Application of gold nanoparticles in the methods of optical molecular absorption spectroscopy: main effecting factors
-
Vladimir V. Apyari
, Viktoria V. Arkhipova
Abstract
Results of studies on the analytical capabilities of gold nanoparticles in the methods of optical molecular absorption spectroscopy are reported. Peculiar effects of the nature of nanoparticle stabilizer, its charge, morphology of nanoparticles, form in which they are present in the system (in a colloidal solution or as a part of a nanocomposite with polyurethane foam) upon the determination of organic compounds (thiols, cationic compounds, catecholamines) and inorganic anions are considered. The determination is based on aggregation of nanoparticles or change in the state of their surface and morphology as a result of silver coating, leading to significant spectral and color changes, which can be monitored both by optical molecular absorption spectroscopy and visually. The ways to increase sensitivity and to control selectivity of the analysis using gold nanoparticles and their nanocomposites are outlined.
Introduction
Gold nanoparticles (AuNPs) have gained particular popularity in the modern analytical chemistry, making a decisive contribution to the emergence and development of such its area as nanoanalytics [1], [2], [3], [4], [5], [6], [7]. Among all analytical methods, AuNPs are the most popular in optical molecular absorption spectroscopy, including colorimetry and naked-eye detection [8], [9], [10]. This is not surprising, since one of the most important advantages of these nanoparticles is their unique optical properties originating form a phenomenon of surface plasmon resonance (SPR). It results in appearance of an intense absorption band in the visible region of the spectrum, position and intensity of which depend strongly on the degree of AuNPs aggregation. It is this property that has become a basis of numerous methods for the determination of substances using AuNPs.
Other advantage of these nanoobjects is the possibility of functionalizing the surface with modifiers of various nature and chemical properties, and the relative ease of preparation. It is also important that an analytical response in the nanosystems is not related to such concepts of the classical spectrophotometry as “chromophore group” or “system of delocalized π-orbitals”. This eliminates a number of structural limitations regarding types of substances being determined. At the same time, an important question arises: what does primarily affect the characteristics of AuNPs as analytical reagents for optical molecular absorption spectroscopy?
In this paper, results of studies pointing out the main factors affecting analytical characteristics of AuNPs have been summarized and discussed.
Experimental
Two types of AuNPs were used in this study: (1) spherical AuNPs stabilized with negatively charged citrate (AuNPs/Cit) and positively charged cetyltrimethylammonium (AuNPs/CTMA) or with 6,6-ionene polycation (AuNPs/Ion); and (2) gold nanorods (AuNRs). These AuNPs were used as either their colloid solutions or their nanocomposites with polyurethane foam (PUF). The AuNPs and their PUF-based nanocomposites were prepared as briefly described below.
Preparation of spherical AuNPs
AuNPs stabilized with citrate
AuNPs/Cit were prepared using a common citrate method as described elsewhere [11], [12]. Briefly, 1 mL of 1 % HAuCl4 was introduced in 250 mL bulb, diluted with 100 mL of deionized water and heated until boiling. 1.4 mL of 1 % sodium citrate was added to the hot solution at stirring. The solution was boiled in 5 min till stable ruby color. The mixture was cooled at stirring and kept in the dark for 24 h to complete stabilization and re-crystallization of AuNPs/Cit. The concentration of AuNPs/Cit in the final solution was 70 μg mL−1 (0.35 mM in terms of gold). The concentration of citrate in the final solution was 0.53 mM.
AuNPs stabilized with CTMA
AuNPs/CTMA were prepared via a borohydride method. Briefly, 10 mL of a 0.05 % HAuCl4 solution was added dropwise to 10 mL of a 0.012 M solution of CTMA chloride, with vigorous stirring, the resulting solution was stirred for 15 min. Then, 20 mL of a 0.05 % NaBH4 solution was added dropwise with stirring. After stirring for 30 min, the solution was kept for 1 day to completely stabilize the NPs and complete the recrystallization processes. The concentration of gold NPs synthesized in this way is 73 μg mL−1 (0.37 mM in gold). The concentration of CTMA in the final solution was 3 mM.
AuNPs stabilized with 6,6-ionene
AuNPs/Ion were prepared according to [13]. Briefly, 0.05 g of 6,6-ionene was placed in a round bottom bulb, dissolved in 18.5 mL of deionized water. Then, 6.5 mL of 0.1 mol L−1 HCl was injected at stirring. After that 25 mL of a solution containing 1.25 mL of 1 % HAuCl4 was introduced dropwise in the bulb. The brown mixture was stirred for 15 min. Then, a solution of 0.025 g NaBH4 in 50 mL of water was added dropwise into the bulb at vigorous stirring. The color of the mixture changed to ruby. The solution was stirred for 30 min and kept for 24 h to achieve re-crystallization and complete stabilization of AuNPs/Ion. The concentration of AuNPs/Ion in final solution was 70 μg mL−1 (0.35 mmol L−1 in terms of gold). The concentration of 6,6-ionene in the final solution was 1.2 mM in terms of the monomeric fragment.
Preparation of gold nanorods
AuNRs were prepared using a seed mediated approach according to [14]. (1) Preliminarily, a seed solution was prepared: 5 mL of 0.5 mmol L−1 HAuCl4, 5 mL of 0.20 mol L−1 CTMA and 0.6 mL of ice-cold 0.010 mol L−1 NaBH4 were added into a round-bottom flask under stirring. Vigorous stirring of the solution was continued for 2 min.
