Abstract
Rapid development of research on the chemistry of boronic acids is connected with their applications in organic synthesis, analytical chemistry, materials’ chemistry, biology and medicine. In many applications Lewis acidity of boron atoms plays an important role. Special group of arylboronic acids are fluoro-substituted compounds, in which the electron withdrawing character of fluorine atoms influences their properties. The present paper deals with fluoro-substituted boronic acids and their derivatives: esters, benzoxaboroles and boroxines. Properties of these compounds, i.e. acidity, hydrolytic stability, structures in crystals and in solution as well as spectroscopic properties are discussed. In the next part examples of important applications are given.
Introduction
Arylboronic acids have huge importance in chemistry. They are applied among others in organic synthesis, electrochemistry, separation techniques, building fluorophores and sensors, polymers, materials’ chemistry and pharmacy [1], [2]. These applications require particular compounds of specific properties, both physical and chemical, e.g. acidity, solubility, stability or possibility of functionalization. Fluoroarylboronic acids and their derivatives play an important role in the above-mentioned applications and their role is still growing.
Introduction of a fluorine substituent into organic compounds significantly changes their physical properties such as: heat of vaporization, critical temperature, density, viscosity and other. Even more important is the influence of fluorine atom on the chemical properties of a given molecule [3]. The extreme electronegativity of fluorine substituent induces a strong withdrawing inductive effect, making fluorine a σ-electron acceptor, whereas the electron-donating resonance effect of its lone-pair electrons allows the fluorine atom to be considered as a π-electron donor as well. These electronic properties originating from the presence of fluorine in the molecule make the effects on, for example, acidity and basicity. Fluorine is inductively electron-withdrawing but electron-donating by resonance, while perfluoroalkyl groups (e.g. CF3) are only electron-withdrawing [4]. Fluorine substituents change also the quadrupole moment in aromatic ring [5], [6] and the lipophilicity of compound. The ability of fluorine to participate in formation of hydrogen bonds is crucial to biological activity of fluorochemicals [3].
History of scientific interest in a fluorophenylboronic acids goes back to the beginning of the nineties of the twentieth century and was connected with Suzuki-Miyaura reaction used to synthesize receptor antagonists [7] or enzyme inhibitors [8]. An interaction between fluorophenylboronic acid and some biologically significant ligands (i.e. NAD+, sorbitol, l-lactate) and enzyme – subtilisin was also investigated [9], [10].
As fluorinated boronic acids are used in various application, their properties are still explored. Basic research of fluorophenylboronic acid are focused on conformational analysis [11], [12], hydrogen bonding [11], [13], [14], [15], [16], [17], [18], [19] and acidity [14], [15], [20]. An important part of investigations is spectroscopic analysis – mainly by NMR [14], [15], [16], [17], [18], [19], [21], [22], [23] and IR [13], [23], which in addition to characterize boronic acids [14], [15], [21], [23] can serve as a tool for studies of their complexation and aggregation [9], [10], [16], [17], [18], [19], [22].
A more detailed discussion of numerous applications of fluoro-substituted phenylboronic acids is given later in this paper. The main areas are as follows:
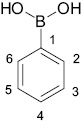
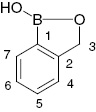
The subject of the present paper are fluoro-substituted phenylboronic acids (1) and their derivatives: benzoxaboroles (2), esters (3) and boroxines (4) (Scheme 1).

Types of the compounds described in the paper (Ar=fluorinated aromatic ring).
In the paper properties and application of these classes of boronic compounds are discussed.
It is worth mentioning that there is a wide field of research related with application of boronic acids as fluoride ion receptors [63], [64], [65], [66], [67], [68], which is not included in the current review.
Properties
Acidity
Boronic acids display Lewis acidic character described by equilibrium reaction (Scheme 2).

Lewis acidity of boronic acids.
The increasing of electrophilic character of boron atom causes the rise of acidity of boronic acids [2], which can be achieved by introduction of electron withdrawing substituents into the aryl group. The pKa values for fluoro-substituted phenylboronic compounds are collected in Table 1.
pKa values for fluoro-substituted phenylboronic compounds compared with unsubstituted compounds.
| Substituents | pKa | Methoda | Reference |
|---|---|---|---|
| Boronic acids |
 |
||
| – | 8.8 | Spectr. | [69] |
| 2-F | 7.89±0.01 | Spectr. | [70] |
| 7.85±0.07 | Pot. | [70] | |
| 7.83±0.02 | Spectr. | [71] | |
| 3-F | 8.09±0.01 | Spectr. | [70] |
| 8.15±0.11 | Pot. | [70] | |
| 7.50±0.02 | Spectr. | [71] | |
| 4-F | 8.77±0.01 | Spectr. | [70] |
| 8.71±0.10 | Pot. | [70] | |
| 8.66±0.05 | Spectr. | [71] | |
| 8.6 | Spectr. | [69] | |
| 9.1 | [72] | ||
| 2,3-F2 | 6.99±0.04 | Spectr. | [70] |
| 6.93±0.10 | Pot. | [70] | |
| 2,4-F2 | 7.75±0.01 | Spectr. | [70] |
| 7.73±0.06 | Pot. | [70] | |
| 7.12±0.02 | Spectr. | [71] | |
| 7.6 | Spectr. | [69] | |
| 2,5-F2 | 7.06±0.03 | Spectr. | [70] |
| 7.01±0.05 | Pot. | [70] | |
| 7.0 | Spectr. | [69] | |
| 2,6-F2 | 7.37±0.01 | Spectr. | [70] |
| 7.41±0.09 | Pot. | [70] | |
| 3,4-F2 | 7.74±0.01 | Spectr. | [70] |
| 3,5-F2 | 7.60±0.06 | Spectr. | [70] |
| 7.52±0.11 | Pot. | [70] | |
| 7.08±0.03 | Spectr. | [71] | |
| 2,3,4-F3 | 6.97±0.01 | Spectr. | [70] |
| 7.01±0.08 | Pot. | [70] | |
| 6.8 | Spectr. | [69] | |
| 2,3,5-F3 | 6.34±0.05 | Spectr. | [70] |
| 6.38±0.11 | Pot. | [70] | |
| 2,3,6-F3 | 5.6 | Spectr. | [70] |
| 6.5 | Pot. | [70] | |
| 2,4,5-F3 | 7.06±0.04 | Spectr. | [70] |
| 6.98±0.10 | Pot. | [70] | |
| 6.7 | Spectr. | [69] | |
| 2,4,6-F3 | 7.1 | Spectr. | [70] |
| 7.6 | Pot. | [70] | |
| 3,4,5-F3 | 7.34±0.02 | Spectr. | [70] |
| 7.32±0.07 | Pot. | [70] | |
| 6.8 | Spectr. | [69] | |
| 2,3,4,5-F4 | 6.23±0.02 | Spectr. | [70] |
| 6.17±0.03 | Pot. | [70] | |
| 2,3,4,6-F4 | 6.17±0.04 | Spectr. | [70] |
| 6.19±0.05 | Pot. | [70] | |
| 2-F, 5-NO2 | 6.0 | Spectr. | [69] |
| 3-F, 2-CHO | 5.74±0.02 | Spectr. | [14] |
| 4-F, 2-CHO | 6.42±0.03 | Spectr. | [14] |
| 5-F, 2-CHO | 6.72±0.03 | Spectr. | [14] |
| 6-F, 2-CHO | 6.05±0.03 | Spectr. | [14] |
| 2,4-F2, 3-CHO | 6.5 | Spectr. | [73] |
| Benzoxaboroles |
 |
||
| – | 7.39 | Spectr. | [74] |
| 4-F | 6.97±0.02 | Spectr. | [75] |
| 5-F | 6.57±0.08 | Spectr. | [75] |
| 6.63 | Spectr. | [76] | |
| 6-F | 6.36±0.04 | Spectr. | [75] |
| 6.45 | Spectr. | [76] | |
| 7-F | 7.42±0.15 | Spectr. | [75] |
| Diboronic acids |
 |
||
| – | 6.0 | Pot. | [15] |
| 4,5-F2 | 5.3 | Pot. | [15] |
| 3,6-F2 | 4.1 | Cond. | [15] |
| 3,4,5,6-F4 | 3.0 | Cond. | [15] |
-
aSpectr., spectrophotometric; pot., potentiometric; cond., conductometric.
The data presented in Table 1 confirm that introduction of a fluorine substituent into phenylboronic compounds increases their acidity: the pKa values for substituted compounds are lower than for unsubstituted ones. This effect is dependent on position of F substituent. The lowest effect, observed for para position, is the result of compensation of the inductive and resonance effects, which have comparable values [4]. For meta position the resonance effect is much weaker, which causes increased acidity. In the case of ortho derivative increased acidity can be caused by the formation of intramolecular B–O–H···F hydrogen bond [77]. It is interesting, that although introduction of the next fluorine substituents results in a further increase of acidity, the 2,6-substituted compounds have lower acidity than their 2,5-isomers. It can be explained by the formation of intramolecular hydrogen bond by only one of the ortho fluorine substituent [77], whereas the second ortho substituent can cause the opposite effect due to the steric hindrance [70].
4-, 5-, and 6-fluoro-substituted benzoxaboroles display considerably higher acidity than the unsubstituted compound. Surprisingly, 7-fluorobenzoxaborole has a higher pKa value, similar to that of fluorine-substituted phenylboronic acids. Decrease of the acidity of this compound can be caused by the difficulty of the tetrahedral boronate ion formation caused by intramolecular hydrogen bond formation between fluorine atom and the neighboring B(OH) group [75].
1,2-Diboronic acids have extremely high acidity. It is explained by the increase stability of the anion resulting from the proximity of two boronic groups [15].
Noteworthy is to remark, that there are few examples of calculations of pKa in literature [78], [79] and that the calculated values significantly differ from the experimental results.
The Lewis acidity of phenylboronic esters can be described by the value of Guttman’s acceptor number [80], [81]. Acceptor number (AN), previously applied by Gutmann for quantitative description of electrophilic properties of solvents, is proportional to the change in 31P NMR shift between complexed and uncomplexed triethylphosphine oxide (TEPO). This approach was used for a quantitative determination of Lewis acidity of fluorinated boronic esters [82]. Similarly as in the case of boronic acids, introduction of fluorine substituents increases the acidity of catechol boronates. In general, introduction of next substituents further increases the acidity. Value for catechol ester of pentafluorophenylboronic acid is even higher than that for the tris(pentafluorophenyl)borate (77.8 vs. 76.3) [82]. The acidity of other cyclic diols’ esters of pentafluorophenylboronic acid was also investigated [83].
Stability of arylboronic acids. Hydrodeboronation reaction
Fluorinated arylboronic acids are susceptible to hydrodeboronation (Scheme 3).

Hydrodeboronation of arylboronic acids.
As early as in 1965 Chambers and Chivers reported, that pentafluorophenylboronic acid rapidly hydrolyses to pentafluorobenzene and boric acid [84]. The rate and mechanism of deboronation of fluoro-substituted was further investigated and it was found, that deboronation can occur both in acidic and alkaline environment – by electrophilic or nucleophilic attack, respectively [24], [85]. Lozada et al. [86] investigated protodeboronation of several fluoro-substituted phenylboronic acids and proved that di-ortho-substituted species underwent facile C−B fission in aqueous basic conditions. Recently, Zarzeczańska et al. [70] reported study on the stability of all fluorinated phenylboronic acids. Noteworthy is, that the increase of acidity is not always followed by the rise in deboronation rate. It was confirmed, that 2,6-difluoro-substituted boronic acids deboronate faster than 3,4-difluoro, 3,5-difluorophenylboronic acid and even 3,4,5-trifluorophenylboronic acid [86].
Crystal structure
Structural data for fluoro-substituted phenylboronic acids and their derivatives are collected in Table 2.
