The human health effects of unconventional oil and gas (UOG) chemical exposures: a scoping review of the toxicological literature
-
Élyse Caron-Beaudoin
, Hélène Akpo
Abstract
Many chemicals associated with unconventional oil and natural gas (UOG) are known toxicants, leading to health concerns about the effects of UOG. Our objective was to conduct a scoping review of the toxicological literature to assess the effects of UOG chemical exposures in models relevant to human health. We searched databases for primary research studies published in English or French between January 2000 and June 2023 on UOG-related toxicology studies. Two reviewers independently screened abstracts and full texts to determine inclusion. Seventeen studies met our study inclusion criteria. Nine studies used solely in vitro models, while six conducted their investigation solely in animal models. Two studies incorporated both types of models. Most studies used real water samples impacted by UOG or lab-made mixtures of UOG chemicals to expose their models. Most in vitro models used human cells in monocultures, while all animal studies were conducted in rodents. All studies detected significant deleterious effects associated with exposure to UOG chemicals or samples, including endocrine disruption, carcinogenicity, behavioral changes and metabolic alterations. Given the plausibility of causal relationships between UOG chemicals and adverse health outcomes highlighted in this review, future risk assessment studies should focus on measuring exposure to UOG chemicals in human populations.
Introduction
Hydraulic fracturing is a fossil fuel extraction technique that consists of injecting large volumes of fracking fluid (a mixture of water, sand or other proppants, and a variety of chemicals used as, for example, biocides, friction reducers, scale inhibitors, clay stabilizers, surfactants, acids, corrosion inhibitors, gelling agents, foaming agents or pH adjustors [1]) in the rock formation to create fractures, freeing the trapped fossil fuel (e.g., natural gas) for extraction [2]. Unconventional oil and gas (UOG) operations generate large quantities of wastewaters (flowback fluids and produced water) whose physical properties vary considerably depending on the rock formation [3]. These wastewaters contain hydraulic fracturing chemicals, and constituents naturally present in the oil and gas deposits such as volatile organic compounds (VOCs; such as benzene, toluene, ethylbenzene, xylenes [commonly referred as BTEX], acetate, acetone, dichloromethane and chloroform [4], [5], [6]), radioactive elements, and trace metals (e.g., arsenic, strontium, barium, manganese) [3], [6], [7], [8], [9], [10], [11]. Local contamination of soil, air and water in proximity to unconventional oil and gas (UOG) operations has been demonstrated in various studies [12], [13], [14], [15], [16], [17], [18].
As highlighted in recent reviews [19], 20], a growing number of epidemiological studies are showing deleterious health effects in communities living in the vicinity of UOG operations. Health effects associated with proximity to UOG include birth outcomes (e.g., preterm birth, low birth weight, and congenital abnormalities) [21], [22], [23], [24], [25], [26], [27], [28], self-reported health symptoms (e.g., rashes/skin problems, nose bleeds, stuffy nose, cough, blocked sinuses and fatigue) [29], 30], asthma exacerbations [31], 32], as well as adverse cardiovascular [33] and mental health [34], 35] outcomes. A number of studies found that exposure to ambient air pollutants (e.g., carbon monoxide, nitrogen dioxide, particulate matter and VOCs) in various areas of the world not necessarily impacted by UOG, is associated with many of these health outcomes, including birth outcomes [36], 37], and respiratory and cardiovascular diseases [38], 39]. Although limited in number, toxicological research initiatives are working towards elucidating some of the underlying mechanisms of toxicity explaining the associations between proximity to UOG and health outcomes. Indeed, some chemicals associated with UOG, such as acrylamide, benzene, bisphenol A, dibutyl phtalate and strontium, are reproductive and developmental toxicants in humans [40], carcinogenic and mutagenic [41], 42], endocrine disruptors [43], [44], [45], [46], and can promote oxidative stress [47], [48], [49], [50], [51], [52], [53]. All of these mechanisms of toxicity are known to be implicated in the etiology of multiple diseases identified in the UOG epidemiological literature. A review used FracFocus data to describe the chemicals used in hydraulic fracturing for oil production in California, their frequency, function, and potential acute toxicity for aquatic and mammalian species. Of the approximately 300 chemicals used for unconventional oil production in California, many of these, including solvents and surfactants, lacked toxicity data. For example, the authors found that toxicity data was unavailable for five of the most frequently used chemicals. For the chemicals with available toxicity data, the data was often incomplete [54].
An assessment of acute and chronic health hazards of hydraulic fracturing chemicals published in 2015 compiled health hazard information for 113 individual chemicals reported to be used in hydraulic fracturing fluids in North Dakota. The health hazard acute endpoints found to be the most associated with hydraulic fracturing fluids constituents included respiratory tract irritation, as well as eye and skin irritation or damage. Chronic toxicity endpoints were not available for the majority of chemicals [55]. It is important to note that health hazards were identified based on the information reported for individual chemicals in Safety Data Sheets and publicly available databases, such as the Agency for Toxic Substances and Disease Registry and the European Chemicals Agency. A review of the toxicological literature published in 2020 was limited to experimental studies evaluating the endocrine disrupting potential of exposure to a mixture of 23 UOG chemicals [46]. Our primary objective was to update and expand on these reviews to include toxicological studies published between January 2000 to June 2023 examining toxicity on multiple endpoints associated with UOG chemicals whose effects were measured specifically in the context of UOG (i.e., using mixtures of UOG chemicals, samples of UOG wastewater) in models (in vivo or in vitro) relevant for human health.
Methods
Our review is a companion paper of our recent scoping review of epidemiological studies regarding the human health effects of UOG [56].
Data sources and searches
We defined UOG using hydraulic fracturing as the injection of fluids under pressure great enough to fracture shale and tight rock formations. An experienced biomedical librarian (MDW) conducted comprehensive searches in MEDLINE, and Embase (through OVID) for all published studies in English or French from 2000 to June 16, 2023. The toxicology search concepts are listed in Supplementary Material Table S1.
Study selection
We included toxicological studies that investigated harmful effects in in vitro (cellular) or animal assays exposed to UOG chemicals in the context of UOG, i.e., that chemicals (either individual or in mixtures) had to mimic the UOG components (e.g., components of hydraulic fracturing fluid; air emissions; water contamination by UOG activities; or UOG wastewaters). We excluded studies that assessed the impact of UOG chemicals (1) individually and in studies not related to UOG; (2) on in vitro and animal models not relevant to human health (e.g., yeast, aquatic species models, birds). We further excluded studies that had no control group and were not peer-reviewed. Reviews and conference abstracts were also excluded.
Titles and abstracts of studies were first screened to determine initial eligibility for full-text review using COVIDENCE [57], a web-based screening and data extraction tool. Both screening and full text review were completed by two independent reviewers. Disagreements at either stage were resolved through discussion until achieving consensus or, if required, the input of a third reviewer.
Due to the wide heterogeneity of models, exposures (i.e., chemicals used, pathways, duration), outcomes and methodological approaches, a formal bias tool evaluating the quality of selected studies was not applied.
Data extraction, synthesis, and analysis
Given the variation in models, exposure (i.e., chemicals, pathways, duration) and outcome definitions, data synthesis was descriptive. Relevant information extracted from in vitro toxicological studies included: first author; publication year; journal; funding source; study objective; model (i.e., cell line, tissue culture, source); UOG chemicals or samples tested; endpoints of interest; main findings. Relevant information extracted from animal toxicological studies included: first author; publication year; journal; funding source; study objective; model (i.e., animal species, strain, sex, age); exposure treatments (UOG chemicals or samples tested; concentrations, route and duration of exposure); endpoints of interest; and main findings.
Data were extracted directly into the Health Assessment Workplace Collaborative (HAWC) platform (https://hawcproject.org). HAWC is a publicly available web-based tool designed for data extraction of animal bioassay and in vitro studies [58], 59]. Data were independently extracted first by one member of the study team for all selected studies. A second member of the team then verified the exactitude of the data extraction process for each study, and summarized the extracted data descriptively in order to highlight main findings. The main findings from each study from each stream (in vitro and animal models) were synthesized in tables.
A PRISMA flow diagram visually summarises the screening and selection process is presented in Figure 1. One screening and selection process was conducted for both this scoping review and our companion paper [56].