(2) 1.25 mL of 0.0040 mol L−1 AgNO3, 25 mL of 0.20 mol L−1 CTMA, 25 mL of 1.0 mmol L−1 HAuCl4 were added into a round-bottom flask under stirring. Then 0.35 mL of 0.0788 mol L−1 ascorbic acid was added dropwise into the solution. Then 60 μL of the seed solution were added. The color of the solution was gradually changed into reddish-brown within 30 min. The temperature of the solution was 50°C during the synthesis.
The obtained solution was kept for 2 weeks, centrifuged for 30 min at 8000 rpm in order to precipitate AuNRs, and the excess of CTAB was removed by decantation of the supernatant. AuNRs were washed in deionized water by redispersion of the sediment at sonication. The procedure was carried out twice. The concentration of AuNRs in the final solution was 71 mg mL−1 (0.36 mmol L−1 in terms of gold).
Preparation of PUF-based nanocomposites
For preparation of PUF-based nanocomposites of various AuNPs, a general approach including adsorption modification of PUF with AuNPs from an aqueous solution under agitation by shaking. This approach has been described earlier [15], [16], [17]. It is quite universal and can be applied to AuNPs of different types.
Results and discussion
Among all parameters affecting properties of AuNPs as the reagents for optical molecular spectroscopy, we could indicate the following main factors. The charge and nature of the stabilizer (it can be an anion, a cation of a surfactant, a polymer), the state of AuNPs in the analytical system (whether they are present in free form in a colloid solution or immobilized on surface of a substrate), their morphology (for example, spherical nanoparticles or gold nanorods) These factors were considered in detail.
Charge and nature of a stabilizer
To investigate this aspect, a systematic study and comparison of the processes of AuNPs aggregation under the influence of compounds of various classes was carried out. The aggregation depends on colloid stability of the nanoparticle dispersion, which is usually also governed by general lows, not only those specific for AuNPs [18], [19], [20]. Three types of AuNPs were synthesized and characterized: negatively charged citrate-stabilized AuNPs, positively charged cetyltrimethylammonium-stabilized AuNPs and 6,6-ionene polycation-stabilized AuNPs. The main difference between nanoparticles is their charge and type of stabilizer (Table 1). Besides charge, these three types of stabilizers were chosen considering the following speculations. Citrate and CTMA are low molecular weight substances that are frequently used in preparation of negatively and positively charged AuNPs, respectively. Therefore, a systematic study on their effects on AuNPs analytical features is important for practice. On the contrary, 6,6-ionene is a rarely used stabilizer. However, it is an important example of high molecular weight polycation that can provide additional steric stabilization of AuNPs.
Properties of prepared gold nanoparticles.
| Stabilizer | Citrate | CTMA | 6,6-Ionene |
|---|---|---|---|
| Formula of a stabilizer | 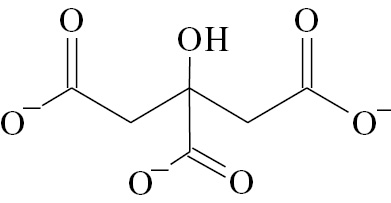 |
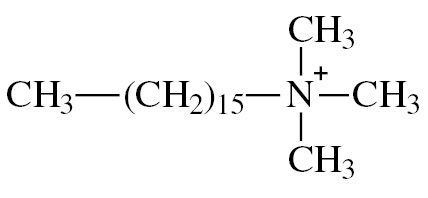 |
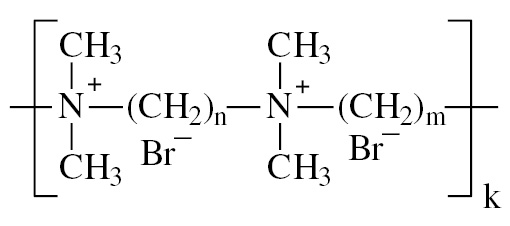 |
| ζ-potential, mV | –19 | +41 | +8 |
| λSPR, nm | 525 | 515 | 520 |
| Molar extinction coefficient, L mol−1·cm−1 | 1.4·109 | 3.0·106 | 5.0·108 |
| Hydrodynamic diameter, nm (DLS method) | 42 | 35 | 42 |
Compounds of three types were considered as determined compounds (Table 2): thiocompounds, the thiol group of which is capable of forming a strong bond with gold; cationic compounds, which can electrostatically interact with negatively charged nanoparticles; and anions, which are capable of interacting with positively charged nanoparticles. When choosing the compounds, the presence of substituents of various nature and the possibility of existence in solution in different charge states, as well as the relevance of developing methods for the determination of certain compounds were taken into account.