Fluoro-substituted phenylboronic acids and their derivatives described in literature.
| Substituents | Molecular structure | B–C distance (Å) | Dihedral anglea (°) | Reference |
|---|---|---|---|---|
| Boronic acids | ||||
| – | Dimer | 1.56/1.56 | 6.6/21.0 | [87] |
| 2-F | Dimer, intramol. BOH···F | 1.57 | 25.8 | [77] |
| 3-F | Dimer | 1.56 | 25.9 | [88] |
| 2,3-F2 | Dimer, intramol. BOH···F | 1.58/1.57 | 24.1/27.6 | [77] |
| 2,4-F2 | Dimer | 1.57 | 5.91 | [77] |
| Dimer | 1.57 | 4.9 | [89] | |
| 2,5-F2 | Dimer, intramol. BOH···F; polymorphs | 1.58/1.58 | 21.8/27.8 | [77] |
| 2,6-F2 | Dimer, intramol. BOH···F | 1.59 | 25.0 | [77] |
| 2,3,4-F3 | Dimer, intramol. BOH···F | 1.58 | 26.8 | [77] |
| 2,4,6-F3 | Dimer, one intramol. BOH···F | 1.58 | 23.6 | [77] |
| 2,3,4,5,6-F5 | Dimer, one intramol. BOH···F | 1.58 | 38.4 | [90] |
| 4-F, 2-CHO | Dimer | 1.58 | 44.6 | [14] |
| 6-F, 2-CHO | With H2O | 1.55 | 74.7 | [14] |
| 2,3-F2, 4-CHO | Intermol. BOH···HCO, intramol. BOH···F | 1.59 | 18.5 | [91] |
| 3-F, 4-NH2 | Dimer | 1.55 | 24.1 | [20] |
| 2-F, 6-CH2N(iPr)2 | Dimer, intramol. BOH···N | 1.60 | 26.0 | [92] |
| 2,6-(CF3)2 | Dimer | 1.60 | 68.2 | [93] |
| 2-CF3, 6-CH2N(iPr)2 | Dimer, intramol. BOH···N | 1.59 | 21.8 | [92] |
| 2-F, 4-Fc | Dimer | 1.57/1.56 | 6.4/5.9 | [94] |
| 3,4,5-F3 * urea | Cocrystal | 1.58 | 1.7 | [95] |
| Benzoxaboroles | ||||
| – | Dimer | 1.55/1.56 | [96] | |
| 5-F | Dimer | 1.55 | [97] | |
| Dimer | 1.55 | [98] | ||
| 6-F | Dimer | 1.55 | [75] | |
| Diboronic acids (DBA) | ||||
| 4,5-F2o-DBA | Dimer | 1.58; 1.59 | 28.4;45.5 | [15] |
| 1.58; 1.58 | 25.9;12.7 | |||
| 3,4,5,6-F4o-DBA | Anhydride with coordinated water molecule and coordination dimer with B4O4 ring | see text | see text | [15] |
| 2-F p-DBA | 1.57 | 34.0 | [99] | |
| 2,3-F2p-DBA | 1.58 | 6.6 | [99] | |
| 2,5-F2p-DBA | 1.57 | 19.0 | [99] | |
| 2,6-F2p-DBA | 1.58 | 37.6 | [99] | |
| 2,3,5,6-F4p-DBA | 1.58 | 30.6 | [99] | |
| Boroxine | ||||
| 4-F * pyridine | Complex with pyridine | 1.56/1.61b | 9.7/49.8b | [100] |
| Phenylboronic catechol esters | ||||
| 4-F | 1.53 | 1.9 | [101] | |
| 2,6-F | 1.55 | 3.5 | [101] | |
| 2,4,6-F3 | 1.55 | 1.6 | [101] | |
| 3,4,5-F3 | 1.55 | 5.6 | [101] | |
| 2,3,4,5,6-F5 | 1.56 | 1.6 | [101] | |
| 4-CF3*picoline | Co-crystal of 5 molecules of the complex with one picoline molecule | 1.60 | [102] | |
-
aDihedral angle between benzene ring and O–B–O planes.
-
bCoordinated boron atom.
-
Numbering of the atoms is the same as in Table 1.
-
*Stands for cocrystal structures or complex.
The most common structure of phenylboronic acid is a dimer with two intermolecular BOH···OB hydrogen bonds, in which boronic group has syn-anti conformation. This synthon is formed in almost all investigated fluoro-substituted phenylboronic acids (Fig. 1a). Fluorine substituents practically do not affect the B–C bond length which is similar for unsubstituted acid and for the investigated compounds. The presence of fluorine substituent at the ortho position enables the formation of the BO–H···F intramolecular hydrogen bond. The formation of such a bond can be assumed for all investigated ortho-fluorophenylboronic acids: the observed H···F distances range from 2.06 to 2.53 Å [77]. Interestingly, the second ortho-fluorine substituent does not form intramolecular hydrogen bond, as was stated for several 2,6-dialkoxy substituted phenylboronic acids [103]. Moreover, the majority of the molecules with ortho-fluorine substituents is not planar, contrary to what was for ortho-alkoxyphenylboronic acids [103]. These facts indicate that intramolecular BO–H···F hydrogen bonds are the weak ones and that they are insignificant compared to the O–H···O ones in dimers in controlling the syn-anti conformation of the boronic group.

Examples of dimeric structures of fluoro-substituted phenylboronic acid (a) and benzoxaborole (b).
The dimeric synthons serve as main building blocks for three-dimensional structures either by their close packing or by the aid of weak secondary interaction such as C–H···π, O–H···F, or C–H···F hydrogen bonds [77]. Dimeric hydrogen-bonded structure is also observed for fluoro-substituted benzoxaboroles (Fig. 1b), in which the B–C distance is the same as for the unsubstituted compound.
The 1,2-phenylenediboronic acids in solution are in equilibrium with their dehydrated forms. Stability of such cyclic semianhydrides is improved by fluorination of the aromatic ring and complexation of one of the boron centers with water, which is observed in two crystalline forms of the anhydrides [15]. For 1,4-phenylenediboronic acids different fluorine substitutions only slightly influence bond lengths between non-hydrogen atoms. Solely the presence of four fluorine atoms at the phenyl ring gives a significant B–C bond elongation [99].
Extensive study on hydrogen bonding in catechol phenylboronic esters was presented by Madura et al. [101]. All the investigated molecules are almost planar. Depending on the number and position of the fluorine substituents, the substantial differentiation of the molecular dipole moment is observed. The supramolecular motif of stacking columns directed by the antiparallel dipole–dipole interactions is observed in the case of highly polar molecules. The presence of ortho-F substituents enhances the proton acceptor character of oxygen atoms, which favors the formation of the intermolecular C–H···O hydrogen bonds.
Conformational analysis and equilibria in solution
Conformational analysis for isolated molecule of 2-fluorophenylboronic acid confirms its syn-anti conformation and the formation of BO–H···F intramolecular hydrogen bond [11], [12].
Phenylboronic acids display several equilibria in solution, one of which is the acid-boroxine equilibrium (Scheme 4) [40], [104], [105], which importance is connected with the formation of COFs systems [40] or self-healing polymers [41]. They can be investigated by various analytical methods, from which 19F NMR seems to be very convenient due to several advantages of this method [21]. That will be discussed further in the next paragraph.

Formation of triarylboroxine from arylboronic acid.
Ortho-formyl substituted phenylboronic acids display the tautomeric equilibria with the formation of the corresponding hydroxybenzoxaboroles (Scheme 5) [106].

Tautomeric equilibrium for ortho-formylphenylboronic acids.
Equilibrium constant (Kcycl) values for this reaction depends on the substituent X and for the fluorine substituent vary from 0.08 to 0.69, depending on its position in the benzene ring [14].
Spectroscopic data
Spectroscopic techniques, especially in connection with computational methods, can serve as a useful tool for structural analysis. Such comprehensive study (FT-IR, Raman, 1H and 13C NMR, UV-Vis and DFT calculations) was carried out for several fluoro-substituted phenylboronic acids by Karabacak et al. [107], [108], [109]. Results of IR and multinuclear (1H, 11B, 13C and 19F) solid state NMR characterization together with DFT calculations were presented by Sene et al. [98] for 5-fluorobenzoxaborole and compared with the results of crystallographic research.
Particular NMR techniques have exceptional significance in characterization of phenylboronic compounds. A detailed 1H, 13C, 11B, 19F and 17O NMR study of the series of all mono- and multi-fluoro substituted phenylboronic acids was carried out by Gierczyk et al. [21]. From the practical point of view the proton, boron and fluorine NMR methods are easy to use methods mainly due to the high natural abundance of the particular isotope. Of these three methods, 19F NMR spectroscopy is of particular importance [110]. The advantages of this method are as follows:
high sensitivity (83% 1H NMR), connected with the high natural abundance (100% 19F),
spin number I=½,
short relaxation time T1 (typical acquisition time 1.0 s, interscan 0.2 s),
small half-width,
range of chemical shifts more than 400 ppm (up to 1000 ppm),
coupling constants: JÓΔ δ, intensity and splitting as in 1H spectra.
The 19F NMR chemical shifts for the boronic acids and their derivatives are collected in Table 3.
19F NMR chemical shifts of fluorinated phenylboronic acids, benzoxaboroles and boroxines.
| Aryl group | Solvent | Chemical shifts, ppm |
Ref. | ||||
|---|---|---|---|---|---|---|---|
| 2-F | 3-F | 4-F | 5-F | 6-F | |||
| Phenylboronic acids | |||||||
| 2-F | Acetone-d6 | −105.99 | – | – | – | – | [21] |
| 3-F | Acetone-d6 | – | −115.01 | – | – | – | [21] |
| 3-F | CD3OD | – | −114.8 | – | – | – | [111] |
| 4-F | Acetone-d6 | – | – | −111.07 | – | – | [21] |
| 4-F | Acetone-d6 | – | – | −110.98 | – | – | [23] |
| 4-F | Ether | – | – | −111.52 | – | – | [23] |
| 2,3-F2 | Acetone-d6 | −132.59 | −140.54 | – | – | – | [21] |
| 2,4-F2 | Acetone-d6 | −101.7 | – | −107.7 | – | – | [21] |
| Acetone-d6 | −101.37 | – | −107.65 | – | – | [23] | |
| CD2Cl2 | −105.97 | – | −105.72 | – | – | [23] | |
| D2O/Py | −101.64 | – | −109.19 | – | – | [85] | |
| 2,5-F2 | Acetone-d6 | −112.2 | – | – | −120.8 | – | [21] |
| 2,6-F2 | Acetone-d6 | −103.10 | – | – | – | −103.10 | [21] |
| Acetone-d6 | −103.16 | – | – | – | −103.16 | [23] | |
| D2O/Py | −103.65 | – | – | – | −103.65 | [85] | |
| 3,4-F2 | Acetone-d6 | – | −140.90 | −137.00 | – | – | [21] |
| 3,5-F2 | Acetone-d6 | – | 111.59 | – | −111.59 | – | [21] |
| 2,3,4-F3 | Acetone-d6 | −128.23 | −164.19 | −133.59 | – | – | [21] |
| 2,3,5-F3 | Acetone-d6 | −138.01 | −136.10 | – | −117.60 | – | [21] |
| 2,3,6-F3 | Acetone-d6 | −127.99 | −144.95 | – | – | −108.49 | [21] |
| 2,4,5-F3 | Acetone-d6 | −106.71 | – | −131.75 | −145.17 | – | [21] |
| 2,4,6-F3 | Acetone-d6 | −100.13 | – | −107.85 | – | −100.13 | [21] |
| Acetone-d6 | −99.95 | – | −107.67 | – | −99.95 | [23] | |
| D2O/Py | −100.79 | – | −109.01 | – | 100.79 | [85] | |
| 3,4,5-F3 | Acetone-d6 | – | −136.99 | −160.05 | −136.99 | – | [21] |
| Acetone-d6 /D2O | – | −136.41 | −159.65 | −136.41 | – | [23] | |
| Ether | – | −136.41 | −159.51 | −136.41 | – | [23] | |
| D2O/Py | – | −139.08 | −162.46 | −139.08 | – | [85] | |
| 2,3,4,5-F4 | Acetone-d6 | −131.99 | −158.05 | −154.72 | −140.94 | – | [21] |
| Acetone-d6/D2O | −131.69 | −158.17 | −155.27 | −141.14 | – | [23] | |
| D2O/Py | −130.66 | −156.80 | −154.64 | −139.50 | – | [85] | |
| <mspace>2,3,4,6-F4 | Acetone-d6 | −126.04 | −168.07 | −132.55 | – | −105.98 | [21] |
| Acetone-d6 | −126.22 | −168.26 | −132.81 | – | −106.12 | [23] | |
| Acetonitrile-d3 | −125.56 | −167.16 | −131.51 | – | −105.80 | [23] | |
| D2O/Py | −125.32 | −166.79 | −132.41 | – | −105.20 | [85] | |
| MeOH | −126.13 | −167.44 | −131.61 | – | −106.20 | [85] | |
| Py | −124.59 | −166.31 | −131.98 | – | −104.65 | [85] | |
| 2,3,5,6-F4 | Acetone-d6 | −133.60 | −140.24 | – | −140.24 | −133.60 | [21] |
| Acetone-d6 | −133.70 | −140.34 | – | −140.34 | −133.70 | [23] | |
| Ether | −133.28 | −140.27 | – | −140.27 | −133.28 | [23] | |
| D2O/Py | −133.48 | −140.30 | – | −140.30 | −133.48 | [85] | |
| MeOH | −133.59 | −139.30 | – | −139.30 | −133.59 | [85] | |
| 2,3,4,5,6-F5 | Acetone-d6 | −132.53 | −163.38 | −154.65 | −163.38 | −132.53 | [21] |
| Acetone-d6 | −132.61 | −163.50 | −154.72 | −163.50 | −132.61 | [23] | |
| Acetone | −132.9 | −164.1 | −155.4 | −164.1 | −132.9 | [84] | |
| Ether | −132.13 | −163.35 | −155.09 | −163.35 | −132.13 | [23] | |
| CH2Cl2 | −133.31 | −162.06 | −150.03 | −162.06 | −133.31 | [23] | |
| CH3CO2H | −131.86 | −162.72 | −153.17 | −162.72 | −131.86 | [23] | |
| MeOH | −132.44 | −162.75 | −154.11 | −162.75 | −132.44 | [85] | |
| H2O/MeOH | −132.14 | −162.30 | −153.69 | −162.30 | −132.14 | [85] | |
| 3-F, 2-CHO | Acetone-d6 | – | −122.39 | – | – | – | [14] |
| −120.81a | |||||||
| 4-F, 2-CHO | Acetone-d6 | – | – | −112.75 | – | – | [14] |
| −111.21a | |||||||
| 5-F, 2-CHO | Acetone-d6 | – | – | – | −106.73 | – | [14] |
| −116.12a | |||||||
| 6-F, 2-CHO | Acetone-d6 | – | – | – | – | −106.03 | [14] |
| −105.52a | |||||||
| Boroxines | |||||||
| 4-F | Ether | – | – | −106.34 | – | – | [23] |
| 2,6-F | Acetonitrile-d3 | −103.0 | – | – | – | −103.0 | [32] |
| 2,4,6-F | Acetonitrile-d3 | −99.9 | – | −106.8 | – | 99.9 | [32] |
| 3,4,5-F | Ether | – | −135.52 | −156.50 | −135.52 | – | [23] |
| 2,3,5,6-F | Ether | −131.60 | −139.94 | – | 139.94 | −131.60 | [23] |
| 2,3,4,5,6-F | Ether | −132.37 | −163.66 | −154.07 | −163.66 | −132.37 | [23] |
| 2,3,4,5,6-F | Acetonitrile-d3 | −132.4 | −163.5 | −153.7 | −163.5 | −132.4 | [32] |
| Catechol esters | |||||||
| 2-F | CDCl3 | −102.61 | – | – | – | – | [82] |
| 3-F | CDCl3 | – | −113.46 | – | – | – | [82] |
| 4-F | CDCl3 | – | – | −106.45 | – | – | [82] |
| 2,4-F | CDCl3 | −98.47 | – | −102.75 | – | – | [82] |
| 2,6-F | CDCl3 | −99.56 | – | – | – | −99.56 | [82] |
| 3,4,5-F | CDCl3 | – | −134.36 | −154.52 | −134.36 | – | [82] |
| 2,4,6-F | CDCl3 | −96.13 | – | −100.68 | – | −96.13 | [82] |
| 2,3,4,5,6-F | CDCl3 | −128.43 | −161.09 | −146.620 | −161.09 | −128.43 | [82] |
| Pinacol esters | |||||||
| 2-F | CDCl3 | −103.06 | – | – | – | – | [82] |
| 3-F | CDCl3 | – | −114.2 | – | – | – | [112] |
| 4-F | CDCl3 | – | – | −115.5 | – | – | [111] |
| 2,3-F | CDCl3 | −129.1 | 139.1 | – | – | – | [112] |
| 2,5-F | CDCl3 | −109.5 | – | – | −120.6 | – | [112] |
| 3,5-F | CDCl3 | – | −110.9 | – | −110.9 | – | [112] |
| 2,3,5-F | CDCl3 | −134.0 | −133.6 | – | −116.2 | – | [112] |
| 2,4,5-F | CDCl3 | −104.2 | – | −128.5 | −144.2 | – | [112] |
| 2,3,4,5-F | CDCl3 | −129.0 | −156.1 | −150.9 | −139.8 | – | [112] |
|
3-F
|
4-F
|
5-F
|
6-F
|
7-F
|
|||
| Benzoxaboroles | |||||||
| 4-F | Acetone-d6 | – | −121.46 | – | – | – | [75] |
| 5-F | Acetone-d6 | – | – | −111.84 | – | – | [75] |
| 6-F | Acetone-d6 | – | – | – | −118.40 | – | [75] |
| 7-F | Acetone-d6 | – | – | – | – | −105.51 | [75] |
Great differences in the chemical shifts makes the 19F NMR spectroscopy an excellent tool in the research of structures, complexation, association as well as reaction kinetics. For instance, the differences in chemical shifts between 4-fluorophenylboronic acid, corresponding boroxine and pinacol ester are in order of 5 ppm. It is worth noting, that, in addition to chemical shifts, coupling constants values provide valuable information on structural parameters [21].
