Abstract
The development of highly sensitive and selective DNA sensors has fuelled applications in a wide range of fields including medical diagnostics, forensics, biodefense, food contamination and environment monitoring. We demonstrate a novel superquenching based DNA sensor with “switch-on” readout using poly(p-phenylenevinylene) (PPV) coated magnetic beads (PPV-MagSi) and quencher functionalized tentacle probes (TP). The sensor design utilizes signal amplification properties of PPV and cooperativity of TPs to monitor hybridization of target oligonucleotides (ONs). The switch-on sensor exhibits excellent sensitivity and selectively discriminates mismatches in the target DNA sequence. Two novel anionic PPVs – poly (6,6′-((2-methyl-5-((E)-4-((E)-prop-1-en-1-yl)styryl)-1,4-phenylene)-bis(oxy) dihexanoic acid) (PMDH) and poly (6,6′-((2-((E)-2,5-bis(2-methoxyethoxy)-4-((E)-prop-1-en-1-yl)styryl)-5-methyl-1,4-phenylene)-bis-(oxy)) di-hexanoic acid) (PDMonoG) were tested and compared against each other as part of the sensor design. The employed hairpin TPs possess further advantages of avoiding labelling of target ON, increased selectivity and sensitivity; faster assay time, and capability of magnetically controlled deployment and separation of PPV-MagSi beads.
Introduction
Conjugated polymers (CPs) with their unique optical and electrical properties, have found extensive use in gene sensing as both passive support for entrapment of DNA as well as a transducer of the hybridization event into an observable and measurable electrical [1–5] or optical [6–9] signal. They also present other desirable properties, including ease of processing, emission colour-tuning, low costs and high environmental stability [10, 11]. CPs such as poly(p-phenylene) (PPP), poly(p-phenylenevinylene) (PPV), poly(p-phenyleneethynylene) (PPE), have been reported to exhibit amplified light quenching abilities, which is termed as “superquenching” [12–14]. Superquenching in polymers occurs due to efficient electron/energy transfer from the excited photoluminescent CP to the quenchers, along with the contribution from electrostatic interactions [13].
Molecular beacons (MBs) are single DNA sequences which form a hairpin-like structure due to complementary bases near the fluorophore and quencher [15, 16]. Driven by the thermodynamic stability, the hairpin opens up only in the presence of the complementary DNA, extending the quencher away from the fluorophore causing the increase in fluorescence from the fluorophore [16]. Although MBs offer high selectivity and sensitivity, challenges have accompanied the use of MBs, such as a change in the melting temperature of MBs with the concentration of MBs [17]. Recently, Satterfield and co-workers [17, 18] have developed a new class of probes, similar to MBs, called tentacle probes (TPs) that overcome the limitations of MBs. In addition to a stem-loop “detection” region of MBs, TPs have a linear oligonucleotide (ON) “capture” region (‘tentacle’) that increase the probability of the hybridization reaction with the detection region [17]. As the result, TPs possess increased selectivity, lower detection limits and faster hybridization times.
Solution based and heterogeneous label-free DNA sensing assays utilizing both CPs and molecular beacons [9, 15, 19–22] have been recently developed [23–25] by a number of groups. Water-soluble PPE-DNA conjugates with quenchers have been most commonly used as derivatised MBs [23–25] whereby PPE acts as the fluorophore. In-depth studies into understanding the molecular parameters (such as stem length, number of quenchers, replacing the quencher with a fluorescent dye) have also been reported [23–25]. CP based MB sensing designs with monoquenchers [24–26] only report up to 58 % quenching efficiency of the CP fluorescence, observed in the hairpin form. This reduction in quenching efficiency was rationalized by the presence of multiple fluorophores in CPs which cannot be fully quenched by the monoquenchers, reducing the quenching efficiency.
Inspired by the successes of employing TP probes, in this communication we report a proof-of-concept of a novel sensing design that incorporates the signal amplification of CPs, advantageous properties of TPs and benefits of easy handling through magnetic deployment. The switch-on sensor (Fig. 1) is based on quencher-functionalized tentacle probes bound to PPV-modified magnetic beads. Our sensing technology differs from other reported CP-based DNA sensing platforms in some key aspects, as outlined below. We use facile EDC/NHS chemistry to covalently immobilize both PPV and TPs on the magnetic beads. The majority of the label-free CP-based heterogeneous sensors utilising MBs reported in the literature use harsh conditions or non-covalent methods to functionalize the substrate with CPs as well as lengthy on-chip DNA synthesis procedures [23–25]. Our sensor distinguishes two-base mismatches in the target ON sequence, demonstrating its high selectivity compared to the reported DNA sensors [23–25].
