Thermal and chemical stability of europium(III) and terbium(III) coordination polymers with 4,4′-stilbenedicarboxylic acid: synthesis, structural transformations and sorption properties
Abstract
Polycrystalline coordination polymers based on 4,4′-stilbenedicarboxylic acid (H2SDC) and selected lanthanide ions were synthesized via a conventional precipitation method in aqueous media, in accordance with the principles of green chemistry. The coordination compounds (Eu2SDC3·5H2O and Tb2SDC3·5.5H2O) were characterized by elemental analysis, powder X-ray diffraction (PXRD), ATR-FTIR spectroscopy, TG-DSC thermal analysis in air, nitrogen sorption measurements at −196 °C, and scanning electron microscopy (SEM). The influence of water, acidic (HCl), basic (NaOH), and hydrothermal conditions, as well as the dehydration process, on their structures and stability was investigated. The results revealed structural changes upon exposure to water and a partial loss of crystallinity due to the removal of solvent molecules. Acidic and basic environments led to degradation of the coordination polymers. As porous materials, their sorption capacities toward organic dyes were also evaluated, demonstrating their potential in environmental remediation. Additionally, corresponding lanthanide oxides were obtained via thermal decomposition of the parent complexes, which acted as structure directing agents.
Introduction
A considerable number of metal-organic frameworks (MOFs) have been synthesized and characterized in the last several deacades. 1 , 2 , 3 They are classified as inorganic-organic compounds and as multidimensional coordination polymers in terms of chemical structure. MOFs based on the broad range of secondary building units (SBUs) have been at the center of attention due to their soaring porosity, quite high durability, effortless changeable functional groups, and molecular-level control over pore structure and dimension. 4 , 5 , 6 One of the most important properties of MOFs is structural variability, e.g., one organic ligand with different metal ions or metal clusters forms structurally versatile coordination polymers with diverse topologies and characteristic.
Metal-organic frameworks are promising candidates for use in a variety branch of science and industry, including gas storage 7 and separation, 8 sensing, 9 drug delivery, 10 removal of contaminates 11 and catalysis. 4 , 5 , 6 A diverse range of their potential applications in various industrial fields require this group of compounds to be stable not only under ambient atmosphere but also under exposition of specific process conditions, which are carried out usually in harsh environments typical of industrial processes such as: elevated temperature, acidic or alkaline media, and very often aqueous medium. Many porous coordination polymers intended for sorption applications require activation to remove guest molecules from their pores or channels, commonly achieved via thermal treatment, which necessitates sufficient thermal robustness of the materials. Despite these remarkable requirements, the knowledge of the chemical and thermal stability of MOFs is still limited, mainly for the most commonly explored metal-organic frameworks. 12 , 13 , 14 , 15 , 16 , 17 Therefore, investigations related to the determination of the different factors impacting the coordination polymer structures, including metal-ligands stability upon exposition to chemical and temperature circumstances, are important for a real assessment of the use of these compounds in practice. The stability of metal-organic frameworks results from the building elements used, i.e., metal ions/clusters and ligands. Lanthanide(III) ions as nodes in MOFs structures exhibit a high and variable coordination numbers forming mainly in their coordination compounds nine (light lanthanides) or eight (heavy lanthanides) coordination bonds in metal complexes. In this work, europium(III) and terbium(III) ions were selected as representative lanthanide centers because they combine high coordination flexibility and strong affinity for oxygen donor ligands with unique physicochemical features that are advantageous for structure-property studies. Both ions possess favourable ionic radius, lying near the middle of the lanthanide contraction, which makes them structurally versatile and comparable in coordination behavior, while still showing subtle differences that can influence framework stability and transformations. In addition, Eu3+ and Tb3+ are well known for their characteristic luminescent properties, which often facilitate the tracking of structural and electronic changes in coordination polymers, providing additional functional value beyond purely structural considerations. Unlike lanthanum(III), which is non-emissive and larger in size, europium(III) and terbium(III) offer a better balance of stability, spectroscopic activity, and relevance to practical applications such as sensing, catalysis, and adsorption. These factors justify their choice as model ions for evaluating the stability and sorption properties of 4,4′-stilbenedicarboxylate-based coordination polymers. They are considered as hard Pearson acids; therefore, they have a high affinity for O donor ligands, including polycarboxylic aromatic acids. The high ionic nature of the Ln–O bonds renders these lanthanide coordination compounds thermally/chemically stable. Many efforts have been made to synthesize and structurally characterize novel lanthanide MOFs, attracted by the special spectroscopic and magnetic properties of lanthanide elements. 18 , 19 , 20 , 21 As there are a limited number of reports regarding the chemical stability of the lanthanide(III) coordination polymers, the study of the behavior of synthesized compounds in aqueous solutions of varying acidities (or temperature and pressure) is of utmost importance. 22 , 23
The 4,4′-stilbenedicarboxylic acid (H2SDC) as an elongated derivative of 4,4′-biphenyldicarboxylic acid due to the presence of a double bond, is a widely explored organic building block for construction of metal-organic frameworks. 24 , 25 Most coordination polymers with 4,4′-stilbenedicarboxylic acid are based on transition metals as nodes, while the number of coordination polymers with lanthanides is much smaller. The composition analysis of the reported coordination polymers with the mentioned above ligand, 26 clearly points out that they are mainly mixed ligands compounds containing N-donor ligands such as 2,2′-bipyridyl, 1,10-phenanthroline, pyridine, or phenylalanine. 27 , 28 , 29 In spite of the fact that their structures and spectroscopic characterization are quite well known the chemical and thermal stability of these metal complexes remains a considerable challenge. 30 , 31 , 32 Without this fundamental attribute, their practical utility may be severely limited, posing a significant obstacle to further advancements in the diverse field. The constant development aimed at synthesis new stable coordination polymers is imperative to fully harness the potential that coordination polymers hold in various technological and scientific realms.
The aim of this paper is the physicochemical studies on europium(III) and terbium(III) coordination polymers with 4,4′-stilbenedicarboxylate ligand, synthesized by the classic precipitation method. The utilization of such a synthesis method is highly conducive to environmentally friendly practices, aligning with the principles of green chemistry. This method, conducted in an aqueous environment, contributes significantly to reducing the use of organic solvents, known for their detrimental environmental impact. 33 , 34 , 35 , 36 , 37 , 38 Additionally, the method’s efficiency in terms of time underscores its sustainable nature. 39 For the determination of the thermal and chemical stability of the obtained lanthanide(III) complexes, we have investigated: the as-synthesized compounds, products of their dehydration and re-dehydration processes, as well as samples obtained after immersion of pristine compounds in acidic/alkaline solutions. The impact of hydrothermal conditions on the chemical/thermal stability of the prepared complexes was also investigated. The characterization of all compounds was carried out by the X-ray powder diffraction method, along with the determination of the unit cell parameters of crystalline samples, FTIR spectroscopy and TG-DSC thermal analysis methods in the air atmosphere. For pristine metal complexes, porosity was investigated by the nitrogen sorption experiments.
This study also reports on the characterization of lanthanide oxides obtained from the thermal decomposition of synthesized europium(III) and terbium(III) 4,4′-stilbenedicarboxylate complexes, highlighting their potential as precursor materials. The morphological variability of the prepared lanthanide oxides is achieved through the careful selection of ligands and reaction conditions, enabling the synthesis of materials with tailored porosity, functionality, and thermal properties. Simultaneously, the conversion of lanthanide coordination compounds into their respective oxides facilitates the efficient recycling of these materials and the subsequent reutilization of the oxides in further synthetic processes, fully aligning with the principles of circular economy and sustainable development. An additional objective of this study is to evaluate the potential of these compounds as effective adsorbents for commonly used dyes, which are frequently present as pollutants in various environmental systems, including wastewater.
Experimental section
4,4′-Stilbenedicarboxylic acid (≥95 %) was obtained from Abcr, while lanthanide salts EuCl3·6H2O and TbCl3·6H2O (≥99.9 %) were purchased from Thermo Scientific. Prior to synthesis, the organic ligand was purified by refluxing in distilled water.
Coordination polymers (Eu-1 and Tb-1) were synthesized as follows: NaOH solution (0.1 mol/L) was dropwise added to the aqueous suspension of 4,4′-stilbenedicarboxylic acid (2 mmol, 0.54 g) with continuously stirring and heating (80 °C) as solution reached pH 6.5. EuCl3·5H2O (1.3 mmol, 0.45 g) was dissolved in 10 mL of water. The reaction of the sodium salt of 4,4′-stilbenedicarboxylic acid with dissolved EuCl3 was carried out on a magnetic stirrer in the continuous stirring mode, at the temperature of 80 °C and lasted 30 min. Afterwards the resulting white suspension was filtered and washed with hot water. The obtained precipitate was left to dry at room temperature. Coordination compound with Tb(III) ions was obtained by the same method and under the same conditions.
In order to investigate the effect of temperature, pressure, and the presence of water (hydrothermal conditions) on the obtained compounds, 50 mL of water was added to the as-synthesized complex (0.1 g of Eu-1 or Tb-1). The resulting suspensions were transferred into Teflon-lined vessels, sealed in autoclaves, and heated at 90 °C for 3 days, yielding Eu-1* and Tb-1*. The dehydrated forms of complexes (Eu-2 and Tb-2) were obtained by thermal activation of the Eu-1 and Tb-1 compounds (0.5 g) at 160 °C under vacuum using an ASAP 2020 automatic volumetric adsorption apparatus (Micromeritics).
The Eu-6 and Tb-6 compounds were obtained by heating of their respective precursors, Eu-1 and Tb-1, in a laboratory furnace at 1000 °C during 2 h resulting in the formation of the corresponding lanthanide oxides.
The elemental analysis of the solid samples was carried out with a CHN 2400 (Perkin-Elmer) analyser (Table S.1).
The ATR-FTIR spectra of the 4,4′-stilbenedicarboxylic acid and all tested samples were recorded in the range of 4000–600 cm−1 by means of a Nicolet 6700 (Thermo Scientific) spectrophotometer equipped with a universal ATR Smart iTR diamond attachment.
The thermal analyses (thermogravimetric-TG and differential scanning calorimetry-DSC) were carried out in flowing air atmosphere (v = 0.75 dm3 h−1) using the SETSYS 16/18 thermal analyser (Setaram). The samples (5–9 mg) were heated in ceramic crucibles up to 1000 °C at a heating rate of 10° min−1.
The X-ray powder diffractions of the examined compounds were registered on an PANalytical Empyrean automated diffractometer (Bragg–Brentano method; Cu-Kα radiation) via continuous scan over the scattering angular range 2θ between 3 and 70° at ambient temperature 40 , 41 .
The nitrogen adsorption-desorption isotherms were measured at −196 °C using an automatic volumetric adsorption ASAP 2020 (Micromeritics) apparatus. The Eu-1 and Tb-1 samples were outgassed at 160 °C.
The morphological characteristics of the solid-state aggregation of the Ln(III) complex compounds and their oxides were investigated using SEM, with images captured at 25000× magnification under a 30-kV electron acceleration voltage.
The adsorption studies of methylene blue (BM1), methyl blue (BM2), and rhodamine B (RB) on the synthesized compounds were monitored by UV–Vis spectroscopy using a GENESYS 10S spectrophotometer (Thermo Scientific). The concentration of the dye in the supernatant was determined from the calibration curve of its maximum absorbance (665 nm, 585 nm and 554 nm, respectively).
For the adsorption experiments, 10 mg of the Eu-1 compound was weighed into separate flasks. Then, 20 mL of dye solutions (BM1, BM2, and RB) each with a concentration of 10 mg/L, were added to the flasks. The mixtures were shaken, and adsorption was monitored at time intervals of 20, 40, 60, and 120 min. After each time point, samples were transferred into centrifuge tubes and centrifuged at 3000 rpm for 10 min. The supernatant was then collected, and the remaining dye concentration was measured to determine the extent of adsorption. The same procedure was followed for the other compounds: Eu-2, Eu-6, Tb-2, and Tb-6. The obtained data were analyzed using the pseudo-first-order (Eq. (1)) and pseudo-second-order (Eq. (2)) adsorption models. 42
where q e is the adsorbed amount at the equilibrium state [mg/g], q t is the adsorbed amount after time t [mg/g], k 1 is the equilibrium rate constant [1/min], and k 2 is the equilibrium rate constant [g/(mg min)].
The morphologies of the samples were analysed using the highresolution scanning electron-ion microscope (Quanta 3D FEG, FEI).
