Abstract
Intrathecal analgesia has increased over the last 30 years. In oncology, it is a real alternative for the treatment of refractory pain. The diversity of the molecules alone or in combination that can be used, the risk related to the route of administration, and the cost of certain molecules are all arguments in favor of centralized preparation within the pharmacy. The purposes of this work are first of all to explain the reasons for centralization of these preparations, and in a second time to describe the circuit developed within our establishment.
Description of intrathecal analgesia
Clinical interest
The management of a patient with cancer goes beyond the borders of the only treatment of cancer pathology. From the patient’s point of view, pain is often considered the most feared symptom [1]. The painkillers prescribed on the scale established by the World Health Organization are effective in relieving pain related to cancer in more than 80 % of cases [2]. However, for 15 % to 20 % of patients, conventional analgesic management is not sufficient (impossibility to relieve pain or undesirable side effects observed [3].
In these cases intrathecal analgesia should be considered for better control of pain as well as for the purpose of improving quality of life [4]. The superiority of intrathecal analgesia over standard therapy was demonstrated in a randomized multicenter clinical trial in 2002 [5], with a 52 % decrease in pain in the implanted group and a significant decrease in adverse events. Studies published since then have also confirmed these results with a comparable level of pain reduction [6, 7, 8, 9, 10, 11, 12, 13].
In France, the indication of intrathecal analgesia exists according to the Société Française d’Anesthésie et de Réanimation (SFAR) in case of refractory chronic cancer pain despite a well-conducted treatment according to the recommendations of the World Health Organization, (WHO) as well as in patients with adverse effects related to analgesics invalidating the quality of life of the patient [4, 14].
Intrathecal analgesia is therefore a technique of analgesia more and more developed in the world and also in France.
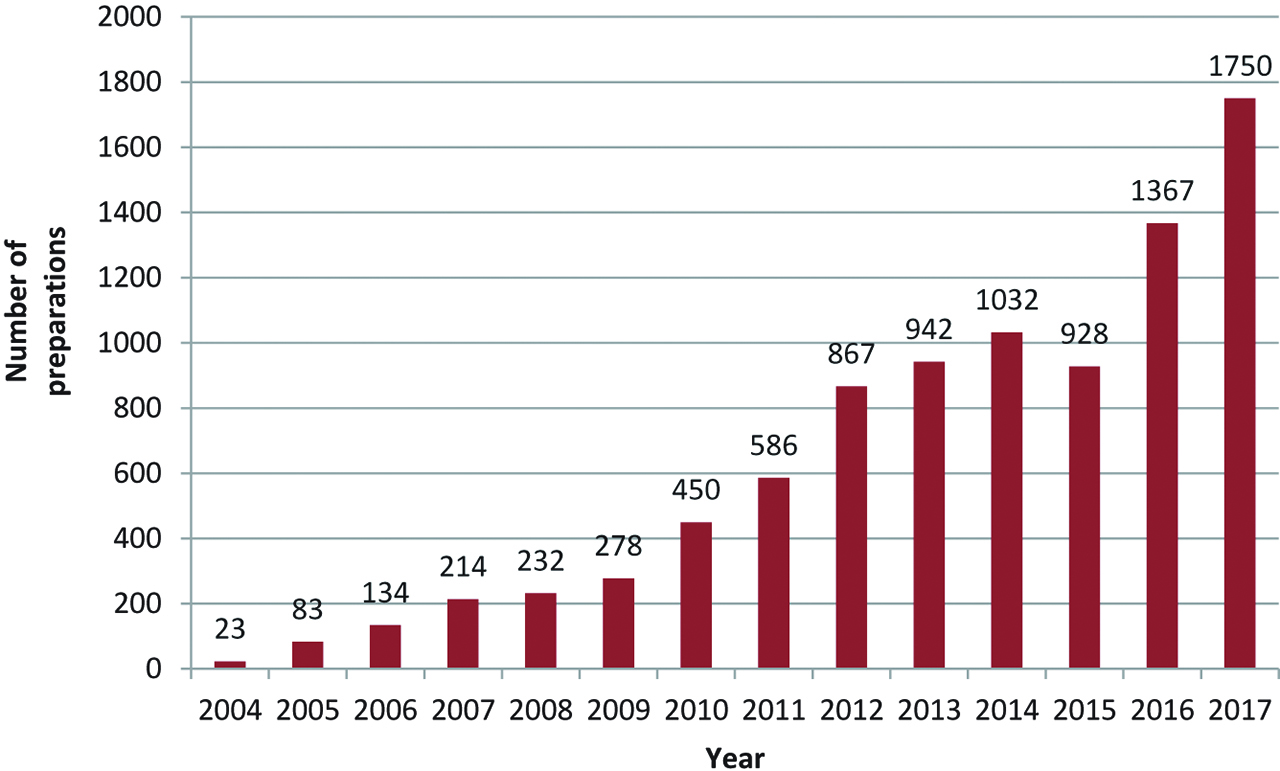
Activity evolution over time.
