Pd(0)-catalyzed amination in the synthesis of chiral derivatives of BINAM and their evaluation as fluorescent enantioselective detectors
-
Alexei D. Averin
, Olga K. Grigorova
Abstract
A mini-review covers recent successes in the synthesis of (S)-1,1′-binaphthyl-2,2′-diamine (BINAM) using Pd(0)-catalyzed amination reactions. As a result, versatile compounds with C2-chiral backbone were synthesized, among them are derivatives bearing additional chiral amino and fluorophore groups like dansyl amide, 7-methoxycoumarin, 6-aminoquinoline, different macrocyclic compounds with oxadiamine and polyamine linkers were obtained as well. BINAM derivatives of various structures were evaluated as fluorescent enantioselective detectors for a series of model amino alcohols. Many of them were shown to be efficient in sensing certain enantiomers of the amino alcohols by selective changes in the emission in the presence of these analytes. Small changes in the structure of the BINAM derivatives lead to serious difference in the recognition ability of the compounds under investigation.
Introduction
The necessity for the development of reliable and express methods for the analysis of optical isomers of the organic compounds stems from the boosting synthesis of medicaments and agrochemicals in enantiomerically pure form, performing enantioselective catalytic reactions which increasingly demand various chiral catalysts, wide-scale monitoring of environment in which biologically relevant, i.e., optically pure compounds play a crucial role. Diverse analytical tools involving spectra of fluorescence are advantageous in sensitivity, selectivity and, in line with the miniaturization of spectrofluorometers, such analyses become more and more handy.
The investigation of the spectra of fluorescence of optically active compounds and their dependence on the presence of individual enantiomers of analytes aimed at the construction of fluorescent enantioselective detectors begins from late 1970s. In 1978 the fluorescence quenching of 1,1′-binaphthalene in the presence of N,N-dimethyl-α-phenylethylamine was reported [1], later the fluorescence of a more complex compound, immobilized BINOLphosphoric acid monoamide (BINOL = 1,1′-bi-2,2′-naphthol), was studied using the same amine [2]. Beginning from 1992 systematic investigations of the influence of chiral amines on the optically pure BINOL [3] laid the basis for the application of this most demanded molecule in the enantiomers recognition using fluorescence spectroscopy. Up to present several reviews dealing with this problem were published [4], [5], [6], [7], they encompass various types of fluorescent detectors, in some works the emphasis is made on the application of macrocyclic compounds [8], [9], other describe the role of synthetic and supramolecular oligomers, polymers, nanoparticles and organometallic compounds in fluorescent sensing of various chiral objects [9], [10]. It should be noted that natural amino acids, their N- and O-derivatives are the preferred analytes almost in all publications.
The detection of individual enantiomers of optically active compounds using fluorescence spectroscopy is based on the systematic changes in the emission spectra (quenching or enhancement) of a chiral detector upon the formation of a molecular complex with a particular enantiomer of the analyte. Two opposite enantiomers form two diastereomers with the detector which is also taken as an individual optical isomer, and as physical properties of two diastereomers differ to much or less extent, the same is true for their spectra of fluorescence. In this case, another enantiomer either does not cause considerable changes in the fluorescence of the detector due to a significantly lower stability constant of the molecular complex, or the complexation is accompanied by a change in the fluorescence with a sign opposite to that caused by another enantiomer. On the other hand, while the alteration of the emission by opposite enantiomers differing in the intensity but with the same sign is not suitable for qualitative observations, it can be employed for quantification, i.d. for the control of optical purity of the analyte, provided the changes are substantial and well distinguishable.
The fact that the majority of studies are dedicated to the use of BINOL derivatives is due to the inherent chirality and fluorescent properties of this unique molecule. As a result of the presence of two close hydroxyl groups in its structure it easily forms molecular complexes with various polar organic compounds. To enhance the selectivity, various modifications of BINOL were elaborated. The introduction of the substituents at positions 3 and 3′ resulted in the creation of chemosensors for N-Boc-alanine and N-Boc-phenylalanine exploiting emission quenching in the presence of one enantiomer [11], [12]. The combination of two BINOL fragments with an amine linker via their oxygen atoms allowed the formation of efficient sensors for mandelic acid, in this case the enhancement of the fluorescence was used for detection [13], [14]. The same analyte can be analyzed by the compound obtained by the introduction of two BINOL groups and two chiral moieties of 1,2-diaminocyclohexane in a macrocycle [15], [16]. Various substituents were tested in 3 and 3′-positions of BINOL, like amines [17], [18], diamines [19], and amino alcohols [20], [21], the resulting compounds were used as chemosensors for N-Boc derivatives of amino acids and α-oxycarboxylic acids. BINOLs modified with chiral ureas and thioureas at the oxygen atoms were employed for the recognition of enantiomers of phenylglycine, tryptophan, mandelic and malic acids []. Heterocycles like imidazoline were used in the synthesis of linear [25] and macrocyclic [26] BINOL derivatives, a complex macrocycle with BINOL, two triazine and two chiral amino acid linkers was useful to increase stereodiscrimination in the formation of complexes with various enantiomers [26].
In some cases the inherent fluorescence of BINOL is thought to be insufficient, thus additional fluorophores are introduced in the molecules to take advantage of the fluorescence at longer wavelengths, e.g., the derivative with two BODIPY groups at 3 and 3′ positions of BINOL was described [27]. To analyze chiral amines and amino alcohols, dimeric, oligomeric, or dendrimeric BINOL derivatives are employed which contain substituents at positions 3,3′ or 6,6′ [28], [29], [30], [31], [32]. An unusual oligoBINOL macrocycle was obtained by self-assembling in which the moieties are linked through rhenium complexes [33]. Different ways exist to couple BINOL with various macrocycles. Thus, to detect anions of the tartaric acid, bis(triazacyclononanyl) derivative of BINOL was tested [34], the side arm of the calix[4]crown ether was modified with BINOL [35]. The enantiomeric recognition of optically active amines and amino acids was carried out using a crown ether derivative of BINOL containing two additional chiral endocyclic carbon centers [36]. The BINOL-containing derivative of the crown ether with an additional dinitrophenyl chromophore group [37], azacrown ether bearing endocyclic BINOL fragment and exocyclic anthracene fluorophore [38] are examples of the peculiar combinations of various receptor and sensing units. The latter can be used not only for the recognition of chiral alkylammonium salts but also for detecting Hg(II), Cu(II) and Zn(II) cations. It was possible even to combine two BINOL and two coplanar porphyrin macrocycles in one polymacrocyclic compound [39], [40]. Recently described BINOL-containing macrocycle can be used for amino acids detection [41], another macrocycle with the same chiral moiety is able to assess enantiomeric excess of chiral carboxylates [42]. An interesting macrocyclic chemosensor combining BINOL and deoxycholic acid units gives a selective response in the presence of Hg(II), while the complex formed allows the recognition of amino acids enantiomers [43].
It is worth noting that though the nitrogen-containing analogue of BINOL, i.e., 2,2′-diamino-1,1′-binapthalene (BINAM) was studied as a potential fluorescent detector quite a long ago and showed its efficiency in the recognition of α-phenylethylamine enantiomers [44], it was not yet developed further. Only scarce information about the application of this compound and its derivatives can be found in literature. Thus, the attempts to recognize tryptophan enantiomers using free BINAM were undertaken [45], BINAM was combined with two residues of chiral thiourea to detect mandelic acid [46]. Chiral polymers were synthesized on the basis of BINOL and BINAM for the detection of phenylalanine by the enhancement of emission intensity [47]. However, further systematic investigations were not carried out in this direction. Taking these facts into consideration we decided to develop the synthesis of BINAM-containing detectors using a powerful synthetic tool, i.e., palladium-catalyzed amination reactions [48], [49]. We acquired good experience in the synthesis of macrocyclic compounds with the help of this catalytic reaction [50], [51], among molecules synthesized by us are those containing endocyclic fluorophore moieties like diaminonaphthalene [52], 2,2′-bipyridine [53], anthracene [54], anthraquinone [55], quinoline [56], 1,10-phenanthroline [57]. Also we managed to obtain planar-chiral macrocycles comprising 1,5-diaminoanthraquinone moiety which possesses fluorescent properties [58], [59]. In this paper our progress in the synthesis of BINAM-based compounds of various architecture is described.
(S)-BINAM substituted with chiral and fluorophore groups
First (S)-BINAM (1) was modified with two 3-bromophenyl substituents using Pd(0)-catalyzed amination reaction and the resulting compound 2 was further used in various transformations (Scheme 1) [60]. Also, under the conditions of the palladium catalysis, by the action of optically pure amines 3–5 it was transformed into derivatives 7–9 each possessing two chiral substituents. This was performed to enhance possible stereodiscrimination of the resulting products due to the presence of additional chiral groups able of forming complexes with polar molecules. The reaction of 2 with 2-methoxyethylamine (6) afforded compound 10 which possesses two short non-chiral N,O-podands. All reactions gave high yields of the corresponding products of diamination 7–10 (79–90%).

Pd(0)-catalyzed modification of BINAM with N- and O-containing groups including chiral ones.
The BINAM derivatives 7, 8, 10 were further transformed into more complex compounds by introducing additional fluorophore groups. It was done in view of the improvement of the fluorescent properties of the detectors by red-shifting the emission maxima, also this could change the coordination properties of these compounds as all tested fluorophore groups (dansylamide, 7-methoxycoumarin and quinoline) possess additional donor sites able to participate in the molecular complex formation. As for the bis(tetrahydrofurfurylamino) substituted compound 7, it was modified with two dansylamide and 7-methoxycoumarin groups according to simple nucleophilic substitution reactions affording corresponding derivatives 14 and 15 in almost quantitative yields (Scheme 2), while to obtain diquinolinyl derivative 16, 4 equivalents of 6-bromoquinoline were needed alongside with high catalyst loadings as the arylation of the secondary amino group as usually is not a simple task [61].