Determined compounds.
| Chemical name | Formula | Form of existence in a solution at pH 4–6 | Abbre-viation |
|---|---|---|---|
| Thiocompounds | |||
| Cysteamine | 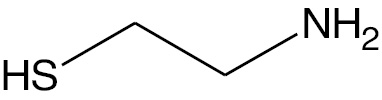 |
Cation | CA |
| Cysteine | 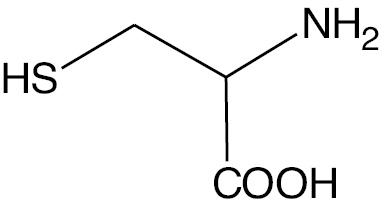 |
Zwitterion | CN |
| Acetylcysteine | 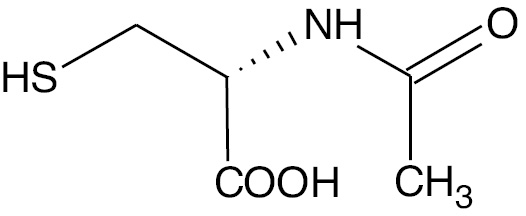 |
Anion | AC |
| Glutathione |  |
Anion containing a zwitterionic part | GLU |
| Mercaptoethanol | 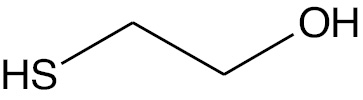 |
Uncharged molecule | ME |
| Mercaptopropionic acid | 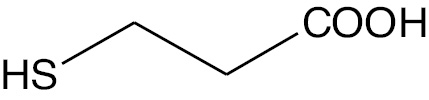 |
Anion | MPA |
| Cationic compounds | |||
| Polyhexamethylene-guanidine hydrochloride | 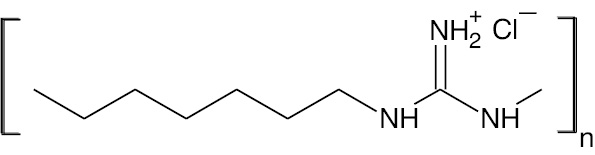 |
Polycation | PHMG |
| Neomycin | 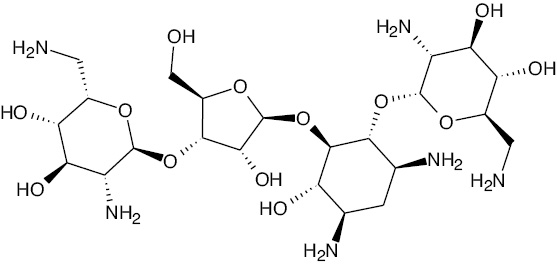 |
Multi-charged cation | NEO |
| Anions | |||
| Sulfate | SO42− | Two-charged anion | – |
| Pyrophosphate | P2O74− | Two-charged anion (dihydro-) | – |
| Oxalate | C2O42− | Two-charged anion | – |
| Other | Cl−, Br−, I−, F−, SO32−, S2O32−, ClO3−, NO3−, NO2−, ClO4−, PO43−, CH3COO−, CO32− | Anion | – |
The AuNPs aggregation degree was evaluated by the ratio of absorbances of the aggregates and the absorbance at 520 nm (Aagg/A520). This parameter was also used as an analytical response during spectrophotometric determination of the compounds. The results of interaction of AuNPs with the compounds are represented in Fig. 1. It can be seen that AuNPs/Cit in an aqueous solution in the absence of additional reagents aggregate only in the presence of cysteamine, neomycin and PHMG. With an increase in the concentration of the compound up to 150 μg mL−1, aggregation by glutathione is also observed. In this case, aggregation is associated with the efficient neutralization of the negative charge of citrate-capped AuNPs by adsorbed cationic species. Also one can consider interparticle electrostatic interaction via positively charged analytes. AuNPs/CTMA having a high value of ζ-potential are very stable and do not aggregate in the presence of compounds of the studied types. This also may be related with high molar concentration of CTMA used in AuNPs/CTMA synthesis. High amounts of CTMA in a solution compete with an analyte on the surface of AuNPs decreasing its aggregative effect. The aggregation of AuNPs/Ion is possible only in the presence of ions that carry a relatively large negative charge: sulfate, pyrophosphate and oxalate. Aggregation in the presence of carbonate, phosphate, and sulfite is not observed, apparently, since they are protonated under experimental conditions, which reduces their charge. It is important to note that the aggregation proceeds only at relatively high amounts of analytes, which can be ascribed to desensitization of AuNPs because of their steric stabilization by 6,6-ionene polymeric chains.
