Abstract
This document summarizes and extends definitions and notations for the description of tactic polymers and the diad structures of which they are composed. It formally recognizes and resolves apparent inconsistencies between terminology used in the polymer field to describe tactic polymers and terminology in more common use in organic chemistry. Specifically, the terms m and r diads are recommended to replace the terms meso and racemo diads. The definitions are also updated from those in the existing Stereochemistry Document to use the term ‘stereogenic centre’, rather than ‘chiral or prochiral atoms’. Further, the terms relating to tacticity have been defined for the constituent macromolecules, rather than for the polymers composed of those macromolecules. Therefore, this document also forms an addendum and corrigendum to the 1981 document, ‘Stereochemical definitions and notations relating to polymers’.
SD-1 Introduction
In 1981, the former IUPAC Commission on Macromolecular Nomenclature published the document, ‘Stereochemical definitions and notations relating to polymers’ [1], which was reprinted in the Compendium of Macromolecular Nomenclature, better known as the first (1991) edition of the IUPAC ‘Purple Book’ [2], and again, with minor editorial corrections, in the second (2008) edition of the ‘Purple Book’ (the Compendium of Polymer Terminology and Nomenclature) [3].
With the current document, it is intended to formally recognize and resolve apparent inconsistencies between terminology in the polymer field and that in more common use in organic chemistry, as described in the ‘Basic terminology of stereochemistry’ [4] and the IUPAC ‘Blue Book’ [5]. Recommendations are provided to minimize any potential confusion. Also, some of the definitions in [1], [2], [3] are inconsistent with those in the ‘Glossary of Basic Terms in Polymer Science’, published in 1996 [6], and hence with those in the Compendium of Chemical Terminology (the IUPAC ‘Gold Book’) [7]. Since both of these documents [1], [6] are reprinted in the ‘Purple Book’ [3], there is also inconsistency within that book.
The current document summarizes, revises, and extends the basic definitions and notations relating to tactic polymers that were introduced in the Stereochemical Document [1], [2], [3]. It not only forms an addendum and corrigendum to the 1981 document [1], [2], [3], which it supersedes in part, but also replaces the corresponding definitions in [6], [7].
Attention is also drawn to the fact that the sections relating to crystalline polymers in the Stereochemical Document [1], [2], [3] have been superseded with the publication of the revised ‘Definitions of terms relating to crystalline polymers’ [8] in 2011.
SD-2 Terms for chirality centres
Centres or atoms referred to in the existing Stereochemical Document [1], [2], [3] as ‘chiral centres’ or ‘chiral atoms’ should be described as ‘chirality centres’, or as ‘stereogenic centres’ or ‘stereogenic units’, as appropriate depending on context [4]. Some relevant definitions from [4] are reproduced below.
SD-2.1 chirality centre
An atom holding a set of ligands in a spatial arrangement that is not superposable on its mirror image [4].
Note: Chirality centres are a subgroup of stereogenic centres, which are in turn a subgroup of stereogenic units.
SD-2.2 stereogenic unit
A grouping of atoms within a molecular entity that may be considered a focus of stereoisomerism [4].
Note 1: At least one of these must be present in every chiral chemical species (conversely, the presence of stereogenic units does not imply that the corresponding chemical species are chiral).
Note 2: Modified from [4].
SD-2.3 diastereoisomerism
Stereoisomerism other than enantiomerism.
Note 1: Diastereoisomers (or diastereomers) are stereoisomers not related as mirror images.
Note 2: Diastereoisomers are characterized by differences in physical properties and by some differences in chemical behaviour towards both achiral and chiral reagents.
Note 3: Definition from [4].
SD-2.4 diastereomorphism
The relationship between objects (or models) analogous to that between diastereoisomeric molecular entities.
Note: Definition from [4].
SD-2.5 enantiomer
One of a pair of molecular entities that are mirror images of each other and non-superposable.
Note: Definition from [4].
SD-2.6 enantiomorph
One of a pair of chiral objects or models that are non-superposable mirror images of each other.
Note 1: The adjective enantiomorphic is also applied to mirror-image related groups within a molecular entity.
Note 2: Definition from [4].
SD-3 Use of rotated Fischer projections in polymer publications
To eliminate possible confusion for those not familiar with the polymer literature, in particular with the increased use of zigzag formulae with wedges to indicate configuration, it is recommended that rotated Fischer projections should not be used in any publication unless (a) it is specified as such at or before its first usage and (b) an appropriate reference (e.g., [1], [2], [3], alone or in combination with this document) is given to explain what is meant by a rotated Fischer projection.
