−4 |
| C4 | 1.157 × 10−4 |
| Bleomycin | 0.513 × 10−4 |
3.12.4 Anti-inflammatory activity
Inflammation can be caused by various factors such as temperature, external stress, and organic solvents, which can disrupt protein structure and lead to pathogenic diseases. Inflammation can be beneficial as a defense mechanism against tissue damage, but it can also contribute to serious illnesses such as asthma, arthritis, etc.(Table12 and Figure9) 30
The study showed that all compounds were highly effective in inhibiting inflammation, and the percentage of inhibition rate increased with the concentration, as shown in (Table 12). The anti-inflammatory activity of the ligand can be observed, which is comparable to its complexes with enhanced efficacy.
It has been observed that the complexes exhibit a trend of C4 > C3 > C2 > H 2 L > C1 in terms of activity. This is due to the property of protein denaturation present in the complexes, which helps prevent the formation of adema and water retention and indicates a decrease in inflammation. 55 Other factors such as hydrophilicity, chelation, and metallic effects may also impact biological activity. 53
Anti-inflammatory activity of ligand and their complexes.
| Symbol | Inhibition % ± SD | ||||
|---|---|---|---|---|---|
| 15 | 25 | 50 | 100 | 200 | |
| H2L | 17.93 ± 0.02 | 20.68 ± 0.05 | 24.97 ± 0.08 | 29.03 ± 0.06 | 34.51 ± 0.05 |
| C1 | 16.59 ± 0.04 | 20.84 ± 0.04 | 25.03 ± 0.02 | 29.77 ± 0.05 | 34.86 ± 0.02 |
| C2 | 18.57 ± 0.08 | 21.07 ± 0.06 | 25.18 ± 0.10 | 30.26 ± 0.05 | 35.18 ± 0.02 |
| C3 | 19.23 ± 0.06 | 21.84 ± 0.09 | 26.15 ± 0.10 | 31.16 ± 0.07 | 35.93 ± 0.02 |
| C4 | 21.07 ± 0.06 | 23.54 ± 0.03 | 27.94 ± 0.08 | 34.75 ± 0.06 | 40.08 ± 0.09 |
| Diclofenac sodium | 23.86 ± 0.02 | 29.57 ± 0.05 | 33.16 ± 0.08 | 38.75 ± 0.02 | 41.63 ± 0.02 |
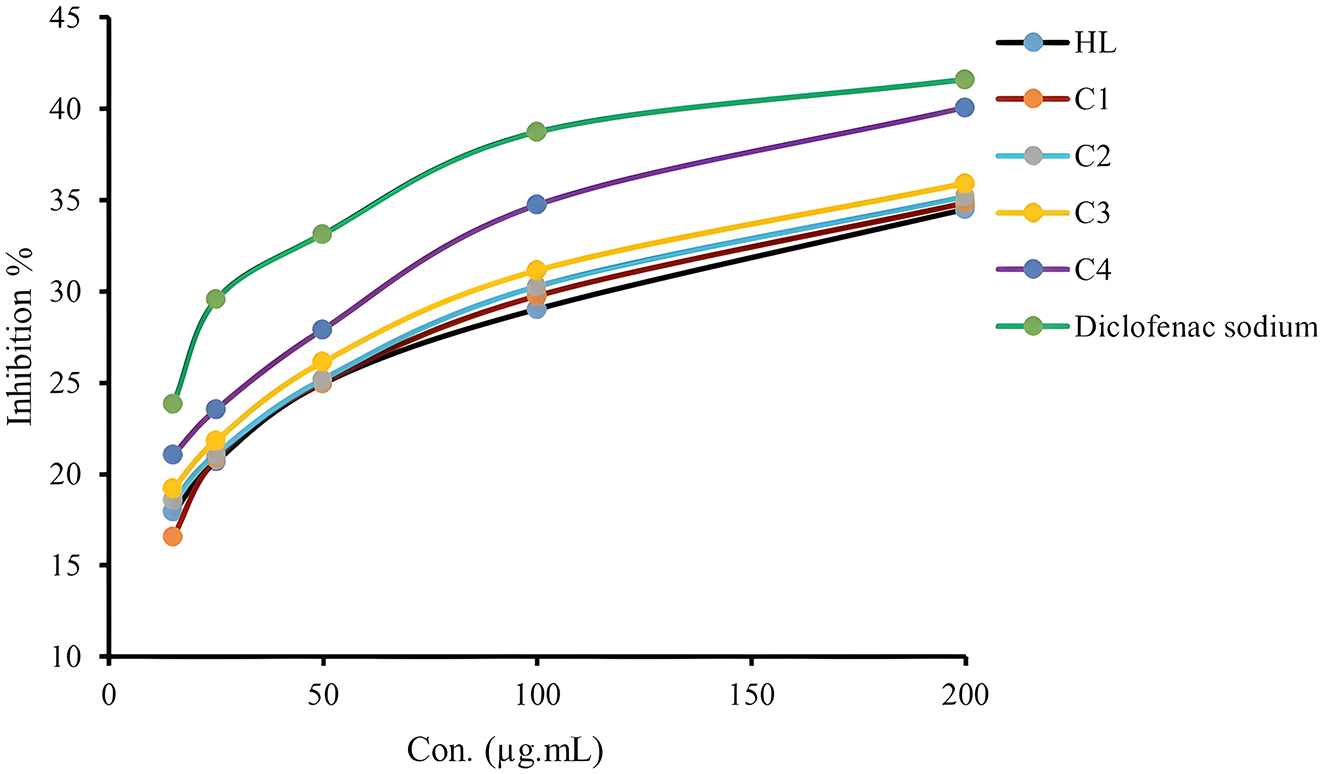
Anti-inflammatory activity of compounds and standard drug as % inhibition.
