Abstract
Pollution of ecosystems with potentially toxic elements (PTEs) has become a global problem with serious consequences for public health. The PTEs are hazardous to humans owing to their longevity, toxicity, and ability to accumulate in the biotic environment. As most PTEs cannot be degraded microbially or chemically, they can persist in soils for a long time. Besides posing a threat to landsphere, they may be transported to surrounding environmental spheres through movement of water, atmospheric circulation, and biological transmission. This can severely affect the ecological equilibrium. Accumulation of PTEs in soils pose serious health hazards to higher organisms leading to various diseases and disorders and significant relationships exist between the occurrence of PTEs and the toxic effects in humans. In natural soils, PTEs accumulate due to weathering of rocks and ores. Furthermore, locally or regionally significant accumulation of PTEs in soils may occur from industrial goods, pesticides and paints, municipal and industrial waste, fertilizer application, mining activities and atmospheric deposition. In response to the growing need to address PTE contamination, remediation methods have been developed employing mechanical, physico-chemical or biological based technologies. In this review, we discuss sources, sinks, pathways and mitigation measures related to natural and anthropogenic PTEs. We focus on As, Cd, Cr, Hg and Pb which are highly toxic and perform no physiological functions in biota. Further, these are the most widely studied PTEs.
Introduction
Potentially toxic elements (PTEs) include heavy metals having density greater than 5 g cm−3 and non-metals (Figure 1). Some of them are persistent and can be highly toxic to plants, animals and humans [1, 2]. Several biologically essential elements, called micronutrients or trace elements, are also categorized as heavy metals (Figure 1). The latter become toxic when they occur at high concentrations and are also bioavailable [3, 4]. Non-metals such as As and Se are classified as PTEs as well. Like other micronutrients, Se at low concentrations performs a number of functions in plants and humans [3].
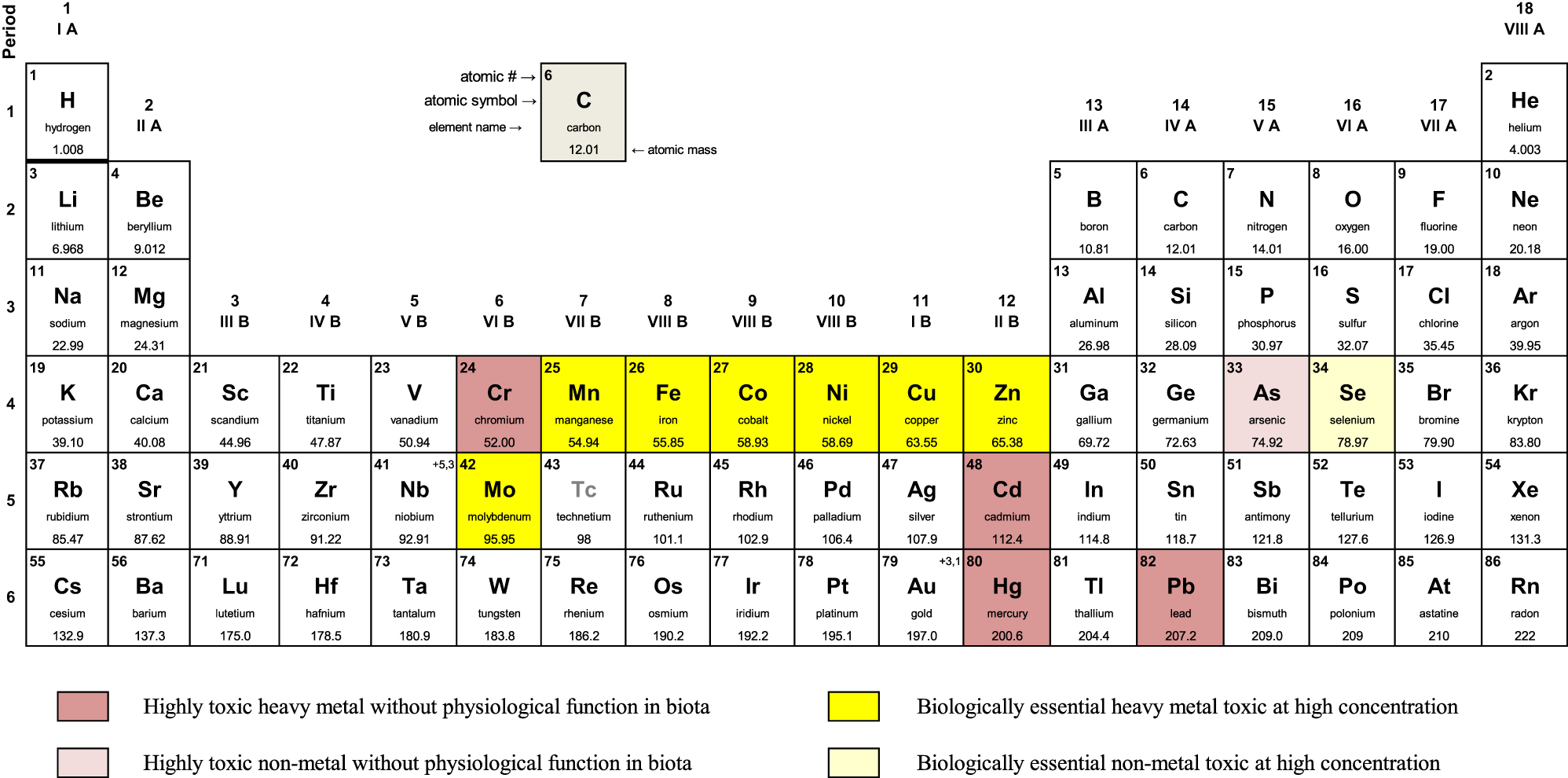
Periodic table of elements highlighting PTEs according to their physiological functions and toxicity.
In uncontaminated soils, trace elements are commonly present in concentrations <1,000 mg kg−1, thus being infrequently toxic [5]. The difference between essential and poisonous concentrations for some of these elements may be a few mg kg−1 only [6]. Most PTEs are widely distributed in the environment and may accumulate in the biosphere.
Potentially toxic elements can accumulate in soils during pedogenesis due to weathering of rocks. The geogenic background of soils is thus determined by PTE concentrations in the underlying parent materials. Besides geogenic and pedogenic processes, PTEs can originate from anthropogenic activities including emissions from industrial areas, disposal of hazardous municipal and mining waste, use of leaded paints and gasoline, application of pesticides, mineral and organic (e.g. animal manures, sewage sludge, compost) fertilizers, wastewater irrigation, spillage of petrochemicals, and atmospheric deposition of dusts from burning of coal [7], [8], [9], [10]. These activities often modify cycling and level of PTEs in atmosphere, pedosphere, hydrosphere and biosphere.
More than 10 million sites were reported to be contaminated with substances potentially harmful and highly relevant for human health worldwide. Over 50 % of them have been polluted with PTEs [11]. In Western Europe alone, about 300,000 sites have been contaminated with PTEs [12]. In the USA, 600,000 PTE-polluted sites require reclamation [13]; about 100,000 ha of arable land, 55,000 ha of pasture land and 50,000 ha of forest area have been deteriorated as a consequence of PTE contamination [14].
About one sixth of the arable land area of China has been polluted with PTEs [15]. In a nationwide soil survey in China, 16.1 % of soil samples were found to exceed recommended standards developed by China’s Ministry of Environmental Protection, which drew public attention to the severity of soil contamination in China [16]. Thirteen areas in China were identified by the “Third National Survey on causes of Death” as so-called cancer villages. In four of these, cancer death rates exceeded the national average [17]. Raw municipal sewage and untreated polluted effluents from industry are often disposed of into agricultural fields in peri‐urban areas in Pakistan, India and Bangladesh. These severely contaminated waste waters are commonly used for growing fodder crops and vegetables [18].
Potentially toxic elements are a serious threat to the functioning of ecosystems, as they are toxic and persistent. Bioavailable PTE fractions are most likely to harm the soil microflora, plants, animals and humans [19]. In soils, the biodegradation of organic contaminants can be strongly inhibited, as exposure to PTEs causes reduction of microbial activity and diversity [20], [21], [22].
Protection and remediation of soils polluted with PTEs require environmental risk assessment including PTE toxicity monitoring, especially for soil reclamation and/or for growing fodder and food crops [23]. Technologies for remediation of PTE-contaminated sites frequently include immobilization, soil washing and phytoremediation [24]. Successful application of these methods necessitates knowledge of the pollution source, basic chemistry, and environmental as well as health risks of PTEs. Environmental risk assessment can help decision makers in managing contaminated sites in a cost-effective way while preserving ecosystem and public health.
Sources of PTEs
Natural sources
Geochemical background in rocks and soils
Concentrations of PTEs in soil depend on the kind of parent materials from which soils have developed [25], [26], [27]. Depending on the mineral composition of the underlying rocks, soils vary widely in total concentrations of PTEs [28]. Table 1 summarizes ranges of PTE concentrations in rocks and uncontaminated naturally occurring soils.
Concentrations (mg kg−1) of PTEs (ranges) in igneous and sedimentary rocks and uncontaminated soils (adapted from [9], compiled from [26], [27], [28], [29], [30]).
| PTE | Basaltic igneous | Granitic igneous | Shales and clays | Black shales | Soils |
|---|---|---|---|---|---|
| As | 0.2–10 | 0.2–13.8 | n.i. | 1–900 | 5–10 |
| Cd | 0.006–0.6 | 0.003–0.18 | <11 | 0.3–8.4 | 0.01–0.7 |
| Co | 24–90 | 1–15 | 5–25 | 7–100 | 1–40 |
| Cr | 40–600 | 2–90 | 30–590 | 2–1,000 | 5-3,000 |
| Cu | 30–160 | 4–30 | 18–120 | 20–200 | 2–100 |
| Mo | 0.9–7 | 1–6 | n.i. | 1–300 | 0.2–5 |
| Ni | 45–410 | 2–20 | 20–250 | 10–500 | 10–100 |
| Pb | 2–18 | 6–30 | 16–50 | 7–150 | 2–200 |
| Zn | 48–240 | 5–140 | 18–180 | 34–1,500 | 10–300 |
-
n.i., not indicated.
Concentrations of As, Pb and Zn in sandstone were reported to range between 0.6 and 9.7, <1–31 and 2–41 mg kg−1, respectively [9]. Potentially toxic elements present in primary and secondary minerals are released by weathering (Figure 2) of the parent material. They may be reprecipitated as, or incorporated into, newly formed minerals like clays and Fe and Mn (hydr)oxides. A part may be adsorbed onto oxides and mineral surfaces, or bound by soil organic matter (SOM) [31]. In soil solution, PTEs become available to soil biota and plants.