SD-3.1 Fischer projection
Projection formula in which vertically drawn bonds are considered to lie below the projection plane and horizontal bonds to lie above that plane. Thus, for the molecule comprising a tetrahedral carbon with substituents a, b, c, and d:
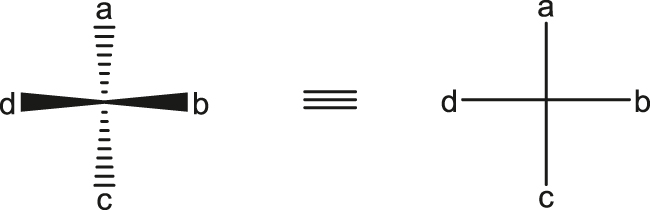
Note: Definition from [4].
SD-3.2 rotated Fischer projection
Projection formula for macromolecules in which horizontal lines denote polymer backbone bonds. At each individual backbone carbon atom, horizontal lines represent bonds directed below the projection plane and vertical lines represent bonds directed above that plane.
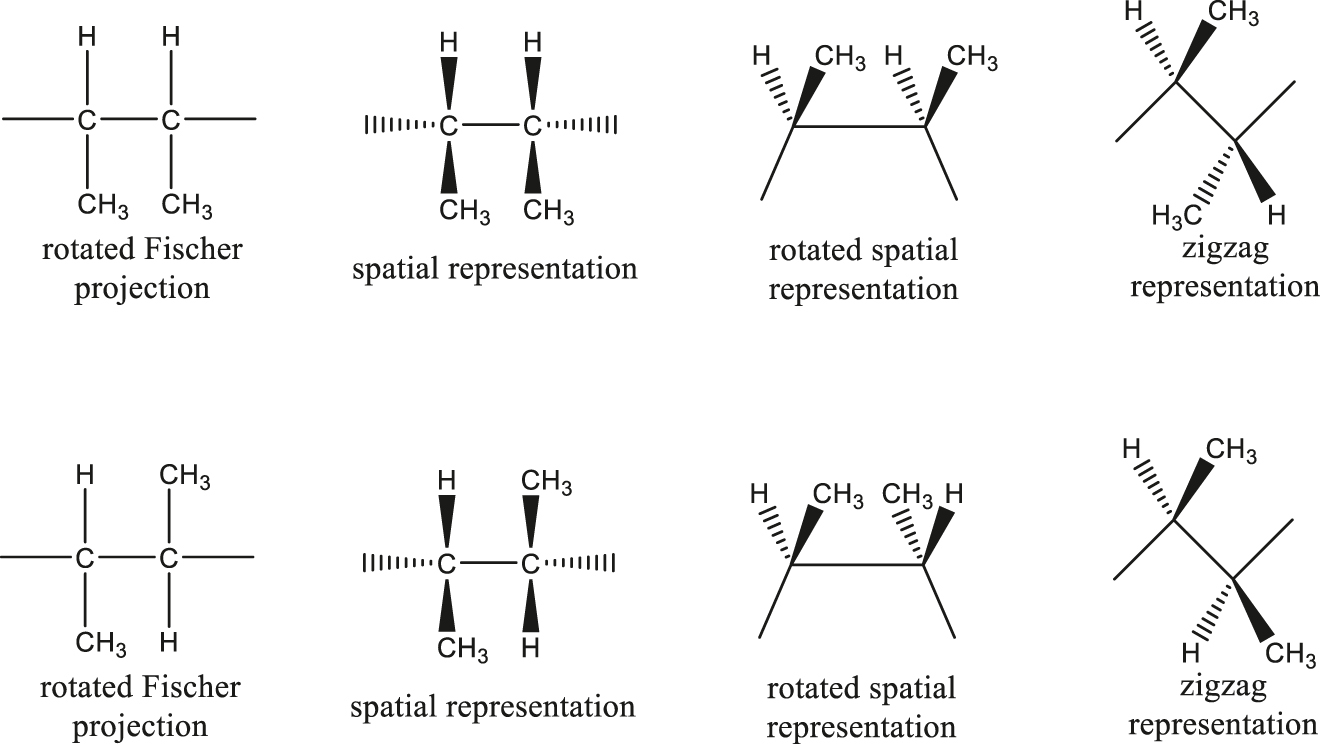
Note: Definition derived from the description in [1].
SD-4 Terms describing relative configuration (m and r structures)
Relative configuration is to be referred to, where appropriate, as m and r structures. The section in the Stereochemistry Document [1], [2], [3] entitled, ‘meso and racemo structures’ should be retitled, ‘m and r structures’.