3.12.5 Anti-tritubercular activity
In vitro, the activity of the compounds and standard drug against M. tuberculosis was evaluated using the proportion method, 27 PZA was used as a positive control, and the results obtained were quite encouraging. The findings are listed in Table 13. Among the compounds examined, C4 was found to be the most effective, followed by C2, which showed higher activity compared to PZA against M. tuberculosis (Figure 10). The rest compounds showed activity similar to that of the standard drug against tuberculosis.
Anti-tuberculosis activity data for compounds.
| Compound | MIC (μg mL−1) |
|---|---|
| H2L | 2.45 ± 0.018 |
| C1 | 3.16 ± 0.07 |
| C2 | 2.68 ± 0.10 |
| C3 | 2.74 ± 0.04 |
| C4 | 4.05 ± 0.05 |
| PZA | 3.2 ± 0.05 |
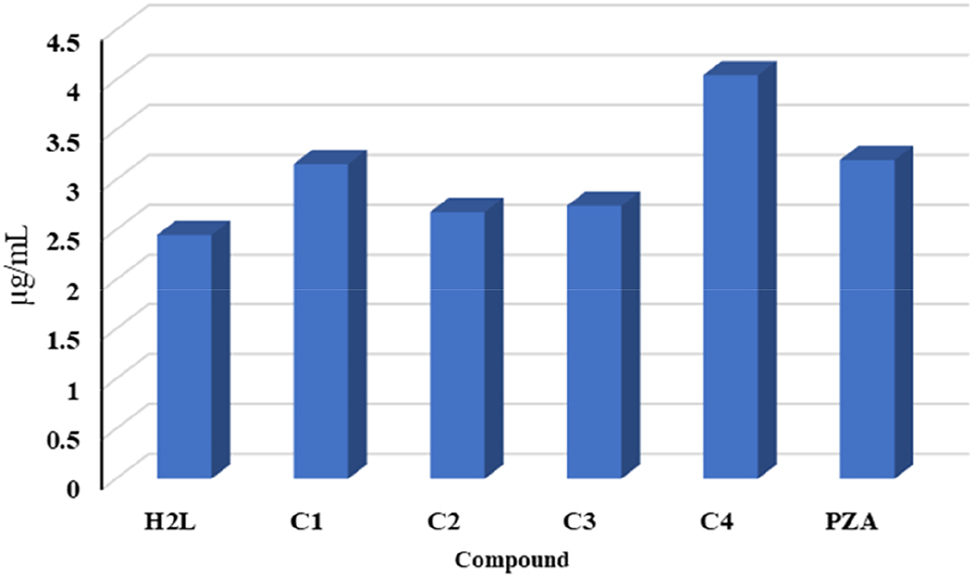
The antituberculosis activity of compounds and (PZA) standard drug.
3.13 Molecular docking
A molecular docking study was carried out using AutodockVina to simulate docking with the target amino acids of the protein (PDB ID:1DQZ) and verify the binding properties of the compounds designed as tuberculosis drugs (Figure 11). 31 Table 14 summarizes the binding affinity and type of binding between compounds with active sites in the amino acids of the protein. The major residues of the diethylamine site and the phenazine moiety’s aromatic ring form hydrophobic interactions via Pi-alkyl bonding, for Instance, VAL-B163 and PRO-B237. Pi-anion binding was only observed in C2, which interacts electrostatically through the aromatic ring of the phenazine moiety with ASP-B234. Based on the binding affinity values of the compounds prepared, we found that the highest value was obtained for compound C4.
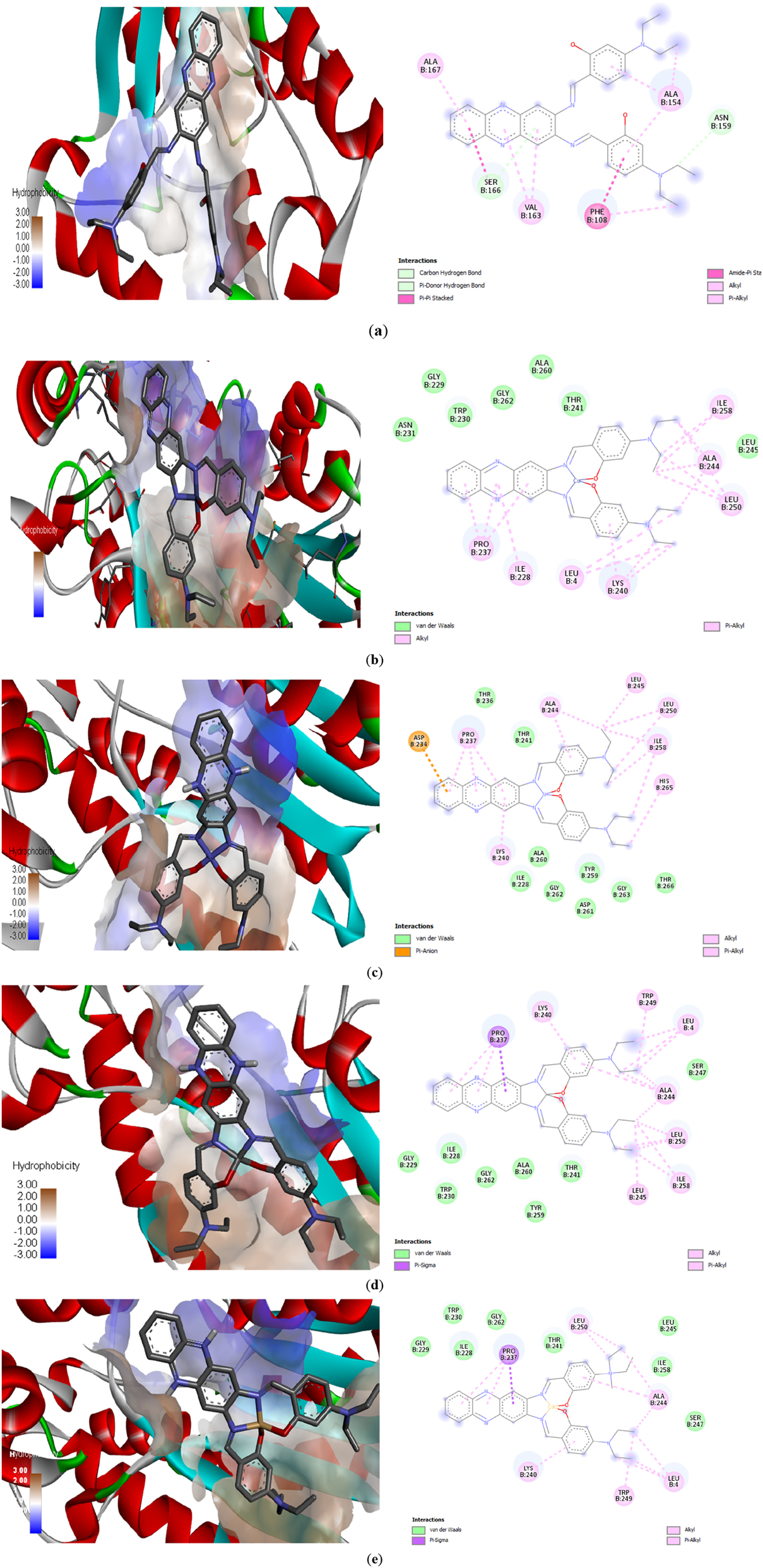
Predicted position of (a) H 2 L; (b) C1; (c) C2; (d) C3; (e) C4 from docking analysis and binding interactions with amino acids.
