Abstract
Thymidylate synthase (TS) is a valid target for treatment of non-small cell lung cancer (NSCLC) and mesothelioma (MPM). The TS inhibitor pemetrexed (PMX) is now commonly used in combination with cisplatin (CDDP) or carboplatin, in the first-line setting for both of these lethal diseases. A combination of another TS inhibitor, Tomudex with CDDP, demonstrated similar activity in MPM patients. However, the efficacy of TS inhibitors is limited by uptake, metabolism and target affinity. While uptake of most antifolates is mediated by the reduced folate carrier, PMX is also a good substrate for the proton-coupled folate transporter (PCFT), which displays optimal activity at the acidic pH of the tumor microenvironment. NSCLC and MPM have a variable expression of PCFT, which might be caused by the differential methylation status of the PCFT promoter, resulting in decreased anticancer activity. PMX and TDX activity also depends on polyglutamylation, catalyzed by folylpolyglutamate synthetase (FPGS), which results in intracellular retention and thus enhanced cytotoxicity. We demonstrated that resistance to the classical antifolate methotrexate in human leukemia cells with a marked loss of FPGS activity was associated with impaired splicing of FPGS pre-mRNA. Moreover, we recently showed that the sensitivity of MPM patients to PMX-carboplatin is related to tumor TS expression. Patients with low TS mRNA levels had a significantly longer overall survival (20 vs. 7 months) compared with patients with high expression. Intriguingly, recent trials demonstrated that histology plays an important role in the sensitivity of NSCLC patients, and PMX-CDDP is now approved only for adenocarcinoma patients. These results might be partly explained by the higher TS expression detected in lung tumors of squamous histology. In summary, the clinical success of TS inhibitors may depend on biomarker-driven patient selection, and further studies should evaluate the genetic/epigenetic factors involved in the modulation of these biomarkers in the preclinical and clinical setting.
Introduction
Thymidylate synthase (TS) inhibitors such as pemetrexed changed the current practice of treatment of advanced non-squamous non-small cell lung cancer (NSCLC) as well as mesothelioma. In both diseases, its combination with cisplatin has become first-line treatment [1–3]. In this review, we highlight molecular determinants of TS inhibitors in NSCLC, as well as new pharmacodynamics and pharmacogenetic biomarkers. The evaluation of these markers could help in the selection of the optimal clinical dose, as well as markers of resistance, which might be used to tailor TS inhibitor-based chemotherapy. This review is an updated, shortened version of a review published previously [4].
Lung cancer represents the leading cause of cancer mortality worldwide with a 5-year survival rate at 10%–15% and <7% of patients alive 10 years after diagnosis. Improvement of treatment remains a major challenge [5]. Surgery is curative only in early stage (I–II) NSCLC, while radiotherapy is an option in some patients. Platinum-doublet treatment is considered the front-line standard therapy in advanced NSCLC [6], improving both disease-free survival and 5-year overall survival (OS) [7] of locally advanced or stage IIIA disease, as well as stages IIB and IV [8]. Platinum-based doublet therapy is the standard of care against these tumors. In patients with good performance status, different combinations with cisplatin, gemcitabine, taxanes, irinotecan, vinorelbine, ifosfamide, mitomycin C, vindesine or vinblastine reached better results than single agent treatment both in terms of response and survival [9]. Three-drug combinations were better than two-drug combinations only in terms of response [10], while single-agent chemotherapy is only applied in patients with poor performance status. Because of the lower incidence of side effects, carboplatin has often replaced cisplatin, although several meta-analyses showed higher response rates for cisplatin compared with carboplatin combinations [11]. Third-generation drugs, such as gemcitabine, taxanes, irinotecan, pemetrexed and vinorelbine, in combination with platinum compounds, now constitute the core of chemotherapy in advanced NSCLC [12]. Non-platinum based combinations of some of these drugs is now considered as an alternative first-line treatment when platinum therapy is contraindicated [13].
In patients with non-squamous NSCLC, the combination of pemetrexed with cisplatin [1] was recently approved as first-line therapy. Only for a small group of NSCLC patients with activating mutations in the epidermal growth factor receptor (EGFR), tyrosine kinase inhibitors such as erlotinib and gefitinib are approved as first-line treatment [14]. However, larger studies are needed to examine the efficacy and safety of different potential third-line regimens in an effort to prolong survival while maintaining quality of life of patients with advanced NSCLC.
Role of thymidylate synthase as therapeutic target
TS is a folate-dependent enzyme that catalyzes the methylation of deoxyuridine-5′-monophosphate (dUMP) using 5,10-methylene-tetrahydrofolate (5,10-CH2-THF) as the methyl donor to form deoxythymidine-5′-monophosphate (dTMP) [15]. This reaction is the only de novo source for dTMP, which is critical for DNA replication and repair, which in turn is essential for cell proliferation (Figure 1). Therefore, inhibition of DNA synthesis through TS is an established successful antimetabolite approach controlling tumor cell growth. Hence, TS inhibitors can be considered one of the first “targeted” drugs because they were specifically designed in order to interfere with a very well-defined molecular target, DNA with essential functions in cell growth and proliferation. Like all “targeted” drugs, the target has essential functions in normal cells. Regarding TS inhibitors, proliferating tissues, such as bone marrow and gastrointestinal mucosa, are also dependent on DNA synthesis and thus sensitive to TS inhibition, leading to myelosuppression, mucositis and diarrhea.
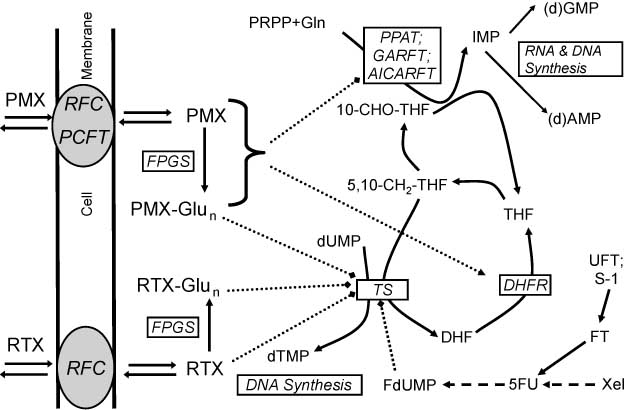
Uptake and metabolism of antifolates.
RTX and MTX are both taken up by the reduced folate carrier (RFC), while PMX is also a substrate for the proton-coupled folate transporter (PCFT). Both RTX and PMX are activated to their polyglutamate forms by folylpolyglutamate synthetase (FPGS); both the monoglutamate and polyglutamate (Glun) forms inhibit dihydrofolate reductase (DHFR) and thymidylate synthase (TS), which converts deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) for which 5,10-methylene-tetrahydrofolate (5,10-CH2-THF) is the methyl donor. Especially PMX-Glun is a more effective inhibitor of TS. 5-Fluoro-deoxyuridine monophosphate (FdUMP) forms a ternary stable covalent complex with TS and 5,10-CH2-THF. PMX and PMX-Glun also inhibit the purine de novo enzymes aminoimidazole carboxamide ribonucleotide transformylase (AICARFT) and glycinamide ribonucleotide formyl transferase (GARFT), for which 10-formyl-tetrahydrofolate (10-CHO-THF) donates the formyl group. Phosphoribosyl pyrophosphate (PRPP) is the initial phosphoribose ring donor for phosphoribosyl pyrophosphate amidotransferase (PPAT) in the first step of the purine de novo pathway, leading to the synthesis of IMP, precursor for the purine (deoxy) nucleotides (d)AMP and (d)GMP, substrates for RNA and DNA synthesis. UFT and S-1 are oral drug formulations which contain ftorafur (FT), which is a prodrug for 5-fluorouracil (5-FU), which inhibits TS via its metabolite FdUMP. 5-FU can also be formed via several enzymatic steps from another 5-FU prodrug capecitabine (Xeloda, Xel). Abbreviations: DHF, dihydrofolate; THF, tetrahydrofolate; Gln, glutamine.