The historical connection of the terms m and r to meso and racemo, respectively, when describing structures with a symmetrically-constituted connecting group between stereogenic centres of a polymer backbone, such as –CH2–, –CH2–CH2–, and –CR2–CH2–CR2–, is recognized. However, the usage of meso and racemo in this context is not acceptable because of the potential for confusion with the similar terms used in organic chemistry [4], [5], in particular since a single molecule cannot be racemic. The terms meso and racemo are clearly incorrect if intended to describe structures where the connecting groups between stereogenic centres are not symmetrically-constituted, such as in, for example, poly(oxypropylene), where the connecting group is –CH2–O–.
It is now recommended that the relative configuration of consecutive, but not necessarily contiguous, constitutionally equivalent stereogenic centres be designated as m or r, as appropriate (Fig. 1). The m- descriptor, formerly an abbreviation for meso, refers to a structure where the configuration of adjacent stereogenic centres is maintained [9]. The r descriptor, previously an abbreviation for racemo, describes a structure in which the relative configuration of adjacent stereogenic centres is reversed [9]. The connecting group need not be symmetrically constituted. An example is provided in Fig. 2.
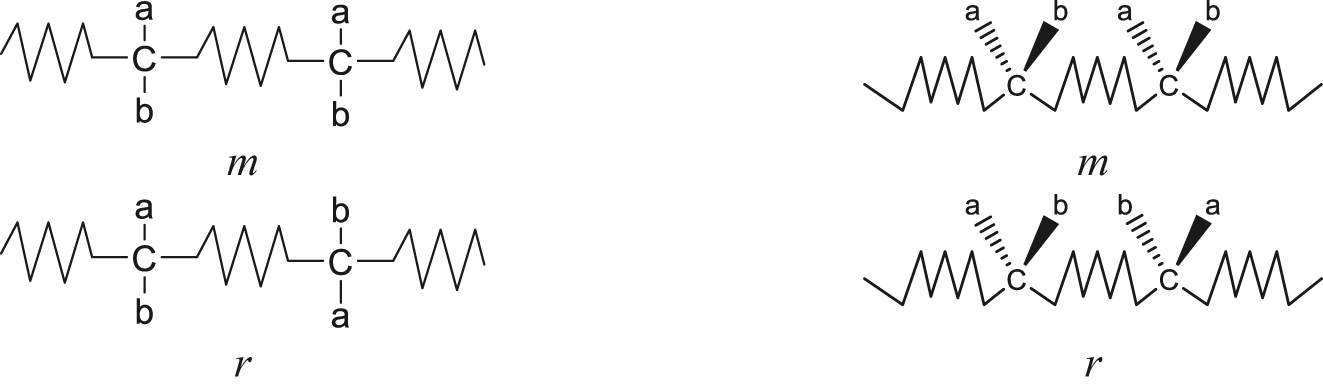
Diagrammatic representations of individual m and r diads as rotated Fischer projections (left) and as zigzag Natta projections (right). The zigzag line –/\/\/\– represents a connecting group or bond, which need not be symmetrically-constituted.
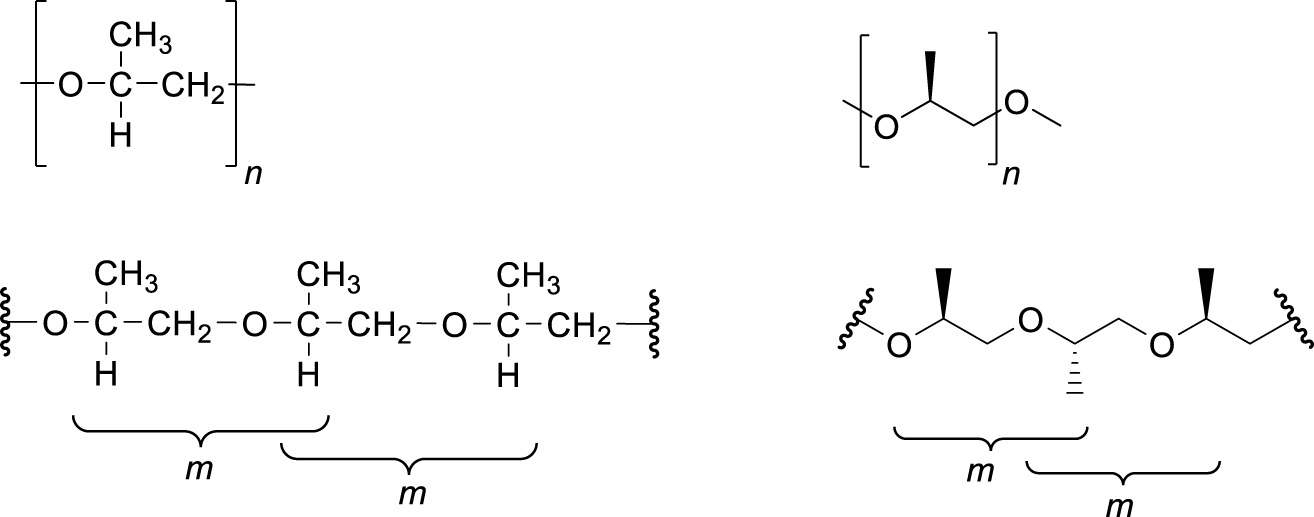
Diagrammatic representations of the configurational repeating unit or stereorepeating unit (upper), and the diad sequence (lower) for isotactic poly(oxypropylene), both as a rotated Fischer projections (left) and as a zigzag projection (right).