Physical features of ligand (HL) along with its complexes.
| Compound | H2L | C1 | C2 | C3 | C4 | |
|---|---|---|---|---|---|---|
| Bonding affinity (kcal mol−1) | −6.9 | −7.8 | −8.0 | −8.0 | −8.4 | |
| Hydrophobic | Pi-alkyl | ALAB154(4.89), VALB163(5.04) | LYSB240(3.57), PROB237(4.43), IEUB258(3.97) | LYSB240(5.39), PROB237(00), ALAB244(3.82), LEUB250(3.98) | LYSB240(4.30) | LYSB240(4.59), ALAB244(4.15) |
| Pi- pi stacked |
PHEB108(4.01) | |||||
| Amide-pi stacked |
PHEB108(4.60) | |||||
| Alkyl | ALAB154(4.24) | ALAB244(4.09), LYSB240(3.60) | ALAB244(4.24), LEUB245(4.09), LEUB250(4.71), IELB258(00) | LEUB250(4.65), LEUB245(4.32), ALAB244(3.48), LEUB4(4.70), TRPB249(4.98) | LEUB250(4.32), LEUB4(3.81), TRPB249(5.16) | |
| Van der waals | ASNB231(5.28), GLYB229(5.28), GLYB262(3.81), TRPB230(4.36), ALAB260(4.10, THRB241(3.59) | TRPB230(3.76), GLYB229(5.03), IELB228(5.02), THRB241(4.59), SERB247(5.16) | ||||
| Pi-sigma | PROB237(3.76) | |||||
| Pi-anion | ASPB234(4.43) | |||||
| Conventional hydrogen bond | ASNB159(3.41), SERB166(4.22) | |||||
The C4 exhibited a higher binding capacity with the protein of M. tuberculosis compared to other compounds, as shown by docking studies, which is consistent with the laboratory results.
3.14 ADMET analysis
Many compounds cannot be used as medicine due to various reasons such as insufficient absorption, distribution, high levels of toxicity, or excretion. These effects are collectively known as ADMET. 30 To determine whether an organic ligand and its synthesized metal complexes have a toxic effect after ingestion or have a good pharmacokinetic profile, they need to be tested thoroughly. To calculate ADMET parameters, the AdmetSAR web server was used. The server calculates pharmacokinetic, pharmacodynamic parameters and human intestinal absorption, etc. 31 The organic Schiff ligand and its metal(II) complexes exhibit promising pharmacological properties for human intestinal absorption (Table 15). Negative values indicate a low P-glycoprotein inhibitor and CYP2D9, while the ligand and its metal complexes also show similar values for oral toxicity. Additionally, it is considered safer than some drugs that disrupt liver function as it exhibits negative results for hepatotoxicity. Although positive nephrotoxicity values are moderate but pose a still risk as a drug design.
ADMET parameter of the ligand and its complexes.
| Parameter | H2L | C1 | C2 | C3 | C4 |
|---|---|---|---|---|---|
| ADMET CYP2D6 | (−)0.7022 | (−)0.7705 | (−)0.7705 | (−)0.7694 | (−)0.7694 |
| ADMET CYP2D9 | (−)0.6265 | (−)0.5707 | (−)0.5707 | (−)0.5710 | (−)0.5710 |
| ADMET hepatoxicity | (−)0.5697 | (−)0.5322 | (−)0.5197 | (−)0.5697 | (+)0.5428 |
| ADMET nephrotoxicity | (+)0.6601 | (+)0.6933 | (+)0.6559 | (+)0.6053 | (+)0.8150 |
| P-Glucoprotien inhibitor | (+) 0.8539 | (+) 0.8772 | (+) 0.8770 | (+) 0.8772 | (+) 0.8772 |
| Acute oral toxicity | (III) 0.6300 | (III) 0.5904 | (III) 0.5903 | (III) 0.5904 | (III) 0.5904 |
| Human intestinal absorption | (+)0.9475 | (+) 0.9580 | (+) 0.9579 | (+) 0.9580 | (+) 0.9580 |
4 Conclusions
In this current research, a new series of metal complexes was synthesized in the study using the Schiff base bond formed from 2,3-diaminophenazine and 4-diethylaminosalicyaldehyde. The organic ligand and its complexes with Co(II), Ni(II), Cu(II), and Cd(II) were then analyzed using various methods, including molar conductivity, melting point, elemental analysis, magnetic moment, 1H and 13C NMR, FT-IR, UV–Vis, ESR, and mass spectra, to confirm their structural identification and other characterization details. These analyses confirmed the square planar geometry of the complexes, and thermal analysis data showed that the compounds are thermally stable up to 253 °C. Moreover, the therapeutic aspects of (H 2 L, C1, C2, C3, and C4) were highlighted by careful screening and serial dilution for anti-tuberculosis activities, antimicrobial activity, antioxidant, anti-inflammatory activity, and cytotoxicity, respectively, and reveal. The biological activity of the compound was enhanced by complexation and followed the activity order of C4 > C3 > C1 > C2. Antimicrobial activity highlights that C4 and C3 are the most active with values comparable to standard drugs. The comparison of antioxidant activity values revealed that C1 and C2 were more susceptible to oxidation defects, displaying similar values to ascorbic acid. In terms of anti-inflammatory activity, the ligand and C4 exhibited higher activity, with a value similar to that of the standard diclofenac sodium. Computational studies, including DFT, MESP, molecular docking, and ADMET analyses, supported the experimental results and indicated strong interaction potential and suitability as therapeutic agents. The Cd(II) complex (C4) demonstrated the highest biological response, highlighted by its binding capacity, electrophilicity, and dipole moment. MESP results corroborated the superior biological efficiency of the complexes compared to the ligand, and molecular docking studies confirmed that all synthesized compounds were more effective against tuberculosis than pyrazinamide, with the Cd(II) complex showing the highest docking affinity. ADMET analyses indicated that the compounds were free from immunological, hepatic toxicity, and mutagenicity concerns, although there was a cautionary indication of potential nephrotoxicity. This research showcases a new direction in the use of Schiff base compounds containing 2,3-diaminophenazine moiety as a potential treatment for tuberculosis.