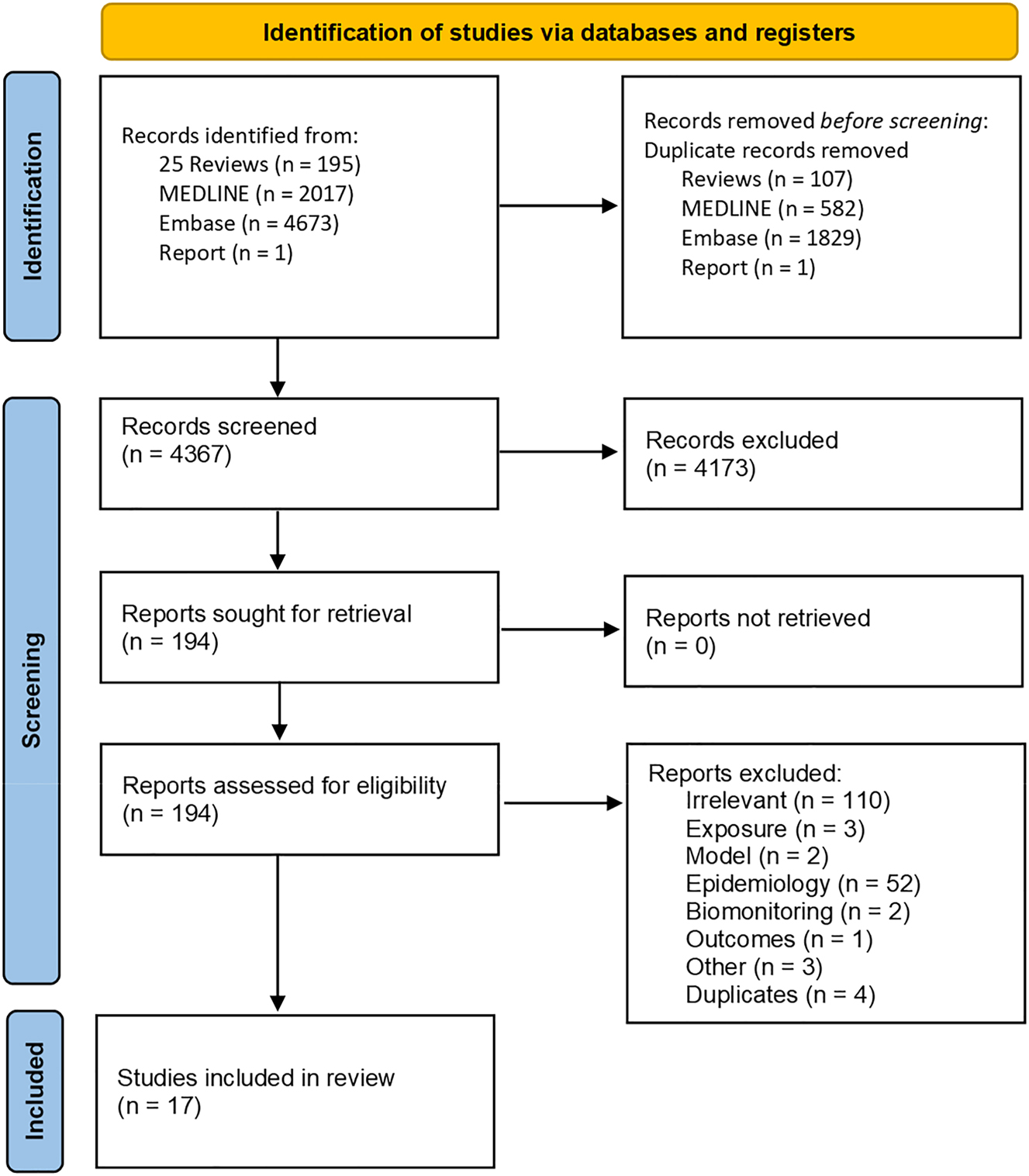
Summary of selection of studies included in this scoping review.
Results
6,886 records were identified through the searches. After duplicates were removed, 4,367 titles and abstracts were reviewed independently by two reviewers, of which 17 met our inclusion criteria. Nine studies used solely in vitro models to study the toxicity of UOG chemicals, while six conducted their investigation solely in mice. Two studies [44], 60] incorporated both in vitro and animal models in their design. All studies were published after 2014.
Relevant information extracted from the 11 in vitro toxicological studies are presented in Table 1. In terms of exposure conditions, four studies used real water samples impacted by UOG (e.g., produced, flowback or wastewater, drinking water, surface water, groundwater) [45], [61], [62], [63]. Three studies used various lab-made mixtures of UOG chemicals [44], 45], 64]. One study investigated the effects of three biocides, a surfactant, a friction reducer and a coal seam geogenic [65]. One recent study used UOG wastewater samples treated by various oxidation processes [66]. Another recent study used drinking water samples containing UOG wastewater and then treated by chlorination or chloramination, two disinfectant treatments used in municipal water treatment plants [61].
Characteristics (UOG chemicals or samples tested; models; endpoints; main findings; deleterious effects) of included in vitro toxicological studies.
| Lead author, year | UOG chemicals/samples tested | Models | Endpoints | Main findings | Deleterious effects |
|---|---|---|---|---|---|
| Abraham (2023) |
|
Toxicity values based on mammalian cell assays (CHO cells; hamster; ovary) |
|
|
|
| Bain (2018) |
|
Luciferase report assays (CALUX®) using U-2 OS cell line (human; epithelial; osteosarcoma) |
|
|
Endocrine disruption |
| Bamberger (2019) |
|
|
|
|
Endocrine disruption |
| Crosby (2018) |
|
|
|
|
|
| Kassotis (2014) |
|
Firefly luciferase reporter gene assay in transfected HepG-2 (human; epithelial; hepatoma) and MCF-7 cells (human; epithelial; adenocarcinoma of the mammary gland) |
|
|
Endocrine disruption |
| Kassotis (2015) |
|
Reporter gene assay in transfected Ishikawa (human; epithelial, endometrial carcinoma) and HepG-2 cells (human; epithelial; hepatoma) |
|
|
Endocrine disruption |
| Kassotis (2016) |
|
Reporter gene assay in transfected Ishikawa cells (human; epithelial, endometrial carcinoma) |
|
|
|
| Kassotis (2018) |
|
|
|
|
Impaired metabolic health |
| Kassotis (2020) |
|
Reporter gene assay in transfected Ishikawa cells (human; epithelial, endometrial carcinoma) |
|
|
Endocrine disruption |
| Yao (2015) |
|
BEAS-2B cells (human; epithelial; lung) |
|
|
Carcinogenic potential |
| Zhuang (2023) |
|
V79 cells (Chinese hamster; fibroblast; lung) |
|
|
|
In vitro models ranged from mammalian cells to reporter assays, and to transfected cells. Most studies used human cells, while a few used hamster and mouse cells. All studies used monocultures. Endpoints included cytotoxicity (6 of 11 studies), genotoxicity (2/11), hormone receptor activity (6/11), gene and protein expression (2/11), wound healing inhibition (1/11), uptake of chemicals in cells (1/11), triglyceride accumulation (1/11), cell proliferation (1/11), carcinogenicity (1/11) and cell migration (1/11).
All studies found deleterious effects associated with exposure to UOG chemicals or samples. In particular, Abraham et al. [61] found significant cytotoxicity and genotoxicity associated with chlorinated or chloraminated drinking water samples containing UOG wastewater using the TIC-Tox method. Bain et al. [65] used the CALUX® assay and reported endocrine disruption on multiple hormone receptors (estrogen, androgen, progesterone, glucocorticoid and PPARγ) following exposure to biocides, surfactant, friction reducer and coal seam geogenic compounds. Bamberger et al. [62] found that surface and groundwater samples collected in a dense UOG region exhibited endocrine disruption activity, including an increase in the activity of the aryl-hydrocarbon receptor (AhR) in samples close to impaired natural gas wells, as well as several endocrine receptor antagonism and agonism. Crosby et al. [63] found that exposure to wastewater samples in liver and kidney cells led to impaired expression of genes implicated in cellular communication. Kassotis et al. [[43], [44], [45, 67] found evidence of endocrine disruption in multiple studies, with impaired receptor activities both in reporter gene assays and in transfected cells exposed to surface and groundwater samples, either from drilling-dense regions or in mixtures of commonly-used UOG chemicals. To our knowledge, Kassotis et al. [45] is the first study investigating the toxicity of UOG, specifically the endocrine disruption potential of UOG. Notably, in Kassotis et al. [43], the antagonist activities of several hormone receptors were seen in surface water at an injection well disposal site and downstream. In Kassotis et al. [64], impaired metabolic health – with triglyceride accumulation and pre-adipocyte proliferation, was observed in mouse fibroblast cells exposed to a mixture of 23 UOG chemicals, UOG wastewater and surface water at a UOG well pad. Yao et al. [60] investigated the carcinogenic effects of flowback water samples in human lung cells and found that exposed cells had higher migration capacity and increased expression of genes implicated in inflammation, proliferation and migration. Genes promoting apoptosis, endocytosis and adherent junctions were inhibited. Additionally, Zhuang et al. [66] measured the cytotoxicity and DNA damage caused by exposure of Chinese hamster lung fibroblast cells to UOG produced water, when treated or not by oxidation. The authors found that treated produced water induced greater DNA damage than untreated produced water.