Higher order sequences are usually described as a sequence of diads. Thus, there are mm, rm, mr and rr triads. The mr and rm triads are not distinguishable structures as long as the connector is symmetric. If the connector is non-symmetric, they may be diastereomeric. The mm diad is often referred to as an isotactic triad, the rr triad as a syndiotactic triad and the mr and rm triads as heterotactic triads.
SD-4.1 m diad
Pair of adjacent constitutionally equivalent stereogenic units that are of like configuration.
Note: Usage of the term meso diad for describing structures with a symmetrically constituted connecting group is no longer acceptable because of the potential for confusion with the similar term used in organic chemistry to describe small molecules.
SD-4.2 r diad
Pair of adjacent constitutionally equivalent stereogenic units that are of unlike configuration.
Note: Usage of the term racemo diad for describing structures with a symmetrically-constituted connecting group is no longer acceptable because of the potential for confusion with the similar term used in organic chemistry to describe small molecules.
SD-5 Terms describing tactic polymers
It is worth recalling a comment from the introduction to the Polymer Stereochemistry Document [1], which should be considered when dealing with the configuration and stereochemistry of polymers. It reads:
“In order to present clear concepts, it is necessary that idealized definitions be adopted, but it is recognized that the realities of polymer science must be faced. Deviations from ideality arise with polymers at both molecular and bulk levels in ways that have no parallel with the ordinary small molecules of organic or inorganic chemistry. Although such deviations are not explicitly taken into account in the definitions below, the nomenclature recommended can usefully be applied to the predominant structural features of real polymer molecules, if necessary with self-explanatory, if imprecise, qualifications, such as ‘almost completely isotactic’ or ‘highly syndiotactic’. Although such expressions lack the rigour beloved of the purist, every experienced polymer scientist knows that communication in this discipline is impossible without them.”
Given its importance, the substance of this comment is now included as notes to the specific terms.
The definitions are also revised from those in the Stereochemistry Document [1], [2], [3] to use the term ‘stereogenic centre’, rather than ‘chiral or prochiral atoms’ (see SD-2). Further, the constituent macromolecules, rather than the polymers composed of those macromolecules, are defined.
The basic definitions of configurational unit, configurational base unit, configurational repeating unit, and stereorepeating unit are reiterated here [1], [2], [3]. They are, however, given in the wording of the ‘Gold Book’ [7] as updated in 1996 [6].
For ease of reference and to assist the reader, in Section SD-5, cross-references to terms also defined in this section are denoted in italic typeface.
SD-5.1 configurational unit
Constitutional unit having at least one site of defined configuration.
Note 1: The term configurational unit can also be used when the only stereogenic units are in side chains.
SD-5.2 configurational base unit
Constitutional repeating unit in a regular macromolecule, a regular oligomer molecule, a regular block, or a regular chain, the configuration of which is defined at least at one site of stereoisomerism in the main chain [3], [6].
Note: In a regular macromolecule, a configurational base unit corresponds to the constitutional repeating unit.
SD-5.3 configurational repeating unit
Smallest set of successive configurational base units that prescribes configurational repetition at one or more sites of stereoisomerism in the main chain of a regular macromolecule, a regular oligomer molecule, a regular block, or a regular chain [3], [6].
Note: For a syndiotactic macromolecule, the configurational repeating unit will be composed of two constitutional repeating units or configurational base units.
SD-5.4 stereorepeating unit
Configurational repeating unit having defined configuration at all sites of stereoisomerism in the main chain of a regular macromolecule, a regular oligomer molecule, a regular block, or a regular chain [3], [6].
