Abstract
Objectives
Cisplatin (DDP) remains to be commonly employed in treating gastric cancer (GC) patients, particularly advanced-stage ones. However, acquired resistance to DDP often occurs, which causes a poor prognosis. This study aimed to understand the potential contribution of tissue inhibitor of metalloproteinase 1 (TIMP1) in acquired resistance to DDP in GC.
Methods
Bioinformatics analysis was performed to explore the relation of TIMP1 expression with stages and survival rate in GC. The TIMP1 expression between the parental and DDP-resistant GC cell lines were detected by quantitative reverse-transcription polymerase chain reaction (qRT-PCR). The effect of TIMP1 on the ability of cells against DDP was elevated by CCK-8, wounding healing, and transwell assays after exposing DDP. The role of TIMP1 in stemness and EMT process was explored through spheres formation assay and detecting stem cell- and EMT-related markers. Finally, the regulation of TIMP1 in Wnt/β-catenin signaling in DDP-resistant GC cells was also analyzed by western blot.
Results
Bioinformatics analysis revealed that TIMP1 is highly expressed and closely related to tumor stage and poor survival in GC. The TIMP1 expression of DDP-resistant GC cell lines was significantly higher than that of the parental one. CCK-8, wounding healing, and transwell assays showed that the tolerance to DDP of DDP-resistant AGS (AGS/DDP) cells was significantly augmented compared with that of parental AGS cells, revealed by increased IC50 and enhanced migration and invasion when exposed to DDP. Stronger stemness and epithelial–mesenchymal transition could be also observed in AGS/DDP cells. These malignant phenotypes were eliminated by silencing TIMP1 but aggravated by overexpressing TIMP1 in AGS/DDP cells. The use of the Wnt/β-catenin inhibitor could effectively reverse the function of TMIP1 overexpression in AGS/DDP cells, which suggested that the role of TIMP1 in DDP resistance relied on the Wnt/β-catenin signaling.
Conclusions
TIMP1 is an essential regulator of DDP resistance in GC, which may be a potential therapeutic target for cases that are refractory to DDP.
Introduction
In China, gastric cancer (GC) is the third most common cancer with 479,000 new cases after lung and colorectal cancer in 2020 [1]. Over the past decades, several chemotherapeutic agents, including cisplatin (DDP), irinotecan, and 5-fluorouracil (5-Fu), were used in the treatment of advanced GC, when surgery is not recommended. DDP induces G2/S arrest in rapidly growing cancer cells to cause cell death by forming platinum–DNA adducts [2]. In addition, DDP increases intracellular reactive oxygen species levels after reacting with cytoplasmic biomolecules and proteins to cause toxically oxidative stress, which contributes to the antitumor response [3]. However, acquired resistance to DDP is common in most patients, which is considered a major cause of treatment failure. Since DDP remains the primary choice for patients with GC during chemotherapy, exploring the mechanisms and solutions to DDP resistance has clinical significance for patients with GC.
As a tumor is a heterogeneous cell mixture with diverse gene expressions and various genetic mutations, the molecular mechanisms underlying drug resistance are also heterogeneous and complex [4]. Numerous mechanisms have been implicated in chemoresistance, which included enhanced DNA repair, detoxification system activation, impaired apoptosis, and altered oncogene expression [5]. Moreover, mounting evidence supported that Wnt/β-catenin signaling is critical in multiple processes related to chemoresistance [6]. In 2013, Wei et al. demonstrated that β-catenin accumulation in the nucleus of DDP-resistant Huh7 cells is implicated in canonical Wnt signaling in DPP resistance [7]. Chemoresistance to interferon-α/5-Fu combination in hepatocellular carcinoma was found to be regulated by this signaling [8]. In GC, Zhang et al. revealed that the aggressive phenotype of adriamycin-resistant GC cells was closely related to Wnt/β-catenin signaling activation [9]. Given the importance of Wnt/β-catenin signaling, the gene with a regulatory role in this pathway may become a promising therapeutic target against chemoresistance in GC.
Tissue inhibitor of metalloproteinase 1 (TIMP1), an endogenous inhibitor of matrix metalloproteinases (MMPs), has been studied intensively in the last two decades [10], [11], [12]. TIMP1 has complex roles in regulating cell processes, which depend on the cell environment and can be classified as MMP-dependent (processes realized by MMP inactivation) and non-MMP-dependent (directly influences cell apoptosis, growth, and angiogenesis) roles. Increasing evidence supported that TIMP1 is involved in the cellular sensitivity/resistance to several drugs that were clinically used in cancer chemotherapy [13], [14], [15]. A recent study showed that TIMP-1 acts as an activator of Wnt/β-catenin signaling to regulate non-MMP-dependently the function of adipose-derived stem cells [16]. Over the last decade, TIMP1 has been identified as a prognostic biomarker in GC [17]. Based on these, our study aimed to gain new insights into the biological functions of TIMP1 in DDP resistance of GC and preliminarily uncover the mechanism underlying these functions.
Materials and methods
Gene expression profiling interactive analysis (GEPIA)
GEPIA (http://gepia.cancer-pku.cn/) [18], a web-based tool to deliver fast and customizable functionalities based on TCGA and GTEx data, was applied to analyze the predictive significance of the TIMP1 expression level on tumor stage and overall survival in GC.
GEO dataset analysis
GSE56807 dataset [19] was utilized to analyze the relative expression level of TIMP1 mRNA in five paired GC and adjacent normal tissues.
Cell culture
AGS cell line was obtained from ATCC, and BGC-823 cell line was kindly provided by Cell Bank, Chinese Academy of Sciences. DDP-resistant GC cell lines (DPP/R) were developed by continuously exposing the parental ones to gradient DDP in a previous study [20]. To maintain DDP resistance, DPP/R cell lines were continuously exposed to 1 μg/mL DDP. DDP-resistant AGS cells, denoted as AGS/DDP, were used to perform further analysis.
During the biological functional experiments in this study, the concentrations of DDP and AR-A014418 (Cat. no A3230, Sigma-Aldrich, MO, USA) were 1 μg/mL and 2.5 μM, respectively.
Reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted as per standard protocols. Afterward, on the 7,500 Real-Time PCR System, RT-PCR was performed with SYBR green reagent (Cat. No. 4309155, Applied Biosystems, CA, USA). This study considered GAPDH an internal reference gene to quantitate the mRNA expression of TIMP1 based on the 2−ΔΔCt method [21]. The primer sequences were as follows: TIMP1 forward primer (F): 5′ -GGGGCCATTCCCTAAATCGT-3′, (R) reverse primer: 5′-CTGGTCCGTCCACAAGCAAT-3′; GAPDH (F): 5′ -AGTTAATGCCGCCCCTTACC-3′, (R): 5′-CAGGGCTGACTACAAACCCA-3′.
