Recent advances on hydrogen generation based on inorganic metal oxide nano-catalyst using water electrolysis approach
-
Muhammad Naeem Ayub
und Mohammed M. Rahman
Abstract
Today world is looking for a cheap, environment friendly and efficient substitute of fossil fuel. Because due to large consumption of the fossil fuels on daily basis in whole world, emission of hazardous gases have produced lethal effects on human being. In this scenario hydrogen energy has emerged in form of clean, renewable and more efficient energy. Now the key challenge is that efficient production of the green hydrogen at commercial scale to meet demand of hydrogen. The electrolysis of water is the best pathway to achieve efficient hydrogen production. For this purpose the synthesis and improvement of low cast, active as well as stable catalysts or electrolysis is prerequisite for hydrogen production by electro-catalytic method for splitting of water. Main focus of this review is that, how we can perform the electrolysis of water by various techniques using novel methods especially electro-catalysts in term of activity, efficiency, large surface area, porosity, and stability. This will be performed by the method of two-half cell reaction one is the Hydrogen Evolution Reaction (HER) other one Oxygen Evolution Reaction (OER), where reaction proceeded in both medium acidic as well as alkaline phases. Particular attention is given to produce green clean hydrogen production from usable water and its physical and chemical storages for further uses for the support of human sustainability. Basically the recent strategy is to prepare, design and development of nanoscale materials/composite with non-noble metals and with also nanostructured with noble-metals will be discussed in this approach. The increased efficiency and utility have been the focal points of the use of diverse materials from different classes. To increase the electro-catalytic efficiency in OER and HER, we will discuss about new analyses methods and insights into studying the chemical compositions, shapes, surface area, porosity, and synergy of catalysts and the active sites of nanostructured electro-catalysts. This review will further provide the picture of current state of developments as well as recent progress for mechanized efficient production of clean hydrogen (i.e., HER) from water by electrocatalytic method using various nanoscale materials in a broad scale.
1 Introduction
There is no wonder that many decades have been passed by researcher to find out the more efficient, cheap, and clean and environment friendly fuel as to replace the fossil fuels 80 % energy is coming from that. There are two main issues of the modern world i-e energy and environment for economic and social development on sustainable level. 1 As per survey about half decade ago in 2018, whole world 79 % of economy energy depends upon old conventional sources of energy like coal, oil, natural gas and major content from petroleum. All these sources are nonrenewable and not environmentally friendly due to emission of hazardous gases. 2 Fortunately, nature provides us with a variety of renewable energy sources-including solar power, tidal power; wind power, biomass power, and water energy-that can help us cope with the severe problems caused by these sources. A couple of decades ago a global drive has been started to find out such type of renewable sources for future need of economic need for sustainable development environment.
Because of its increased energy density and the wonderful benefit of being free of contamination after combustion, hydrogen is seen as a clean energy carrier and a potential option for sustainable development. Green hydrogen technologies are gradually replacing fossil fuel-based hydrogen production and industrial by product hydrogen production technologies, which co-occur with environmental contamination from carbon pollution emissions. 3 Electrolytic water hydrogen production technology is expected to become the mainstream development direction in the future. But these all renewable sources are limited and times constrain intermittent availability due to seasonal and regional availability. 4 This need drives a efficient energy production techniques. Innovations in energy storage and other forms of energy innovation were compelled by this requirement. Various systems have garnered significant interest in recent decades for applications such as electrolysis-based (Figure 1).
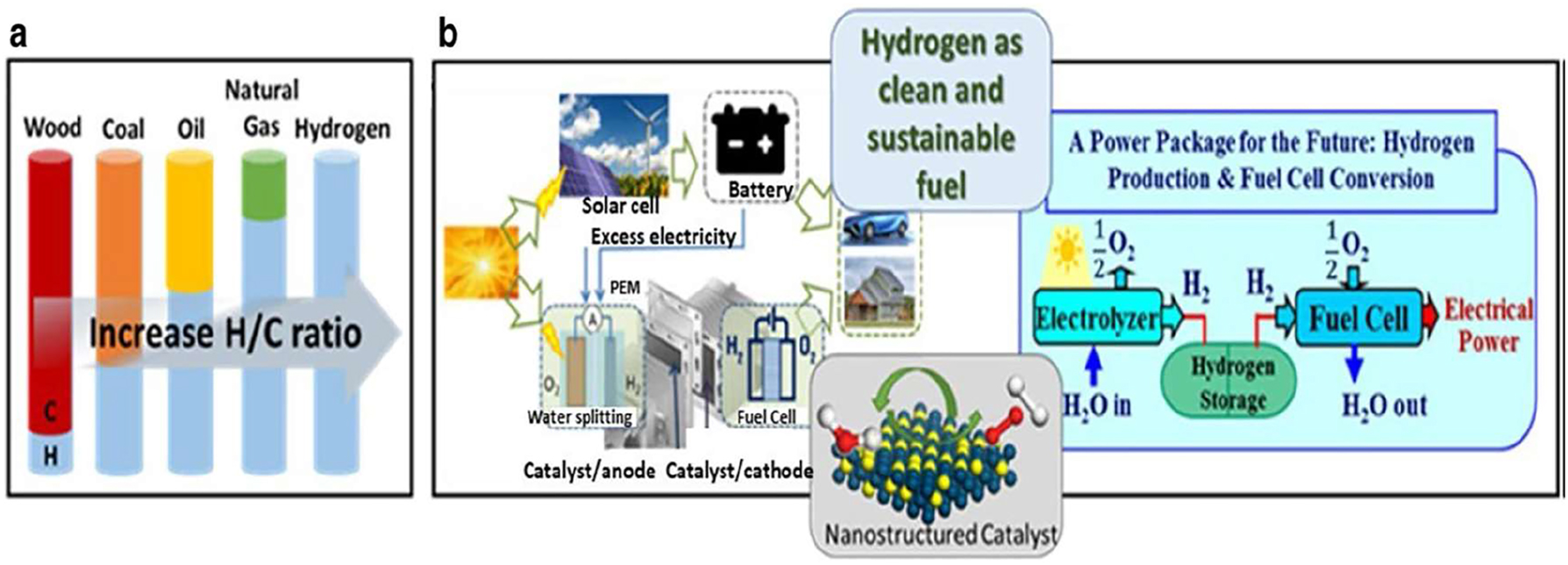
Hydrogen fuel cells. (a) Illustration of hydrogen fuel generation with respect to the carbon-to-hydrogen ratio. (b) The future prospects and function of catalyst are demonstrated via the elaboration of a dual cell accomplishment as H2 synthesis by splitting water on or after solar energy and the fuel cell scheme to convert hydrogen energy into electricity. Copyright from Wang et al. Nano Convergence 8:4 – (2021).
Hydrogen production, fuel cells for hydrogen utilization, lithium-ion batteries, and metal air batteries for long-term fuel or charge storage. 5 , 6 , 7 If we talk about the charge-storage capacity of developed batteries of any type are not have such enough capacity for large scale industrial usage. For a large scale it will be very expensive. Solar energy and wind power at large scale produced excess electricity, so there is need to search for substitute pathway for storage of hydrogen. The production of hydrogen by splitting of water splitting by electrical energy from renewable sources to produce clean fuel-hydrogen (H2).
2 Advancement of electrolytic water (alkaline) technology for producing hydrogen
As of right now, photo-catalysis, electrocatalysis, biomass fuel hydrogen synthesis, and fossil fuel reforming are the primary industrial methods of hydrogen production. The creation of hydrogen from fossil fuels releases CO2 and other harmful gases into the atmosphere, and the hydrogen produced from biomass fuels needs to go through a difficult purification procedure in order to be called “grey hydrogen.” “Green hydrogen,” or hydrogen produced without pollution, should be the industry’s acknowledged development direction. 8 , 9 , 10 , 11 Among these, the light source serves as the primary catalyst for photocatalytic hydrogen synthesis.
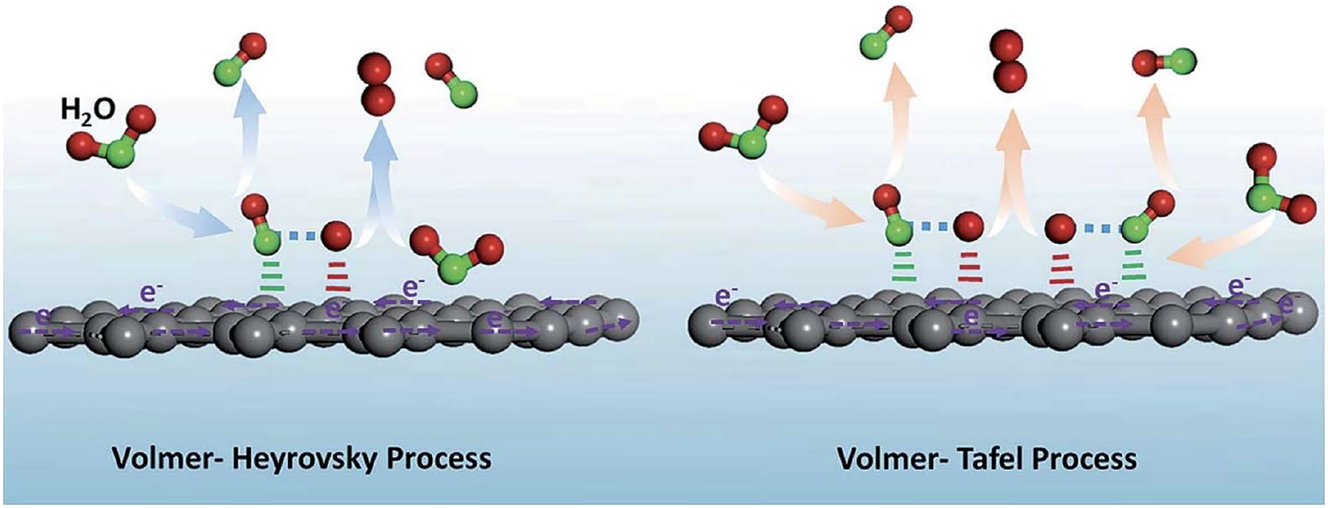
The two ways the hydrogen evolution reaction may proceed illustrated. Copy right from ‘The Royal Society of Chemistry (ACS-Omega-2022)’.
Hydrogen is a safe and high potential energy source because hydrogen has maximum gravimetric energy mass. If it be used in fuel cell as a fuel it has high effectiveness in energy conversion and also produce zero pollution, water is produced as byproduct during electrolysis. Now efforts has been making to move toward the selection modification, of catalyst used in electrocatalytic process for the production of hydrogen by splitting of water. 12 , 13 At the cathode, reduction takes place to produce H2, whereas at the anode, oxidation produces O2. These two reactions, which are sometimes combined into the acronym OER and HER, take place in acidic environments and follow these chemical equations (1)–(3):
There are a number of kinetic energy barriers that must be overcome in the electrochemical reaction between water, oxygen, and hydrogen in order to split water in a fuel cell. In hydrolysis, there are two distinct cell reactions: the hydrogen evolution reaction (HER) as well as the oxygen evolution reaction (OER). The process splits water into hydrogen at the cathode as well as oxygen at the anode. In splitting of water process reduction carried out at cathode and oxidized at anode. The main energy barrier during electrocatalytic method is the high over potential. 4 For this purpose highly suitable and effective catalyst are required to reduce the energy barrier between reaction and possibility chances. The design of catalyst and its activity, efficiency depend upon the chemical reaction conditions and on medium in which reaction proceed.
There are three techniques are being adopted to carry out this reaction in different medium. Out of three technologies first is protein exchange membrane (PEM), second one is alkaline electrolysis process, third is high temperature solid oxide water electrolysis (SOE). The third one is not most suitable there is high energy consumption required due to high temperature. To proceed Reaction according to (PEM) acidic medium is required, this method is quiet suitable in term of low gas permeability and high efficiency in production of hydrogen speedily in short limit time. But on other hand its flaw is that is slow in OER process especially in noble metal and other noble metal electrolysis. So the splitting of the water in alkaline media provide a vast choice if catalyst for the production of hydrogen with noble metal complex and other non-noble metal oxide. But in alkaline media the efficiency rate of HER is 2–3 time lower than acidic medium. 14 , 15
Depending on the pH of the electrolyte and the base used, alkaline electrolyzers can use a variety of anion-permeable separators, such as asbestos or zirfon, or anionic polymer membranes. The following chemical reaction (Equations (4)–(6)) occurs at each electrode:
Basically there are limited review number in case of in sighting into mechanistic report on the catalytic structure and morphology as well as composition and also active site plus their correlation with activity and stability of HER and OER in both acidic as well as alkaline medium. Need of age is that we focus on the developing such novel method and techniques that produce clean, safe plus active, stable and low-cast electrocatalyst to produce hydrogen gas. Typically, yttrium-stabilized zirconium or oxygen anions or hydrogen protons can be carried by the thick ceramic electrolyte layer in solid oxide electrolyzers when operated at high temperatures (500–1,000 °C). 16 , 17
Aside from these significant obstacles to hydrogen production, storage has drawn a lot of attention. The scientific community in the field of energy materials continues to face a formidable challenge in its quest for an economical, safe, and effective storage solution. Although the concept of using hydrogen as an energy vector in stationary applications could be visualized, the possibility of replacing fossil fuel in portable devices, especially in the transportation sector, is currently the main drive to use hydrogen as an energy vector, which emphasizes the concern about safe and efficient hydrogen storage. But as of right now, crude oil accounts for 97 % of transportation fuel and produces around 25 % of the world’s greenhouse gas emissions. Furthermore, the situation will be even worse in the near future as worldwide car use is experiencing exponential growth. As for the overall distribution (approximate percentage) of the world’s primary energy supply, carbon-based fossil fuels like natural gas and oil make up more than 50 % of the supply, while the distribution of other energy sources – such as hydropower (2 %) and renewable energy (11 %), for examples far less significant. 18 , 19
As we talked first that there are numerous challenges in the production of hydrogen by a stable, active and efficient catalyst. The first challenge is that in most cases the Ir and Ru during OER process these catalysts shows the elevated dissolution confrontation in acidic medium, so the of noble metal based catalyst do not perform well in these condition. So there is a great challenge for the scientist to develop a novel, efficient and suitable catalysts in term of greater efficiency during OER, especially with reference of non-noble metal catalyst. Second challenge is that non noble metal based catalyst during electrocatalytic process such as carbides, chalcogens have got greater attention due to good performance in OER process and their suitability, ability of structural transformation, and chemical composition in alkaline medium during OER condition. In this case a need to form such catalyst that has ability to identify its active sites which guide during designing the optimal catalyst. Third is about the mechanistic approach of transition metal complex electro-catalyst for HER process in alkaline medium. But in acidic medium its performance is not so well. So there is most important challenge to identify that all factors that control the catalytic method to HER in alkaline medium. 20 , 21 Hydrogen is a useful energy vector for transportation applications since it has a number of advantages. It can be produced from renewable sources from a range of non-fossil feed stocks, has the highest energy density by weight of any commercial fuel, and can be used in fuel cells to create power with only water as a byproduct. Hydrogen storage can be physical or chemical, depending on whether it is stored as molecular hydrogen (H2) or as hydridic/protonic H combined with other elements (e.g., B, C, and N), in which pressurized H2 and H2O are the most typical examples of these storage methods.
In this article we will provide the summary about performance index in introduction for the understanding and evaluation the concept of efficiency, activity and stability of various catalysts for the production of green fuel (hydrogen) in HER reaction mechanism. In review we will describe the recent advancement in developing techniques and advance tool that are being used in the production of hydrogen to save the environment which stand on the verge of chaos gradually toward dexterous corner of dustiness. By improving the best electrocatalyst for HER process (Scheme 1), which will be derived from the noble metals and from non-noble metal-based carbides, metal-based chalcogenides and metal-based sulphides. In review it will also be discussed about new catalysts insghiting, in this process and new mechanistic approaches for the various reaction conditions and the new outlook for new reaction pathways. 22 , 23 , 24 By highlighting the role of activity of active sites will also be discussed how activity if active sites can be enhanced by different techniques with help of Nano-structured base noble metals and on Non-noble metal-based catalysts in the OER procedure (Figure 3).
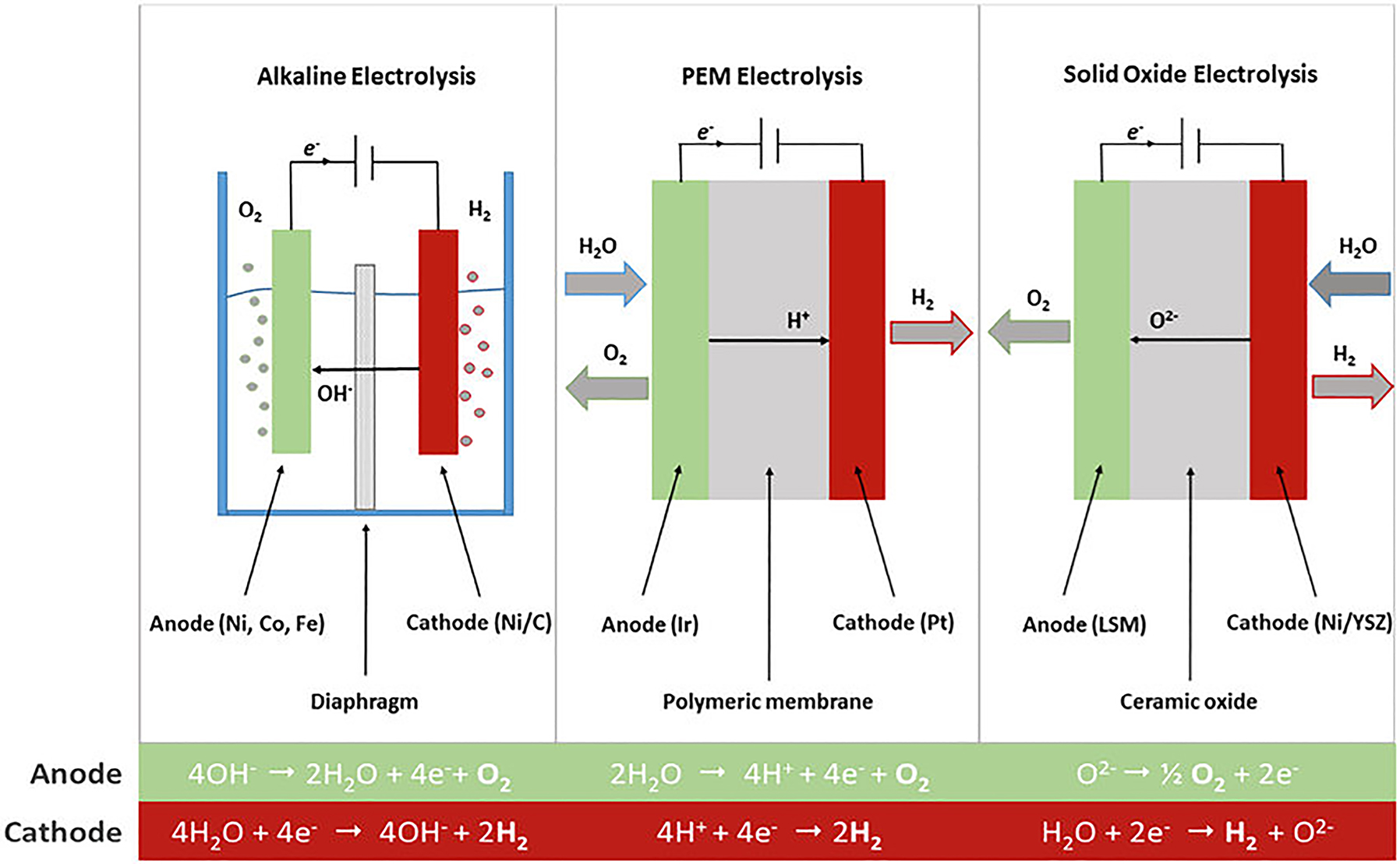
Mechanism of HER/OER. Operation principles of alkaline, PEM (proton-exchange membrane) and solid oxide water electrolysis. The overall reaction is H2O → H2 + 1/2 O2. Oxygen evolution occurs at the anode, hydrogen evolves at the cathode. Copy right from Progress in energy and combustion science 2017.
3 Performance evaluation index (PEI) for electrocatalysts
The positive rate of the Gibbs free energy indicates that electrolytic water splitting is an upward process, and there is a large kinetic barrier to overcome as well. As seen in Figure 2(a), catalysts substantially assist in lowering the kinetic barrier. Electrocatalytic water splitting relies on a number of important properties, such as activity, stability, and efficiency, to determine a catalyst’s efficacy (Figure 4). The activity’s features, such as the overpotential, Tafel slope, and exchange current density, can be retrieved from these polarization graph curves. When the over potential or current varies with time, it means that things are stable. To compare theoretical predictions with practical results, we use the Faraday efficiency and turnover frequency to characterize efficiency.
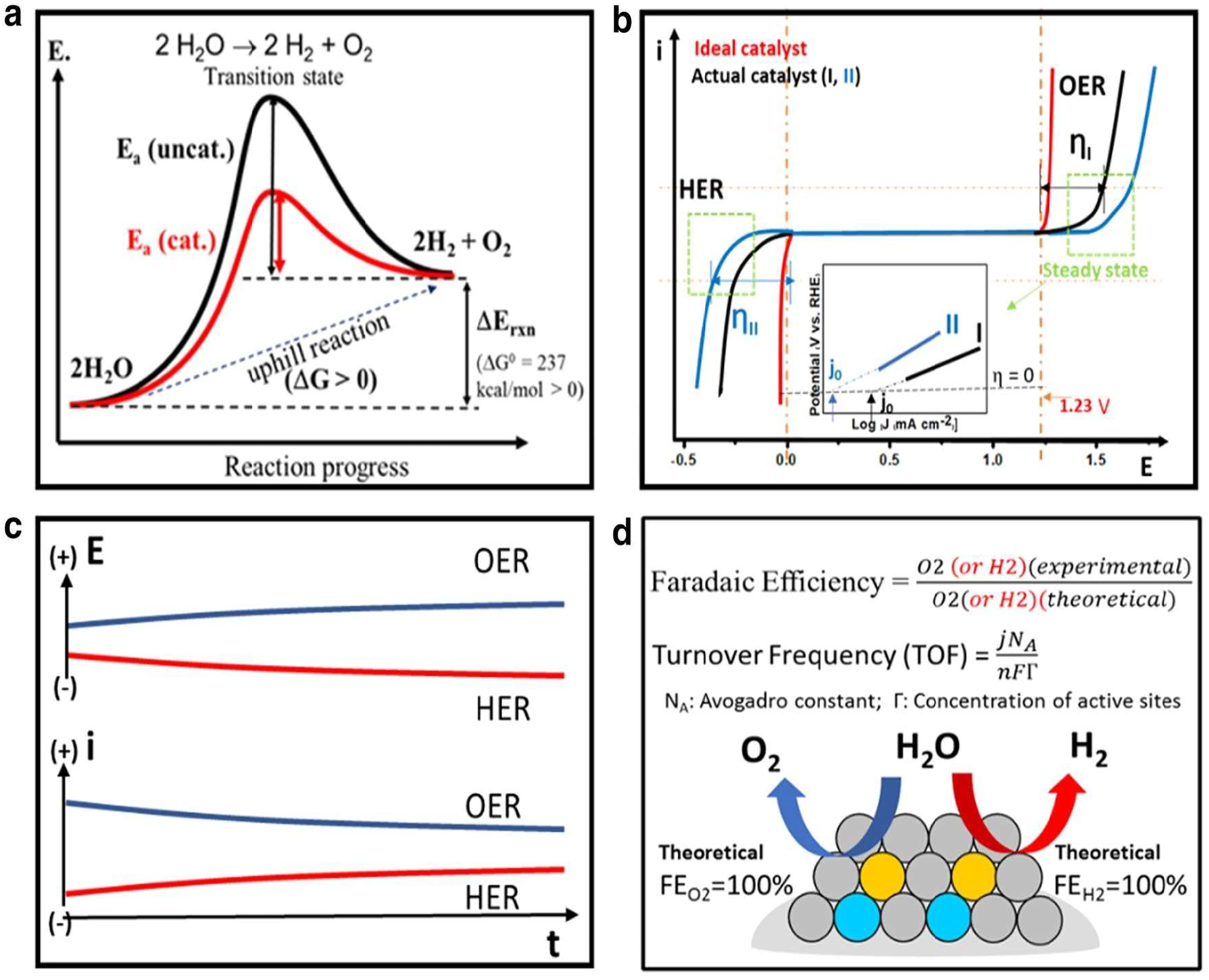
Performances of catalysts. (a) Design for sinking of energy footraces by numerous catalysts. (b) In terms of the over-potential, exchange current-density, Tafel slope and activity (c) according to (d) electrocatalytic reaction performance evaluation criteria, including current stability and potential time curves, 25 Copyright from Wang et al. Nano Convergence (2021).