Note 1: Two configurational units that correspond to the same constitutional unit are considered to be enantiomorphic if they are non-superposable mirror images. Two non-superposable configurational units that correspond to the same constitutional unit are considered to be diastereomorphic if they are not mirror images.
SD-5.5 tactic macromolecule
Regular macromolecule in which essentially all configurational repeating units are identical.
Note 1: The prefix ta- is recommended to indicate a tactic macromolecule.
SD-5.6 tactic polymer
Polymer composed of tactic macromolecules [6], [7].
Note 1: The prefix ta- is recommended to indicate a tactic polymer.
SD-5.7 isotactic macromolecule
Regular macromolecule having only one species of configurational base unit, which has stereogenic units in the main chain in a unique arrangement with respect to its adjacent constitutional units.
Note 1: In an isotactic macromolecule, the configurational repeating unit is identical with the configurational base unit.
Note 2: A macromolecule consisting only of m diads is isotactic.
Note 3: It is common practice to use the term isotactic macromolecule to refer to macromolecules with predominantly (i.e. larger than 95 %) m diads. When a such a macromolecule has a measurable quantity of r diads, it should be explicitly mentioned and, where possible, the tacticity should be quantitatively described. Use of the term isotactic to describe a macromolecule that contains a significant fraction of r diads (i.e. larger than 5 %) is not acceptable.
Note 4: The prefix it- is recommended to indicate an isotactic macromolecule.
Note 5: Isotactic polypropylene can be depicted by a rotated Fischer Projection (left) or a zigzag projection (right) as follows. The configurational repeating unit is shown within brackets.
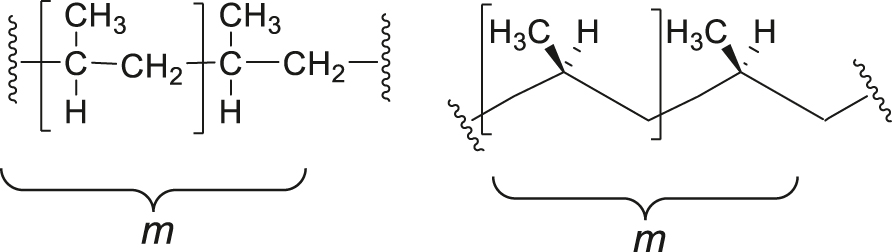
SD-5.8 isotactic polymer
Polymer composed of isotactic macromolecules [6], [7].
Note 1: The prefix it- is recommended to indicate an isotactic polymer.
SD-5.9 syndiotactic macromolecule
Regular macromolecule consisting of alternating enantiomorphic configurational base units, which have stereogenic centres in the main chain in a unique arrangement with respect to their adjacent constitutional units.
Note 1: In a syndiotactic macromolecule, the configurational repeating unit consists of two configurational base units that are enantiomorphic.
Note 2: A macromolecule consisting only of r diads is syndiotactic.
Note 3: It is common practice to use the term syndiotactic macromolecule to refer to macromolecules with predominantly (i.e. larger than 95 %) r diads. When a such a macromolecule has a measurable quantity of m diads, it shouldbe explicitly mentioned and, where possible, the tacticity should be quantitatively described. Use of theterm syndiotactic to describe a macromolecule that contains a significant fraction of m diads (i.e. larger than 5 %) is not acceptable.
Note 4: The prefix st- is recommended to indicate a syndiotactic macromolecule.
Note 5: Syndiotactic polypropylene can be depicted by a rotated Fischer Projection (left) and zigzag projection (right) as follows. The configurational repeating unit is shown within brackets.
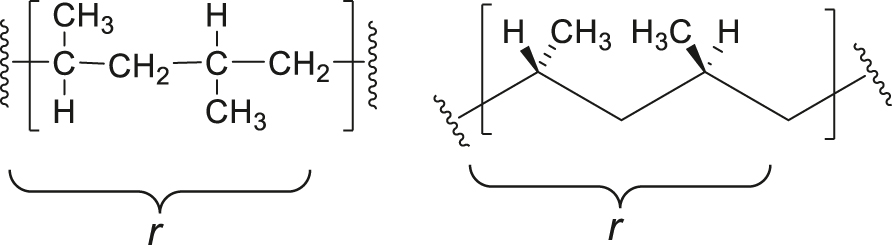
SD-5.10 syndiotactic polymer
Polymer composed of syndiotactic macromolecules [6], [7].
Note 1: The prefix st- is recommended to indicate a syndiotactic polymer.
SD-5.11 heterotactic macromolecule
Regular macromolecule having a configurational repeating unit comprising four configurational base units, the first two stereogenic units of which are identical and enantiomorphic to the last two.
