Abstract
Based on field studies of the snow cover and systematization and analysis of scientific data and technical literature data, the distributions of fluorine, sodium, and lithium, as elements included in the raw materials used for aluminum production, in the snow cover in areas proximal to Siberian aluminum smelters were considered. The results showed that the changes in concentrations of fluorine, sodium, and lithium in the snow cover near various plants have the same dispersion pattern, which can be described by an exponential relationship. Exponential relationships of diminishing concentration with distance from the emission source had high correlation coefficients. From the examples established by these relationships, an assumption was made that the behavior of these aerosols in the atmosphere is determined by the general physical and chemical properties, irrespective of the technologies and natural climatic regions of the plant locations. It is suggested that deposition of aerosols from industrial aluminium production can be achieved at a minimum distance from the plants or within the plant area through particle enlargement by various technological methods in aluminium production or by changing the atmospheric scattering capacity.
Introduction
Snow cover is an effective accumulator of gaseous substances and aerosols derived from the atmospheric air [1], [2], [3], [4]. Thus, local industrial emissions of industrial centers play a significant role in the formation of the chemical composition of the snow cover. During the cold period of the year, some emissions enter the snow cover by wet deposition with snow, dry aerosol deposition, gas exchange in the atmosphere–snow cover system, and gas exchange in the soil–snow cover system. The chemical composition of the snow cover forms under the influence of physical–chemical processes occurring in the sub-cloud layer and the underlying layer, in the snow cover, in the contact zone with the underlying surface, etc. In the future, it will be possible to reduce fluoride emissions through the use of lithium additives.
This paper considers the peculiarities of distributions of such elements as fluorine, sodium, and lithium in snow cover in different regions of Siberia. In Siberia, enterprises for primary aluminum production are located in the following cities: Krasnoyarsk (Krasnoyarsk Aluminum Smelter), Bratsk, Irkutsk region (Bratsk Aluminum Smelter), Shelekhov, Irkutsk region (Irkutsk Aluminum Smelter), and Sayanogorsk, Republic of Khakassia (Sayanogorsk Aluminum Smelter). The plants use “wet” gas purification of electrolytic gases (soda-bicarbonate purification method) and “dry” gas purification based on adsorption of gaseous hydrogen fluoride by aluminum oxide (alumina).
The cities under consideration, that is, Krasnoyarsk, Bratsk, and Shelekhov (as well as Irkutsk, a suburb), have many industrial enterprises that are sources of air pollution. For example, in addition to the primary aluminum production at the Bratsk Aluminum Smelter, industrial emissions in Bratsk occur through ferroalloy production, cellulose production at the timber processing complex, power plants, and motor transport. A major problem associated with emission reduction at the Irkutsk Aluminum Smelter in Shelekhov is that the town is located within about 80 km of Lake Baikal.
We focused to study fluorine, sodium, and lithium content in the snow samples because they are marker elements for source identification. These elements are the compounds in the usage raw materials for aluminum production. Moreover, these elements are emitted into the air from the aluminum smelters.
The technology of aluminum production by the electrolytic method [5] includes dissolution of alumina Al2O3 in cryolite Na3AlF6 at the temperature of 1233 K. Alumina, fluoride salts, anode mass, soda ash, and caustic soda are consumed in the aluminum production. The total consumption of sodium in this process is 15–25 kg Na/t Al.
The consumption of sodium is needed to purify the electrolysis gases with the soda-bicarbonate method. For example, the production of 1,000,000 ton/year of aluminum needs the 15,000–25 ton/year or 41–68 ton/day of sodium consumption.
Some elements that are part of the feedstock used for production of metallic aluminum by the electrolytic method are also included in the composition of the solid and gaseous emissions from primary aluminum production. Lithium fluoride, lithium carbonate, or lithium-containing alumina can be used [6, 7] as an additive to cryolite-alumina electrolyte to reduce the melting temperature of the melt and also reduce fluoride emissions. However, the specific features of the aluminum production technology with additives are a commercial secret.