Modification of the compound 7 with fluorophore groups.
The same reactions with di(N-Boc-pyrrolidinyl) compound 8 produced didansylated derivative 17 in almost quantitative yield, its decoration with two 7-methoxycoumarin groups was also efficient, however, catalytic transformation to introduce quinoline fluorophore carried out with 6-bromoquinoline was somewhat less efficient (Scheme 3) [60]. Probably, this was due to difficulties in the separation of the target product 19 from resting bromoquinolines taken in 2-fold excess.

Modification of the compound 8 with fluorophore groups.
The attempts to fulfill the same reactions in the case of the bis(benzyloxycyclopentylamino) derivative 9 were totally unsuccessful due to the steric hindrances at the secondary amino groups. Even simple nucleophilic substitution with the most active dansyl chloride 11 did not proceed at all. Thus this compound should be used as a possible detector as it is, using the inherent fluorescence of the BINAM unit.
The modifications of the bis(2-methoxyethylamino) BINAM derivative 10 were carried out using dansyl chloride 11, 7-methoxy- and 6,7-dimethoxy-4-bromomethylcoumarins 12 and 20, 6-bromo- and 3-bromoquinolines 13 and 21 (Scheme 4). Except for the last reaction, in all other syntheses the yields of the target products 22–25 were high (71–98%). Thus, a substantial variety of BINAM derivatives bearing different additional chiral and fluorophore groups was synthesized by the combination of the catalytic and non-catalytic approaches.

Modification of the compound 10 with fluorophore groups.
Macrocyclic derivatives of (S)-BINAM
The next approach to BINAM modification is the introduction of this fluorescent chiral unit into macrocyclic structures. For this purpose we carried out a series of Pd(0)-catalyzed macrocyclization reactions of N,N′-di(3-bromophenyl) substituted BINAM 2 with various oxadiamines 27–31 which gave corresponding chiral macrocyclic compounds 32–36 (Scheme 5) [62]. Their yields were dependent on the nature of the starting oxadiamines (chain length, number of oxygen atoms and methylene groups between N and O atoms), and in the best case (compound 32) exceeded 50%. Also cyclic dimers 37 and 38 were isolated in individual state as second products. The formation of the macrocycles with naphthalene spacers 40–44 was also successful, though the yields were lower and did not surpass 34%.

Synthesis of BINAM-containing macrocycles with phenylene and napththalene spacers.
Interesting macrocycles were obtained in the reactions of isomeric 1,8- and 1,5-dichloroanthraquinones with (S)-BINAM followed by the catalytic macrocyclization processes (Scheme 6) [63]. The resulting molecules 47–52 possess diaminoanthraquinone moieties which exhibit more intensive fluorescence in comparison with that of BINAM, but with the emission maximum blue-shifted. Compounds 47–49 and 50–52 differ by the reciprocal positions of aromatic and aliphatic parts of these sophisticated macrocycles, their yields proved to be seriously dependent on the nature of starting compound and ranged from 16 to 40%.

Synthesis of BINAM-containing macrocycles with anthraquinone spacers.
Macrocycles with phenylene and naphthalene spacers were modified with dansyl amide exocyclic fluorophore groups (Scheme 7). The reactions with dansyl chloride (11) were found to be crucially dependent on the structure of starting compounds. Unexpectedly the yields of the products 53–58 varied from very low 16% (product 54) up to 97-99% (compounds 53, 57). Probably, the reason is the difference in the nucleophilicity of the nitrogen atoms caused by the conformational features arising from the different structures of oxadiamine linkers in these molecules. The application of excess dansyl chloride or running the reactions at elevated temperature did not improve the results.

Introduction of exocyclic dansylamide fluorophores into BINAM-containing macrocycles.
The modifications of the macrocycles with another fluorophore group, i.e., 7-methoxycoumarin, was not a simple task, it demanded carrying out the process at 50 °C to ensure better consumption of the starting bromide 12, however, the isolation of the target products 61 and 62 in pure state was quite tedious and modest yields 47 and 28% well correspond to this fact (Scheme 8). In contrast, the catalytic heteroarylation reactions of the selected macrocycles 32, 36, 40, 44 with 6-bromoquinoline or 3-bromoquinoline turned to be generally more efficient, providing 36–73% yields of the corresponding diquinolinyl derivatives 63–68 (Scheme 9). As in the case with simpler BINAM derivatives described above, application of 4 equivalents of bromoquinolines was inevitable to ensure better conversion of the macrocycles into desirable derivatives.

Introduction of exocyclic 7-methoxycoumarin fluorophores into BINAM-containing macrocycles.

Introduction of exocyclic quinoline fluorophores into BINAM-containing macrocycles.
We also attempted the synthesis of BINAM-containing macrocycles with tri- and tetraamino linkers in order to diversify the nature of the coordination sites of the detectors (Scheme 10). It was crucial to proceed the reactions using 1.5 excess of corresponding polyamines 69–72 as was found to be helpful in our previous works on the 1,4,7-triazacyclononane-based cryptands synthesis [64], [65], [66]. The yields of the desirable products 73–76 were dramatically dependent on the polyamine structure, and in the case of the most capricious tris(3-aminopropyl)amine 72, the right choice of the ligand (Josiphos) was important to ensure a better yield of 76. Compounds 74 and 76 were modified with the dansyl amide fluorophore groups, in both cases the application of small excess of dansyl chloride was important to ensure full substitution at all nitrogen atoms. Thus, tetra- and tridansylated compounds 77 and 78, respectively, were obtained. As a result, a series of various macrocycles with the central chiral BINAM moiety was elaborated and many of them were further equipped with additional exocyclic fluorophore groups.

Synthesis of the BINAM-containing macrocyclic with polyamine linkers.
Cryptands possessing (S)-BINAM moiety
The variety of the structures of macrocyclic compounds incorporating BINAM moiety was expanded to a family of chiral cryptands. It was thought initially that the more rigid organization of the donor sites (nitrogen and oxygen atoms) could provide more selectivity for coordinating certain aminoalcohols. For this purpose, (S)-BINAM was reacted with N,N′-di(bromobenzyl) substituted diazacrown ethers 79–82 to give corresponding macrobicycles 83–86 (Scheme 11) [67]. The yields were obviously higher in the case of diaza-18-crown-6 derivatives 81, 82, probably, due to more favorable conformations of these compounds. A similar approach using N,N′-di(bromobenzyl) derivatives of tetraazamacrocycles like cyclen and cyclam 87–90 was also successful and allowed the synthesis of the chiral cryptands 91–94 though the yields were modest (13–34%) (Scheme 12) [68]. These compounds were further converted into didansyl derivatives 95–97; as in the case of above mentioned macrocycles (cf. Scheme 7).
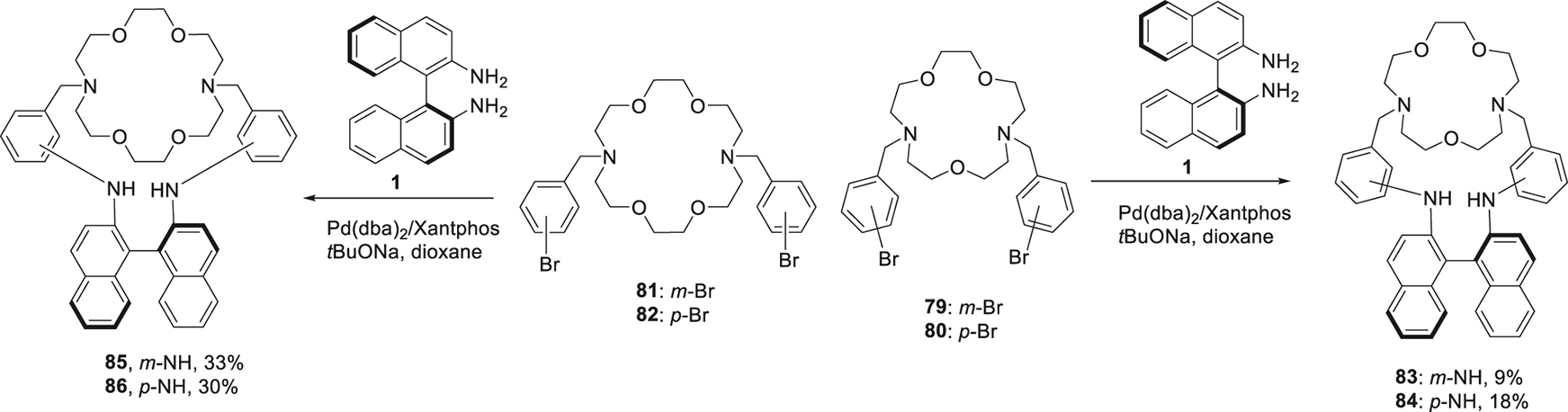
Formation of the cryptands incorporating BINAM and diazacrown moieties.