Cell transfection
The cells were divided into six groups to receive different transfections as follows: si-NC group, cells transfected with an irrelevant nucleotide (si-NC (F): 5′-UUCUCCGAACGUGUCACGUTT-3′, (R): 5′-ACGUGACACGUUCGGAGAATT-3′) to act as a negative control; (2) si-TIMP1 groups (#1, #2, and #3),cells transfected with a short interfering RNA (siRNA) that specifically targeted TIMP1 (si-TIMP1#1 (F): 5′-GGAACGGAAAUUUGCACAUTT-3′, (R): 5′- AUGUGCAAAUUUCCGUUCCTT-3′; si-TIMP1#2 (F): 5′-GCACAGUGUUUCCCUGUUU-3′, (R): 5′-AAACAGGGAAACACUGUGC-3′; si-TIMP1#3 (F): 5′-CCACCCUUAUACCAGCGUUA-3′, (R): 5′-UAACGCUGGUAUAAGGUGG-3′); (3) vector group, cells transfected with empty vector; (4) TIMP1 OE group, cells transfected with the overexpression vector of TMIP1 (TIMP1 OE) that was synthesized by GenePharma (Shanghai, China). Briefly, both AGS cells were transfected using Lipofectamine® 2000 (Invitrogen, CA, USA) following the manufacturer’s manuals, and then harvested 48 h post-transfection to evaluate the transfection efficiency using RT-PCR and Western blot. The siRNA with the highest efficiency was chosen for the subsequent experiment.
Western blot
Western blot is often used in research to separate and identify proteins [22]. After finishing the treatments according to the study design, cells were harvested and subsequently lysed with RIPA buffer on ice for 20 min at 4 °C to acquire the total protein. Nuclear fractions were prepared using a nuclear extraction kit (Cat. No. ab113474; Abcam, MA, USA). Equivalent amounts of protein were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and subsequently electrotransferred onto polyvinylidene fluoride membranes (Millipore Sigma, MA, USA). Then, the membranes were blocked and then incubated with primary antibodies against TIMP1 (Cat. No. ab211926, Abcam, MA, USA), CD44 (Cat. No. ab19857, Abcam, MA, USA), CD47 (Cat. No. AF6423, Beyotime, Beijing, China), Nanog (Cat. No. AF1912, Beyotime, Beijing, China), Oct4 (cat.no ab189524, Abcam, MA, USA), E-cadherin (cat.no AF6759, Beyotime, Beijing, China), N-cadherin (Cat. No. AF5237, Beyotime), GSK3β (Cat. No. AF1543, Beyotime, Beijing, China), phospho-GSK3β (Cat. No. AF1531, Beyotime, Beijing, China), β-catenin (Cat. No. AF1189, Beyotime, Beijing, China), and GAPDH (Cat. no. ab8245, Abcam, MA, USA). Then, the membranes were rinsed thrice with a secondary antibody before incubation for 1 h (Cat. no ab6721, Abcam, MA, USA). The protein bands were visualized using an ECL kit (Cat. no. ab65623, Abcam, MA, USA).
CCK-8 assay
CCK-8 assay was exploited to determine cell viability as previously reported [23]. Briefly, GC cells were seeded in 96-well plates after being digested and resuspended. After complete adherence, cells were treated with DMEM (Gibco, CA, USA) containing diverse concentrations of DDP for three time points (24, 48, and 72 h). The cells added with 0.1% dimethyl sulfoxide were used as the experimental control, and wells containing only DMEM were set as the blank group. After the incubation, the medium was replaced with the CCK-8 reagent (Dojindo, Tokyo, Japan) (10%, v/v, dissolved in DMEM). After incubating for 2 h, the absorbance was examined with a microplate reader at 450 nm to determine the cell viability. To determine the resistance of cells to DDP, the median inhibitory concentration (IC50) of DDP for 24, 48, and 72 h on cells was calculated based on the cell viability curve.
Sphere formation assay
The sphere formation assay provides a useful tool to assess the stem cell population residing in tumors or cancerous cell lines [24]. Cells were plated on 6-well ultra-low attachment plates at a density of 1,000/well and grown in a medium supplemented with 5 μg/mL insulin, 20 ng/mL basic fibroblast growth factor, and 20 ng/mL epidermal growth factor. The plates were further incubated for 15 days. Finally, under a light microscope (Olympus, Tokyo, Japan), the number of spheroids with a diameter of >50 μm in each well was counted.
Wound healing assay
To explore the directional cell migration of cells from diverse groups, the wound healing assay (also called scratch assay), a well-developed and simple laboratory technique [25], was performed. In brief, cells were seeded into six-well plates to form a monolayer. The 200-μL pipette tip was utilized to create a scratch in the cell layer. After scratched cells were removed by rinsing with PBS, the remaining cells were cultured in DMEM in a 5% CO2 incubator at 37 °C for 24 h. A BX51 microscope (Olympus, Tokyo, Japan) was utilized to photograph the same position of scratches at 0 and 24 h after scratching.
Transwell assay
The cell invasion ability was determined using transwell chambers with Matrigel-coated membrane (Corning, NY, USA) [26]. Briefly, DMEM-containing serum and cell suspension were added in the lower and upper chambers, respectively. Twenty-four hours later, cells that adhered to the membrane of the upper chamber were wiped out, whereas cells crossing the membrane were fixed before staining with 0.1% crystal violet. Finally, under a light microscope, the stained cells were imaged and counted.
Statistical analysis
Data were expressed as mean ± standard deviation. The differences were analyzed by analysis of variance using GraphPad Prism 8.0.1 software (GraphPad Software, CA, USA). Graphpad Prism was also applied to calculate IC50 values based on the cell viability curve. p <0.05 was considered statistically significant.
Results
TIMP1 upregulation is closely related to the tumor stage, poor survival, and DDP chemoresistance in GC
Based on GEPIA, STAD, which means stomach adenocarcinoma, represents GC. TIMP1 was highly expressed in GC (p <0.05) (Figure 1A). In the analysis of the GSE56807 dataset, the expression of TIMP1 was significantly higher in gastric cancerous tissues than in adjacent normal tissues, (p <0.05) (Figure 1B). GEPIA showed that the high TIMP1 expression was positively associated with tumor stage (p <0.001) and poor survival (p =0.017) in GC (Figure 1C and D). To investigate whether TIMP1 expression also correlated with the DDP chemoresistance of GC, two DPP/R GC cell lines (AGS/DDP and BGC-803/DDP) were established. RT-PCR analysis showed that TIMP1 expressions in two DPP/R GC cell lines were both higher than that in the parental ones (Figure 1E), which suggested a potential role of TIMP1 in DDP-resistant GC. Among the two paired GC cell lines, parental AGS and AGS/DDP exhibited the most difference in TIMP1 expression (8.70-fold, p <0.001). Hence, we chose AGS and AGS/DDP cells for further analysis on the role of TIMP1 in GC.