Because the technological cycle is not closed, technological losses occur, which include compounds of fluorine, sodium, and lithium. It should be noted that the presence of lithium in the emissions and, subsequently, in the snow cover can also be attributable to the presence of microquantities of lithium in the initial raw materials.
Lately, cities such as Bratsk and Shelekhov were included in the Priority List of Russian cities with the highest levels of atmospheric air pollution in 2014 [8] due to the presence of solid and gaseous fluorides in the atmosphere from primary aluminum production.
This study aimed to establish general trends of the concentrations of fluorine, sodium, and lithium in the snow cover in the emission areas of aluminum smelters in Siberia.
Methods
The study was conducted in the dispersion areas of aluminum smelters located in Krasnoyarsk, Bratsk (Irkutsk region), Shelekhov (Irkutsk region), and Sayanogorsk (Republic of Khakassia), all within Siberia. The plants are located in regions with various geographical and climatic conditions, have different emission rates, and either different or common methods of emission purification (Table 1). Samples of snow were collected in the direction of main air mass transport. In the territory of Siberia, western transport of air masses is common. Air masses are directed from west to east toward the Baikal region. However, a detailed consideration of the long-range transport is beyond the scope of this paper.
Characteristics of Siberian aluminum smelters: primary aluminum production, technologies, and specific fluoride emissions.
| Aluminum smelter | Launch year | Al production, Mt/year | Main technology | Main gas cleaning | Specific fluoride emissions, kg/t(Al) [5] |
|---|---|---|---|---|---|
| Bratsk | 1966 | 1007 (2015) | Søderberg | Wet | 2.73 |
| Irkutsk (Shelekhov) | 1962 | 412.6 (2015) | Søderberg | Wet, dry | 3.27 |
| Sayanogorsk | 1985 | 514 (2014) | Anodes | dry | 0.35 |
| Krasnoyarsk | 1964 | 1005 (2014) | Søderberg | Wet | 2.48 |
Samples of snow were collected in the vicinities of Bratsk, Shelekhov, and Krasnoyarsk, in the areas affected by the Bratsk, Irkutsk, and Krasnoyarsk aluminum smelters, respectively. Sampling and sample preparation were performed in accordance with the Atmospheric Pollution Control Guide [9].
Snow samples were collected in the north-eastern direction from Krasnoyarsk aluminum smelter in 2016. The sample preparation was made in laboratories of Tomsk Polytechnic University. Sample preparation included melting snow cover samples in plastic containers at room temperature. Fluorine content in the samples of snowmelt water was determined by potentiometric technique using the device Anion 4100 with fluoride-selective electrode. The low detection limit of fluorine amounts to 0.05 mg/dm3. The samples were also measured with ICP-MS to identify sodium and lithium content. The detection limit of ICP-MS for sodium and lithium quantification in snowmelt water was 0.01 and 0.001 mg/dm3, respectively.
Snow samples were taken in the vicinities of Bratsk and Shelekhov aluminum smelters in 2014 and 2015 by researchers from Irkutsk State Polytechnic University. Snow water was filtered through cellulose acetate membrane filters with a pore diameter of 0.2 µm. To determine sodium and lithium contents, the prepared samples were examined on a quadrupole ICP-MS (Agilent 7500). Determination of fluorine content in the liquid phase was carried out by capillary electrophoresis using a capillary electrophoresis system (Kapel 105M).
The results obtained from our field studies of snow cover were compared with data of other authors [10–12].
Results and discussion
Data on the distribution of some components of aluminum plant emissions in the snow cover have been published [10–16], but no reports have generalized such information. We performed a comparison of the elemental composition of snow cover in the areas affected by industrial emissions from Siberian aluminum smelters. Analyses of the data from in situ measurements and data from the scientific and technical literature allowed us to conclude that, in the areas of aluminum plant emissions, changes in sodium concentration in the snow cover with distance from the source of emissions are described by exponential curves with high correlation coefficients (Fig. 1).

Variation of sodium concentration in the snow cover with distance from the industrial source.
The determined correlation shows the equal sources of sodium aerosol emissions into the air. They are aluminum smelters, which are located in Bratsk, Krasnoyarsk and Shelekhov (suburb of Irkutsk).