Acknowledgments
The authors express acknowledgement to Departments for providing research facilities.
-
Research ethics: Not applicable.
-
Author contributions: Conceptualization, S.B. , M.E. and A.A; methodology, R.E., M.B., H.A and A.A. .; software, M.B. D.T and F.B.; validation, S.B., D.T. and L.M.; formal analysis, R.E., L.E., R.A., and M.B; investigation, S.B., R.K. and D.T; resources, R.E. and S.B; data curation, S.B.; writing—original draft preparation, S.B. and M.E and D.T; writing—review and editing, R. E., F.B; visualization, S.B., D.T, F.B; supervision, S.B., M.E and F.B; project administration, R.E, L.M and A.A.; funding acquisition, R.E. and M.B. All authors have read and agreed to the published version of the manuscript.
-
Competing interests: The authors states no conflict of interest.
-
Research funding: None declared.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Gilham, E. L.; Pearce-Smith, N.; Carter, V.; Ashiru-Oredope, D. Assessment of Global Antimicrobial Resistance Campaigns Conducted to Improve Public Awareness and Antimicrobial Use Behaviours: a Rapid Systematic Review. BMC Publ. Health 2024, 24, 396; https://doi.org/10.1186/s12889-024-17766-w.Suche in Google Scholar PubMed PubMed Central
2. Ahmed, S. K.; Hussein, S.; Qurbani, K.; Ibrahim, R. H.; Fareeq, A.; Mahmood, K. A.; Mohamed, M. G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg Public Health 2024, 2, 100081; https://doi.org/10.1016/j.glmedi.2024.100081.Suche in Google Scholar
3. Sharma, V.; Das, R.; Mehta, D. K.; Sharma, D.; Aman, S.; Khan, M. Quinolone Scaffolds as Potential Drug Candidates against Infectious Microbes: a Review. Mol. Divers. 2024, 1–27; https://doi.org/10.1007/s11030-024-10862-4.Suche in Google Scholar PubMed
4. Yakobi, S. H.; Magibile, Y. B.; Pooe, O. J. A Systematic Review of Neisseria Gonorrhoeae Drug Resistance Development in South Africa. Braz. J. Microbiol. 2024, 1–11; https://doi.org/10.1007/s42770-024-01281-6.Suche in Google Scholar PubMed PubMed Central
5. Pardo, L.; Mota, M. I.; Parnizari, A.; Varela, A.; Algorta, G.; Varela, G. Detection of Vancomycin Resistance Among Methicillin-Resistant Staphylococcus aureus Strains Recovered from Children with Invasive Diseases in a Reference Pediatric Hospital. Antibiotics 2024, 13, 298; https://doi.org/10.3390/antibiotics13040298.Suche in Google Scholar PubMed PubMed Central
6. Okaiyeto, S. A.; Sutar, P. P.; Chen, C.; Ni, J.-B.; Wang, J.; Mujumdar, A. S.; Zhang, J.-S.; Xu, M.-Q.; Fang, X.-M.; Zhang, C.; Zhang, J. S.; Xu, M. Q.; Fang, X. M.; Xiao, H. W. Antibiotic Resistant Bacteria in Food Systems: Current Status, Resistance Mechanisms, and Mitigation Strategies. Agri. Commun. 2024, 100027; https://doi.org/10.1016/j.agrcom.2024.100027.Suche in Google Scholar
7. Dheda, K.; Mirzayev, F.; Cirillo, D. M.; Udwadia, Z.; Dooley, K. E.; Chang, K.-C.; Omar, S. V.; Reuter, A.; Perumal, T.; Horsburgh, C. R.Jr; Murray, M.; Lange, C. Multidrug-resistant Tuberculosis. Nat. Rev. Dis. Prim. 2024, 10, 22; https://doi.org/10.1038/s41572-024-00504-2.Suche in Google Scholar PubMed
8. Kumari, N.; Sharma, R.; Ali, J.; Chandra, G.; Singh, S.; Krishnan, M. Y. The Use of Mycobacterium tuberculosis H37Ra-Infected Immunocompetent Mice as an In Vivo Model of Persisters. Tuberculosis 2024, 145, 102479; https://doi.org/10.1016/j.tube.2024.102479.Suche in Google Scholar PubMed
9. Hu, X.; Liu, J.; Shao, Y.; Li, G.; Song, H.; Liu, Q.; Chen, C.; Zhu, L. Smoking Exposure and the Risk of Latent Tuberculosis Infection: Results from NHANES 2011–2012. Toxics 2024, 12, 94; https://doi.org/10.3390/toxics12010094.Suche in Google Scholar PubMed PubMed Central
10. Hussein, H. A.; Ahmed, J. M.; Musse, A. H.; Gizaw, Y. Prevalence and Risk Factors of Bovine Tuberculosis in Cattle in Selected Districts of Fafan Pastoral Settings, Eastern Ethiopia. Heliyon 2024, 10; https://doi.org/10.1016/j.heliyon.2024.e24998.Suche in Google Scholar PubMed PubMed Central
11. Kouengoua, A. P. K.; Tsissa, Y. L.; Noudeke, N. D.; Chimi, R. N.; Njayou, A.; Youssao, A. K. I.; Dahouda, M.; Boko, C.; Dougnon, V.; Awah-Ndukum, J.; Souaibou, F. Prevalence and Zoonotic Risk Factors of Mycobacterium Bovis Tuberculosis in Cattle at the Cattle-Wildlife-Human Interface in South and East Cameroon. Vet. World 2024, 17, 8; https://doi.org/10.14202/vetworld.2024.8-16.Suche in Google Scholar PubMed PubMed Central