Relevant information extracted from the eight toxicological studies using animal assays are presented in Table 2. In terms of exposure conditions, seven studies used lab-made mixtures of UOG chemicals [44], [68], [69], [70], [71], [72], [73] and one study used BEAS-2B cells exposed to flowback water and then injected subcutaneously in female mice [60]. Animals were exposed to lab-made mixtures of UOG chemicals in drinking water for varied amounts of time.
Characteristics (UOG chemicals or samples tested; models; endpoints; main findings; deleterious effects) of included animal toxicological studies.
| Lead author, year | UOG chemicals/samples tested | Models | Endpoints | Main findings | Deleterious effects |
|---|---|---|---|---|---|
| Animal assays | |||||
|
|
|||||
| Balise (2019) | Mixture of 23 commonly used UOG chemicals at 1.5, 150 of 1,500 ug/kg/day in drinking water for 5 weeks prior to mating, and between gestational day 0 until PND21. | C57BL/6J female mice offspring aged to 7 months |
|
|
Behavioral changes |
| Balise (2019) | Mixture of 23 commonly used UOG chemicals at 1.5, 15, 150 and 1,500 μg/kg/day in drinking water for 5 weeks prior to mating, and between gestational day 0 until PND21. | C57BL/6J female mice offspring aged to 12 months and given a 3 day high fat, high sugar diet (HFHSD) challenge |
|
|
|
| Boule (2018) | Mixture of 23 commonly used UOG chemicals at 30 and 300 μg/kg/day in drinking water from gestational day 0 until PND21. | Male and female C57BL/6J mice offspring challenged with infectious agents (house dust mite (HDM) extract-induced allergic airway disease; influenza A virus (IAV); autoimmune encephalomyelitis (EAE)) after exposure |
|
|
Immune dysregulation |
| Kassotis (2015) | Mixture of 23 commonly used UOG chemicals at 3, 30, 300, and 3,000 μg/kg/day in drinking water, from gestational day 11 until birth | Male C57BL/6J mice offspring |
|
|
Endocrine disruption |
| Kassotis (2016) | Mixture of 23 commonly used UOG chemicals at 3, 30, 300, and 3,000 μg/kg/day in drinking water, from gestational day 11 until birth | Female C57Bl/6 mice offspring |
|
|
|
| O’Dell (2021) | Mixture of 23 commonly used UOG chemicals in drinking water at a final concentration of 0.1 μg/mL for each chemical for at least 8 weeks | Adult (6–8 weeks) male and female C57Bl/6 mice challenged with infectious agents (house dust mite (HDM) extract-induced allergic airway disease; influenza A virus (IAV); autoimmune encephalomyelitis (EAE)) after exposure |
|
|
Altered immune system |
| Sapouckey (2018) | Mixture of 23 commonly used UOG chemicals at 3, 30, 300, and 3,000 μg/kg/day in drinking water, from gestational day 11 to birth | Female C57Bl/6 mice offspring |
|
|
Mammary gland development |
| Yao (2015) | BEAS-2B cells transformed by flowback water injected subcutaneously in left and rank flanks | Female athymic nude mice (Nu/J) aged 6–8 weeks with BEAS-2B cells tumor xenografts |
|
|
Carcinogenic potential |
All studies used mice, including C57BL/6 J (7 of eight studies) or athymic nude mice (1/8). Two studies used both males and females, while five and one studies used only females and males, respectively. Six studies used offspring, while two studies used adult mice. Endpoints included body weight (3/8); energy, activity and behavior (2/8); metabolic health endpoints such as glucose tolerance, liver triacylglycerol, insulin and pancreas analysis (2/8); immune cells populations and immune response (2/8); endocrine endpoints such as anogenital distances, serum hormones, sperm and ovarian follicle assessments and mammary gland development (3/8); heart assessment (1/8); and tumor formation (1/8).
All studies found deleterious effects associated with exposure to UOG chemicals. In particular, Balise et al. [68] found that exposure to a mixture of 23 commonly used UOG chemicals at concentrations from 1.5 to 1,500 μg/kg/day in drinking water prior to mating, and between gestational day 0 until postnatal day 21 (PND21), led to disruption of energy expenditure in female mice offspring. A follow-up study in adult female mice challenged with a high fat, high sugar diet also showed impaired behavior (decreased sleep, increased exploratory behavior) and metabolic health (decreased fat pad weight) [69]. Results from Boulé et al. [70] showed that exposure to a mixture of 23 commonly used UOG chemicals at 30 and 300 μg/kg/day in drinking water from gestational day 0 to PND21 in male and female mice offspring challenged with various infectious agents led to disruptions in immune cell populations. A follow-up study conducted by O’Dell et al. [72] in adult male and female mice challenged with the same infectious agents also noted altered immune cells populations, with distinct effects in males and females. Kassotis et al. [44] found that exposure to the same mixture at concentrations from 30 to 3,000 μg/kg/day in drinking water from gestational day 11 until birth led to disruption in testis and heart weight, and a decrease in sperm count and anogenital distance in male offspring. A study from the same group conducted in female offspring showed altered levels of various hormones such as FSH, LH, prolactin, GH and TSH, as well as disrupted folliculogenesis and collagen deposition in the heart [71]. Using the same experimental design, Sapouckey et al. [73] also noted an increase in mammary ducts and volume of the mammary epithelium in these female offspring. The study conducted by Yao et al. [60] injected human lung epithelial cells exposed to UOG flowback water into female athymic nude mice to generate solid human tumor xenografts. The authors found that five out of six mice injected with the flowback water-treated cells developed tumor xenografts. As well, one control mouse was injected with normal cells, which did not form any tumors.
Discussion
A scoping review published in 2020 that examined the human health outcomes associated with exposure to UOG activity highlighted that very few studies investigated mechanisms of toxicity underpinning the associations between UOG exposure and health outcomes [20]. Our review included only 17 studies, comprising nine using solely in vitro models, six conducted solely in animal models, and two using both in vitro and animal models. Notably, among the in vitro studies included in the review, all found deleterious effects associated with exposure to UOG chemicals or samples on a variety of endpoints, such as cytotoxicity, endocrine disruption, triglyceride accumulation and carcinogenesis. In terms of exposure, most studies used drinking, surface or groundwater samples from UOG-intensive regions [43], 45], 61], 62], 64], 67], while five studies used wastewater or produced water samples [60], 63], 64], 66], 67] and four studies were conducted with lab-made mixtures of UOG chemicals [44], 45], 64], 65]. All animal studies included in this review also reported significant impacts of UOG exposure on a range of endpoints, including behavioral changes, metabolic health, immune dysregulation, endocrine disruption, developmental disruption, carcinogenesis and mammary gland development. In terms of exposure, most studies used lab-made mixtures of UOG chemicals to exposure animals through drinking water [44], [68], [69], [70], [71], [72], [73]. One study used BEAS-2B cells previously exposed to flowback water to induce tumor xenografts in mice [60].
More than a 1,000 different chemicals have been identified as commonly-used compounds in UOG operations for a variety of functions, such as proppants, biocides, friction reducers, scale inhibitors, clay stabilizers, surfactants, acids, corrosion inhibitors, gelling agents, foaming agents and pH adjustors [1]. Assessing the toxicity of UOG chemicals is a complicated enterprise, in part due to the lack of toxicological information for the majority of chemicals used in this industry [40] and the withholding of information on proprietary or trade secret grounds. Elliott et al. [40] evaluated 1,021 chemicals used in hydraulic fracturing for their reproductive and developmental toxicity and found that for the 24 % of chemicals with available toxicity information, 43 and 40 % of them were reproductive and developmental toxicants, respectively. Trickey et al. [74] investigated disclosure forms submitted to FracFocus, the US hydraulic fracturing chemical registry, and determined that 18 % of the chemicals imported in FracFocus were not identifiable and marked as confidential, proprietary or trade secret. Yost et al. (2016) used a list of 1,173 chemicals associated with the UOG industry (1,076 chemicals used in the hydraulic fluid, and 134 chemicals identified in wastewaters) and compiled the available chronic oral reference values (i.e., amount of the chemical that can be ingested every day without significantly increasing the risk of adverse health effects over a lifetime) for these 1,173 chemicals. Their analysis revealed chronic oral reference values were available for only 90 of the 1,076 chemicals reported in hydraulic fracturing fluids and 83 of the 134 chemicals reported in wastewaters. Chemicals for which chronic oral reference values were available included well studied heavy metals (e.g., arsenic, cobalt, cadmium), organic compounds (e.g., benzene, phosphine, benzyl chloride), polycyclic aromatic hydrocarbons (e.g., benzo [a] pyrene) and pesticides (e.g., heptachlor, aldrin, dieldrin). The authors also identified 36 chemicals frequently used in UOG operations: chronic oral reference values were not available for 28 of these chemicals (including, but not limited to, quartz-alpha, hydrochloric acid, isopropanol, diammonium peroxydisulfate, guar gum, sodium hydroxide, glutaraldehyde, sodium chloride, potassium hydroxide, ethanol, solvent naphtha, ammonium chloride) [75].