![Figure 2:
Interactions between different forms of PTEs in soils (adapted from [9]).](/document/doi/10.1515/reveh-2022-0161/asset/graphic/j_reveh-2022-0161_fig_002.jpg)
Interactions between different forms of PTEs in soils (adapted from [9]).
Some pedogenetic processes modify the regolith and thus redistribute PTEs in soils. The influence of the parent material on PTEs decreases with increasing soil development [32]. Soils may be affected by displacement processes within the profile (in particular soil colloids such as SOM, phyllosilicates and (hydr)oxides), by surface runoff, leaching, deposition, as well as turbation processes [33]. Each of these processes may have an influence on distribution and concentration of PTEs in soils [34]. As a consequence of such processes, PTE concentrations and forms in soils to some extent may differ from those in the soil forming rocks.
Airborne sources
The most important natural airborne input of PTEs to soils is from deposition of volcanic ash and mineral dust. Volcanoes were reported to emit particles containing high levels of Al, As, Cu, Hg, Ni, Pb and Zn, together with toxic gases [35]. Sahara dusts have high amounts of Fe and lower levels of Cr, Ni, Pb and Zn [36]. Steppe and forest fires as well as marine aerosols also contribute to the transport of some PTEs in ecosystems. Table 2 gives an overview of the emissions of PTEs from natural sources.
| PTE | Volcanic particles | Windblown dust | Vegetation | Forest fires | Sea salt | Total |
|---|---|---|---|---|---|---|
| Cd | 0.5 | 0.25 | 0.2 | 0.01 | 0.002 | 0.96 |
| Co | 1.4 | 4 | n.i. | n.i. | n.i. | 5.4 |
| Cu | 4 | 12 | 2.5 | 0.3 | 0.1 | 18.9 |
| Cr | 3.9 | 5 | n.i. | n.i. | n.i. | 8.9 |
| Hg | 0.03 | 0.03 | n.i. | 0.1 | 0.003 | 0.16 |
| Ni | 3.8 | 20 | 1.6 | 0.6 | 0.04 | 26 |
| Pb | 6.4 | 10 | 1.6 | 0.5 | 0.1 | 18.6 |
| Zn | 10 | 25 | 10 | 0.5 | 0.02 | 45.5 |
-
n.i., not indicated.
Anthropogenic sources
Airborne sources
Besides natural sources, anthropogenic airborne sources of PTEs also exist. Refuse and coal burning, metal smelting, and emissions from automobiles are the most important anthropogenic sources [32, 38]. Potentially toxic elements from airborne sources are usually released as particulate matter. Cadmium, As and Pb are volatile at high temperatures and may convert to oxides and subsequently precipitate as fine particulates [39]. Emissions from chimneys (stack emissions) may be spread over large areas by air flow until precipitation (dry or wet) removes them from the atmosphere. Fugitive emissions are often distributed over smaller areas. Compared to stack emissions, contaminant concentrations are generally lower in fugitive ones [24].
Particles in smoke from fires and other emissions from chimneys can be deposited on land or sea. Fossil fuels generally contain some PTEs. Very high Cd, Pb, and Zn concentrations have been found in soils and plants next to smelting works [24, 40]. Another source of pollutant deposition is the emission of lead from burning of petrol containing Pb which increases concentrations of the pollutant in soils of urban areas and in the vicinity of roads. Zinc and Cd contained in lubricant oils and in tyres may also be deposited on soils at smaller distances from roads [41]. The median values of global emissions of Cd, Cu, Pb, and Zn into soils were estimated 22, 954, 796 and 1,372 × 103 Mg year−1, respectively [9]. Over half of these amounts may be associated with smelting activities and metal mining [42, 43].
Arsenic in the atmosphere usually occurs in particulate form emanating from natural, (forest fires and volcanic eruptions) as well as anthropogenic sources, such as tobacco smoke, burning of fossil fuels and automobile exhaust. The residence time of these particles (with a diameter of commonly <2 µm) is approximately 9 days, during which period they are transported by winds until they reach the earth. Volcanic and microbial activity, and the burning of fossil fuels were estimated to release 3,000, 20,000, and 80,000 Mg of As year−1 to the atmosphere, respectively [44].
Mining
Civilization is strongly dependent on minerals containing metals which are available in the earth’s crust. Inter alia, these include gold (Au), silver (Ag), platinum (Pt), Fe, Cu, Cr, Pb, Ni, tin (Sn), vanadium (V), Zn, beryllium (Be), niobium (Nb), tungsten (W), zirconium (Zr), boron (B), antimony (Sb), As, germanium (Ge), carbon (C), sulphur (S), bismuth (Bi), uranium (U) and rare earth metals comprising 14 elements of the lanthanide series (from lanthanum to ytterbium) [45]. Wherever sufficiently large deposits occur, exploitable minerals are extracted through surface, marine and underground mining activities.
Mining activities including crushing, grinding, washing and smelting of mining materials and other metal extraction and treatment processes produce high amounts of mining wastes. The latter in many cases contain high concentrations of PTEs, including Al, As, Cd, Cu, Hg, Mo, U and Zn [9, 46]. Deposits located outside the mining area can have detrimental effects on soils and on the environment [47]. The negative impact is mainly due to the presence of high volumes of mine tailings that excert adverse effect on plant species growing on them. The waste material frequently exhibits (extremely) low pH [48], low levels of plant nutrients [23], high metal concentrations [49] and low water holding capacity [50]. Levels of PTEs at concentrations exceeding drinking water criteria were found in wells located near mining sites [51]. Release of PTEs from mining sites occurs mainly through water erosion and drainage of waste deposits. Potentially toxic elements reach surface waters by mine drainage, discharge of mining or processing waste, tailings dam failures and remobilization of metals in mining-contaminated alluvial soils next to rivers. Important point sources of soil pollution with PTEs (e.g. As, Cd and Cr) also include contamination from smelter emissions as well as wind-blown dust from mine tailings and smelter slag dumps [52, 53]. Artisanal small-scale gold mining, often carried out under hazardous conditions, has expanded during the last decades in some world regions. In many developing countries, extraction of Au by using Hg has been a way of poverty reduction for millions of people. In this procedure, Hg is mixed with Au-containing ores which forms Hg-Au amalgam. By subsequent heating, Hg is vaporized to obtain Au and a part of Hg reaches surface waters and soils. An estimated 1,000 Mg of Hg year−1 from at least 70 different countries is released into the environment due to Au mining, corresponding to about a third of the total global anthropogenic Hg release [54]. Of this amount, about 400 Mg year−1 is present as airborne elemental Hg [55].
Fertilizers
Inorganic and organic fertilizers are the most important sources of PTEs in agricultural land. Each year, high amounts of fertilizers are applied to soils in intensive farming systems to provide adequate nutrients (mainly N, P, K) for crop production. Many fertilizers contain trace amounts of PTEs (Table 3) and continued fertilizer application may lead to their significant accumulation in soils [56]. Liming also increases PTE levels of soils.
Concentration ranges (mg kg−1) of PTEs present in nitrogen and phosphate fertilizers and lime (adapted from [9, 36]).
| PTE | Nitrogen | Phosphate | Lime |
|---|---|---|---|
| Cr | 3.2–19 | 66–245 | 10–15 |
| Ni | 7–34 | 7–38 | 10–20 |
| Cu | n.i. | 1–300 | 2–125 |
| Zn | 1–42 | 1–1,450 | 10–450 |
| Cd | 0.05–8.5 | 1.1–190 | 0.04–0.1 |
| Pb | 2–120 | 4–1,000 | 20–1,250 |
-
n.i., not indicated.
Most of the organic fertilizers are considered valuable nutrient source which may partly substitute mineral fertilizers. In addition, they are important sources for preservation and build up of SOM. However, application of organic fertilizers (e.g., livestock manures, composts, municipal sewage sludge) may also lead to PTE accumulation in soils [24]. Manures from pig, cattle and poultry are commonly applied either as slurries or solids. There are also cases of direct disposal of liquid manures into surface waters which can severely affect the quality of potential drinking water. In pig and poultry industry, Cu and Zn are added to diets which may have the potential of causing PTE accumulation in soils [57]. The use of As and Cr as feed additives is also well-known [58, 59]. Generally, regions with intensive livestock farming and excessive animal waste disposal are particularly concerned by PTE pollution of soils [58].
Waste waters and sewage sludge
Waste water arising from domestic and commercial activities is perhaps the largest source of PTEs in surface waters worldwide [46]. Domestic effluents consist of untreated waste substances passed over sewage outfalls, mechanically treated waste waters and water which has passed through biological treatment plants. Application of detergents creates a possible PTE accumulation risk, as most enzyme detergents contain trace amounts of boron (B), Co, Cr, Fe, Sr and Zn [60]. It is estimated that globally 20 × 106 ha of arable land are irrigated with waste water [9]. Agriculture based on wastewater irrigation in several cities of Africa and Asia accounts for about 50 % of the vegetable supply to urban areas [61]. Even though PTE concentrations in wastewater effluents are relatively low, long-term irrigation of soils with such irrigation water can result in hazardous PTE accumulation. There is an increasing awareness that runoff from urban areas presents a major source of PTEs to surface waters [46].
Sewage sludge, which is produced by wastewater treatment processes, is another important source of PTE accumulation in soils. For example, in the USA more than half of the total amount of sewage sludge (5.6 × 106 Mg dry matter) produced per year is applied to land [24] and in the EU, more than 30 % of the sewage sludge is used for fertilization of soils in agriculture [62]. In Australia, over 175,000 Mg year−1 (dry matter) of sewage sludge is produced, most of it is applied to agricultural land [63].
Pesticides
Several pesticides, containing PTEs are used to control diseases of vegetables, cereals and fruit crops. Copper-containing fungicides such as Cu sulphate (Bordeaux mixture) and Cu oxychloride, also used in organic farming, are typical examples among others [64]. These fungicides are used most frequently in orchard plantations. Due to the use of PTE-containing pesticides, soils of orchard plantations may be highly contaminated, in particular with As, Cu, Fe, Hg, Pb and Zn [36]. In the UK about 10 % of the substances that have been approved for use as pesticides were based on chemicals containing Cu, Hg, Mn, Pb, or Zn. In orchards in Canada, for many decades Pb arsenate was used to control of harmful insects; correspondingly, soils were found to be highly polluted with As, Pb and Zn [46]. Arsenic containing chemicals were also applied extensively to control cattle ticks and pests in banana [24]. In Australia and New Zealand, timbers have been treated with formulations of As, Cr and Cu, and there are now numerous areas where soil concentrations of these elements greatly exceed tolerable concentrations. Part of the soil pollution with PTEs may also originate from irrigation with contaminated river, lake, deep well or canal water [36].