3.1 Activity in reference of over potential, tafel slope, current density
At 25.0 °C and 1 atm, the electrochemical breakdown of water reaction has a thermodynamic potential of 1.24 V. But a higher potential than the thermodynamic potential (1.24 V) is needed for electrolysis of water. So as to make up for the reaction’s kinetic obstacles. The over potential, or surplus potential, is mostly caused by the inherent activation barriers that exist on both the anode and the cathode of the electrode. The word “over potential” is essential for evaluating the activity of the electrocatalysts. It is common practice to use the over potential value, which is equal to 10 mA cm2, when comparing the behavior of different catalysts. The current density results in a 12.3 % efficiency in converting solar energy into hydrogen. The equation: = a + b log j, where “j” is the current density, states the relationship between the over potential and the kinetic current. Additionally, the Tafel slope and exchange current can be used to assess the output of this connection. Two major kinetic parameters for the Tafel diagram are produced by the linear relationship. By dividing the zero-current-over-potential equation by two, we get the exchange current density; the other is the Tafel slope-b. The catalytic reaction system is related to the Tafel slope-b in terms of electron-transfer kinetics. An example of this would be a current density that increases more rapidly as a function of over potential shift, as shown by a lower Tafel slope. 26 , 27 , 28
3.2 Stability of catalyst in terms of current plus potential time curves
When deciding whether the catalyst is suitable for use in water splitting cells in practical situations, stability is an important consideration. There are usually two ways that electrocatalyst stability is described. Methods such as chrono-potentiometry (E-t curve) and chronoamperometry (I-t curve) can be used to detect the potential change or current fluctuation over time at a fixed current. The accuracy of a measurement is common practice to conduct a 10 h test with a current density higher than 10 mA cm2 so that results can be compared to those of other research groups. Another method is known as iso-cyclic voltammetry (CV), and it measures current cycles one at a time at a scan rate of 50 mVs−1 by counting the number of potential cycles. It is usual practice to employ linear sweep volta-metry (LSV) to examine the over potential shift prior to and subsequent to CV cycling at a specific current density. Minimizing the shift of over potential enhances the electrocatalysts’ stability directly proportional to the length of time that the measured current or potential stays constant.
3.3 Faradaic efficiency and turnover frequency
The efficiency of the electrons in external circuit in electrolysis that are transferred from the surface of electrode for electrochemical reaction is described quantitatively as Faradic efficiency. The ratio between the amount of H2 or oxygen (O2) that can be measured empirically and the amount that can be predicted theoretically is known as the faradaic efficiency. Calculations based on integration to chrono-ampherometric or chrono-potentiometric analysis can yield theoretical results. The experimental figures are determined by analyzing the gas generation with either the gas chromatography method or the water-gas displacement technique. A useful metric for describing reaction rates in relation to the catalytic active sites-the catalyst’s intrinsic catalytic activity is turnover frequency (TOF). The rate of reactant-to-product conversion at each catalytic site in a specific time period is commonly referred to as TOF. The precise amount of active sites on an electrode surface is, however, not always known. 29 , 30 , 31 When working with a big number of heterogeneous electrocatalysts, it can be difficult to pin down precise TOF values. Regardless of its relative impression, TOF is still a useful method for comparing the catalytic activity of different catalysts, especially when applied to the same system or under the same conditions.
3.4 Mechanism of electro catalytic water splitting
Hydrogen can currently be obtained using a variety of processes, including the synthesis of hydrogen from fossil fuels, water gas reaction, and hydrolysis of ammonia borane, methane reforming, electro-catalytic water splitting, and petroleum gas cracking. Direct conversion of electrical energy into chemical energy is achieved by the method of electro-catalytic hydrogen generation, which has the advantages of low equipment requirements, high hydrogen yield, high hydrogen purity, and environmental friendliness. Furthermore, there are abundant natural water resources, and the primary method used in industry to create hydrogen is electrocatalytic technology. 32 , 33 , 34 At the cathode, a hydrogen evolution reaction (HER) called electrocatalytic water splitting takes place. Figure depicts the primary apparatus, which is made up of an external power source, electrolyte solution, and cathode and anode electrode plates. The electrolyte’s pH has an impact. The specific formula is as follows:
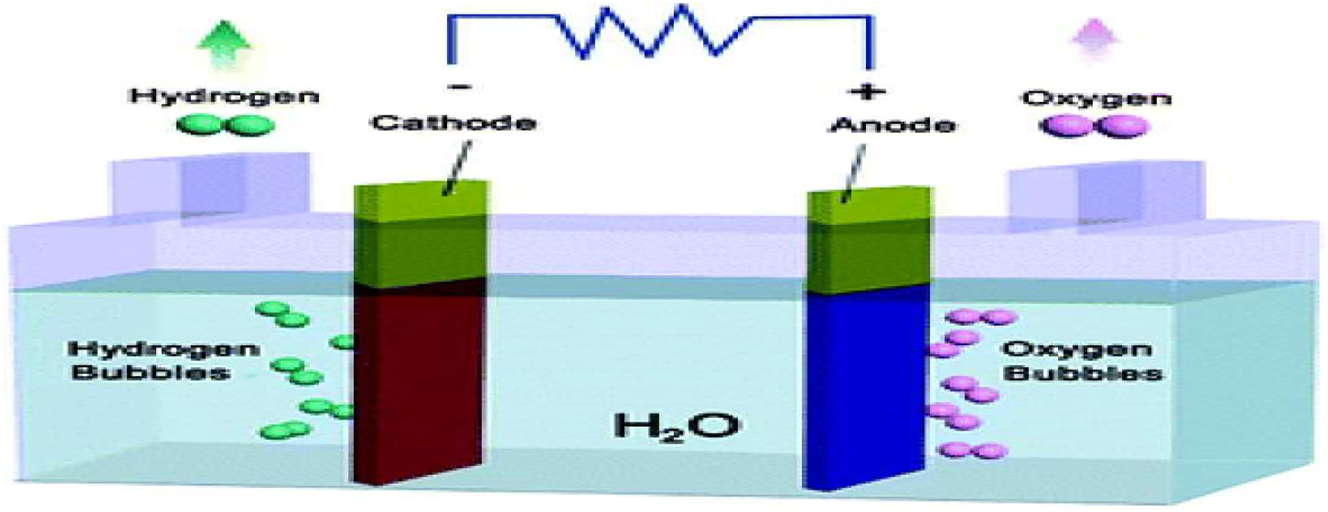
Hydrogen production device using electrocatalytic water splitting. Copyright from The Royal Society of Chemistry (chem.soc.rev 2015). 33
4 Electrocatalysts for hydrogen evolution reaction (HER)
4.1 Reaction steps in the process of HER
In the two-electron transfer process of water electrolysis, HER is the crucial half-reaction that produces hydrogen at the cathode. The environment has a significant impact on this HER’s system. There are three potential reaction steps for the HER response in acidic media. The methods employed to assess the catalysts’ activity, stability, and efficiency will be defined by the specific goals of the research and development. In terms of the particular focus, the present research of activity, stability, and efficiency can be divided into three categories (Equation (7)–(9)) in addition to production and manufacture of the electrocatalysts for HER process. 35 , 36 , 37
For the synthesis of adsorbed hydrogen, Volmer step (1a) is the initial step. Afterwards, the evolution of hydrogen can be achieved by either the Heyrovsky step (1b) or the Tafel step (1c), or even both, as previously mentioned. Regarding the reaction of HER in acidic environments. A Volmer step (2a) and a Heyrovsky step (2b) are two potential reaction routes that can be determined by the following equations. Finding a happy medium between hydroxy adsorption (OHad), as well as water dissociation is essential for the HER action in acidic conditions. Theoretical simulations have demonstrated a link between HER activity. The free energy of hydrogen adsorption (GH) is a well-established metric for describing materials that undergo hydrogen evolution. HER process benefits from a modest degree of binding energy of hydrogen. As revealed in Figure 6(a), 37 and in Figure 6(b). 38
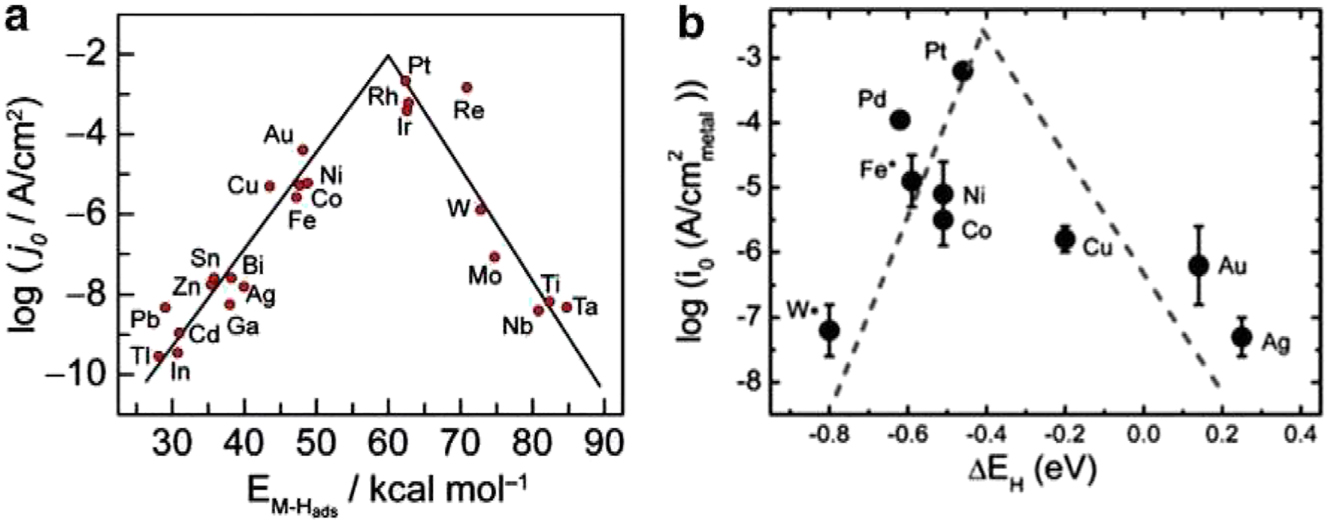
Diagram of HER. (a) Volcano diagrams are used to illustrate the relationship between current density and metal-hydrogen binding energy for different metal surfaces in an acidic liquid. (b) The relationship between the area of monometallic surfaces and the current density as a function of the predicted HBE in alkaline media, 38 Copyright from Energy Envi. (2013).
Volcano figure in Figure 7 was created by measuring ECDs as a function of hydrogen bond strengths using density functional theory (DFT). This demonstrates how the strength of the hydrogen bond affects the hydrogen evolution activity. The common transitional metals on the left (Ni, Mo, Co, and W) bind hydrogen too strongly, filling the surface and leaving fewer sites available for additional hydrogen adsorption, which lowers reaction speed. Noble metals like Au and Ag, on the other hand, bond hydrogen far too weakly. The unstable bond in the surface-adsorbed hydrogen makes proton transfer more difficult as the hydrogen-metal bond strength drops. Because every step of the HER proceeds at a nearly thermoneutral overpotential, Pt and Ir live at the top. 4 , 12 In general, it can be concluded that the majority of practical materials would not be good catalysts for the alkaline HER. In order to get over this problem, one can combine two or more elements, which will improve the stability and electrocatalytic activity because of the ligand effect that results from the formation of heteroatom bonds and the strain effect caused by altered bond lengths. It is evident from Figure 3 that, in terms of HBE, combining two metals from opposing sides of the plot may produce an alloy that is centred on elements like Pt and Ir, a finding that has been validated. 39 This is probably the outcome of a mass electron transfer between the active sites of the two metal atoms, which makes it a very efficient way to alter the electronic structure of DFT calculations revealed that 5 at.% Ti produces near-optimal HBEs at two Cu–Cu–Ti hollow sites, and that additional increments/reductions of the Ti-percentage introduce inactive Cu–Ti–Ti sites and decrease the amount of the active Cu–Cu–Ti sites, as Figures 7 and 8.
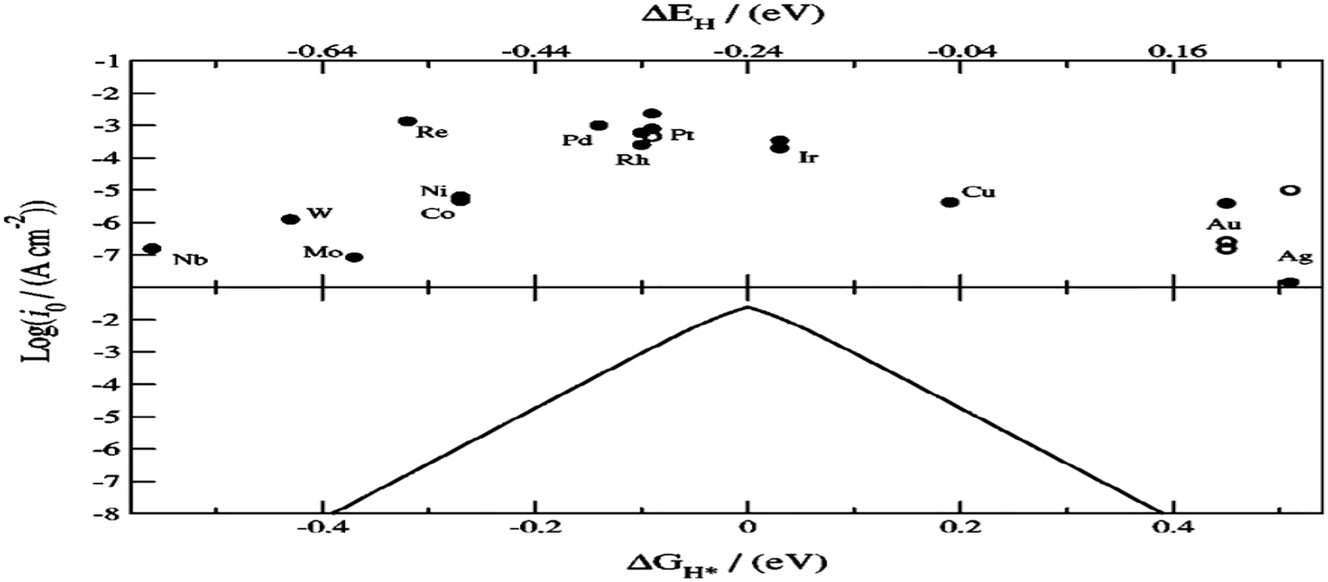
Exchange current densities (ECDs) measured experimentally for the HER on various metal surfaces as a function of the DFT calculated hydrogen chemisorption energy per atom (∆E H ) (upper axis). Single crystal data are indicated by open circles. Gibbs free energy for hydrogen adsorption (∆G H ∗) determined from a simple kinetic model (bottom axis), ∆G H ∗ = ∆E H + 0.24 eV. Copyright from the Electrochemical Society. 12
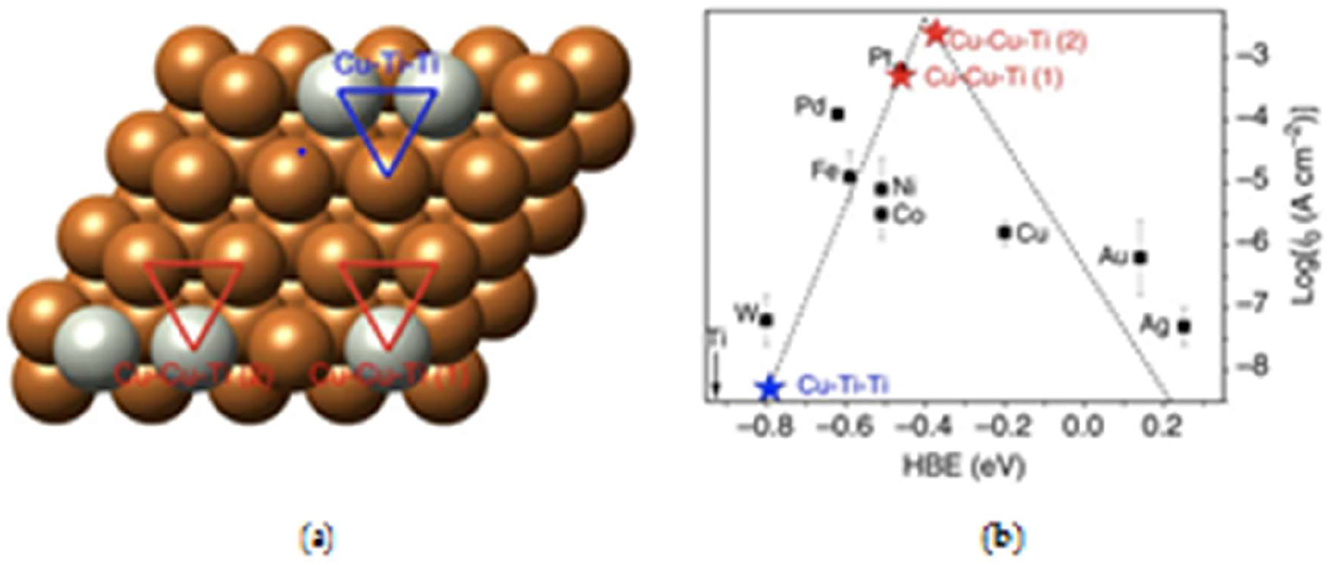
Schematic diagram of bimetallic catalysts. (a) Possible bimetallic sites on the surface of copper modified with titanium. (b) Corresponding HBEs showing the activity of the different Cu–Ti sites relative to other common elements. Copyright from Nat. Commun. 2015. 40
The volcano curve is a useful tool for comparing the activities of different metals in acidic and alkaline conditions. With the highest exchange current density and the best hydrogen adsorption energy, Pt is the best catalyst (HER) in both media. The activity of HER is often reduced in acidic media compared to alkaline ones. 41 This is partly due to reaction being hampered by the slow water dissociation phase, which causes a 2–3 order of magnitude decrease in reaction rate. In industrial operations, alkaline electrolysis is more preferred. It is essential for electrocatalysts to have the ability to bind hydrogen species and dissociate water to rationally develop electrocatalysts with high alkaline HER performance.
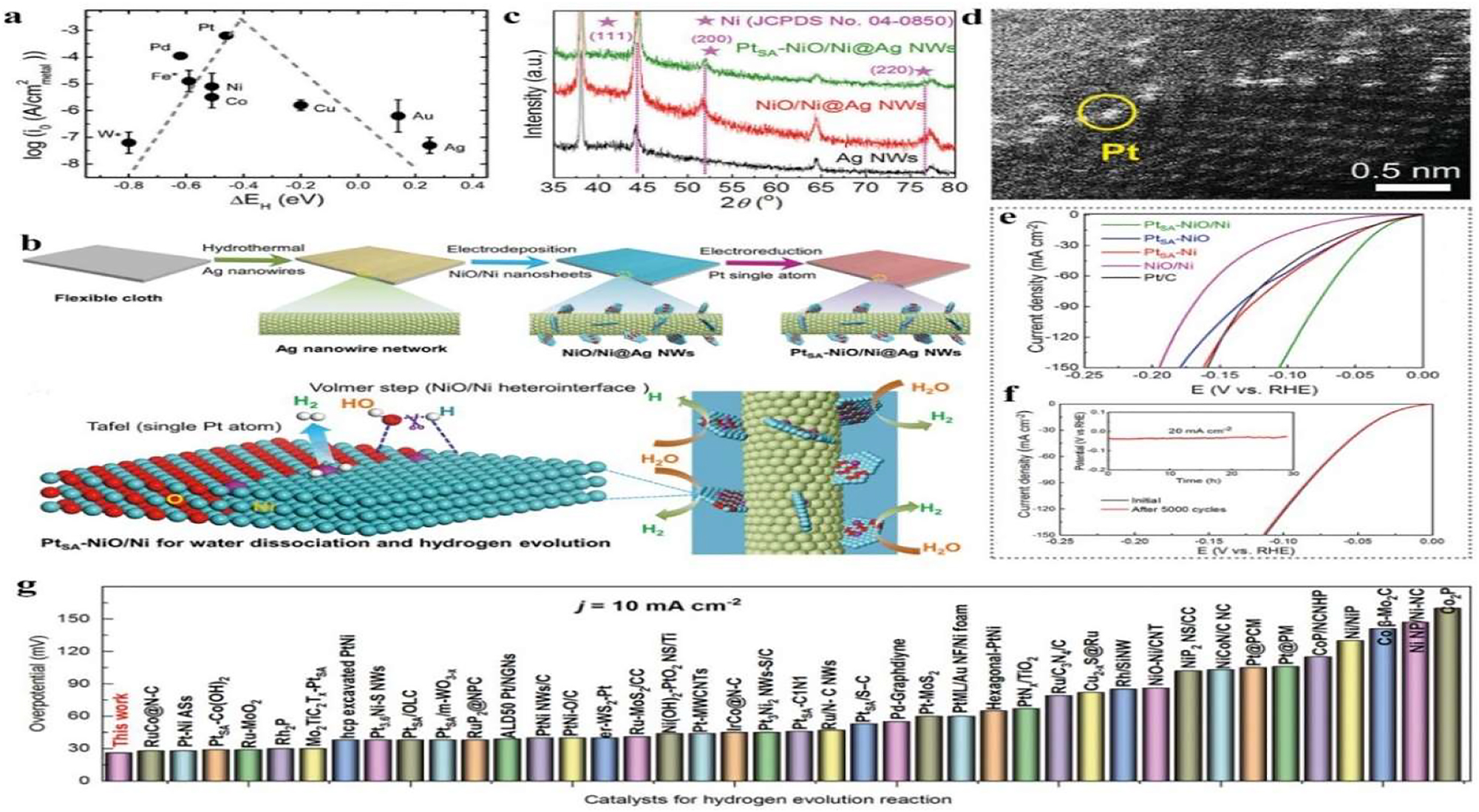
Synthesis and characterization of catalysts for HER. (a) HER volcano plot of single metals under alkaline conditions. Reprinted with permission from reference. 30 Copyright 2013 The Royal Society of Chemistry. (b) Synthesis illustration of PtSA-NiO/Ni catalyst. (c) XRD patterns of Ag nanowires, NiO/Ni@Ag, and PtSA-NiO/Ni. (d) HAADF-STEM images of PtSA-NiO/Ni. (e) Comparison of linear scan curves of different catalysts. (f) V − t stability and cyclic voltammetry stability tests. (g) Comparison of HER activities of PtSA-NiO/Ni and reported catalysts. Copyright from Springer Nature 2021. 41
4.2 HER electrocatalysts
A number of current instances of research studies to create efficient HER catalysts are listed in Table 1. The electrocatalytic efficiency and kinetic character of such type’s catalysts are compared under various reaction circumstances. There are two main types of HER electrocatalysts: those based on noble metals and those based on non-noble metals. It is the goal of many researchers to find ways to make “Pt” based catalysts and other noble metal electrocatalysts more affordable while also improving their effectiveness in HERs. For example, alloying with Pt or with other inexpensive transition metals may enhance Pt’s efficiency, and the alloy’s synergistic effects may change the electronic surroundings to increase activity. Additionally, combining Pt among other water dissociation promoters is a crucial tactic for enhancing alkaline HER actions, which is highly significant for actual industrial exercise. The development of HER electrocatalysts based on non-noble metals has received a lot of attention, partly because of their low cost and earth abundance properties. 26 , 42 , 43 We’ll kick off the discussion of a few chosen examples of noble metal catalysts in the sections that follow. Moving on, we will focus on other non-noble metal based electrocatalysts classes that have seen significant advancement in electrocatalytic HER, including as transition metal carbides, transition metal phosphides, and transition chalcogenides.