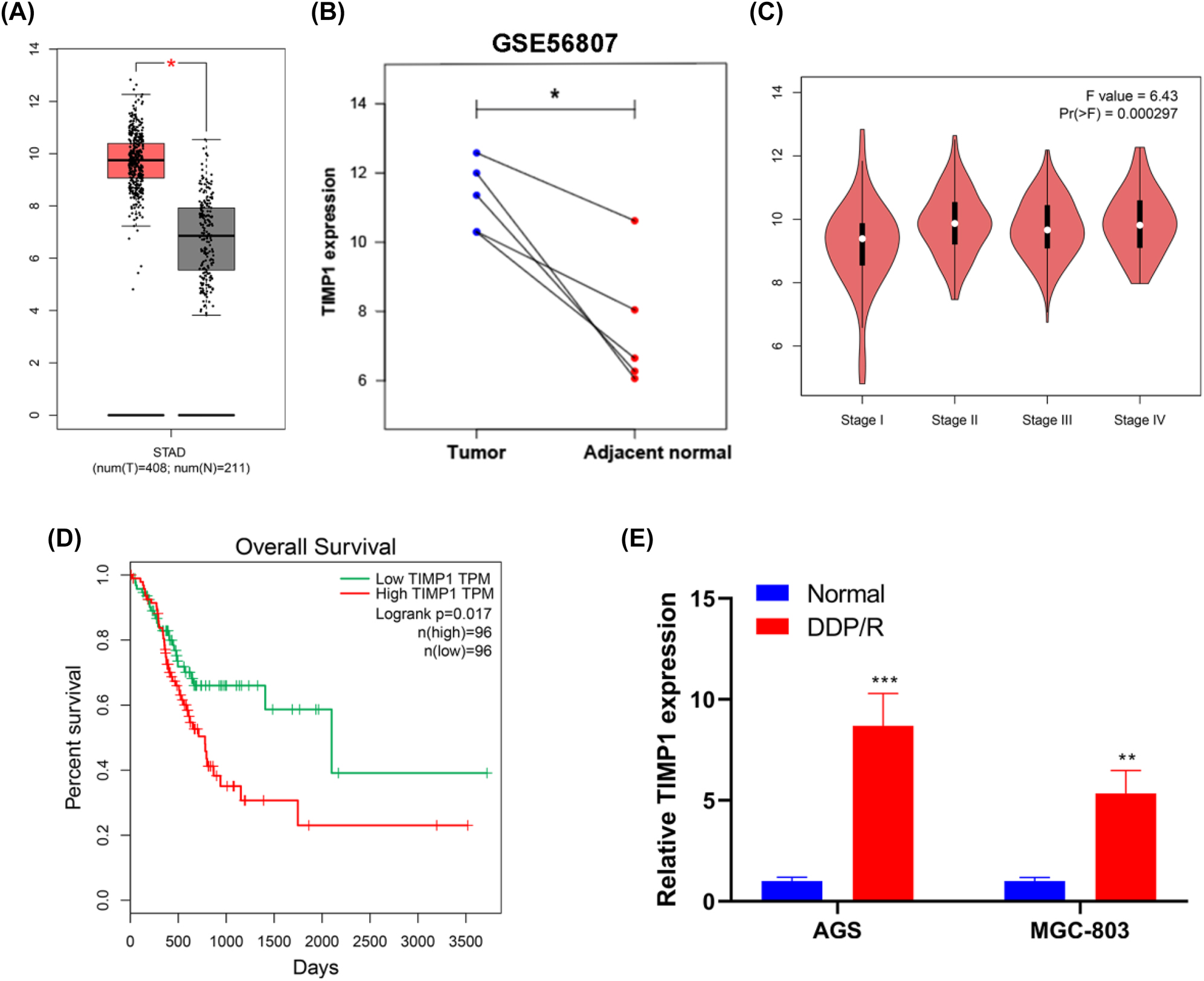
TIMP1 is highly expressed and closely related to the tumor stage, poor survival, and DDP chemoresistance in GC. (A) The expression analysis for TIMP1 between GC (n=408) and normal samples (n=211) based on the GEPIA database. (B) Expression analysis for TIMP1 on five pairs of GC and adjacent normal tissue samples from the GSE56807 dataset. (C) Expression analysis for TIMP1 on different stages of GC based on the GEPIA database. (D) By using GEPIA data, survival analysis for patients with GC (n=192) was performed based on TIMP1 expression. (E) Relative expression levels of TIMP1 in two GC cell lines (normal) and their DDP-resistant ones (DDP/R) were detected using RT-PCR. * p<0.05, ** p<0.01, and *** p<0.001 vs. the normal group.
Resistance to DDP and stemness in DDP-resistant AGS cells is mediated by TIMP1
Initially, siRNAs against TIMP1 were transfected into AGS/DDP cells. Then, the efficiency of transfection was measured by RT-PCR and Western blot (Figure 2A and B). Notably, si-TIMP1#2 led to the most efficient suppression of TIMP1 expression in cells. Thus, si-TIMP1#2 (hereafter defined as “si-TIMP1”) was chosen for subsequent experiments. The CCK-8 assay was conducted to evaluate cell viability after treatment with a series of DDP concentrations for 24, 48, and 72 h. The viability curve showed that AGS/DDP cells exerted significantly stronger resistance against DDP than AGS cells (Figure 2C), as evidenced by the increased IC50 value (Table 1). AGS/DDP transfecting si-TIMP1 showed significantly enhanced sensitivity to DDP in comparison with AGS/DDP cells or AGS/DDP cells delivered with si-NC (Figure 2C and Table 1). These results suggested a critical role of TIMP1 in regulating the response of GC cells to DDP.
