Abstract
In recent years, a significant increase in the amount of research published about the application of eggshells for the removal of metal ions from aqueous solutions has been observed. The paper presents different aspects of metal adsorption from aqueous solutions on untreated eggshells. Pretreatment procedures and tested parameters for the adsorption differ significantly across all the reviewed data, providing a source of variance for the results. For untreated eggshells, the range of the reported BET surface area is from 0.07 m2/g to 8.941 m2/g. Correlation between particle size and BET surface area has been highlighted. Reported removal efficiencies for the untreated eggshell have been compared. Reported results show that eggshell is most employed for the removal of Pb(II), Cd(II), and Cu(II) from aqueous solutions. Eggshell capacity to remove metal ions from the main group elements has also been demonstrated. While results look promising, not enough data are present to make reliable conclusions about its efficiency with other (mainly transition) metal ions – which makes it a possible research direction. Based on the reported data, multiple removal pathways are involved. Several eggshell modification methods and possibilities of creating new adsorbents using eggshells only as a part of the raw material have been assessed. Finally reported eggshell modification methods have been assessed and it is clear that to compare different material’s effectiveness as an adsorbent, comparing only materials adsorption capacities is insufficient. Certain environmental water pollution removal studies using adsorption demand further study, such as metal ion specification in aqueous solution, in different processing water, and even in wastewater.
1 Introduction
Technological advancements bring a plethora of benefits; however, a number of challenges are created simultaneously as well. The increasing volume of toxic waste is one of those challenges. In the European Union, the amount of nonferrous metal waste has increased by 63.5 % in recent years – from 6.96 million tons in 2010 up to 10.95 million tons in 2018 (Eurostat 2022). Since toxicity of metals in aqueous solutions is an everlasting environmental concern, development of efficient water purification methods remains a popular research direction.
Out of available purification methods, the intrinsic advantage of adsorption is the ability to transfer and concentrate the contaminant onto different media, thus improving its potential for future utilization. Calcium carbonate is one such adsorbent. Being one of the most abundant minerals in the Earth’s crust, with its common geological forms being chalk and limestone (Al Omari et al. 2016), its adsorption properties have come into the limelight in recent years.
One of the first studies on calcite adsorption properties toward copper was conducted in 1959 (Heydemann 1959). Later on, (Comans and Middleburg 1987) explored the applicability of a surface precipitation model, originally proposed by (Farley et al. 1985), thereby describing the adsorption process via a mathematical model. Despite this, however, exploration of these properties has been slow until the beginning of 21 century.
The beginning of the new millennium has seen principles of sustainability and circular economy gaining more traction (Geissdoerfer et al. 2017). One of the founding principles of circular economy is focus on reintegration of waste material flows back into the production process, or finding utilization for materials commonly considered waste. Because of that, various production process wastes have been evaluated and some have been found to be a perspective source of calcium carbonate – such as eggshells.
Eggshells according the waste type definitions, used in practice in EU countries, according to (EU 1999) on the landfilling of waste, are biodegradable waste (any waste capable of aerobic or anaerobic decomposition). The industrial eggshell waste resulting from egg breakage is considered an animal by-product (ABP) and thus some constraints may limit eggshells application (Quina et al. 2017). The legal ways of disposing of egg shells in the European Union sets out in the (EC 2008) on waste and repeating certain Directives. The costs associated with the eggshell disposal (mainly on landfilling sites) are significant and expected to continue to increase as landfilling taxes increase (Ahmed et al. 2021).
Applications of the eggshells grouped into two groups: raw material and as a part of operating supply. As part of operating supply, eggshells may be used as sorbents. Although the environmental benefit of those potential applications is generally high, the likelihood of economic profit should be assessed on a case-by-case basis (Quina et al. 2017).
Regarding using eggshells as sorbents for a potential source for removal of metals, no special and sophisticated treatment is required (only washing, drying milling, sieving). Therefore, the development of low-cost adsorbents derived from eggshells could decrease the problem concerning their utilization. However, eggshell has been applied not only in the adsorption field. Eggshells have also been used as a growth promoter (Vu et al. 2022), as well as a raw material for the production of calcium salts and ultrafine calcium carbonate sources – moreover, other potential markets for eggshell waste have been identified, such as steel and paper manufacture (Ahmed et al. 2021), and a very broad and perspective application in production of cement-based materials (Yang et al. 2022) and in the same time reduce the open waste (eggshell) disposal. A comprehensive analysis of the perspective application of eggshells in material science (including producing new catalysts for different applications and other perspective opportunities for use) has been done by (Baláž et al. 2021). The authors (Sarder et al. 2019) show the potential for soil-improving material to be obtained by properly and responsibly organizing the collection of eggshells in small communities (university campuses) from student cafeterias, dormitories, and other locations and storing them properly.
During the last decade, research in utilization of hen eggshells as a low-cost adsorbent has increased significantly. The reason for that is two-fold: hen eggshell consists of 95 % of inorganic materials, of which 98.4 % is calcium carbonate (Gautron et al. 2021), therefore, making its chemical composition similar to abiotic calcium carbonate. Simultaneously, global egg production reached 86.67 million metric tons in 2021 (Shahbandeh 2022). Additionally, modern literature has multiple examples of calcium carbonate from multiple other sources being tested: microbiologically derived calcium carbonate (Liu et al. 2018), coral remains (Ahmad et al. 2012), and oyster shells (Santos et al. 2019a,b; Zhou et al. 2017). However, none of those sources are as widespread and produced in such volumes as hen eggshell.
There is a great deal of variance in reported data about the adsorption process of metals on hen eggshells in recent literature. The main purpose of this work is to agglomerate and compare the available data to identify the sources of this variance, while cross-referencing results, where possible. Priority has been given to studying and comparing characterization methods, experiment designs, and reported results. Following that, the biggest differences have been compared and analyzed.
2 Methodology of the literature search
Figure 1 shows trends in publication tags over recent years in the online search engine Scopus. The use of tags “Calcium AND Carbonate AND Adsorption” and “Eggshell AND Adsorption” in research articles has been increasing over the last three decades; however, the frequency of application of the “Eggshell” tag is lagging behind the application of the “Calcium AND Carbonate” tag. This correlation highlights the graduality of the scientific process involved – research of adsorptive properties of calcium carbonate has been gaining since 1990. Research in the application of eggshell as an adsorption medium began a decade later. The overall growth trend has been linear until 2010, with an exponential surge during 2010–2020.
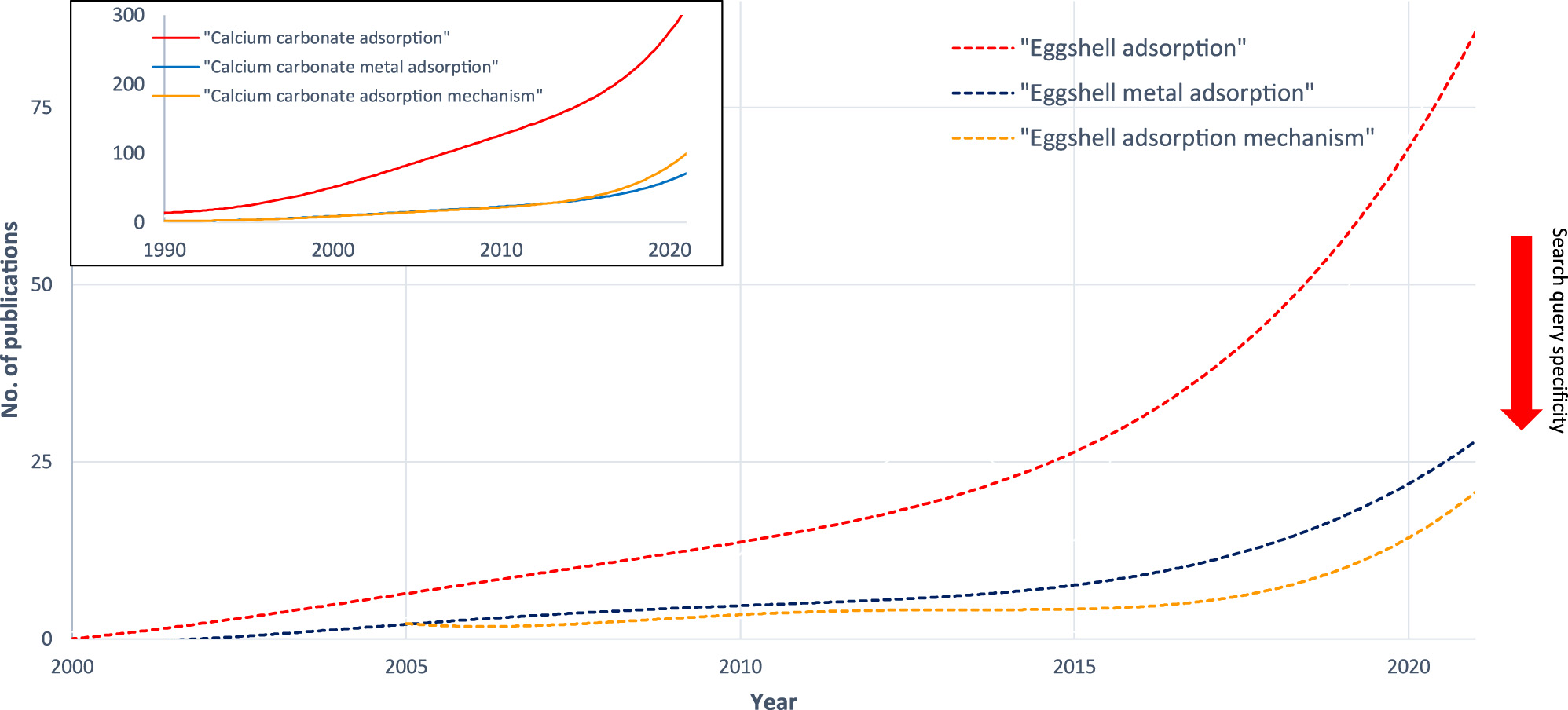
Trends in article tags.
It is common to use extra keywords as a way to specify the research field and the lower number of publications found via the search engine. Providing additional tags to specify search query (“Metal” or “Mechanism”), significantly less results are provided. However, the proportion between general search queries (“Calcium AND Carbonate AND Adsorption” and “Eggshell AND Adsorption”) and specific ones (with additional tags) is not preserved over the years – in fact, the proportion of publications found via the use of specified search tags gets smaller with time. Such correlation may indicate that the effect of a quantitative increase in volume of research has not been properly converted into a qualitative increase and evaluation of existing research is in order.
The original purpose of the research has been focused on collecting information and trying to understand the adsorption mechanism and compare results for the adsorption of metal ions on the unmodified eggshell. However, during the research process, it has been identified that the term “unmodified eggshell” defines different materials between different works. For example, (Abatan et al. 2020; Abbas et al. 2021; Sabah et al. 2018) all use term “pulverized eggshells” to describe their adsorbent, but the preparation method is different in every case: in first case, eggshells were washed, boiled, and then grinded; in second case, they were grinded twice with intermittent washing; in last case, they were simply washed and grinded.
Different preparation procedures leave different amount of eggshell membrane on potential adsorbent, which complicates evaluation of adsorption properties of eggshell itself. Such differences in preparation procedures can impact the amount of remaining eggshell membrane that is left in the material. Since structure of eggshell membrane and eggshell itself is significantly different (Hincke et al. 2012), they should be studied separately to better understand their respective adsorption mechanisms – but large amount of reviewed literature does not specify how much eggshell membrane is remaining in the adsorbent after adsorbent preparation. Eggshell membrane presence should be described as a potential factor that can influence adsorption results and parameters of the adsorbent (e.g., BET area).
Similarly, eggshell treatments that significantly modify the original material also should be identified and separated – since altered material properties may impact the adsorption mechanism. While most such treatments are easily distinguishable (like, for example, in (Basaleh et al. 2020), where eggshells are chemically modified with various aqueous solutions during adsorbent preparation), ball milling in particular presents a special case. Short treatments with ball milling show no impact on the structure of the eggshell, while long treatments start to modify eggshell structure, as described in (Baláž et al. 2015b). This is why, similarly to eggshell, ball milling is mentioned in the review – when ball milling is employed shortly, resulting material is not altered significantly, and can be considered as an eggshell original structure and the data can be compared with other untreated eggshells; but when material have been treated via ball milling for extended periods of time, the original structure of the material has been changed and results cannot be compared to untreated eggshell for our purposes.
Being a popular theme, a number of reviews have been made about adsorption processes on an eggshell. Some prominent examples include (Guru and Dash 2014) discussing common applications of eggshells, including adsorption; (Mittal et al. 2016) describing applications of eggshell and eggshell membrane as an adsorbent; (Singh et al. 2018) depicting general trends in application of adsorbents in water purification, where eggshell has been mentioned; and (Ahmed et al. 2021), which reviews modern biotechnological applications of eggshell.
3 Eggshell origin and its impact on its adsorptive properties
3.1 Factors influencing adsorption capacity of hen eggshell and other biogenic CaCO3
When discussing adsorption of metals on calcium carbonate, the desired property will be adsorption capacity. The origin of the material has a significant influence on the desired property of the material. Some sources have claimed that CaCO3 from biological sources has shown significantly better adsorption capacities of various metals (Liu and Lian 2019a; Zhou et al. 2017) than abiogenic material. In turn, differences in adsorption capacities of material, which uses biogenic CaCO3 to adsorb various pollutants from aqueous media, are caused by such factors as crystallinity of the material (Zhou et al. 2017), the presence of residual functional groups (Sankaran et al. 2020), and material of different crystal structures (Liu et al. 2012). Morphology (crystal size, shape, and surface) is often mentioned as a potential factor that increases adsorption properties of material (Liu et al. 2018; Liu and Lian 2019a; Zhou et al. 2017). It is also common to enhance adsorption properties of material via application of additional organic matter (Soares et al. 2016; Zhou et al. 2018). In calcium carbonate of biogenic origin, most of the mentioned factors are either present naturally or the material is modified before the adsorption process to positively influence the adsorption capacity of the material.
Differences in the crystalline structure of biogenic CaCO3 are caused via its origin. Different animal organisms have different CaCO3 generation pathways (Kahil et al. 2021), often dictated by the environment and evolutionary pressure that species experience. Generally, microbial species produce less stable forms of CaCO3, such as vaterite (Liu et al. 2018; Liu and Lian 2019b); oysters have been shown to produce a mix of aragonite and calcite (Santos et al. 2019a,b) and avian species employ mainly calcite in their biology (Gautron et al. 2021). The distinctive features of an avian eggshell, as compared to bone or teeth, are the nature of the mineral deposit – calcium carbonate in the form of calcite, as well as the absence of cell-directed assembly during its fabrication (Gautron et al. 2021). Chemical and physical structures of eggshells vary significantly between different animal species as well, due to the different CaCO3 forms present and different biochemical compositions of the membrane. The root of such differences lies within the formation process of the egg: different species have different eggshell formation mechanisms, which create different eggshell material (Hincke et al. 2012). The structure of the singular eggshell is not homogenous either. The overall structure of the chicken eggshell is considered to be polycrystalline CaCO3, with the eggshell physical structure classified in layers by depth. From innermost to outermost, those layers are eggshell membranes, consisting of interlacing protein fibers; mammillary layer, consisting of array of calcite crystal cones protruding into membrane layer; palisade layer, made of large columnar units of calcite; vertical crystal layer, perpendicular to shell surface and cuticle layer, consisting of hydroxyapatite crystals and superficial eggshell pigments (Gautron et al. 2021). While chicken eggshell mineral part is about 320 µm thick and is made of dense columnar units arranged perpendicular to the eggshell outer surface, during eggshell formation many eggshell proteins are preferentially adsorbed on calcite crystal faces, thus affecting eggshell microstructure (Gautron et al. 2021). A recent study has also identified a lack of chemical homogeneity – a number of metals have been found to accumulate differently in various eggshell regions in some avian species (Orłowski et al. 2019). It has also been identified that different genotypes of laying hens show different parameters of eggshell quality (Ketta and Tůmová 2016). The presence and impact of these factors on the adsorption capacities of material, produced from hen eggshell, have not been studied. However, due to the scale of these factors, their influence on adsorption properties of the material is unlikely.