Note 1: A macromolecule consisting only of m and r diads in alternating sequence (i.e. mr and rm triads) is heterotactic. The configurational repeating unit consists of four configurational base units.
Note 2: The prefix ht- is recommended to indicate a heterotactic macromolecule.
Note 3: A heterotactic polypropylene can be depicted by a rotated Fischer Projection (top) and zigzag projection (bottom) as follows. The configurational repeating unit, a mrm tetrad, is shown within brackets. The configurational repeating unit can be equally well defined as a rmr terad.
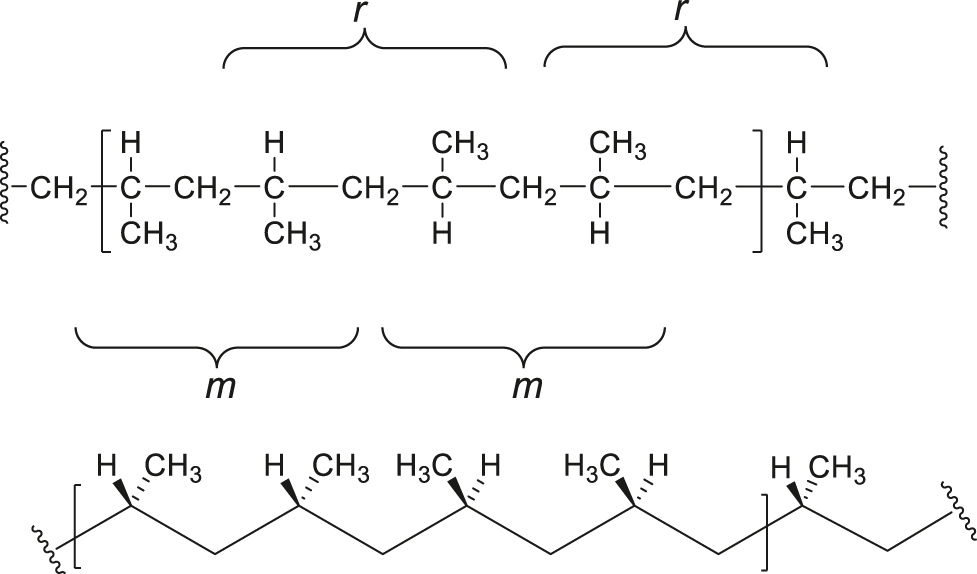
SD-5.12 heterotactic polymer
Polymer composed of heterotactic macromolecules.
Note: The prefix ht- is recommended to indicate a heterotactic polymer.
SD-5.13 atactic macromolecule
Regular macromolecule having essentially equal numbers of the possible enantiomorphic configurational base units that have stereogenic units in the main chain in a random sequence distribution.
Note 1: A macromolecule consisting of an essentially equal number of m and r diads in random sequence is atactic.
Note 2: It is common practice to use the term atactic macromolecule to refer to macromolecules where the number of possible configurational base units is almost equal, for example, macromolecules formed by radical polymerization of monosubstituted monomers (they usually contain a small [2 to 5 %] but experimentally verifiable excess of r diads). When a such a macromolecule has a measurable imbalance of m and r diads, it should be explicitly mentioned and, where possible, the tacticity should be described quantitatively. Use of the term atactic to describe a macromolecule which departs significantly (i.e., by more than 5 %) from a 1:1 ratio of m and r diads is not acceptable.
Note 3: The use of the term atactic to refer to a macromolecule where the stereochemical composition is simply unknown or not determined is not acceptable.
Note 4: The prefix at- is recommended to indicate an atactic macromolecule.
Note 5: Adapted from [1], [2], [3]. A different definition was given in [6].
Note 6: An atactic polypropylene can be depicted by a rotated Fischer Projection (top) and zigzag projection (bottom) as follows. The quantitative diad description follows from that used to describe lactic acid-based polymers [10].
