Abstract
After production and before the use in different applications, hydrogen may need to be purified, transported, compressed and stored. Hydrogen is conventionally stored in high pressure gas cylinders and, as a liquid phase at low temperatures, in opened tanks. These methods present several economic and security problems. So, hydrogen storage in liquid or solid carriers is a suitable method for future applications. Hydrogen absorption and desorption in metal and complex hydrides will be discussed. Examples are provided, including the role of additives in promoting hydrogen sorption reactions. Some case studies using metal hydrides as hydrogen carrier are presented. The HyCARE project, focussed on the development of an efficient metal hydride-based system for the storage of renewables energies is presented, giving evidence of about 50 kg of hydrogen stored in metal hydrides. A small-scale hydrogen refuelling station developed to provide hydrogen for a fuel cell driven drone will be described. The Life Cycle Assessment (LCA) methodology to evaluate the environmental impacts associated with developed systems is also shortly described. Finally, main open challenges will be outlined, suggesting possible approaches for their overcoming.
Introduction
To get rid of pollution and global temperature increase due to climate change, it is fundamental to look for alternatives to fossil fuel, especially in transport and energy sectors. A solution is the use of renewable sources (i.e. solar, wind, water), if their energy can be efficiently stored. The management of renewable energies can be obtained using hydrogen as energy vector, which shows several advantages [1]. It is a secondary energy vector, so it can be produced from other primary energy sources, resulting unlimited and it can be stored for a long period of time. The interest of hydrogen as an energy vector is mainly due to its highly exothermic reaction with oxygen to produce only water. Compared to fossil fuels, 1 kg of H2 has the same energy as 2.4 kg of CH4 or 2.8 kg of gasoline [2]. This means that hydrogen has a higher energy-to-weight ratio compared to other fuels. On the other hand, for the energy-to-volume ratio, the situation is inverted. Liquid hydrogen has 8.5 M J L−1 against 32.6 M J L−1 of gasoline. This is a drawback for hydrogen use, because a much higher volume of hydrogen is required to have the same amount of energy provided by most of fossil fuels.
Hydrogen can be obtained from a variety of possible sources, i.e. proton exchange membrane and alkaline electrolyzers, biogas reforming, or from photoelectrochemical production. Its transformation back to energy can be obtained by using fuel cells or internal combustion engines. The use of hydrogen as energy vector implies the development of the necessary infrastructures for its handling. In fact, after production, hydrogen needs to be purified, in order to eliminate possible contaminations in the gas. Then, hydrogen needs to be transported and stored, with an amount strongly dependent on the specific application. If necessary, hydrogen may need also to be compressed [3].
Conventionally, hydrogen is handled as compressed gas or in the liquid phase. It requires significant energy consumption to reach hyperbaric pressures and cryogenic temperatures, resulting non-economically advantageous. On the contrary, hydrogen carriers can be considered, since they allow handling hydrogen at low pressure and close to room temperature, achieving large volumetric densities compared to compressed or liquid hydrogen [4]. Moreover, handling hydrogen with carriers results to be safer than liquid and compressed gas. Nowadays, one of the main bottlenecks towards an affordable and efficient storage of renewables through hydrogen is still related to the development of suitable hydrogen handling, to be used in integrated systems. The aim of research in hydrogen sector is then aimed to overcome this problem, increasing energy density, but limiting systems volume.
Hydrogen sorption
Liquid hydrogen carriers have been investigated since a long time, and commercial applications are already present in the market [5]. On the other hand, the use of solid carriers has been also considered, being suitable for large scale applications [6]. Materials to be used as solid hydrogen carriers require high mass and volumetric capacity, coupled with a fast kinetics of gas uptake and release. In addition, the reversible hydrogenation reaction should take place close to ambient pressures and temperatures. These properties can be reached when proper thermodynamic and kinetic properties are merged, together a suitable gravimetric and volumetric density for the hydrogen carrier.
A huge number of materials for hydrogen handling have been investigated in recent years [7]. Hydrogen can be linked to the carrier by physisorption in highly porous materials (e.g. zeolites, MOFs, nanotubes, graphene) [8]. However, these materials have been investigated up to now at a research level and they have been never tested in large prototypes. More interesting is the process of chemisorption, forming solid solutions or hydrides [9]. In this way, hydrogen forms ionic bonds with element of s-block, covalent bonds with p-block, and metallic bond, but also ionic and covalent ones, with d and f-block elements.
The interaction of hydrogen (H2) in the gas phase (g) with a carrier (M) in a solid phase (s) can bring to the formation of a solid solution, according to
In this case, the process is driven by the Sievert law:
where H/M is the hydrogen-to-carrier ratio in the solid solution, K s is a constant and p is the pressure. The temperature (T) dependence of K s can be expressed as
where ΔH mix is the enthalpy and ΔS mix is the entropy of the mixing process and R is the gas constant.
On the other hand, the reaction between H2 and M can be written as:
where MH x is a solid hydride and Q is the heat of the reaction. In this case, the thermodynamics of the equilibrium is characterized by the interrelation between equilibrium pressure (p eq), the concentration of hydrogen in the solid phase (H/M) and T, which is described in the so called PCT-diagram. In the two-phase region, p eq is related to temperature according to the Van’t Hoff equation:
where the ΔH for and ΔS for are, respectively, the enthalpy and entropy of formation of the hydride. For practical applications, ΔH for corresponds to Q, defining the main parameter for the thermal management of the system. From this relation, the variation in enthalpy and entropy of both absorption and desorption reactions can be experimentally obtained by plotting ln p eq as a function of 1/T, in the as called Van’t Hoff plot.
With a reference pressure p eq = 1 bar, the decomposition temperature (T dec) can be calculated according to:
A scheme of the free energy versus composition at constant p and T is reported for both cases in Fig. 1(a), and the corresponding behaviour of p as a function of H/M at constant T is shown in Fig. 1(b). The hydrogen sorption capacity of materials can be expressed as atomic ratio (H/M), as gravimetric capacity (H2 wt%), or as volumetric capacity (gH2 L−1). The plateau width provides the reversibility capacity, Δ(H/M), which is an important parameter for hydrogen handling applications.
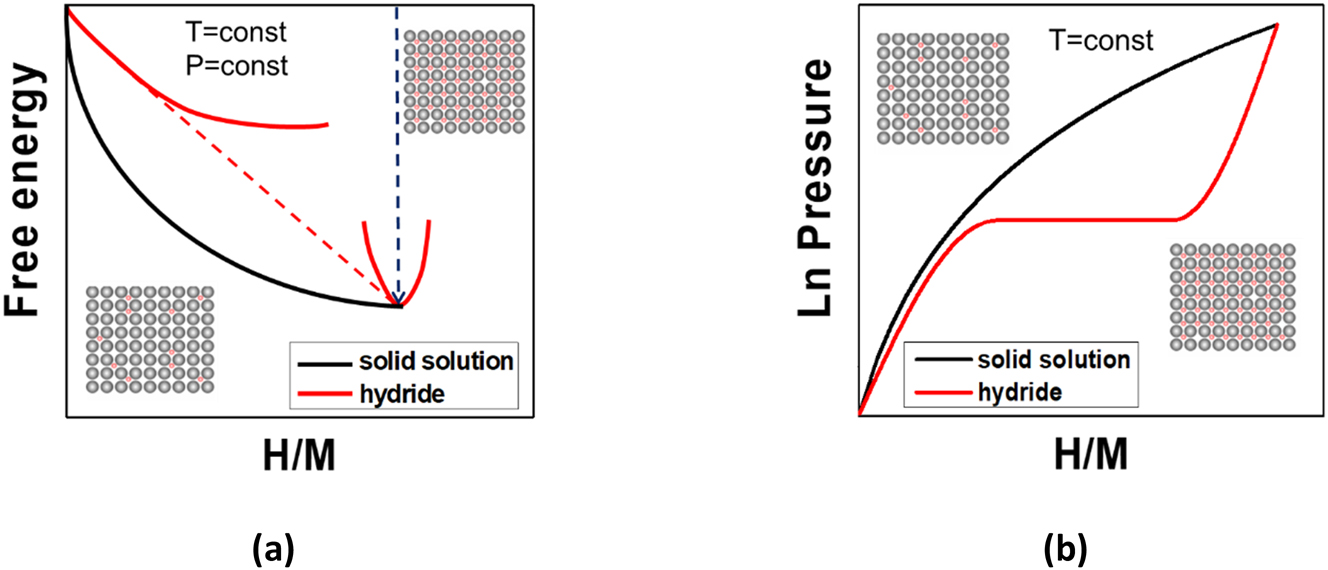
Scheme of the free energy (a) and pressure (b) as a function of composition for the formation of a solid solution (black curves) and a hydride (red curves). The red dashed line in (a) shows the common tangent, defining the equilibrium compositions, whereas the dashed blue line represents the free energy of formation of the hydride.