Useful tool in the investigation of oxygen-containing organoboron compounds is 17O NMR spectroscopy [113]. However, the limitation of this method is very low natural abundance of 17O nuclei (3.7*10−2%). In earlier works isotope enriched compounds were used. Contemporary spectrometers allow for a substantial acceleration of the spectral acquisition making the method more useful [114]. Selected 17O NMR data for fluorinated phenylboronic compounds are collected in Table 4.
17O NMR chemical shifts of fluorinated phenylboronic acids and benzoxaboroles.
| Aryl group | Solvent | Chemical shifts, ppm | Ref. | |
|---|---|---|---|---|
| Phenylboronic acids | ||||
| 2-F | Acetone-d6 | 82.0 | [21] | |
| 3-F | Acetone-d6 | 77.3 | [21] | |
| 4-F | Acetone-d6 | 74.6 | [21] | |
| 2,3-F2 | Acetone-d6 | 83.2 | [21] | |
| 2,4-F2 | Acetone-d6 | 80.9 | [21] | |
| 2,5-F2 | Acetone-d6 | 83.2 | [21] | |
| 2,6-F2 | Acetone-d6 | 91.9 | [21] | |
| 3,4-F2 | Acetone-d6 | 76.1 | [21] | |
| 3,5-F2 | Acetone-d6 | 78.4 | [21] | |
| 2,3,4-F3 | Acetone-d6 | 82.2 | [21] | |
| 2,3,5-F3 | Acetone-d6 | 84.3 | [21] | |
| 2,3,6-F3 | Acetone-d6 | 92.7 | [21] | |
| 2,4,5-F3 | Acetone-d6 | 82.1 | [21] | |
| 2,4,6-F3 | Acetone-d6 | 90.8 | [21] | |
| 3,4,5-F3 | Acetone-d6 | 76.7 | [21] | |
| 2,3,4,5-F4 | Acetone-d6 | 82.7 | [21] | |
| 2,3,4,6-F4 | Acetone-d6 | 91.4 | [21] | |
| 2,3,5,6-F4 | Acetone-d6 | 93.5 | [21] | |
| 2,3,4,5,6-F5 | Acetone-d6 | 92.1 | [21] | |
|
B–O–H
|
B–O–C
|
|||
| Benzoxaboroles | ||||
| 4-F | Acetone-d6 | 68.0 | 96.3 | [75] |
| 5-F | Acetone-d6 | 66.3 | 99.1 | [75] |
| 6-F | Acetone-d6 | 67.2 | 100.9 | [75] |
| 7-F | Acetone-d6 | 68.3 | 97.5 | [75] |
-
Numbering of the atoms is the same as in Table 1.
It is worth noting that 17O chemical shifts are affected not only by electronic effect of fluorine substituents but also by their steric factors, which result in higher values for the ortho-substituted compounds [114].
1H, 13C and 11B NMR data for fluoro-substituted boronic acids and their derivatives are collected in Supplementary material.
The molecular structure and adsorption mode onto the silver surface of the two groups of fluoro-substituted phenylboronic acids were investigated by experimental (FT-Raman, FT-IR, and SERS) and theoretical [DFT, B3LYP/6-311++G(d,p)] methods. The most stable structure of these absorbed molecules is a cyclic dimer. Conformational analysis for the absorbed molecules was also done [13].
Synthesis
Introduction of the boronic group
The fluorine-carbon bond is a stable one and does not react with common reagents used in the synthesis of boronic acids. Hence, typical reaction sequence shown in Scheme 6 can be applied.

Introduction of boronic group by aryl bromide metalation.
The most common metalating agents are n-butyllithium or lithium diisopropylamide (LDA) solutions, although an alternative method by Grignard reagent can be also used. Introduction of the boryl moiety can be achieved with trialkyl borates or cyclic pinacol esters. The synthesis of various fluorine-substituted phenylboronic acids was extensively investigated by Frohn et al. [23], [115]. Recently, the continuous process in a cryogenic flow reactor was described with the use of n-BuLi and B(OMe)3 [116] or PinBOiPr [117]. Fluorinated phenylboronic acids with various functional groups in the phenyl ring can be synthesized in the same manner with optional protection of the functional group if necessary. For instance, several fluoroformylphenylboronic acids were synthesized with the protection of formyl group by acetal formation [14], [97].
The acidity of arenes is enhanced by fluorine substituent due to its electron-withdrawing inductive effect. Hence, direct metalation of fluorobenzene can be achieved at the ortho position. Thermodynamic and kinetic parameters of this reaction were discussed [118], [119]. Long range substituent effect was also investigated [120]. This effect is utilized in preparation of fluorophenylboronic acids by direct metalation, which is most often used for the syntheses of multi-fluoro derivatives, e.g. 2,3,5,6-tetrafluorophenylboronic acid (Scheme 7).

Introduction of boronic group by direct metalation.
Similarly as in the reactions of aryl bromides, this approach can be applied in the synthesis of fluorinated phenylboronic acids containing functional groups [120].
Transformations of boronic acids
Derivatives of fluoro-substituted phenylboronic acids can be synthesized by typical reactions, e.g. esterification, dehydration, amination followed by dehydration, or reduction of the parent acids leading to esters, boroxines or benzoxaboroles, respectively. For instance, 3,3′-piperazine bis(5-fluorobenzoxaborole) was obtained from 4-fluoro-2-formylphenylboronic acid (Scheme 8) [121].

Synthesis of bis-benzoxaborole from formylphenylboronic acid.
In addition to the simple transformation of boronic acids, particular methods can be applied for the straightforward syntheses of these derivatives from different substrates. Scheme 9 shows various methods of preparation 5-fluorobenzoxaborole, which has been widely studied due to its antifungal properties and its recent use as the drug Kerydin.
The modification of this method is metalation of protected boronic acids (Scheme 10).

Metalation of protected phenylboronic acids ElY: electrophile.
This method was applied to introduce additional functional groups (El: formyl, methoxycarbonyl, silyl, boronic) into phenyl ring [125]. Other isomers of difluorophenylboronic acids were also used in this approach [125]. Iodo-substituted compounds can be obtained from silyl derivatives by iododesilylation [126].
Applications
Organic synthesis
Suzuki-Miyaura cross-coupling
Suzuki-Miyaura cross-coupling (SMC) is the most widely used reaction to create bond between two sp2 carbons [24], [127], [128]. SMC is regarded as the most significant discovery in chemistry of boronic acid [129]. Meaningful issue of using polyfluorinated boronic acids, which are easy to deboronate in SMC was described by Kinzel et al. [24]. In this article new palladium pre-catalyst was presented (Scheme 11), which enables carrying out of reaction in mild conditions – important for avoiding the deboronation reaction.
![Scheme 11:
(a) structure of palladium pre-catalyst, which is used in SMC of easily deboronating boronic acids, (b) structures of investigated fluorinated boronic acids [24].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_045.jpg)
(a) structure of palladium pre-catalyst, which is used in SMC of easily deboronating boronic acids, (b) structures of investigated fluorinated boronic acids [24].
Recently published paper by Kohlmann et al. [130] shows an interesting example of SMC between pinacol 3,4,5,6-tetrafluoro-2-pyridinylboronate and phenylalanine precursors (Scheme 12). A variety of highly fluorinated amino acid derivatives were synthesized in this manner. These conversions gave access to building blocks with important functional moieties, such as 2-C5F4N, 4-C6H4SF5, and 2-C6H4SCF3.
![Scheme 12:
SMC of pinacol 3,4,5,6-tetrafluoro-2-pyridinylboronate with phenylalanine derivative [130].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_046.jpg)
SMC of pinacol 3,4,5,6-tetrafluoro-2-pyridinylboronate with phenylalanine derivative [130].
Regioselectivity of SMC reaction was investigated by Chung et al. [131] and by Cai et al. [132] for the reaction of 2,4-difluorophenylboronic acid with 1,6-naphthyridone dichloride, leading to an efficient intermediate in kinase inhibitor synthesis (Scheme 13).
The extensive study of SMC between 4-fluorophenylboronic compounds and 3,5-bis(trifluoromethyl)phenyl bromide was presented by Butters et al. [25] (Scheme 14). Authors compare yields of the main product and side-products of protodeboronation, homocoupling and oxidation reactions.
![Scheme 14:
SMC of 4-fluorophenylboronic acid with fluorinated phenyl bromide [25].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_048.jpg)
SMC of 4-fluorophenylboronic acid with fluorinated phenyl bromide [25].
Wide investigation has been carried on the SMC between aromatic amides and boronic acids resulting in diaryl ketones [26] (Scheme 15). Among others, fluoro and trifluoromethyl-substituted boronic acids were studied.
![Scheme 15:
SMC of fluorophenylboronic acids with amides [26].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_049.jpg)
SMC of fluorophenylboronic acids with amides [26].
Interesting case of applying SMC is a site-specific introduction of 4-[18]fluorophenyl group into polypeptide [133]. This is an efficient way to obtain a tracer for the positron emission tomography (PET) imaging.
Reactions of primary and secondary alkyl chlorides with fluorophenylboronic acids can be accomplished through the use of nickel/amino alcohol-based catalysts (Scheme 16) [134].
![Scheme 16:
SMC of fluorinated phenylboronic acids and alkyl chlorides [134].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_050.jpg)
SMC of fluorinated phenylboronic acids and alkyl chlorides [134].
Transformations of boronic group in fluorinated boronic acids
Fluorinated arylboronic acids have been transformed into various organic compounds by substitution of the boronic unit by other functional groups. Scheme 17 shows several examples of such reactions: hydroxylation of 2-fluoro-4-pyridinylboronic acid [135], fluorination with caesium fluoroxysulfate (CFS) [136], nitration by simple nitrates [137], creation of azides [138], [139], introduction of trifluoromethyl group [140] and bromination [141]. Generally, the presented transformations proceed with high yields.

Examples of fluorinated boronic acids’ conversion into other organic compounds. NMDE, N-methyldiethanolamine; TMS-Cl, chlorotrimethylsilane; DBDMH, 1,3-dibromo-5,5-dimethylhydantoin.
Fluorinated boronic acids in covalent organic frameworks formation
Since boroxine-based COF-1 was synthesized [142] and similar COF-5 with p-diboronic acid as a linker, several COFs with the use of fluorinated phenylboronic acids were reported. Chen et al. [42] presented the HHTP-DFFPBA-TATTA COF with the use of DFFPBA as a linker, HHTP-FFPBA-TATTA COF with use FFPBA as a linker and boroxine-TATTA-based TATTA-DFFPBA COF and TATTA-FFPBA COF (Scheme 18). Porosity and gas adsorption in COFs, among others, was investigated. Li et al. [43] presented boroxine-based three-dimensional DL-COF-2 with use FFPBA and 1,3,5,7-tetraaminoadamantane (TAA). Gas adsorption in COF and catalytic properties in the cascade reaction with Knoevenagel-type condensation was investigated.