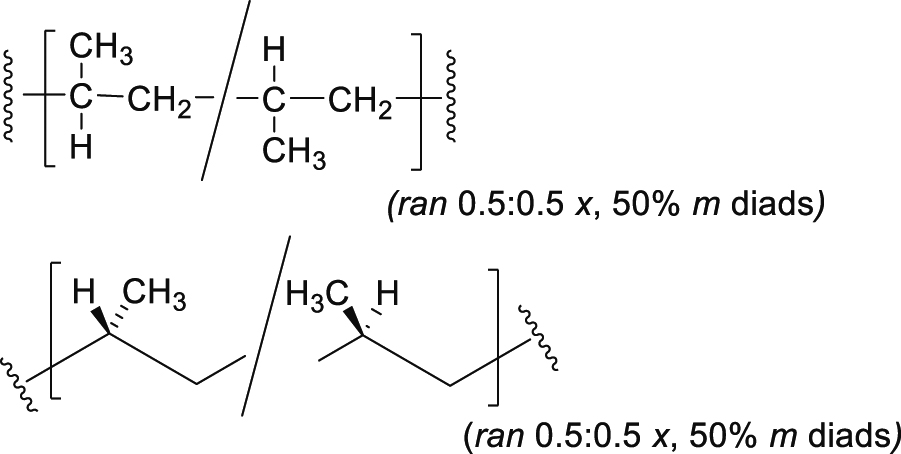
SD-5.14 atactic polymer
Polymer composed of atactic macromolecules [6], [7].
Note 1: The prefix at- is recommended to indicate an atactic polymer.
SD-6 Memberships of sponsoring bodies
Membership of the IUPAC Division of Chemical Nomenclature and Structure Representation for the period 2018–2019 is as follows:
President: A. T. Hutton (South Africa); Past President: K.-H. Hellwich (Germany); Secretary: R. S. Laitinen (Finland); Titular Members: M. A. Beckett (UK); E. C. Constable (Switzerland); T. Damhus (Denmark); R. T. Macaluso (USA); E. Nordlander (Sweden); A. P. Rauter (Portugal); M. M. Rogers (USA); Associate Members: E. Mansfield (USA); J. Nagy (Hungary); M. A. Strausbaugh (USA); K. T. Taylor (USA); C. A. Tovee (UK); J. Vohlídal (Czech Republic); National Representatives: F. Aricò (Italy); N. Burford (Canada); A. M. da Costa Ferreira (Brazil); S. Erdem (Turkey); S. Koo (Korea); R. Kruszyński (Poland); L. Meesuk (Thailand); M. A. Petrova (Bulgaria); E. Szabó (Slovakia); A. Yerin (Russia); Ex Officio: R. M. Hartshorn (New Zealand); G. P. Moss (UK).
Membership of the IUPAC Polymer Division Committee for the period 2018–2019 is as follows:
President: G. T. Russell (New Zealand); Vice President: C. K. Luscombe (USA); Secretary: M. G. Walter (USA); Past President: M. Buback (Germany); Titular Members: C. Fellows (Australia); R. C. Hiorns (France); R. Hutchinson (Canada); I. Lacík (Slovakia); N. Stingelin (USA); P. D. Topham (UK); Y. Yagci (Turkey); Associate Members: S. Beuermann (Germany); M. C. H. Chan (Malaysia); C. dos Santos (Brazil); D. S. Lee (South Korea); G. Moad (Australia); P. Théato (Germany); National Representatives: R. Adhikari (Nepal); J. He (China); M. Hess (Germany); V. Hoven (Thailand); C.-S. Hsu (Taipei, China); P. Mallon (South Africa); O. Philippova (Russia); M. Sawamoto (Japan); A. Sturcova (Czech Republic); J. van Hest (Netherlands).
Membership of the Subcommittee on Polymer Terminology during the preparation of these Recommendations (2009–2018) was as follows:
Chair: R. G. Jones (UK), 2006-2013; R. C. Hiorns (France), from 2014; Secretary: T. Kitayama (Japan), 2008-2009; R. C. Hiorns (France), 2010-2013; C. K. Luscombe (USA), 2014-2015; P. D. Topham (UK), from 2016; Members: V. Abetz (Germany); R. Adhikari (Nepal); G. Allegra (Italy); M. Barón (Argentina); R. Boucher (UK); P. Carbone (UK); M. C. H. Chan (Malaysia); T. Chang (Korea); J. Chen (USA); C. Fellows (Australia); A. Fradet (France); C. F. O. Graeff (Brazil); J. He (China); K.-H. Hellwich (Germany); M. Hess (Germany); P. Hodge (UK); W. Hu (China); A. D. Jenkins (UK); J.-I. Jin (Korea); J. Kahovec (Czech Republic); T. Kitayama (Japan); P. Kratochvíl (Czech Republic); P. Kubisa (Poland); M. Malinconico (Italy); J. B. Matson (USA); S. V. Meille (Italy); J. Merna (Czech Republic); G. Moad (Australia); W. Mormann (Germany); T. Nakano (Japan), C. K. Ober (USA); M. Peeters (Netherlands); S. Penczek (Poland); O. Philippova (Russia); M. D. Purbrick (UK); G. Raos (Italy); G. Russell (USA); C. dos Santos (Brazil); C. Scholz (USA); F. Schué‡ (France); S. Słomkowski (Poland); D. W. Smith (USA), R. F. T. Stepto‡ (UK); N. Stingelin (UK); A. Sturcova (Czech Republic); D. Tabak (Brazil); P. Théato (Germany); J.-P. Vairon (France); M. Vert (France); J. Vohlídal (Czech Republic); M. G. Walter (USA); E. S. Wilks (USA); W. J. Work (USA); M.-H. Yoon (Korea).