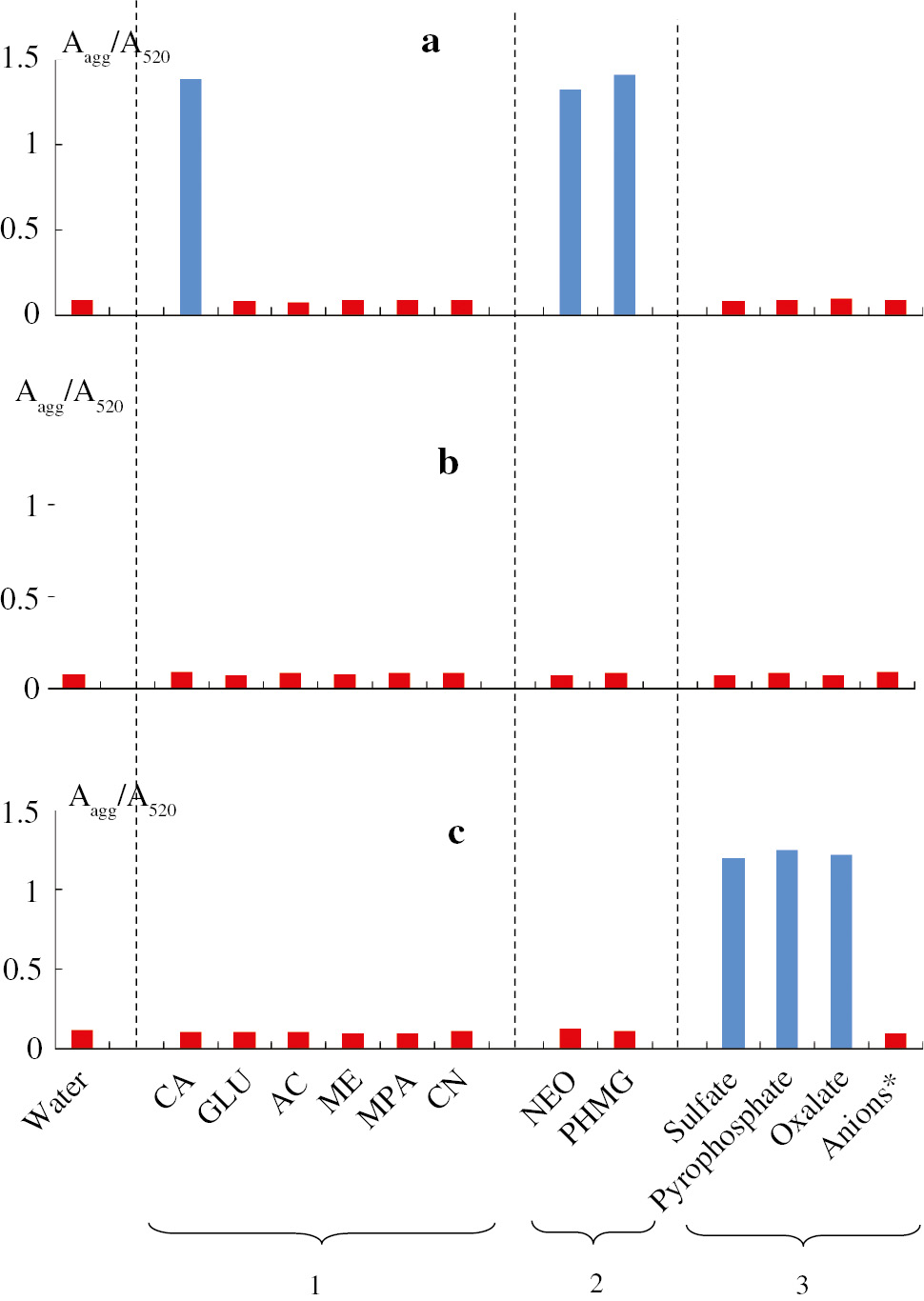
Aggregation degree of AuNPs stabilized with citrate (a), CTMA (b) and 6,6-ionene (c) in the presence of thiocompounds, c=1 μg mL−1 (1), cationic compounds, c=1 μg mL−1 (2), and anions, c=1 mg mL−1 (3). * Cl−, Br−, I−, F−, SO32−, S2O32−, ClO3−, NO3−, NO2−, ClO4−, PO43−, CO32−, CH3COO−.
The aggregation effect can be used to develop methods for determining compounds that exist under certain conditions in the form of multiply charged particles. In addition, thiocompounds and certain anions can be determined in this way. However, sometimes this is possible only in the presence of substances that reduce aggregative stability of AuNPs. E.g. addition of ethylenediaminetetraacetic acid (EDTA) effectively decreases aggregative stability of AuNPs/Cit, simultaneously playing the role of a masking agent preventing interferences from metal cations [12], [21]. Analytical features of merit for the spectrophotometric determination of different compounds using AuNPs are represented in Table 3. The limits of detection (LODs) depend on the nature of the compounds determined and can reach down to 0.01 μg mL−1. The developed methods can be used in the analysis of pharmaceuticals, urine, food additives of disinfectants and water.
Features of merit for spectrophotometric determination of compounds using AuNPs.
| Type of determined compounds | Determined compounds | Conditions of determination | Determination range, μg mL−1 | LOD, μg mL−1 |
|---|---|---|---|---|
| Thiocompounds | Cysteamine | AuNPs/Cit, cEDTA=0.005 M | 0.02–0.05 | 0.01 |
| Cysteine | AuNPs/Cit, cEDTA=0.01 M | 0.06–0.5 | 0.05 | |
| Glutathione | AuNPs/Cit | 150–400 | 100 | |
| Cationic compounds | PHMG | AuNPs/Cit, cEDTA=0.005 M | 0.04–0.08 | 0.03 |
| Neomycin | AuNPs/Cit, cEDTA=0.005 M | 0.03–0.05 | 0.02 | |
| Anions | Sulfate | AuNPs/Ion | 70–150 | 60 |
| Pyrophosphate | AuNPs/Ion | 30–50 | 20 |
State in the analytical system
A remarkable effect on the characteristics of AuNPs as analytical reagents is exerted by their state in the analytical system – whether they are present in free form in a solution or immobilized on surface of a solid support. In our work, polyurethane foam (PUF) is considered as such a support. This polymeric material has been well studied in chemical analysis as a sorbent for preconcentration of substances from water and air [22], [23], [24]. It is known that PUF contains functional groups of different polarity and electronic properties, which favors the immobilization of various types of nanoparticles [15], [16]. In addition, it is an easy-to-use durable and chemically stable monolithic material containing an open pore system.
PUF-based nanocomposites of AuNPs were prepared using the approach proposed earlier [15], [16], [17]. It has been shown that AuNPs are able to aggregate on the surface of PUF under the influence of thiocompounds [15], as can be seen from the TEM images represented in Fig. 2. In this case, a change in the optical characteristics of the samples is observed, which can be used for the determination of the compounds by solid-phase spectroscopic methods, e.g. diffuse reflectance spectroscopy.