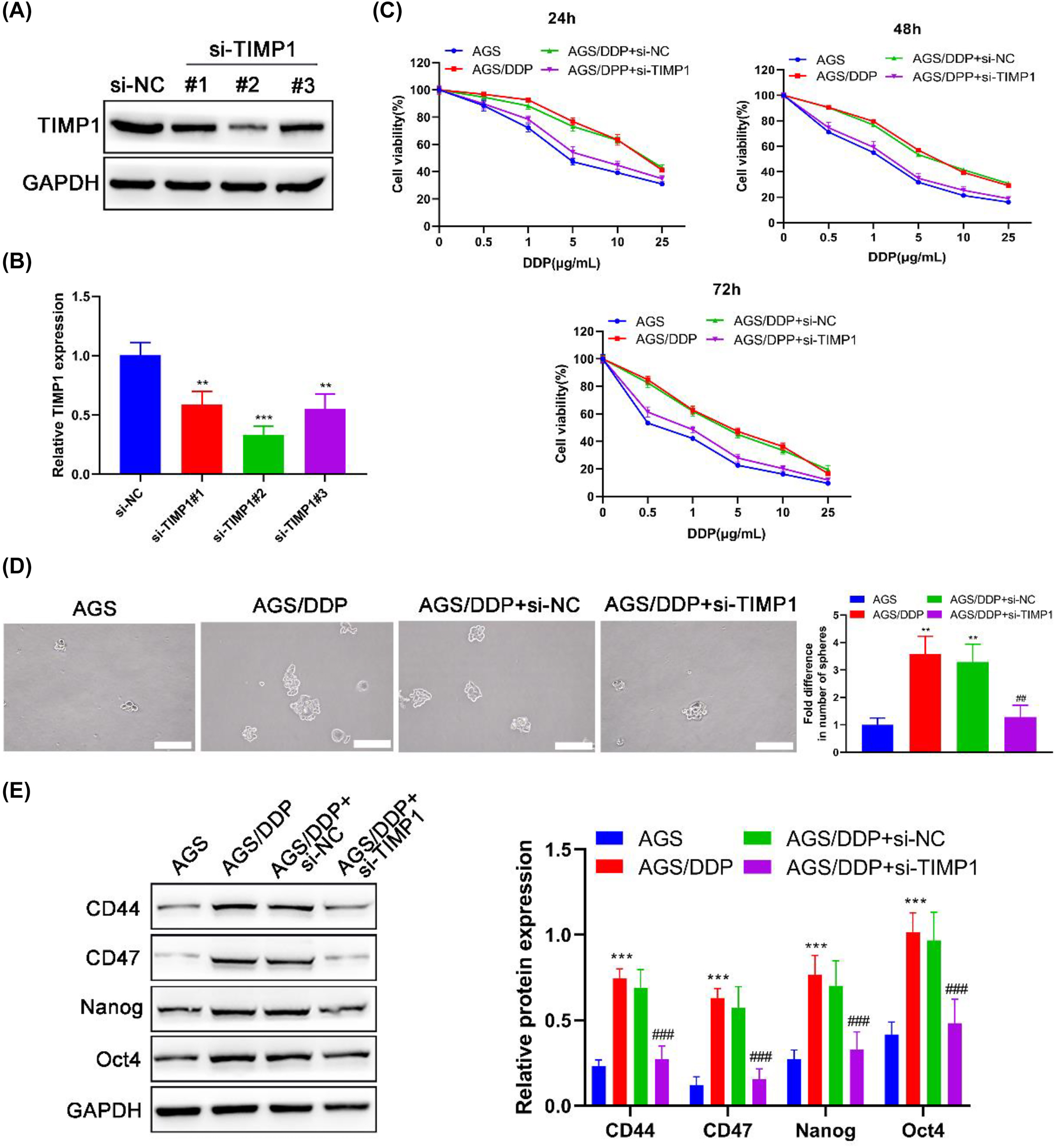
Resistance to DDP and stemness of DDP-resistant AGS cells are restrained by TIMP1 silencing. AGS/DDP cells were transfected with/without si-NC or si-TIMP1; parental AGS cells were defined as the positive control. The protein and mRNA expression levels of TIMP1 were examined by (A) Western blot and (B) RT-PCR after the transfection of si-NC, si-TIMP1#1, si-TIMP1#2, and si-TIMP1#3; si-RNA with the highest silencing effect on TIMP1 expression was chosen for further analysis. After treating with DDP for 24, 48, and 72 h, (C) cell viability in different groups was evaluated by the CCK-8 assay. (D) The self-renewal of cells was determined by the sphere formation assay. (E) Western blot detected the expression levels of stem cell markers in cells. ** p<0.01 and *** p<0.001 vs. the AGS group; ## p<0.01 and ### p<0.001 vs. the AGS/DDP group.
IC50 values of each group of cells at different culture times (μg/mL).
| Time(h) Cell line | 24 | 48 | 72 |
|---|---|---|---|
| AGS | 5.37 ± 0.40 | 1.60 ± 0.28 | 0.61 ± 0.06 |
| AGS/DDP | 17.22 ± 1.79 | 6.00 ± 0.51 | 3.05 ± 0.25 |
| AGS/DDP + si-NC | 18.36 ± 2.01 | 6.72 ± 1.61 | 3.30 ± 0.73 |
| AGS/DPP + si-TIMP1 | 6.06 ± 1.23 | 2.10 ± 0.56 | 0.79 ± 0.17 |
| p1 value | <0.001 | <0.001 | <0.001 |
| p2 value | <0.001 | <0.001 | <0.001 |
-
p1, AGS vs. AGS/DDP; p2, AGS/DDP vs. AGS/DPP + si-TIMP1.
Sphere formation assay showed that AGS/DDP cells formed approximately 2.57-fold (p=0.002) greater numbers of spheres per field than AGS cells (Figure 2D), suggesting a stronger stemness in AGS/DDP cells. Western blot detecting stem cell markers further confirmed this point, which showed that CD44, CD47, Nanog, and Oct4 expression levels in AGS/DDP cells were obviously elevated compared with those in AGS cells (all p<0.001) (Figure 2E). Moreover, TIMP1 silencing significantly restrained the stem properties in AGS/DDP cells (Figure 2D and E), which signposted that the stemness of AGS/DDP cells was mediated by TIMP1. The numbers of formed spheres from the AGS/DDP + si-TIMP1 group were markedly less than those from the AGS/DDP group (p=0.003) (Figure 2D). Additionally, the expression levels of CD44, CD47, Nanog, and Oct4 in AGS/DDP cells were significantly decreased after transfecting with si-TIMP1 (all p<0.001) (Figure 2E).
TIMP1 is involved in migration, invasion, EMT process, and Wnt/β-catenin signaling pathway in DDP-resistant AGS cells
We further evaluated cell migration and invasive capacities in the AGS cell line and its DDP-resistant counterpart (AGS/DDP). Results revealed that both the migration and invasive abilities of AGS/DDP cells were markedly stronger than those of AGS cells (all p<0.001), which were significantly restrained by knocking down TIMP1 (p<0.001) (Figure 3A and B). As known, EMT activation frequently occurred in drug resistance, stemness, and invasiveness of cancer cells [27]. Compared with AGS cells, E-cadherin upregulation and N-cadherin downregulation (both p<0.001) were observed in AGS/DDP cells (Figure 3C), which suggested EMT activation occurred in AGS/DDP cells. Then, our study further examined whether TIMP1 participates in the EMT process in AGS/DDP cells. Si-TIMP1 transfection suppressed the epithelial converse to mesenchymal phenotype in AGS/DDP cells, as evidenced by the reduction of E-cadherin (p<0.001) and elevation of N-cadherin (p<0.001) (Figure 3C).