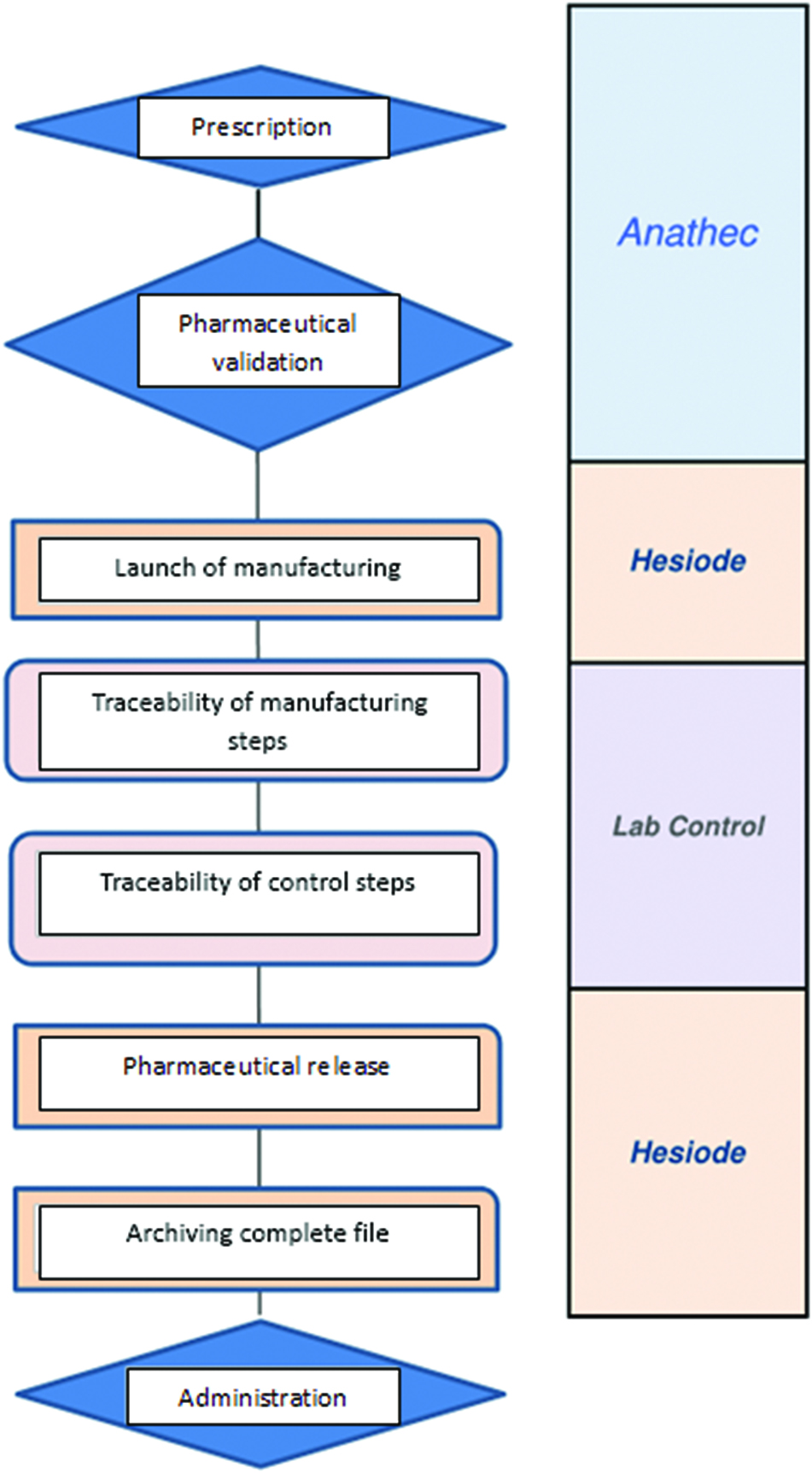
Circuit of intrathecal preparations.