If ΔS for = −130 J molH2 −1 K−1 is considered, corresponding to the entropy change due to a mole of a gas which transforms into a solid [10], the Van’t Hoff plot can be calculated for different values of ΔH for. As an example, the equilibrium pressure and temperature are shown in Fig. 2(a) for ΔH for values of −30, −40 and −50 kJ molH2 −1. It is clear that an equilibrium at room conditions (e.g. T = 300 K and p eq = 1 bar) is obtained for ΔH for ∼ −40 kJ molH2 −1. If a constant pressure of 1 bar is fixed, a variation of ΔH for of about 10 kJ molH2 −1 shifts T dec of about 100 K. Similarly, if a constant temperature of 300 K is taken, a variation of p eq of about two orders of magnitude is obtained for the same value of ΔH for. So, it is quite clear that small variations in ΔH for can modify significantly the hydrogen sorption conditions. In order to estimate the combined effect of ΔH for and ΔS for, the equilibrium conditions have been calculated at p eq = 1 bar for different values of T dec and the results are shown in Fig. 2(b). It appears that, in order to maintain a constant T dec = 300 K, a value of ΔH for ∼ −50 kJ molH2 −1 has to be associated with a ΔS for value of about −165 J molH2 −1 K−1. Similarly, a value of ΔH for ∼ −30 kJ molH2 −1, has to be associated with a ΔS for value of about −100 J molH2 −1 K−1 in order to maintain the same value of T dec. Of course, these conditions would be related to a change in the entropy content of the M and MH x solid phases du to the hydrogen sorption reaction.
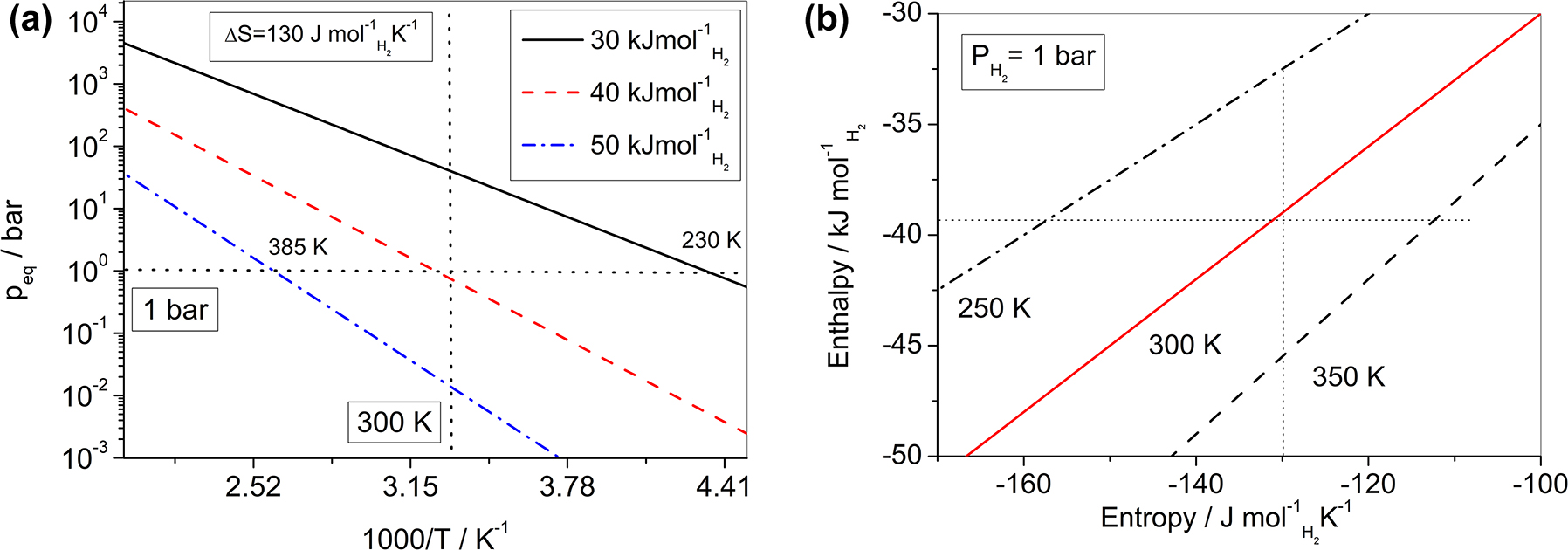
(a) Calculated Van’t Hoff plot for a hydrogen sorption reaction with ΔS for = −130 J molH2 −1 K−1 and ΔH for values of −30, −40 and −50 kJ molH2 −1. Equilibrium temperatures (at p eq = 1 bar) and pressures (at T = 300 K) are indicated by dotted lines. (b) Standard enthalpy and entropy for the hydrogen sorption reaction considering different values of equilibrium decomposition temperature. Values corresponding to ΔS for = −130 J molH2 −1 K−1 and ΔH for = −40 kJ molH2 −1 are indicated by dotted lines.
Ideally, PCT-diagrams in absorption and desorption should follow the same trend. However, most of real systems display the absorption and desorption plateau pressure at different values, in which absorption is higher than desorption, showing and hysteresis effect. Hysteresis is linked to sample parameters (preparation conditions, microstructure, etc.) and it is not unique for the same material. It can be controlled with suitable treatments after synthesis or by alloying with specific additives. Hysteresis is a very important parameter for handling purposes, and it should be as little as possible for applications, in which reversibility and no loss of efficiency are required.
The plateau pressure should be flat, but it could happen to have a slope, because of compositional fluctuations due to impurities and/or fluctuations of stoichiometry or to para-equilibrium phenomena [11]. Also in this case, suitable treatments or additives can reduce the slope. The plateau cannot be unique, but for some materials (e.g. TiFe), it can show multiple plateaux for different H/M ratio, because of formation of different hydride phases [12].
The kinetic properties determine the rate at which a practical hydrogen sorption reaction can be realised in the material [13]. So, the time of solid solution or hydride formation and decomposition can be crucial in the choice of the proper hydrogen carrier. Hydrogen sorption reactions usually occur in several steps. In first stage, the hydrogen physisorption at the surface is followed by the dissociation of the molecule into atoms (i.e. chemisorption), the diffusion of the hydrogen atoms into the metal sub-surface and bulk lattice, and the formation of a solid solution (α). In case of formation of a hydride, nucleation and growth processes are following in a two phases (α + β) region, until a single β phase is present. The process is reversed during desorption, so that the metallic phase nucleates from the hydride while hydrogen atoms diffuse in the bulk, recombining into molecules at the surface and then desorbing. For the study of the kinetics of the sorption reaction it is necessary to determine the rate-limiting/determining step, which is the slowest step of the process. If hydrogen adsorption, dissociation and diffusion are fast enough, the rate-limiting process is the hydride formation at the α/β interface. On the contrary, if the formation at the interface is not the rate-limiting step, the kinetics follows a diffusion-based mechanism. However, often there is not a single rate-determine step [14]. Similar considerations can be done for desorption step.
To determine the reaction mechanisms, different models have been developed, which describe experimental kinetic data. Considering the hydride formation/degradation as rate-determine step, the best-known Avrami model is used:
in which α is the reacted fraction, t is time, n is an exponent linked to phase growth mechanism and k is the rate constant. Different variation of this model exists, e.g. the Johnson-Mehl-Avrami (JMC). These models used to apply the linear form of the Avrami’s model and are used to model isobaric measurements of amount of hydrogen uptakes or released in isothermal conditions. Indeed, kinetic curves can be obtained at constant pressure, reporting the amount of hydrogen absorbed or desorbed as a function of time [9]. Curves are often reported at constant pressure, with different temperatures. This approach implies a different driving force (ΔG) of reaction. A proper study should imply a constant driving force that involves a different back pressure for every temperature used in the measurements [15].
The reaction rate changes with temperature, since the rate constant follows the Arrhenius relation. One of the most important kinetic parameters is the activation energy (E a), which expresses the energy barrier necessary to activate the sorption process. Different methods have been developed to determine the E a, e.g. the Kissinger method, usually based on results from thermal analysis. In general, during a kinetic study, it should be taken into account that experimental conditions and artefacts can affect the results of measurements.