Recent study has shown that various eggshell defects have a potential to significantly influence some eggshell characteristics (density, thickness, and shell permeability) during eggshell storage (Cheng and Ning 2023; Wengerska et al. 2023). While impact of this factor may be minimal, due to typical waste eggshell collection procedure, at the present moment, this research direction is not explored.
The main component of hen eggshell is calcite (Ahmad et al. 2012), providing eggshell with its adsorptive properties. While the content of calcium carbonate in eggshell is approximately 95 %, 3.5 % of eggshell is proteins, proteoglycans, and glycoproteins (Guru and Dash 2014). Proteins are arranged into an organic matrix that is composed of roughly 10 % collagen (types I, V, and X) and 70–75 % of other proteins and glycoproteins (such as matrix proteins Ovocleidin-17, Ovocleidin-116, Ovocalyxin-32, Ovocalyxin-36, and Osteopontin) containing lysine-derived cross-links, which are incorporated into the base of the eggshell during its formation process (Hincke et al. 2012). Notably, some sources suggested that embedded proteins inhibit eggshell dissolution in acidic environments, compared to pure calcite (Elabbas et al. 2016).
Utilization of eggshell waste in adsorption processes uncovered some unique properties and provided some new research directions, which go beyond emulation of adsorption process on calcium carbonate. While sharing most of the chemical properties with abiotic calcium carbonate, eggshells may also employ additional separation mechanisms, for example, via eggshell membrane (or its residue) (Santos et al. 2019a,b; Wang et al. 2013).
In Table 3, pH influence on adsorption of metal ions from aqueous solutions onto unmodified eggshell adsorbents can be seen, and in Table 4, influence of other conditions on adsorption of metal ions from aqueous solutions onto unmodified eggshell adsorbents can be seen.
3.2 Eggshell pretreatment
When comparing properties of different materials, it is important to note their differences and similarities. In particular, the preparation procedure of the adsorbent can influence its adsorption capacity and other properties significantly. This is especially important in the case of eggshell application as an adsorbent, due to the eggshell membrane being naturally present. Since eggshell membrane has shown different adsorption properties when compared to the eggshell (Baláž et al. 2016; Ho et al. 2014), the pretreatment procedure can influence the amount of membrane in the final material, while also impacting other physical properties, thereby influencing adsorption properties of the material.
Pretreatment procedures are different across reviewed works, with four common approaches that can be separated. The most common way to treat eggshell before its application as an adsorbent is to wash the eggshell with water, then dry, and mill the eggshell (with separation of a desired fraction using a set of standardized sieves). The second approach involves adding an intermittent milling step with an additional water wash to the previously described procedure, in an attempt to completely remove residual eggshell membrane. The third approach involves the addition of chemical and/or thermal treatment to the procedure described above, aimed at improved eggshell membrane removal. Finally, in some works, pretreatment procedures are kept to a minimum.
The most important impact of the difference in these procedures is the amount of remaining eggshell membrane in the adsorption material. As mentioned before, the adsorption properties of eggshell and eggshell membranes are different; therefore, varying residual amounts of eggshell membrane can significantly affect adsorption properties of the resulting material. The difference in reported drying parameters is also noticeable (analyzed data can be seen in supplemental material, in Table S1). Residual water in the adsorbent can influence its weight, thereby altering the calculated adsorption capacity of the material. However, the overall impact of this is insignificant, because most drying has been either conducted until no change of mass has been observed or the selected parameters guarantee residual water evaporation.
Separated eggshell membranes have been used as an unmodified adsorbent for heavy metals from aqueous solutions (Baláž 2014). Also, similarly to eggshell, eggshell membrane can be modified to improve the adsorption process, with such methods as esterification between thioglycolic acid and oxygen-containing functional groups on eggshell membrane, to prepare thiol-functionalized eggshell membrane for removal of mercury (Cheng et al. 2010); preparation of new hybrid material in which eggshell membranes act as nucleation sites for magnetite nanoparticles, for adsorption of Pb(II) from aqueous solutions (Peigneux et al. 2020); and modification of eggshell membrane with Na2S for adsorption of Cu(I) and Ag(I) ions from aqueous solutions (Xin et al. 2018).
While in all of the cited works, the product of described pretreatment procedures has been considered an untreated eggshell adsorbent, and the preparation procedures differ significantly across the sample size. This fact complicates result comparison and agglomeration and introduces increased uncertainty to the overall reported results. This uncertainty multiplicatively adds up with the fact that eggshell, as biomaterial, has natural variance of its properties.
4 Reported properties of unmodified eggshells during adsorption of metal ions form aqueous solutions
4.1 Adsorption experiment design
Similarly to the eggshell pretreatment conditions, adsorption experiment conditions are important in revealing properties of material. The importance of rigorous reporting on experimental conditions is also exacerbated by the fact that metal removal from solution via eggshell appears to involve several mechanisms in the case of some metals (Hamouda et al. 2020; Hess et al. 2018); the impact and importance of which are influenced by the conditions of the adsorption experiment. Most of the reviewed data are acquired by shaking adsorption material in the solution of adsorbate. Some of the works also describe and/or analyze further application of eggshell adsorbent: for example, Sasikala et al. (2021) describe adsorption experiments, using column filtration; (Pettinato et al. 2015) describe application of eggshell adsorbent in membrane biological reactor; and (Elabbas et al. 2022) describe utilization of eggshell adsorbent coupled with electrocoagulation cell.
In Table 1, batch adsorption experiments conditions from a number of works are collected. In all cases, adsorption experiments include a sample of untreated eggshells; however, the difference in adsorption experiment conditions complicates their comparison. The table shows that nitrates and sulfates are the two most popular sources of metal ions for adsorption experiments. Common eggshell particle sizes tested are below 1 mm. The most common adsorbent range tested is from 1 to 10 g/L, while tested adsorbate concentrations are commonly under 200 mg/L. Impact of the low pH (<7) has been studied more extensively than impact of the high pH. However, significantly fewer experiments have been performed to test the effects of varying temperatures and of different agitation intensities and regimes.
Adsorption experiment conditions for unmodified and modified eggshell adsorbents.
| Metal | Metal source (name) | Eggshell particle size (µm)c | Eggshell dosage tested (g/L)c | Adsorbate concentration tested (mg/L)c | pH tested | Adsorption time (h) | Mixing parameters | Temperature (°C) | Source | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Regime | Mixing intensity (rpm) | Mixing regime (type) | ||||||||
| Al(III) | KAl(SO4)2·12H2O | 0–1000 | 10 | 6 | 3 | Monitored | 2 | 500 | Stirring | 25 | Pettinato et al. (2015) |
| Coal mine impacted watera | 1 | 0.76–9.24 | 19.5 | 3.1 | Monitored | 48 | 30–170 | Shaking | 25 | Jeremias et al. (2020) | |
| Cr(III) | Cr(NO3)3·9H2O | 100 | 1–15 | 100–300 | 3–5 | Initial adjustment | 2 | 150 | Shaking | 20–60 | Chojnacka (2005) |
| Cr(NO3)3 | 74–149 | 2–20 | 25–200 | X | X | 4 | 200 | Shaking | 25 | Kim et al. (2019) | |
| Tanning impacted water | 158.64 | 4–50 | 25–200 | 2–5 | Initial adjustment | 24 | 250 | Shaking | 25–55 | Elabbas et al. (2016) | |
| X | <354 | 10–100 | 0.5–30 | 5 | Initial adjustment | 6 | 120 | Stirring | 25 | Zonato et al. (2022) | |
| Cr(III) | K2Cr2O7 | 80–210 | 25–125 | 50–200 | 2–10 | Initial adjustment | 2.5 | 150 | Stirring | r.t. | Abatan et al. (2020) |
| K2CrO4 | <250 | 10–80 | 10–30 | 3–11 | Initial adjustment | 2 | X | Shaking | r.t. | Alomari (2020) | |
| Mntotal | Coal mine impacted watera | 1 | 0.76–9.24 | 1.2 | 3.1 | Monitored | 48 | 30–170 | Shaking | 25 | Jeremias et al. (2020) |
| Fe(II) | FeSO4·7H2O | 0–1000 | 10 | 6.5 | X | Monitored | 2 | 500 | Stirring | 25 | Pettinato et al. (2015) |
| Fetotal | Coal mine impacted watera | 1 | 0.76–9.24 | 20.1 | 3.1 | Monitored | 48 | 30–170 | Shaking | 25 | Jeremias et al. (2020) |
| Ni(II) | Ni(NO3)2 | 80–210 | 5–90 | 30–200 | 2–10 | Initial adjustment | 3.5 | No agitation | No agitation | 24 | Mashangwa et al. (2017) |
| NiCl2 | <75 | 5–40 | 25–200 | 1–9 | Initial adjustment | 72 | 100 | Shaking | 15–45 | Ho et al. (2014) | |
| Xa | <100 | 5 | 0.531–3.1388 | 5 | Monitored | 3 | 500 | Shaking | X | Makuchowska–Fryc (2019) | |
| Cu(II) | Cu(NO3)2·5H2O | <1000 | 25 | 10–60 | 5.5 | Continuous adjustment | 24 | 120 | Shaking | 25 | Ahmad et al. (2012) |
| Cu(NO3)2·3H2O | 53 | 3–9 | 20–100 | 2–9 | Initial adjustment | 1.5 | 180 | Shaking | 10–50 | Anantha and Kota (2016) | |
| Cu(NO3)2 | 80–210 | 5–90 | 30–200 | 2–10 | Initial adjustment | 3.5 | No agitation | X | 24 | Mashangwa et al. (2017) | |
| Cu + HNO3 | <1000 | 5–15 | 0–80 | 2–9 | Continuous adjustment | 240 | 350 | Stirring | 20–50 | Hess et al. (2018) | |
| CuSO4 | 250–10 000 | 1000 | 5–100 | 3–10 | Initial adjustment | 1 | 80 | Shaking | 25–70 | Khaskheli et al. (2021) | |
| CuSO4·5H2O | 12–14.8 | 5 | 5–60 | 2–6 | Initial adjustment | 2 | 200 | Shaking | X | Mohammad et al. (2022) | |
| CuCl2 | 106–250 | 0.5–5 | 10–50 | 2–5 | Initial adjustment | 48 | 150 | Shaking | 25–50 | Polat and Aslan (2014) | |
| Xa | <100 | 5 | 0.4416–4.3136 | 5 | Monitored | 3 | 500 | Shaking | r.t. | Makuchowska-Fryc (2019) | |
| Zn(II) | Zn(NO3)2 | 80–210 | 5–90 | 30–200 | 2–10 | Initial adjustment | 3.5 | No agitation | No agitation | 24 | Mashangwa et al. (2017) |
| ZnCl2 | 0–1000 | 10 | 12 | X | Monitored | 2 | 500 | Stirring | 25 | Pettinato et al. (2015) | |
| 250–10 000 | 1000 | 5–100 | 3–10 | Initial adjustment | 1 | 80 | Shaking | 25–70 | Khaskheli et al. (2021) | ||
| As(V) | As2O5 | 1500–3000 | 0.5–10 | 0.1 | 2–12 | Initial adjustment | 2 | X | Shaking | 10–45 | Çelebi et al. (2021) |
| X | 132–148 | 0.5 | 1–5 | X | X | 16 | 200 | Stirring | 25 | Alamillo-López et al. (2020) | |
| Sr(II) | SrCl2·6H2O | 22.2 | 10 | 1–100 | 3–11 | Initial adjustment | 3 | X | Shaking | 25–65 | Metwally et al. (2017) |
| Ag(I) | AgNO3 | Xb | 1 | 10–200 | 5 | Initial adjustment | 4 | X | Shaking | r.t. | Baláž et al. (2016) |
| <75 | 5–40 | 25–200 | 1–9 | Initial adjustment | 72 | 100 | Shaking | 15–45 | Ho et al. (2014) | ||
| Cd(II) | Cd(NO3)2 | 80–210 | 25–125 | 50–200 | 2–10 | Initial adjustment | 2.5 | 150 | Stirring | r.t. | Abatan et al. (2020) |
| 80–120 | 1.16–2.84 | 200–400 | 6 | Continuous adjustment | 2.36 | X | Stirring | 23 | Tizo et al. (2018) | ||
| Cd(NO3)2·4H2O | <1000 | 25 | 10–60 | 5.5 | Continuous adjustment | 24 | 120 | Shaking | 25 | Ahmad et al. (2012) | |
| 200–1000 | 0.3–8 | 5–200 | 2.5–7 | Initial adjustment | 2 | 250 | Stirring | 25 | Annane et al. 2023 | ||
| 212 | 0.08–24 | 50–250 | 3–7 | Initial adjustment | 2 | 500 | Shaking | 25 | Sabah et al. (2018) | ||
| 75–250 | 10 | 40–240 | 2–7 | Continuous adjustment | 2 | 150 | X | r.t. | Harripersadth et al. (2020) | ||
| <710 | 1–8 | 3–20 | 2–10 | Initial adjustment | 1.33 | 0–200 | Stirring | 30 | Zadeh et al. (2018) | ||
| 74–149 | 2–20 | 25–200 | X | X | 4 | 200 | Shaking | 25 | Kim et al. (2019) | ||
| 950 | 5 | 20–400 | 4–6 | Continuous adjustment | 168 | Manual | Shaking | 15–35 | Flores-Cano et al. (2013) | ||
| 3CdSO4·8H2O | Xb | 0.5–50 | 100 | 2–6 | Initial adjustment | 2.5 | 250 | Shaking | 20 | Özcan et al. (2018) | |
| Xa | <100 | 5 | 0.3696–8.736 | 5 | Monitored | 3 | 500 | Stirring | X | Makuchowska-Fryc (2019) | |
| X | <354 | 10–100 | 0.5–30 | 7.5 | Initial adjustment | 6 | 120 | Stirring | 25 | Zonato et al. (2022) | |
| Hg(II) | X | 142–159 | 0.5 | 1–5 | X | X | 16 | 200 | Stirring | 25 | Alamillo-López et al. (2020) |
| Pb(II) | Pb(NO3)2 | <1000 | 25 | 10–150 | 5.5 | Continuous adjustment | 24 | 120 | Shaking | 25 | Ahmad et al. (2012) |
| 80–210 | 5–90 | 30–200 | 2–10 | Initial adjustment | 3.5 | No agitation | No agitation | 24 | Mashangwa et al. (2017) | ||
| 75–250 | 10 | 40–240 | 2–7 | Continuous adjustment | 2 | 150 | X | r.t. | Harripersadth et al. (2020) | ||
| 250–10 000 | 1000 | 5–100 | 3–10 | Initial adjustment | 1 | 80 | Shaking | 25–70 | Khaskheli et al. (2021) | ||
| 74–149 | 2–20 | 25–200 | X | X | 4 | 200 | Shaking | 25 | Kim et al. (2019) | ||
| 0.1 M Pb(ClO4)2 | 0–800 | 0.5–10 | 0–70 | 5.5–5.7 | Monitored | 2 | 150 | Shaking | 25 | Hamouda et al. (2020) | |
| X | 25–500 | 10 | 100–1500 | 2–5.5 | Initial adjustment | 3 | X | Stirring | 25 | Soares et al. (2016) | |
| 142–159 | 0.04–0.5 | 1–5 | X | X | 16 | 200 | Stirring | 25 | Alamillo-López et al. (2020) | ||
| Bi(V) | [4BiNO5H2·BiO(OH)] | <0.45 | 1.25–6.25 | 5–50 | 2–12 | Continuous adjustment | 3 | X | Shaking | 20–50 | Abbas et al. (2021) |
-
X, parameter not mentioned in the publication;a, adsorption of multiple metals simultaneously during experiment;b, employed ball milling;c, converted parameters reported in different units.