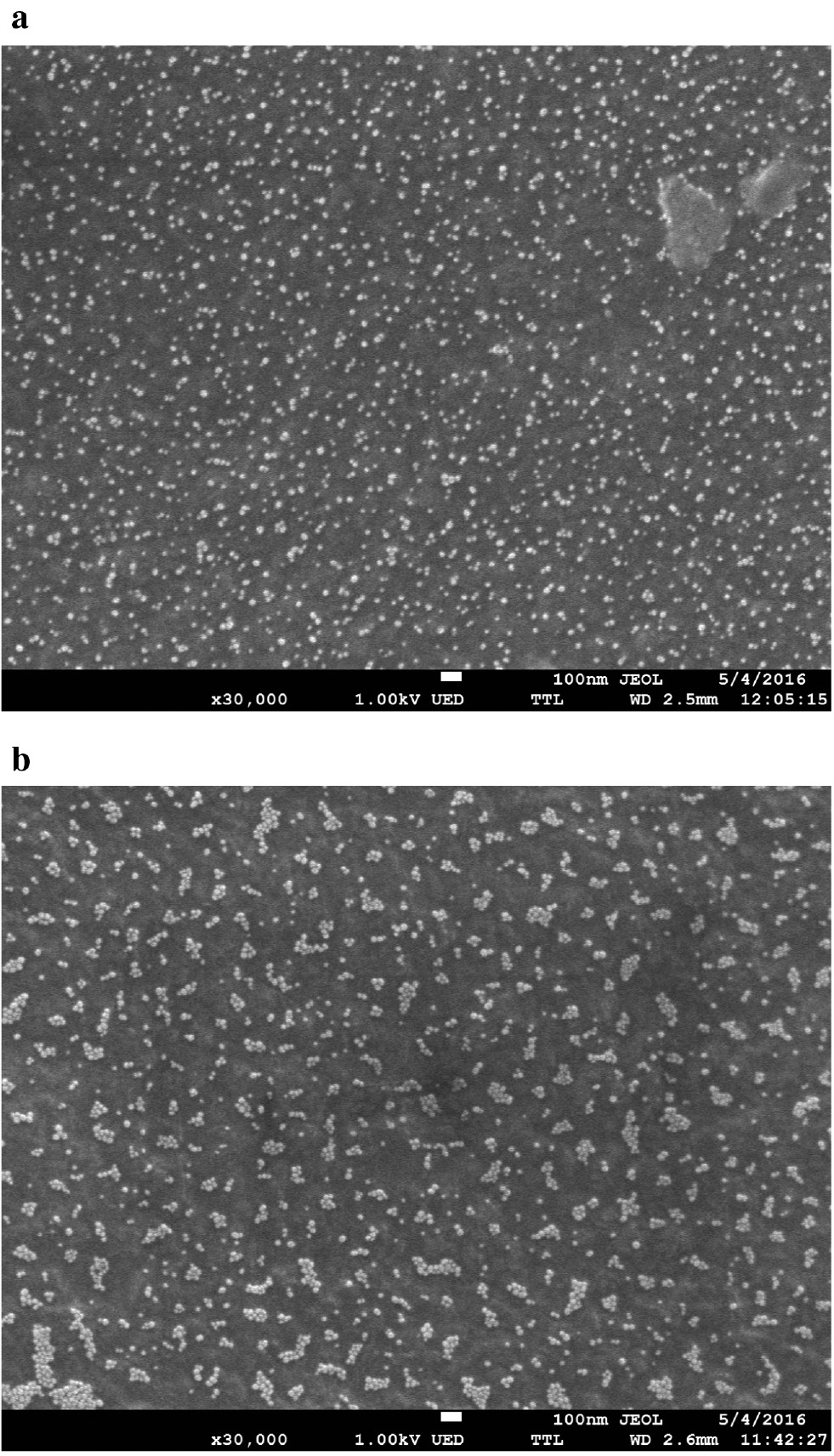
A TEM image of PUF–AuNPs/Cit nanocomposite before (a) and after (b) interaction with 1 μg mL−1 solution of cysteine.
It should be stressed that selectivity of AuNPs aggregation on the surface of PUF is essentially different compared with an aqueous solution (Fig. 3). Aggregation of AuNPs on PUF is caused by thiocompounds both in the case of AuNPs/Cit and in the case of AuNPs/Ion. This is quite different from the behavior of the same AuNPs in a solution that has been discussed earlier. A particularly strong difference is noticeable with respect to the cationic compounds and anions, which cause aggregation of the corresponding AuNPs in solution and do not cause it on PUF. Aggregation of AuNPs on PUF seems to be associated with some peculiarities. First of all, AuNPs on PUF have closer location to each other. In other words, they are “pre-ordered” to aggregation. This improves sensitivity of the system. Another factor facilitating aggregation is absence of excess of a stabilizer compared to a solution, since the main amount of a stabilizer is not adsorbed by PUF. However, at the same time, AuNPs on PUF are lower mobile. In addition, an important role affecting selectivity can be ascribed to the PUF adsorption properties, since this polymer is unable to absorb highly charged compounds. Thus, it can be concluded that aggregation of AuNPs on PUF depends mainly on the specific interactions, whereas aggregation in an aqueous solution is affected by the charge state of a compound.