Natural and anthropogenic sources
Several natural together with anthropogenic sources are deemed responsible for As pollution of groundwater [65]. Arsenic occurs as a major constituent of more than 200 minerals [66]. The desorption and dissolution of naturally occurring As-bearing minerals and alluvial sediments can result in high As concentration in groundwater in alluvial plains and river deltas even if the concentration of the metalloid in the solid phase of the sediment is not high [67]. The presence of As in groundwater in high concentrations may be associated with ore deposits because As occurs predominantly in sulfidic minerals like pyrite and arsenopyrite [68]. The most important anthropogenic sources of groundwater contamination are mining activities, use of As-containing pesticides in agriculture, application of wood preservatives, and burning of fossil fuels [69].
Elevated levels of As in groundwater have been documented in Argentina, Chile, China, Mexico, in the USA [70], [71], [72], Bangladesh [73], the Indian State of West Bengal [9], and Vietnam [74]. Major reports of As-contaminated drinking water have also emerged from countries in Europe and Asia Minor such as Slovakia, Hungary, Croatia, Serbia, Spain, Romania, Greece and Turkey [75].
Pathways and sinks of PTEs
Potentially toxic elements may contaminate soils and plants, the atmosphere, rainwater, freshwater bodies such as rivers, lakes and streams, and groundwater. In case of contaminated freshwater bodies, PTEs may bioaccumulate in fish. In terrestrial ecosystems, such pollution leads to PTE bioaccumulation in soil biota, plants and animals. Since most humans are omnivorous they may be at risk through consumption of PTE-polluted plant products and meat. A schematic diagram representing the major pathways of PTEs is presented in Figure 3.
![Figure 3:
Major pathways of PTEs in the environment (adapted from [9]).](/document/doi/10.1515/reveh-2022-0161/asset/graphic/j_reveh-2022-0161_fig_003.jpg)
Major pathways of PTEs in the environment (adapted from [9]).
The soil–to human transfer of PTEs via the food chain strongly depends on a number of soil properties and processes. The major exposure route by which humans can take up PTEs is through soil and water movement to food plants for consumption [76]. Humans may also be endangered through the consumption of PTE-contaminated meat and meat products. Examples of PTE concentrations in selected terrestrial food products are presented in Table 4.
Concentrations (mg kg−1) of PTEs found in selected food products (compiled from [9]).
| PTE | Cereal products | Lettuce | Other vegetables | Apples | Other fruits | Meat and meat products |
|---|---|---|---|---|---|---|
| As | 0.1–3.53 | 0.01–3.78 | 0.01–2.95 | 0.04–1.72 | 0.10–1.20 | 0.008–0.32 |
| Cd | 0.03 | n.i. | 0.067 | n.i. | 0.0039 | 0.0974 |
| Cr | 0.04–0.22 | n.i. | 0.03–0.23 | n.i. | 0.02–0.19 | 0.11–0.23 |
| Hg | 0.0001–0.030 | 0.0005–0.0028 | 0.0001–0.008 | 0.0004–0.003 | n.i. | 0.001–0.058 |
| Pb | 0.020–0.047 | 0.034–0.042 | 0.007–0.023 | 0.010–0.015 | n.i. | 0.002–0.136 |
-
n.i., not indicated.
The ranges of PTE concentrations in the products generally correlate with contamination levels of soils. More details of exposure routes to humans, as well as risk assessment, clinical effects and therapy of PTE-associated diseases have been recently reviewed by [76].
Soils are the major sink for most PTEs released to the environment [24]. The majority of PTEs of geogenic origin may have low mobility and bioavailability in soil, as they are sparingly soluble. Soil pollution is commonly defined on the basis of national or regional environmental laws and regulations [77]. At contaminated sites As, Cd, Cr, Cu, Hg, Ni, Pb and Zn are frequently found [78]. Background PTE concentrations in young or immature soils are determined by their concentrations in the underlying geologic materials [25]. In more developed soils, pedogenetic processes commonly make the correlation less obvious. Solubility and bioavailability of PTEs in soil determine their soil-to plant (–to animal) -to human transfer. However, PTE bioavailability in soil is highly dependent on processes such as adsorption/desorption, precipitation/dissolution, and PTE ligand formation [77]. Among the factors and soil properties that govern these processes are elemental speciation, pH, SOM content, clay content and clay mineral quality, cation and/or anion exchange capacity, oxide type and content, and redox potential [79]. Plant species and root exudates may also influence PTE availability [80]. Potentially toxic elements in soils are commonly adsorbed by SOM and clay (in particular three-layer clay minerals), and are not microbially or chemically degradable. Accordingly, they persist in soils for a long time [81], with residence times up to thousands of years. However, if changes in their valence and chemical form occur, small portions may become soluble under certain conditions. The soil–to human transfer of PTEs via the food chain strongly depends on a number of soil properties and processes [80]. Added PTEs of anthropogenic origin tend to be more mobile initially, with decreasing bioavailability over time [82]. However, PTE mobility may increase due to decreasing soil pH which may cause increased cationic metal solubility, and thus increased plant PTE uptake. Soil organic matter could also enhance PTE solubility. The organic component of soil provides colloidal phases that increase PTE binding capacity especially in soils with high sand and poor clay content. In case of fast decomposition of SOM (e.g. global warming or land use changes that induce SOM decomposition), soil binding capacity will be reduced, and in turn PTEs previously adsorbed by SOM may be released into soil solution and thus easily be taken up by plants [82].
Numerous plant species (about 450) act as hyperaccumulators of PTEs. They represent only <0.2 % of all known species but have a wide taxonomic range [83]. Crops can be negatively affected by PTEs because of their interference with metabolic processes within the plant tissue, leading sometimes to death [84]. Since the elements As, Hg and Pb are strongly bound to soil colloids, they may be absorbed particularly by subsoil plant organs (i.e. plant roots) and are not readily translocated to aboveground plant tissues. Therefore, they pose risks to human health only when root vegetables are grown on polluted sites. In contrast, elements such as Cd, Cu, Mn, Ni and Zn are readily taken up by plants and thus also affect aboveground plant organs [9].
The accumulation of PTEs in aquatic sediments and environments is of major global concern, because of toxicological effects on aquatic biota and biomagnification in the food chain. A diverse range of PTEs occur in aquatic environments including As, Cd, Cr, Hg and Pb [85]. Industrial and domestic effluents, mining waste water, agricultural run-off as well as atmospheric deposition are sources of PTEs in lakes and rivers [86]. The release of untreated industrial effluents into aquatic bodies is a major source of pollution of surface water and groundwater [87]. Aquatic environments may serve as either a direct or indirect sink of PTEs, which may be labile and vary between the dissolved, particulate, and biological phases [88]. Sediments act as the main pool of PTEs in aquatic systems. Adsorption, desorption and concentrations of PTEs in sediments are affected by processes and factors such as hydrodynamic interactions, redox reactions, temperature, pH, mineralogy, particle size and organic matter content [89].
Mitigation measures
Reduction of PTE production and emission
Reduction of PTE production and emission, including recycling of used metals are important building blocks for minimizing human exposure to PTEs. Some important measures are summarized in Table 5.
Measures for reducing PTE production and emission (adapted from [90]).
| Measure | Target PTE, s |
|---|---|
| Minimizing emissions and discharges from mining, industry and waste management | Cd, Cr, Pb |
| Safe and effective handling and recycling | Cd, Cr, Pb, Hg |
| Eliminating production and use in mining and industry as well as in thermometers and sphygmomanometers | Hg |
| Eliminating the use in toys, jewlery and plastics | Cd |
| Restricting use of (in)organic fertilizers containing PTEs | Several PTEs |
| Eliminating non-essential uses (e.g. in paint); reductions in the use in petrol | Pb |
| Promoting the use of clean energy sources that do not rely on burning of coal | As and Cd |
Educating the public about the importance of safe disposal of batteries, computers and mobile phones are measures especially for reducing production of several PTEs and rare earth metals. If applied successfully, these measures can result in significant reduction in the use and release of PTEs. Besides the measures listed in Table 5, it is essential to promote reduction of occupational exposures and safe working conditions for workers engaged in the production and treatment of PTE-containing goods. Reduction in human exposure to As can be achieved by identifying water supplies that exceed the WHO provisional guideline or national permissible limits and prohibiting water withdrawl from such sources [91]. Safe groundwater, the use of rainwater and treated surface water provide an alternative to the use of contaminated water sources. Other options include the use of As removal technologies and dilution of high As-content water with low As-content water that must be microbiologically safe [90]. Minimizing PTE application to soils with (in)organic fertilizers and pesticides would result in substanial reduction of PTE accumulation in agricultural soils [9].
Measures to reduce PTE accumulation in food crops
Large crop production areas are affected by PTE pollution worldwide. Hot spots are located around industrial sites, in and close to big cities and in the vicinity of mining areas and smelting plants. Agriculture in such areas faces major problems due to potential PTE uptake by forage plants and food crops and subsequent transfer to the food chain [91]. The consumption of plants grown in local fields polluted with PTEs may present a serious health risk for animals and humans. It is obvious that selection of food crops is effective for reducing the transfer to the human food chain [92]. An overview of the relative PTE uptake by some important food crops is given in Table 6.
| Relative uptake | Crops |
|---|---|
| Very low | Beans, melon, pea, pepper, tomato |
| Low | Broccoli, brussels sprouts, cauliflower, celery, maize |
| Medium | Cabbage, potato, radish, red beet, turnip |
| High | Carrot, chicory, lettuce, mangold, spinach |
A number of studies focused on Cd accumulation in soils due to it’s toxicity and high level of bioavailability. Uptake of Cd by crop species increases in the order: grain crops <root vegetables <leaf vegetables [93]. For demonstrating the relationship between the PTE content of soils and crops growing on them, the transfer factor can be used to assess plants’ capability to take up PTEs from the soil [94]. Transfer factors for Cd are commonly low for bean seeds and winter rye grain (Table 7).
| Crop | Cd | Cu | Ni | Pb | Zn |
|---|---|---|---|---|---|
| Bean seeds | 0.08 | 0.14 | 0.28 | 0.04 | 0.25 |
| Winter rye grain | 0.16 | 0.12 | 0.10 | 0.01 | 0.61 |
| Maize cob | 0.30 | 0.11 | 0.15 | 0.01 | 0.68 |
| Maize straw | 1.09 | 0.10 | 0.06 | 0.09 | 1.53 |
| Potato tuber | 0.33 | 0.18 | 0.14 | 0.05 | 0.21 |
| Tomato fruit | 0.38 | 0.18 | 0.15 | 0.03 | 0.21 |
| Onion tuber | 0.47 | 0.06 | 0.09 | 0.01 | 0.54 |
| Beet leaves | 5.55 | 0.25 | 0.52 | 0.07 | 6.04 |
| Beet body | 0.84 | 0.23 | 0.28 | 0.02 | 1.18 |
| Spinach leaves | 5.00 | 0.22 | 0.25 | 0.13 | 1.27 |
| Celery leaves | 2.82 | 0.15 | 0.15 | 0.04 | 1.22 |
| Celery roots | 2.09 | 0.32 | 0.18 | 0.04 | 0.74 |
| Lucerne shoot | 1.73 | 0.18 | 0.60 | 0.02 | 1.66 |
-
Transfer factor, PTE concentration in plant/PTE concentration in soil (concentrations are expressed in mg kg−1).