Parameters like adsorbate and adsorbent dosages, pH, time, and temperature are explicitly reported and taken into account in further analysis. However, such parameters as pH and its control during the adsorption, mixing regime, mixing intensity, and the presence of interfering ions in solution are often overlooked in further analysis, despite their potential impact on the adsorption process. This diversity in conditions contributes negatively when comparing results from different studies, further complicating the evaluation.
4.2 Influence of surface area, particle size, and milling procedures on properties of unmodified eggshell adsorbents
Surface area is often correlated with adsorption capacity of the material and is commonly determined via the BET adsorption method. The results for unmodified, micronized eggshells are presented below in Table 2. Precision and uncertainty errors are represented as in the original publications. All of the presented data have been acquired using nitrogen as an adsorbate. However, only three sources report on the degassing parameters for the method: 150 °C for 1 h (Hamouda et al. 2020), 200 °C for 2 h (Granados-Correa and Jiménez-Reyes 2013), and 100 °C for 24 h (Jeremias et al. 2020).
Reported surface area, pore volume, pore size, and particle size of unmodified eggshell.
| BET surface (m2/g) | Pore volume (cm3 × 103)/g | Pore size (nm) | Particle size (µm) | Source |
|---|---|---|---|---|
| 0.07 | 0.22 | 13.2 | 950 | Flores-Cano et al. (2013) |
| 0.12 | 0.98 | X | 25–500 | Soares et al. (2016) |
| 0.056 | X | X | 150–500 | Hamouda et al. (2020) |
| 0.256 | 1.53 | 25.8 | 500–700 | Sankaran et al. (2020) |
| 0.273 | 1.76 | 23.9 | 300–500 | Sankaran et al. (2020) |
| 0.38 | X | X | 158.64 | Elabbas et al. (2022) |
| 0.406 | 1.00 | X | X | Smirnova et al. (2016) |
| 0.511 | 3.19 | 20 | 200–300 | Sankaran et al. (2020) |
| 0.6563 | 2.373 | 14.4649 | 75–150 | Kim et al. (2019) |
| 0.693 | 3.37 | 19.5 | 100–200 | Sankaran et al. (2020) |
| 0.801 | 3.24 | X | 200 | Markovski et al. (2014) |
| 0.977 | 3.544 | 14.8 | <150 | Hamouda et al. (2020) |
| 1.023 ± 0.339 | 6.5 ± 2.5 | X | 78 | Tsai et al. (2006) |
| 1.09 | 47 | 169.7 | 50–300 | Granados-Correa and Jimenez-Reyes (2013) |
| 1.44 | 1200 | X | X | Mohammad et al. (2022) |
| 1.7114 | 16.32 | 14.27 | 75–250 | Harripersadth et al. (2020) |
| 1.9 | 3.20 | X | 22.2 | Metwally et al. (2017) |
| 1.91 ± 0.12 | 190 | 3.27 | <35 | Choi (2019) |
| 2.33 | 13.473 | 11.567 | <75 | Awogbemi et al. (2020) |
| 2.35 | 13.21 | 22.61 | 200–1000 | Annane et al. (2023) |
| 2.37 | 20 | X | 12–14.8 | Mohammad et al. (2022) |
| 2.5769 | 4.674 | X | 40–100 | Basaleh et al. (2020) |
| 3.26a | 29.482 | 13.8 | <75 | Awogbemi et al. (2020) |
| 4.2 | 10 | X | <150 | Guo et al. (2017) |
| 5.24 | 3.63 | 19.2 | 212.3 | Sabah et al. (2018) |
| 5.692 | 56.7 | X | 1 | Jeremias et al. (2020) |
| 8.941 | 39.5 | 10.9 | 100–300 | Dayanidhi et al. (2020) |
-
a, eggshell has been additionally boiled;X, parameter not mentioned.
As can be seen in Table 2, the reported BET surface area of eggshell varies from 0.070 to 8.9 m2/g.
Figure 2 shows the relation between reported eggshell surface area and average particle size. Few of the cited publications have performed particle size measurements after treatment. In most cases, sieving was used as a separation technique from which the particle size fraction has been assumed. Therefore, in cases where average particle size has not been measured, error bars represent which particle fraction has been analyzed after sieving. Importantly, a common trend can be seen: as particle sizes decrease, the surface area of the material obviously increases.

Reported eggshell surface area to particle size.
A number of treatment methods are reported to have a significant effect on the measured BET surface area of the eggshell. Firstly, (Awogbemi et al. 2020) have reported a significant increase in surface area of an eggshell after boiling in water. Secondly, when ball milling (the classical method for obtaining colloid sized particles) for powder preparation has been employed, (Baláž et al. 2015b) reported a rapid increase in surface areas after prolonged milling, with a maximum observed surface area of 28.4 m2/g after 30 min of milling.
Similarly, modification procedures upon eggshells usually significantly increase the surface volume of the adsorbent as well. For example, after treatment of eggshell powder with a KMnO4 solution, the BET surface area increased significantly from 2.58 to 90.40 m2/g (Basaleh et al. 2020). A similar effect was observed after eggshell calcination as well, with some sources reporting surface areas of 9.80 m2/g (Gurav and Samant 2021).
The surface area of eggshell membrane has also been reported via several sources. While some sources claim it to be significantly larger than untreated eggshell surface area (13.2 m2/g for untreated membrane) (Baláž et al. 2016), others have reported different results, with specific surface area, for most avian eggshell membranes being in a range from 1 to 2 m2/g (Badillo-Camacho et al. 2020). Being an integral part of the egg, the remaining content of the eggshell membrane can significantly affect the surface area of the resulting adsorbent.
Based on the literature review (Table 2), particle size influences the absorbent surface area; however, the adsorption capacity can be differently affected. (Hamouda et al. 2020; Harripersadth et al. 2020) conducted experiments with different particle sizes, comparing the influence of different particle sizes in a range of 75–500 µm, and did not identify any noticeable difference in adsorption capacity. However, comparison of larger particles, as in the case of (Pettinato et al. 2015) (0–1000 µm) and (Khaskheli et al. 2021) (0.25–10 mm), showed noticeable differences in adsorption capacity of similar metal ions, namely, a decrease in adsorption capacity correlated with an increase in particle size. Based on the available data, a sufficiently small (<500 µm) eggshell particle size did not influence the removal efficiency, while still influencing the total surface area of the eggshell.
Different mechanical treatment of eggshell waste appears to influence the efficiency of the adsorption process as well. On the one hand, eggshell milling increased the surface area of CaCO3 (eggshell) at the beginning of milling. (Mohammad et al. 2022) reported differences in Cu(II) adsorption caused by employing different milling methods. Using the ball mill technique for preparation of nanoparticle, eggshell adsorbent increased material adsorption capacity toward Cu(II) slightly, and the rate of Cu(II) adsorption increased significantly. Similarly, (Baláž et al. 2016) reported that intense ball milling increased the surface area of the eggshell, but not the adsorption capacity. However, after prolonged milling (>60 min), calcite in the eggshell began to change to aragonite (Baláž et al. 2016). This, in turn, changed the effectiveness of the adsorption process, so that after 360 min of ball milling, the ability of eggshell material to adsorb Ag(I) ions increased two-fold, while its aragonite content increased to 54.9 % (Baláž et al. 2016). Similarly, in (Balaž et al. 2015a), it has been demonstrated that the phase transformation from calcite to aragonite is a key process leading to an increase adsorption ability of cadmium. Originally, this application of ball milling to the eggshell have been described by (Tsai et al. 2008) – to create porous network in eggshell agrowaste by high-energy ball milling. However, while adsorption capacity of the material has been improved, its structure was altered from its original state, which means it cannot be considered untreated eggshell after such treatment. Therefore, while adsorption capacity of the material has been improved, its structure was significantly altered. On the other hand, milling eggshell membrane (the other part of eggshell waste), the SBET value of starting membrane was 13.2 m2/g, after milling for 5 min, it dropped to 2.4 m2/g, and after 30 min of treatment, it further decreased to 0.7 m2/g (Baláž et al. 2016). After intensive milling of the eggshell, the calcite phase is transformed into aragonite, whereas milling of the eggshell membrane leads only to the deterioration of the fibrous structure (Baláž 2018).
Other successful applications of ball mills to produce very small (even nano-sized) particles are also reported in the literature. A nanoscale sorbent from eggshell using a small ball mill with increased adsorption ability for sorption of Hg(II) and methyl violet (MV) from real wastewaters produced by (Foroutan et al. 2019). Using a small ball mill, (Hassan et al. 2020) obtained eggshell particles that showed enhanced adsorption capacity, removing (Co(III), Pb(II), Hg(II), and Zn(II)) from aqueous solutions and real wastewaters.
4.3 Influence of pH on properties of unmodified eggshell adsorbents
Adsorption capacity of metals depending on pH of the media is a well-documented fact. In Table 3, pH influence on the adsorption process is summarized. In the table, correlation between change in pH and change in adsorption capacity of the material is represented. The green color shows the pH range in which pH increase positively influences adsorption capacity of the material; the red color shows the pH range in which pH increase negatively affects adsorption capacity; the yellow color shows the pH range in which there is no significant impact from pH increase onto adsorption capacity; and the gray color represents the pH range that has not been tested.
pH influence on adsorption of metal ions from aqueous solutions onto unmodified eggshell adsorbents.
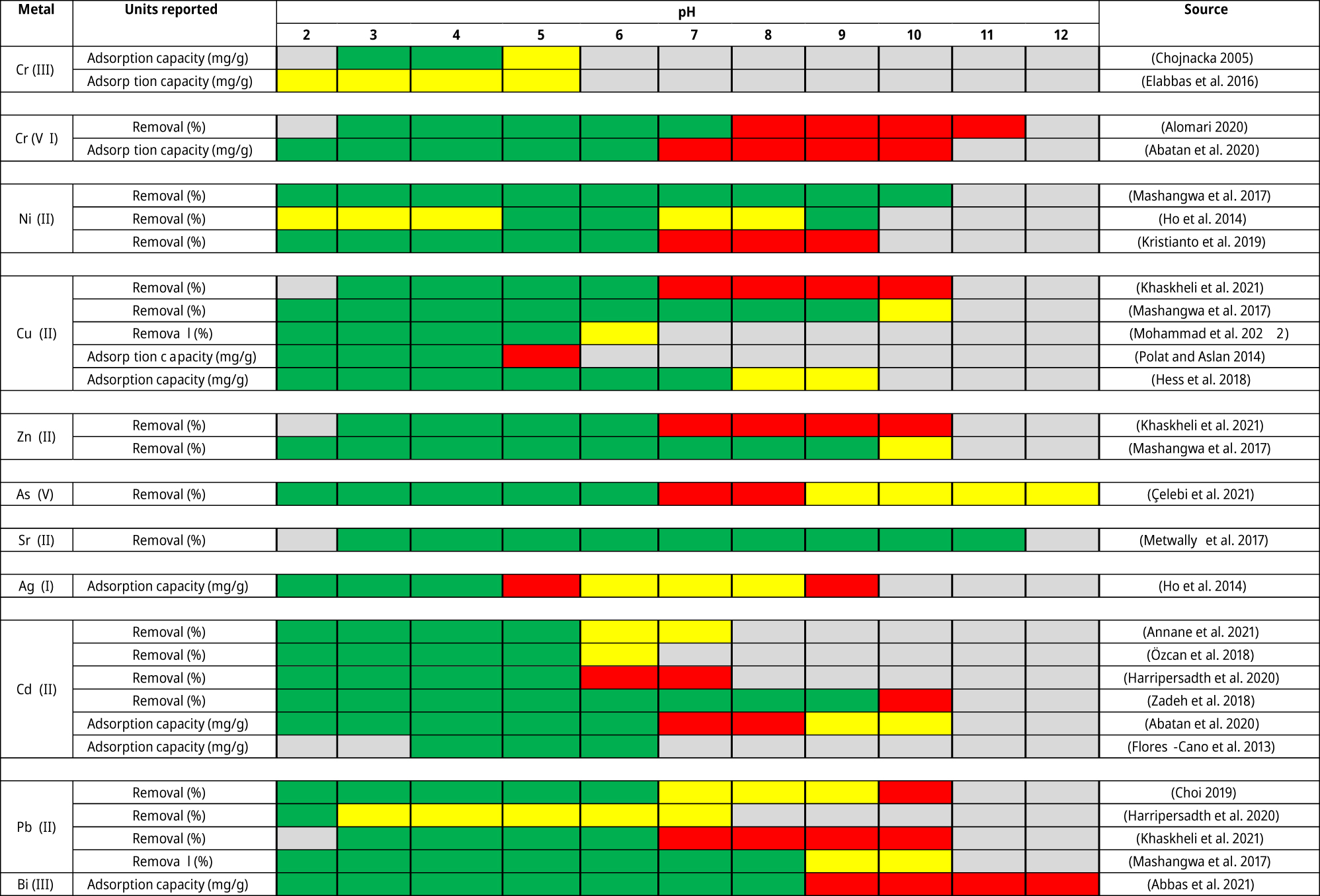
|
-
Green, positive correlation between pH value and eggshell adsorption capacity;Red, negative correlation between pH value and adsorption capacity;Yellow, no pronounced correlation between pH value and eggshell adsorption capacity.
It can be seen that more data are present on the acidic pH range (pH < 7) than the basic pH range. All of the evaluated data have shown an increase in adsorption capacity with an increase of pH in the acidic range (<7). It is impossible to compare quantitative data about adsorption capacities from the presented data, due to the variance in adsorption capacities reported, adsorption experiment design considerations, studied ranges of other parameters, differences in preparation procedures for the adsorbate, etc. Nevertheless, a correlation between pH and adsorption capacity can be observed.
Several common threads emerge in the reviewed data. The following two mechanisms of pH influence on the adsorption process can be identified:
Inhibition of the adsorption process via charge modification of adsorbate surface, through protonation of the adsorbent;
Inhibition of adsorption via the charge modification of metal ions, due to their speciation in aqueous solution at different pH values.
Adsorption of all metal ions at low pH values involves a large amount of protonation (more H+ ions) on the surface of the adsorbent, thus hindering the adsorption capacity of a sorbent (Alomari 2020; Guru and Dash 2014). At a low solution pH, the eggshell surface acquires more positive charge via formation of calcite complexes on the surface via protonation, which in turn hinders adsorption of cations (Flores-Cano et al. 2013; Kobiraj et al. 2012). On the other hand, increasing pH values create negative charges on the surface of the eggshell particle to the media (carbonate groups) (Abatan et al. 2020).
The solution pH values have a particularly important role in the sorption process of chromium, including cationic Cr(III) and anionic Cr(VI) (Table 3). In addition, pH-dependent speciation of Cr(VI) in aqueous solutions (Alomari 2020) has to be considered: at pH 1–6, hydrogen chromate (HCrO4−) is dominant, while at higher pH chromate (CrO42−) is dominant. This suggests that speciation of metal ions in solutions strongly influences removal efficiencies of metals (Chojnacka 2005). However, not all reviewed information correlates with these mechanisms. Some of the reviewed data show no significant change in adsorption capacity with changing pH of Cr(III) (Elabbas et al. 2016). A potential reason for this can be the design of adsorption experiments and the influence of other adsorption parameters, such as equilibrium time, adsorbate/adsorbent correlation, temperature, etc. Similarly, when evaluating adsorption efficiency of Cd(II) on eggshell, (Annane et al. 2023) noted that at pH over 7, Cd(OH)2 can precipitate, thus limiting their evaluation range to the pH lower than 7. However, (Zadeh et al. 2018) tested a higher pH and reported decreased adsorption capacity.