12. El-Sonbati, A.; Omar, N.; Abou-Dobara, M.; Diab, M.; El-Mogazy, M.; Morgan, S. M.; Hussien, M.; El-Ghettany, A. Structural, Molecular Docking Computational Studies and In-Vitro Evidence for Antibacterial Activity of Mixed Ligand Complexes. J. Mol. Struct. 2021, 1239, 130481; https://doi.org/10.1016/j.molstruc.2021.130481.Suche in Google Scholar
13. Bufarwa, S.; El-Seifat, R.; Binhamad, H.; Hesien, R. Synthesis, Characterization, Thermal, Theoretical Studies, Antimicrobial, Antioxidant Activity, Superoxide Dismutase-like Activity and Catalase Mimetics of Metal (II) Complexes Derived from Sugar and Schiff Base. Rev. Inorg. Chem. 2024, 44, 1–13; https://doi.org/10.1515/revic-2023-0028.Suche in Google Scholar
14. Binhamad, H. A.; El-seifat, R. M.; Hesien, R. A.; Bufarw, S. M. Synthesis, Characterization (I.R, Elemental Analysis, Molar Conductivity), and Antibacterial Investigation of Complex Produced by the Reaction between Co(II) Ion with Mixed Ligands of (Amoxicillin and Salen). Al-Mukhtar J. Basic Sci. 2023, 21; https://doi.org/10.54172/4kk1ad33.Suche in Google Scholar
15. Almáši, M.; Vilková, M.; Bednarčík, J. Synthesis, Characterization and Spectral Properties of Novel Azo-Azomethine-Tetracarboxylic Schiff Base Ligand and its Co(II), Ni(II), Cu(II) and Pd(II) Complexes. Inorg. Chim. Acta. 2021, 515, 120064; https://doi.org/10.1016/j.ica.2020.120064.Suche in Google Scholar
16. Anacona, J.; Santaella, J.; Al-Shemary, R. K. R.; Amenta, J.; Otero, A.; Ramos, C.; Celis, F. Ceftriaxone-based Schiff Base Transition Metal(II) Complexes. Synthesis, Characterization, Bacterial Toxicity, and DFT Calculations. Enhanced Antibacterial Activity of a Novel Zn(II) Complex against S. aureus and E. coli. J. Inorg. Biochem. 2021, 223, 111519; https://doi.org/10.1016/j.jinorgbio.2021.111519.Suche in Google Scholar PubMed
17. Majumdar, D.; Philip, J. E.; Gassoumi, B.; Ayachi, S.; Abdelaziz, B.; Tüzün, B.; Roy, S. Supramolecular Clumps of μ2-1, 3-acetate Bridges of Cd(II)-Salen Complex: Synthesis, Spectroscopic Characterization, Crystal Structure, DFT Quantization’s, and Antifungal Photodynamic Therapy. Heliyon 2024, 10, 1–21; https://doi.org/10.1016/j.heliyon.2024.e29856.Suche in Google Scholar PubMed PubMed Central
18. Sandhu, Q.-U.-A.; Pervaiz, M.; Majid, A.; Younas, U.; Saeed, Z.; Ashraf, A.; Khan, R. R. M.; Ullah, S.; Ali, F.; Jelani, S. Schiff Base Metal Complexes as Anti-inflammatory Agents. J. Coord. Chem. 2023, 76, 1094–1118; https://doi.org/10.1080/00958972.2023.2226794.Suche in Google Scholar
19. Mohapatra, D.; Patra, S. A.; Pattanayak, P. D.; Sahu, G.; Sasamori, T.; Dinda, R. Monomeric Copper(II) Complexes with Unsymmetrical Salen Environment: Synthesis, Characterization and Study of Biological Activities. J. Inorg. Biochem. 2024, 253, 112497; https://doi.org/10.1016/j.jinorgbio.2024.112497.Suche in Google Scholar PubMed
20. Sanjurani, T.; Paul, S.; Barman, P. Indole-based NNN Donor Schiff Base Ligand and its Complexes: Sonication-Assisted Synthesis, Characterization, DNA Binding, Anti-cancer Evaluation and In-Vitro Biological Assay. Bioorg. Chem. 2024, 107281; https://doi.org/10.1016/j.bioorg.2024.107281.Suche in Google Scholar PubMed
21. Kobus, M.; Friedrich, T.; Zorn, E.; Burmeister, N.; Maison, W. Medicinal Chemistry of Drugs with N-Oxide Functionalities. J. Med. Chem. 2024, 67, 5168–5184; https://doi.org/10.1021/acs.jmedchem.4c00254.Suche in Google Scholar PubMed PubMed Central
22. Chaudhary, A.; Khurana, J. M. Synthetic Routes for Phenazines: an Overview. Res. Chem. Intermed. 2018, 44, 1045–1083; https://doi.org/10.1007/s11164-017-3152-8.Suche in Google Scholar
23. Abdolmaleki, A.; Tavakol, H.; Molavian, M. R.; Firouz, K. Synthesis, FT-IR, NMR and DFT Analysis of a New Salophen Based on Diaminophenazine Moiety. J. Mol. Struct. 2014, 1062, 44–47; https://doi.org/10.1016/j.molstruc.2014.01.012.Suche in Google Scholar
24. Saravanan, P.; Dusthackeer, V. A.; Rajmani, R.; Mahizhaveni, B.; Nirmal, C. R.; Rajadas, S. E.; Bhardwaj, N.; Ponnuraja, C.; Bhaskar, A.; Hemanthkumar, A.; Ramachandran, G.; Tripathy, S. P. Discovery of a Highly Potent Novel Rifampicin Analog by Preparing a Hybrid of the Precursors of the Antibiotic Drugs Rifampicin and Clofazimine. Sci. Rep. 2021, 11, 1029; https://doi.org/10.1038/s41598-020-80439-2.Suche in Google Scholar PubMed PubMed Central