Sodium compounds may be present in the snow cover of inland industrial cities [10, 17]. In other cities, the presence of sodium is may be attributable to the use of deicing agents [18], [19], [20]. Sodium compounds in snow cover may also be attributable to natural causes. For example, the results of studies of Antarctic snow cover [21, 22] suggest that sodium compounds in the snow have a marine (oceanic) origin, which is typical of coastal regions [23]. All studied aluminum smelters are located far from the seas and oceans. However, we considered the sources of precipitations in Bratsk using data of isotopic method and back scarred trajectories [24]. In Bratsk, precipitation associated with western transport of air masses from the Atlantic, with arrival of moisture from the Arctic sector, from continental sources, and from the water area of the Sea of Okhotsk has been noted [24]. However, the exact proportion of marine-origin sodium in Bratsk was not discussed in this paper [24] we did not consider the portion of marine sodium in Bratsk. Nonetheless, in the salts daily sodium consumption amounts 41–68 ton/year as it was mentioned above.
Soda liquor is used for particulate catchment and purify the electrolysis fluorine content gases in aluminum production and as a result sodium is loss (Fig. 2). Sodium in snow cover as other ions influence the snow cover pH (Fig. 2), as was established previously [25]. Water pH values from snow cover samples collected in Bratsk reach 7.80–7.94 [26], whereas the pH of normal atmospheric precipitation in background areas is 5.5–6.5 [27]. It has been reported [12, 25] that pH values of 7.65–7.94 occur in areas affected by emissions from cellulose production facilities, where sodium hydroxide and sodium carbonate are used as inputs.
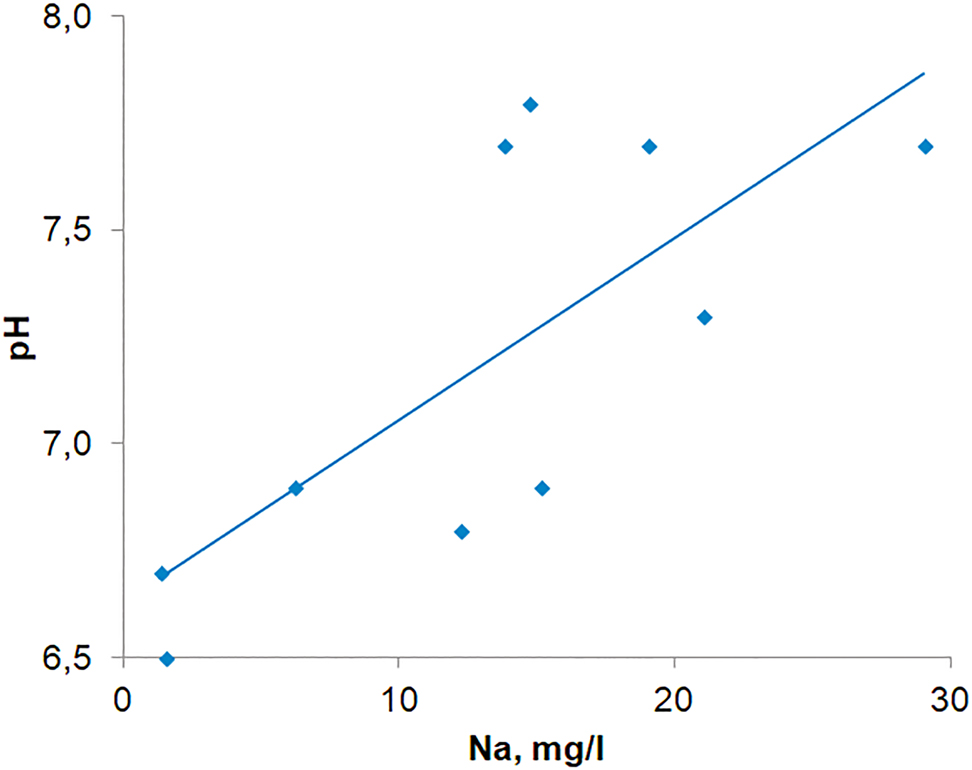
Correlation of pH and sodium content in snow cover at Bratsk.