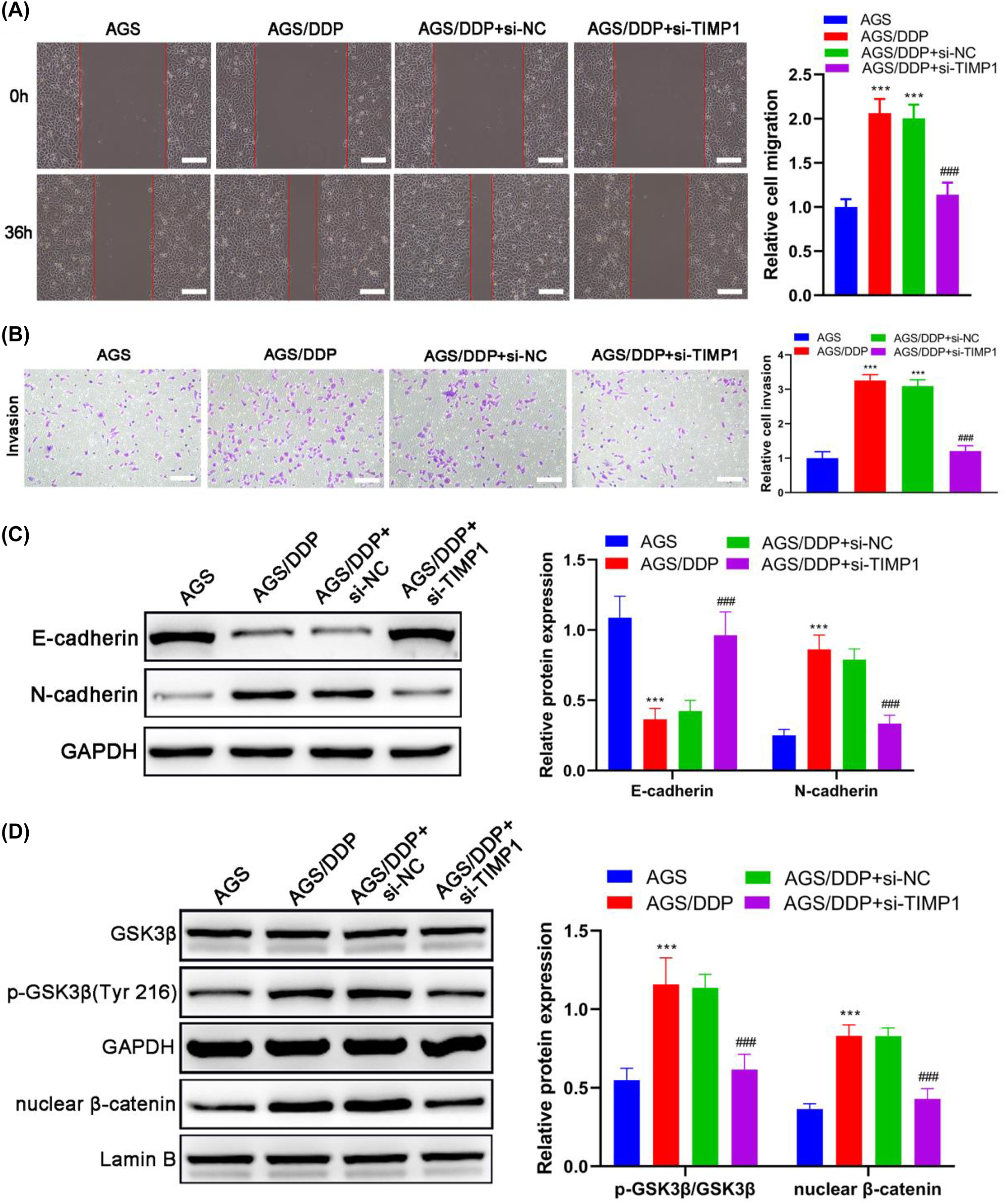
TIMP1 is involved in the migration, invasion, and EMT of DDP-resistant GC cells. AGS/DDP cells were transfected with si-NC or si-TIMP1; AGS/DDP cells without transfection were considered the control, whereas parental AGS cells were defined as the positive control. (A) The wound healing assay evaluated cell migration; scale bar=100 μm. (B) The transwell assay detected cell invasion; scale bar=100 μm. (C) The expression of EMT-related markers was detected by Western blot. (D) The expression and phosphorylation levels of GSK3β and expression of nuclear β-catenin were detected by Western blot. *** p<0.001 vs. the AGS group; ### p<0.001 vs. the AGS/DDP group.
To further explore the mechanisms involved in the effect of TIMP1 on DDP-resistant GC, the expressions of GSK3β and β-catenin were analyzed by Western blot. The phosphorylation level of GSK3β and the accumulation of nuclear β-catenin were significantly increased in AGS/DDP cells compared with AGS cells (both p<0.001) (Figure 3D). In the meantime, the transfection of si-TIMP1 led to a significant decrease in GSK3β phosphorylation and nuclear accumulation of β-catenin in AGS/DDP (both p<0.001) (Figure 3D), which suggested that TIMP1 contributes to activating Wnt/β-catenin in DDP-resistant GC. Taken together, TIMP1 knockdown impaired the cell migration and invasion capacities and blocked the EMT process in AGS/DDP cells, which might relies on the repression of Wnt/β-catenin signaling.
TIMP1 contributes to the malignant behaviors and EMT of DDP-resistant AGS cells via Wnt/β-catenin signaling
To determine whether the effect of TIMP1 on the malignant behaviors and EMT of DDP-resistant AGS cells relies on its role in Wnt/β-catenin signaling, AGS/DDP cells were treated with 2.5 μM AR-A014418 (a selective GSK-3 inhibitor) after being transfected with TIMP1 overexpression vector (TIMP1 OE). The result of the Western blot confirmed that TIMP1 OE transfection successfully overexpressed TIMP1 in AGS/DDP cells (Figure 4A). As expected, overexpressing TIMP1 led to aggravation in the malignant phenotypes of AGS/DDP cells (Figure 4B–F), which is revealed by the significant elevation in the IC50 value for DDP (17.48–28.54 μg/mL), numbers of formed spheres, stemness-related marker expression, and capacities of migration and invasion. In the meantime, E-cadherin expression was markedly reduced, and N-cadherin expression was dramatically increased in TIMP1-overexpressing AGS/DDP cells (Figure 4G), revealing that TIMP1 promotes the EMT process of AGS/DDP cells. After treatment with AR-A014418, the effects induced by TIMP1 OE transfection on the malignant behaviors and EMT of AGS/DDP cells were almost blocked (Figure 4B–G), which demonstrated that TIMP1 contributes to the malignant behaviors and EMT of DDP-resistant AGS cells by suppressing Wnt/β-catenin signaling.