The main deleterious effects identified in the toxicological studies included in our review are aligned with the current epidemiological literature on the associations between exposure to UOG activity and human health outcomes. A recent review conducted by our team identified 52 studies where investigated health outcomes were plausibly attributable to exposure to UOG chemicals, with birth outcomes being the most widely studied health effects. The majority of studies found significant deleterious effects, including on maternal, birth and infant outcomes, respiratory and cardiovascular outcomes, childhood cancer, hospital admissions, self-reported health symptoms, and mortality [56].
Endocrine disruption, as identified in multiple toxicological studies included in this review, is a plausible mechanism that could explain the higher odds of negative maternal and birth outcomes observed in multiple epidemiological studies conducted in the UOG context [21], 23], [25], [26], [27], [28, 76], 77]. Toxicological data on individual chemicals known to be used or emitted by UOG operations can be used to contextualize the results of the studies included in this review, and the plausibility of the epidemiological literature findings. For example, certain VOCs (e.g., BTEX) and trace elements (e.g., barium, strontium, arsenic) are emitted by UOG operations [3], 6], 8], 10], 11], 14], 78]–85] with some congeners being known or suspected endocrine disruptors and human carcinogens [86], [87], [88], [89], [90], [91].
Limitations and future opportunities
The wide range of models, exposures and outcomes measured in the various studies included in our review made it challenging to apply a formal bias tool to evaluate the quality of studies, which is a limitation of our review. Furthermore, our review focuses on the toxicity of UOG mixtures and environmental samples and does not provide a comprehensive overview of the toxicological data relevant to all individuals chemicals. All in vitro studies identified in this review used monocultures. This is an important limitation of the identified studies, since cell-cell communication and interactions with the extracellular matrix, which cannot be evaluated in monocultures systems, play important roles in cellular function, behavior, and the development of disease [92]. More complex in vitro systems, such as co-cultures and organ-on-chip systems, are more physiologically-relevant and accurate to study how cells interact with each other and their environment and should be prioritized in future research endeavors [93], [94], [95], [96], [97].
Rodents are not always an accurate model to use in toxicity testing. For example, studies have shown that the metabolism of environmental contaminants, including endocrine disruptors, differ between rodent species and humans [98], [99], [100]. Researchers should ensure that the animal models used are relevant for the human disease of interest and share similar mechanisms of action [100]. Another important limitation of the animal studies included in the review is the fact that they all used drinking water as the pathway of exposure to UOG chemicals or samples, therefore not considering other relevant pathways of exposure. Indeed, studies have demonstrated impacts of UOG activity on local air quality [79], [101], [102], [103], [104], making inhalation an appropriate additional exposure pathway to use in future studies. Future toxicological studies could focus on the inhalation pathway by using innovative cellular models at the Air-Liquid Interface (ALI). Indeed, pulmonary cell systems at the ALI allow for a better replication of the airway physiology, the possibility of repeating and chronic exposure scenarios and the delivery of contaminants in aerosol similar to inhalation in humans [105]. In addition, animal models can be exposed to air pollutants in various types of exposure chambers [106]. Other considerations to improve the appropriate selection of models include: selection of the most sensitive species possible, accounting for species differences in metabolism of contaminants (for example, by using humanized transgenic rodents; conducting prior testing of contaminants metabolism in cellular models of various species, and using physiologically based pharmacokinetic [PBPK] modeling to predict the contaminants metabolism and behavior in humans [107], 108]); ensuring that the animal model presents the relevant target and outcome; ensuring animals of both sexes are included when relevant; and consideration of the latency period between exposure and effect in the design of the study [100].
The studies we identified offered little background on their choice of exposure concentrations, highlighting the need for more toxicological studies using physiologically-relevant models, environmentally-relevant concentrations of UOG chemicals based on biomonitoring studies, and exposure pathways relevant to real-life exposure scenarios.
Conclusions
There is a growing body of evidence of an association between proximity to UOG operations and adverse human health outcomes. Our review highlighted multiple mechanisms of toxicity associated with UOG chemicals, including endocrine disruption, genotoxicity, impaired cellular communication, impaired metabolic health biomarkers, behavioral changes, immune dysregulation, and carcinogenesis. This review also highlights the sparsity of studies using mixtures of UOG chemicals and environmentally-relevant samples, and suggests models and exposure methods that could be prioritized in future research.
Funding source: Rural Coordination Centre of British Columbia
Award Identifier / Grant number: N/A
Funding source: University of British Columbia Centre for Rural Health Research
Award Identifier / Grant number: N/A
Acknowledgments
The authors would like to thank Tamera Panjalingam, Anna Thompson, Siddharthan Lakshmanan and Ruchika Gautam for research support. We thank Dr. Reza Afshari for his assistance with study selection.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Élyse Caron-Beaudoin: Conceptualization, Methodology, Validation, Investigation, Data Curation, Formal analysis, Visualization, Writing – original draft, Project administration. Hélène Akpo: Investigation, Data Curation, Formal analysis, Visualization. Mary M. Doyle-Waters: Conceptualization, Methodology, Validation, Investigation, Visualization, Writing – review & editing. Lisa Ronald: Conceptualization, Methodology, Validation, Writing – review & editing. Michael Friesen: Conceptualization, Methodology, Validation. Tim Takaro: Conceptualization, Methodology, Writing – review & editing. Karen Levin: Conceptualization. Ulrike Meyer: Conceptualization, Methodology, Funding acquisition. Margaret J. McGregor: Conceptualization, Methodology, Investigation, Writing – review & editing, Project administration, Funding acquisition.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: This work was supported by the Rural Coordination Centre of BC–Rural Physician Research Grant and the UBC Department of Family Practice Centre for Rural Health Research.
-
Data availability: Not applicable.
References
1. Wollin, KM, Damm, G, Foth, H, Freyberger, A, Gebel, T, Mangerich, A, et al.. Critical evaluation of human health risks due to hydraulic fracturing in natural gas and petroleum production. Arch Toxicol 2020;94:967–1016. https://doi.org/10.1007/s00204-020-02758-7.Search in Google Scholar PubMed PubMed Central
2. Norris, JQ, Turcotte, DL, Moores, EM, Brodsky, EE, Rundle, JB. Fracking in tight shales: what is it, what does it accomplish, and what are its consequences? Annu Rev Earth Planet Sci 2016;44:321–51. https://doi.org/10.1146/annurev-earth-060115-012537.Search in Google Scholar
3. Pichtel, J. Oil and gas production wastewater: soil contamination and pollution prevention. Appl Environ Soil Sci 2016;2016:24. https://doi.org/10.1155/2016/2707989.Search in Google Scholar
4. Goldstein, BD, Brooks, BW, Cohen, SD, Gates, AE, Honeycutt, ME, Morris, JB, et al.. The role of toxicological science in meeting the challenges and opportunities of hydraulic fracturing. Toxicol Sci 2014;139:271–83. https://doi.org/10.1093/toxsci/kfu061.Search in Google Scholar PubMed PubMed Central
5. Hecobian, A, Clements, AL, Shonkwiler, KB, Zhou, Y, MacDonald, LP, Hilliard, N, et al.. Air toxics and other volatile organic compound emissions from unconventional oil and gas development. Environ Sci Technol Lett 2019;6:720–6. https://doi.org/10.1021/acs.estlett.9b00591.Search in Google Scholar