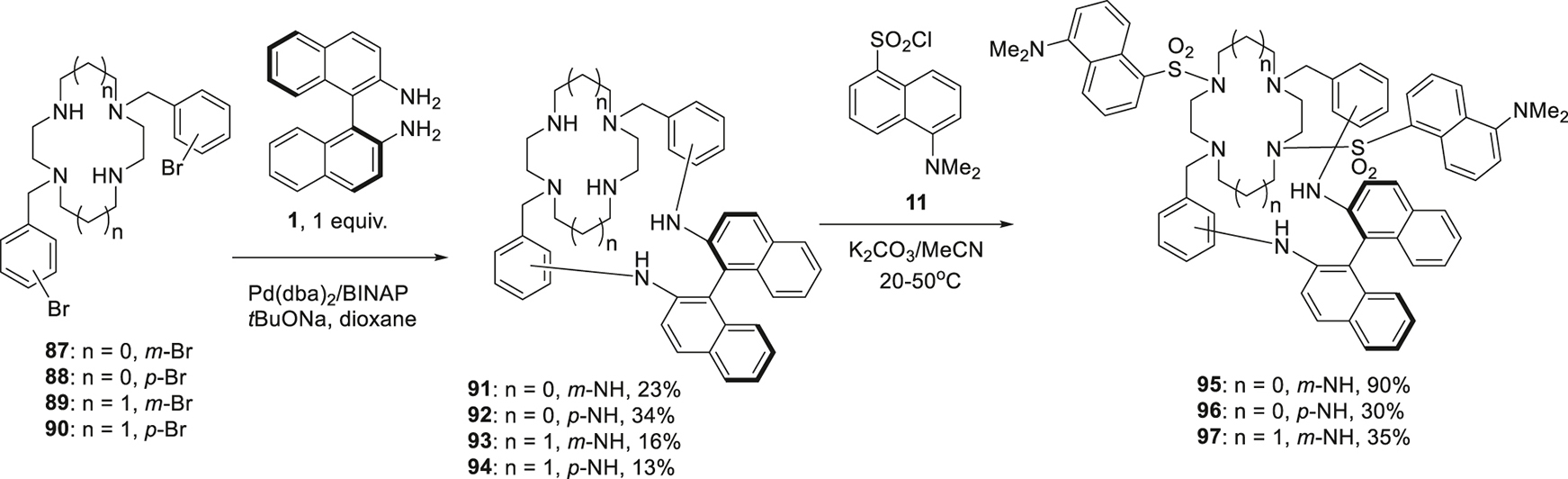
Synthesis of the cryptands on the basis of BINAM and cyclen or cyclam.
It was possible also to combine BINAM with the calix[4]arene macrocyclic unit (Scheme 13). The catalytic macrocyclization reaction was carried out with diaminocalix[4]arenes 98, 99 and N,N′-di(3-bromobiphenyl) substituted BINAM 2 and produced desirable cryptands 100, 101 in 39 and 27% yields, respectively. After this the macrobicycles were modified with two dansylamide or two 7-methoxycoumarin fluorophore groups to give compounds 102–104 in 41–46% yields (Scheme 13).
![Scheme 13:
Synthesis of the cryptands comprising BINAM and calix[4]arene moieties.](/document/doi/10.1515/pac-2020-0205/asset/graphic/j_pac-2020-0205_scheme_013.jpg)
Synthesis of the cryptands comprising BINAM and calix[4]arene moieties.
Introduction of exocyclic chiral groups to macrocyclic derivatives of (S)-BINAM
Not only the additional substituents with fluorescent properties were introduced in the macrocyclic compounds based on (S)-BINAM. We also attempted the synthesis of more complex compounds with exocyclic chiral groups in order to alter the ability for complexation with chiral analytes and presumably to enhance stereodiscrimanation by the combination of two types of chirality in one molecule, i.e., C2 chirality of the BINAM moiety and central chirality of the N,O-containing substituents. For this reason, model macrocycles 33 and 37 were transformed into corresponding N,N′-di(3-bromobenzyl) derivatives 105 and 106, then the Pd(0)-catalyzed reactions with the optically pure (S)-tetrahydrofurfurylamine were accomplished to produce compounds 107 and 108, and one of them, with the trioxadiamine chain, was converted into didansylated derivative 109 (Scheme 14).

Modification of the BINAM-containing macrocycles with exocyclic chiral and fluorophore groups.
Quite similar procedure was carried out for the modification of the same macrocycles with the chiral N-Boc-pyrrolidinylmethyl substituents (Scheme 15). For this purpose, compounds 105, 106 were introduced in the catalytic reaction with the amine 4 and the resulting product was further modified with the 7-methoxycoumarin fluorophore moiety to produce compound 112.

Modification of the BINAM-containing macrocycles with exocyclic chiral and fluorophore groups.
In order to diversify the structures of the potential enantioselective receptors, the macrocycle 105 was modified with a chiral substituent featuring N-Boc-piperidine moiety using the reaction with the amine 113 (Scheme 16). The resulting compound 114 was further decorated with two dansylamide fluorophore groups to give compound 115. The same idea of structural diversity governed the synthesis of N,N′-di(2-bromobenzyl)substituted macrocycle 116, 117 (Scheme 17). Only the compound with a larger macrocyclic cavity could be further transformed into bis(tetrahydrofurfurylmethylamino) derivative 118, probably, due to steric demands in such compounds with unpredictable conformational peculiarities. This can also be the reason for modest yields (30–40%) both in catalytic and non-catalytic reactions of the macrocycles 105, 106 described here.

Modification of the BINAM-containing macrocycle with exocyclic chiral and fluorophore groups.
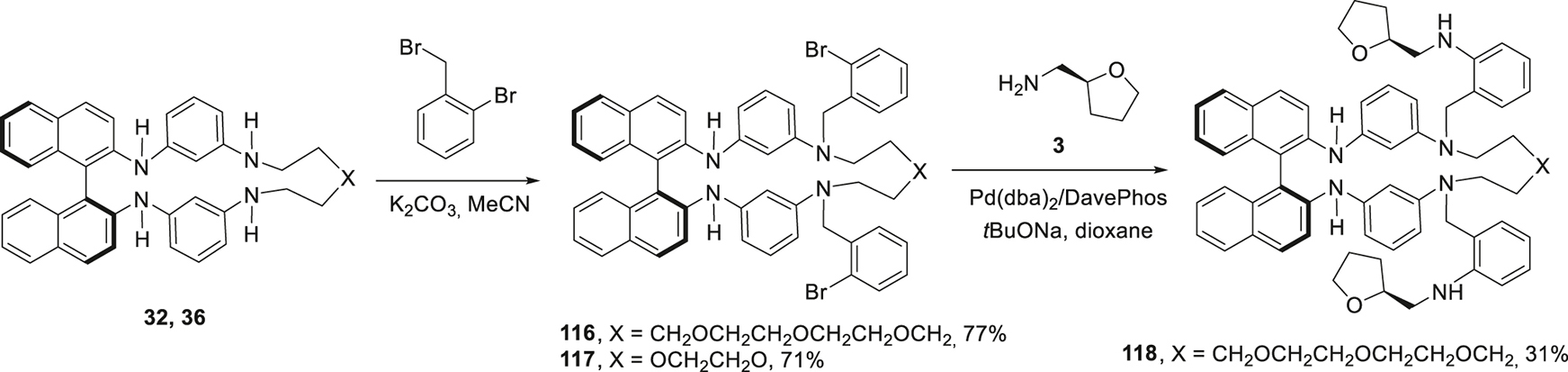
Modification of the BINAM-containing macrocycles with exocyclic chiral and fluorophore groups.
Detection properties of (S)-BINAM derivatives
The majority of the BINAM derivatives described above were tested as potential enantioselective fluorescent detectors using a panel of amino alcohols taken as individual enantiomers (Fig. 1). These compounds were chosen as model analytes in view of their structural similarity to corresponding amino acids, perfect solubility in various organic solvents and because they do not form ionic species and thus their coordination properties are not sensitive to pH of the media. Standard experiments included measurements of the emission spectra of the free BINAM derivatives in MeCN and after successive addition of 100, 200, 500 and 1000 equiv. of analytes. In the case when the change of the fluorescence was quite different for two enantiomers, or when in the presence of one enantiomer no change was observed while the second enantiomer led to emission quenching or enhancement, the compound can be judged as a detector for this certain pair of amino alcohols. It is obvious that the comprehensive description of the significant fluorescence changes for each BINAM derivative and each amino alcohol is going far beyond the scope of this mini-review, and we demonstrate here some characteristic examples for various types of BINAM derivatives to assess and compare their usefulness.
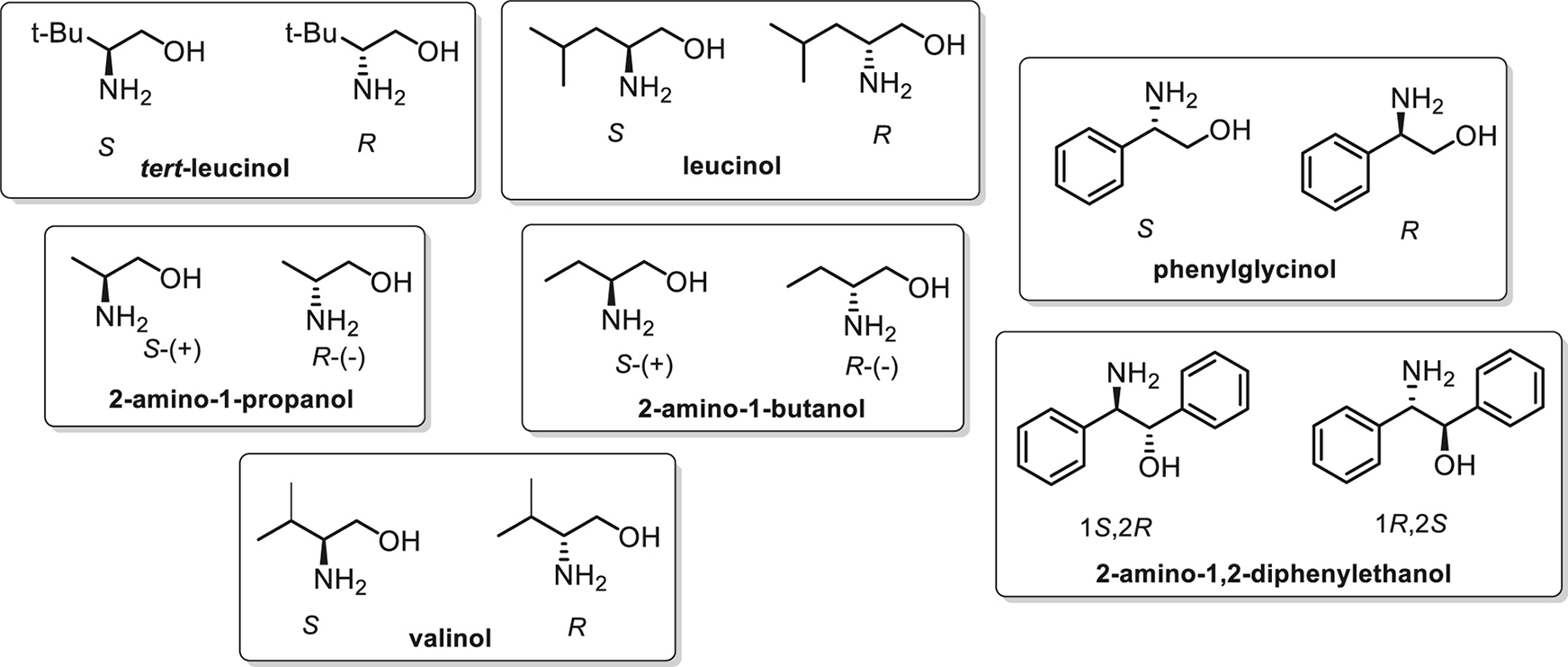
The panel of amino alcohols employed for the evaluation of the detecting abilities of the synthesized BINAM derivatives.