Comparison of selectivity of AuNPs/Cit (a, b) and AuNPs/Ion (c, d) aggregation in a solution (a, c) and on PUF (b, d) in presence of cysteamine, cysteine, PHMG and sulfate.
Analytical capabilities in some thiocompounds determination by diffuse reflectance spectroscopy using PUF-based nanocomposites are illustrated in Table 4. The methods can achieve low limits of detection. It should also be noted that, in contrast to nanoparticles in solution, in the case of nanocomposites, an additional tool that increases sensitivity of the determination is increasing volume of the analyzed solution.
Features of merit for determination of thiocompounds using PUF–AuNPs nanocomposites and diffuse reflectance spectroscopy
| Determined thiocompound | Conditions of determination | Determination range, μg mL−1 | LOD, μg mL−1 |
|---|---|---|---|
| Cysteine | PUF-AuNPs/Cit, V=10 mL | 0.05–0.3 | 0.04 |
| PUF-AuNPs/Cit, V=50 mL | 0.02–0.15 | 0.01 | |
| PUF-AuNPs/Ion, V=10 mL | 0.03–0.10 | 0.02 | |
| Cysteamine | PUF-AuNPs/Ion, V=10 mL | 0.04–0.10 | 0.03 |
| Acetylcysteine | PUF-AuNPs/Ion, V=10 mL | 0.04–0.2 | 0.03 |
| Mercaptopropionic acid | PUF-AuNPs/Ion, V=10 mL | 0.06–0.2 | 0.03 |
Morphology
Another factor in this system which should be considered is the morphology of AuNPs. To compare AuNPs of different shape, we consider here capabilities of spherical AuNPs and AuNRs. Since we aim to discuss on the aspect of morphology, these two kinds of AuNPs should be considered in processes leading to change in their shape. One of such processes is covering of nanoparticles by metal layers. As an example, we have considered an interaction of catecholamines (dopamine, noradrenaline, adrenaline, and dobutamine) with silver nitrate in the presence of AuNRs. As a result of this interaction, a hypsochromic shift of absorption bands of AuNRs occurs. Along with this, the color of the solutions changes significantly. It was proposed by us earlier to use this effect for spectrophotometric determination of catecholamines [14]. The mechanism of these changes involves reduction of silver ions by catecholamines and deposition of metallic silver on the surface of AuNRs forming Au@Ag core-shell structures.
Comparison of AuNRs and spherical AuNPs in this process (by the example of interaction with adrenaline under various conditions) allows one to conclude that spectral changes in the case of AuNRs are much more abundant than for spherical AuNPs (Fig. 4). A pronounced shift of absorption bands can be noted for AuNRs whereas only some increase of their intensity can be found in the case of spherical AuNPs. The color changes of solutions are also more remarkable for AuNRs. The consequence of all this is the better analytical characteristics of AuNRs compared to spherical AuNPs, which is especially clearly seen for nanocomposites based on PUF (Table 5).
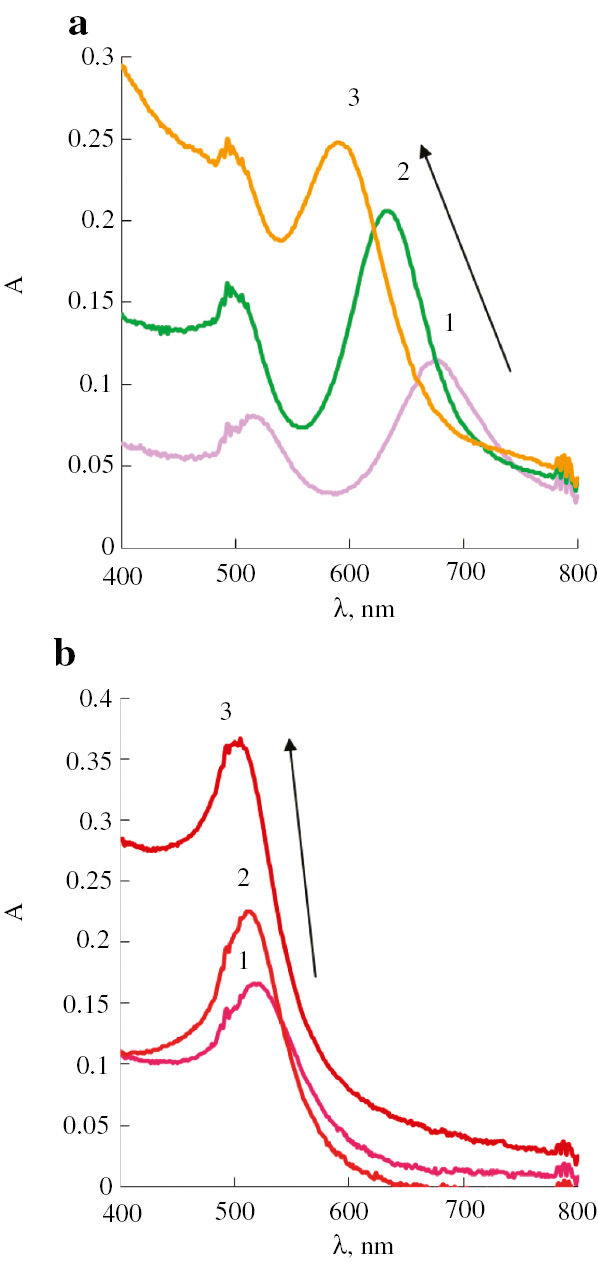
Absorption spectra of AuNRs (a) and spherical AuNPs (b) before (1) and after interaction with 2 μM adrenaline solution at 25°C (2) and 70°C (3). The color changes are illustrated by color of corresponding lines.