![Scheme 18:
Compounds used to create COFs: HHTP, 2,3,6,7,10,11-hexahydroxytriphenylene; TATTA, 4,4′,4″-(1,3,5-triazine-2,4,6-triyl)trianiline; DFFPBA, 2,3-difluoro-4-formyl-phenylboronic acid; FFPBA, 3-fluoro-4-formylphenylboronic acid [42].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_052.jpg)
Compounds used to create COFs: HHTP, 2,3,6,7,10,11-hexahydroxytriphenylene; TATTA, 4,4′,4″-(1,3,5-triazine-2,4,6-triyl)trianiline; DFFPBA, 2,3-difluoro-4-formyl-phenylboronic acid; FFPBA, 3-fluoro-4-formylphenylboronic acid [42].
Other reactions
4-Fluorophenylboronic acid was used in palladium-catalyzed reaction of 1,1-fluoroarylation of aminoalkenes with addition of Selectfluor [143].
Fluoro-substituted phenylboronic acids were used in nickel-catalyzed cross-coupling with 2,2-difluoro-1-iodoethenyl tosylate to obtain difluoroalkylated arenes [144].
Reaction of ipso-iodination of silylated fluorophenylboronic acids was investigated [126].
Efficient two step synthesis of indoles with aryl and trifluoromethyl groups from substituted alkynes and various substituted 2-nitrophenylboronic acids – among others 4-fluoro-2-nitrophenylboronic acid – was reported [145].
Synthesis of bis- and tris(2,6-difluorophenylboronic) acids with the methylsilane core was reported by Gontarczyk et al. [146].
Enantioselective rhodium-catalyzed 1,4-addition of 3,5-difluorophenylboronic acid to α,β-unsaturated ester was reported. This is the key step of the multikilogram-scale synthesis of important pharmaceutical ingredients [147].
Binding to diols
General considerations
Ability of binding diols by phenylboronic acids is well known for many years. In 1959 Lorand and Edwards presented important paper about complexes between phenylboronic acid and cis diols, e.g. monosaccharides [148]. Westmark et al. discussed the mechanism and efficiency of sugars transport through lipid layer. Authors remarked, that in aqueous solution tetrahedral boronate anion is created, but in lipid layer it takes the trigonal form. Among others 4-fluorophenylboronic acid was investigated [72]. London and Gabel [9] showed changes in 19F NMR chemical shifts depending on changes in pH of 4-fluorophenylboronic acid and 3-chloro-4-fluorophenylboronic acid. Moreover, it was remarked, that additional signal in the 19F NMR spectra of boronic acid bound with diol or phosphate appears. It means, that 19F NMR spectrometry could be useful to diol and phosphate binding monitoring for fluorinated boronic acids.
A detailed mechanism of diols binding and kinetics of binding was presented by Furikado et al. [149]. The influence of pH on reaction between 2,4-difluorophenylboronic acid and chromotropic acid was investigated in details.
Important insight into the binding affinity of diols to boronic acids depending on pKa of the acids and pH of a solution was presented by Yan et al. [69]. Mono- and multifluorinated boronic acids were reacted with diols: glucose, fructose, catechol and Alizarin Red S (ARS). ARS is commonly used as spectrophotometric indicator of boronic acid binding affinity to diols [150], [151]. In the paper by Yan et al. [69] it was stated, that pKa of acids and pH of a solution are not the only significant factors, but the presence of buffer and steric hindrance in boronic acid also influence the binding affinity. Significant conclusion from this paper is that increasing the acidity of boronic acid is not always followed by raise in the binding affinity. For example, at physiological pH (7.5) 3,4,5-trifluorophenyloboronic acid displays lower binding constant for glucose than 2,5-difluorophenylboronic acid despite higher pKa of the latter. However, for fructose 3,4,5-trifluorophenylboronic acid has higher binding constant than 2,5-difluorophenylboronic acid. Introduction of nitro group to phenylboronic acids often diminishes affinity of diols, also to fluorinated acids, in spite of decreasing effect for pKa [69], [152].
Binding of diols by boronic compounds is crucial from the point of view of their biological activity. It is discussed in the next chapter.
Applications in analytical chemistry – solid-phase extraction, enrichment of analytes, sensors and receptors
As it was stated earlier, introduction of fluorine substituents into phenylboronic acids decreases the pKa value. In many cases it allows to make sensors suitable for sugar sensing at physiological pH [20], [30], [153].
In order to connect boronic acid to polymer matrix or other unit, additional functional group is required. It can be the amine [20], [29], [30], carboxyl [30] or formyl group [73], [154], [155], [156], [157], [158].
Important class of sensors is that based on hydrogels. In the paper of Zhang et al. [29] the binding of glucose by aminophenylboronic acids attached with polymerized crystalline colloidal arrays (PCCA) was described. Immobilization of boronic acids on PCCA was followed by creation of the amide bond between free carboxylic group from polyacrylic acid and amine group from boronic acid. Authors showed two mechanisms of glucose binding by immobilized boronic acids. In instance of shortage of glucose, the 2:1 complex boronic acid-glucose is formed, resulting in shrinking of the hydrogel. If the concentration of glucose in solution is higher – the 1:1 complex is formed, resulting in swelling of the hydrogel. In consequence, a huge shift in diffracted wavelength was observed. Aminophenylboronic acids with electron withdrawing groups (EWG) such as fluorine group were also investigated. However, introduction of EWG, which decreases pKa, is not always a good way for optimization of response.
Similar example of a hydrogel sensing material is the photonic colloidal crystal based on polyacrylamide-poly(ethylene glycol) with attached 4-amino- and 4-carboxy-3-fluorophenylboronic acids and then amplified by applying 4-carboxy-3-fluorophenylboronic acid [153].
Interesting example is the use of 2,4-difluoro-3-formylphenylboronic acid (DFFPBA) attached to various materials, firstly reported in 2013 by Li et al. [73] Authors presented a column for selective enrichment of nucleosides – also from urine sample. The preparation of this type of columns has been described in details [158]. Important application of DFFPBA is also the capillary electrophoresis, which is a fast, accurate and precise way for measuring association constant of binding monosaccharides by boronic acids. Binding with fructose, mannose, N-acetylneuraminic acid (Neu5Ac), xylose and fucose was investigated [159]. DFFPBA has significantly higher association constant for fructose than others boronic acids. Also for other sugars – xylose, fucose, mannose – stronger affinity was observed [159]. Therefore monolithic column (copolymer poly(MBAA-co-GMA) covered by PEI) with use of DFFPBA for enrichment of trace glycoproteins from urine samples was prepared. MALDI-TOF MS spectrum shows, that this method of enrichment by solid-phase extraction succeeded. Lowered binding pH of DFFPBA profitably influenced the selectivity of binding glycoproteins [156]. Similar method is the enrichment of nucleosides by the use of aminated magnetic attapulgite as a bottom support for DFFPBA [154].
Interesting way was to create a hybrid method of selection of aptamers for binding glycoproteins – alkaline phosphatase (ALP) and ribonuclease B (RNase B) was investigated. Magnetic beads with attached DFFPBA enable enriching binding sequences, which is followed by capillary electrophoresis separation [160].
Novel amplification in creating specific receptors for glycans, glycoproteins and monosaccharides is creating molecularly imprinted polymers (MIPs) with use of DFFPBA as binding agent for template and analyte [157]. Xing et al. described the process of preparation and evaluations of molecularly imprinted receptor (MIR). MIR based on 3D arrays was created for specific glycoprotein recognition (RNase B) [155], which is part of a sensor, connected with display electrode. On the latter a reduction of Prussian blue to Prussian white takes place. The idea assumes absorbance measuring of fading blue color, which indicates quantitatively the concentration of RNase B in solution. DFFPBA is also used as a linker between the base surface and template or analyte.
Interesting examples of receptors for fluoride ion recognition are macrocycles, one of which is made of two particles of DFFPBA, 3-aminophenylboronic acid and pentaerythritol. It was found, that such macrocycles can include some small compounds, e.g. chloroform, benzene and tetrabutylammonium fluoride (TBAF). TBAF was used to investigate receptor properties of macrocycles. Fluoride ion inclusion was observed by significant changes in 11B NMR spectrum [161].
2-Fluoro-4-formylphenylboronic acid can be used as linker – converter between diol (analyte) and anthracene (amplifier) (Scheme 19). Amplifier and converter noncovalently interact with synthetic pores. This sensing system was used to detect polyphenols in green tea [162].
![Scheme 19:
Applying 2-fluoro-4-formylphenylboronic acid as converter in sensing system for polyphenols [162].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_053.jpg)
Applying 2-fluoro-4-formylphenylboronic acid as converter in sensing system for polyphenols [162].
Important achievement in the research of boronic receptors was a computational design of receptor based on phenylboronic acid for recognition of dopamine. It was possible to predict distances between molecules and to project three binding parts involving: (i) covalent bonding with creating boronic ester with analyte, (ii) π–π stacking between aromatic ring in receptor and dopamine, (iii) and ionic interaction between amine group of dopamine and geminal diol in receptor (Scheme 20). One of the discussed boronic acids was 2-amino-5-fluorophenylboronic acids [31].
![Scheme 20:
Computer-designed complex of receptor and dopamine [31].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_054.jpg)
Computer-designed complex of receptor and dopamine [31].
Biological activity
Inhibitory properties of fluoroarylboronic acids
The above-mentioned ability to bind diols molecules by arylboronic acids is also the basis of their biological activity, which depends on enzyme inhibition. London and Gabel investigated interaction of 4-fluorophenylboronic acid and 3-chloro-4-fluorophenylboronic acid, with the active site of subtilisin Carlsberg. The reaction was monitored by 19F NMR spectrometry [10]. 2-Fluoro-, 4-fluorophenylboronic acid and 4-fluoro-3-pyridinylboronic acid, among others, were investigated as inhibitors for fatty acid amide hydrolase. Fluoro- and trifluoromethylphenylboronic acids were found to be potent inhibitors. However, these acids are far from the best one investigated compound, 4-nonylphenylboronic acid [163].
Medical applications
Substantial increase of interest in benzoxaborole chemistry is associated with the discovery of biological activity of 5-fluorobenzoxaborole, AN2690 (tavaborole) [122]. AN2690 is a novel antifungal drug, which is applied for the topical treatment of toenail onychomycosis [164], [165], [166]. Improved nail penetration by this compound in comparison with earlier applied drugs is connected with its small molar mass. Tavaborole is described as a safe, non-toxic substance [166]. It is available as a 5% solution, brand name – Kerydin [46], [166]. Potential formulation of drugs containing AN2690 in inorganic Mg–Al double hydroxide matrix and in poly-l-lactic acid matrix was described by Sene et al. [167], [168]. The mechanism of AN2690 antifungal activity is connected with enzyme inhibition: trapping tRNA by creation stable adduct tRNALeu in the editing site of the leucyl-tRNA synthetase [44]. Other active fluorobenzoxaborole is the 5-fluoro-6-ethylamino-derivative, AN3018. Its interaction with enzyme is based on the formation of additional hydrogen bond to ethylamine group and was proved by crystallography of the complex [45]. Another fluorinated benzoxaborole, AN5568, is active against human African trypanosomiasis [98], [169] (Scheme 21).

Fluorinated benzoxaboroles with biological activity.
Biological activity of 2,4,6-trifluorophenylboronic acid and its catechol ester against several fungi and bacteria was investigated and compared with that of AN2690 [170]. Results show significant advantage of AN2690 over others compounds. Other extensive comparison of biological activity between diversely fluoro-substituted benzoxaboroles was presented by Baker et al. [122]. Again, AN2690 had the lowest minimum inhibitory concentrations. Rock et al. [44] investigated several fluorinated boronic compounds and showed significant difference in the inhibitory concentration between AN2690 and investigated compounds.
AN2690 was used also as a part of more complex compounds. AN2690-containing molecules were investigated as HCV NS3 protease inhibitors (Scheme 22) [47]. Noteworthy is, that this potential inhibitors have 6-amino group as in AN3018. The best inhibition properties is displayed by compounds with R1 and R3 moieties (from similar 10 compounds studied). Akin AN2690-containing macrocycles were also investigated as HCV NS3 protease inhibitors [48].
![Scheme 22:
AN2690-containing potential HCV NS3 inhibitors [47].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_056.jpg)
AN2690-containing potential HCV NS3 inhibitors [47].
Among 22 benzoxaborole-based compounds investigated against malarial parasite Plasmodium falciparum, three monofluoro-substituted isomers (Scheme 23) have the lowest IC50 values. The most effective was the 4-fluorosubstituted isomer [49].
![Scheme 23:
Fluoro-substituted benzoxaboroles (three isomers) – compounds investigated for possible treatment disease caused by malarial parasite Plasmodium falciparum [49].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_057.jpg)
Fluoro-substituted benzoxaboroles (three isomers) – compounds investigated for possible treatment disease caused by malarial parasite Plasmodium falciparum [49].
Boron neutron capture therapy. Positron emission tomography
In contrast to traditional radiotherapy, the boron neutron capture therapy (BNCT) is a biologically-targeted one. It means, that BNCT saves normal tissue around tumor [171]. 10B atom captures a neutron and converts it to an unstable 11B isotope, than decays to 7Li and α particle (Scheme 24) [50], [171]. The penetration range of particles is as short as dimension of a cell. For efficient delivery of radionuclides to tumour issue, suitable carrier is needed. Approved for clinical use are mercapto[10B]borane (BSH) [172], [173], [174] and p-[10B]boronophenylalanine (PBA) [170], [173]. To monitor by positron emission tomography (PET) Imaging pharmacokinetics, incorporation in cells and metabolism of PBA, 4-[10B]borono-2-[18F]fluoro-l-phenylalanine (18F-PBA, Scheme 24) was investigated [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60].
![Scheme 24:
The fission reaction of 10B atom. Basic process in BNCT [50].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_058.jpg)
The fission reaction of 10B atom. Basic process in BNCT [50].