Variation in PTE accumulation can be observed between different plant organs. Compared to vegetative plant parts, generative organs are commonly less concerned by PTE accumulation. Accordingly, high Cd values were found in leaves of beet, spinach and celery. Besides leaves, high PTE concentrations are also found in roots, whereas the lowest are commonly observed in seeds [95]. Results from a field study demonstrate that the transfer factor of Cd from soil to plant was twice as high for straw compared to grain [96]. Differences in transfer factors for Cu, Ni and Pb with respect to various plant organs were not as pronounced compared to Cd. As leaffy vegetables including spinach have generally high transfer factors [9], it should be concluded that these species are not to be cultivated on contaminated sites.
For agricultural use of PTE-polluted soils, the cultivation of industrial plants has been considered as a reasonable option. Especially fibre plants such as cotton, hemp and flax, and energy crops like reed canary grass and Salix trees were found to represent a meaningful use on such sites [9, 97].
PTE immobilization in soils
Inorganic and organic soil amendments have been widely used as agents for immobilizing PTEs [98]. Mechanisms reducing PTE bioavailability in soils include precipitation, complexation, redox reactions, ion exchange, and electrostatic interaction. Soil properties such as pH, clay, SOM and sesquioxides content as well as processes such as sorption/desorption and redox reactions, are important factors influencing the amendments’ efficiency for immobilizing PTEs in soil.
Immobilization with inorganic amendments
Limes, phosphates, and industrial co-products are suitable PTE fixation agents among numerous inorganic treatment options [9]. Liming is a common practice to restore and maintain soil pH and thus to overcome problems related to soil acidification. Liming materials including CaO, Ca(OH)2, CaMgCO3, and CaCO3, and phosphate-containing substances such as CaHPO3, Ca(H2PO3)2, K2HPO4, H3PO4, and (NH4)HPO4 are used for the in situ immobilization PTEs and to reduce PTE plant uptake. CaO was found to be more effective in immobilizing PTEs compared to other liming materials because of its high reactivity and the distinct pH effect [99]. For the remediation of As-contaminated soil and water, oxides of Fe and Mn have been used; Fe oxides act as absorbents and Mn oxides as oxidants. As the oxidation of extremely toxic As3+ by Fe3+ is slow, the use of Fe2+ (instead of Fe3+) can help to accelerate the process [100].
Fly ash and slag from thermal power plants are other inorganic amendments suitable for PTE immobilization. In addition, clay minerals such as bentonite, zeolite, sepiolite and palygorskite can be applied [101]. Clay minerals would have better PTE immobilizing effects by increasing soil pH due to generation of elevated negative surface electric charge [102, 103].
Immobilization with organic amendments
Organic soil amendments are known to increase SOM content, thereby enhancing soil’s ability to hold moisture by increasing field capacity and to store higher amounts of plant nutrients. Biosolids, compost, biochar, farmyard manure, and/or crop residues may effectively reduce the bioavailability of PTEs in soils by enhancing processes such as adsorption, complexation and precipitation [98, 104]. An additional advantage of this treatment is that most amendments are available in large quantities and inexpensive [105]. With high SOM contents, PTEs may also be retained against crop uptake and leaching from soil [106]. The use of organic soil amendments is thus an opportunity to reclaim contaminated soils. Another positive effect is that organic materials are effectively reused and are simultaneously removed from the waste stream [107].
Remediation of PTE-contaminated soils
The methods used for PTE removal are partly physico-chemical or mechanically-based, including soil replacement, soil washing, thermal desorption, electrochemical remediation, solidification and vitrification. In contrast, phytoremediation makes use of natural processes by which plants and their microbial rhizosphere organisms sequester or immobilize PTEs. The cost differ widely for different technologies (Figure 4).
Physico-chemically or mechanically-based methods
Soil replacement is an in situ method by which PTE-contaminated soil is removed and replaced by uncontaminated soil material. The soil removed needs to be further treated or safely deposited. This method is suitable only for severely polluted sites with small area, as the labour costs are very high [109].
Soil washing is an ex situ remediation technology, involving washing with water to remove PTEs from the polluted soil [9]. To improve the efficacy of soil washing different additives including H2SO4 and HNO3, chelating agents like EDTA (ethylenediaminetetraacetic acid), DTPA (diethylenetriamine pentaacetic acid) and EDDS (ethylenediaminedisuccinic acid) or surfactants (e.g. rhamnolipid) can be applied [110]. In contact with the soil surfaces, the additives can enhance dispersal and desorption as well as solubilization of PTEs. Due to these mechanisms, this technology is most appropriate for weakly bound PTEs occurring as exchangeable ions, hydroxides, reducible oxides and carbonates. A limitation of the washing technology is that residual fractions are not affected [111]. EDTA was found to effectively remove Cd, Cu, Pb and Zn. Depending on the soil, removal efficiencies ranged between 65 and 86 % [112]. Chelators, however, need to be handled with care due to possible enhancement of PTEs leaching to the groundwater, particularly in coarse-textured soils.
Thermal desorption is based on the volatility of several pollutants (e.g. Hg, As). The contaminated soil is heated to make the elements volatile, using steam, microwave or infrared radiation. Finally, the elements are collected using a carrier gas or the vacuum negative pressure [113]. Thermal desorption can be categorized as low temperature desorption (90–320 °C) and high temperature desorption (320–560 °C). This method has advantages including a simple process, devices with mobility and the remediated soil suitable for reuse. However, a long desorption time and high cost of devices limit its application [110].
Electrochemical remediation involves electrodes being inserted into the soil and encompassing the contaminated zone. Migration of charged ions occurs when electric fields are applied. Negative ions are attracted by the positively charged anode and positive ions move to the negatively charged cathode. For example, the hexavalent anionic Cr (CrO4 2−) moves towards the anode, while the cationic Cd2+, Cr3+ and Ni2+ move towards the cathode when an electric potential is being induced [110]. The contaminants accumulated at the electrodes can be extracted by different methods including complexing with ion-exchange resins, electroplating, precipitation/co-precipitation or pumping water near the electrodes [114]. This method is particularly suited for soils with high clay content, as the electric conductivity is the highest in the fine particles.
Solidification is a suitable method for treatment of extremely contaminated sites by involving cements, polymer modified cements, inorganic fixing agents (e.g. solid silica polymers) and organic polymers that each produces an inert matrix [9]. Using this method the volume of waste is usually increased and the material requires disposal. Therefore, this method is of less value when large volumes of material require treatment.
Vitrification is equivalent to solidification involving higher temperatures. The method includes “melting” the soil at 1,600–2,000 °C at which all the organic substances are incinerated. The PTEs are trapped in the melt which after cooling forms a vitreous rock. In-situ remediation is also possible by applying the heat through electrodes inserted into the contaminated soil. Although this technology can immobilize contaminants in an efficient manner it needs to be considered that the process is cost-and energy-intensive. Energy can be supplied from heating electrodes or fossil fuel for ex-situ remediation. Due to the high energy demand, this method is more suitable for low volumes of contaminated soil [115].
Phytoremediation
Phytoremediation is an ecofriendly bioremediation technique for removal of PTEs from soils, sediments and water. There are different categories of phytoremediation involving varied mechanisms: phytoextraction (extraction of PTEs from soil/water and transfer from the roots to other plant parts), phytostabilisation (immobilisation of PTEs in plant tissue and conversion in less toxic form by precipitation, complexation or reduction of pollutants), and phytovolatisation (plant uptake of PTEs and transfer to the environment in volatile, less toxic forms via transpiration) [116].
“Hyperaccumulator plants” are known to have a high capacity for PTE accumulation. The use of these plants has become a promising technique to remediate PTE-contaminated sites [117]. Compared to nonaccumulator plants PTE levels in shoots of hyperaccumulators can be more than 100-fold higher. A hyperaccumulator plant can exhibit concentrations of >10 mg kg −1 Hg, >100 mg kg −1 Cd, >1,000 mg kg −1 Co, Cr, Cu, and Pb, and >10,000 mg kg −1 Zn and Ni [24]. More than 500 plant species are known to hyperaccumulate PTEs [9, 21, 118], [119], [120]. Some examples of PTE accumulation levels are given in Table 8.
Extremely high PTE accumulation levels of some plant species growing on contaminated soils (adapted from [9]).
| Plant species | PTE | Accumulation (mg kg−1) |
|---|---|---|
| Aeollanthus subacaulis | Cu | 13,700 |
| Agrostis tenuis | Pb | 13,400 |
| Arabis paniculata | Cd | 1,127 |
| Armeria maritime | Pb | 1,600 |
| Astragalus racemosus | Se | 14,920 |
| Berkheya codii | Ni | 5,500 |
| Brassica juncea | Ni | 3,916 |
| Dichapetalum gelonioides | Zn | 30,000 |
| Ipomoea alpine | Cu | 12,300 |
| Potentilla griffithii | Zn | 19,600 |
| Pteris vittata | As | 23,000 |
| Sedum alfredii | Cd | 2,183 |
| Sedum alfredii | Zn | 13,799 |
| Sesbania drummondi | Cd | 1,687 |
| Sorghum sudanense | Cu | 5,330 |
| Streptanthus polygaloydes | Ni | 14,800 |
| Thlaspi caerulescens | Zn | 19,410 |
| Thlaspi praecox | Cd | >1,000 |
| Thlaspi tatrense | Zn | 20,100 |
| Viola calamanaria | Zn | 10,000 |
| Zea mays | Cr | 2,538 |
Hypertolerance of plants to high levels of PTEs makes hyperaccumulation feasible; hypertolerant species have effective protection mechanisms that counteract PTE toxicity. They are capable of compartmentalizing metal ions (i.e., sequestrating them in cell walls or the vacuolar compartment), which excludes PTEs from cellular sites where processes take place like respiration and cell division [118, 121]. A sequence of processes is involved during hyperaccumulation. First, PTEs are adsorbed at root surfaces and move into root cells across the cellular membrane. Part of the PTEs taken up by the root cells is immobilized in cell walls or in the vacuole. Another part, consisting of intracellular mobile PTEs crosses cellular membranes into the xylem and is transported from the root to stems and leaves. After reaching the target organ of the plant, most PTEs become insoluble and are precipitated in apoplastic or symplastic compartments by formation of phosphates, carbonates, or sulphates [9]. Genetic improvements, new knowledges about plant responses to mycorrhizae, bacteria and chelators, PTE plant uptake and plant tolerance, as well as appropriate ways of application of these approches are examples of great efforts in recent years to improve phytoextraction. Major results have been reported by [122], [123], [124], [125].