Features of merit for determination of adrenaline using AuNRs and spherical AuNPs.
| AuNRs | Spherical AuNPs | |
|---|---|---|
| Colloidal solutions of nanoparticles | ||
| LOD, μg mL−1 | 0.02 | 0.04 |
| 0.02a | 0.02a | |
| Determination range, μg mL−1 | 0.05–0.92 | 0.12–0.92 |
| 0.06–0.92a | 0.07–0.55a | |
| PUF-based nanocomposites | ||
| LOD, μg mL−1 | 0.07 | 0.6 |
| Determination range, μg mL−1 | 0.2–1.8 | – |
aDetermination was carried out at 70°C.
Conclusion
Based on the results of studies on the analytical capabilities of AuNPs in the methods of optical molecular absorption spectroscopy, we can conclude that the main factors affecting analytical characteristics and performance of AuNPs are the nature of nanoparticle stabilizer, its charge, morphology of nanoparticles, and state in which they are present in the system. Selectivity and sensitivity of charged analytes determination can be effectively changed by varying the nature of nanoparticle stabilizer. For analytes participating in specific interactions with AuNPs, these parameters depend strongly on the state in which AuNPs are present in the system. Morphology of nanoparticles is an effective tool to manage their analytical performance in those methods which utilize properties of AuNPs as a function of their geometry.
Article note
A collection of invited papers based on presentations at 21st Mendeleev Congress on General and Applied Chemistry (Mendeleev-21), held in Saint Petersburg, Russian Federation, 9–13 September 2019.
Funding source: Russian Science Foundation
Award Identifier / Grant number: 18-73-10001
Funding statement: This work was financially supported by the Russian Science Foundation (Funder Id: http://dx.doi.org/10.13039/501100006769, grant N 18-73-10001).
References
[1] S. N. Shtykov (Ed.) Nanoanalytics. Nanoobjects and Nanotechnologies in Analytical Chemistry, De Gruyter, Germany (2018).10.1515/9783110542011Suche in Google Scholar
[2] L. Wang, S. Song, D. Pan, D. Li, C. Fan. Pure Appl. Chem. 82, 81 (2010).10.1351/PAC-CON-09-01-20Suche in Google Scholar
[3] C. Zeng. Pure Appl. Chem. 90, 1409 (2018).10.1515/pac-2018-0511Suche in Google Scholar
[4] S. Manivannan, R. Ramaraj. Pure Appl. Chem. 83, 2041 (2011).10.1351/PAC-CON-11-03-04Suche in Google Scholar
[5] Y. A. Zolotov. J. Anal. Chem. 65, 1207 (2010).10.1134/S1061934810120014Suche in Google Scholar
[6] C.-C. Chang, C.-P. Chen, T.-H. Wu, C.-H. Yang, C.-W. Lin, C.-Y. Chen. Nanomaterials 9, 861 (2019) DOI: 10.3390/nano9060861.10.3390/nano9060861Suche in Google Scholar PubMed PubMed Central
[7] C. Coutinho, Á. Somoza. Anal. Bioanal. Chem. 411, 1807 (2019).10.1007/s00216-018-1450-7Suche in Google Scholar PubMed
[8] A. Y. Olenin. J. Anal. Chem. 74, 355 (2019).10.1134/S1061934819040099Suche in Google Scholar
[9] V. V. Apyari, S. G. Dmitrienko, M. V. Gorbunova, A. A. Furletov, Y. A. Zolotov. J. Anal. Chem. 74, 21 (2019).10.1134/S1061934819010052Suche in Google Scholar
[10] V. V. Apyari, V. V. Arkhipova, S. G. Dmitrienko, Y. A. Zolotov. J. Anal. Chem. 69, 1 (2014).10.1134/S1061934814010031Suche in Google Scholar
[11] G. Frens. Nat. Phys. Sci. 241, 20 (1973).10.1038/physci241020a0Suche in Google Scholar
[12] V. V. Apyari, S. G. Dmitrienko, V. V. Arkhipova, A. G. Atnagulov, M. V. Gorbunova, Y. A. Zolotov. Spectrochim. Acta A. 115, 416 (2013).10.1016/j.saa.2013.06.043Suche in Google Scholar PubMed
[13] V. V. Apyari, A. N. Ioutsi, V. V. Arkhipova, S. G. Dmitrienko, E. N. Shapovalova. Adv. Nat. Sci.: Nanosci. Nanotechnol. 6, 025002 (2015) DOI:10.1088/2043-6262/6/2/025002.10.1088/2043-6262/6/2/025002Suche in Google Scholar
[14] M. V. Gorbunova, V. V. Apyari, S. G. Dmitrienko, A. V. Garshev. Anal. Chim. Acta. 936, 185 (2016).10.1016/j.aca.2016.07.038Suche in Google Scholar PubMed
[15] V. V. Apyari, V. V. Arkhipova, M. V. Gorbunova, P. A. Volkov, A. I. Isachenko, S. G. Dmitrienko, Y. A. Zolotov. Talanta 161, 780 (2016).10.1016/j.talanta.2016.09.037Suche in Google Scholar PubMed
[16] M. V. Gorbunova, M. A. Matveeva, V. V. Apyari, A. V. Garshev, P. A. Volkov, S. G. Dmitrienko, Y. A. Zolotov. Nanotechnologies in Russia. 12, 185 (2017).10.1134/S1995078017020069Suche in Google Scholar
[17] M. V. Gorbunova, V. V. Apyari, I. I. Zolotov, S. G. Dmitrienko, A. V. Garshev, P. A. Volkov, V. E. Bochenkov. Gold Bull. 52, 115 (2019).10.1007/s13404-019-00267-9Suche in Google Scholar