The fluorescence of many non-macrocyclic derivatives of BINAM was found to be less sensitive to the addition of the amino alcohols, in many cases the changes were either insufficient or of the same sign for both enantiomers. However, in some cases the compounds do behave as real detectors, e.g., the BINAM derivative 7 can distinguish between two enantiomers of tert-leucinol (Fig. 2a). Macrocyclic compounds were more sensitive and cyclic dimer 37 can be used for the recognition of leucinol enantiomers (Fig. 2b). Macrocycle 55 bearing two dansyl amide fluorophore groups is able to distinguish between 2-phenylglycinol isomers (Fig. 2c). In all these cases one enantiomer leads to the emission quenching while another one does not change the spectrum. In the case of 7-methoxycoumarin-substituted macrocycle 62 the enhancement of the emission was observed, and in the case of tert-leucinol the difference caused by two enantiomers was the most pronounced. Also, in the presence of the enantiomer which led to more significant increase in the emission notable hypsochromic shift was noted (Fig. 2d). As for quinolinyl-substituted macrocycles 63, 64, the signs of the fluorescence changes were different for the enantiomers, and again the hypsochromic shift was evident in the case of the emission enhancement (Fig. 2e,f). We may propose the scheme of the plausible coordination patterns for amino alcohols with an exemplary ligand 7 (Fig. 3).
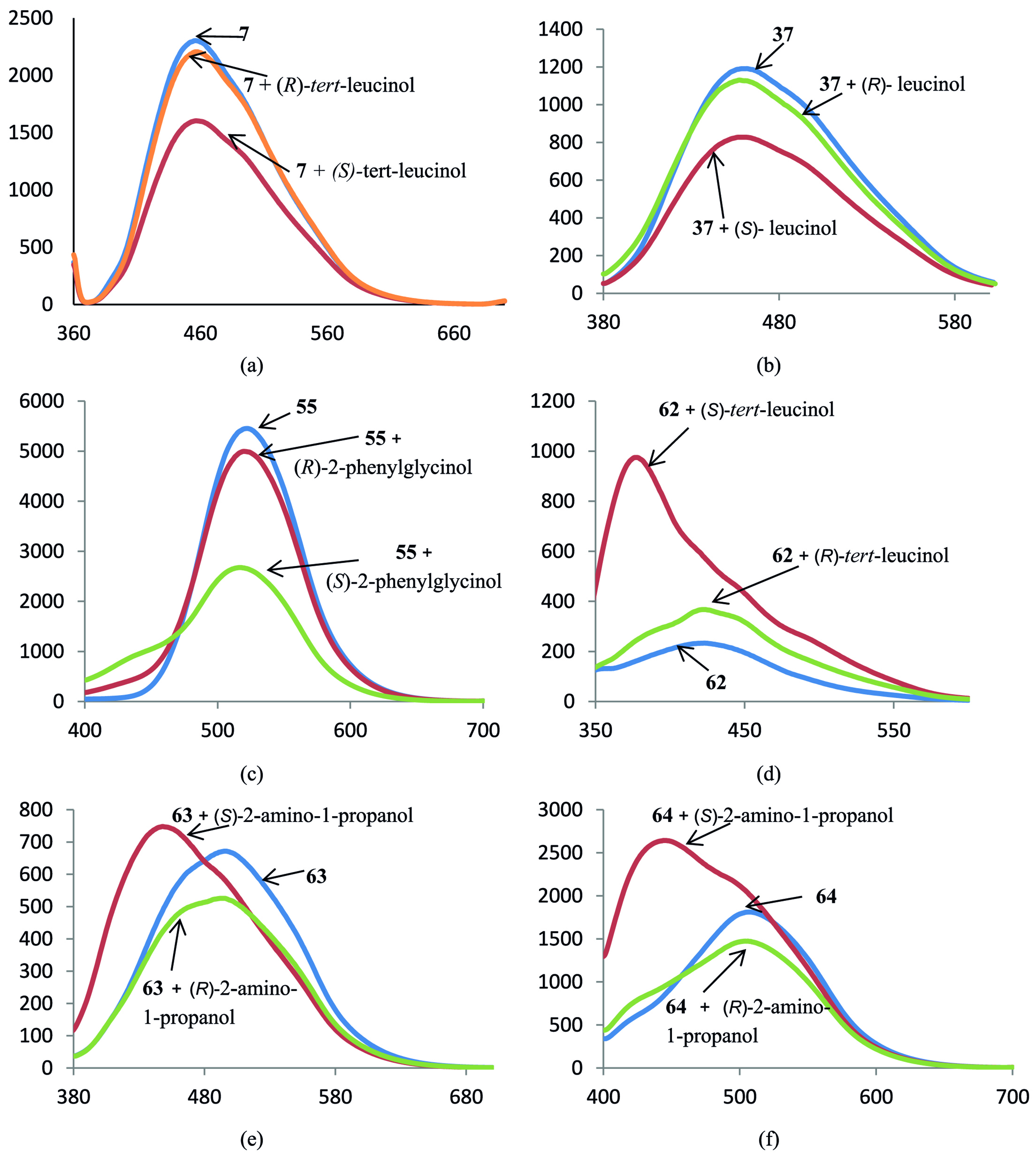
Spectra of fluorescence of BINAM derivatives 7 (a), 37 (b), 55 (c), 62 (d), 63 (e), 64 (f) in the presence of 1000 equiv. of enantiomers of corresponding amino alcohols; X-axis: wavelength, nm, Y-axis: relative intensity of emission.
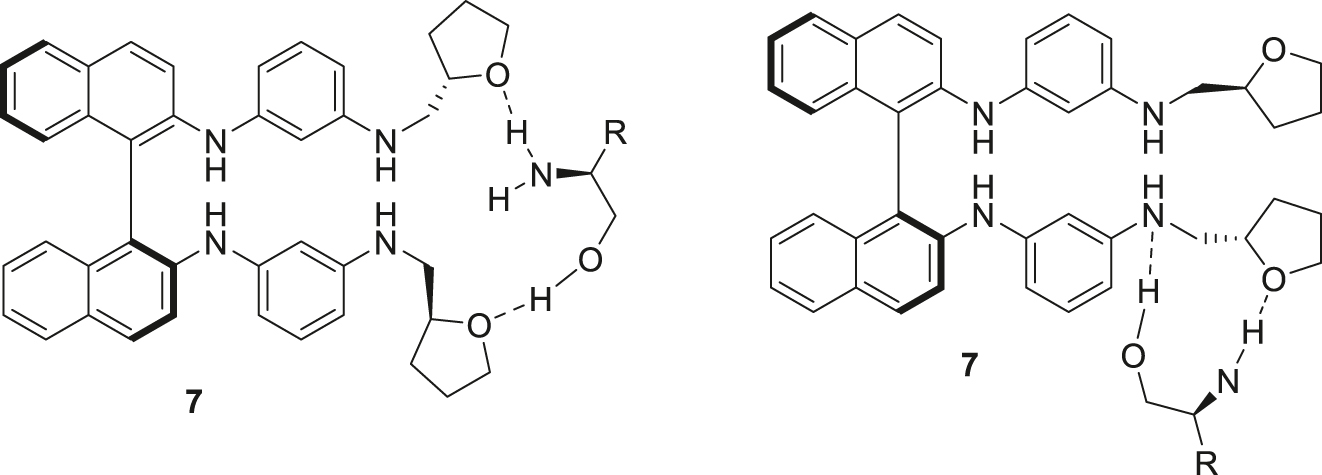
Plausible coordination patterns for amino alcohols with the BINAM-based ligands.
Figure 4 shows the possibilities of the anthraquinone-containing macrocycle 49 to recognize the enantiomers of leucinol and 2-amino-1-propanol, in both cases one enantiomer did not change the fluorescence while the second led to an increase in the fluorescence intensity.