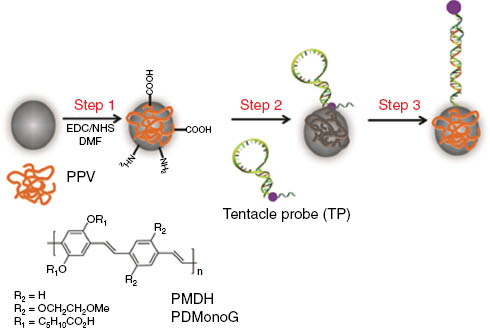
Step 1 – PPV is covalently bound to magnetic beads (PPV-MagSi). PPV-MagSi is then immobilized with tentacle probes (TP) modified with BHQ-1 quencher (purple dot) in Step 2 causing quenching of fluorescence from the PPV-MagSi. Step 3 – PPV-MagSi-TP forms duplexes with target ON and increases the distance between the BHQ-1 quencher and the emitting PPV, thereby increasing the fluorescence emission from PPV-MagSi. The structure of PPVs – PMDH and PDMonoG used are also included.
This work follows on from our reported DNA sensing designs based on an optical Fluorescence Resonance Energy Transfer (FRET) type sensor based on interactions of a cationic PPV with anionic DNA [7], and a more recent report on a novel switch-on/switch-off sensor [8] utilizing sandwich hybridization between anionic PPV bound captureprobe coated onto magnetic beads, target and the signalling probe. The hybridization-readout was monitored by either FRET or superquenching depending on the type of signalling probe used. It should be noted that, to our knowledge there have been no reports of heterogeneous highly selective and sensitive DNA sensors using PPVs and TPs. The PPVs PMDH and PDMonoG (Fig. 2) recently synthesized in our group [27] were selected for this study due to the presence of carboxylic acids for bioconjugation and attachment on MagSi beads. Furthermore, PDMonoG was chosen here because the mono-ethylene glycol groups are expected to improve the polymer solubility in aqueous systems and contribute towards prevention of non-specific biomolecule adsorption.
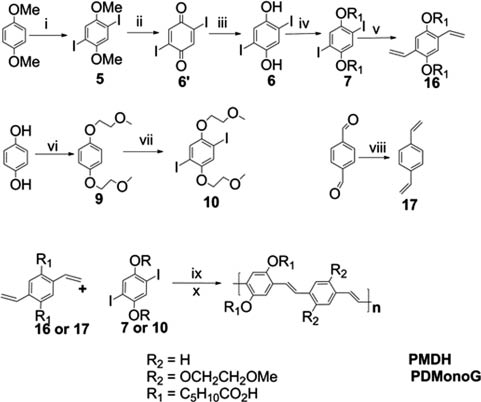
Synthesis of PMDH and PDMonoG. (i) I2, KIO3, AcOH/H2SO4/H2O, 81 % (ii) BBr3, DCM, 60 % (iii) Na2S2O3, Et2O, 97 % (iv) KOH, BrC5H10COOCH3, DMSO, 73 % (v) Pd(PPh3)4, vinyl tributyltin, DMF, 62 % (vi) 9 (KOH, KI, 1,4-hydroquinone, 2-bromomethyl ether, EtOH, 55 %), (vii) 10 (ICl, MeOH, 55 %), (viii) KtBuOH, methyltriphenylphosphonium bromide, dry THF, 63 %, (ix) Palladium acetate, tri-ortho-tolylphosphine, tributylamine, DMF, 65 % (x) 4M NaOH, THF, MeOH.
Materials and methods
1-Ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC), N-hydroxy succinimide (NHS, 97 %), phosphate buffered saline (PBS, pH 7.4), tris(hydroxymethyl)aminomethane (Tris) buffer and tween-20 were commercially obtained from Sigma-Aldrich and used without purification. Tris(hydroxymethyl)aminomethane-hydrochloride (Tris-HCl) buffer (10 mM, 200 mM NaCl, 0.1 % Tween-20, pH 8) were prepared using Milli-Q water (18.2 Ω cm resistivity). HCl or NaOH were added to adjust the pH to the desired value. All other chemicals were purchased from Sigma Aldrich and used as received unless otherwise mentioned. Silica coated magnetic beads (MagSi, 100–600 nm) functionalized with amines were purchased from MagnaMedics (http://www.magnamedics.com/), Netherlands [24–27]. The DNA ON sequences were synthesized by Alpha DNA (Quebec, Canada) and Biosearch Technologies (California, USA), the sequences (Table 1) used in the sensor designs are listed below.