Funding source: International Union of Pure and Applied Chemistry
Award Identifier / Grant number: 2009-047-1-400
Acknowledgements
The role of Aubrey D. Jenkins, Tatsuki Kitayama, and the late Robert F. T. Stepto at the inception of the project is gratefully acknowledged. We are also grateful for the input of Andrey Yerin for his assistance in the finalization of this project.
-
Funding: This work was prepared under project 2009-047-1-400 of IUPAC (Funder ID: 10.13039/100006987).
References
[1] A. D. Jenkins. Pure Appl. Chem.53, 733 (1981); reprinted as chapter 2 in [2]; reprinted as chapter 2 in [3].Suche in Google Scholar
[2] IUPAC. in Compendium of Macromolecular Nomenclature (the ‘Purple Book’). W. V. Metanomski (Ed.), Blackwell Scientific Publications, Oxford, UK (1991).Suche in Google Scholar
[3] IUPAC. Compendium of Polymer Terminology and Nomenclature (IUPAC Recommendations 2008) (the ‘Purple Book’). Prepared for publication by R. G. Jones, J. Kahovec, R. Stepto, E. S. Wilks, M. Hess, T. Kitayama, W. V. Metanomski, with advice from A. Jenkins and P. Kratochvíl, RSC Publishing, Cambridge, UK (2008).Suche in Google Scholar
[4] G. P. Moss. Pure Appl. Chem.68, 2193 (1996).10.1351/pac199668122193Suche in Google Scholar
[5] IUPAC. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (the ‘Blue Book’). Prepared for publication by H. A. Favre and W. H. Powell, Royal Society of Chemistry, Cambridge, UK (2014); errata: https://www.qmul.ac.uk/sbcs/iupac/bibliog/BBerrors.html.Suche in Google Scholar
[6] A. D. Jenkins, P. Kratochvíl, R. F. T. Stepto, U. W. Suter. Pure Appl. Chem.68, 2287 (1996); reprinted with some changes as chapter 1 in [3].10.1351/pac199668122287Suche in Google Scholar
[7] IUPAC. Compendium of Chemical Terminology (the ‘Gold Book’). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Science, Oxford, UK, 2nd ed. (1997); XML on-line corrected version: http://goldbook.iupac.org (2006) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. D. Jenkins. (https://doi.org/10.1351/goldbook).Suche in Google Scholar
[8] S. V. Meille, G. Allegra, P. H. Geil, J. He, M. Hess, J.-I. Jin, P. Kratochvíl, W. Mormann, R. Stepto. Pure Appl. Chem.83, 1831 (2011).10.1351/PAC-REC-10-11-13Suche in Google Scholar
[9] M. Farina. in Topics in Stereochemistry, E. L. Eliel and S.H. Wilen (Eds.), Vol. 17, J. Wiley & Sons, New York, pp. 1–111 (1987); from a suggestion by P. Sozzani (see note 114).Suche in Google Scholar
[10] M. Vert, J. Chen, K.-H. Hellwich, P. Hodge, T. Nakano, C. Scholz, S. Slomkowski, J. Vohlidal. Pure Appl. Chem.92, 193 (2020).10.1515/pac-2017-1007Suche in Google Scholar
© 2020 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- IUPAC Technical Report
- Global occurrence, chemical properties, and ecological impacts of e-wastes (IUPAC Technical Report)
- IUPAC Recommendations
- Definitions and notations relating to tactic polymers (IUPAC Recommendations 2020)
- Glossary of methods and terms used in surface chemical analysis (IUPAC Recommendations 2020)
- Terminology of polymers in advanced lithography (IUPAC Recommendations 2020)
Artikel in diesem Heft
- Frontmatter
- IUPAC Technical Report
- Global occurrence, chemical properties, and ecological impacts of e-wastes (IUPAC Technical Report)
- IUPAC Recommendations
- Definitions and notations relating to tactic polymers (IUPAC Recommendations 2020)
- Glossary of methods and terms used in surface chemical analysis (IUPAC Recommendations 2020)
- Terminology of polymers in advanced lithography (IUPAC Recommendations 2020)