Results and discussion
Polycrystalline coordination polymers based on Eu(III) and Tb(III) ions and 4,4′-stilbenedicarboxylic acid of the formula Eu2SDC3·5H2O (Eu-1) and Tb2SDC3·5.5H2O (Tb-1) (where SDC2− = C16H10O4 2−) were synthesized using the green precipitation method in the reaction of sodium 4,4′-stilbenedicarboxylate (Na2SDC) and LnCl3 (Ln(III) = Eu or Tb) in the aqueous solutions (Fig. 1). The compounds were obtained in the form of white polycrystalline powders hardly soluble in water and common organic solvents such as: DMF, DMSO, and ethanol. The first stage of the study included the characteristics of the as-synthesized complexes which were designated as Eu-1 and Tb-1. In the next stage of the study, the influence of various factors on the chemical stability of the obtained compounds was determined. The influence of simultaneous exposure to water, high temperature, and pressure, using hydrothermal conditions was determined. The obtained samples were named as Eu-1* and Tb-1*. In order to evaluate the impact of water molecule removal from the structure of as-synthesized complexes, a dehydration process was conducted at a temperature of 160 °C under vacuum before nitrogen sorption measurements. The dehydrated forms of the investigated complexes were assigned as Eu-2 and Tb-2. To evaluate the stability of as-synthesized compounds in water, the Eu-1 and Tb-1 samples were immersed in water at room temperature, yielding samples designated as Eu-3 and Tb-3. Subsequent immersion of the parent compounds in acidic and alkaline media resulted in the formation of samples Eu-4 (Tb-4) and Eu-5 (Tb-5), respectively. In most cases, the resulting powdered products were subjected to further analyses. The complete degradation of complexes was observed solely in the cases of samples that were treated with NaOH solution. The resulted solid residues (Eu-5/Tb-5) were insufficient for further PXRD and thermal analysis. The final stage of the research involved the synthesis and characterization of the corresponding lanthanide oxides (Eu-6 and Tb-6) obtained as a result of the thermal decomposition of the synthesized metal complexes in air atmosphere.
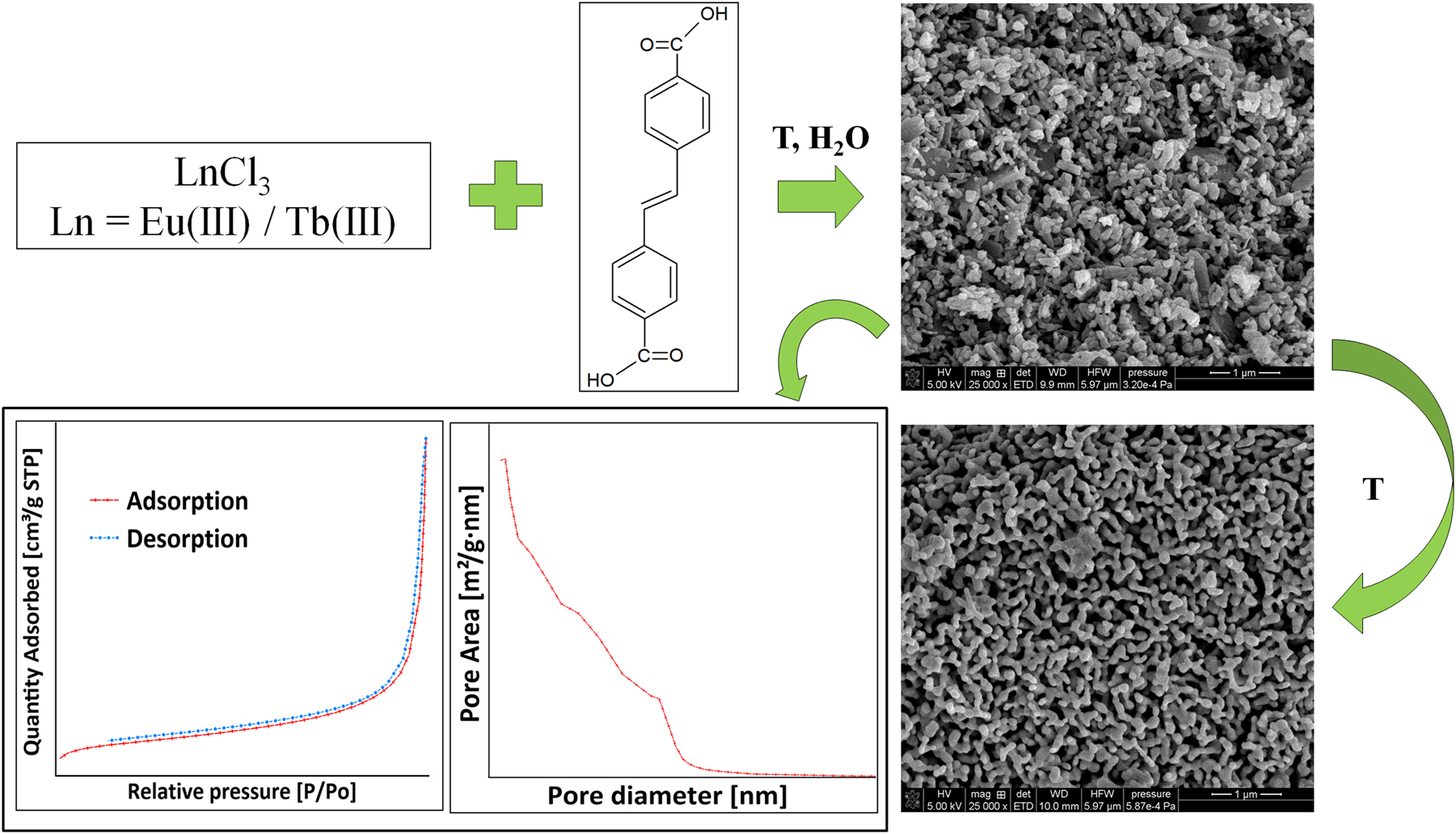
Schematic representation of the synthesis of Ln(III) complexes.
PXRD analysis
Powder X-ray diffraction patterns were recorded for free 4,4′-stilbenedicarboxylic acid, pristine Eu-1 and Tb-1 complexes and their products after exposure to hydrothermal conditions, temperature, water and acidic/alkaline conditions. The XRD patterns shown in Fig. 2 do not reflect the actual reflection intensities, but provide only qualitative information about the studied samples. The XRD patterns of the Eu-1 and Tb-1 samples confirm the formation of pure crystalline phases of the metal complexes and further indicate that the two compounds adopt distinct crystal structures. As can be seen from the powder diffraction patterns of Eu-1* and Tb-1*, the hydrothermal conditions induced structural changes in the europium(III) complex while structure of terbium(III) complex was unchanged. A crystal-to-crystal transformation from the original Eu-1 structure to that of Tb-1 was observed. In the europium complex Eu-1*, the diffraction peaks are shifted to lower 2θ angles compared to those observed in Tb-1. Heating of the starting compounds under reduced pressure causes complete (Eu-2) or partial (Tb-2) loss of water molecules from the structures of the parent complexes affect their crystallinity. As can be seen from the comparison of the XRD patterns of Eu-1 and Eu-2, the positions of the reflection peaks remain unchanged, which indicates the preservation of the crystalline structure of the metal-organic framework after the removal of water molecules. In contrast, the partial dehydration of Tb-1 leads to structural changes and partial amorphization of the sample and some crystal-to-crystal transformations. Immersion of the parent samples in water induced a recrystallization process in the Eu-1 complex, leading to a structural transformation into a form identical to that observed for Tb-1 complex. In contrast, the terbium(III) complex retained its original structure upon exposure to water. The XRD pattern of the Eu-3 sample exhibits diffraction peaks at the same positions as those of Eu-1*, while the XRD pattern of Tb-3 closely resembles that of the Tb-1 complex. These observations suggest that in both cases, crystal-to-crystal transformations occurred, and ultimately, both Eu-3 and Tb-3 exhibit the same crystalline structure. The effect of water molecules on the structural properties of lanthanide-based coordination compounds was likewise observed in metal-organic frameworks constructed from the quinoline-2,4-dicarboxylic acid. 43
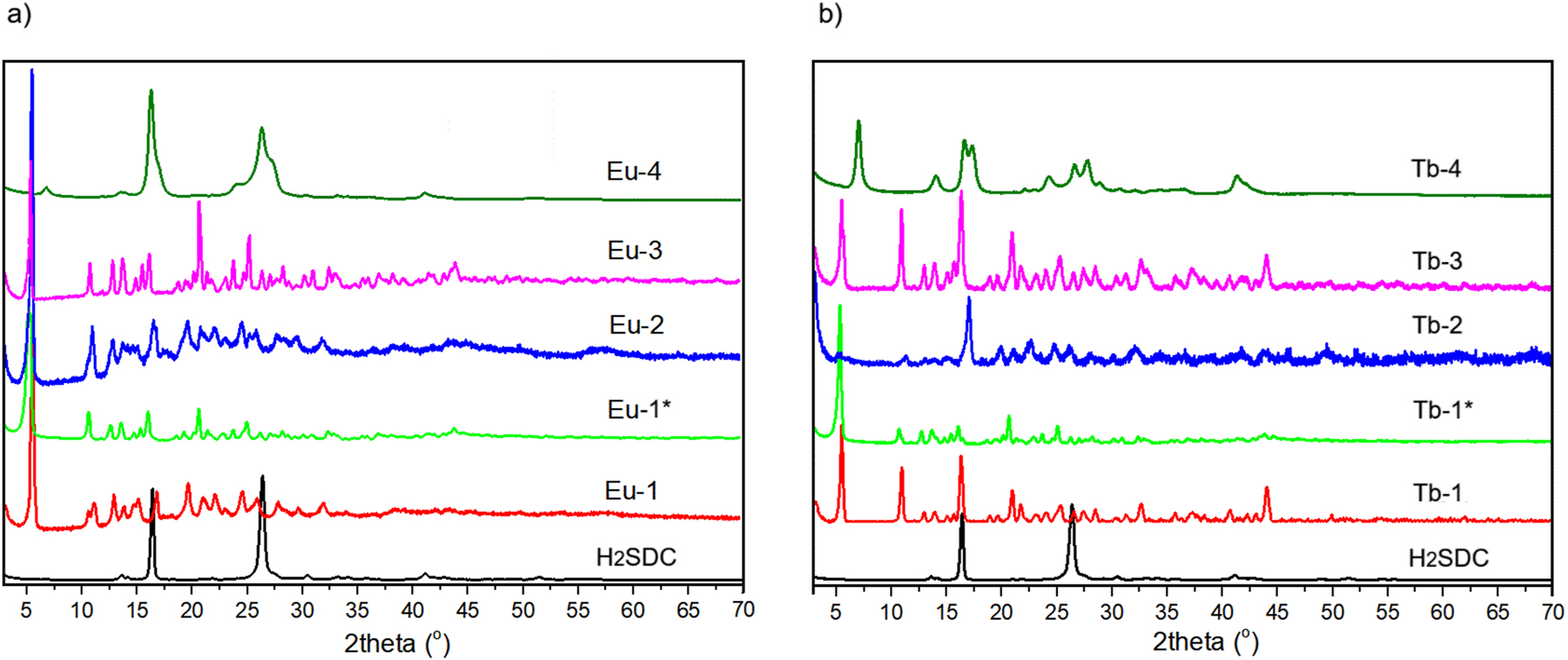
XRD patterns of (a) H2SDC, Eu-1, Eu-1*, Eu-2, Eu-3, and Eu-4; (b) H2SDC, Tb-1, Tb-1*, Tb-2, Tb-3, and Tb-4.
In an acidic environment, partial hydrolysis of metal complexes took place, producing most probably hardly soluble in water 4,4′-stilbenedicarboxylic acid and other unidentified phases as can be seen from Eu-4 and Tb-4 diffractograms.
PXRD analyses were also conducted for the lanthanide oxides (Eu-6 and Tb-6), obtained directly from thermal decomposition of the Eu-1 and Tb-1 complexes. The corresponding diffraction patterns are displayed in Fig. S.1. The Eu-6 sample was identified as phase-pure europium(III) oxide (Eu2O3), based on semi-quantitative phase analysis. In contrast, the Tb-6 sample was found to comprise a mixture of terbium(III) oxide (Tb2O3, 30 %) and terbium(III, IV) oxide.