If the adsorption process on eggshells begins in acidic conditions, the solution pH values have been reported to rise. Pettinato et al. 2015 reported an increase in pH from 3 to 7 during the adsorption process. Similar effects were also identified by (Jeremias et al. 2020), underlying the correlation of this effect with eggshell dosage. With sufficient increase in dosage, the solution pH value was reported to rise to ∼8, while lower eggshell dosages did not significantly alter the solution pH. The authors attribute this effect to the CaCO3 capacity of neutralization, with similar neutralization properties being noted in limestone (Hammarstrom et al. 2003). Low pH of the solution has been proven to dissolve part of the CaCO3 of the eggshell (Flores-Cano et al. 2013; Mashangwa et al. 2017). In particular, (Flores-Cano et al. 2013) reported that CaCO3 solubility sharply decreases from 1.8 % of eggshells at pH of 3 to solubility of 0.5 % of eggshell in the pH range from 5 to 10.
It should be noted that measuring only the pH and total concentration of the particular metal ions ignores potential speciation of the mentioned metal in the solution – the metal’s capacity to exist in water in more than one form, for example, chromate (in which Cr is as Cr(VI) as an anion (Alomari 2020) and Cr(III) as an cation (Chojnacka 2005)). Depending on the water solution pH, both water and metal properties, redox chemistry, valence state of heavy metals, and the presence of oxygen and/or other ligands (mainly organic complexing ligands, which undergo reactions with metals ions and change the activity, charge and size of molecule), metal speciation varies significantly (Levina et al. 2017). Historically accepted for describing metal behavior in the aqua is also the pE/pH diagram, also called a Pourbaix diagram. For metals (including transient elements), pE/pH diagrams will define the ox–red situation of the water solution (Green and Perry 2008).
4.4 Reported adsorption capacity of unmodified eggshell adsorbents
Most of the reviewed literature considers the adsorption capacity of unmodified eggshells under different conditions. Table 4 shows a compilation of data on adsorption experiments using unmodified eggshells, showing the highest removal percentage at the highest concentration of the adsorbate reported for each source. The data selection for Table 4 was performed with the view of potential future application of the eggshell material. While in some cases, higher adsorption capacities have been reported under different conditions, and for the adsorption purification method to be economically competitive on an industrial scale, it is necessary to achieve sufficiently low adsorbate concentrations using the lowest amount of adsorbent.
Optimal conditions for metal ions adsorption with unmodified eggshell.
| Metal | Removal efficiency (%) | Adsorbate concentration (mg/L) | Particle size (µm) | Adsorbent dosage (g) | Experiment volume (L) | Temperature (oC) | Agitation (rpm) | Time (h) | Initial pH | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Al(III) | 100 | 6 | 0–400 | 10 | 1 | 25 | 500 | 2 | 3 | Pettinato et al. (2015) |
| 100 | 19.5a | 1 | 1.318 | 0.2 | 25 | 95 | 24 | 3.1 | Jeremias et al. (2020) | |
| Cr(III) | 98.4 | 50 | 74–149 | 2.5 | 0.5 | 25 | 200 | 4 | X | Kim et al. (2019) |
| 99 | 3210 | 158.64 | 1 | 0.05 | 25 | 250 | 24 | 5 | Elabbas et al. (2016) | |
| X | 200 | 100 | 0.5 | 0.1 | 20 | 150 | 2 | 5 | Chojnacka (2005) | |
| 95.65 | 0.5 | <354 | 1.0 | 0.05 | 25 | 120 | 6 | 5 | Zonato et al. (2022) | |
| Cr(VI) | 88.4 | 50 | 80–210 | 5 | 0.2 | r.t. | 150 | 2 | 6 | Abatan et al. (2020) |
| X | 10 | <250 | 2 | 0.05 | r.t. | X | 1 | 7 | Alomari (2020) | |
| 70.7 | 38.28 | <500 | 2 | 0.05 | r.t. | 150 | 0.5 | 5 | Katha et al. (2021) | |
| Mntotal | 58.33 | 1.2a | 1 | 5.6 | 0.2 | 25 | 280 | 24 | 3.1 | Jeremias et al. (2020) |
| Mn(II) | X | 100 | 150 | 0.5 | X | 25 | 185 | 1 | 6 | Altameemi and Thuraya (2021) |
| Fe(II) | 74 | 6.5 | 0–400 | 10 | 1 | 25 | 500 | 2 | 3 | Pettinato et al. (2015) |
| Fetotal | 100 | 20.1a | 1 | 1.318 | 0.2 | 25 | 95 | 24 | 3.1 | Jeremias et al. (2020) |
| Ni(II) | 70 | 3.1388a,b | <100 | 1 | 0.2 | r.t. | 500 | 3 | 5 | Makuchowska-Fryc (2019) |
| 94 | 100 | 80–210 | 7 | 0.1 | 24 | No agitation | 6 | 7 | Mashangwa et al. (2017) | |
| 73.1 | 100 | <75 | 0.2 | 0.02 | 45 | 100 | 24 | 5.76 | Ho et al. (2014) | |
| Cu (II) | 94 | 100 | 250 | 20 | 0.02 | 25 | 80 | 1 | 6 | Khaskheli et al. (2021) |
| 90 | 4.28a,b | <100 | 1 | 0.2 | r.t. | 500 | 3 | 5 | Makuchowska-Fryc (2019) | |
| 99.73 | 20b | <1000 | 1 | 0.04 | 25 | 120 | 24 | 5.5 | Ahmad et al. (2012) | |
| 95 | 100 | 80–210 | 7 | 0.1 | 24 | No agitation | 6 | 7 | Mashangwa et al. (2017) | |
| X | 40 | <1000 | 1 | 0.1 | 20 | 350 | 240 | 2.2 | Hess et al. (2018) | |
| 97.21 | 20 | 12–14.8d | 0.5 | 0.1 | X | 200 | 2 | 5 | Mohammad et al. (2022) | |
| 89 | 25 | 106–250 | 0.5 | 0.1 | 25 | 150 | 24 | 4 | Polat and Aslan (2014) | |
| 95.2 | 25 | <250 | 0.01 | 0.006 | 25 | 100 | 24 | 5 | Chou et al. (2023) | |
| Zn(II) | 83 | 100 | 250 | 20 | 0.02 | 25 | 80 | 1 | 6 | Khaskheli et al. (2021) |
| 59 | 12 | 0–400 | 10 | 1 | 25 | 500 | 2 | 3 | Pettinato et al. (2015) | |
| 80 | 100 | 80–210 | 7 | 0.1 | 24 | No agitation | 6 | 7 | Mashangwa et al. (2017) | |
| As(V) | 91.43 | 0.1 | 1500–3000 | X | X | 25 | X | 2 | 5.97 | Çelebi et al. (2021) |
| Sr(II) | 49.9 | 10 | 22.2 | 0.05 | 0.005 | 25 | X | 3 | 6 | Metwally et al. (2017) |
| Ag(I) | 59.8 | 100 | <75 | 0.2 | 0.02 | 45 | 100 | 24 | 4 | Ho et al. (2014) |
| X | 80 | Xd | 1 | X | r.t. | X | 4 | 5 | Baláž et al. (2016) | |
| Cd(II) | 90 | 0.8736a,b | <100 | 1 | 0.2 | r.t. | 500 | 3 | 5 | Makuchowska-Fryc (2019) |
| 97.98 | 10b | <1000 | 1 | 0.04 | 25 | 120 | 24 | 5.5 | Ahmad et al. (2012) | |
| 98.76 | 36.74 | 212 | 2.98 | 0.1 | 44 | 500 | 2 | 7 | Sabah et al. (2018) | |
| 85 | 50 | 80–210 | 5 | 0.2 | r.t. | 150 | 1.5 | 6 | Abatan et al. (2020) | |
| 96 | 100 | Xd | 3.5 | 0.1 | 20 | 250 | 60 | 5 | Özcan et al. (2018) | |
| 75 | 40 | 75 | 1 | 0.1 | r.t. | 150 | 2 | 5.5 | Harripersadth et al. (2020) | |
| X | 350 | 950 | 0.2 | 0.04 | 25 | Manual | 168 | 6 | Flores-Cano et al. (2013) | |
| 96 | 20 | <710 | 0.5 | 0.1 | 30 | 200 | 1.33 | 9 | Zadeh et al. (2018) | |
| 52 | 50 | 74–149 | 2.5 | 0.5 | 25 | 200 | 4 | X | Kim et al. (2019) | |
| 99.5 | 100 | 200–1000 | 0.02 | 0.025 | 25 | 250 | 0.17 | 5 | Annane et al. 2023 | |
| 73 | 150 | 80–120 | 0.75 | X | 23 | X | 1.25 | 6 | Tizo et al. (2018) | |
| 99.82 | 0.5 | <354 | 1.0 | 0.05 | 25 | 120 | 6 | 7.5 | Zonato et al. (2022) | |
| Pb(II) | 97 | 100 | 250 | 20 | 0.02 | 25 | 80 | 1 | 6 | Khaskheli et al. (2021) |
| 96 | 100 | 80–210 | 7 | 0.1 | 24 | No agitation | 6 | 7 | Mashangwa et al. (2017) | |
| 97.97 | 60b | <1000 | 1 | 0.04 | 25 | 120 | 24 | 5.5 | Ahmad et al. (2012) | |
| 99 | 30 | 150–500 | 0.5 | 0.1 | 25 | 150 | 2 | 5.5 | Hamouda et al. (2020) | |
| 99.6 | 50 | 74–149 | 2.5 | 0.5 | 25 | 200 | 4 | X | Kim et al. (2019) | |
| 100 | 100 | 25–500 | 1 | 0.1 | 25 | X | 3 | 5 | Soares et al. (2016) | |
| 99.55 | 240 | 75 | 1 | 0.1 | r.t. | 150 | 2 | 5.5 | Harripersadth et al. (2020) | |
| Bi(II) | X | 50 | <0.45 | 0.25 | 0.04 | 40 | X | 0.75 | 8 | Abbas et al. (2021) |
-
a - Simultaneous adsorption;b - values converted from mmol to mg;X – parameter not mentioned;r.t. – room temperature.
It can be seen that most of the available adsorption data of unmodified eggshell in aqueous solutions are about removal of Cd(II) (11 publications), Pb(II) (7 publications), and Cu(II) (7 publications) (see Table 4). Considering that none of the other elements (Al(III), Cr(III), Cr(VI), Mntotal, Fe(II), Fetotal, Ni(II), Zn(II), As(V), Sr(II), Ag(I), Bi(II)) have more than three publications, this highlights those elements as point of interest. In most cases, the highest removal efficiency has been achieved at metal concentrations below 100 mg/L, with adsorption capacity not exceeding 10 mg/g. With few exceptions, pH of the adsorption procedure was acidic or neutral (pH < 7). Similarly, temperature is reported to be in the range of 20–25 °C. No distinguishable trend can be identified when analyzing adsorption time and agitation intensity employed.
Further conclusions can be made based on the data shown in Table 4. Data available on Pb(II) have shown that eggshell material can provide almost complete Pb(II) removal from aqueous solutions with concentrations up to 100 mg/L and even higher under optimal conditions. Similarly, the upper concentration limit for complete Cd(II) removal was around 100 mg/L, while for Cu(II), most literature reports 20 mg/L, with only a few sources mentioning higher values. Eggshell materials have also shown the capacity to remove Ni(II) and Cr(III) species with relatively high efficiency (up to 100 mg/L). Various other metals, such as Al(III), Ag(I), As(V), Cr(VI), Zn(II), Sr(II), Bi(II), and all Fe and Mn species, have also shown the ability to be absorbed onto eggshell. In some of these cases reported results look promising, such as reported adsorption capacity of Bi(II) on eggshell of 891.29 mg/g (Abbas et al. 2021). However, the amount of reviewed data for adsorption of those metals on untreated eggshell is insufficient to make any conclusions.
While it is compelling to draw additional conclusions from the presented data, due to the fact that many adsorption parameters differ significantly, few overarching conclusions can be made. Various factors complicate reliable evaluation and comparison of reviewed data:
In some cases, the reported pH of the adsorption process has been adjusted only at the beginning of adsorption (Annane et al. 2023), while in other cases, continuous adjustment of the pH has been done (Ahmad et al. 2012);
Reported adsorption time varies significantly: some experiments report on adsorption experiments with an adsorption time of 1 h (Khaskheli et al. 2021), while in some cases, adsorption takes several days (48 h (Polat and Aslan 2014));
Reported mixing intensity also varies significantly: no agitation (Mashangwa et al. 2017), manual agitation (Flores-Cano et al. 2013), and agitation up to 500 rpm (Pettinato et al. 2015) have all been reported;
As mentioned before, pretreatment methods for eggshell also differ: in some cases, eggshell has only been washed, dried, and pulverized before adsorption (Chojnacka 2005), while in others, additional cleaning steps have been employed – like additional treatment of eggshell with sodium hypochloride solution (1.3 %) during the pretreatment procedure (Alamillo-López et al. 2020).
Finally, while most experiments report on metal adsorption from a solution with one type of metal ions as a pollutant, in some cases, adsorptions have been performed on a solution with multiple metal ions present (Jeremias et al. 2020).
4.5 Influence of temperature on properties of unmodified eggshell adsorbents
For most of the reported data, experiments designed to evaluate the effect of the temperature on the adsorption capacity and have shown an increase in adsorption capacity of the material with increasing temperature of the experiment (Flores-Cano et al. 2013; Granados-Correa and Jiménez-Reyes 2013; Hess et al. 2018; Khaskheli et al. 2021). For example, increasing adsorption temperature from 10 °C to 45 °C increased As(V) adsorption efficiency from 76.51 % to 87.21 % (Çelebi et al. 2021). Additionally, (Polat and Aslan 2014) also reported that temperature increase (from 25 °C to 50 °C) caused a two-fold increase in the release of Ca(II) ions into adsorbate solution during the adsorption process in acidic conditions. Notably, (Hess et al. 2018) underlined a change in adsorption isotherm (Langmuir) parameters with the temperature increase. Regardless of the metal adsorbed, thermodynamic analysis of adsorbing metal ions from aqueous solutions on unmodified eggshell have been described as endothermic, based on positive enthalpy (ΔH) of the process: 49.4 kJ/mol for Cd(II) (Flores-Cano et al. 2013); 25 kJ/mol for Cu(II) (Hess et al. 2018); 28.2 kJ/mol for Eu(III) (Granados-Correa and Jiménez-Reyes 2013); and 23.589 kJ/mol for As(V) (Çelebi et al. 2021). On the other hand, reported changes in Gibbs energy (ΔG) are not as uniform: while for As(V) and Eu(III) Gibbs energy, change is reported to be negative (Çelebi et al.2021; Granados-Correa and Jiménez-Reyes 2013), which identifies adsorption process as spontaneous, evaluation of Cu(II) adsorption have revealed positive change in ΔG (Hess et al. 2018).
The most studied temperature range for the adsorption process on eggshell is 20–40 °C, with only a few sources having tested the impact of higher temperatures on the adsorption process. While commonly the temperature increase correlates with the adsorption capacity increase, (Abbas et al. 2021) reported a slight decrease in Bi(III) adsorption capacities at temperatures >40 °C, explaining the efficiency decrease through polymerization of functional groups on the adsorbent. Similarly, (Khaskheli et al. 2021) reported that for Pb(II), Zn(II), and Cu(II), the best adsorption capacities were obtained at 65 °C, with further temperature increase lowering adsorption efficiency.
4.6 Potential adsorption mechanism of unmodified eggshell adsorbents
Based on the reviewed literature, adsorption of metals on eggshell occurs through multiple mechanisms, both nonreversible and reversible. The complete interactions between mechanisms of removal remain a theme for the debate at large, with multiple theories proposed.