As TS has two substrates, dUMP and 5,10-CH2-THF, inhibition can be mediated by a dUMP or a folate analog (Figures 1 and 2). 5-Fluorouracil (5-FU) was introduced into the clinical setting more than 50 years ago [16]. 5-FU is a precursor of fluoro-dUMP (FdUMP), which inhibits TS by the formation of a covalent ternary complex with TS and CH2-THF, resulting in a depletion of dTMP and inhibition of DNA synthesis leading to thymine-less death. TS inhibition also results in an increase in 2′-deoxyuridine- 5′-triphosphate (dUTP), leading to misincorporation of dUTP into DNA: its excision, catalyzed by uracil-DNA glycosylase, results in DNA damage. Moreover, a specific interaction exists between oncogenes and TS, by binding of TS protein to p53 and c-myc RNA [17, 18], while wild-type p53 can also inhibit TS promoter activity [19].
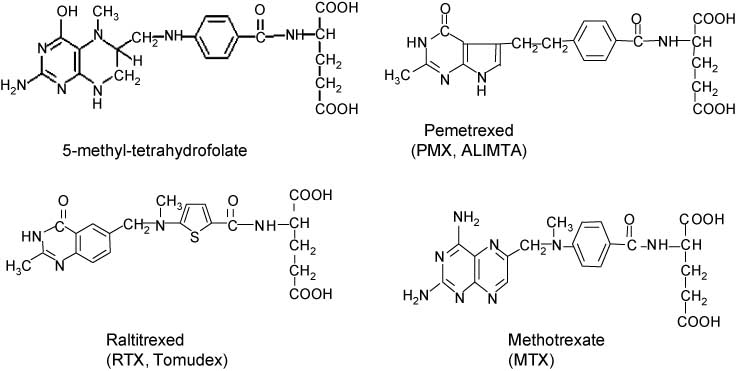
Structural formulas.
5-Methyl-tetrahydrofolate (5-CH3-THF), the physiological precursor for 5,10-CH2-THF in a reaction catalyzed by methylene tetrahydrofolate (MTHFR), methotrexate (MTX), pemetrexed (PMX, ALIMTA) and Raltitrexed (RTX, Tomudex).
At transcriptional level, TS expression is activated by the transcription factor E2F1, which plays a key role in cell cycle progression [20]. In addition, TS protein functions as an RNA-binding protein repressing the translation of its own mRNA [21, 22]. When TS inhibitors bind to TS, the complex cannot interact with its cognate mRNA, which results in increased TS protein expression [23]. Hence, elevated expression of TS after exposure to TS inhibitors was, in part, attributable to increased protein stability [24, 25].
Thymidylate synthase inhibitors in NSCLC
Pemetrexed
As pemetrexed not only inhibits TS but also dihydrofolate reductase (DHFR) and the purine de novo pathway, it is often referred to as a multitargeted antifolate. Pemetrexed was first registered for the treatment of malignant pleural mesothelioma, based on a randomized, Phase III, single-blind, multicenter trial which compared cisplatin alone versus cisplatin plus pemetrexed. In that trial, the addition of pemetrexed to cisplatin significantly improved both the survival, by 3 months, and the overall response rate [26]. Because pemetrexed was also active as a single agent in Phase II and Phase III trials with NSCLC patients previously treated with chemotherapy, it was also approved for second-line treatment of NSCLC [27]. In that trial, pemetrexed (500 mg/m2 every 3 weeks, with vitamin B12 and folic acid supplementation) was equally as effective as docetaxel (75 mg/m2 every 3 weeks), with a median survival of 8.3 vs. 7.9 months, respectively. However, side effects of pemetrexed were significantly lower than with docetaxel.
In first-line therapy of advanced NSCLC patients, the combinations of pemetrexed plus cisplatin or carboplatin were equally as effective as gemcitabine plus cisplatin or carboplatin [2], while carboplatin plus pemetrexed or gemcitabine were also equally effective. In this study with 436 patients, the overall median survival was 7.3 months for the pemetrexed/carboplatin arm and 7.0 months for the gemcitabine/carboplatin arm, but pemetrexed/carboplatin caused less toxicity [28]. In a randomized Phase III trial enrolling 1725 chemotherapy-naive patients, cisplatin plus gemcitabine or cisplatin plus pemetrexed showed similar efficacy with better tolerability and more convenient administration than cisplatin/gemcitabine [1], with an OS of 10.3 months in both arms and a response rate of 30.6% in the pemetrexed/cisplatin arm and 28.2% in the gemcitabine/cisplatin arm. For cisplatin/pemetrexed, the rates of grade 3 or 4 neutropenia, anemia and thrombocytopenia (p≤0.001), febrile neutropenia (p=0.002) and alopecia (p<0.001) were significantly lower [1].
In the same study, patients with non-squamous NSCLC performed statistically superior in the cisplatin/pemetrexed treatment arm, whereas patients with squamous cell histology performed better with cisplatin/gemcitabine [1]. Other Phase II studies and retrospective review of two large, randomized, Phase III trials supported the predictive role of histology for pemetrexed [2], which was confirmed in a multicenter, double-blind Phase III trial evaluating pemetrexed versus placebo in stage IIIB/IV NSCLC patients who did not progress after four cycles of first-line platinum-based chemotherapy [29]. Pemetrexed showed a statistically significant superior activity in non-squamous (n=482; 4.5 months vs. 2.6 months) than in squamous histology (n=182; 2.8 months vs. 2.6 months). Therefore, pemetrexed was approved for use in first-line treatment of advanced non-squamous NSCLC.
Thymidylate synthase expression as determinant of pemetrexed activity
One potential explanation of the differential activity with pemetrexed treatment was related to the baseline expression of TS gene and protein, which were significantly higher in squamous cell carcinoma compared with adenocarcinoma patients (p<0.0001) [29].
Pemetrexed is a multitargeted agent that enters the cell via the reduced folate carrier (RFC) and is converted by folylpolyglutamate synthetase (FPGS) to a series of active polyglutamate derivatives able to inhibit several folate-dependent enzymes such as TS, DHFR, glycinamide ribonucleotide formyl transferase (GARFT) and, to a lesser extent, aminoimidazole carboxamide ribonucleotide transformylase (AICARFT) [30] (Figure 1). This mechanism of action leads to depletion of fully reduced folates and results in disruption of both purine and pyrimidine nucleotides. These polyglutamates are retained intracellularly longer than the parent compound, resulting in more prolonged cytotoxic effects. Whereas GARFT is inhibited just weakly by pemetrexed, TS represents the main target of this drug and its inhibition leads to the interruption of tetrahydrofolate oxidation with consequential lack of DHFR activity [31]. However, GARFT inhibition may become important in the case of TS overexpression or mutation, and the multitargeted effects of pemetrexed explain its broader spectrum of cytotoxic activity when compared in preclinical studies with other antimetabolites such as 5-FU and antifolates such as methotrexate or raltitrexed [32, 33].