25. Soekamto, N. H.; Okino, T.; Rasyid, H.; Pudjiastuti, P.; Hadisaputri, Y. E.; Zainul, R. Chemotherapeutic Prospects of Organic Extracts of Bornetella Nitida from Selayar Island. Kuwait J. Sci. 2024, 51, 100223; https://doi.org/10.1016/j.kjs.2024.100223.Suche in Google Scholar
26. Nithyabalaji, R.; Ramya, R. M.; Kavitha, R.; Radhakrishnan, K.; Kumar, J. V.; Al-Asbahi, B. A.; Dhananjaya, M.; Joo, S. W. Molecular Structure, Characterization, In Vitro and In-Silico Studies of N, N-Dimethyl Aminophenyl Schiff’s Base-Chalcone Hybrid. Chem. Phys. Impact 2024, 8, 100422; https://doi.org/10.1016/j.chphi.2023.100422.Suche in Google Scholar
27. Dueke-Eze, C. U.; Fasina, T. M.; Oluwalana, A. E.; Familoni, O. B.; Mphalele, J. M.; Onubuogu, C. Synthesis and Biological Evaluation of Copper and Cobalt Complexes of (5-Substituted-Salicylidene) Isonicotinichydrazide Derivatives as Antitubercular Agents. Sci. Afr. 2020, 9, e00522; https://doi.org/10.1016/j.sciaf.2020.e00522.Suche in Google Scholar
28. Morgan, S. M.; Diab, M.; El‐Sonbati, A. Supramolecular Assembly of Hydrogen Bonding, ESR Studies and Theoretical Calculations of Cu(II) Complexes. Appl. Organomet. Chem. 2018, 32, e4504; https://doi.org/10.1002/aoc.4504.Suche in Google Scholar
29. Abou‐Dobara, M. I.; Omar, N. F.; Diab, M. A.; El‐Sonbati, A. Z.; Morgan, S. M.; El‐Mogazy, M. A. Allyl Rhodanine Azo Dye Derivatives: Potential Antimicrobials Target D‐alanyl Carrier Protein Ligase and Nucleoside Diphosphate Kinase. J. Cell. Biochem. 2019, 120, 1667–1678; https://doi.org/10.1002/jcb.27473.Suche in Google Scholar PubMed
30. Kumar, B.; Devi, J.; Dubey, A.; Tufail, A.; Sharma, S. Exploring the Antimalarial, Antioxidant, Anti-inflammatory Activities of Newly Synthesized Transition Metal(II) Complexes Bearing Thiosemicarbazone Ligands: Insights from Molecular Docking, DFT, MESP and ADMET Studies. Inorg. Chem. Commun. 2024, 159, 111674; https://doi.org/10.1016/j.inoche.2023.111674.Suche in Google Scholar
31. Junaid, M.; Alam, M. J.; Hossain, M. K.; Halim, M. A.; Ullah, M. O. Molecular Docking and Dynamics of Nickel-Schiff Base Complexes for Inhibiting β-lactamase of Mycobacterium tuberculosis. Silico Pharmacol. 2018, 6, 1–11; https://doi.org/10.1007/s40203-018-0044-6.Suche in Google Scholar PubMed PubMed Central
32. Al-Shemary, R. K.; Mohapatra, R. K.; Kumar, M.; Sarangi, A. K.; Azam, M.; Tuli, H. S.; Ansari, A.; Mohapatra, P. K.; Dhama, K. Synthesis, Structural Investigations, XRD, DFT, Anticancer and Molecular Docking Study of a Series of Thiazole Based Schiff Base Metal Complexes. J. Mol. Struct. 2023, 1275, 134676; https://doi.org/10.1016/j.molstruc.2022.134676.Suche in Google Scholar
33. Shebl, M.; Adly, O. M.; El-Shafiy, H. F.; Khalil, S. M.; Taha, A.; Mahdi, M. A. Structural Variety of Mono-And Binuclear Transition Metal Complexes of 3-[(2-Hydroxy-Benzylidene)-Hydrazono]-1-(2-Hydroxyphenyl)-Butan-1-One: Synthesis, Spectral, Thermal, Molecular Modeling, Antimicrobial and Antitumor Studies. J. Mol. Struct. 2017, 1134, 649–660; https://doi.org/10.1016/j.molstruc.2017.01.012.Suche in Google Scholar
34. Amer, S.; El-Wakiel, N.; El-Ghamry, H. Synthesis, Spectral, Antitumor and Antimicrobial Studies on Cu(II) Complexes of Purine and Triazole Schiff Base Derivatives. J. Mol. Struct. 2013, 1049, 326–335; https://doi.org/10.1016/j.molstruc.2013.06.059.Suche in Google Scholar
35. Gaber, M.; El-Ghamry, H. A.; Fathalla, S. K.; Mansour, M. A. Synthesis, Spectroscopic, Thermal and Molecular Modeling Studies of Zn2+, Cd2+ and UO22+ Complexes of Schiff Bases Containing Triazole Moiety. Antimicrobial, Anticancer, Antioxidant and DNA Binding Studies. Mater. Sci. Eng. C 2018, 83, 78–89; https://doi.org/10.1016/j.msec.2017.11.004.Suche in Google Scholar PubMed
36. Tian, W.; Zhong, W.; Yang, Z.; Chen, L.; Lin, S.; Li, Y.; Wang, Y.; Yang, P.; Long, X. Synthesis, Characterization and Discovery of Multiple Anticancer Mechanisms of Dibutyltin Complexes Based on Salen-like Ligands. J. Inorg. Biochem. 2024, 251, 112434; https://doi.org/10.1016/j.jinorgbio.2023.112434.Suche in Google Scholar PubMed
37. Al-Resayes, S. I.; Laria, F. Y.; Miloud, M. M.; El-ajaily, M. M.; El-Barasi, N. M.; Sarangi, A. K.; Verma, S.; Azam, M.; Seidel, V.; Mohapatra, R. K. Synthesis, Characterization, Biological Applications, and Molecular Docking Studies of Amino-Phenol-Derived Mixed-Ligand Complexes with Fe(III), Cr(III), and La(III) Ions. J. Saudi Chem. Soc. 2023, 27, 101622; https://doi.org/10.1016/j.jscs.2023.101622.Suche in Google Scholar