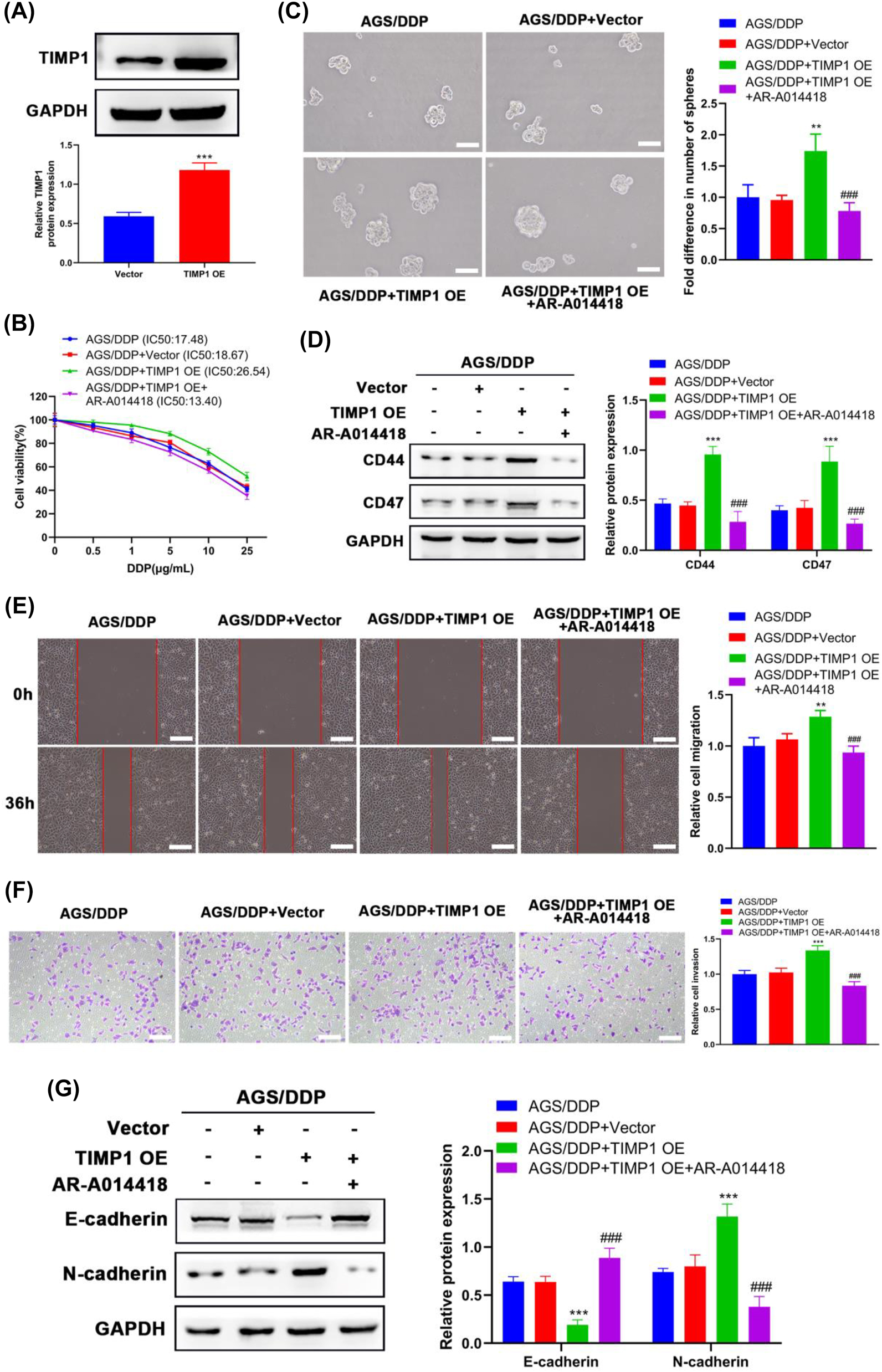
TIMP1 regulated the Wnt/β-catenin signaling pathway in DDP-resistant GC cells. After being transfected with a vector or TIMP1 OE, AGS/DDP cells were treated with or without 2.5 μM AR-A014418 (a selective GSK-3 inhibitor). (A) The protein expression levels of TIMP1 were examined by Western blot after the transfection of a vector or TIMP1 OE. (B) Cell viability in different groups was evaluated by the CCK-8 assay after treatment with DDP for 24 h. (C) The self-renewal of cells was determined by the sphere formation assay. (D) Western blot detected the expression levels of stem cell markers in cells. (E) The wound healing assay evaluated cell migration; scale bar=100 μm. (F) The transwell assay detected cell invasion; scale bar=100 μm. (G) The EMT-related marker expression was detected by Western blot. ** p<0.01 and *** p<0.001 vs. the AGS/DDP group; ### p<0.001 vs. the AGS/DDP + TIMP1 OE group.
Discussion
Despite considerable progress in the development of chemotherapy for patients with advanced GC, acquired resistance remains a major obstacle during therapy [28]. Considering that DDP is the most important chemotherapeutic agent widely used in GC, identifying mechanisms of DDP resistance and developing strategies to overcome resistance is significant for patients with advanced GC. Previous clinical reports have revealed that TIMP1 expression is significantly increased in both clinical serum and tissue samples in GC [29, 30]. The expression of TIMP1 exhibited a positive correlation with the poor outcome of patients with GC [31]. In agreement with the abovementioned reports, bioinformatics analysis based on public data in our study showed that TIMP1 is highly expressed and closely related to tumor stage and poor survival in GC. Moreover, TIMP1 upregulation enhanced not only cell migration, invasion, and proliferation but also tumorigenesis in GC [32, 33], hinting that TIMP1 might be a potential target for GC therapy. TIMP1 has also been considered a chemoresistant factor in colorectal [15], ovarian [14], and breast [13] cancers. Hence, this study attempted to uncover the role of TIMP1 in DDP-resistant GC.
In this study, two DDP-resistant GC cell lines were constructed. Our data showed that the mRNA expression levels of TMIP1 in DDP-resistant GC cells were observably higher than those in parental ones. To determine whether TIMP1 is a mediator of the malignant behaviors and EMT of DDP-resistant GC cells, this study transfected siRNAs against TIMP1 into DDP-resistant AGS (AGS/DDP) cells. The CCK-8 assay revealed that the resistance against DDP in AGS/DDP cells was abrogated after TIMP1 silencing.
A plethora of evidence supports the hypothesis that cancer stem cells could be a potential mechanism that causes chemotherapy failure and tumor relapse [34]. Assays for distinctive stem cell properties, such as sphere-forming assay and stem cell marker detection, revealed that the self-renewal ability of AGS/DDP cells was significantly stronger than that of AGS cells, whereas si-TIMP1 transfection reversed this trend to some extent and TIMP1 OE transfection significantly aggravated this trend. Moreover, our data showed that both the migration and invasive abilities of AGS/DDP cells were strongly strengthened after TIMP1 OE transfection but undermined after si-TIMP1 transfection. All these data suggested the involvement of TIMP1 in regulating DDP resistance, stemness, migration, and invasion of AGS/DDP cells.
EMT has been considered a critical regulator of the cancer stem cell phenotype, which is closely linked with the increased migration and invasion capacities of cancer cells [27]. More importantly, EMT has also been implicated in DDP resistance in cancer [35]. In our study, compared with AGS cells, EMT activation was observed in AGS/DDP cells, which was suppressed by si-TIMP1 and further enhanced by TIMP1 OE. This means that the functions of TIMP1 in regulating DDP resistance, stemness, migration, and invasion of AGS/DDP cells were probably attributed to its regulatory role in EMT.
Despite the lack of a clear mechanism underlying the DDP resistance of GC, the Wnt/β-catenin signaling pathway is essential for tumor initiation, tumor growth, metastasis, and chemoresistance in GC [36]. As a key molecule in this pathway, β-catenin acts as a transcriptional regulator after translocating to the nucleus. In inactive situations, β-catenin is rendered inactive because of its phosphorylation by GSK3. Phosphorylated GSK3β at Ser9 sites led to GSK3 activity suppression, rendering to the accumulation of β-catenin, thereby activating the transcription of genes that regulate malignant behaviors of cancer cells [37]. In this study, GSK3β phosphorylation and nuclear β-catenin levels were significantly increased in AGS/DDP cells, suggesting that Wnt/β-catenin signaling has been activated in DDP-resistant cells. Moreover, knocking down TIMP1 could block the activation in AGS/DDP cells; thus, we can suppose that TIMP1 abnormal expression modulates the malignant behaviors and EMT of AGS/DDP cells via the Wnt/β-catenin signaling. More importantly, our data showed that the role of TIMP1 in the malignant behaviors and EMT of AGS/DDP cells was nearly blocked after suppressing the Wnt/β-catenin signaling by a selective GSK-3 inhibitor, confirming that TIMP1 confers AGS/DDP cells to malignant behaviors and EMT by activating Wnt/β-catenin signaling. A study limitation is a lack of in vivo functional experiments. Further studies in vivo DDP-resistant model are needed to validate the functions and mechanism of TIMP1 in GC.