Thermal analysis (TG-DSC) in air
The thermogravimetric method (TG) along with differential scanning calorimetry (DSC) were used to determine the thermal stability and pathways of the decomposition of the investigated metal complexes during heating in air atmosphere. The TG-DSC curves were recorded for the as-synthesized metal complexes (Eu-1 and Tb-1), their dehydrated forms (Eu-2 and Tb-2) and pristine samples immersed in water (Eu-3 and Tb-3). The profiles of the TG-DSC curves suggest two-stage thermal decomposition of the Eu-1 and Tb-1 complexes (Fig. 3) related to the dehydration process and combustion of the organic part of Ln(III) complexes. The initial mass losses of 7.5 % for Eu2SDC3·5H2O and 8.4 % for Tb2L3·5.5H2O observed during heating correspond to the removal of water molecules. These losses occur within the temperature ranges of 58–199 °C for Eu-1 and 31–163 °C for Tb-1, respectively. This process is accompanied by an endothermic effects observed on the DSC curves. The anhydrous forms of both complexes are thermally stable up to about 415 °C. Further mass losses observed in the TG curves are associated with the decomposition of the anhydrous complexes and the combustion of their organic parts. These processes are accompanied by strong exothermic effects recorded in the DSC curves within the temperature ranges of 430–558 °C (mass loss: 67 %) for Eu-1 and 433–569 °C (mass loss: 66.7 %) for Tb-1. The final solid decomposition products are lanthanide oxides Eu2O3 and Tb4O7 formed at about 600 °C. 43 , 44
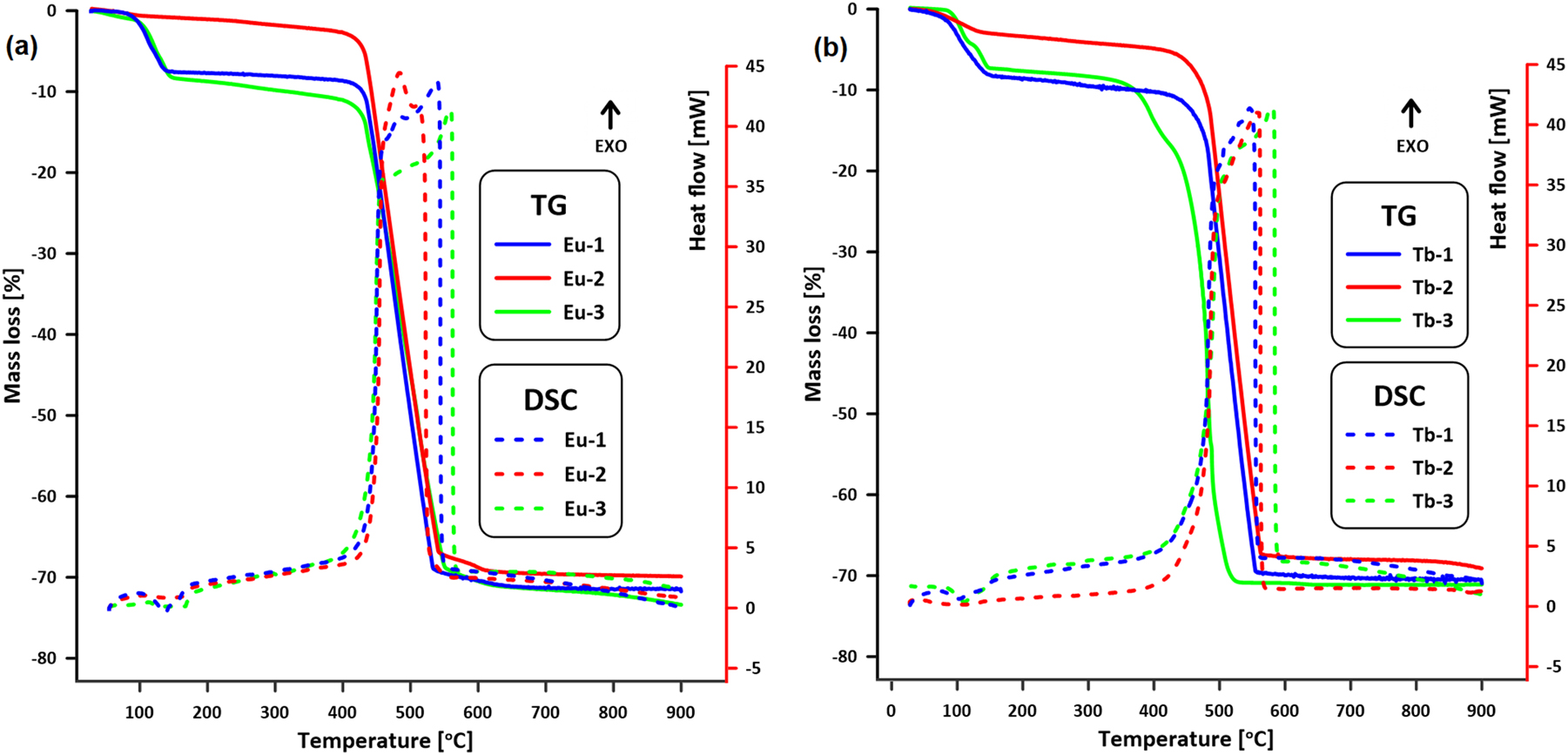
TG-DSC curves of (a) Eu-1; Eu-2; Eu-3 and (b) Tb-1; Tb-2; Tb-3 complexes in air atmosphere.
The thermal profiles of the dehydrated complexes, Eu-2 and Tb-2, differ notably from those of the as-synthesized complexes. The complete removal of solvent molecules from the structure of Eu-1 was confirmed by the lack of characteristic mass loss in the temperature range of dehydration (approximately 60–200 °C). The TG curve of Tb-2 exhibits a mass loss of about 2 % within this temperature range, which is most likely associated with strongly bound water molecules. This observation suggests that the dehydration process did not result in the complete removal of water from the structure of Tb-1 complex. The major decomposition steps for Eu-2 and Tb-2 occur at 390–589 °C (mass loss of 69.1 %) and 397–614 °C (mass loss of 67.7 %), respectively. As can be seen from Fig. 3, the TG curves of pristine and corresponding dehydrated form of complexes in those temperature ranges perfectly overlapped that indicates the same mechanism of the degradation. These results indicate that the main metal-organic frameworks in as-synthesized and dehydrated forms of complexes remain the same. The thermal stability of compounds after prior dehydration did not change significantly compared to the pristine compounds (Eu-1 and Tb-1). 45 , 46 The course of the TG curve for the terbium complex (Tb-2) above 600 °C indicates the formation of a stable intermediate inorganic compound, most likely a carbonate or oxycarbonate, which subsequently transforms into the corresponding oxide at temperature above 800 °C. 46
The thermal behavior of the Eu-3 and Tb-3 samples (Fig. 3), obtained by immersing Eu-1 and Tb-1 in water, differs slightly from that of the pristine complexes. For Eu-3 and Tb-3, the first mass losses recorded due to the dehydration are higher, being 10.5 % and 8.8 %, respectively. The release of water molecules took place in very similar temperature range as in the as-synthesized complexes. At higher temperature, profiles of the TG curves of Eu-3 and Tb-3 samples deviate from the TG curves of the Eu-1 and Tb-1 samples. The thermal stability of the dehydrated form of Eu-3 is similar to the stability of Eu-1 while dehydrated Tb-3 exhibit lower stability in comparison to the Tb-1. Eu-3 decomposes in the temperature range of 380–620 °C with a mass loss of 64.7 %, while Tb-3 degrades in the temperature range of 336–579 °C with a mass loss of 71.2 %. The observed changes in the thermal behavior of the Eu-3 and Tb-3 samples, in comparison to the pristine ones, can be attributed to the changes in the crystalline structure of the compounds, as discussed in the above section. The water treatment resulted in a phase containing a slightly increased number of water molecules in the europium(III) complex and a crystal structure analogous to that formed upon hydrothermal treatment, which induced a crystal-to-crystal phase transition in the europium complex. In contrast, for the terbium complex, immersion in water led to an increase in crystallinity. The distinct thermal behavior of the Tb-3 complex relative to Tb-1 is likely a consequence of differences in molecular packing, influenced by variations in weak intermolecular forces, particularly hydrogen bonding, arising from the rearrangement of water molecules in the coordination network.
FTIR-ATR studies
The infrared spectra of the investigated compounds Eu-1, Eu-1*, Eu-2, Eu-3, Tb-1, Tb-1*, Tb-2 and Tb-3 in comparison to the spectrum of free 4,4′-stilbenedicarboxylic acid do not show the presence of characteristic bands from stretching vibrations of ν(C=O) at 1670 cm−1 and ν(COH) at 1320 cm−1, together with deformation vibrations β(OH) at 946 cm−1 (Fig. 5). 47 , 48 This is a confirmation that the deprotonation process of both carboxylic groups of the used ligand H2SDC took place during formation of metal complexes. The infrared spectra of Eu-1 and Tb-1 show broad band in the range of 3650–3000 cm−1 with narrow peak at 3619 cm−1 and at 3590 cm−1 from the stretching vibrations of hydrogen bonded and free OH groups from water molecules. Subsequently, these bands are almost absent from the FTIR-ATR spectra of Eu-2 and Tb-2 compounds that is indicative for anhydrous metal complexes. The infrared spectra of Eu-3 and Tb-3 compounds exhibit also diagnostic bands originating from the water molecules. The FTIR spectra of all tested metal complexes show distinctive stretching vibrations of carboxylate groups due to formation of Ln(III)–Ocarb bonds. The asymmetric νas(COO) and symmetric νs(COO) stretching vibrations of carboxylate groups appear at 1522 and 1523 as well as 1417, 1397 and 1421, 1398 cm−1 for the as-synthesized Eu-1 and Tb-1 complexes, respectively (Table 1). The wavenumbers of stretching vibrations of carboxylate groups in the europium complexes Eu-1*, Eu-2, Eu-3 and corresponding terbium samples are very close to each other and to vibrations in the pristine compounds. This fact point out to very similar coordination modes of carboxylate groups of SDC2− ligands in the investigated samples. The very small differences in the positions of the bands are more likely due to variations in the involvement of oxygen atoms from the carboxylate groups in hydrogen bonding rather than actual differences in the coordination modes of these groups toward the central metal atoms. The only notable difference in the positions of the COO stretching vibration bands is the presence of an unsplit symmetric stretching band at 1402 cm−1 in the Tb-2 complex, which may suggest a different nature of this group in the given complex. Considering the structure of the 4,4′-stilbenedicarboxylate ligand (Fig. 4), it can be assumed that the obtained complexes adopt the structure of coordination polymers, in which the organic ligand acts as a bridging building block. The exact arrangement of water molecules within their structures cannot be determined unambiguously. They may be located in either the inner or outer coordination sphere of the central metal atoms. Otherwise, the location of ν(COO) bands in the Eu(III) and Tb(III) complexes is different in comparison to the sodium complex with 4,4′-stilbenedicarboxylic acid that may point out on different coordination behavior of COO groups in these complexes. The hydrothermal conditions, removal of water molecules as well as reintroducing of water molecules in their structures do not significantly change geometry of carboxylate groups caused by changing of metal-COO bonds (Fig. 5).
Wavenumbers of the most characteristic vibrations.
| Compound | νOH [cm−1] | νas(COO) [cm−1] | νs(COO) [cm−1] | ν(CArCAr) [cm−1] |
|---|---|---|---|---|
| Eu-1 | 3619 | 1522 | 1417, 1397 | 1607 |
| Eu-1* | 3598 | 1519 | 1415, 1397 | 1607 |
| Eu-2 | – | 1529 | 1411, 1393 | 1608 |
| Eu-3 | 3620 | 1522 | 1417, 1396 | 1607 |
| Tb-1 | 3614 | 1523 | 1421, 1398 | 1609 |
| Tb-1* | 3612 | 1523 | 1421, 1397 | 1606 |
| Tb-2 | – | 1522 | 1421, 1402 | 1607 |
| Tb-3 | 3618 | 1522 | 1421, 1398 | 1606 |
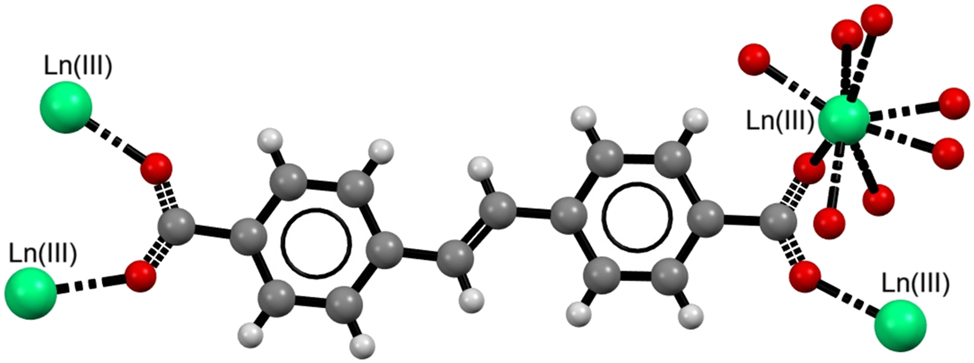
Proposed coordination polymer structure of Ln(III) with 4,4′-stilbenedicarboxylate as a bridging linker.