38. Bufarwa, S.; Abdel-Latif, S. Spectroscopic, Thermal, and Conductometric Studies of Some (Arylazo) Quinolin-8-Ol and Their Complexes with the Divalent Ions of Mn, Ni, Cu, and Zn. Eur. Chem. Bull. 2023, 12, 187–197.10.21203/rs.3.rs-2210817/v1Suche in Google Scholar
39. Adly, O. M.; Shebl, M.; Abdelrhman, E. M.; El‐Shetary, B. Structural Variety of Cu(II), Ni(II), and Co(II) Complexes of a Hydrazone Based on 5-acetyl-4-hydroxy-2H-1, 3-thiazine-2, 6 (3H)-dione: Synthesis, Spectroscopic, Density Functional Theory, Antitumor, and Docking Studies. Appl. Organomet. Chem. 2023, 37, e7036; https://doi.org/10.1002/aoc.7036.Suche in Google Scholar
40. Khalil, M. H.; Abdullah, F. O. Synthesis, Characterisation, and Anticancer and Antioxidant Activities of Novel Complexes of Palladium and an Organic Schiff-Base Ligand. Bull. Chem. Soc. Ethiop. 2024, 38, 605–613; https://doi.org/10.4314/bcse.v38i3.5.Suche in Google Scholar
41. Turomsha, I. S.; Gvozdev, M. Y.; Osipovich, N. P.; Staravoitava, V. A.; Shiman, D. I.; Loginova, N. V. Copper(II) Complexes of Sterically Hindered Phenolic Schiff Bases: Synthesis, Characterization, Interaction with Biomolecules, and Antioxidant and Antimicrobial Activity. New J. Chem. 2024; https://doi.org/10.1039/d4nj00430b.Suche in Google Scholar
42. Sousa, J. M.; Braz, E. M.; Bezerra, R. D.; Morais, A. I.; Vieira, A. C.; Costa, M. P.; Rizzo, M. S.; Chaves, L. L.; Barreto, H. M.; Osajima, J. A.; Silva-Filho, E. C. Study of the Antibacterial and Cytotoxic Activity of Chitosan and its Derivatives Chemically Modified with Phthalic Anhydride and Ethylenediamine. Int. J. Biol. Macromol. 2024, 130292; https://doi.org/10.1016/j.ijbiomac.2024.130292.Suche in Google Scholar PubMed
43. Al-Noor, T. H.; Mohapatra, R. K.; Azam, M.; Karem, L. K. A.; Mohapatra, P. K.; Ibrahim, A. A.; Parhi, P. K.; Dash, G. C.; El-ajaily, M. M.; Al-Resayes, S. I.; Raval, M. K.; Pintilie, L. Mixed-ligand Complexes of Ampicillin Derived Schiff Base Ligand and Nicotinamide: Synthesis, Physico-Chemical Studies, DFT Calculation, Antibacterial Study and Molecular Docking Analysis. J. Mol. Struct. 2021, 1229, 129832; https://doi.org/10.1016/j.molstruc.2020.129832.Suche in Google Scholar
44. P. G. Lacroix, S. Di Bella, I. Ledoux, Synthesis and second-order nonlinear optical properties of new copper(II), nickel(II), and zinc(II) Schiff-base complexes. Toward a role of inorganic chromophores for second harmonic generation, Chem. Mater. 1996, 8, 541–545, https://doi.org/10.1021/cm950426q.Suche in Google Scholar
45. El‐Sonbati, A. Z.; Diab, M. A.; Eldesoky, A. M.; Morgan, S. M.; Salem, O. L. Polymer Complexes. LXXVI. Synthesis, Characterization, CT-DNA Binding, Molecular Docking and Thermal Studies of Sulfoxine Polymer Complexes. Appl. Organomet. Chem. 2019, 33, e4839; https://doi.org/10.1002/aoc.4839.Suche in Google Scholar
46. El-Sonbati, A.; Diab, M.; Morgan, S. M.; Abou-Dobara, M.; El-Ghettany, A. Synthesis, Characterization, Theoretical and Molecular Docking Studies of Mixed-Ligand Complexes of Cu(II), Ni(II), Co(II), Mn(II), Cr(III), UO2(II) and Cd(II). J. Mol. Struct. 2020, 1200, 127065; https://doi.org/10.1016/j.molstruc.2019.127065.Suche in Google Scholar
47. Abdelrhman, E. M.; El-Shetary, B.; Shebl, M.; Adly, O. M. Coordinating Behavior of Hydrazone Ligand Bearing Chromone Moiety towards Cu(II) Ions: Synthesis, Spectral, Density Functional Theory (DFT) Calculations, Antitumor, and Docking Studies. Appl. Organomet. Chem. 2021, 35, e6183; https://doi.org/10.1002/aoc.6183.Suche in Google Scholar
48. Adly, O. M.; El-Shafiy, H. F.; Shebl, M. Synthesis, Spectroscopic Studies, DFT Calculations, Antimicrobial and Antitumor Activity of Tridentate NNO Schiff Base Metal Complexes Based on 5-Acetyl-4-Hydroxy-2h-1, 3-thiazine-2, 6 (3H)-Dione. J. Mol. Struct. 2019, 1196, 805–818; https://doi.org/10.1016/j.molstruc.2019.07.010.Suche in Google Scholar
49. Abbass, L. M.; Sadeek, S. A.; Zordok, W. A.-a.; Aziz, M. A. E.-R.; El-Attar, M. S. Mixed Ligand 4‑hydroxy Acetanilide-Febuxostat Complexes of Co (II),-Ni (II), Cu (II) and Zr (IV): Synthesis,Structural Characterization, DFT Calculations, Antibacterial, Antioxidant and Molecular Docking Studies. J. Mol. Struct. 2024, 1308, 138115.10.1016/j.molstruc.2024.138115Suche in Google Scholar