Most authors have found that metal ion removal from aqueous solutions is a physical process, happening on the surface of the material. (Liu et al. 2012; Liu and Lian 2019a,b; Ahmad et al. 2012) suggested that adsorption of metals onto eggshell is dependent of available binding sites, and decrease in adsorption capacity is caused by a decreasing number of available binding sites. However, when comparing adsorption efficiencies of oyster shells CaCO3 versus adsorption of geological CaCO3, (Zhou et al. 2017) proposed that adsorption of metal ions is not only a physicochemical process that takes place on the surface but metal removal also happens through permeation and diffusion of metal ions into the bulk of the material, which have been determined via microstructure study. Alternatively, chemisorption is often discussed as a potential pathway for the adsorption on the surface of the material for most metal ions, often correlated with theorized monolayer adsorption (Ahmad et al. 2012; Charazińska et al. 2022).
There is no unity in describing the adsorption process on eggshell: some describe it as a physical process (Çelebi et al. 2021), some as chemisorption (Flores-Cano et al. 2013), and some as a combination of physical and ion-exchange processes (Hess et al. 2018).
Solubility of CaCO3 in acidic conditions appears to play a substantial role in removal mechanisms. Multiple sources confirm the presence of Ca(II) ions and an increase of pH of aqueous solutions after treating them with eggshell (Jeremias et al. 2020; Wen et al. 2020). Moreover, both cited sources reported higher concentrations of Ca(II) ions in solutions after adsorption in acidic conditions. This indicates that a portion of CaCO3 is dissolved, introducing carbonates into the solution (with more calcium carbonate being dissolved at a lower pH). Effectiveness of the removal mechanism is then attributed to the solubility of hydroxides and carbonates of the adsorbate. For example, (Makuchowska-Fryc 2019) identified metal precipitation in the form of carbonates and hydroxides as the dominant removal mechanism for Cd(II), Cu(II), and Ni(II), citing low solubility values for carbonates and hydroxide of respective metals: (NiCO3/Ni(OH)2 ⇒ 10−7/10−16; CuCO3/Cu(OH)2 ⇒ 10−10/10−20; CdCO3/Cd(OH)2 ⇒ 10−12/10−15).
However, some of the research points to ion exchange as a potential adsorption mechanism (Song et al. 2021; Wen et al. 2020). Some sources claim that ion exchange is more pronounced when adsorption of metal ions with a higher atomic number (Cd(II), Pb(II)) takes place (Wang et al. 2020; Zhou et al. 2017); similarly, Usman 2008 proposed that selectivity of the adsorption process in the case of metal ions is related to their properties, such as ionic radii, atomic weight, electronegativity, the hydrolysis constant, and softness. (Wen et al. 2020) concluded that calcium carbonate adsorbs Cu(II), Mn(II), Zn(II), and Ni(II) substitutionally, releasing Ca(II) and distorting the calcite lattice. In the case of adsorption of Cu(II) and Zn(II), distortion became severe enough to qualify for mineral phase change – from CaCO3 to posnjakite (Cu4(SO4)(OH)6·H2O) and hydrozincite (Zn5(CO3)2(OH)6). On the other hand, (Mohammad et al. 2022) proposed that interaction of Cu(II) ions with CaCO3 would involve electrostatic physical forces such as van der Waal’s and hydrogen bonding, where Cu(II) ions are held onto the surface via outer-sphere complexation.
During Cd(II) removal from solution, (Flores-Cano et al. 2013) noticed formation of crystals on the surface of the adsorbent after the adsorption process. Elemental analysis of surface crystals determined that mass percentages of Cd(II) and Ca(II) were 41.9 % and 12.0 %, respectively. However, when analyzing the eggshell surface without crystals, the mass percentages of Cd(II) and Ca(II) were 19.8 % and 21.3 %, respectively. These results indicate that the process of Cd(II) adsorption involves both precipitation and ion exchange pathways, in agreement with the conclusion of (Song et al. 2021). Moreover, the precipitation pathway was also proven experimentally by (Hamouda et al. 2020). When treating Pb(II) ion solution with water that was previously treated with eggshell, Pb(II) precipitation was observed without any direct contact with the eggshell. These results show that precipitation is a major contributor to Pb(II) removal effectiveness.
The described effects have been identified previously on abiogenic CaCO3 as well. The fact that divalent metal ions are incorporated into the CaCO3 lattice by adsorption and precipitation has been known for some time (Papadopoulos and Rowell 1989; Schosseler et al. 1999).
When adsorbed on the CaCO3 surface, metal ions are rapidly dehydrated and coordinated into monodentate complexes on the surface. Zn(II)/Cu(II) ions can form what was described as surface-solid solutions; however, that process is disrupted by precipitation of more stable hydroxides or hydroxycarbonates (Papadopoulos and Rowell 1989). In the case of Cu(II) specifically, its strong surface binding is caused by its ability to coordinate between carbonate surface ions on the surface of the eggshell. The resulting local lattice distortions are expected to destabilize the Cu x Ca(12x)CO3 solid solution. Finally, upon recrystallization, the Cu(II) ions are integrated into the calcite lattice (Schosseler et al. 1999). Some recent research suggests that the adsorption mechanism is in fact separated into two parts: metal ions initially precipitate via CaCO3 present in the sorbent particles, followed by adsorption of the metal carbonate precipitate from the eggshell surface (Soares et al. 2016).
Theoretically, this mechanism applied to different metal ions would result in different interactions and structures. This has been identified by (Wen et al. 2020), who postulates that during the adsorption of Cu(II), Mn(II), Zn(II), and Ni(II), changes in Ca(II) concentration, pH value, and adsorption capacity have proven different migration rules for different metal ions. The release of Ca(II) and Mg(II) ions into solution during adsorption of Pb(II) on eggshell, which have been identified by (Hamouda et al. 2020), supports this point as well.
The presence of the remnant functional groups on the surface of the biogenic CaCO3 have also been considered as a potential removal pathway (Kınaytürk et al. 2021; Zhou et al. 2017). While the effectiveness of already present functional groups as an adsorption mechanism have been debated (Abbas et al. 2021; Baláž et al. 2016), some efforts in modifying existing functional groups for better adsorption have been successful (Zhou et al. 2018).
Adsorption of different metal ions has been proven to be a competitive mechanism. (Abbas et al. 2021) have reported decreased adsorbance of a target ion, Bi(III) in the presence of increased concentrations of Zn(II) and Mg(II) in solution. (Jeremias et al. 2020) identified that Fetotal and Al(III) ions are outcompeting Mntotal ions during adsorption onto eggshell. Similar effects have been identified by (Baláž et al. 2016), who attributed this effect to competition for adsorption sites. This correlates with the cation competition effect, which (Farley et al. 1985) have observed in surface precipitation model. (Zadeh et al. 2018) have also noted that there is competition between hydrogen cations and Cd(II) to occupy active sites of the adsorbent, which lead to lower adsorption at lower pH, during adsorption of Cd(II) on unmodified eggshell. Similar effect is also observed by (Abatan et al. 2020; Ho et al. 2014; Khaskheli et al. 2021; Kristianto et al. 2019; Metwally et al. 2017) during adsorption of various metal ions from aqueous solutions on eggshell.
4.7 Reported adsorption isotherms and kinetic models of unmodified eggshell
Certain similarities can be defined, when comparing reported results of applying isotherms and kinetic models to the adsorption data. In the reviewed literature, kinetics data of the adsorption process of metal ions on untreated eggshell are most often described best with pseudo-second-order kinetics. This adsorption model is reported as the best fit for following metal ion adsorption kinetic data: Cr(III) on unmodified eggshell (Chojnacka 2005; Elabbas et al. 2016; Kim et al. 2019); Cr(VI) on unmodified eggshell (Abatan et al. 2020) and on eggshell, coated with Fe2O3 (Alomari 2020); Pb(II) on eggshell-sericite adsorbent (Choi 2019) and unmodified eggshell (Kim et al. 2019); Eu(III) on unmodified eggshell (Granados-Correa and Jiménez-Reyes 2013); As(V) on eggshell with eggshell membrane residue (Çelebi et al. 2021); Bi(III) on unmodified eggshell (Abbas et al. 2021); Cd(II) on unmodified eggshell (Annane et al. 2023; Kim et al. 2019; Tizo et al. 2018; Zadeh et al. 2018); Cu(II) on unmodified eggshell (Hess et al. 2018; Polat and Aslan 2014; Mohammad et al. 2022); and Sr(II) on eggshell, modified with phosphotungstic acid (Metwally et al. 2017).
Most of the reported data utilize a linearized equation, with only two (Çelebi et al. 2021; Hess et al. 2018) using a nonlinearized equation. During linearization of adsorption data, it is assumed that variance of amount of adsorbate adsorbed by the adsorbent is equal for all measurements. When variance of the data is not equal for all data points, linearization may result in overestimating the goodness of fit as measured by the correlation coefficient (R), which translates into error in the coefficient of determination (R2) and also in the adjusted coefficient of determination (Lima et al. 2015). Such prevalence of a pseudo-second-order model is explained by (Revellame et al. 2020). Due to commonly applied linearization techniques onto kinetic adsorption data, the pseudo-second-order model becomes highly favored, especially when evaluating prevalence of model validation through the coefficient of determination (R2).
Reported results about adsorption isotherms are not as homogenous as kinetics data. While some reports indicate that their experimental data for metal ion adsorption on eggshells are better described by Freundlich model, others identify Langmuir as a better-fitting adsorption isotherm. Langmuir isotherm assumes that adsorption happens in a monomolecular layer, while Freundlich isotherm assumes formation of multiple layers of adsorbent (Lima et al. 2015). As it can be seen in Table S2, in most cases, Langmuir isotherm model is identified as a best fitting model, which would imply that the adsorption of metal from aqueous solution on eggshell is similar to monolayer adsorption.
It is worth noting, however, that in most cases, the R2 values for both isotherms in question are relatively close. In some cases, application of combined Langmuir and Freundlich isotherms yields a better correlation. For example, (Granados-Correa and Jiménez-Reyes) 2013 described adsorption of Eu(III) on eggshells using the Langmuir–Freundlich isotherm; according to (Alamillo-López et al. 2020), the adsorption process for Pb(II) and As(V) ions on modified eggshell is best described by using the Redlich–Peterson isotherm. Finally, (Hess et al. 2018 employed the Dubinin–Radushkevich (D–R) isotherm to describe the adsorption of Cu(II) ions on eggshells (analyzed data can be seen in Supplementary Material, Table S2).
The same trend persists in the description of isotherms and kinetic data for modified eggshell and eggshell-derived adsorbents. For instance, Pb(II) adsorption on calcinated eggshell (Choi 2019); Cd(II) and Pb(II) adsorption on eggshell-derived aragonite (Habte et al. 2020); Cu(II) adsorption on eggshell-chitosan adsorbent (Anantha and Kota 2016); Cu(II) and Ni(II) adsorption on eggshell-derived hydroxyapatite (Ayodele et al. 2021); and Pb(II) adsorption on KMnO4-modified eggshell (Basaleh et al. 2020) all exhibited pseudo-second-order kinetics. At the same time, no such uniformity was observed for adsorption isotherms. For Cd(II) and Pb(II) adsorption on aragonite (Habte et al. 2020) and Pb(II) adsorption on modified eggshell (Basaleh et al. 2020), the Langmuir isotherm was reported as the best fitting model, while Cu(II) adsorption on eggshell-chitosan adsorbent (Anantha and Kota 2016), Cu(II) and Ni(II) adsorption of on eggshell-derived hydroxyapatite (Ayodele et al. 2021), and Pb(II) adsorption on calcinated eggshell (Choi 2019) were best described by using Freundlich isotherm.
4.8 Desorption experiments with unmodified eggshell
Of the reviewed literature, only a few of the studies included desorption studies in the reported results. Similarly to the adsorption data reported, a great deal of variance in the desorption experiment design is present between different works, with such parameters as desorption effluent, adsorbate concentration, mixing regime, contact time, etc., all being significantly different, which further complicates evaluating and comparing the reported results. This coupled with the great variance already existing in adsorption starting conditions and results in the ability to draw only very few conclusions on the matter.
In the available literature, nitric acid is reported to have the highest desorption rates: for Cd(II), with 96 % recovery was achieved using nitric acid with a concertation of 0.1 M (Annane et al. 2023), and for Sr(II), with 96.4 % recovery using nitric acid with a concertation of 1.0 mol/L (Metwally et al. 2017; Mohammad et al. 2022) also reported an efficient application of EDTA (0.1 M) as a desorption effluent for Cu(II) ions, with desorption rates of over 90 % under optimum conditions (2 h contact time). Simultaneously, desorption experiments that use water as an effluent all have been reported to have low recovery rates with an average value of desorption of 32 % for Cd(II), with a contact time of 7 days (Flores-Cano et al. 2013), and 3.5 % and 6.3 % for Ag(I) and Cd(II), respectively, with a contact time of 6 h (Baláž et al. 2016). Notably, several studies have reported the application of CaCl2 solutions as effluent in desorption experiments: in the case of Cd(II), the maximal recovery rate was 45.6 % at optimum conditions, with a CaCl2 concentration of 1.0 mol/L (Sabah et al. 2018), and with Cu(II) only up to 4 % of present metal content was recovered, with a CaCl2 concentration of 140 mg/L (Hess et al. 2018).
5 Reported eggshell modification methods
5.1 Eggshell calcination
One of the popular pretreatment methods for eggshell adsorbents is the generation of lime via thermal treatment of the eggshell. The process is known as calcination and is widely applied in cement production. Calcination includes the treatment of eggshell at temperatures over 700 °C and has been commonly applied in modification of adsorbent material for the improvement of adsorption properties (Gurav and Samant 2021; Kristianto et al. 2019; Park et al. 2007; Yusuff 2017). Due to the high treatment temperatures, this method alters the original structure of the eggshell, in which the remaining organic matter is destroyed, the porosity of the material is increased (Park et al. 2007), and chemical composition changes mostly to CaO (Yusuff et al. 2017).
Figure 3 shows the scanning electron microscopy images for raw (Figure 3a and b) and calcined (Figure 3c) eggshells pore size distribution and surface morphology. Raw egg shells are porous, but the pores are very narrow and tiny, which also explains the material’s small specific surface area and small total pore volume. As a result of the calcination process, the specific surface area and porosity of the sorbent increases.

SEM micrographs of untreated (a and b) and calcined (c) eggshell powders. Scale bars: (a and c) 2 µm and (b) 10 µm. The morphology of both materials was also investigated by scanning electron microscopy (Mira/LMU, Tescan, Czech Republic) using an accelerating voltage of 15 kV. Eggshell powders were spread on a double-sided carbon tape adhered to the SEM sample holder. Before imaging, the samples were coated with a thin layer of gold.
All these changes lead to alterations in the metal ion adsorption mechanisms. The adsorption process of metal ions on calcinated eggshell is reported to be exothermic and achieving equilibrium much faster than for untreated eggshell (Gurav and Samant 2021; Kristianto et al. 2019), with the maximum achievable amount of adsorbed metal significantly increasing (Kristianto et al. 2019; Park et al. 2007). Detected changes in the adsorption process suggest a change of the adsorption mechanism to chemisorption.
Calcinated eggshell is also employed as a precursor material for generation of complex adsorbents. For example, (Ayodele et al. 2021) described a method for creation of hydroxyapatite for metal ion removal from aqueous solutions; (Markovski et al. 2014) utilized a mixture of calcinated eggshell and goethite as an adsorbent; and (Choi 2019) performed the calcination procedure on mixtures of eggshell with sericite to produce efficient adsorbent for Pb(II) removal. All these examples highlight the fact that eggshell calcination increases reactivity and surface area of the material. Generally, calcinated eggshell and its derivatives provide better adsorption capacities than untreated eggshell (Gurav and Samant 2021; Kristiano et al. 2019). However, addition of the extra treatment step multiplicatively complicates an already convoluted evaluation process of different adsorbents. Moreover, calcination is an energy demanding process, which diminishes chances of its implementation on an industrial scale due to financial and ecological concerns. Finally, change in the adsorption mechanism suggests that the material has undergone significant change, altering the material’s original properties with a material no longer being eggshell.