[18] P. Rouster, M. Pavlovic, T. Cao, B. Katana, I. Szilagyi. J. Phys. Chem. C. 123, 12966 (2019).10.1021/acs.jpcc.9b03983Suche in Google Scholar
[19] S. Sáringer, R. A. Akula, A. Szerlauth, I. Szilagyi. J. Phys. Chem. B. 123, 9984 (2019).10.1021/acs.jpcb.9b08799Suche in Google Scholar PubMed
[20] S. Sáringer, P. Rouster, I. Szilágyi. Langmuir. 35, 4986 (2019).10.1021/acs.langmuir.9b00242Suche in Google Scholar PubMed
[21] V. V. Apyari, V. V. Arkhipova, A. I. Isachenko, P. A. Volkov, S. G. Dmitrienko, I. I. Torocheshnikova. Sens. Actuat. B. 260, 953 (2018).10.1016/j.snb.2018.01.118Suche in Google Scholar
[22] S. G. Dmitrienko, Y. A. Zolotov. Russ. Chem. Rev. 71, 159 (2002).10.1070/RC2002v071n02ABEH000703Suche in Google Scholar
[23] L. Sprunger, W. E. Acree Jr., M. H. Abraham. Anal. Chem. 79, 6891 (2007).10.1021/ac071384fSuche in Google Scholar PubMed
[24] Z. Zhang, L. Zhu, J. Jin. Cailiao Daobao/Materials Review 31, 34 (2017).Suche in Google Scholar
©2020 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
Artikel in diesem Heft
- Frontmatter
- In this issue
- Preface
- Research papers from the 21st Mendeleev Congress on General and Applied Chemistry
- Conference papers of the 21st Mendeleev Congress on General and Applied Chemistry
- Unusual behavior of bimetallic nanoparticles in catalytic processes of hydrogenation and selective oxidation
- Soft chemistry of pure silver as unique plasmonic metal of the Periodic Table of Elements
- Catalytic hydrogenation with parahydrogen: a bridge from homogeneous to heterogeneous catalysis
- Azidophenylselenylation of glycals towards 2-azido-2-deoxy-selenoglycosides and their application in oligosaccharide synthesis
- Bis-γ-carbolines as new potential multitarget agents for Alzheimer’s disease
- Octafluorobiphenyl-4,4′-dicarboxylate as a ligand for metal-organic frameworks: progress and perspectives
- Some aspects of the formation and structural features of low nuclearity heterometallic carboxylates
- Particular kinetic patterns of heavy oil feedstock hydroconversion in the presence of dispersed nanosize MoS2
- Concentration profiles around and chemical composition within growing multicomponent bubble in presence of curvature and viscous effects
- Application of gold nanoparticles in the methods of optical molecular absorption spectroscopy: main effecting factors
- Membrane materials for energy production and storage
- New types of the hybrid functional materials based on cage metal complexes for (electro) catalytic hydrogen production
- Conference paper of the 15th Eurasia Conference on Chemical Sciences
- Discovery of bioactive drug candidates from some Turkish medicinal plants-neuroprotective potential of Iris pseudacorus L.
Artikel in diesem Heft
- Frontmatter
- In this issue
- Preface
- Research papers from the 21st Mendeleev Congress on General and Applied Chemistry
- Conference papers of the 21st Mendeleev Congress on General and Applied Chemistry
- Unusual behavior of bimetallic nanoparticles in catalytic processes of hydrogenation and selective oxidation
- Soft chemistry of pure silver as unique plasmonic metal of the Periodic Table of Elements
- Catalytic hydrogenation with parahydrogen: a bridge from homogeneous to heterogeneous catalysis
- Azidophenylselenylation of glycals towards 2-azido-2-deoxy-selenoglycosides and their application in oligosaccharide synthesis
- Bis-γ-carbolines as new potential multitarget agents for Alzheimer’s disease
- Octafluorobiphenyl-4,4′-dicarboxylate as a ligand for metal-organic frameworks: progress and perspectives
- Some aspects of the formation and structural features of low nuclearity heterometallic carboxylates
- Particular kinetic patterns of heavy oil feedstock hydroconversion in the presence of dispersed nanosize MoS2
- Concentration profiles around and chemical composition within growing multicomponent bubble in presence of curvature and viscous effects
- Application of gold nanoparticles in the methods of optical molecular absorption spectroscopy: main effecting factors
- Membrane materials for energy production and storage
- New types of the hybrid functional materials based on cage metal complexes for (electro) catalytic hydrogen production
- Conference paper of the 15th Eurasia Conference on Chemical Sciences
- Discovery of bioactive drug candidates from some Turkish medicinal plants-neuroprotective potential of Iris pseudacorus L.