Anesthesiologists of our establishment are all trained in this technique [15]. From the beginning, clinicians and pharmacists worked together to provide the patient with optimal care and thus to set up a suitable circuit.
Equipment
The principle of the technique is to distribute the analgesics closer to the medullary receptors, in the cerebrospinal fluid, to reduce the doses of the analgesics and to reduce the side effects related to the treatments systemically. A catheter is placed directly in the cerebrospinal fluid, and is tunneled to the pump or to an implantable site connected to an external pump. Choosing the location of the catheter post at the vertebrae is important and depends on the type of pain.
Implantable pump has a tank of 20 or 40 ml. The flow is continuous and bolus can be generated by the patient via a remote control. The implanted pump is filled at regular intervals, depending on the flow rate of each patient. The filling of the pump reservoir is done via a septum placed under the skin.
Mix used and indication
Elements on Cerebro Spinal Fluid (CSF) diffusion are important to understand the use of certain molecules intrathecally. CSF circulation is passive and pulsatile. It is modulated mainly by changes in blood and trans-thoracic pressure [16]. In addition, CSF is a very hydrophilic medium, while its environment is highly vascularized and lipophilic.
For instance, hydrophilic drugs such as morphine will have a slow blood resorption with a longer duration of action intrathecally.
Different molecules can be used, alone or in combination. They must meet several criteria to be used: diffusion in CSF, not to be toxic for the spinal cord, to be chemically and physically stable in pump whatever the mixture used.
The 2017 Polyanalgesic Consensus Conference has established a therapeutic strategy based on the type of pain (nociceptive or neuropathic) [17].
The most used molecules are
Opioids: morphine remains the reference. Fentanyl and sufentanil can also be used with a faster onset of action. Hydromorphone is also cited, principally in case of diffuse nociceptive or neuropathic pain.
Local anesthetics by blocking sodium channels: bupivacaine or ropivacaine. Ropivacaine is the only one currently marketed in France.
N-type calcium channel blocker voltages inhibitors: ziconotide.
Alpha 2 adrenergic receptor agonist: clonidine. Its action on the secretion of substance P and its membrane hyperpolarization allows an action on neuropathic pain.
GABA-B receptor agonist: baclofen. This molecule is widely used in spinal spasticity.
Synergistic combinations are often used in oncology in view of the intensity of the pain and its mixed characteristics. The combination morphine – ziconotide, associated or not with ropivacaine, has already showed clinical benefits in cancer pain [18]. However, it is necessary to check the physicochemical compatibility as well as the stability of the associations used. First, in the syringes and then, in the implanted pumps.
Description of medical circuit
Centralization of preparations
The centralization of the intrathecal analgesic preparations was done in collaboration with the anesthesia-intensive care team on several arguments:
The route of administration: the infectious risk must be controlled.
The use of drugs with a narrow therapeutic margin such as ziconotide with its psychiatric adverse effects profile.
The use of several molecules in combination makes emerge then more risk during the preparation: confusion of molecules or concentrations.
The cost: centralization makes it possible to group the manufacture of preparations containing ziconotide and thus the use of a vial for several preparations in complete safety.
The control of the preparation: there is no possible antidote by the intrathecal route, a quality control of the preparation makes it possible to reduce the risk of error. Only the pharmacy department has the skills and the materials resources to perform those controls.
Centralization at the pharmacy was therefore adopted with this following circuit (Figure 1): a computerized prescription with pharmaceutical validation, a manufacturing under a laminar flow hood dedicated for non-toxic sterile preparation and a liberating analytical control. The constitution of a batch file with traceability of all stages of manufacture is carried out.