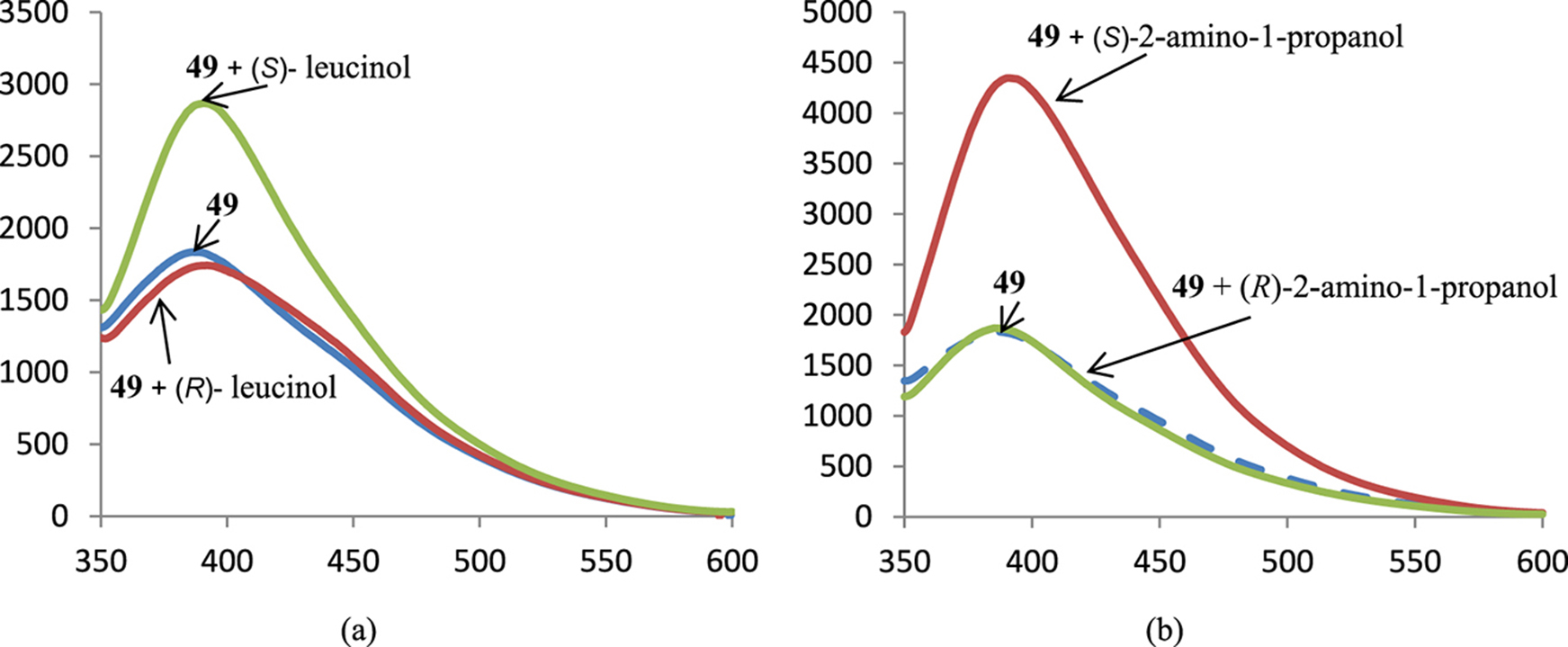
Spectra of fluorescence of BINAM derivative 49 in the presence of 1000 equiv. of enantiomers of leucinol (a) and 2-amino-1-propanol (b); X-axis: wavelength, nm, Y-axis: relative intensity of emission.
Different behavior of the spectra of fluorescence after the introduction of additional fluorophore groups is demonstrated by Fig. 5. The parent tetraazamacrocycle 74 changes its emission in different ways upon the addition of 2-phenylglycinol enantiomers, and the fluorescence of its tetradansylated derivative 77 quenches in the presence of (R)-tert-leucinol while the addition of (S)-isomer does not lead to any change of the emission.
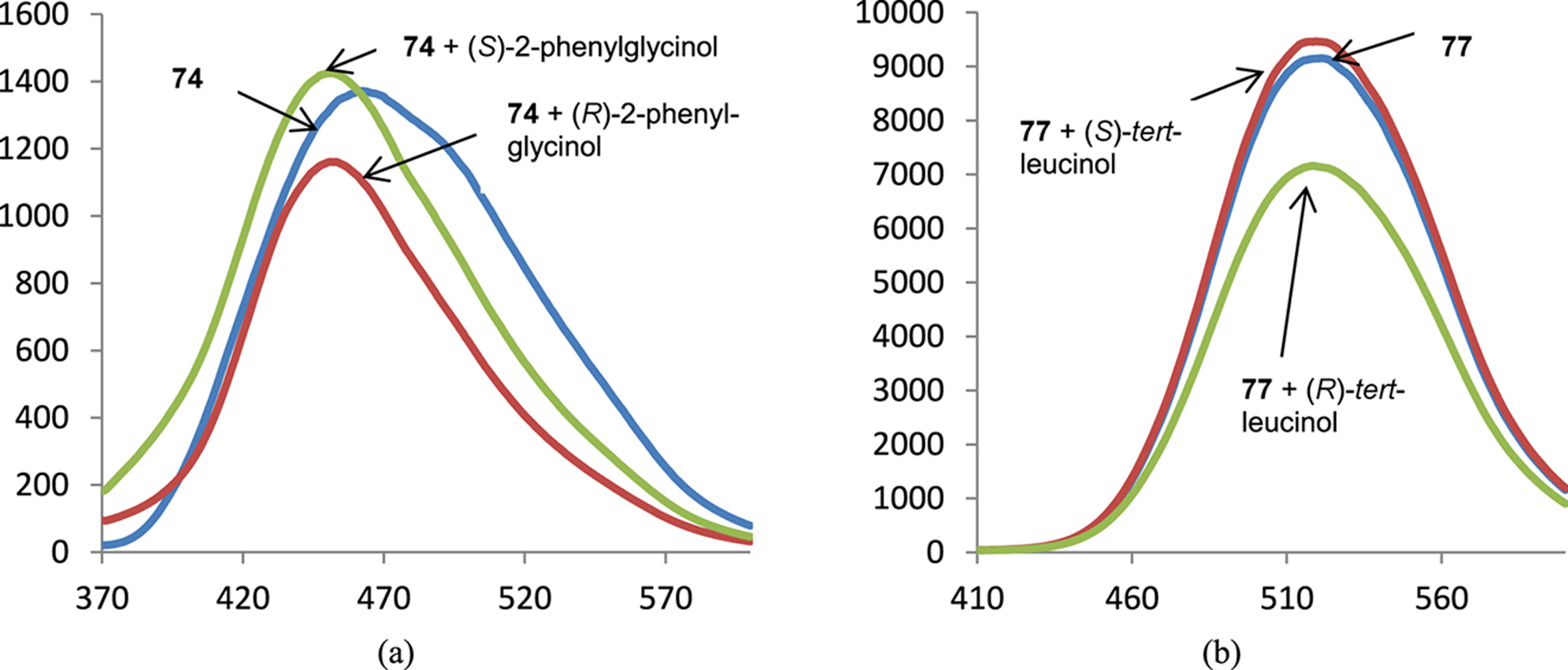
Spectra of fluorescence of BINAM derivative 74 (a) and 77 (b) in the presence of 1000 equiv. of enantiomers of 2-phenylglycinol (a) and tert-leucinol (b); X-axis: wavelength, nm, Y-axis: relative intensity of emission.
BINAM-derived cryptands can detect certain amino alcohols, as it is depicted on Fig. 6, however the changes in fluorescence are often not important. In some cases the addition of only one enantiomer leads to the change of the emission intensity (Fig. 6b,c), in other cases the signs of the changes are opposite (Fig. 6a,d).
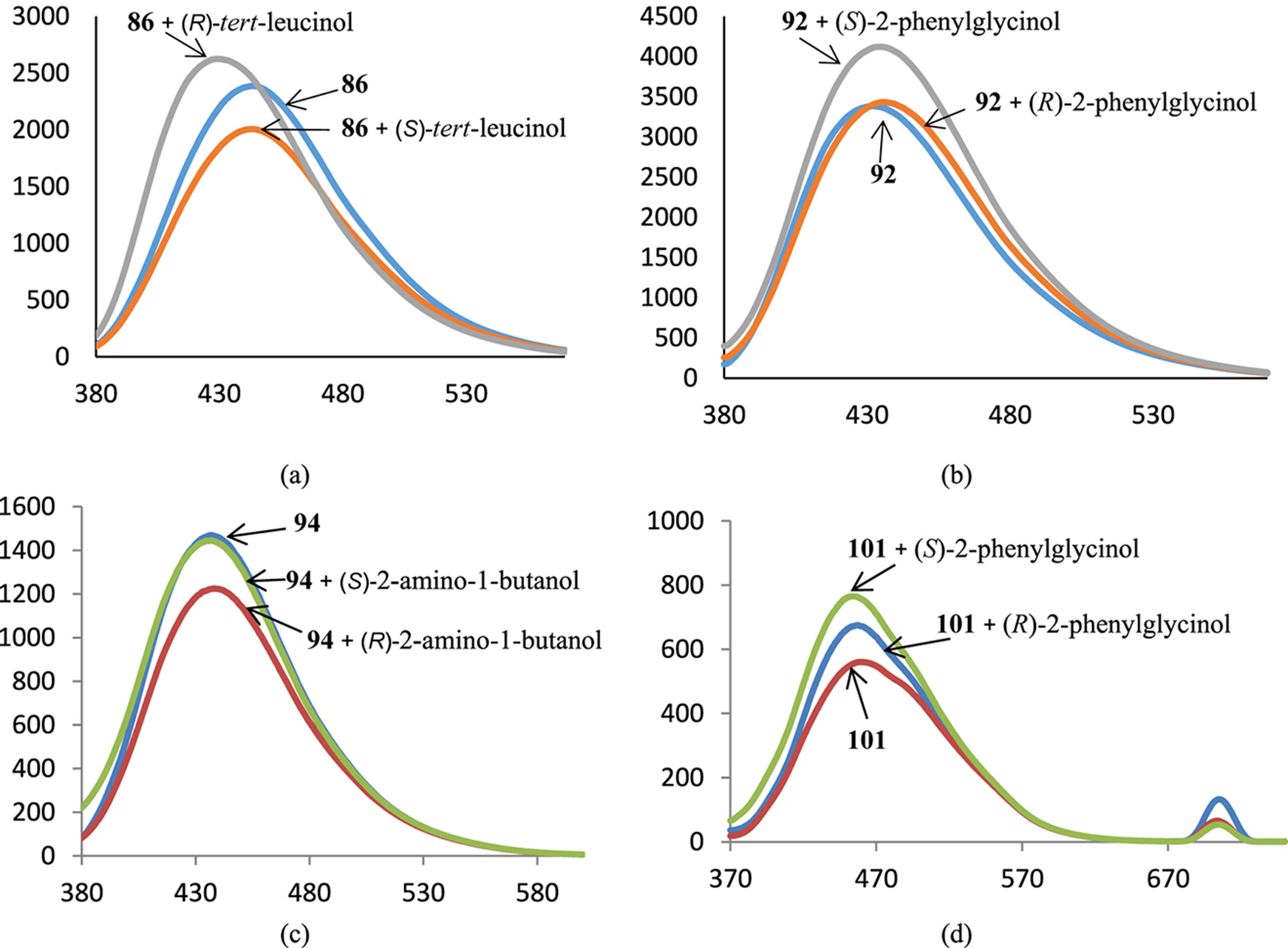
Spectra of fluorescence of BINAM-containing cryptands 86 (a), 92 (b), 94 (c), 101 (d) in the presence of 1000 equiv. of enantiomers of corresponding amino alcohols; X-axis: wavelength, nm, Y-axis: relative intensity of emission.