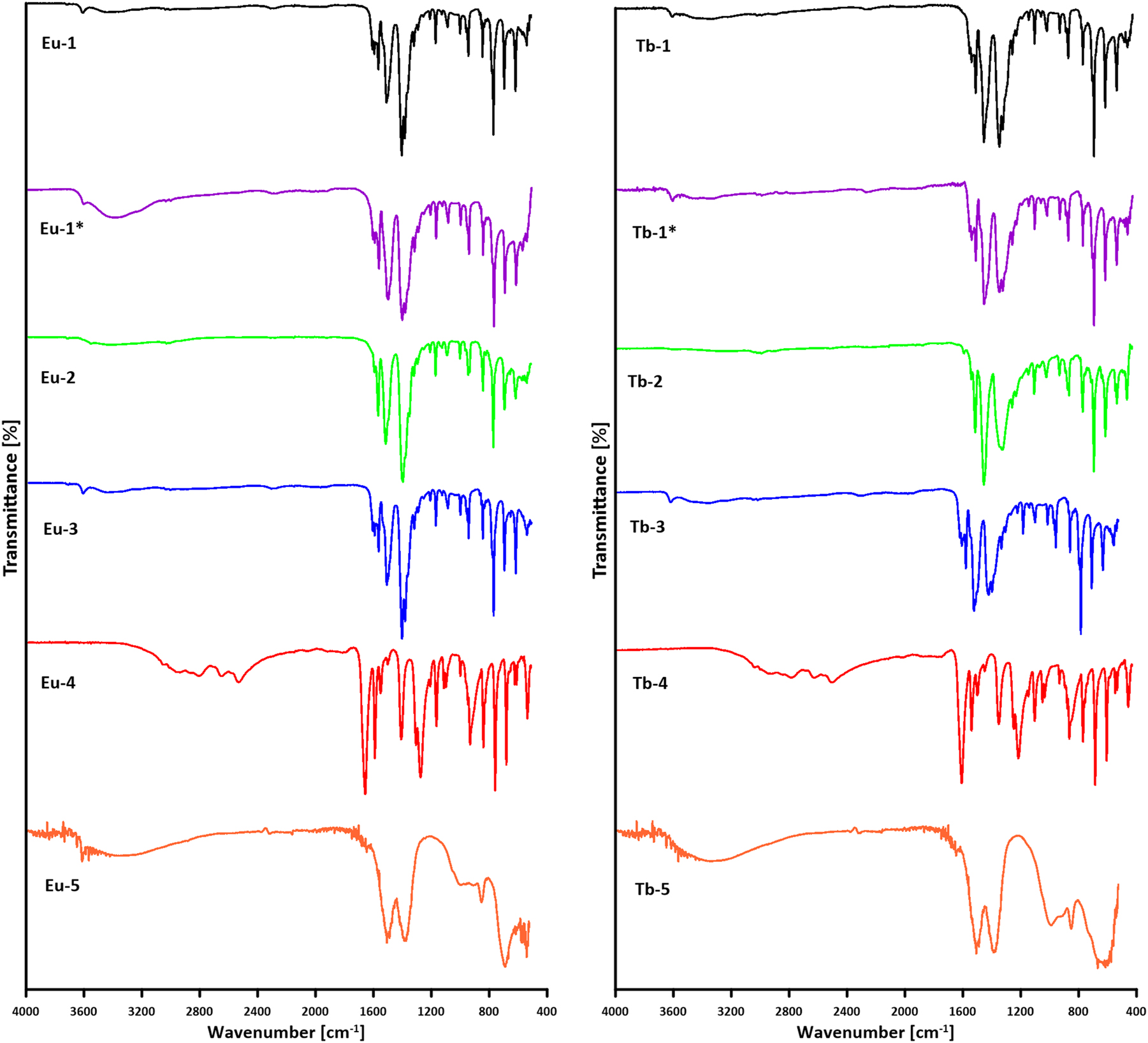
ATR-FTIR spectra of the tested solid samples.
The FTIR-ATR spectra of the Eu-4 and Tb-4 samples are very similar to the infrared spectrum of free 4,4′-stilbenedicarboxylic acid. This observation confirm that acidic solution causes degradation of metal-organic framework connected with precipitation of H2SDC hardly soluble in water. Besides of COOH groups, the FTIR spectra of the tested samples (with the exception of Eu-5 and Tb-5) exhibit also bands diagnostic for stilbene core. At wavenumber 1607 cm−1 appear in-plane stretching vibrations ν(CArCAr), while deformation bands β(CArH) occur at 1333, 1223, 1184, 1139 and 1015 cm−1. Out-of-plane bands from the skeletal system take place at 775, 709, 632 cm−1. 47 , 48
Immersion of the Eu-1 and Tb-1 samples in the alkaline solution led to the chemical decomposition of the parent compounds accompanied by the formation of sodium 4,4′-stilbenedicarboxylate soluble in water and lanthanide(III) carbonates due to absorption of carbon dioxide from air by the applied aqueous solution of NaOH. 49 The FTIR spectra of Eu-5 and Tb-5 samples are dominated by very strong bands at 1507 and 1386 cm−1 assigned to the asymmetric and symmetric stretching vibrations (ν3) of europium(III) or terbium(III) carbonates. The FTIR spectra also show bands 991, 853 and 689 cm−1 corresponding to the carbonate stretching modes ν2, ν8 and ν6 respectively. 45 , 46 , 47 , 48 , 49
Physicochemical measurements
Nitrogen adsorption-desorption isotherms at −196 °C were measured to assess the surface area and porosity characteristics of the obtained europium(III) and terbium(III) 4,4′-stilbenedicarboxylates. Nitrogen isotherms were recorded for the Eu-2 and Tb-2 samples, which were obtained after the degassing process of Eu-1 and Tb-1 at a temperature of 160 °C. The Brunauer–Emmett–Teller (BET) method was used to determine the specific surface area and average pore widths. For the Eu-2 and Tb-2 compounds, the values were 19.94 m2/g and 17.86 nm, and 17.19 m2/g and 14.67 nm, respectively. Total pore volume of pores at p/p o = 0.992 is 0.089056 cm3/g for Eu-2 while for Tb-2 is 0.063063 cm3/g. As evident from the pore size distribution determined by the Barrett-Joyner-Halenda (BJH) method (adsorption branch) (Fig. 6), both samples exhibit a broad pore size distribution, with a significant proportion of pores in the range of 2–30 nm, reflecting their predominantly mesoporous character and only minor microporosity (micropore volume: ∼0.0013 cm3/g). 50 The nitrogen adsorption-desorption isotherms of the Eu-2 and Tb-2 samples, presented in Fig. 7, correspond to type IV according to the IUPAC classification. 51 These isotherms exhibit slight hysteresis loops, which are characteristic of mesoporous materials. However, a precise assignment of the hysteresis loop type remains difficult due to the broad distribution of pore sizes and the structural heterogeneity observed in the studied samples. This complexity is attributed to the intrinsic nature of the coordination polymers under investigation, which consist of diverse network topologies formed through specific coordination interactions between metal centers and organic ligands. As a result, the materials contain various types of channels, voids, and interlayer spaces that give rise to a wide range of pore shapes and dimensions. Such structural features contribute to the overall mesoporous character, while also complicating the interpretation of the adsorption-desorption behavior. Given its mesoporous character and moderate surface area, the material may be suitable for the adsorption of organic dyes, especially in aqueous environments where pore accessibility and diffusion are crucial. 52
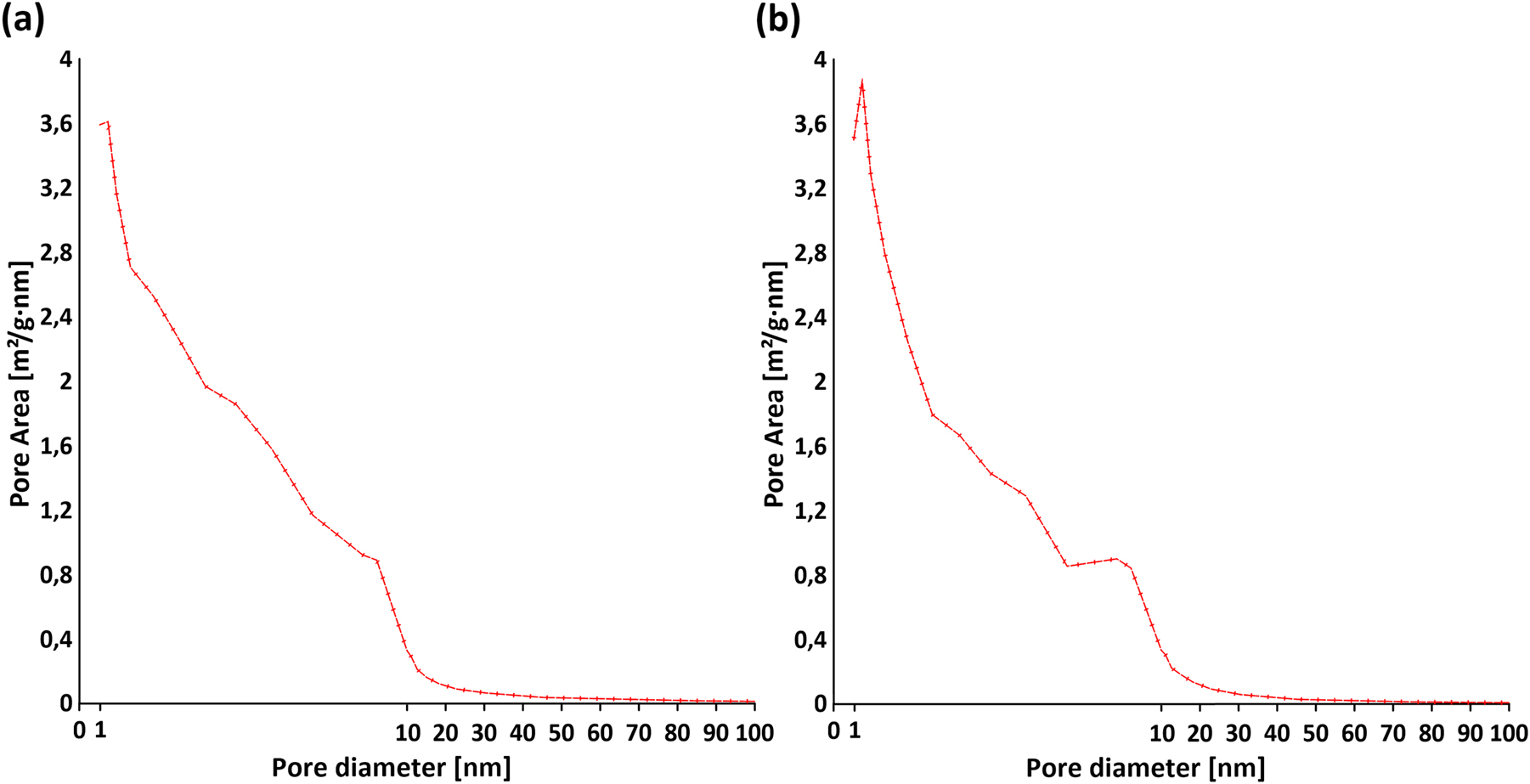
Surface area-based pore size distribution (BJH method) of (a) Eu-2 and (b) Tb-2.