Conclusions
Collectively, our findings demonstrated that TIMP1 contributes to DDP resistance, stemness, migration, invasion, and EMT in DDP-resistant GC cells through Wnt/β-catenin signaling activation, which might provide scientific information for the potential use of TIMP1 in the treatment of patients with advanced stage and DDP resistance.
-
Research funding: None declared.
-
Author contributions: Study conception and design: Feng Zhu; data collection: Feng Zhu, Xiaogu He, Fen Shuang, Xiangming Fang, Jinxia Jiang; analysis and interpretation of results: Feng Zhu, Jinxia Jiang; draft manuscript preparation: Jinxia Jiang. All authors reviewed the results and approved the final version of the manuscript.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Not applicable.
-
Ethical approval: The local Institutional Review Board deemed the study exempt from review.
References
1. Cao, W, Chen, HD, Yu, YW, Li, N, Chen, WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J 2021;134:783–91. https://doi.org/10.1097/cm9.0000000000001474.Search in Google Scholar PubMed PubMed Central
2. Michalke, B. Platinum speciation used for elucidating activation or inhibition of Pt-containing anti-cancer drugs. J Trace Elem Med Biol 2010;24:69–77. https://doi.org/10.1016/j.jtemb.2010.01.006.Search in Google Scholar PubMed
3. Tchounwou, PB, Dasari, S, Noubissi, FK, Ray, P, Kumar, S. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J Exp Pharmacol 2021;13:303–28. https://doi.org/10.2147/jep.s267383.Search in Google Scholar PubMed PubMed Central
4. Dagogo-Jack, I, Shaw, AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 2018;15:81–94. https://doi.org/10.1038/nrclinonc.2017.166.Search in Google Scholar PubMed
5. Bukowski, K, Kciuk, M, Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 2020;21:3233. https://doi.org/10.3390/ijms21093233.Search in Google Scholar PubMed PubMed Central
6. Mohammed, MK, Shao, C, Wang, J, Wei, Q, Wang, X, Collier, Z, et al.. Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis 2016;3:11–40. https://doi.org/10.1016/j.gendis.2015.12.004.Search in Google Scholar PubMed PubMed Central
7. Wei, Y, Shen, N, Wang, Z, Yang, G, Yi, B, Yang, N, et al.. Sorafenib sensitizes hepatocellular carcinoma cell to cisplatin via suppression of Wnt/β-catenin signaling. Mol Cell Biochem 2013;381:139–44. https://doi.org/10.1007/s11010-013-1695-6.Search in Google Scholar PubMed
8. Noda, T, Nagano, H, Takemasa, I, Yoshioka, S, Murakami, M, Wada, H, et al.. Activation of Wnt/beta-catenin signalling pathway induces chemoresistance to interferon-alpha/5-fluorouracil combination therapy for hepatocellular carcinoma. Br J Cancer 2009;100:1647–58. https://doi.org/10.1038/sj.bjc.6605064.Search in Google Scholar PubMed PubMed Central
9. Zhang, B, Yang, Y, Shi, X, Liao, W, Chen, M, Cheng, AS, et al.. Proton pump inhibitor pantoprazole abrogates adriamycin-resistant gastric cancer cell invasiveness via suppression of Akt/GSK-β/β-catenin signaling and epithelial-mesenchymal transition. Cancer Lett 2015;356:704–12. https://doi.org/10.1016/j.canlet.2014.10.016.Search in Google Scholar PubMed
10. Würtz, SO, Schrohl, AS, Mouridsen, H, Brünner, N. TIMP-1 as a tumor marker in breast cancer-an update. Acta Oncol 2008;47:580–90. https://doi.org/10.1080/02841860802022976.Search in Google Scholar PubMed
11. Oh, WK, Vargas, R, Jacobus, S, Leitzel, K, Regan, MM, Hamer, P, et al.. Elevated plasma tissue inhibitor of metalloproteinase-1 levels predict decreased survival in castration-resistant prostate cancer patients. Cancer 2011;117:517–25. https://doi.org/10.1002/cncr.25394.Search in Google Scholar PubMed
12. Porter, JF, Shen, S, Denhardt, DT. Tissue inhibitor of metalloproteinase-1 stimulates proliferation of human cancer cells by inhibiting a metalloproteinase. Br J Cancer 2004;90:463–70. https://doi.org/10.1038/sj.bjc.6601533.Search in Google Scholar PubMed PubMed Central
13. Hekmat, O, Munk, S, Fogh, L, Yadav, R, Francavilla, C, Horn, H, et al.. TIMP-1 increases expression and phosphorylation of proteins associated with drug resistance in breast cancer cells. J Proteome Res 2013;12:4136–51. https://doi.org/10.1021/pr400457u.Search in Google Scholar PubMed
14. Sonego, M, Poletto, E, Pivetta, E, Nicoloso, MS, Pellicani, R, Vinciguerra, GLR, et al.. TIMP-1 is overexpressed and secreted by platinum resistant epithelial ovarian cancer cells. Cells 2019;9:6. https://doi.org/10.3390/cells9010006.Search in Google Scholar PubMed PubMed Central
15. Sørensen, NM, Byström, P, Christensen, IJ, Berglund, A, Nielsen, HJ, Brünner, N, et al.. TIMP-1 is significantly associated with objective response and survival in metastatic colorectal cancer patients receiving combination of irinotecan, 5-fluorouracil, and folinic acid. Clin Cancer Res 2007;13:4117–22. https://doi.org/10.1158/1078-0432.ccr-07-0186.Search in Google Scholar
16. Egea, V, Zahler, S, Rieth, N, Neth, P, Popp, T, Kehe, K, et al.. Tissue inhibitor of metalloproteinase-1 (TIMP-1) regulates mesenchymal stem cells through let-7f microRNA and Wnt/β-catenin signaling. Proc Natl Acad Sci USA 2012;109:E309–16. https://doi.org/10.1073/pnas.1115083109.Search in Google Scholar PubMed PubMed Central
17. Grunnet, M, Mau-Sørensen, M, Brünner, N. Tissue inhibitor of metalloproteinase 1 (TIMP-1) as a biomarker in gastric cancer: a review. Scand J Gastroenterol 2013;48:899–905. https://doi.org/10.3109/00365521.2013.812235.Search in Google Scholar PubMed
18. Tang, Z, Li, C, Kang, B, Gao, G, Li, C, Zhang, Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 2017;45:W98–102. https://doi.org/10.1093/nar/gkx247.Search in Google Scholar PubMed PubMed Central
19. Wang, J, Ni, Z, Duan, Z, Wang, G, Li, F. Altered expression of hypoxia-inducible factor-1α (HIF-1α) and its regulatory genes in gastric cancer tissues. PLoS One 2014;9:e99835. https://doi.org/10.1371/journal.pone.0099835.Search in Google Scholar PubMed PubMed Central