Phytostabilization involves the establishment of a plant cover with species that are tolerant to high levels of pollutants. The aim is to reduce PTE bioavailability in the soil through accumulation by roots or immobilization within the rhizosphere. The method is useful for the remediation of Pb, As, Cd, Cr, Cu, and Zn polluted sites [125]. By increasing SOM and nutrient levels, biological activity and cation exchange capacity, phytostabilisation improves the biological and chemical characteristics of polluted soils [126]. Phytostabilization can be enhanced by using soil amendments immobilizing PTEs. The choice of adequate plant species is an important aspect in phytostabilisation-based methods [127]. Plants should be able to develop extended root systems and keep the transport of PTEs from roots to shoots at a low level [128]. Especially perennial species are capable of storing a significant part of PTEs in the rooting zone [129]. Although this method is effective in PTE immobilization, the polluted sites require regular monitoring. Soil amendments applied to enhance containment may need periodic application to maintain their effectiveness. A major disadvantage of this technology is that the pollutant is not removed from the system and remains in the soil as it is [129].
Phytovolatisation makes use of plants that take up PTEs from the rhizosphere, transforming the pollutants into volatile form and emitting them into the atmosphere in the gas phase. Several pollutants can pass through the plants to the leaves and volatilize at relatively low concentrations [130]. Phytovolatilization in particular has been used for the removal of Hg. In the plant, the Hg ion is transformed into the less toxic elemental form Hg0 [131].
Conclusions
In this review, we presented generalities on sources, sinks, pathways and mitigation measures related to man-made and naturally occurring PTEs in the environment, focusing on As, Cd, Cr, Hg and Pb. In nature PTEs result from release from geologic materials, soil forming processes, volcanic eruptions, as well as wind dust and marine aerosol deposition. Man-made sources include emissions from industrial areas, mine tailings, waste deposits, use of leaded gasoline and paints, application of mineral fertilizers, animal manures, sewage sludge and pesticides, wastewater irrigation, coal combustion and atmospheric deposition. The production and use of PTEs continues to grow worldwide, particularly in developing countries. Multisectoral action is increasingly needed to protect terrestrial and aquatic ecosystems and human health from the harmful effects of improperly managed PTEs. Primary efforts should aim at reducing PTE production and emission, and recycling of used metals. Because of crop-specific differences in PTE transfer from soil to plants, including food crops, the choice of crop is vital to reduce their transfer to the human food chain. For immobilizing PTEs in soils, inorganic and organic soil amendments have been successfully applied. Although immobilization techniques can minimize the bioavailability and toxicity of these pollutants, but the major drawback is that the total PTE concentration remains unchanged. Therefore, attaining effective remediation efficiency must be accompanied by continuous monitoring to avoid undesired PTE mobilization or leaching.
Currently used remediation technologies are partly physico-chemical- or mechanically-based, including soil replacement, soil washing, thermal desorption, electrochemical remediation, solidification and vitrification. The major disadvantages of the latter are that they are expensive, energy-intensive (e.g. thermal desorption and vitrification), and are not environment friendly and soil disturbing. Phytoremediation, using the mechanisms of phytoextraction, phytostabilization and phytovolatilization, is an ecofriendly remediation technique for removal of PTEs from the environment. Efforts have been made to improve phytoextraction, e.g. using chelate-assisted phytoextraction. However, some concerns have been expressed regarding the potential risk of leaching of PTEs to groundwater which may also arise when chelators are used as additives for soil washing. Phytovolatilization for the removal of mercury has the disadvantage that Hg released into the atmosphere is likely to be recycled by precipitation and redeposition into the ecosystem. The major weakness of phytostabilization is that the contaminant remains in the soil system which requires regular soil monitoring. For heavily PTE-polluted soils, the cultivation of industrial plants, such as fibre plants as well as energy crops may be a suitable option. In summary, the choice of the appropriate method depends, inter alia, on the kind and the degree of pollution, the site characteristics as well as the targeted land use.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no competing of interest.
-
Informed consent: Not applicable.
-
Ethical approval: The conducted research is not related to either human or animal use. The authors herewith confirm that all research reviewed complies with all the relevant national regulations, institutional policies, and was performed in accordance with the tenets of the Helsinki Declaration, and has been approved with the authors‘ institutional review board or equivalent committee.
References
1. Pourret, O, Bollinger, JC. “Heavy metal”-what to do now: to use or not to use. Sci Total Environ 2018;610–611:419–20. https://doi.org/10.1016/j.scitotenv.2017.08.043.Search in Google Scholar PubMed
2. Wu, W, Wu, P, Yang, F, Sun, DL, Zhang, DX, Zhou, YK. Assessment of heavy metal pollution and human health risks in urban soils around an electronics manufacturing facility. Sci Total Environ 2018;630:53–61. https://doi.org/10.1016/j.scitotenv.2018.02.183.Search in Google Scholar PubMed
3. Alloway, BJ. Sources of heavy metals and metalloids in soils. Heavy metals in soils. Dordrecht: Springer; 2013:11–50 pp.10.1007/978-94-007-4470-7_2Search in Google Scholar
4. Li, G, Sun, GX, Ren, Y, Luo, XS, Zhu, YG. Urban soil and human health: a review. Eur J Soil Sci 2018;69:196–215. https://doi.org/10.1111/ejss.12518.Search in Google Scholar
5. Kabata-Pendias, A, Pendias, H. Trace metals in soils and plants, 2nd ed. Boca Raton: CRC Press; 2001.10.1201/9781420039900Search in Google Scholar
6. Oliver, MA. Soil and human health: a review. Eur J Soil Sci 1997;48:573–92. https://doi.org/10.1046/j.1365-2389.1997.00124.x.Search in Google Scholar
7. Khan, S, Cao, Q, Zheng, YM, Huang, YZ, Zhu, YG. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pol 2008;152:686–92. https://doi.org/10.1016/j.envpol.2007.06.056.Search in Google Scholar PubMed
8. Liu, G, Wang, J, Liu, X, Li, X, Ren, Y, Wang, J, et al.. Partitioning and geological fractions of heavy metals from geogenic and anthropogenic sources in various particle size fractions. Geoderma 2018;312:104–13. https://doi.org/10.1016/j.geoderma.2017.10.013.Search in Google Scholar
9. Nieder, R, Benbi, DK, Reichl, FX. Soil components and human health. Dordrecht: Springer; 2018.10.1007/978-94-024-1222-2Search in Google Scholar
10. Rinklebe, J, Antoniadis, V, Shaheen, SM, Rosche, O, Altermann, M. Health risk assessmant of potentially oxic elements in soils along the Elbe River, Germany. Environ Int 2019;126:76–88. https://doi.org/10.1016/j.envint.2019.02.011.Search in Google Scholar PubMed
11. Khalid, S, Shahid, M, Niazi, NK, Murtaza, B, Bibi, I, Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J Geochem Explor 2017;182:247–68. https://doi.org/10.1016/j.gexplo.2016.11.021.Search in Google Scholar
12. Mahmood, T. Phytoextraction of heavy metals – the process and scope for remediation of contaminated soils. Soil Environ 2010;29:91–109.Search in Google Scholar
13. Mc Keehan, P. The financial, legislative and social aspects of the redevelopment of contaminated commercial and industrial properties; 2000. Available from: http://www.casa.com/discoveryguides/brown/overview.Search in Google Scholar
14. Ragnarsdottir, KV, Hawkins, D. Trace metals in soils and their relationship with scrapie occurrence. Geochem Cosmochim Acta 2005;69:A194–6.Search in Google Scholar
15. Liu, Y. Shrinking arable lands jeopardizing China’s food security; 2006. Available from: http://www.worldwatch.org/node/3912.Search in Google Scholar
16. Hou, D, Li, F. Complexities surrounding China’s soil action plan. Land Degrad Dev 2017;28:2315–20. https://doi.org/10.1002/ldr.2741.Search in Google Scholar
17. Liu, L. Made in China: cancer villages. Environment 2010;52:8–21. https://doi.org/10.1080/00139151003618118.Search in Google Scholar
18. Iqbal, HH, Taseer, R, Anwar, S, Mumtaz, M, Qadir, A, Shahid, N. Human health risk assessment: heavy metal contamination of vegetables in Bahawalpur, Pakistan. Bull Environ Stud 2016;1:10–7.Search in Google Scholar
19. Giller, KE, Witter, E, McGrath, SP. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 1998;30:1389–414. https://doi.org/10.1016/s0038-0717(97)00270-8.Search in Google Scholar
20. Castaldi, S, Rutigliano, FA, Virzo de Santo, A. Suitability of soil microbial parameters as indicators of heavy metal pollution. Water Air Soil Pollut 2004;158:21–35.10.1023/B:WATE.0000044824.88079.d9Search in Google Scholar
21. Lasat, MM. Phytoextraction of toxic metals: a review of biological mechanism. J Environ Qual 2002;31:109–20. https://doi.org/10.2134/jeq2002.0109.Search in Google Scholar
22. McGrath, SP, Zhao, FJ, Lombi, EL. Plant and rhizosphere process involved in phytoremediation of metal contaminated soils. Plant Soil 2001;232:207–14. https://doi.org/10.1023/a:1010358708525.10.1007/978-94-010-0566-1_20Search in Google Scholar
23. Nieder, R, Weber, TKD, Paulmann, I, Muwanga, A, Owor, M, Naramabuye, FX, et al.. The geochemical signature of rare-metal pegmatites in the Central Africa Region: soils, plants, water and stream sediments in the Gatumba tin-tantalum mining district, Rwanda. J Geochem Explor 2014;144:539–51. https://doi.org/10.1016/j.gexplo.2014.01.025.Search in Google Scholar