Performances of HER catalysts based on various nanostructure materials.
| Name of catalysts | Electrolyte | Value of η (mV) | Value of I (mA cm−2) | Tafel slope value | Time of stability | Ref |
|---|---|---|---|---|---|---|
| PtNi–Ni NA/CC | 0.1 M KOH | 38 | 10 | 42 | 90 h | 4 , 40 |
| PtNi–O/C | 1 M KOH | 39.8 | 10 | 78.8 | 10 h | 12 , 36 |
| PtNi(N) NW | 1 M KOH | 13 | 10 | 29 | 10 h | 40 |
| Mo 2 C–R | 1 M KOH | 200 | 30 | 45 | 41 | |
| 0.5 M H2SO4 | 200 | 32 | 58 | 2,000 cycle | ||
| Mo 2 C–GNR | 0.5 M H2SO4 | 167 | 10 | 63 | 3,000 cycles | 44 |
| 1 M NaOH | 217 | 10 | 64 | 3,000 cycles | ||
| 1 M PBS | 266 | 10 | 74 | 3,000 cycles | ||
| Ni 2 P/Ti | 0.5 M H2SO4 | 130 | 20 | 46 | 500 cycles | 42 |
| NiCo 2 P x | 1 M KOH | 58 | 10 | 34.3 | 5,000 cycles | 43 |
| 1 M PBS | 63 | 10 | 63.3 | 5,000 cycles | ||
| 0.5 M H2SO4 | 104 | 10 | 59.6 | 5,000 cycles | ||
| Defect-rich MoS 2 | 0.5 M H2SO4 | 200 | 13 | 50 | 10,000 s | 26 |
| CoS 2 NW | 0.5 M H2SO4 | 145 | 10 | 51.6 | 3 h | 27 |
| CoS 2 MW | 0.5 M H2SO4 | 158 | 10 | 58 | 41 h | |
| Oxygenated MoS 2 | 0.5 M H2SO4 | 120 | Onset | 55 | 3,000 cycles | 27 |
4.2.1 Noble-metal based electrocatalysts
PGMs, which include Pt, Pd, Ru, Ir, and Rh, are examples of noble metals that exhibit exceptional HER catalytic activity. In Figure 3, Pt is positioned near the peak of the volcanic curve. However, the limited storage and high price of these noble metal based catalysts prevent their practical utilization. A thoughtful design and shape of catalysts with low metal loading, good metal utilization is required to meet this problem. When Pt is alloyed with transition metals, it can be used much more effectively. The synergistic effects of these alloys can change the electrical environment, which in turn increases the electroactivity of HERs. At a current density of 10 mA cm−2, Sun et al. demonstrated excellent HER activity with a modest over-potential of 37 mV in 0.1 M KOH, using carbon cloth and an ultralow loading Pt concentration of 7.7 % to create an in-situ array of ultrafine Pt–Ni nanoparticles decorated with Ni nanosheets.
The results of the long-term durability test up to 90 h of catalytic activity are quite remarkable. It is reasonable to assume that the improved HER performance of PtNi–Ni NA/CC is related to the downshift relative to Pt’s d-band center, which lowers the adsorption energy of oxygenated species such (OH) on the surface Pt atom. 4 , 45 As a general rule, platinum electrocatalysts have decreased HER activity in acidic media compared to alkaline ones. Hydrolysis on the surface of Pt is inefficient, so the HER activity is modest. One common method for increasing the alkaline HER activity is to combine Pt with water dissociation promoters. 40 Improving the electrocatalytic activity of different platinum-based electrocatalysts for HER requires the capacity to modify the surface metal composition. 46 , 47 , 48
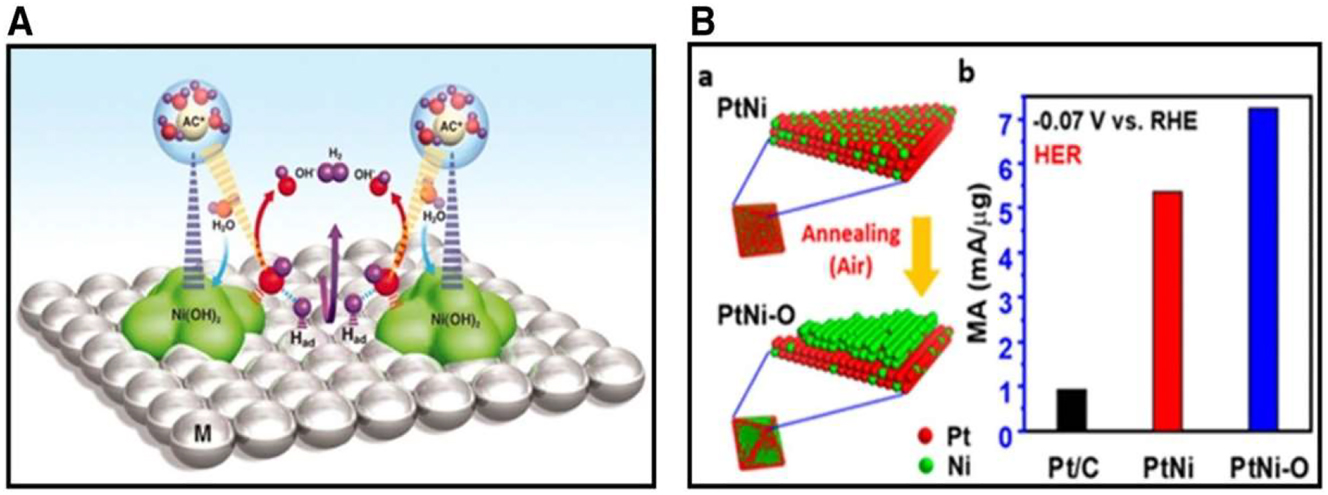
Mechanism of electrocatalysis in HER. (A) Depiction of the nanoscales mechanism of the HER process Ni(OH)2 on the surface of a Pt electrode, (B) annealing PtNi/C to PtNi/–O/C using air with HER mass activities 0.07 versus HER causes illustrations. Copyright from Advance Material (2019).
Markovic et al. 49 recently shown an eight-fold increase in HER activity compared to Pt of the highest quality by controlling the formation of nanometer-scale Ni(OH)2 clusters on Pt electrode surfaces. Figure 4A shows the HER mechanism. The Ni(OH)2 cluster’s periphery on Pt promotes water dissociation, leading to the formation of M–Had intermediates.
H2 is produced by the reaction of the adsorbate hydrogen intermediates. Drawing inspiration from the synergy between Ni(OH)2 and Pt(111), Huang et al. developed the concept of surface-engineered PtNi–O nanoparticles with an enhanced NiO/PtNi interface. In conditions with a high pH, this surface makeup changes to Ni(OH)2, resulting in an interface that resembles Ni(OH)2/Pt(111). With just 5.1 gpt cm2 of Pt added, the catalyst displayed a small HER overpotential of 39.8 mV at 10 mA cm2. The structural change from PtNi/C to Pt–Ni–O/C is seen in Figure 4(a) throughout the annealing process. Pt–Ni–O/C exhibits the greatest mass activity at an overpotential of 70 mV against the reversible hydrogen electrode (RHE), surpassing both Pt–Ni (5.35 mA/gpt) and commercial Pt/C (0.92 mA/gpt), as shown in Figure 4(b). The graphic shows that the Tafel-slope. Another efficient method to increase the HER catalytic capacity with less Pt is to dope platinum-based materials with other metal components. Wang et al. 40 created a catalyst with Pt–Ni nanowires that were modified with N. An extraordinarily low overpotential of 13 mV at 10 mA cm2 in acidic conditions was achieved as a consequence of nitrogen-induced orbital alteration, which improved hydrolysis kinetics. Pt–Ni, Pt–Ni(N), Pt–Ni/Ni4N, and retail Pt/C linear remove voltamogram (LSV) curves (a) are shown in Figure 5(a)–(d) to illustrate the HER catalytic evidence of PtNi(N) NWs in an alkaline medium. Pt–Ni(N) NWs exhibit the most favorable overpotential, measuring 13 mV, when the current density is 10 mA cm2 s can be used to investigate the rate-determining phases of the alkaline HER process. Data on the intrinsic activity of the catalysts are also uncovered by studying TOF data, as shown in Figure 11(c).
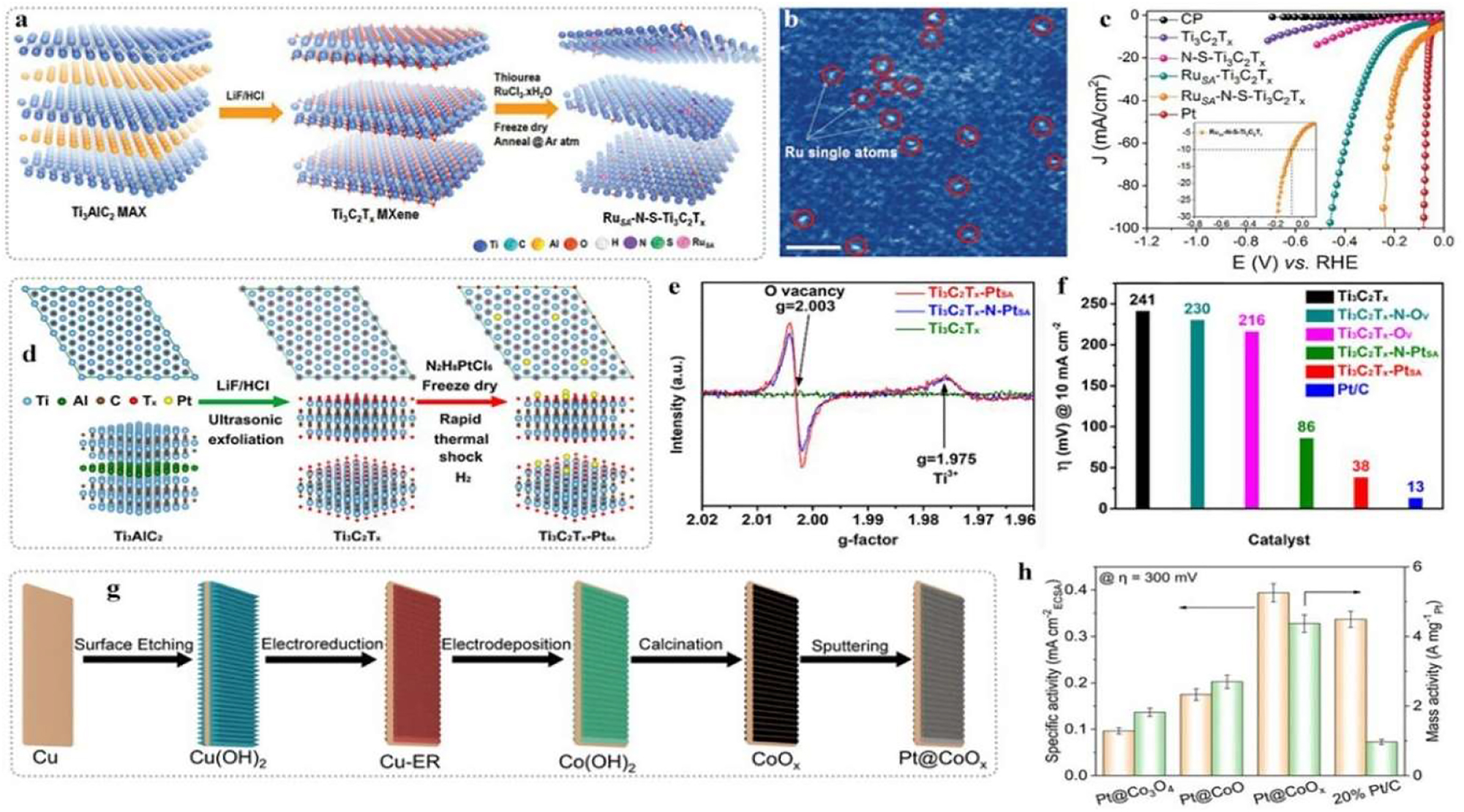
Design of noble-metal-based electrocatalyst. (a) Synthetic route of Ru-based catalysts with Ru–N–S coordination. (b) HAADF-STEM image of Ru–N–S–Ti3C2T x . (c) HER curves of Ru-based catalysts. Copyright 2019 Wiley-VCH. (d) Synthetic route of Pt–Ti3C2T x with O vacancy. (e) EPR of catalysts. (f) HER activity comparison of catalysts. Reprinted from reference. 49 Copyright 2022 American Chemical Society. (g) Synthetic route of Pt@CoOx. (h) Intrinsic activity comparison of Pt-based catalysts. 50 Copyright 2022 Wiley-VCH GmbH.
A greater TOF is achieved by Pt–Ni(N) compared to Pt/C and Pt–Ni alone. Lastly, the chrono-potentiometric durability test of Pt–Ni(N) shows that after 10 h at a current density of 40 mA cm2, no noticeable change in potential is seen. Through the use of density function theory (DFT) calculations, the modulation essence of nitrogen in the Pt–Ni nanowire was examined (Figure 12(e)–(g)). Nitrogen may cause a decrease in electron density surrounding the Ni site as a consequence of strong interaction between the two elements, as shown by analysis of the surface electron density difference in (Figure 12(e)). The dz2 empty orbital with high N–Ni connection and has ideal orientation for the dissociation of water is confirmed by the orbital analysis.
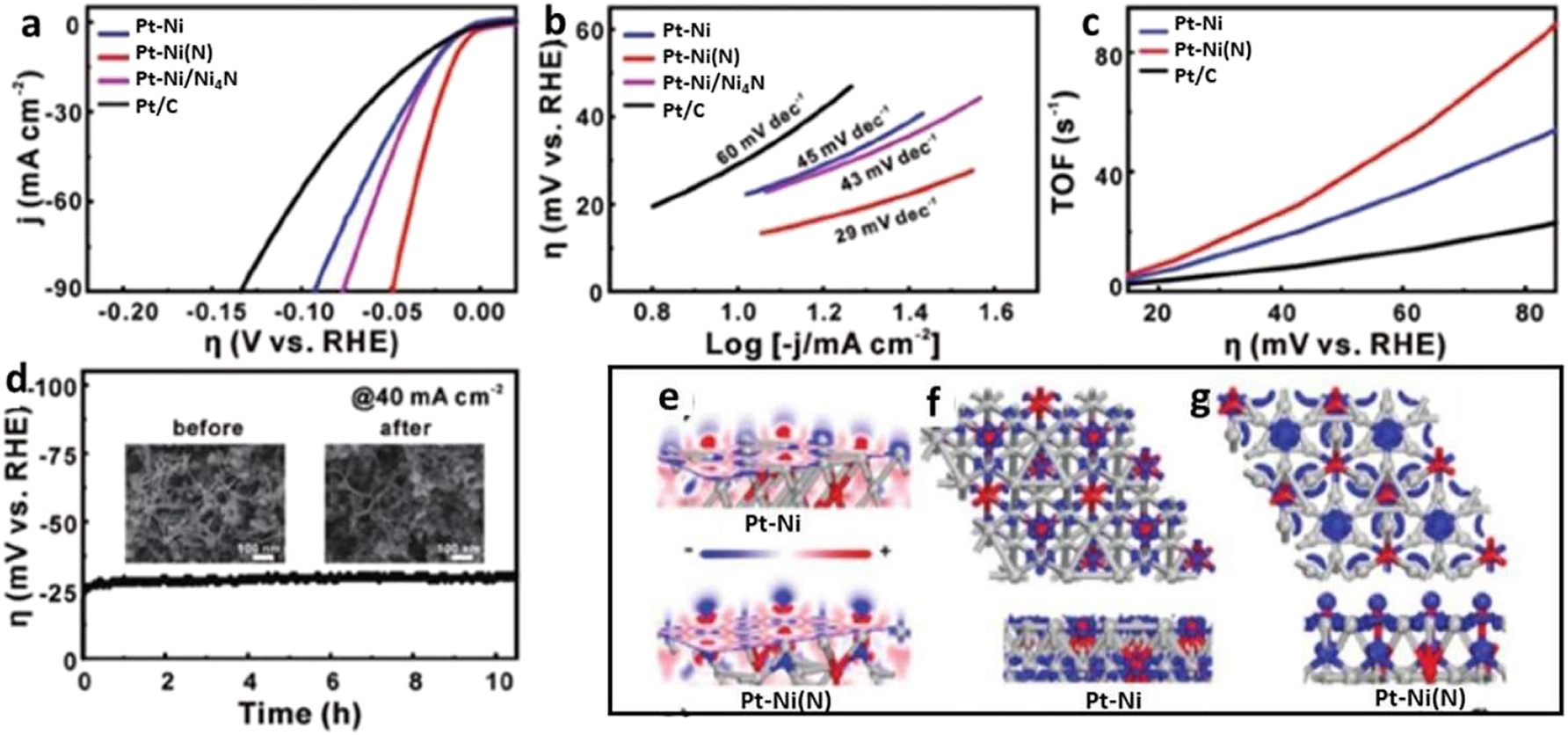
Electrochemical analysis of HER. (a) Shows the results of infrared correction applied to the HER presentation, with LSV curves plotted against different catalysts in 1.0 M KOH. The picture (b) shows Tafel plots, and curves c and d represent total organic solvents and Pt–Ni(N) NWs, respectively. Results from the insets e-DFT calculation: By comparing the electron densities of Pt–Ni and Pt–Ni(N) slices observed from the top and side views of the orbital above the fermi level, we were able to calculate the difference in electron densities. 40 Copyright from Advance Material (2019).
4.2.2 Platinum group metals-based electrocatalysts
It is commonly known that, according to the Sabatier principle, the binding energy between the intermediate Had and the catalyst establishes the rate-determining stage of the reaction process. 51 , 52 Reaction speed decreases due to excessively strong Had binding that restricts H2 release. Conversely, had is more difficult to adsorb due to its lower binding energy. Consequently, the electrocatalytic reaction process benefits from the mild adsorption and desorption energy (Figure 13). 53 , 54 , 55
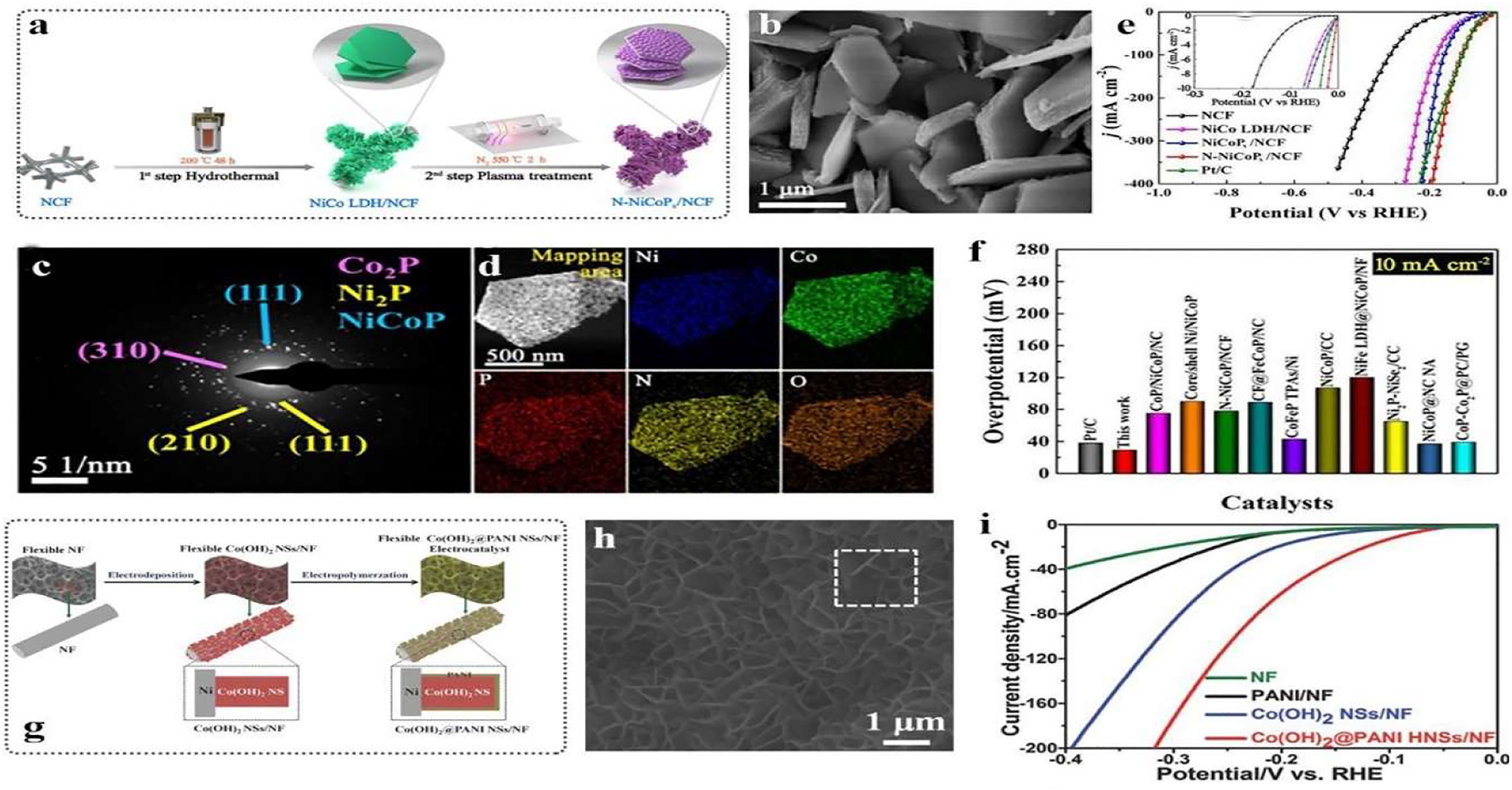
Metal based electrocatalysts. (a) Synthesis routes of N-NiCoP x . (b) SEM of N–NiCoP x . (c) Selected area electron diffraction (SAED) of N–NiCoP x . (d) EDS mapping of N–NiCoP x . (e) HER curves of catalysts. (f) Comparison of N–NiCoP x with previous reported catalysts. 56 Copyright 2020 Elsevier B.V. (g) Synthesis of Co(OH)2@PANI. (h) SEM of Co(OH)2@PANI. (i) HER curves of Co(OH)2@PANI. 57 Copyright 2015 Wiley-VCH.