Connection between BNCT and magnetic resonance imaging (MRI) was also proposed [62] basing on 4-[10B]borono-2,6-fluorophenylalanine, 4-[10B]borono-2,6-fluorophenylalaninol (racemate) and on 2-trifluoromethyl analogs (all with [19F]fluorine). The 4-[10B]borono-2,6-fluorophenylalaninol has over eight-fold solubility in water than 4-[10B]borono-2,6-fluorophenylalanine and 4-[10B]boronophenylalanine. Moreover the former compound has the lowest IC50–0.8. For comparison, 4-[10B]borono-2,6-fluorophenylalanine and 4-[10B]boronophenylalanine have IC50 value over 6 and 4, respectively and the best incorporation values into tumour cells (over 1.0 μg/1.0×107 of [10B]boron concentration).
Although various similar to 18F-PBA radiocompounds are obtained by activation of nitro groups (such as radiofluorinated DOPA and phenylalanine), nucleophilic approaches to 18F-PBA are not appropriately efficient because of Lewis acidic character of boron [175]. Radiosynthesis of 18F-PBA with use of acetyl [18F]hypofluorite in trifluoroacetic acid (TFA) was reported (Scheme 25) [171], [176], [177], [178]. Another approach is applying gas [18F]fluorine ([18F]F2) in freon-11 (CCl3F) and TFA [175]. The desired product was isolated from by-products by semi-preparative RP-HPLC. Similar method was applied into automated module, in which [18F]F2 is added into 4-[10B]borono-l-phenylalanine dissolved in TFA [179].
![Scheme 25:
Radiosynthesis of 4-[10B]borono-2-[18F]fluoro-l-phenylalanine (18F-PBA) [171].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_059.jpg)
Radiosynthesis of 4-[10B]borono-2-[18F]fluoro-l-phenylalanine (18F-PBA) [171].
Pinacol and benzopinacol 2,6-difluoro-4-carboxyphenylboronates’ and a biotin derivatives’ conversion to corresponding [18F]-labeled trifluorophenylborates was reported [180]. Stability of benzopinacol esters is appropriate for PET imaging (stability over two-fold of the half-life of the 18F). Condensation of benzopinacol 3-carboxy-2,4,6-trifluorophenylboronate with biotinylated piperazine and conversion to the corresponding [18F]-labeled trifluoroborane yielded a novel radiotracer. Tests of distribution of radiotracer in mice were presented [181]. Conversion to labeled trifluoroboranes was also investigated for chromophoric BODIPY derivative of benzopinacol 2,4,6-trifluoroboronate [182]. Benzopinacol 3-carboxy-2,4,6-trifluorophenylboronate was also linked with marimastat – drug for breast cancer – by diether chain and converted to [18F]fluoro-[19F]difluoroborane [183]. The final compound enables matrix metalloproteases’ activity in vivo monitoring by PET, which would be helpful in breast cancer treatment.
It was established, that isotope 18F introduces some difficulties, such as time constrains, recombination and fragmentation [174]. Therefore, 4-[10B]borono-2-fluorophenylalanine with [19F]fluorine (2-F PBA) was investigated. To improve solubility of 2-F PBA in water, the complex with fructose was created by boronic group.
Apart from applying boronic acid in BNCT, 18F-radiolabeling of various peptides by Suzuki-Miyaura cross-coupling (SMC) was reported [133]. SMC of chemically modified subtilisin from Bacillus lentus containing p-iodobenzylcysteine [183] with 4-[18F]fluorophenylboronic acid was investigated. SMC was successfully carried out also with synthesized decamer polypeptide called comprehensive carcinoma homing peptide (CCHP) [133], containing 4-iodo-l-phenylalanine. CCHP was characterized as widely cancer-targeting [184].
Fluorophenylboronic acids as catalysts
3,4,5-Trifluorophenylboronic acid was found to be a catalyst in the condensation reaction of carboxylic acids and ureas (Scheme 26) [186].
![Scheme 26:
Phenylboronic acid-catalyzed amide condensation [186].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_060.jpg)
Phenylboronic acid-catalyzed amide condensation [186].
Another application of fluorinated phenylboronic acids is the catalysis of dipeptide synthesis. 3,4,5-Trifluorophenylboronic acid and o-nitrophenylboronic acid – among others investigated boronic acids – gave good or excellent yields [187].
Other example is catalysis of Friedel-Crafts prenylation and alkylation with use of perfluorophenylboronic acid, 2,3,4,5-tetrafluorophenylboronic acid and 2,3-difluoro-4-pyridiniumboronic acid chloride – two last gave the best yields (80%) [188].
Fluorinated phenylboronic acid in electrochemistry – anion complexing properties
Important application of fluorinated phenylboronic acids chemistry is their use in electrochemistry as anion complexing agents. Additives to lithium-ion battery increase solubility of salts in non-aqueous solution, with increases conductivity [33], [34], [35], [36], [37], [38], [39]. As additives for battery electrolytes phenylboronic esters [33], [37], [38], [39], borates [34], [35] and borinates can be used [36]. Nair et al. [32] reported, that introduction of fluorine substituents into phenyl rings in boroxines increases the fluoride anion binding energy. Similar tendency can be observed for esters [82], where increase of conductivity was also remarked [38]. The influence of other fluoro-substituted phenylboronic esters on conductivity of polymer electrolytes was also investigated [33], [39].
The concept of a fluorine tag
High sensitivity of 19F NMR chemical shift on the changes in the organic molecule allows to use the fluorine substituent as “fluorine tag”. 2-Formyl-4-fluorophenylboronic acid was used as a derivatizating agent in a three-component coupling reaction with (rac)-α-methyl-benzylamine with diol to afford diastereomeric imino-boronate esters. This method was applied to determine enantiomeric purity of chiral diols [189]. Recently, Axthelm et al. [190] described an array of three water-soluble boronic acid receptors in combination with 19F NMR spectroscopy to discriminate diol-containing bioanalytes: catechol, dopamine, fructose, glucose, glucose-1-phosphate, glucose-6-phosphate, galactose, lactose, and sucrose at low mM concentrations. Another example is the use of fluorinated boronic acid appended pyridinium salts in combination with 19F NMR spectroscopy to screen a pool of 59 analytes [191].
Article note
A collection of invited papers based on presentations at the 16th International Meeting on Boron Chemistry (IMEBORON-16), Hong Kong, 9–13 July 2017.
Acknowledgement
This work was supported by the National Science Centre of Poland, Funder Id: 10.13039/501100004281, Project Number 2016/23/B/ST5/02847.
References
[1] L. Meng, J. S. Fossey, T. D. James, eds. Boron. Sensing, Synthesis and Supramolecular Self-Assembly, Royal Society of Chemistry, Cambridge (2015).Search in Google Scholar
[2] D. G. Hall, ed. Boronic Acids. Preparation and Applications in Organic Synthesis, Medicine and Materials, 2nd ed., Wiley-VCH, Weinheim (2011).10.1002/9783527639328Search in Google Scholar
[3] P. Kirsch. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd ed., Wiley-VCH, Darmstadt (2013).10.1002/9783527651351Search in Google Scholar
[4] T. Siodła, W. P. Ozimiński, M. Hoffmann, H. Koroniak, T. M. Krygowski. J. Org. Chem.79, 7321 (2014).10.1021/jo501013pSearch in Google Scholar PubMed
[5] J. H. Williams. Acc. Chem. Res.26, 593 (1993).10.1021/ar00035a005Search in Google Scholar
[6] T. Dahl. Acta Chem. Scand.48, 95 (1994).10.3891/acta.chem.scand.48-0095Search in Google Scholar
[7] J. S. Sawyer, R. F. Baldwin, M. J. Sofia, P. Floreancig, P. Marder, D. L. Saussy, L. L. Froelich, S. A. Silbaugh, P. W. Stengel, S. L. Cockerham, W. T. Jackson. J. Med. Chem.36, 3982 (1993).10.1021/jm00076a030Search in Google Scholar PubMed
[8] D. B. Reitz, J. J. Li, M. B. Norton, E. J. Reinhard, J. T. Collins, G. D. Anderson, S. A. Gregory, C. M. Koboldt, W. E. Perkins. J. Med. Chem.37, 3878 (1994).10.1021/jm00049a005Search in Google Scholar PubMed
[9] R. E. London, S. A. Gabel. J. Am. Chem. Soc.116, 2562 (1994).10.1021/ja00085a041Search in Google Scholar
[10] R. E. London, S. a. Gabel. J. Am. Chem. Soc.116, 2570 (1994).10.1021/ja00085a042Search in Google Scholar
[11] J. M. Silla, R. A. Cormanich, R. Rittner, M. P. Freitas. Beilstein J. Org. Chem.9, 1127 (2013).10.3762/bjoc.9.125Search in Google Scholar PubMed PubMed Central
[12] N. Z. Rao, J. D. Larkin, C. W. Bock. Struct. Chem.28, 945 (2017).Search in Google Scholar
[13] N. Piergies, E. Proniewicz, Y. Ozaki, Y. Kim, L. M. Proniewicz. J. Phys. Chem. A.117, 5693 (2013).10.1021/jp404184xSearch in Google Scholar PubMed
[14] K. Kowalska, A. Adamczyk-Woźniak, P. Gajowiec, B. Gierczyk, E. Kaczorowska, Ł. Popenda, G. Schroeder, A. Sikorski, A. Sporzyński. J. Fluor. Chem.187, 1 (2016).10.1016/j.jfluchem.2016.05.001Search in Google Scholar
[15] K. Durka, S. Luliński, J. Serwatowski, K. Woźniak. Organometallics.33, 1608 (2014).10.1021/om401146pSearch in Google Scholar
[16] T. Beringhelli, G. D’Alfonso, D. Donghi, D. Maggioni, P. Mercandelli, A. Sironi. Organometallics.26, 2088 (2007).10.1021/om061167pSearch in Google Scholar
[17] D. Donghi, D. Maggioni, T. Beringhelli, G. D’Alfonso, P. Mercandelli, A. Sironi. Eur. J. Inorg. Chem.1, 1645 (2008).10.1002/ejic.200701210Search in Google Scholar
[18] D. Donghi, D. Maggioni, T. Beringhelli, G. D. Alfonso. Eur. J. Inorg. Chem.2008, 3606 (2008).10.1002/ejic.200800280Search in Google Scholar
[19] T. Beringhelli, D. Donghi, D. Maggioni, G. D’Alfonso. Coord. Chem. Rev.252, 2292 (2008).10.1016/j.ccr.2008.01.018Search in Google Scholar
[20] S. Das, V. L. Alexeev, A. C. Sharma, S. J. Geib, S. A. Asher. Tetrahedron Lett.44, 7719 (2003).10.1016/j.tetlet.2003.08.094Search in Google Scholar
[21] B. Gierczyk, M. Kaźmierczak, Ł. Popenda, A. Sporzyński, G. Schroeder, S. Jurga. Magn. Reson. Chem.52, 202 (2014).10.1002/mrc.4051Search in Google Scholar PubMed
[22] T. Kliś, J. Serwatowski. Tetrahedron Lett.48, 5223 (2007).10.1016/j.tetlet.2007.05.149Search in Google Scholar
[23] H. J. Frohn, N. Y. Adonin, V. V. Bardin, V. F. Starichenko. Z. Anorg. Allg. Chem.628, 2827 (2002).10.1002/1521-3749(200213)628:13<2827::AID-ZAAC2827>3.0.CO;2-NSearch in Google Scholar
[24] T. Kinzel, Y. Zhang, S. L. Buchwald. J. Am. Chem. Soc.132, 14073 (2010).10.1021/ja1073799Search in Google Scholar
[25] M. Butters, J. N. Harvey, J. Jover, A. J. J. Lennox, G. C. Lloyd-jones, P. M. Murray. Angew. Chemie – Int. Ed.49, 5156 (2010).10.1002/anie.201001522Search in Google Scholar