Computerized prescription
The circuit is integrated in a single software consisting of three modules BP PREP® (Anathec, Hesiode, LabControl, Alma Accos®, France).
The prescription is made using the Anathec® software. For the clinician, the intrathecal prescription is based on a rate of administration of the analgesic mixture and the daily dose of each drug. Depending on the evaluation of the pain it is also possible to set boluses (number and volume) as well as a refractory period. An optimization of the mixture is possible to keep the same doses per day by making a concession on the flow. Then for a given volume of preparation, the software calculates a volume for each product, the concentration and therefore the total amount to be present in the refill. The algorithm is constructed to take into account the feasibility of preparation.
The clinicians and the pharmacist are informed of the evolution of the daily dose of each compound compared to the previous load.
For a 40 ml pump, clinicians prescribe a refill of 50 ml, allowing taking a sample for the control and purging the pump system. Once the prescription is completed, it is sent to the pharmacy.
Pharmaceutical analysis
The prescription then appears in the Hesiode® planning tool which allows to see for each prescription, the progress of its state: “Prescribed”, “Ok pharmacist”, “in preparation”, “Available”.
Level II pharmaceutical validation is performed for each prescription before starting the preparation [19].
For each preparation, the software adjusts the vials of morphine used according to the concentration of the desired mixture. The preparation is then sent to the LabControl® traceability software in which the batch file data will be entered: batch number, analytical control, visual inspection.
Preparation
The preparation is then carried out in a class B room, under a vertical laminar flow hood (HERAsafe KS, Thermoelectron LED GmbH, Langensenbold, Germany) to ensure the sterility of the preparation.
Each product is added one after the other in a 60 ml syringe (BD, Le Pont de Chaix, France). Then a volume of sodium chloride 0.9 % is added to reach the final volume of preparation desired (30, 50, 100, 250 mL). The preparation is then packaged in a sterile field.
A 2 ml sample of the mixture is taken for the analytical control.
Analytical control
In order to secure our production process and thus provide a real control certificate to our different customers, several methods of analytical control has been developed over time. The first method allowed the control of preparations containing only morphine ropivacaine and ziconotide [20]. The second method allowed the control of preparations containing sufentanil and ziconotide [21]. Finally, the use of different molecules, alone or in combination, has forced us to develop a new method for the control of 8 molecules: morphine, ropivacaine, bupivacaine, baclofen, clonidine, fentanyl, sufentanil and ziconotide [22].
The analyzes are performed with an Acquity Hclass® UPLC coupled to a diode array detector (Waters, Guyancourt, France). The column used is an Acquity UPLC® BEH C18 1.7 μm 2.1 * 50 mm heated to 50 °C. The mobile phase used is a gradient of water and acetonitrile acidified to 0.1 % with trifluoroacetic acid. Two chromatographic methods are used because of a scale of different concentrations for all molecules. For morphine, ropivacaine, bupivacaine, baclofen and clonidine 0.3 μL are injected into the system while 10 μL are injected for ziconotide, fentanyl and sufentanil [22].
The detection takes place at
280 nm for morphine,
230 nm for ropivacaine and bupivacaine,
220 nm for baclofen,fentanyl and sufentanil
204 nm for ziconotide.
201 nm for clonidin.
pH control is also performed with a micro pH electrode(Model HI 2210, Hanna, Tanneries, France).
Stability
The literature is not rich in stability data for intrathecal analgesic mixtures in syringe. The ropivacaine morphine combination has been studied and appears to be stable at 30 °C [23]. However, the concentrations of morphine (0.02 mg/ml at 0.1 mg/ml) and ropivacaine (2 mg/ml max) are very low. The stability of the morphine clonidine combination in polypropylene syringe has been demonstrated by an American team [24] with a stability of 60 days at 4 °C.
In order to enrich the stability data of these intrathecal mixtures, several stability studies have been carried out within the pharmacy.
The first stability study concerns the morphine–ropivacaine-ziconotide mixture in polypropylene syringes stored at 5 °C, 21 °C, 31 °C and in polyolefin infusion bags stored at 21 °C. This study showed a stability of the mixture during three days at 5 °C in polypropylene syringes and fourtenn days at 21 °C in the polyolefin infusion bags. Beyond this, a decrease in ziconotide concentrations is observed [25].