6. Luek, JL, Gonsior, M. Organic compounds in hydraulic fracturing fluids and wastewaters: a review. Water Res 2017;123:536–48. https://doi.org/10.1016/j.watres.2017.07.012.Search in Google Scholar PubMed
7. Brown, VJ. Radionuclides in fracking wastewater: managing a toxic blend. Environ Health Perspect 2014;122:A50–5. https://doi.org/10.1289/ehp.122-a50.Search in Google Scholar PubMed PubMed Central
8. Chittick, EA, Srebotnjak, T. An analysis of chemicals and other constituents found in produced water from hydraulically fractured wells in California and the challenges for wastewater management. J Environ Manag 2017;204:502–9. https://doi.org/10.1016/j.jenvman.2017.09.002.Search in Google Scholar PubMed
9. Fontenot, BE, Hunt, LR, Hildenbrand, ZL, Carlton, JDD, Oka, H, Walton, JL, et al.. An evaluation of water quality in private drinking water wells near natural gas extraction sites in the Barnett Shale Formation. Environ Sci Technol 2013;47:10032–40. https://doi.org/10.1021/es4011724.Search in Google Scholar PubMed
10. Lester, Y, Ferrer, I, Thurman, EM, Sitterley, KA, Korak, JA, Aiken, G, et al.. Characterization of hydraulic fracturing flowback water in Colorado: implications for water treatment. Sci Total Environ 2015;512–513:637–44. https://doi.org/10.1016/j.scitotenv.2015.01.043.Search in Google Scholar PubMed
11. Srebotnjak, T, Rotkin-Ellman, M. Fracking fumes: air pollution from hydraulic fracturing threatens public health and communities. Natural Resources Defense Council; 2014.Search in Google Scholar
12. Crowe, E, Patton, S, Thomas, D, Thorpe, B. When the wind blows: tracking toxic chemicals in gas fields and impacted communities. Battleboro, VT: Coming Clean Inc.; 2016. [Internet]. Available from: https://aglaw.psu.edu/wp-content/uploads/2020/06/document_gw_01.pdf.Search in Google Scholar
13. Gilman, JB, Lerner, B, Kuster, W, De Gouw, J. Source signature of volatile organic compounds from oil and natural gas operations in northeastern Colorado. Environ Sci Technol 2013;47:1297–305. https://doi.org/10.1021/es304119a.Search in Google Scholar PubMed
14. Macey, GP, Breech, R, Chernaik, M, Cox, C, Larson, D, Thomas, D, et al.. Air concentrations of volatile compounds near oil and gas production: a community-based exploratory study. Environ Health 2014;13:82. https://doi.org/10.1186/1476-069x-13-82.Search in Google Scholar PubMed PubMed Central
15. Vengosh, A, Jackson, RB, Warner, N, Darrah, TH, Kondash, A. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ Sci Technol 2014;48:8334–48. https://doi.org/10.1021/es405118y.Search in Google Scholar PubMed
16. Werner, AK, Vink, S, Watt, K, Jagals, P. Environmental health impacts of unconventional natural gas development: a review of the current strength of evidence. Sci Total Environ 2015;505:1127–41. https://doi.org/10.1016/j.scitotenv.2014.10.084.Search in Google Scholar PubMed
17. Wisen, J, Chesnaux, R, Wendling, G, Werring, J, Barbecot, F, Baudron, P. Assessing the potential of cross-contamination from oil and gas hydraulic fracturing: a case study in northeastern British Columbia, Canada. J Environ Manag 2019;246:275–82. https://doi.org/10.1016/j.jenvman.2019.05.138.Search in Google Scholar PubMed
18. Wisen, J, Chesnaux, R, Werring, J, Wendling, G, Baudron, P, Barbecot, F. A portrait of wellbore leakage in northeastern British Columbia, Canada. Proc Natl Acad Sci 2019;117:913–22.10.1073/pnas.1817929116Search in Google Scholar PubMed PubMed Central
19. Bamber, AM, Hasanali, SH, Nair, AS, Watkins, SM, Vigil, DI, Van Dyke, M, et al.. A systematic review of the epidemiologic literature assessing health outcomes in populations living near oil and natural gas operations: study quality and future recommendations. Int J Environ Res Publ Health 2019;16:2123. https://doi.org/10.3390/ijerph16122123.Search in Google Scholar PubMed PubMed Central
20. Deziel, NC, Brokovich, E, Grotto, I, Clark, CJ, Barnett-Itzhaki, Z, Broday, D, et al.. Unconventional oil and gas development and health outcomes: a scoping review of the epidemiological research. Environ Res 2020;182:109124. https://doi.org/10.1016/j.envres.2020.109124.Search in Google Scholar PubMed
21. Caron-Beaudoin, É, Whitworth, KW, Bosson-Rieutort, D, Wendling, G, Liu, S, Verner, MA. Density and proximity to hydraulic fracturing wells and birth outcomes in Northeastern British Columbia, Canada. J Expo Sci Environ Epidemiol 2020;31:53–61. https://doi.org/10.1038/s41370-020-0245-z.Search in Google Scholar PubMed
22. Casey, JA, Savitz, DA, Rasmussen, SG, Ogburn, EL, Pollak, J, Mercer, DG, et al.. Unconventional natural gas development and birth outcomes in Pennsylvania, USA. Epidemiology 2016;27:163–72. https://doi.org/10.1097/ede.0000000000000387.Search in Google Scholar
23. Currie, J, Greenstone, M, Meckel, K. Hydraulic fracturing and infant health: new evidence from Pennsylvania. Sci Adv 2017;3:e1603021. https://doi.org/10.1126/sciadv.1603021.Search in Google Scholar PubMed PubMed Central
24. Gonzalez, DJ, Sherris, AR, Yang, W, Stevenson, DK, Padula, AM, Baiocchi, M, et al.. Oil and gas production and spontaneous preterm birth in the San Joaquin Valley, CA: a case – control study. Environ Epidemiol 2020;4:7. https://doi.org/10.1097/ee9.0000000000000099.Search in Google Scholar
25. Hill, EL. Shale gas development and infant health: evidence from Pennsylvania. J Health Econ 2018;61:134–50. https://doi.org/10.1016/j.jhealeco.2018.07.004.Search in Google Scholar PubMed PubMed Central
26. Whitworth, KW, Marshall, AK, Symanski, E. Drilling and production activity related to unconventional gas development and severity of preterm birth. Environ Health Perspect 2018;126:8. https://doi.org/10.1289/ehp2622.Search in Google Scholar PubMed PubMed Central
27. Whitworth, KW, Marshall, AK, Symanski, E. Maternal residential proximity to unconventional gas development and perinatal outcomes among a diverse urban population in Texas. PLoS One 2017;12:e0180966. https://doi.org/10.1371/journal.pone.0180966.Search in Google Scholar PubMed PubMed Central
28. Willis, MD, Hill, EL, Boslett, A, Kile, ML, Carozza, SE, Hystad, P. Associations between residential proximity to oil and gas drilling and term birth weight and small-for-gestational-age infants in Texas: a difference-in-differences analysis. Environ Health Perspect 2021;129:077002. https://doi.org/10.1289/ehp7678.Search in Google Scholar PubMed PubMed Central
29. Rabinowitz, PM, Slizovskiy, IB, Lamers, V, Trufan, SJ, Holford, TR, Dziura, JD, et al.. Proximity to natural gas wells and reported health status: results of a household survey in Washington Country, Pennsylvania. Environ Health Perspect 2015;123:21–6. https://doi.org/10.1289/ehp.1307732.Search in Google Scholar PubMed PubMed Central
30. Tustin, AW, Hirsch, AG, Rasmussen, SG, Casey, JA, Bandeen-Roche, K, Schwartz, BS. Associations between unconventional natural gas development and nasal and sinus, migraine headache, and fatigue symptoms in Pennsylvania. Environ Health Perspect 2017;125:189. https://doi.org/10.1289/ehp281.Search in Google Scholar PubMed PubMed Central
31. Rasmussen, SG, Ogburn, EL, McCormack, M, Casey, JA, Bandeen-Roche, K, Mercer, DG, et al.. Association between unconventional natural gas development in the Marcellus Shale and asthma exacerbations. JAMA Intern Med 2016;176:1334–43. https://doi.org/10.1001/jamainternmed.2016.2436.Search in Google Scholar PubMed PubMed Central
32. Willis, M, Hystad, P, Denham, A, Hill, E. Natural gas development, flaring practices and paediatric asthma hospitalizations in Texas. Int J Epidemiol 2020;49:1883–96. https://doi.org/10.1093/ije/dyaa115.Search in Google Scholar PubMed PubMed Central
33. Denham, A, Willis, MD, Croft, DP, Liu, L, Hill, EL. Acute myocardial infarction associated with unconventional natural gas development: a natural experiment. Environ Res 2021;195:7. https://doi.org/10.1016/j.envres.2021.110872.Search in Google Scholar PubMed PubMed Central
34. Aker, AM, Whitworth, KW, Bosson-Rieutort, D, Wendling, G, Ibrahim, A, Verner, MA, et al.. Proximity and density of unconventional natural gas wells and mental illness and substance use among pregnant individuals: an exploratory study in Canada. Int J Hyg Environ Health 2022;242. https://doi.org/10.1016/j.ijheh.2022.113962.Search in Google Scholar PubMed