The absorption and desorption rate can be linked to the material microstructure [16]. Indeed, materials with small grains or crystallites (dimension of the order of nanometres) show fast sorption rates, due to the fast diffusion along grain boundaries. Moreover, grain boundaries act as favourable nucleation sites for the decomposition of hydride phases [17]. Thus, the synthesis and processing of materials can influence the microstructure, which in turn can affect the kinetic processes. An example is shown by mechanical alloying methods, which allow to obtain nano-crystallites, enhancing the kinetics of hydrogen sorption reactions. In addition, also the starting powder particle dimension and the specific surface area, can affect the kinetics, which is enhanced when the contact surface with the gas increases. Finally, some additives can be used to enhance the kinetics, because they can reduce the hydride nucleation barrier. Additives can act as a catalyst or they can be an element of the hydride former alloy, without having a significant impact on hydride thermodynamic behaviour [7].
Hydrogen sorption has effects on the crystal structure of the carrier, and therefore on its volume (V). Indeed, during the hydride formation (i.e. absorption), the crystal structure of the metal changes, forming that of the hydride phase. The corresponding unary cell is usually bigger than that of starting intermetallic compound, implying an increase in volume (ΔV). On the contrary, during the metal formation (i.e. desorption), the volume decreases, since, releasing hydrogen, the crystal structure comes back to the original one. These changes in material volume during hydrogen uptake and release must be considered for the tank design, since a dead volume must be planned to allow the carrier to expand during absorption. It is worth to note that cell volume variations (i.e. contraction–expansion) taking place during hydrogen sorption can lead to ΔV/V values of the order of 20 %. As an example, LaNi5H6 has a volume expansion of 18.9 % [7].
In real applications, carrier parameters that should be considered are its activation, the possibility to compact the powder and the thermal conductivity. Moreover, also the purity of the inlet hydrogen has an important role. The reversible capacity can be less than the maximum plateau width, since the material can lose its properties during cycling, because of material degradation or impurities introduced during hydrogen sorption cycling.
Generally, metal surfaces could be covered by an oxide layer that hinders hydrogen diffusion. So, an activation process is necessary before the absorption. As an example, the effect of possible oxide layers (e.g. TiO2, Fe2O3, Fe3O4 and TiFeO3) on hydrogen sorption in TiFe compound has been recently investigated [9]. Commonly, the activation involves processing the material at high temperatures and pressures, to facilitate hydrogen diffusion through the oxide layer. By alternating cycles of absorption and desorption, the crystal lattice is expanded during absorption and shrunk during dehydrogenation. This results in cracking of the carrier particles and allows to expose fresh surfaces and reduce particle sizes. In this way, there is also the advantage to create a fine microstructure which contributes to enhance the kinetic of hydrogen absorption and desorption, as explain previously. The number of cycles and operative conditions depends on material. Also, ball milling is interesting as activation process. Indeed, milling can reduce the oxide layer and the microstructure, but it results to be not easily used at industrial scale.
Solid hydrogen carriers can be used as powder, but also as pellet. Pelletized powder has the advantage to decrease the empty spaces of the storage medium, increasing the volumetric capacity [6]. The pressure used to pelletize affects the compactness of the pellet, and so the final volumetric capacity of the carrier. Pellets can be mechanically stabilized using some additives, like Expanded Natural Graphite (ENG) [18] or polymers [19]. However, the use of an additive which cannot absorb hydrogen, decreases the gravimetric capacity of the system. Pellets have to be mechanically stable upon cycling, avoiding being transformed back into the powder form. An important benefit in using pellets is the facilitation of the hydrogen tank loading process. In addition, since pellets need a physical empty space for the expansion in volume during absorption, a suitable design is necessary to avoid tank mechanical instabilities.
The thermal conductivity is fundamental for the management of hydrogen carriers, and it often defines the reaction rate. In fact, even if suitable kinetic properties have been obtained in the material, it can become the rate determining step during the hydrogen sorption reactions. Indeed, thermal conductivity affects the ability to uptake and release hydrogen, in a homogenous way through the material. Generally, hydrides have low thermal conductivity, and, in order to enhance it, it is necessary to design a proper heat exchanger to maximize the removal of heat during absorption and the supplying of it during desorption. Additives, e.g. ENG, can also increase thermal conductivity on powder and in pellets, having benefits in the handling process rate.
Impurities of the inlet gas may cause some losses in hydrogen sorption properties of the carrier, like reducing kinetic properties or reducing hydrogen storage capacity, because they can affect the surface or degrade the bulk of the alloy. Oxygen and/or water are possible impurities of a hydrogen flow produced by an electrolyser. The ability to resist to impurities depends on the material but, if poisoned, it could be possible re-activate it. Restoration can be obtained either with activation cycles of absorption and desorption or by using a chemical treatment through a proper chemical solution [20].
Materials for hydrogen storage and handling
It has been evidenced that, for the use of metal hydrides as carriers for hydrogen handling, different fundamental aspects of materials have to be taken into account, starting for the thermodynamic and kinetics of the hydrogen sorption reaction, moving to the selection of non-Critical Raw Materials (CRM) and finally considering different application parameters (activation, compaction, thermal conductivity, resistance to hydrogen impurities). The synthesis of solid-state hydrogen carriers is usually performed by melting processes or by ball milling, which promotes the formation of nanostructures. Structural, microstructural, thermal and volumetric characterisations are usually performed by a combination of several experimental techniques (i.e. XRD, SEM-EDS, DSC, TPD, PCI).
Considering pure metals, MgH2 is an excellent material for hydrogen handling, due to its high hydrogen storage capacity (i.e. volumetric H2 density about 130 kgH2 m−3 and gravimetric H2 density around 7.6 wt%) [9]. Unfortunately, according to available thermodynamic data (ΔH for = −75 kJ/molH2 and ΔS for = −130 J/molH2/K), the equilibrium dehydrogenation temperature is rather high (i.e. around 300 °C under 1 bar of H2) and the sorption kinetics is undesirably slow. Another promising elemental metal hydride is aluminium hydride (AlH3), characterized by a theoretical gravimetric density of 10.1 wt%, but suffering for very limited reversibility [21]. Other hydrides of pure metals are characterized by extreme conditions for hydrogen sorption reactions (e.g. Ti, Zr) or by very high costs (e.g. Pd).
Considering the periodic table of elements, A can be stated as an element which forms stable hydrides (AHy), such as transition metals of the left side of the periodic table or a rare earth metal. On the contrary, B can be defined as an element that forms unstable hydrides (BHw), like transition metals of the right side of the period table. For intermetallic compounds, particularly favourable are those based on A2B, AB, AB2 and AB5 stoichiometry, which form metal hydrides absorbing and releasing hydrogen close to normal conditions of temperature and pressure. In this way, the resulting AmBnHz hydrides will have properties intermediate between AHy and BHw. Examples of composition for intermetallic compounds of interest for hydrogen handling are reported in Table 1 [7, 9].
Examples of intermetallic compounds for solid-state hydrogen storage.
| A | B | AB | AB2 | AB5 |
|---|---|---|---|---|
| Mg, Ca, Zr, Ti, V, Y, La, rare earth | Ni, Fe, Co, Cu, Cr, Mn | TiNi, TiFe, ZrNi | LaNi2, YNi2, YMn2, ZrCr2, ZrMn2, ZrV2, TiMn2 | CaNi5, LaNi5, CeNi5, LaCu5 |
A huge variety of AB compounds has been studied, in particular Ti- and Zr-based. The most known AB compound for hydrogen storage application is the TiFe and its modifications, obtained by substituting Ti or Fe with other metals [22]. AB2 compounds for hydrogen storage include usually Ti and/or Zr as A-type element, while B is a transition metal [23]. AB2 compounds have usually a crystal structure of Laves-phases, mainly a hexagonal MgZn2-type (C14), cubic MgCu2-type (C15) and hexagonal MgNi2-type (C36). Thanks to this type of structures, they have the advantage to show high gravimetric capacity (up to about 2H2 wt%), fast kinetics in absorption and desorption and easy activation. However, they do not show high resistance to gas impurities, because of possible reactions with constituent elements. AB5 compounds involves rare earth metals and transition metals, and the most representative composition is LaNi5 [24], which displays fast activation, high reversibility at low temperatures, fast kinetics, good resistance to gas impurities and quite good cycling stability. LaNi5 has a hydrogen volumetric capacity of 115 kgH2m−3 and a maximum gravimetric capacity of 1.5 H2 wt%. Several body centered cubic solid solutions have been identified as potential carriers for hydrogen handling [25]. Recently, high entropy alloys have been suggested for hydrogen handling. The presence in the alloy of elements with different affinity to hydrogen leads to promising gravimetric capacities, with sorption reactions occurring at working temperatures close to normal conditions [26].