In those areas of Bratsk, where there may be a predominant influence of aluminum plant emissions, the pH value range of snow cover is 6.0–7.5 [12]. It is possible that these snow-cover pH values are attributable to the combined presence in the atmosphere of sulfur, nitrogen and carbon oxides, and sodium compounds, which are used in the “wet” gas emissions purification technology at the Bratsk aluminum smelter. It should also be noted that emissions of sodium compounds probably prevent acidification of atmospheric precipitation and environmental components, which is typically associated with emissions of sulfur and nitrogen and carbon oxides formed during coal combustion at thermal power plants and during combustion of hydrocarbon fuels (gasoline, diesel, etc.) during transport [12].
Distribution results of fluorine in the snow cover in Krasnoyarsk obtained from field studies were compared with data from the available literature for Sayanogorsk [13–16], Bratsk [11], and Shelekhov [10] (Fig. 3). For all cases considered, there was an exponential relationship between fluorine concentration and distance from the emission source. It was assumed that the dispersion of emissions and the transfer distance are determined by the aerosol form of fluorides in the atmosphere and the general physical and chemical nature of the aluminum production aerosols [28], rather than the capacity of fluoride emissions (Table 1), which allows us to propose general approaches for emissions reduction.

Variation of fluorine concentration in snow cover with distance from an industrial source (fluorine concentration in Sayanogorsk, Shelekhov, Krasnoyarsk, in mg/L, and in Bratsk in kg/km2 month).
By examining the distribution of lithium, it was found that changes in lithium concentration in the snow cover of Krasnoyarsk and Bratsk with distance from the emission source were also described by exponential relationships with high correlation coefficients (Fig. 4), as was established previously for changes in fluorine and sodium concentrations. Consideration of lithium distribution is important, first, because of its use as a marker of components of aluminum smelter emissions and secondly in terms of its impact on biota and other environmental components.

Variation of lithium concentration in snow cover with distance from the industrial source.
The results showed that the distributions of fluorine, sodium, and lithium in the snow cover in the area of emissions of the aluminum smelters in Siberia (Fig. 1, 3 and 4) had the same characteristic of change, an exponential dependence with high correlation coefficient (R > 0.9). The fluctuations in concentrations of fluorine, sodium, and lithium (Fig. 1, 3 and 4) in the snow cover before active snow melting in different years or in different study areas are related to both technological factors and weather conditions, such as albedo, amount of precipitation, and the number of days with thawing.
Using the behaviors of fluorine, sodium, and lithium as examples, it was demonstrated that more efficient removal of aerosol particles requires the development and adoption of practical measures that can be applied both within industrial production and also off-site.
One possible solution within the scope of plant technology could be reducing the height of organized emission sources (pipes), which reduces the transport range of the emissions. In addition, the technological scheme can be modified to enlarge particles of emitted aerosols and thus deposit them at a minimal distance from the source (e.g., in the sanitary protection zone or within the territory of the plant).
Reduction of fluoride emissions, in theory, is possible with the application of lithium salts as an additive to the electrolyte [6, 7, 29, 30]. We assume that lithium is already present in the raw material, which is evidenced by the established exponential relationship (Fig. 3) of diminished lithium concentration with distance from the emission source. The use of lithium salts as an additive to the electrolyte to reduce fluoride emissions is especially relevant for the territories under consideration because brines on the Siberian Platform contain lithium in sufficient quantities to be used as a source of lithium. Technologies for extraction of lithium from brines and production of salts for aluminum production already exist [30].
As an additional measure, approaches such as landscaping and improving the industrial territory by creating hedge rows or high-rise non-residential production areas on the path of transfer of plant emissions and at an acceptable distance from them can lead to changes in the circulation of air masses [31] and, consequently, to a reduction in the range of emissions transfer.
The environmental problems of aluminum smelters in Siberia associated with the impact of gas and dust emissions are simultaneously caused by many factors: limitations of improvement of emission reduction technologies, low dispersion capacity of the atmosphere, low mineralization of Siberian natural waters, and low atmospheric temperatures during the period of stable snow cover.