Summary of the commercially synthesized ON sequences used.
| Name | Functionality | Purification | Sequence (5′-3′) |
|---|---|---|---|
| Tentacle probe | 5′Amino (C6 alkane)Linker BHQ-1 PEG9 Internally modified | RP/AX HPLC | CGA TGA CCA TAT TGT CAT CG – BHQ1-Spacer9-(PEG) – AAT AAA GGG AAA GTA TA |
| Tentacle Target | None | AX HPLC | ACG GTA TAC TTT CCC TTT ATT AGT GAA GAA TAG AAT ATG GTC AT |
| Tentacle NonC | None | AX HPLC | ACG GAT ATA AAA TTT AAA TAA AGT GAA GAA TAG TTA AAC CAT TA |
| Tentacle 2BMisM | None | AX HPLC | ACG GTA TAC TTT CCC CTT ATT AGT GAA GAA TAG AAT ATG ATC AT |
| Tentacle 1BMisM-loop | None | AX HPLC | ACG GTA TAC TTT CCC TTT ATT AGT GAA GTT GAC AAT ATG ATC AT |
| Tentacle 1BMisM-Cap | None | AX HPLC | ACG GTA TAC TTT CCC CTT ATT AGT GAA GTT GAC AAT ATG GTC AT |
The mis-matches in the sequences are underlined.
Two newly synthesized PPVs, PMDH and PDMonoG were tested as part of the sensing design: poly(6,6′-((2-methyl-5-((E)-4-((E)-prop-1-en-1-yl)styryl)-1,4-phenylene)bis(oxy))dihexanoic acid) (PMDH), and poly(6,6′-((2-((E)-2,5-bis(2-methoxyethoxy)-4-((E)-prop-1-en-1-yl)styryl)-5-methyl-1,4-phenylene)-bis-(oxy)) di-hexanoic acid) (PDMonoG) – (Fig. 2). The syntheses of PPVs were carried out using standard Heck reaction conditions with a catalytic system containing palladium acetate, tributylamine, tri-ortho-tolylphosphine (Fig. 2). The full details of the synthesis, characterization, optical and electrochemical properties of PMDH and PDMonoG have been recently reported (Table 2) [27].
Molecular weights and polydispersity (PD) of synthesized PPVs.
| Namea | Molecular Weight (Mw, gmol−1) | PD (Mw/Mn) |
|---|---|---|
| PMDH | 383 200 | 1.15 |
| PDMonoG | 22 060 | 1.05 |
aAll of the polymers had a dimethyl ester protecting group.
A procedure similar to the one described by Shavel et al. [28] was followed for covalently attaching PPV on to MagSi beads. A solution of PPV (1 mg) in DMF (100 μL) was added to a solution of EDC (7 mg, 50 mM)/NHS (9 mg, 80 mM) also in DMF (1000 μL). This mixture was allowed to react for 5 min before adding a suspension of MagSi beads (0.4 mg) in DMF (500 μL). The mixture was sonicated for 2 min after which it was mechanically shaken overnight. The PPV coated beads were isolated using centrifugation (3.5 min, 14 680 rpm) and washed thrice with DMF to remove unbound polymer. To cap the residual amines on the MagSi beads, PPV-MagSi beads were gently shaken for 30 min at room temperature in a 1:3:5 (v/v) with acetic anhydride/pyridine/THF mixtures [29]. The beads were then thoroughly washed 3–4 times with THF and DMF using centrifugation (3.5 min, 14 680 rpm) and the UV-Visible spectra (Fig. 3) were measured.
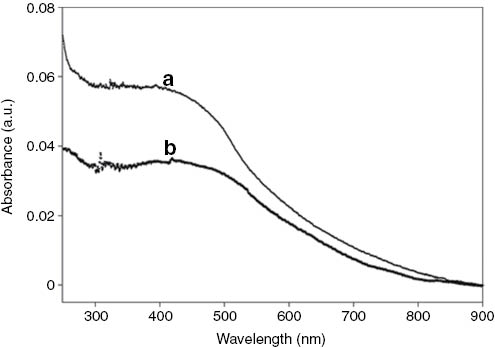
The UV-Visible spectra of (a) PMDH-MagSi and (b) PDMonoG-MagSi beads in Tris-HCl buffer.
TP based DNA sensing with PMDH-MagSi and PDMonoG-MagSi were conducted by covalently attaching 5′-end amine-modified quencher-modified tentacle probe (1248 nM, 1300 μL) on PPV-MagSi beads using a solution of EDC (7 mg, 50 mM)/NHS (9 mg, 80 mM) in PBS buffer (pH 5.4). This solution was then mechanically shaken overnight. The PPV coated beads immobilized with TPs were extracted with centrifugation (3.5 min, 14 680 rpm) and washed thrice with Tris-HCl buffer (pH 8) to remove unbound tentacle probes.