24. Wuana, RA, Okieimen, FE. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Commun Soil Sci Plant Anal 2011;42:111–22. https://doi.org/10.5402/2011/402647.Search in Google Scholar
25. Mitchell, RL. Trace elements in soils. In: Bear, FE, editor. Chemistry of the soil. New York: AACS Monograph Series; 1964:320–68 pp.Search in Google Scholar
26. Alloway, BJ. Heavy metals in soils. London: Blackie Academic and Professional; 1995.10.1007/978-94-011-1344-1Search in Google Scholar
27. Cannon, HL, Connally, GG, Epstein, JB, Parker, JG, Thornton, I, Wixson, G. Rocks: geological sources of most trace elements. In: report to the workshop at south plantation Captiva Island, FL, US. Geochem Environ 1978;3:17–31.Search in Google Scholar
28. Prabhakaran, KP, Cottenie, A. Parent material – soil relationship in trace elements – a quantitative estimation. Geoderma 1971;5:81–97. https://doi.org/10.1016/0016-7061(71)90014-0.Search in Google Scholar
29. Allaway, WH. Agronomic control over the environmental cycling of trace elements. Adv Agron 1968;20:235–74.10.1016/S0065-2113(08)60858-5Search in Google Scholar
30. Feng, L, Yuan-Ming, Z, Ji-Zheng, H. Microbes influence the fractionation of arsenic in paddy soils with different fertilization regimes. Sci Total Environ 2009;407:2631–40.10.1016/j.scitotenv.2008.12.021Search in Google Scholar PubMed
31. Mc Laren, RG. Micronutrients and toxic elements. In: Benbi, DK, Nieder, R, editors. Handbook of processes and modeling in soil-plant system. New York: Haworth Press Inc; 2003:589–625 pp.10.1201/9781003578543-19Search in Google Scholar
32. Zhang, XP, Deng, W, Yang, XM. The background concentrations of 13 soil trace elements and their relationships to parent materials and vegetation in Xizang (Tibet), China. J Asian Earth Sci 2002;21:167–74. https://doi.org/10.1016/s1367-9120(02)00026-3.Search in Google Scholar
33. Bronger, A, Bruhn-Lobin, N. Paleopedology of mediterranean soils – case studies from NW Morocco. In: Abstracts of the 2nd international meeting on red mediterranean soils. Adana, Turquía; 1993:183 p.Search in Google Scholar
34. Bech, J, Tobias, FJ, Roca, N, Rustullet, J. Trace elements in some mediterranean red soils from the NE of Spain. Agrochimica 1998;XLII:26–40.Search in Google Scholar
35. Seaward, MRD, Richardson, DHS. Atmospheric sources of metal pollution and effects on vegetation. In: Shaw, AJ, editor. Heavy metal tolerance in plants evolutionary aspects. Boca Raton: CRC Press; 1990.Search in Google Scholar
36. Ross, SM. Toxic metals in soil-plant systems. Chichester: Wiley; 1994.Search in Google Scholar
37. Pacyna, JM. Atmospheric trace elements from natural and anthropogenic sources. In: Nriagu, JO, Davidson, CI, editors. Toxic metals in the atmosphere, chap 2. New York: Wiley; 1986.Search in Google Scholar
38. Fergusson, JE. The heavy elements: chemistry, environmental impact and health effects. Oxford: Pergamon Press; 1990.Search in Google Scholar
39. Smith, LA, Means, JL, Chen, A. Remedial options for metals-contaminated sites. Boca Raton: Lewis Publishers; 1995.Search in Google Scholar
40. Altfelder, S, Beyer, C, Duijnisfeld, WHM, Schneider, J, Streck, T. Distribution of Cd in the vicinity of a metal smelter: interpolation of soil Cd concentrations with regard to regulative limits. J Plant Nutr Soil Sci 2002;165:697–705. https://doi.org/10.1002/jpln.200290006.Search in Google Scholar
41. US EPA (United States Environmental Protection Agency). Report: recent developments for in situ treatment of metals contaminated soils. Washington DC: U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response; 1996.Search in Google Scholar
42. Nriagu, JO, Pacyna, JM. Quantitative assessment of worldwide contamination of air water and soils by trace metals. Nature 1988;333:134–9. https://doi.org/10.1038/333134a0.Search in Google Scholar PubMed
43. Lone, MI, He, Z, Stoffella, PJ, Yang, X. Phytoremediation of heavy metal polluted soils and water: progress and perspectives. J Zhejiang Univ – Sci B 2008;9:210–20. https://doi.org/10.1631/jzus.b0710633.Search in Google Scholar
44. Van den Enden, E. Arsenic poisoning; 1999. Available from: http://www.itg.be/evde/Teksten/sylabus/49_Arsenicism.doc.Search in Google Scholar
45. Pohl, WL. Economic geology. Principles and practice, 2nd ed. Stuttgart: Schweizerbart Science Publishers; 2020.Search in Google Scholar
46. Nagajyoti, PC, Lee, KD, Sreekanth, TVM. Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 2010;8:199–216. https://doi.org/10.1007/s10311-010-0297-8.Search in Google Scholar
47. Dudka, S, Adriano, DC. Environmental impacts of metal ore mining and processing: a review. J Environ Qual 1997;26:590–602. https://doi.org/10.2134/jeq1997.00472425002600030003x.Search in Google Scholar
48. Obrador, A, Alvarez, JM, Lopez-Valdivia, LM, Gonzalez, D, Novillo, J, Rico, MI. Relationships of soil properties with Mn and Zn distribution in acidic soils and their uptake by a barely crop. Geoderma 2007;137:432–43. https://doi.org/10.1016/j.geoderma.2006.10.001.Search in Google Scholar
49. Norland, MR, Veith, DL. Revegetation of coarse taconite iron ore tailing using municipal waste compost. J Hazard Mater 1995;41:123–34. https://doi.org/10.1016/0304-3894(94)00115-w.Search in Google Scholar
50. Henriques, FS, Fernandez, C. Metal uptake and distribution in rush (Juncus conglomerates L.) plants growing in pyrites mine tailings at Lousal, Portugal. Sci Total Environ 1991;102:253–60. https://doi.org/10.1016/0048-9697(91)90319-a.Search in Google Scholar
51. Peplow, D. Environmental impacts of mining in Eastern Washington. Center for water and watershed studies fact sheet. Seattle: University of Washington; 1999.Search in Google Scholar
52. Ettler, V, Johan, Z, Kribek, B, Sebek, O, Mihaljevic, M. Mineralogy and environmental stability of slags from the Tsumeb smelter, Namibia. Appl Geochem 2009;24:1–15. https://doi.org/10.1016/j.apgeochem.2008.10.003.Search in Google Scholar
53. Kribek, B, Majer, V, Veselovsky, F, Nyambe, I. Discrimination of lithogenic and anthropogenic sources of metals and sulphur in soils of the central northern part of the Zambian copper belt mining district: a topsoil vs. subsurface soil concept. J Geochem Explor 2010;104:69–86. https://doi.org/10.1016/j.gexplo.2009.12.005.Search in Google Scholar
54. Telmer, KH, Veiga, MM. World emissions of mercury from artisanal and mall scale gold mining. In: Pirrone, N, Mason, RP, editors. Mercury fate and transport in the global atmosphere: emissions, measurements and models. Dordrecht: Springer; 2009:131–73 pp.10.1007/978-0-387-93958-2_6Search in Google Scholar
55. US EPA (Environmental Protection Agency). Reducing mercury pollution from gold mining; 2011. Available from: http://www.epa.gov/oia/toxics/asgm.html.Search in Google Scholar
56. Verkleji, JAS. The effects of heavy metals stress on higher plants and their use as bio- monitors. In: Markert, B, editor. Plant as bioindicators: indicators of heavy metals in the terrestrial environment. New York: VCH; 1993:415–24 pp.Search in Google Scholar
57. Sumner, ME. Beneficial use of effluents, wastes and biosolids. Commun Soil Sci Plant Anal 2000;31:1701–15. https://doi.org/10.1080/00103620009370532.Search in Google Scholar
58. Ostermann, A, Gao, J, Welp, G, Siemens, J, Roelcke, M, Heimann, L, et al.. Identification of soil contamination hotspots with veterinary antibiotics using heavy metal concentrations and leaching data - a field study in China. Environ Monit Assess 2014;186:7693–707. https://doi.org/10.1007/s10661-014-3960-x.Search in Google Scholar PubMed
59. Zhang, S, Zhang, F, Liu, X, Wang, Y, Zou, S, He, X. Determination and analysis on main harmful composition in excrement of scale livestock and poultry feedlots. Plant Nutr Fert Sci 2005;11:822–9. (in Chinese).Search in Google Scholar
60. Angino, EE, Magnuson, LM, Waugh, TC, Galle, OK, Bredfeldt, J. Arsenic in detergents – possible danger and pollution hazard. Science 1970;168:389–92. https://doi.org/10.1126/science.168.3929.389.Search in Google Scholar PubMed
61. Bjuhr, J. Trace metals in soils irrigated with waste water in a periurban area downstream Hanoi City, Vietnam, Institutionen for markvetenskap. Uppsala: Sveriges Lantbruksuniversitet; 2007.Search in Google Scholar
62. Silveira, MLA, Alleoni, LRF, Guilherme, LRG. Biosolids and heavy metals in soils. Sci Agric 2003;60:64–111. https://doi.org/10.1590/s0103-90162003000400029.Search in Google Scholar
63. Mc Laughlin, MJ, Hamon, RE, McLaren, RG, Speir, TW, Rogers, SL. Review: a bioavailability-based rationale for controlling metal and metalloid contamination of agricultural land in Australia and New Zealand. Aust J Soil Res 2000;38:1037–86. https://doi.org/10.1071/sr99128.Search in Google Scholar
64. Jones, LHP, Jarvis, SC. The fate of heavy metals. In: Green, DJ, Hayes, MHB, editors. The chemistry of soil processes. New York: John Wiley and Sons; 1981:593 p.Search in Google Scholar
65. Shankar, S, Shanker, U, Shikha. Arsenic contamination of groundwater: a review of sources, prevalence, health risks, and strategies for mitigation. Sci World J 2014;2014:304524. https://doi.org/10.1155/2014/304524.Search in Google Scholar PubMed PubMed Central
66. Bissen, M, Frimmel, FH. Arsenic: a review – part I: occurrence, toxicity, speciation, mobility. Acta Hydrochim Hydrobiol 2003;31:9–18. https://doi.org/10.1002/aheh.200390025.Search in Google Scholar
67. Polizzotto, ML, Harvey, CF, Li, G, Badruzzman, B, Ali, A, Newville, M, et al.. Solid-phases and desorption processes of arsenic within Bangladesh sediments. Chem Geol 2006;228:97–111. https://doi.org/10.1016/j.chemgeo.2005.11.026.Search in Google Scholar