The second study concerns the morphine – baclofen combination. Two mixtures were studied: a weakly concentrated mixture and a highly concentrated mixture. Two storage conditions were tested: 5 °C ± 3 °C and 25 °C ± 2 °C. This combination is stable for seven days in both temperature conditions [26].
Many studies have yet to be carried out to enrich the stability database of these mixtures and thus ensure proper storage and transport for these preparations.
Evolution
It was in 2004 that the first Institut de Cancérologie de l’Ouet (ICO) patients benefited from intrathecal analgesia. Activity increased over time (Figure 2) and was multiplied by a factor 1 to 10 in the last ten years. In 2017 the activity of intrathecal preparations represents more than 1700 annual preparations.
The subcontracting activity
Currently, only a few centers in France are trained in this method of analgesia because it requires a consistent technical environment and specific medical and pharmaceutical skills. For patients receiving intrathecal preparations, each filling requires that the patients be transported from their home to a referral center. These repeated trips can be difficult for patients to experience.
It is in this spirit that our institution has turned to the outsourcing of these preparations. The first step is to establish an agreement between the two institutions. The agreement specifies in particular the following points: the routing of the prescriptions to the pharmacy, the conditions of transport and schedules of availability of the preparations. This is an important step determining the modalities of cooperation between the two establishments.
In parallel, the ICO has submitted a complete evidence file on the process to the Regional Health Agency (RHA) for authorization to carry out masterful preparations on behalf of other health establishments. The RHA has given its approval via a decree authorizing the modification of the hospital pharmacy of the ICO for carrying out antalgic compounding for other establishments.
The RHA of the Pays de Loire authorized in 2015 an agreement between the Hospital Center of La Roche-sur-Yon and ICO Paul Papin for a manufacture of preparations by the pharmacy of the ICO Paul Papin and a routing up to in the center of La Roche-sur-Yon. This authorization has made it possible to offer this type of treatment to more patients likely to benefit from it.
Conclusion
The interest of intrathecal analgesia in the management of refractory cancer pain is certain. ICO Paul Papin, with more than 10 years of practice in the field saw the number of these intrathecal preparations for analgesic purposes increase. The daily practice and the means to supervise the manufacturing allowed acquiring an expertise (support of prescription and preparation, formation, analytical control) always in a will of quality and transparency for our customers. The opening to centralization of other preparations used intravenously, such as antibiotics, PCA morphine, could then be considered.
Conflict of interest statement: The authors state no conflict of interest. The authors have read the journal's Publication ethics and publication malpractice statement available at the journal's website and hereby confirm that they comply with all its parts applicable to the present scientific work. The study was in part granted by the company Loccioni Group, however study design and writing of the paper were under full responsibility of the authors.
References
1. Bhatia G, Lau ME, Koury KM, Gulur P. Intrathecal drug delivery (ITDD) systems for cancer pain. F1000Research. 2014 Jul;2. doi: https://doi.org/10.12688/f1000research.2-96.v4.Suche in Google Scholar
2. Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of world health organization guidelines for cancer pain relief: a 10-year prospective study. Pain 1995;63:65–76.10.1016/0304-3959(95)00017-MSuche in Google Scholar
3. Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, Grond S. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 2001;93:247–57.10.1016/S0304-3959(01)00324-4Suche in Google Scholar
4. Association Francophone des Soins Oncologiques de Support. Référentiels en soins oncologiques de support - Prise en charge de la douleur : antalgie intrathécale. 2014. Available at: http://www.afsos.org/liste-complete-referentiels/. Accessed: 01 Nov 2017.Suche in Google Scholar
5. Davis MP, Walsh D, Lagman R, LeGrand SB. Randomized clinical trial of an implantable drug delivery system. J Clin Oncol 2003;21:2800–01.10.1200/JCO.2003.99.003Suche in Google Scholar
6. Rauck RL, Cherry D, Boyer MF, Kosek P, Dunn J, Alo K. Long-term intrathecal opioid therapy with a patient-activated, implanted delivery system for the treatment of refractory cancer pain. J Pain 2003;4:441–7.10.1067/S1526-5900(03)00730-2Suche in Google Scholar
7. Burton AW, Rajagopal A, Shah HN, Mendoza T, Cleeland C, Hassenbusch III SJ. Epidural and intrathecal analgesia is effective in treating refractory cancer pain. Pain Med 2004;5:239–47.10.1111/j.1526-4637.2004.04037.xSuche in Google Scholar PubMed
8. Mercadante S, Intravaia G, Villari P, Ferrera P, Riina S, David F. Intrathecal treatment in cancer patients unresponsive to multiple trials of systemic opioids. Clin J Pain 2007;23:793–8.10.1097/AJP.0b013e3181565d17Suche in Google Scholar PubMed
9. Pasutharnchat K, Tan K-H, Hadi MA, Ho K-Y. Intrathecal analgesia in patients with cancer pain–an audit in a tertiary. Ann Acad Med Singap 2009;38:943–6.10.47102/annals-acadmedsg.V38N11p943Suche in Google Scholar
10. Brogan SE, Winter NB. Patient-controlled intrathecal analgesia for the management of breakthrough cancer pain: a retrospective review and commentary. Pain Med 2011;12:1758–68.10.1111/j.1526-4637.2011.01262.xSuche in Google Scholar PubMed
11. Dupoiron D. Analgésie intrathécale en cancérologie. Principes – état des lieux et perspectives. Douleurs Eval Diagn Trait 2016;17:128–34.10.1016/j.douler.2016.03.010Suche in Google Scholar
12. Dupoiron D, Lefebvre-kuntz D, Brenet O, de Bourmont S, Grelon F, Dixmeria F. Douleur chronique cancéreuse et analgésie intrathécale : expérience de trois centres de lutte contre le cancer. Douleurs Eval Diagn Trait 2011;12:140–6.10.1016/j.douler.2011.05.001Suche in Google Scholar
13. Huang Y, Li X, Zhu T, Lin J, Tao G. Efficacy and safety of ropivacaine addition to intrathecal morphine for pain management in intractable cancer. Mediators Inflamm 2015;2015:1–6.10.1155/2015/439014Suche in Google Scholar
14. Beloeil H, Viel E, Navez M-L, Fletcher D, Peronnet D. Techniques analgésiques locorégionales et douleur chronique. Ann Fr Anesth Réanimation 2013;32:275–84.10.1016/j.annfar.2013.02.021Suche in Google Scholar
15. Dupoiron D, Bore F, Lefebvre-Kuntz D, Brenet O, Debourmont S, Dixmerias F. Ziconotide adverse events in patients with cancer pain: a multicenter observational study of a slow titration, multidrug protocol. Pain Physician 2012;15:395–403.10.1016/S1754-3207(11)70349-2Suche in Google Scholar
16. Hettiarachchi HDM, Hsu Y, Harris TJ, Linninger AA. The effect of pulsatile flow on intrathecal drug delivery in the spinal canal. Ann Biomed Eng 2011;39:2592–602.10.1007/s10439-011-0346-xSuche in Google Scholar PubMed
17. Deer TR, Pope JE, Hayek SM, Bux A, Buchser E, Eldabe S. The polyanalgesic consensus conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines: intrathecal therapy best practices and guidelines. Neuromodulation Technol Neural Interface 2017;20:96–132.10.1111/ner.12538Suche in Google Scholar PubMed
18. Alicino I, Giglio M, Manca F, Bruno F, Puntillo F. Intrathecal combination of ziconotide and morphine for refractory cancer pain: a rapidly acting and effective choice. Pain 2012;153:245–9.10.1016/j.pain.2011.10.002Suche in Google Scholar PubMed
19. Conort O, Allenet B, Bedouch P, Charpiat B, , Juste M, Rose F-X. Recommandation de Bonne Pratique en Pharmacie Clinique - Société Française de Pharmacie Clinique. Sep 2012.Suche in Google Scholar