PPV-MagSi beads immobilized with TPs were then incubated with solutions of tentacle target (6240 nM, 250 μL) for 1 h in Tris-HCl buffer under continuous mechanical shaking at room temperature. The mixture was then thoroughly washed with Tris-HCl buffer (pH 8) followed by re-dispersing in Tris-HCl buffer (2 mL) for superquenching measurements. The PPV polymers were selectively excited at 400 nm and fluorescence spectra were recorded from 440 to 700 nm. Control experiments with non-complementary (Tentacle-NonC) (6240 nM, Tris-HCl buffer) and 2-base mismatched ONs (Tentacle-2BMisM-PPVend) (6240 nM, SSC buffer) were performed under the same conditions as described above. The change in fluorescence intensity (ΔI, %) was calculated based on the difference in integrated intensities before and after hybridization with the ONs.
The emission quantum yields (Φ) of PPV-MagSi (Table 3) were measured according to the established procedure where absolute values were calculated according to the following equation:
The emission quantum yields (Φ) of PPV-MagSi beads in DMF and water.
| Polymer | λ em (nm) | Φ DMF | Φ Water |
|---|---|---|---|
| PMDH-MagSi | 533 | 0.045 | 0.0022 |
| PDMonoG-MagSi | 575 | 0.055 | 0.0034 |
where Φ is the emission quantum yield, I is the measured integrated emission intensity, and n is the refractive index of the solvents. The subscript R refers to the reference fluorophore, fluorescein dissolved in ethanol (emission quantum yield, Φ = 0.79). Absorbances (A) were kept below 0.05 to minimize the self-quenching and re-absorption effects.
A procedure similar to one described in the literature was used for calculating the number of carboxylic acids on PPV-MagSi beads [8, 30]. Briefly, toluidine blue solution (2 × 10−4 M, 2 mL) in NaOH (0.1 × 10−3 M) was added to PPV-MagSi beads (0.4 mg). The solution was incubated for 1 h at 40 °C and washed with NaOH (0.1 × 10−3 M) thoroughly by centrifuging and magnetic separation. Acetic acid (50 %, 2 mL) was added to the PPV-MagSi beads and the mixture was incubated at 40 °C for 30 min after which supernatant was magnetically decanted into a quartz cuvette to measure the UV-Visible absorbance. The absorbance of toluidine blue in acetic acid (50 %) at different concentrations (2 × 10−4 M to 2 × 10−8 M) was measured to obtain a calibration graph. The number of carboxylic acids was calculated using the calibration graph.
PMDH-MagSi beads were activated with EDC (7 mg, 50 mM)/NHS (9 mg, 80 mM) in PBS buffer (970 μL, pH 5.3) and mechanically shaken for 2 h at room temperature. They were washed four times with PBS buffer to remove unreacted EDC/NHS. UV-Visible absorbance of TP was measured at 260 nm before adding it to the activated beads. The mixture was mechanically shaken at room temperature overnight after which the supernatant was collected by magnetically separating the PMDH-MagSi beads. The UV-Visible absorbance of the supernatant was measured to determine the concentration of the TP (pmol/μL) after immobilization with PMDH-MagSi beads using the following equation where A is the optical density, NA, NC, NG and NT are the number of adenine, cytosine, guanine and thymine bases respectively [31]:
Results and discussion
The characterization of the PPV-MagSi beads (UV-Visible spectra, fluorescence quantum yields (Φ), number of carboxylic acids and tentacle probes attached) were carried out using similar previously reported methods and are described below [8].
Confirmation of the attachment of PPVs PMDH and PDMonoG onto the magnetic beads was established by UV-Visible spectra with the appearance of a new broad peak in the 450–480 nm range (Fig. 3) characteristic of the presence of PPVs [27]. The amount of carboxylic acid functional groups on PPV-MagSi beads using the toluidine blue method [3, 30] indicated about 1.7 × 108 and 5.2 × 107 carboxylic acids (in 0.4 mg of beads) on PMDH-MagSi and PDMonoG-MagSi beads (Table 4) respectively. The polymers with carboxylic acids have pKa values between 3.5 and 4.5 and as a result PMDH and PDMonoG would be deprotonated at pH 5.4.
The concentration (c, (M)), number of moles (n, (mol)) and number of carboxylic acids per MagSi bead (N/bead) with PPVs PMDH and PDMonoG.
| Polymer | c (M) | n (mol) | N/bead |
|---|---|---|---|
| PMDH-MagSi | 4.18 × 10−5 | 8.36 × 10−8 | 1.7 × 108 |
| PDMonoG-MagSi | 1.06 × 10−5 | 0.24 × 10−8 | 5.2 × 107 |
The fluorescence quantum yields of the PMDH-MagSi and PDMonoG-MagSi beads were further measured in solvents DMF and water. PMDH-MagSi and PDMonoG-MagSi had 0.045 and 0.055 in DMF and 0.0022 and 0.0034 in water respectively (Table 3) against fluorescein as the standard. The errors of the quantum yield measurements were within 5 %. The loss in quantum efficiency upon addition of water is attributed to the aggregated state of the polymer on the surface of the beads as well as the reduced solubility of CPs in water compared to DMF [32]. As observed by the increase in quantum yield in DMF and water for PDMonoG-MagSi, the mono-ethylene glycol groups increase the solubility of the polymer. The difference in UV-Visible absorbance before and after immobilization was used to calculate the number of TP on PMDH-MagSi beads as 4.8 × 104 [31]. Furthermore, the charges on the ONs help in preventing further aggregation of the beads.