Looking for suitable solid-state hydrogen carriers, attention has been also focused on complex hydrides, which are interesting because of their light weight and their high number of hydrogen atoms per metal atoms [27]. Complex hydrides can be described by the general chemical formula: L x Q y H z . The L elements are mainly light elements of the first and second groups of the periodic table, which forms the cation. The Q element could be aluminium, boron, nitrogen, or a transition-metal, which, bonded to hydrogen, forms the complex anion. Alanates are composed of a complex anion [AlH4]− or [AlH6]3− and respective metal cation, which are salt-like, insulating materials [28]. Depending on the metal cation, theoretical gravimetric capacity are up to 10.5 wt% for LiAlH4, 5.5 wt% for NaAlH4, 5.7 wt% for KAlH4, 9.3 wt% for Mg(AlH4)2 have been observed. Also borohydrides are inorganic ionic compounds suitable as hydrogen carrier [29]. The theoretical hydrogen content of LiBH4 is 18.5 wt%, i.e. the highest among metal borohydrides, while it is equal to 10.7 wt% for NaBH4, 7.5 wt% for KBH4, 14.9 wt% for Mg(BH4)2 and 11.6 wt% for Ca(BH4)2. Amides and imides of alkali and alkaline-earth metals have also the potential to meet the needs of hydrogen carriers [30]. Additionally, the formulation of mixtures of metal hydrides and complex hydrides (mainly borohydrides or amide/imide), the so-called Reactive Hydride Composites (RHC), enables reversible and kinetically/thermodynamically favourable hydrogen desorption/absorption under moderate temperature and hydrogen pressure conditions [27].
Applications of hydrogen storage and handling
The use of carriers for hydrogen storage systems integrated with a fuel cell has been widely investigated [31], but they are mainly available at the lab-scale. Most of them are based on intermetallic compounds and are demonstrative, prototype or simulated devices. For hydrogen storage applications at an industrial scale (i.e. units of kilos of hydrogen and even more) metal hydride powders are produced at a ton level. Hydrogen storage systems based on hydrides are available in the market, even if real applications are still limited.
There is growing interest in the use of carriers for solid state compression of hydrogen [6]. Advantages over mechanical compressors include no hydrogen contamination, reduced footprint, no moving parts, thus eliminating mechanical wear and vibration, reduced maintenance costs and noise, and, by using waste heat from external sources, having a very low electrical energy consumption for the systems control [32]. In order to reach the hydrogen compression, the system converts a thermal energy into compressed hydrogen gas. The effect of hydrogen compression, in fact, exploits the reversible heat driven reaction between hydrogen and a metal able to produce metal hydrides, Q in eq. (4). The scheme of a hydrogen compressor based on solid carrier is shown in Fig. 3(a). The input hydrogen is stored at low pressure (p 1) at the input temperature (T low) in a hydride placed in a tank. By using a suitable thermal fluid, the hydride is heated at a higher temperature (T high), so that the desorption pressure is increased (p 1′).
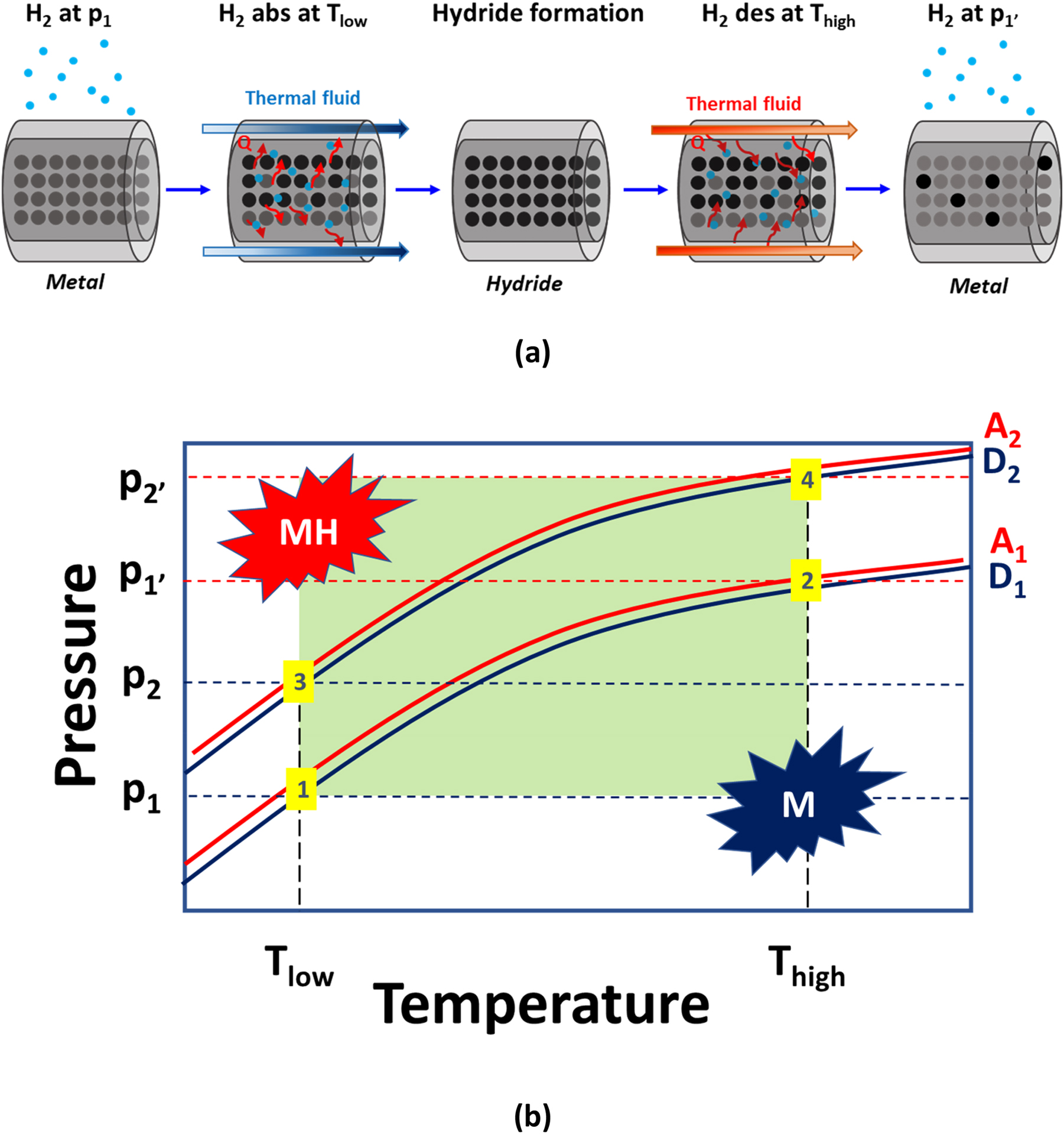
(a) Generic scheme illustrating the operation of a hydride hydrogen compressor. (b) Scheme of a 2 stages metal hydride compressor.
By exploiting the thermodynamic characteristics of a series of hydrides, it is possible to further increase the release pressure of hydrogen, as shown in Fig. 3(b). After absorption at low pressure (p 1) at the input temperature (T low) in the first hydride (point 1), the hydride is heated at a higher temperature (T high), so that the desorption pressure is increased (p 1′) (point 2). The hydrogen released by the first hydride is absorbed at T low by the second hydride (point 3), that has a higher absorption pressure (p 2) with respect to the first one. By increasing the temperature to T high, hydrogen desorbs at p 2′ from the second hydride (point 4), that is higher than p 1′. If useful, the scheme is continued for n stages, delivering the required output pressure to the tank. This approach requires that equilibrium pressure of the first hydride at T high (p 1′) is sufficient for allowing the hydrogen absorption from the second hydride at T low. The same description could be done over n stages, in order to reach in the final stage at T high the targeted pressure. Current compressors based on carriers are for pressures up to 300 bar [6], so there is the need for the development of new alloys with suitable thermodynamic properties.