[26] G. Meng, S. Shi, M. Szostak. ACS Catal.6, 7335 (2016).10.1021/acscatal.6b02323Search in Google Scholar
[27] Y. Chen, M. C. Willis. Chem. Sci.8, 3249 (2017).10.1039/C6SC05483HSearch in Google Scholar
[28] G. Gómez-Jaimes, V. Barba. J. Mol. Struct.1075, 594 (2014).10.1016/j.molstruc.2014.06.078Search in Google Scholar
[29] C. Zhang, M. D. Losego, P. V. Braun. Chem. Mater.25, 3239 (2013).10.1021/cm401738pSearch in Google Scholar
[30] V. L. Alexeev, S. Das, D. N. Finegold, S. A. Asher. Clin. Chem.50, 2353 (2004).10.1373/clinchem.2004.039701Search in Google Scholar
[31] S. Jin, M. Li, C. Zhu, V. L. Tran, B. Wang. ChemBioChem.9, 1431 (2008).10.1002/cbic.200700663Search in Google Scholar
[32] N. G. Nair, M. Blanco, W. West, F. C. Weise, S. Greenbaum, V. R. Prakash. J. Phys. Chem. A.113, 5918 (2009).10.1021/jp901952tSearch in Google Scholar
[33] G. Żukowska, M. Szczechura, M. Marcinek, A. Żubrowska, A. Adamczyk-Woźniak, A. Sporzyński, W. Wieczorek. ECS Trans.16, 105 (2009).10.1149/1.3123132Search in Google Scholar
[34] H. S. Lee. J. Electrochem. Soc.145, 2813 (1998).10.1149/1.1838719Search in Google Scholar
[35] J. McBreen, H. S. Lee, X. Q. Yang, X. Sun. J. Power Sources.89, 163 (2000).10.1016/S0378-7753(00)00425-0Search in Google Scholar
[36] X.-Q. Yang, H. S. Lee, W.-S. Yoon, J. McBreen, S. Hossain, Y.-K. Kim. Meet. Abstr. - Electrochem. Soc.702, 727 (2007).10.1149/MA2007-02/10/727Search in Google Scholar
[37] H. S. Lee, X.-Q. Yang, X. Sun, J. McBreen. J. Power Sources.97–98, 566 (2001).10.1016/S0378-7753(01)00535-3Search in Google Scholar
[38] H. S. Lee, Z. F. Ma, X. Q. Yang, X. Sun, J. McBreen. J. Electrochem. Soc.151, A1429 (2004).10.1149/1.1779407Search in Google Scholar
[39] J. Kryczka, S. Drzewiecki, J. Szczypińska, Z. Żukowska, A. Adamczyk Woźniak, J. S. Syzdek, M. Marcinek, A. Sporzyński, W. Wieczorek. ECS Trans. 25, 61 (2010).10.1149/1.3393840Search in Google Scholar
[40] R. Nishiyabu, Y. Kubo, T. D. James, J. S. Fossey. Chem. Commun.47, 1124 (2011).10.1039/C0CC02921ASearch in Google Scholar
[41] S. Delpierre, B. Willocq, J. De Winter, P. Dubois, P. Gerbaux, J. M. Raquez. Chem. Eur. J.23, 6730 (2017).10.1002/chem.201700333Search in Google Scholar PubMed
[42] X. Chen, M. Addicoat, E. Jin, H. Xu, T. Hayashi, F. Xu, N. Huang, S. Irle, D. Jiang. Sci. Rep.5, 14650 (2015).10.1038/srep14650Search in Google Scholar PubMed PubMed Central
[43] H. Li, Q. Pan, Y. Ma, X. Guan, M. Xue, Q. Fang, Y. Yan, V. Valtchev, S. Qiu. J. Am. Chem. Soc.138, 14783 (2016).10.1021/jacs.6b09563Search in Google Scholar PubMed
[44] F. L. Rock, W. Mao, A. Yaremchuk, M. Tukalo, T. Crepin, H. Zhou, Y.-K. Zhang, V. Hernandez, T. Akama, S. J. Baker, J. J. Plattner, L. Shapiro, S. A. Martinis, S. J. Benkovic, S. Cusack, M. R. K. Alley. Science316, 1759 (2007).10.1126/science.1142189Search in Google Scholar PubMed
[45] E. Seiradake, W. Mao, V. Hernandez, S. J. Baker, J. J. Plattner, M. R. K. Alley, S. Cusack. J. Mol. Biol.390, 196 (2009).10.1016/j.jmb.2009.04.073Search in Google Scholar PubMed
[46] A. Adamczyk-Woźniak, K. M. Borys, A. Sporzyński. Chem. Rev.115, 5224 (2015).10.1021/cr500642dSearch in Google Scholar PubMed
[47] X. Li, S. Zhang, Y. K. Zhang, Y. Liu, C. Z. Ding, Y. Zhou, J. J. Plattner, S. J. Baker, W. Bu, L. Liu, W. M. Kazmierski, M. Duan, R. M. Grimes, L. L. Wright, G. K. Smith, R. L. Jarvest, J. J. Ji, J. P. Cooper, M. D. Tallant, R. M. Crosby, K. Creech, Z. J. Ni, W. Zou, J. Wright. Bioorg. Med. Chem. Lett.21, 2048 (2011).10.1016/j.bmcl.2011.02.006Search in Google Scholar PubMed
[48] C. Z. Ding, Y. K. Zhang, X. Li, Y. Liu, S. Zhang, Y. Zhou, J. J. Plattner, S. J. Baker, L. Liu, M. Duan, R. L. Jarvest, J. Ji, W. M. Kazmierski, M. D. Tallant, L. L. Wright, G. K. Smith, R. M. Crosby, A. A. Wang, Z. J. Ni, W. Zou, J. Wright. Bioorg. Med. Chem. Lett.20, 7317 (2010).10.1016/j.bmcl.2010.10.071Search in Google Scholar PubMed
[49] Y. K. Zhang, J. J. Plattner, Y. R. Freund, E. E. Easom, Y. Zhou, L. Ye, H. Zhou, D. Waterson, F. J. Gamo, L. M. Sanz, M. Ge, Z. Li, L. Li, H. Wang, H. Cui. Bioorg. Med. Chem. Lett.22, 1299 (2012).Search in Google Scholar
[50] S. Chandra, G. W. Kabalka, D. R. Lorey, D. R. Smith, J. A. Coderre. Clin. Cancer Res.8, 2675 (2002).Search in Google Scholar
[51] K. Ehara, N. Tamaki, Y. Hara, Y. Imahori, H. Horij, J. S. Ueda, J. Hiratsuka, Y. Mishima. “Selective detection of brain metastasis of malignant melanoma using 18F-10B-L-borono-phenylalanine with positron emission tomography”, in Cancer Neutron Capture Ther., Y. Mishima (Ed.), pp. 829–833, Springer, Boston, MA, New York (1996).10.1007/978-1-4757-9567-7_117Search in Google Scholar
[52] C. Grunewald, M. Sauberer, T. Filip, T. Wanek, J. Stanek, S. Mairinger, S. Rollet, P. Kudejova, O. Langer, C. Schütz, M. Blaickner, C. Kuntner. Nucl. Med. Biol.44, 83 (2017).10.1016/j.nucmedbio.2016.08.012Search in Google Scholar PubMed
[53] K. Havu-Aurén, J. Kiiski, K. Lehtiö, O. Eskola, M. Kulvik, V. Vuorinen, V. Oikonen, J. Vähätalo, J. Jääskeläinen, H. Minn. Eur. J. Nucl. Med. Mol. Imaging.34, 87 (2007).10.1007/s00259-006-0154-ySearch in Google Scholar PubMed
[54] C. Hsieh, Y. Chen, F. Chen, J. Hwang, J. Chen, R. Liu, J. Kai, C. Chang, H. Wang. J. Nucl. Med.46, 1858 (2016).Search in Google Scholar
[55] K. Kobayashi, H. Kurihara, Y. Watanabe, N. Murakami, K. Inaba, S. Nakamura, A. Wakita, H. Okamoto, R. Umezawa, K. Takahashi, H. Igaki, Y. Ito, S. Yoshimoto, N. Shigematsu, J. Itami. Appl. Radiat. Isot.115, 138 (2016).10.1016/j.apradiso.2016.05.026Search in Google Scholar PubMed
[56] R. Kubota, S. Yamada, K. Ishiwata, M. Tada, T. Ido, K. Kubota. Br. J. Cancer.67, 701 (1993).10.1038/bjc.1993.129Search in Google Scholar PubMed PubMed Central
[57] H. Tani, H. Kurihara, K. Hiroi, N. Honda, M. Yoshimoto, Y. Kono, R. Murakami, S. Kumita, Y. Arai, J. Itami. Radiother. Oncol.113, 193 (2014).10.1016/j.radonc.2014.11.001Search in Google Scholar PubMed
[58] B. Wingelhofer, K. Kreis, S. Mairinger, V. Muchitsch, J. Stanek, T. Wanek, O. Langer, C. Kuntner. Appl. Radiat. Isot.118, 67 (2016).10.1016/j.apradiso.2016.08.026Search in Google Scholar PubMed
[59] M. Yoshimoto, H. Kurihara, N. Honda, K. Kawai, K. Ohe, H. Fujii, J. Itami, Y. Arai. Nucl. Med. Biol.40, 625 (2013).10.1016/j.nucmedbio.2013.02.010Search in Google Scholar PubMed
[60] T. Watanabe, Y. Hattori, Y. Ohta, M. Ishimura, Y. Nakagawa, Y. Sanada, H. Tanaka, S. Fukutani, S. Masunaga, M. Hiraoka, K. Ono, M. Suzuki, M. Kirihata. BMC Cancer.16, 859 (2016).10.1186/s12885-016-2913-xSearch in Google Scholar PubMed PubMed Central
[61] K. Ishiwata, T. Ito, M. Kawamura, K. Kubota, M. Ichihashi, Y. Mishima. Nucl. Med. Biol.18, 745 (1991).10.1016/0883-2897(91)90013-BSearch in Google Scholar
[62] Y. Hattori, K. Kurihara, H. Kondoh, T. Asano, M. Kirihata, Y. Yamaguchi, T. Wakamiya. Protein Peptide Lett.14, 269 (2007).10.2174/092986607780090856Search in Google Scholar PubMed
[63] N. DiCesare, J. R. Lakowicz. Anal. Biochem.301, 111 (2002).10.1006/abio.2001.5476Search in Google Scholar PubMed PubMed Central
[64] Z. Xu, S. K. Kim, S. J. Han, C. Lee, G. Kociok-Kohn, T. D. James, J. Yoon. Eur. J. Org. Chem.2009, 3058 (2009).10.1002/ejoc.200900120Search in Google Scholar
[65] Y. Kubo, T. Ishida, A. Kobayashi, T. D. James. J. Mater. Chem.15, 2889 (2005).10.1039/b501243kSearch in Google Scholar
[66] W. Tan, D. Zhang, D. Zhu. Bioorg. Med. Chem. Lett.17, 2629 (2007).10.1016/j.bmcl.2007.01.099Search in Google Scholar PubMed
[67] M. Nicolas, B. Fahre, J. Simonet. Electrochim. Acta.46, 1179 (2001).10.1016/S0013-4686(00)00694-0Search in Google Scholar
[68] C. Bresner, J. K. Day, N. D. Coombs, I. A. Fallis, S. Aldridge, S. J. Coles, M. B. Hursthouse. Dalton Trans. 3660 (2006).10.1039/b605031jSearch in Google Scholar PubMed
[69] J. Yan, G. Springsteen, S. Deeter, B. Wang. Tetrahedron60, 11205 (2004).10.1016/j.tet.2004.08.051Search in Google Scholar
[70] D. Zarzeczańska, A. Adamczyk-Woźniak, A. Kulpa, T. Ossowski, A. Sporzyński. Eur. J. Inorg. Chem. 4493 (2017).10.1002/ejic.201700546Search in Google Scholar
[71] Y. Yamamoto, T. Matsumura, N. Takao, H. Yamagishi, M. Takahashi, S. Iwatsuki, K. Ishihara. Inorg. Chim. Acta.358, 3355 (2005).10.1016/j.ica.2005.05.026Search in Google Scholar
[72] P. R. Westmark, S. J. Gardiner, B. D. Smith. J. Am. Chem. Soc.118, 11093 (1996).10.1021/ja961264hSearch in Google Scholar
[73] Q. Li, C. Lü, Z. Liu. J. Chromatogr. A.1305, 123 (2013).10.1016/j.chroma.2013.07.007Search in Google Scholar PubMed
[74] A. Adamczyk-Woźniak, K. M. Borys, I. D. Madura, A. Pawełko, E. Tomecka, K. Żukowski. New J. Chem.37, 188 (2013).10.1039/C2NJ40687JSearch in Google Scholar
[75] A. Adamczyk-Woźniak, M. K. Cabaj, P. M. Dominiak, P. Gajowiec, B. Gierczyk, J. Lipok, Ł. Popenda, G. Schroeder, E. Tomecka, P. Urbański, D. Wieczorek, A. Sporzyński. Bioorg. Chem.60, 130 (2015).10.1016/j.bioorg.2015.05.004Search in Google Scholar PubMed
[76] J. W. Tomsho, A. Pal, D. G. Hall, S. J. Benkovic. ACS Med. Chem. Lett.3, 48 (2012).10.1021/ml200215jSearch in Google Scholar PubMed PubMed Central
[77] I. D. Madura, K. Czerwińska, D. Sołdańska. Cryst. Growth Des.14, 5912 (2014).10.1021/cg501132dSearch in Google Scholar
[78] A. Minkkila, S. M. Saario, H. Ka, A. Minkkilä, H. Käsnänen, J. Leppänen, A. Poso, T. Nevalainen. J. Med. Chem.1, 7057 (2008).Search in Google Scholar
[79] S. Kheirjou, A. Abedin, A. Fattahi. Comput. Theor. Chem.1000, 1 (2012).10.1016/j.comptc.2012.08.012Search in Google Scholar
[80] V. Gutmann. Electrochim. Acta.21, 661 (1976).10.1016/0013-4686(76)85034-7Search in Google Scholar
[81] U. Mayer, V. Gutmann, W. Gerger. Monatsh. Chem.106, 1235 (1975).10.1007/BF00913599Search in Google Scholar
[82] A. Adamczyk-Woźniak, M. Jakubczyk, A. Sporzyński, G. Żukowska. Inorg. Chem. Commun.14, 1753 (2011).10.1016/j.inoche.2011.08.002Search in Google Scholar
[83] A. Adamczyk-Woźniak, M. Jakubczyk, P. Jankowski, A. Sporzyński, P. M. Urbański. J. Phys. Org. Chem.26, 415 (2013).10.1002/poc.3102Search in Google Scholar
[84] R. D. Chambers, T. J. Chivers. J. Chem. Soc. 3933 (1965).10.1039/jr9650003933Search in Google Scholar
[85] H. J. Frohn, N. Y. Adonin, V. V. Bardin, V. F. Starichenko. Z. Anorg. Allg. Chem.628, 2834 (2002).10.1002/1521-3749(200213)628:13<2834::AID-ZAAC2834>3.0.CO;2-2Search in Google Scholar
[86] J. Lozada, Z. Liu, D. M. Perrin. J. Org. Chem.79, 5365 (2014).10.1021/jo500734zSearch in Google Scholar