The electronic structure of the active centre can be efficiently controlled by transition metal catalysts that are doped with non-metal atoms, such as N, P, S, or Cl.67–69 N-doped Ni–Co phosphide catalyst was produced by using hydrothermal and plasma treatment techniques (Figure 14(a)). The shape of the NiCo hydroxide nanosheet is visible in the SEM image (Figure 14(b)). Large surface areas and an abundance of catalytic active sites are features of this type of morphology. Energy-dispersive spectroscopy (EDS) has been used to characterise the component (Figure 6(d)). Additionally, the HER curves demonstrate that NiCoP (111) activity (Figure 6(c)) is comparable to commercial Pt/C and earlier studies (Figure 14(e) and (f)). Consequently, it makes sense to populate an electrocatalyst that is efficient, clean, and beneficial to the environment. Additionally, in order to address the issue of high stability, composite catalysts have been produced because (Figure 14(h) and (i)).
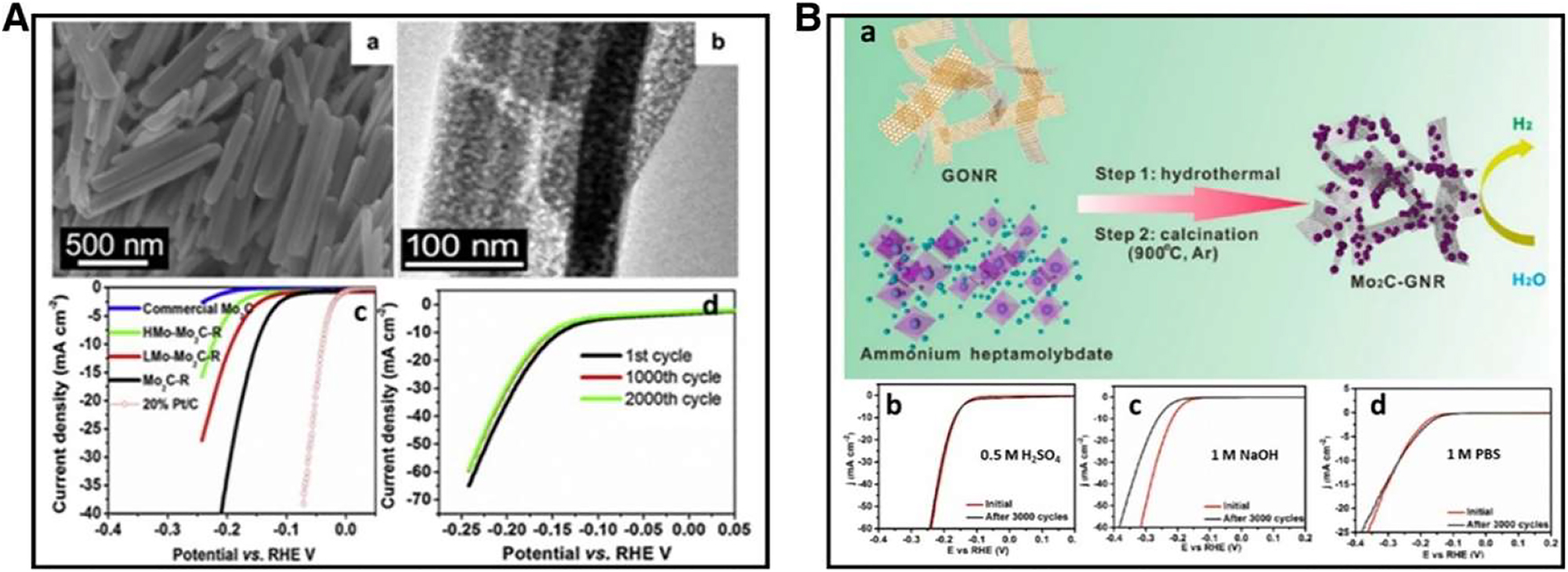
Shows the solution of Mo2C–R in 0.5 M H2SO4, we find the following: (a) The process of creating molybdenum carbide (Mo2C) nano-particles that attach to graphene nano-ribbons (GNRs); and (b–d) the effects of acidic, basic, and neutral conditions on the activity and longevity of Mo2C-GNR. Copyright from Appl. Catal. (2014), and Chem. Eng. (2016).
4.2.3 Electrocatalysts based on non-noble metals
TMCs are transition metal-carbides. The development of electrocatalysts based on non-noble metals has generated significant interest in TMCs. For instance, it has been demonstrated that molybdenum carbide micro particles (Mo2C) has strong catalytic activity towards HER. Their hydrogen adsorption properties and d-band electronic density state that similar to that of Platinum also demonstrate an ideal grouping, which is thought the primary cause of the high HER activity that has been found. In addition to their elevated electrical conductivity. In 1973, Levy and Boudart made the initial discovery that tungsten carbide had platinum-like catalytic behavior because it has d-band electronic thickness states that were comparable to those of Pt specialties. 58 , 59 Additionally, Chen et al. used DFT calculations to examine a series of transition metals’ physical, chemical, and electronic structure features. According to their findings, adding carbon atoms to the lattice interstitials gives them d-band electronic density states that are comparable to the Pt benchmark. Experimental data first confirmed the theoretical prediction in 2012. In acidic as well as alkaline conditions, commercially available molybdenum carbide micro particles (com–Mo2C) have well HER catalytic activity, according to Hu et al. To achieve a cathodic current of 10 mA cm2, there is a relatively high overpotential (190–230 mV), though. Researchers followed various strategies for the Mo2C catalyst optimization by nano-engineering the materials to expose additional active areas in response to the groundbreaking investigations. By easily carburizing anilinium-molybdate effectively synthesized Mo2C nanorods having a permeable structure.
Transmission electron microscopy (TEM) and field emission scanning electron microscope (FE-SEM) pictures of nanorods morphological features having a even surface and a spongy structure are shown in Figure 14A(a) and (b) of this article. Mo2C nanorods catalyst has improved HER electrocatalytic performance, as demonstrated. As can be seen from Figure 14A(c), the Mo2C nanorods outperform the commercial Mo2C in terms of activity, according to the results of the linear scan voltammetry (LSV) in 0.5 M H2SO4. The stability test results for the Mo2C are also shown in Figure 14A(d). Based on the results, it appears that the activity remains constant even after 2,000 cycles, suggesting a longer cycling life. The Mo2C nano-rod outperforms commercial Mo2C in 1 M KOH, according to additional studies conducted in acidic environments.
Due to their high conductivity and clearly defined, porous architecture, the Mo2C nanorods exhibit very competitive presentation for HER in the acidic as well as alkaline media. Ni nanoparticles addition could enhance the catalytic activity even more. Another technique to enhance HER’s efficiency as a hybrid nano-electrocatalyst is the accumulation of molybdenum carbides (Mo2C) on the surface of materials having carbon based properties.
Molybdenum carbide (Mo2C) materials nano-particles attached on the graphene nano-ribbons (GNRs) were produced by hydrothermally forming carbides on a GNR template in situ, followed by high temperature calcinations process, as illustrated in Figure 14B(a). In acidic and basic, as well as neutral environments, the Mo2C-GNR hybrid demonstrates exceptional electrocatalytic action and durability (Figure 14B(b)–(d)). Phosphide of transitional metals one of the quickly expanding fields in the improvement of electrocatalysts with high catalytic activity and stability in both acid as well as basic (pH universal) environments is the study of transition metal phosphides (TMP). It has been suggested that the P atom’s strong conductivity and distinctive electrical structure contribute significantly to TMP. Ni2P catalysts were first identified as one of the finest real-world HER catalysts in 2005. A number of electrocatalysts were investigated by Liu and Rodriguez using density functional theory (DFT). 60
The hydrogen species would require more energy to desorbs from the metal surface due to the tight connection. Because P atoms dilute the amount of highly active Ni sites, a reasonable bonding to intermediates and products is achieved, or what is called the “ensemble effect.” The first direct experimental evidence in favor of the catalytic synergy was shown, who demonstrated that a Ti foil substrate bearing a Ni2P nanoparticle exhibited outstanding HER activity, with an exchange density of 3.3 × 10–5 mA cm2 and a Tafel slope of 46 m V dec1. 36 Having said that, the stability of the Ni2P/Ti electrode was lacking, especially in an electrolyte with a high pH. Hu et al.’s follow-up research involved the creation of NiCo2P x , as a bimetal ordered phosphide electrocatalyst. It has been shown that this specific catalyst performs well as a pH-universal catalyst for HER and shows great durability and long-term stability in various electrolytes.
On commercial carbon felt (CF), the self support NiCo2P x nanowire arrangements were created using a wet chemical-hydrothermal process and an in-place phosphorization reaction. Figure 15(a) demonstrates NiCo2P x ’s superior HER performance in neutral, alkaline, and acidic environments. NiCo2P x , as opposed to CoP x , NiP x , and commercial Pt, had the lowest overpotential in the alkaline electrolyte at 58 mV at a current density of 10 mA cm2 Figure 15(b) illustrates the shape of NiCo2P x following in term of HER activity test and indicates that, even after 5,000 cycles under various circumstances, the catalyst structure of NiCo2P x is still well-preserved. According to Figure 15(c), NiCo2P x in an alkaline electrolyte is thought to have a synergistic impact. 2 , 61 , 62
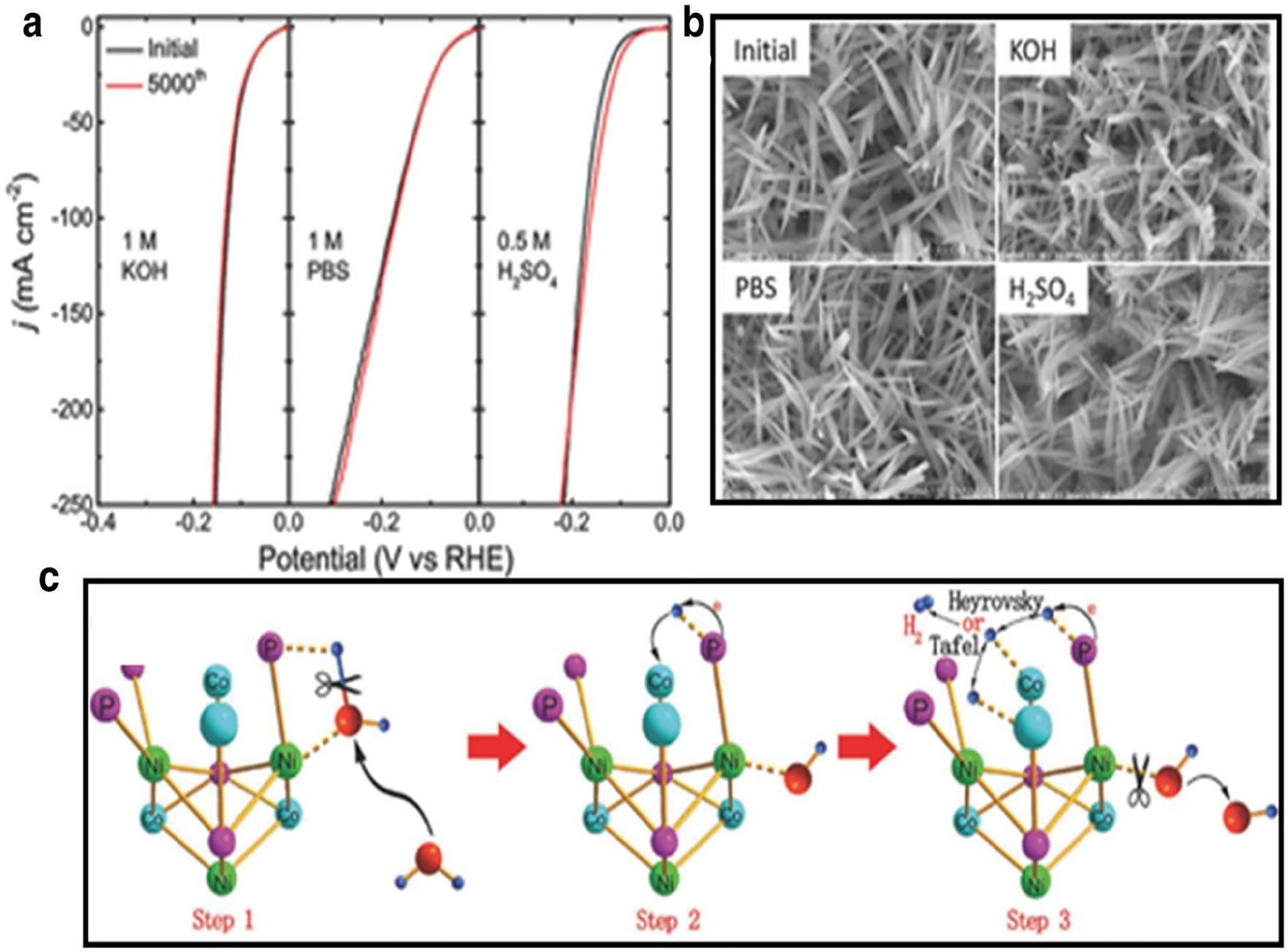
Shows the NiCo2P X catalyst in action: (a) The catalytic reaction (HER) in 1 M KOH, PBS, and 0.5 M H2SO4; (b) scanning electron micrographs taken before and after the HER long-term stability test in various media; and (c) a simplified representation of the water dissociation process on NiCo2P X in an alkaline solution, as described in reference. 63 Copyright from Advance Material (2017).
There are two significant interactions: the one between atom (P) and the H atom, and the other between the under coordinated metal centre (M+, M = Ni, Co) as well as the oxygen atom. The H–OH bond is weakened by the combination of these two interactions, which causes the water molecule to split into hydrogen atom and an OH molecule. The (H) atom is subsequently moved to a close by open metal site, where it anchors as adsorbed H. Molecular H2 is created by the combination of the adsorbed H atoms. Chalcogenides of transition metals (selenides and sulphides) in 2005, 62 et al. [54] grown triangular MoS2 single crystals on the active site of the MoS2 structure 54 demonstrated that the free energy of the atomic hydrogen bonding to the MoS2 edging is similar to Pt. This finding suggests that MoS2 could be used as an electrocatalyst for HER.
They proved that the amount of corner points on the MoS2 catalyst linearly affects the electrocatalytic HER activity. MoS2 has extremely active catalytic sites at its edges. This understanding has led to the development of numerous methods, such as nanostructure tuning and morphology tuning, to expose the active areas and increase HER activity. An approach to design flaws in MoS2 ultra thin nano-sheets, for instance, was disclosed by Xie et al. and was demonstrated to significantly enhance MoS2’s electrocatalytic HER performance. This high activity was attributable to the high fault structure’s additional active edge sites, which were created by partially breaking the basal plane, which is catalytically inert. CoS2 with tunable film, micro-wire (MW), and nano-wire (NW) shape was successfully synthesized 60 , 64 (Figure 16A(b)–(d)).
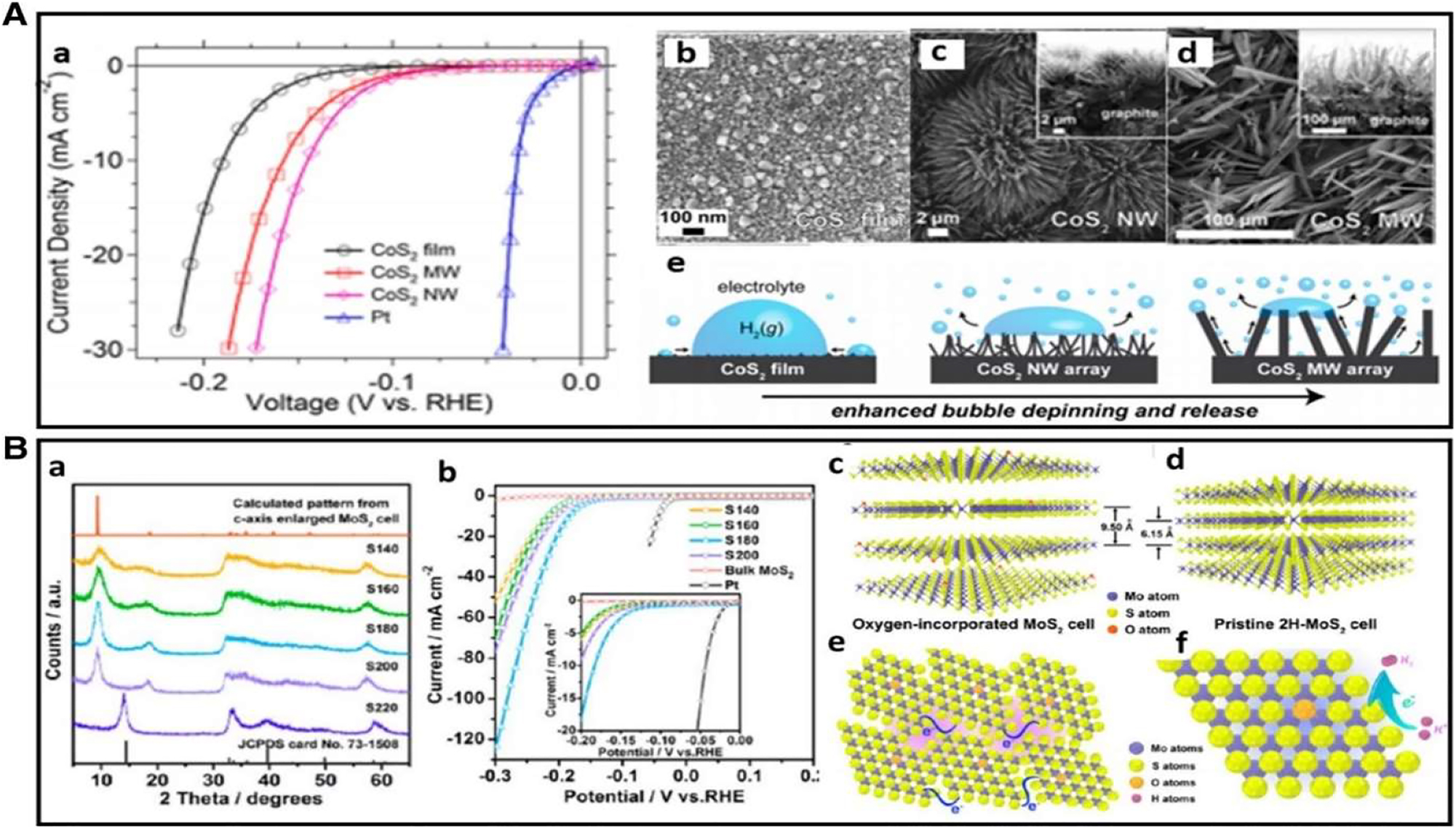
A shows polarization curves for the CoS2 film, MW array, and NW array electrode for HER. Then, from b to d, there are scanning electron micrographs of the film, array, and graphite. Finally, from e to f, there are schematic representations of the surface of the film, NWs, and MWs, as well as the produced H2(g) bubbles. This figure was reprinted with permission from reference. 65 , 66 , 67 , 68 The XRD pattern of the products obtained at different temperatures and the HER performance of the catalysts with varied oxygen-incorporated MoS2 ultrathin nanosheets are shown in graph B. (c, d) two-dimensional structural models of 2H–MoS2 and oxygen-incorporated MoS2 with increased interlayer gap. (e, f) visual depiction of the disorganized structure in ultrathin MoS2 nanosheets with oxygen incorporation. The purple shadings show the enrichment impact of active sites caused by the disordered structure, while the blue lines show the fast electron transport between the nano-domains that are quasi-periodically aligned. In reference. 69 Copyright from J. Am. Chem. Soc. (2013).
They established the shape dependent improvement of both activity as well as stability by methodically studying their structures, activities, and stabilities. Due to their large efficient electrode surface area and quick release of gas bubbles from the electrode surface (Figure 16A(e)), CoS2 NWs exhibit the best HER catalytic performance, stability among the three distinct morphologies (Figure 16A(a)). In order to maximize the HER electrocatalytic activity, significant work has been put towards tailoring metal chalcogenides’ electronic conductivity in addition to their active sites. The use of heteroatom doping to increase HER activity is efficient. According to, 46 adding oxygen atoms and engineering adjustable disorder can successfully manage the electrical structure of MoS2 ultrathin nano-sheets, increasing conductivity.
A variety of XRD patterns for catalysts grown at various temperatures are displayed in Figure 8B(a), revealing that MoS2 ultrathin nano-sheets made at 220 °C have numerous flaws. A novel structure with an enlarged interlayer spacing of 9.5 instead of 6.15 in ideal 2H–MoS2 is suggested by the appearance of two new peaks at the low-diffraction angle area when the synthesis temperature decreases to 200 °C or lower. In Figure 8B(c) and (d), the suggested structure models are displayed. The MoS2 structures from the various temperatures can be controlled to have varying degrees of disorder, as seen by HRTEM pictures and associated FFT patterns. The ultrathin MoS2 nanosheet with oxygen incorporates was subjected to electrochemical tests with varying degrees of disorder.
5 Electrocatalysts for oxygen evolution reaction (OER)
As we have discussed prior that OER is half-cell reaction of water splitting process, it occurs at anode and it also require a large overpotential as compared to HER, due to four electron transfer process. OER is main key step in improving the efficiency of electrochemical breakage of water. The hunt for a super electrochemical catalyst that can lower the kinetic barriers in electrocatalytic processing is of the utmost importance for improving reaction efficiency. Regarding this, there have been a lot of noteworthy advancements in our understanding of the mechanism of OER for improved processing of electrocatalyst reactions in the recent past. Both the adsorbent evolution mechanism (AEM) and the lattice-oxygen mediated mechanism (LOM) provide useful frameworks for understanding the OER process.