Separation and purification of hydrogen using hydrides is based on the selective absorption and release of hydrogen by the carrier under a certain temperature and pressure. If the hydrogen content in the raw gas is higher than 50–60 %, hydrides can be used to separate the components of the raw gas and produce high purity hydrogen from it. But even if the hydrogen content merely exceeds 15 %, hydrogen separation with hydrides still is feasible. Furthermore, carrier-based hydrogen purification has the advantages of simple device layout, low energy consumption, simple and safe operations, and a high recovery ratio of hydrogen [33]. Early reports on possible applications of hydrogen purification by hydrides at industrial scale pointed out the needs of proper process management. The application of hydride purification has been proven in several case studies, mainly focusing on La-based AB5 type carriers, showing purification of different types of gas mixtures. Studied gas mixtures were H2 containing CO, CH4, CO2, O2 and N2, and syngas. Though the principles of carrier-based hydrogen purification are known for many years, so far they have been implemented nearly only at prototype scale. Main reasons are cost and stability of the selected alloys, non-optimal reactor designs and missing knowledge about optimal integration into suitable applications.
Case studies
As an example of applications of hydrogen storage by solid carriers, results from HyCARE project can be considered (see Fig. 4) [34]. The aim of the HyCARE project was to realize an innovative hydrogen storage tank based on solid-state carriers, using metal hydrides, to exploit the use of hydrogen as energy vector for stationary energy storage. The plant has been implemented at LabCrigen of ENGIE, in France, near Paris. The main advantages in using carriers for hydrogen storage in stationary applications are the following: (i) large quantities of hydrogen stored in small volume (i.e. low footprint); (ii) improved energy efficiency of the whole process and (iii) high safety of the storage. The energy produced by a renewable source (e.g. sun and wind) is used to produce hydrogen from water through an electrolyser. The gas is then stored in the designed tank using a carrier. The main innovation of the HyCARE project is related to the presence of a heat storage system, connected to the hydrogen tanks, aimed to collect the heat produced from the hydride during hydrogen absorption. The collected heat is then used during the desorption event to release hydrogen from the carrier. Finally, the released hydrogen is used to supply a fuel cell, producing electricity.

Hydrogen storage system developed in HyCARE project: Cylinders at the top contain the metal hydride for hydrogen storage and boxes at the bottom contain the PCM for heat storage.
Moving from basic research to the implementation of hydrogen storage system based on metal hydride, the industrial production of the active material is fundamental. The alloy TiFe0.85Mn0.05 was selected as hydrogen carrier for a storage plant of about 50 kg of hydrogen. For testing purposes, a preliminary batch of 5 kg of the selected alloy was synthesized at industrial level and characterized to determine the structure and phase abundance [35]. The hydrogen sorption properties were investigated, performing studies on long-term cycling and resistance to poisoning. The alloy absorbs and desorbs hydrogen between 25 bar and 1 bar at 328 K, storing 1.0H2 wt%, displaying fast kinetic, good resistance to gas impurities, and storage stability over 250 cycles.
The industrial production promoted the formation of a passive layer and a high amount of secondary phases, observing differences in the hydrogen sorption behaviour compared to samples prepared at laboratory scale. These differences are caused by the synthesis itself and have a strong effect on the activation procedure, sorption capacities and kinetics. In particular, the activation was harder in the sample prepared at industrial scale, as the powder needed to be heated and cooled in hydrogen atmosphere, and the storage capacity was significantly decreased. Observed results can be linked to the amount of oxygen introduced during the synthesis, either from the use of staring Ti with limited purity or from the material processing procedure, like a low grade of vacuum and the processing of the powder in air. Oxygen contaminations promoted the formation of a significant fraction of oxides as secondary phases, Ti4(Fe,Mn)2O0.4, and Ti3Fe3O as a passive layer.
From results obtained in HyCARE project, it is clear that metal hydrides are strongly influenced by the synthesis method and confirmed that the industrial production results in a different hydrogen sorption behaviour with respect to materials prepared al the laboratory scale. The comparison between properties observed in laboratory and industrially prepared materials suggests that the amount of oxygen introduced in the synthesis should be limited, e.g. using high purity raw materials, that however might results in higher cost of production.
A two-stage metal hydride compressor was developed and integrated in a small-scale hydrogen refuelling station at prototype level in the frame of the CleanDronhy project (see Fig. 5) [36]. The compressor employs two commercial alloys, i.e. a La0.9Ce0.1Ni5 from Labtech in the first stage and the Hydralloy-C5 from GfE in the second one. Working between room temperature for absorption and 573 K for desorption, the hydrogen produced by an electrolyser at 28 bar was compressed up to 250 bar, resulting in a compression ratio of about 9. The compressor contains less than 1 kg of powder in each stage and an average hydrogen flowrate of 104 NL/min was observed. The metal hydride compressor had a final power consumption of 614 W, of which 85 W are linked to the hydrogen sorption reactions, while other contributions came from the pumps involved in the plant and the heat dissipations. The performances of the plant were optimized and maintained for a working time of about 245 h. The principles behind the compression work resulted in a variable flow of hydrogen that decreased cycle after cycle while filling the cylinder, and also in a variable flow by changing the filling volume. The mentioned case study demonstrated the feasibility to use commercial metal hydrides to achieve high pressure of hydrogen, i.e. 250 bar. The main advantage is the limited amount of power required and the easy scale-up of the entire refuelling station, encouraging its spread into the market. Nevertheless, results pointed out the limited pressure that can be reached with compounds available on the market, implying a narrow range of applications.
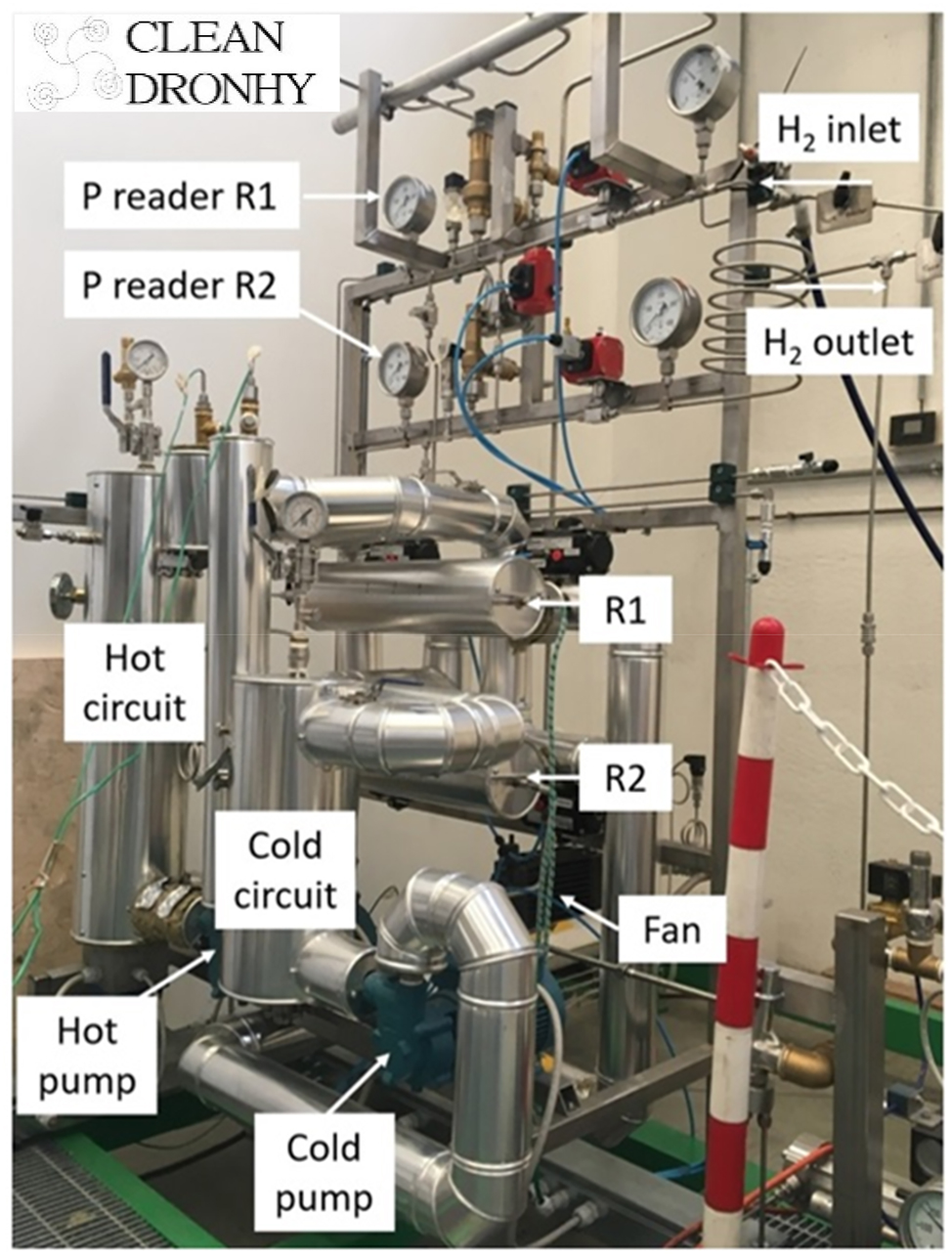
Hydrogen compressor system developed in CleanDronhy project: two reactors (R1 and R2) are linked to a thermal management system.