The decoration of the cryptands on the basis of cyclen or cyclam with dansylamide fluorophores was useless, on the other hand, this procedure enhanced the sensititvity of the calix[4]arene-based cryptands in comparison with the parent cryptand 101. In the case of dansylamide- and 7-methoxycoumarin-modified macrobicycles 102 and 104 the fluorescence notably enhanced in the presence of one enantiomer of amino alcohols and did not change upon the addition of the another enantiomer (Fig. 7). The introduction of the additional exocyclic chiral substituents in the BINAM-containing macrocycles modified their ability to recognize amino alcohol enantiomers. In many cases only one enantiomer led to emission quenching while the second one did not alter the fluorescence intensity (Fig. 8a,c,d), but also there are examples when both enantiomers changed the intensity with opposite signs (Fig. 8b).
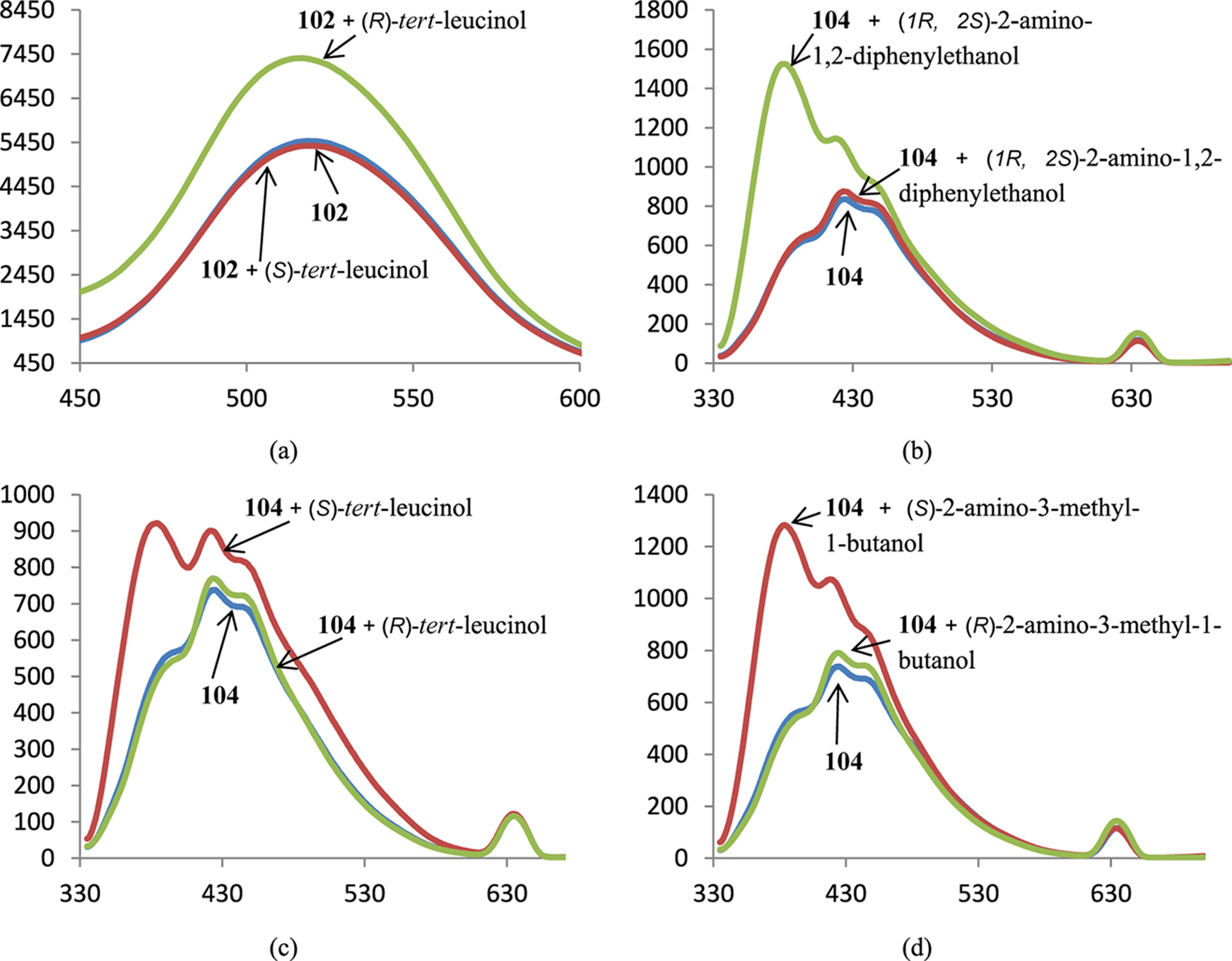
Spectra of fluorescence of BINAM-containing cryptands 102 (a), 104 (b-d) in the presence of 1000 equiv. of enantiomers of corresponding amino alcohols; X-axis: wavelength, nm, Y-axis: relative intensity of emission.
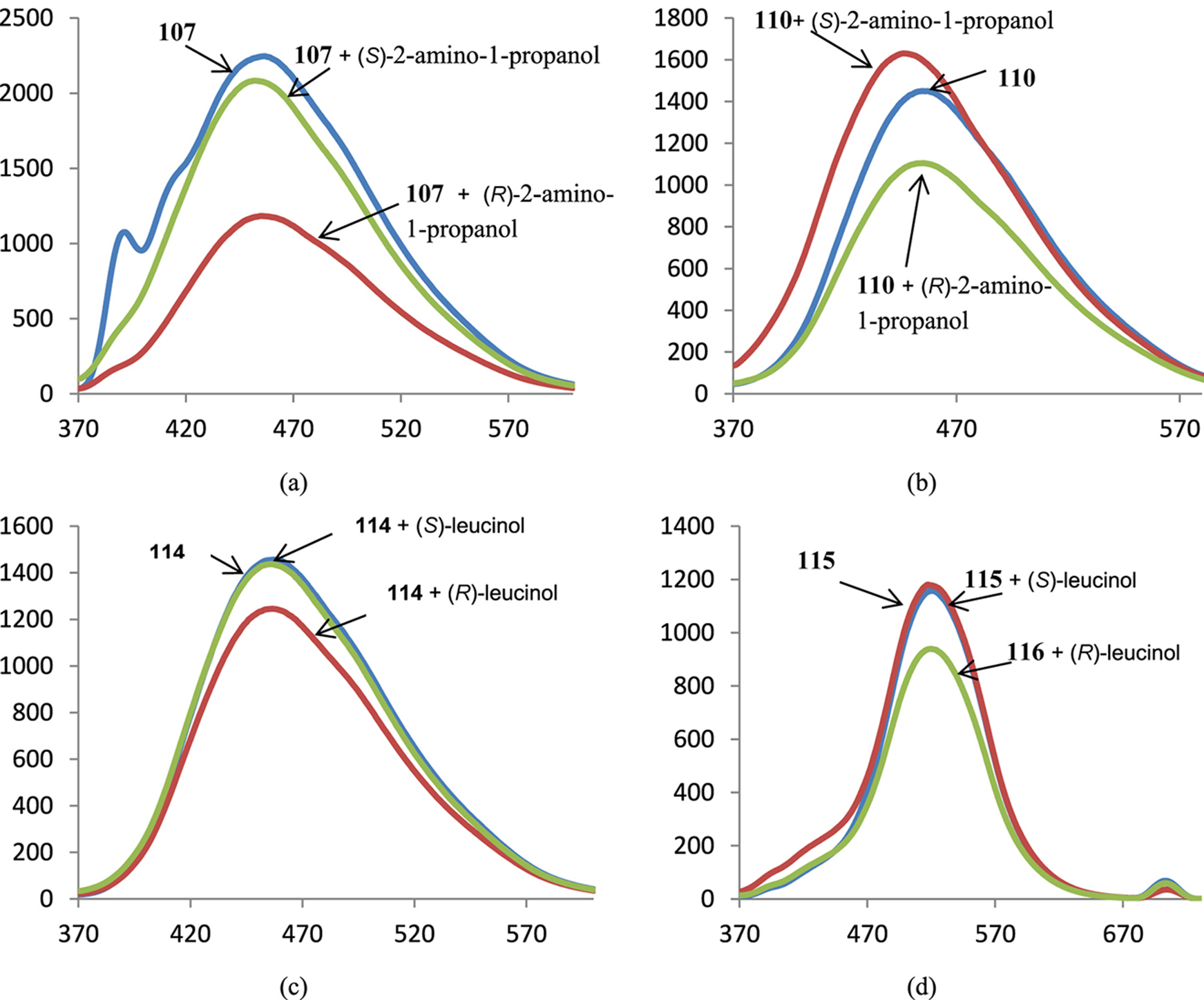
Spectra of fluorescence of BINAM-containing macrocycles 107 (a), 110 (b), 114 (c), 115 (d) in the presence of 1000 equiv. of enantiomers of corresponding amino alcohols; X-axis: wavelength, nm, Y-axis: relative intensity of emission.