Due to the proximal distances between the polymer fluorophores and the quencher molecules on the TPs, the fluorescence emission of the CPs is quenched when the TPs are in the hairpin conformation. Hybridization of both the “capture” and “detection” regions of the TPs with the complementary target ONs forces the TPs to open up and form a stiffer duplex, forcing the quencher molecules away from the polymer. In such a configuration, the fluorescence emission from the PPV-MagSi beads is ‘switched-on’ indicating the presence of complementary target ONs.
The dark quencher, N-(4-(4-(2-Nitro-4-methylphenylazo)-2-methoxy-5-methylphenylazo)-phenyl)-N- (2-(4,4′-dimethoxy- trityloxy)-ethyl)-2-aminoethyl-1-oxy-glycolate (Black Hole Quencher, BHQ-1) with absorption between 480 and 580 nm and an absorption maximum at 535 nm was chosen for this study. BHQ-1 has advantages of good spectral overlap with PPVs, and reduced background signal with no native fluorescence (https://www.biosearchtech.com). The sensor response, i.e., the change in fluorescence intensity (ΔI, %) was calculated based on the difference in integrated intensities before and after hybridization with the ONs in Tris-HCl buffer (10 mM, 200 mM NaCl, 0.1 % Tween-20, pH 8).
The change in fluorescence intensity of the sensing system with PPVs – PMDH and PDMonoG, as target ON hybridized with TP were evaluated (for raw data see Section S1.1 Figure S1.1) and the results are shown in Fig. 4, Inset. PMDH-MagSi demonstrated the best switch-on fluorescence response (ΔI = 24.76 %), much stronger than PDMonoG-MagSi (ΔI = 8.60 %). The observed differences in switch-on responses can be rationalized by the extent of the overlap of fluorescence emission of PPVs with the absorption of BHQ-1 (Fig. 4). Emission of PMDH-MagSi overlaps better with the absorption of BHQ-1 than PDMonoG-MagSi, thereby fulfilling one of the important requirements for efficient quenching, resulting in higher efficiency of quenching in the case of PMDH-MagSi. Thus, the sensing system with PMDH-MagSi beads was explored further and is described in the following sections.
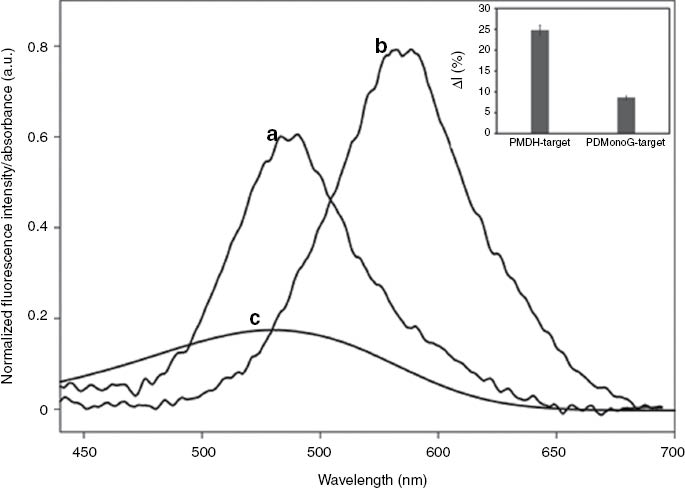
Normalized fluorescence spectra of PMDH-MagSi beads immobilized with tentacle probes (TP) (a), PDMonoG-MagSi beads immobilized with TP (b) in Tris-HCl buffer when excited at 400 nm and the UV-Visible absorption spectrum of the quencher BHQ-1 (c).
Spectra were normalized to PDMonoG-MagSi’s emission. Inset: Sensor response with PPVs bound to MagSi beads – PMDH-MagSi and PDMonoG-MagSi.