68. Borba, RP, Figueiredo, BR, Matschullat, J. Geochemical distribution of arsenic in waters, sediments and weathered gold mineralized rocks from Iron Quadrangle, Brazil. Environ Geol 2003;44:39–52. https://doi.org/10.1007/s00254-003-0766-5.Search in Google Scholar
69. Nriagu, J, Bhattacharya, P, Mukherjee, A, Bundschuh, J, Zevenhoven, R, Loeppert, R. Arsenic in soil and groundwater: an overview. In: Bhattacharya, P, Mukherjee, A, Bundschuh, J, Zevenhoven, R, Loeppert, R, editors. Arsenic in soil and groundwater environment. Amsterdam: Elsevier; 2007:3–60 pp.10.1016/S0927-5215(06)09001-1Search in Google Scholar
70. Bhattacharya, P, Jacks, G, Ahmed, KM, Routh, J, Khan, AA. Arsenic in groundwater of the bengal delta plain aquifers in Bangladesh. Bull Environ Contam Toxicol 2002;69:538–45. https://doi.org/10.1007/s00128-002-0095-5.Search in Google Scholar PubMed
71. Smedley, PL, Kinniburgh, DG. A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 2002;17:517–68. https://doi.org/10.1016/s0883-2927(02)00018-5.Search in Google Scholar
72. Parga, JR, Cocke, DL, Jesus, LV, Gomes, JA, Kesmez, M, George, I, et al.. Arsenic removal via lector coagulation of heavy metal contaminated ground water in La Comarca Lagurera, Mexico. J Hazard Mater 2005;124:247–54. https://doi.org/10.1016/j.jhazmat.2005.05.017.Search in Google Scholar PubMed
73. Shakoor, MB, Niazi, NK, Bibi, I, Shahid, M, Saqib, ZA, Nawaz, MF, et al.. Exploring the arsenic removal potential of various biosorbents from water. Environ Int 2019;123:567–79. https://doi.org/10.1016/j.envint.2018.12.049.Search in Google Scholar PubMed
74. Berg, M, Trans, HC, Nguyeu, TC, Pham, MV, Scherteuleib, R, Giger, W. Arsenic contamination of groundwater and drinking water in Vietnam: a human health threat. Environ Sci Technol 2001;35:2621–6. https://doi.org/10.1021/es010027y.Search in Google Scholar PubMed
75. Ahmad, A, van der Wens, P, Baken, K, de Waal, L, Battacharya, P, Stuyfsand, P. Arsenic reduction to <1 µg/L in Dutch drinking water. Environ Int 2020;134:105253. https://doi.org/10.1016/j.envint.2019.105253.Search in Google Scholar PubMed
76. Nieder, R, Benbi, DK. Integrated review of the nexus between toxic elements in the environment and human health. AIMS Public Health 2022;9:758–89. https://doi.org/10.3934/publichealth.2022052.Search in Google Scholar PubMed PubMed Central
77. Antoniadis, V, Shaheen, SM, Levizou, E, Shahid, M, Niazi, NK, Vithanage, M, et al.. Soil amendments for immobilization of potentially toxic elements in contaminated soils: a critical review. Environ Int 2020;134:105046. https://doi.org/10.1016/j.envint.2019.105046.Search in Google Scholar PubMed
78. GWRTAC. Remediation of metals-contaminated soils and groundwater, tech rep TE-97-01. Pittsburgh: GWRTAC-E Series; 1997.Search in Google Scholar
79. Gregor, M. Metal availability, uptake, transport and accumulation in plants. In: Prasad, MNV, editor. Heavy metal stress in plants – from biomolecules to ecosystems. Berlin: Springer; 2004:1–27 pp.10.1007/978-3-662-07743-6_1Search in Google Scholar
80. Antoniadis, V, Levizou, E, Shaheen, SM, Ok, YS, Sebastian, A, Baum, C, et al.. Trace elements in the soil-plant interface: phytoavailability, translocation, and phytoremediation – a review. Earth Sci Rev 2017;171:621–45. https://doi.org/10.1016/j.earscirev.2017.06.005.Search in Google Scholar
81. Adriano, DC. Trace elements in terrestrial environments: biogeochemistry, bioavailability and risks of metals, 2nd ed. New York: Springer; 2003.Search in Google Scholar
82. Antoniadis, V, Shaheen, SM, Levizou, E, Shahid, M, Niazi, NK, Vithanage, M, et al.. A critical prospective analysis of the potential toxicity of trace element regulation limits in soils worldwide: are they protective concerning health risk assessment? – a review. Environ Int 2019;127:819–47. https://doi.org/10.1016/j.envint.2019.03.039.Search in Google Scholar PubMed
83. Visioli, G, Marmiroli, N. The proteomics of heavy metal hyperaccumulation by plants. J Proteonomics 2013;79:133–45. https://doi.org/10.1016/j.jprot.2012.12.006.Search in Google Scholar PubMed
84. Pal, R, Rai, JPN. Phytochelatins: peptides involved in heavy metal detoxification. Appl Biochem Biotechnol 2010;160:945–63. https://doi.org/10.1007/s12010-009-8565-4.Search in Google Scholar PubMed
85. Lu, S, Ren, L, Fang, J, Ji, J, Liu, G, Zhang, J, et al.. Trace elements are associated with urinary 8-hydroxy-2′-deoxyguanosine level: a casestudy of college students in Guangzhou, China. Environ Sci Pollut Res 2016;23:8484–91. https://doi.org/10.1007/s11356-016-6104-8.Search in Google Scholar PubMed
86. Elkady, AA, Sweet, ST, Wade, TL, Klein, AG. Distribution and assessment of heavy metals in the aquatic environment of Lake Manzala, Egypt. Ecol Indicat 2015;58:445–57. https://doi.org/10.1016/j.ecolind.2015.05.029.Search in Google Scholar
87. Rajaei, G, Mansouri, B, Jahantigh, H, Hamidian, AH. Metal concentration in the water of Chah nimeh reservoirs in Zabol, Iran. Bull Environ Contam Toxicol 2012;89:495–500. https://doi.org/10.1007/s00128-012-0738-0.Search in Google Scholar PubMed
88. Tang, W, Zhang, H, Zhang, W, Shan, B, Zhu, X, Song, Z. Dynamics of heavymetals and phosphorus in the pore water of estuarine sediments following agri-cultural intensification in chao lake valley. Environ Sci Pollut Res 2015;22:7948–53. https://doi.org/10.1007/s11356-014-3945-x.Search in Google Scholar PubMed
89. Azadi, NA, Mansouri, B, Spada, L, Sinkakarimi, MH, Hamesadeghi, Y, Mansouri, A. Contamination of lead (Pb) in the coastal sediments of North and South of Iran: a review. Chem Ecol 2018;34:884–900.10.1080/02757540.2018.1508462Search in Google Scholar
90. WHO (World Health Organization). 10 chemicals of public health concern; 2020. Available from: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern.Search in Google Scholar
91. Puschenreiter, M, Horak, O, Friesl, W, Hartl, W. Low-cost agricultural measures to reduce heavy metal transfer into the food chain – a review. Plant Soil Environ 2005;51:1–11. https://doi.org/10.17221/3549-pse.Search in Google Scholar
92. Kloke, A, Sauerbeck, DR, Vetter, H. The contamination of plants and soils with heavy metals and the transport of metals in terrestrial food chains. In: Nriagu, editor. Changing metal cycles and human health. Berlin, Heidelberg, New York: Springer; 1984.10.1007/978-3-642-69314-4_7Search in Google Scholar
93. Page, AL, Logan, TJ, Ryan, JA. Land application of sludge: food chain implications. Chelsea: Lewis Publications; 1987.Search in Google Scholar
94. Kabata-Pendias, A, Pendias, H. Trace elements in soils and plants, 2nd ed. Boca Raton: CRC Press; 1992.Search in Google Scholar
95. Machelett, B, Metz, R, Bergmann, H. Schwermetalltransferuntersuchungen an landwirtschaftlichen und gärtnerischen nutzpflanzen unter gleichen anbaubedingungen. VDLUFA – Schriftenr 1992;37:579–82.Search in Google Scholar
96. Puschenreiter, M, Horak, O. Influence of different soil parameters on the transfer factor soil to plant of Cd, Cu and Zn for wheat and rye. Bodenkultur 2000;51:3–10.Search in Google Scholar
97. Börjesson, P. Environmental effects of energy crop cultivation in Sweden – I: identification and quantification. Biomass Bioenergy 1999;16:137–54. https://doi.org/10.1016/s0961-9534(98)00080-4.Search in Google Scholar
98. Palansooriya, KN, Shaheen, SM, Chen, SS, Tsang, DCW, Hashimoto, Y, Hou, D, et al.. Soil amendments for immobilization of potentially toxic elements in contaminated soils: a critical review. Environ Int 2020;134:105046. https://doi.org/10.1016/j.envint.2019.105046.Search in Google Scholar PubMed
99. Dermatas, D, Meng, XG. Stabilization/Solidification (S/S) of heavy metal contaminated soils by means of a quicklime-based treatment approach. In: Stab solidif hazard radioact mix wastes, ASTM STP 1240, Vol. 1. Philadelphia: American Society for Testing and Materials; 1996;449–513.10.1520/STP14134SSearch in Google Scholar
100. Yuan, CL, Li, Q, Sun, ZY, Zhang, WJ, Chen, JR, Chen, Z, et al.. Chemical oxidation of arsenic in the environment and applications: A mini review. Pedosphere 2023;33:185–93.10.1016/j.pedsph.2022.06.033Search in Google Scholar
101. Feizi, M, Jalali, M, Antoniadis, V, Shaheen, SM, Ok, YS, Rinklebe, J. Geo-and nano-materials affect the mono-metal and competitive sorption of Cd, Cu, Ni, and Zn in a sewage sludge-treated alkaline soil. J Hazard Mater 2019;379:120567. https://doi.org/10.1016/j.jhazmat.2019.04.050.Search in Google Scholar PubMed
102. Lahori, AH, Zhang, Z, Shaheen, SM, Rinklebe, J, Guo, Z, Li, R, et al.. Mono-and co-applications of Ca-bentonite with zeolite, Ca-hydroxide, and tobacco biochar affect phytoavailability and uptake of copper and lead in a gold mine-polluted soil. J Hazard Mater 2019;374:401–11. https://doi.org/10.1016/j.jhazmat.2019.04.057.Search in Google Scholar PubMed
103. Yi, X, Liang, X, Yingming, X, Xu, Q, Huang, Q, Lin, W, et al.. Remediation of heavy metal-polluted agricultural soils using clay minerals: a review. Pedosphere 2017;27:193–204. https://doi.org/10.1016/s1002-0160(17)60310-2.Search in Google Scholar
104. Adriano, DC, Wenzel, WW, Vangronsveld, J, Bolan, NS. Role of assisted natural remediation in environmental cleanup. Geoderma 2004;122:121–42. https://doi.org/10.1016/j.geoderma.2004.01.003.Search in Google Scholar
105. Guo, GL, Zhou, QX, Ma, LQ. Availability and assessment of fixing additives for the in situ remediation of heavy metal contaminated soils: a review. Environ Monit Assess 2006;116:513–28. https://doi.org/10.1007/s10661-006-7668-4.Search in Google Scholar PubMed
106. Jones, KC, Johnston, AE. Cadmium in cereal grain and herbage from long-term experimental plots at Rothamsted, UK. Environ Pollut 1989;57:199–216. https://doi.org/10.1016/0269-7491(89)90012-2.Search in Google Scholar PubMed
107. Gadepalle, VP, Ouki, SK, Van Herwijnen, R, Hutchings, T. Immobilization of heavy metals in soil using natural and waste materials for vegetation establishment on contaminated sites. Soil Sediment Contam 2007;16:233–51. https://doi.org/10.1080/15320380601169441.Search in Google Scholar