Conclusions
Based on the analysis of our results from field studies of snow cover and literature data, the distributions of fluorine, sodium, and lithium in the snow cover in the emission zones of the Bratsk, Irkutsk, Sayanogorsk, and Krasnoyarsk aluminum smelters were considered. The results showed that the changes of fluorine, sodium, and lithium concentrations in the snow cover of all considered regions have the same character, an exponential relationship of decreasing concentration with distance from the emission source with a high correlation coefficient. Based on the established dependencies, it is suggested that the behavior of these aerosols in the atmosphere is determined by their similar physical and chemical properties, irrespective of the technologies and the climatic region of Siberia. As a possible solution to the problem of emissions reduction, a proposal to precipitate industrial aerosols of aluminum production at a minimal distance from the plant by reducing the height of organized emission sources, enlarging the particle sizes of emitted aerosols, and changing the atmospheric dispersion capacity was developed. It was also proposed that reductions of fluorine compound emissions could be achieved through the application of lithium salt additives to the cryolite-alumina melt.
Article note:
Snow cover, atmospheric precipitation, aerosols: chemistry and climate: reports of the 3rd Baikal international scientific conference endorsed by IUPAC (March 23–27, 2020).
Acknowledgments
The authors are grateful to Prof. A. N. Baranov (Irkutsk National Research Technical University) and to Prof. E. G. Yazikov and PhD Osipova N. A. (National Research Tomsk Polytechnic University). The experimental procedures for Krasnoyarsk aluminum plant were carried out at Tomsk Polytechnic University within the framework of Tomsk Polytechnic University Competitiveness Enhancement Program Grant in the Group of Top Level World Research and Academic Institutions.
References
[1] V. N. Vasilenko, I. M. Nazarov, S. D. Fridman. Snow pollution monitoring, p. 182, Hydrometeoizdat, Leningrad (1985). (in Russian).Search in Google Scholar
[2] V. P. Shevchenko, S. N. Vorobyev, I. V. Krickov, A. G. Boev, A. G. Lim, A. N. Novigatsky, D. P. Starodymova, O. S. Pokrovsky. Atmosphere 11, 1184 (2020), https://doi.org/10.3390/atmos11111184.Search in Google Scholar
[3] D. Vlasov, J. Vasil’chuk, N. Kosheleva, N. Kasimov. Atmosphere 11, 907 (2020), https://doi.org/10.3390/atmos11090907.Search in Google Scholar
[4] M. Gaberšek, M. Gosar. Environ. Geochem. Health 43, 2583 (2021), https://doi.org/10.1007/s10653-020-00609-z.Search in Google Scholar
[5] B. I. Zelberg, L. V. Ragozin, A. G. Barantsev, O. I. Yasevich, V. G. Grigoryev, A. N. Baranov. Steel Worker’s Guide. Aluminum and Aluminum Alloys Production, p. 764, IRSTU, Itkutsk (2015). (in Russian).Search in Google Scholar
[6] N. I. Ianchenko, O. G. Larionova. Tsvetnye Met. 9–10, 60 (2001).Search in Google Scholar
[7] N. P. Kotsupalo, A. D. Ryabtsev, V. V. Boldyrev. Chem. Technol. 1, 36 (2011). (in Russian).Search in Google Scholar
[8] The State of Air Pollution in Cities on the Territory of Russia for 2014. Yearbook, p. 288. Voeikov Main Geophysical Observatory, Saint Petersburg (2015). (in Russian).Search in Google Scholar
[9] RD 52.04.186-89 Atmospheric Pollution Control Guide, p. 531. USSR State Committee for Hydrometeorology, USSR Ministry of Health, Moscow (1991). (in Russian).Search in Google Scholar
[10] L. M. Filimonova, A. V. Parshin, V. A. Bychinskii. Russ. Meteorol. Hydrol. 40, 691 (2015), https://doi.org/10.3103/s1068373915100076.Search in Google Scholar