5.1 Reactions steps in the OER-in term of adsorbate evolution mechanism (AEM)
Using a diagrammatic explanation of the alkaline pathway, this technique concentrates proton transfers with metal electrons as an active site to produce oxygen molecules from water using the water-splitting method in both acidic and alkaline media. This evaluation mechanism has traditionally been used in the field of electrochemistry to describe various electrochemical reactions. If we talked about in production of oxygen molecule during this process, basically there are route for the production of oxygen molecule during water splitting. The one ways that hydroxide anions absorb on the surface of metal where active sites are available in first method oxygen atoms reacts with metal surface active site of metal where they form an intermediate MOOH molecule to produce season molecule through the protonation of MOOH molecule oxygen produced. the second method as illustrated by green route as in Figure 17 in which two molecules of more reacts to each other to form oxygen molecule along the active site in metal during this process there are large number of activation barriers.
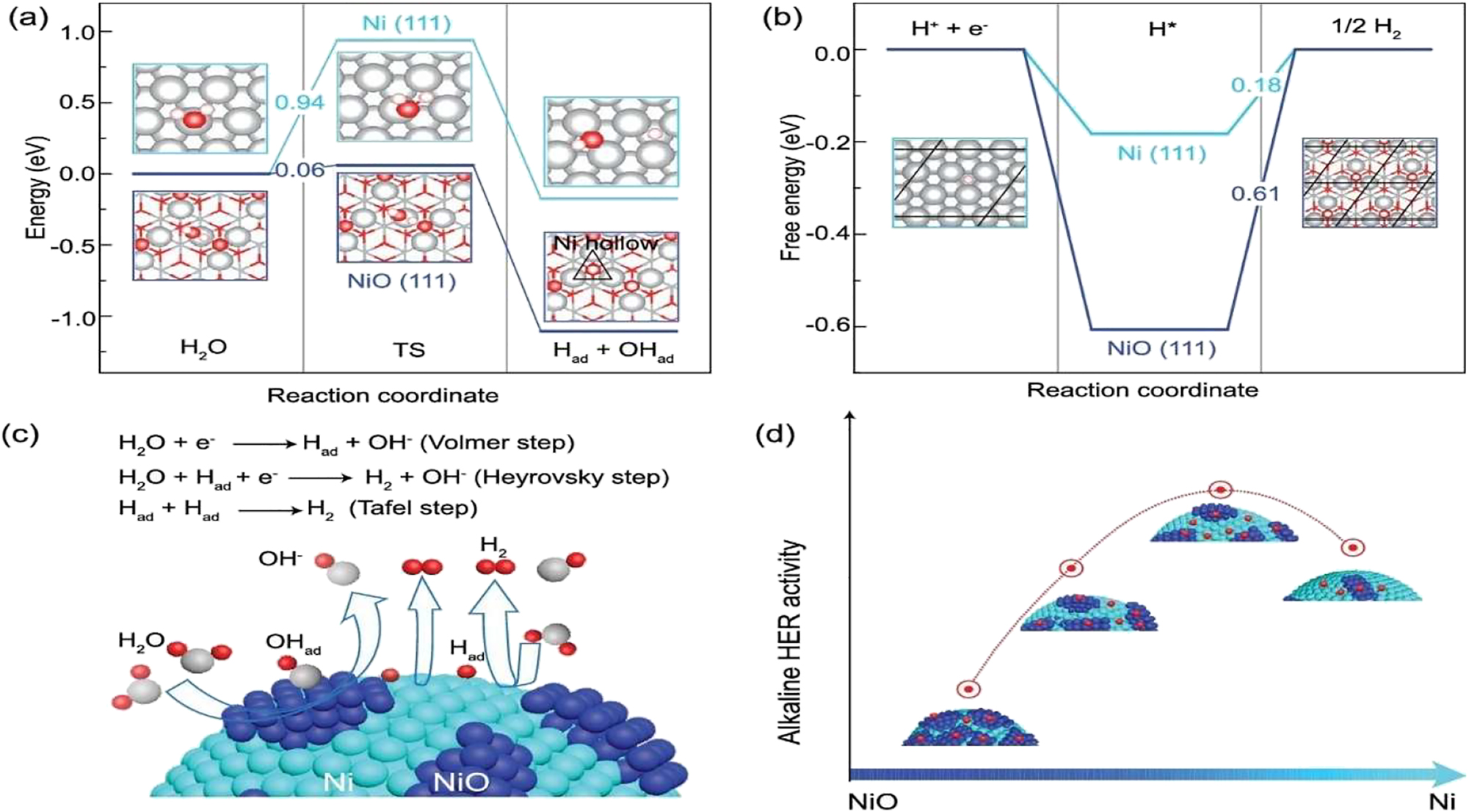
DFT calculated reaction energies for (a) dissociation of water and (b) adsorption of hydrogen atoms on Ni and NiO surfaces. (c) A schematic of complete reaction on dual Ni/NiO surfaces and (d) catalytic activity as a function of Ni/NiO ratio. 70
5.2 Creating the specific sites for hydrogen adsorption
Both the ratio of Ni/NiO was carefully regulated to maximize the alliance between the two surfaces handling the initial phase of water dissociation and the subsequent adsorption of hydrogen. 70 Ni/NiO heterostructures with a range of crystal sizes were proven to have formed using high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM), with most of the heterostructures having core–shell architecture. As schematically shown in Figure 17(a)–(c), DFT calculations indicate that the most effective reaction pathway is through water dissociation on the NiO {111} surface, leaving OH− to adsorb onto the oxygen-vacancy of NiO {111} and the hydrogen atom to adsorb onto the Ni {111} site. The activity of the material was highly correlated to the ratio of Ni/NiO, where it peaked around 0.2 and aggressively degraded upon further rise/fall as shown in Figure 17(d).
The other mechanism of acidic OER reaction the same intermediate as M–OH, MOOH, M–O are concerned in electrocatalysis a detailed understanding of these intermediate has not been yet completely revealed due to the activation barriers during the binding mechanism of Hydroxide ion did metal active sites in OER reactions because the binding strength of hydroxide ion is the key parameter for performance of the reaction over potential. As shown by the energy diagram there is no difference reaction steps No there is no potential difference needed between steps there is no energy gap between the steps remain same. 25 , 71 The OER energy potential is calculated by rate determining steps (RDS), which comes from the energy of the intermediate has shown in figure the binding energy how these intermediate show a constant difference of 3.2 eV this is due to that Hydroxide anions HO, HOO read for bonding will catalyst surface throw similar pattern of adsorption configuration with oxygen atom via a single bond this all is represented by spectacular volcano shaped relationship which is being used traditionally to explain the activity trends of metal oxides we acidic as well as in basic media as shown by the results (Equations (10) and (11)) latest research has revealed that the best catalyst in reference of lowest hypothetical over potential of OER, IrO2 as well as RuO2 that show the maximum binding potential with metal exercise on the surface of catalyst.
First, M–OH is created by the adsorbed hydroxide anions on the surface of metal’s active site. Metal-hydroxide then undergoes deprotonation to produce M–O. There are then two distinct pathways that lead to the formation of O2 molecules. A potential pathway involves the reaction of metal-hydroxide and hydroxide to form an intermediate, followed by the deprotonation of M–OOH during active site regeneration to produce O2. Figure 9(a) shows an alternate path that uses two M–O types, adapts them to O2, and regenerates the metal active site, which is thought to have a high activation energy blockage. The majority of scientists believe that the acidic oxygen evolution reaction (OER) mechanism involves intermediates (Equations (12)–(14)) such as M–OH, M–O, and M–OOH. A comprehensive knowledge of the binding force of the reaction intermediates on the electrode surface is essential for improving the overall performance of OER electrocatalysis, since the binding power is a significant component determining the reaction overpotential. If the free energy gap for each of the fundamental processes remains constant at 1.23 eV, as shown in the perfect free energy graph of the several OER reaction levels in Figure 17(b), 72 then OER can take place without the need for excess potential. Nevertheless reaching this perfect state is really challenging.
Difference of 3.2 eV between GHOO and GHO. This is due to the fact that both HOO− and HO− combine to catalyst surfaces using common adsorption topologies that involve oxygen atom connected by a solo link. 57 The minimal theoretical overpotential, which corresponds to the distinction between the constant gap in binding energy (3.2 eV) and the ideal value of 2.46 eV, is calculated using the scaling relation to be 0.37 eV ((3.2 × 1.23 × 2 eV)/2). Most OER catalysts are believed to have their RDS in the second and third phases, respectively, of their development the production of M–O and M–OOH, so it is helpful to use the difference between GO and GHO in broad descriptions to predict their OER activity. This is exemplified by the Sabatier’s volcano-shaped correlation, which has long been employed to describe patterns in OER activity on M–O in acidic and alkaline media. iridium dioxide and ruthenium dioxide, which exhibit an ideal binding strength on the catalyst’s surface which shows the moderate strength not too strong or not too week as illustrated in Figure 17(d), 73 are the most excellent catalysts having the minimum hypothetical overpotential for OER.
5.3 OER electrocatalyst
In reference to their performance description there is a list of some OER catalyst (Table 2). We will discussed further in later with reference to their specification. There are the two primary categories of OER electro-catalyst techniques one is noble-metal based and the second one is non-noble metal based. Ru as well as Ir base catalysts are regarded the most advanced noble-metal electrocatalysts for the OER, particularly in the acid electrolyte, that has a higher disbanding struggle than other metals. There are numerous ways to optimize the catalyst chemical composition, configuration, as well as morphology in order to lower the high cost, increase electro-catalyst action, strength, and even implement the dissolving resistance in acidic conditions. 74 , 75 In addition to Ir and Ru, bi- or tri-functional electrocatalysts based on additional noble-metals, such as Rh, Au as well as Pt plus Pd, have also been produced.
Performances of OER catalyst based on various nanomaterials.
| Catalysts | Electrolyte | η (mV) | I (mA cm−2) | Tafel slope | Stability | Refs |
|---|---|---|---|---|---|---|
| Cu-doped RuO 2 | 0.5 M H2SO4 | 187 | 10 | 42.86 | 10,000 cycles | 76 |
| IrNi NPNW | 0.1 M HClO4 | 288 | 10 | 55.8 | 200 min | 77 |
| IrCo NPNW | 0.1 M HClO4 | 292 | 10 | 61.3 | ||
| IrFe NPNW | 0.1 M HClO4 | 300 | 10 | 67.4 | ||
| IrNiCu DNF | 0.1 M HClO4 | 298 | 10 | 49 | 2,500 cycles | 78 |
| IrO 2 NN | 1 M H2SO4 | 310 | 10 | 58 | 200 h | 50 |
| Au 40 Co 60 NPs | 0.1 M KOH | 177 | 10 | 68 | 1 h | 79 |
| G-FeCoW | 1 M KOH | 195 | 10 | 500 h | 80 | |
| Plasma-engraved Co 3 O 4 | 0.1 M KOH | 149 | 10 | 69 | 2,000 cycles | 81 |
| NiFe-LDH NPs | 0.1 M KOH | 148 | 30 | 48 | 10 h | 82 |
| Ni 0.83 Fe 0.17 (OH) 2 | 1 M KOH | 238 | 10 | 59 | 10 h | 83 |
| Ni x Fe 1−x Se 2 -DO | 1 M KOH | 197 | 10 | 27 | 24 h | 84 |
5.3.1 Electrocatalysts based on nobel metals
The mainly effective electrode resources in OER have long been thought to be noble-metal and M–O electrocatalysts. Examples include the cutting-edge electrocatalysts for OER, RuO2 and IrO2, which are frequently cited as examples. However, the significant dissolution of RuO2 and IrO2 and their expensive cost are the main issues, which focus a lot of attention on the change of the catalysts’ chemical composition and configuration as well as shape. Numerous methods have suggested increasing the activity and constancy of electrocatalysts as well as lower their high cost. There is a lot of interest in heteroatom doping as a means of modification the chemical composition of IrO2 base OER electrocatalysts. However, host systems diverse guest atoms produced unique energy domains. By thermally decomposing derivatives of Ru exchanged metal organic framework (MOF) materials, Chen created Cu-doped RuO2 with empty porous polyhedral shape made up of extremely small nanocrystals. 65 , 78 , 85 , 86 Figure 10(a) shows the high index surface facet of the Cu-doped RuO2 phase, which was created by incorporating Cu into the RuO2 lattice. This information was collected by high resolution transmission electron microscopy (TEM) and XRD statistics. Figure 10(b) shows that the synthesis of OOH on RuO2(110) is caused by the RDS. Cu-dopant RuO2 can enhance OER activity by changing the electronic arrangement, which results in a wide binding area closer to the Fermi level of the p-band center. Additionally, Cu dopant generates O positions on the surface and induces the formation of unsaturated Ru sites. An innovative method to create OER catalysts that can successfully change the electrical structure and optimize the chemical reaction (Figure 18). 2 , 26 , 87 , 88 , 89
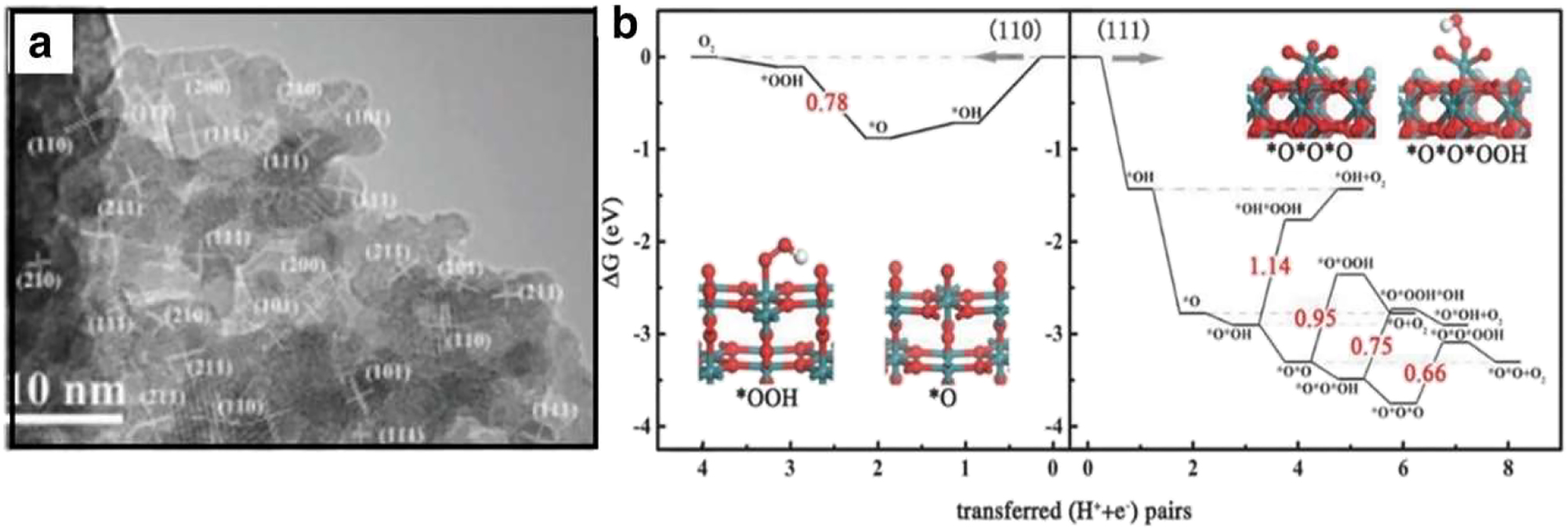
Electrocatalysis of nobel metals. (a) Shows a high-resolution transmission electron microscopy (HR-TEM) image of Cu-doped RuO2 showing raised surface features, and Figure 10(b) shows the free energy profile of optical emission spectra (OER) on surfaces (110) and (111) according to reference. 90 Copyright from Advance Materials (2018).
5.3.2 Self-supported bi-functional electrocatalysts for overall water splitting
In order to reduce the overpotential during the actual electrolysis of water, the HER and OER catalysts are used in the same electrolyte, which is a strongly acidic or basic solution. The majority of HER catalysts that are based on nonprecious metals, such as carbides, nitrides, phosphides, and chalcogens, exhibit their best catalytic activity in acidic media. Conversely, OER catalysts, like transition metal oxides and oxy/hydroxides, are only effective in alkaline electrolytes. Finding two electrocatalysts that couple well in a single electrolyzers for overall water splitting (OWS) is made challenging by the mismatching of operating conditions. 66 , 80 , 91 , 92 It is preferred to develop a bifunctional electrocatalyst that can efficiently catalyze both HER and OER at the same time in order to reduce device costs, simplify the design of the electrolyzer, and prevent the negative crossover effect of different electrocatalysts at two electrodes. Thus, great efforts have been made to create high-performance bifunctional catalysts for the electrolysis of water, primarily concentrating on establishing the bifunctionality of the HER and OER catalysts that are now under development (Figure 19).
Water electrolysis using a self-supported electrode as a bifunctional catalyst for HER/OER. the following are reproduced with permission: (a) SEM pictures; (b) Electrocatalytic water splitting performance; and (c) Schematic electrolyzer application of nanoporous copyright from Royal Society of Chemistry, Copyright 2016. (d) SEM pictures, (e) Water electrolysis results, and (f) Nitrogen-doped tungsten carbide nanoarrays applied to an electrolyzer. (d–f) Used with authorization. 93 (2018) All rights reserved.
By using a eutectic directed self-templating approach, 94 created a group of Ir–M (M = Ni, Co & Fe) type of catalysts with a distinctive system topology made up of interconnecting nonporous nanowires. The findings reveal a property that depends on the transition metal. Ir–Ni display the OER activity in comparison to Ir–Fe and Ir–Co NWs and have the minimum overpotential at 274 mV at 09 mA cm2. The DFT calculation results (Figure 11) provided an explanation for the increased activity of IrNi NW. Ir as well as M are oxidized at maximum potential to create Ir–MOx during OER process. IrO2, IrFeO x , IrCoO x , and IrNiO x had d-band centers of 3.61, 3.72, 4.09, and 4.34 eV, respectively (Figure 20(a)). The density of state (DOS) has changed in a negative way. The d-band electron distribution gone out the Fermi scale is induced by the ligands that effect after alloying, according to the down-shift of Ir d-band centre.
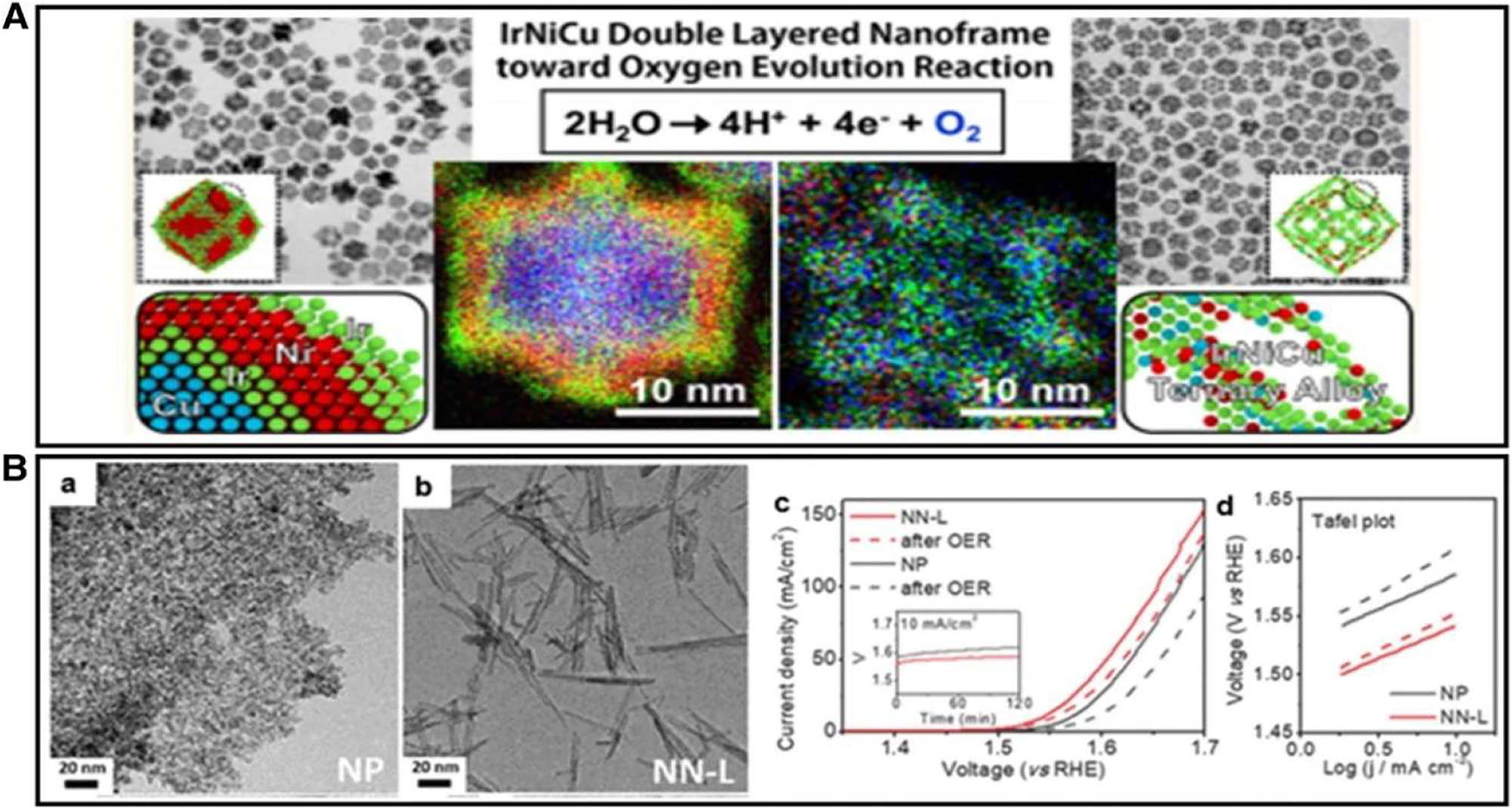
IrNiCu DNF structures. (A) Illustration of the HAADF-STEM, transmission electron microscope (TEM) as well as HRTEM, and elemental map of preferred IrNiCu DNF structure. 95 Copyright ACS Nano (2017) (B) (a, b) transmission electron microscope (TEM) imagery of IrO2 nano-particles along with IrO2 nano-needles, (c, d) OER presentation before plus after 2 h galvano-static operation, (c) depiction of LSV curves of IrO2 NN and amorphous nano-particles, (d) Tafel slope 96 Copyright from Advance. Funct. Material (2018).