There is the need to develop commercially available alloys able to compress hydrogen at high pressures, for the possible development of hydrogen refuelling stations based on hydride compressor, where 400 bar or more than 700 bar are required. To achieve the goal, new Laves (C14) intermetallic compounds, with Ti1.1(Cr,Mn,V)2 and Ti1.1(Cr,Mn,V,Fe)2 compositions, were synthetized and characterized [37]. Results showed the influence of the substitution of Cr with Mn and Fe on the crystal structure, and its correlation with the hydrogen sorption properties, detecting an increment in the plateau pressure and the hysteresis gap. Thanks to suitable thermodynamics, developed alloys are good candidates for a high pressure hydrogen compression. Moreover, they present an easy activation, that can be performed at room temperature, and a fast reaction rate, with few seconds required to absorb about 90 % of the hydrogen content in the alloy. Results highlighted how small changes in the composition can have a high impact in the sorption equilibrium pressure. The most promising composition turned out to be Ti1.1Cr0.9Mn0.8V0.1Fe0.2, that was estimated to release hydrogen at about 700 bar at 573 K.
Life cycle assessment
The evaluation of the environmental impact lined to the use of solid-state carriers for hydrogen handling can be obtained through a Life Cycle Analysis (LCA) [6].
The environmental impacts of a solid-state hydrogen storage tank, to be linked with a fuel cell system, was evaluated for an Auxiliary Power Unit (APU) for a light duty vehicle [38]. Following an eco-design approach, processes mostly contributing to the environmental impacts have been identified, and possible improvements have been suggested. By performing an LCA, it was found that, when the electricity consumption for hydrogen gas compression is included into the analysis, a solid-state hydrogen storage tank has similar greenhouse gas emissions and primary energy demand than gas tanks of type III and IV. However, the resources depletion is higher for the solid-state system, even though the inclusions of the end of life of the APU and the recycling of the materials may result in different conclusions. To improve the environmental performances of the APU, a reduction of structural materials for both the solid-state hydrogen tank and Balance of Plant was recommended.
An environmental analysis of a metal hydride compressor and of competing technologies (i.e. air booster and commercial hydrogen compressor) was performed for an application to fuel cell driven forklifts [39]. For the selected function (i.e. compression of hydrogen) and functional unit considered in the study (i.e. 1.79 kg H2 at 200 bar in 7 h), it was found that the best solution to compress hydrogen, from an environmental perspective, is to use a system based on a booster coupled to an air compressor. The use of the hydride compressor, which requires a dedicated energy source (like heat from natural gas, electricity from the grid or electricity from a photovoltaic system), has a greater impact when compared to all the other solutions analysed. The hydride compressor showed limited environmental impacts only when a source of waste heat is available for hydrogen desorption. In this case, impacts would be similar to a generic compressor, but larger than those generated by an air booster. An interesting result is the significant environmental impacts attributable to the stainless steel to be used for the hydride compressor, mainly because of the large amount used. Considering an optimized design, with an increased alloys-steel ratio, environmental impacts of alloys for hydrogen sorption to be used in the metal hydride system become dominant. Although the system based on metal hydrides currently did not prove to be the most suitable from an environmental point of view, it has a large room for improvement. In fact, the hydride compressor has been modelled making some assumptions that probably led to an overestimation of the environmental impacts (i.e. low alloy-steel ratio and substitution of alloys over a short period of time). Taking into consideration the previous limitations and assuming a further optimization of the compressor structure, as well as the use of alloys with optimized effectiveness, could allow to considerably reduce the impacts on the environment and increase the competitive advantage of this compression technology.
Since the flying time of drones currently in commerce is too short for mobile crane inspections, proton exchange membrane fuel cells and Li-ion batteries are considered as alternative power systems to extend the flying time. Both systems have been analysed and an LCA was performed to identify the main contributors to the environmental impacts [40]. Results showed that the battery has a very low environmental burdens if compared to other components of the same system, and the main bottleneck in this case is the source of the electricity used for battery charging. The main drawback of a battery-powered drone is the weight of the batteries, which increases with their size in order to extend the flying time becoming a limiting factor. In the case of a fuel cell powered system, on the other hand, since the fuel cell size is fixed as it depends only on the features of the drone, the limiting factor is given by the amount of hydrogen stored, which is determined by the volume of the pressure vessels and by their operating pressures. In this case, an operating pressure of 300 bar has been chosen due to the scarcity of refuelling stations that allow to reach higher pressures. An operating pressure of 700 bar, already possible in refuelling stations, could increase about twice the flying range. If use of drones is considered, it is worth noting that lifetime for fuel cells is about three times that of batteries. So, the environmental impact of production of a fuel cell-powered system is similar to that of the battery-powered one, if the life-time of the two systems is considered.
Conclusions and challenges
In conclusion, main open challenges for hydrogen handling using solid-state carriers can be outlined, suggesting possible approaches for their overcoming. Considering hydrogen handling, first of all the maximum quantity of hydrogen to be managed must be considered. In fact, for applications currently requested, hydrogen quantities at the ton level need often to be afforded. Gravimetric and volumetric density (i.e. the amount of hydrogen per kg and m3 of the handling system) are strongly related to case study, being the first more important in mobility and the second more important in stationary applications. In same case, because of limited space available, the shape conformability of hydrogen tanks becomes crucial. Being hydrogen considered an energy carrier, the efficiency of the whole handing process is fundamental for the selection. In particular, the thermal management needed for handling processes might play an important role in the management. Short charging and discharging times, as well as a full reversibility, are requested, in particular for mobile applications. Because of the possible presence of contaminants in the hydrogen produced by electrolysis or by a biomass, the sensitivity of the handling system to the gas quality is also to be taken into account. The whole life cycle of the handling process has to be monitored, starting from the selection of materials to be used for tanks and components, moving to their availability, and then considering their stability over time. Finally, safety and cost of the selected solution plays a key role in the acceptance of the solution, either from an economical and a social point of view.
Low pressure (i.e. around 30 bar) gas phase is definitely the most affordable solution. Hydrogen obtained by water electrolysis can be simply moved from the production site to the handling system, without any further treatment. Of course, this solution requires a large volume occupancy. Compressed gas is typically used for mobile applications, considering a pressure of about 350 bar for trains and up to 700 bar for cars. This solution definitely reduces the volume of the handling system, but it requires significant costs for compression. Large quantities of hydrogen can be handled if it is liquified, but in this case high costs for the low temperature cooling process are necessary. Liquid carriers (e.g. ammonia or liquid organic hydrogen carriers) are a suitable solution for hydrogen transportation, but specific reactors and catalysis needed to be developed. Solid carriers exploiting hydrogen physisorption usually are characterized by a rather limited volumetric density, and they can be used only at low temperatures. The use of absorption reaction in solid carrier has been demonstrated to a be suitable solution for hydrogen handling, being possible an easy management in the pressure range from about 30 bar to around 2 bar (i.e. the typical pressure of hydrogen to feed a fuel cell). In this case, a proper thermal management of the system is necessary. If metal hydrides are considered as energy carriers, the temperature range for hydrogen handling is rather close to standard conditions, making this solution affordable. In this case, the gravimetric density is quite limited (typically below 2 wt%), but the volumetric density is much higher than current solutions. So, this approach is mainly oriented to stationary applications. If complex hydrides are considered, a higher gravimetric density can be reached (even up to about 10 wt%), but the temperature range is also higher, making the thermal management more challenging.
Handling remains a bottleneck for hydrogen technologies, and it has to be properly managed for a wide spreading of the use of this energy carrier. Hydrides might play a role on that, likely limited but beneficial to specific applications.