[11] Pollution of Soils of the Irkutsk Region with Toxicants of Industrial Origin: Yearbook (2000–2007), p. 101. Irkutsk Department for Hydrometeorology and Environmental Monitoring, Irkutsk (2008). (in Russian).Search in Google Scholar
[12] N. I. Ianchenko, A. N. Baranov, V. A. Ershov, E. P. Chebykin, E. N. Vodneva, E. V. Timkina. Syst. Methods Technol. 4, 164 (2013). (in Russian).Search in Google Scholar
[13] N. D. Davydova. Adv. Mod. Nat. Sci. 5, 186 (2014). (in Russian).Search in Google Scholar
[14] N. D. Davydova, T. I. Znamenskaya, D. A. Lopatkin. Contemp. Probl. Ecol. 6, 228 (2013), https://doi.org/10.1134/s1995425513020029.Search in Google Scholar
[15] L. A. Nikolaev, V. F. Turchaninova. Nat. Sci. Human. 6, 58 (2010). (in Russian).Search in Google Scholar
[16] N. D. Davydova, T. I. Znamenskaya. Geogr. Nat. Resour. 1, 55 (2016). (in Russian).Search in Google Scholar
[17] M. I. Vasilevich, R. S. Vasilevich, D. N. Gabov, B. M. Kondratenok. Geoecology. Engineering Geol. Hydrogeol. Geocryol. 6, 94 (2019). https://doi.org/10.31857/s0869-78092019694-105 (in Russian).Search in Google Scholar
[18] V. R. Kelly, G. M. Lovett, K. C. Weathers, S. E. Findlay, D. L. Strayer, D. J. Burns, G. E. Likens. Environ. Sci. Technol. 42, 410 (2007).10.1021/es071391lSearch in Google Scholar PubMed
[19] V. R. Kelly, S. E. Findlay, S. K. Hamilton, G. M. Lovett, K. C. Weathers. Water Air Soil Pollut. 230, 13 (2019), https://doi.org/10.1007/s11270-018-4060-2.Search in Google Scholar
[20] K. R. Kolesara, C. N. Mattsona, P. K. Peterson, N. W. Maya, R. K. Prendergast, K. A. Pratt. Atmos. Environ. 177, 195 (2018), https://doi.org/10.1016/j.atmosenv.2018.01.008.Search in Google Scholar
[21] M. M. Frey, S. J. Norris, I. M. Brooks, P. S. Anderson, K. Nishimura, X. Yang, A. E. Jones, M. G. Nerentorp Mastromonaco, D. H. Jones, E. W. Wolff. Atmos. Chem. Phys. 20, 2549 (2019).10.5194/acp-20-2549-2020Search in Google Scholar
[22] M. Legrand, S. Preunkert, E. Wolff, R. Weller, B. Jourdain, D. Wagenbach. Atmos. Chem. Phys. 17, 14039 (2017), https://doi.org/10.5194/acp-17-14039-2017.Search in Google Scholar
[23] E. I. Kotova, V. B. Korobov, V. P. Shevchenko. Mod. Probl. Sci. Educ. 6, 631 (2012). (in Russian).Search in Google Scholar
[24] Y. N. Chizhova, N. I. Ianchenko, N. A. Budantseva. Arctic Antarct. 2, 1 (2016). (in Russian).Search in Google Scholar
[25] N. I. Ianchenko. Chem. Chem. Technol. 325, 23 (2014). (in Russian).Search in Google Scholar
[26] O. V. Ignatenko, M. V. Senchenko, N. A. Meshcherova. Syst. Methods Technol. 3, 138 (2012). (in Russian).Search in Google Scholar
[27] P. F. Svistov, A. I. Polishchuk, N. A. Pershina, T. M. Pavlova. Annual Data on the Chemical Composition of Atmospheric Precipitation for 2006–2010 (Data Review), p. 100, Voeikov Main Geophysical Observatory, Saint Petersburg (2013). (in Russian).Search in Google Scholar
[28] D. Cheng. J. Atmos. Chem. 75, 1 (2018), https://doi.org/10.1007/s10874-017-9359-7.Search in Google Scholar
[29] O. S. Ignatyev, O. A. Bragazina. Russ. J. Non-Ferrous Metals 2, 13 (1997). (in Russian).Search in Google Scholar
[30] A. N. Baranov, A. G. Vakhromeev, N. I. Ianchenko. Obtaining Lithium Products from Siberian Brines for the Greening of Aluminum Production, p. 125, IRSTU, Itkutsk (2004). (in Russian).Search in Google Scholar
[31] K. Y. Kondratyev. Opt. Atmos. Ocean 15, 301 (2002). (in Russian).Search in Google Scholar
© 2021 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
Articles in the same Issue
- Frontmatter
- In this issue
- Preface
- Foreword to the special issue dedicated to the 3rd Baikal International IUPAC Conference on chemistry of snow cover, atmospheric precipitation, aerosols and climate
- Conference papers
- Chemical composition of atmospheric particulate matter in the winter season as indicator of environment quality within urban areas