[87] M. K. Cyrański, A. Jezierska, P. Klimentowska, J. J. Panek, A. Sporzyński. J. Phys. Org. Chem.21, 472 (2008).10.1002/poc.1389Search in Google Scholar
[88] Y. M. Wu, C. C. Dong, S. Liu, H. J. Zhu, Y. Z. Wu. Acta Crystallogr. Sect. E Struct. Reports Online62, 4236 (2006).10.1107/S1600536806034763Search in Google Scholar
[89] P. Rodríguez-Cuamatzi, H. Tlahuext, H. Höpfl. Acta Crystallogr. Sect. E Struct. Reports Online65, o44 (2009).10.1107/S1600536808040646Search in Google Scholar PubMed PubMed Central
[90] P. N. Horton, M. B. Hursthouse, M. A. Beckett, M. P. Rugen-Hankey. Acta Crystallogr. Sect. E Struct. Reports Online60, o2204 (2004).10.1107/S1600536804022408Search in Google Scholar
[91] T. Kliś, S. Luliński, J. Serwatowski. Acta Crystallogr. Sect. C Cryst. Struct. Commun.63, o145 (2007).10.1107/S1744309107004939Search in Google Scholar
[92] K. Arnold, A. S. Batsanov, B. Davies, A. Whiting. Green Chem.10, 124 (2008).10.1039/B712008GSearch in Google Scholar
[93] S. M. Cornet, K. B. Dillon, C. D. Entwistle, M. A. Fox, A. E. Goeta, H. P. Goodwin, T. B. Marder, A. L. Thompson. Dalton Trans.2, 4395 (2003).Search in Google Scholar
[94] V. N. Okulov, I. V. Ananyev, E. R. Milaeva, D. A. Lemenovskii, V. P. Dyadchenko. Russ. Chem. Bull.64, 2244 (2015).10.1007/s11172-015-1145-6Search in Google Scholar
[95] K. Kopczyńska, P. H. Marek, B. Banaś, I. D. Madura. Acta Crystallogr. Sect. C Struct. Chem.73, 889 (2017).10.1107/S2053229617013675Search in Google Scholar PubMed
[96] A. Adamczyk-Woźniak, M. K. Cyrański, M. Jakubczyk, P. Klimentowska, A. Koll, J. Kołodziejczak, G. Pojmaj, A. Zubrowska, G. Z. Zukowska, A. Sporzyński. J. Phys. Chem. A.114, 2324 (2010).10.1021/jp9086283Search in Google Scholar PubMed
[97] I. D. Madura, A. Adamczyk-Woźniak, M. Jakubczyk, A. Sporzyński. Acta Crystallogr. Sect. E Struct. Reports Online67, o414 (2011).10.1107/S1600536811001632Search in Google Scholar PubMed PubMed Central
[98] S. Sene, B. Donnadieu, J. Vezzani, D. Granier, H. Mutin, C. Gervais, D. Laurencin. CrystEngComm16, 4999 (2014).10.1039/c4ce00313fSearch in Google Scholar
[99] K. Durka, K. N. Jarzembska, R. Kamiński, S. Luliński, J. Serwatowski, K. Woźniak. Cryst. Growth Des.12, 3720 (2012).10.1021/cg3005272Search in Google Scholar
[100] W. H. Pearson, S. Lin, P. M. Iovine. Acta Crystallogr. Sect. E Struct. Reports Online64, o235 (2008).10.1107/S1600536807064367Search in Google Scholar PubMed PubMed Central
[101] I. D. Madura, K. Czerwińska, M. Jakubczyk, A. Pawełko, A. Adamczyk-Woźniak, A. Sporzyński. Cryst. Growth Des.13, 5344 (2013).10.1021/cg4012026Search in Google Scholar
[102] N. J. Wilcox, W. R. Kwochka, R. D. Pike. J. Chem. Crystallogr.46, 28 (2016).Search in Google Scholar
[103] M. K. Cyrański, P. Klimentowska, A. Rydzewska, J. Serwatowski, A. Sporzyński, D. K. Stępień. CrystEngComm14, 6282 (2012).10.1039/c2ce25657fSearch in Google Scholar
[104] J. Beckmann, D. Dakternieks, A. Duthie, A. E. Lim, E. R. Tiekink. J. Organomet. Chem.633, 149 (2001).10.1016/S0022-328X(01)01060-9Search in Google Scholar
[105] C. P. Brock, R. P. Minton, K. Niedenzu. Acta Crystallogr. Sect. C Cryst. Struct. Commun.43, 1775 (1987).10.1107/S010827018709022XSearch in Google Scholar
[106] S. Luliński, I. Madura, J. Serwatowski, H. Szatyłowicz, J. Zachara. New J. Chem.31, 144 (2007).10.1039/B611195ESearch in Google Scholar
[107] M. Karabacak, E. Kose, A. Atac, M. Ali Cipiloglu, M. Kurt. Spectrochim. Acta – Part A Mol. Biomol. Spectrosc.97, 892 (2012).10.1016/j.saa.2012.07.077Search in Google Scholar PubMed
[108] M. Karabacak, E. Kose, A. Atac, A. M. Asiri, M. Kurt. J. Mol. Struct.1058, 79 (2014).10.1016/j.molstruc.2013.10.064Search in Google Scholar
[109] M. Karabacak, E. Kose, E. B. Sas, M. Kurt, A. M. Asiri, A. Atac. Spectrochim. Acta – Part A Mol. Biomol. Spectrosc.136, 306 (2015).10.1016/j.saa.2014.08.141Search in Google Scholar PubMed
[110] W. R. Dolbier Jr. Guide to Fluorine NMR for Organic Chemists, 2nd ed., Wiley, New Jersey (2016).10.1002/9781118831106Search in Google Scholar
[111] A. M. Mfuh, J. D. Doyle, B. Chhetri, H. D. Arman, O. V. Larionov. J. Am. Chem. Soc.138, 2985 (2016).10.1021/jacs.6b01376Search in Google Scholar PubMed PubMed Central
[112] J. Zhou, M. W. Kuntze-Fechner, R. Bertermann, U. S. D. Paul, J. H. J. Berthel, A. Friedrich, Z. Du, T. B. Marder, U. Radius. J. Am. Chem. Soc.138, 5250 (2016).10.1021/jacs.6b02337Search in Google Scholar PubMed
[113] J.-P. Kintzinger. “Oxygen NMR. Characteristic parameters and applications”, in NMR Basic Princ. Prog., P. Diehl, E. Fluck, R. Kosfeld (Eds.), Springer-Verlag, Berlin, Heidelberg, New York (1981).Search in Google Scholar
[114] B. Gierczyk, M. Kaźmierczak, G. Schroeder, A. Sporzyński. New J. Chem.37, 1056 (2013).10.1039/c3nj40903aSearch in Google Scholar
[115] H.-J. Frohn, H. Franke, P. Fritzen, V. V. Bardin. J. Organomet. Chem.598, 127 (2000).10.1016/S0022-328X(99)00690-7Search in Google Scholar
[116] A. Hafner, M. Meisenbach, J. Sedelmeier. Org. Lett.18, 3630 (2016).10.1021/acs.orglett.6b01681Search in Google Scholar PubMed
[117] D. L. Browne, M. Baumann, B. H. Harji, I. R. Baxendale, S. V. Ley, Org. Lett.13, 3312 (2011).10.1021/ol2010006Search in Google Scholar PubMed
[118] H. H. Büker, N. M. M. Nibbering, D. Espinosa, F. Mongin, M. Schlosser. Tetrahedron Lett.38, 8519 (1997).10.1016/S0040-4039(97)10303-3Search in Google Scholar
[119] M. Schlosser. Angew. Chemie – Int. Ed.110, 1496 (1998).Search in Google Scholar
[120] T. Kliś, S. Luliński, J. Serwatowski. Curr. Org. Chem.12, 1479 (2008).10.2174/138527208786241475Search in Google Scholar
[121] A. Adamczyk-Woźniak, K. M. Borys, I. D. Madura, S. Michałek, A. Pawełko. Tetrahedron69, 8936 (2013).10.1016/j.tet.2013.07.102Search in Google Scholar
[122] S. J. Baker, Y.-K. Zhang, T. Akama, A. Lau, H. Zhou, V. Hernandez, W. Mao, M. R. K. Alley, V. Sanders, J. J. Plattner. J. Med. Chem.49, 4447 (2006).10.1021/jm0603724Search in Google Scholar PubMed
[123] D. S. Gunasekera, D. J. Gerold, N. S. Aalderks, J. S. Chandra, C. A. Maanu, P. Kiprof, V. V. Zhdankin, M. V. R. Reddy. Tetrahedron63, 9401 (2007).10.1016/j.tet.2007.06.099Search in Google Scholar
[124] S. Cao, R. Christiansen, X. Peng. Chem. Eur. J.19, 9050 (2013).10.1002/chem.201300539Search in Google Scholar PubMed
[125] K. Durka, P. Kurach, S. Luliński, J. Serwatowski. Eur. J. Org. Chem. 4325 (2009).10.1002/ejoc.200900526Search in Google Scholar
[126] K. Durka, J. Górka, P. Kurach, S. Luliński, J. Serwatowski. J. Organomet. Chem.695, 2635 (2010).10.1016/j.jorganchem.2010.08.016Search in Google Scholar
[127] N. Miyaura. J. Organomet. Chem.653, 54 (2002).10.2307/3853324Search in Google Scholar
[128] N. Miyaura, K. Yamada, A. Suzuki. Tetrahedron Lett.36, 3437 (1979).10.1016/S0040-4039(01)95429-2Search in Google Scholar
[129] D. G. Hall. “Structure, properties and preparation of boronic acid derivatives”, in Boronic Acids. Preparation and Applications in Organic Synthesis, Medicine and Materials, Vol. 1, 2nd ed., D. G. Hall (Ed.), p. 85, Wiley-VCH, Weinheim (2011).10.1002/9783527639328.ch1Search in Google Scholar
[130] J. Kohlmann, T. Braun, R. Laubenstein, R. Herrmann. Chem. Eur. J.23, 12218 (2017).10.1002/chem.201700549Search in Google Scholar PubMed
[131] J. Y. L. Chung, C. Cai, J. C. McWilliams, R. A. Reamer, P. G. Dormer, R. J. Cvetovich. J. Org. Chem.70, 10342 (2005).10.1021/jo0514927Search in Google Scholar PubMed
[132] C. Cai, J. Y. L. Chung, J. C. McWilliams, Y. Sun, C. S. Shultz, M. Palucki. Org. Process Res. Dev.11, 328 (2007).10.1021/op060215eSearch in Google Scholar
[133] Z. Gao, V. Gouverneur, B. G. Davis. J. Am. Chem. Soc.135, 13612 (2013).10.1021/ja4049114Search in Google Scholar PubMed PubMed Central
[134] F. González-Bobes, G. C. Fu. J. Am. Chem. Soc.128, 5360 (2006).10.1021/ja0613761Search in Google Scholar PubMed
[135] A. Affrose, I. A. Azath, A. Dhakshinamoorthy, K. Pitchumani. J. Mol. Catal. A Chem.395, 500 (2014).10.1016/j.molcata.2014.09.016Search in Google Scholar
[136] L. J. Diorazio, D. A. Widdowson, J. M. Clough. Tetrahedron48, 8073 (1992).10.1016/S0040-4020(01)80478-6Search in Google Scholar
[137] G. K. S. Prakash, C. Panja, T. Mathew, V. Surampudi, N. A. Petasis, G. A. Olah. Org. Lett.8, 11 (2004).Search in Google Scholar
[138] J. Morgan, J. T. Pinhey. J. Chem. Soc., Perkin Trans.1, 715 (1990).10.1039/P19900000715Search in Google Scholar
[139] M.-L. Huber, J. T. Pinhey. J. Chem. Soc. Perkin Trans.1, 721 (1990).10.1039/p19900000721Search in Google Scholar
[140] H. Serizawa, K. Aikawa, K. Mikami. Chem. Eur. J.19, 17692 (2013).10.1002/chem.201303828Search in Google Scholar PubMed
[141] R. H. Szumigala, P. N. Devine, D. R. Gauthier, R. P. Volante. J. Org. Chem.69, 566 (2004).10.1021/jo035184pSearch in Google Scholar PubMed
[142] A. P. Côté, A. I. Benin, N. W. Ockwig, M. O’Keeffe, A. J. Matzger, O. M. Yaghi. Science310, 1166 (2005).10.1126/science.1120411Search in Google Scholar PubMed
[143] Y. He, Z. Yang, R. T. Thornbury, F. D. Toste. J. Am. Chem. Soc.137, 12207 (2015).10.1021/jacs.5b07795Search in Google Scholar PubMed PubMed Central
[144] J. H. Kim, S. J. Choi, I. H. Jeong. Beilstein J. Org. Chem.9, 2470 (2013).10.3762/bjoc.9.286Search in Google Scholar PubMed PubMed Central
[145] Y. Yamamoto, E. Ohkubo, M. Shibuya. Adv. Synth. Catal.359, 1747 (2017).10.1002/adsc.201700122Search in Google Scholar
[146] K. Gontarczyk, K. Durka, P. Klimkowski, S. Luliński, J. Serwatowski, K. Woźniak. J. Organomet. Chem.783, 1 (2015).10.1016/j.jorganchem.2015.01.024Search in Google Scholar
[147] S. Brock, D. R. J. Hose, J. D. Moseley, A. J. Parker, I. Patel, A. J. Williams. Org. Process Res. Dev.12, 496 (2008).10.1021/op700246gSearch in Google Scholar
[148] J. P. Lorand, J. O. Edwards. J. Org. Chem.24, 769 (1959).10.1021/jo01088a011Search in Google Scholar
[149] Y. Furikado, T. Nagahata, T. Okamoto, T. Sugaya, S. Iwatsuki, M. Inamo, H. D. Takagi, A. Odani, K. Ishihara. Chem. – A Eur. J.20, 13194 (2014).10.1002/chem.201403719Search in Google Scholar PubMed
[150] G. Springsteen, B. Wang. Chem. Commun. 1608 (2001).10.1039/b104895nSearch in Google Scholar PubMed
[151] G. Springsteen, B. Wang. Tetrahedron58, 5291 (2002).10.1016/S0040-4020(02)00489-1Search in Google Scholar
[152] M. Dowlut, D. G. Hall. J. Am. Chem. Soc.128, 4226 (2006).10.1021/ja057798cSearch in Google Scholar PubMed