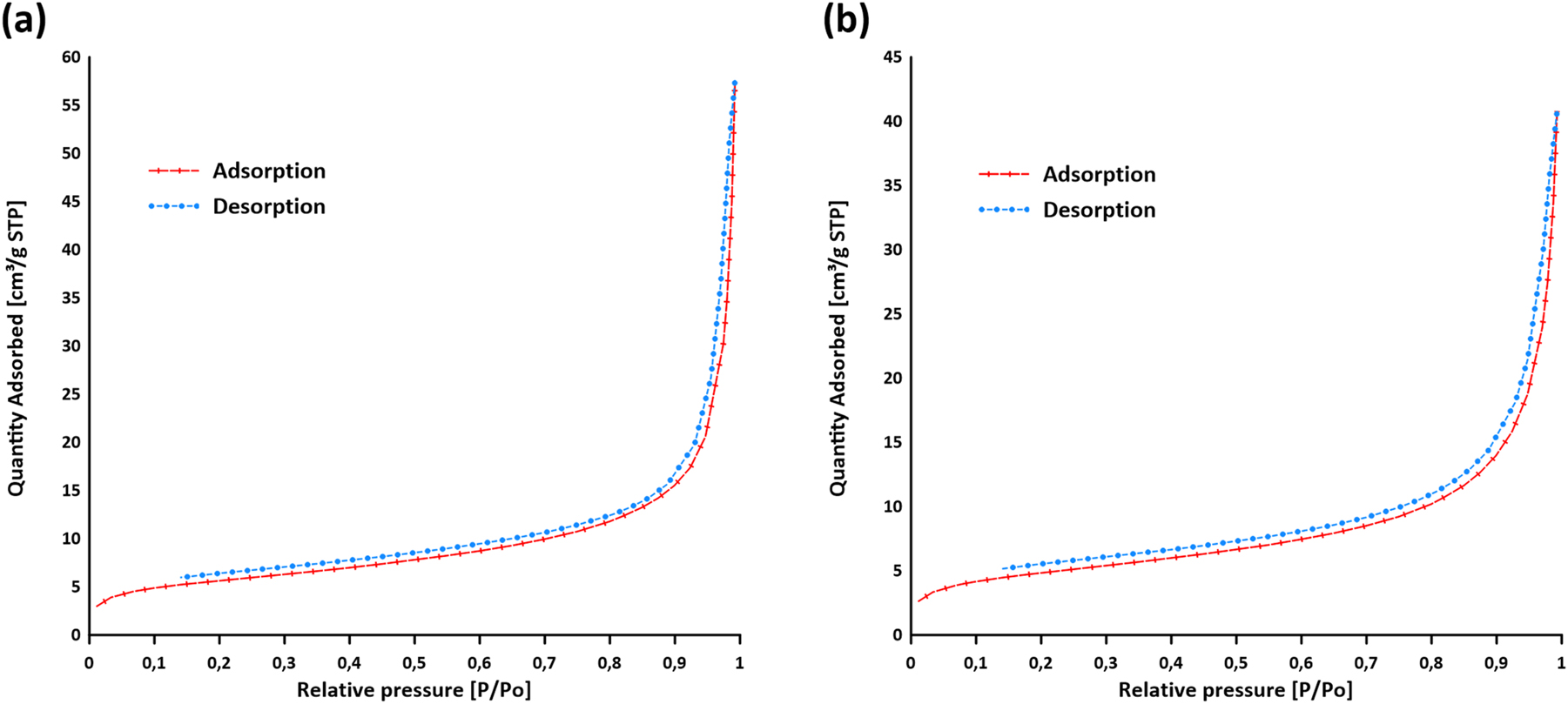
Adsorption/desorption isotherms of (a) Eu-2 and (b) Tb-2.
From the nitrogen adsorption-desorption isotherms, the specific surface areas of the oxides obtained from the thermal decomposition of the initial complexes were determined using the BET method, and were found to be 3.82 m2/g and 2.12 m2/g for Eu-6 and Tb-6, respectively (Table S.2). The differential pore volume analysis reveals a broad distribution of pore diameters, encompassing both mesoporous and macroporous regions, which is indicative of complex pore architectures (Fig. S.2).
Morphology by SEM
The morphological characteristics of the solid-state aggregation of the europium(III) and terbium(III) 4,4′-stilbenedicarboxylates (Eu-1 and Tb-1) and their oxides (Eu-6 and Tb-6) were investigated using SEM, with images captured at 250 00× magnification (Fig. 8). The SEM/EDX analysis (Table 2) revealed the elemental composition of the samples, showing distinct variations in the atomic percentages (at.%) of carbon (C), oxygen (O), and the respective lanthanide elements (Eu, Tb). For instance, the Eu-1 and Tb-1 samples exhibited higher carbon content (70.78 % and 72.60 %, respectively), while the Eu-6 and Tb-6 samples showed a significant increase in oxygen content (52.46 % and 54.77 %, respectively), along with higher lanthanide concentrations (27.29 % Eu and 22.59 % Tb) due to their chemical composition as well as the inhomogeneity of the analyzed surface. These compositional differences correlate with the observed morphological variations in the SEM images, suggesting that the aggregation state and crystalline tendencies of the compounds are influenced by the lanthanide coordination and the surrounding ligand environment. 53 , 54 The data provide valuable insights into the structural and compositional properties of these materials, which are critical for their potential applications in catalysis and material science. 55 The SEM images of Eu-1 and Tb-1 show an irregular, highly agglomerated morphology composed of randomly arranged angular crystallites of varying sizes. The surfaces appear rough and heterogeneous, with no well-defined particle shapes. The aggregates vary in size and seem to consist of plate-like or blocky structures. These morphologies are characteristic of materials synthesized without stringent control over nucleation and growth processes, confirming a fast and non-templated precipitation route. The morphology of the resulting lanthanide oxides (Eu-6 and Tb-6) is distinctly different from that of the coordination polymers. The resulting agglomerates are better separated and exhibit improved homogeneity, as well as reduced particle aggregation. Their architecture is more defined, and the particles display a more rounded shape, somewhat reminiscent of tetrapods. Additionally, the agglomerates of europium oxide appear smaller in size compared to those of terbium oxide. The smooth surface texture and uniform particle size suggest a slow, diffusion-controlled growth mechanism, likely facilitated by the thermal decomposition of the highly ordered crystal structures of the parent Eu-1 and Tb-1 compounds, which act as structure-directing agents. 56 , 57 , 58
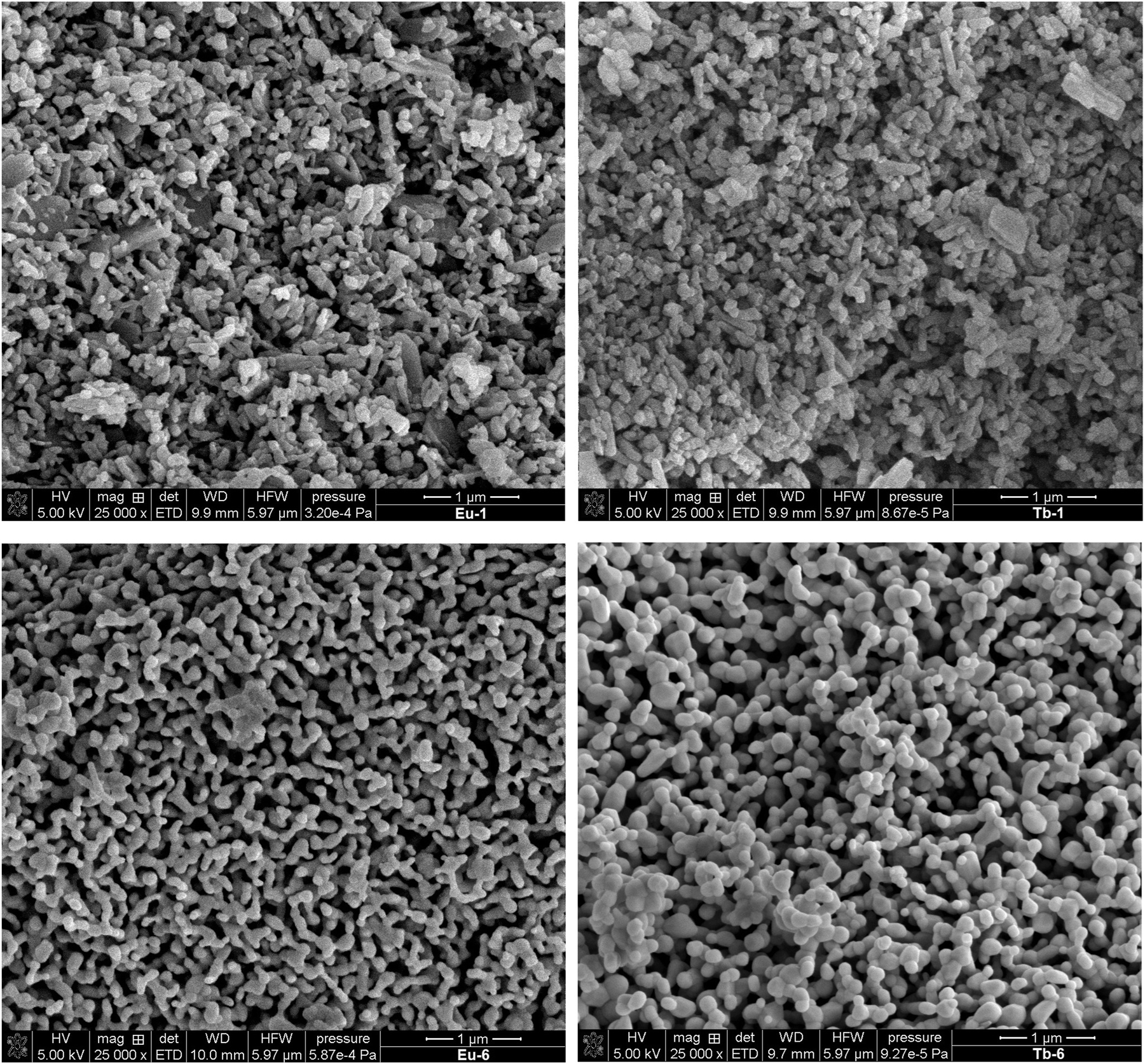
SEM images of Eu-1 and Tb-1 complex compounds and Eu-6 and Tb-6 lanthanide oxides.
Qualitative analysis SEM/EDX of the obtained Ln(III) complex compounds and Ln(III) oxides.
| Element [at.%] | Eu-1 | Tb-1 | Eu-6 | Tb-6 |
|---|---|---|---|---|
| C | 70.78 | 72.60 | 19.68 | 22.43 |
| O | 28.34 | 25.65 | 52.46 | 54.77 |
| Eu | 0.75 | 27.29 | ||
| Tb | 1.42 | 22.59 |
Adsorption studies
Preliminary studies were also conducted to evaluate the sorption properties of the as-synthesized coordination complexes (Eu-1 and Tb-1), as well as to investigate the effect of the activation process i.e., partial or complete removal of water molecules from their structures on their sorption properties. Additionally, sorption measurements were performed for the corresponding lanthanide oxides (Eu-6 and Tb-6) obtained during their thermal decomposition. This study focuses on the investigations of tested samples sorption characteristics towards three different dye molecules: methylene blue (BM1) (Methylthioninium chloride, ([7-(dimethylamino)phenothiazin-3-ylidene]-dimethylazanium chloride)), methyl blue (BM2) (Cotton blue, (Methyl blue ([[4-[Bis[4-[(sulfophenyl)amino]phenyl]methylene]-2,5-cyclohexadien-1-ylidene]amino]-benzenesulfonic acid disodium salt))), and rhodamine B (RB). In addition to the determination of adsorption capacities, this study also investigates the kinetics of the adsorption process by monitoring the variation in dye concentration over time (Table 3). Such an analysis is essential to understanding the dynamic nature of the adsorption, including the rate at which these compounds can remove the dyes from aqueous solutions. The time-dependent adsorption measurements provide valuable insights into the mechanisms governing the interaction between the dyes and the adsorbents, which is crucial for optimizing the use of these materials in practical applications.