In order toward expose catalytically active areas and benefit from the interfacial effect, surface structure alterations are crucial. One component of the surface structure change is morphology regulation, which has received. There has been a negative change in the density-of-state (DOS). The down-shift for Ir’s d-band centre indicates that the ligand effect after alloying induces the d-band electron distribution further from the Fermi level. According to Figure 11(b)–(d), the binding powers of various species like O, OH, plus OOH having a prominent impact on OER activity. Surface chemical composition modifications are essential to expose catalytically active regions and gain from the interfacial effect. A single electro-catalyst could be created using straightforward one-step production, and the change in surface structure is.
According to findings from following instruments HAADF-STEM, TEMas well as HRTEM, and from elemental mapping, the intended Ir–Ni–Cu DNF arrangement (ternary alloy) retains a rhombic dodecahedral morphology following intense acidic etching and has a consistent factor distribution throughout, whole DNF structure. Ir–Ni–Cu catalysts for oxygen evolution reactions have exceptional electrocatalytic activity and durability because its frame structure prevents particles from becoming agglomerated and coarse and allows for the in situ creation of a durable rutile IrO2 phase during the OER process. Electrical conductance of catalyst is also demonstrated to be impacted by morphology management, which enhances OER electrocatalytic activity.
IrO2 nano-needles were created by Lee et al. using a scale-able molten salt technique, and they exhibit greater OER activity and stability than IrO2 nanoparticles Figure 12B(a) and (b). According to Figure 12B(c) and (d), which compares the electro-chemical activity of IrO2 NPs as well as IrO2 nano-needles for OER, the IrO2 nano-needles have higher activity and stability than IrO2 NPs do. The electrical conductance of ultra-thin IrO2 nano-needles is 318.3 S cm, which is higher than the electrical conductivity of IrO2 unshaped nanoparticles, which is 25.9 S cm. 96 This suggests that the form of the catalyst is critical for the high activity for OER caused by electron transfer. In addition to Ir and Ru, additional Nobel metals like (Rh, Au, and Pt as well as Pd) have also been showing promising results for OER electrocatalysts. Hydrogen evolution reaction (HER), oxygen reduction reaction (ORR), and oxygen evolution reaction (OER) catalyst design entails building bi- or tri-functional electrocatalysts from Pt, Pd, Ru, or Au. It is common practice to study the OER behaviors of Rh, Pt, Au, and Pd in alkaline solutions since their dissolving resistances are lower than Ir’s and Ru’s in acidic electrolytes with a high over-potential. To achieve the appropriate electrocatalytic capabilities, the catalysts’ morphology and composition must be under control.
To achieve the appropriate electrocatalytic capabilities, the catalysts’ morphology and composition must be under control. Structure of Au–Co nano-particles as OER catalysts in basic conditions was demonstrated by. 97 The Au–Co nanoparticles have a core shell shape and a homogeneous size distribution.
5.3.3 Electrocatalysts based on non-noble metals
OER electrocatalysts without noble metals have generated a lot of research attention due to their affordability and availability. The search for effective noble-metal free OER electrocatalysts has been become more intense. In the previous few decades, significant progress has been made in exhibiting excellent catalytic activity that is similar to the catalyst constructed of noble metals. For the purpose of logically designing highly effective OER electrocatalysts, we will highlight some recent approaches to produce more active sites by controlling various reaction factors like morphology, chemical composition of intermediates, tuning electronic shape as well as binding energy through elemental doping method an also by inducing hybrid materials into composites. Recently, Zhang et al. 98 used sol-gel fabrication to create a gelled version of Fe–Co–W oxyhydroxide with uniform metal allocation. The lowest overpotential was 190 mV for the Fe–Co–W oxyhydroxide at 10 mA cm2 current density and 502 h cycle stability. This result shows that of the benchmark catalyst made of NiFe. The outcome demonstrates that on various substrates, G–FeCoW has a much better catalytic activity than anneal A–Fe–Co–W, gelled FeCo rather than W (G–Fe–Co), with LDH Fe–Co. The two dimensional (2D) volcano plot (Figure 14(c)) shows that the computed theoretical OER overpotential has dramatically increased catalytic activity towards OER. The local electrical and geometrical surroundings are modulated to obtain a hypothetical overpotential of just 0.4 V.
Another efficient method for controlling the structural and electrical characteristics of electrocatalysts is defect engineering. By varying the intermediate adsorption energy, the OER activity can be increased, which occasionally results in unanticipated active sites. A technique to produce enough oxygen vacancies using a plasma engraving process on a Co3O4 nanosheet was described by Dai et al. According to X-ray photoelectron spectroscopy (XPS), Ar plasma treatment partially reduces Co3+ to Co2+ and creates oxygen vacancies. By manipulating the Co2+/Co3+ ratio, by this technique we can change the surface area of material but also alters the electronic structure of material, resulting in good OER process catalytic action having over-potential of 154 eV at current density of 10 mA cm2. For enhancing OER activity, tuning the electron transit is just as important as the fundamental change for ideal absorption energy of the intermediate materials. On nickel foams, carried out in situ formation of the 3D spongy films made of perpendicularly aligned Ni–Fe–LDH nano-plates in (Figure 21(a)).
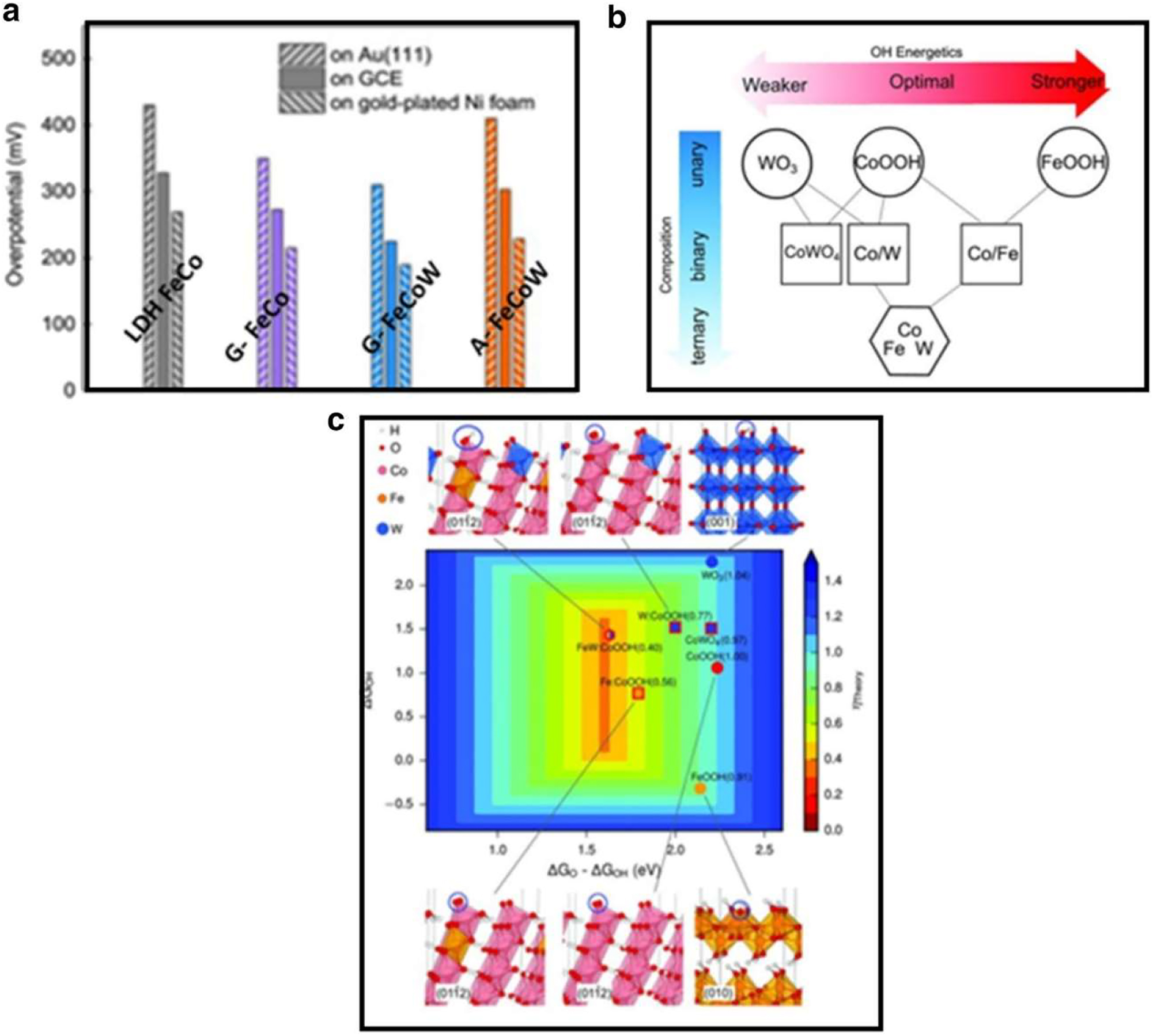
Performances of non-nobel metals in OER. (a) The following potentials were determined using density-functional theory (DFT): oxyhydroxides and W, Fe-doped Co oxyhydroxides, cobalt tungstate, and W oxides; and the surface of Au-(111) and GCE (glass carbon electrode). The measurements were taken at 10 mA cm−2. 96 Copyright from Science (2016).
The catalyst displays a noticeable endurance and a minor over potential of 152 mV at 31 mA cm2 better by 20 wt% of Ir/C catalyst. 96 The 3D spongy arrangement, which offers with high density of active sites of the large surface area, was said to have a synergistic impact that led to the significant electrocatalytic activity that was found. The porous features and metallic electronic conductivity of Ni foam substrate, as depicted in Figure 22A(b), are thought to make it an ideal substrate for OER because they speed up the electron transport process. The active phase was investigated using in situ Raman technology (Figure 22A(c)). The newly discovered bands at oxygen evolution potential show that NiO2H was the active part for OER by proving the conversion of LDH into NiO2H.
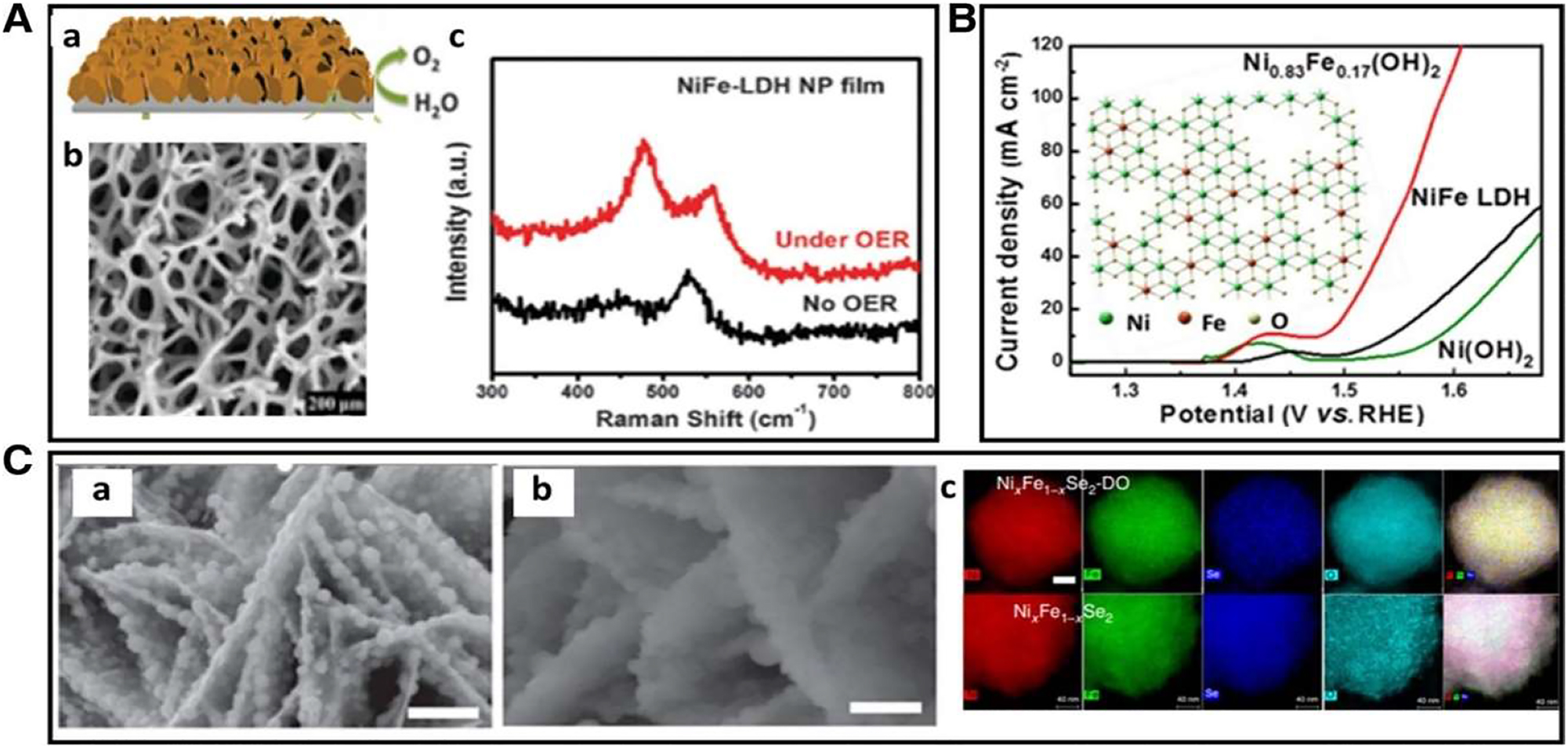
Electrocatalytic activity of OER. (a) A representation of NiFe-LDH nano-plates grown on nickel foam, (b) a scanning electron micrograph of nickel fluff, and (c) an in situ Raman spectrum of Ni–Fe–LDH films with and without oxygen evolution reaction (OER) applied. 50 The publication by Chemical Communication in 2014. Potential energy surface polarization curves of Ni(OH)2 and Ni–Fe LDH and Ni0.83Fe0.17 (OH)2 after correction for infrared light. The number 66 published in ACS Catalysis in 2018 by C (a) SEM pictures of Ni x Fe1–xSe2 (b) pictures of Ni x Fe1–xSe2–Do (c) evaluation of Ni x Fe1–xSe2 and Ni x Fe1–xSe2 in images of elemental mapping. 80 , 90 Copyright from the edition of Natural Communication 2016.
By using a simple and universal cation-exchange technique 96 created Fe doped Ni(OH)2 nano-sheets with numerous defects and a nono-porous surface structure. There is an increase in OER activity in these nano-sheets. A defect-rich Ni(0.83), Fe(0.17) nano-sheet exhibits the lowest over-potential of 246 mV at a current density of 10 mA cm2 when contrasted with the conventional Ni–Fe layered double hydroxide (LDH) nanosheet. Several factors, such as a high number of flaws, an enhancement in surface wet ability, and an increase in active surface sites, contribute to the remarkable OER activity. A new approach to making highly effective OER catalysts has been unveiled by the successful utilization of the cation exchange method in the fabrication of the active Fe doped Co(OH)2 nanosheet.
Metal oxides as well as hydroxides are amongst the non-precious metal catalysts that are most commonly used. Recently, numerous more interesting electrocatalysts, which include transition-metal phosphides, sulphides, and selenides, have shown excellent OER catalytic activity. However, these compounds exhibit unsatisfactory stability in an alkaline solution with a high oxidative potential. As a result, a lot of emphasis has been paid to understanding the chemical makeup of real active sites in the development of catalysts for OER. In order to provide an in situ creation of a high active Ni–Fe oxide catalyst, Hu and coworkers synthesized nanostructured nickel iron di-selenide (Ni x Fe1xSe2). The OER activity of this catalyst was outstanding, with over-potential of just 195 mV for 10 mV cm2.
5.4 Mechanism of OER by involving oxygen (lattice) species
Typical AEM involves a single metal active site for the whole chemical reaction, with a minimum possible over-potential of 0.36 eV due to the scaling relation and the presence of OER intermediates. A novel OER mechanism utilizing lattice oxygen species, the lattice oxygen mediated mechanism (LOM), has lately been suggested. The oxygen evolution reaction in LOM involves the catalyst’s lattice oxygen. Lattice oxygen is involved in gas phase catalytic oxidation processes, according to recent research with alloy catalysts. 96 That several reaction pathways are thought of as this phenomenon is intriguing. On occasion, the most beneficial aspects of oxygen evolution reaction electrocatalysis have been achieved, thanks to substantial progress in our knowledge of the process involving oxygen species, particularly lattice species. One DFT study proposed a basic reaction route for OER by lattice oxygen through the reversible production of surface oxygen vacancies. Their presentation of several cobaltite Perovskites highlights the interdependence of oxygen vacancies, metal oxygen covalency, and OER activity. This correlation between oxygen vacancy content and Co–O bond covalency is illustrated in Figure 23A(a).
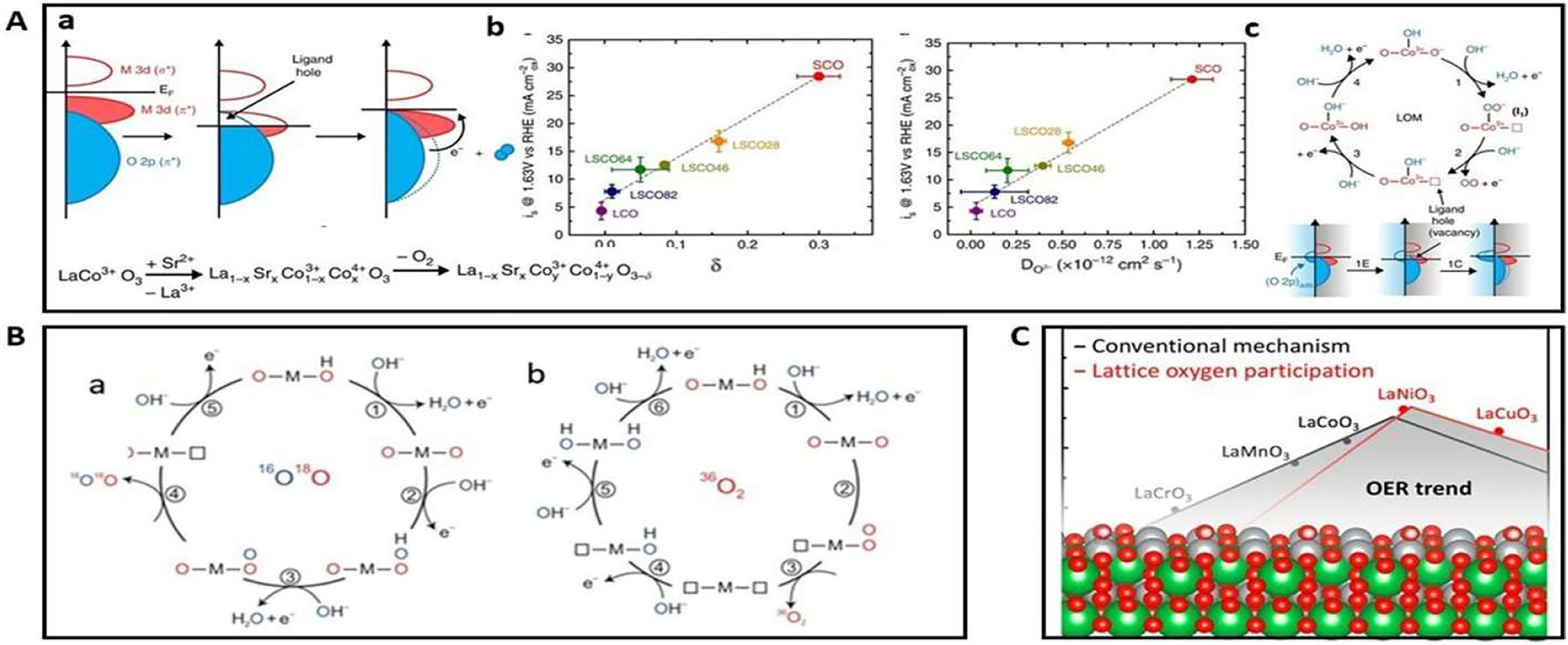
A depiction of the oxygen vacancy concentration and the covalency of the CO bond (a). (b) The CO2 vacancies, oxygen diffusivity, and OER activity are associated. (c) Copyright from Natural Communication (2016) for the predicted LOM approach. A likely B OER pathway that releases (a) 34O2(16O18O) and (b) 36O2(18O18O) via coordinated proton-electron transfer on oxygen sites 70 credit from Natural Chemistry (2016). C for OER on certain perovskites including AEM and LOM, a volcano strategy is proposed. 74 Copyright from ACS Catalysis (2018).
The structural arrangement reduces the energy required to attain a stable state by causing oxygen vacancies when O2 is formed and released. A lower valence Sr2+ can be substituted into the La1xSr x CoO3 (LSCO) structure to manage the increased vacancy concentration in the catalyst caused by the enhanced covalency between metal and oxygen bonds. According to DFT modeling and experimental data, vacancies of oxygen and also diffusion rate of oxygen, and OER activity are all directly correlated with one another Figure 23A(b) Higher OER activity is associated with faster vacancy and surface exchange kinetics, the results show. The response mechanism of the AEM is illustrated in Figure 23A(c). A LOM technique is suggested as a comparable one based on correlation. The formation of an oxygen vacancy in the lattice is caused by the interaction of adsorbed oxygen at the metal site with the oxygen in the lattice.
This differs from the generally-proposed adsorbed oxygen species-based AEM process. The respective stabilities of these two intermediates are crucial in deciding whether the OER carried out through AEM or else LOM for a specific LSCO composition. The energy needed to produce O vacancies will decrease and bulk stability will decrease as a result of the predicted shift from the AEM to LOM in the La1xSr x CoO3 system. Research by Shao-Horn utilizing in situ 18O-isotope-labeling mass spectrometry provides experimental proof that lattice oxygen is involved in OER oxygen molecule production. They demonstrated that in high covalent metal oxides such as La(0.5) Sr(0.5) CoO3 and SrCoO3 (where vacancy parameter is represented), the OER activity of this process is pH-independent, and that lattice oxygen aids in oxide production during OER. Consequently, the OER process is activated by increasing the metal-oxygen covalency. The purpose of this online electro-chemical mass spectrometry (OLEMS) analysis is to find out whether the lattice oxygen has been provided to the oxidation reaction by the different metal oxygen covalencies of the Cobalt based perovskites La0.5Sr0.5CoO3, SrCoO3, and Pr0.5Ba0.5CoO3. A real-time measurement of oxygen gas was taken using mass spectroscopy. As depicted in Figure 16B, the production of 34O2(16O18O) and 36O2(18O18O) is thought to result from the interaction of two distinct lattice oxygen species. The chemical process to create molecular oxygen (O2) and the oxygen vacancies activity carried out on the surface of specific oxygenated places were both implicated in these processes. Additionally, as depicted in Figure 23C, volcano graph activity has been created by Kolpak et al. of AEM as well as LOM by reducing the thermodynamically necessary overpotential, the LOM displays greater OER activity as compared to AEM. 99 , 100 AEM has a minimal hypothetical over-potential of 0.37 eV based on the scaling relation. The relative constant G for LOM, which is roughly 1.5–1.7 eV for VO + OO VO + OH, is significantly lower than G, which is 3.1 eV.