50. Omara, W.; Abdel-Latif, S. A.; Azzazy, H. M.; Abdel-Kader, N. S. Exploring Polyaniline Nanofilaments for Enhanced Optical Recognition of Lead in Water: An Integrated Approach of Experimental and Theoretical Studies. Appl. Organomet. Chem. 2024, 38, e7439; https://doi.org/10.1002/aoc.7439.Suche in Google Scholar
51. El-Sonbati, A.; Diab, M.; El-Bindary, A.; Morgan, S. M. Supramolecular Spectroscopic and Thermal Studies of Azodye Complexes. Spectrochim. Acta Mol. Biomol. Spectrosc. 2014, 127, 310–328; https://doi.org/10.1016/j.saa.2014.02.037.Suche in Google Scholar PubMed
52. Mahmoud, W. H.; Sayed, F. N.; Mohamed, G. G. Azo Dye with Nitrogen Donor Sets of Atoms and its Metal Complexes: Synthesis, Characterization, DFT, Biological, Anticancer and Molecular Docking Studies. Appl. Organomet. Chem. 2018, 32, e4347; https://doi.org/10.1002/aoc.4347.Suche in Google Scholar
53. Taxak, B.; Devi, J.; Dubey, A.; Kumar, B.; Tufail, A.; Pachwania, S.; Boora, A. Investigation of Anti‐inflammatory and Antimicrobial Activities of Hydrazone-based Diorganotin (IV) Complexes: Synthesis, Spectroscopic Characterization, and Computational Studies. Appl. Organomet. Chem. 2024, 38, e7323; https://doi.org/10.1002/aoc.7323.Suche in Google Scholar
54. Keypour, H.; Zeynali, H.; Fatemikia, H.; Ranjbar, N.; Karamian, R.; Rezaei, M. T.; Gable, R. W. Anticancer, Antioxidant, and Antimicrobial Studies and Molecular Docking of a New Hexanuclear Zn(II) Complex, Together with its X-Ray Crystal Analysis. Dalton Trans. 2024, 53, 4512–4525; https://doi.org/10.1039/d3dt03327a.Suche in Google Scholar PubMed
55. Mamta, P.; Chaudhary, A. Novel Zn(II) Heterobimetallic Complexes: Synthesis, Characterization, Density Functional Theory Calculations, In Vitro Antimicrobial, Anti-inflammatory and Anticancer Activities. Res. Chem. Intermed. 2024, 1–31.10.1007/s11164-024-05255-zSuche in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/revic-2024-0007).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- A review of coordination compounds: structure, stability, and biological significance
- Effluent wastewater technologies for textile industry: a review
- Efficient removal of Cr(VI) ions from industrial wastewater using carbon-based adsorbents functionalized with boronic acid
- Exploring inorganic phosphors: basics, types, fabrications and their luminescence properties for LED/WLED/displays
- Recent advances on hydrogen generation based on inorganic metal oxide nano-catalyst using water electrolysis approach
- Antituberculosis, antimicrobial, antioxidant, cytotoxicity and anti-inflammatory activity of Schiff base derived from 2,3-diaminophenazine moiety and its metal(II) complexes: structural elucidation, computational aspects, and biological evaluation
- Carbon-based nanomaterials: synthesis, types and fuel applications: a mini-review
- Recent advances in carbon quantum dots for antibiotics detection
- Modern innovations in the provision and efficient application of 2D inorganic nanoscale materials
- Bioinorganic metal nanoparticles and their potential applications as antimicrobial, antioxidant and catalytic agents: a review
Artikel in diesem Heft
- Frontmatter
- A review of coordination compounds: structure, stability, and biological significance
- Effluent wastewater technologies for textile industry: a review
- Efficient removal of Cr(VI) ions from industrial wastewater using carbon-based adsorbents functionalized with boronic acid
- Exploring inorganic phosphors: basics, types, fabrications and their luminescence properties for LED/WLED/displays
- Recent advances on hydrogen generation based on inorganic metal oxide nano-catalyst using water electrolysis approach
- Antituberculosis, antimicrobial, antioxidant, cytotoxicity and anti-inflammatory activity of Schiff base derived from 2,3-diaminophenazine moiety and its metal(II) complexes: structural elucidation, computational aspects, and biological evaluation
- Carbon-based nanomaterials: synthesis, types and fuel applications: a mini-review
- Recent advances in carbon quantum dots for antibiotics detection
- Modern innovations in the provision and efficient application of 2D inorganic nanoscale materials
- Bioinorganic metal nanoparticles and their potential applications as antimicrobial, antioxidant and catalytic agents: a review