Comparison of the adsorption properties of lanthanide(III) compounds with various dyes – 10 mg/L.
| Adsorbent | Adsorption capacity [mg/g] | Time [minutes] |
|---|---|---|
| BM1 | ||
|
|
||
| Eu-1 | 8.61 | 40 |
| Eu-2 | 8.00 | 60 |
| Eu-6 | – | – |
| Tb-1 | 3.52 | 60 |
| Tb-2 | 7.35 | 60 |
| Tb-6 | – | – |
|
|
||
| BM2 | ||
|
|
||
| Eu-1 | 9.66 | 20 |
| Eu-2 | 2.62 | 20 |
| Eu-6 | 17.31 | 60 |
| Tb-1 | 3.83 | 20 |
| Tb-2 | 5.22 | 40 |
| Tb-6 | 7.84 | 20 |
|
|
||
| RB | ||
|
|
||
| Eu-1 | 12.23 | 40 |
| Eu-2 | 7.92 | 40 |
| Eu-6 | 8.62 | 40 |
| Tb-1 | 6.54 | 20 |
| Tb-2 | 7.18 | 20 |
| Tb-6 | 4.85 | 40 |
Figure 9 presents the adsorption isotherms of the selected dyes: (a) BM1, (b) BM2, and (c) RB on the most promising compounds (Eu-1, Tb-1, Eu-6, Tb-6). In the case of BM1, Eu-1 exhibited the highest adsorption capacity, reaching approximately 9 mg/g, while Tb-1 showed the lowest uptake, not exceeding 3.5 mg/g. For BM2, Eu-6 proved to be the most efficient adsorbent with a capacity of nearly 18 mg/g, clearly outperforming the other tested materials. Tb-6 displayed moderate adsorption efficiency, whereas Eu-1 and Tb-1 adsorbed considerably less of this dye. In the case of RB, Eu-1 again achieved the best performance, with adsorption capacities above 12 mg/g, followed by Eu-6 and Eu-2, while the terbium-based adsorbents demonstrated the weakest adsorption (6–7.5 mg/g). These results indicate that europium-containing compounds, particularly Eu-1 and Eu-6, possess superior adsorption properties compared to their terbium analogues.
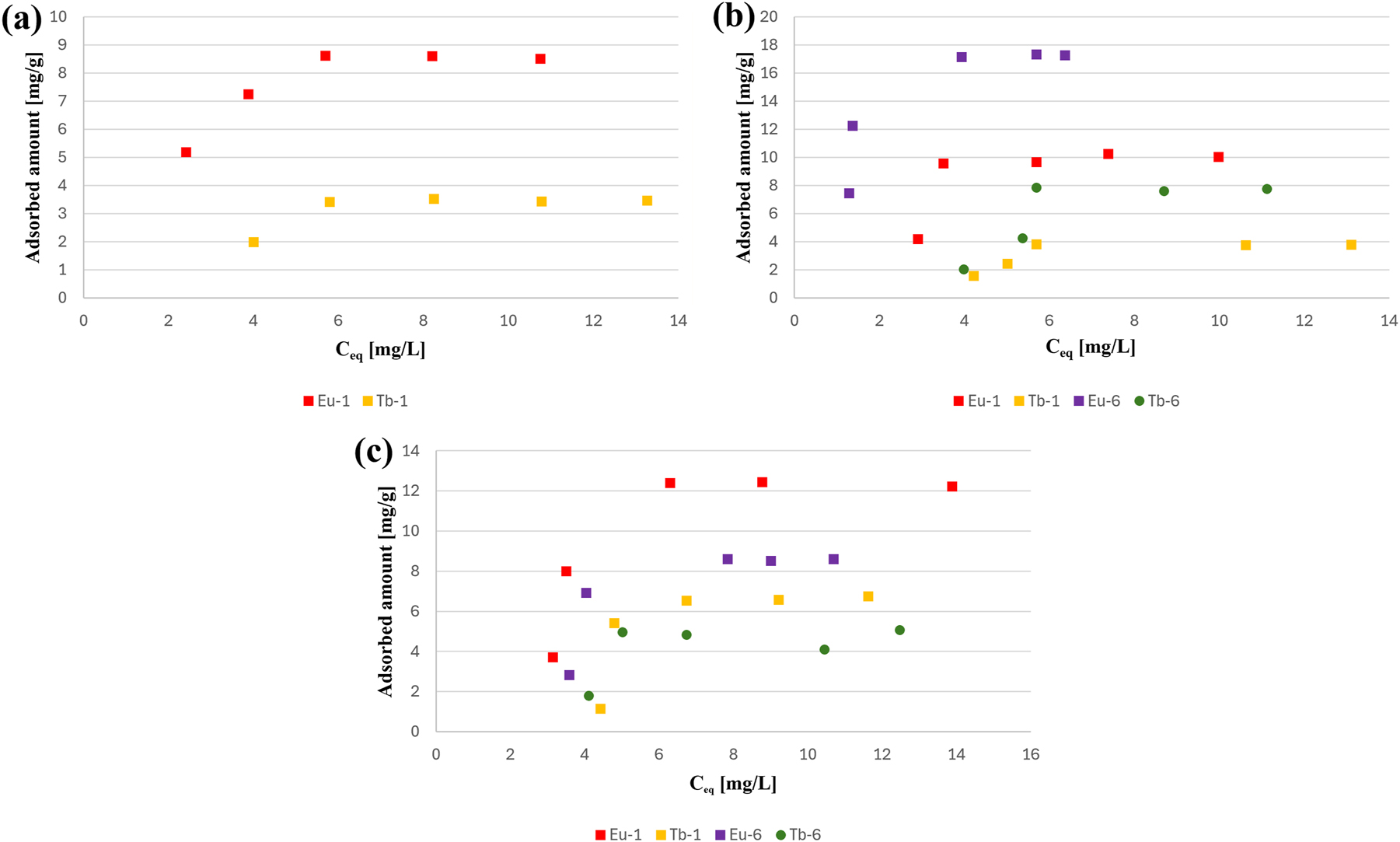
Adsorption isotherms of dyes (a) BM1, (b) BM2, and (c) RB on the most promising compounds Eu-1, Tb-1, Eu-6, and Tb-6.
The adsorption behavior of the organic dyes BM1, BM2, and RB on the Tb-1 (Table 4) sorbent was evaluated using the Langmuir and Freundlich isotherm models. The Langmuir model, which assumes monolayer adsorption on a homogeneous surface, showed a good fit to the experimental data for BM1 and BM2, as indicated by the high correlation coefficients (R 2 = 0.8922 and 0.9874, respectively). The maximum adsorption capacities (q m ) were 4.54 mg/g for BM1 and 7.93 mg/g for BM2, while RB exhibited a lower correlation (R 2 = 0.5349) with q m of 5.27 mg/g. The Freundlich model, which accounts for adsorption on heterogeneous surfaces, showed weaker correlations overall, with R 2 values of 0.5941, 0.7744, and 0.1597 for BM1, BM2, and RB, respectively. These results suggest that adsorption of BM1 and BM2 onto Tb-1 is better described by the Langmuir model, indicating predominantly monolayer adsorption, whereas the behavior of RB deviates from this trend, suggesting more complex interactions. 59 , 60
Calculated parameters of Tb-1 adsorption isotherms.
| Isotherm parameters | BM1 | BM2 | RB | |
|---|---|---|---|---|
| Experimental | q exp [mg/g] | 3.51 | 3.83 | 6.75 |
| Langmuir | q m [mg/g] | 4.54 | 7.93 | 5.27 |
| K L [cm3/mg] | 0.2948 | 0.5252 | 0.2290 | |
| R 2 | 0.8922 | 0.9874 | 0.5349 | |
| Freundlich | n F | 2.5201 | 4.3010 | 2.8089 |
| K F [mg/g·(mg/cm3)1/n ] | 0.1166 | 0.0873 | 0.1155 | |
| R 2 | 0.5941 | 0.7744 | 0.1597 |
Figure 10 presents the adsorption kinetics of three dyes: methylene blue (BM1), methyl blue (BM2), and rhodamine B (RB) onto six different adsorbents, highlighting the time required to reach maximum adsorption capacity. The adsorption behavior of cationic BM1 is shown on Fig. 10(a), where adsorption increases over time and reaches equilibrium after approximately 120 min. Among the tested materials, Eu-1 exhibits the highest adsorption capacity for BM1 (∼9 mg/g), followed by Eu-2 (8 mg/g). In contrast, the terbium-based adsorbents show much lower efficiency: Tb-2 reaches about 7 mg/g, while Tb-1 performs the weakest, with capacity below 3.5 mg/g. Figure 10(b) displays the adsorption kinetics of anionic BM2 (methyl blue). Eu-6 appears to be the most efficient in this series, achieving a capacity of over 17 mg/g. Tb-6 demonstrates moderate efficiency (∼7 mg/g), while Tb-1 again records the lowest uptake (3.8 mg/g). The adsorption of RB, another cationic dye is illustrated on Fig. 10(c) where Eu-1 once again achieves the best performance (∼12 mg/g), followed by Eu-2 and Eu-6 (∼8 mg/g). The Tb-based adsorbents (Tb-1 and Tb-2) show the weakest adsorption of RB, with capacities between 6 and 7.5 mg/g. Overall, Eu-1 emerges as the most effective adsorbent for BM1 and RB, while Eu-6 performs best for BM2. Tb-1 consistently shows the lowest adsorption capacity for all dyes.
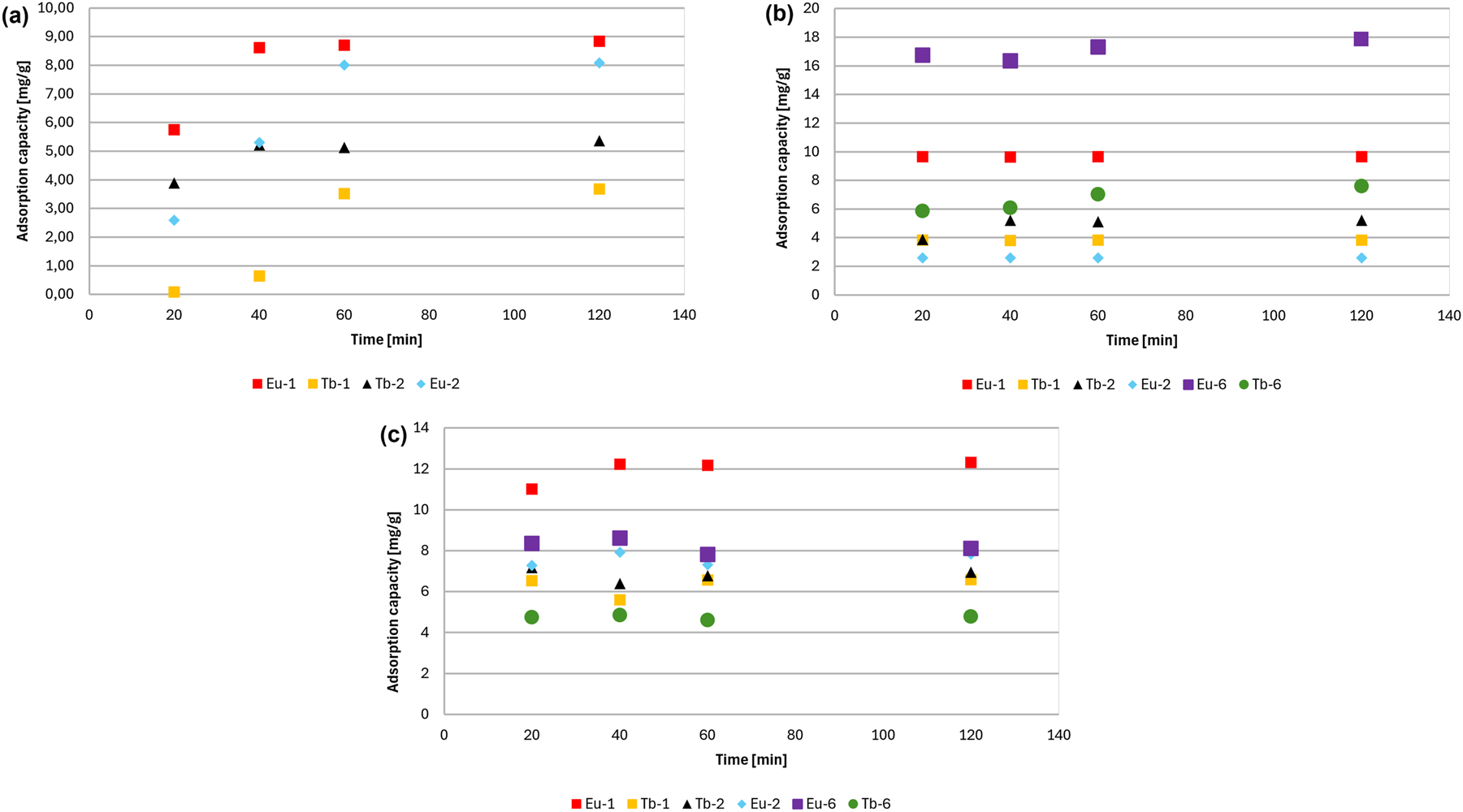
Adsorption kinetics of (a) BM1, (b) BM2 and (c) RB.