6 Summary and perspectives
By expanding the atomic, molecular, and nanoscales material production methodologies, major advancements have been designed in term of electro-catalysts for water splitting to produce hydrogen. It has been proved that hydrogen has the maximum gravimetric energy density and produces no emissions; it is a possible alternative to fossil fuels since it offers clean, renewable energy. Hydrogen production greatly benefits from the advancement in splitting of water as an effective energy alteration and storage system. However, the slow reaction kinetics of OER and HER caused by large overpotential, which result in just 4 % of the world’s hydrogen production from water splitting at present, restrict the energy efficiency of water electrolysis. To achieve high-current density in industry, the challenge of alkaline water electrocatalysis for hydrogen production must be addressed. One way to increase the number of H atoms produced is by increasing the number of electrocatalytic active sites. Conversely, the inherent cause of achieving a high current density is the interaction between H atoms and active sites. As a result, it’s critical to create fundamental electrocatalysts using chemical and physical techniques. Given the importance of H2 as a fuel, greater research into the creation of electrocatalysts is worthwhile. Despite all of the progress, there are still several obstacles in the way of making this technology truly helpful.
In order to reduce the overpotential and increase energy efficiencies, effective catalyst design is crucial in both OER and HER. This will make it easier for industries to use water splitting practically. We have summarized the data concerned about the production of green hydrogen and role of various electrocatalysts as well as recent advancements to design the noble-metal based and non noble-metal based electro-catalysts for OER and HER can be seen in (Tables 1 and 2). These catalysts exhibit high performance that is comparable to that of the benchmark electro-catalysts Pt along with IrO2/RuO2 for the HER and OER with low cost. Basic understandings of the synergistic structure, morphology, as well as chemical composition of the catalysts for HER and OER in various mediums have been attained. AEM by opening up a fresh path for the creation of superior OER electrocatalysts. Utilizing HER/OER catalysts that are affordable, reliable, and efficient is essential for water-splitting technology that produces hydrogen energy. Self-supported electrodes that integrate in situ grown catalytically active phase have several advantages over conventional electrodes in powdery paste form, including simplified electrode preparation, reduced interface resistance, increased stability, and exposure of abundant active sites. These advantages hold promise for practical applications in electrolyzers. First, the demands of practical electrolyzers, which demand capable electrocatalysts to afford a substantial current density beyond 500 mA cm2 at overpotentials under 300 mV, are difficult for nonprecious catalysts’ catalytic activity and stability to achieve. The slow kinetics are the primary bottleneck. Strategies like compositional modulation, downsizing, active facet exposure, or single site catalysis can increase the intrinsic activity and density of catalytically active sites; 2) improving charge transfer and mass transport through cation/anion doping, defect control, interface engineering, porosity design, and other means can be used in tandem for improvement.
By opening a novel path for the development of superior OER electro-catalysts, the present thoughtful of the reaction processes for HER as well as OER, particularly the upcoming LOM for OER, may enable significant advancements in overcoming the limitations of the conventional AEM. Despite the enormous advancements in our understanding of the OER and HER electrocatalytic processes, there are still a number of obstacles in the way of commercially viable, mass-scale hydrogen production via water splitting electrolysis. To begin, studies and developments of OER electro-catalysts based on non-noble metals that exhibit maximum activity and long-term stability in acidic environments continue to face significant challenges. The vast majority of efficient OER catalysts, however, are Ir and Ru based electro-catalysts due to their greater dissolution resistance in acidic environments. Such conditions are too much for most electrocatalysts based on non-noble metals. Consequently, it is evident that OER electro-catalysts based on non-noble metals must be developed in order to provide stability and reliability. Proved a reasonable method for creating acid-tolerant, non-noble metal electrocatalysts by isolating the two properties of mixed metal oxides: activity and stability.
In the CoMnO x film, for instance, Mn has used as a structural stabilizing agent that is connected to the catalytically active Cobalt center. Second, there is a lack of understanding of the precise catalytic pathways, particularly for HER and OER electrocatalysts based on transition metals. Based solely on the descriptor of TOF, the original active sites of different electrocatalysts cannot be fully identified. Due to their excellent OER performance in alkaline conditions, nonnoble metal-based carbides, phosphides, as well as chalcogenides have recently received a lot of interest. However, throughout the reaction proceed along OER conditions, the nanostructured electrocatalysts go through structural and compositional changes. To ascertain the actual active phases and places, it is necessary to comprehend structural trans-formation. For the rational design, understanding the intricate mechanism, structural transformation, and genuine active locations is essential.
Additionally, more focus needs to be placed on a trustworthy assessment of the catalytic efficacy of various electrocatalysts. Merely contrasting the current density according to the geometric area of the electrodes may obscure significant influencing elements including the size of the catalyst, mass loading 101 , 102 substrate influence, and testing conditions. Therefore, an assessment of comprehensive indexes including overpotential, Tafel slope, exchange current density, TOF value, specific activity based on mass and electrochemically active surface area, and Faradaic efficiency should be taken into account for convincing comparison.
An advanced strategy to learn more about the transformation of pattern of structural configuration, reaction intermediates, as well as reaction mechanisms of the catalysts is the integration of in situ chemical characterization of compound by different analytical tools and techniques as well as theoretical modeling. Thirdly, it is difficult to directly compare different nanostructured catalyst materials based on performance descriptors because of the different substrate materials and the specific mass loadings of the catalysts on the electrode surface. These factors can impact the electron transfer rate measured by different electro-chemical methods. We need more efficient screening procedures for electrocatalysts if we’re going to have a standard way to compare the performance of different catalysts developed by different research groups. However, the recent rise in interest in catalyst engineering including nanostructures and lattice oxygen is anticipated to result in new advancements in the design of active and the development of dynamic, long term stable, and affordable OER as well as HER electro-catalysts for the widespread commercial level water splitting based hydrogen production is anticipated to benefit from the recent upsurge in interest in nano-structure and lattice oxygen engineering of catalysts.
Award Identifier / Grant number: CEAMR
Acknowledgment
Center of Excellence for Advanced Materials Research (CEAMR), Chemistry Department, King Abdulaziz University, Jeddah, Saudi Arabia is highly acknowledged for financial supports and research facilities.
-
Research ethics: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Li, A.; Sun, Y.; Yao, T.; Han, H. Earth-Abundant Transition-Metal-Based Electrocatalysts for Water Electrolysis to Produce Renewable Hydrogen. Chem. Eur. J. 2018, 24 (69), 18334–18355; https://doi.org/10.1002/chem.201803749.Suche in Google Scholar PubMed
2. Zhu, J.; Hu, L.; Zhao, P.; Lee, L. Y. S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2019, 120 (2), 851–918; https://doi.org/10.1021/acs.chemrev.9b00248.Suche in Google Scholar PubMed
3. Roses, L.; Manzolini, G.; Campanari, S.; De Wit, E.; Walter, M. Techno-Economic Assessment of Membrane Reactor Technologies for Pure Hydrogen Production for Fuel Cell Vehicle Fleets. Energy Fuels 2013, 27 (8), 4423–4431; https://doi.org/10.1021/ef301960e.Suche in Google Scholar
4. Tang, T.; Ding, L.; Yao, Z.; Pan, H.; Hu, J.; Wan, L. Synergistic Electrocatalysts for Alkaline Hydrogen Oxidation and Evolution Reactions. Adv. Funct. Mater. 2022, 32 (2), 2107479; https://doi.org/10.1002/adfm.202107479.Suche in Google Scholar
5. Li, G.; Ma, Y.; Liu, X.; Li, J.; Li, D.; Ma, J. Current Situation and Development Suggestions of Hydrogen Energy and Hydrogen Fuel Cell Vehicle Industry in Henan Province. Henan Sci. Technol. 2022, 9, 137–139.Suche in Google Scholar
6. Yan, J.; Jing, J.; Li, Y. Hydrogen Fuel Cell Commercial Vehicles in China: Evaluation of Carbon Emission Reduction and Its Economic Value. Int. J. Hydrogen Energy 2024, 52, 734–749; https://doi.org/10.1016/j.ijhydene.2023.04.164.Suche in Google Scholar
7. Saeed, M.; Shahzad, U.; Rabbee, M. F.; Manzar, R.; Al-Humaidi, J. Y.; Siddique, A.; Sheikh, T. A.; Althomali, R. H.; Qamar, T.; Rahman, M. M. Potential Development of Porous Carbon Composites Generated from the Biomass for Energy Storage Applications. Chem. Asian J. 2024, e202400394; https://doi.org/10.1002/asia.202400394.Suche in Google Scholar PubMed
8. Song, J.; Wei, C.; Huang, Z.-F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z. J. A Review on Fundamentals for Designing Oxygen Evolution Electrocatalysts. Chem. Soc. Rev. 2020, 49 (7), 2196–2214; https://doi.org/10.1039/c9cs00607a.Suche in Google Scholar PubMed
9. Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H. M. Electrocatalysis for the Oxygen Evolution Reaction: Recent Development and Future Perspectives. Chem. Soc. Rev. 2017, 46 (2), 337–365; https://doi.org/10.1039/c6cs00328a.Suche in Google Scholar PubMed
10. Yu, F.; Yu, L.; Mishra, I. K.; Yu, Y.; Ren, Z. F.; Zhou, H. Q. Recent Developments in Earth-Abundant and Non-Noble Electrocatalysts for Water Electrolysis. Mater. Today Phys. 2018, 7, 121–138; https://doi.org/10.1016/j.mtphys.2018.11.007.Suche in Google Scholar
11. Shahzad, U.; Saeed, M.; Marwani, H. M.; Al-Humaidi, J. Y.; ur Rehman, S.; Althomali, R. H.; Awual, M. R.; Rahman, M. M. Recent Progress on Potentiometric Sensor Applications Based on Nanoscale Metal Oxides: A Comprehensive Review. Crit. Rev. Anal. Chem. 2024, 1–18; https://doi.org/10.1080/10408347.2024.2337876.Suche in Google Scholar PubMed
12. Zou, X.; Zhang, Y. Noble Metal-free Hydrogen Evolution Catalysts for Water Splitting. Chem. Soc. Rev. 2015, 44 (15), 5148–5180; https://doi.org/10.1039/c4cs00448e.Suche in Google Scholar PubMed
13. Saeed, M.; Shahzad, U.; Marwani, H. M.; Asiri, A. M.; ur Rehman, S.; Althomali, R. H.; Rahman, M. M. Recent Advancements on Sustainable Electrochemical Water Splitting Hydrogen Energy Applications Based on Nanoscale Transition Metal Oxide (TMO) Substrates. Chem. Asian J. 2024, e202301107; https://doi.org/10.1002/asia.202301107.Suche in Google Scholar PubMed
14. Hu, C.; Zhang, L.; Gong, J. Recent Progress Made in the Mechanism Comprehension and Design of Electrocatalysts for Alkaline Water Splitting. Energy Environ. Sci. 2019, 12 (9), 2620–2645; https://doi.org/10.1039/c9ee01202h.Suche in Google Scholar
15. Shahzad, U.; Saeed, M.; Marwani, H. M.; Al-Humaidi, J. Y.; ur Rehman, S.; Althomali, R. H.; Rahman, M. M. Transition Metal-Based Chalcogenides as Electrocatalysts for Overall Water Splitting in Hydrogen Energy Production. Int. J. Hydrogen Energy 2024, 65, 215–224; https://doi.org/10.1016/j.ijhydene.2024.03.275.Suche in Google Scholar
16. Guo, B.; Huo, H.; Zhuang, Q.; Ren, X.; Wen, X.; Yang, B.; Huang, X.; Chang, Q.; Li, S. Iron Oxyhydroxide: Structure and Applications in Electrocatalytic Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33 (25), 2300557; https://doi.org/10.1002/adfm.202300557.Suche in Google Scholar
17. Saeed, M.; Marwani, H. M.; Shahzad, U.; Asiri, A. M.; Rahman, M. M. Nanoscale Silicon Porous Materials for Efficient Hydrogen Storage Application. J. Energy Storage 2024, 81, 110418; https://doi.org/10.1016/j.est.2024.110418.Suche in Google Scholar
18. Wang, H.; Ren, X.; Chen, J.; Xu, W.; He, Q.; Wang, H.; Zhan, F.; Chen, L. Recent Advances of Emerging Oxyhydroxide for Electrochemical Energy Storage Applications. J. Power Sources 2023, 554, 232309; https://doi.org/10.1016/j.jpowsour.2022.232309.Suche in Google Scholar
19. Zhang, R.; Cheng, L.; Wang, Z.; Kong, F.; Tsegazab, Y.; Lv, W.; Wang, W. Ni3S2-Co9S8 Heterostructure Nanowires Supported on Ni Foam as Highly Efficient and Stable Electrocatalyst for Oxygen Evolution Reaction. Appl. Surf. Sci. 2020, 526, 146753; https://doi.org/10.1016/j.apsusc.2020.146753.Suche in Google Scholar
20. Wu, Z.; Lu, X. F.; Zang, S.; Lou, X. W. Non-Noble-Metal-Based Electrocatalysts Toward the Oxygen Evolution Reaction. Adv. Funct. Mater. 2020, 30 (15), 1910274; https://doi.org/10.1002/adfm.201910274.Suche in Google Scholar
21. Fabbri, E.; Schmidt, T. J. Oxygen Evolution Reaction—The Enigma in Water Electrolysis. ACS Catal. 2018, 9765–9774; https://doi.org/10.1021/acscatal.8b02712.Suche in Google Scholar
22. Yan, Y.; Xia, B. Y.; Zhao, B.; Wang, X. A Review on Noble-Metal-Free Bifunctional Heterogeneous Catalysts for Overall Electrochemical Water Splitting. J Mater Chem A Mater 2016, 4 (45), 17587–17603; https://doi.org/10.1039/c6ta08075h.Suche in Google Scholar
23. Zhong, C.-J.; Luo, J.; Fang, B.; Wanjala, B. N.; Njoki, P. N.; Loukrakpam, R.; Yin, J. Nanostructured Catalysts in Fuel Cells. Nanotechnology 2010, 21 (6), 062001; https://doi.org/10.1088/0957-4484/21/6/062001.Suche in Google Scholar PubMed
24. Loukrakpam, R.; Luo, J.; He, T.; Chen, Y.; Xu, Z.; Njoki, P. N.; Wanjala, B. N.; Fang, B.; Mott, D.; Yin, J Nanoengineered PtCo and PtNi Catalysts for Oxygen Reduction Reaction: An Assessment of the Structural and Electrocatalytic Properties. J. Phys. Chem. C 2011, 115 (5), 1682–1694; https://doi.org/10.1021/jp109630n.Suche in Google Scholar
25. Zhao, L.; Zhang, Y.; Zhao, Z.; Zhang, Q.-H.; Huang, L.-B.; Gu, L.; Lu, G.; Hu, J.-S.; Wan, L.-J. Steering Elementary Steps Towards Efficient Alkaline Hydrogen Evolution via Size-dependent Ni/NiO Nanoscale Heterosurfaces. Natl. Sci. Rev. 2020, 7 (1), 27–36.10.1093/nsr/nwz145Suche in Google Scholar PubMed PubMed Central
26. Nørskov, J. K.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density Functional Theory in Surface Chemistry and Catalysis. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (3), 937–943.10.1073/pnas.1006652108Suche in Google Scholar PubMed PubMed Central
27. Wang, X.; Zhu, Y.; Vasileff, A.; Jiao, Y.; Chen, S.; Song, L.; Zheng, B.; Zheng, Y.; Qiao, S.-Z. Strain Effect in Bimetallic Electrocatalysts in the Hydrogen Evolution Reaction. ACS Energy Lett. 2018, 3 (5), 1198–1204.10.1021/acsenergylett.8b00454Suche in Google Scholar
28. Wang, W.; Wang, Z.; Wang, J.; Zhong, C.; Liu, C. Highly Active and Stable Pt–Pd Alloy Catalysts Synthesized by Room-Temperature Electron Reduction for Oxygen Reduction Reaction. Adv. Sci. 2017, 4 (4), 1600486.10.1002/advs.201600486Suche in Google Scholar PubMed PubMed Central
29. Zhong, C.-J.; Luo, J.; Njoki, P. N.; Mott, D.; Wanjala, B.; Loukrakpam, R.; Lim, S.; Wang, L.; Fang, B.; Xu, Z. Fuel Cell Technology: Nano-Engineered Multimetallic Catalysts. Energy Environ. Sci. 2008, 1 (4), 454–466; https://doi.org/10.1039/b810734n.Suche in Google Scholar
30. Manzar, R.; Saeed, M.; Shahzad, U.; Al-Humaidi, J. Y.; ur Rehman, S.; Althomali, R. H.; Rahman, M. M. Recent Advancements in Boron Carbon Nitride (BNC) Nanoscale Materials for Efficient Supercapacitor Performances. Prog. Mater. Sci. 2024, 101286; https://doi.org/10.1016/j.pmatsci.2024.101286.Suche in Google Scholar
31. Shahzad, U.; Saeed, M.; Rabbee, M. F.; Marwani, H. M.; Al-Humaidi, J. Y.; Altaf, M.; Althomali, R. H.; Baek, K.-H.; Awual, M. R.; Rahman, M. M. Recent Progress in Two-Dimensional Metallenes and Their Potential Application as Electrocatalyst. J. Energy Chem. 2024, 94, 577–598; https://doi.org/10.1016/j.jechem.2024.02.068.Suche in Google Scholar
32. Liang, C.; Zou, P.; Nairan, A.; Zhang, Y.; Liu, J.; Liu, K.; Hu, S.; Kang, F.; Fan, H. J.; Yang, C. Exceptional Performance of Hierarchical Ni–Fe Oxyhydroxide@ NiFe Alloy Nanowire Array Electrocatalysts for Large Current Density Water Splitting. Energy Environ. Sci. 2020, 13 (1), 86–95; https://doi.org/10.1039/c9ee02388g.Suche in Google Scholar
33. Jiang, R.; on Tung, S.; Tang, Z.; Li, L.; Ding, L.; Xi, X.; Liu, Y.; Zhang, L.; Zhang, J. A Review of Core-Shell Nanostructured Electrocatalysts for Oxygen Reduction Reaction. Energy Storage Mater. 2018, 12, 260–276; https://doi.org/10.1016/j.ensm.2017.11.005.Suche in Google Scholar
34. Zhang, C.; Shen, X.; Pan, Y.; Peng, Z. A Review of Pt-Based Electrocatalysts for Oxygen Reduction Reaction. Front. Energy 2017, 11, 268–285; https://doi.org/10.1007/s11708-017-0466-6.Suche in Google Scholar
35. Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C. A Comprehensive Review of Pt Electrocatalysts for the Oxygen Reduction Reaction: Nanostructure, Activity, Mechanism and Carbon Support in PEM Fuel Cells. J Mater Chem A Mater 2017, 5 (5), 1808–1825; https://doi.org/10.1039/c6ta08580f.Suche in Google Scholar
36. Wu, Z.-P.; Caracciolo, D. T.; Maswadeh, Y.; Wen, J.; Kong, Z.; Shan, S.; Vargas, J. A.; Yan, S.; Hopkins, E.; Park, K. Alloying–Realloying Enabled High Durability for Pt–Pd-3d-Transition Metal Nanoparticle Fuel Cell Catalysts. Nat. Commun. 2021, 12 (1), 859; https://doi.org/10.1038/s41467-021-21017-6.Suche in Google Scholar PubMed PubMed Central
37. Chang, F.; Bai, Z.; Li, M.; Ren, M.; Liu, T.; Yang, L.; Zhong, C.-J.; Lu, J. Strain-Modulated Platinum–Palladium Nanowires for Oxygen Reduction Reaction. Nano Lett. 2020, 20 (4), 2416–2422; https://doi.org/10.1021/acs.nanolett.9b05123.Suche in Google Scholar PubMed
38. Kong, Z.; Maswadeh, Y.; Vargas, J. A.; Shan, S.; Wu, Z.-P.; Kareem, H.; Leff, A. C.; Tran, D. T.; Chang, F.; Yan, S. Origin of High Activity and Durability of Twisty Nanowire Alloy Catalysts Under Oxygen Reduction and Fuel Cell Operating Conditions. J. Am. Chem. Soc. 2019, 142 (3), 1287–1299; https://doi.org/10.1021/jacs.9b10239.Suche in Google Scholar PubMed
39. Shahzad, U.; Marwani, H. M.; Saeed, M.; Asiri, A. M.; Repon, R.; Althomali, R. H.; Rahman, M. M. Progress and Perspectives on Promising Covalent-Organic Frameworks (COFs) Materials for Energy Storage Capacity. Chem. Rec. 2023, 24, e202300285; https://doi.org/10.1002/tcr.202300285.Suche in Google Scholar PubMed
40. Zhao, X.; Yin, F.; He, X.; Chen, B.; Li, G. Enhancing Hydrogen Evolution Reaction Activity on Cobalt Oxide in Alkaline Electrolyte by Doping Inactive Rare-Earth Metal. Electrochim. Acta 2020, 363, 137230; https://doi.org/10.1016/j.electacta.2020.137230.Suche in Google Scholar
41. Ayub, M. N.; Shahzad, U.; Saeed, M.; Rabbee, M. F.; Al-Humaidi, J. Y.; Althomali, R. H.; Baek, K.-H.; Rahman, M. M. Modern Innovations in the Provision and Efficient Application of 2D Inorganic. Nanoscale Mater. 2025, 45, 175–206.10.1515/revic-2023-0036Suche in Google Scholar