108. Glass, DJ. U.S. and international markets for phytoremediation, 1999–2000. Needham: D. Glass Associates; 1999.Search in Google Scholar
109. Yao, ZT, Li, JH, Xie, HH, Yu, CH. Review on remediation technologies of soil contaminated by heavy metals. Proc Environ Sci 2012;16:722–9. https://doi.org/10.1016/j.proenv.2012.10.099.Search in Google Scholar
110. Peng, JF, Song, YH, Yuan, P, Cui, XY, Qiu, GL. The remediation of heavy metals contaminated sediment. J Hazard Mater 2009;161:633–40. https://doi.org/10.1016/j.jhazmat.2008.04.061.Search in Google Scholar PubMed
111. Ortega, LM, Lebrun, R, Blais, JF, Hauslerd, R, Drogui, P. Effectiveness of soil washing, nanofiltration and electrochemical treatment for the recovery of metal ions coming from a contaminated soil. Water Res 2008;42:1943–52. https://doi.org/10.1016/j.watres.2007.11.025.Search in Google Scholar PubMed
112. Polettini, A, Pomi, R, Rolle, E, Ceremigna, D, Propris, LD, Gabellini, M, et al.. A kinetic study of chelant-assisted remediation of contaminated degraded sediment. J Hazard Mater 2006;137:1458–65. https://doi.org/10.1016/j.jhazmat.2006.04.022.Search in Google Scholar PubMed
113. Li, J, Zhang, GN, Li, Y. Review on the remediation technologies of POPs. Hebei: Hebei Environ Sci; 2010:65–8 pp.Search in Google Scholar
114. Krishna, RR, Xu, CY, Supraja, C. Assessment of electrokinetic removal of heavy metals from soils by sequential extraction analysis. J Hazard Mater 2001;84:279–96. https://doi.org/10.1016/s0304-3894(01)00237-0.Search in Google Scholar PubMed
115. Fu, JH. The research status of soil remediation in China. In: 2008 annual meeting of Chinese society for environmental sciences; 2008:1056–60 pp.Search in Google Scholar
116. Mahajan, P, Kaushal, J. Role of phytoremediation in reducing cadmium toxicity in soil and water. J Toxicol 2018;2018:1–16. https://doi.org/10.1155/2018/4864365.Search in Google Scholar PubMed PubMed Central
117. Ingwersen, J, Bücherl, B, Neumann, G, Streck, T. Cadmium leaching from micro-lysimeters planted with the hyperaccumulator Thlaspi caerulescens: experimental findings and modeling. J Environ Qual 2006;35:2055–65. https://doi.org/10.2134/jeq2005.0461.Search in Google Scholar PubMed
118. Sarma, H. Metal hyperaccumulation in plants: a review focusing on phytoremediation technology. J Environ Sci Technol 2011;4:118–38. https://doi.org/10.3923/jest.2011.118.138.Search in Google Scholar
119. Marques, APGC, Rangel, AOSS, Castro, PML. Remediation of heavy metal contaminated soils: phytoremediation as a potentially promising clean-up technology. Crit Rev Environ Sci Technol 2009;39:622–54. https://doi.org/10.1080/10643380701798272.Search in Google Scholar
120. Salt, DE, Smith, RD, Raskin, I. Phytoremediation. Annu Rev Plant Biol 1998;49:643–68. https://doi.org/10.1146/annurev.arplant.49.1.643.Search in Google Scholar PubMed
121. Chaney, RL, Malik, M, Li, YM, Brown, SL, Brewer, EP, Angle, JS, et al.. Phytoremediation of soil metals. Curr Opin Biotechnol 1997;8:279–84. https://doi.org/10.1016/s0958-1669(97)80004-3.Search in Google Scholar PubMed
122. Marques, APGC, Oliveira, RS, Samardjieva, KA, Pissarra, J, Rangel, AOSS, Castro, PML. Solanum nigrum in contaminated soil: effect of arbuscular mycorrhizal fungi on zinc accumulation and histolocalisation. Environ Pollut 2007;145:691–9. https://doi.org/10.1016/j.envpol.2006.06.029.Search in Google Scholar PubMed
123. Marques, APGC, Oliveira, RS, Samardjieva, KA, Rangel, AOSS, Pissarra, J, Castro, PML. EDDS and EDTA-enhanced zinc accumulation by Solanum nigrum inoculated with arbuscular mycorrhizal fungi grown in contaminated soil. Chemosphere 2007;70:1002–14. https://doi.org/10.1016/j.chemosphere.2007.08.045.Search in Google Scholar PubMed
124. Vamerali, T, Bandiera, M, Mosca, G. Field crops for phytoremediation of metal-contaminated land. A review. Environ Chem Lett 2010;8:1–17. https://doi.org/10.1007/s10311-009-0268-0.Search in Google Scholar
125. Jadia, CD, Fulekar, MH. Phytoremediation of heavy metals: recent techniques. Afr J Biotechnol 2009;8:921–8.Search in Google Scholar
126. Arienzo, M, Adamo, P, Cozzolino, V. The potential of Lolium perenne for revegetation of contaminated soils from a metallurgical site. Sci Total Environ 2004;319:13–25. https://doi.org/10.1016/s0048-9697(03)00435-2.Search in Google Scholar PubMed
127. Rizzi, L, Petruzelli, G, Poggio, G, Guidi, GV. Soil physical changes and plant availability of Zn and Pb in a treatability test of phytostabilization. Chemosphere 2004;57:1039–46. https://doi.org/10.1016/j.chemosphere.2004.08.048.Search in Google Scholar PubMed
128. Mendez, MO, Maier, RM. Phytostabilization of mine tailings in arid and semiarid environments - an emerging remediation technology. Environ Health Perspect 2008;116:278–83. https://doi.org/10.1289/ehp.10608.Search in Google Scholar PubMed PubMed Central
129. Ghosh, M, Singh, SP. A review on phytoremediation of heavy metals and utilization of its byproducts. Appl Ecol Environ Res 2005;3:1–18. https://doi.org/10.15666/aeer/0301_001018.Search in Google Scholar
130. Mueller, B, Rock, S, Gowswami, D, Ensley, D. Phytoremediation decision tree. Washington DC: Interstate Technology and Regulatory Cooperation Work Group; 1999:1–36 pp.Search in Google Scholar
131. Henry, JR. An overview of phytoremediation of lead and mercury. Washington DC: NNEMS Report; 2000.Search in Google Scholar
© 2023 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Reviews
- E-waste in Vietnam: a narrative review of environmental contaminants and potential health risks
- Knowledge mapping and research trends of the social determinants of health (SDoH): a scientometric analysis
- Hospital wastewater treatment methods and its impact on human health and environments
- Associated health risk assessment due to exposure to BTEX compounds in fuel station workers
- Associations between fine particulate matter and colorectal cancer: a systematic review and meta-analysis
- Health effects of air pollutant mixtures (volatile organic compounds, particulate matter, sulfur and nitrogen oxides) – a review of the literature
- Status and frontier analysis of indoor PM2.5-related health effects: a bibliometric analysis
- Relationship between parental exposure to radiofrequency electromagnetic fields and primarily hematopoietic neoplasms (lymphoma, leukemia) and tumors in the central nervous system in children: a systematic review
- Blood and hair copper levels in childhood autism spectrum disorder: a meta-analysis based on case-control studies
- Cellular and molecular effects of non-ionizing electromagnetic fields
- Benzo (a) pyrene in infant foods: a systematic review, meta-analysis, and health risk assessment
- Relationship between exposure to heavy metals on the increased health risk and carcinogenicity of urinary tract (kidney and bladder)
- The nexus between economic growth, health expenditure, environmental quality: a comparative study for E7 countries
- Potentially toxic elements in the environment – a review of sources, sinks, pathways and mitigation measures
- Assessment of medical waste generation rate in Viet Nam
- A scoping review of waterborne and water-related disease in the Florida environment from 1999 to 2022
- Effects of man-made electromagnetic fields on heart rate variability parameters of general public: a systematic review and meta-analysis of experimental studies
- Letter to the Editor
- Environmental perspectives of monkeypox virus: correspondence
Articles in the same Issue
- Frontmatter
- Reviews
- E-waste in Vietnam: a narrative review of environmental contaminants and potential health risks
- Knowledge mapping and research trends of the social determinants of health (SDoH): a scientometric analysis
- Hospital wastewater treatment methods and its impact on human health and environments
- Associated health risk assessment due to exposure to BTEX compounds in fuel station workers
- Associations between fine particulate matter and colorectal cancer: a systematic review and meta-analysis
- Health effects of air pollutant mixtures (volatile organic compounds, particulate matter, sulfur and nitrogen oxides) – a review of the literature
- Status and frontier analysis of indoor PM2.5-related health effects: a bibliometric analysis
- Relationship between parental exposure to radiofrequency electromagnetic fields and primarily hematopoietic neoplasms (lymphoma, leukemia) and tumors in the central nervous system in children: a systematic review
- Blood and hair copper levels in childhood autism spectrum disorder: a meta-analysis based on case-control studies
- Cellular and molecular effects of non-ionizing electromagnetic fields
- Benzo (a) pyrene in infant foods: a systematic review, meta-analysis, and health risk assessment
- Relationship between exposure to heavy metals on the increased health risk and carcinogenicity of urinary tract (kidney and bladder)
- The nexus between economic growth, health expenditure, environmental quality: a comparative study for E7 countries
- Potentially toxic elements in the environment – a review of sources, sinks, pathways and mitigation measures
- Assessment of medical waste generation rate in Viet Nam
- A scoping review of waterborne and water-related disease in the Florida environment from 1999 to 2022
- Effects of man-made electromagnetic fields on heart rate variability parameters of general public: a systematic review and meta-analysis of experimental studies
- Letter to the Editor
- Environmental perspectives of monkeypox virus: correspondence

![Figure 4:
Remediation cost for various methods (redrawn from [9, 108]). White fields in columns indicate minimum costs; grey fields give ranges of total costs.](/document/doi/10.1515/reveh-2022-0161/asset/graphic/j_reveh-2022-0161_fig_004.jpg)