5.2 Other eggshell modifications
Modification of eggshell to improve its adsorptive properties is a common research direction in modern literature. In some cases, it is performed to enhance eggshell’s natural ability for removal of metal ions. One of the approaches is to modify eggshell by enhancing its structure via an additional component, which is designed to improve the adsorption of the target element. Effectively, this involves adsorbing an additional component on the surface of the eggshell before adsorption of the target element. Several examples of this approach include the following:
Modification of eggshell surface with KMnO4 for improved adsorption of Pb(II) via oxidation of OH groups on the adsorbent to the aldehyde or carboxyl groups by KMnO4 (Basaleh et al. 2020);
Coating eggshell with Fe3O4 to improve removal ability of Cr(VI) ions due to release of Fe(II) ions, which reduce Cr(VI) ions to Cr(III) ions followed by the coprecipitation of Cr(III) and Fe(III) hydroxides (Alomari 2020);
Enhancing the eggshell surface with Ag–Fe nanoparticles (which have shown good adsorption capacity toward heavy metals) for improved removal of Pb(II), As(V), and Hg(II) (Alamillo-López et al. 2020);
Coating eggshell with MnCl2 to improve removal efficiency of As(III) via enhancing adsorbent structure with manganese dioxide (Mubarak et al. 2015);
Modifying eggshell with phosphotungstic acid for improved removal of Sr(II) ions via enhancing surface area and pore volume of the adsorbent (Metwally et al. 2017);
To synthesize SnO2–ZnO–eggshell nanocomposite for removal of Hg(II) ions (Honarmand et al. 2020);
In these cases, eggshell modification includes preliminary eggshell treatment via aqueous solutions of a modifying agent. While such an approach provides better adsorption properties, the modifying agent only enhances already existing adsorption properties of eggshell, without dramatically altering the structure of the eggshell surface. However, compared to untreated eggshell, additional effort and time have to be spent on preparation procedures.
Another approach involves utilization of eggshell as a source material, with proposed treatment completely altering the structure of the material. Examples include the following:
Creating calcium-enhanced carbon from chicken feathers (Rahmani-Sani et al. 2020), in which eggshells are utilized as a source of Ca(II) for material modification;
Creation of aragonite crystals from eggshell, both chemically (Habte et al. 2020) or physically (Baláž et al. 2016);
Creating a mixture of magnetite, eggshell, and fly ash and mechanically alloying it in a ball mill for Cd(II) removal from aqueous solution (Segneanu et al. 2022);
Composting eggshell with other organic materials (Soares et al. 2016);
Modifying eggshell with chitosan, to create a new adsorbent (Anantha and Kota 2016);
Creation of hydroxyapatite via chemical route, using eggshell as a starting material (Mignardi et al. 2020);
Obtaining amorphous calcium phosphate (Ma et al. 2023);
Treatment of eggshells with strong acids, such as HCl, to create nanoporous material (Setiawan et al. 2018).
In these approaches, existing properties of the eggshells are omitted, and eggshell is utilized only as a source of necessary raw material for the adsorbent. Adsorbents produced with these methods often show dramatically better properties and adsorption characteristics at the expense of significantly more complicated preparation methods.
Finally, the combination of eggshell adsorption with some other physical purification process is another potential modification direction. Examples include removal of Cr(III) via electrocoagulation with simultaneous eggshell adsorption (Elabbas et al. 2022) and combination of a membrane biological reactor with supplemental eggshell adsorption (Pettinato et al. 2015). While no modifications are applied to the eggshell itself, the already existing adsorption process is enhanced via simultaneous execution of an additional separation process.
6 Conclusions
A review of recent literature on the subject of eggshell application as an adsorbent for removal of metal ions from aqueous solutions has been performed. Recent publications on the topic have been analyzed, underlying common trends in reported results and methods.
A great deal of diversity in eggshell pretreatment methods is present, with preparation procedures differing significantly. Similarly, diversity is present in adsorption experiment design and tested experiment conditions.
Similar conclusions can be made about the analytical and characterization methods employed. The result is that the term “untreated eggshell” often describes different material across different works, which are analyzed under different conditions. To minimize confusion and streamline material evaluation, a set of standard definitions should be developed, with the potential of developing standardized guidelines as well.
For untreated eggshell, the range of the reported BET surface area is from 0.07 m2/g to 8.941 m2/g. While particle size does influence eggshell surface area, it does not significantly improve adsorption capacity when sufficiently small particle sizes are employed, unless phase change of the material occurs during adsorbent preparation.
While the exact adsorption mechanism is unclear, a significant part of some metal ion removal from aqueous solution via eggshell appears to occur through precipitation, while the pH of the adsorption media significantly influences eggshell adsorption capacity for all metal ions. Influence of residual organic groups, present in eggshell, on metal ion removal does not seem significant.
Based on the reviewed literature, removal of Pb(II), Cd(II), and Cu(II) are most studied directions for adsorption of metal ions on eggshell from aqueous solutions. Eggshell capacity to remove metal ions from the main group elements has also been demonstrated. While the results look promising, not enough data are present to make reliable conclusions about its efficiency with other (mainly transition) metal ions, which makes this a potential research direction.
Different modifications and transformations of calcium carbonate change its morphological parameters and adsorption properties, which provides an opportunity for further investigation. Eggshell modification via calcination is a commonly employed method. While its advantages include enhanced adsorption properties of calcinated material, evidence suggests that the modification process alters material in such a way that a new adsorption mechanism is employed. Moreover, energy costs (together with CO2 emission) are an important factor for assessing an environmentally friendly production process and should be considered as well when selecting an eggshell modification method.
The analyzed data show that to compare the effectiveness of different materials as adsorbents, comparing only the materials adsorption capacities is insufficient. Adsorption capacity of materials significantly varies based on a multitude of parameters, which can represent a problem during industrial application of the method. When evaluating different adsorbents, their application parameters need to be considered as well.
Desorption and recovery of eggshell adsorbent remains a sparsely researched direction as well. Research in adsorbent recovery is a perspective direction in increasing viability of the developed adsorbents and their application on an industrial scale. There is practically no analysis of the chemical bonding and how tightly the metal is bound to the adsorbent. This is a critical parameter for determining an effective way of disposal of used adsorbent.
Our findings identify that metal speciation in aqua solutions, different processing water, and even in wastewater in studies about pollution removal from environmental water using adsorption often demand further research. Information on the speciation and coordination complexes of metals is usually missing; thus, a comprehensive and thorough analysis of the speciation distribution, the role of each species, and discovering the adsorption configuration are strongly encouraged in future studies by the authors (Xu et al. 2022). Particularly, it is desirable to reveal the mechanism of a self-enhancement or self-inhibition effect during the complexation and/or stability of ligands. Despite the fact that eggshells can be used as an effective and inexpensive tool for adsorbing metals from various aqua media, not enough data are available to compare its efficiency between different metal ions.
Due to the low costs and simplicity in operation, the adsorption technique is quite a promising method for removing metal complexes from different water/processing and water/wastewater matrices. The availability and large volumes of eggshells make the question of their efficient use a matter for systematic research – only then eggshell will change from waste to a material, which could lead to the rational use of eggshells as adsorbents.
Funding source: RÄ«ga Technical University
Award Identifier / Grant number: Doctoral Grant programme
Funding source: ERAF
Award Identifier / Grant number: 1.1.1.5./18.I/008 (B3664.14400)
-
Research ethics: Not applicable.
-
Author contributions: PS: writing-original draft, data analysis, elaborated tables and figures. DK: writing – review & editing. AL: conceptualization, writing – review & editing. All co-authors have contributed to the significant way to the final format of the review. The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: We thank Riga Technical University (PS) for Doctoral Grant program and ERAF 1.1.1.5./18.I/008 (B3664.14400; DK) for funding of this work.
-
Data availability: Not applicable.
References
Abatan, O.G., Alaba, P.A., Oni, B.A., Akpojevwe, K., Efeovbokhan, V., and Abnisa, F. (2020). Performance of eggshells powder as an adsorbent for adsorption of hexavalent chromium and cadmium from wastewater. SN Appl. Sci. 2: 1996, https://doi.org/10.1007/s42452-020-03866-w.Suche in Google Scholar
Abbas, A., Chen, L., Liao, Y., Wu, Z., Yu, Y., and Yang, J. (2021). Removal of bismuth ion from aqueous solution by pulverized eggshells. Desalin. Water Treat. 213: 395–405, https://doi.org/10.5004/dwt.2021.26724.Suche in Google Scholar
Ahmad, M., Usman, A.R.A., Lee, S.S., Kim, S., Joo, J., Yang, J.E., and Ok, Y.S. (2012). Eggshell and coral wastes as low cost sorbents for the removal of Pb2+, Cd2+ and Cu2+ from aqueous solutions. J. Ind. Eng. Chem. 18: 198–204, https://doi.org/10.1016/j.jiec.2011.11.013.Suche in Google Scholar
Ahmed, T.A.E., Wu, L., Younes, M., and Hincke, M. (2021). Biotechnological applications of eggshell: recent advances. Front. Bioeng. Biotechnol. 9: 675364, https://doi.org/10.3389/fbioe.2021.675364.Suche in Google Scholar PubMed PubMed Central
Alamillo-López, V.M., Sánchez-Mendieta, V., Olea-Mejía, O.F., González-Pedroza, M.G., and Morales-Luckie, R.A. (2020). Efficient removal of heavy metals from aqueous solutions using a bionanocomposite of eggshell/Ag-Fe. Catalysts 10: 727, https://doi.org/10.3390/catal10070727.Suche in Google Scholar
Alomari, A.A. (2020). Effect of modified eggshell on adsorption capacity of chromium(VI) from aqueous solution. Asian J. Chem. 32: 1549–1556, https://doi.org/10.14233/ajchem.2020.22651.Suche in Google Scholar
Al Omari, M.M.H., Rashid, I.S., Qinna, N.A., Jaber, A.M., and Badwan, A.A. (2016). Calcium carbonate. In: Brittain, H. (Ed.). Profiles of drug substances, excipients and related methodology, 41. Elsevier, London, pp. 31–132.10.1016/bs.podrm.2015.11.003Suche in Google Scholar PubMed
Altameemi, I.A. and Thuraya, M.A. (2021). Removal of manganese (Mn2+) from aqueous solution by low-cost adsorbents and study the adsorption thermodynamics and kinetics. J. Phys. Conf. Ser. 1773: 012038, https://doi.org/10.1088/1742-6596/1773/1/012038.Suche in Google Scholar
Anantha, R.K. and Kota, S. (2016). An evaluation of the major factors influencing the removal of copper ions using the egg shell (Dromaius novaehollandiae): chitosan (Agaricus bisporus) composite. 3 Biotech 6: 83, https://doi.org/10.1007/s13205-016-0381-2.Suche in Google Scholar PubMed PubMed Central
Annane, K., Lemlikchi, W., and Tingry, S. (2023). Efficiency of eggshell as a low-cost adsorbent for removal of cadmium: kinetic and isotherm studies. Biomass Conv. Bioref. 13: 6163–6174.10.1007/s13399-021-01619-2Suche in Google Scholar
Awogbemi, O., Inambao, F., and Onuh, E.I. (2020). Modification and characterization of chicken eggshell for possible catalytic applications. Heliyon 6: e05283, https://doi.org/10.1016/j.heliyon.2020.e05283.Suche in Google Scholar PubMed PubMed Central
Ayodele, O., Olusegun, S.J., Oluwasina, O.O., Okoronkwo, E.A., Olanipekun, E.O., Mohallem, N.D.S., Guimarães, W.G., Gomes, B.L.F.M., Souza, G.O., and Duarte, H.A. (2021). Experimental and theoretical studies of the adsorption of Cu and Ni ions from wastewater by hydroxyapatite derived from eggshells. Environ. Nanotechnology, Monit. Manag. 15: 100439, https://doi.org/10.1016/j.enmm.2021.100439.Suche in Google Scholar
Badillo-Camacho, J., Orozco-Guareño, E., Carbajal-Arizaga, G.G., Manríquez-Gonzalez, R., Barcelo-Quintal, I.D., and Gomez-Salazar, S. (2020). Cr(VI) adsorption from aqueous streams on eggshell membranes of different birds used as biosorbents. Adsorpt. Sci. Technol. 38: 413–434, https://doi.org/10.1177/0263617420956893.Suche in Google Scholar
Baláž, M. (2014). Eggshell membrane biomaterial as a platform for applications in materials science. Acta Biomater. 10: 3827–3843, https://doi.org/10.1016/j.actbio.2014.03.020.Suche in Google Scholar PubMed
Baláž, M. (2018). Ball milling of eggshell waste as a green and sustainable approach: a review. Adv. Colloid Interface Sci. 256: 256–275, https://doi.org/10.1016/j.cis.2018.04.001.Suche in Google Scholar PubMed
Baláž, M., Boldyreva, E.V., Rybin, D., Pavlović, S., Rodríguez-Padrón, D., Mudrinić, T., and Luque, R. (2021). State-of-the-art of eggshell waste in materials science: recent advances in catalysis, pharmaceutical applications, and mechanochemistry. Front. Bioeng. Biotechnol. 8: 612567, https://doi.org/10.3389/fbioe.2020.612567.Suche in Google Scholar PubMed PubMed Central