42. Wu, Z.-P.; Shan, S.; Zang, S.-Q.; Zhong, C.-J. Dynamic Core–Shell and Alloy Structures of Multimetallic Nanomaterials and Their Catalytic Synergies. Acc. Chem. Res. 2020, 53 (12), 2913–2924.10.1021/acs.accounts.0c00564Suche in Google Scholar PubMed
43. Lee, J.; Muya, J. T.; Chung, H.; Chang, J. Unraveling V (V)-V (IV)-V (III)-V (II) Redox Electrochemistry in Highly Concentrated Mixed Acidic Media for a Vanadium Redox Flow Battery: Origin of the Parasitic Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11 (45), 42066–42077.10.1021/acsami.9b12676Suche in Google Scholar PubMed
44. Xie, Y.; Yang, Y.; Muller, D. A.; Abruña, H. D.; Dimitrov, N.; Fang, J. Enhanced ORR Kinetics on Au-Doped Pt–Cu Porous Films in Alkaline Media. ACS Catal. 2020, 10 (17), 9967–9976.10.1021/acscatal.0c02690Suche in Google Scholar
45. Saeed, M.; Shahzad, U.; Rabbee, M. F.; Al-Humaidi, J. Y.; Marwani, H. M.; Rehman, S. U.; Shabbir, A.; Ayub, M. N.; Althomali, R. H.; Asghar, M. N.; Rahman, M. M. Comprehensive Reviews on the Potential Applications of Inorganic Metal Sulfide Nanostructures in Biological. Environ. Healthcare Energy Gener. Storage 2024; https://doi.org/10.1515/revic-2024-0016.Suche in Google Scholar
46. Jin, H.; Ruqia, B.; Park, Y.; Kim, H. J.; Oh, H.; Choi, S.; Lee, K. Nanocatalyst Design for Long-Term Operation of Proton/Anion Exchange Membrane Water Electrolysis. Adv. Energy Mater. 2021, 11 (4), 2003188; https://doi.org/10.1002/aenm.202003188.Suche in Google Scholar
47. Lu, Q.; Hutchings, G. S.; Yu, W.; Zhou, Y.; Forest, R. V.; Tao, R.; Rosen, J.; Yonemoto, B. T.; Cao, Z.; Zheng, H. Highly Porous Non-Precious Bimetallic Electrocatalysts for Efficient Hydrogen Evolution. Nat. Commun. 2015, 6 (1), 6567; https://doi.org/10.1038/ncomms7567.Suche in Google Scholar PubMed PubMed Central
48. Zhou, K. L.; Wang, Z.; Han, C. B.; Ke, X.; Wang, C.; Jin, Y.; Zhang, Q.; Liu, J.; Wang, H.; Yan, H. Platinum Single-Atom Catalyst Coupled with Transition Metal/Metal Oxide Heterostructure for Accelerating Alkaline Hydrogen Evolution Reaction. Nat. Commun. 2021, 12 (1), 3783; https://doi.org/10.1038/s41467-021-24079-8.Suche in Google Scholar PubMed PubMed Central
49. Suib, S. L. New and Future Developments in Catalysis: Batteries, Hydrogen Storage and Fuel Cells; New and Future Developments in Catalysis, Elsevier: Newnes, 2013. https://www.sciencedirect.com/book/9780444538802/new-and-future-developments-in-catalysis#book-description.Suche in Google Scholar
50. Shan, S.; Luo, J.; Wu, J.; Kang, N.; Zhao, W.; Cronk, H.; Zhao, Y.; Joseph, P.; Petkov, V.; Zhong, C.-J. Nanoalloy Catalysts for Electrochemical Energy Conversion and Storage Reactions. RSC Adv. 2014, 4 (80), 42654–42669; https://doi.org/10.1039/c4ra05943c.Suche in Google Scholar
51. Sardar, K.; Petrucco, E.; Hiley, C. I.; Sharman, J. D.; Wells, P. P.; Russell, A. E.; Kashtiban, R. J.; Sloan, J.; Walton, R. I. Angew. Chem. Int. Ed. 2014, 126 (41), 11140–11144; https://doi.org/10.1002/ange.201406668.Suche in Google Scholar
52. Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.-C.; Uchimura, M.; Paulikas, A. P.; Stamenkovic, V.; Markovic, N. M. Enhancing Hydrogen Evolution Activity in Water Splitting by Tailoring Li+–Ni (OH) 2-Pt Interfaces. Science (1979) 2011, 334 (6060), 1256–1260; https://doi.org/10.1126/science.1211934.Suche in Google Scholar PubMed
53. Huynh, M.; Ozel, T.; Liu, C.; Lau, E. C.; Nocera, D. G. Design of Template-Stabilized Active and Earth-Abundant Oxygen Evolution Catalysts in Acid. Chem. Sci. 2017, 8 (7), 4779–4794.10.1039/C7SC01239JSuche in Google Scholar PubMed PubMed Central
54. Skúlason, E.; Karlberg, G. S.; Rossmeisl, J.; Bligaard, T.; Greeley, J.; Jónsson, H.; Nørskov, J. K. Density Functional Theory Calculations for the Hydrogen Evolution Reaction in an Electrochemical Double Layer on the Pt (111) Electrode. Phys. Chem. Chem. Phys. 2007, 9 (25), 3241–3250.10.1039/B700099ESuche in Google Scholar PubMed
55. Markovića, N. M.; Sarraf, S. T.; Gasteiger, H. A.; Ross, P. N. Hydrogen Electrochemistry on Platinum Low-Index Single-Crystal Surfaces in Alkaline Solution. J. Chem. Soc., Faraday Trans. 1996, 92 (20), 3719–3725.10.1039/FT9969203719Suche in Google Scholar
56. Trasatti, S. Work Function, Electronegativity, and Electrochemical Behaviour of Metals: III. Electrolytic Hydrogen Evolution in Acid Solutions. J. Electroanal. Chem. Interfacial Electrochem. 1972, 39 (1), 163–184.10.1016/0368-1874(72)85118-9Suche in Google Scholar
57. Cook, T. R.; Dogutan, D. K.; Reece, S. Y.; Surendranath, Y.; Teets, T. S.; Nocera, D. G. Solar Energy Supply and Storage for the Legacy and Nonlegacy Worlds. Chem. Rev. 2010, 110 (11), 6474–6502.10.1021/cr100246cSuche in Google Scholar PubMed
58. Zhang, J.; Wang, E.; Cui, S.; Yang, S.; Zou, X.; Gong, Y. Single-Atom Pt Anchored on Oxygen Vacancy of Monolayer Ti3C2T X for Superior Hydrogen Evolution. Nano Lett. 2022, 22 (3), 1398–1405; https://doi.org/10.1021/acs.nanolett.1c04809.Suche in Google Scholar PubMed
59. Zhai, L.; She, X.; Zhuang, L.; Li, Y.; Ding, R.; Guo, X.; Zhang, Y.; Zhu, Y.; Xu, K.; Fan, H. J. Modulating Built-in Electric Field via Variable Oxygen Affinity for Robust Hydrogen Evolution Reaction in Neutral Media. Angew. Chem. 2022, 134 (14), e202116057; https://doi.org/10.1002/ange.202116057.Suche in Google Scholar
60. Jin, R.; Huang, J.; Chen, G.; Chen, W.; Ouyang, B.; Chen, D.; Kan, E.; Zhu, H.; Li, C.; Yang, D. Water-Sprouted, Plasma-Enhanced Ni–Co Phospho-Nitride Nanosheets Boost Electrocatalytic Hydrogen and Oxygen Evolution. Chem. Eng. J. 2020, 402, 126257.10.1016/j.cej.2020.126257Suche in Google Scholar
61. Ye, G.; Wang, H.; Chen, W.; Chu, H.; Wei, J.; Wang, D.; Wang, J.; Li, Y. In Situ Implanting of Single Tungsten Sites into Defective UiO-66 (Zr) by Solvent-Free Route for Efficient Oxidative Desulfurization at Room Temperature. Angew. Chem. 2021, 133 (37), 20481–20487.10.1002/ange.202107018Suche in Google Scholar
62. Hansen, J. N.; Prats, H.; Toudahl, K. K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is There Anything Better Than Pt for HER? ACS Energy Lett. 2021, 6 (4), 1175–1180.10.1021/acsenergylett.1c00246Suche in Google Scholar PubMed PubMed Central
63. Shan, S.; Li, J.; Maswadeh, Y.; O’Brien, C.; Kareem, H.; Tran, D. T.; Lee, I. C.; Wu, Z.-P.; Wang, S.; Yan, S. Surface Oxygenation of Multicomponent Nanoparticles Toward Active and Stable Oxidation Catalysts. Nat. Commun. 2020, 11 (1), 4201.10.1038/s41467-020-18017-3Suche in Google Scholar PubMed PubMed Central
64. Feng, J.; Ding, L.; Ye, S.; He, X.; Xu, H.; Tong, Y.; Li, G. Co (OH) 2@ PANI Hybrid Nanosheets with 3D Networks as High-Performance Electrocatalysts for Hydrogen Evolution Reaction. Adv. Mater. 2015, 27 (44), 7051–7057.10.1002/adma.201503187Suche in Google Scholar PubMed
65. Liu, P.; Rodriguez, J. A. Catalysts for Hydrogen Evolution from the [NiFe] Hydrogenase to the Ni2P (001) Surface: The Importance of Ensemble Effect. J. Am. Chem. Soc. 2005, 127 (42), 14871–14878; https://doi.org/10.1021/ja0540019.Suche in Google Scholar PubMed
66. Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L. Homogeneously Dispersed Multimetal Oxygen-Evolving Catalysts. Science (1979) 2016, 352 (6283), 333–337, https://doi.org/10.1126/science.aaf1525.Suche in Google Scholar PubMed
67. Xie, Y.; Cai, J.; Wu, Y.; Zang, Y.; Zheng, X.; Ye, J.; Cui, P.; Niu, S.; Liu, Y.; Zhu, J. Boosting Water Dissociation Kinetics on Pt–Ni Nanowires by N-induced Orbital Tuning. Adv. Mater. 2019, 31 (16), 1807780; https://doi.org/10.1002/adma.201807780.Suche in Google Scholar PubMed
68. Xiao, P.; Yan, Y.; Ge, X.; Liu, Z.; Wang, J.-Y.; Wang, X. Investigation of Molybdenum Carbide Nano-Rod as an Efficient and Durable Electrocatalyst for Hydrogen Evolution in Acidic and Alkaline Media. Appl. Catal., B 2014, 154, 232–237; https://doi.org/10.1016/j.apcatb.2014.02.020.Suche in Google Scholar
69. Sheng, W.; Myint, M.; Chen, J. G.; Yan, Y. Correlating the Hydrogen Evolution Reaction Activity in Alkaline Electrolytes with the Hydrogen Binding Energy on Monometallic Surfaces. Energy Environ. Sci. 2013, 6 (5), 1509–1512; https://doi.org/10.1039/c3ee00045a.Suche in Google Scholar
70. Zhao, Z.; Liu, H.; Gao, W.; Xue, W.; Liu, Z.; Huang, J.; Pan, X.; Huang, Y. Surface-Engineered PtNi–O Nanostructure with Record-High Performance for Electrocatalytic Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2018, 140 (29), 9046–9050; https://doi.org/10.1021/jacs.8b04770.Suche in Google Scholar PubMed
71. Popczun, E. J.; McKone, J. R.; Read, C. G.; Biacchi, A. J.; Wiltrout, A. M.; Lewis, N. S.; Schaak, R. E. Nanostructured Nickel Phosphide as an Electrocatalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135 (25), 9267–9270; https://doi.org/10.1021/ja403440e.Suche in Google Scholar PubMed
72. Zhang, R.; Wang, X.; Yu, S.; Wen, T.; Zhu, X.; Yang, F.; Sun, X.; Wang, X.; Hu, W. Ternary NiCo2Px Nanowires as PH-Universal Electrocatalysts for Highly Efficient Hydrogen Evolution Reaction. Adv. Mater. 2017, 29 (9), 1605502; https://doi.org/10.1002/adma.201605502.Suche in Google Scholar PubMed
73. Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X. W.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, (40), 5807–5813; https://doi.org/10.1002/adma.201302685.Suche in Google Scholar PubMed
74. Faber, M. S.; Dziedzic, R.; Lukowski, M. A.; Kaiser, N. S.; Ding, Q.; Jin, S. High-Performance Electrocatalysis Using Metallic Cobalt Pyrite (CoS2) Micro- and Nanostructures. J. Am. Chem. Soc. 2014, 136 (28), 10053–10061.10.1021/ja504099wSuche in Google Scholar PubMed
75. Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable Disorder Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2013, 135 (47), 17881–17888.10.1021/ja408329qSuche in Google Scholar PubMed
76. Lim, J.; Park, D.; Jeon, S. S.; Roh, C.; Choi, J.; Yoon, D.; Park, M.; Jung, H.; Lee, H. Ultrathin IrO2 Nanoneedles for Electrochemical Water Oxidation. Adv. Funct. Mater. 2018, 28 (4), 1704796.10.1002/adfm.201704796Suche in Google Scholar
77. Huang, Z.-F.; Song, J.; Du, Y.; Xi, S.; Dou, S.; Nsanzimana, J. M. V.; Wang, C.; Xu, Z. J.; Wang, X. Chemical and Structural Origin of Lattice Oxygen Oxidation in Co–Zn Oxyhydroxide Oxygen Evolution Electrocatalysts. Nat. Energy 2019, 4 (4), 329–338.10.1038/s41560-019-0355-9Suche in Google Scholar
78. Kitchin, J. R.; Nørskov, J. K.; Barteau, M. A.; Chen, J. G. Trends in the Chemical Properties of Early Transition Metal Carbide Surfaces: A Density Functional Study. Catal. Today 2005, 105 (1), 66–73.10.1016/j.cattod.2005.04.008Suche in Google Scholar
79. Mefford, J. T.; Rong, X.; Abakumov, A. M.; Hardin, W. G.; Dai, S.; Kolpak, A. M.; Johnston, K. P.; Stevenson, K. J. Water Electrolysis on La1−xSrxCoO3−δ Perovskite Electrocatalysts. Nat. Commun. 2016, 7 (1), 11053.10.1038/ncomms11053Suche in Google Scholar PubMed PubMed Central
80. Lu, A.; Sun, H.; Zhang, N.; Che, L.; Shan, S.; Luo, J.; Zheng, J.; Yang, L.; Peng, D.-L.; Zhong, C.-J. Surface Partial-Charge-Tuned Enhancement of Catalytic Activity of Platinum Nanocatalysts for Toluene Oxidation. ACS Catal. 2019, 9 (8), 7431–7442.10.1021/acscatal.9b01776Suche in Google Scholar
81. Zhang, J.; Yao, W.; Huang, C.; Shi, P.; Xu, Q. High Efficiency and Stable Tungsten Phosphide Cocatalysts for Photocatalytic Hydrogen Production. J Mater Chem A Mater 2017, 5 (24), 12513–12519.10.1039/C7TA02297BSuche in Google Scholar
82. Shahzad, U.; Marwani, H. M.; Saeed, M.; Asiri, A. M.; Althomali, R. H.; Rahman, M. M. Exploration of Porous Metal-Organic Frameworks (MOFs) for an Efficient Energy Storage Applications. J. Energy Storage 2023, 74, 109518.10.1016/j.est.2023.109518Suche in Google Scholar
83. Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; Van Der Vliet, D.; Paulikas, A. P.; Stamenkovic, V. R.; Markovic, N. M. Improving the Hydrogen Oxidation Reaction Rate by Promotion of Hydroxyl Adsorption. Nat. Chem. 2013, 5 (4), 300–306.10.1038/nchem.1574Suche in Google Scholar PubMed
84. Levy, R. B.; Boudart, M. Platinum-Like Behavior of Tungsten Carbide in Surface Catalysis. Science (1979) 1973, 181 (4099), 547–549.10.1126/science.181.4099.547Suche in Google Scholar PubMed
85. Morales-Guio, C. G.; Tilley, S. D.; Vrubel, H.; Grätzel, M.; Hu, X. Hydrogen Evolution from a Copper (I) Oxide Photocathode Coated with an Amorphous Molybdenum Sulphide Catalyst. Nat. Commun. 2014, 5 (1), 3059; https://doi.org/10.1038/ncomms4059.Suche in Google Scholar PubMed
86. Hinnemann, B.; Moses, P. G.; Bonde, J.; Jørgensen, K. P.; Nielsen, J. H.; Horch, S.; Chorkendorff, I.; Nørskov, J. K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127 (15), 5308–5309; https://doi.org/10.1021/ja0504690.Suche in Google Scholar PubMed
87. Nørskov, J. K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J. R.; Bligaard, T.; Jonsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108 (46), 17886–17892.10.1021/jp047349jSuche in Google Scholar PubMed
88. Man, I. C.; Su, H.; Calle-Vallejo, F.; Hansen, H. A.; Martínez, J. I.; Inoglu, N. G.; Kitchin, J.; Jaramillo, T. F.; Nørskov, J. K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3 (7), 1159–1165.10.1002/cctc.201000397Suche in Google Scholar
89. Su, J.; Ge, R.; Jiang, K.; Dong, Y.; Hao, F.; Tian, Z.; Chen, G.; Chen, L. Assembling Ultrasmall Copper-Doped Ruthenium Oxide Nanocrystals into Hollow Porous Polyhedra: Highly Robust Electrocatalysts for Oxygen Evolution in Acidic Media. Adv. Mater. 2018, 30 (29), 1801351.10.1002/adma.201801351Suche in Google Scholar PubMed
90. Xie, C.; Wang, Y.; Hu, K.; Tao, L.; Huang, X.; Huo, J.; Wang, S. In Situ Confined Synthesis of Molybdenum Oxide Decorated Nickel–Iron Alloy Nanosheets from MoO42− Intercalated Layered Double Hydroxides for the Oxygen Evolution Reaction. J Mater Chem A Mater 2017, 5 (1), 87–91.10.1039/C6TA08149ESuche in Google Scholar
91. Park, J.; Kwon, T.; Kim, J.; Jin, H.; Kim, H. Y.; Kim, B.; Joo, S. H.; Lee, K. Hollow Nanoparticles as Emerging Electrocatalysts for Renewable Energy Conversion Reactions. Chem. Soc. Rev. 2018, 47 (22), 8173–8202; https://doi.org/10.1039/c8cs00336j.Suche in Google Scholar PubMed
92. Lee, S.; Ashwin Kishore, M. R.; Kim, D.; Kang, H.; Chun, J.; Oh, L. S.; Park, J. H.; Kim, H. J.; Yoo, J. S.; Lim, E. Direct O–O Coupling Promoted the Oxygen Evolution Reaction by Dual Active Sites from Ag/LaNiO3 Interfaces. ACS Appl. Energy Mater. 2022, 5 (12), 14658–14668; https://doi.org/10.1021/acsaem.2c01232.Suche in Google Scholar
93. Xu, X.; Li, S.; Wang, Y.; Fan, T.; Jiang, Y.; Huang, L.; He, Q.; Ao, T. Silicon Nanowires Prepared by Electron Beam Evaporation in Ultrahigh Vacuum. Nanoscale Res. Lett. 2012, 7, 1–7; https://doi.org/10.1186/1556-276x-7-243.Suche in Google Scholar
94. Lu, Z.; Xu, W.; Zhu, W.; Yang, Q.; Lei, X.; Liu, J.; Li, Y.; Sun, X.; Duan, X. Three-Dimensional NiFe Layered Double Hydroxide Film for High-Efficiency Oxygen Evolution Reaction. Chem. Commun. 2014, 50 (49), 6479–6482; https://doi.org/10.1039/c4cc01625d.Suche in Google Scholar PubMed
95. Zhao, G.; Rui, K.; Dou, S. X.; Sun, W. Boosting Electrochemical Water Oxidation: The Merits of Heterostructured Electrocatalysts. J Mater Chem A Mater 2020, 8 (14), 6393–6405; https://doi.org/10.1039/d0ta00708k.Suche in Google Scholar
96. Zhu, Y.; Tahini, H. A.; Hu, Z.; Chen, Z.; Zhou, W.; Komarek, A. C.; Lin, Q.; Lin, H.; Chen, C.; Zhong, Y. Boosting Oxygen Evolution Reaction by Creating Both Metal Ion and Lattice-Oxygen Active Sites in a Complex Oxide. Adv. Mater. 2020, 32 (1), 1905025.10.1002/adma.201905025Suche in Google Scholar PubMed
97. Wang, Z.; Ren, X.; Luo, Y.; Wang, L.; Cui, G.; Xie, F.; Wang, H.; Xie, Y.; Sun, X. An Ultrafine Platinum–Cobalt Alloy Decorated Cobalt Nanowire Array with Superb Activity Toward Alkaline Hydrogen Evolution. Nanoscale 2018, 10 (26), 12302–12307.10.1039/C8NR02071JSuche in Google Scholar
98. Li, Q.; Wu, L.; Wu, G.; Su, D.; Lv, H.; Zhang, S.; Zhu, W.; Casimir, A.; Zhu, H.; Mendoza-Garcia, A. New Approach to Fully Ordered Fct-FePt Nanoparticles for Much Enhanced Electrocatalysis in Acid. Nano Lett. 2015, 15 (4), 2468–2473.10.1021/acs.nanolett.5b00320Suche in Google Scholar PubMed
99. Fu, W.; Wang, Y.; Hu, J.; Zhang, H.; Luo, P.; Sun, F.; Ma, X.; Huang, Z.; Li, J.; Guo, Z. Surface-Electron Coupling for Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2019, 58 (49), 17709–17717; https://doi.org/10.1002/anie.201908938.Suche in Google Scholar PubMed
100. Shahzad, U.; Marwani, H. M.; Saeed, M.; Asiri, A. M.; Rahman, M. M. Two-Dimensional MXenes as Emerging Materials: A Comprehensive Review. ChemistrySelect 2023, 8 (25), e202300737; https://doi.org/10.1002/slct.202300737.Suche in Google Scholar
101. Saeed, M.; Marwani, H. M.; Shahzad, U.; Asiri, A. M.; Hussain, I.; Rahman, M. M. Utilizing Nanostructured Materials for Hydrogen Generation, Storage, and Diverse Applications. Chem. Asian J. 2023, e202300593; https://doi.org/10.1002/asia.202300593.Suche in Google Scholar PubMed
102. Saeed, M.; Marwani, H. M.; Shahzad, U.; Asiri, A. M.; Rahman, M. M. Recent Advances, Challenges, and Future Perspectives of ZnO Nanostructure Materials Towards Energy Applications. Chem. Rec. 2024, 24 (1), e202300106; https://doi.org/10.1002/tcr.202300106.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- A review of coordination compounds: structure, stability, and biological significance
- Effluent wastewater technologies for textile industry: a review
- Efficient removal of Cr(VI) ions from industrial wastewater using carbon-based adsorbents functionalized with boronic acid
- Exploring inorganic phosphors: basics, types, fabrications and their luminescence properties for LED/WLED/displays
- Recent advances on hydrogen generation based on inorganic metal oxide nano-catalyst using water electrolysis approach
- Antituberculosis, antimicrobial, antioxidant, cytotoxicity and anti-inflammatory activity of Schiff base derived from 2,3-diaminophenazine moiety and its metal(II) complexes: structural elucidation, computational aspects, and biological evaluation
- Carbon-based nanomaterials: synthesis, types and fuel applications: a mini-review
- Recent advances in carbon quantum dots for antibiotics detection
- Modern innovations in the provision and efficient application of 2D inorganic nanoscale materials
- Bioinorganic metal nanoparticles and their potential applications as antimicrobial, antioxidant and catalytic agents: a review
Artikel in diesem Heft
- Frontmatter
- A review of coordination compounds: structure, stability, and biological significance
- Effluent wastewater technologies for textile industry: a review
- Efficient removal of Cr(VI) ions from industrial wastewater using carbon-based adsorbents functionalized with boronic acid
- Exploring inorganic phosphors: basics, types, fabrications and their luminescence properties for LED/WLED/displays
- Recent advances on hydrogen generation based on inorganic metal oxide nano-catalyst using water electrolysis approach
- Antituberculosis, antimicrobial, antioxidant, cytotoxicity and anti-inflammatory activity of Schiff base derived from 2,3-diaminophenazine moiety and its metal(II) complexes: structural elucidation, computational aspects, and biological evaluation
- Carbon-based nanomaterials: synthesis, types and fuel applications: a mini-review
- Recent advances in carbon quantum dots for antibiotics detection
- Modern innovations in the provision and efficient application of 2D inorganic nanoscale materials
- Bioinorganic metal nanoparticles and their potential applications as antimicrobial, antioxidant and catalytic agents: a review