The analysis of kinetic models (Table 5) indicated a clear divergence in adsorption mechanisms. For adsorbent BM1, the process was best fitted by the pseudo-second-order model (R 2 = 0.8318), suggesting a chemisorption mechanism. This conclusion was further strengthened for adsorbents BM2 and RB, where the pseudo-second-order model yielded an excellent fit with near-unity correlation coefficients (R 2 = 0.9998 and R 2 = 0.9962, respectively). 61
Kinetic parameters of the BM1, BM2 and RB adsorption on the representative compound Tb-1.
| Calculated parameters | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|
| q e [mg/g] | k 1 [1/min] | R 2 | q e [mg/g] | K 2 [g/(mg⸱min)] | R 2 | |
| Tb-1 | ||||||
|
|
||||||
| BM1 | 1.038830 | 4.573758 | 0.4422 | 0.631712 | 0.013710 | 0.8318 |
| BM2 | 1.008839 | 2.800218 | 0.9233 | 3.968254 | 0.174318 | 0.9998 |
| RB | 1.026136 | 1.168312 | 0.6916 | 6.954103 | 0.022984 | 0.9962 |
Furthermore, the study extends its investigation to the desorption process, wherein the adsorbed dyes are released from the surface of the adsorbents under specific conditions (Table 6). The efficiency of this desorption process was thoroughly evaluated for the most effective adsorbents identified in the previous adsorption tests. Desorption studies are particularly important for assessing the reusability of the adsorbents, as they determine the potential for recovering the adsorbed dyes and regenerating the adsorbents for multiple cycles of use. The results of the desorption experiments provide a comprehensive understanding of the overall performance of the adsorbents, including their capacity for dye retention and recovery.
Percentage desorption of BM1, BM2 and RB from the selected obtained lanthanide compounds using 0.01 M HCl solution in 50 % MeOH/H2O (v/v).
| Dyes | Compound | Desorption [%] |
|---|---|---|
| BM1 | Eu-1 | 67.06 |
| BM2 | Eu-6 | 84.67 |
| RB | Eu-1 | 38.37 |
By combining adsorption capacity, kinetics, and desorption efficiency, this research contributes to the broader understanding of the potential applications of Eu- and Tb-based compounds in environmental remediation. The findings are particularly relevant for designing more efficient materials for the removal of dye pollutants from industrial effluents and wastewater, with the potential for further optimization of the adsorption and desorption processes to enhance their practical applicability.
Conclusions
The precipitation method was employed to the synthesis of polycrystalline coordination polymers of Eu(III) and Tb(III) ions with 4,4′-stilbenedicarboxylic acid from aqueous solution. The crystallinity of the tested samples was monitored by XRD patterns after chemical/hydrothermal treatment. The synthesized compounds are stable in water, whereas acidic and alkaline solutions exert destructive impact on the frameworks of the prepared complexes yielding different solid products. The removal of water molecules from the structures of complexes lead to the crystal-to-crystal transformations for europium complex while for terbium complex also partial amorphization was observed. The parent compounds maintained their crystallinity under hydrothermal conditions and in the presence of water; however, the europium(III) complex exhibited subtle structural modifications under these conditions. Based on the infrared spectra analysis it can be concluded that obtained complexes form coordination polymers with coordination of metal centers through carboxylate oxygen atoms. Some observed crystal-to-crystal transformations are rather connected with rearrangement of molecules than changes in coordination modes of COO groups. Additionally, adsorption studies revealed that the europium-based coordination polymers exhibit higher affinity towards cationic dyes such as methylene blue and rhodamine B, while their performance towards anionic methyl blue was more variable. These results suggest that the charge of the dye and structural stability of the adsorbents are key factors governing their adsorption efficiency, further indicating their potential application in dye removal from aqueous solutions.
Our investigations have contributed to a deeper understanding of the chemical and thermal stability of coordination polymers and have identified the conditions under which they can be effectively applied. Moreover, the corresponding lanthanide oxides were successfully obtained and characterized, demonstrating that lanthanide coordination compounds can serve as templates for the synthesis of lanthanide oxides with tailored morphologies. These findings underscore the potential of such materials for diverse applications, particularly as precursors for advanced oxide materials.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: All other authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Chen, J.; Xin, Y.; Wu, J.; Liao, P.; Chen, L.; Zhang, J. Supported Metal Nanoparticles in Metal-Organic Monoliths for Assembly of a Catalytic Microfluidic Reactor. ChemNanoMat 2021, 7 (4), 334–340. https://doi.org/10.1002/cnma.202000587.Suche in Google Scholar
2. De, D.; Sahoo, P. The Impact of MOFs in pH-Dependent Drug Delivery Systems: Progress in the Last Decade. Dalton Trans. 2022, 51 (26), 9950–9965. https://doi.org/10.1039/D2DT00994C.Suche in Google Scholar
3. Furukawa, H.; Cordova, K. E.; O’Keeffe, M.; Yaghi, O. M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341 (6149), 1230444. https://doi.org/10.1126/science.1230444.Suche in Google Scholar PubMed
4. Zhang, X.; Chen, Z.; Liu, X.; Hanna, S. L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O. K. A Historical Overview of the Activation and Porosity of Metal-Organic Frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. https://doi.org/10.1039/D0CS00997K.Suche in Google Scholar
5. Kalmutzki, M. J.; Hanikel, N.; Yaghi, O. M. Secondary Building Units as the Turning Point in the Development of the Reticular Chemistry of MOFs. Sci. Adv. 2018, 4. https://doi.org/10.1126/sciadv.aat9180.Suche in Google Scholar PubMed PubMed Central
6. Hwang, J.; Ejsmont, A.; Freund, R.; Goscianska, J.; Schmidt, B. V. K. J.; Wuttke, S. Controlling the Morphology of Metal–Organic Frameworks and Porous Carbon Materials: Metal Oxides as Primary Architecture-Directing Agents. Chem. Soc. Rev. 2020, 49, 3348–3422. https://doi.org/10.1039/C9CS00871C.Suche in Google Scholar
7. Zou, R.; Abdel-Fattah, A. I.; Xu, H.; Zhao, Y.; Hickmott, D. D. Storage and Separation Applications of Nanoporous Metal–Organic Frameworks. CrystEngComm 2010, 12, 1337–1353. https://doi.org/10.1039/B909643B.Suche in Google Scholar
8. Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2011, 112, 869–932. https://doi.org/10.1021/cr200190s.Suche in Google Scholar PubMed
9. Xie, Z.; Ma, L.; deKrafft, K. E.; Jin, A.; Lin, W. Porous Phosphorescent Coordination Polymers for Oxygen Sensing. J. Am. Chem. Soc. 2010, 132, 922–923. https://doi.org/10.1021/ja909629f.Suche in Google Scholar PubMed
10. Horcajada, P.; Serre, C.; Vallet‐Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–Organic Frameworks as Efficient Materials for Drug Delivery. Angew. Chem., Int. Ed. 2006, 45, 5974–5978. https://doi.org/10.1002/anie.200601878.Suche in Google Scholar PubMed
11. Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M. B.; Ji, S.-W.; Jeon, B.-H. Metal–Organic Frameworks (MOFs) for the Removal of Emerging Contaminants from Aquatic Environments. Coord. Chem. Rev. 2019, 380, 330–352. https://doi.org/10.1016/j.ccr.2018.10.003.Suche in Google Scholar
12. Bunzen, H. Chemical Stability of Metal-Organic Frameworks for Applications in Drug Delivery. ChemNanoMat 2021, 7, 998–1007. https://doi.org/10.1002/cnma.202100226.Suche in Google Scholar
13. Wu, D.; Liu, C.; Tian, J.; Jiang, F.; Yuan, D.; Chen, Q.; Hong, M. Microporous Metal–Organic Framework Stabilized by Balanced Multiple Host – Couteranion Hydrogen-Bonding Interactions for High-Density CO2 Capture at Ambient Conditions. Inorg. Chem. 2015, 54, 13542–13550. https://doi.org/10.1021/acs.inorgchem.5b02316.Suche in Google Scholar PubMed
14. Healy, C.; Patil, K. M.; Wilson, B. H.; Hermanspahn, L.; Harvey-Reid, N. C.; Howard, B. I.; Kleinjan, C.; Kolien, J.; Payet, F.; Telfer, S. G.; Kruger, P. E.; Bennett, T. D. The Thermal Stability of Metal-Organic Frameworks. Coord. Chem. Rev. 2020, 419, 213388. https://doi.org/10.1016/j.ccr.2020.213388.Suche in Google Scholar
15. Cogswell, C.; Xie, Z.; Choi, S. Tuning of Metal–Organic Frameworks by Pre- and Post-Synthetic Functionalization for Catalysis and Separations. In Metal‐Organic Frameworks: Applications in Separations and Catalysis; García, H., Navalón, S., Eds.; Wiley‐VCH Verlag GmbH & Co. KGaA, 2018; pp. 297–327.10.1002/9783527809097.ch10Suche in Google Scholar
16. Liu, J. Potential Industrial Applications of Metal–Organic Frameworks for Gas Separations. In Gas Adsorption in Metal–Organic Frameworks: Fundamentals and Applications; Glover, T. G., Mu, B., Eds.; CRC Press: Boca Raton, 2018; pp. 478–508.10.1201/9780429469770-11Suche in Google Scholar
17. Hu, Z.; Sun, Y.; Zeng, K.; Zhao, D. Structural-Failure Resistance of Metal–Organic Frameworks Toward Multiple-Cycle CO2 Sorption. Chem. Commun. 2017, 53, 8653–8656. https://doi.org/10.1039/C7CC04313A.Suche in Google Scholar
18. Lustig, W. P.; Mukherjee, S.; Rudd, N. D.; Desai, A. V.; Li, J.; Ghosh, S. K. Metal–Organic Frameworks: Functional Luminescent and Photonic Materials for Sensing Applications. Chem. Soc. Rev. 2017, 46, 3242–3285. https://doi.org/10.1039/C6CS00930A.Suche in Google Scholar
19. Bünzli, J. C. G. On the Design of Highly Luminescent Lanthanide Complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. https://doi.org/10.1016/j.ccr.2014.10.013.Suche in Google Scholar
20. Bünzli, J. C. G. Review: Lanthanide Coordination Chemistry: From Old Concepts to Coordination Polymers. J. Coord. Chem. 2014, 67, 3706–3733. https://doi.org/10.1080/00958972.2014.957201.Suche in Google Scholar
21. Bünzli, J. C. G.; Piguet, C. Taking Advantage of Luminescent Lanthanide Ions. Chem. Soc. Rev. 2005, 34, 1048–1077. https://doi.org/10.1039/B406082M.Suche in Google Scholar
22. Amiaud, T.; Stephant, N.; Dessapt, R.; Serier-Brault, H. Microwave-Assisted Synthesis of Anhydrous Lanthanide-Based Coordination Polymers Built upon Benzene-1,2,4,5-Tetracarboxylic Acid. Polyhedron 2021, 204, 115261. https://doi.org/10.1016/j.poly.2021.115261.Suche in Google Scholar
23. Li, Q.; Qian, J.; Zhou, J.; Du, L.; Zhao, Q. Highly Chemically and Thermally Stable Lanthanide Coordination Polymers for Luminescent Probes and White Light Emitting Diodes. CrystEngComm 2020, 22, 2667–2674. https://doi.org/10.1039/D0CE00228C.Suche in Google Scholar