Preclinical data showed a significant correlation between overexpression of TS mRNA or protein with reduced sensitivity to pemetrexed in NSCLC cell lines [34, 35]. The inhibition of DNA synthesis and induction of apoptotic cell death by pemetrexed were also significantly reduced by overexpression of TS [36]. Messenger RNA expression of genes involved in the mechanism of action of pemetrexed was correlated with in vitro sensitivity of 61 freshly explanted human tumor specimens, and low gene expression levels of TS, as well as of GARFT and DHFR, significantly correlated with chemosensitivity to pemetrexed [37]. Moreover, xenografts with TS overexpression were more resistant to pemetrexed [36]. Hence, patients with “low” baseline TS were more likely to respond to pemetrexed than patients with “high” baseline TS [38]. Two recent studies on mesothelioma samples reported that TS protein and mRNA expression significantly correlated with survival and response rates in patients treated with pemetrexed-based regimens [39, 40]. Similarly, the immunohistochemical analysis of TS expression in 24 NSCLC specimens from pemetrexed non-responding patients was significantly higher than that in those of responders [36]. In another study, the analysis of 49 tumor specimens from NSCLC patients treated with pemetrexed showed that low TS expression was correlated with longer median PFS (4.8 vs. 3.4 months; p=0.01), whereas in patients with adenocarcinoma, the low TS patient group also had a longer median survival as compared with patients with high TS expression [41].
However, there may be a difference in the potential prognostic and predictive role of TS. Although several studies suggested that TS levels may be associated with stage of disease, lymph node metastasis, tumor differentiation, prognosis and tumor cell proliferation, many results were controversial [42–44]. A high cytoplasm tumoral TS analyzed by automated in situ protein quantification (AQUA) was associated with improved survival for NSCLC patients [44], but in colon cancer improved survival was found for patients with low tumoral TS expression as detected by immunohistochemistry [45]. However, standard immunohistochemistry with visual scoring in an attempt to quantify protein expression has significant technical limitations, including the non-quantitative chemistry of routine immunoperoxidase stains and the subjective light intensity perception of the human eye [45]. Additional studies, with consistent methodology, and broader validation cohorts are needed to define the precise value of TS.
Although overexpression of TS is the most studied mechanism involved in resistance to pemetrexed, multiple other mechanisms of antifolate resistance might reduce pemetrexed activity in vitro and in vivo, such as (i) impaired uptake due to loss of transporter function of either the RFC or the proton-coupled folate transporter (PCFT); (ii) overexpression of ATP-driven multidrug resistance (MDR) transporters which increase drug efflux; (iii) defective antifolate polyglutamylation due to decreased FPGS expression, inactivating mutations or altered splicing; (iv) increased expression of γ-glutamyl hydrolase (GGH); (v) overexpression of DHFR and mutations that decrease its affinity; and (vi) expansion of intracellular 5,6,7,8-tetrahydrofolate (THF) cofactor pools [46].
Previous preclinical findings showed that multidrug resistance protein 1 (MRP1)–MRP5 and BCRP can transport folates and antifolates including methotrexate, raltitrexed and pemetrexed. Polymerase chain reaction (PCR) analysis in NSCLC samples showed an increased MRP4 (significant) or MRP5 (trend) in resistant tumors [37], as well as a higher gene and protein expression of ABCC11/MRP8 [47]. However, in 13 lung adenocarcinoma cells there was no correlation between ABCC11 gene expression and pemetrexed sensitivity, whereas in most antifolate-resistant selected cell lines no overexpression of any of the MRPs or BCRP has been reported [48]. By contrast, 14 human leukemia cell lines were made resistant against various polyglutamatable inhibitors of DHFR, TS and GARFT, using a repeated high-dose intermittent schedule mimicking the chemotherapeutic treatment. The vast majority of these antifolate-resistant cell lines had a 90%–99% loss of FPGS activity, but without any substantial decrease in FPGS mRNA levels. Cross-resistance was observed in polyglutamylation-dependent antifolates including pemetrexed up to five orders of magnitude, while retaining sensitivity to polyglutamylation-independent antifolates [49]. Although inactivating FPGS mutations exist, they are not a frequent cause for the loss of FPGS function [50]. However, multiple antifolate-resistant tumor cell lines showed a marked suppression of FPGS activity in the absence of decreased FPGS mRNA levels [51]. An alternative explanation for the post-transcriptional loss of FPGS activity in the absence of decreased FPGS mRNA levels is the alternative splicing of FPGS, as demonstrated in leukemia cells [52].
The impact of an increase in the intracellular THF cofactor pool size on the cytotoxic activity of multiple antifolates was shown by a marked increase of the 50% inhibitory concentrations for the polyglutamatable antifolates pemetrexed and raltitrexed. The high intracellular folate pool resulted in a significant suppression of the formation of antifolate polyglutamates, whereas folate depletion resulted in enhanced pemetrexed inhibition of purine synthesis [53, 54]. Of note, impaired RFC function is an important mechanism of resistance to methotrexate and other antifolates in vitro [55], and may be associated with clinical resistance to this agent in the treatment of acute lymphoblastic leukemia [56]. The transport for pemetrexed is very different, because an RFC-independent, pH-dependent pathway serves as an alternative transport route for this drug and is mediated by the PCFT [57]. A high PCFT increased the growth inhibitory activity of pemetrexed, illustrating its unique role in the transport and pharmacological activity of pemetrexed. Because of the ubiquitous expression of PCFT in human tumors, and the ability of PCFT to sustain pemetrexed activity even in the absence of RFC, tumor cells are unlikely to become resistant to pemetrexed as a result of impaired transport because of the redundancy of these genetically distinct routes [58].
However, the promoter of the PCFT is highly methylated [59], leading to reduced activity in cancer cell systems. Promoter silencing through methylation and gene copy loss accounted for the loss of PCFT activity in antifolate-resistant HeLa R1-11 cells [60]. A plausible modality to overcome antifolate resistance in cells that are devoid of PCFT activity due to promoter methylation might be the combination with relatively well-tolerated demethylating agents, such as 5-Aza-dC [59]. Therefore, data about the possible association of PCFT promoter methylation and clinical outcome in NSCLC patients treated with pemetrexed can be very useful to offer alternative therapies to resistant patients.
In conclusion, resistance to pemetrexed in NSCLC is likely to be related to TS and a transporter, but other mechanisms need further clarification. However, the clinical safety profile of pemetrexed makes this drug an attractive agent for polychemotherapy regimens, because it can overcome tumor resistance and improve clinical outcome.
UFT and S-1
As primary lung cancers are characterized by high expression and activity of dihydropyrimidine dehydrogenase (DPD), which is the key enzyme of 5-FU degradation, studies on the activity of oral 5-FU derivatives preventing 5-FU degradation appeared of interest for NSCLC treatment. The first oral 5-FU derivatives were tegafur [61], and 5′-deoxy-5-fluorouridine (5-DFUR), which yielded a response rate lower than 15% [62]. Other rationally engineered and metabolically activated DPD inhibiting fluoropyrimidines (DIFs), such as UFT [63] and S-1 [64], proved to be effective in several randomized controlled studies in NSCLC patients.
UFT combines uracil, a competitive inhibitor of DPD, with the 5-FU prodrug tegafur in a 4:1 molar ratio (Figure 1). In Japan, UFT is approved for several tumors, including NSCLC [65], while in the UK, the combination of UFT and folinic acid is approved as first-line treatment for metastatic colorectal cancer and was active in second- or third-line therapy of Caucasian NSCLC patients [66], and because of its favorable toxicity profile warranted further investigation. Another more promising oral 5-FU prodrug formulation is S-1, in which a more potent DPD inhibitor is combined. S-1 combines the 5-FU prodrug tegafur (ftorafur, FT) with two enzyme inhibitors, CDHP (5-chloro-2,4-dihydroxypyridine) and OXO (potassium oxonate), in a molar ratio of 1(FT):0.4(CDHP):1(OXO). CDHP is a reversible very potent competitive inhibitor of DPD, preventing the degradation of FT-derived 5-FU. Therefore, 5-FU remains in plasma and tumor tissue longer and at higher levels than when low dose 5-FU is continuously infused intravenously. OXO is a reversible competitive inhibitor of orotate phosphoribosyltransferase, which is expressed at high levels in the gastrointestinal tract. After oral S-1 administration, OXO will prevent activation of 5-FU in gastrointestinal endothelial cells, resulting in a reduction in diarrhea and mucositis caused by 5-FU. A recent Phase III trial showed that treatment with carboplatin plus S-1 was non-inferior than carboplatin plus paclitaxel with regard to OS in 564 chemotherapy-naive Japanese patients [67]. Median OS was 15.2 months in patients in the carboplatin and S-1 arm and 13.3 months in the carboplatin/paclitaxel arm, respectively, suggesting that S-1 might be a valid treatment option in patients with advanced NSCLC.