Baláž, M., Bujňáková, Z., Baláž, P., Zorkovská, A., Danková, Z., and Briančin, J. (2015a). Adsorption of cadmium(II) on waste biomaterial. J. Colloid Interface Sci. 454: 121–133, https://doi.org/10.1016/j.jcis.2015.03.046.Suche in Google Scholar PubMed
Baláž, M., Ficeriová, J., and Briančin, J. (2016). Influence of milling on the adsorption ability of eggshell waste. Chemosphere 146: 458–471, https://doi.org/10.1016/j.chemosphere.2015.12.002.Suche in Google Scholar PubMed
Baláž, M., Zorkovská, A., Fabián, M., Girman, V., and Briančin, J. (2015b). Eggshell biomaterial: characterization of nanophase and polymorphs after mechanical activation. Adv. Powder Technol. 26: 1597–1608, https://doi.org/10.1016/j.apt.2015.09.003.Suche in Google Scholar
Basaleh, A.A., Al-Malack, M.H., and Saleh, T.A. (2020). Metal removal using chemically modified eggshells: preparation, characterization, and statistical analysis. Desalin. Water Treat. 173: 313–330, https://doi.org/10.5004/dwt.2020.24690.Suche in Google Scholar
Çelebi, Ö., Şimşek, İ., and Çelebi, H. (2021). Escherichia coli inhibition and arsenic removal from aqueous solutions using raw eggshell matrix. Int. J. Environ. Sci. Technol. 18: 3205–3220, https://doi.org/10.1007/s13762-021-03216-2.Suche in Google Scholar
Charazińska, S., Burszta-Adamiak, E., and Lochyński, P. (2022). Recent trends in Ni(II) sorption from aqueous solutions using natural materials. Rev. Environ. Sci. Biotechnol. 21: 105–138, https://doi.org/10.1007/s11157-021-09599-5.Suche in Google Scholar
Cheng, X.Z., Hu, C.J., Cheng, K., Wei, B.M., Hu, S.C. (2010). Removal of mercury from wastewater by adsorption using thiol-functionalized eggshell membrane. Adv. Mat. Res. 113–116: 22–26, https://doi.org/10.4028/www.scientific.net/amr.113-116.22.Suche in Google Scholar
Cheng, X. and Ning, Z. (2023). Research progress on bird eggshell quality defects: a review. Poult. Sci. 102: 102283, https://doi.org/10.1016/j.psj.2022.102283.Suche in Google Scholar PubMed PubMed Central
Choi, H. (2019). Assessment of the adsorption kinetics, equilibrium and thermodynamic for Pb(II) removal using a hybrid adsorbent, eggshell and sericite, in aqueous solution. Water Sci. Technol. 79: 1922–1933, https://doi.org/10.2166/wst.2019.191.Suche in Google Scholar PubMed
Chojnacka, K. (2005). Biosorption of Cr(III) ions by eggshells. J. Hazard. Mater. 121: 167–173, https://doi.org/10.1016/j.jhazmat.2005.02.004.Suche in Google Scholar PubMed
Chou, M., Lee, T., Lin, Y., Hsu, S., Wang, M., Li, P., Huang, P., Lu, W., and Ho, J. (2023). On the removal efficiency of copper ions in wastewater using calcined waste eggshells as natural adsorbents. Sci. Rep. 13: 437, https://doi.org/10.1038/s41598-023-27682-5.Suche in Google Scholar PubMed PubMed Central
Comans, R.N.J. and Middelburg, J.J. (1987). Sorption of trace metals on calcite: applicability of the surface precipitation model. Geochim. Cosmochim. Acta 51: 2587–2591, https://doi.org/10.1016/0016-7037(87)90309-7.Suche in Google Scholar
Dayanidhi, K., Vadivel, P., Jothi, S., and Eusuff, N. (2020). White eggshells: a potential biowaste material for synergetic adsorption and naked-eye colorimetric detection of heavy metal ions from aqueous solution. ACS Appl. Mater. Interfaces 12: 1746–1756, https://doi.org/10.1021/acsami.9b14481.Suche in Google Scholar PubMed
Elabbas, S., Adjeroud, N., Mandi, L., Berrekhis, F., Pons, M.N., Leclerce, J.P., and Ouazzani, N. (2022). Eggshell adsorption process coupled with electrocoagulation for improvement of chromium removal from tanning wastewater. Int. J. Environ. Anal. Chem. 102: 2966–2978, https://doi.org/10.1080/03067319.2020.1761963.Suche in Google Scholar
Elabbas, S., Mandi, L., Berrekhis, F., Pons, M.N., Leclerc, J.P., and Ouazzani, N. (2016). Removal of Cr(III) from chrome tanning wastewater by adsorption using two natural carbonaceous materials: eggshell and powdered marble. J. Environ. Manage. 166: 589–595, https://doi.org/10.1016/j.jenvman.2015.11.012.Suche in Google Scholar PubMed
European Union (1999). Council directive 1999/31/EC of 26 April 1999 on the landfill of waste. Off. J. Eur. Comm. 42: 1–19.Suche in Google Scholar
European Union (2008). Council directive 2008/98/EC of 19 November 2008 on waste and repealing certain directives. Off. J. Eur. Comm. 51: 3–30.Suche in Google Scholar
Eurostat (2022). Generation of waste by waste category, hazardousness and NACE Rev. 2 activity, Available at: https://ec.europa.eu/eurostat/databrowser/view/env_wasgen/default/table?lang=en (Accessed 15 August 2022).Suche in Google Scholar
Farley, K.J., Dzombak, D.A., and Morel, F.M.M. (1985). A surface precipitation model for the sorption of cations on metal oxides. J. Colloid Interface Sci. 106: 226–242, https://doi.org/10.1016/0021-9797(85)90400-x.Suche in Google Scholar
Flores-Cano, J.V., Leyva-Ramos, R., Mendoza-Barron, J., Guerrero-Coronado, R.M., Aragón-Piña, A., and Labrada-Delgado, G.J. (2013). Sorption mechanism of Cd(II) from water solution onto chicken eggshell. Appl. Surf. Sci. 276: 682–690, https://doi.org/10.1016/j.apsusc.2013.03.153.Suche in Google Scholar
Foroutan, R., Mohammadi, R., Farjadfard, S., Esmaeili, H., Ramavandi, B., and Sorial, G.A. (2019). Eggshell nano-particle potential for methyl violet and mercury ion removal: surface study and field application. Adv. Powder Technol. 30: 2188–2199, https://doi.org/10.1016/j.apt.2019.06.034.Suche in Google Scholar
Gautron, J., Stapane, L., Roy, N., Nys, Y., Rodriguez-Navarro, A., and Hincke, M. (2021). Avian eggshell biomineralization: an update on its structure, mineralogy and protein tool kit. BMC Mol. Cell Biol. 22: 11, https://doi.org/10.1186/s12860-021-00350-0.Suche in Google Scholar PubMed PubMed Central
Geissdoerfer, M., Savaget, P., Bocken, N.M.P., and Hultink, E.J. (2017). The Circular Economy – a new sustainability paradigm? J. Cleaner Prod. 143: 757–768, https://doi.org/10.1016/j.jclepro.2016.12.048.Suche in Google Scholar
Granados-Correa, F. and Jiménez-Reyes, M. (2013). Kinetic, equilibrium and thermodynamic studies on the adsorption of Eu(III) by eggshell from aqueous solutions. Adsorpt. Sci. Technol. 31: 891–902, https://doi.org/10.1260/0263-6174.31.10.891.Suche in Google Scholar
Green, D. and Perry, R. (Eds.) (2008). Perry’s chemical engineers’ handbook. McGraw Hill, New York.Suche in Google Scholar
Guo, Z., Li, J., Guo, Z., Guo, Q., and Zhu, B. (2017). Phosphorus removal from aqueous solution in parent and aluminum-modified eggshells: thermodynamics and kinetics, adsorption mechanism, and diffusion process. Environ. Sci. Pollut. Res. 24: 14525–14536, https://doi.org/10.1007/s11356-017-9072-8.Suche in Google Scholar PubMed
Gurav, V.L. and Samant, R.A. (2021). Application of waste egg shell for adsorption of Cd(II) and Pb(II) ions to protect environment: equilibrium, kinetic and adsorption studies. Orient. J. Chem. 37: 128–135, https://doi.org/10.13005/ojc/370117.Suche in Google Scholar
Guru, P.S. and Dash, S. (2014). Sorption on eggshell waste—a review on ultrastructure, biomineralization and other applications. Adv. Colloid Interface Sci. 209: 49–67, https://doi.org/10.1016/j.cis.2013.12.013.Suche in Google Scholar PubMed
Habte, L., Shiferaw, N., Khan, M.D., Thriveni, T., and Ahn, J.W. (2020). Sorption of Cd2+ and Pb2+ on aragonite synthesized from eggshell. Sustainability 12: 1174, https://doi.org/10.3390/su12031174.Suche in Google Scholar
Hammarstrom, J.M., Sibrell, P.L., and Belkin, H.E. (2003). Characterization of limestone reacted with acid-mine drainage in a pulsed limestone bed treatment system at the Friendship Hill National Historical Site, Pennsylvania, USA. Appl. Geochem. 18: 1705–1721, https://doi.org/10.1016/s0883-2927(03)00105-7.Suche in Google Scholar
Hamouda, M.A., Sweidan, H., Maraqa, M.A., and El-Hassan, H. (2020). Mechanistic study of Pb2+ removal from aqueous solutions using eggshells. Water 12: 2517, https://doi.org/10.3390/w12092517.Suche in Google Scholar
Harripersadth, C., Musonge, P., Isa, Y.M., Morales, M.G., and Sayago, A. (2020). The application of eggshells and sugarcane bagasse as potential biomaterials in the removal of heavy metals from aqueous solutions. South African J. Chem. Eng. 34: 142–150, https://doi.org/10.1016/j.sajce.2020.08.002.Suche in Google Scholar
Hassan, E.R.E., Rostom, M., Farghaly, F.E., and Khalek, M.A.A. (2020). Bio-sorption for tannery effluent treatment using eggshell wastes; kinetics, isotherm and thermodynamic study. Egypt. J. Pet. 29: 273–278, https://doi.org/10.1016/j.ejpe.2020.10.002.Suche in Google Scholar
Hess, B.J., Kolar, P., Classen, J.J., Knappe, D., and Cheng, J.J. (2018). Evaluation of waste eggshells for adsorption of copper from synthetic and swine wastewater. Trans. ASABE 61: 967–976, https://doi.org/10.13031/trans.12599.Suche in Google Scholar
Heydemann, A. (1959). Adsorption aus sehr verdünnten Kupferlösungen an reinen Tonmineralen. Geochim. Cosmochim. Acta 15: 305–308.10.1016/0016-7037(59)90064-XSuche in Google Scholar
Hincke, M.T., Nys, Y., Gautron, J., Mann, K., Rodriguez-Navarro, A.B., and McKee, M.D. (2012). The eggshell: structure, composition and mineralization. Front. Biosci. 17: 1266–1280, https://doi.org/10.2741/3985.Suche in Google Scholar PubMed
Ho, J., Yeh, Y., Wang, H., Khoo, S.K., Chen, Y., and Chow, C. (2014). Removal of nickel and silver ions using eggshells with membrane, eggshell membrane, and eggshells. Food Sci. Technol. Res. 20: 337–343, https://doi.org/10.3136/fstr.20.337.Suche in Google Scholar
Honarmand, M., Mirzadeh, M., and Honarmand, M. (2020). Green synthesis of SnO2-ZnO-eggshell nanocomposites and study of their application in removal of mercury (II) ions from aqueous solution. J. Environ. Health Sci. Eng. 18: 1581–1593, https://doi.org/10.1007/s40201-020-00576-8.Suche in Google Scholar PubMed PubMed Central
Jeremias, T.C., Pineda-Vásquez, T., Lapolli, F.R., and Lobo-Recio, M.Á. (2020). Use of eggshell as a low-cost biomaterial for coal mine-impacted water (MIW) remediation: characterization and statistical determination of the treatment conditions. Water. Air. Soil Pollut. 231: 562, https://doi.org/10.1007/s11270-020-04919-x.Suche in Google Scholar
Kahil, K., Weiner, S., Addadi, L., and Gal, A. (2021). Ion pathways in biomineralization: perspectives on uptake, transport, and deposition of calcium, carbonate, and phosphate. J. Am. Chem. Soc. 143: 21100–21112, https://doi.org/10.1021/jacs.1c09174.Suche in Google Scholar PubMed PubMed Central
Katha, P.S., Ahmed, Z., Alam, R., Saha, B., Acharjee, A., and Rahman, M.S. (2021). Efficiency analysis of eggshell and tea waste as low cost adsorbents for Cr removal from wastewater sample. S. Afr. J. Chem. Eng. 37: 186–195, https://doi.org/10.1016/j.sajce.2021.06.001.Suche in Google Scholar
Ketta, M. and Tůmová, E. (2016). Eggshell structure, measurements, and quality-affecting factors in laying hens: a review. Czech J. Anim. Sci. 61: 299–309, https://doi.org/10.17221/46/2015-cjas.Suche in Google Scholar
Khaskheli, M.A., Abro, M.I., Chand, R., Elahi, E., Khokhar, F.M., Majidano, A.A., Aaoud, H., and Rekik, N. (2021). Evaluating the effectiveness of eggshells to remove heavy metals from wastewater. Desalin. Water Treat. 216: 239–245, https://doi.org/10.5004/dwt.2021.26807.Suche in Google Scholar
Kim, D., Hwang, S., Kim, Y., Jeong, C.H., Hong, Y.P., and Ryoo, K.S. (2019). Removal of heavy metals from water using chicken egg shell powder as a bio-adsorbent. Bull. Korean Chem. Soc. 40: 1156–1161, https://doi.org/10.1002/bkcs.11884.Suche in Google Scholar
Kınaytürk, N.K., Tunalı, B., and Altuğ, D.T. (2021). Eggshell as a biomaterial can have a sorption capability on its surface: a spectroscopic research. R. Soc. Open Sci. 8: 210100, https://doi.org/10.1098/rsos.210100.Suche in Google Scholar PubMed PubMed Central
Kobiraj, R., Gupta, N., Kushwaha, A.K., and Chattopadhyaya, M.C. (2012). Determination of equilibrium, kinetic and thermodynamic parameters for the adsorption of Brilliant Green dye from aqueous solutions onto eggshell powder. Indian J. Chem. Technol. 19: 26–31.Suche in Google Scholar
Kristianto, H., Daulay, N., and Arie, A.A. (2019). Adsorption of Ni(II) ion onto calcined eggshells: a study of equilibrium adsorption isotherm. Indones. J. Chem. 19: 143–150, https://doi.org/10.22146/ijc.29200.Suche in Google Scholar
Levina, A., Crans, D., and Lay, P. (2017). Speciation of metal drugs, supplements and toxins in media and bodily fluids controls in vitro activities. Coord. Chem. Rev. 352: 473–498, https://doi.org/10.1016/j.ccr.2017.01.002.Suche in Google Scholar
Lima, E.C., Adebayo, M.A., and Machado, F.M. (2015). Kinetic and equilibrium models of adsorption. In: Bergmann, C.P. and Machado, F.M. (Eds.), Carbon nanomaterials as adsorbents for environmental and biological applications. Springer Cham, Switzerland, pp. 33–69.10.1007/978-3-319-18875-1_3Suche in Google Scholar
Liu, Q., Guo, L., Zhou, Y., Dai, Y., Feng, L., Zhou, J., Zhao, J., Liu, J., and Qian, G. (2012). Phosphate adsorption on biogenetic calcium carbonate minerals: effect of a crystalline phase. Desalin. Water Treat. 47: 78–85, https://doi.org/10.1080/19443994.2012.696798.Suche in Google Scholar
Liu, R., Guan, Y., Chen, L., and Lian, B. (2018). Adsorption and desorption characteristics of Cd2+ and Pb2+ by micro and nano-sized biogenic CaCO3. Front. Microbiol. 9: 41, https://doi.org/10.3389/fmicb.2018.00041.Suche in Google Scholar PubMed PubMed Central
Liu, R. and Lian, B. (2019a). Immobilisation of Cd(II) on biogenic and abiotic calcium carbonate. J. Hazard. Mater. 378: 120707, https://doi.org/10.1016/j.jhazmat.2019.05.100.Suche in Google Scholar PubMed
Liu, R. and Lian, B. (2019b). Non-competitive and competitive adsorption of Cd2+, Ni2+, and Cu2+ by biogenic vaterite. Sci. Total Environ. 659: 122–130, https://doi.org/10.1016/j.scitotenv.2018.12.199.Suche in Google Scholar PubMed
Ma, Q., Rubenis, K., Sigurjónsson, Ó.E., Hildebrand, T., Standal, T., Zemjane, S., Locs, J., Loca, D., and Haugen, H.J. (2023). Eggshell-derived amorphous calcium phosphate: synthesis, characterization, and bio-functions as bone graft materials in novel 3D osteoblastic spheroids model. Smart Mater. Med. 4: 522–537, https://doi.org/10.1016/j.smaim.2023.04.001.Suche in Google Scholar
Makuchowska-fryc, J. (2019). Use of the eggshells in removing heavy metals from wastewater – the process kinetics and efficiency. Ecol. Chem. Eng. S 26: 165–174, https://doi.org/10.1515/eces-2019-0012.Suche in Google Scholar