The selectivity of PMDH-MagSi (for raw data see Section S1.2 Figure S1.2) with non-complementary and mismatched DNA was investigated further (Fig. 5). When random, non-complementary (NonC) DNA was added, PMDH-MagSi functionalized with BHQ-1 labelled TP demonstrated good selectivity (ΔI = 1.76 %) (7 % signal of the fully complementary ON). Furthermore, the ability of the sensor to distinguish one-base mismatch and two-base mismatches in the target ON sequence was investigated. The sensor demonstrated an increase in signal (Fig. 5) when the mismatch was placed in the “capture region” (1BMisM-cap, ΔI = 9.21 %) when compared to the mismatch placed in the ‘detection’ region (1BMisM-loop, ΔI = 8.10 %). Additionally, we chose a two-base mismatched sequence (2BMisM) with one mismatch in the “capture region” and the second mismatch in the “detection region” in the hairpin. The sensor demonstrated good selectivity as only 24.8 % signal (ΔI = 6.14 %) of the fully complementary target ON was observed. The difference in signal observed with 1BMisM-cap, 1BMisM-loop and 2BMisM can be explained by the lower stability of the hybrid DNA duplex influencing the hybridization efficiency. The tentacle probes can react with target DNA via two pathways, namely via the detection “loop” region or the “capture” region. Placement of mismatches in either or both the detection regions decreases the stability of the hybrid causing loss of signal. The errors for the selectivity of the sensor were between 5 and 7 % of the measured signal response.
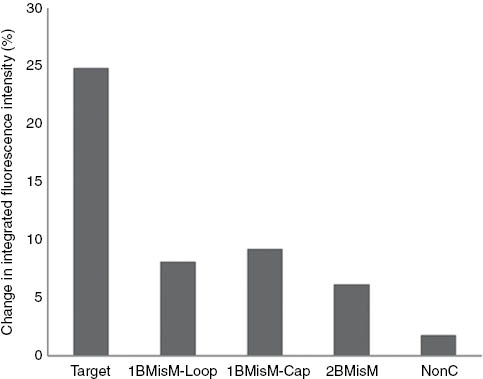
The sensor response expressed as the change in integrated fluorescence intensity (%) of PMDH-MagSi DNA sensor with (a) 1BMisM-loop, (b) 1BMisM-Cap and (c) 2BMisM in Tris-HCl buffer.
These hybridization results suggest that BHQ-1 effectively quenches the fluorescence emission of PMDH–MagSi in the hairpin conformation and the TP-grafted to PPV-MagSi acts as a good TP reporter highly selective of the fully complementary target ON but not to the mismatched ON sequence. However, the PMDH-MagSi sensing system demonstrated lower quenching efficiencies (24.8 %) compared to other heterogeneous CP-based MB designs. Further improvements to the quenching efficiency could be brought about by using a spacer between the TP and PMDH-MagSi surface to allow sufficient mobility of the hairpin probe, increasing the quantum efficiency of the PMDH-MagSi beads while also increasing surface concentration of TP, thereby number of BHQ-1 quenchers on PMDH-MagSi (values up to a concentration of 2.8 × 1012 molecules cm−2 has been reported using direct on-chip ON synthesis) [20].
The detection limit of the ON sensor was investigated using target ON concentrations in the range from 1.88 × 10−5 M (18.8 μM) to 1.88 × 10−12 M (1.88 pM). The trend indicated a gradual increase in the polymer intensity until 10−8 M after which a linear increase until ca. 10−5 M was observed (Fig. 6). A theoretical detection limit of 18.8 pM (376 fmol in 2 mL) of target ON was observed, calculated based on 3× the standard deviation of the background non-complementary control. This detection limit demonstrates the functionality of our sensor, and importantly surpasses previously reported detection limits of 2 nM [26], 5 nM [33], 10 nM [21], for other MB-based sensors utilizing fluorescence for the detection of ONs. The sensitivity measurements were conducted using a non-titration method (involving use of multiple samples of PPV-MagSi beads from a big batch of PPV-MagSi beads, to which different concentrations of target ON were added) compared to the conventional titration method (involving monitoring the increase in signal as the target ON concentration is increased on the same sample of PPV-MagSi beads). This method of non-titration was used to minimize the degradation of PPV attached on MagSi beads and errors in measurement due to variables in parameters such as room temperature, humidity and concentration of probe attached on PPV-MagSi (which in turn affects hybridization efficiency). The errors of the limit of detection were between 8 and 10 %. The increase in quenching efficiency was plotted against the target ON concentration (M) for the switch-on sensor.
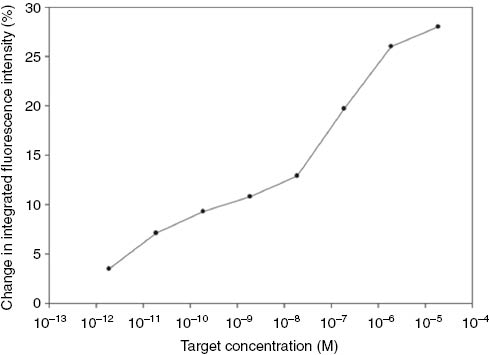
Calibration graph for the superquenching based PMDH-MagSi DNA sensor. The solid line is a guide for the eye only.