Preclinical studies showed a synergistic antitumor effect of S-1 with the EGFR tyrosine kinase inhibitor (TKI) gefitinib in NSCLC cell lines. Importantly, gefitinib reduced the expression of the transcription factor E2F1, downregulating TS at both mRNA and protein levels, favoring the activity of S-1 [68]. Furthermore, the combination of S-1 and gefitinib synergistically inhibited the growth of NSCLC gefitinib-resistant NSCLC cells and xenografts with MET amplification, suggesting that the addition of S-1 to EGFR TKIs is a promising strategy to overcome EGFR TKI resistance in NSCLC with MET amplification [69].
Although a recent study showed that tumor expression levels of TS and DPD were predictive of response to S-1-carboplatin chemotherapy [70], a pooled analysis of S-1 trials in NSCLC demonstrated that the activity of S-1 was independent of tumor histology [71].
Other thymidylate synthase inhibitors
Other TS inhibitors in NSCLC include the quinazoline folate analog raltitrexed (Figures 1 and 2), which is a direct and specific TS inhibitor. In a Phase I trial, the dose-escalation study of the combination of raltitrexed and cisplatin was studied in 19 patients with previously untreated metastatic NSCLC. In this trial, 3 patients achieved a partial response, 13 had stable disease and 3 progressed [72]. However, withdrawal of the sponsor’s support has left raltitrexed (i.e., Tomudex) without the level of continuing investigation afforded to pemetrexed.
Raltitrexed was also evaluated in the treatment of mesothelioma in combination with cisplatin versus cisplatin alone, reaching an overall survival of 11.4 and 8.8 months, respectively [73]. Evaluation of raltitrexed as an alternative for pemetrexed in mesothelioma is ongoing.
Capecitabine is another oral 5-FU prodrug, for which thymidine phosphorylase (TP) mediates the final step of its activation pathway. A high TP expression could provide a rationale for the use of this drug in NSCLC. High TP expression in lung cancer cells and stroma was associated with response to capecitabine/docetaxel chemotherapy [74], and might be a useful predictor of NSCLC response to capecitabine-based chemotherapy. Moreover, the high response rate observed in a Phase II trial of docetaxel plus capecitabine in pretreated NSCLC patients [75] encourage further evaluation of capecitabine in NSCLC. However, the prognostic significance of TP expression in NSCLC as well as the possible role of capecitabine in NSCLC treatment need further evaluation.
Conclusions and future perspectives
TS is a validated target in NSCLC and mesothelioma, resulting in the widespread use of the TS inhibitor pemetrexed as first/second-line and maintenance therapy for both non-squamous NSCLC and mesothelioma. However, benefits from conventional chemotherapy in NSCLC have plateaued, while much more cost-effective results should be obtained with individualized patient treatment. Accordingly, the clinical success for TS inhibitors may ultimately be dependent upon our ability to correctly administer these agents following appropriate biomarker-driven patient selection, including TS genotype and expression, and using the right combination therapy.
The levels of TS expression have been correlated with resistance to therapy with pemetrexed, and a prospective validation on the role of TS for pemetrexed-based chemotherapy is ongoing in a Phase III randomized study [4]. Further studies should also evaluate the genetic and epigenetic factors involved in the regulation of TS. The promoter of the human TS gene contains a 28-bp tandem repeat near the initiation codon in its 5′-untranslated region. This tandem repeat region functions as an enhancer element for the TS gene promoter, the TS enhancer region (TSER). TSER has been shown to be polymorphic: (i) a variable number of the tandem repeat of a 28-bp sequence (2R/3R) and (ii) a G>C single nucleotide substitution within the repeats, altering the E-box sequence binding an upstream stimulatory factor (USF-1) [76]. In vitro expression studies have suggested an association between the number of repeats and TS expression: the presence of a triple repeat (3R/3R) results in 2.6-fold greater TS expression than a double repeat [77]. However, the relationship between these polymorphisms, regulation of TS expression and patient response to fluoropyrimidine treatment has been inconsistent, and no correlation between TS polymorphisms and survival was observed in patients enrolled in a randomized Phase II study of pemetrexed compared with pemetrexed plus carboplatin in pretreated patients with advanced NSCLC [78].
Negative results were also reported in a study on TS gene copy number, which was increased more frequently in squamous cell carcinomas, but was not associated with prognosis. These results are consistent with studies in colorectal cancer which showed that tandem repeats were associated with TS activity in normal colon tissue, but not in tumors [4]. Therefore, it seems unlikely that the TS gene copy number will predict clinical benefit from treatment with pemetrexed, although this can only be validated in prospective clinical studies. Another promising genetic marker consists of a polymorphism in methylene tetrahydrofolate reductase (MTHFR), for which a number of polymorphisms have been described. In a combined analysis of 206 patients treated with either pemetrexed or pemetrexed plus carboplatin, an increased progression-free and overall survival were observed for patients harboring the TT genotype [79]. This genotype results in an altered MTHFR activity affecting the reduced folate pools, which are important for sensitivity to pemetrexed.
A recent study evaluated two microRNA targeting TSs, miR-192 and miR-215. However, downregulation of TS by miR-192/215 did not increase 5-FU sensitivity. By contrast, overexpression of both microRNAs resulted in a reduction of cell proliferation and therefore diminished the effectiveness of S-phase specific drugs such as 5-FU, suggesting that miR-192 and miR-215 can still play a role in resistance to TS inhibitors [80]. Therefore, further studies should evaluate whether miRNA expressed in NSCLC cells might regulate resistance mechanisms towards pemetrexed, as reported for other chemotherapeutic agents [81]. Additional information will also be gained with the integration of data from “next-generation” or “massively parallel” sequencing platforms.
Regarding drug combinations (Figure 3), another intriguing observation is the downregulation of TS by several targeted drugs, such as the EGFR TKI erlotinib which decreased TS expression and activity, showing synergistic interaction with pemetrexed in NSCLC cells [40]. Similar results were observed with the novel EGFR TKI BIBW2992, providing a scientific rationale for testing the combination of BIBW2992 with pemetrexed in patients who develop acquired resistance after harboring the EGFR T790M mutation [82].