- Snow samples from settlements of the Murmansk region-genotoxic effects in Drosophila germ cells
- Comparative assessment of fluorine, sodium, and lithium distributions in snow cover in Siberia
- Elemental composition of dust aerosols near cement plants based on the study of samples of the solid phase of the snow cover
- Results of AAS-measurements of atmospheric deposition of copper and lead in the snow cover of Almaty agglomeration
- Climatology of transport and deposition of atmospheric substances of different intensity on the southern Primorye territory by using the meteorological reanalysis data and observations at EANET monitoring station
- Composition of rainfall in the coastal zone of the Kaliningrad region of the Russian Federation (based on data from 2019)
- Chemical and algological composition of the snow cover at the mouth of the Onega river (White Sea basin)
- Assessment of the quality of atmospheric air in woodlands of natural areas based on the intensity analysis of the process of dry deposition of impurities on an artificial underlying surface
- Methodological aspects of snow cover sampling for chemical analysis
- Organic carbon in atmospheric precipitation in the urbanized territory of the South of Western Siberia, Russia
- Monitoring-based assessment of environmental pollution in regions of the Russian Federation
- Estimated acceptable critical load values for the ecosystem at the Russian Far East using EANET monitoring data
- IUPAC Technical Report
- Seabed mining and blue growth: exploring the potential of marine mineral deposits as a sustainable source of rare earth elements (MaREEs) (IUPAC Technical Report)
Articles in the same Issue
- Frontmatter
- In this issue
- Preface
- Foreword to the special issue dedicated to the 3rd Baikal International IUPAC Conference on chemistry of snow cover, atmospheric precipitation, aerosols and climate
- Conference papers
- Chemical composition of atmospheric particulate matter in the winter season as indicator of environment quality within urban areas
- Snow samples from settlements of the Murmansk region-genotoxic effects in Drosophila germ cells
- Comparative assessment of fluorine, sodium, and lithium distributions in snow cover in Siberia
- Elemental composition of dust aerosols near cement plants based on the study of samples of the solid phase of the snow cover
- Results of AAS-measurements of atmospheric deposition of copper and lead in the snow cover of Almaty agglomeration
- Climatology of transport and deposition of atmospheric substances of different intensity on the southern Primorye territory by using the meteorological reanalysis data and observations at EANET monitoring station
- Composition of rainfall in the coastal zone of the Kaliningrad region of the Russian Federation (based on data from 2019)
- Chemical and algological composition of the snow cover at the mouth of the Onega river (White Sea basin)
- Assessment of the quality of atmospheric air in woodlands of natural areas based on the intensity analysis of the process of dry deposition of impurities on an artificial underlying surface
- Methodological aspects of snow cover sampling for chemical analysis
- Organic carbon in atmospheric precipitation in the urbanized territory of the South of Western Siberia, Russia
- Monitoring-based assessment of environmental pollution in regions of the Russian Federation
- Estimated acceptable critical load values for the ecosystem at the Russian Far East using EANET monitoring data
- IUPAC Technical Report
- Seabed mining and blue growth: exploring the potential of marine mineral deposits as a sustainable source of rare earth elements (MaREEs) (IUPAC Technical Report)