24. Li, J.; Ren, Y.; Zhang, M.; Jiang, H. Full Meta-Substituted 4,4′-Biphenyldicarboxylate Based MOFs: Synthesis, Structures and Catalytic Activities. Eur. J. Inorg. Chem. 2017, 11, 1485–1492. https://doi.org/10.1002/ejic.201601242.Suche in Google Scholar
25. Wang, X.-Z.; Zhu, D.-R.; Xu, Y.; Yang, J.; Shen, X.; Zhou, J.; Fei, N.; Ke, X.-K.; Peng, L.-M. Three Novel Metal-Organic Frameworks with Different Topologies Based on 3,3′-Dimethoxy-4,4′-biphenyldicarboxylic Acid: Syntheses, Structures, and Properties. Cryst. Growth Des. 2010, 10 (2), 887–894. https://doi.org/10.1021/cg9012262.Suche in Google Scholar
26. Allen, F. H. The Cambridge Structural Database: A Quarter of a Million Crystal Structures and Rising. Acta Crystallogr., Sect. B: Struct. Sci. 2002, B58, 380–388. https://doi.org/10.1107/S0108768102003890.Suche in Google Scholar PubMed
27. Maiwald, M. M.; Wagner, A. T.; Kratsch, J.; Skerencak-Frech, A.; Trumm, M.; Geist, A.; Roesky, P. W.; Panak, P. J. 4,4′-Di-tert-butyl-6-(1H-tetrazol-5-yl)-2,2′-bipyridine: Modification of a Highly Selective N-Donor Ligand for the Separation of Trivalent Actinides from Lanthanides. Dalton Trans. 2017, 46 (30), 9192–9201. https://doi.org/10.1039/C7DT01864A.Suche in Google Scholar
28. Rannulu, N. S.; Rodgers, M. T. Noncovalent Interactions of Ni+ with N-Donor Ligands (Pyridine, 4,4′-Dipyridyl, 2,2′-Dipyridyl, and 1,10-Phenanthroline): Collision-Induced Dissociation and Theoretical Studies. J. Phys. Chem. A 2009, 113 (16), 4534–4548. https://doi.org/10.1021/jp8112045.Suche in Google Scholar PubMed
29. Meng, R.; Naren, G.; Qiu, Z.; Liang, R. Preparation and Luminescent Properties of Europium-Praseodymium Bis-Rare Earth Phenylalanine Complexes. J. Mol. Struct. 2025, 1327, 141174. https://doi.org/10.1016/j.molstruc.2024.141174.Suche in Google Scholar
30. Ding, M.; Jiang, H.-L. Improving Water Stability of Metal–Organic Frameworks by a General Surface Hydrophobic Polymerization. CCS Chem. 2021, 3, 2740–2748. https://doi.org/10.31635/ccschem.020.202000515.Suche in Google Scholar
31. Howarth, A. J.; Liu, Y.; Li, P.; Li, Z.; Wang, T. C.; Hupp, J. T.; Farha, O. K. Chemical, Thermal and Mechanical Stabilities of Metal–Organic Frameworks. Nat. Rev. Mater. 2016, 1, 15018. https://doi.org/10.1038/natrevmats.2015.18.Suche in Google Scholar
32. Li, B.; Wen, H.-M.; Cui, Y.; Qian, G.; Chen, B. Multifunctional Lanthanide Coordination Polymers. Prog. Polym. Sci. 2015, 48, 40–84. https://doi.org/10.1016/j.progpolymsci.2015.04.008.Suche in Google Scholar
33. Elgarahy, A. M.; Maged, A.; Eloffy, M. G.; Zahran, M.; Kharbish, S.; Elwakeel, K. Z.; Bhatnagar, A. Geopolymers as Sustainable Eco-Friendly Materials: Classification, Synthesis Routes, and Applications in Wastewater Treatment. Sep. Purif. Technol. 2023, 324, 124631. https://doi.org/10.1016/j.seppur.2023.124631.Suche in Google Scholar
34. Mehrabadi, Z.; Ahmadi, S.; Gutierrez, A.; Karimi, M.; Hayati, P.; Sharafi-Badr, P.; Moaser, A. G.; Rostamnia, S.; Hasanzadeh, A.; Khaksar, S.; Rouhani, S.; Msagati, T. A. M. Morphologically Controlled Eco-Friendly Synthesis of a Novel 2D Hg(II) Metal-Organic Coordination Polymer: Biological Activities and DFT Analysis. J. Mol. Struct. 2021, 1226, 129335. https://doi.org/10.1016/j.molstruc.2020.129335.Suche in Google Scholar
35. Gonçalves, R. A.; Toledo, R. P.; Joshi, N.; Berengue, O. M. Green Synthesis and Applications of ZnO and TiO2 Nanostructures. Molecules 2021, 26, 2236. https://doi.org/10.3390/molecules26082236.Suche in Google Scholar PubMed PubMed Central
36. Kumar, S.; Jain, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kim, K.-H. Green Synthesis of Metal–Organic Frameworks: A State-of-the-Art Review of Potential Environmental and Medical Applications. Coord. Chem. Rev. 2020, 423, 213407. https://doi.org/10.1016/j.ccr.2020.213407.Suche in Google Scholar
37. Friščić, T.; Mottillo, C.; Titi, H. M. Mechanochemistry for Synthesis. Angew. Chem., Int. Ed. 2020, 59 (3), 1018–1029. https://doi.org/10.1002/anie.201906755.Suche in Google Scholar PubMed
38. Li, Z. Q.; Qiu, L. G.; Xu, T.; Wu, Y.; Wang, W.; Wu, Z.-Y.; Jiang, X. Ultrasonic Synthesis of the Microporous Metal–Organic Framework Cu3(BTC)2 at Ambient Temperature and Pressure: An Efficient and Environmentally Friendly Method. Mater. Lett. 2009, 63, 78–80. https://doi.org/10.1016/j.matlet.2008.09.010.Suche in Google Scholar
39. Dubé, M. A.; Salehpour, S. Applying the Principles of Green Chemistry to Polymer Production Technology. Macromol. React. Eng. 2014, 8, 7–28. https://doi.org/10.1002/mren.201300103.Suche in Google Scholar
40. Boultif, A.; Louër, D. Powder Pattern Indexing with the Dichotomy Method. J. Appl. Crystallogr. 2004, 37, 724–731. https://doi.org/10.1107/S0021889804014876.Suche in Google Scholar
41. Smith, G. S.; Snyder, R. L. FN: A Criterion for Rating Powder Diffraction Patterns and Evaluating the Reliability of Powder-Pattern Indexing. J. Appl. Crystallogr. 1979, 12, 60–65. https://doi.org/10.1107/S002188987901178X.Suche in Google Scholar
42. Ho, Y. S.; McKay, G. Sorption of Dye from Aqueous Solution by Peat. Chem. Eng. J. 1998, 70, 115–124. https://doi.org/10.1016/S0923-0467(98)00076-1.Suche in Google Scholar
43. Vlasyuk, D.; Łyszczek, R.; Mazur, L.; Pladzyk, A.; Hnatejko, Z.; Woźny, P. A Series of Novel 3D Coordination Polymers Based on the Quinoline-2,4-dicarboxylate Building Block and Lanthanide(III) Ions – Temperature Dependence Investigations. Molecules 2023, 28 (17), 6360. https://doi.org/10.3390/molecules28176360.Suche in Google Scholar PubMed PubMed Central
44. Brayshaw, P. A.; Hall, A. K.; Harrison, W. T. A.; Harrowfield, J. M.; Pearce, D.; Shand, T. M.; Skelton, B. W.; Whitaker, C. R.; White, A. H. Structural Studies of Rare Earth/Transition Metal Complex Ion Systems as a Basis for Understanding Their Thermal Decomposition to Mixed Oxides. Eur. J. Inorg. Chem. 2005 (6), 1127–1141. https://doi.org/10.1002/ejic.200400863.Suche in Google Scholar
45. Głuchowska, H.; Łyszczek, R.; Jusza, A.; Piramidowicz, R. Effect of N,N′-Dimethylformamide Solvent on Structure and Thermal Properties of Lanthanide(III) Complexes with Flexible Biphenyl-4,4′-dioxydiacetic Acid. J. Therm. Anal. Calorim. 2022, 147, 1187–1200. https://doi.org/10.1007/s10973-020-10435-1.Suche in Google Scholar
46. Łyszczek, R.; Vlasyuk, D.; Podkościelna, B.; Głuchowska, H.; Piramidowicz, R.; Jusza, A. A Top-Down Approach and Thermal Characterization of Luminescent Hybrid BPA.DA-MMA@Ln2L3 Materials Based on Lanthanide(III) 1H-Pyrazole-3,5-Dicarboxylates. Materials 2022, 15 (24), 8826. https://doi.org/10.3390/ma15248826.Suche in Google Scholar PubMed PubMed Central
47. Max, J. J.; Chapados, C. Infrared Spectroscopy of Aqueous Carboxylic Acids: Comparison Between Different Acids and Their Salts. J. Phys. Chem. A 2004, 108 (16), 3324–3337; https://doi.org/10.1021/jp036401t.Suche in Google Scholar
48. Silverstein, R. M.; Webster, F. X.; Kiemle, D. J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Hoboken, NJ, 2005.Suche in Google Scholar
49. Liu, S.; Ma, R. Synthesis and Structure of Hydrated Europium Carbonate. J. Cryst. Growth 1996, 169, 190–192. https://doi.org/10.1016/0022-0248(96)00290-4.Suche in Google Scholar
50. Walton, K. S.; Snurr, R. Q. Applicability of the BET Method for Determining Surface Areas of Microporous Metal–Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. https://doi.org/10.1021/ja071174k.Suche in Google Scholar PubMed
51. De Lange, M. F.; Vlugt, T. J. H.; Gascon, J.; Kapteijn, F. Adsorptive Characterization of Porous Solids: Error Analysis Guides the Way. Microporous Mesoporous Mater. 2014, 200, 199–215. https://doi.org/10.1016/j.micromeso.2014.08.048.Suche in Google Scholar
52. Haque, E.; Jun, J. W.; Jhung, S. H. Adsorptive Removal of Methyl Orange and Methylene Blue from Aqueous Solution with a Metal-Organic Framework Material, Iron Terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. https://doi.org/10.1016/j.jhazmat.2010.09.035.Suche in Google Scholar PubMed
53. Hasegawa, M.; Ohmagari, H.; Tanaka, H.; Machida, K. Luminescence of Lanthanide Complexes: From Fundamental to Prospective Approaches Related to Water- and Molecular-Stimuli. J. Photochem. Photobiol., C 2022, 50, 100484. https://doi.org/10.1016/j.jphotochemrev.2022.100484.Suche in Google Scholar
54. Zhu, Q.-Y.; Sun, Q.; Guo, M.-C.; Gao, X.-S.; Liu, W.-L.; Ren, X.-M. Influence of Solvent Coordination on Crystal Structure and Luminescent Property in Lanthanide MOFs. Polyhedron 2023, 237, 116386. https://doi.org/10.1016/j.poly.2023.116386.Suche in Google Scholar
55. Allendorf, M. D.; Bauer, C. A.; Bhakta, R. K.; Houk, R. J. T. Luminescent Metal–Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. https://doi.org/10.1039/B802352M.Suche in Google Scholar PubMed
56. Dzhardimalieva, G. I.; Uflyand, I. E. Design and Synthesis of Coordination Polymers with Chelated Units and Their Application in Nanomaterials Science. RSC Adv. 2017, 7, 42242–42288. https://doi.org/10.1039/C7RA05302A.Suche in Google Scholar
57. Carp, O. Materials Obtained by Solid-State Thermal Decomposition of Coordination Compounds and Metal–Organic Coordination Polymers. In Reactions and Mechanisms in Thermal Analysis of Advanced Materials; Tiwari, A.; Raj, B., Eds.; Wiley: Hoboken, 2015; pp. 63–84.10.1002/9781119117711.ch3Suche in Google Scholar
58. Głuchowska, H.; Łyszczek, R.; Mazur, L.; Kirillov, A. M. Structural and Thermal Investigations of Co(II) and Ni(II) Coordination Polymers Based on Biphenyl-4,4′-Dioxydiacetate Linker. Materials 2021, 14 (13), 3545. https://doi.org/10.3390/ma14133545.Suche in Google Scholar PubMed PubMed Central
59. Ramakrishnaiah, H.; Rajasimman, M.; Shanmugapriya, S.; Rajeshkumar, S. Insights into Isotherms, Kinetics, and Thermodynamics of AB113 Biosorbent. Sci. Rep. 2023, 13, 22423. https://doi.org/10.1038/s41598-023-49471-w.Suche in Google Scholar PubMed PubMed Central
60. Murphy, O. P.; Vashishtha, M.; Palanisamy, P.; Kumar, K. V. A Review on the Adsorption Isotherms and Design Calculations for the Optimization of Adsorbent Mass and Contact Time. ACS Omega 2023, 8, 17407–17430. https://doi.org/10.1021/acsomega.2c08155.Suche in Google Scholar PubMed PubMed Central
61. Tran, H. N. Applying Linear Forms of Pseudo-Second-Order Kinetic Model for Feasibly Identifying Errors in the Initial Periods of Time-Dependent Adsorption Datasets. Water 2023, 15, 1231. https://doi.org/10.3390/w15061231.Suche in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/pac-2025-0559).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