35. Casey, JA, Wilcox, HC, Hirsch, AG, Pollak, J, Schwartz, BS. Associations of unconventional natural gas development with depression symptoms and disordered sleep in Pennsylvania. Sci Rep 2018;8:1–10. https://doi.org/10.1038/s41598-018-29747-2.Search in Google Scholar PubMed PubMed Central
36. Bergstra, AD, Brunekreef, B, Burdorf, A. The influence of industry-related air pollution on birth outcomes in an industrialized area. Environ Pollut 2021;269:7. https://doi.org/10.1016/j.envpol.2020.115741.Search in Google Scholar PubMed
37. Stieb, DM, Chen, L, Eshoul, M, Judek, S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 2012;117:100–11. https://doi.org/10.1016/j.envres.2012.05.007.Search in Google Scholar PubMed
38. Hoek, G, Krishnan, RM, Beelen, R, Peters, A, Ostro, B, Brunekreef, B, et al.. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health 2013;12:1–16. https://doi.org/10.1186/1476-069x-12-43.Search in Google Scholar PubMed PubMed Central
39. Vawda, S, Mansour, R, Takeda, A, Funnell, P, Kerry, S, Mudway, I, et al.. Associations between inflammatory and immune response genes and adverse respiratory outcomes following exposure to outdoor air pollution: a HuGE systematic review. Am J Epidemiol 2014;179:432–42. https://doi.org/10.1093/aje/kwt269.Search in Google Scholar PubMed
40. Elliott, EG, Ettinger, AS, Leaderer, BP, Bracken, MB, Deziel, NC. A systematic evaluation of chemicals in hydraulic-fracturing fluids and wastewater for reproductive and developmental toxicity. J Expos Sci Environ Epidemiol 2016;27:90–9. https://doi.org/10.1038/jes.2015.81.Search in Google Scholar PubMed
41. Elliott, EG, Trinh, P, Ma, X, Leaderer, BP, Ward, MH, Deziel, NC. Unconventional oil and gas development and risk of childhood leukemia: assessing the evidence. Sci Total Environ 2017;576:138–47. https://doi.org/10.1016/j.scitotenv.2016.10.072.Search in Google Scholar PubMed PubMed Central
42. Xu, X, Zhang, X, Carrillo, G, Zhong, Y, Kan, H, Zhang, B. A systematic assessment of carcinogenicity of chemicals in hydraulic-fracturing fluids and flowback water. Environ Pollut 2019;251:128–36. https://doi.org/10.1016/j.envpol.2019.04.016.Search in Google Scholar PubMed
43. Kassotis, CD, Iwanowicz, LR, Akob, DM, Cozzarelli, IM, Mumford, AC, Orem, WH, et al.. Endocrine disrupting activities of surface water associated with a West Virginia oil and gas industry wastewater disposal site. Sci Total Environ 2016;557:901–10. https://doi.org/10.1016/j.scitotenv.2016.03.113.Search in Google Scholar PubMed
44. Kassotis, CD, Klemp, KC, Vu, DC, Lin, CH, Meng, CX, Besch-Williford, CL, et al.. Endocrine-disrupting activity of hydraulic fracturing chemicals and adverse health outcomes after prenatal exposure in male mice. Endocrinology 2015;156:4458–73. https://doi.org/10.1210/en.2015-1375.Search in Google Scholar PubMed
45. Kassotis, CD, Tillitt, DE, Davis, JW, Hormann, AM, Nagel, SC. Estrogen and androgen receptor activities of hydraulic fracturing chemicals and surface and ground water in a drilling-dense region. Endocrinology 2014;155:897–907. https://doi.org/10.1210/en.2013-1697.Search in Google Scholar PubMed
46. Nagel, S, Kassotis, CD, Vandenberg, L, Lawrence, B, Robert, J, Balise, V. Developmental exposure to a mixture of unconventional oil and gas chemicals: a review of experimental effects on adult health, behavior, and disease. Mol Cell Endocrinol 2020;513:11. https://doi.org/10.1016/j.mce.2020.110722.Search in Google Scholar PubMed PubMed Central
47. Andersson, H, Piras, E, Demma, J, Hellman, B, Brittebo, E. Low levels of the air pollutant 1-nitropyrene induce DNA damage, increased levels of reactive oxygen species and endoplasmic reticulum stress in human endothelial cells. Toxicology 2009;262:57–64. https://doi.org/10.1016/j.tox.2009.05.008.Search in Google Scholar PubMed
48. Bayil, S, Cicek, H, Geyikli Cimenci, I, Hazar, M. How volatile organic compounds affect free radical and antioxidant enzyme activity in textile workers. Arh Hig Rad Toksikol 2008;59:283–7. https://doi.org/10.2478/10004-1254-59-2008-1918.Search in Google Scholar PubMed
49. Huang, W, Wang, G, Lu, SE, Kipen, H, Wang, Y, Hu, M, et al.. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med 2012;186:1150–9. https://doi.org/10.1164/rccm.201205-0850oc.Search in Google Scholar PubMed PubMed Central
50. Kim, JH, Moon, JY, Park, EY, Lee, KH, Hong, YC. Changes in oxidative stress biomarker and gene expression levels in workers exposed to volatile organic compounds. Ind Health 2010;49:8–14. https://doi.org/10.2486/indhealth.ms1112.Search in Google Scholar PubMed
51. Kim, SS, Meeker, JD, Keil, AP, Aung, MT, Bommarito, PA, Cantonwine, DE, et al.. Exposure to 17 trace metals in pregnancy and associations with urinary oxidative stress biomarkers. Environ Res 2019;179:9. https://doi.org/10.1016/j.envres.2019.108854.Search in Google Scholar PubMed PubMed Central
52. Lu, CY, Ma, YC, Lin, JM, Li, CY, Lin, RS, Sung, FC. Oxidative stress associated with indoor air pollution and sick building syndrome-related symptoms among office workers in Taiwan. Inhal Toxicol 2007;19:57–65. https://doi.org/10.1080/08958370600985859.Search in Google Scholar PubMed
53. Peluso, M, Munnia, A, Ceppi, M, Giese, RW, Catelan, D, Rusconi, F, et al.. Malondialdehyde–deoxyguanosine and bulky DNA adducts in schoolchildren resident in the proximity of the Sarroch industrial estate on Sardinia Island, Italy. Mutagenesis 2013;28:315–21. https://doi.org/10.1093/mutage/get005.Search in Google Scholar PubMed PubMed Central
54. Stringfellow, WT, Camarillo, MK, Domen, JK, Sandelin, WL, Varadharajan, C, Jordan, PD, et al.. Identifying chemicals of concern in hydraulic fracturing fluids used for oil production. Environ Pollut 2017;220:413–20. https://doi.org/10.1016/j.envpol.2016.09.082.Search in Google Scholar PubMed
55. Wattenberg, EV, Bielicki, JM, Suchomel, AE, Sweet, JT, Vold, EM, Ramachandran, G. Assessment of the acute and chronic health hazards of hydraulic fracturing fluids. J Occup Environ Hyg 2015;12:611–24.10.1080/15459624.2015.1029612Search in Google Scholar PubMed
56. Aker, AM, Friesen, M, Ronald, LA, Doyle-Waters, MM, Takaro, TJ, Thickson, W, et al.. The human health effects of unconventional oil and gas development (UOGD): a scoping review of epidemiologic studies. Can J Public Health 2024;1–22. https://doi.org/10.17269/s41997-024-00860-2.Search in Google Scholar PubMed PubMed Central
57. Covidence. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation; 2021. [Internet] Available from: www.covidence.org.Search in Google Scholar
58. Shapiro, A, Antoni, S, Guyton, K, Lunn, R, Loomis, D, Rusyn, I, et al.. HAWC: health assessment Workspace collaborative. Research Triangle Park, NC: National Toxicology Program; 2015.Search in Google Scholar
59. Shapiro, AJ, Antoni, S, Guyton, KZ, Lunn, RM, Loomis, D, Rusyn, I, et al.. Software tools to facilitate systematic review used for cancer hazard identification. Environ Health Perspect 2018;126:104501. https://doi.org/10.1289/ehp4224.Search in Google Scholar
60. Yao, Y, Chen, T, Shen, SS, Niu, Y, DesMarais, TL, Linn, R, et al.. Malignant human cell transformation of marcellus shale gas drilling flow back water. Toxicol Appl Pharmacol 2015;288:121–30. https://doi.org/10.1016/j.taap.2015.07.011.Search in Google Scholar PubMed PubMed Central
61. Abraham, DG, Liberatore, HK, Aziz, MT, Burnett, DB, Cizmas, LH, Richardson, SD. Impacts of hydraulic fracturing wastewater from oil and gas industries on drinking water: quantification of 69 disinfection by-products and calculated toxicity. Sci Total Environ 2023;882:7. https://doi.org/10.1016/j.scitotenv.2023.163344.Search in Google Scholar PubMed
62. Bamberger, M, Nell, M, Ahmed, AH, Santoro, R, Ingraffea, AR, Kennedy, RF, et al.. Surface water and groundwater analysis using aryl hydrocarbon and endocrine receptor biological assays and liquid chromatography-high resolution mass spectrometry in Susquehanna County, PA. Environ Sci: Process Impacts 2019;21:988–98. https://doi.org/10.1039/c9em00112c.Search in Google Scholar PubMed PubMed Central
63. Crosby, L, Tatu, CA, Varonka, M, Charles, KM, Orem, WH. Toxicological and chemical studies of wastewater from hydraulic fracture and conventional shale gas wells. Environ Toxicol Chem 2018;37:2098–111. https://doi.org/10.1002/etc.4146.Search in Google Scholar PubMed