20. Sun, MY, Xu, B, Wu, QX, Chen, WL, Cai, S, Zhang, H, et al.. Cisplatin-resistant gastric cancer cells promote the chemoresistance of cisplatin-sensitive cells via the exosomal RPS3-mediated PI3K-Akt-Cofilin-1 signaling axis. Front Cell Dev Biol 2021;9:618899. https://doi.org/10.3389/fcell.2021.618899.Search in Google Scholar PubMed PubMed Central
21. Livak, KJ, Schmittgen, TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402–8. https://doi.org/10.1006/meth.2001.1262.Search in Google Scholar PubMed
22. Mahmood, T, Yang, PC. Western blot: technique, theory, and trouble shooting. N Am J Med Sci 2012;4:429–34. https://doi.org/10.4103/1947-2714.100998.Search in Google Scholar PubMed PubMed Central
23. Hwang, BS, Jeong, YT, Lee, S, Jeong, EJ, Rho, JR. Densazalin, a new cytotoxic diazatricyclic alkaloid from the marine sponge haliclona densaspicula. Molecules 2021;26:3164. https://doi.org/10.3390/molecules26113164.Search in Google Scholar PubMed PubMed Central
24. Bahmad, HF, Cheaito, K, Chalhoub, RM, Hadadeh, O, Monzer, A, Ballout, F, et al.. Sphere-formation assay: three-dimensional in vitro culturing of prostate cancer stem/progenitor sphere-forming cells. Front Oncol 2018;8:347. https://doi.org/10.3389/fonc.2018.00347.Search in Google Scholar PubMed PubMed Central
25. Rodriguez, LG, Wu, X, Guan, JL. Wound-healing assay. Methods Mol Biol 2005;294:23–9. https://doi.org/10.1385/1-59259-860-9:023.10.1385/1-59259-860-9:023Search in Google Scholar PubMed
26. Marshall, J. Transwell(®) invasion assays. Methods Mol Biol 2011;769:97–110. https://doi.org/10.1007/978-1-61779-207-6_8.Search in Google Scholar PubMed
27. Shibue, T, Weinberg, RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 2017;14:611–29. https://doi.org/10.1038/nrclinonc.2017.44.Search in Google Scholar PubMed PubMed Central
28. Köberle, B, Tomicic, MT, Usanova, S, Kaina, B. Cisplatin resistance: preclinical findings and clinical implications. Biochim Biophys Acta 2010;1806:172–82. https://doi.org/10.1016/j.bbcan.2010.07.004.Search in Google Scholar PubMed
29. Mroczko, B, Lukaszewicz-Zajac, M, Groblewska, M, Czyzewska, J, Gryko, M, Guzińska-Ustymowicz, K, et al.. Expression of tissue inhibitors of metalloproteinase 1 (TIMP-1) in gastric cancer tissue. Folia Histochem Cytobiol 2009;47:511–6. https://doi.org/10.2478/v10042-009-0071-6.Search in Google Scholar PubMed
30. Wang, CS, Wu, TL, Tsao, KC, Sun, CF. Serum TIMP-1 in gastric cancer patients: a potential prognostic biomarker. Ann Clin Lab Sci 2006;36:23–30.Search in Google Scholar
31. Szász, AM, Lánczky, A, Nagy, Á, Förster, S, Hark, K, Green, JE, et al.. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget 2016;7:49322–33. https://doi.org/10.18632/oncotarget.10337.Search in Google Scholar PubMed PubMed Central
32. Liu, H, Xiang, Y, Zong, QB, Zhang, XY, Wang, ZW, Fang, SQ, et al.. miR-6745-TIMP1 axis inhibits cell growth and metastasis in gastric cancer. Aging 2021;13:24402–16. https://doi.org/10.18632/aging.203688.Search in Google Scholar PubMed PubMed Central
33. Omar, OM, Soutto, M, Bhat, NS, Bhat, AA, Lu, H, Chen, Z, et al.. TFF1 antagonizes TIMP-1 mediated proliferative functions in gastric cancer. Mol Carcinog 2018;57:1577–87. https://doi.org/10.1002/mc.22880.Search in Google Scholar PubMed
34. Toh, TB, Lim, JJ, Chow, EK. Epigenetics in cancer stem cells. Mol Cancer 2017;16:29. https://doi.org/10.1186/s12943-017-0596-9.Search in Google Scholar PubMed PubMed Central
35. Ashrafizadeh, M, Zarrabi, A, Hushmandi, K, Kalantari, M, Mohammadinejad, R, Javaheri, T, et al.. Association of the epithelial-mesenchymal transition (EMT) with cisplatin resistance. Int J Mol Sci 2020;21:4002. https://doi.org/10.3390/ijms21114002.Search in Google Scholar PubMed PubMed Central
36. Chiurillo, MA. Role of the Wnt/β-catenin pathway in gastric cancer: an in-depth literature review. World J Exp Med 2015;5:84–102. https://doi.org/10.5493/wjem.v5.i2.84.Search in Google Scholar PubMed PubMed Central
37. Gao, C, Xiao, G, Hu, J. Regulation of Wnt/β-catenin signaling by posttranslational modifications. Cell Biosci 2014;4:13. https://doi.org/10.1186/2045-3701-4-13.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Articles
- Breast cancer status, grading system, etiology, and challenges in Asia: an updated review
- The role of acetylation of histone H3 and H4 in oral squamous cell carcinoma
- Research Articles
- Temporal trends of cervical cancer between 1990 and 2019, in Asian countries by geographical region and socio-demographic index, and comparison with global data
- Human EVI2B acts as a Janus-faced oncogene/antioncogene by differently affecting as per cancer type neoplastic cells growth and immune infiltration
- Wnt/β-catenin signaling activation by TIMP1 confers cisplatin-resistant gastric cancer cells to malignant behaviors and epithelial–mesenchymal transition
- Hsa_circ_0079598 acts as a potential diagnostic and prognostic biomarker for gastric cancer
- The inhibitory function of GDF11/BMP11 in liver cancer by inducing apoptosis and ROS–JNK pathway
- Identification of cancer stem cells derived from U118MG and the involvement of LncRNA-DC and STAT3 in promoting their malignant transformation
Articles in the same Issue
- Frontmatter
- Review Articles
- Breast cancer status, grading system, etiology, and challenges in Asia: an updated review
- The role of acetylation of histone H3 and H4 in oral squamous cell carcinoma
- Research Articles
- Temporal trends of cervical cancer between 1990 and 2019, in Asian countries by geographical region and socio-demographic index, and comparison with global data
- Human EVI2B acts as a Janus-faced oncogene/antioncogene by differently affecting as per cancer type neoplastic cells growth and immune infiltration
- Wnt/β-catenin signaling activation by TIMP1 confers cisplatin-resistant gastric cancer cells to malignant behaviors and epithelial–mesenchymal transition
- Hsa_circ_0079598 acts as a potential diagnostic and prognostic biomarker for gastric cancer
- The inhibitory function of GDF11/BMP11 in liver cancer by inducing apoptosis and ROS–JNK pathway
- Identification of cancer stem cells derived from U118MG and the involvement of LncRNA-DC and STAT3 in promoting their malignant transformation