In the majority of experiments, except for the titrations with the compounds 62–64 (Fig. 2d–f), we observed the changes in the intensities of fluorescence without any notable shift of the emission maxima. Such behavior is usual in the case when PET mechanism of fluorescence is applicable [69]. In the cases of detectors 62–64 the hypsochromic shifts of the emission maxima were noted upon the addition of the amino alcohols what may imply that in these cases the PCT mechanism might be considered. This mechanism is well documented for such fluorophore groups like coumarin (present in 62) or quinoline (present in 63, 64) which possess electron donor and electron withdrawing sites to achieve the charge transfer in the fluorophore moiety. PCT mechanism demands that an interaction of the detector with the analyte takes place which alters this intramolecular charge transfer. Somewhat smaller hypsochromic shifts were also noted for compounds 74 (Fig. 5a) and 86 (Fig. 6a) though they possess only aminonapthyl moieties less prone to act as PCT fluorophores.
Conclusions
As a result of our investigations in the field of the synthesis of perspective enantioselective fluorescent detectors we obtained several families of compounds on the basis of C2-chiral BINAM: non-cyclic derivatives of BINAM comprising chiral N,O-substituents, macrocyclic compounds with oxadiamine and polyamine linkers which also may contain additional exocyclic chiral and fluorophore moieties, BINAM-based cryptands. Optically active amines were used to modify the BINAM derivatives with chiral substituents while the following fluorophore groups were introduced to enhance emission of the detectors: dansylamide, 7-methoxycoumarin, 6- and 3-aminoquinolines. Moreover, these substituents also could change the coordination properties of the detectors thus modifying their recognition abilities. The crucial steps in the syntheses of all these compounds were accomplished using Pd(0)-catalyzed amination reactions. Many of the synthesized BINAM derivatives were tested as potential enantioselective fluorescent detectors with a series of amino alcohols and many of them were found to be able to distinguish between two enantiomers of such analytes. The addition of amino alcohols caused either quenching or enhancement of the emission of the detectors, in some cases hypsochromic shifts of the maxima of fluorescence were also noted. BINAM-containing macrocyclic compounds in general were found to be more suitable for the fluorescent enantioselective detection of amino alcohols.
Funding source: Russian Foundation for Basic Research
Award Identifier / Grant number: 18-03-00709
-
Funding: This research was funded by the Russian Foundation for Basic Research (RFBR grant N 18-03-00709).
References
[1] M. Irie, T. Yorozu, K. Hayashi. J. Am. Chem. Soc. 100, 2236–2237 (1978).10.1021/ja00475a047Search in Google Scholar
[2] D. Avnir, E. Wellner, M. Ottolenghi. J. Am. Chem. Soc. 111, 2001–2003 (1989).10.1021/ja00188a008Search in Google Scholar
[3] W. Iwanek, J. Mattay. J. Photochem. Photobiol. A 67, 209–226 (1992).10.1016/1010-6030(92)85230-RSearch in Google Scholar
[4] L. Pu. Chem. Rev. 104, 1687–1716 (2004).10.1021/cr030052hSearch in Google Scholar PubMed
[5] L. Pu. Acc. Chem. Res. 45, 150–163 (2012).10.1021/ar200048dSearch in Google Scholar PubMed
[6] X. Zhang, J. Yin, J. Yoon. Chem. Rev. 114, 4918–4959 (2014).10.1021/cr400568bSearch in Google Scholar PubMed
[7] T. Ema. J. Incl. Phenom. Macrocycl. Chem. 74, 41–55 (2012).10.1007/s10847-012-0136-6Search in Google Scholar
[8] D.-C. Zhong, N.-B. Lu. Chem. Commun. 52, 10322–10337 (2016).10.1039/C6CC03660KSearch in Google Scholar
[9] Z. Chen, Q. Wang, X. Wu, Z. Li, Y.-B. Jiang. Chem. Soc. Rev. 44, 4249–4263 (2015).10.1039/C4CS00531GSearch in Google Scholar
[10] J. Wang, H.-B. Liua, Z. Tong, C.-S. Ha. Coord. Chem. Rev. 303, 139–157 (2015).10.1016/j.ccr.2015.05.008Search in Google Scholar
[11] K. Xu, Z. Qiu, J. Zhao, J. Zhao, C. Wang. Tetrahedron: Asymmetry 20, 1690–1696 (2009).10.1016/j.tetasy.2009.06.016Search in Google Scholar
[12] K. Xu, L. Yang, Y. Wang, J. Zhao, C. Wang. Supramol. Chem. 22, 563–570 (2010).10.1080/10610271003713920Search in Google Scholar
[13] J. Lin, Q. Hu, M. Xu, L. Pu. J. Am. Chem. Soc. 124, 2088–2089 (2002).10.1021/ja011971xSearch in Google Scholar PubMed
[14] M. Xu, J. Lin, Q. Hu, L. Pu. J. Am. Chem. Soc. 124, 14239–14246 (2002).10.1021/ja020989kSearch in Google Scholar PubMed
[15] J. Lin, H. Zhang, L. Pu. Org. Lett. 4, 3297–3300 (2002).10.1021/ol026565cSearch in Google Scholar PubMed
[16] Z. Li, J. Lin, H. Zhang, M. Sabat, M. Hiacinth, L. Pu. J. Org. Chem. 69, 6284–6293 (2004).10.1021/jo049366aSearch in Google Scholar PubMed
[17] J. Lin, A. R. Rajaram, L. Pu. Tetrahedron 60, 11277–11281 (2004).10.1016/j.tet.2004.08.050Search in Google Scholar
[18] X. He, X. Cui, M. Li, L. Lin, X. Liu, X. Feng. Tetrahedron Lett. 50, 5853–5856 (2009).10.1016/j.tetlet.2009.08.006Search in Google Scholar
[19] X. Yang, K. Shen, X. Liu, C. Zhu, Y. Cheng. Tetrahedron Lett. 52, 4611–4614 (2011).10.1016/j.tetlet.2011.06.084Search in Google Scholar
[20] H. Liu, X. Hou, L. Pu. Angew. Chem. Int. Ed. 48, 382–385 (2009).10.1002/anie.200804538Search in Google Scholar PubMed
[21] H. Liu, Q. Zhao, X. Hou, L. Pu. Chem. Commun. 47, 3646–3648 (2011).10.1039/c0cc05514jSearch in Google Scholar PubMed
[22] C. Hu, Y. He, Z. Chen, X. Huang. Tetrahedron Asymmetry 20, 104–110 (2009).10.1016/j.tetasy.2008.11.038Search in Google Scholar
[23] L. Tang, G. Wei, R. Nandhakumar, Z. Guo. Bull. Korean Chem. Soc. 32, 3367–3371 (2011).10.5012/bkcs.2011.32.9.3367Search in Google Scholar
[24] Q. Lu, L. Dong, J. Zhang, J. Li, L. Jiang, Y. Huang, S. Qin, C. Hu, X. Yu. Org. Lett. 11, 669–672 (2009).10.1021/ol8027303Search in Google Scholar PubMed
[25] L. Yang, S. Qin, X. Su, F. Yang, J. You, C. Hu, R. Xie. J. Lan. Org. Biomol. Chem. 8, 339–348 (2010).10.1039/B908540HSearch in Google Scholar
[26] K. Xu, S. Jiao, W. Yao, E. Xie, B. Tang, C. Wang. Chirality 24, 646–651 (2012).10.1002/chir.22059Search in Google Scholar PubMed
[27] G. Beer, K. Rurack, J. Daub. J. Chem. Soc. Chem. Commun. 1138–1139 (2001). https://doi.org/10.1039/B102376B.Search in Google Scholar
[28] Q.-S. Hu, V. Pugh, M. Sabat, L. Pu. J. Org. Chem. 64, 7528–7536 (1999).10.1021/jo990856qSearch in Google Scholar
[29] V. Pugh, Q.-S. Hu, X.-B. Zuo, F. D. Lewis, L. Pu. J. Org. Chem. 66, 6136–6140 (2001).10.1021/jo010479tSearch in Google Scholar PubMed
[30] T. J. Liu, Y. J. Chen, K. S. Zhang, D. Wang, D. W. Guo, X. Z. Yang. Chirality 13, 595–600 (2001).10.1002/chir.1183Search in Google Scholar PubMed
[31] D. Wang, T.-J. Liu, W.-C. Zhang, W. T. SlavenIV, C.-J. Li. J. Chem. Soc. Chem. Commun. 1747–1748 (1998). https://doi.org/10.1039/A802855I.Search in Google Scholar
[32] L. Ma, P. S. White, W.-B. Lin. J. Org. Chem. 67, 7577–7586 (2002).10.1021/jo0255680Search in Google Scholar PubMed
[33] S. J. Lee, W.-B. Lin. J. Am. Chem. Soc. 124, 4554–4555 (2002).10.1021/ja0256257Search in Google Scholar PubMed
[34] A. Bencini, C. Coluccini, A. Garau, C. Giorgi, V. Lippolis, L. Messori, D. Pasini, S. Puccioni. Chem. Commun. 48, 10428–10430 (2012).10.1039/c2cc35383kSearch in Google Scholar PubMed
[35] J. Luo, Q. Zheng, C. Chen, Z. Huang. Tetrahedron 61, 8517–8528 (2005).10.1016/j.tet.2005.06.015Search in Google Scholar
[36] H. Wang, X. Tian, D. Yang, Y. Pan, Q. Wu, C. He. Tetrahedron Asymmetry 22, 381–386 (2011).10.1016/j.tetasy.2011.02.014Search in Google Scholar
[37] E. N. R. Cho, Y. Li, H. J. Kim, M. H. Hyun. Chirality 23, 349–353 (2011).10.1002/chir.20928Search in Google Scholar PubMed
[38] K. S. Kim, E. J. Jun, S. K. Kim, H. J. Choi, J. Yoo, C. Lee, M. H. Hyun, J. Yoon. Tetrahedron Lett. 48, 2481–2484 (2007).10.1016/j.tetlet.2007.02.028Search in Google Scholar
[39] T. Ema, N. Ura, K. Eguchi, Y. Ise, T. Sakai. Chem. Commun. 47, 6090–6092 (2011).10.1039/c1cc11572cSearch in Google Scholar PubMed