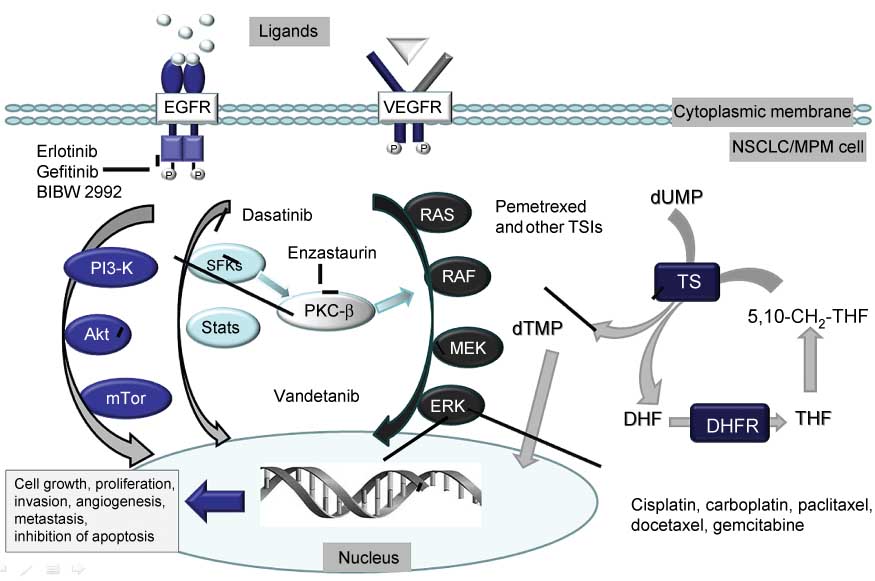
Interaction between PMX and signaling and DNA targeted drugs.
PMX and other thymidylate synthase inhibitors (TSIs) cause DNA damage through the depletion of dTMP, which is a basis for the interaction with DNA targeted drugs, such as cisplatin, carboplatin, paclitaxel docetaxel and gemcitabine. Signaling pathways, such as the Ras-RAF-MEK-ERK pathway, the PI3-K, Akt, mTor pathway, and the Src family kinases (SFKs) pathway are often activated during cytotoxic stress in order to support cellular growth, proliferation, angiogenesis and inhibition of apoptosis. These signaling pathways can be inhibited by relatively specific drugs such as erlotinib, gefitinib and BIB2992, which inhibit epidermal growth factor receptor (EGFR), leading to inhibition of other signaling pathways. Antagonists of natural ligands can inhibit the EGFR and vascular endothelial growth factor receptor (VEGFR). Multitargeted drugs such as vandetanib inhibit Mek and Akt; dasatinib inhibits the SFK pathway, while enzastaurin inhibits the serine-theonine kinase PKXβ. Hence, inhibition of one (more or all) of these survival signaling pathways forms a basis for synergistic interaction with PMX.
Other targeted agents modulating TS included the PKCβ inhibitor enzastaurin and the Src inhibitor dasatinib, which synergistically enhanced pemetrexed cytotoxicity in NSCLC cells [83, 84]. In mesothelioma cells, the multiple targeted drug vandetanib also enhanced sensitivity to pemetrexed [85].
These data suggest innovative combinations improving the efficacy of TS-inhibiting agents for the treatment of NSCLC, which should be tested and validated in the clinical setting.
References
1. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51.10.1200/JCO.2007.15.0375Search in Google Scholar
2. Scagliotti G, Hanna N, Fossella F, Sugarman K, Blatter J, Peterson P, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist 2009;14:253–63.10.1634/theoncologist.2008-0232Search in Google Scholar
3. Lehman NL. Future potential of thymidylate synthase inhibitors in cancer therapy. Expert Opin Investig Drugs 2002;11:1775–87.10.1517/13543784.11.12.1775Search in Google Scholar
4. Galvani E, Giovannetti E, Peters GJ. Thymidylate synthase inhibitors for non-small cell lung cancer. Expert Opinion Invest Drugs 2011;20:1343–56.10.1517/13543784.2011.617742Search in Google Scholar
5. Sant M, Aareleid T, Berrino F, Bielska LM, Carli PM, Faivre J, et al. EUROCARE-3: survival of cancer patients diagnosed 1990–94 – results and commentary. Ann Oncol 2003;14(Suppl 5):v61–118.10.1093/annonc/mdg754Search in Google Scholar
6. Qiao X, Tullgren O, Lax I, Sirzen F, Lewensohn R. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer 2003;41:1–11.10.1016/S0169-5002(03)00152-1Search in Google Scholar
7. Keller SM, Adak S, Wagner H, Herskovic A, Komaki R, Brooks BJ, et al. A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern Cooperative Oncology Group. N Engl J Med 2000;343:1217–22.10.1056/NEJM200010263431703Search in Google Scholar
8. Crino L, Weder W, van Meerbeeck J, Felip E. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21(Suppl 5):v103–15.10.1093/annonc/mdq207Search in Google Scholar
9. Marino P, Preatoni A, Cantoni A, Buccheri G. Single-agent chemotherapy versus combination chemotherapy in advanced non-small cell lung cancer: a quality and meta-analysis study. Lung Cancer 1995;13:1–12.10.1016/0169-5002(95)00477-ISearch in Google Scholar
10. Delbaldo C, Michiels S, Syz N, Soria JC, Le CT, Pignon JP. Benefits of adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non-small-cell lung cancer: a meta-analysis. J Am Med Assoc 2004;292:470–84.10.1001/jama.292.4.470Search in Google Scholar PubMed
11. Ardizzoni A, Boni L, Tiseo M, Fossella FV, Schiller JH, Paesmans M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst 2007;99:847–57.10.1093/jnci/djk196Search in Google Scholar PubMed
12. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92–8.10.1056/NEJMoa011954Search in Google Scholar PubMed
13. D’Addario G, Pintilie M, Leighl NB, Feld R, Cerny T, Shepherd FA. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol 2005;23:2926–6.10.1200/JCO.2005.03.045Search in Google Scholar PubMed
14. Gutierrez ME, Kummar S, Giaccone G. Next generation oncology drug development: opportunities and challenges. Nat Rev Clin Oncol 2009;6:259–65.10.1038/nrclinonc.2009.38Search in Google Scholar PubMed PubMed Central
15. Carreras CW, Santi DV. The catalytic mechanism and structure of thymidylate synthase. Annu Rev Biochem 1995;64:721–62.10.1146/annurev.bi.64.070195.003445Search in Google Scholar PubMed
16. Muggia FM, Peters GJ, Landolph JR Jr. XIII International Charles Heidelberger Symposium and 50 Years of Fluoropyrimidines in Cancer Therapy held on September 6 to 8, 2007 at New York University Cancer Institute, Smilow Conference Center. Mol Cancer Ther 2009;8:992–9.10.1158/1535-7163.MCT-08-0731Search in Google Scholar PubMed PubMed Central
17. Ju J, Pedersen-Lane J, Maley F, Chu E. Regulation of p53 expression by thymidylate synthase. Proc Natl Acad Sci USA 1999;96:3769–774.10.1073/pnas.96.7.3769Search in Google Scholar PubMed PubMed Central
18. Chu E, Takechi T, Jones KL, Voeller DM, Copur SM, Maley GF, et al. Thymidylate synthase binds to c-myc RNA in human colon cancer cells and in vitro. Mol Cell Biol 1995;15:179–85.10.1128/MCB.15.1.179Search in Google Scholar PubMed PubMed Central
19. Giovannetti E, Backus HH, Wouters D, Peters GJ. Functional inactivity and mutations of p53 differentially affect sensitivity to 5-fluorouracil and antifolate inhibitors of thymidylate synthase (TS) by altering TS levels in colorectal cancer cells. Nucleosides Nucleotides Nucleic Acids 2008;27:740–5.10.1080/15257770802145512Search in Google Scholar PubMed
20. DeGregori J, Kowalik T, Nevins JR. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol 1995;15:4215–24.10.1128/MCB.15.8.4215Search in Google Scholar PubMed PubMed Central
21. Chu E, Voeller D, Koeller DM, Drake JC, Takimoto CH, Maley GF, et al. Identification of an RNA binding site for human thymidylate synthase. Proc Natl Acad Sci USA 1993;90:517–21.10.1073/pnas.90.2.517Search in Google Scholar PubMed PubMed Central
22. Lin X, Parsels LA, Voeller DM, Allegra CJ, Maley GF, Maley F, et al. Characterization of a cis-acting regulatory element in the protein coding region of thymidylate synthase mRNA. Nucleic Acids Res 2000;28:1381–9.10.1093/nar/28.6.1381Search in Google Scholar PubMed PubMed Central
24. Zhang Y, Yang S, Liu M, Song C, Wu N, Ling P, et al. Interaction between thymidylate synthase and its cognate mRNA in zebrafish embryos. PLoS One 2010;5:e10618.10.1371/journal.pone.0010618Search in Google Scholar PubMed PubMed Central