64. Kassotis, CD, Nagel, SC, Stapleton, HM. Unconventional oil and gas chemicals and wastewater-impacted water samples promote adipogenesis via PPARγ-dependent and independent mechanisms in 3T3-L1 cells. Sci Total Environ 2018;640:1601–10. https://doi.org/10.1016/j.scitotenv.2018.05.030.Search in Google Scholar PubMed PubMed Central
65. Bain, PA, Kumar, A. In vitro nuclear receptor inhibition and cytotoxicity of hydraulic fracturing chemicals and their binary mixtures. Chemosphere 2018;198:565–73. https://doi.org/10.1016/j.chemosphere.2017.12.057.Search in Google Scholar PubMed
66. Zhuang, Y, Ji, Y, Kuang, Q, Zhang, Z, Li, P, Song, J, et al.. Oxidation treatment of shale gas produced water: molecular changes in dissolved organic matter composition and toxicity evaluation. J Hazard Mater 2023;452:11. https://doi.org/10.1016/j.jhazmat.2023.131266.Search in Google Scholar PubMed
67. Kassotis, CD, Harkness, JS, Vo, PH, Vu, DC, Hoffman, K, Cinnamon, KM, et al.. Endocrine disrupting activities and geochemistry of water resources associated with unconventional oil and gas activity. Sci Total Environ 2020;748:16. https://doi.org/10.1016/j.scitotenv.2020.142236.Search in Google Scholar PubMed PubMed Central
68. Balise, VD, Cornelius-Green, JN, Kassotis, CD, Rector, RS, Thyfault, JP, Nagel, SC. Preconceptional, gestational, and lactational exposure to an unconventional oil and gas chemical mixture alters energy expenditure in adult female mice. Front Endocrinol 2019;10:10. https://doi.org/10.3389/fendo.2019.00323.Search in Google Scholar PubMed PubMed Central
69. Balise, VD, Cornelius-Green, JN, Parmenter, B, Baxter, S, Kassotis, CD, Rector, RS, et al.. Developmental exposure to a mixture of unconventional oil and gas chemicals increased risk-taking behavior, activity and energy expenditure in aged female mice after a metabolic challenge. Front Endocrinol 2019;10:13. https://doi.org/10.3389/fendo.2019.00460.Search in Google Scholar PubMed PubMed Central
70. Boulé, LA, Chapman, TJ, Hillman, SE, Kassotis, CD, O’Dell, C, Robert, J, et al.. Developmental exposure to a mixture of 23 chemicals associated with unconventional oil and gas operations alters the immune system of mice. Toxicol Sci 2018;163:639–54. https://doi.org/10.1093/toxsci/kfy066.Search in Google Scholar PubMed PubMed Central
71. Kassotis, CD, Bromfield, JJ, Klemp, KC, Meng, CX, Wolfe, A, Zoeller, RT, et al.. Adverse reproductive and developmental health outcomes following prenatal exposure to a hydraulic fracturing chemical mixture in female C57Bl/6 mice. Endocrinology 2016;157:3469–81. https://doi.org/10.1210/en.2016-1242.Search in Google Scholar PubMed PubMed Central
72. O’Dell, CT, Boule, LA, Robert, J, Georas, SN, Eliseeva, S, Lawrence, BP. Exposure to a mixture of 23 chemicals associated with unconventional oil and gas operations alters immune response to challenge in adult mice. J Immunot 2021;18:105–17. https://doi.org/10.1080/1547691x.2021.1965677.Search in Google Scholar
73. Sapouckey, SA, Kassotis, CD, Nagel, SC, Vandenberg, LN. Prenatal exposure to unconventional oil and gas operation chemical mixtures altered mammary gland development in adult female mice. Endocrinology 2018;159:1277–89. https://doi.org/10.1210/en.2017-00866.Search in Google Scholar PubMed PubMed Central
74. Trickey, K, Hadjimichael, N, Sanghavi, P. Public reporting of hydraulic fracturing chemicals in the USA, 2011–18: a before and after comparison of reporting formats. Lancet Planet Health 2020;4:e178–85. https://doi.org/10.1016/s2542-5196(20)30076-0.Search in Google Scholar
75. Yost, EE, Stanek, J, DeWoskin, RS, Burgoon, LD. Overview of chronic oral toxicity values for chemicals present in hydraulic fracturing fluids, flowback, and produced waters. Environ Sci Technol 2016;50:4788–97. https://doi.org/10.1021/acs.est.5b04645.Search in Google Scholar PubMed
76. Cairncross, ZF, Couloigner, I, Ryan, MC, McMorris, C, Muehlenbachs, L, Nikolaou, N, et al.. Association between residential proximity to hydraulic fracturing sites and adverse birth outcomes. JAMA Pediatr 2022;176:585–92. https://doi.org/10.1001/jamapediatrics.2022.0306.Search in Google Scholar PubMed PubMed Central
77. Stacy, SL, Brink, LL, Larkin, JC, Sadovsky, Y, Goldstein, BD, Pitt, BR, et al.. Perinatal outcomes and unconventional natural gas operations in Southwest Pennsylvania. PLoS One 2015;10:15. https://doi.org/10.1371/journal.pone.0126425.Search in Google Scholar PubMed PubMed Central
78. Brown, VJ. Industry issues: putting the heat on gas. Environ Health Perspect 2007;115:A76. https://doi.org/10.1289/ehp.115-a76.Search in Google Scholar PubMed PubMed Central
79. Caron-Beaudoin, É, Whyte, KP, Bouchard, MF, Chevrier, J, Haddad, S, Copes, R, et al.. Volatile organic compounds (VOCs) in indoor air and tap water samples in residences of pregnant women living in an area of unconventional natural gas operations: findings from the EXPERIVA study. Sci Total Environ 2022;805:150242. https://doi.org/10.1016/j.scitotenv.2021.150242.Search in Google Scholar PubMed
80. Caron-Beaudoin, É, Valter, N, Chevrier, J, Ayotte, P, Frohlich, K, Verner, MA. Gestational exposure to volatile organic compounds (VOCs) in Northeastern British Columbia, Canada: a pilot study. Environ Int 2018;110:131–8. https://doi.org/10.1016/j.envint.2017.10.022.Search in Google Scholar PubMed
81. Ferrer, I, Thurman, EM. Chemical constituents and analytical approaches for hydraulic fracturing waters. Trends Environ Anal Chem 2015;5:18–25. https://doi.org/10.1016/j.teac.2015.01.003.Search in Google Scholar
82. Franklin, M, Chau, K, Cushing, LJ, Johnston, JE. Characterizing flaring from unconventional oil and gas operations in South Texas using satellite observations. Environ Sci Technol 2019;53:2220–8. https://doi.org/10.1021/acs.est.8b05355.Search in Google Scholar PubMed PubMed Central
83. Johnston, JE, Chau, K, Franklin, M, Cushing, L. Environmental justice dimensions of oil and gas flaring in south Texas: disproportionate exposure among hispanic communities. Environ Sci Technol 2020;54:6289–98. https://doi.org/10.1021/acs.est.0c00410.Search in Google Scholar PubMed PubMed Central
84. Sun, Y, Wang, D, Tsang, DC, Wang, L, Ok, YS, Feng, Y. A critical review of risks, characteristics, and treatment strategies for potentially toxic elements in wastewater from shale gas extraction. Environ Int 2019;125:452–69. https://doi.org/10.1016/j.envint.2019.02.019.Search in Google Scholar PubMed
85. Vengosh, A, Kondash, A, Harkness, J, Lauer, N, Warner, N, Darrah, TH. The geochemistry of hydraulic fracturing fluids. Proc Earth Planet Sci 2017;17:21–4. https://doi.org/10.1016/j.proeps.2016.12.011.Search in Google Scholar
86. Caserta, D, Graziano, A, Monte, GL, Bordi, G, Moscarini, M. Heavy metals and placental fetal-maternal barrier: a mini-review on the major concerns. Eur Rev Med Pharmacol Sci 2013;17:2198–206.Search in Google Scholar
87. Faniband, M, Lindh, CH, Jönsson, BA. Human biological monitoring of suspected endocrine-disrupting compounds. Asian J Androl 2014;16:5. https://doi.org/10.4103/1008-682x.122197.Search in Google Scholar
88. Iavicoli, I, Fontana, L, Bergamaschi, A. The effects of metals as endocrine disruptors. J Toxicol Environ Health, Part B 2009;12:206–23. https://doi.org/10.1080/10937400902902062.Search in Google Scholar PubMed
89. Loomis, D, Guyton, KZ, Grosse, Y, El Ghissassi, F, Bouvard, V, Benbrahim-Tallaa, L, et al.. Carcinogenicity of benzene. Lancet Oncol 2017;18:1574–5. https://doi.org/10.1016/s1470-2045(17)30832-x.Search in Google Scholar
90. Reutman, SR, LeMasters, GK, Knecht, EA, Shukla, R, Lockey, JE, Burroughs, GE, et al.. Evidence of reproductive endocrine effects in women with occupational fuel and solvent exposures. Environ Health Perspect 2002;110:805–11. https://doi.org/10.1289/ehp.02110805.Search in Google Scholar PubMed PubMed Central
91. Webb, E, Bushkin-Bedient, S, Cheng, A, Kassotis, CD, Balise, V, Nagel, SC. Developmental and reproductive effects of chemicals associated with unconventional oil and natural gas operations. Rev Environ Health 2014;29:307–18. https://doi.org/10.1515/reveh-2014-0057. 2014/12/06 ed.Search in Google Scholar PubMed
92. Miki, Y, Ono, K, Hata, S, Suzuki, T, Kumamoto, H, Sasano, H. The advantages of co-culture over mono cell culture in simulating in vivo environment. J Steroid Biochem Mol Biol 2012;131:68–75. https://doi.org/10.1016/j.jsbmb.2011.12.004.Search in Google Scholar PubMed