[40] T. Ema, N. Ura, K. Eguchi, T. Sakai. Bull. Chem. Soc. Jpn. 85, 101–109 (2012).10.1246/bcsj.20110230Search in Google Scholar
[41] C. Fraschetti, A. Filippi, M. E. Crestoni, T. Ema, M. Speranza. ChemPlusChem 78, 979–987 (2013).10.1002/cplu.201300086Search in Google Scholar
[42] A. Akdeniz, T. Minami, S. Watanabe, M. Yokoyama, T. Ema, P. Anzenbacher. Chem. Sci. 7, 2016–2022 (2016).10.1039/C5SC04235FSearch in Google Scholar
[43] J. Wu, J. Lu, J. Liu, C. Zheng, Y. Gao, J. Hu, Y. Ju. Sensors Actuators B 241, 931–937 (2017).10.1016/j.snb.2016.10.117Search in Google Scholar
[44] K. S. Parker, A. Townshend, S. J. Bale. Anal. Commun. 33, 265–267 (1996).10.1039/ac9963300265Search in Google Scholar
[45] K. Murakoshi, T. Azechi, H. Hosokawa, Y. Wada, S. Yanagida. J. Electroanal. Chem. 473, 117–124 (1999).10.1016/S0022-0728(99)00149-7Search in Google Scholar
[46] L. Wei, Y. He, K. Xu, S. Liu, L. Meng. Chin. J. Chem. 23, 757–761 (2005).10.1002/cjoc.200590757Search in Google Scholar
[47] J. Meng, G. Wei, X. Huang, Yu. Dong, Y. Cheng, C. Zhu. Polymer 52, 363–367 (2011).10.1016/j.polymer.2010.12.011Search in Google Scholar
[48] A. S. Abel, A. D. Averin, I. P. Beletskaya. New J. Chem. 40, 5818–5828 (2016).10.1039/C5NJ03231HSearch in Google Scholar
[49] A. S. Abel, A. Y. Mitrofanov, Y. Rousselin, F. Denat, A. Bessmertnykh-Lemeune, A. D. Averin, I. P. Beletskaya. ChemPlusChem 81, 35–39 (2016).10.1002/cplu.201500390Search in Google Scholar PubMed
[50] A. D. Averin, S. M. Kobelev, M. V. Anokhin, A. G. Bessmertnykh Lemeune, R. Guilard, I.P. Beletskaya. In Targets in Heterocyclic Systems: Chemistry and Properties, Vol. 15. O. A. Attanasi, D. Spinelli (Eds), pp. 193–225, RSC, Cambridge (2011).Search in Google Scholar
[51] I. P. Beletskaya, A. D. Averin, A. G. Bessmertnykh, F. Deant, R. Guilard. Russ. J. Org. Chem. 46, 947–967 (2010).10.1134/S1070428010070018Search in Google Scholar
[52] A. D. Averin, A. N. Uglov, I. P. Beletskaya. Chem. Lett. 37, 1074–1075 (2008).10.1246/cl.2008.1074Search in Google Scholar
[53] A. D. Averin, A. N. Uglov, A. K. Buryak, A. G. Bessmertnykh, R. Guilard, I. P. Beletskaya. Heterocycles 80, 957–975 (2010).10.3987/COM-09-S(S)68Search in Google Scholar
[54] I. P. Beletskaya, A. G. Bessmertnykh, A. D. Averin, F. Denat, R. Guilard. Eur. J. Org Chem. 281–305 (2005).10.1002/ejoc.200400455Search in Google Scholar
[55] A. D. Averin, E. R. Ranyuk, A. K. Buryak, I. P. Beletskaya. Chem. Lett. 37, 160–161 (2008).10.1246/cl.2008.160Search in Google Scholar
[56] A. S. Abel, A. D. Averin, I. P. Beletskaya. New J. Chem. 40, 5818–5828 (2016).10.1039/C5NJ03231HSearch in Google Scholar
[57] A. S. Abel, A. Yu. Mitrofanov, Y. Rousselin, F. Denat, A. Bessmertnykh-Lemeune, A. D. Averin, I. P. Beletskaya. ChemPlusChem 81, 35–39 (2016).10.1002/cplu.201500390Search in Google Scholar PubMed
[58] E. R. Ranyuk, A. D. Averin, I. P. Beletskaya. Adv. Synth. Catal. 352, 2299–2305 (2010).10.1002/adsc.201000181Search in Google Scholar
[59] O. K. Grigorova, A. D. Averin, O. A. Maloshitskaya, V. B. Rybakov, I. P. Beletskaya. Macroheterocycles 9, 418–424 (2016).10.6060/mhc161069aSearch in Google Scholar
[60] A. V. Shaferov, A. S. Malysheva, A. D. Averin, O. A. Maloshitskaya, I. P. Beletskaya. Sensors 20, 3234 (2020).10.3390/s20113234Search in Google Scholar PubMed PubMed Central
[61] I. P. Beletskaya, A. G. Bessmertnykh, A. D. Averin, F. Denat, R. Guilard. Eur. J. Org Chem. 261–280 (2005).10.1002/ejoc.200400456Search in Google Scholar
[62] O. K. Grigorova, A. D. Averin, O. A. Maloshitskaya, I. P. Beletskaya. Macroheterocycles 9, 425–432 (2016).10.6060/mhc161071aSearch in Google Scholar
[63] O. K. Grigorova, A. D. Averin, O. A. Maloshitskaya, I. P. Beletskaya. Macroheterocycles 10, 446–453 (2017).10.6060/mhc170937aSearch in Google Scholar
[64] N. M. Chernichenko, V. N. Shevchuk, A. D. Averin, O. A. Maloshitskaya, I. P. Beletskaya. Synlett 28, 2800–2806 (2017).10.1055/s-0036-1590883Search in Google Scholar
[65] N. M. Chernichenko, A. D. Averin, I. P. Beletskaya. Lett. Org. Chem. 15, 425–430 (2018).10.2174/1570178615666171222151821Search in Google Scholar
[66] A. D. Averin, N. M. Chernichenko, V. N. Shevchuk, O. A. Maloshitskaya, F. Denat, I. P. Beletskaya. Macroheterocycles 11, 141–149 (2018).10.6060/mhc171255aSearch in Google Scholar
[67] O. K. Grigorova, D. I. Gusev, A. D. Averin, O. A. Maloshitskaya, I. P. Beletskaya. Russ. Chem. Bull. 68, 848–854 (2019).10.1007/s11172-019-2495-2Search in Google Scholar
[68] O. K. Grigorova, A. D. Averin, O. A. Maloshitskaya, F. Denat, I. P. Beletskaya. Macroheterocycles 12, 312–318 (2019).10.6060/mhc190337aSearch in Google Scholar
[69] B. Valeur, I. Leray. Coord. Chem. Rev. 205, 3–40 (2000).10.1016/S0010-8545(00)00246-0Search in Google Scholar
© 2020, IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
Articles in the same Issue
- Frontmatter
- In this issue
- Conference papers of the 21st Mendeleev Congress on General and Applied Chemistry
- Recent achievements in copper catalysis for C–N bond formation
- Novel selective anticancer agents based on Sn and Au complexes. Mini-review
- Novel colchicine conjugate with unusual effect on the microtubules of cancer cells
- Surface chemistry of structural materials subjected to corrosion
- Redox-conducting polymers based on metal-salen complexes for energy storage applications
- Thermodynamic approach for prediction of oxide materials properties at high temperatures
- Competitive ways for three-component cyclization of polyfluoroalkyl-3-oxo esters, methyl ketones and amino alcohols
- Fragment-based approach to novel bioactive purine derivatives
- Reaction of 1,3-dimethylimidazolium dimethylphosphate with elemental sulfur
- Synthetic models of hydrogenases based on framework structures containing coordinating P, N-atoms as hydrogen energy electrocatalysts – from molecules to materials
- Macrokinetic investigation of the interaction mechanism of the pyrophoric iron nanopowder compacts with air
- Polylactide-based stent coatings: biodegradable polymeric coatings capable of maintaining sustained release of the thrombolytic enzyme streptokinase
- 3D printing in analytical chemistry: current state and future
- High purity substances – prototypes of elements of Periodic Table
- Pd(0)-catalyzed amination in the synthesis of chiral derivatives of BINAM and their evaluation as fluorescent enantioselective detectors
Articles in the same Issue
- Frontmatter
- In this issue
- Conference papers of the 21st Mendeleev Congress on General and Applied Chemistry
- Recent achievements in copper catalysis for C–N bond formation
- Novel selective anticancer agents based on Sn and Au complexes. Mini-review
- Novel colchicine conjugate with unusual effect on the microtubules of cancer cells
- Surface chemistry of structural materials subjected to corrosion
- Redox-conducting polymers based on metal-salen complexes for energy storage applications
- Thermodynamic approach for prediction of oxide materials properties at high temperatures
- Competitive ways for three-component cyclization of polyfluoroalkyl-3-oxo esters, methyl ketones and amino alcohols
- Fragment-based approach to novel bioactive purine derivatives
- Reaction of 1,3-dimethylimidazolium dimethylphosphate with elemental sulfur
- Synthetic models of hydrogenases based on framework structures containing coordinating P, N-atoms as hydrogen energy electrocatalysts – from molecules to materials
- Macrokinetic investigation of the interaction mechanism of the pyrophoric iron nanopowder compacts with air
- Polylactide-based stent coatings: biodegradable polymeric coatings capable of maintaining sustained release of the thrombolytic enzyme streptokinase
- 3D printing in analytical chemistry: current state and future
- High purity substances – prototypes of elements of Periodic Table
- Pd(0)-catalyzed amination in the synthesis of chiral derivatives of BINAM and their evaluation as fluorescent enantioselective detectors