25. Kitchens ME, Forsthoefel AM, Rafique Z, Spencer HT, Berger FG. Ligand-mediated induction of thymidylate synthase occurs by enzyme stabilization. Implications for autoregulation of translation. J Biol Chem 1999;274:12544–7.10.1074/jbc.274.18.12544Search in Google Scholar PubMed
26. Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636–44.10.1200/JCO.2003.11.136Search in Google Scholar PubMed
27. Hanna N, Shepherd FA, Fossella FV, Pereira JR, de Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589–97.10.1200/JCO.2004.08.163Search in Google Scholar PubMed
28. Gronberg BH, Bremnes RM, Flotten O, Amundsen T, Brunsvig PF, Hjelde HH, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:3217–24.10.1200/JCO.2008.20.9114Search in Google Scholar PubMed
29. Ceppi P, Volante M, Saviozzi S, Rapa I, Novello S, Cambieri A, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 2006;107:1589–96.10.1002/cncr.22208Search in Google Scholar PubMed
30. Shih C, Chen VJ, Gossett LS, Gates SB, MacKellar WC, Habeck LL, et al. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res 1997;57:1116–23.Search in Google Scholar
31. Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther 2007;6:404–17.10.1158/1535-7163.MCT-06-0343Search in Google Scholar PubMed
32. Chen VJ, Bewley JR, Andis SL, Schultz RM, Iversen PW, Shih C, et al. Preclinical cellular pharmacology of LY231514 (MTA): a comparison with methotrexate, LY309887 and raltitrexed for their effects on intracellular folate and nucleoside triphosphate pools in CCRF-CEM cells. Br J Cancer 1998;78(Suppl 3):27–34.10.1038/bjc.1998.751Search in Google Scholar PubMed PubMed Central
33. Takimoto CH. New antifolates: pharmacology and clinical applications. Oncologist 1996;1:68–81.10.1634/theoncologist.1-1-68Search in Google Scholar
34. Giovannetti E, Mey V, Nannizzi S, Pasqualetti G, Marini L, Del TM, et al. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol 2005;68:110–8.10.1124/mol.104.009373Search in Google Scholar
35. Giovannetti E, Lemos C, Tekle C, Smid K, Nannizzi S, Rodriguez JA, et al. Molecular mechanisms underlying the synergistic interaction of erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, with the multitargeted antifolate pemetrexed in non-small-cell lung cancer cells. Mol Pharmacol 2008;73:1290–300.10.1124/mol.107.042382Search in Google Scholar
36. Takezawa K, Okamoto I, Okamoto W, Takeda M, Sakai K, Tsukioka S, et al. Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. Br J Cancer 2011;104:1594–601.10.1038/bjc.2011.129Search in Google Scholar
37. Hanauske AR, Eismann U, Oberschmidt O, Pospisil H, Hoffmann S, Hanauske-Abel H, et al. In vitro chemosensitivity of freshly explanted tumor cells to pemetrexed is correlated with target gene expression. Invest New Drugs 2007;25:417–23.10.1007/s10637-007-9060-9Search in Google Scholar
38. Gomez HL, Santillana SL, Vallejos CS, Velarde R, Sanchez J, Wang X, et al. A phase II trial of pemetrexed in advanced breast cancer: clinical response and association with molecular target expression. Clin Cancer Res 2006;12:832–8.10.1158/1078-0432.CCR-05-0295Search in Google Scholar
39. Righi L, Papotti MG, Ceppi P, Bille A, Bacillo E, Molinaro L, et al. Thymidylate synthase but not excision repair cross-complementation group 1 tumor expression predicts outcome in patients with malignant pleural mesothelioma treated with pemetrexed-based chemotherapy. J Clin Oncol 2010;28:1534–9.10.1200/JCO.2009.25.9275Search in Google Scholar
40. Zucali PA, Giovannetti E, Destro A, Mencoboni M, Ceresoli GL, Gianoncelli L, et al. Thymidylate synthase and excision repair cross-complementing group-1 as predictors of responsiveness in mesothelioma patients treated with pemetrexed/carboplatin. Clin Cancer Res 2011;17:2581–90.10.1158/1078-0432.CCR-10-2873Search in Google Scholar
41. Chen CY, Chang YL, Shih JY, Lin JW, Chen KY, Yang CH, et al. Thymidylate synthase and dihydrofolate reductase expression in non-small cell lung carcinoma: the association with treatment efficacy of pemetrexed. Lung Cancer 2011;74:132–8.10.1016/j.lungcan.2011.01.024Search in Google Scholar
42. Higashiyama M, Kodama K, Yokouchi H, Takami K, Fukushima M, Minamigawa K, et al. Thymidylate synthase and dihydropyrimidine dehydrogenase activities in non-small cell lung cancer tissues: relationship with in vitro sensitivity to 5-fluorouracil. Lung Cancer 2001;34:407–16.10.1016/S0169-5002(01)00248-3Search in Google Scholar
43. Nakagawa T, Tanaka F, Otake Y, Yanagihara K, Miyahara R, Matsuoka K, et al. Prognostic value of thymidylate synthase expression in patients with p-stage I adenocarcinoma of the lung. Lung Cancer 2002;35:165–70.10.1016/S0169-5002(01)00407-XSearch in Google Scholar
44. Zheng Z, Li X, Schell MJ, Chen T, Boulware D, Robinson L, et al. Thymidylate synthase in situ protein expression and survival in stage I nonsmall-cell lung cancer. Cancer 2008;112:2765–73.10.1002/cncr.23491Search in Google Scholar
45. Edler D, Kressner U, Ragnhammar P, Johnston PG, Magnusson I, Glimelius B, et al. Immunohistochemically detected thymidylate synthase in colorectal cancer: an independent prognostic factor of survival. Clin Cancer Res 2000;6:488–92.Search in Google Scholar
46. Assaraf YG. Molecular basis of antifolate resistance. Cancer Metastasis Rev 2007;26:153–81.10.1007/s10555-007-9049-zSearch in Google Scholar
47. Uemura T, Oguri T, Ozasa H, Takakuwa O, Miyazaki M, Maeno K, et al. ABCC11/MRP8 confers pemetrexed resistance in lung cancer. Cancer Sci 2010;101:2404–10.10.1111/j.1349-7006.2010.01690.xSearch in Google Scholar
48. Assaraf YG. The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resist Updat 2006;9:227–46.10.1016/j.drup.2006.09.001Search in Google Scholar
49. Liani E, Rothem L, Bunni MA, Smith CA, Jansen G, Assaraf YG. Loss of folylpoly-gamma-glutamate synthetase activity is a dominant mechanism of resistance to polyglutamylation-dependent novel antifolates in multiple human leukemia sublines. Int J Cancer 2003;103:587–99.10.1002/ijc.10829Search in Google Scholar
50. Zhao R, Titus S, Gao F, Moran RG, Goldman ID. Molecular analysis of murine leukemia cell lines resistant to 5,10-dideazatetrahydrofolate identifies several amino acids critical to the function of folylpolyglutamate synthetase. J Biol Chem 2000;275:26599–606.10.1074/jbc.M002580200Search in Google Scholar
51. Pizzorno G, Moroson BA, Cashmore AR, Russello O, Mayer JR, Galivan J, et al. Multifactorial resistance to 5,10-dideazatetrahydrofolic acid in cell lines derived from human lymphoblastic leukemia CCRF-CEM. Cancer Res 1995;55:566–73.Search in Google Scholar
52. Stark M, Wichman C, Avivi I, Assaraf YG. Aberrant splicing of folylpolyglutamate synthetase as a novel mechanism of antifolate resistance in leukemia. Blood 2009;113:4362–9.10.1182/blood-2008-08-173799Search in Google Scholar