93. Berg, EL, Hsu, YC, Lee, JA. Consideration of the cellular microenvironment: physiologically relevant co-culture systems in drug discovery. Adv Drug Deliv Rev 2014;69:190–204. https://doi.org/10.1016/j.addr.2014.01.013.Search in Google Scholar PubMed
94. Bogdanowicz, DR, Lu, HH. Studying cell-cell communication in co-culture. Biotechnol J 2013;8:395. https://doi.org/10.1002/biot.201300054.Search in Google Scholar PubMed PubMed Central
95. Clift, MJ, Fytianos, K, Vanhecke, D, Hočevar, S, Petri-Fink, A, Rothen-Rutishauser, B. A novel technique to determine the cell type specific response within an in vitro co-culture model via multi-colour flow cytometry. Sci Rep 2017;7:1–15. https://doi.org/10.1038/s41598-017-00369-4.Search in Google Scholar PubMed PubMed Central
96. Danoy, M, Shinohara, M, Rizki-Safitri, A, Collard, D, Senez, V, Sakai, Y. Alteration of pancreatic carcinoma and promyeloblastic cell adhesion in liver microvasculature by co-culture of hepatocytes, hepatic stellate cells and endothelial cells in a physiologically-relevant model. Integr Biol 2017;9:350–61. https://doi.org/10.1039/c6ib00237d.Search in Google Scholar PubMed
97. Wikswo, JP. The relevance and potential roles of microphysiological systems in biology and medicine. Exp Biol Med 2014;239:1061–72. https://doi.org/10.1177/1535370214542068.Search in Google Scholar PubMed PubMed Central
98. Burkina, V, Rasmussen, MK, Pilipenko, N, Zamaratskaia, G. Comparison of xenobiotic-metabolising human, porcine, rodent, and piscine cytochrome P450. Toxicology 2017;375:10–27. https://doi.org/10.1016/j.tox.2016.11.014.Search in Google Scholar PubMed
99. Martignoni, M, Groothuis, GM, de Kanter, R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expet Opin Drug Metabol Toxicol 2006;2:875–94. https://doi.org/10.1517/17425255.2.6.875.Search in Google Scholar PubMed
100. Patisaul, HB, Fenton, SE, Aylor, D. Animal models of endocrine disruption. Best Pract Res Clin Endocrinol Metabol 2018;32:283–97. https://doi.org/10.1016/j.beem.2018.03.011.Search in Google Scholar PubMed PubMed Central
101. Colborn, T, Schultz, K, Herrick, L, Kwiatkowski, C. An exploratory study of air quality near natural gas operations. Hum Ecol Risk Assess 2014;20:86–105. https://doi.org/10.1080/10807039.2012.749447.Search in Google Scholar
102. Litovitz, A, Curtright, A, Abramzon, S, Burger, N, Samaras, C. Estimation of regional air-quality damages from Marcellus Shale natural gas extraction in Pennsylvania. Environ Res Lett 2013;8:9. https://doi.org/10.1088/1748-9326/8/1/014017.Search in Google Scholar
103. Swarthout, RF, Russo, RS, Zhou, Y, Miller, BM, Mitchell, B, Horsman, E, et al.. Impact of Marcellus Shale natural gas development in southwest Pennsylvania on volatile organic compound emissions and regional air quality. Environ Sci Technol 2015;49:3175–84. https://doi.org/10.1021/es504315f.Search in Google Scholar PubMed
104. Vinciguerra, T, Yao, S, Dadzie, J, Chittams, A, Deskins, T, Ehrman, S, et al.. Regional air quality impacts of hydraulic fracturing and shale natural gas activity: evidence from ambient VOC observations. Atmos Environ 2015;110:144–50. https://doi.org/10.1016/j.atmosenv.2015.03.056.Search in Google Scholar
105. Upadhyay, S, Palmberg, L. Air-liquid interface: relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol Sci 2018;164:21–30. https://doi.org/10.1093/toxsci/kfy053.Search in Google Scholar PubMed
106. Chen, L, Lippmann, M. Inhalation toxicology methods: the generation and characterization of exposure atmospheres and inhalational exposures. Curr Protoc Toxicol 2015;63:24–4. https://doi.org/10.1002/0471140856.tx2404s63.Search in Google Scholar PubMed PubMed Central
107. Indorf, P, Patzak, A, Lichtenberger, F. Drug metabolism in animal models and humans: translational aspects and chances for individual therapy. Acta Physiol 2021;233:5. https://doi.org/10.1111/apha.13734.Search in Google Scholar PubMed
108. Zhuang, X, Lu, C. PBPK modeling and simulation in drug research and development. Acta Pharm Sin B 2016;6:430–40. https://doi.org/10.1016/j.apsb.2016.04.004.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/reveh-2024-0076).
© 2024 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Reviews
- Analytical methods, source, concentration, and human risks of microplastics: a review
- Solid fuel use and low birth weight: a systematic review and meta-analysis
- The human health effects of unconventional oil and gas (UOG) chemical exposures: a scoping review of the toxicological literature
- WHO to build neglect of RF-EMF exposure hazards on flawed EHC reviews? Case study demonstrates how “no hazards” conclusion is drawn from data showing hazards
- The role of environmental pollution in the development of pulmonary exacerbations in cystic fibrosis: a narrative review
- Semi-IPN polysaccharide-based hydrogels for effective removal of heavy metal ions and dyes from wastewater: a comprehensive investigation of performance and adsorption mechanism
- A structured review of the associations between breast cancer and exposures to selected organic solvents
- A review of the potential adverse health impacts of atrazine in humans
- Comprehensive approach to clinical decision-making strategy, illustrated by the Gulf War
- A systematic review and quality assessment of estimated daily intake of microplastics through food
- Adapting to heat-health vulnerability in temperate climates: current adaptation and mitigation responses and future predictions in Aotearoa New Zealand
- Evaluation of the impact of environmental pollutants on the sex ratio: a systematic review
- A critical review on the toxicological and epidemiological evidence integration for assessing human health risks to environmental chemical exposures
- The association between screen exposure and autism spectrum disorder in children: meta-analysis
- The association between maternal perfluoroalkylated substances exposure and neonatal birth weight: a system review and meta-analysis
- School built environment and children’s health: a scientometric analysis
- Letter to the Editors
- Underground power lines as a confounding factor in observational studies concerning magnetic fields and childhood leukemia
- A critical appraisal of the WHO 2024 systematic review of the effects of RF-EMF exposure on tinnitus, migraine/headache, and non-specific symptoms
Articles in the same Issue
- Frontmatter
- Reviews
- Analytical methods, source, concentration, and human risks of microplastics: a review
- Solid fuel use and low birth weight: a systematic review and meta-analysis
- The human health effects of unconventional oil and gas (UOG) chemical exposures: a scoping review of the toxicological literature
- WHO to build neglect of RF-EMF exposure hazards on flawed EHC reviews? Case study demonstrates how “no hazards” conclusion is drawn from data showing hazards
- The role of environmental pollution in the development of pulmonary exacerbations in cystic fibrosis: a narrative review
- Semi-IPN polysaccharide-based hydrogels for effective removal of heavy metal ions and dyes from wastewater: a comprehensive investigation of performance and adsorption mechanism
- A structured review of the associations between breast cancer and exposures to selected organic solvents
- A review of the potential adverse health impacts of atrazine in humans
- Comprehensive approach to clinical decision-making strategy, illustrated by the Gulf War
- A systematic review and quality assessment of estimated daily intake of microplastics through food
- Adapting to heat-health vulnerability in temperate climates: current adaptation and mitigation responses and future predictions in Aotearoa New Zealand
- Evaluation of the impact of environmental pollutants on the sex ratio: a systematic review
- A critical review on the toxicological and epidemiological evidence integration for assessing human health risks to environmental chemical exposures
- The association between screen exposure and autism spectrum disorder in children: meta-analysis
- The association between maternal perfluoroalkylated substances exposure and neonatal birth weight: a system review and meta-analysis
- School built environment and children’s health: a scientometric analysis
- Letter to the Editors
- Underground power lines as a confounding factor in observational studies concerning magnetic fields and childhood leukemia
- A critical appraisal of the WHO 2024 systematic review of the effects of RF-EMF exposure on tinnitus, migraine/headache, and non-specific symptoms