Markovski, J.S., Marković, D.D., Ðokić, V.R., Mitrić, M., Ristić, M.Ð., Onjia, A.E., and Marinković, A.D. (2014). Arsenate adsorption on waste eggshell modified by goethite, α-MnO2 and goethite/α-MnO2. Chem. Eng. J. 237: 430–442, https://doi.org/10.1016/j.cej.2013.10.031.Suche in Google Scholar
Mashangwa, T.D., Tekere, M., and Sibanda, T. (2017). Determination of the efficacy of eggshell as a low-cost adsorbent for the treatment of metal laden effluents. Int. J. Environ. Res. 11: 175–188, https://doi.org/10.1007/s41742-017-0017-3.Suche in Google Scholar
Metwally, S.S., Rizk, H.E., and Gasser, M.S. (2017). Biosorption of strontium ions from aqueous solution using modified eggshell materials. Radiochim. Acta 105: 1021–1031, https://doi.org/10.1515/ract-2016-2729.Suche in Google Scholar
Mignardi, S., Archilletti, L., Medeghini, L., and Vito, C.D. (2020). Valorization of eggshell biowaste for sustainable environmental remediation. Sci. Rep. 10: 2436, https://doi.org/10.1038/s41598-020-59324-5.Suche in Google Scholar PubMed PubMed Central
Mittal, A., Teotia, M., Soni, R.K., and Mittal, J. (2016). Applications of egg shell and egg shell membrane as adsorbents: a review. J. Mol. Liq. 223: 376–387, https://doi.org/10.1016/j.molliq.2016.08.065.Suche in Google Scholar
Mohammad, S.G., Ahmed, S.M., and El-Sayed, M.M.H. (2022). Removal of copper (II) ions by eco-friendly raw eggshells and nano-sized eggshells: a comparative study. Chem. Eng. Commun. 209: 83–95, https://doi.org/10.1080/00986445.2020.1835875.Suche in Google Scholar
Mubarak, S., Zia-Ur-Rehman, M., and Chaudhry, M.N. (2015). Modified eggshells as cost effective adsorbent for the treatment of arsenic(III) contaminated industrial effluents. Asian J. Chem. 27: 1995–2000, https://doi.org/10.14233/ajchem.2015.17634.Suche in Google Scholar
Orłowski, G., Siekiera, J., Karg, J., Tobolka, M., Wuczyński, A., Kaługa, I., Siekiera, A., Cyga-Döhner, R., and Dudzik, E. (2019). Calcium and metals are not evenly distributed in avian eggshells over their longitudinal section. Auk 136: 1–14, https://doi.org/10.1093/auk/ukz026.Suche in Google Scholar
Özcan, S., Çelebi, H., and Özcan, Z. (2018). Removal of heavy metals from simulated water by using eggshell powder. Desalin. Water Treat. 127: 75–82, https://doi.org/10.5004/dwt.2018.22580.Suche in Google Scholar
Papadopoulos, P. and Rowell, D.L. (1989). The reactions of copper and zinc with calcium carbonate surfaces. J. Soil Sci. 40: 39–48, https://doi.org/10.1111/j.1365-2389.1989.tb01252.x.Suche in Google Scholar
Park, H.J., Jeong, S.W., Yang, J.K., Kim, B.G., and Lee, S.M. (2007). Removal of heavy metals using waste eggshell. J. Environ. Sci. 19: 1436–1441, https://doi.org/10.1016/s1001-0742(07)60234-4.Suche in Google Scholar PubMed
Peigneux, A., Puentes-Pardo, J.D., Rodríguez-Navarro, A.B., Hincke, M.T., and Jiménez-Lópeza, C. (2020). Development and characterization of magnetic eggshell membranes for lead removal from wastewater. Ecotoxicol. Environ. Saf. 192: 110307, https://doi.org/10.1016/j.ecoenv.2020.110307.Suche in Google Scholar PubMed
Pettinato, M., Chakraborty, S., Arafat, H.A., and Calabro, V. (2015). Eggshell: a green adsorbent for heavy metal removal in an MBR system. Ecotoxicol. Environ. Saf. 121: 57–62, https://doi.org/10.1016/j.ecoenv.2015.05.046.Suche in Google Scholar PubMed
Polat, A. and Aslan, S. (2014). Kinetic and isotherm study of cupper adsorption from aqueous solution using waste eggshell. J. Environ. Eng. Landsc. Manag. 22: 132–140, https://doi.org/10.3846/16486897.2013.865631.Suche in Google Scholar
Quina, M.J., Soares, M.A.R., and Quinta-Ferreira, R. (2017). Applications of industrial eggshell as a valuable anthropogenic resource. Resour. Conserv. Recycl. 123: 176–186, https://doi.org/10.1016/j.resconrec.2016.09.027.Suche in Google Scholar
Rahmani-Sani, A., Singh, P., Raizada, P., Lima, E.C., Anastopoulos, I., Giannakoudakis, D.A., Sivamani, S., Dontsova, T.A., and Hosseini-Bandegharaei, A. (2020). Use of chicken feather and eggshell to synthesize a novel magnetized activated carbon for sorption of heavy metal ions. Bioresour. Technol. 297: 122452, https://doi.org/10.1016/j.biortech.2019.122452.Suche in Google Scholar PubMed
Revellame, E.D., Fortela, D., Sharp, W., Hernandez, R., and Zappi, M.E. (2020). Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: a review. Clean. Eng. Technol. 1: 100032, https://doi.org/10.1016/j.clet.2020.100032.Suche in Google Scholar
Sabah, H., Thouraya, T., Melek, H., and Nadia, M. (2018). Application of response surface methodology for optimization of cadmium ion removal from an aqueous solution by eggshell powder. Chem. Res. Chinese Univ. 34: 302–310, https://doi.org/10.1007/s40242-015-7163-9.Suche in Google Scholar
Sankaran, R., Show, P.L., Ooi, C., Ling, T.C., Shu-Jen, C., Chen, S., and Chang, Y. (2020). Feasibility assessment of removal of heavy metals and soluble microbial products from aqueous solutions using eggshell wastes. Clean Technol. Environ. Policy 22: 773–786, https://doi.org/10.1007/s10098-019-01792-z.Suche in Google Scholar
Santos, C.R., Fernández, J.B., Hernández, G.P., Rivera, M.Á.H., and Flores, L.L.D. (2019a). Adsorption of copper (II) and cadmium (II) in aqueous suspensions of biogenic nanostructured CaCO3. Bol. la Soc. Esp. Ceram. y Vidr. 58: 2–13.10.1016/j.bsecv.2018.05.003Suche in Google Scholar
Santos, L.B., Oliveira, D.M., Souza, A.O., and Lemos, V.A. (2019b). A new method for the speciation of arsenic species in water, seafood and cigarette samples using an eggshell membrane. J. Iran. Chem. Soc. 16: 1879–1889, https://doi.org/10.1007/s13738-019-01665-8.Suche in Google Scholar
Sarder, M.R., Hafiz, N.A., and Alamgir, M. (2019). Study on the effective reuse of eggshells as a resource recovery from municipal solid waste. In: Ghosh, S.K. (Ed.). Proceedings of 6th IconSWM, 2016: waste management and resource efficiency. Springer Nature, Singapore, pp. 71–79.10.1007/978-981-10-7290-1_7Suche in Google Scholar
Sasikala, V., Koteswara, Ch., Kumar, A., Sruthi, T., Prakash, S., Nissy, M., and Vangalapati, M. (2021). Extraction and removal of nickel from battery waste, using nano sized activated carbon of Egg shell powder in a column. Mater. Today: Proc. 44: 2296–2299, https://doi.org/10.1016/j.matpr.2020.12.393.Suche in Google Scholar
Schosseler, P.M., Wehrli, B., and Schweiger, A. (1999). Uptake of Cu2+ by the calcium carbonates vaterite and calcite as studied by continuous wave (cw) and pulse electron paramagnetic resonance. Geochim. Cosmochim. Acta 63: 1955–1967, https://doi.org/10.1016/s0016-7037(99)00086-1.Suche in Google Scholar
Segneanu, A., Marin, C.N., Vlase, G., Cepan, C., Mihailescu, M., Muntean, C., and Grozescu, I. (2022). Highly efficient engineered waste eggshell-fly ash for cadmium removal from aqueous solution. Sci. Rep. 12: 9676, https://doi.org/10.1038/s41598-022-13664-6.Suche in Google Scholar PubMed PubMed Central
Setiawan, B.D., Rizqi, O., Brilianti, N.F., and Wasito, H. (2018). Nanoporous of waste avian eggshell to reduce heavy metal and acidity in water. Sustain. Chem. Pharm. 10: 163–167, https://doi.org/10.1016/j.scp.2018.10.002.Suche in Google Scholar
Shahbandeh, M. (2022). Egg production worldwide 1990–2020, Available at: https://www.statista.com/statistics/263972/egg-production-worldwide-since-1990/.Suche in Google Scholar
Singh, N.B., Nagpal, G., Agrawal, S., and Rachna (2018). Water purification by using adsorbents: a Review. Environ. Technol. Innov. 11: 187–240, https://doi.org/10.1016/j.eti.2018.05.006.Suche in Google Scholar
Smirnova, A., Kalnina, D., and Locs, J. (2016). Removal of phosphates from water using eggshell bio sorbents. Key Eng. Mater. 721: 149–153, https://doi.org/10.4028/www.scientific.net/kem.721.149.Suche in Google Scholar
Soares, M.A.R., Marto, S., Quina, M.J., Gando-Ferreira, L., and Quinta-Ferreira, R. (2016). Evaluation of eggshell-rich compost as biosorbent for removal of Pb(II) from aqueous solutions. Water. Air. Soil Pollut. 227: 150, https://doi.org/10.1007/s11270-016-2843-x.Suche in Google Scholar
Song, X., Cao, Y., Bu, X., and Luo, X. (2021). Porous vaterite and cubic calcite aggregated calcium carbonate obtained from steamed ammonia liquid waste for Cu2+ heavy metal ions removal by adsorption process. Appl. Surf. Sci. 536: 147958, https://doi.org/10.1016/j.apsusc.2020.147958.Suche in Google Scholar
Tizo, M.S., Blanco, L.A.V., Cagas, A.C.Q., Cruz, B.R.B.D., Encoy, J.C., Gunting, J.V., Arazo, R.O., and Mabayo, V.I.F. (2018). Efficiency of calcium carbonate from eggshells as an adsorbent for cadmium removal in aqueous solution. Sustain. Environ. Res. 28: 326–332, https://doi.org/10.1016/j.serj.2018.09.002.Suche in Google Scholar
Tsai, W., Yang, J., Hsu, H., Lin, C., Lin, K., and Chiu, C. (2008). Development and characterization of mesoporosity in eggshell ground by planetary ball milling. Microporous Mesoporous Mater. 111: 379–386, https://doi.org/10.1016/j.micromeso.2007.08.010.Suche in Google Scholar
Tsai, W.T., Yang, J.M., Lai, C.W., Cheng, Y.H., Lin, C.C., and Yeh, C.W. (2006). Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour. Technol. 97: 488–493, https://doi.org/10.1016/j.biortech.2005.02.050.Suche in Google Scholar PubMed
Usman, A.R.A. (2008). The relative adsorption selectivities of Pb, Cu, Zn, Cd and Ni by soils developed on shale in New Valley, Egypt. Geoderma 144: 334–343, https://doi.org/10.1016/j.geoderma.2007.12.004.Suche in Google Scholar
Vu, N., Dinh, T., Le, T., Vu, T., Nguyen, T., Pham, T., Vu, N., Koji, S., Hama, S., Kim, I., et al.. (2022). Eggshell powder as calcium source on growth and yield of groundnut (Arachis hypogaea L.). Plant Prod. Sci. 25: 413–420, https://doi.org/10.1080/1343943x.2022.2120506.Suche in Google Scholar
Wang, P., Shen, T., Li, X., Tang, Y., and Li, Y. (2020). Magnetic mesoporous calcium carbonate-based nanocomposites for the removal of toxic Pb(II) and Cd(II) ions from water. ACS Appl. Nano Mater. 3: 1272–1281, https://doi.org/10.1021/acsanm.9b02036.Suche in Google Scholar
Wang, S., Wei, M., and Huang, Y. (2013). Biosorption of multifold toxic heavy metal ions from aqueous water onto food residue eggshell membrane functionalized with ammonium thioglycolate. J. Agric. Food Chem. 61: 4988–4996, https://doi.org/10.1021/jf4003939.Suche in Google Scholar PubMed
Wen, T., Zhao, Y., Zhang, T., Xiong, B., Hu, H., Zhang, Q., and Song, S. (2020). Selective recovery of heavy metals from wastewater by mechanically activated calcium carbonate: inspiration from nature. Chemosphere 246: 125842, https://doi.org/10.1016/j.chemosphere.2020.125842.Suche in Google Scholar PubMed
Wengerska, K., Batkowska, J., and Drabik, K. (2023). The eggshell defect as a factor affecting the egg quality after storage. Poult. Sci. 102: 102749, https://doi.org/10.1016/j.psj.2023.102749.Suche in Google Scholar PubMed PubMed Central
Xin, Y., Li, C., Liu, J., Liu, J., Liu, Y., He, W., and Gao, Y. (2018). Adsorption of heavy metal with modified eggshell membrane and the in situ synthesis of Cu–Ag/modified eggshell membrane composites. R. Soc. Open. Sci. 5: 180532, https://doi.org/10.1098/rsos.180532.Suche in Google Scholar PubMed PubMed Central
Xu, Z., Zhang, Q., Li, X., and Huang, X. (2022). A critical review on chemical analysis of heavy metal complexes in water/wastewater and the mechanism of treatment methods. Chem. Eng. J. 429: 131688, https://doi.org/10.1016/j.cej.2021.131688.Suche in Google Scholar
Yang, D., Zhao, J., Ahmad, W., Amin, M., Aslam, F., Khan, K., and Ahmad, A. (2022). Potential use of waste eggshells in cement-based materials: a bibliographic analysis and review of the material properties. Constr. Build. Mater. 344: 128143, https://doi.org/10.1016/j.conbuildmat.2022.128143.Suche in Google Scholar
Yusuff, A.S. (2017). Preparation and characterization of composite anthill-chicken eggshell adsorbent: optimization study on heavy metals adsorption using response surface methodology. J. Environ. Sci. Technol. 10: 120–130, https://doi.org/10.3923/jest.2017.120.130.Suche in Google Scholar
Yusuff, A.S., Olateju, I.I., and Ekanem, S.E. (2017). Equilibrium, kinetic and thermodynamic studies of the adsorption of heavy metals from aqueous solution by thermally treated quail eggshell. J. Environ. Sci. Technol. 10: 245–257, https://doi.org/10.3923/jest.2017.245.257.Suche in Google Scholar
Zadeh, B.S., Esmaeili, H., and Foroutan, R. (2018). Cadmium(II) removal from aqueous solution using microporous eggshell: kinetic and equilibrium studies. Indones. J. Chem. 18: 265–271, https://doi.org/10.22146/ijc.28789.Suche in Google Scholar
Zhou, X., Liu, W., Tian, C., Mo, S., Liu, X., Deng, H., and Lin, Z. (2018). Mussel-inspired functionalization of biological calcium carbonate for improving Eu(III) adsorption and the related mechanisms. Indones. J. Chem. 351: 816–824, https://doi.org/10.1016/j.cej.2018.06.142.Suche in Google Scholar
Zhou, X., Liu, W., Zhang, J., Wu, C., Ou, X., Tian, C., Lin, Z., and Dang, Z. (2017). Biogenic calcium carbonate with hierarchical organic–inorganic composite structure enhancing the removal of Pb(II) from wastewater. ACS Appl. Mater. Interfaces 9: 35785–35793, https://doi.org/10.1021/acsami.7b09304.Suche in Google Scholar PubMed
Zonato, R.O., Estevam, B.R., Perez, I.D., Ribeiro, V.A., and Boina, R.F. (2022). Eggshell as an adsorbent for removing dyes and metallic ions in aqueous solutions. Cleaner Chem. Eng. 2: 100023, https://doi.org/10.1016/j.clce.2022.100023.Suche in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/revce-2023-0025).
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Reviews
- Pattern identification in data about unmodified waste eggshell application as an adsorbent for metal ion removal from aqueous media
- Biogenic potassium: sources, method of recovery, and sustainability assessment
- Sustainable approach for the treatment of dye-containing wastewater – a critical review
- Experimental research of paraffin deposition with flow loops
Artikel in diesem Heft
- Frontmatter
- Reviews
- Pattern identification in data about unmodified waste eggshell application as an adsorbent for metal ion removal from aqueous media
- Biogenic potassium: sources, method of recovery, and sustainability assessment
- Sustainable approach for the treatment of dye-containing wastewater – a critical review
- Experimental research of paraffin deposition with flow loops