53. Zhao R, Gao F, Goldman ID. Marked suppression of the activity of some, but not all, antifolate compounds by augmentation of folate cofactor pools within tumor cells. Biochem Pharmacol 2001;61:857–65.10.1016/S0006-2952(01)00532-9Search in Google Scholar
54. Zhao R, Zhang S, Hanscom M, Chattopadhyay S, Goldman ID. Loss of reduced folate carrier function and folate depletion result in enhanced pemetrexed inhibition of purine synthesis. Clin Cancer Res 2005;11:1294–301.10.1158/1078-0432.1294.11.3Search in Google Scholar
55. Matherly LH, Goldman DI. Membrane transport of folates. Vitam Horm 2003;66:403–56.10.1016/S0083-6729(03)01012-4Search in Google Scholar
56. Gorlick R, Goker E, Trippett T, Steinherz P, Elisseyeff Y, Mazumdar M, et al. Defective transport is a common mechanism of acquired methotrexate resistance in acute lymphocytic leukemia and is associated with decreased reduced folate carrier expression. Blood 1997;89:1013–8.10.1182/blood.V89.3.1013Search in Google Scholar
57. Zhao R, Gao F, Hanscom M, Goldman ID. A prominent low-pH methotrexate transport activity in human solid tumors: contribution to the preservation of methotrexate pharmacologic activity in HeLa cells lacking the reduced folate carrier. Clin Cancer Res 2004;10:718–27.10.1158/1078-0432.CCR-1066-03Search in Google Scholar PubMed
58. Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter: impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Mol Pharmacol 2008;74:854–62.10.1124/mol.108.045443Search in Google Scholar PubMed PubMed Central
59. Gonen N, Bram EE, Assaraf YG. PCFT/SLC46A1 promoter methylation and restoration of gene expression in human leukemia cells. Biochem Biophys Res Commun 2008;376: 787–92.10.1016/j.bbrc.2008.09.074Search in Google Scholar PubMed
60. Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Mol Cancer Ther 2009;8:2424–31.10.1158/1535-7163.MCT-08-0938Search in Google Scholar PubMed PubMed Central
61. Ansfield FJ, Kallas GJ, Singson JP. Phase I–II studies of oral tegafur (ftorafur). J Clin Oncol 1983;1:107–10.10.1200/JCO.1983.1.2.107Search in Google Scholar PubMed
62. Ota K, Taguchi T, Kimura K. Report on nationwide pooled data and cohort investigation in UFT phase II study. Cancer Chemother Pharmacol 1988;22:333–8.10.1007/BF00254241Search in Google Scholar
63. Keicho N, Saijo N, Shinkai T, Eguchi K, Sasaki Y, Tamura T, et al. Phase II study of UFT in patients with advanced non-small cell lung cancer. Jpn J Clin Oncol 1986;16:143–6.10.1093/oxfordjournals.jjco.a039130Search in Google Scholar PubMed
64. Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 1996;7:548–57.10.1097/00001813-199607000-00010Search in Google Scholar PubMed
65. Tanaka F, Wada H, Fukushima M. UFT and S-1 for treatment of primary lung cancer. Gen Thorac Cardiovasc Surg 2010;58:3–13.10.1007/s11748-009-0498-xSearch in Google Scholar PubMed
66. Surmont V, Aerts JG, Pouw E, Tan KY, Vernhout R, Gras J, et al. Oral UFT, etoposide and leucovorin in recurrent non-small cell lung cancer: a non-randomized phase II study. Lung Cancer 2009;66:333–7.10.1016/j.lungcan.2009.02.016Search in Google Scholar
67. Okamoto I, Yoshioka H, Morita S, Ando M, Takeda K, Seto T, et al. Phase III trial comparing oral S-1 plus carboplatin with paclitaxel plus carboplatin in chemotherapy-naive patients with advanced non-small-cell lung cancer: results of a west Japan oncology group study. J Clin Oncol 2010;28:5240–6.10.1200/JCO.2010.31.0326Search in Google Scholar
68. Okabe T, Okamoto I, Tsukioka S, Uchida J, Iwasa T, Yoshida T, et al. Synergistic antitumor effect of S-1 and the epidermal growth factor receptor inhibitor gefitinib in non-small cell lung cancer cell lines: role of gefitinib-induced down-regulation of thymidylate synthase. Mol Cancer Ther 2008;7:599–606.10.1158/1535-7163.MCT-07-0567Search in Google Scholar
69. Okabe T, Okamoto I, Tsukioka S, Uchida J, Hatashita E, Yamada Y, et al. Addition of S-1 to the epidermal growth factor receptor inhibitor gefitinib overcomes gefitinib resistance in non-small cell lung cancer cell lines with MET amplification. Clin Cancer Res 2009;15:907–13.10.1158/1078-0432.CCR-08-2251Search in Google Scholar
70. Takeda M, Okamoto I, Hirabayashi N, Kitano M, Nakagawa K. Thymidylate synthase and dihydropyrimidine dehydrogenase expression levels are associated with response to S-1 plus carboplatin in advanced non-small cell lung cancer. Lung Cancer 2011;73:103–9.10.1016/j.lungcan.2010.10.022Search in Google Scholar
71. Yamamoto N, Yamanaka T, Ichinose Y, Kubota K, Sakai H, Gemma A, et al. Pooled analysis of S-1 trials in non-small cell lung cancer according to histological type. Anticancer Res 2010;30:2985–90.Search in Google Scholar
72. Manegold C, Buchholz E, Kloeppel R, Kreisel C, Smith M. Phase I dose-escalating study of raltitrexed (‘Tomudex’) and cisplatin in metastatic non-small cell lung cancer. Lung Cancer 2002;36:183–9.10.1016/S0169-5002(01)00491-3Search in Google Scholar
73. van Meerbeeck JP, Gaafar R, Manegold C, Van Klaveren RJ, Van Marck EA, Vincent M, et al.; European Organisation for Research and Treatment of Cancer Lung Cancer Group; National Cancer Institute of Canada. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 2005;23:6881–9.10.1200/JCO.20005.14.589Search in Google Scholar PubMed
74. Han JY, Hong EK, Lee SY, Yoon SM, Lee DH, Lee JS. Thymidine phosphorylase expression in tumour cells and tumour response to capecitabine plus docetaxel chemotherapy in non-small cell lung cancer. J Clin Pathol 2005;58:650–4.10.1136/jcp.2004.022764Search in Google Scholar PubMed PubMed Central
75. Kindwall-Keller T, Otterson GA, Young D, Neki A, Criswell T, Nuovo G, et al. Phase II evaluation of docetaxel-modulated capecitabine in previously treated patients with non-small cell lung cancer. Clin Cancer Res 2005;11:1870–6.10.1158/1078-0432.CCR-04-1727Search in Google Scholar PubMed
76. Mandola MV, Stoehlmacher J, Muller-Weeks S, Cesarone G, Yu MC, Lenz HJ, et al. A novel single nucleotide polymorphism within the 5’ tandem repeat polymorphism of the thymidylate synthase gene abolishes USF-1 binding and alters transcriptional activity. Cancer Res 2003;63:2898–904.Search in Google Scholar
77. Horie N, Aiba H, Oguro K, Hojo H, Takeishi K. Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5′-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Funct 1995;20:191–7.10.1247/csf.20.191Search in Google Scholar PubMed
78. Smit EF, Burgers SA, Biesma B, Smit HJ, Eppinga P, Dingemans AM, et al. Randomized phase II and pharmacogenetic study of pemetrexed compared with pemetrexed plus carboplatin in pretreated patients with advanced non-small-cell lung cancer. J Clin Oncol 2009;27:2038–45.10.1200/JCO.2008.19.1650Search in Google Scholar PubMed
79. Tiseo M, Giovannetti E, Tibaldi C, Camerini A, Di Costanzo F, Barbieri F, et al. Pharmacogenetic study of patients with advanced non-small cell lung cancer treated with second-line pemetrexed or pemetrexed-carboplatin. Lung Cancer 2012;78:92–9.10.1016/j.lungcan.2012.07.009Search in Google Scholar PubMed