Conclusions
In conclusion, we have demonstrated proof-of-concept of a novel optical DNA sensor with “switch-on” superquenching-based readout with PPV-MagSi beads and modified hairpin probes termed TPs. Our sensor design utilizes signal amplification properties of PPV and cooperativity of hairpin-TPs to monitor hybridization of ONs. The measured emitted fluorescence from the PPV upon hybridization changes, due to increased distances between the PPV and quencher to detect ONs. We tested two carboxylic acid functionalized PPVs – PMDH and PDMonoG in the sensor design. The “switch-on” sensor based on PMDH-MagSi demonstrated selectivity down to two-base mismatches in the target ON sequence. This simple and rapid technology has enabled the detection of ONs in the picomolar range (18.8 pM, 376 fmol in 2 mL). This sensing platform has further advantages of simplicity, fast assay time, being label-free (labelling of target ON is avoided), utilising magnetic silica beads for simple and easy separation of unbound ONs and other components of the hybridization medium, whilst using only small volumes of target (250 μL). A kinetic study investigating the dependence of the sensor response on the incubation time with target ONs and comparison with MBs will be explored in the future work.
Article note
A collection of invited papers based on presentations at the 12th International Conference on Frontiers of Polymers and Advanced Materials (ICFPAM 2013), Auckland, New Zealand, 8–13 December 2013.
Acknowledgments
We thank Industrial Research Limited (IRL) for funding ARGS scholarship through the NERF Subcontract grant.
References
[1] S. R. Mikkelsen. Electroanalysis8, 15 (1996).10.1002/elan.1140080104Search in Google Scholar
[2] H. Peng, L. Zhang, C. Soeller, J. Travas-Sejdic. Biomaterials30, 2132 (2009).10.1016/j.biomaterials.2008.12.065Search in Google Scholar
[3] B. Kannan, D. E. Williams, M. A. Booth, J. Travas-Sejdic. Anal. Chem.83, 3415 (2011).10.1021/ac1033243Search in Google Scholar
[4] S. Cosnier. Anal. Lett.40, 1260 (2007).10.1080/00032710701326643Search in Google Scholar
[5] T. G. Drummond, M. G. Hill, J. K. Barton. Nat. Biotechnol.21, 1192 (2003).10.1038/nbt873Search in Google Scholar
[6] H. Peng, L. Zhang, T. H. M. Kjaellman, C. Soeller, J. Travas-Sejdic. J. Am. Chem. Soc.129, 3048 (2007).10.1021/ja0685452Search in Google Scholar
[7] H. Peng, C. Soeller, J. Travas-Sejdic. Chem. Commun.35, 3735 (2006).10.1039/b607293cSearch in Google Scholar
[8] A. R. Gulur Srinivas, H. Peng, D. Barker, J. Travas-Sejdic. Biosens. Bioelectron.35, 498 (2012).10.1016/j.bios.2012.03.022Search in Google Scholar
[9] B. Liu, G. C. Bazan. Chem. Mater.16, 4467 (2004).10.1021/cm049587xSearch in Google Scholar
[10] H. Jiang, P. Taranekar, J. R. Reynolds, K. S. Schanze. Angew. Chem., Int. Ed.48, 4300 (2009).10.1002/anie.200805456Search in Google Scholar
[11] F. Feng, F. He, L. An, S. Wang, Y. Li, D. Zhu. Adv. Mater.20, 2959 (2008).10.1002/adma.200800624Search in Google Scholar
[12] C. Tan, E. Atas, J. G. Mueller, M. R. Pinto, V. D. Kleiman, K. S. Schanze. J.Am. Chem. Soc.126, 13685 (2004).10.1021/ja046856bSearch in Google Scholar
[13] K. E. Achyuthan, T. S. Bergstedt, L. Chen, R. M. Jones, S. Kumaraswamy, S. A. Kushon, K. D. Ley, L. Lu, D. McBranch, H. Mukundan, F. Rininsland, X. Shi, W. Xia, D. G. Whitten. J.Mater. Chem.15, 2648 (2005).10.1039/b501314cSearch in Google Scholar
[14] M. E. A. Downs, S. Kobayashi, I. Karube. Anal. Lett.20, 1897 (1987).10.1080/00032718708078036Search in Google Scholar
[15] T. J. Drake, W. Tan. Appl. Spectrosc.58, 269A (2004).10.1366/0003702041959406Search in Google Scholar
[16] S. Tyagi, F. R. Kramer. Nat. Biotechnol.14, 303 (1996).10.1038/nbt0396-303Search in Google Scholar
[17] B. C. Satterfield, J. A. A. West, M. R. Caplan. Nucleic Acids Res.35, e76 (2007).10.1093/nar/gkm113Search in Google Scholar