[153] V. L. Alexeev, A. C. Sharma, A. V. Goponenko, S. Das, I. K. Lednev, C. S. Wilcox, D. N. Finegold, S. A. Asher. Anal. Chem.75, 2316 (2003).10.1021/ac030021mSearch in Google Scholar PubMed
[154] T. Cheng, H. Li, Y. Ma, X. Liu, H. Zhang. Anal. Bioanal. Chem.407, 3525 (2015).10.1007/s00216-015-8550-4Search in Google Scholar PubMed
[155] C. Gao, Y. Wang, S. Yuan, J. Xue, B. Cao, J. Yu. Biosens. Bioelectron.90, 336 (2017).10.1016/j.bios.2016.12.002Search in Google Scholar PubMed
[156] D. Li, Y. Li, X. Li, Z. Bie, X. Pan, Q. Zhang, Z. Liu. J. Chromatogr. A.1384, 88 (2015).10.1016/j.chroma.2015.01.050Search in Google Scholar PubMed
[157] R. Xing, S. Wang, Z. Bie, H. He, Z. Liu. Nat. Protoc.12, 964 (2017).10.1038/nprot.2017.015Search in Google Scholar PubMed
[158] Q. Li, Z. Liu. “Preparation and characterization of fluorophenylboronic acid-functionalized affinity monolithic columns for the selective enrichment of cis-diol-containing biomolecules”, in Affin. Chromatogr., 3rd ed. S. Reichelt (Ed.), pp. 159–169, Humana Press, Leipzig (2015).10.1007/978-1-4939-2447-9_13Search in Google Scholar PubMed
[159] C. Lü, H. Li, H. Wang, Z. Liu. Anal. Chem.85, 2361 (2013).10.1021/ac3033917Search in Google Scholar PubMed
[160] X. Li, Y. He, Y. Ma, Z. Bie, B. Liu, Z. Liu. Anal. Chem.88, 9805 (2016).10.1021/acs.analchem.6b02907Search in Google Scholar PubMed
[161] N. A. Celis, C. Godoy-Alcántar, J. Guerrero-Álvarez, V. Barba. Eur. J. Inorg. Chem. 1477 (2014).10.1002/ejic.201301450Search in Google Scholar
[162] S. Hagihara, H. Tanaka, S. Matile. J. Am. Chem. Soc.130, 5656 (2008).10.1021/ja801094pSearch in Google Scholar PubMed
[163] A. Minkkilä, S. M. Saario, H. Käsnänen, J. Leppänen, A. Poso, T. Nevalainen. J. Med. Chem.51, 7057 (2008).10.1021/jm801051tSearch in Google Scholar PubMed
[164] B. E. Elewski, R. Aly, S. L. Baldwin, R. F. González Soto, P. Rich, M. Weisfeld, H. Wiltz, L. T. Zane, R. Pollak. J. Am. Acad. Dermatol.73, 62 (2015).10.1016/j.jaad.2015.04.010Search in Google Scholar PubMed
[165] A. K. Gupta, D. Daigle. Expert Rev. Anti. Infect. Ther.12, 735 (2014).10.1586/14787210.2014.915738Search in Google Scholar PubMed
[166] S. Jinna, J. Finch. Drug Des. Devel. Ther.9, 6185 (2015).Search in Google Scholar
[167] S. Sene, S. Bégu, C. Gervais, G. Renaudin, A. Mesbah, M. E. Smith, P. H. Mutin, A. Van Der Lee, J. M. Nedelec, C. Bonhomme, D. Laurencin. Chem. Mater.27, 1242 (2015).10.1021/cm504181wSearch in Google Scholar
[168] S. Sene, J. McLane, N. Schaub, S. Begu, P. H. Mutin, L. Ligon, R. J. Gilbert, D. Laurencin. J. Mater. Chem. B.4, 257 (2016).10.1039/C5TB02258DSearch in Google Scholar PubMed
[169] S. Wring, E. Gaukel, B. Nare, R. Jakobs, B. Beaudet, T. Bowling, L. Mercer, C. Bacchi, N. Yarlett, R. Randolph, R. Parham, C. Rewerts, J. Platner, R. Don. Parasitology.141, 104 (2014).10.1017/S003118201300098XSearch in Google Scholar PubMed PubMed Central
[170] A. Adamczyk-Woźniak, O. Komarovska-Porokhnyavets, B. Misterkiewicz, V. P. Novikov, A. Sporzyński. Appl. Organomet. Chem.26, 390 (2012).10.1002/aoc.2880Search in Google Scholar
[171] L. Evangelista, G. Jori, D. Martini, G. Sotti. Appl. Radiat. Isot.74, 91 (2013).10.1016/j.apradiso.2013.01.001Search in Google Scholar PubMed
[172] P. Bendel, N. Koudinova, Y. Salomon. Magn. Reson. Med.46, 13 (2001).10.1002/mrm.1154Search in Google Scholar PubMed
[173] J. Capala, M. S. Makar, J. A. Coderre. Radiat. Res.146, 554 (1996).10.2307/3579556Search in Google Scholar
[174] P. Porcari, S. Capuani, R. Campanella, A. La Bella, L. M. Migneco, B. Maraviglia. Phys. Med. Biol.51, 3141 (2006).10.1088/0031-9155/51/12/010Search in Google Scholar PubMed
[175] J. K. Vähätalo, O. Eskola, J. Bergman, S. Forsback, P. Lehikoinen, J. Jääskeläinen, O. Solin. J. Label. Compd. Radiopharm.45, 697 (2002).10.1002/jlcr.600Search in Google Scholar
[176] Y. Imahori, S. Ueda, Y. Ohmori, T. Kusuki, K. Ono, R. Fujii, I. Tatsuo. J. Nucl. Med.39, 325 (1998).Search in Google Scholar
[177] Y. Imahori, S. Ueda, Y. Ohmori, K. Sakae, T. Kusuki, T. Kobayashi, M. Takagaki, K. Ono, T. Ido, R. Fujii. Clin. Cancer Res.4, 1825 (1998).Search in Google Scholar
[178] Y. Imahori, S. Ueda, Y. Ohmori, K. Sakae, T. Kusuki, T. Kobayashi, M. Takagaki, K. Ono, T. Ido, R. Fujii. Clin. Cancer Res.4, 1833 (1998).Search in Google Scholar
[179] S. Mairinger, J. Stanek, T. Wanek, O. Langer, C. Kuntner. Appl. Radiat. Isot.104, 124 (2015).10.1016/j.apradiso.2015.06.034Search in Google Scholar PubMed
[180] C. W. Harwig, R. Ting, M. J. Adam, T. J. Ruth, D. M. Perrin. Tetrahedron Lett.49, 3152 (2008).10.1016/j.tetlet.2008.03.021Search in Google Scholar
[181] R. Ting, C. Harwig, U. Auf Dem Keller, S. McCormick, P. Austin, C. M. Overall, M. J. Adam, T. J. Ruth, D. M. Perrin. J. Am. Chem. Soc.130, 12045 (2008).10.1021/ja802734tSearch in Google Scholar PubMed
[182] R. Ting, J. Lo, M. J. Adam, T. J. Ruth, D. M. Perrin. J. Fluor. Chem.129, 349 (2008).10.1016/j.jfluchem.2008.01.011Search in Google Scholar
[183] Y. Li, R. Ting, C. W. Harwig, C. L. Bellac, P. F. Lange, J. A. H. Inkster, P. Schaffer, M. J. Adam, T. J. Ruth, M. Overall, D. M. Perrin. Med. Chem. Commun.2, 942 (2011).10.1039/c1md00117eSearch in Google Scholar
[184] J. M. Chalker, C. S. C. Wood, B. G. Davis. J. Am. Chem. Soc.131, 16346 (2009).10.1021/ja907150mSearch in Google Scholar PubMed
[185] J. Cutrera, D. Dibra, X. Xia, A. Hasan, S. Reed, S. Li. Mol. Ther.19, 1468 (2011).10.1038/mt.2011.38Search in Google Scholar PubMed PubMed Central
[186] K. Ishihara, S. Ohara. J. Org. Chem.61, 4196 (1996).10.1021/jo9606564Search in Google Scholar PubMed
[187] S. Liu, Y. Yang, X. Liu, F. K. Ferdousi, A. S. Batsanov, A. Whiting. Eur. J. Org. Chem. 5692 (2013).10.1002/ejoc.201300560Search in Google Scholar
[188] C. L. Ricardo, X. Mo, J. A. McCubbin, D. G. Hall. Chemistry21, 4218 (2015).10.1002/chem.201500020Search in Google Scholar PubMed
[189] S. L. Yeste, M. E. Powell, S. D. Bull, T. D. James. J. Org. Chem.74, 427 (2009).10.1021/jo8019187Search in Google Scholar PubMed
[190] J. Axthelm, H. Görls, U. S. Schubert, A. Schiller. J. Am. Chem. Soc.137, 15402 (2015).10.1021/jacs.5b10934Search in Google Scholar PubMed
[191] J. Axthelm, S. H. C. Askes, M. Elstner, U. R. G, H. Görls, P. Bellstedt, A. Schiller. J. Am. Chem. Soc.139, 11413 (2017).10.1021/jacs.7b01167Search in Google Scholar PubMed
Supplemental Material
The online version of this article offers supplementary material (https://doi.org/10.1515/pac-2017-1009).
©2018 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
Articles in the same Issue
- Frontmatter
- In this issue
- Preface
- 16th International Meeting on Boron Chemistry (IMEBORON XVI)
- Conference papers
- Palladium-promoted sulfur atom migration on carboranes: facile B(4)−S bond formation from mononuclear Pd-B(4) complexes
- When diazo compounds meet with organoboron compounds
- Transition-metal complexes with oxidoborates. Synthesis and XRD characterization of [(H3NCH2CH2NH2)Zn{κ3O,O′,O′′-B12O18(OH)6-κ1O′′′}Zn(en)(NH2CH2CH2NH3)]·8H2O (en=1,2-diaminoethane): a neutral bimetallic zwiterionic polyborate system containing the ‘isolated’ dodecaborate(6−) anion
- Novel sulfur containing derivatives of carboranes and metallacarboranes
- Metal–metal bonding in deltahedral dimetallaboranes and trimetallaboranes: a density functional theory study
- Nanostructured boron compounds for cancer therapy
- Heterometallic boride clusters: synthesis and characterization of butterfly and square pyramidal boride clusters*
- Influence of fluorine substituents on the properties of phenylboronic compounds
- Copper-catalyzed asymmetric dearomative borylation: new pathway to optically active heterocyclic compounds
- Borenium and boronium ions of 5,6-dihydro-dibenzo[c,e][1,2]azaborinine and the reaction with non-nucleophilic base: trapping of a dimer and a trimer of BN-phenanthryne by 4,4′-di-tert-butyl-2,2′-bipyridine
- The electrophilic aromatic substitution approach to C–H silylation and C–H borylation
- Recent advances in B–H functionalization of icosahedral carboranes and boranes by transition metal catalysis
- closo-Dodecaborate-conjugated human serum albumins: preparation and in vivo selective boron delivery to tumor
- IUPAC Technical Report
- Risk assessment of effects of cadmium on human health (IUPAC Technical Report)
Articles in the same Issue
- Frontmatter
- In this issue
- Preface
- 16th International Meeting on Boron Chemistry (IMEBORON XVI)
- Conference papers
- Palladium-promoted sulfur atom migration on carboranes: facile B(4)−S bond formation from mononuclear Pd-B(4) complexes
- When diazo compounds meet with organoboron compounds
- Transition-metal complexes with oxidoborates. Synthesis and XRD characterization of [(H3NCH2CH2NH2)Zn{κ3O,O′,O′′-B12O18(OH)6-κ1O′′′}Zn(en)(NH2CH2CH2NH3)]·8H2O (en=1,2-diaminoethane): a neutral bimetallic zwiterionic polyborate system containing the ‘isolated’ dodecaborate(6−) anion
- Novel sulfur containing derivatives of carboranes and metallacarboranes
- Metal–metal bonding in deltahedral dimetallaboranes and trimetallaboranes: a density functional theory study
- Nanostructured boron compounds for cancer therapy
- Heterometallic boride clusters: synthesis and characterization of butterfly and square pyramidal boride clusters*
- Influence of fluorine substituents on the properties of phenylboronic compounds
- Copper-catalyzed asymmetric dearomative borylation: new pathway to optically active heterocyclic compounds
- Borenium and boronium ions of 5,6-dihydro-dibenzo[c,e][1,2]azaborinine and the reaction with non-nucleophilic base: trapping of a dimer and a trimer of BN-phenanthryne by 4,4′-di-tert-butyl-2,2′-bipyridine
- The electrophilic aromatic substitution approach to C–H silylation and C–H borylation
- Recent advances in B–H functionalization of icosahedral carboranes and boranes by transition metal catalysis
- closo-Dodecaborate-conjugated human serum albumins: preparation and in vivo selective boron delivery to tumor
- IUPAC Technical Report
- Risk assessment of effects of cadmium on human health (IUPAC Technical Report)

![Scheme 9:
Reported pathways in the synthesis of Kerydin [48], [97], [98], [111], [122], [123], [124].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_043.jpg)
![Scheme 13:
SMC between 2,4-difluorophenylboronic acid and 1,6-naphthyridone [131], [132].](/document/doi/10.1515/pac-2017-1009/asset/graphic/j_pac-2017-1009_fig_047.jpg)