20. Rossignol E, Sorrieul J, Beaussart H, Kieffer H, Folliard C, Dupoiron D. Validation study of uplc method for determination of morphine, ropivacaïne and ziconotide in combination for intrathecal analgesia. J Anal Bioanal Tech 2016;7. doi:https://doi.org/10.4172/2155-9872.1000310.Suche in Google Scholar
21. Sorrieul J, Gibory V, Dinh CP, Kieffer H, Folliard C, Dupoiron D. Simultaneous determination of sufentanil and ziconotide in combination for intrathecal analgesia by UPLC-UV. Pharm Technol Hosp Pharm 2016;1:203–9.10.1515/pthp-2016-0020Suche in Google Scholar
22. Sorrieul J, Robert J, Gibory V, Collet M, Boutet M, Kieffer H. Validated chromatographic method for the simultaneous determination of eight drugs (morphine, ropivacaine, bupivacaine, baclofen, clonidine, sufentanil, fentanyl and ziconotide) for intrathecal analgesia. Ann Pharm Fr 2018;76:201–9.10.1016/j.pharma.2018.01.006Suche in Google Scholar PubMed
23. Öster Svedberg K, McKenzie J, Larrivee-Elkins C. Compatibility of ropivacaine with morphine, sufentanil, fentanyl, or clonidine. J Clin Pharm Ther 2002;27:39–45.10.1046/j.1365-2710.2002.00386.xSuche in Google Scholar PubMed
24. Xu QA, Trissel LA, Pham L. Physical and chemical stability of low and high concentrations of morphine sulfate with clonidine hydrochloride packaged in plastic syringes. Int J Pharm Compd 2002;6:66–9.Suche in Google Scholar
25. Robert J, Sorrieul J, Rossignol E, Beaussart H, Kieffer H, Folliard C. Chemical stability of morphine, ropivacaine, and ziconotide in combination for intrathecal analgesia. Int J Pharm Compd 2017;21:347–51.Suche in Google Scholar
26. Robert J, Sorrieul J, Kieffer H, Folliard C, Dupoiron D, Devys C. Stability study of morphine and baclofen solution in polypropylene syringes. Pharm Technol Hosp Pharm 2017;2:173–80.10.1515/pthp-2017-0028Suche in Google Scholar
© 2018 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- After Ten Issues Our Journal Has Found Its Audience and Main Topics
- Research Articles
- HPLC – Quality by Design Approach for Simultaneous Detection of Torsemide, Spironolactone and Their Degradant Impurities
- Physico-Chemical Stability of Sodium Thiosulfate Infusion Solutions in Polyolefin Bags at Room Temperature over a Period of 24 Hours
- Long-Term Stability Comparison between an Original and a Generic Version of Piperacillin/Tazobactam in Dextrose 5 % Infusion Polyolefin Bags at 5 ± 3 °C after Microwave Freeze-Thaw Treatment
- Environmental and Product Contamination during the Preparation of Antineoplastic Drugs with Robotic Systems
- Qualification and Performance Evaluation of an Automated System for Compounding Injectable Cytotoxic Drugs
- Short Communication
- Feedback on the Centralization of Intrathecal Analgesic Preparations in Hospital Pharmacy
- Opinion Paper
- Pharmaceutical Technology in Practice: A Personal View
Artikel in diesem Heft
- Frontmatter
- Editorial
- After Ten Issues Our Journal Has Found Its Audience and Main Topics
- Research Articles
- HPLC – Quality by Design Approach for Simultaneous Detection of Torsemide, Spironolactone and Their Degradant Impurities
- Physico-Chemical Stability of Sodium Thiosulfate Infusion Solutions in Polyolefin Bags at Room Temperature over a Period of 24 Hours
- Long-Term Stability Comparison between an Original and a Generic Version of Piperacillin/Tazobactam in Dextrose 5 % Infusion Polyolefin Bags at 5 ± 3 °C after Microwave Freeze-Thaw Treatment
- Environmental and Product Contamination during the Preparation of Antineoplastic Drugs with Robotic Systems
- Qualification and Performance Evaluation of an Automated System for Compounding Injectable Cytotoxic Drugs
- Short Communication
- Feedback on the Centralization of Intrathecal Analgesic Preparations in Hospital Pharmacy
- Opinion Paper
- Pharmaceutical Technology in Practice: A Personal View