[18] B. C. Satterfield, M. R. Caplan, J. A. A. West. Nucleic Acids Res.36, e129/1 (2008).10.1093/nar/gkn564Search in Google Scholar
[19] J. R. Epstein, I. Biran, D. R. Walt. Anal. Chim. Acta469, 3 (2002).10.1016/S0003-2670(02)00030-2Search in Google Scholar
[20] B. D. Malhotra, A. Chaubey, S. P. Singh. Anal. Chim. Acta578, 59 (2006).10.1016/j.aca.2006.04.055Search in Google Scholar PubMed
[21] E. Palecek. Trends Biotechnol. 22, 55 (2004).10.1016/j.tibtech.2003.11.009Search in Google Scholar PubMed
[22] J. Zhai, C. Hong, R. Yang. Biotechnol. Adv.15, 43 (1997).10.1016/S0734-9750(97)00003-7Search in Google Scholar
[23] C. J. Yang, M. Pinto, K. Schanze, W. Tan. Angew. Chem. Int. Ed.44, 2572 (2005).10.1002/anie.200462431Search in Google Scholar
[24] K. Lee, J.-M. Rouillard, B.-G. Kim, E. Gulari, J. Kim. Adv. Funct. Mater.19, 3317 (2009).10.1002/adfm.200901175Search in Google Scholar
[25] K. Lee, L. K. Povlich, J. Kim. Adv. Funct. Mater.17, 2580 (2007).10.1002/adfm.200700218Search in Google Scholar
[26] X. Feng, X. Duan, L. Liu, L. An, F. Feng, S. Wang. Langmuir24, 12138 (2008).10.1021/la802932tSearch in Google Scholar PubMed
[27] A. R. Gulur Srinivas, T. E. Kerr-Phillips, H. Peng, D. Barker, J. Travas-Sejdic. Polym. Chem.4, 2506 (2013).10.1039/c3py21090aSearch in Google Scholar
[28] A. Shavel, N. Gaponik, A. Eychmueller. ChemPhysChem6, 449 (2005).10.1002/cphc.200400516Search in Google Scholar PubMed
[29] H. Salo, A. Guzaev, H. Loennberg. Bioconjugate Chem.9, 365 (1998).10.1021/bc970194gSearch in Google Scholar
[30] J. Drews, H. Launay, C. M. Hansen, K. West, S. Hvilsted, P. Kingshott, K. Almdal. Appl. Surf. Sci.254, 4720 (2008).10.1016/j.apsusc.2008.01.085Search in Google Scholar
[31] M. J. Cavaluzzi, P. N. Borer. Nucleic Acids Res.32, e13/1 (2004).10.1093/nar/gnh015Search in Google Scholar PubMed PubMed Central
[32] K. Ogawa, S. Chemburu, G. P. Lopez, D. G. Whitten, K. S. Schanze. Langmuir23, 4541 (2007).10.1021/la0630108Search in Google Scholar PubMed
[33] X. Fang, X. Liu, S. Schuster, W. Tan. J. Am. Chem. Soc.121, 2921 (1999).10.1021/ja9837809Search in Google Scholar
Supplemental Material
The online version of this article (DOI: https://doi.org/10.1515/pac-2014-1114) offers supplementary material, available to authorized users.
Electronic Supplementary Information (ESI) available: Details of the raw superquenching spectra before and after hybridization for PMDH-MagSi and PDMonoG-MagSi is avaliable.
©2015 IUPAC & De Gruyter
Articles in the same Issue
- Frontmatter
- Preface
- XXVth IUPAC Symposium on Photochemistry (XXV IUPAC Photochemistry)
- Conference papers
- Discovery and development of photochromic diarylethenes
- Highly efficient electroluminescence from purely organic donor–acceptor systems
- Singlet oxygen and natural substrates: functional polyunsaturated models for the photooxidative degradation of carotenoids
- Photophysical, electrochemical, and spectroelectrochemical investigation of electronic push–pull benzothiadiazole fluorophores
- The history of the IUPAC Symposia on photochemistry – a success story
- ‘Switch-on’ DNA sensor based on poly (p-phenylene vinylenes) bound tentacle probes
Articles in the same Issue
- Frontmatter
- Preface
- XXVth IUPAC Symposium on Photochemistry (XXV IUPAC Photochemistry)
- Conference papers
- Discovery and development of photochromic diarylethenes
- Highly efficient electroluminescence from purely organic donor–acceptor systems
- Singlet oxygen and natural substrates: functional polyunsaturated models for the photooxidative degradation of carotenoids
- Photophysical, electrochemical, and spectroelectrochemical investigation of electronic push–pull benzothiadiazole fluorophores
- The history of the IUPAC Symposia on photochemistry – a success story
- ‘Switch-on’ DNA sensor based on poly (p-phenylene vinylenes) bound tentacle probes