Funding source: Fuel Cells and Hydrogen Joint Undertaking
Award Identifier / Grant number: 826352
Funding source: Regione Piemonte
Award Identifier / Grant number: POR-FESR 2014/2020
Funding source: Ministero dell’Università e della Ricerca
Award Identifier / Grant number: D13C22003520001
Award Identifier / Grant number: NODES ECS00000036
Acknowledgments
Support from the Project CH4.0 under the MUR program “Dipartimenti di Eccellenza 2023–2027” (CUP: D13C22003520001) is acknowledged. HyCARE project leading to this publication has received funding from the Fuel Cells and Hydrogen 2 Joint Undertaking (now Clean Hydrogen Partnership) under Grant Agreement No 826352. This Joint Undertaking receives 2 support from the European Union’s Horizon 2020 Research and Innovation program, Hydrogen Europe and Hydrogen Europe Research. Authors acknowledge the Regione Piemonte (Italy) for the financial support at the project Clean-DronHy, POR-FESR 2014/2020. This publication is part of the project NODES which has received funding from the MUR e M4C2 1.5 of PNRR with grant agreement no. ECS00000036.
References
[1] A. S. Strub, G. Imarisio (Eds.) Hydrogen as an energy vector. In Proceedings of the International Seminar, Brussels, 12–14 February 1980, Bruxelles, Springer Verlag (2014).10.1007/978-94-009-9042-5Search in Google Scholar
[2] M. Ball, M. Wietschel. The Hydrogen Economy, Opportunities and Challenges, Cambridge, UK, Cambridge Press (2009).10.1017/CBO9780511635359Search in Google Scholar
[3] R. Neugebauer. Hydrogen Technologies, Cham, Springer Nature (2023).10.1007/978-3-031-22100-2Search in Google Scholar
[4] T. He, P. Pachfule, H. Wu, X. Qiang Xu, P. Chen. Nat. Rev. Mater. 1, 16059 (2016), https://doi.org/10.1038/natrevmats.2016.59.Search in Google Scholar
[5] T. H. Ulucan, S. A. Akhade, A. Ambalakatte, T. Autrey, A. Cairns, P. Chen, Y. Whan Cho, F. Gallucci, W. Gao, J. B. Grinderslev, K. Grubel, T. R. Jensen, P. E. de Jongh, J. Kothandaraman, K. E. Lamb, Y. Lee, C. Makhloufi, P. Ngene, P. Olivier, C. J. Webb, B. Wegman, B. C. Wood, C. Weidenthaler. Progr. Energy 5, 012004 (2023), https://doi.org/10.1088/2516-1083/acac5c.Search in Google Scholar
[6] M. Dornheim, L. Baetcke, E. Akiba, J. Ares, T. Autrey, J. Barale, M. Baricco, K. Brooks, N. Chalkiadakis, V. Charbonnier, S. Christensen, J. Bellosta von Colbe, M. Costamagna, E. Dematteis, J. F. Fernández, T. Gennett, D. Grant, T. W. Heo, M. Hirscher, K. Hurst, M. Lototskyy, O. Metz, P. Rizzi, K. Sakaki, S. Sartori, E. Stamatakis, A. Stuart, A. Stubos, G. Walker, C. J. Webb, B. Wood, V. Yartys, E. Zoulias. Progr. Energy 4, 042005 (2022), https://doi.org/10.1088/2516-1083/ac7cb7.Search in Google Scholar
[7] M. Hirscher, V. A. Yartys, M. Baricco, J. Bellosta von Colbe, D. Blanchard, R. C. BowmanJr., D. P. Broom, C. E. Buckley, F. Chang, P. Chen, Y. Whan Cho, J. Crivello, F. Cuevas, W. I.F. David, P. E. de Jongh, R. V. Denys, M. Dornheim, M. Felderhoff, Y. Filinchuk, G. E. Froudakis, D. M. Grant, E. Gray, B. C. Hauback, T. He, T. D. Humphries, T. R. Jensen, S. Kim, Y. Kojima, M. Latroche, H. Li, M. V. Lototskyy, J. W. Makepeace, K. T. Møller, Lu. Naheed, P. Ngene, D. Noreus, M. Moe Nygård, S. Orimo, M. Paskevicius, L. Pasquini, D. B. Ravnsbæk, M. V. Sofianos, T. J. Udovic, T. Vegge, G. S. Walker, C. J. Webb, C. Weidenthaler, Cl. Zlotea. J. Alloys Compd. 827, 153548 (2020), https://doi.org/10.1016/j.jallcom.2019.153548.Search in Google Scholar
[8] L. Zhang, M. D. Allendorf, R. l. Balderas-Xicohténcatl, D. P. Broom, G. S. Fanourgakis, G. E. Froudakis, T. Gennett, K. E. Hurst, S. Ling, C. Milanese, P. A. Parilla, D. Pontiroli, M. Riccò, S. Shulda, V. Stavila, T. A. Steriotis, C. J. Webb, M. Witman, M. Hirscher. Prog. Energy 4, 042013 (2022), https://doi.org/10.1088/2516-1083/ac8d44.Search in Google Scholar
[9] L. Pasquini, K. Sakaki, E. Akiba, M. D. Allendorf, E. Alvares, J. R. Ares, D. Babai, M. Baricco, J. Bellosta von Colbe, M. Bereznitsky, C. E. Buckley, Y. Whan Cho, F. Cuevas, P. de Rango, E. M. Dematteis, R. V. Denys, M. Dornheim, J. F. Fernández, A. Hariyadi, B. C. Hauback, T. W. Heo, M. Hirscher, T. D. Humphries, J. Huot, I. Jacob, T. R. Jensen, P. Jerabek, S. Y. Kang, N. Keilbart, H. Kim, M. Latroche, F. Leardini, H. Li, S. Ling, M. V. Lototskyy, R. Mullen, S. Orimo, M. Paskevicius, C. Pistidda, M. Polanski, J. Puszkiel, E. Rabkin, M. Sahlberg, S. Sartori, A. Santhosh, T. Sato, R. Z. Shneck, M. H. Sørby, Y. Shang, V. Stavila, J. Suh, S. Suwarno, L. T. Thu, L. F. Wan, C. J. Webb, M. Witman, C. Wan, B. C. Wood, V. A. Yartys. Progr. Energy 4, 032007 (2022), https://doi.org/10.1088/2516-1083/ac7190.Search in Google Scholar
[10] A. Züttel. Naturwissenschaften 91, 157 (2004), https://doi.org/10.1007/s00114-004-0516-x.Search in Google Scholar PubMed
[11] T.B. Flanagan, W.A.J. Oates. J. Less Common Met. 100, 299 (1984), https://doi.org/10.1016/0022-5088(84)90070-5.Search in Google Scholar
[12] T. Tanaka, M. Keita, D. E. Azofeifa. Phys. Rev. B 4, 1771 (1981), https://doi.org/10.1103/physrevb.24.1771.Search in Google Scholar
[13] Q. Li, X. Lin, Q. Luo, Y. Chen, J. Wang, B. Jiang, F. Pan. Int. J. Miner. Metall. Mater. 29, 32 (2022), https://doi.org/10.1007/s12613-021-2337-8.Search in Google Scholar
[14] A. Borgschulte, R. Gremaud, R. Griessen. Phys. Rev. B 78, 094 (2008).10.1103/PhysRevB.78.094106Search in Google Scholar
[15] T. Huang, H. Liu, C. Zhou. Int. J. Hydrogen Energy 46, 37986 (2021), https://doi.org/10.1016/j.ijhydene.2021.09.044.Search in Google Scholar
[16] E. David. J. Mater. Process. Technol. 162-163, 169 (2005).10.1016/j.jmatprotec.2005.02.027Search in Google Scholar
[17] M. Dorheim, S. Doppiu, G. Barkhordarian, U. Boesenberg, T. Klassen, O. Gutfleisch, R. Bormann. Scr. Mater. 56, 841 (2007).10.1016/j.scriptamat.2007.01.003Search in Google Scholar
[18] K. Herbrig, L. Röntzsch, C. Pohlmann, T. Weißgärber, B. Kieback. Int. J. Hydrogen Energy 38, 7026 (2013), https://doi.org/10.1016/j.ijhydene.2013.03.104.Search in Google Scholar
[19] C. Pohlmann, L. Röntzsch, F. Heubner, T. Weißgärber, B. Kieback. J. Power Sources 231, 97 (2013), https://doi.org/10.1016/j.jpowsour.2012.12.044.Search in Google Scholar
[20] S. Miura, A. Fujisawa, M. Ishida. Int. J. Hydrogen Energy 37, 2794 (2012), https://doi.org/10.1016/j.ijhydene.2011.03.150.Search in Google Scholar
[21] J. Graetz, J.J. Reilly, V.A. Yartys, J.P. Maehlen, B.M. Bulychev, V.E. Antonov, B.P. Tarasov, I.E. Gabis. J. Alloys Compd. 509, S517 (2011), https://doi.org/10.1016/j.jallcom.2010.11.115.Search in Google Scholar