80. Boni V, Bitarte N, Cristobal I, Zarate R, Rodriguez J, Maiello E, et al. miR-192/miR-215 influence 5-fluorouracil resistance through cell cycle-mediated mechanisms complementary to its post-transcriptional thymidilate synthase regulation. Mol Cancer Ther 2010;9:2265–75.10.1158/1535-7163.MCT-10-0061Search in Google Scholar PubMed
81. Giovannetti E, Erozenci A, Smit J, Danesi R, Peters GJ. Molecular mechanisms underlying the role of microRNAs (miRNAs) in anticancer drug resistance and implications for clinical practice. Crit Rev Oncol Hematol 2012;81:103–22.10.1016/j.critrevonc.2011.03.010Search in Google Scholar PubMed
82. Takezawa K, Okamoto I, Tanizaki J, Kuwata K, Yamaguchi H, Fukuoka M, et al. Enhanced anticancer effect of the combination of BIBW2992 and thymidylate synthase-targeted agents in non-small cell lung cancer with the T790M mutation of epidermal growth factor receptor. Mol Cancer Ther 2010;9:1647–56.10.1158/1535-7163.MCT-09-1009Search in Google Scholar PubMed
83. Tekle C, Giovannetti E, Sigmond J, Graff JR, Smid K, Peters GJ. Molecular pathways involved in the synergistic interaction of the PKCβ inhibitor enzastaurin with the antifolate pemetrexed in non-small cell lung cancer cells. Br J Cancer 2008;99:750–9.10.1038/sj.bjc.6604566Search in Google Scholar PubMed PubMed Central
84. Ceppi P, Rapa I, Lo IM, Righi L, Giorcelli J, Pautasso M, et al. Expression and pharmacological inhibition of thymidilate synthase and Src kinase in non-small cell lung cancer. Int J Cancer 2012;130:1777–86.10.1002/ijc.26188Search in Google Scholar PubMed
85. Giovannetti E, Zucali PA, Assaraf YG, Leon LG, Smid K, Alecci C, et al. Preclinical emergence of vandetanib as a potent antitumor agent in mesothelioma: molecular mechanisms underlying its synergistic interaction with pemetrexed and carboplatin. Br J Cancer 2011;105:1542–53.10.1038/bjc.2011.400Search in Google Scholar PubMed PubMed Central
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Editorial
- New developments in the publication of Pteridines
- Chemistry
- First synthesis of asperopterin A, an isoxanthopterin glycoside from Aspergillus oryzae
- Tetrahydrobiopterin
- Three classes of tetrahydrobiopterin-dependent enzymes
- Tetrahydrobiopterin attenuates ischemia-reperfusion injury following organ transplantation by targeting the nitric oxide synthase: investigations in an animal model
- Inflammatory diseases
- Folates and antifolates in rheumatoid arthritis
- Immune activation and inflammation increase the plasma phenylalanine-to-tyrosine ratio
- Tryptophan degradation and neopterin levels by aging
- Spot analyses of serum neopterin, tryptophan and kynurenine levels in a random group of blood donor population
- Endothelial dysfunction, cardiovascular diseases
- Tetrahydrobiopterin protects soluble guanylate cyclase against oxidative inactivation
- Immune activation and inflammation in patients with cardiovascular disease are associated with elevated phenylalanine-to-tyrosine ratios
- Malignant diseases treatment
- Thymidylate synthase inhibitors for thoracic tumors
- Polymorphisms correlated with the clinical outcome of locally advanced or metastatic colorectal cancer patients treated with ALIRI vs. FOLFIRI
- Folate homeostasis of cancer cells affects sensitivity to not only antifolates but also other non-folate drugs: effect of MRP expression
- Enzymology folates
- Crystal structures of thymidylate synthase from nematodes, Trichinella spiralis and Caenorhabditis elegans, as a potential template for species-specific drug design
- Crystal structures of complexes of mouse thymidylate synthase crystallized with N4-OH-dCMP alone or in the presence of N5,10-methylenetetrahydrofolate
- Enzymology pterins
- First insights into structure-function relationships of alkylglycerol monooxygenase
- Fatty aldehyde dehydrogenase, the enzyme downstream of tetrahydrobiopterin-dependent alkylglycerol monooxygenase
- Expression of full-length human alkylglycerol monooxygenase and fragments in Escherichia coli
- Enzyme occurrence and function in model organisms
- The diverse biological functions of glutathione S-transferase omega in Drosophila
- Uncommon and parallel developmental patterns of thymidylate synthase expression and localization in Trichinella spiralis and Caenorhabditis elegans
Articles in the same Issue
- Masthead
- Masthead
- Editorial
- New developments in the publication of Pteridines
- Chemistry
- First synthesis of asperopterin A, an isoxanthopterin glycoside from Aspergillus oryzae
- Tetrahydrobiopterin
- Three classes of tetrahydrobiopterin-dependent enzymes
- Tetrahydrobiopterin attenuates ischemia-reperfusion injury following organ transplantation by targeting the nitric oxide synthase: investigations in an animal model
- Inflammatory diseases
- Folates and antifolates in rheumatoid arthritis
- Immune activation and inflammation increase the plasma phenylalanine-to-tyrosine ratio
- Tryptophan degradation and neopterin levels by aging
- Spot analyses of serum neopterin, tryptophan and kynurenine levels in a random group of blood donor population
- Endothelial dysfunction, cardiovascular diseases
- Tetrahydrobiopterin protects soluble guanylate cyclase against oxidative inactivation
- Immune activation and inflammation in patients with cardiovascular disease are associated with elevated phenylalanine-to-tyrosine ratios
- Malignant diseases treatment
- Thymidylate synthase inhibitors for thoracic tumors
- Polymorphisms correlated with the clinical outcome of locally advanced or metastatic colorectal cancer patients treated with ALIRI vs. FOLFIRI
- Folate homeostasis of cancer cells affects sensitivity to not only antifolates but also other non-folate drugs: effect of MRP expression
- Enzymology folates
- Crystal structures of thymidylate synthase from nematodes, Trichinella spiralis and Caenorhabditis elegans, as a potential template for species-specific drug design
- Crystal structures of complexes of mouse thymidylate synthase crystallized with N4-OH-dCMP alone or in the presence of N5,10-methylenetetrahydrofolate
- Enzymology pterins
- First insights into structure-function relationships of alkylglycerol monooxygenase
- Fatty aldehyde dehydrogenase, the enzyme downstream of tetrahydrobiopterin-dependent alkylglycerol monooxygenase
- Expression of full-length human alkylglycerol monooxygenase and fragments in Escherichia coli
- Enzyme occurrence and function in model organisms
- The diverse biological functions of glutathione S-transferase omega in Drosophila
- Uncommon and parallel developmental patterns of thymidylate synthase expression and localization in Trichinella spiralis and Caenorhabditis elegans