[22] E. M. Dematteis, N. Berti, F. Cuevas, M. Latroche, M. Baricco. Mater. Adv. 2, 2524 (2021), https://doi.org/10.1039/d1ma00101a.Search in Google Scholar
[23] N.A.A. Rusman, M. Dahari. Int. J. Hydrogen Energy 41, 12108 (2016), https://doi.org/10.1016/j.ijhydene.2016.05.244.Search in Google Scholar
[24] J. Joubert, V. Paul-Boncour, F. Cuevas, J. Zhang, M. Latroche. J. Alloys Compd. 862, 158163 (2021), https://doi.org/10.1016/j.jallcom.2020.158163.Search in Google Scholar
[25] E. Akiba, M. Okada. MRS Bull. 27, 699 (2002), https://doi.org/10.1557/mrs2002.225.Search in Google Scholar
[26] F. Marques, M. Balcerzak, F. Winkelmann, G. Zepon, M. Felderhoff. Energy Environ. Sci. 14, 5191 (2021), https://doi.org/10.1039/d1ee01543e.Search in Google Scholar
[27] E. M. Dematteis, M. B. Amdisen, T. Autrey, J. Barale, M. E. Bowden, C. E. Buckley, Y. Whan Cho, S. Deledda, M. Dornheim, P. de Jongh, J. B. Grinderslev, G. Gizer, V. Gulino, B. C. Hauback, M. Heere, T. Wook Heo, T. D. Humphries, T. R. Jensen, S. Y. Kang, Y. Lee, H. Li, S. Li, K. T. Møller, P. Ngene, S. Orimo, M. Paskevicius, M. Polanski, S. Takagi, L. Wan, B. C. Wood, M. Hirscher, M. Baricco. Progr. Energy 4, 032009 (2022), https://doi.org/10.1088/2516-1083/ac7499.Search in Google Scholar
[28] C. Milanese, S. Garroni, F. Gennari, A. Marini, T. Klassen, M. Dornheim, C. Pistidda. Metals 8, 567 (2018), https://doi.org/10.3390/met8080567.Search in Google Scholar
[29] L.H. Rude, T.K. Nielsen, D.B. Ravnsbæk, U. Bösenberg, M.B. Ley, B. Richter, L.M. Arnbjerg, M. Dornheim, Y. Filinchuk, F. Besenbacher, T.R. Jensen. Phys. Status Solidi A 208, 1754 (2011), https://doi.org/10.1002/pssa.201001214.Search in Google Scholar
[30] S. Garroni, A. Santoru, H. Cao, M. Dornheim, T. Klassen, C. Milanese, F. Gennari, C. Pistidda. Energies 11, 1027 (2018), https://doi.org/10.3390/en11051027.Search in Google Scholar
[31] M. V. Lototskyy, I. Tolja, L. Pickering, C. Sita, F. Barbir, V. Yartys. Progr. Nat. Sci. Mater. Intern. 27, 3 (2017), https://doi.org/10.1016/j.pnsc.2017.01.008.Search in Google Scholar
[32] M.V. Lototskyy, V.A. Yartys, B.G. Pollet, R.C. Bowman. Int. J. Hydrogen Energy 39, 5818 (2014), https://doi.org/10.1016/j.ijhydene.2014.01.158.Search in Google Scholar
[33] X. Chen, L. Wei, L. Deng, F. Yang, Z. Zhang. Appl. Mech. Mater. 448–453, 3027 (2013), https://doi.org/10.4028/www.scientific.net/amm.448-453.3027.Search in Google Scholar
[34] HyCARE, HyCARE Project – Hydrogen Carrier for Renewable Energy Storage , https://hycare-project.eu/.Search in Google Scholar
[35] J. Barale, E. M. Dematteis, G. Capurso, B. Neumann, S. Deledda, P. Rizzi, F. Cuevas, M. Baricco. Int. J. Hydrogen Energy 47, 29866 (2022), https://doi.org/10.1016/j.ijhydene.2022.06.295.Search in Google Scholar
[36] J. Barale, F. Nastro, D. Violi, P. Rizzi, C. Luetto, M. Baricco. Int. J. Hydrogen Energy 48, 34105 (2023), https://doi.org/10.1016/j.ijhydene.2023.05.155.Search in Google Scholar
[37] J. Barale, J. R. Ares, P. Rizzi, M. Baricco, J. F. Fernandez Rios. J. Alloys Compd. 947, 169497 (2023), https://doi.org/10.1016/j.jallcom.2023.169497.Search in Google Scholar
[38] A. Agostini, N. Belmonte, A. Masala, J. Hu, P. Rizzi, M. Fichtner, P. Moretto, C. Luetto, M. Sgroi, M. Baricco. Appl. Energy 215, 1 (2018), https://doi.org/10.1016/j.apenergy.2018.01.095.Search in Google Scholar
[39] M. Costamagna, J. Barale, C. Carbone, C. Luetto, A. Agostini, M. Baricco, P. Rizzi. Int. J. Hydrogen Energy 47, 10122 (2022), https://doi.org/10.1016/j.ijhydene.2022.01.098.Search in Google Scholar
[40] N. Belmonte, S. Staulo, C. Luetto, P. Rizzi, M. Baricco. Appl. Energy 215, 556 (2018), https://doi.org/10.1016/j.apenergy.2018.02.072.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Frontmatter
- In this issue
- Preface
- Avogadro Colloquia in Rome on “Vision and Opportunities of a Sustainable Hydrogen Society”
- Conference papers
- H2 in the energy transition
- Watching atoms at work during reactions
- Hydrogen production and conversion to chemicals: a zero-carbon puzzle?
- Rethinking chemical production with “green” hydrogen
- Hydrogen as an energy carrier: constraints and opportunities
- Shaping the future of green hydrogen: De Nora’s electrochemical technologies for fueling the energy transition
- In-situ and operando Grazing Incidence XAS: a novel set-up and its application to model Pd electrodes for alcohols oxidation
- Hydrogen storage and handling with hydrides
- Advanced polymer electrolyte membrane water electrolysis for power to gas applications
- Inkjet printed acrylate-urethane modified poly(3,4-ethylenedioxythiophene) flexible conductive films
- Cu(II) complexes using acylhydrazones or cyclen for biocidal antifouling coatings
- Randomly cross-linked amphiphilic copolymer networks of n-butyl acrylate and N,N-dimethylacrylamide: synthesis and characterization
- Roles of electrostatics and intermolecular electronic motions in the structural and spectroscopic features of hydrogen- and halogen-bonded systems
- The accurate assessment of the chemical speciation of complex systems through multi-technique approaches
Articles in the same Issue
- Frontmatter
- In this issue
- Preface
- Avogadro Colloquia in Rome on “Vision and Opportunities of a Sustainable Hydrogen Society”
- Conference papers
- H2 in the energy transition
- Watching atoms at work during reactions
- Hydrogen production and conversion to chemicals: a zero-carbon puzzle?
- Rethinking chemical production with “green” hydrogen
- Hydrogen as an energy carrier: constraints and opportunities
- Shaping the future of green hydrogen: De Nora’s electrochemical technologies for fueling the energy transition
- In-situ and operando Grazing Incidence XAS: a novel set-up and its application to model Pd electrodes for alcohols oxidation
- Hydrogen storage and handling with hydrides
- Advanced polymer electrolyte membrane water electrolysis for power to gas applications
- Inkjet printed acrylate-urethane modified poly(3,4-ethylenedioxythiophene) flexible conductive films
- Cu(II) complexes using acylhydrazones or cyclen for biocidal antifouling coatings
- Randomly cross-linked amphiphilic copolymer networks of n-butyl acrylate and N,N-dimethylacrylamide: synthesis and characterization
- Roles of electrostatics and intermolecular electronic motions in the structural and spectroscopic features of hydrogen- and halogen-bonded systems
- The accurate assessment of the chemical speciation of complex systems through multi-technique approaches

